Abstract
Background: Numerous studies have documented the negative effects of maternal alcohol consumption during pregnancy on risk of pregnancy loss, yet whether prepregnancy alcohol intake affects the risk of spontaneous abortion is still unclear.
Objective: This study aimed to assess prepregnancy alcohol intake and risk of spontaneous abortion and stillbirth.
Methods: Our prospective cohort study included 27,580 pregnancies reported by 17,929 women in the Nurses’ Health Study II between 1990 and 2009. Alcohol intake was assessed in 1989 and 1991 and every 4 y thereafter with the use of a validated questionnaire. Women were classified into 5 categories of consumption: 0, 0.1–1.9, 2–4.9, 5–9.9, and ≥10 g/d (1 serving = ∼12 g). Pregnancies were self-reported, with case pregnancies lost spontaneously (spontaneous abortion after gestation of <20 wk and stillbirth after gestation of ≥20 wk) and comparison pregnancies not ending in fetal loss (live birth, ectopic pregnancy, or induced abortion). Multivariable log-binomial regression models with generalized estimating equations were used to estimate RRs and 95% CIs.
Results: Incident spontaneous abortion and stillbirth were reported in 4326 (15.7%) and 205 (0.7%) pregnancies, respectively. Prepregnancy alcohol intake was not associated with spontaneous abortion. Compared with women who did not consume alcohol, the multivariable RRs (95% CIs) for increasing categories of alcohol intake among women who did consume alcohol were 1.04 (0.97, 1.12) for 0.1–1.9 g/d, 1.02 (0.94, 1.11) for 2–4.9 g/d, 1.01 (0.92, 1.10) for 5–9.9 g/d, and 0.98 (0.88, 1.09) for ≥10 g/d (P-trend = 0.45). Women who consumed ≥2 servings beer/wk before pregnancy had a 9% (95% CI: 1%, 17%) lower risk of spontaneous abortion than did women who consumed <1 serving beer/mo; however, this association did not persist in various sensitivity analyses. Prepregnancy consumption of wine and liquor were not associated with spontaneous abortion. Total alcohol and specific alcohol beverage intake before pregnancy were not associated with stillbirth.
Conclusion: Prepregnancy alcohol intake was not related to risk of incident spontaneous abortion or stillbirth in women with no history of pregnancy loss. Our results provide reassuring evidence that low to moderate alcohol intake (≤12 g/d) before pregnancy initiation does not affect risk of pregnancy loss.
Keywords: alcohol, ethanol, miscarriage, pregnancy loss, stillbirth, spontaneous abortion
Introduction
Pregnancy loss is estimated to affect up to 30% of pregnancies (1, 2). Although many of these early losses are not recognized by women, clinically recognized losses account for ∼15% of recognized pregnancies, making it the most frequent adverse pregnancy outcome (1). Nutritional exposures offer promise as potential modifiable risk factors for pregnancy loss, with numerous studies having demonstrated their impact on fertility, gametogenesis, embryonic development, and pregnancy outcomes (3).
Alcohol intake has received much attention in the context of pregnancy loss because of its proposed role as a reproductive toxicant. Numerous studies have documented the adverse consequences of maternal alcohol consumption during pregnancy on pregnancy loss (4–10), yet there is conflicting evidence on the impact of prepregnancy alcohol intake on pregnancy loss. Prepregnancy alcohol intake is a particularly relevant exposure not only because of its high prevalence [50% of reproductive age women report consuming alcohol (11)], but also because it may represent a more relevant time window of exposure, with most losses occurring early in pregnancy.
In an animal model, rats that received ethanol for 16 wk but that were not fed alcohol during pregnancy had just as many pups per litter as controls that were fed no alcohol when allowed to mate (12). However, in a primate model, binge ethanol consumption for 6 mo before controlled ovarian stimulation and oocyte retrieval altered follicle cell gene expression, reduced preimplantation embryo development, and resulted in an elevated rate of spontaneous abortion (13). In humans, prepregnancy alcohol intake was reported to be positively related (14, 15), inversely related (16), and unrelated (9, 17–19) to spontaneous abortion. The majority of these previous studies, however, relied on a retrospective report of prepregnancy alcohol consumption (9, 15, 18, 19), were unable to include early pregnancy losses (9, 14, 15, 18, 19), had limited control over other lifestyle factors (9, 14–17, 19), and relied on small sample sizes (e.g., <60 cases) (14, 15, 18). Our study aimed to expand on previous studies by assessing total alcohol and specific alcoholic beverage intake in relation to spontaneous abortion and stillbirth in a large prospective cohort of women.
Methods
Design and study population.
Women in this study were participants in the Nurses’ Health Study II, an ongoing prospective cohort of 116,480 female nurses aged 24–44 y at the study’s inception in 1989. Questionnaires are distributed every 2 y to update lifestyle and medical characteristics and to capture incident health outcomes. Alcohol intake was first assessed in 1989, was updated in 1991, and has since been updated every 4 y thereafter. Response rates for each questionnaire cycle have exceeded 90%. Women were eligible for this analysis if they had no history of pregnancy loss in 1989 and reported at least one pregnancy during 1990–2009. Eligible participants contributed pregnancies until their first pregnancy loss or the end of follow-up. Women were censored after their first pregnancy loss to prevent reverse causation, because of behavioral changes in response to an adverse outcome. Of the 29,257 eligible pregnancies, we excluded from the analysis those with missing data on alcohol intake in 1989 (n = 288), implausible or missing gestational age (n = 215), or missing year of pregnancy (n = 721), as well as those in women with a diagnosis of type 2 diabetes (n = 113), cardiovascular disease (n = 125), or nonskin cancers (n = 215) before the pregnancy. The final sample consisted of 27,580 pregnancies from 17,929 women. This study was approved by the institutional review board of the Partners Health Care System, Boston, Massachusetts, with the participants’ consent implied by the return of the questionnaires.
Alcohol assessment.
Alcohol intake was assessed in 1989 by 3 validated questions included on the baseline questionnaire (20). Women were asked to report their average intake of beer, wine, and liquor over the previous year from 9 categories ranging from “none or less than one per month” to “≥6 per day.” In 1991 and every 4 y thereafter, alcohol intake was assessed as part of a validated 131-item FFQ (21, 22). On the FFQ, women were asked to report how often they consumed regular beer, light beer, red wine, white wine, and liquor during the previous year with the use of the same 9 categories for frequency of consumption. The alcohol content of beverages was derived from USDA food composition sources supplemented with other sources (23–25). The estimated alcohol content of each beverage was 11.3 g/bottle or can of light beer (355 mL), 12.8 g/bottle or can of regular beer (355 mL), 11.0 g/4-oz glass of wine (118 mL), and 14.0 g/shot of liquor (44 mL). We calculated the total intake of alcohol by summing the alcohol content for specific items multiplied by weights proportional to the frequency of use of each item. In a similar population of female health professionals, high correlations were found between alcoholic beverage intake and total alcohol intake assessed with the FFQ and four 1-wk diet records (total alcohol, r = 0.90; beer, r = 0.87; wine, r = 0.85; and liquor, r = 0.80) (22, 26). To maintain a strictly prospective analysis of prepregnancy alcohol intake in relation to pregnancy loss, alcohol information from 1989 was related to pregnancies in 1990 and 1991; the 1991 alcohol information was used for pregnancies in 1992–1995; the 1995 alcohol information was used for 1996–1999; and so forth. If a woman was missing information on her most recent alcohol intake before pregnancy (<5% of women), the most recent previous alcohol data were carried forward.
Outcome assessment.
Women were asked to report their pregnancies at baseline and in each biennial follow-up questionnaire. On the 2009 questionnaire, women also reported information on the year, length, complications, and outcomes of all previous pregnancies. Options for pregnancy outcomes were a singleton live birth, multiple birth, miscarriage or stillbirth, tubal or ectopic pregnancy, or induced abortion. Gestational lengths were reported in the following categories: <8, 8–11, 12–19, 20–27, 28–31, 32–36, 37–39, 40–42, and ≥43 wk. Self-reported pregnancy outcome and gestation length previously have been found to be validly reported (27). The main outcome in this study was spontaneous abortion, defined as a fetal loss occurring before 20 completed weeks of gestation. We also considered early first to mid first trimester losses (<8 wk), late first trimester losses (8–11 wk), early second trimester losses (12–19 wk), and stillbirths (≥20 wk) as separate outcomes. The validity of maternal recall of pregnancy loss was not assessed in this population; however, the sensitivity of reporting a loss when one actually occurred was estimated to be ∼75% (28, 29). In the validation study by Wilcox et al. (28), spontaneous abortions at <6 wk were recalled in 54% of cases, whereas spontaneous abortions occurring after 13 wk were recalled in 93% of cases. The comparison group for our analyses was all pregnancies that did not end in fetal loss [live births (n = 21,728), induced abortions (n = 1062), or tubal/ectopic pregnancies (n = 259)].
Covariate assessment.
Information on potential confounding variables was assessed at baseline and during follow-up. For variables that were updated over follow-up, the most recent value before pregnancy was used. Maternal age was computed as the difference between year of birth and the year of pregnancy. Physical activity was ascertained in 1989, 1991, 1997, 2001, and 2005 with the use of a previously validated questionnaire (30) from which metabolic equivalent task–hrs/wk were derived. Weight, smoking status, multivitamin use, hormonal contraceptive use, and history of infertility were self-reported at baseline and updated every 2 y thereafter. Marital status was reported in 1989, 1993, and 1997. Race and height were reported in 1989. Prepregnancy BMI was calculated as weight in kilograms divided by self-reported height in meters squared. In a previous validation study, self-reported weight was highly correlated with weight measured by a technician in a similar group of nurses (r = 0.97) (31). Caffeine intake was assessed on the FFQ by summing the caffeine content of caffeinated coffee (137 mg caffeine/237-mL cup) and tea (47 mg caffeine/237-mL cup), caffeinated soft drinks (46 mg caffeine/355-mL bottle or can) and chocolate (7 mg caffeine/28 g 1-oz serving) multiplied by weights proportional to the frequency of use of each item. For pregnancies in 1990 and 1991, caffeine intake before pregnancy was estimated in 1998 with the use of a semiquantitative 124-item high school-FFQ that included food items typically consumed between 1960 and 1982, when the nurses were in high school. The reproducibility and validity (compared with the maternal report) of high school caffeine intake as assessed by this FFQ was assessed in a subsample of women and found to be high (r = 0.74 and 0.47 respectively) (32).
Statistical analysis.
Baseline characteristics were derived from the 1989 questionnaire for all women contributing eligible pregnancies. For the primary analyses, we divided women into 5 groups according to average prepregnancy intake of alcohol, beer, wine, and liquor (0, 0.1–1.9, 2–4.9, 5–9.9, and ≥10 g/d). We also explored a finer classification of alcohol intake with the use of 10 predetermined categories.
The RR of spontaneous abortion in relation to prepregnancy alcohol intake was estimated with the use of log-binomial regression. Generalized estimating equations with an exchangeable working correlation structure were used to account for the within-person correlation between pregnancies. In all models, the RR was computed as the risk of spontaneous abortion in a specific category compared with the risk in the lowest intake category. Tests for linear trend across categories were conducted by using the median values in each category as a continuous variable. In addition to age- and year-adjusted models, multivariable models were further adjusted for a priori selected prepregnancy covariables. These included prepregnancy BMI, smoking status, physical activity, history of infertility, marital status, race, multivitamin use, and caffeine intake. Specific beverages also were mutually adjusted for each other in fully adjusted models. Fully adjusted models were run both with and without adjusting for nulliparity, because adjusting for reproductive history might lead to overadjustment if ongoing dietary habits are related to the inability to carry a pregnancy to term, which in this case could manifest as nulliparity (33, 34). Categorical covariables included an indicator for missing data, if necessary.
Next, we investigated whether the relation of prepregnancy alcohol intake with pregnancy loss differed by gestational age at loss (<8, 8–11, 12–19, and ≥20 wk). This analysis was done to investigate potential biological mechanisms driving any associations between alcohol intake and spontaneous abortion and to evaluate the potential impact of outcome misclassification on our results. We also performed various sensitivity analyses. First, we restricted our analysis to pregnancies from women ≤40 y, pregnancies from never smokers, pregnancies with no history of infertility, and first-eligible pregnancies to address the potential of residual confounding by factors strongly related to risk of pregnancy loss. To minimize uncontrolled confounding by behaviors related to pregnancy planning and pregnancy recognition, we also performed analyses restricted to married women not taking oral contraception. Last, to address the potential of misclassification of exposure due to the interval between alcohol assessments, we restricted analyses to pregnancies in the years closest to alcohol assessment (1990, 1992, 1996, 2000, and 2004) (n = 11,198 pregnancies).
Effect modification by well-characterized risk factors for pregnancy loss, including prepregnancy BMI (in kg/m2) (<25 and ≥25), prepregnancy smoking status (current and never/former smokers), maternal age (<35 y compared with ≥35 y), and caffeine intake (median intake <104 mg/d compared with ≥104 mg/d), was tested with the use of cross-product terms in the final multivariate model. All tests of statistical significance were 2-sided, and a significance level of 0.05 was used. All data were analyzed with the use of SAS 9.3.
Results
Overall, 17,929 women met our inclusion criteria, contributing 27,580 pregnancies to this analysis during 20 y of follow-up. Of these pregnancies, 4326 (15.7%) ended in spontaneous abortion and 205 (0.7%) ended in stillbirth. On average, women who were in the highest category of prepregnancy alcohol intake reported more physical activity; were more likely to be white, former or current smokers, current users of oral contraceptives, and nulliparous; and were less likely to be married or report a history of infertility (Table 1). Women with higher alcohol consumption also were more likely to consume higher amounts of caffeine (Spearman correlation between 1991 alcohol and caffeine intake: r = 0.29). The biggest contributor to alcohol intake was white wine (29%) followed by light beer (24%), liquor (19%), beer (16%), and red wine (11%).
TABLE 1.
Baseline demographic characteristics by amount of alcohol intake in 1989 (n = 17,929 women): Nurses’ Health Study II, 1989–20091
| Baseline alcohol intake category |
|||
| Lowest | Middle | Highest | |
| Range of intake, g/d | 0 | 2–4.9 | ≥10 |
| Women, n | 5937 | 2345 | 1705 |
| Maternal age, y | 30.0 (28.0, 33.0) | 30.0 (28.0, 33.0) | 30.0 (28.0, 33.0) |
| Prepregnancy BMI, kg/m2 | 22.1 (20.4, 25.0) | 21.6 (20.1, 23.7) | 21.8 (20.3, 23.8) |
| Physical activity, MET-h/wk | 12.3 (4.5, 28.8) | 19.8 (8.2, 40.3) | 20.9 (9.0, 43.0) |
| Smoking status | |||
| Never smoker | 4803 (80.9) | 1563 (66.7) | 832 (48.8) |
| Former smoker | 783 (13.2) | 548 (23.4) | 518 (30.4) |
| Current smoker | 342 (5.8) | 230 (9.8) | 354 (20.8) |
| White | 5360 (90.3) | 2245 (95.7) | 1640 (96.2) |
| Married | 5048 (85.0) | 1678 (71.6) | 1050 (61.6) |
| Oral contraceptive use | |||
| Never | 1304 (22.0) | 354 (15.1) | 191 (11.2) |
| Past | 3618 (60.9) | 1325 (56.5) | 934 (54.8) |
| Current | 1007 (17.0) | 665 (28.4) | 579 (34.0) |
| History of infertility | 880 (14.8) | 249 (10.6) | 192 (11.3) |
| Parity | |||
| Nulliparous | 2050 (34.5) | 1330 (56.7) | 1151 (67.5) |
| 1 | 2149 (36.2) | 577 (24.6) | 323 (18.9) |
| 2 | 1245 (21.0) | 334 (14.2) | 190 (6.1) |
| ≥3 | 493 (8.3) | 104 (4.4) | 41 (2.4) |
| Multivitamin use | 2498 (42.1) | 1158 (49.4) | 829 (48.6) |
| Alcohol intake, g/d | 0.0 (0.0, 0.0) | 2.7 (2.6, 3.5) | 12.2 (11.0, 15.9) |
| Caffeine intake,2 mg/d | 77.0 (24.0, 184) | 152.0 (63.0, 351) | 185.0 (92.0, 378) |
Values are medians (25th percentiles, 75th percentiles) or n (%). MET-h, metabolic equivalent task hours.
Assessed in 1991.
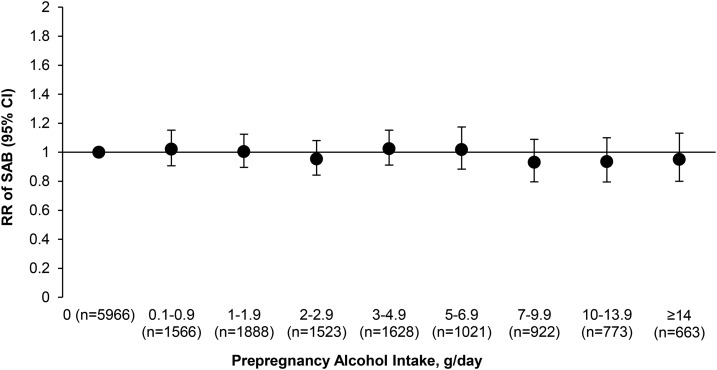
Prepregnancy alcohol intake was not associated with risk of spontaneous abortion (Figure 1). Compared with women who did not consume alcohol, the multivariable RRs (95% CIs) for increasing categories of alcohol intake for those who did consume alcohol were 1.04 (0.97, 1.12) for 0.1–1.9 g/d, 1.02 (0.94, 1.11) for 2–4.9 g/d, 1.01 (0.92, 1.10) for 5–9.9 g/d, and 0.98 (0.88, 1.09) for ≥10 g/d (P-trend = 0.45) (Table 2). When specific alcoholic beverages were examined, only beer intake was significantly associated with spontaneous abortion after multivariable adjustment. Specifically, women who consumed ≥2 servings beer/wk before pregnancy had a 9% (95% CI: 1%, 17%) lower risk of pregnancy loss than did women who consumed <1 serving beer/mo before pregnancy (P-trend = 0.04). Adjustment for caffeine intake and wine and liquor intake resulted in the largest effect estimate changes. Although both wine and liquor intake had positive associations with spontaneous abortion in age- and year-adjusted models, these associations became nonsignificant after adjustment for other demographic and lifestyle characteristics. For the wine intake models, adjustment for caffeine intake resulted in the most attenuation; for liquor intake, adjustment for marital status resulted in the most attenuation.
FIGURE 1.
Adjusted RRs (95% CIs) for spontaneous abortion by fine categories of prepregnancy alcohol intake: Nurses’ Health Study II, 1989–2009. The n shown is the number of pregnancies. Analyses were conducted with the use of a log-binomial generalized linear model with an exchangeable working correlation structure to compute RR estimates. The model was adjusted for age (continuous), total energy intake (continuous), year (continuous), BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, ≥30, or missing), smoking status (never, former, current, or missing), physical activity (in MET-h/wk; <3, 3–8.9, 9–17.9, 18–26.9, 27–41.9, >42, or missing), history of infertility (no, yes, or missing), marital status (married or not married), race (white or other), caffeine intake (quintiles), and multivitamin use (yes, no, or missing). MET-h, metabolic equivalent task hours; SAB, spontaneous abortion.
TABLE 2.
Prepregnancy alcohol and alcoholic beverage intake and RRs of spontaneous abortion: Nurses’ Health Study II, 1989–20091
| RR (95% CI) |
||||
| Categories of intake | Cases, n/total pregnancies, n | % | Age- and year-adjusted2 | Multivariable-adjusted3 |
| Alcohol, g/d | ||||
| 0 | 1513/10,021 | 15.1 | 1.00 (ref) | 1.00 (ref) |
| 0.1–1.9 | 1107/7116 | 15.6 | 1.05 (0.98, 1.13) | 1.04 (0.97, 1.12) |
| 2–4.9 | 769/4673 | 16.5 | 1.04 (0.97, 1.13) | 1.02 (0.94, 1.11) |
| 5–9.9 | 537/3354 | 16.0 | 1.03 (0.95, 1.13) | 1.01 (0.92, 1.10) |
| ≥10 | 400/2416 | 16.6 | 1.02 (0.92, 1.12) | 0.98 (0.88, 1.09) |
| P-trend | 0.87 | 0.45 | ||
| Beer, servings | ||||
| <1/mo | 2779/17,566 | 15.8 | 1.00 (ref) | 1.00 (ref) |
| 1–3/mo | 569/3738 | 15.2 | 0.97 (0.90, 1.06) | 0.95 (0.87, 1.03) |
| 1/wk | 424/2651 | 16.0 | 1.01 (0.92, 1.11) | 0.96 (0.87, 1.05) |
| ≥2/wk | 554/3625 | 15.3 | 0.98 (0.91, 1.07) | 0.91 (0.83, 0.99) |
| P-trend | 0.74 | 0.04 | ||
| Wine, servings | ||||
| <1/mo | 2251/14,947 | 15.1 | 1.00 (ref) | 1.00 (ref) |
| 1–3/mo | 931/5939 | 15.7 | 1.08 (1.01, 1.16) | 1.08 (1.00, 1.16) |
| 1/wk | 509/3208 | 15.9 | 1.02 (0.93, 1.11) | 1.00 (0.91, 1.10) |
| ≥2/wk | 539/3486 | 18.2 | 1.10 (1.01, 1.19) | 1.08 (0.99, 1.18) |
| P-trend | 0.03 | 0.13 | ||
| Liquor, servings | ||||
| <1/mo | 3400/21,831 | 15.6 | 1.00 (ref) | 1.00 (ref) |
| 1–3/mo | 624/3992 | 15.6 | 1.05 (0.98, 1.14) | 1.04 (0.96, 1.12) |
| ≥1/wk | 302/1757 | 17.2 | 1.11 (1.00, 1.24) | 1.09 (0.98, 1.22) |
| P-trend | 0.03 | 0.12 | ||
Analyses conducted with the use of a log-binomial generalized linear model with an exchangeable working correlation structure to compute RR estimates. Tests for linear trend were conducted by using the median values in each category as a continuous variable. MET-h, metabolic equivalent task hours; ref, reference.
Continuous variables.
Age- and year-adjusted model further adjusted for BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, ≥30, or missing), smoking status (never, former, current, or missing), physical activity (in MET-h/wk; <3, 3–8.9, 9–17.9, 18–26.9, 27–41.9, >42, or missing), history of infertility (no, yes, or missing), marital status (married or not married), race (white or other), caffeine intake (quintiles), and multivitamin use (yes, no, or missing). All beverages were adjusted for one another.
When the association of prepregnancy alcohol intake with spontaneous abortion was evaluated separately for early to mid first trimester losses (<8 wk), late first trimester losses (8–11 wk), and early second trimester losses (12–19 wk), no significant heterogeneity was observed (Table 3). There was also no association between prepregnancy alcohol intake and stillbirth (pregnancy loss at ≥20 wk). The protective association between prepregnancy beer intake and spontaneous abortion appeared to be driven by an effect on early second trimester losses, because there was no association at other time points. There was some suggestion of an increased risk of spontaneous abortion at <8 wk in women who consumed ≥2 servings of wine/wk compared with those who consumed <1 serving/mo (P-trend = 0.05); however, across all other time points, the association was null. Prepregnancy liquor intake was not associated with spontaneous abortion at any points across gestation.
TABLE 3.
Prepregnancy alcohol and alcoholic beverage intake and RRs of spontaneous abortion and stillbirth stratified by gestational length: Nurses’ Health Study II, 1989–20091
| Pregnancy loss <8 wk |
Pregnancy loss 8–11 wk |
Pregnancy loss 12–19 wk |
Pregnancy loss ≥20 wk |
|||||
| Categories of intake | Cases, n/total, n | Adjusted RR (95% CI)2 | Cases, n/total, n | Adjusted RR (95% CI)2 | Cases, n/total, n | Adjusted RR (95% CI)2 | Cases, n/total, n | Adjusted RR (95% CI)2 |
| Alcohol, g/d | ||||||||
| 0 | 558/10,021 | 1.00 (ref) | 609/9262 | 1.00 (ref) | 346/8549 | 1.00 (ref) | 68/8162 | 1.00 (ref) |
| 0.1–1.9 | 393/7116 | 1.02 (0.90, 1.16) | 458/6521 | 1.08 (0.96, 1.22) | 256/5961 | 1.07 (0.91, 1.25) | 53/5676 | 1.11 (0.77, 1.59) |
| 2–4.9 | 259/4673 | 0.95 (0.82, 1.10) | 328/4279 | 1.06 (0.93, 1.21) | 182/3892 | 1.07 (0.89, 1.28) | 35/3681 | 1.08 (0.71, 1.64) |
| 5–9.9 | 188/3354 | 0.98 (0.83, 1.16) | 221/3058 | 1.00 (0.86, 1.17) | 128/2778 | 1.06 (0.86, 1.30) | 24/2634 | 0.99 (0.61, 1.61) |
| ≥10 | 163/2416 | 1.09 (0.92, 1.30) | 167/2130 | 1.02 (0.86, 1.22) | 70/1900 | 0.81 (0.62, 1.05) | 25/1818 | 1.36 (0.84, 2.19) |
| P-trend | 0.48 | 0.84 | 0.14 | 0.34 | ||||
| Beer, servings | ||||||||
| <1/mo | 1002/17,566 | 1.00 (ref) | 1122/16,114 | 1.00 (ref) | 655/14,776 | 1.00 (ref) | 121/14,042 | 1.00 (ref) |
| 1–3/mo | 213/3738 | 0.98 (0.85, 1.13) | 236/3411 | 0.98 (0.85, 1.12) | 120/3120 | 0.86 (0.70, 1.04) | 26/2979 | 1.05 (0.68, 1.62) |
| 1/wk | 133/2651 | 0.83 (0.70, 1.00) | 193/2450 | 1.06 (0.91, 1.24) | 98/2213 | 0.91 (0.74, 1.14) | 28/2103 | 1.61 (1.05, 2.49) |
| ≥2/wk | 213/3625 | 0.96 (0.82, 1.12) | 232/3275 | 0.95 (0.82, 1.10) | 109/2971 | 0.76 (0.61, 0.94) | 30/2847 | 1.25 (0.81, 1.94) |
| P-trend | 0.51 | 0.52 | 0.01 | 0.27 | ||||
| Wine, servings | ||||||||
| <1/mo | 797/14,947 | 1.00 (ref) | 927/13,776 | 1.00 (ref) | 527/12,670 | 1.00 (ref) | 112/12,069 | 1.00 (ref) |
| 1–3/mo | 338/5939 | 1.11 (0.98, 1.26) | 385/5427 | 1.09 (0.97, 1.22) | 208/4964 | 1.07 (0.91, 1.26) | 47/4740 | 1.04 (0.73, 1.48) |
| 1/wk | 179/3208 | 1.00 (0.85, 1.18) | 215/2935 | 1.01 (0.87, 1.17) | 115/2667 | 1.01 (0.82, 1.25) | 19/2534 | 0.68 (0.41, 1.14) |
| ≥2/wk | 247/3486 | 1.18 (1.01, 1.38) | 256/3112 | 1.04 (0.89, 1.20) | 132/2779 | 1.05 (0.86, 1.29) | 27/2628 | 0.85 (0.53, 1.35) |
| P-trend | 0.05 | 0.75 | 0.68 | 0.43 | ||||
| Liquor, servings | ||||||||
| <1/mo | 1224/21,831 | 1.00 (ref) | 1414/20,072 | 1.00 (ref) | 762/18,381 | 1.00 (ref) | 154/17,523 | 1.00 (ref) |
| 1–3/mo | 215/3992 | 0.97 (0.84, 1.12) | 246/3641 | 0.99 (0.87, 1.14) | 163/3326 | 1.29 (1.09, 1.53) | 38/3139 | 1.38 (0.96, 2.00) |
| ≥1/wk | 122/1757 | 1.17 (0.97, 1.41) | 123/1537 | 1.11 (0.92, 1.33) | 57/1373 | 1.07 (0.82, 1.41) | 13/1309 | 1.03 (0.57, 1.86) |
| P-trend | 0.11 | 0.29 | 0.46 | 0.83 | ||||
Analyses conducted with the use of a log-binomial generalized linear model with an exchangeable working correlation structure to compute RR estimates. Tests for linear trends were conducted by using the median values in each category as a continuous variable. The reference group for fetal losses at <8 wk was all pregnancies, the reference group for fetal losses at 8–11 wk was all pregnancies lasting beyond 8 wk, the reference group for fetal losses at 12–19 wk was all pregnancies lasting beyond 12 wk, and the reference group for fetal losses at ≥20 wk was all pregnancies lasting beyond 20 wk. MET-h, metabolic equivalent task hours; ref, reference.
Adjusted for age (continuous), energy intake (continuous), BMI (in kg/m2; <18.5, 18.5–24.9, 25–29.9, ≥30, or missing), smoking status (never, former, current, or missing), physical activity (in MET-h/wk; <3, 3–8.9, 9–17.9, 18–26.9, 27–41.9, > 42, or missing), year of pregnancy (continuous), history of infertility (no, yes, or missing), marital status (married or not married), race (white or other), multivitamin use (yes, no, or missing), and caffeine intake (quintiles). All beverages were adjusted for one another.
To assess the robustness of our findings and reduce residual confounding, we did a variety of sensitivity analyses and subanalyses (Supplemental Tables 1–4). There was no relation between prepregnancy alcohol intake and spontaneous abortion when the analyses excluded high risk pregnancies (women >40 y of age and women with a history of infertility) or when analyses were restricted to never smokers or the most likely pregnancy planners in our cohort (married women who were not taking oral contraception). Moreover, the association with alcohol intake remained null when the analysis was restricted to pregnancies in the year immediately after alcohol assessment, suggesting that exposure misclassification was not a likely explanation for the lack of findings.
Some interesting differences emerged when comparing the main results and sensitivity analyses for specific beverages. For instance, the protective association between beer intake before pregnancy and spontaneous abortion lost statistical significance in many sensitivity analyses, particularly those restricted to never smokers. On the other hand, the association between liquor intake before pregnancy and spontaneous abortion became significant in analyses restricted to pregnancies in the year immediately after diet assessment, suggesting that exposure misclassification could be one explanation for these null results.
No significant differences in effect estimates were seen when assessing prepregnancy alcohol intake and risk of spontaneous abortion in overweight compared with nonoverweight women, in current compared with never or former smokers, and in younger compared with older women (<35 y compared with ≥35 y) (P-interaction > 0.05). Analyses also were similar after further adjustment for nulliparity.
Discussion
In this prospective cohort of 27,580 pregnancies, we found no harmful association between low to moderate prepregnancy alcohol intake and risk of incident spontaneous abortion or stillbirth in women with no history of pregnancy loss. Although the consequences of alcohol intake during pregnancy are well recognized, these results provide reassurance that alcohol intake in moderation before pregnancy does not negatively affect a woman’s fertility.
The majority of previous research on prepregnancy alcohol intake and spontaneous abortion is in line with our findings suggesting no association (9, 17–19). A nested case-control study that used prospective data from a population-based cohort comprising 11,088 Danish women found the multivariable OR (95% CI) for miscarriage (defined as a pregnancy loss at a gestation of <28 wk) was 0.92 (0.64, 1.32) for 1–3 drinks/wk, 0.98 (0.67, 1.45) for 7–13 drinks/wk, and 1.28 (0.71, 2.32) for ≥14 drinks/wk compared with <1 drink/wk (17). Similarly, a cohort study of 5342 women from the United States who were asked to retrospectively report their prepregnancy alcohol intake found that the number of drinks a woman consumed before pregnancy was not associated with spontaneous abortion at a gestation of ≤12 wk (9). In general, the 2 studies that reported a positive association between prepregnancy alcohol intake and spontaneous abortion were small (n = 302 and n = 186 pregnancies), had limited to no control over potential confounders, and aimed to assess alcohol intake around conception rather than habitual prepregnancy consumption (14, 15). For instance, the study by Chiodo et al. (15) reported an unadjusted OR of 1.39 (95% CI: 1.08, 1.79) for an increase in intake of 2 alcoholic drinks around conception. Weighing our evidence with previous literature, the overwhelming evidence suggests that moderate alcohol intake even at amounts up to 2 servings/d before pregnancy is not associated with excess risk of spontaneous abortion.
Although we did find a slight protective association between beer intake before pregnancy and lower risk of spontaneous abortion, this association did not remain significant in several sensitivity analyses, specifically those restricted to never smokers, suggesting that this finding could be due to chance or residual confounding rather than due to a true biological effect. The associations between wine intake before pregnancy and spontaneous abortion were null in all sensitivity analyses; however, the associations between prepregnancy liquor intake and spontaneous abortion became significant in certain sensitivity analyses, specifically those restricted to pregnancies immediately after diet assessment. Thus, it is possible that the lack of association between prepregnancy liquor intake and spontaneous abortion found in the main analyses could reflect exposure misclassification. Only one study to date, to our knowledge, has reported associations between consumption of specific alcoholic beverages before pregnancy and spontaneous abortion. In an Italian case-control study that included 462 cases and 814 controls, Parazzini et al. (19) reported multivariable RRs (95% CIs) of 1.0 (0.7, 1.3) and 0.5 (0.4, 0.8) for women who consumed 1–7 and >7 servings of wine/wk compared with women who consumed 0 servings of wine/wk before conception. Although this study found a protective association between wine consumption and spontaneous abortion, these significant findings should be interpreted with caution, because all women retrospectively reported their wine consumption after the outcome occurred, and controls in this study were women who gave birth to healthy term infants (rather than all initiated pregnancies not ending in spontaneous abortion). Currently, there is no evidence to suggest that specific alcoholic beverages consumed in moderation before pregnancy have differential effects on risk of spontaneous abortion, although clearly more research is needed.
Although our study expands on previous research, it is important to consider the limitations in light of the null findings. First, misclassification of alcohol intake is likely because diet information was updated every 2–4 y. Although this type of misclassification is likely nondifferential, it would tend to attenuate effects to the null and could be one explanation for our lack of significant findings. However, when we limited our analyses to pregnancies in the years closest to alcohol assessment (1990, 1992, 1996, 2000, and 2004) the associations between total alcohol, wine, and beer intake and spontaneous abortion remained null. There is also concern that many early losses were unrecognized and that this could be differential with respect to pregnancy intention or prepregnancy alcohol intake. For instance, pregnancy planners might be more likely to have healthy lifestyles and to recognize a loss, particularly early losses (35). Because alcohol consumption is a strong predictor of unintended pregnancy, and women attempting to conceive may limit alcohol intake (36), it is possible that women who consumed alcohol were less likely to recognize miscarriages. We tried to address this potential bias by restricting our population to the most likely pregnancy planners, and the results remained similar. Moreover, if this bias was present, it would be most apparent in analyses focusing on early losses (i.e., gestation of <8 wk), which we did not find to be the case. Nevertheless, because we lack specific information on pregnancy planning, it is possible that bias still remains. As in all observational studies, despite our adjustment and stratification for a variety of potential confounders, we cannot rule out the possibility of residual confounding. Our study also does not distinguish chromosomally normal from abnormal miscarriages. If prepregnancy alcohol intake affects chromosomally abnormal miscarriages differently from normal miscarriages, then this heterogeneity in outcome would tend to attenuate our results toward the null. Finally, the alcohol intake of our cohort of women (∼0.3 drinks/d) was somewhat lower than that reported in a nationally representative sample of US women (0.4 drinks/d) (37). We also had very few women who consumed high amounts of alcohol (e.g., <2% of these women consumed >1 drink/d). Thus, it is possible that a high intake of alcohol before pregnancy is associated with spontaneous abortion, but we were unable to assess this in our study.
Despite these limitations, our study had many strengths, including a large number of pregnancies, the prospective design, a nearly complete follow-up over the 20 y, an ability to assess specific alcoholic beverages, a validated diet assessment (including caffeine consumption), and the inclusion of early pregnancy losses. Our cohort’s reported rates of spontaneous abortion and stillbirth also are similar to that of other cohorts and the US population (38, 39).
In conclusion, our results indicate that prepregnancy alcohol intake was not associated with risk of incident spontaneous abortion or stillbirth in women with no history of pregnancy loss. Of the specific alcoholic beverages, prepregnancy beer intake was associated with a small reduced risk of spontaneous abortion; however, in secondary analyses, these associations were not robust. Although alcohol intake during pregnancy clearly has been linked to adverse pregnancy outcomes, including spontaneous abortion, our results provide reassurance that low to moderate alcohol intake before pregnancy is not associated with an increased risk of spontaneous abortion or stillbirth.
Acknowledgments
JWR-E, SAM, and JEC acquired the data; AJG and JEC analyzed and interpreted the data; AJG, JWR-E, PLW, TLT, SAM, and JEC provided critical revision of the manuscript for important intellectual content; AJG performed the statistical analysis; and AJG and JEC had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967;12:77–126. [PubMed] [Google Scholar]
- 2.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–94. [DOI] [PubMed] [Google Scholar]
- 3.Gaskins AJ, Toth TL, Chavarro JE. Prepregnancy nutrition and early pregnancy outcomes. Curr Nutr Rep 2015;4:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avalos LA, Roberts SC, Kaskutas LA, Block G, Li DK. Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst Use Misuse 2014;49:1437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feodor Nilsson S, Andersen PK, Strandberg-Larsen K, Nybo Andersen AM. Risk factors for miscarriage from a prevention perspective: a nationwide follow-up study. BJOG 2014;121:1375–84. [DOI] [PubMed] [Google Scholar]
- 6.Andersen AM, Andersen PK, Olsen J, Gronbaek M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol 2012;41:405–13. [DOI] [PubMed] [Google Scholar]
- 7.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand 2003;82:182–8. [DOI] [PubMed] [Google Scholar]
- 8.Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol Alcohol 2002;37:87–92. [DOI] [PubMed] [Google Scholar]
- 9.Windham GC, Von Behren J, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology 1997;8:509–14. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health 1992;82:85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Alcohol use and binge drinking among women of childbearing age—United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2012;61:534–8. [PubMed] [Google Scholar]
- 12.Krueger WA, Bo WJ, Rudeen PK. Female reproduction during chronic ethanol consumption in rats. Pharmacol Biochem Behav 1982;17:629–31. [DOI] [PubMed] [Google Scholar]
- 13.VandeVoort CA, Grimsrud KN, Midic U, Mtango N, Latham KE. Transgenerational effects of binge drinking in a primate model: implications for human health. Fertil Steril 2015;103:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriksen TB, Hjollund NH, Jensen TK, Bonde JP, Andersson AM, Kolstad H, Ernst E, Giwercman A, Skakkebaek NE, Olsen J. Alcohol consumption at the time of conception and spontaneous abortion. Am J Epidemiol 2004;160:661–7. [DOI] [PubMed] [Google Scholar]
- 15.Chiodo LM, Bailey BA, Sokol RJ, Janisse J, Delaney-Black V, Hannigan JH. Recognized spontaneous abortion in mid-pregnancy and patterns of pregnancy alcohol use. Alcohol 2012;46:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi BV, Berry KF, Hornstein MD, Cramer DW, Ehrlich S, Missmer SA. Effect of alcohol consumption on in vitro fertilization. Obstet Gynecol 2011;117:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolstrup JS, Kjaer SK, Munk C, Madsen LB, Ottesen B, Bergholt T, Gronbaek M. Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum Reprod 2003;18:2704–10. [DOI] [PubMed] [Google Scholar]
- 18.Cavallo F, Russo R, Zotti C, Camerlengo A, Ruggenini AM. Moderate alcohol consumption and spontaneous abortion. Alcohol Alcohol 1995;30:195–201. [PubMed] [Google Scholar]
- 19.Parazzini F, Tozzi L, Chatenoud L, Restelli S, Luchini L, La Vecchia C. Alcohol and risk of spontaneous abortion. Hum Reprod 1994;9:1950–3. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 1991;133:810–7. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Lenart E. Chapter 6: Reproducibility and validity of food frequency questionnaires. In: Nutritional Epidemiology, Second Edition. Edited by Willett WC. New York: Oxford University Press; 1998. [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 23.Agricultural Research Service, US Department of Agriculture. Composition of foods—raw, processed, and prepared. IWashington (DC): Goverment Printing Office; 1993. [Google Scholar]
- 24.Agricultural Research Service, US Department of Agriculture. USDA nutrient database for standard reference release 11. Washington (DC): Government Printing Office; 1996. [Google Scholar]
- 25.Agricultural Research Service, US Department of Agriculture. USDA Nutrient database for standard reference release 14. Washington (DC): Government Printing Office; 2001. [Google Scholar]
- 26.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 27.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. Am J Epidemiol 1997;145:58–67. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox AJ, Horney LF. Accuracy of spontaneous abortion recall. Am J Epidemiol 1984;120:727–33. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen P, Irgens LM. Maternal reproductive history: a registry based comparison of previous pregnancy data derived from maternal recall and data obtained during the actual pregnancy. Acta Obstet Gynecol Scand 2000;79:471–7. [PubMed] [Google Scholar]
- 30.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 32.Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC. Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol 2005;161:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol 1993;137:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Howards PP, Schisterman EF, Heagerty PJ. Potential confounding by exposure history and prior outcomes: an example from perinatal epidemiology. Epidemiology 2007;18:544–51. [DOI] [PubMed] [Google Scholar]
- 35.Dott M, Rasmussen SA, Hogue CJ, Reefhuis J. Association between pregnancy intention and reproductive-health related behaviors before and after pregnancy recognition, National Birth Defects Prevention Study, 1997–2002. Matern Child Health J 2010;14:373–81. [DOI] [PubMed] [Google Scholar]
- 36.Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC. Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics 2003;111:1136–41. [PubMed] [Google Scholar]
- 37. Guenther PM, Bowman SA, Goldman JD [Internet]. Alcoholic beverage consumption by adults 21 years and over in the United States: results from the National Health and Nutrition Examination Survey, 2003–2006: technical report. 2010 [cited 2016 Jan 5]. Center for Nutrition Policy and Promotion, and Agricultural Research Service, US Department of Agriculture. Available from: http://www.cnpp.usda.gov/DGAs2010-Meeting5.htm.
- 38.Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol 2012;94:417–23. [DOI] [PubMed] [Google Scholar]
- 39.MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep 2012;60:1–22. [PubMed] [Google Scholar]