This article describes a complete xeno-free protocol for extracting, culturing, and cryopreserving human adipose-derived stem cells that can be safely implemented in clinical studies.
Keywords: Adipose, Adult stem cells, Xeno-free production, Human platelet lysate, Explant culture, Cryopreservation
Abstract
Molecules of animal or bacterial origin, which pose a risk for zoonoses or immune rejection, are commonly used for extraction, culture, and cryopreservation of mesenchymal stem cells. There is no sequential and orderly protocol for producing human adipose-derived stem cells (hASCs) under xeno-free conditions. After standardizing a human platelet lysate (hPL) production protocol, four human adipose tissue samples were processed through explants with fetal bovine serum (FBS)-supplemented or hPL-supplemented media for extracting the adipose-derived stem cells. The cells were cultivated in cell culture medium + hPL (5%) or FBS (10%). The cellular replication rate, immunophenotype, and differentiation potential were evaluated at fourth passage. Cellular viability was evaluated before and after cryopreservation of the cells, with an hPL-based solution compared with an FBS-based solution. The explants cultured in hPL-supplemented media showed earlier and faster hASC proliferation than did those supplemented with FBS. Likewise, cells grown in hPL-supplemented media showed a greater proliferation rate, without losing the immunophenotype. Osteogenic differentiation of xeno-free hASC was higher than the hASC produced in standard conditions. However, adipogenic differentiation was reduced in xeno-free hASC. Finally, the cells cryopreserved in an hPL-based solution showed a higher cellular viability than the cells cryopreserved in an FBS-based. In conclusion, we have developed a complete xeno-free protocol for extracting, culturing, and cryopreserving hASCs that can be safely implemented in clinical studies.
Significance
This study was performed to standardize a complete ordered protocol to produce xeno-free human adipose-derived mesenchymal stem cells (hASCs) as a safe therapeutic alternative. Cells were extracted by adipose tissue explants and then cultured and cryopreserved using human platelet lysate (hPL). Different scientific journals have published data regarding the use of hPL as a safe fetal bovine serum substitute for hASC culture, using heparin to avoid clot formation. This article reports the use of hPL for extracting, culturing, and cryopreserving hASCs without anticoagulant.
Introduction
The proliferation and differentiation potential of mesanchymal stem cells (MSCs) has attracted worldwide attention in search of an alternative to replace lost cells in injured tissues [1]. MSCs can self-renew and differentiate into mesodermal and nonmesodermal cell lineages, including chondrogenic, osteogenic, adipogenic, myogenic, and neurogenic lineages [2]. They were initially isolated from bone marrow (bone marrow mesenchymal stem cells [BMSCs]) but can be isolated from various tissues [3], including adipose tissue (adipose-derived stem cells [ASCs]) [4].
BMSCs and ASCs have similar proliferation and differentiation potentials [5–7]. However, ASCs have certain characteristics that make them more attractive than BMSCs for therapeutic applications. They show greater morphological and genetic stability, retain their multipotentiality in culture and in vitro longer, have greater proliferation capability [8], are less prone to reach senescence [5], and support hematopoiesis more efficiently compared with BMSCs [9].
Moreover, more MSCs can be extracted from a primary sample of adipose tissue [10]. MSCs are rare in the bone marrow (approximately 1/100,000) [11], whereas in adipose tissue they are equivalent to nearly 2% of the nucleated cells [12]. In addition, the quantity of primary sample that can be obtained from bone marrow never exceeds a few milliliters, whereas it is very easy to obtain hundreds of milliliters of lipoaspirate. All these elements make it relatively easy to extract more than 5 × 105 ASCs per milliliter of adipose tissue sample [10].
Commonly used human ASC (hASC) extraction, culture, and cryopreservation protocols use molecules of animal or bacterial origin, which pose different kinds of risks for humans [13, 14], thereby affecting the potential of these cells as a treatment option for regenerative medicine.
It has been suggested that ASCs are part of the nucleated cell population of the perivascular adipose tissue stroma [15–17]. Thus, enzymes such as collagenase (usually from animal or bacterial origin) are used to degrade the extracellular matrix and release the ASCs. A protocol for ASC extraction that does not use any enzyme but rather uses an adipose tissue explant technique has been reported [18–20]; this protocol can be used in a xeno-free protocol to produce hASCs.
MSC culture requires the use of a nutritional supplement, usually fetal bovine serum (FBS), in the culture medium. However, there are several concerns about FBS safety in the production of human therapeutic products. Immune rejection of FBS may be an important cause of treatment failures and adverse reactions reported in clinical trials [21]. In addition, it represents a risk for the transmission of infectious agents, such as the transmissible spongiform encephalopathies [22].
In recent years, human platelet lysate (hPL) has been proposed as a substitute for FBS in MSC production [23, 24]. In addition to lacking the risk of xenogeneic immune rejection or zoonosis transmission associated with FBS, hPL can be produced under good manufacturing practices at much lower costs than FBS [25, 26].
Previous studies have shown that hPL supports cellular viability, enhances proliferation [27, 28], delays senescence [29, 30], ensures cellular genomic stability [23], and preserves hASC phenotype. MSCs grown in media supplemented with hPL express surface markers commonly used to identify MSCs, even in advanced cell passages [31], in contrast to what happens with cells grown in media supplemented with FBS [32, 33]. It has also been shown that hASCs grown in media supplemented with hPL retain their adipogenic and osteogenic differentiation potential [24, 28, 31], although cells cultured in media supplemented with hPL may have less adipogenic differentiation potential [24] and greater capacity for osteogenic differentiation [34, 35].
Platelets are small enucleated structures of hematopoietic origin produced by the fragmentation of megakaryocytes in the bone marrow [36]. They have three different intracellular granules that store different soluble molecules, including proteins such as growth factors, cytokines, and chemokines [37]. Different protocols have been proposed for the production of hPL, but the complete set of platelet-released factors has not been described [38]. However, it is known that hPL contains substantial amounts of trophic factors such as transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF), in addition to the three isoforms of platelet-derived growth factor (PDGF) (AA, AB, and BB) [31, 39]. It has been suggested that the effect of hPL on MSCs depends mainly on the activity of PDGF-BB, TGF-β, and bFGF [39]. The expression of TGF-β receptor in MSCs has been documented and is known to influence their proliferation, differentiation, and migration capacity [40, 41] and their angiogenic potential [42]. However, assays with mixtures of recombinant proteins have suggested that additional factors are responsible for the effects of hPL on MSCs [39, 43].
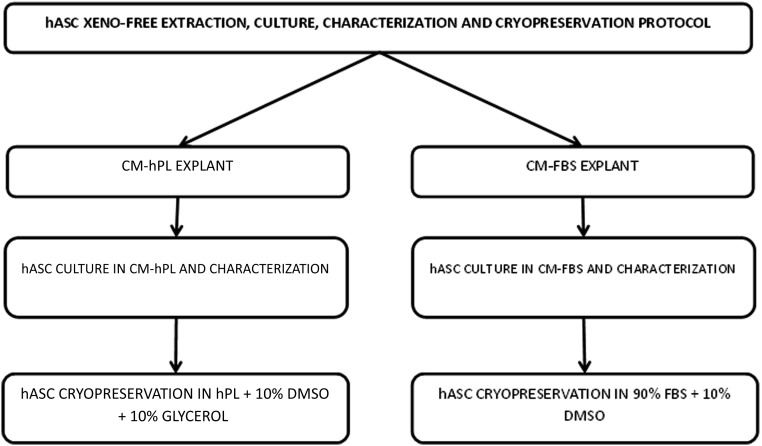
Finally, the implementation of MSC therapy for regenerative medicine requires the establishment of cryopreservation methods that can ensure cell viability and phenotypic stability for long periods. Cryopreservation protocols are based on cryoprotectants, such as dimethyl sulfoxide (DMSO) or glycerol and FBS. Although there are some commercial solutions for xeno-free cryopreservation, a cryopreservation protocol based on hPL has not been described [44]. Here we describe a protocol for xeno-free extraction, culture, and cryopreservation of hASCs, which could be implemented in clinical trials to evaluate the therapeutic potential of these cells (Fig. 1).
Figure 1.
Flowchart of extraction, culture, characterization, and cryopreservation of adipose-derived stem cells with FBS and hPL. Abbreviations: CM-FBS, complete media fetal bovine serum; CM-hPL, complete media human platelet lysate; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; hASC, human adipose-derived stem cell; hPL, human platelet lysate.
Materials and Methods
Preparation of hPL
In a modification of a previously reported protocol [27], 10 units of platelets from adult donors of both sexes and different blood groups donated by the Hemocentro Distrital of the Secretaría Distrital de Salud de Bogotá (Blood, Tissue and Stem Cell Bank of the Secretariat of Health of Bogotá) were centrifuged at 1200 g for 15 minutes. The supernatant was discarded, and the pellets were frozen at −80°C after resuspension in the same volume of phosphate-buffered saline (PBS). This solution was then thawed at 4°C, homogenized, and centrifuged at 4,000g for 15 minutes; the supernatant was filtered through a 0.22-μm pore. The filtration product was separated into 10-ml aliquots and stored at −20°C. The hPL protein concentration was measured following the Micro BCA Protein Assay Kit standard protocol (Thermo Scientific Pierce, Rockford, IL, https://www.thermofisher.com) and the absorbance was measured at 660 nm on a plate reader Ultramark, Bio-Rad, Hercules, CA, http://www.bio-rad.com).
Protein Array
Two different hPL batches and one FBS batch were evaluated for the detection of 43 human proteins, including cytokines, growth factors, proteases, and soluble receptors, using the Human Angiogenesis Array C1000 (RayBiotech, Norcross, GA, http://www.raybiotech.com), according to the manufacturer’s instructions. Briefly, 1 ml of solution was incubated with arrayed antibody membranes for 2 hours at room temperature; membranes were then washed and incubated with the mix of biotin-conjugated antibodies for another hour at room temperature. After washing, horseradish peroxidase-conjugated streptavidin was added to the membranes for 1 hour at room temperature. The signal was developed with detection buffer, and membranes were exposed to autoradiographic films. Signal optical densities were quantified using a program for digital image processing (ImageJ 1.410; National Institutes of Health, Bethesda, MD, https://www.nih.gov). Signal densities (pixels) were normalized against total protein concentration and then expressed as arbitrary units (A.U.) per milligram of protein.
Extraction of hASCs
In a modification of a previously described protocol [19], four biopsy specimens, approximately 5 g each, of abdominal adipose tissue from healthy donors (one man and three women) of different blood groups and age 10–50 years were washed twice with PBS supplemented with penicillin (100 μg/ml), streptomycin (100 μg/ml), and neomycin (200 mg/ml) and cut into pieces of about 1 mm3. The fragments were seeded into 40-mm flat-bottom wells and incubated in a humidified atmosphere at 37°C with 5% CO2 for 1 hour. Then, 500 µl of Opti-MEM (Life Technologies, ThermoFisher Scientific, Grand Island, NY, https://www.thermofisher.com, Grand Island, NY) supplemented with penicillin (50 mg/ml), streptomycin (50 mg/ml), and neomycin (100 mg/ml) (Life Technologies, ThermoFisher Scientific), and 5% hPL (complete media hPL [CM-hPL]) or 10% FBS (complete media FBS [CM-FBS]) was added; the solution was incubated for 24 hours in a humidified atmosphere at 37°C with 5% CO2. Finally, 500 µl of CM-hPL or CM-FBS was added to continue the incubation in a humidified atmosphere at 37°C with 5% CO2; the culture media was changed twice a week with fresh medium.
Two weeks later, the explants were removed, and the cells were recovered with TrypLE Select (Life Technologies, ThermoFisher Scientific) after the cell monolayers were washed twice with PBS. The recovered cells were seeded in 750-mm2 surface culture flasks and cultured in CM-hPL or CM-FBS.
Cell Proliferation Assay
The hASCs were seeded at 2,000 cells per well in triplicate by using 24-well flat-bottom plates and cultured in CM-hPL or CM-FBS for 12 hours, 1, 2, 7 and 14 days. After the culturing period, the hASCs per well were quantified with the CyQuant NF Cell Proliferation Assay Kit (Life Technologies, ThermoFisher Scientific), following the manufacturer's instructions. The absorbance was measured at 530 nm on a plate reader (Ultramark). The results were compared with a standard curve of known cellular concentrations in CM-hPL or CM-FBS.
The population doubling (PD) was calculated by using the following equation [45]:
where NH corresponds to the final cell number in every time point and N1 corresponds to the initial cell-plated number.
hASC In Vitro Differentiation Assay
To compare the xeno-free and standard-produced hASC differentiation potential (adipogenic and osteogenic), cells of the fourth pass were seeded at 70% of confluence, in triplicate, and cultured for 2 weeks (the culture media was changed three times per week) in StemPro Adipogenesis and Osteogenesis differentiation media (Gibco, Life Technologies, ThermoFisher Scientific), following the manufacturer’s protocols. After the differentiation induction period, the cellular monolayers were stained with Oil Red O or alizarin red to identify the intracellular lipids deposits (adipogenic differentiation) or the interstitial calcium deposits (osteogenic differentiation). The percentage of the area covered by intracellular lipid deposits or extracellular calcium deposits was quantified by densitometric analysis using ImageJ 1.4 software as previously described [46].
hASC Immunophenotypification
Fourth-passage hASCs were recovered with TrypLE Select (Life Technologies, ThermoFisher Scientific) and 5 × 105 cells were incubated with monoclonal anti-human CD34-APC-conjugated (clone AC 136/ isotype IgG2A/Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com), monoclonal anti-human CD45-RPE-Cy5-conjugated (clone T29/33/isotype IgG1/Dako Cytomation, Glostrup, Denmark, http://www.dako.com), monoclonal anti-human CD73-PE-conjugated (clone D2/ isotype IgG1/BD Pharmingen, San Jose, CA, http://www.bdbiosciences.com), monoclonal anti-human CD-90 APC-conjugated (clone F15-42-1/isotype IgG1/AbD Serotec, Kidlington, U.K., https://www.abdserotec.com), monoclonal anti-human CD105 PE-conjugated (clone SN6/isotype IgG1, eBioscience, San Diego, CA, http://www.ebioscience.com), monoclonal anti-human HLA-ABC-FITC-conjugated (clone w6/32/isotype IgG2A/AbD Serotec), monoclonal anti-human HLA-DR-RPE-conjugated (clone AB3/isotype IgG2A/Dako Cytomation), or specific isotype-negative controls (IgG2a/APC, IgG1/RPE-Cy5, IgG1/PE, IgG1/APC, IgG2A/FITC, IgG2A/RPE, Dako Cytomation). Readings and analysis were performed on an FACSCanto cytometer (BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com) at the Banco de Sangre de Cordón Umbilical del Hemocentro Distrital de Bogotá.
hASC Cryopreservation in hPL
After recovery of the cells with TrypLE Select (Life Technologies, ThermoFisher Scientific), the hASCs were cryopreserved at −80°C at a concentration of 1 × 106 cells per milliliter. Different DMSO and glycerol (Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com) concentrations (10%–30%) were assayed as hPL supplements for the cryopreservation solution. The solution of FBS (90%) and DMSO (10%) was used as a control.
Statistical Analysis
The protein quantification and cellular proliferation data are expressed as mean ± SEM of independent triplicates in every experiment. The statistical significance of the differences among groups was tested by using the Student's t test. The differences were considered to be significant at p ≤ .05.
Results
hPL Characterization
The hPL produced with the described protocol is translucent and does not form a clot at 37°C. Thus, it was not necessary to add heparin or any other anticoagulant to the cell culture. The protein concentration was 2.53 ± 0.28 mg/ml.
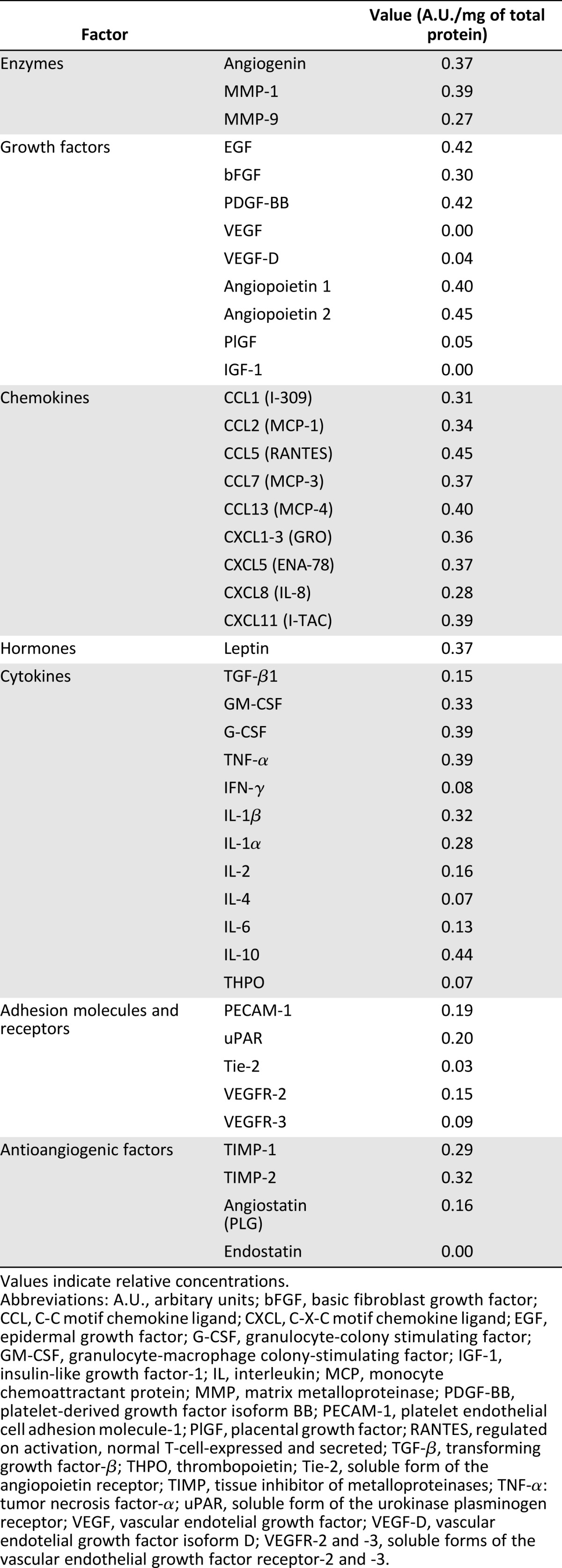
The relative concentration of 43 different proteins, including EGF, bFGF, PDGF-BB, monocyte chemoattractant protein-1 (MCP-1), TGF-β1, granulocyte-macrophage colony-stimulating factor, and granulocyte colony-stimulating factor (G-CSF), was quantified. The presence of IGF-1, VEGF, and endostatin was not detected. On the other hand, some other proteins, such as angiogenin, EGF, bFGF, MCP-1, angiopoietin, G-CSF, matrix metalloproteinase-9 (MMP-9), C-X-C motif chemokine ligand-5 (CXCL-5), C-C motif chemokine ligand-1, and platelet endothelial cell adhesion molecule-1 were found in high concentrations, with values between 0.12 and 0.45 A.U./mg of total protein (Table 1).
Table 1.
Relative concentration of angiogenic factors, detected in the human platelet lysate with a human angiogenesis antibody array
Isolation and Culture of hASC
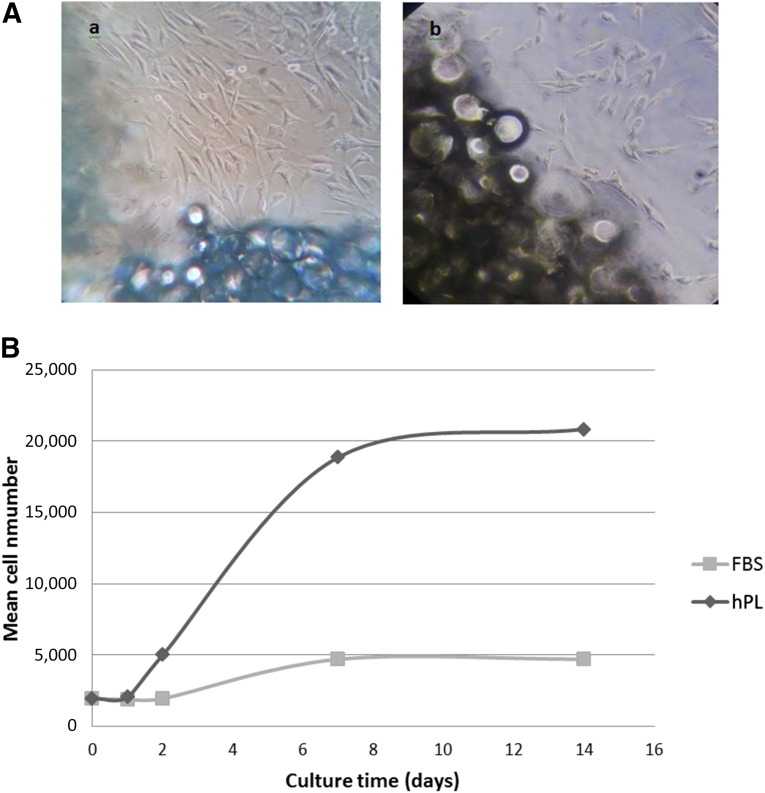
Spindle-shaped cells began to proliferate from the explants after 2 days of culture and reached 80% confluence after 1 or 2 weeks of culture. These cells were uniform in shape and size and exhibited the characteristic morphology of hASC. However, migration and cell proliferation were greater in the explants cultured in CM-hPL (Fig. 2A).
Figure 2.
Proliferation of human adipose-derived stem cells (hASCs). (A): Proliferation of hASCs from adipose tissue explant. (Aa): Two weeks of incubation in complete media human platelet lysate (CM-hPL). (Ab): Two weeks of incubation in complete media fetal bovine serum (CM-FBS). Magnification, ×20. (B): Curves for hASC proliferation. The values correspond to the average of three replicates of hASC cultured in CM-hPL or CM-FBS. Abbreviations: FBS, fetal bovine serum; hPL, human platelet lysate.
Cell morphology was preserved as the cells were replicated throughout the study, and no differences were evident between the samples. Cells isolated from a sample, randomly selected, were used for the proliferation evaluation. The cellular differentiation potential was evaluated in cells from all samples, and the immunophenotype was evaluated in cells from two randomly selected samples.
hASC Characterization
After 7 days of culture, the cumulated number of population doublings had reached 3.39 for hASCs cultured in CM-hPL and 1.23 for hASCs cultured in CM-FBS. The population doubling time after 7 days of culture was 2.16 days for hASCs cultured in CM-hPL and 5.65 days for hASCs cultured in CM-FBS (Fig. 2B).
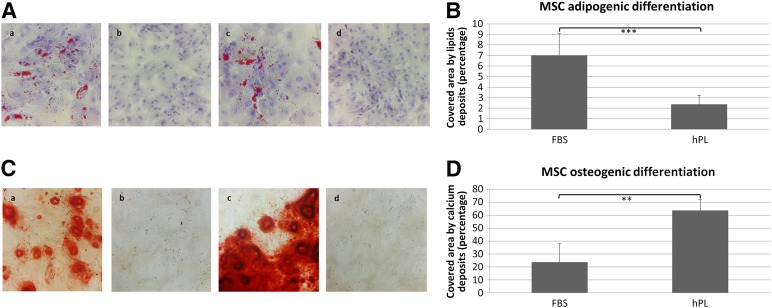
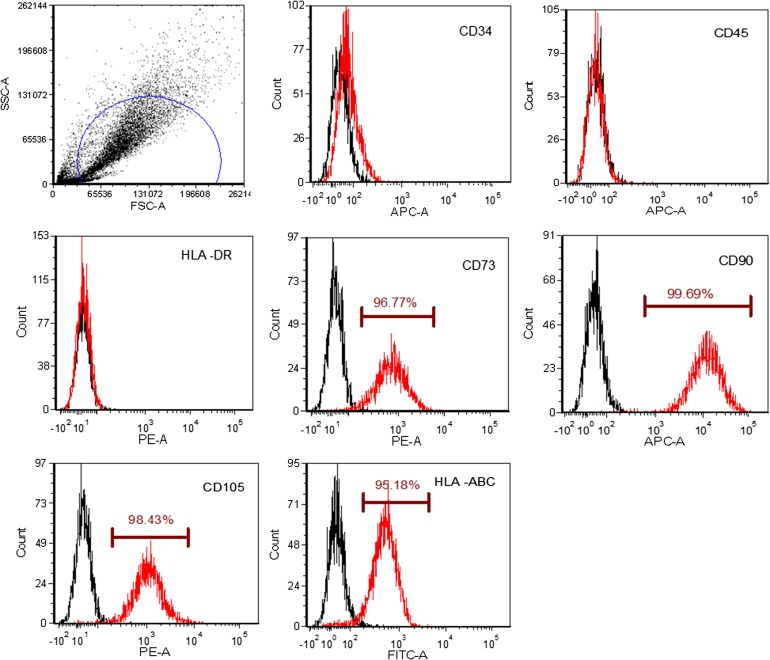
Both the xeno-free hASCs (CM-hPL) and the standard conditions (CM-FBS) produced hASC and showed osteogenic and adipogenic differentiation potential (Fig. 3A, 3C), but the xeno-free hASCs showed a significantly greater osteogenic differentiation (Fig. 3C, 3D; p = .000001) and less adipogenic differentiation than the hASCs produced under standard conditions (Fig. 3A, 3B; p = .003). Finally, a high percentage of the xeno-free hASCs expressed CD73 (96.77%), CD90 (99.69%), CD105 (98.43%), and HLA-ABC (95.18%), without CD34, CD45, or HLA-DR expression, a characteristic hASC immunophenotype (Fig. 4).
Figure 3.
Differentiation potential of human adipose-derived stem cells (hASCs). (A): Adipogenic differentiation of fourth-passage hASCs was evaluated in cells cultured for 2 weeks in adipocyte differentiation medium stained with Oil Red O to detect intracellular fat droplets (red). All experiments (n = 4) were performed in triplicate. Shown are hASCs produced under standard conditions (Aa), negative control (Ab), xeno-free-produced hASCs (Ac), and negative control (Ad). Magnification, ×20. (B): Quantification of the adipogenic differentiation. Percentage of surface area covered by lipid intracellular deposits in hASCs, stained with O Red Oil. ∗∗, p ≤ 0.01. (C): Osteogenic differentiation of fourth-passage hASCs was evaluated in cells cultured for 2 weeks in osteogenic differentiation medium stained with alizarin red to detect interstitial calcium deposits (red). All experiments (n = 4) were performed in triplicate. Shown are hASCs produced under standard conditions (Ca), negative control (Cb), xeno-free-produced hASCs (Cc), and negative control (Cd). Magnification, ×20. (D): Quantification of the osteogenic differentiation. Percentage of surface area covered by interstitial calcium deposits in hASC cultures, stained with alizarin red. ∗∗∗, p ≤ 0.001. Abbreviations: FBS, fetal bovine serum; hPL, human platelet lysate; MSC, mesenchymal stem cell.
Figure 4.
Immunophenotyping of human adipose-derived stem cells (hASCs). Representative flow cytometry analysis of fourth-passage hASCs. The cells were cultured in complete media human platelet lysate and incubated with monoclonal antibodies (CD34-APC, CD45-RPECy5, HLA II-RPE, CD73-PE, CD90-APC, CD105-PE, HLA I-FITC) or their respective isotype controls.
hASCs Showed Higher Viability After Cryopreservation in hPL
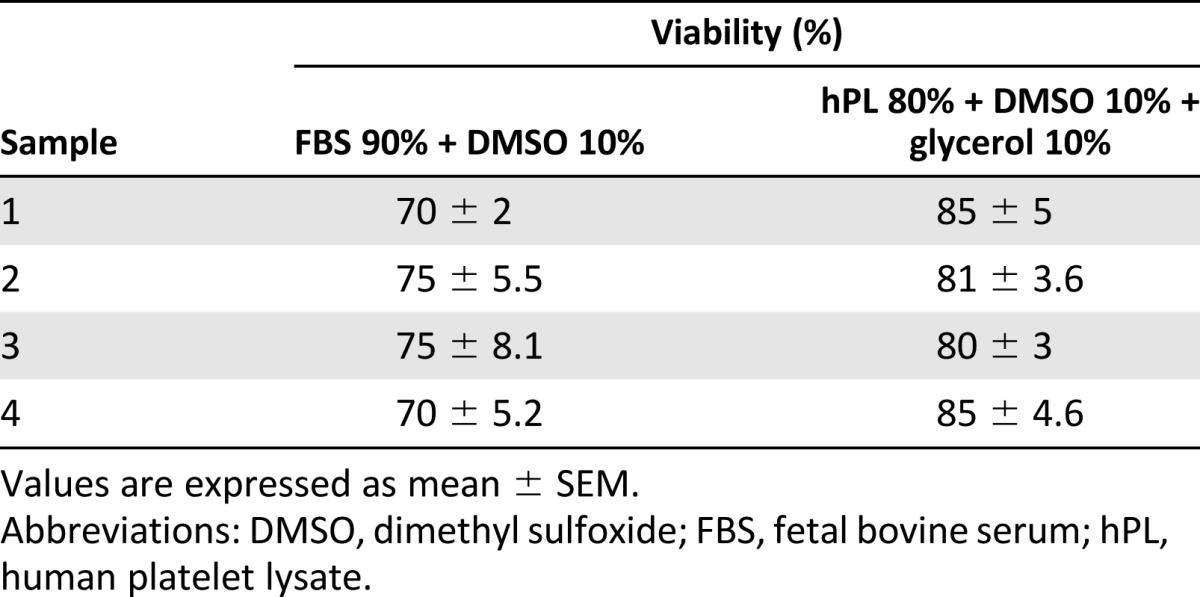
The hPL produced with our protocol could also be used for hASC cryopreservation. The hASCs cryopreserved in the hPL 80% + DMSO 10% + glycerol 10% solution showed 82.5% ± 2.88% viability after cryopreservation compared with 72.5% ± 2.88% after cryopreservation in an FBS-based solution (FBS 90% + DMSO 10%) (Table 2).
Table 2.
Cellular viability of human adipose-derived stem cells cryopreserved in human platelet lysate-based or fetal bovine serum-based solutions
Discussion
The production of xeno-free hASCs has been a factor hindering evaluation of its therapeutic efficacy. FBS has been used as supplement for the MSC production, including cells for clinical trials. There are several problems with FBS as a supplement for MSC production for human applications [21] besides the risk for zoonosis transmission [22, 47]. The MSCs can internalize components of the cell culture medium and those molecules could induce an immune rejection [14, 48, 49]. Stem cells cultured in FBS express a nonhuman immunogenic sialic acid form (Neu5Gc) [50], and the cell lysis is mediated by human antibodies against Neu5Gc [39]. Moreover, the immune reaction against FBS antigens in individuals who received several dose of MSC produced with FBS supplement [48] and the development of a diffuse urticariform reaction [13, 49] have been reported.
A xeno-free protocol to produce BMSCs using hPL [24] has already been reported, but there are no reports of alternatives to produce xeno-free ASCs. A previous study showed hPL is a feasible substitute for FBS [45]. hPL facilitates ASC proliferation more efficiently than does FBS [27, 28], favors genomic stability [23], and maintains the differentiation potential and expression of characteristic surface markers [29, 30].
Clot formation is the main problem seen with replacing FBS by hPL. There are different alternatives to avoid the fibrin clot in the culture medium. Perhaps the most used strategy is addition of heparin, but this anticoagulant has adverse effects on the cells, evidenced by a reduction of their proliferation [51]. In addition, seroconversion of the hPL has been proposed, inducing the coagulation and recovering the soluble proteins [52]. However, although the proliferation and phenotype conservation of the MSCs cultured in seroconverted hPL has been shown, a significant reduction in PDGF-BB concentration has been documented; this could have important effects on MSC phenotype in long-term cell cultures [40–42].
We have modified the hPL production protocol, replacing the plasma with PBS and avoiding the clot formation. Using this hPL, we have developed a xeno-free extraction, culture, and cryopreservation protocol for hASCs, which could be used for the production of BMSCs or umbilical cord blood-derived stem cells.
There is no standard protocol for hPL production, but it is known that growth factors such as PDGF-BB, EGF, TGF-β, bFGF, VEGF, and IGF-1 are among the most important components. However, assays with different combinations of the recombinant proteins have not reproduced hPL effects [39, 43].
Our results show some similarities and differences with previous reports. We have found high concentrations of proteins such as EGF, angiogenin, angiopoyetin-1, G-CSF, MMP-9, and CXCL-5 in the hPL; however, have not found IFG-1, VEGF, or endostatin [31, 39]. The absence of IGF-1 its not surprising, considering that it is mainly a plasma protein [53], and the platelet α granules have a very low concentration [54]. On the other hand, the absence of VEGF and endostatin could be a consequence of the level of sensitivity of the detection method or a different lysis efficiency for some subpopulations of α granules [55]
It has been reported that hASCs extracted from adipose tissue explants meet the identification criteria for MSCs, including their differentiation potential and the expression of commonly used markers for their identification [18, 19]. In addition, studies have documented that the hASCs cultured in hPL-supplemented medium retain their phenotype characteristics [32, 33]. Here we have shown that the hASCs extracted by explant and cultured in hPL-supplemented media retain all their phenotypic characteristics.
The xeno-free hASCs produced with our protocol have adipogenic and osteogenic differentiation potential. However, they vary in their differentiation potential in comparison with the hASCs produced under standard conditions. We documented a higher osteogenic differentiation of xeno-free hASCs, confirming previous reports [34, 35]. We also observed a lower adipogenic differentiation potential of xeno-free hASCs, which has also been suggested previously [24].
Conclusion
We have developed a sequential and ordered protocol for extraction, culture, and cryopreservation of xeno-free hASCs that can be safely applied as a therapeutic alternative for regenerative medicine.
Acknowledgments
This work was supported by the Universidad Nacional de Colombia, División de Investigaciones (QUIPU 201010018594), and the Fundación Universitaria de Ciencias de la Salud, División de Investigaciones. We thank the Hemocentro Distrital de Bogotá for excellent support in the implementation of this research.
Author Contributions
C.H.E.: financial support, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing; O.C.: conception and design, financial support, administrative support, provision of study material, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.H.E. has compensated employment and research funding from Fundación Universitaria de Ciencias de la Salud. O.C. has compensated employment and has uncompensated intellectual property rights from Universidad Nacional de Colombia.
References
- 1.Phinney DG, Sensebe L.Mesenchymal stromal cells: Misconceptions and evolving concepts Cytotherapy 2013;15:140–145. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 6.Peng L, Jia Z, Yin X, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761–773. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 7.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Toni F, Poglio S, Youcef AB, et al. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: A key step for therapeutic studies. Stem Cells Dev. 2011;20:2127–2138. doi: 10.1089/scd.2011.0044. [DOI] [PubMed] [Google Scholar]
- 10.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 12.Konno M, Hamabe A, Hasegawa S, et al. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ. 2013;55:309–318. doi: 10.1111/dgd.12049. [DOI] [PubMed] [Google Scholar]
- 13.Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89:776–779. [PubMed] [Google Scholar]
- 14.Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 16.Quirici N, Scavullo C, de Girolamo L, et al. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 2010;19:915–925. doi: 10.1089/scd.2009.0408. [DOI] [PubMed] [Google Scholar]
- 17.Murray IR, West CC, Hardy WR, et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing W, Xiao J, Xiong Z, et al. Explant culture: An efficient method to isolate adipose-derived stromal cells for tissue engineering. Artif Organs. 2011;35:105–112. doi: 10.1111/j.1525-1594.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 19.Linero IM, Doncel A, Chaparro O. [Proliferation and osteogenic differentiation of mesenchymal stem cells in hydrogels of human blood plasma] Biomedica. 2014;34:67–78. doi: 10.1590/S0120-41572014000100010. [DOI] [PubMed] [Google Scholar]
- 20.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014;9:e107001. doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown P, Will RG, Bradley R, et al. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: Background, evolution, and current concerns. Emerg Infect Dis. 2001;7:6–16. doi: 10.3201/eid0701.010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trojahn Kølle SF, Oliveri RS, Glovinski PV, et al. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086–1097. doi: 10.1016/j.jcyt.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 24.Warnke PH, Humpe A, Strunk D, et al. A clinically-feasible protocol for using human platelet lysate and mesenchymal stem cells in regenerative therapies. J Craniomaxillofac Surg. 2013;41:153–161. doi: 10.1016/j.jcms.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Fekete N, Rojewski MT, Furst D, et al. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7:e43255. doi: 10.1371/journal.pone.0043255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinzebach S, Bieback K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. Adv Biochem Eng Biotechnol. 2013;129:33–57. doi: 10.1007/10_2012_134. [DOI] [PubMed] [Google Scholar]
- 27.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 28.Schallmoser K, Bartmann C, Rohde E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 29.Jonsdottir-Buch SM, Lieder R, Sigurjonsson OE. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2013;8:e68984. doi: 10.1371/journal.pone.0068984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths S, Baraniak PR, Copland IB, et al. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–1483. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Crespo-Diaz R, Behfar A, Butler GW, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 34.Chevallier N, Anagnostou F, Zilber S, et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31:270–278. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 35.van den Dolder J, Mooren R, Vloon AP, et al. Platelet-rich plasma: Quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 36.Harrison P. Platelet function analysis. Blood Rev. 2005;19:111–123. doi: 10.1016/j.blre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Zufferey A, Fontana P, Reny JL, et al. Platelet proteomics. Mass Spectrom Rev. 2012;31:331–351. doi: 10.1002/mas.20345. [DOI] [PubMed] [Google Scholar]
- 38.Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Fekete N, Gadelorge M, Furst D, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fierro F, Illmer T, Jing D, et al. Inhibition of platelet-derived growth factor receptorbeta by imatinib mesylate suppresses proliferation and alters differentiation of human mesenchymal stem cells in vitro. Cell Prolif. 2007;40:355–366. doi: 10.1111/j.1365-2184.2007.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim WS, Park HS, Sung JH. The pivotal role of PDGF and its receptor isoforms in adipose-derived stem cells. Histol Histopathol. 2015;30:793–799. doi: 10.14670/HH-11-598. [DOI] [PubMed] [Google Scholar]
- 42.Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chieregato K, Castegnaro S, Madeo D, et al. Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor-bb can substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tissue. Cytotherapy. 2011;13:933–943. doi: 10.3109/14653249.2011.583232. [DOI] [PubMed] [Google Scholar]
- 44.Zeisberger SM, Schulz JC, Mairhofer M, et al. Biological and physicochemical characterization of a serum- and xeno-free chemically defined cryopreservation procedure for adult human progenitor cells. Cell Transplant. 2011;20:1241–1257. doi: 10.3727/096368910X547426. [DOI] [PubMed] [Google Scholar]
- 45.Bieback K, Hecker A, Kocaomer A, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 46.Fan J, Park H, Tan S, et al. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2. PLoS One. 2013;8:e72474. doi: 10.1371/journal.pone.0072474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Medicines Agency, Committee for Medicinal Products for Human Use. Guideline on the use of bovine serum in the manufacture of human biological medicinal products (EMA/CHMP/BWP/457920/2012 rev 1). Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/06/WC500143930.pdf. Accessed September 4, 2015 [Google Scholar]
- 48.Sundin M, Ringden O, Sundberg B, et al. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 49.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 51.Hemeda H, Kalz J, Walenda G, et al. Heparin concentration is critical for cell culture with human platelet lysate. Cytotherapy. 2013;15:1174–1181. doi: 10.1016/j.jcyt.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Mojica-Henshaw MP, Jacobson P, Morris J, et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy. 2013;15:1458–1468. doi: 10.1016/j.jcyt.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Le Roith D. The insulin-like growth factor system. Exp Diabesity Res. 2003;4:205–212. doi: 10.1155/EDR.2003.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karey KP, Marquardt H, Sirbasku DA. Human platelet-derived mitogens. I. Identification of insulinlike growth factors I and II by purification and N alpha amino acid sequence analysis. Blood. 1989;74:1084–1092. [PubMed] [Google Scholar]
- 55.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]