Endothelial colony-forming cells (ECFCs) collected from placentas were isolated and characterized. In vitro, macrovascular ECFCs, which were of fetal origin, had a greater potential to generate high-proliferative colonies and formed more complex capillary-like networks on Matrigel. In contrast, in vivo assessment demonstrated that microvascular ECFCs, which were of maternal origin, had a greater potential to form vessels.
Keywords: Placental vasculature, Angiogenesis, Preeclampsia, Stem cell, Endothelial progenitor cell
Abstract
Alterations in the development of the placental vasculature can lead to pregnancy complications, such as preeclampsia. Currently, the cause of preeclampsia is unknown, and there are no specific prevention or treatment strategies. Further insight into the placental vasculature may aid in identifying causal factors. Endothelial colony-forming cells (ECFCs) are a subset of endothelial progenitor cells capable of self-renewal and de novo vessel formation in vitro. We hypothesized that ECFCs exist in the micro- and macrovasculature of the normal, term human placenta. Human placentas were collected from term pregnancies delivered by cesarean section (n = 16). Placental micro- and macrovasculature was collected from the maternal and fetal side of the placenta, respectively, and ECFCs were isolated and characterized. ECFCs were CD31+, CD105+, CD144+, CD146+, CD14−, and CD45−, took up 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein, and bound Ulex europaeus agglutinin 1. In vitro, macrovascular ECFCs had a greater potential to generate high-proliferative colonies and formed more complex capillary-like networks on Matrigel compared with microvascular ECFCs. In contrast, in vivo assessment demonstrated that microvascular ECFCs had a greater potential to form vessels. Macrovascular ECFCs were of fetal origin, whereas microvascular ECFCs were of maternal origin. ECFCs exist in the micro- and macrovasculature of the normal, term human placenta. Although macrovascular ECFCs demonstrated greater vessel and colony-forming potency in vitro, this did not translate in vivo, where microvascular ECFCs exhibited a greater vessel-forming ability. These important findings contribute to the current understanding of normal placental vascular development and may aid in identifying factors involved in preeclampsia and other pregnancy complications.
Significance
This research confirms that resident endothelial colony-forming cells (ECFCs) exist in the micro- and macrovasculature of the normal, term human placenta. Their isolation from two different anatomical locations yields two functionally different ECFC populations. Investigation of these ECFC populations during placental pathologies, such as preeclampsia, may lead to a better understanding of the disease process and aid in developing new therapies.
Introduction
The placenta is the organ that connects the developing fetus to the uterine wall, allowing nutrient uptake, waste elimination, and gas exchange via the mother’s blood supply. Vascular adaptation in pregnancy comprises maternal uterine artery remodeling and fetal placental development. Vasculogenesis, the process of de novo blood vessel formation, occurs during the first 4 weeks of gestation [1]. Angiogenesis, the process of blood vessel formation by sprouting of preexisting vessels, occurs after vasculogenesis and provides continuous vascular adaptation until term [2]. Any impairment in vascular development can lead to poor placentation and a decrease in blood flow to the fetus. This can lead to various pregnancy complications, one of which is preeclampsia (PE).
PE, defined as hypertension and proteinuria arising after 20 weeks of gestation with reversal of hypertension and proteinuria after delivery, complicates approximately 7% of all pregnancies worldwide [3, 4]. It significantly increases maternal and neonatal morbidity and mortality and is responsible for more than 60,000 maternal deaths each year in developing countries [5, 6]. PE is also associated with low birth weight and intrauterine fetal growth restriction [7]. The cause of PE is unknown, and there are currently no specific prevention or treatment strategies [8, 9]. Therefore, further insight into the development of the placental vasculature is necessary to increase the current knowledge of normal placental physiology and aid in identifying potential factors involved in PE.
Endothelial progenitor cells (EPCs), a population of vascular precursor cells, have been shown to give rise to differentiated endothelial cells in vivo and incorporate at sites of injury and repair [10, 11]. Two distinct subset populations of EPCs have been identified: (a) early-outgrowth EPCs that contain colony-forming units and circulating angiogenic cells, and (b) late-outgrowth EPCs known as endothelial colony-forming cells (ECFCs) [12]. Unlike colony-forming units and circulating angiogenic cells, which cannot develop into mature endothelial cells, ECFCs are capable of self-renewal and de novo vessel formation in vitro [13–15]. Although studies have explored the role of circulating EPCs in the peripheral blood during pregnancy [16–19] and the umbilical cord blood of neonates [20, 21], the existence and role of resident ECFCs in the placenta remain unknown. Therefore, we hypothesized that ECFCs exist in the normal human placenta and that their function is dependent on their location (the micro- or macrovasculature) within the placenta.
Materials and Methods
All procedures were approved by the Human Research Ethics Board of the University of Alberta (Edmonton, Alberta, Canada), the Animal Care and Veterinary Service, University of Ottawa (Ottawa, Ontario, Canada), and the Indiana University Institutional Animal Care and Use Committee (Indianapolis, Indiana, USA).
Study Population
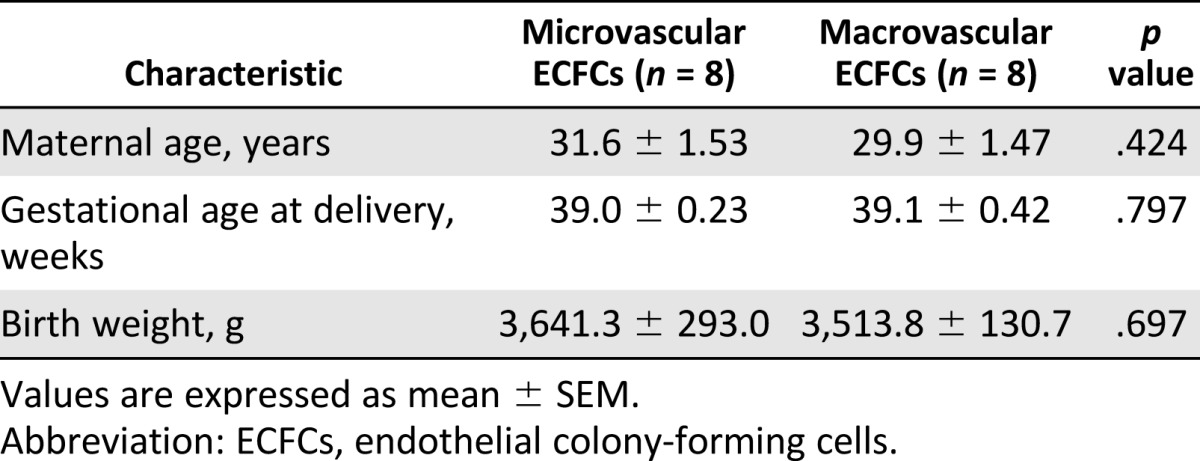
Term pregnant women were recruited on admission to the hospital, and written informed consent was obtained before collection of the placenta. Only placentas from term births delivered by cesarean section were collected. Placentas from 16 deliveries were collected for analysis of the microvasculature (n = 8) and macrovasculature (n = 8). Clinical characteristics are shown in Table 1. Exclusion criteria of the pregnant women were known history of chronic hypertension, diabetes, renal disease, cardiovascular disease, hepatic disease, infections (defined as fever and premature rupture of membranes or established bacterial infection), autoimmune disorders, other significant preexisting metabolic disorders, recent history of illicit drug use, and current multiple gestation or fetal malformation.
Table 1.
Clinical characteristics of micro- and macrovascular endothelial colony-forming cell groups
Collection of Placental Microvasculature
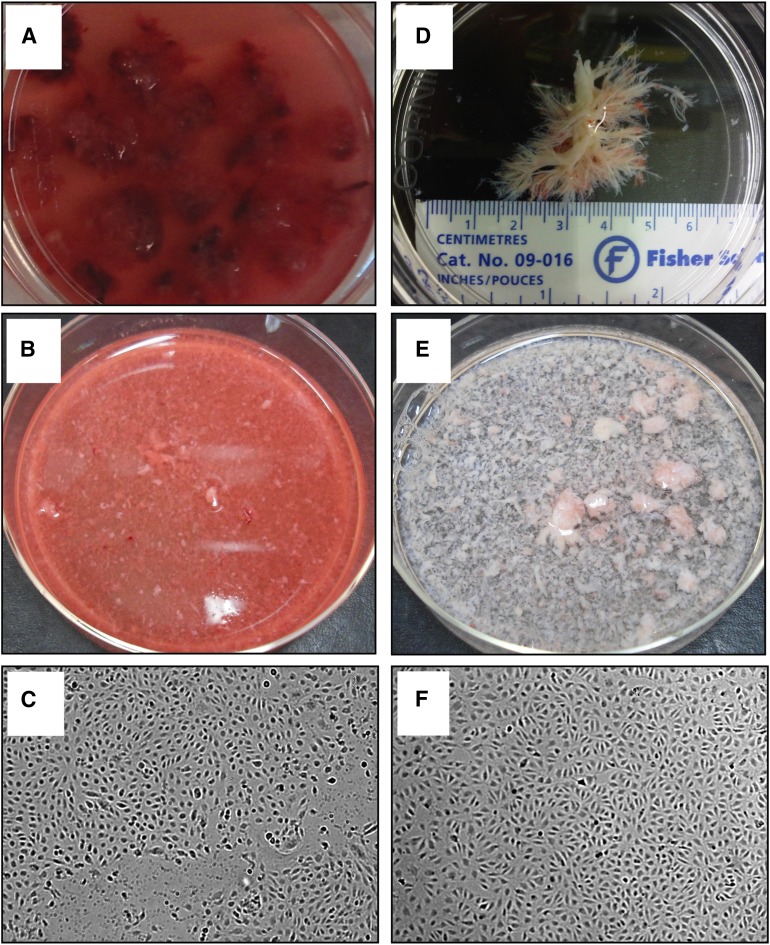
Placentas were transported to the laboratory within 90 minutes postpartum. After removal of the amnion, three tissue samples from the maternal side of the placenta were collected. Each sample contained a placental cotyledon and was one-third of the total placental thickness. Samples were placed in sterile phosphate buffered saline (PBS) (Fig. 1A–1C).
Figure 1.
Isolation process of micro- and macrovascular endothelial colony-forming cells (ECFCs). (A–C): Microvascular ECFC isolation. (A): Placental tissue samples from maternal side, washed in phosphate-buffered saline. (B): Chopped placental tissue samples in collagenase/dispase digestion solution. (C): Microvascular ECFC colonies with cobblestone appearance at day 14 in culture. (D–F): Macrovascular ECFC isolation. (D): Placental proximal vessels from fetal side, previously flushed with saline and cleaned of adherent tissue. (E): Chopped proximal vessels in collagenase/dispase digestion solution. (F): Macrovascular ECFC colonies with cobblestone appearance at day 14 in culture. Magnification, ×100 (C, F).
Collection of Placental Macrovasculature
Within 10 minutes postpartum, the amnion was removed from the placenta, and a depth of one-third from the maternal side was completely removed. Saline (50 ml) was injected into the placenta via the umbilical cord to flush the proximal vessels. Three tissue samples containing proximal vessels from the fetal side of the placenta were collected and placed in saline. The samples were then transported to the laboratory within 90 minutes postpartum, where the proximal vessels were dissected from the adjacent tissue and placed in sterile PBS (Fig. 1D–1F).
ECFC Isolation and Culture
The micro- and macrovascular tissue samples were finely chopped using a McIlwain Mechanical Tissue Chopper (Ted Pella, Redding, CA, http://www.tedpella.com/). The tissue was then incubated in collagenase/dispase digestive solution (0.1 U collagenase, 0.8 U dispase/ml) (Roche Applied Science, Laval, QC, Canada, http://www.roche.com/index.htm) for 60 minutes at 37°C with intermittent shaking (Fig. 1B, 1E). Dulbecco’s modified Eagle medium containing 10% fetal bovine serum (FBS) was added, and the digested sample was filtered through 100- and 70-μm sterile cell strainers. The cell suspension was centrifuged (300g, 10 minutes, 4°C) and resuspended in sterile PBS containing 0.1% bovine serum albumin. To positively select cells for CD31, they were incubated with streptavidin-coated magnetic beads (Dynabeads; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) that were pretreated with biotinylated anti-human CD31 (#560983; BD Pharmingen, Franklin Lakes, NJ, http://www.bdbiosciences.com) for 30 minutes at 4°C. CD31+ cells were selected using a magnetic separator and were plated in six-well plates precoated with rat tail collagen type I. Cells were plated at various cell densities ranging from 1,500 to 400,000 cells per well using the limited dilution assay and placed in a humidified incubator (37°C, 5% CO2). After 24 hours, nonadherent cells and debris were aspirated, adherent cells were washed with sterile PBS, and serum-containing endothelial basal medium 2 (10% FBS, 1% penicillin-streptomycin-fungizone) supplemented with EGM-2 Singlequots (Lonza, Basel, Switzerland, http://www.lonza.com/) was added. The medium was changed daily for the first 7 days, and thereafter every other day until completion of culture.
ECFC Characterization
From day 14 to 21, micro- and macrovascular ECFC colonies were morphologically identified as having a well-circumscribed monolayer of cobblestone-appearing cells (Fig. 1C, 1F). Individual ECFC colonies were isolated using glass rings. The colonies were then plated in T25 flasks and later expanded in T75 flasks.
Immunophenotyping
After cell expansion, micro- and macrovascular ECFCs at passages 4–6 were characterized by identifying endothelium-specific surface markers previously described for ECFCs [15]. Cells were trypsinized and washed twice with flow buffer (PBS containing 0.05% sodium azide and 0.1% bovine serum albumin). Next, 150,000 ECFCs per tube were incubated with anti-human CD14, CD31, CD45, CD105, CD144, and CD146 for 60 minutes in the dark at 4°C. Cells were washed twice with sterile PBS, resuspended in 300 μl flow buffer, and analyzed by fluorescence-activated cell sorting (FACS).
Endothelium-Specific Staining
Micro- and macrovascular ECFCs were also assessed for their capacity to incorporate endothelium-specific staining, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate-labeled acetylated low density lipoprotein (Dil-acLDL), and Ulex europaeus agglutinin 1 (UEA-1), as previously described [15].
Single-Cell Clonogenic Assay
Micro- and macrovascular ECFCs from passages 5–8 were used to assess colony-forming capacity when plated at single-cell density. Cells were sorted, singly plated in 96-well plates using the FACS Aria Cell Sorter (BD Biosciences Canada, Mississauga, ON, Canada, https://m.bdbiosciences.com/ca/home), and incubated at 37°C, 5% CO2. Medium was changed every other day, and wells were analyzed at day 14. Human umbilical vein endothelial cells (HUVECs) were prepared in the same manner for use as control cells. Wells were classified according to a previously described hierarchy, which is dependent on the number of cells per well that a single cell can generate: (a) low proliferative potential (LPP) colonies containing subgroups of 2–50, 50–500, or 500–2,000 cells per well and (b) high proliferative potential (HPP) colonies containing >2,000 cells per well [12, 22]. After classification, an HPP well was trypsinized and used for second-generation clone formation, where cells were replated at a single-cell level in 96-well plates and analyzed at day 14, as described above.
Cord Network Formation In Vitro
The formation of cord-like network structures by micro- and macrovascular ECFCs was assessed on Matrigel-coated wells in a 96-well plate. ECFCs were seeded at 10,000 cells per well and incubated at 37°C, 5% CO2 for 6–8 hours. Cord-like structures were observed with an inverted microscope at 100× magnification and quantified by measuring cord length and the number of intersections in three random fields from each well using OpenLab software (Quorum Technologies, Guelph, ON, Canada, http://www.quorumtechnologies.com/).
Genetic Analysis of ECFCs
ECFCs from male-only pregnancies were used to establish the genetic origin of the micro- and macrovascular ECFCs. The Y-chromosome markers sY127, sY84, sY255, sY86, SRY, sY134, and sY254 and the X-chromosome marker ZFY were identified by multiplex polymerase chain reaction (PCR) with fluorescently labeled primers. Pooled PCR products were run on the AB 3130 Genetic Analyzer. Three multiplex PCR reactions were run, each including a normal male control, a normal female control, and a no-template control.
In Vivo Assessment of ECFC Function
Implantation of ECFCs Into NOD/SCID Mice
Cellularized gel implants were cast as previously described [13, 23]. Cultured placental micro- and macrovascular ECFCs (2 × 106 cells/ml) were suspended in a solution containing 1.5 mg/ml rat tail collagen I (BD Biosciences Pharmingen), 100 μg/ml human fibronectin (Chemicon, EMD Millipore, Billerica, MA, http://www.emdmillipore.com), 1.5 mg/ml sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 25 mM HEPES (Cambrex, East Rutherford, NJ, http://www.cambrex.com), 10% FBS, and 30% serum-free medium, pH-adjusted to 7.4. Thereafter, 250 μl of the cell suspension was added per well of a 48-well plate, allowed to polymerize at 37°C for 30 minutes, and covered with 500 μl of serum-reduced medium for overnight incubation (37°C, 5% CO2). Gels were implanted into the flanks of anesthetized 6- to 9-week-old NOD/SCID mice. After 14 days, mice were killed (n = 6 per group), and the grafts were excised and analyzed by histology and immunohistochemistry as previously described [13, 23]. Briefly, paraffin-embedded tissue sections were stained with hematoxylin and eosin or incubated with an anti-human CD31 antibody (clone JC70/A; Abcam, San Francisco, CA, http://www.abcam.com). CD31 antibody was detected using LASB2 link-biotin and streptavidin-horseradish peroxidase (Vector Laboratories, Burlingame, CA, http://vectorlabs.com) and developed with 3,3′-diaminobenzidine (DAB) (Vector Laboratories). Vessel number and size were analyzed by light microscopy at 40× magnification.
Model of Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a common pulmonary complication in prematurely born infants that is characterized by reduced lung vasculature growth [24]. Experimental BPD was induced as previously described [25, 26]. NOD/SCID mice were exposed to normoxia (21% oxygen) or hyperoxia (95% oxygen, experimental model) from postnatal day (P) 4 to P14 in sealed Plexiglas chambers with continuous oxygen monitoring. Neonatal pups were allocated into four groups: (a) normoxia (21% oxygen, control group, n = 8), (b) hyperoxia (95% oxygen, BPD group, n = 5), (c) hyperoxia plus microvascular ECFCs (n = 6), and (d) hyperoxia plus macrovascular ECFCs (n = 7). Cultured placental micro- and macrovascular ECFCs were administered on P14 via an intrajugular injection (105 cells in 25 μl). Lungs were harvested at P28. Lungs were pressure-fixed (20 cm·H2O) via the trachea with 10% formaldehyde solution, paraffin-embedded, sectioned 5 μm thick, and immunohistochemically stained for von Willebrand Factor (vWF) for vessel density analysis, as previously described [26, 27]. Briefly, for vWF staining, nonspecific binding was blocked and sections were incubated overnight with a vWF antibody (A0082; Dako, Glostrup, Denmark, http://www.dako.com). Sections were then incubated with a biotinylated goat anti-rabbit IgG secondary antibody (B2770, Invitrogen). Antibody signal was amplified by incubation with streptavidin-horseradish peroxidase complex (S911, Invitrogen) and visualized with DAB staining. Using light microscopy at ×40 magnification, the pulmonary vasculature was quantified by calculating the vessel density.
Statistical Analysis
Values are expressed as the mean ± SEM. Statistical comparisons were made with one-way analysis of variance (ANOVA). If ANOVA showed a significant difference, a least significant difference post hoc test was conducted. A value of p < .05 was considered statistically significant.
Results
Limited Dilution Assays Determine Optimal Seeding Density
To establish the seeding density that yields the most viable ECFC colonies, we used a limited dilution assay. We show that seeding 100,000 cells per well yields the greatest number of well-defined colonies in conjunction with limited contaminating cells. Higher seeding densities gave rise to overconfluent wells, and lower densities produced fewer or no ECFC colonies (supplemental online Fig. 1).
Micro- and Macrovascular ECFCs Exhibit Endothelial Cell Characteristics
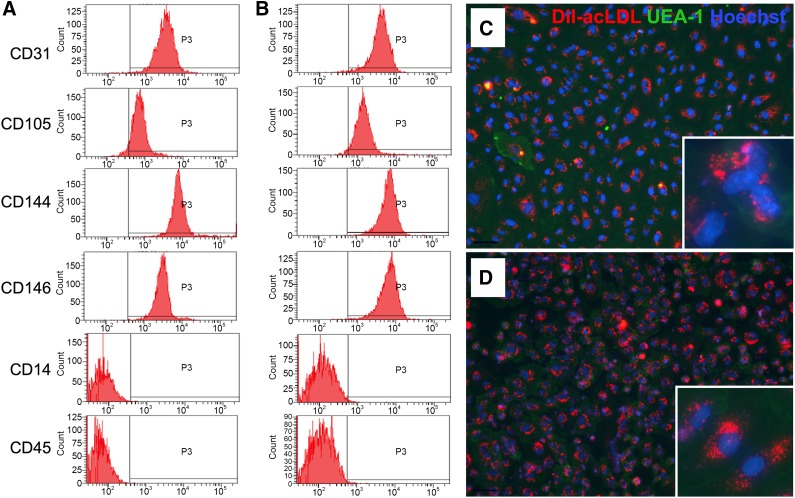
ECFC populations isolated from both the micro- and macrovasculature strongly expressed the endothelium-specific cell surface antigens CD31, CD105, CD144, and CD146. Furthermore, neither of the ECFC populations expressed the hematopoietic cell specific marker CD45 or the monocyte/macrophage marker CD14 (Fig. 2A, 2B). FACS analysis confirmed a pure population of isolated ECFCs with no contaminating cells. Furthermore, both populations of ECFCs exhibited basic endothelial cell characteristics, such as ingestion of Dil-acLDL and binding to UEA-1 (Fig. 2C, 2D).
Figure 2.
Phenotypic characterization by fluorescence-activated cell sorting and micro- and macrovascular endothelial colony-forming cell (ECFC) staining. Microvascular (A) and macrovascular (B) ECFCs were positive for endothelial-specific cell surface markers CD31, CD105, CD144, and CD146 and negative for monocyte/macrophage-specific marker CD14 and hematopoietic cell specific marker CD45. Microvascular (C) and macrovascular (D) ECFCs incorporated the endothelium-specific stains Dil-acLDL (red) and UEA-1 (green). Counterstaining with Hoechst 33258 (blue). Magnification, ×100; inset, ×400. Abbreviations: Dil-acLDL, 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein; UEA-1, Ulex europaeus agglutinin 1.
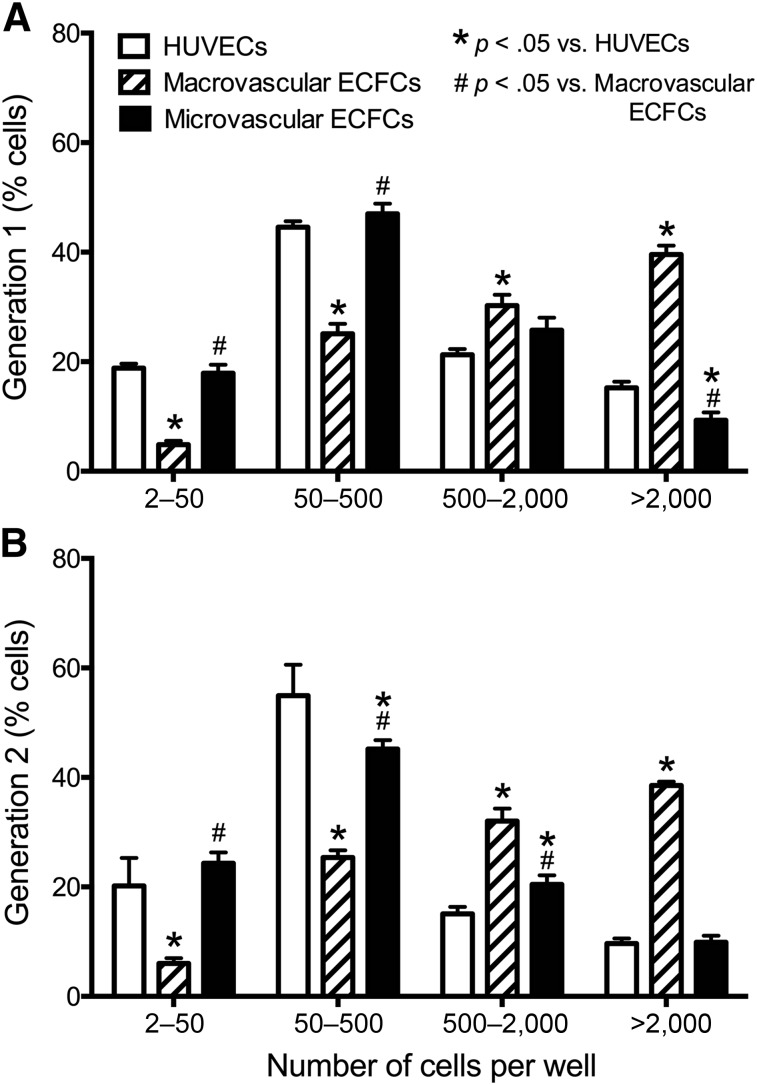
Macrovascular ECFCs Give Rise to Highly Proliferative Colonies
Macrovascular ECFCs exhibited a significantly higher percentage of HPP colonies in the first-generation clone compared with both microvascular ECFCs and control HUVECs (p < .05) (Fig. 3A). After replating and production of the second generation, macrovascular ECFCs responded in a similar manner by producing a high percentage of HPP colonies compared with the other cells (Fig. 3B). This shows that macrovascular ECFCs are able to maintain a high proliferation potential compared with microvascular ECFCs.
Figure 3.
Single-cell clonogenic potential. Percentage of low proliferative potential and high proliferative potential (HPP) colonies in micro- and macrovascular ECFCs and control HUVECs generated in the single-cell clonogenic assay 14 days after single-cell plating. Macrovascular ECFCs demonstrate more potency by generating significantly more HPP colonies than microvascular ECFCs and HUVECs in both the first-generation (A) and second-generation (B) plating. Results represent the mean ± SEM of eight individual experiments. ∗, #, Significant differences. Abbreviations: ECFC, endothelial colony-forming cell; HUVEC, human umbilical vein endothelial cell.
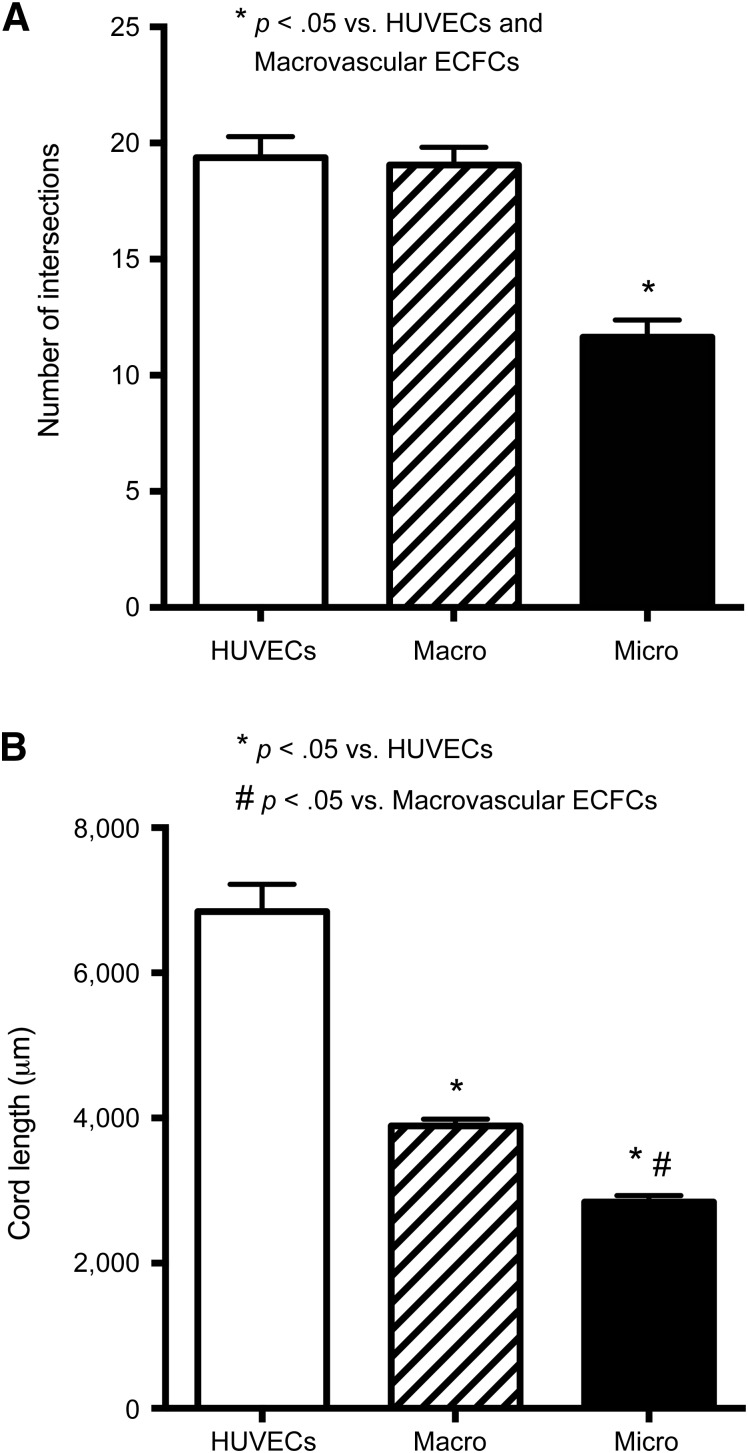
Macrovascular ECFCs Form More Complex Cord Networks In Vitro
When plated on Matrigel, microvascular ECFCs formed significantly fewer cord intersections compared with HUVECs and macrovascular ECFCs (p < .05) (Fig. 4A). There was no significant difference in the number of intersections between HUVECs and macrovascular ECFCs. Although macrovascular ECFCs formed a similar number of cord intersections as HUVECs, their cord length was significantly shorter than that of HUVECs (p < .05, Fig. 4B). However, the cord length of macrovascular ECFCs was significantly greater than that of microvascular ECFCs (p < .05, Fig. 4B).
Figure 4.
Capillary-like cord formation in vitro. The number of cord intersections (A) and cord length (B) in micro- and macrovascular ECFCs and HUVECs in a Matrigel assay. Results represent the mean ± SEM of eight individual experiments. ∗, #, Significant differences. Abbreviations: ECFC, endothelial colony-forming cell; HUVEC, human umbilical vein endothelial cell.
Macrovascular ECFCs Are of Fetal Origin; Microvascular ECFCs Are of Maternal Origin
Genetic analysis of micro- and macrovascular ECFCs from male pregnancies identified two different origins. Macrovascular ECFCs expressed the X-chromosome marker (ZFY) as well as the Y-chromosome markers (sY127, sY84, sY255, sY86, SRY, sY134, sY254), indicating that macrovascular ECFCs are of fetal origin (supplemental online Fig. 2A). Conversely, the microvascular ECFCs expressed only the X-chromosome marker and none of the Y-chromosome markers (supplemental online Fig. 2B), indicating that these cells are of maternal origin.
Microvascular ECFCs Form More Vessels in an In Vivo Graft Implant Model
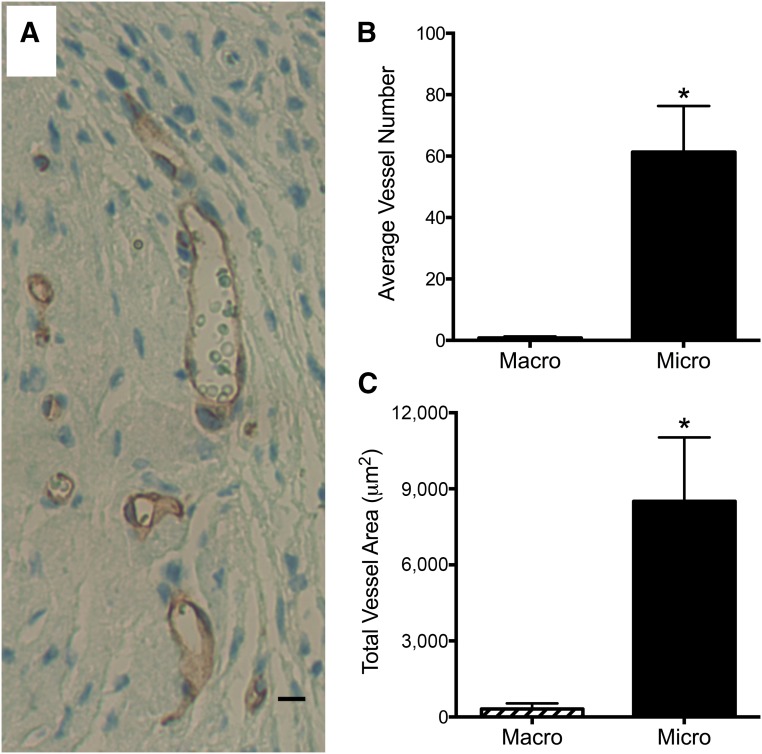
Assessment of the gel implants containing the ECFCs showed that microvascular ECFCs were able to form significantly more vessels in vivo compared with macrovascular ECFCs (p < .05, Fig. 5B). Microvascular ECFCs formed CD31-expressing endothelial lined vessels that connected with the mouse vasculature. This was evident through the presence of mouse red blood cells within the vessels derived from the human cells. Furthermore, microvascular ECFCs produced vessels with a significantly greater total vascular area compared with macrovascular ECFCs (p < .05, Fig. 5C).
Figure 5.
In vivo vessel formation in Matrigel implants. Human placental microvascular endothelial colony-forming cell (ECFCs) formed blood vessels in vivo (A). This image depicts microvascular ECFCs forming CD31-expressing endothelial lined vessels that have connected with the mouse vasculature to perfuse the human vessels (note the mouse red blood cells within the vessels). Scale bar = 10 μm. Only vessels containing mouse red blood cells were quantified. The microvascular ECFCs formed significantly more vessels (B) with a significantly greater total vascular area (C) compared with macrovascular ECFCs. Results represent the mean ± SEM. ∗, Significant difference.
Microvascular ECFCs Promote Vascular Growth in an In Vivo Model of Neonatal Lung Injury
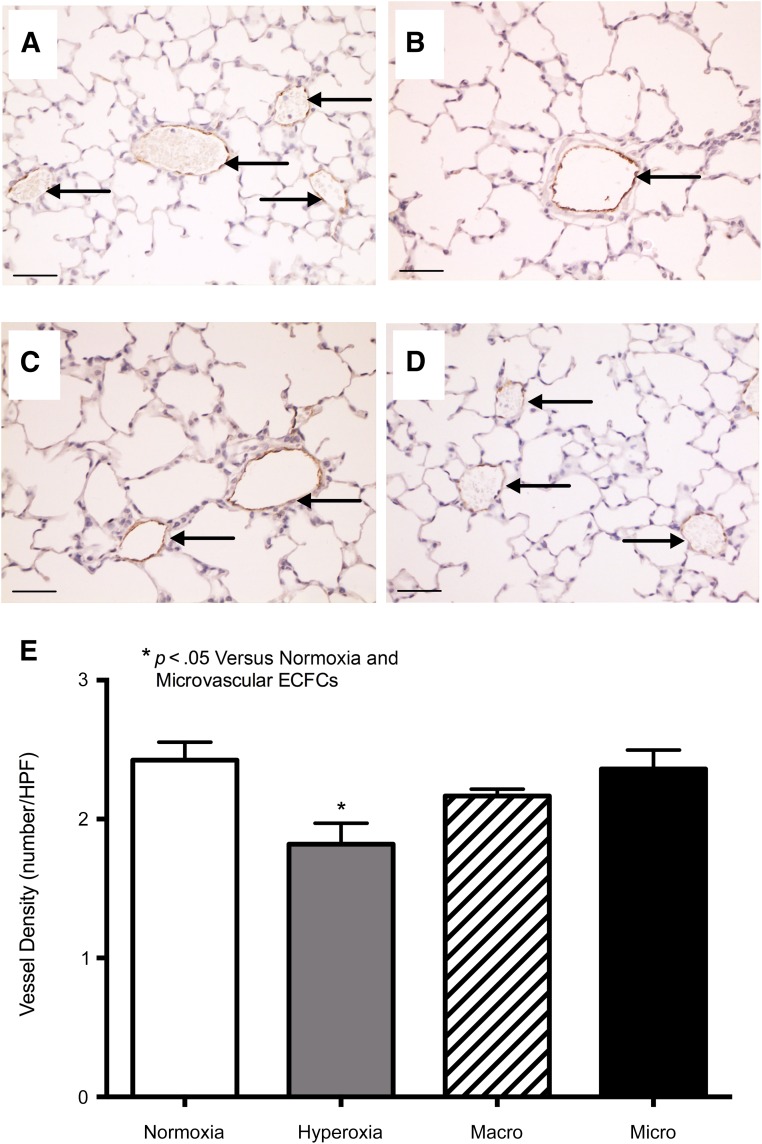
Administration of ECFCs as a rescue therapy after neonatal lung injury showed that microvascular ECFCs were able to promote vascular growth, whereas macrovascular ECFCs exhibited a limited therapeutic potential. Exposure to hyperoxia led to an arrest in lung vascular growth, as assessed by a significant decrease in vWF-positive lung vessels (p < .05, Fig. 6). Microvascular ECFC treatment rescued lung angiogenesis to the level of normoxia controls. Although macrovascular ECFC treatment partially attenuated the loss of vWF-positive vessels, it was not to the level of normoxia controls.
Figure 6.
In vivo vessel formation in lung injury model. (A–D): Representative photomicrographs of the vessel density in normoxia controls (A), hyperoxia controls (B), hyperoxia plus macrovascular ECFCs (C), and hyperoxia plus microvascular ECFCs (D). Data for the number of vessels per high power field. (E). Arrows indicate von Willebrand factor-positive vessels. Results represent the mean ± SEM. ∗, Significant difference. Scale bars = 50 μm. Abbreviations: ECFC, endothelial colony-forming cell; HPF, high-power field.
Discussion
We show that ECFCs are present in both the micro- and macrovasculature of the human placenta. These two populations of ECFCs, which were isolated from two distinct locations in the placenta, exhibited different origins and functional abilities. Specifically, the fetal macrovascular ECFCs were more potent in vitro, as they gave rise to the highest percentage of HPP wells and formed more complex networks on Matrigel compared with the maternal microvascular ECFCs. However, in vivo, the microvascular ECFCs were able to form more vessels in a Matrigel plug assay and were also able to attenuate vascular alterations after neonatal lung injury compared with macrovascular ECFCs.
EPCs represent at least two distinct cell populations, which can be categorized as early-outgrowth EPCs and late-outgrowth EPCs, or ECFCs. ECFCs are easily identified in culture from endothelial cells, because of their high proliferative potential, capacity for self-renewal, and de novo vessel formation in vitro [13–15]. Many studies have explored the role of circulating late-outgrowth EPCs in the peripheral blood during pregnancy [16] and the umbilical cord blood of neonates [21, 28–30]. Gussin et al. showed that late-outgrowth EPCs are present in the peripheral blood of pregnant women and absent in nonpregnant women; however, the functional aspects of these cells were not assessed [16]. Baker et al. demonstrated that ECFCs from the umbilical cord blood of preterm neonates (28–35 weeks’ gestation) yielded significantly more ECFC colonies than term cord blood; however, they also have an increased susceptibility to hyperoxia compared with term ECFCs [28]. Javed et al. in turn showed that cord blood of extremely premature infants (24–28 weeks’ gestation) had fewer ECFCs than cord blood from term infants [29]. More recently, Muñoz-Hernandez et al. investigated the presence of ECFCs in umbilical cord blood from neonates of normal and PE pregnancies. Although they showed that umbilical cord blood of PE pregnancies yielded a lower number of ECFCs and the cells required more time to emerge in culture compared with control pregnancies, ECFC function was similar between the groups [21]. Another recent study that investigated ECFCs in umbilical cord blood found a positive correlation between prepregnancy maternal body mass index and ECFC content; however, ECFC phenotype and function were similar between groups of differing body mass index [30]. In spite of the various studies on ECFCs from maternal peripheral and umbilical cord blood, knowledge on the existence of ECFCs in the placenta is currently limited. A recent study by Patel et al. reported the isolation of ECFCs from the human term placenta and showed that the ECFC population was of fetal origin and displayed characteristics similar to umbilical cord blood-derived ECFCs [31]. Furthermore, Patel et al. highlighted the clinical translational benefits of placental ECFC isolations by demonstrating that the placenta yields much larger quantities of ECFCs compared with the cord blood [31].
Here we report a technique to isolate ECFCs from two different anatomical locations within the human placenta and demonstrate the existence of ECFCs in both the micro- and macrovasculature. The characteristics used to define our ECFC populations are comparable to those of the above studies. We purified our cell isolation by positively selecting for the cell surface marker CD31. We then demonstrated that the cells displayed stringent characteristics of ECFCs, including the ability to generate colonies with 500 or more daughter cells derived from a single cultured cell, capability of clonogenic cell self-renewal, and in vitro formation of capillary-like structures.
ECFCs isolated from both the micro- and macrovasculature demonstrated comparable surface antigen expression and endothelium-specific stain incorporation. However, functional differences between the two ECFC groups were apparent in vitro in both the clonogenic assay and capillary-like cord formation on Matrigel. Compared with microvascular ECFCs, we showed that macrovascular ECFCs exhibit greater potency through their ability to maintain HPP colonies (>2,000 cells) from a cultured single cell and their ability to produce more capillary-like cord intersections of greater length. Interestingly, when we investigated the in vivo effects of the two ECFC populations, we found results that conflicted with those exhibited in vitro. Microvascular ECFCs demonstrated a greater ability to form vessels in a Matrigel plug assay. These ECFCs were able to form vessels that connected with the mouse vasculature, as shown by the perfusion of the microvascular ECFC-derived vessels with mouse red blood cells. Also, in a lung injury model, the microvascular ECFCs were able to rescue the lung vasculature to that of the uninjured controls. In these in vivo models, the macrovascular ECFCs did not demonstrate the same vessel-forming ability. Furthermore, using genetic analysis, we showed that the micro- and macrovascular ECFCs are of different origins. Microvascular ECFCs were positive only for the X-chromosome markers, giving it the maternal XX phenotype, and macrovascular ECFCs were positive for both X- and Y-chromosome markers, giving it the fetal XY phenotype.
It was initially thought that the increased potency of the macrovascular ECFCs seen in vitro was caused by the higher turnover state of their fetal cell origin that would be required to create and sustain the developing fetus. However, this increased potency was not translated to the in vivo experiments. It is likely that the microenvironment plays an important role in modulating the effects of ECFCs. In environments with no or low vessel density, as seen in the two in vivo models, it is possible that the microvascular ECFCs respond better than the macrovascular ECFCs, thus resulting in revascularization. Interestingly, we have previously shown that ECFCs with high proliferative potential are enriched in umbilical cord blood compared with adult peripheral blood, and these cord blood-derived ECFCs displayed a rapid population doubling time, higher telomerase activity, and longer telomeres compared with adult blood-derived ECFCs [12]. Similar to the cord blood-derived ECFCs in Patel et al., these cord blood-derived ECFCs display therapeutic benefits in experimental lung disease [32]. Furthermore, we have previously shown that HUVEC-derived ECFCs display higher telomerase and more HPP activity than human adult aortic ECFCs [14].
Similar to the observations by Patel et al., our results also indicate that the placenta can be used as a reliable source of readily available ECFCs. However, it may also be important to consider the anatomic site for the isolation of ECFCs. According to our in vitro results, the macrovasculature seemed promising because of its higher vasculogenic and clonogenic potential, but the microvascular ECFCs outperformed the macrovascular ECFCs in the in vivo setting.
Conclusion
We show that resident ECFCs exist in the normal human placenta, and their isolation from two different anatomical locations yields two different ECFC populations, fetal and maternal. Investigation of these ECFC populations during placental pathologies, such as preeclampsia, may lead to a better understanding of the disease process and aid in developing new therapies.
Supplementary Material
Acknowledgments
We acknowledge Shirley Beauchamp and Stacey Hume for their help in the collection of the placentas and for their technical assistance. This work was supported by a grant from the Canadian Institutes for Health Research (B.T.) and the Riley Children’s Foundation (M.C.Y.). M.O. was the recipient of a Molly Towell Fellowship in Perinatal Research. R.S.A. was the recipient of an Alberta Innovate Health Solutions (AIHS) stipend. B.T. was supported by a Canada Research Chair and AIHS clinical investigator award and by the Canadian Foundation for Innovation.
Author Contributions
I.S.: conception and design, provision of study materials or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.O.: provision of study materials or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.I., R.S.A., S.R., S.Z., A.V., and W.C.S.: provision of study materials or patients, data analysis and interpretation, final approval of manuscript; M.C.Y. and B.T.: conception and design, provision of study materials or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Kingdom J, Huppertz B, Seaward G, et al. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 2.Demir R, Kayisli UA, Cayli S, et al. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–539. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jameil N, Aziz Khan F, Fareed Khan M, et al. A brief overview of preeclampsia. J Clin Med Res. 2014;6:1–7. doi: 10.4021/jocmr1682w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–270. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia—A pressing problem: An executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14:508–523. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 6.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 7.Xiong X, Mayes D, Demianczuk N, et al. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999;180:207–213. doi: 10.1016/s0002-9378(99)70176-6. [DOI] [PubMed] [Google Scholar]
- 8.Sibai BM. Prevention of preeclampsia: A big disappointment. Am J Obstet Gynecol. 1998;179:1275–1278. doi: 10.1016/s0002-9378(98)70146-2. [DOI] [PubMed] [Google Scholar]
- 9.Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209–215. doi: 10.1016/S0140-6736(00)03599-6. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 12.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 13.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 15.Mead LE, Prater D, Yoder MC, et al. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2:Unit 2C 1. [DOI] [PubMed] [Google Scholar]
- 16.Gussin HA, Bischoff FZ, Hoffman R, et al. Endothelial precursor cells in the peripheral blood of pregnant women. J Soc Gynecol Investig. 2002;9:357–361. doi: 10.1016/s1071-5576(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 17.Lin C, Rajakumar A, Plymire DA, et al. Maternal endothelial progenitor colony-forming units with macrophage characteristics are reduced in preeclampsia. Am J Hypertens. 2009;22:1014–1019. doi: 10.1038/ajh.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubara K, Abe E, Matsubara Y, et al. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2006;56:79–85. doi: 10.1111/j.1600-0897.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Sugawara J, Mitsui-Saito M, Hoshiai T, et al. Circulating endothelial progenitor cells during human pregnancy. J Clin Endocrinol Metab. 2005;90:1845–1848. doi: 10.1210/jc.2004-0541. [DOI] [PubMed] [Google Scholar]
- 20.Monga R, Buck S, Sharma P, et al. Effect of preeclampsia and intrauterine growth restriction on endothelial progenitor cells in human umbilical cord blood. J Matern Fetal Neonatal Med. 2012;25:2385–2389. doi: 10.3109/14767058.2012.697228. [DOI] [PubMed] [Google Scholar]
- 21.Muñoz-Hernandez R, Miranda ML, Stiefel P, et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Critser PJ, Grimes BR, et al. Human umbilical cord blood plasma can replace fetal bovine serum for in vitro expansion of functional human endothelial colony-forming cells. Cytotherapy. 2011;13:712–721. doi: 10.3109/14653249.2010.548380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thébaud B, Abman SH. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thébaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 26.Vadivel A, Alphonse RS, Ionescu L, et al. Exogenous hydrogen sulfide (H2S) protects alveolar growth in experimental O2-induced neonatal lung injury. PLoS One. 2014;9:e90965. doi: 10.1371/journal.pone.0090965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadivel A, Alphonse RS, Collins JJP, et al. The axonal guidance cue semaphorin 3C contributes to alveolar growth and repair. PLoS One. 2013;8:e67225. doi: 10.1371/journal.pone.0067225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker CD, Ryan SL, Ingram DA, et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javed MJ, Mead LE, Prater D, et al. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res. 2008;64:68–73. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Luna R, Muñoz-Hernandez R, Lin RZ, et al. Maternal body-mass index and cord blood circulating endothelial colony-forming cells. J Pediatr. 2014;164:566–571. doi: 10.1016/j.jpeds.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel J, Seppanen E, Chong MS, et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Translational Medicine. 2013;2:839–847. doi: 10.5966/sctm.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alphonse RS, Vadivel A, Fung M, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation. 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.