This study compared the effects of different types of media on adipose-derived stem cell (ASC) cultures. It was found that culture in fetal calf serum versus human platelet lysate had a significant effect on the degree of expression of stem cell-associated surface markers. These results underscore the need to carefully investigate the effect of culture media on ASC behavior before a medium is selected for clinical use.
Keywords: Adipose-derived stem cells, Culture media, Xenogeneic free, Cell proliferation, Phenotype, Cell therapy
Abstract
Adipose-derived stem cells (ASCs) are being tested in clinical trials related to cell-based regenerative therapies. Although most of the current expansion protocols for ASCs use fetal calf serum (FCS), xenogeneic-free medium supplements are greatly desired. This study aims to compare the effect of FCS, human platelet lysate (hPL), and a fully defined medium on the initiation and maintenance of ASC cultures. ASCs obtained from five donors were cultured in five different media: StemPro, Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% hPL, or α-minimum essential medium (A-MEM) supplemented with 5% hPL, 10% hPL, or 10% FCS. The effect of media on proliferation, colony-forming units (CFUs), attachment, and morphology was assessed along with cell size, granularity, and immunophenotype. StemPro greatly compromised the initiation of ASC cultures, which could not survive more than a few passages. Cells cultured in A-MEM proliferated at a faster rate than in DMEM, and hPL significantly enhanced cell size, granularity, and proliferation compared with FCS. All media except StemPro supported CFUs equally well. Analysis of surface markers revealed higher levels of CD73 and CD105 in FCS-cultured ASCs, whereas increased levels of CD146 were found in hPL-cultured cells. Multiparametric flow cytometric analysis performed after seven passages revealed the existence of four distinct ASC subpopulations, all positive for CD73, CD90, and CD105, which mainly differed by their expression of CD146 and CD271. Analysis of the different subpopulations might represent an important biological measure when assessing different medium formulations for a particular clinical application.
Significance
In most clinical trials using adipose-derived stem cells (ASCs), the cells have been expanded in culture media supplemented with fetal calf serum. However, there is much interest in replacing fetal calf serum with human platelet lysate or using completely serum- and xenogeneic-free media. This study found that culture in fetal calf serum versus human platelet lysate had a significant effect on the degree of expression of stem cell–associated surface markers. These results underscore the need to carefully investigate the effect of culture media on ASC behavior before committing to one medium type for clinical use.
Introduction
Adipose-derived stem cells (ASCs) are a heterogeneous population of adult multipotent stem cells that can be isolated with relative ease and in substantial amounts from the stromal vascular fraction (SVF) of adipose tissue using the protocol of Zuk et al. [1]. In a joint statement by the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy, it was proposed that ASCs should be characterized by their capacity for plastic adherence; their ability to differentiate into bone, cartilage, and adipose tissue; and their expression of a selected set of surface markers including CD73, CD90, and CD105 [2]. On review of 15 recent clinical trials on ASCs, however, we found that in a majority of trials, ASCs have been characterized based on a limited set of surface markers, most often including CD73, CD90, and CD105, and in only one trial was trilineage differentiation potential assessed (supplemental online Table 1).
Initially, the multilineage potential of ASCs garnered much attention, as it was proposed that ASCs could be used for ex vivo tissue engineering of osteochondral tissue; indeed, in animal models and human case reports, it has been demonstrated that ASCs may be used to construct functional tissue, such as bone and trachea, that is suitable for transplantation [3, 4]. However, recent years have seen a shift in focus, and today ASCs are increasingly being recognized as having strong regenerative capacity because of their paracrine activity facilitated by soluble mediators, immunomodulatory properties, and capacity to home to injured tissues [5]. This shift has increased their attraction, as they now are seen not as building blocks but as initiators and regulators of tissue regeneration. Research on the potential clinical usefulness of ASCs, for example in cardiac regeneration, has moved from promising in vitro and animal studies [6–8] to clinical trials [9, 10]. In addition to these studies, clinical trials are completed or under way for the use of ASCs to treat critical limb ischemia [11], perianal fistula [12], Crohn’s fistula [13, 14], osteoarthritis [15], and acute respiratory distress syndrome [16], to name but a few.
For most if not all clinical indications, after the isolation of SVF, cells must be expanded in vitro to achieve the necessary therapeutic dose [17, 18], which also results in a more homogeneous and less immunogenic product [17]. Optimal cell growth depends on a variety of microenvironmental factors including oxygen tension in the culture system [6, 19–22], topography of the culture surfaces [23, 24], mechanical factors [25, 26], and most notably, cell culture medium composition [27, 28]. Basically, the culture medium has to supply all essential nutrients, hormonal factors, transport proteins, minerals, lipids, attachment and spreading factors, and stabilizing and detoxifying factors to reach optimal cell metabolism, growth, and proliferation [29]; often medium compositions that promote maximal proliferation are perceived as superior.
For historical, practical, and economic reasons, ASCs are typically expanded in basal medium supplemented with fetal calf serum (FCS), and ex vivo expanded ASCs have been used in several clinical trials [9, 13, 14, 30–35]. However, even Good Manufacturing Practice-approved FCS is a complex mixture of essential nutrients and bioactive molecules with high lot-to-lot variability. Furthermore, there is concern that the use of FCS increases the risk of contamination and immunization because of the presence of xenogeneic components and possibly viruses, which can lead to transplantation failure [36–40]. To address this issue, it has been proposed that human blood-derived products, such as autologous platelet-derived products [41, 42], should be used; however, apart from being an impractical solution, advanced donor age has been shown to have a negative effect on the multipotential of mesenchymal stem cells (MSCs) [43]. The use of allogeneic human platelet lysate (hPL) has been proposed as an alternative to FCS and has been shown to be a safe option [29, 44]. hPL is generated from common platelet units by a simple freeze-thaw procedure and contains a variety of growth factors, proteins, and enzymes to support attachment, growth, and proliferation; yet it is still poor in antibodies and has been shown to promote the growth of various cell lines including ASCs [38, 44]. Furthermore, it has been convincingly documented that during and after expansion in medium supplemented with hPL, ASCs retain their capacity for trilineage differentiation [45, 46]. Potential caveats on the use of hPL are that it is not fully defined as a supplement, there are lot-to-lot variations, and there is a risk of pathogen carryover, albeit small.
To overcome these disadvantages, chemically defined, serum-free, and xenogeneic-free media have been developed. Because the growth factors essential for mesenchymal stem cell (MSC) isolation and expansion have not yet been identified, several synthetic media have proven unsuitable for ASC expansion; they have been shown to support ASC proliferation and at the same time fail to support ASC properties in terms of differentiation potential [28, 47]. Novel synthetic media, however, show more promise than the earlier types in terms of promoting proliferation while maintaining multipotentiality, and we and others have previously shown that expansion of ASCs in synthetic serum- and xenogeneic-free StemPro media supports trilineage differentiation [27, 48].
In this study, we compared culture in media supplemented with hPL, FCS, and synthetic media with respect to ASC isolation from SVF and expansion in terms of attachment, colony-forming capacity, growth rate, and immunophenotype. We found that synthetic medium was unsuitable for initiation of ASC cultures from SVF, and that whereas culture in either FCS or hPL yielded ASCs displaying the characteristic phenotype defined by positive expression of surface markers CD73, CD90, and CD105, there was a difference between the two supplements in the degree to which the individual ASCs expressed several stem cell-associated surface markers.
Materials and Methods
Isolation and Culture of ASCs
For harvesting ASCs, excess subcutaneous adipose tissue was obtained from five donors undergoing elective liposuction surgery at Teres Private Hospital, Skejby, Denmark. All patients gave written informed consent, and the protocol was approved by the regional committee on biomedical research ethics of Northern Jutland, Denmark (project no. VN 2005/54). A summary of patient data is found in supplemental online Table 2. This study complied with the principles of the Declaration of Helsinki.
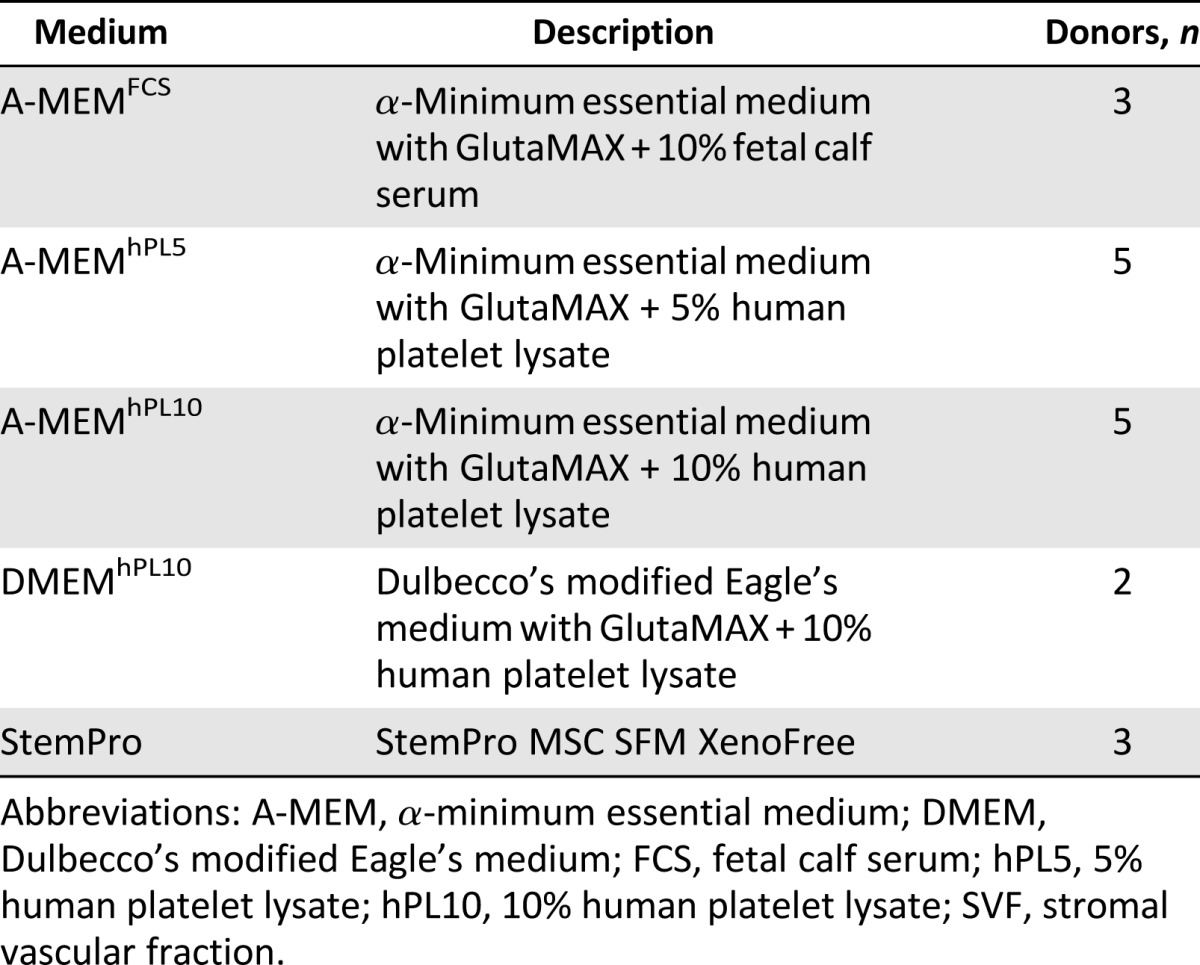
ASCs were isolated according to methods previously described by our laboratory [49], with minor modifications. Briefly, adipose tissue was washed repeatedly with sterile phosphate-buffered saline (PBS) (Invitrogen, Taastrup, Denmark, https://www.thermofisher.com/) and then dissociated enzymatically for 60 min at 37°C with 0.6 U/ml Collagenase NB 4 Standard Grade (Serva Electrophoresis, Heidelberg, Germany, http://www.serva.de) in Hanks balanced salt solution (HBSS; Invitrogen) under continuous, gentle agitation. The SVF within the dissociated tissue was filtered through a 100-μm filter and pelleted by centrifugation at 400g for 10 min. The pellet was resuspended and filtered through a 60-μm filter and pelleted again by centrifugation at 400g for 10 min, forming the SVF. The cells were resuspended in PBS, and the cell yield was determined with a Nucleocounter NC-200 cell counter (Chemometec, Allerod, Denmark, http://chemometec.com/). Cells were divided into aliquots to allow for parallel experiments with different media. The culture media were α-minimum essential medium (A-MEM) with GlutaMAX (Invitrogen) supplemented with 10% FCS (Invitrogen), A-MEM supplemented with 10% hPL (Stemulate; Cook Medical, Bloomington, IN, https://www.cookmedical.com/), A-MEM supplemented with 5% hPL, Dulbecco’s modified Eagle’s Medium (DMEM) with GlutaMAX (Invitrogen) supplemented with 10% hPL, or StemPro MSC SFM XenoFree (Invitrogen) supplemented with l-glutamine (Invitrogen). They were all supplemented 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen) and cultured on tissue culture propylene (TCP; Greiner Bio-One, Fredensborg, Denmark, http://www.greinerbioone.com). Explanations of medium abbreviations are given in Table 1. The tissue culture surface for the cells cultured in StemPro were additionally coated with CellStart CTS (Invitrogen) according to the manufacturer’s protocol. Because of limitations of the resulting SVF cell number, parallel cultures of at most four different culture media were possible for each of the five donors (Table 1). To compensate for interdonor variations and facilitate comparisons between all media, A-MEMhPL5 and A-MEMhPL10 were included in the experimental setups for each donor. The abbreviation SVF is used throughout the study for cells not yet passaged, and the term ASC denotes cells after first passage.
Table 1.
Compositions of the different media used in this study
Proliferation
To determine the effect of medium composition on the proliferation rate of ASCs, SVFs were seeded at a density of 150,000 cells per cm2 in T25 Cellstar Tissue Culture Flasks (Greiner Bio-One), and after overnight incubation, thoroughly washed with PBS to remove unattached cells. ASCs were cultured in a standard Steri-Cycle CO2 incubator in a humidified atmosphere containing 20% O2 and 5% CO2 at 37°C, with medium changes twice a week. When the first of the parallel cultures reached 80% confluence, all cultures were subcultured using TrypLe (Invitrogen), and the number of ASCs per flask was counted using a hemocytometer. The cells were cultured for up to four passages in which ASCs were seeded at a density of 2,000 cells per cm2 in T25 tissue culture flasks, maintained with medium changes twice a week, and passaged and counted when the first culture reached 80% confluence. Cultures of SVFs were performed in quadruplicate and ASCs in triplicate for each donor. Accumulated cell number (N) was calculated from N(t) = c ∙ 2t/d, where N(t) is the number of cells at time t, d is doubling time, and c is total number of cells after previous passage. Doubling time was calculated from the following: t ∙ log(2)/log(e/s), where e is harvested number of cells and s is seeding density. Population doubling was calculated for each passage according to the equation PD = 3.32(log Xe − log Xb), where Xe is the cell number at the end of the passage and Xb is the cell number at the beginning of the passage [50].
Attachment
The effect of the different media on cell attachment was assessed by seeding SVFs at a density of 150,000 cells per cm2 and ASCs in passages 1 and 2 at 2,000 cells per cm2 in T25 Cellstar Tissue Culture Flasks, incubating overnight, and thoroughly washing the culture vessels with PBS to remove unattached cells. After washing, phase-contrast pictures were taken at three random locations for each culture flask using an Olympus CKX41 microscope (Olympus Life Science Solutions, Chelmsford, MA, http://www.olympus-lifescience.com) with a PixeLINK PL-A782 camera. The number of cells attached to the culture surface was determined for a fixed surface area for each picture.
CFUs
To determine the frequency of colony-forming units (CFUs) in the cultures grown in the different medium compositions, SVFs and ASCs were seeded in limiting dilution in a 96-well plate (Costar, Corning Life Sciences, Tewksbury, MA, http://www.corning.com) according to Schellenberg et al. [51] with minor changes. In brief, ASCs were seeded at densities of 10–1,000 cells per well for SVF and 1–30 cells per well for ASCs in passages 1–3 and cultured with medium changes twice a week. At day 12, the cells were fixed in 10% formalin (Ampliqon, Odense, Denmark, http://www.ampliqon.com/) and stained using 0.05% Crystal Violet (Sigma Aldrich Denmark, Brøndby, Denmark, http://www.sigmaaldrich.com/denmark/om-danmark.html) in H2O. Wells containing one or more colonies were noted, and L-Calc Software (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com/) was used to calculate the proportion of CFUs using Poisson statistics. Data not following the Poisson distribution were excluded from analysis.
Surface Marker Phenotypic Analysis
Flow cytometric analysis was performed on a MOFLO Astrios EQ (Beckman Coulter, Birkerød, Denmark, https://www.beckmancoulter.com) using a six-color stain setup that included the following antibodies (BD Bioscience, Albertslund, Denmark, http://www.bd.com) and channels: anti-CD73 fluorescein isothiocyanate on 488-513/26, anti-CD90 peridinin chlorophyll protein-cyanine 5.5 on 488-710/45, anti-CD105 allophycocyanin on 640-664/22, anti-CD146 phycoerythrin-CF594 on 561-614/20, and anti-CD271 phycoerythrin-cyanine 7 on 561-795/70. Analysis of the culture surface marker phenotype was based on SVFs collected immediately after isolation and ASCs collected at passages 1 and 7 grown in the five different medium compositions. Before staining the SVFs, erythrocytes were lysed in distilled water for 10 seconds, after which a physiological salt balance was restored. Before, between, and after staining, cells were washed in staining buffer (0.1% bovine serum albumin in PBS). The cells were first stained with the five antibodies for 30 min and afterward stained with Live/Dead Fixable Aqua Dead Cell Stain (Molecular Probes, Taastrup, Denmark, http://www.thermofisher.com) for another 30 min. Next, the cell sample suspended in staining buffer was dissociated using a cell strainer-capped tube to ensure single cells (BD Falcon; BD Biosciences, Erembodegem, Belgium, http://www.bdbiosciences.com). Stream alignment was optimized using Sphero Ultra Rainbow Fluorescent Particles (Spherotech, Lake Forest, IL, http://www.spherotech.com/). The voltage and gains of the photomultiplier tubes were adjusted inside the linear range so that an unstained sample produced a bell-shaped emission with a median fluorescence of 101 and no double-negative peaks apparent in the red channels. Compensations were made with BD CompBeads Set Anti-Mouse Ig, κ (BD Bioscience) to standardize compensation despite differences in marker expression and to ensure sufficient bright populations when markers were vaguely expressed. The resulting compensation matrix was individually fitted to each sample based on dot-plots depicting the combination of channels in question. Fluorescence minus one (FMO) controls were included for each fluorochrome, donor, and passage. Flow-Set Pro Fluorospheres (Beckman Coulter) were analyzed in regard to coefficient of variation and mean fluorescence intensity with each sample to ensure sample comparability. Cell viability was assessed on 355-448/59. Data were visualized and analyzed using Kaluza version 1.3 (Beckman Coulter). Cells were first gated on a forward scatter (FSC) (height)/side scatter (height) contour plot to gate out noise and debris, then a FSC (width)/FSC (height) contour plot was used to discriminate doublets. Subsequently, viable cells were gated out. The resulting cells were then plotted in histograms corresponding to the above markers, and overlays between FMOs and the stained sample were created. Positivity was defined by an overlay marker whose lower boundary was set to include the top 2.5% of the FMO.
Statistical Analysis
All data have been normalized to the level of A-MEMhPL10 at first passage for each individual donor because of large interdonor variation. All statistical analysis was performed using SigmaPlot 12.0 (Systat Software, Erkrath, Germany, http://www.systat.com). The normal distribution of each group was assessed by means of the Shapiro-Wilk test. Additionally, variance was tested using an equal variance test. Data are represented as mean ± SEM, except for the quantitative flow cytometry data, where data are presented as mean ± SD. A p value < .05 was considered statistically significant. For comparison of two groups, a Student t test or a Mann-Whitney rank sum test was used. When comparing more than two groups, one-way analysis of variance with Bonferroni’s post hoc test was used.
Results
Effect of Medium Composition on Establishment of ASC Cultures
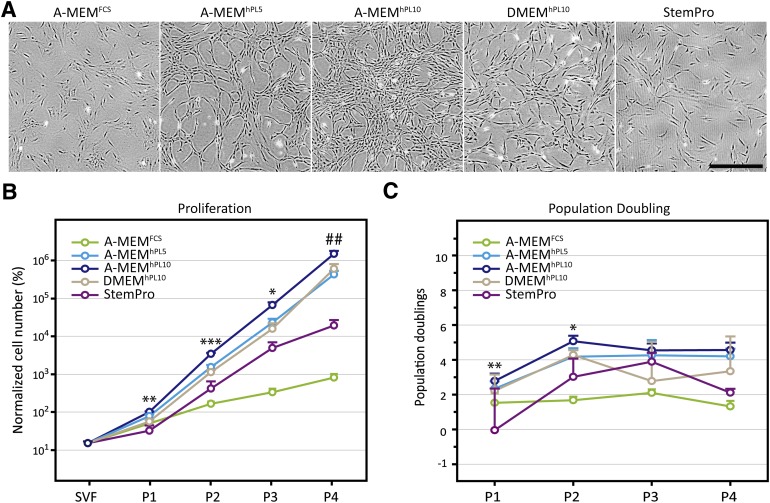
To determine the effect of medium composition on cell growth, freshly isolated cells were cultured for up to three passages in different media. From visual inspection of the cultures, it was apparent that media supplemented with hPL supported growth of ASCs better than either StemPro or media supplemented with FCS (Fig. 1A). Quantitative assessment of cell growth for up to four passages confirmed the superiority of hPL as a medium supplement and revealed a significantly higher cumulative number of cells in all three media supplemented with hPL at the end of passages 2, 3, and 4 compared with A-MEMFCS (Fig. 1B). Data are expressed on a log 10 scale to optimally visualize the exponential growth. As explained in the section on statistical analysis, the cell numbers are normalized and therefore are expressed as percentages. The higher cumulative cell number is a result of shorter population doubling time (supplemental online Fig. 1). Most of the media supported stable cell growth during serial passages (Fig. 1C). Interestingly, however, StemPro did not consistently support cell growth, and we observed that for two of three donors, the corresponding cell cultures could not be satisfactorily maintained for more than three passages, and for the third donor, no more than five passages.
Figure 1.
Assessment of proliferation of adipose-derived stem cells (ASCs). (A): ASC cultures reached different cell densities during expansion depending on media, as evident at passage 1 after 4 days of culture. (B): Accumulative cell number after a series of passages, where a clear effect of media is shown. (C): Number of population doublings that each population underwent during each passage. The same tendency as in (B) was seen. The results are presented as mean ± SEM. Cell number was normalized to the cell number of each individual donor for -MEMhPL10 at passage 1. A-MEMhPL10 was statistically different from all other media at p < .05 (∗), p < .01 (∗∗), and p < .001 (∗∗∗), and statistically different from all other media except DMEM at p < .007 (##). Scale bar = 500 µm. Abbreviations: A-MEM, α-minimum essential medium; DMEM, Dulbecco’s modified Eagle’s medium; FCS, fetal calf serum; hPL5, 5% human platelet lysate; hPL10, 10% human platelet lysate; P, passage; SVF, stromal vascular fraction.
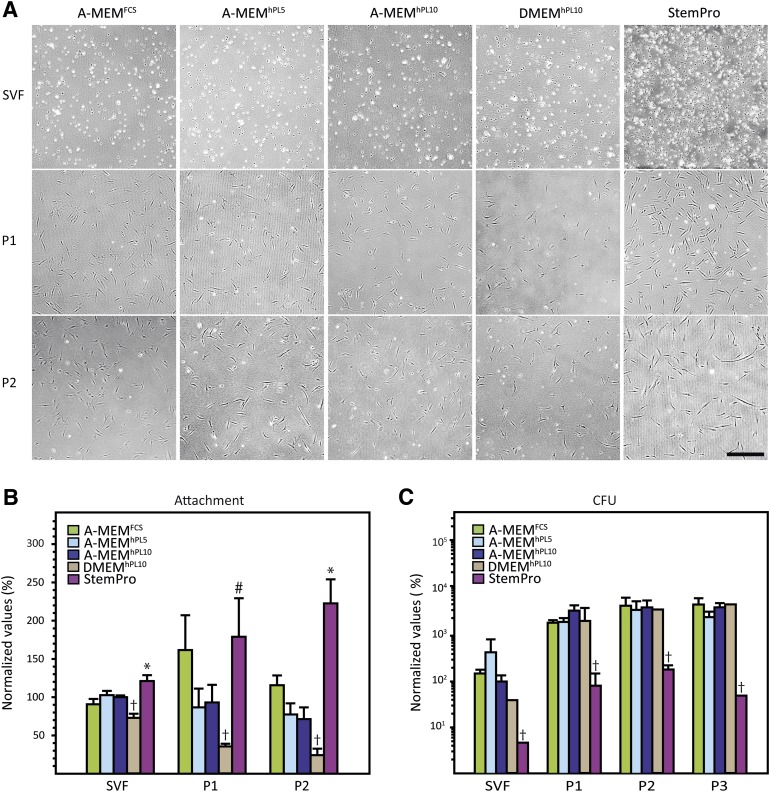
The effect of the media on cell attachment and support of CFUs was determined for SVFs and ASCs in early passages (Fig. 2). Overnight culture of SVFs and subsequent removal of nonadherent cells resulted in mixed populations of mostly spheric and a few slightly elongated cells, with no obvious morphologic differences between cells grown in the different media (Fig. 2A). For all media, passaging (both passages 1 and 2) resulted in changes in cell morphology and spreading, with the cells looking larger and more spindle shaped. For cells in passages 1 and 2, it appeared that culture in StemPro promoted cell attachment. A quantitative measure of the cell attachment within 16 hours after seeding (Fig. 2B) confirmed that StemPro did promote cell attachment to a higher degree than the other media, in particular higher than the hPL-supplemented media. The DMEM-based media, on the other hand, consistently supported cell attachment to a lower degree than the other media.
Figure 2.
Cell morphology, attachment (A, B), and clonogenicity (C). (A): Differences in the degree of cell attachment were observed after seeding and overnight incubation. Additionally, morphologic differences were seen between passages. (B): Quantification of cell attachment revealed significant differences between media. (C): Colony-forming potential of the different media. It can be seen that StemPro significantly underperformed compared with the other media. The results are presented as mean ± SEM. Cell number was normalized to the cell number of each individual donor for A-MEMhPL10 at passage 1. †, Statistically different from all other media at p < .05. ∗, StemPro statistically different from all other media. p < .05. #, StemPro statistically different from all other media except A-MEMFCS at p < .05. Scale bar = 500 µm. Abbreviations: A-MEM, α-minimum essential medium; CFU, colony-forming unit; DMEM, Dulbecco’s modified Eagle’s medium; FCS, fetal calf serum; hPL5, 5% human platelet lysate; hPL10, 10% human platelet lysate; P, passage; SVF, stromal vascular fraction.
To determine whether medium composition influenced the proportion of highly proliferative and clonogenic cells in the cultures, a CFU assay was performed (Fig. 2C). Not surprisingly, for all medium compositions the proportion of CFUs was higher in the passaged cells than in the SVFs. Also, comparing the frequency of CFUs across all passages, StemPro significantly underperformed, and there were no consistent differences between the other media. Furthermore, an obvious difference in colony size and confluence was observed, with the hPL-supplemented media being superior to both FCS and StemPro (data not shown).
Effect of Expansion in Different Media on ASC Morphology and Phenotype
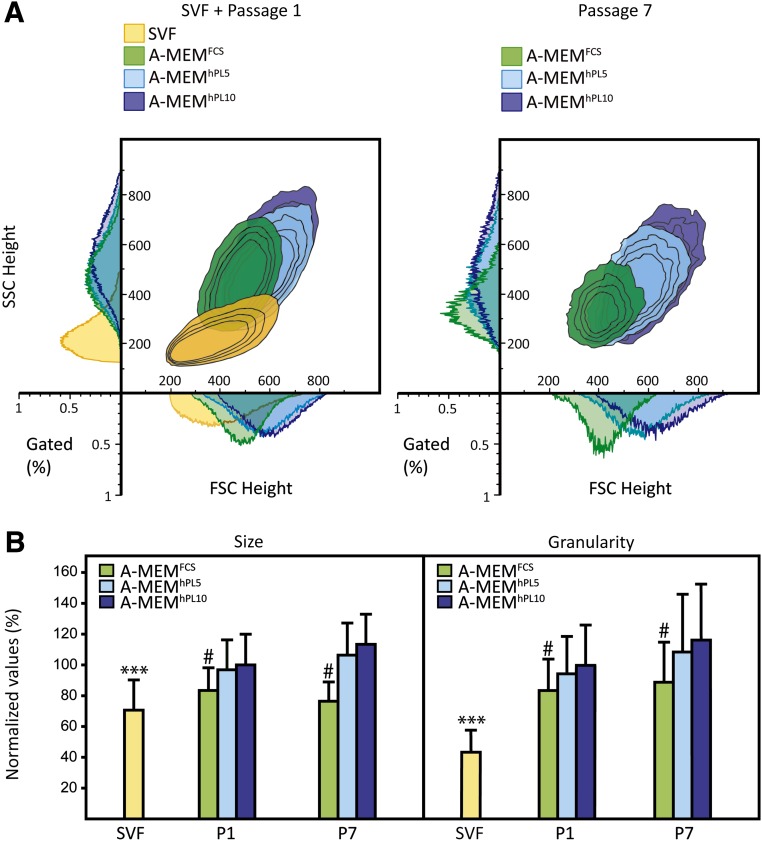
The effect of expansion on cell size and granularity was determined by flow cytometry. Figure 3 displays the analysis of cells from a representative donor. Regardless of supplement, cells cultured in A-MEM for one passage were larger and more granular than the cells composing the SVFs (Fig. 3A). Also it appeared that A-MEMhPL5/10 promoted subpopulations of cells that were larger and with a more granular phenotype than A-MEMFCS. At passage 7, this difference was even more prominent, with the cells cultured in A-MEMFCS representing a smaller, less granular, and more homogeneous population than cells in A-MEMhPL5 or A-MEMhPL10. These observations were consistent across donors (Fig. 3B). Interestingly, whereas cells cultured in DMEM appeared to have characteristics similar to cells cultured in A-MEM, cells cultured in StemPro seemed less granular (supplemental online Fig. 2).
Figure 3.
Size and granularity of adipose-derived stem cells (ASCs) cultured in different medium compositions. (A): Representative example from donor 1 showing the changes in size and granularity between the stromal vascular fraction, passage 1, and passage 7. Size was measured by mean of forward scatter height, and granularity was measured by mean of side scatter height using flow cytometry. The number of cells is presented as the percentage of total number of gated cells. (B): Average size and granularity for all donors reveal an increase in both size and granulation by first passage. The results are presented as mean ± SD. Cell size and granularity were normalized to the cell size and granularity of each individual donor for A-MEMhPL10 at passage 1. ∗∗∗, Statistically different from all other groups at p < .001. #, A-MEMFCS statistically different from all hPL-supplemented media at p < .001. Abbreviations: A-MEM, α-minimum essential medium; FCS, fetal calf serum; FSC, forward scatter; hPL5, 5% human platelet lysate; hPL10, 10% human platelet lysate; P, passage; SSC side scatter; SVF, stromal vascular fraction.
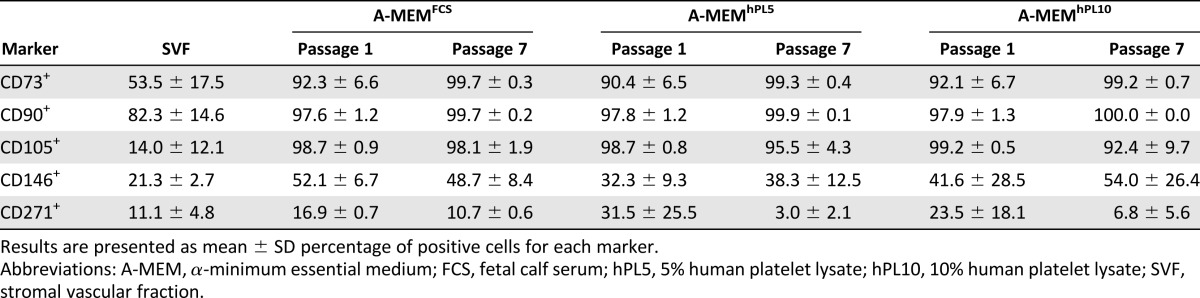
A qualitative assessment of selected surface markers showed that for the SVFs, >40% of the cells were positive for CD73 and CD90 for all donors (Table 2; supplemental online Table 3). As for the expression of CD105, on average only 14% of the cells were positive, and there was large interdonor variation. After just one passage in any A-MEM-based medium, >80% of the cells from all compositions were CD73+, CD90+, and CD105+. The average proportion of CD146+ and CD271+ cells in the SVF was relatively low, 21% and 11%, respectively. The average number of CD146+ cells increased with passaging to almost 50% at passage 7, and the amount of CD271+ cells decreased and almost disappeared for some donors.
Table 2.
Effect of media on expression of surface markers
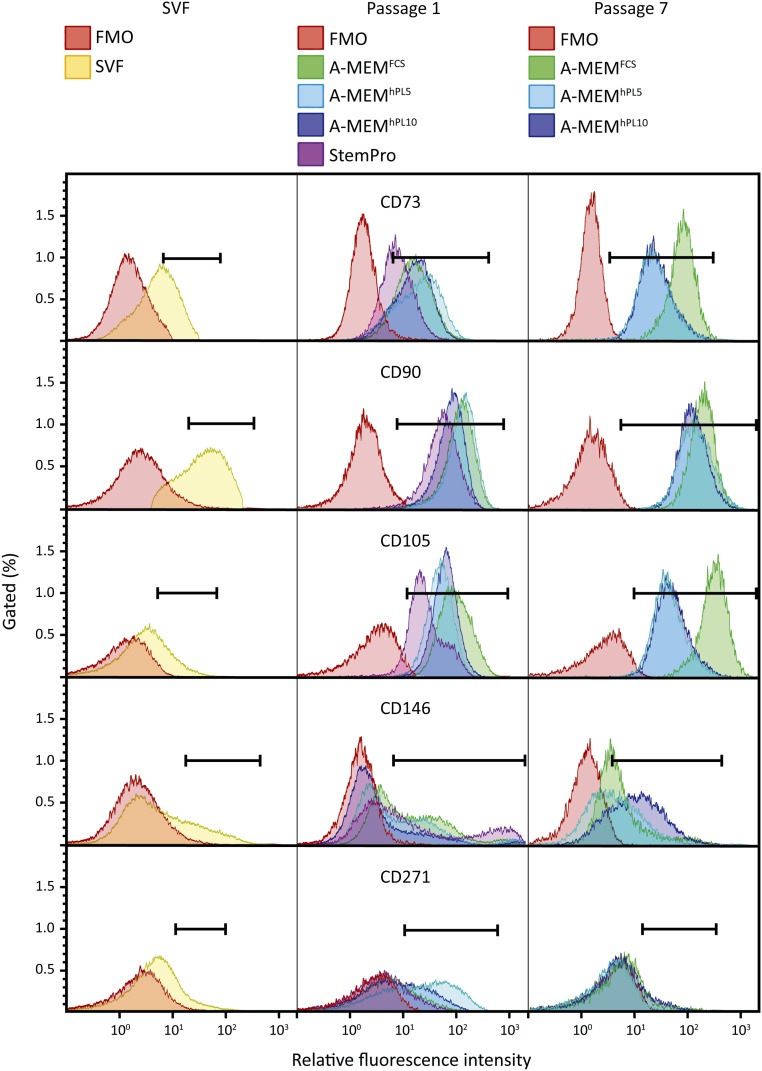
A comparative analysis of surface marker levels revealed substantial differences between cells cultured in the different media. This is illustrated in Figure 4, which depicts the intensity levels for all analyzed markers from a representative donor. It was evident that the number of CD73+ cells increased with passaging, especially from SVF to passage 1. However, this effect was less pronounced for the cells cultured in StemPro. At passage 7, all cells were positive for CD73; interestingly, though, culture in FCS seemed to stimulate expression levels more than culture in hPL, resulting in a more bright CD73+ population in A-MEMFCS. No differences between cells cultured in either 5% or 10% hPL were found. Also, we did not detect a difference between cells cultured in A-MEMhPL and DMEMhPL (supplemental online Fig. 3). We observed no effect of media on the expression level of CD90. The expression of CD105 mirrored the profile of CD73, with the cells becoming increasingly positive with passaging, and with an even larger difference in the antigen expression level between cells cultured in either FCS or hPL. In contrast, regarding CD146 expression, although the proportion of bright cells decreased from passage 1 to passage 7, culture in A-MEMhPL10 appeared to maintain a relatively higher expression level. These tendencies were observed for all donors.
Figure 4.
Flow cytometric profiles of surface markers obtained from SVF and adipose-derived stem cells (ASCs). ASCs were cultured in different medium compositions for one or seven passages. Differences between passages and medium compositions were found using flow cytometric analysis. Representative data are shown for donor 1. Abbreviations: A-MEM, α-minimum essential medium; FCS, fetal calf serum; FMO, fluorescence minus one; hPL5, 5% human platelet lysate; hPL10, 10% human platelet lysate; SVF, stromal vascular fraction.
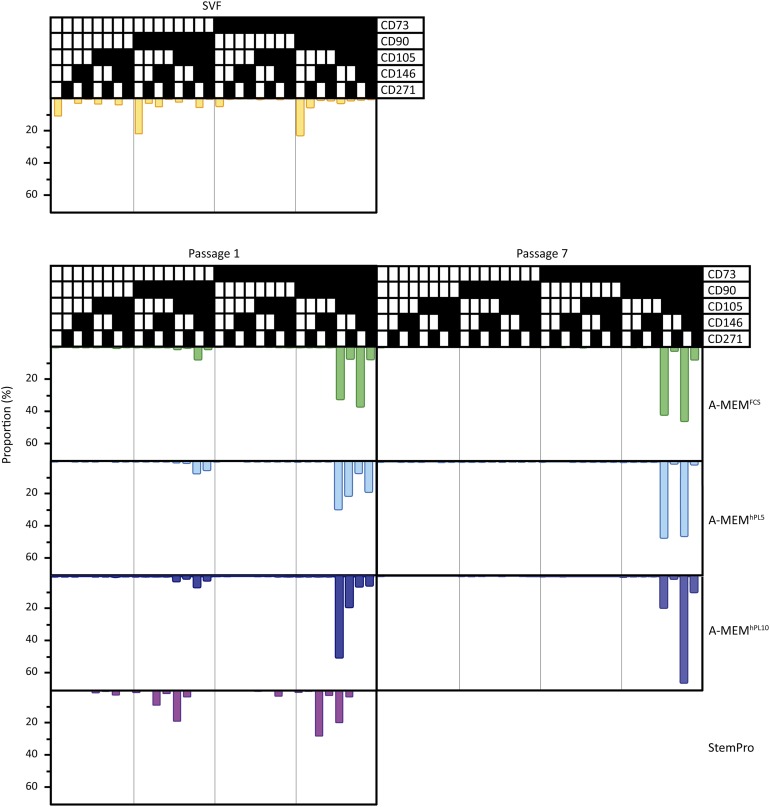
Figure 5 shows multiparametric analysis of the surface markers performed on cells from a representative donor. In the SVF sample, a very heterogeneous distribution with a variety of cell subpopulations was found. Populations with all combinations of the used markers were identified. The two largest subpopulations were CD90+, CD105−, CD146−, and CD271− and then either CD73+ or CD73−, comprising 22% and 21% of the total cell number, respectively. At passage 1, cells cultured in StemPro retained relatively more of the heterogeneous phenotype. However, cells cultured in A-MEMFCS/hPL were considerably more homogeneous. The CD105− populations had almost disappeared, and the majority of the cells fell in two categories that were CD90+, CD105+, and either CD73+ or CD73−. At passage 7, the populations were even more homogeneous compared with passage 1, as the CD73− populations were no longer found. Most of the cells were of the phenotype CD73+, CD90+, CD105+, CD271−, and either CD146+ or CD146−. This general pattern was observed for cells from all donors; however, the relative size of the CD146+ or CD146− populations varied between donors. Finally, it did not appear that culture in media supplemented with either hPL or FCS promoted a particular immunophenotypic profile, upon qualitatively assessing the populations. Neither was any difference between A-MEM and DMEM observed (supplemental online Fig. 4).
Figure 5.
Flow cytometric multiparametric analysis of adipose-derived stem cell (ASC) subpopulations for each medium composition. By the first passage, ASCs cultured in all medium compositions became more homogeneous. Homogeneity was greater by passage 7. Overall, subpopulations had the same characteristics, but differences were found in the size of the different subpopulations. Representative data are shown for donor 1. ▪, the marker was expressed; □, the marker was not expressed. Abbreviations: A-MEM, α-minimum essential medium; FCS, fetal calf serum; FMO, fluorescence minus one; hPL5, 5% human platelet lysate; hPL10, 10% human platelet lysate; SVF, stromal vascular fraction.
Discussion
When choosing media for culture of ASCs, selection is often based on a set of supportive characteristics as expansion, morphology, and multilineage differentiation. Traditionally, ASCs have been cultured in DMEM supplemented with FCS, which has been found to support establishment and maintenance of ASCs while preserving their stem cell characteristics [1]. However, when expanding cells for subsequent clinical applications, regulatory perspectives should also be taken into consideration [52]. For this purpose, expansion in chemically defined media is appealing, as it ensures both a xenogeneic-free and a highly reproducible environment. We found, however, that StemPro, the chemically defined and xenogeneic-free medium used in this study, did not support the establishment of a viable culture of ASCs from SVF, although it was previously shown to satisfactorily support ASC expansion [27]. This effect has also been described in previous studies [53]. The failure of StemPro to support establishment of ASC cultures could not be explained by inhibition of cell attachment to growth surface, as this medium was found to support attachment the most, probably supported by the CELLstart coating used for this medium as recommended by the manufacturer. However, in spite of supporting attachment of cells, StemPro failed to support both cell proliferation and generation of CFUs.
From a clinical viewpoint, the choice of a basal medium supplemented with hPL is another attractive option, to avoid the issues associated with the use of FCS. In this study, we compared A-MEM supplemented with FCS versus A-MEM supplemented with either 5% or 10% hPL. Regardless of the concentration, hPL supported ASC proliferation significantly better than FCS, which is in agreement with observations by others [38, 44, 54, 55]. The increase in cell yield did not appear to stem from higher numbers of cells attaching after seeding, nor from better yield of CFUs. However, larger colonies were observed in all hPL-supplemented medium compositions, which probably was a result of the faster proliferation in these compositions. Additionally, ASCs cultured in hPL-supplemented media were larger and more granular than ASCs cultured in FCS-supplemented counterparts. Others have reported the opposite effects of hPL on ASCs’ morphologic parameters, but using DMEM as basal medium [38, 55]. This shows the profound impact that a particular medium formulation has on the morphologic characteristics of ASCs. It has been suggested that morphologic properties may be linked to defined subpopulations within MSC cultures that display defined biological properties [56].
In agreement with earlier studies and the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy criteria for ASCs [2, 20, 57–59], >80% of the cells expressed CD73, CD90, and CD105 with passaging. As already mentioned, the limited amount of cells resulting from the SVF preparations restricted the number of conditions that could be assessed in parallel for each donor. Therefore, as StemPro has been proven unsuitable for initiation of ASC cultures, and DMEM supports ASC proliferation to a lesser degree than A-MEM, we chose to focus the analysis of surface markers on the effect of hPL and FCS supplementation of A-MEM. In addition to the presence of selected surface markers, the lack of expression of surface antigens CD31 and CD45 should be demonstrated. This lack of expression of CD31 and CD45 by ASCs cultured in conditions described in this article has been conclusively demonstrated by us and others in previous studies [27, 38, 46, 48, 53, 60, 61]. Thus, because of limitations on the number of different surface markers that could be simultaneously detected by flow cytometry, we chose not to perform an analysis of expression of CD31 and CD45.
Interestingly, when quantifying surface marker expression after seven passages, ASCs cultured in FCS were more bright in the expression of CD73 and CD105 and dim in CD146 compared with ASCs cultured in hPL. Also, continuous culture in either FCS or hPL affected the distribution of ASCs within the subcategories of CD146+ and/or CD271+ cells. Whether and how this altered expression of the classic stem cell markers CD73 and CD105 reflects on the functionality of the ASCs is still uncertain, but CD73 has been suggested to be involved in differentiation [62] and regulation of the immune modulatory pathway together with CD105 [63, 64].
We also chose to investigate the effect of culture media on the expression of CD146 and CD271, which are not routinely included in ASC characterization. A high expression level of CD146 has been correlated with multipotency and possible a microvascular pericyte phenotype [65, 66]. Additionally, these cells have been shown to be more angiogenic than their CD146-negative counterparts, making them very attractive for regenerative therapy [67]. Compared with SVF, there is a higher proportion of CD146+ cells in the ASCs. This proportion of approximately 40% positive and very positive cells remained relatively constant from passage 1 to passage 7. However, although the amount of highly expressing cells declined for all media, hPL preserved relatively higher expression levels than FCS, which may have implications for use of ASCs for treatment of ischemic diseases. The marker CD271 has been suggested to be a subpopulation of resting primitive MSCs [68]. In agreement with earlier studies by others [68], we found that the proportion of CD271+ cells decreased with passaging, and we did not observe differential effects of hPL and FCS.
Conclusion
In this work, we compared different types of media on the establishment and maintenance of ASC cultures. It was found that the chemically defined medium StemPro was unsuitable for initiation of ASC cultures from SVF. In addition, culture in hPL favored a high proliferation rate, and both FCS and hPL were appropriate for maintaining stem cell characteristics. However, culture in either FCS or hPL favored specific subpopulations within the ASCs in terms of the intensity of specific surface antigen expression. Apart from the classic markers for ASCs, we identified a number of subpopulations within the ASCs that mainly differed by their expression of CD146 and CD271. Thus, an important biologic measure to take into account when choosing culture system and medium composition, besides the classic measures of proliferation and stem cell characteristics, might be the resulting composition of subpopulations, as these are affected by culture system and might be linked to regenerative potential. Further investigation of the regenerative potential of the different subpopulations is needed to reach more definite conclusions.
Supplementary Material
Acknowledgments
We acknowledge provision of adipose tissue samples by the Teres Private Hospital and the technical assistance of O. Jensen. The work was supported in part by funds from Lily Benthine Lunds Fond (SR Grant 5652) and the Obelske Family Foundation (TF Grant 20114-18036). The funding sources had no influence on study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication.
Author Contributions
S.R.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; F.M.N.: collection and assembly of data, data analysis and interpretation; C.P.P.: manuscript writing, final approval of manuscript; V.Z.: data analysis and interpretation, final approval of manuscript; T.F.:conception and design, manuscript writing, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sándor GK, Numminen J, Wolff J, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Translational Medicine. 2014;3:530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batioglu-Karaaltin A, Karaaltin MV, Ovali E, et al. In vivo tissue-engineered allogenic trachea transplantation in rabbits: A preliminary report. Stem Cell Rev. 2015;11:347–356. doi: 10.1007/s12015-014-9570-8. [DOI] [PubMed] [Google Scholar]
- 5.Kokai LE, Marra K, Rubin JP. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399–408. doi: 10.1016/j.trsl.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen JG, Frøbert O, Pilgaard L, et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen JG, Frøbert O, Holst-Hansen C, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23:195–206. doi: 10.3727/096368912X659871. [DOI] [PubMed] [Google Scholar]
- 8.Shafy A, Fink T, Zachar V, et al. Development of cardiac support bioprostheses for ventricular restoration and myocardial regeneration. Eur J Cardiothorac Surg. 2013;43:1211–1219. doi: 10.1093/ejcts/ezs480. [DOI] [PubMed] [Google Scholar]
- 9.Qayyum AA, Haack-Sørensen M, Mathiasen AB, et al. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): Study design. Regen Med. 2012;7:421–428. doi: 10.2217/rme.12.17. [DOI] [PubMed] [Google Scholar]
- 10.Perin EC, Sanz-Ruiz R, Sánchez PL, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Guadalajara H, Herreros D, De-La-Quintana P, et al. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595–600. doi: 10.1007/s00384-011-1350-1. [DOI] [PubMed] [Google Scholar]
- 13.Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: A phase I clinical study. Cell Transplant. 2013;22:279–285. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 14.Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 15.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 16.Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikuła M, Marek-Trzonkowska N, Wardowska A, et al. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther. 2013;13:1357–1370. doi: 10.1517/14712598.2013.823153. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 19.Fink T, Lund P, Pilgaard L, et al. Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol. 2008;9:98. doi: 10.1186/1471-2199-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilgaard L, Lund P, Duroux M, et al. Effect of oxygen concentration, culture format and donor variability on in vitro chondrogenesis of human adipose tissue-derived stem cells. Regen Med. 2009;4:539–548. doi: 10.2217/rme.09.28. [DOI] [PubMed] [Google Scholar]
- 21.Prasad M, Zachar V, Fink T, et al. Moderate hypoxia influences potassium outward currents in adipose-derived stem cells. PLoS One. 2014;9:e104912. doi: 10.1371/journal.pone.0104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen JG, Riis SE, Frøbert O, et al. PLoS One. Activation of protease-activated receptor 2 induces VEGF independently of HIF-1. 2012;7:e46087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foldberg S, Petersen M, Fojan P, et al. Patterned poly(lactic acid) films support growth and spontaneous multilineage gene expression of adipose-derived stem cells. Colloids Surf B Biointerfaces. 2012;93:92–99. doi: 10.1016/j.colsurfb.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Pennisi CP, Zachar V, Fink T, et al. Patterned polymeric surfaces to study the influence of nanotopography on the growth and differentiation of mesenchymal stem cells. Methods Mol Biol. 2013;1058:77–88. doi: 10.1007/7651_2013_10. [DOI] [PubMed] [Google Scholar]
- 25.Huang S-C, Wu T-C, Yu H-C, et al. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol. 2010;11:18. doi: 10.1186/1471-2121-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen JI, Juhl M, Nielsen T, et al. Uniaxial cyclic strain enhances adipose-derived stem cell fusion with skeletal myocytes. Biochem Biophys Res Commun. 2014;450:1083–1088. doi: 10.1016/j.bbrc.2014.06.124. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Pilgaard L, Chase LG, et al. Defined xenogeneic-free and hypoxic environment provides superior conditions for long-term expansion of human adipose-derived stem cells. Tissue Eng Part C Methods. 2012;18:593–602. doi: 10.1089/ten.TEC.2011.0592. [DOI] [PubMed] [Google Scholar]
- 28.Lund P, Pilgaard L, Duroux M, et al. Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy. 2009;11:189–197. doi: 10.1080/14653240902736266. [DOI] [PubMed] [Google Scholar]
- 29.Rauch C, Feifel E, Amann E-M, et al. Alternatives to the use of fetal bovine serum: Human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Olmo D, Herreros D, Pascual M, et al. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: A comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24:27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
- 31.García-Olmo D, García-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 32.de la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 33.Herreros MD, Garcia-Arranz M, Guadalajara H, et al. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762–772. doi: 10.1097/DCR.0b013e318255364a. [DOI] [PubMed] [Google Scholar]
- 34.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 35.Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ J. 2012;76:1750–1760. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- 36.Bal-Price A, Coecke S. Guidance on good cell culture practice (GCCP) Cell Cult Tech. 2011;56:1–25. [Google Scholar]
- 37.Shih DT-B, Chen J-C, Chen W-Y, et al. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51:770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- 38.Trojahn Kølle SF, Oliveri RS, Glovinski PV, et al. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy. 2013;15:1086–1097. doi: 10.1016/j.jcyt.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 39.Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Cholewa D, Stiehl T, Schellenberg A, et al. Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant. 2011;20:1409–1422. doi: 10.3727/096368910X557218. [DOI] [PubMed] [Google Scholar]
- 41.Atashi F, Jaconi MEE, Pittet-Cuénod B, et al. Autologous platelet-rich plasma: A biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods. 2015;21:253–262. doi: 10.1089/ten.tec.2014.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blande IS, Bassaneze V, Lavini-Ramos C, et al. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion. 2009;49:2680–2685. doi: 10.1111/j.1537-2995.2009.02346.x. [DOI] [PubMed] [Google Scholar]
- 43.Abdallah BM, Haack-Sørensen M, Fink T, et al. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone. 2006;39:181–188. doi: 10.1016/j.bone.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 44.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 45.Witzeneder K, Lindenmair A, Gabriel C, et al. Human-derived alternatives to fetal bovine serum in cell culture. Transfus Med Hemother. 2013;40:417–423. doi: 10.1159/000356236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CY, Wu XY, Tong JB, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink T, Rasmussen JG, Lund P, et al. Isolation and expansion of adipose-derived stem cells for tissue engineering. Front Biosci (Elite Ed) 2011;3:256–263. doi: 10.2741/e241. [DOI] [PubMed] [Google Scholar]
- 48.Lindroos B, Boucher S, Chase L, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958–972. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 49.Zachar V, Rasmussen JG, Fink T. Isolation and growth of adipose tissue-derived stem cells. Methods Mol Biol. 2011;698:37–49. doi: 10.1007/978-1-60761-999-4_4. [DOI] [PubMed] [Google Scholar]
- 50.Hayflick L. Tissue Culture Methods and Applications. New York, NY: Academic Press, 1973:220. [Google Scholar]
- 51.Schellenberg A, Hemeda H, Wagner W. Tracking of replicative senescence in mesenchymal stem cells by colony-forming unit frequency. Methods Mol Biol. 2013;976:143–154. doi: 10.1007/978-1-62703-317-6_11. [DOI] [PubMed] [Google Scholar]
- 52.Riis S, Zachar V, Boucher S, et al. Critical steps in the isolation and expansion of adipose-derived stem cells for translational therapy. Expert Rev Mol Med. 2015;17:e11. doi: 10.1017/erm.2015.10. [DOI] [PubMed] [Google Scholar]
- 53.Patrikoski M, Juntunen M, Boucher S, et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4:27. doi: 10.1186/scrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castegnaro S, Chieregato K, Maddalena M, et al. Effect of platelet lysate on the functional and molecular characteristics of mesenchymal stem cells isolated from adipose tissue. Curr Stem Cell Res Ther. 2011;6:105–114. doi: 10.2174/157488811795495440. [DOI] [PubMed] [Google Scholar]
- 55.Naaijkens BA, Niessen HWM, Prins HJ, et al. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348:119–130. doi: 10.1007/s00441-012-1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haasters F, Prall WC, Anz D, et al. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J Anat. 2009;214:759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Harmelen V, Skurk T, Röhrig K, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- 58.Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 59.Jurgens WJFM, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008;332:415–426. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mojica-Henshaw MP, Jacobson P, Morris J, et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy. 2013;15:1458–1468. doi: 10.1016/j.jcyt.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 61.Jang Y, Koh YG, Choi Y-J, et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev Biol Anim. 2015;51:142–150. doi: 10.1007/s11626-014-9814-6. [DOI] [PubMed] [Google Scholar]
- 62.Ode A, Schoon J, Kurtz A, et al. CD73/5′-ecto-nucleotidase acts as a regulatory factor in osteo-/chondrogenic differentiation of mechanically stimulated mesenchymal stromal cells. Eur Cell Mater. 2013;25:37–47. doi: 10.22203/ecm.v025a03. [DOI] [PubMed] [Google Scholar]
- 63.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sempere JM, Martinez-Peinado P, Arribas MI, et al. Single cell-derived clones from human adipose stem cells present different immunomodulatory properties. Clin Exp Immunol. 2014;176:255–265. doi: 10.1111/cei.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell KC, Phinney DG, Lacey MR, et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 66.Pierantozzi E, Badin M, Vezzani B, et al. Human pericytes isolated from adipose tissue have better differentiation abilities than their mesenchymal stem cell counterparts. Cell Tissue Res. 2015;361:769–778. doi: 10.1007/s00441-015-2166-z. [DOI] [PubMed] [Google Scholar]
- 67.Blocki A, Wang Y, Koch M, et al. Not all MSCs can act as pericytes: Functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev. 2013;22:2347–2355. doi: 10.1089/scd.2012.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuevas-Diaz Duran R, González-Garza MT, Cardenas-Lopez A, et al. Age-related yield of adipose-derived stem cells bearing the low-affinity nerve growth factor receptor. Stem Cells Int. 2013;2013:372164. doi: 10.1155/2013/372164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.