Abstract
Background: Consumption of cocoa-derived polyphenols has been associated with several health benefits; however, their effects on the intestinal microbiome and related features of host intestinal health are not adequately understood.
Objective: The objective of this study was to determine the effects of eating flavanol-enriched cocoa powder on the composition of the gut microbiota, tissue metabolite profiles, and intestinal immune status.
Methods: Male pigs (5 mo old, 28 kg mean body weight) were supplemented with 0, 2.5, 10, or 20 g flavanol-enriched cocoa powder/d for 27 d. Metabolites in serum, urine, the proximal colon contents, liver, and adipose tissue; bacterial abundance in the intestinal contents and feces; and intestinal tissue gene expression of inflammatory markers and Toll-like receptors (TLRs) were then determined.
Results: O-methyl-epicatechin-glucuronide conjugates dose-dependently increased (P < 0.01) in the urine (35- to 204-fold), serum (6- to 186-fold), and adipose tissue (34- to 1144-fold) of pigs fed cocoa powder. The concentration of 3-hydroxyphenylpropionic acid isomers in urine decreased as the dose of cocoa powder fed to pigs increased (75–85%, P < 0.05). Compared with the unsupplemented pigs, the abundance of Lactobacillus species was greater in the feces (7-fold, P = 0.005) and that of Bifidobacterium species was greater in the proximal colon contents (9-fold, P = 0.01) in pigs fed only 20 or 10 g cocoa powder/d, respectively. Moreover, consumption of cocoa powder reduced TLR9 gene expression in ileal Peyer’s patches (67–80%, P < 0.05) and mesenteric lymph nodes (43–71%, P < 0.05) of pigs fed 2.5–20 g cocoa powder/d compared with pigs not supplemented with cocoa powder.
Conclusion: This study demonstrates that consumption of cocoa powder by pigs can contribute to gut health by enhancing the abundance of Lactobacillus and Bifidobacterium species and modulating markers of localized intestinal immunity.
Keywords: cocoa flavanols, porcine model, gut microbiota, metabolites, Toll-like receptors
Introduction
Cocoa is a dry, powdered, nonfat plant-derived product prepared from seeds of the Theobroma cacao tree and is considered a common ingredient in many food products, especially chocolate. The primary flavonoids in cocoa are the flavan-3-ols, epicatechin and catechin (monomeric units) and their polymers, and the proanthocyanidins or procyanidins. The concentration of bioactive components in cocoa varies depending on the variety and ripeness of the cocoa bean, the growing region, harvesting practices, and processing steps. Fresh and fermented cocoa beans contain ∼10% flavanols (100 mg/g), ∼3.6% cocoa powder, and ∼5% cocoa-rich dark chocolate (1). Because of the large variation in the flavanol content of chocolate and cocoa products, it is critical to determine the concentration of flavanols in the foods used to evaluate the health effects of cocoa flavonoids.
Meta-analysis of epidemiologic studies of humans fed cocoa products has shown improved cardiovascular health by reducing blood pressure (2) and maintaining normal endothelium-dependent vasodilatation (3). Short-term intake of cocoa can improve measures of oxidative stress and inflammation in obese adults (4). Moreover, consumption of cocoa-derived flavanols has a prebiotic effect that stimulates the growth of beneficial Bifidobacterium and Lactobacillus species while inhibiting potentially pathogenic Clostridium species in healthy humans (5).
The scientific evidence indicates that the beneficial effects of eating foods enriched with phytochemicals are more likely due to metabolites derived from the colon microbiota rather than to the high-molecular-mass compounds originally present in foods that are minimally absorbed by the intestine (6, 7). The colonic metabolism of polyphenols consists of biotransformation into lactones and simple phenolic acids with different-length side chains and hydroxylation patterns that are absorbed and eventually excreted in urine and feces. Recently, Choy et al. (8) demonstrated that phenolic metabolites and bacterial populations in pig feces can be changed by ingesting grape seed proanthocyanidins. However, the composition of the intestinal microbiota and related effects on host gastrointestinal health remain largely unknown.
It has been observed that circulating concentrations of some microbial-derived phenolics exceed those of the parent flavonoids in food after consumption of a flavonoid-rich diet (9). Thus, accurate estimation of the polyphenol content in the food and quantification of their metabolites in biological fluids after consumption are important for determining health benefits of a particular group of polyphenols. Appropriate analytical methods are based on solid-phase extraction and LC-MS/MS (10). NMR and MS-based metabolic profiling have also been used as complementary techniques to provide a comprehensive modeling of the metabolites associated with a biological response to daily consumption of polyphenol-enriched foods (11).
The gastrointestinal tract is continuously in contact with luminal bacteria and their products. Intestinal epithelial cells actively participate in innate immune defense in addition to their protective role as a physical barrier (12). Toll-like receptors (TLRs)9 are crucial components of innate immunity that recognize signature pathogen-associated microbial compounds such as lipoteichoic acids (TLR2), LPS (TLR4), flagellin (TLR5), and nucleic acids (TLR3, TLR7, TLR8, TLR9) (12, 13). After recognition of microbial patterns, TLRs trigger intracellular signaling pathways that result in the induction of inflammatory cytokines, type I interferon, and chemokines (14). The mechanisms by which TLR signaling is regulated in the intestinal mucosa include differential gene expression of TLRs and their cellular localization and positioning within the tissue so that responses against pathogens are favored, whereas responses to commensals are dampened to maintain tissue homeostasis (15). Many clinical studies have used expression of TLRs to evaluate host immune response as a measure of disease severity (16–18).
The pig is considered a useful in vivo model of human food consumption and metabolism because of similarities between the physiology and microbial composition of the gastrointestinal tract. The pig cecum has been used in studies targeting delivery of metabolites to the colon and has been shown to be suitable for studies of the metabolism of several classes of flavonoids (19–21). Similarly, pig urinary metabolomic studies have detected several metabolites commonly found in humans (22, 23). In the present study, pig diets were supplemented with cocoa powder at a comparable amount as reported in humans (5, 24) to determine its effect on changes in the composition of the gut microbiome, metabolites, and intestinal immune status.
Methods
Cocoa powder.
Acticoa is a high-flavanol cocoa powder that was used in the study and was kindly provided by Barry-Callebaut. Detection of major phenolic compounds was performed as described in the Supplemental Methods.
Animals and diets.
All animal experiments and procedures were conducted in accordance with guidelines established and approved by the Beltsville Area Animal Care and Use Committee. Twenty-four 5-mo-old male pigs were obtained from the experimental farm at the Beltsville Agricultural Research Center in Beltsville, Maryland. Pigs were randomly allocated by weight into 4 groups of 6 animals. Cocoa powder was provided to pigs every morning by mixing with a standard corn- and soy-based diet prepared on site for 27 d (Supplemental Table 1). Pigs were fed 2070 kcal/d supplemented with cocoa powder and/or dextrin and cellulose so that all pigs had the same intake of fiber (Supplemental Table 1). The 0-, 2.5-, 10-, and 20-g dose of cocoa powder contained 0, 51, 205, and 410 mg flavanols, respectively.
Sample collection.
Fecal samples were collected before the start of the study (day 0) and after 27 d of feeding cocoa powder. All other samples, including serum, urine, liver, visceral adipose tissue, and intestinal tissue and contents, were collected at the necropsy after 27 d of feeding cocoa powder. Pigs were killed via intravenous injection with Euthasol (50 mg sodium pentobarbital/kg of body weight) (Virbac Animal Health, Inc.). Intestinal contents were collected from the cecum and the proximal and distal colon for analysis of microbial composition. Intestinal tissue sections from the proximal colon, distal colon, ileal Peyer’s patches (ILPPs), and mesenteric lymph nodes (MLNs) were collected for gene expression of selected immune markers. Serum, urine, liver, visceral adipose tissue, and proximal colon contents were collected for metabolite analysis. All samples were initially frozen in liquid nitrogen and then kept at −80°C until further analysis.
Metabolite analysis.
The phenolic metabolites in biofluids and tissues were analyzed by using ultra-HPLC tandem with high-resolution accurate MS (Supplemental Table 2). Tissues (liver and visceral adipose) and proximal colon contents were lyophilized, ground into powder, solubilized in methanol, and centrifuged. Serum was mixed with methanol to precipitate proteins and centrifuged. Urine was diluted with an equal volume of water. All samples for metabolite analysis were filtered with a 0.45-μm polytetrafluoroethylene filter (Thermo Fisher Scientific) into a 2-mL HPLC vial. Then, 2 μL was injected into ultra-HPLC tandem with high-resolution accurate MS, the conditions of which are described in the Supplemental Methods.
Microbiome analysis.
Bacterial abundance was measured by real-time PCR with the use of species-specific primers and probes. DNA from fecal and intestinal contents was isolated by using the QIAamp DNA stool kit (Qiagen) according to the manufacturer’s instructions (25). DNA concentration was determined by NanoDrop (Thermo Fisher Scientific). Briefly, 40 ng fecal DNA/sample was used as a template for real-time PCR amplification by using primers and probes that differentially amplify variable regions within the 16S ribosomal DNA specific for Eubacteria (26), Bifidobacterium species (27), and Lactobacillus species from the casei (28) and non-casei subgroups (27). The CT values generated for different bacterial species were compared with CT values from a standard curve constructed by using a series of dilutions of purified target gene fragments to determine the target gene copy numbers. The values were expressed as log10 target gene copy number/g of sample (29). A fixed amount of a synthetically designed plasmid pUC57-Kan containing a 219-bp snake venom fragment was mixed into samples and used as a positive control for efficiency of DNA extraction. All molecular assays were performed by duplicates in the 7500-ABI PRISM (PerkinElmer). Mean copy numbers of bacterial species in feces and intestinal contents were calculated and compared among treatment groups. Change in bacterial abundance in feces after consumption of cocoa powder was calculated by comparing copy numbers/g of bacterial species before and after feeding cocoa powder.
RNA extraction, cDNA synthesis, and real-time PCR analysis.
Frozen (−80°C) 1-mm3 tissue sections of proximal colon, distal colon, ILPPs, and MLNs were rapidly homogenized in Trizol (Invitrogen). The RNA extraction and cDNA synthesis were performed as previously described (30). The sequence of probes and primers and running conditions of real-time PCR were obtained from the DGIL Porcine Translational Research database (31). Real-time PCR was performed by using 15 ng cDNA/well in 15 μL on an ABI-7900 sequence detector system (Applied Biosystems). Data for gene expression were normalized to the housekeeping gene RPL32 and expressed as ΔCT and a fold change compared with the unsupplemented group of pigs, which was designated as 1 fold change. The fold change was calculated by using the mean difference of the treatment group.
Statistical analysis.
For metabolite and microbiota variables, data were statistically analyzed by fitting a negative binomial analysis of variance with log (32) by using SAS PROC GLIMMIX to accurately model the typical increase in variance with the increased amount of metabolite or microbiota content. For gene expression data, within-dose variance of observed ΔCT values was homogeneous across doses, so a normal-distribution ANOVA model was fit. Pairwise t test means comparisons among doses were conducted only when the ANOVA indicated a significant dose effect (α = 0.05), to protect against false positives.
Results
Cocoa polyphenols.
The major phenolic compounds in cocoa powder were identified as theobromine, caffeine, purine alkaloids, catechin, epicatechin, B-type proanthocyanidins, dimers, trimers, tetramers, and pentamers (Table 1). The total flavanol concentration of the cocoa powder was 20.5 mg/g.
TABLE 1.
Concentrations of the major phenolic compounds in Acticoa
| Compound of cocoa powder | Concentration, mg/g |
| Theobromine | 21.6 |
| Caffeine | 3.84 |
| Unknown purine alkaloids | 1.65 |
| (+) Catechin | 3.51 |
| Epicatechin | 7.11 |
| Procyanidin B2 | 0.51 |
| Procyanidin C1 | 4.81 |
| Other B-type dimer | 2.92 |
| Other B-type trimer | 0.53 |
| Other B-type tetramer | 0.84 |
| Other B-type pentamer | 0.29 |
| Quercetin 3-O-pentoside | 0.27 |
| Total flavanols | 20.5 |
Body weights.
Body weight was measured on days 0 and 27. There were no differences among the 4 treatment groups (data not shown).
Detection of phase II and colonic microbial metabolites of cocoa-derived flavanols.
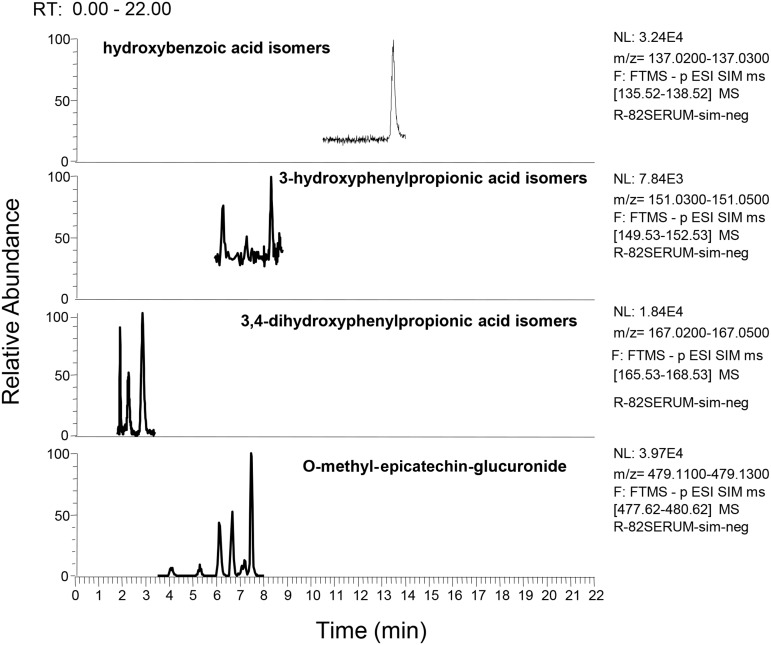
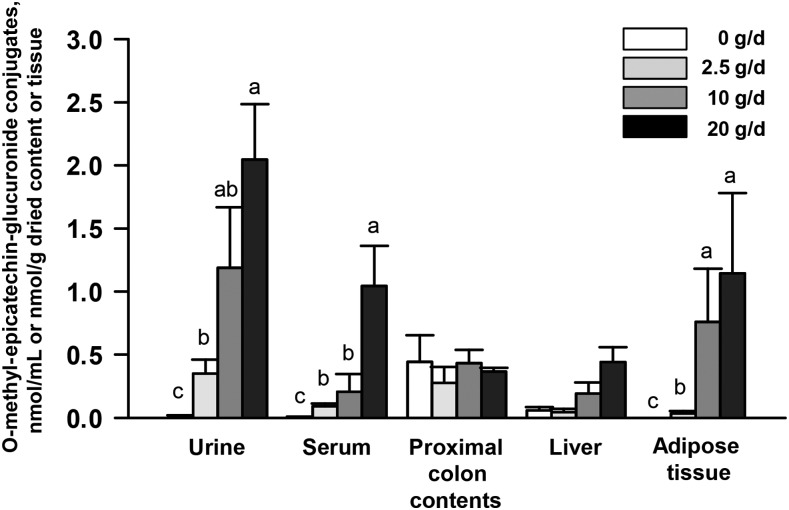
Products of microbial metabolism that included phenylpropionic acids, phenylacetic acids, benzoic acids, conjugated benzoic acids, cinnamic acids, and phase II metabolites were detected in serum (Figure 1 and Table 2), urine (Supplemental Table 3), liver (Supplemental Table 4), proximal colon contents (Supplemental Table 5), and visceral adipose tissue (Supplemental Table 6) of pigs fed 0–20 g cocoa powder/d. The concentration of a few metabolites significantly increased or decreased as the dose of cocoa powder fed to pigs increased as described below. 3,4-Dihydroxyphenylpropionic acid was 95% lower in the serum of pigs fed 10 g cocoa powder/d than in the unsupplemented group (P = 0.04) (Table 2). Compared with the unsupplemented group, 3-hydroxyphenylpropionic acid isomers (85% decrease, P = 0.001), phenylacetic isomers (87% decrease, P = 0.02), 3,4-dihydroxyphenylacetic acid isomers (68% decrease, P = 0.002), and hydroxybenzoic acid isomers (82% decrease, P = 0.02) decreased in the urine of pigs fed 20 g cocoa powder/d (Supplemental Table 3). Several phase II metabolites increased in serum (Table 2), urine (Supplemental Table 3), liver (Supplemental Table 4), and visceral adipose tissue (Supplemental Table 6). For example, consumption of 20 g cocoa powder/d increased dihydropxyphenyl-γ-valerolactone-O-sulfate conjugates, epicatechin-O-sulfate conjugates, O-methyl-epicatechin-sulfate conjugates, and epicatechin-O-glucuronide conjugates in urine (Supplemental Table 3) and epicatechin-O-glucuronide conjugates in the liver (Supplemental Table 4). Notably, we found significant increases in O-methyl-epicatechin-glucuronide conjugates consistently in serum, urine, and visceral adipose tissue (Figure 2 and Supplemental Figure 1). No significant changes of metabolites in proximal colon contents were detected for any cocoa-fed groups (Supplemental Table 5). Detection of some of these metabolites in pigs fed no cocoa powder could be derived from proanthocyanidins found in soybean, which was a major component of the pig diet (Supplemental Table 1) (33).
FIGURE 1.
UHPLC-HRAM trace chromatograms of colonic microbial and phase II metabolites of cocoa flavanols in serum of male pigs fed 0–20 g cocoa powder/d for 27 d. Hydroxybenzoic acid isomers, 3-hydroxyphenylpropionic acid isomers, 3,4-dihydroxyphenylpropionic acid isomers, and O-methyl-epicatechin-glucuronide were detected in serum of pigs fed 0–20 g cocoa powder/d by using UHPLC-HRAM on a Q-Exactive high-resolution mass spectrometer. F, function; FTMS, fourier transform mass spectrometry; NL, normalized level; p ESI SIM, positive mode electrospray ionization selective ion monitoring; RT, retention time; UHPLC-HRAM, ultra-HPLC tandem with high-resolution accurate MS.
TABLE 2.
Concentrations of colonic microbial and phase II metabolites of cocoa flavanols in serum of male pigs fed 0–20 g cocoa powder/d for 27 d1
| Concentration |
||||
| Metabolite | 0 g/d | 2.5 g/d | 10 g/d | 20 g/d |
| Phenylpropionic acids, nmol/mL | ||||
| 3-Hydroxyphenylpropionic acid isomers | 2.46 ± 1.34 (0.57–4.51) | 4.28 ± 2.33 (0.54–4.08) | 1.33 ± 0.72 (0–2.95) | 0.99 ± 0.54 (0–3.28) |
| 3,4-Dihydroxyphenylpropionic acid | 3.31 ± 3.04a (0.57–4.51) | 2.00 ± 1.83a,b (0–6.27) | 0.18 ± 0.16b (0–1.05) | 0.80 ± 0.74a,b (0–2.16) |
| Phenylacetic acids, pmol/mL | ||||
| Phenylacetic acid isomers | 15.3 ± 22.5 (0–55.9) | 0 | 7.61 ± 11.2 (0–45.6) | 3.76 ± 5.56 (0–15.9) |
| 3-Hydroxyphenylacetic acid isomers | 25.2 ± 22.3 (0–62.5) | 40.2 ± 35.6 (0–78.9) | 14.7 ± 13.1 (0–50.6) | 22.2 ± 19.7 (0–64) |
| 3,4-Dihydroxyphenylacetic acid | 163 ± 82.3 (59.9–386) | 95.2 ± 48.1 (0–259) | 68.2 ± 34.5 (0–120) | 87.7 ± 44.4 (0–179) |
| Benzoic acid derivatives, pmol/mL | ||||
| Hydroxybenzoic acid isomers | 0 | 0 | 1.17 ± 1.5 (0–7.05) | 5.80 ± 7.3 (0–22.5) |
| Vanillic acid | 3.37 ± 2.1 (0–10.5) | 9.18 ± 5.5 (0–6.8) | 2.61 ± 1.6 (0–6.5) | 7.24 ± 4.3 (0–14.5) |
| Phase II metabolites, pmol/mL | ||||
| Epicatechin-O-sulfate conjugates | 0 | 1.39 ± 1.44 (0–8.3) | 7.30 ± 7.23 (0–34.4) | 7.12 ± 7.06 (0–25) |
| Epicatechin-O-glucuronide conjugates | 0b | 5.09 ± 7.64a,b (0–30.5) | 446 ± 664a (0–1740) | 0b |
| O-methyl-epicatechin-glucuronide conjugates | 4.70 ± 3.34c (0–14.4) | 91.9 ± 63.3b (19.2–148) | 206 ± 141b (0–878) | 1040 ± 718a (0–2000) |
Values are means ± SEMs (ranges), n = 6. Labeled means without a common superscript letter differ, P < 0.05.
FIGURE 2.
O-methyl-epicatechin-glucuronide conjugates in tissues and fluids from male pigs fed 0–20 g cocoa powder/d for 27 d. O-methyl-epicatechin-glucuronide conjugates were measured by UHPLC-HRAM on a Q-Exactive high-resolution mass spectrometer. Values are means ± SEMs, n = 6. Labeled means without a common letter differ, P < 0.05. UHPLC-HRAM, ultra-HPLC tandem with high-resolution accurate MS.
Microbiota analysis in feces and large intestine contents.
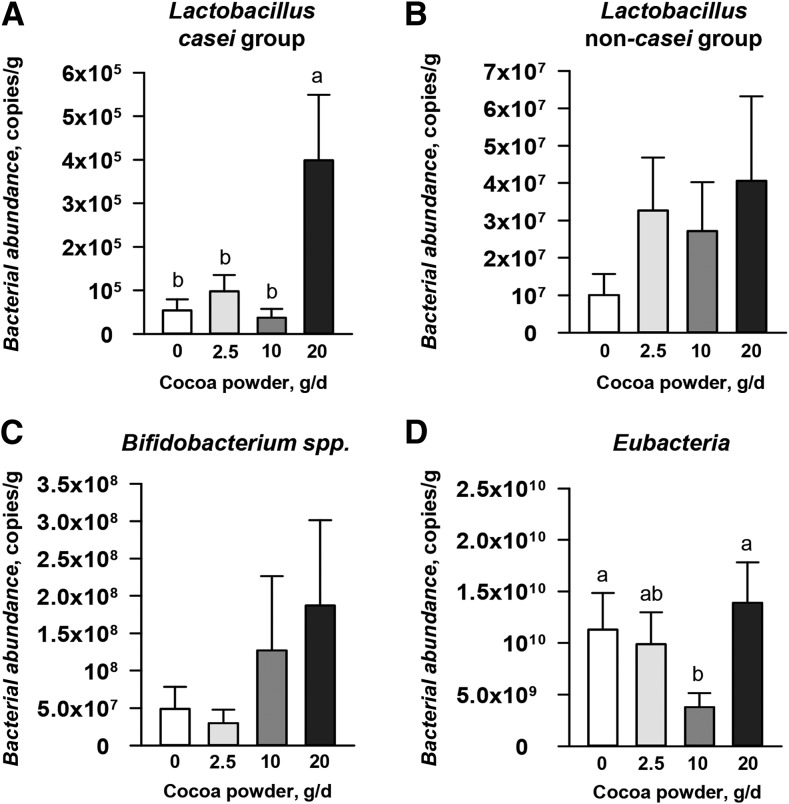
Consumption of 20 g cocoa powder/d induced a significant increase (700% increase, P = 0.005) in Lactobacillus species (casei group) abundance in the feces compared with the unsupplemented group (Figure 3A). No significant changes in abundance for Lactobacillus species (non-casei group) (Figure 3B) or Bifidobacterium species (Figure 3C) were detected for any cocoa powder–fed group compared with controls. Eubacteria in the group fed 10 g cocoa powder/d decreased (66% decrease, P = 0.04) compared with the unsupplemented group (Figure 3D). The abundance of microbiota in each animal is also demonstrated in Supplemental Figure 2 by using dot plot graphs.
FIGURE 3.
Bacterial abundance in feces of male pigs fed 0–20 g cocoa powder/d for 27 d. Bacterial abundance of feces was measured by real-time PCR with the use of species-specific probes. Lactobacillus casei group (A), Lactobacillus non-casei group (B), Bifidobacterium species (C), and Eubacteria (D). Values are means ± SEMs, n = 6. Labeled means without a common letter differ, P < 0.05.
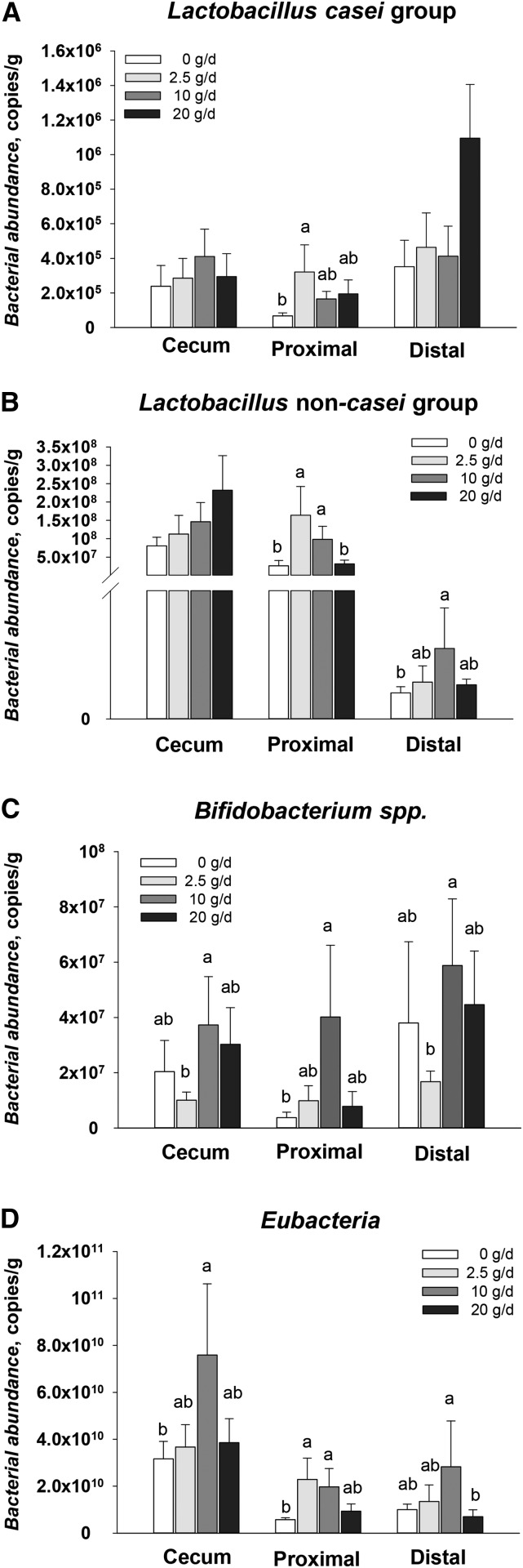
Bacterial abundance in the cecum, proximal colon, and distal colon contents collected after 27 d of feeding cocoa powder was also analyzed. Consumption of 2.5 g cocoa powder/d induced an increase (470% increase, P = 0.01) of Lactobacillus species (casei group) abundance in proximal colon contents (Figure 4A). Non-casei Lactobacillus species were more abundant in proximal colon contents (620% increase, P = 0.002) of pigs fed 2.5 g cocoa powder/d and distal colon contents (270% increase, P = 0.03) of pigs fed 10 g cocoa powder/d (Figure 4B) than the unsupplemented group. Compared with the unsupplemented group, consumption of 10 g cocoa powder/d significantly increased Bifidobacterium species in proximal colon contents (890% increase, P = 0.01) and Eubacteria species in the cecum (230% increase, P = 0.04) and proximal colon contents (340% increase, P = 0.02) (Figure 4C and 4D).
FIGURE 4.
Bacterial abundance in large intestine contents of male pigs fed 0–20 g cocoa powder/d for 27 d. Bacterial abundance of intestinal contents from the cecum, proximal colon, and distal colon was measured by real-time PCR with the use of species-specific probes. Lactobacillus casei group (A), Lactobacillus non-casei group (B), Bifidobacterium species (C), and Eubacteria (D) abundance levels are shown. Values are means ± SEMs, n = 6. Labeled means without a common letter differ, P < 0.05.
Gene expression of intestinal tissues.
Decreased tumor necrosis factor-α (TNF-α) expression was found in pigs fed 2.5 g (70% decrease, P = 0.003) and 10 g cocoa powder/d (60% decrease, P = 0.006) in ILPPs and 2.5 g cocoa powder/d (30% decrease, P = 0.01) in MLNs compared with the control group (Table 3). The expression of TLR2 was reduced >70% in ILPPs of pigs fed the 2.5-g/d (P = 0.02) and 10-g/d (P = 0.04) doses and in MLNs of pigs fed the 2.5-g/d dose (80% decrease, P = 0.02) (Table 3). The consumption of 10 g cocoa/d reduced (60% decrease, P = 0.04) TLR4 gene expression in the proximal colon (Table 3) compared with the unsupplemented pigs. TLR9 expression was significantly reduced in ILPPs and MLNs of pigs fed all 3 doses of cocoa powder and in the proximal colon of pigs fed 2.5 g and 10 g cocoa powder/d (Table 3) compared with unsupplemented pigs. The mRNA expression of other inflammatory genes such as IL-1β and IL-6 was not significantly different in all intestinal tissues measured (data not shown).
TABLE 3.
TNF-α and TLR mRNA expression in intestinal tissues of male pigs fed 0–20 g cocoa powder/d for 27 d1
| 0 g/d |
2.5 g/d |
10 g/d |
20 g/d |
|||||
| Gene | ΔCT | Fold of 0 g/d | ΔCT | Fold of 0 g/d | ΔCT | Fold of 0 g/d | ΔCT | Fold of 0 g/d |
| TNF-α | ||||||||
| ILPPs | 12.3 ± 0.4c | 1.0 | 14.0 ± 0.4a | 0.3 | 13.9 ± 0.4a,b | 0.4 | 12.8 ± 0.4b,c | 0.7 |
| Proximal colon | 12.1 ± 0.4 | 1.0 | 12.8 ± 0.4 | 0.6 | 13.0 ± 0.3 | 0.5 | 12.8 ± 0.4 | 0.6 |
| Distal colon | 12.4 ± 0.6 | 1.0 | 13.0 ± 0.6 | 0.6 | 13.4 ± 0.6 | 0.5 | 13.1 ± 1.28 | 0.6 |
| MLNs | 11.0 ± 0.4b | 1.0 | 12.4 ± 0.4a | 0.7 | 11.8 ± 0.3a,b | 4.0 | 12.0 ± 0.3a,b | 1.9 |
| TLR2 | ||||||||
| ILPPs | 12.3 ± 0.6b | 1.0 | 14.3 ± 0.6a | 0.2 | 14.1 ± 0.6a | 0.3 | 13.2 ± 0.6a,b | 0.5 |
| Proximal colon | 10.6 ± 0.5 | 1.0 | 10.5 ± 0.5 | 1.1 | 11.1 ± 0.5 | 0.7 | 10.5 ± 0.5 | 1.1 |
| Distal colon | 10.9 ± 0.7 | 1.0 | 12.1 ± 0.7 | 0.7 | 11.5 ± 0.7 | 0.6 | 11.3 ± 0.7 | 0.9 |
| MLNs | 10.6 ± 0.6b | 1.0 | 12.6 ± 0.6a | 0.2 | 11.1 ± 0.5a,b | 0.7 | 11.7 ± 0.5a,b | 0.4 |
| TLR4 | ||||||||
| ILPPs | 10.1 ± 0.5 | 1.0 | 11.1 ± 0.5 | 0.5 | 10.5 ± 0.5 | 0.8 | 9.7 ± 0.5 | 1.3 |
| Proximal colon | 8.9 ± 0.4b | 1.0 | 9.1 ± 0.4a,b | 0.9 | 10.1 ± 0.4a | 0.4 | 9.3 ± 0.4a,b | 0.8 |
| Distal colon | 8.1 ± 0.6 | 1.0 | 8.1 ± 0.6 | 1.0 | 8.5 ± 0.6 | 0.8 | 8.0 ± 0.6 | 1.0 |
| MLNs | 8.9 ± 0.4 | 1.0 | 10.0 ± 0.4 | 0.5 | 9.2 ± 0.4 | 0.8 | 9.4 ± 0.4 | 0.7 |
| TLR9 | ||||||||
| ILPPs | 12.3 ± 0.5b | 1.0 | 14.6 ± 0.5a | 0.2 | 13.9 ± 0.5a | 0.3 | 14.0 ± 0.5a | 0.3 |
| Proximal colon | 12.6 ± 0.3b | 1.0 | 13.7 ± 0.3a | 0.5 | 13.8 ± 0.3a | 0.4 | 13.3 ± 0.3a,b | 0.6 |
| Distal colon | 12.3 ± 0.4 | 1.0 | 12.9 ± 0.4 | 0.7 | 13.0 ± 0.4 | 0.6 | 12.5 ± 0.4 | 0.9 |
| MLNs | 10.8 ± 0.3c | 1.0 | 12.6 ± 0.3a | 0.3 | 11.6 ± 0.2b | 0.5 | 12.1 ± 0.2a,b | 0.4 |
Values for ΔCT are means ± SEMs, n = 6. Labeled means without a common superscript letter differ, P < 0.05. ILPP, ileal Peyer’s patch; MLN, mesenteric lymph node; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
Discussion
There is growing evidence that the consumption of fruit and plant-derived polyphenols is correlated with gastrointestinal health (34, 35). Given that cocoa is a food relatively rich in polyphenols, including flavanols, anthocyanidins, and theobromine, and that consumption of cocoa has increased because of the purported health benefits, we investigated the effects of consuming cocoa powder on changes in the composition of selected species of gut microbiota, the metabolomic profile of several biofluids and tissues, and changes in the gene expression of inflammatory markers in pig intestinal tissue. We found that consumption of cocoa powder modulated colonic microbial metabolites and increased the abundance of Lactobacillus species from the casei group in feces and Bifidobacterium species in proximal colon contents. In addition, gene expression of the proinflammatory cytokine TNF-α and TLR2, TLR4, and TLR9 was reduced in the ILPPs, MLNs, and proximal colon of pigs fed cocoa powder.
Monomers of proanthocyanidins (i.e., catechin and epicatechin) are absorbed in the small intestine after ingestion, whereas oligomers and polymers pass intact to the colon without absorption (36). It is known that the microbiota metabolizes polyphenols in the colon to simpler structures, which are more easily absorbed and can modulate biological activities that are more beneficial than the original forms found in foods (6, 7). Our metabolite analysis showed that there was a cocoa powder–dependent increase in phase II metabolites of cocoa-derived flavanols such as epicatechin-O-glucuronide, epicatechin-O-sulfate, O-methyl-epicatechin-glucuronide, and O-methyl-epicatechin-sulfate in most body fluids and tissue samples isolated from pigs fed cocoa powder. However, there was variation among individual pigs in the expression of certain metabolites. For example, epicatechin-O-glucuronide conjugates in serum were detected in only a few pigs fed 2.5 g and 10 g cocoa powder/d but not 20 g cocoa powder/d. This is an unexplained anomaly because the analysis of all other metabolites showed changes in concentration that were generally dose dependent. Interestingly, the absence of intact procyanidins or phase II conjugated dimers and trimers in our analysis indicated that they were fully metabolized within 14 h after feeding cocoa powder to pigs.
Our metabolite analysis showed that 3-hydroxyphenylpropionic acid, 3,4-dihydroxyphenylpropionic acid, phenylacetic acid, 3,4-dihydroxyphenylacetic acid, hydroxybenoic acid, and vanillic acid were detected in 14-h fasting serum (Table 2) and urine (Supplemental Table 3) of pigs fed cocoa powder for 27 d. These are the same metabolites detected in plasma and urine of humans fed cocoa (37). The current data found that 3,4-dihydroxyphenylpropionic acid was decreased in the serum and 3-hydroxyphenylpropionic acid isomers, 3,4-dihydroxyphenylpropionic acid, phenylacetic acid isomers, and 3,4-dihydroxyphenylacetic acid were decreased in urine after consumption of >10 g cocoa powder/d. The decrease in 3,4-dihydroxyphenylpropionic acid was also found in urine from human subjects fed cocoa (37). Similarly, Gu et al. (38) reported a decrease in the colonic metabolite, hippuric acid, in urine of rats fed a high dose of sorghum bran procyanidins. These metabolites are substrates for gut bacteria (7), and the decrease can be explained by alternations in the composition of the gut microbiota following consumption of a polyphenol-rich diet such as a flavanol-enriched cocoa powder. As mentioned earlier, 3,4-dihydroxyphenylpropionic acid in serum and urine decreased significantly in pigs fed 10 g cocoa powder/d but not 20 g/d. There was large individual variation in the detectable metabolites, with some pigs showing no detectable concentrations and others high concentrations. A power analysis suggested that more replicates would be needed to be confident that feeding >10 g cocoa powder/d would show a statistically significant decrease in 3,4-dihydroxyphenylpropionic acid.
Growing evidence suggests that the gut microbiota influences many facets of human health, including the etiology of gastrointestinal disorders (39). The changes in gut microbiota—specifically, an increase in pathogenic bacteria and a decrease in health-promoting bacteria, such as Bifidobacterium species and Lactobacillus species—contribute to intestinal inflammation (40). Despite substantial interindividual variation in the composition of the microbial community, human and animal studies have shown that the diet has an important effect on the gut microbiota. For instance, the fecal microbiota of rats fed a diet supplemented with a proanthocyanidin-rich red wine extract shifted from a microbiota composed predominantly of Clostridium and Propionibacterium species to one with abundant Lactobacillus and Bifidobacterium species (41). Other studies carried out by Smith et al. (42) found that rats fed a tannin-rich diet had the Bacteroides group significantly increased, whereas the Clostridium leptum cluster decreased. Moreover, Massot-Cladera et al. (43) reported that feeding 10% cocoa to young rats for 42 d significantly decreased the proportion of Bacteroides species, Staphylococcus genus, and Clostridium histolyticum subgroups. Because previous animal and human studies reported an increase in fecal Lactobacillus and Bifidobacterium species after consumption of cocoa powder, we examined the potential prebiotic effect of flavanol-enriched cocoa powder on the concentration of prebiotic-type bacteria such as Lactobacillus and Bifidobacterium species in pig feces and intestinal contents and also measured Eubacteria as an indicator of total bacteria abundance (26). Consumption of 20 g or 10 g cocoa powder/d significantly increased the Lactobacillus casei group in feces and Bifidobacterium species in proximal colon contents, respectively. These results are supported by a previous report in humans in which daily consumption of 494 mg cocoa flavanols in a high-cocoa flavanol drink for 28 d significantly increased fecal Lactobacillus and Bifidobacterium compared with daily consumption of 29 mg cocoa flavanols in a low-cocoa flavanol drink (5). Moreover, studies have shown that an increased abundance of Lactobacillus and Bifidobacterium can reduce bacterial pathogens by acidification of the intestinal contents (44), competition for receptors and adhesion molecules on epithelial cells (45), and production of antimicrobial compounds (46). Thus, the increase in cocoa-induced Lactobacillus and Bifidobacterium species would likely be associated with improved gut health.
Our data showed that consumption of cocoa powder induced a generalized downregulation in TLR9 gene expression in ILPPs, MLNs, and proximal colon of pigs, with no significant changes detected in the distal colon (Table 3). TLR2 gene expression was downregulated in ILPPs and MLNs with a mild reduction in TLR4 expression in the proximal colon (Table 3). These results are supported by previous observations by Massot-Cladera et al. (43) in which a 10% cocoa diet induced a nonsignificant change in TLR2 gene expression and 4-fold downregulation in TLR9 gene expression in the rat colon. Peripheral blood mononuclear cells have been used to screen for immune activity of probiotic strains (47) and their derived products (48). However, peripheral blood mononuclear cells are viewed as peripheral indicators of stimulation of the mucosal immune system. Thus, the direct analysis of TLR gene expression in intestinal tissues would provide information on the local effects of diet-induced changes in gut microbiota and their metabolites on the innate immune system, although studies of the functional activity of TLRs in intestinal tissues are needed to support changes in gene expression. We speculate that the generally lower levels of TNF-α and TLR gene expression in the intestinal tissues of pigs fed cocoa powder could indirectly lower the intensity of inflammation in the intestine through increased concentrations of Lactobacillus and Bifidobacterium species (49, 50).
Thus, we found that consumption of cocoa powder modulated colonic microbial metabolites in biofluids and tissues, increased the abundance of L. casei in feces and Bifidobacterium in proximal colon contents, and induced a reduction of TNF-α and TLR gene expression in intestinal tissues. These results are noteworthy because they suggest that consumption of physiologically relevant doses of cocoa in the diet can provide potential benefits to gut health. Although further studies are needed to determine the role of colonic microbial-derived metabolites on the interaction of gut microbiota and health, the current study suggests that cocoa can support intestinal health.
Acknowledgments
SJ, JMH, CDD, and GS-A designed the research; SJ, JS, SL, AM, JFU, and GS-A conducted the research; SJ, JS, PC, and BTV analyzed the data; SJ, JS, PC, JFU, CDD, and GS-A wrote the article; SJ and GS-A had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ILPP, ileal Peyer’s patch; MLN, mesenteric lymph node; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
References
- 1.Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J Agric Food Chem 2010;58:10518–27. [DOI] [PubMed] [Google Scholar]
- 2.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev 2012;8:CD008893. [DOI] [PubMed] [Google Scholar]
- 3.Arranz S, Valderas-Martinez P, Chiva-Blanch G, Casas R, Urpi-Sarda M, Lamuela-Raventos RM, Estruch R. Cardioprotective effects of cocoa: clinical evidence from randomized clinical intervention trials in humans. Mol Nutr Food Res 2013;57:936–47. [DOI] [PubMed] [Google Scholar]
- 4.Stote KS, Clevidence BA, Novotny JA, Henderson T, Radecki SV, Baer DJ. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur J Clin Nutr 2012;66:1153–9. [DOI] [PubMed] [Google Scholar]
- 5.Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr 2011;93:62–72. [DOI] [PubMed] [Google Scholar]
- 6.Neilson AP, Ferruzzi MG. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu Rev Food Sci Technol 2011;2:125–51. [DOI] [PubMed] [Google Scholar]
- 7.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem 2009;57:6485–501. [DOI] [PubMed] [Google Scholar]
- 8.Choy YY, Quifer-Rada P, Holstege DM, Frese SA, Calvert CC, Mills DA, Lamuela-Raventos RM, Waterhouse AL. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct 2014;5:2298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechner AR, Kuhnle G, Hu H, Roedig-Penman A, van den Braak MH, Moore KP, Rice-Evans CA. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic Res 2002;36:1229–41. [DOI] [PubMed] [Google Scholar]
- 10.Urpi-Sarda M, Monagas M, Khan N, Lamuela-Raventos RM, Santos-Buelga C, Sacanella E, Castell M, Permanyer J, Andres-Lacueva C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal Bioanal Chem 2009;394:1545–56. [DOI] [PubMed] [Google Scholar]
- 11.Martin FP, Rezzi S, Pere-Trepat E, Kamlage B, Collino S, Leibold E, Kastler J, Rein D, Fay LB, Kochhar S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J Proteome Res 2009;8:5568–79. [DOI] [PubMed] [Google Scholar]
- 12.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. TLR signaling. Cell Death Differ 2006;13:816–25. [DOI] [PubMed] [Google Scholar]
- 14.McClure R, Massari P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol 2014;5:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Gonzales-Navajas JM, Raz E. The “polarizing-tolerizing” mechanism of intestinal epithelium: its relevance to colonic homeostasis. Semin Immunopathol 2008;30:3–9. [DOI] [PubMed] [Google Scholar]
- 16.Duan D, Zhang S, Li X, Guo H, Chen M, Zhang Y, Han J, Lv Y. Activation of the TLR/MyD88/NF-kappaB signal pathway contributes to changes in IL-4 and IL-12 production in piglet lymphocytes infected with porcine circovirus type 2 in vitro. PLoS One 2014;9:e97653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesar V, Odin JA. Toll-like receptors and liver disease. Liver Int 2014;34:184–96. [DOI] [PubMed] [Google Scholar]
- 18.Broering R, Lu M, Schlaak JF. Role of Toll-like receptors in liver health and disease. Clin Sci (Lond) 2011;121:415–26. [DOI] [PubMed] [Google Scholar]
- 19.Labib S, Erb A, Kraus M, Wickert T, Richling E. The pig caecum model: a suitable tool to study the intestinal metabolism of flavonoids. Mol Nutr Food Res 2004;48:326–32. [DOI] [PubMed] [Google Scholar]
- 20.Van’t Slot G, Mattern W, Rzeppa S, Grewe D, Humpf HU. Complex flavonoids in cocoa: synthesis and degradation by intestinal microbiota. J Agric Food Chem 2010;58:8879–86. [DOI] [PubMed] [Google Scholar]
- 21.Engemann A, Hubner F, Rzeppa S, Humpf HU. Intestinal metabolism of two A-type procyanidins using the pig cecum model: detailed structure elucidation of unknown catabolites with Fourier transform mass spectrometry (FTMS). J Agric Food Chem 2012;60:749–57. [DOI] [PubMed] [Google Scholar]
- 22.Merrifield CA, Lewis M, Claus SP, Beckonert OP, Dumas ME, Duncker S, Kochhar S, Rezzi S, Lindon JC, Bailey M, et al. A metabolic system-wide characterisation of the pig: a model for human physiology. Mol Biosyst 2011;7:2577–88. [DOI] [PubMed] [Google Scholar]
- 23.Rzeppa S, Bittner K, Doll S, Danicke S, Humpf HU. Urinary excretion and metabolism of procyanidins in pigs. Mol Nutr Food Res 2012;56:653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RR, Carson L, Kwik-Uribe C, Evans EM, Erdman JW Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J Nutr 2008;138:725–31. [DOI] [PubMed] [Google Scholar]
- 25.Solano-Aguilar G, Dawson H, Restrepo M, Andrews K, Vinyard B, Urban JF Jr. Detection of Bifidobacterium animalis subsp. lactis (Bb12) in the intestine after feeding of sows and their piglets. Appl Environ Microbiol 2008;74:6338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002;148:257–66. [DOI] [PubMed] [Google Scholar]
- 27.Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D. Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res 2008;163:663–70. [DOI] [PubMed] [Google Scholar]
- 28.Haarman M, Knol J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl Environ Microbiol 2006;72:2359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solano-Aguilar G, Fernandez KP, Ets H, Molokin A, Vinyard B, Urban JF, Gutierrez MF. Characterization of fecal microbiota of children with diarrhea in 2 locations in Colombia. J Pediatr Gastroenterol Nutr 2013;56:503–11. [DOI] [PubMed] [Google Scholar]
- 30.Dawson H, Solano-Aguilar G, Beal M, Beshah E, Vangimalla V, Jones E, Botero S, Urban JF Jr. Localized Th1-, Th2-, T regulatory cell-, and inflammation-associated hepatic and pulmonary immune responses in Ascaris suum–infected swine are increased by retinoic acid. Infect Immun 2009;77:2576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson H, editor. Porcine Immunology and Nutrition [Internet]. Beltsville (MD): USDA North East Area. [cited 2015 Aug 26]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=6065. [Google Scholar]
- 32.Stroup WW. Generalized linear mixed models—modern concepts, methods, and applications. Boca Raton (FL); London; and New York: CRC Press; 2013. [Google Scholar]
- 33.Malencić D, Maksimovic Z, Popovic M, Miladinovic J. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresour Technol 2008;99:6688–91. [DOI] [PubMed] [Google Scholar]
- 34.Denev P, Kratchanova M, Ciz M, Lojek A, Vasicek O, Nedelcheva P, Blazheva D, Toshkova R, Gardeva E, Yossifova L, et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem 2014;157:37–44. [DOI] [PubMed] [Google Scholar]
- 35.Etxeberria U, Fernandez-Quintela A, Milagro FI, Aguirre L, Martinez JA, Portillo MP. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J Agric Food Chem 2013;61:9517–33. [DOI] [PubMed] [Google Scholar]
- 36.Monagas M, Urpi-Sarda M, Sanchez-Patan F, Llorach R, Garrido I, Gomez-Cordoves C, Andres-Lacueva C, Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct 2010;1:233–53. [DOI] [PubMed] [Google Scholar]
- 37.Urpi-Sarda M, Monagas M, Khan N, Llorach R, Lamuela-Raventos RM, Jauregui O, Estruch R, Izquierdo-Pulido M, Andres-Lacueva C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography–tandem mass spectrometry. J Chromatogr A 2009;1216:7258–67. [DOI] [PubMed] [Google Scholar]
- 38.Gu L, House SE, Rooney L, Prior RL. Sorghum bran in the diet dose dependently increased the excretion of catechins and microbial-derived phenolic acids in female rats. J Agric Food Chem 2007;55:5326–34. [DOI] [PubMed] [Google Scholar]
- 39.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andoh A, Fujiyama Y. Therapeutic approaches targeting intestinal microflora in inflammatory bowel disease. World J Gastroenterol 2006;12:4452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolara P, Luceri C, De Filippo C, Femia AP, Giovannelli L, Caderni G, Cecchini C, Silvi S, Orpianesi C, Cresci A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res 2005;591:237–46. [DOI] [PubMed] [Google Scholar]
- 42.Smith AH, Zoetendal E, Mackie RI. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb Ecol 2005;50:197–205. [DOI] [PubMed] [Google Scholar]
- 43.Massot-Cladera M, Perez-Berezo T, Franch A, Castell M, Perez-Cano FJ. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys 2012;527:105–12. [DOI] [PubMed] [Google Scholar]
- 44.Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 2000;66:2001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 2004;28:405–40. [DOI] [PubMed] [Google Scholar]
- 46.Elmer GW. Probiotics: “living drugs. ” Am J Health Syst Pharm 2001;58:1101–9. [DOI] [PubMed] [Google Scholar]
- 47.Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, Gionchetti P, Schrezenmeir J, Foelsch UR, Schreiber S, et al. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol 2006;12:5978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashraf R, Vasiljevic T, Smith SC, Donkor ON. Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J Dairy Sci 2014;97:2542–58. [DOI] [PubMed] [Google Scholar]
- 49.Magrone T, Jirillo E. The interplay between the gut immune system and microbiota in health and disease: nutraceutical intervention for restoring intestinal homeostasis. Curr Pharm Des 2013;19:1329–42. [DOI] [PubMed] [Google Scholar]
- 50.Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol 2011;60:817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]