A simple, low-cost system to enhance the preservation of human adipose-derived stem cells stored at hypothermic temperatures, while maintaining their normal function, is presented. This system has great potential for extending the time windows for quality assurance and efficacy testing, distribution between the sites of manufacture and the clinic, and reducing the wastage associated with the limited shelf life of cells stored in their liquid state.
Keywords: Adipose tissue, Alginate, Biological preservation, Cell therapy, Mesenchymal stem cell
Abstract
Despite considerable progress within the cell therapy industry, unmet bioprocessing and logistical challenges associated with the storage and distribution of cells between sites of manufacture and the clinic exist. We examined whether hypothermic (4°C–23°C) preservation of human adipose-derived stem cells could be improved through their encapsulation in 1.2% calcium alginate. Alginate encapsulation improved the recovery of viable cells after 72 hours of storage. Viable cell recovery was highly temperature-dependent, with an optimum temperature of 15°C. At this temperature, alginate encapsulation preserved the ability for recovered cells to attach to tissue culture plastic on rewarming, further increasing its effect on total cell recovery. On attachment, the cells were phenotypically normal, displayed normal growth kinetics, and maintained their capacity for trilineage differentiation. The number of cells encapsulated (up to 2 × 106 cells per milliliter) did not affect viable cell recovery nor did storage of encapsulated cells in a xeno-free, serum-free,current Good Manufacturing Practice-grade medium. We present a simple, low-cost system capable of enhancing the preservation of human adipose-derived stem cells stored at hypothermic temperatures, while maintaining their normal function. The storage of cells in this manner has great potential for extending the time windows for quality assurance and efficacy testing, distribution between the sites of manufacture and the clinic, and reducing the wastage associated with the limited shelf life of cells stored in their liquid state.
Significance
Despite considerable advancement in the clinical application of cell-based therapies, major logistical challenges exist throughout the cell therapy supply chain associated with the storage and distribution of cells between the sites of manufacture and the clinic. A simple, low-cost system capable of preserving the viability and functionality of human adipose-derived stem cells (a cell with substantial clinical interest) at hypothermic temperatures (0°C–32°C) is presented. Such a system has considerable potential for extending the shelf life of cell therapy products at multiple stages throughout the cell therapy supply chain.
Introduction
The medical applications of cell-based therapies have expanded considerably in recent years, with an ever-increasing number of clinical trials being registered globally, many of which are in the third phase of development. These encompass treatments for a wide range of conditions, including cardiovascular disease, neurodegenerative diseases, cancer, liver disease, diabetes, skeletal disorders, and eye diseases [1–3]. Accompanying this increasing intensity in cell-based clinical research is the emergence of a number of Food and Drug Administration (FDA)- and European Medicines Agency-approved cell therapy products (CTPs) to the market [4–9].
Hypothermic storage involves maintaining a living biologic agent in a suspended state at temperatures above 0°C but below the mammalian normothermic range of 32°C–37°C [10]. Owing to the biological and technical issues associated with cryopreservation, including the required use of cryoprotective agents, such as dimethyl sulfoxide, which can be costly to remove [11], and the risk of thawing, with a hold time as short as 2 hours before a loss in cell viability occurs [12], most approved CTPs have adopted hypothermic storage. This means they are manufactured “just in time” in relatively small unit numbers rather than being stockpiled; keeping production costs low [13]. It does, however, mean that the storage time is restricted before the cells will experience considerable loss in viability and function. This could not only affect the therapeutic potency of the CTP, but also introduce variability in the final product—an unfavorable situation for current Good Manufacturing Practice (cGMP) compliance.
Methods that improve the hypothermic preservation of cell viability, function, and therapeutic potency offer a number of benefits throughout the complex CTP supply chain: (a) extending the period for quality, safety, and efficacy testing; (b) allowing distribution from the donor or bank to sites of processing; and (c) allowing delivery to the clinic and reducing the wastage associated with inadequate scheduling between different sites [14–16].
Human adipose-derived stem cells (hASCs) have received much attention as a source of mesenchymal stem cells (MSCs) for cell therapy applications in recent years, with more than 130 clinical trials registered between February 2007 and April 2015 [17]. Despite this growing interest, the effect of hypothermic storage on hASC viability and function has been limited. We examined encapsulation in alginate hydrogels as a method to support hASCs during hypothermic storage.
Alginate is a natural polysaccharide derived from seaweed that polymerizes rapidly in the presence of cations to form a biocompatible hydrogel [18] and, as such, has gained considerable interest as a biomaterial for cell therapy applications [18–21]. Alginate encapsulation has previously been investigated for its protective effect on the hypothermic storage of rat hepatocytes, recombinant baby hamster kidney cells, human bone marrow-derived MSCs, mouse embryonic stem cells (ESCs), and human limbal epithelial stem cells [22–25]. Its potential to heighten the preservation of cells during hypothermic storage has been postulated to result from its contribution to membrane stabilization and subsequent protection against the osmotic shock and mechanical stress experienced during storage and recovery [23].
We describe the encapsulation of hASCs in alginate hydrogels. We investigated the conditions necessary for cell survival and function over short-term periods in hypothermic storage and have demonstrated the effectiveness of alginate encapsulation in preserving cell viability, revealing the importance of controlling the storage temperature for maximal viable cell recovery. Using an optimal storage temperature, we have demonstrated the effectiveness of alginate encapsulation to preserve the ability of hASCs to attach and regain metabolic activity on return to normothermia. We propose that this should be considered when defining viable cell recovery. We have further demonstrated that hypothermic storage maintains the expression of key stem cell markers and does not affect the capacity for cells to proliferate and differentiate into osteo-, adipo-, and chondrogenic lineages. Finally, we explored scalability and adaptation to a serum-free, xeno-free storage system.
Taken together, we present alginate encapsulation as a low-cost, simple method to improve the hypothermic preservation of hASCs, which has considerable potential in extending the shelf life of hypothermically stored CTPs for distribution throughout the cell therapy supply chain.
Materials and Methods
hASC Culture
hASCs obtained from the subcutaneous fat of 3 healthy donors (Invitrogen, Glasgow, U.K., http://www.invitrogen.com) were used for all the experiments. Cells were isolated from both male and female subjects (aged 45–63 years), and purity was determined by the manufacturer as cells being ≥95% positive for CD29, CD44, CD73, CD90, CD105, and CD166 and ≤2% positive for CD14, CD31, and CD45 surface antigen expression. Following recovery from cryostorage, the cells were seeded at 800 cells per cm2 and maintained in reduced-serum (RS) growth medium (MesenPRO RS medium containing 2 mM GlutaMAX and 1% [vol/vol] antibiotic-antimycotic [all from ThermoFisher Scientific, Cramlington, U.K., http://www.thermofisher.com]) in a humidified incubator at 37°C, 5% CO2 with medium changes every 3–4 days until approximately 80% confluence was reached. Cells were harvested using TrypLE Express enzyme (ThermoFisher Scientific).
Encapsulation, Storage, and Release of hASCs
The harvested cells were incubated with 1 μM Calcein-AM (eBioscience, Holmfirth, U.K., http://www.ebioscience.com) for 15 minutes before counting viable (stained) cells using a Countess II FL automated cell counter (Invitrogen). A 0.25-ml suspension of 0.5–2 × 106 cells in growth medium was mixed with 0.25 ml of 2.4% (wt/vol) sodium alginate (Sigma-Aldrich, Gillingham, U.K., http://www.sigmaaldrich.com) in phosphate-buffered saline (PBS) (Invitrogen) before casting into calcium alginate discs using 102 mM calcium chloride, as described previously [22]. Following a brief wash in PBS, the gelled discs were transferred into 2-ml cryovials containing 1 ml of growth medium, sealed, and placed in a polystyrene box before transferring to an actively cooled incubator set to a specified temperature (11°C–23°C) or refrigerator (4°C). Control (nonencapsulated) cell suspensions (0.25 ml) were added directly to 2-ml cryovials containing 1 ml of growth medium before storing in the same manner as the encapsulated samples. Following 72 hours of storage (during which time the cells were left undisturbed), the cells were allowed to equilibrate to room temperature before dissolving the calcium alginate hydrogel encapsulating them using 3 ml of 100 mM trisodium citrate (Sigma-Aldrich) for 5 minutes at room temperature, with control samples treated in the same manner. The effect of trisodium citrate treatment on the control samples was additionally compared against nontreated cells. Trisodium citrate was diluted using PBS before separating the cells by centrifugation and dissociating them with TrypLE Express enzyme for 5 minutes (necessary because of aggregation of the control samples). An equal volume of growth medium was added, and the cells were thoroughly resuspended before counting. For serum-free conditions, storage was set up as above but growth medium was substituted for either StemPro MSC serum-free medium (SFM) or HypoThermosol-FRS (H-FRS; Sigma-Aldrich). For 4°C experiments in H-FRS, cells were added directly to chilled H-FRS and were not allowed to equilibrate to room temperature before release, as per the manufacturer’s instructions.
Assessment of Viable Cell Recovery
The number of viable cells released from hypothermic storage was enumerated by incubating cells with 1 μM Calcein-AM for 15 minutes at 37°C before counting the viable (stained) cells using a Countess II FL automated cell counter (Invitrogen).
Assessment of Apoptosis and Cell Death
Cells recovered from hypothermic storage were assessed for apoptosis and cell death using an Annexin V-FITC Apoptosis Detection Kit (eBioscience) according to the manufacturer’s instructions. Staining was analyzed using a Canto II flow cytometer (BD Biosciences, Oxford, U.K., http://www.bdbiosciences.com), and the cells were classified as viable (unstained), early apoptotic (Annexin V-positive), or late-apoptotic/dead (Annexin V- and/or propidium iodide-positive).
Functional Assessment of hASCs Before and After Storage
hASCs were examined for their capacity to reattach, regain metabolic activity, proliferate, and differentiate into osteo-, adipo-, and chondrogenic lineages. Stored samples were compared with nonstored control (NSC) samples obtained from the same cultures harvested before storage that were plated, incubated, and assessed using the exact same method as detailed below.
Assessment of Attachment and Metabolic Activity
Following release from storage, the cells were plated at 20,000 cells per cm2 in 24-well plates and incubated for 24 hours at 37°C and 5% CO2 in a humidified incubator. Metabolic activity was then assessed using the alamarBlue assay as described in [26]. After incubation at 37°C for 3 hours, fluorescence was read at an excitation wavelength of 530–560 nm and emission wavelength of 590 nm using a Fluoroskan Ascent FL plate reader (Thermo Scientific, Loughborough, U.K., http://www.thermoscientific.com). The same cultures were then assessed for the attached cell number using the methylene blue-based assay for proliferation as described in [27]. Following elution of the methylene blue stain, absorption was read at 650 nm using an Infinite F50 plate reader (Tecan, Weymouth, U.K., http://www.tecan.com), and the attached cell number was determined using a standard curve.
Assessment of Proliferative Capacity
Cells were plated at 5,000 cells per cm2 in 24-well plates and incubated for 3, 6, 9, 12, and 15 days at 37°C, 5% CO2 in a humidified incubator. The cell number was determined using the methylene blue-based assay for proliferation (as above). Absorption was read at 650 nm using an Infinite F50 plate reader (Tecan), and the cell number was determined using a standard curve.
Trilineage Differentiation Induction
For osteo- and adipogenic differentiation, the previously stored cells were plated at 5,000 cells per cm2 in 96-well plates in growth medium for 4 days at 37°C, 5% CO2 in a humidified incubator, changing the medium after 2 days, before replacing the growth medium with either osteogenic or adipogenic media (StemPro Osteogenesis or Adipogenesis Differentiation Kits containing 1% [vol/vol] antibiotic-antimycotic [all from ThermoFisher Scientific]) and maintaining for up to 31 days, with media changes every 2–3 days. For chondrogenic differentiation, 5 μl of growth medium containing 80,000 cells was seeded into the center of 96-well round-bottomed plates and incubated for 2 hours at 37°C. Next, 200 μl of chondrogenic medium (StemPro Chondrogenesis Differentiation Kit containing 1% [vol/vol] antibiotic-antimycotic [both from ThermoFisher Scientific]) was added, and cultures were maintained at 37°C, 5% CO2 in a humidified incubator for up to 35 days with media changes every 2–3 days. Noninduced controls for all cultures were maintained in growth medium for the same period.
Assessment of Differentiation
All differentiation cultures were fixed with 10% (vol/vol) formaldehyde for 1 hour at room temperature and washed three times with double-distilled H2O (ddH2O) before staining. Osteogenic differentiation was examined by staining mineralized matrices with 1 mg/ml Alizarin Red S (pH 5.5; ammonium hydroxide) as described in [28]. Adipogenic differentiation was assessed by staining lipid droplets with 1.8 mg/ml Oil Red O in isopropanol as described in [29], both at room temperature for 30 minutes with gentle agitation. Excess stain was removed with four sequential washes with ddH2O before capturing the images. Alizarin Red S stain was eluted with 150 μl 10% (vol/vol) acetic acid (as adapted from [28]) for 30 minutes at room temperature with gentle agitation before removing 100-μl aliquots and determining absorbance at 405 nm. Oil Red O stain was washed for 5 minutes with 60% isopropanol before eluting with 150 μl of isopropanol containing 4% IGEPAL CA-630 (Sigma-Aldrich) for 30 minutes at room temperature, removing 100-μl aliquots, and determining absorbance at 520 nm. Alizarin Red S and Oil Red O staining were quantified using a standard curve of known concentrations, and absorbance was read using an Infinite F50 plate reader (Tecan). Chondrogenic differentiation was assessed by staining cartilage-associated glycosaminoglycans with 0.1 mg/ml Alcian Blue 8GX (Sigma-Aldrich) in six parts ethanol to two parts acetic acid at room temperature overnight in the dark. Nonspecific staining was washed four times with six parts ethanol to four parts acetic acid before capturing images. All components for staining and elution were purchased from Sigma-Aldrich.
Assessment of Cell Surface Marker Expression
Following release from storage, the cells were resuspended in sterile PBS and labeled by incubation with the fluorochrome-conjugated primary antibodies for 25 minutes at room temperature in the dark. After incubation, the samples were washed with sterile PBS and centrifuged at 1,500 rpm for 5 minutes. The cells were then resuspended in 300 μl of PBS and analyzed with a Canto II flow cytometer (BD Biosciences). The antibody panel used was composed of antibodies against CD14 and CD45 as negative MSC markers, CD90 and CD73 as positive MSC markers, CD29, CD44, and CD166 as adhesion markers present on the surface of MSCs, and human leukocyte antigen (HLA)-ABC and HLA-DR. The samples were run at a medium flow rate (60 μl/min). Isotype controls were performed with antibodies against IgG1 and 10,000 events, excluding cellular debris, were analyzed for each sample. All antibodies were obtained from BD Biosciences, with the exception of the IgG1 isotype control (Beckman-Coulter, High Wyecombe, U.K., http://www.beckmancoulter.com) and anti-CD90 (Dako, Cambridgeshire, U.K., http://www.dako.com) antibodies.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism, version 4.00 (GraphPad Software, San Diego, CA, http://www.graphpad.com). Data are expressed as the mean of values from three separate donors ± SEM. Statistical comparisons were made using one-way or two-way repeated measures analysis of variance with Bonferroni post hoc tests, with the exception of comparisons between two single variables for which paired two-tailed t tests were used. Values of p < .05 were considered significant (∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001).
Results
Effect of Storage Temperature on Viable Cell Recovery
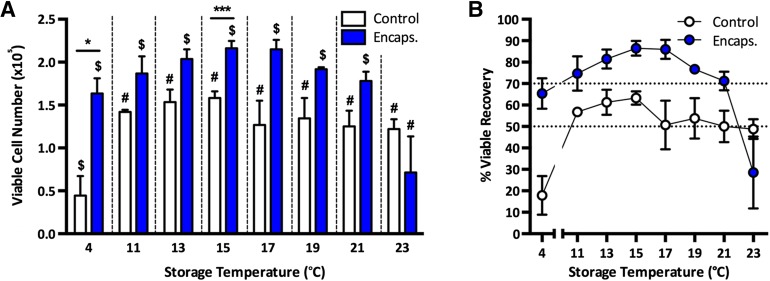
In order to elucidate the storage temperature that would achieve the greatest recovery of cells, we encapsulated hASCs in 1.2% alginate discs and stored them for 72 hours at various temperatures, comparing viable cell recovery with nonencapsulated controls. We found that hASCs were surprisingly sensitive to deviations in changes in storage temperature over 72 hours. At 4°C, nonencapsulated (control) samples demonstrated a dramatic decrease in viable recovery, yielding only 17.8% ± 15.6% of viable cells initially stored; a significantly lower recovery compared with any other temperature examined (Fig. 1A, 1B). In contrast, encapsulated cells exhibited a 3.7 ± 0.7-fold increase in the number of viable cells recovered compared to control (p = .0224). Temperature had no significant effect on the viable cell recovery of control samples at any other temperature tested but did increase from 11°C, reaching an optimum storage temperature at 15°C (63% ± 5% viable recovery) before demonstrating greater variability between 17°C and 23°C, with an average viable recovery of approximately 50% (Fig. 1A, 1B). Similarly, 15°C was the optimum temperature for storage of alginate-encapsulated cells, achieving a viable cell recovery of 86% ± 6%, a significantly higher recovery compared with that of the control samples (p = .0002; Fig. 1A, 1B). At temperatures below or above 15°C, viable cell recovery did decrease slightly after storage in alginate; however, only at 23°C was a significant decrease in cell recovery found compared with all other temperatures tested, with a heightened level of variability in the percentage viable recovery (29% ± 29%; Fig. 1A, 1B). The storage of encapsulated hASCs at 13°C–19°C consistently delivered a percentage of viable recovery greater than 70%, the minimum acceptable viability specification generally set by the FDA for cellular products [30], and this was clearly not achieved without alginate encapsulation (Fig. 1A, 1B). As 15°C storage resulted in the greatest viable cell recovery in both control and encapsulated samples, subsequent experiments were conducted at this storage temperature.
Figure 1.
The effect of storage temperature on viable cell recovery. Human adipose-derived stem cells were stored at different temperatures, either encapsulated or control, for 72 hours before assessing the viable cell number (A) and percentage of viable recovery (B). Values are expressed as mean ± SEM from 3 separate donors, with asterisks representing significance from control values (∗∗∗, p < .001; ∗, p < .05) and symbols representing significance between temperatures ($, #, p < .05). Abbreviations: Control, nonencapsulated; Encaps., encapsulated.
Assessment of Apoptosis and Cell Death in Stored-Cell Populations
As well as determining viable cell recovery after 72 hours of hypothermic storage, we aimed to assess the contribution of apoptosis and death in recovered cell populations. Although nonencapsulated (control) samples predominantly exhibited a viable population of cells (Fig. 2Ai, 2B), this varied (77% ± 14.2%) with contribution of propidium iodide-stained dead cells, and propidium iodide and Annexin V costained late-apoptotic cells (11.3% ± 7.2% and 8.6% ± 7.1%, respectively). Neither control (Fig. 2Ai) nor encapsulated (Fig. 2Aii) samples exhibited a large population of Annexin V-stained early apoptotic cells (2.7% ± 0.4% and 2.7% ± 0.9%, respectively). Encapsulated cells consistently exhibited a high level of viability (92% ± 2.3%), confirming a healthy population of recovered cells with little contribution of apoptosis or death (Fig. 2Aii, 2B). Thus, encapsulation both enhanced viability and reduced variability in the health of the cell populations recovered from hypothermic storage.
Figure 2.
Assessment of apoptosis and cell death in stored cell populations. Human adipose-derived stem cells were stored at 15°C for 72 hours before assessing the percentage of cells positive for Annexin V and/or propidium iodide in control (Ai) and encapsulated (Aii) cell populations. Cells were classified as viable (unstained), early apoptotic (Annexin V-positive), or late-apoptotic/dead (Annexin V- and/or propidium iodide-positive) (B). Values are expressed as mean ± SEM from 3 separate donors. Abbreviations: Control, nonencapsulated; Encaps., encapsulated.
Attachment, Metabolic Activity, and Total Viable Recovery of Stored Cells
In addition to examining the viability of cells after release from storage, it was also important to perform subsequent tests to ensure the cells were functional. We, therefore, examined the ability for stored cells to reattach to tissue culture plastic and recover metabolic activity 24 hours after returning to a cell culture environment. Although nonencapsulated (control) hASCs exhibited a significant decrease in attached cell number compared with the NSC, the encapsulated cells did not (Fig. 3A). A similar trend was observed with the percentage of metabolic activity of cultures, although the difference was not significant (Fig. 3B). Furthermore, when normalized to cell number and expressed as the fold change relative to the NSC (Fig. 3C), no change in metabolic activity per cell was evident. Using these data, a value can be calculated that represents the total percentage of recovery (i.e., the percentage of viable cells able to attach and recover metabolic activity [total % recovery = % viable recovery × (fold change in attachment × fold change in metabolic activity/cell)]). Using this equation, the protective effect of encapsulation in preserving functional cell recovery is highlighted (Fig. 3D), and control conditions only yielded a 45% ± 3% recovery, the alginate-encapsulated samples achieved a significantly higher 80% ± 3% recovery (p = .0053). As trisodium citrate was required for the dissolution of alginate for cell release, and this variable was matched between the control and encapsulated samples, it was also important to determine whether citrate itself could have influenced the outcome with regard to the control. We determined that trisodium citrate had no significant effect on cell recovery, attachment, metabolic activity, or total percentage recovery compared with nontreated controls (supplemental online Fig. 1A–1D). Both yielded a total percentage of recovery similar to the control presented in the present study of 42% ± 1% and 43% ± 4% in citrate-treated and nontreated samples, respectively.
Figure 3.
Attachment, metabolic activity, and total cell recovery following storage. Human adipose-derived stem cells were stored at 15°C for 72 hours before returning to a cell culture environment. After 24 hours, cell attachment (A) and metabolic activity (B, C) were assessed. These values were used to calculate the total percentage recovery [% viable recovery × (fold change in attachment × fold change in metabolic activity per cell)] (D). Values are expressed as mean ± SEM from 3 separate donors, with asterisks representing significance from control values (∗∗, p < .01; ∗, p < .05). Abbreviations: Control, nonencapsulated; Encaps., encapsulated; ns, not significant; NSC, nonstored control.
Morphology and Proliferative Potential of hASCs After Storage
Following their subsequent attachment to tissue culture plastic, stored hASCs exhibited a normal spindle-shaped fibroblast-like morphology, indistinguishable between any of the conditions, forming tightly packed confluent monolayers by 12 days in culture (Fig. 4A). The proliferative potential of hASCs was unaffected by storage and, although the control samples exhibited a cell number lower than encapsulated samples at every time point, this was not significant (Fig. 4B). Growth kinetics were normal in all samples, demonstrating a rapid exponential period of growth up to day 6, which plateaued after this point (Fig. 4C).
Figure 4.
Morphology, proliferation, and multilineage differentiation potential following storage. Human adipose-derived stem cells were stored at 15°C for 72 hours before returning to a cell culture environment. Cells were cultured in growth medium, with images captured after 1 and 12 days (A), and the cell number was assessed every 3 days for 15 days (B, C). Values are expressed as mean ± SEM from 3 separate donors. For differentiation, cells were cultured in osteo-, adipo-, or chondrogenic media for up to 35 days. Cultures were stained with Alizarin Red S (osteogenic), Oil Red O (adipogenic), or Alcian Blue 8GX (chondrogenic) and images captured (D). Osteogenic (Ei) and adipogenic (Eii) stains were quantified. Values are expressed as mean ± SEM from 3 separate donors. Scale bars = 100 μm (A) and 50 μm (D). Abbreviations: Control, nonencapsulated; Encaps., encapsulated; ns, not significant; NSC, nonstored control.
Differentiation Potential of Cells Following Storage
When induced to do so, hASCs have the capacity to differentiate into osteogenic, adipogenic and chondrogenic lineages. In order to investigate whether this differentiation potential was maintained after alginate encapsulation, hypothermic storage, and release, hASCs were induced to differentiate to appropriate lineages for up to 35 days. All samples were able to differentiate into osteocytes, adipocytes, and chondrocytes, as demonstrated by Alizarin Red S, Oil Red O, and Alcian blue staining, respectively (Fig. 4D). Although quantification of Alizarin Red S (Fig. 4Ei) and Oil Red O (Fig. 4Eii) staining suggested a slight conservation of differentiation capacity by alginate encapsulation, this was not significant.
Immunophenotype of Cells Following Storage
In order to determine whether storage at 15°C for 72 hours affected the immunophenotype of encapsulated hASCs, flow cytometric analysis was performed and compared with nonstored cells maintained in culture at 37°C. In terms of size (forward scatter) and granularity (side scatter), a similar profile was observed between cells obtained from culture (Fig. 5A) and from storage (Fig. 5B), with density plots indicating two distinct populations of different cell sizes. Nonstored hASCs exhibited a high level of expression of the MSC-associated markers CD73 and CD90 (Fig. 5C) that was maintained following storage, albeit at a slightly lower percentage (Fig. 5D). Of the MSC-associated markers that have key roles in cell-cell interaction and cell adhesion, CD29 expression was high in both nonstored and stored cell populations, and expression of CD44 and CD166 was considerably decreased following storage (Fig. 5C, 5D). The expression of the negative MSC markers CD14 and CD45 and of HLA-DR and HLA-ABC remained unchanged (Fig. 5C, 5D).
Figure 5.
Assessment of size, granularity, and immunophenotype of encapsulated cells following storage. Human adipose-derived stem cells were assessed for size and granularity after no storage (A) and storage for 72 hours at 15°C (B). Similarly, immunophenotype was assessed after no storage (C) and storage for 72 hours at 15°C (D). Size and granularity plots denote representative images, and values for the percentage of expression of cell surface markers are expressed as mean ± SEM from 3 separate donors. Abbreviations: FSC, forward scatter; HLA, human leukocyte antigen; SSC, side scatter.
Effect of Cell Density on Viable Cell Recovery
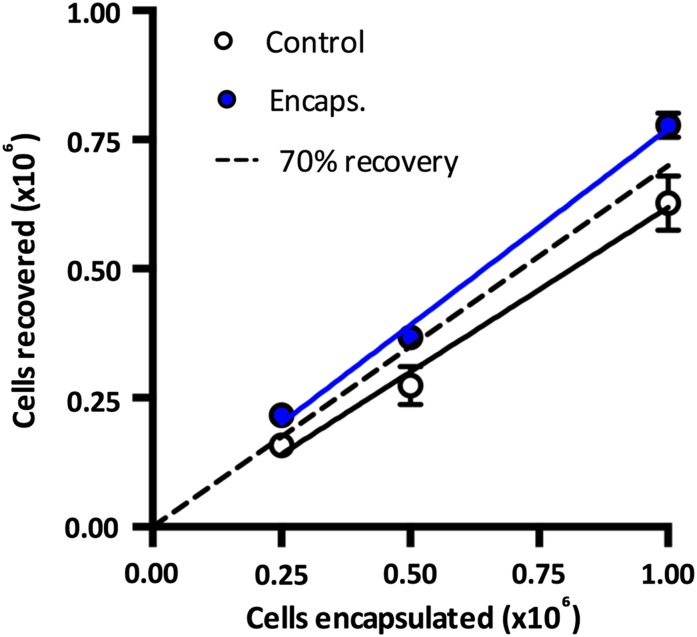
For the purpose of scalability in the number of cells that could be preserved in calcium alginate, we investigated the viable recovery of cells after storage at up to 2 × 106 cells per milliliter, representing a fourfold increase in cell density. We found that encapsulation up to this density (1 × 106 cells in a 0.5-ml calcium alginate disc) did not compromise the recovery of cells, as the encapsulated samples consistently yielded an above 70% viable recovery and control samples resulted in a less than 70% viable recovery (Fig. 6). Alginate encapsulation at the highest cell concentration tested (2 × 106 cells per milliliter) significantly improved viable cell recovery compared with the control (p = .0484).
Figure 6.
The effect of cell load on viable cell recovery after storage. Human adipose-derived stem cells were stored at 15°C for 72 hours at a range of densities before assessing viable cell recovery. Values are expressed as mean ± SEM, with the level for 70% viable cell recovery indicated by a dotted line. Abbreviations: Control, nonencapsulated (R2 = 0.9268); Encaps., encapsulated (R2 = 0.9859).
Examination of Storage in Serum-Free Conditions
For the purposes of adoption of a cell storage system into cell therapy logistics, it is important to demonstrate that any such system can be adapted to be serum-free, xeno-free, and cGMP-compliant. Therefore, we examined hypothermic storage in two commercially available serum-free, cGMP-compliant media: StemPro MSC SFM medium, formulated for MSC expansion, and HypoThermosol-FRS, a biopreservation medium designed for hypothermic storage at 4°C (H-FRS). These were compared against storage in the reduced-serum growth medium (RSM). Although storage of cells at 15°C in SFM did not significantly compromise viable cell recovery compared with RSM in either control or encapsulated conditions, storage in H-FRS resulted in a significant decrease in the number of viable cells recovered (Fig. 7A). As H-FRS is formulated for 4°C, we also examined storage at this temperature and again observed a lower viable cell recovery compared with 15°C. This surprising result confirmed the unsuitability of H-FRS for storage of hASCs in suspension. In contrast, storage in SFM resulted in a comparable total percentage recovery to that with RSM (73% ± 12%; Fig. 7B) and maintained the cytoprotection elicited by alginate encapsulation (p = .0188), confirming its suitability for hASC storage in serum-free, xeno-free conditions.
Figure 7.
Cell storage in serum-free, xeno-free, Current Good Manufacturing Process-grade media. Human adipose-derived stem cells were stored at 15°C for 72 hours in growth medium (RSM or SFM or at 15°C or 4°C in H-FRS) before assessing viable cell recovery (A). Total percentage of recovery (B) was calculated with attachment and metabolic activity data using the following equation [% viable recovery × (fold change in attachment × fold change in metabolic activity per cell)]. Values are expressed as mean ± SEM from 3 separate donors, with asterisks representing significance from control values (∗∗∗, p < .001; ∗∗, p < .01; ∗, p < .05) and symbols representing significance between storage media ($, #, p < .05). Abbreviations: Control, nonencapsulated; Encaps., encapsulated; H-FRS, HypoThermosol-FRS; ns, not significant; RSM, reduced-serum medium; SFM, serum-free medium.
Discussion
Despite extensive progress being made within the cell therapy industry, major logistical complications exist for cell storage and distribution. In this report, we present a simple, low-cost system capable of heightening the preservation of cells stored at hypothermic temperatures. Alginate encapsulation has previously been demonstrated to be efficacious in the preservation of human bone marrow-derived MSCs, mouse ESCs, and human limbal epithelial stem cells at an ambient temperature [22, 25] and rat hepatocytes and recombinant baby hamster kidney cells at 4°C [23, 24]. In the present study, we explored this system further, elucidating the optimal storage conditions for cell survival and more fully characterizing the cells on release from storage.
The examination of hypothermic preservation of MSCs has previously been reported on cells derived from different tissues and at different storage temperatures, including (a) bone marrow at 4°C [31–38], 24°C [33, 35], ambient temperature [22, 36], and 37°C [35]; (b) adipose tissue at 4°C [39, 40], 8°C, 25°C, and 37°C [40]; and (c) umbilical cord at 4°C [41]. Although these studies presented promising findings, most had been conducted at 4°C. Where direct comparisons between temperatures other than 4°C were investigated, they have involved relatively large (greater than 12°C) steps in the temperatures studied. We have demonstrated that small changes in temperature can have a considerable effect on the viable recovery of alginate-encapsulated cells with an optimal temperature in the range of 13°C–19°C and the greatest level of recovery at 15°C in both nonencapsulated and encapsulated samples. The importance of temperature on enzyme activity and the subsequent metabolic rate of cells is likely to be key to this observation, such that an optimal point exists at which cell metabolism is slowed sufficiently for relative quiescence, before lower temperatures induce a phase transition in the cell membrane that results in the segregation of membrane proteins and the uncontrolled entry of ions and subsequent cellular edema [42]. Previously, when protein leakage from liposomes was examined during chilling at a rate of 0.5°C per minute, leakage was initiated below 15°C [43], which could explain why viable cell recovery decreases at temperatures lower than this in our system. Although alginate encapsulation elicited its highest level of cytoprotection at 4°C, this did not result in recovery better than that shown at 13°C–19°C.
At temperatures as high as 23°C, viable cell recovery decreases considerably, likely owing to the temperature being too high to suppress enzyme activity and subsequent metabolism of cells. The result of this was activation of dormant cells with ensuing metabolic requirements and a further sensing of the suboptimal environment for survival. Furthermore, the combination of higher hypothermic temperatures and alginate encapsulation appears to worsen viable recovery. Alginate is an inert hydrogel in which cells are suspended and separated from each other, resulting in little or no physical cell-cell interaction, which might offer protection at higher temperatures. Although the effect of higher temperatures being detrimental for hypothermic preservation is perhaps unsurprising, it is pertinent that this occurs at what might be considered a low ambient temperature of 23°C. This emphasizes the importance of controlling the temperature during storage. We have, however, demonstrated that a range of temperatures are suitable (13°C–19°C), with viable recovery consistently greater than 70%, suggesting a degree of tolerance to possible deviations during storage and distribution. This optimal temperature range for hypothermic preservation is consistent with the findings from studies of adherent retinal pigment epithelial cells [44] and cultured conjunctival epithelium [45] and Chinese hamster ovary cells stored in suspension [46]. These studies similarly demonstrated that the storage of cells at 4°C or the upper range of hypothermic temperatures was highly detrimental for cell survival. The application of nonchilled (above 4°C) hypothermic temperatures for CTP preservation is also reflective of the storage conditions used by approved CTPs, including ChondroCelect (15°C–22°C; TiGenex, NV, Leuven, Belgium, http://www.tigenex.com), Epicel (13°C–23°C; Genzyme Corp., Cambridge, MA, http://www.genzyme.com), and Apligraf (20°C–23°C; Organogenesis Inc., Canton, MA, http://www.organogenesis.com) [4, 6, 8]. Taken together, controlled, nonchilled conditions are optimal for cell preservation in this system.
With CTP consistency and lack of variability important concerns for cell delivery, we examined the contribution of early apoptotic, late-apoptotic, and dead cells in populations released from storage at 15°C. In addition to the increased viable cell recovery effected by encapsulation, it also resulted in a consistently high viability in the cell population recovered, with a low incidence of apoptosis or death. This, however, was highly variable in the nonencapsulated cell populations. With the major cause of cell death during hypothermic preservation being a result of membrane destabilization, ultimately resulting in cellular edema and lysis [42], we were not able to account for all cells present before storage, likely owing to the nature of cell death, resulting in full membrane disintegration. With this common effect of cell preservation, the presentation of the percentage of viability as a measure of storage success can often be anomalous. These results support the fact that the encapsulation of hASCs is able to yield a consistently high recovery of viable cells compared with those present before storage, and the end product has minimal contribution from death and/or apoptosis.
Although it is of great importance to examine viable cell recovery as a quality control measure for CTPs, it is also critical to assess cellular function after a period of rewarming. Such an assessment is important owing to the possibility of the activation of stress pathways up to 24 hours after return to normothermia, as previously described following cryopreservation [47], and in the reported effects of attenuated attachment of bone marrow-derived MSCs after hypothermic storage [38]. We have demonstrated that encapsulation protected against the decrease in attached cell number exhibited by nonencapsulated samples after 24 hours in culture, with no change in metabolic activity per cell. We believe it important to consider these measurements when calculating a value for the “total percentage of viability” (i.e., the number of viable cells released from storage that are able to attach and recover normal metabolic activity). Using this approach (multiplying the viable cell recovery by the fold change in attachment multiplied by the metabolic activity per cell) for every donor, we can account for all permutations that represent a final recovery of cells on return to tissue culture. Consequently, any deviations in attachment or metabolic activity will be represented. There is also a degree of normalization, with those samples that achieve the highest level of viable recovery also having the lowest level of attachment. This suggests that a population of cells that are counted as viable might already be damaged, but this only becomes apparent when the cells are rewarmed on return to normal tissue culture conditions. Thus, we can exclude those cells that would not survive on return to physiological conditions, producing a reliable, consistent measure of recovery. This approach has also highlighted the increased functional cell yield effected by alginate.
On cell reattachment, morphology was shown to be unaffected compared with the nonstored controls. Moreover, the released cells exhibited a normal pattern of growth, an important concern considering previous reports of attenuated growth of bone marrow-derived MSCs after hypothermic storage [34]. In addition to attachment and growth characteristics, one of the most important functional characteristics of MSCs is their ability to differentiate into osteoblasts/osteocytes, adipocytes, and chondroblasts/chondrocytes [48], a capacity reported to be diminished after short-term hypothermic storage [36]. However, under the conditions we tested, we observed no difference in the capacity for trilineage differentiation after storage compared with nonstored controls. Although encapsulation did appear to elicit greater protection against reduced proliferation and differentiation compared with nonencapsulated controls, this was only slight and not significant after 72 hours of storage. Thus, alginate encapsulation elicited a 1.79 ± 0.15-fold increase in the total percentage of recovery of hASCs that maintained a normal phenotype and function after release from storage at 15°C for 72 hours.
In support of hypothermic storage in alginate being able to hold cells in an unaltered state, we have demonstrated that a high expression of most MSC-associated markers was maintained after storage, importantly, CD90 and CD73, albeit at a lower level. We did, however, observe a considerable decrease in the expression of two MSC markers associated with adhesion (CD44 and CD166). This is perhaps unsurprising, because alginate is an inert polymer, and, as such, the interaction of encapsulated cells with their surrounding environment would be minimal, which might have resulted in their expression being lost. It has previously been reported that the expression of a number of cell surface markers, including CD44, CD90, and CD166, are diminished during the culture of human bone marrow-derived MSCs in alginate; however, these are recoverable after a return to monolayer culture [49]. Thus, certain MSC-associated markers might not be appropriate for assessing cells released directly from alginate.
With the clinical application of MSCs requiring high cell numbers, typically 1–2 × 106 cells per kilogram, depending on the treatment [50], it was necessary to examine whether viable cell recovery was compromised at higher cell densities. The cytoprotection offered by alginate encapsulation was maintained in cell densities of up to 2 × 106 cells per milliliter, resulting in viable cell recovery consistently greater than 70%. This suggests that scaling up to higher cell numbers is feasible and, accompanied by scalable platforms for cell encapsulation in alginate beads [51], makes scalable process development of large numbers of therapeutic cells achievable.
Because fetal bovine serum could be a source of prion, viral, and zoonotic diseases [52], it was also desirable to examine whether the cytoprotection offered by alginate encapsulation could be recapitulated when hydrogels were placed in serum-free, xeno-free, and cGMP-grade storage media. Although HypoThermosol-FRS has demonstrable efficacy in the preservation of adherent bone marrow-derived MSCs at 4°C [32, 37] and offers cytoprotection to hypothermically stored hepatocytes in suspension [53], we found it unsuitable for storage of hASCs in suspension at either 4°C or 15°C. In contrast, commercially available StemPro MSC SFM medium affected no significant difference in viable cell recovery or the total percentage of recovery of cells compared with MesenPro-reduced serum (RS) medium. This demonstrated that the storage system we have described could be easily adapted to be clinically safe and cGMP-compliant.
Although we have provided convincing evidence that alginate encapsulation improves the hypothermic preservation of hASCs, the mechanism by which it exerts its effect remains unclear. It has been suggested, during the hypothermic preservation of hepatocytes, that alginate encapsulation functions to stabilize the membrane of suspended cells in the absence of an extracellular matrix and thus minimize the effects of osmotic shock and mechanical stress during cell storage and recovery [23]. Alginate also has a strong influence on ion and solute diffusion throughout the hydrogel matrix [54], as well as having considerable swelling capacity. This could contribute to modulating the diffusion rates of ions and solutes and, therefore, water [42], in the environment surrounding encapsulated cells, further reducing the impact of osmotic shock and cellular edema. Thus, a number of mechanisms exist by which alginate could offer cytoprotection during hypothermic storage.
Conclusion
In a recent assessment by experts in the field, it was considered that the development of preservation and transport-enabling technologies were critical in the cell therapy industry to eliminate cGMP-regulated steps of preservation removal and cell thawing associated with cryopreservation [14]. Although liquid-state storage avoids these issues, concern remains over the limited time windows associated with the transfer of cells to the clinic owing to a restricted shelf life with which high levels of material wastage can result [55]. Alginate encapsulation represents a method whereby the hypothermic preservation of cells can be markedly improved while maintaining cell functionality. As a material with demonstrable biocompatibility, application in cell therapy, and scalability, its integration into CTP bioprocessing has considerable potential.
Supplementary Material
Acknowledgments
This work was jointly funded by the Biotechnology and Biological Sciences Research Council and Engineering and Physical Sciences Research Council as part of a Bioprocessing Research Industry-enabled grant (reference no. BB\K011111\1). A.C. was funded by Defence Science and Technology Laboratory (reference no. CDE38047).
Author Contributions
S.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.C.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.J.C.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Li MD, Atkins H, Bubela T. The global landscape of stem cell clinical trials. Regen Med. 2014;9:27–39. doi: 10.2217/rme.13.80. [DOI] [PubMed] [Google Scholar]
- 2.Foley L, Whitaker M. Concise review: cell therapies: The route to widespread adoption. Stem Cells Translational Medicine. 2012;1:438–447. doi: 10.5966/sctm.2011-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed BA, Evans JB. Stem cell therapy market. Nat Rev Drug Discov. 2013;12:185–186. doi: 10.1038/nrd3953. [DOI] [PubMed] [Google Scholar]
- 4.Apligraf: Factsheet APG, last updated June 2011, and Prescribing Information, last updated December, 2010. Available at http://www.apligraf.com. Accessed September 17, 2014.
- 5.Carticel: Prescribing information, last updated June 2010. Available at http://www.carticel.com. Accessed September 18,2014.
- 6.ChondroCelect. EPAR-product information, last updated November 20, 2013. Available at http://www.ema.europa.eu. Accessed August 22,2014.
- 7.Dermagraft: Directions for use, 2013. Available at http://www.dermagraft.com. Accessed September 18,2014.
- 8.Epicel: Directions for use, last updated June 2014. Available at http://www.epicel.com. Accessed September 18,2014.
- 9.Provenge: Prescribing information, last updated June 2011. Available at http://www.provenge.com. Accessed September 18,2014.
- 10.Baust JG. Concepts in biopreservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Boca Raton, FL: CRC Press; 2007. pp. 1–14. [Google Scholar]
- 11.Thirumala S, Goebel WS, Woods EJ. Manufacturing and banking of mesenchymal stem cells. Expert Opin Biol Ther. 2013;13:673–691. doi: 10.1517/14712598.2013.763925. [DOI] [PubMed] [Google Scholar]
- 12.Pal R, Hanwate M, Totey SM. Effect of holding time, temperature and different parenteral solutions on viability and functionality of adult bone marrow-derived mesenchymal stem cells before transplantation. J Tissue Eng Regen Med. 2008;2:436–444. doi: 10.1002/term.109. [DOI] [PubMed] [Google Scholar]
- 13.Kemp P. History of regenerative medicine: Looking backwards to move forwards. Regen Med. 2006;1:653–669. doi: 10.2217/17460751.1.5.653. [DOI] [PubMed] [Google Scholar]
- 14.Hourd P, Ginty P, Chandra A, et al. Manufacturing models permitting roll out/scale out of clinically led autologous cell therapies: Regulatory and scientific challenges for comparability. Cytotherapy. 2014;16:1033–1047. doi: 10.1016/j.jcyt.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Pamphilon DH, Selogie E, Szczepiorkowski ZM. Transportation of cellular therapy products: Report of a survey by the cellular therapies team of the Biomedical Excellence for Safer Transfusion (BEST) collaborative. Vox Sang. 2010;99:168–173. doi: 10.1111/j.1423-0410.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson NJ, Picken A, Coopman K. Low temperature cell pausing: An alternative short-term preservation method for use in cell therapies including stem cell applications. Biotechnol Lett. 2014;36:201–209. doi: 10.1007/s10529-013-1349-5. [DOI] [PubMed] [Google Scholar]
- 17. ClinicalTrials.gov. Available at http://www.clinicaltrials.gov. Accessed May 20,2015. [Google Scholar]
- 18.Veiseh O, Doloff JC, Ma M, et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccaldi C, Fullana SG, Alfarano C, et al. Alginate scaffolds for mesenchymal stem cell cardiac therapy: Influence of alginate composition. Cell Transplant. 2012;21:1969–1984. doi: 10.3727/096368912X647252. [DOI] [PubMed] [Google Scholar]
- 20.Balyura M, Gelfgat E, Ehrhart-Bornstein M, et al. Transplantation of bovine adrenocortical cells encapsulated in alginate. Proc Natl Acad Sci USA. 2015;112:2527–2532. doi: 10.1073/pnas.1500242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright EJ, Farrell KA, Malik N, et al. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Translational Medicine. 2012;1:759–769. doi: 10.5966/sctm.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Wright B, Sahoo R, et al. A novel alternative to cryopreservation for the short-term storage of stem cells for use in cell therapy using alginate encapsulation. Tissue Eng Part C Methods. 2013;19:568–576. doi: 10.1089/ten.TEC.2012.0489. [DOI] [PubMed] [Google Scholar]
- 23.Mahler S, Desille M, Frémond B, et al. Hypothermic storage and cryopreservation of hepatocytes: The protective effect of alginate gel against cell damages. Cell Transplant. 2003;12:579–592. doi: 10.3727/000000003108747181. [DOI] [PubMed] [Google Scholar]
- 24.Mayer FQ, Baldo G, de Carvalho TG, et al. Effects of cryopreservation and hypothermic storage on cell viability and enzyme activity in recombinant encapsulated cells overexpressing alpha-l-iduronidase. Artif Organs. 2010;34:434–439. doi: 10.1111/j.1525-1594.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 25.Wright B, Cave RA, Cook JP, et al. Enhanced viability of corneal epithelial cells for efficient transport/storage using a structurally modified calcium alginate hydrogel. Regen Med. 2012;7:295–307. doi: 10.2217/rme.12.7. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien J, Wilson I, Orton T, et al. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 27.Oliver MH, Harrison NK, Bishop JE, et al. A rapid and convenient assay for counting cells cultured in microwell plates: Application for assessment of growth factors. J Cell Sci. 1989;92:513–518. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 28.Gregory CA, Gunn WG, Peister A, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim YK, Choi HY, Kim NH, et al. Reversine stimulates adipocyte differentiation and downregulates Akt and p70(s6k) signaling pathways in 3T3-L1 cells. Biochem Biophys Res Commun. 2007;358:553–558. doi: 10.1016/j.bbrc.2007.04.165. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration. Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). Last updated April 2008. Available at http://www.fda.gov. Accessed May 28,2015.
- 31.Corwin WL, Baust JM, Baust JG, et al. Characterization and modulation of human mesenchymal stem cell stress pathway response following hypothermic storage. Cryobiology. 2014;68:215–226. doi: 10.1016/j.cryobiol.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453–463. doi: 10.1089/ten.TEC.2011.0395. [DOI] [PubMed] [Google Scholar]
- 33.Gorodetsky R, Levdansky L, Gaberman E, et al. Fibrin microbeads loaded with mesenchymal cells support their long-term survival while sealed at room temperature. Tissue Eng Part C Methods. 2011;17:745–755. doi: 10.1089/ten.tec.2010.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane TA, Garls D, Mackintosh E, et al. Liquid storage of marrow stromal cells. Transfusion. 2009;49:1471–1481. doi: 10.1111/j.1537-2995.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 35.Muraki K, Hirose M, Kotobuki N, et al. Assessment of viability and osteogenic ability of human mesenchymal stem cells after being stored in suspension for clinical transplantation. Tissue Eng. 2006;12:1711–1719. doi: 10.1089/ten.2006.12.1711. [DOI] [PubMed] [Google Scholar]
- 36.Sohn HS, Heo JS, Kim HS, et al. Duration of in vitro storage affects the key stem cell features of human bone marrow-derived mesenchymal stromal cells for clinical transplantation. Cytotherapy. 2013;15:460–466. doi: 10.1016/j.jcyt.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Biolife Solutions. HypoThermosol Biopreservation Efficacy. Available at http://biolifesolutions.com/biopreservation-media/HypoThermosol-Biopreservation-Efficacy.pdf. Accessed August 14, 2014.
- 38.Veronesi E, Murgia A, Caselli A, et al. Transportation conditions for prompt use of ex vivo expanded and freshly harvested clinical-grade bone marrow mesenchymal stromal/stem cells for bone regeneration. Tissue Eng Part C Methods. 2014;20:239–251. doi: 10.1089/ten.tec.2013.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gálvez P, Martín MJ, Calpena AC, et al. Enhancing effect of glucose microspheres in the viability of human mesenchymal stem cell suspensions for clinical administration. Pharm Res. 2014;31:3515–3528. doi: 10.1007/s11095-014-1438-8. [DOI] [PubMed] [Google Scholar]
- 40.Gálvez-Martín P, Hmadcha A, Soria B, et al. Study of the stability of packaging and storage conditions of human mesenchymal stem cell for intra-arterial clinical application in patient with critical limb ischemia. Eur J Pharm Biopharm. 2014;86:459–468. doi: 10.1016/j.ejpb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Di G, Wang J, Liu M, et al. Development and evaluation of a trehalose-contained solution formula to preserve hUC-MSCs at 4°C. J Cell Physiol. 2012;227:879–884. doi: 10.1002/jcp.23066. [DOI] [PubMed] [Google Scholar]
- 42.Rubinsky B. Principles of low temperature cell preservation. Heart Fail Rev. 2003;8:277–284. doi: 10.1023/a:1024734003814. [DOI] [PubMed] [Google Scholar]
- 43.Tomczak MM, Hincha DK, Estrada SD, et al. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys J. 2002;82:874–881. doi: 10.1016/S0006-3495(02)75449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasovic L, Utheim TP, Maria R, et al. Optimization of storage temperature for cultured ARPE-19 cells. J Ophthalmol. 2013;2013:216359. doi: 10.1155/2013/216359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eidet JR, Utheim OA, Islam R, et al. The impact of storage temperature on the morphology, viability, cell number and metabolism of cultured human conjunctival epithelium. Curr Eye Res. 2015;40:30–39. doi: 10.3109/02713683.2014.909497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt L, Hacker DL, Grosjean F, et al. Low-temperature pausing of cultivated mammalian cells. Biotechnol Bioeng. 2005;89:157–163. doi: 10.1002/bit.20320. [DOI] [PubMed] [Google Scholar]
- 47.Bissoyi A, Nayak B, Pramanik K, et al. Targeting cryopreservation-induced cell death: A review. Biopreserv Biobank. 2014;12:23–34. doi: 10.1089/bio.2013.0032. [DOI] [PubMed] [Google Scholar]
- 48.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 49.Lee HJ, Choi BH, Min BH, et al. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis Rheum. 2009;60:2325–2332. doi: 10.1002/art.24786. [DOI] [PubMed] [Google Scholar]
- 50.Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: Optimization of cell preparation protocols. BioMed Res Int. 2014;2014:951512. doi: 10.1155/2014/951512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prüsse U, Bilancetti L, Bucko M, et al. Comparison of different technologies for alginate beads production. Chem Pap. 2008;62:364–374. [Google Scholar]
- 52.Mannello F, Tonti GA. Concise review: No breakthroughs for human mesenchymal and embryonic stem cell culture: Conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603–1609. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 53.Ostrowska A, Gu K, Bode DC, et al. Hypothermic storage of isolated human hepatocytes: A comparison between University of Wisconsin solution and a hypothermosol platform. Arch Toxicol. 2009;83:493–502. doi: 10.1007/s00204-009-0419-x. [DOI] [PubMed] [Google Scholar]
- 54.Golmohamadi M, Wilkinson KJ. Diffusion of ions in a calcium alginate hydrogel-structure is the primary factor controlling diffusion. Carbohydr Polym. 2013;94:82–87. doi: 10.1016/j.carbpol.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 55.Hernon CA, Dawson RA, Freedlander E, et al. Clinical experience using cultured epithelial autografts leads to an alternative methodology for transferring skin cells from the laboratory to the patient. Regen Med. 2006;1:809–821. doi: 10.2217/17460751.1.6.809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.