The objective was to determine whether A disintegrin and metalloproteinase domain 17 (ADAM17) regulates the cancer stem cell (CSC) phenotype in colorectal cancer (CRC) and elucidate the downstream signaling mechanism mediating cancer stem-ness. The results showed that ADAM17 has a role in regulating the CSC phenotype and chemoresistance in CRC cells. Drugs that inhibit ADAM17 activity might increase the therapeutic benefit to metastatic CRC and, potentially, other solid malignancies.
Keywords: Colorectal cancer, Cancer stem cells, A disintegrin and metalloproteinase domain 17, Notch1, Chemosensitivity, Jagged-1
Abstract
Evidence is accumulating for the role of cancer stem cells (CSCs) in mediating chemoresistance in patients with metastatic colorectal cancer (mCRC). A disintegrin and metalloproteinase domain 17 (ADAM17; also known as tumor necrosis factor-α-converting enzyme [TACE]) was shown to be overexpressed and to mediate cell proliferation and chemoresistance in CRC cells. However, its role in mediating the CSC phenotype in CRC has not been well-characterized. The objective of the present study was to determine whether ADAM17 regulates the CSC phenotype in CRC and to elucidate the downstream signaling mechanism that mediates cancer stemness. We treated established CRC cell lines and a newly established human CRC cell line HCP-1 with ADAM17-specific small interfering RNA (siRNA) or the synthetic peptide inhibitor TAPI-2. The effects of ADAM17 inhibition on the CSC phenotype and chemosensitivity to 5-fluorouracil (5-FU) in CRC cells were examined. siRNA knockdown and TAPI-2 decreased the protein levels of cleaved Notch1 (Notch1 intracellular domain) and HES-1 in CRC cells. A decrease in the CSC phenotype was determined by sphere formation and ALDEFLUOR assays. Moreover, TAPI-2 sensitized CRC cells to 5-FU by decreasing cell viability and the median lethal dose of 5-FU and increasing apoptosis. We also showed the cleavage and release of soluble Jagged-1 and -2 by ADAM17 in CRC cells. Our studies have elucidated a role of ADAM17 in regulating the CSC phenotype and chemoresistance in CRC cells. The use of drugs that inhibit ADAM17 activity might increase the therapeutic benefit to patients with mCRC and, potentially, those with other solid malignancies.
Significance
The present study has demonstrated the role of A disintegrin and metalloproteinase domain 17 (ADAM17) in regulating cancer stemness and chemosensitivity in colorectal cancer (CRC) cells. In addition, a previously unknown cleavage of the Notch ligands Jagged-1 and -2 by ADAM17 in CRC cells is reported. These findings will have an impact on future studies of the regulation of cancer stem cells in CRC and, potentially, other cancer types.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States [1]. Although several new drugs have received regulatory approval for the treatment of patients with metastatic CRC (mCRC) in the past 3 years, the prognosis remains very poor (2.5 years median overall survival), and most patients will develop drug resistance to systemic therapy within 1 year [1–3]. Developing novel treatments based on sound preclinical data is essential to improving the outcomes of patients with mCRC.
Evidence is accumulating of the existence of cancer stem cells (CSCs) in mCRC, and these cells are believed to mediate the development of chemoresistance [4–6]. Several signaling pathways (including Wnt, Notch, Hedgehog, and Nanog) have been identified as key mediators of the CSC phenotype [7]. Our previous study demonstrated a strong correlation between Notch1 activation and CSCs in CRC [8]. As a member of the Notch receptor family, Notch1 can be activated by the binding of ligands (Jagged-1 and -2, and Delta-like 1, 3, and 4) from adjacent cells by juxtacrine or paracrine signaling [9, 10]. On ligand binding, the Notch1 receptor is activated and undergoes conformational changes, leading to cleavage at the S2 site (within the extracellular juxtamembrane region) and then subsequently at the S3 site to release the Notch1 intracellular domain (NICD), allowing its transcription activity [11]. A disintegrin and metalloproteinase domain 17 (ADAM17; also known as tumor necrosis factor-α [TNF-α]-converting enzyme [TACE]) is responsible for the S2 site cleavage and is essential for exposing the S3 site for γ-secretase cleavage [12].
In addition to activating Notch1, ADAM17 is known to mediate the maturation and secretion of a large number of substrates, including TNF-α, cytokines, and growth factors from cell membranes [13]. In CRC and other cancers, ADAM17 is overexpressed in tumor tissues compared with normal tissues and mediates cancer cell proliferation, metastasis, and chemoresistance [14, 15]. Others have shown that blocking ADAM17 activity or expression in cancer cell lines (including ovarian cancer, head and neck cancer, CRC, and breast cancer) resulted in decreased cancer cell proliferation and chemoresistance [16–20]. Although ADAM17 is important in mediating CSC-associated Notch1 activation and promoting the CSC phenotype in glioblastoma, lung cancer, and head and neck cancer [18, 21, 22], the role of ADAM17 in mediating the CSC phenotype in CRC has not been fully elucidated. The aim of the present study was to determine whether ADAM17 regulates the CSC phenotype in CRC and to identify the downstream signaling mechanism that mediates cancer stemness. We have demonstrated that blocking ADAM17 in CRC cells leads to a decrease in the CSC phenotype and sensitizes CRC cells to chemotherapy. These findings suggest a potential therapeutic strategy of targeting CSCs by inhibiting ADAM17 in patients with mCRC.
Materials and Methods
Cell Culture
The CRC cell line HT29 was purchased from American Type Culture Collection (Manassas, VA, http://www.atcc.org). The human CRC primary cell line HCP-1 was established in our laboratory [8]. All CRC cells were cultured within 15 passages in minimal essential medium supplemented with 5% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA, http://www.atlantabio.com), vitamins (1×), nonessential amino acids (1×), penicillin-streptomycin antibiotics (1×), sodium pyruvate (1×), and l-glutamine (1×) (all from Invitrogen, Carlsbad, CA, http://www.thermofisher.com).
Reagents
The ADAM17 inhibitor TAPI-2 and γ-secretase inhibitor (GSI) were purchased from Calbiochem (San Diego, CA, http://www.calbiochem.com). All chemotherapy agents were obtained from the pharmacy at The University of Texas MD Anderson Cancer Center. ADAM17-specific small interfering RNAs (siRNAs; GGUUACAACUCAUGAAUUG and GCAAAGAAACAGAGUGCUA) and a validated control siRNA were obtained from Sigma-Aldrich (St. Louis, MO, http://www.sigmaldrich.com) and were transiently transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Unless mentioned otherwise, all experiments were performed using 20 μM TAPI-2, 100 nM GSI, or 150 pmol per well of siRNA for cell transfection in six-well plates.
Western Blotting
Cell lysates or concentrated conditioned medium (CM) was processed and run in SDS-polyacrylamide gel electrophoresis gels, as described previously [8, 23]. The antibodies used to detect α-tubulin, Delta-like 4 N-terminal, and Nanog were from Santa Cruz Biotechnology (Santa Cruz, CA, http://www.scbt.com), β-actin was from Sigma-Aldrich, β-catenin was from BD Biosciences (Franklin Lakes, NJ, http://www.bdbiosciences.com), Delta-like 1 N-terminal was from Abcam (Cambridge, MA, http://www.abcam.com). All other antibodies were from Cell Signaling (Beverly, MA, http://www.cellsignal.com). The antibodies for the Notch ligands were all specific to the extracellular domain of the proteins. 0.1% Ponceau S in 5% acetic acid was added to polyvinylidene fluoride membranes for Ponceau S staining as the loading control of CM.
Sphere Formation Assay
CRC cells were treated with TAPI-2 or siRNAs for 48 hours. Single suspended cells were then plated 100 per well in ultra-low-attachment 96-well plates (BD Biosciences) with standard sphere-forming medium [24] (serum-free Dulbecco’s modified Eagle’s medium/F-12 supplemented with 1xB27 serum substitute, 20 ng/ml human recombinant epidermal growth factor [EGF], and 20 ng/ml basic fibroblast growth factor; all from Invitrogen). The cells were then cultured for 7–14 days without changing the medium, and spheres larger than 50 μm in diameter in each well were counted.
ALDEFLUOR Assay
The CSC phenotype was assessed by detecting high intracellular aldehyde dehydrogenase (ALDH) enzyme activity using the ALDEFLUOR assay kit (STEMCELL Technologies, Vancouver, BC, Canada, http://www.stemcell.com). In brief, the cells were treated with TAPI-2 or siRNAs for 48 hours. After the incubation, single suspended cells were washed and treated according to the manufacturer’s instructions. Cells with a strong fluorescence signal were detected using the BD FACSCanto II flow cytometry system (BD Biosciences), with 488 nm excitation and 545 nm emission wavelengths and standardized by negative controls.
ADAM17 Activity Assay
ADAM17 enzyme activity was measured using a TACE activity kit (Calbiochem) following the manufacturer’s instructions. In brief, the cells were treated with TAPI-2 or siRNAs for 48 hours. Cell lysates were harvested using sample buffer, followed by sonication and the assay. The fluorescent signal generated by active ADAM17 was measured at wavelengths of 324 nm excitation and 405 nm emission and standardized with blank controls.
MTT Assay
Cells were cultured with or without TAPI-2 for 48 hours and then seeded at 3,000 cells per well in 96-well plates. After pretreatment, increasing doses of 5-fluorouracil (5-FU) that were relevant to the recommended clinical dose (up to 2 μg/ml) [25] were added, with or without TAPI-2, for 72 hours. Cell viability was assessed by adding MTT substrate (0.25% in phosphate-buffered saline [PBS]; Sigma-Aldrich) in growth medium (1:5 dilution) to cells for 1 hour at 37°C. The cells were washed with PBS, and 100 μl of dimethyl sulfoxide was added. Optical density was measured at 570 nm, and relative MTT is presented as a percentage of control.
Conditioned Medium
Equal numbers of CRC cells were cultured in growth medium with 1% FBS for 48 hours. The medium was harvested, centrifuged at 4,000g for 5 minutes to remove cell debris, and concentrated using Amicon Ultra-10K centrifugal filter units (EMD Millipore, Billerica, MA, http://www.emdmillipore.com).
Statistical Analysis
All quantitative data were reproduced in at least three independent experiments, with multiple measures in each replicate. The resulting data are expressed as the mean ± SEM and were considered significantly different at p < .05 by two-tailed Student’s t test.
Results
Blocking ADAM17 Expression Decreased the Cancer Stem Cell Phenotype of CRC Cells
To determine the role of endogenous ADAM17 in regulating the CSC phenotype of CRC cells, we used two siRNAs specifically targeting ADAM17 (si-1 and si-2) to knock down ADAM17 expression in a recently established human CRC cell line (HCP-1) and the established HT29 CRC cell line in vitro. As shown in Figure 1, 48 hours after the transient transfection of siRNAs, ADAM17 knockdown was confirmed by Western blotting. Moreover, the protein levels of cleaved NICD and its downstream target HES-1 were also decreased by ADAM17 knockdown. However, the levels of proteins in other CSC-associated pathways (Nanog, Sonic Hedgehog, and Wnt) were not altered (supplemental online Fig. 1A). HT29 showed higher basal levels of NICD and HES-1 compared with HCP-1, suggesting a higher capacity of the Notch-driven CSC phenotype. The effect of siRNA knockdown on the enzyme activity of ADAM17 was assessed by the TACE protease activity kit to measure ADAM17 cleavage activity after 24 hours of siRNA transfection. In both cell lines, ADAM17-specific siRNAs caused a significant decrease (∼50%) in the protease activity of ADAM17 (Fig. 1B). The effects of ADAM17 knockdown on the CSC phenotype were assessed by sphere formation (Fig. 1C, 1D) and ALDEFLUOR assays (Fig. 1E, 1F). The results showed that ADAM17 siRNAs significantly decreased the number of spheres formed by CRC cells and the percentage of cells with high ALDH activity (ALDH+) by ∼40% in HCP-1 cells and ∼45% in HT29 cells. Consistent with the finding that HT29 cells exhibited higher Notch1 activity than HCP-1 cells, control HT29 cells formed significantly more spheres (14 compared with 6 in HCP-1 cells) and ALDH+ cells (26% compared with 13% in HCP-1 cells).
Figure 1.
Knockdown of ADAM17 expression decreased the cancer stem cell phenotype in colorectal cancer (CRC) cells. CRC cells were transiently transfected with control siRNA or ADAM17-specific siRNAs (si-1 or si-2). (A): Western blotting showed decreased protein levels of ADAM17, cleaved Notch1 (NICD), and HES-1. α-Tubulin was used as the loading control. (B): Decreased ADAM17 enzyme activity was determined by the TACE activity assay and is presented as a percentage relative to the controls. (C, D): Decreased sphere formation per 100 cells per well. (E, F): A decrease in the numbers of cells with high ALDH activity was demonstrated by the ALDEFLUOR assay and is presented as a percentage of the total population. Data are presented as mean ± SEM; ∗, p < .05, Student's t test. Abbreviations: ADAM17, A disintegrin and metalloproteinase domain 17; ALDH, aldehyde dehydrogenase; Ctrl, control; NICD, Notch1 intracellular domain; si-1 and si-2, ADAM17-specific small interfering RNAs; si-Ctrl, control small interfering RNA.
To further validate the effects of blocking ADAM17 on the CSC phenotype in CRC, we used the ADAM17 inhibitor TAPI-2 to block ADAM17 activity in vitro. The effects of TAPI-2 on the protein levels of ADAM17, NICD, and HES-1 were determined by Western blotting after 48 hours of treatment (Fig. 2A). Data showed that without affecting ADAM17 expression, the inhibitor dramatically decreased the protein levels of NICD and its downstream target HES-1 in both HCP-1 and HT29 cells. Consistent with our experiments using siRNA, the ADAM17 inhibitor did not affect any other CSC-associated pathways examined (supplemental online Fig. 1B). As expected, the protease activity of ADAM17 was significantly decreased by the inhibitor by ∼50% in both cell lines (Fig. 2B). Moreover, treating cells with TAPI-2 significantly decreased the CSC phenotype by ∼50% in both CRC cell lines, measured by sphere formation and ALDEFLUOR assays (Fig. 2C, 2D). The dose-dependent effects of TAPI-2 on the sphere formation and protein levels of NICD and HES-1 (supplemental online Fig. 2) confirmed that the concentration we used (20 μM) was within the effective dose range of TAPI-2 (5–40 μM). Compared with the cells transfected with control siRNA (Fig. 1), untransfected CRC cells in the control groups had lower protein levels of NICD and HES-1, coupled with less sphere formation and lower numbers of ALDH+ cells (Fig. 2). We speculate that the transient elevation in Notch activity and CSC phenotype was caused by the stress induced by transfection with Lipofectamine. Therefore, we used the ADAM17 inhibitor in the remaining experiments to avoid the artificial effect caused by transfection.
Figure 2.
ADAM17 inhibitor TAPI-2 decreased the cancer stem cell phenotype in CRC cells. CRC cells were treated with (+ or TAPI-2) or without (- or Ctrl) the ADAM17 inhibitor TAPI-2. (A): Western blotting determined the protein levels of ADAM17 and showed decreased cleaved NICD and HES-1. α-Tubulin was used as the loading control. (B): Decreased ADAM17 enzyme activity was determined by the TACE activity assay and is presented as the percentage relative to the control. (C): Decreased sphere formation per 100 cells per well. (D): ALDEFLUOR assay showed decreased numbers of cells with high ALDH activity, presented as a percentage of the total population. Data are presented as mean ± SEM; ∗, p < .05, Student's t test. Abbreviations: ADAM17, A disintegrin and metalloproteinase domain 17; ALDH, aldehyde dehydrogenase; Ctrl, control; NICD, Notch1 intracellular domain.
Blocking ADAM17 Sensitized CRC Cells to Chemotherapy
To determine whether ADAM17 mediates the CRC cell response to chemotherapy, we treated HCP-1 and HT29 cells with 5-FU, with or without TAPI-2, and cell viability and apoptosis were measured. In the control groups, 5-FU alone caused steady decreases in cell viability in both cell lines. When a fixed dose of TAPI-2 was added with 5-FU, a ∼20% decrease in cell viability was observed even at low doses of 5-FU (0.2 and 0.5 μg/ml; Fig. 3A, 3B). Similar results were found when the cells were treated with TAPI-2 and SN38 (the active metabolite of irinotecan), but not with oxaliplatin (supplemental online Fig. 3). To further validate the effect of TAPI-2 sensitizing CRC cells to 5-FU, we estimated the median lethal dose (LD50) of 5-FU (Fig. 3C, 3D) based on another set of MTT assays with a wider range of 5-FU doses (data not shown). Compared with the control groups, TAPI-2 treatment decreased the LD50 of 5-FU by approximately threefold (from ∼1.5 to 0.5 μg/ml) in HCP-1 cells and close to fourfold (from ∼2.7 to 0.7 μg/ml) in HT29 cells. To determine whether the decreased cell viability by TAPI-2 at low doses of 5-FU was caused by increased cell death, we treated CRC cells with 5-FU (0.5 μg/ml), with or without TAPI-2, and measured the protein levels of apoptotic markers by Western blotting (Fig. 3E). When the cells were treated with low-dose 5-FU alone, no increase in cleaved poly(ADP-ribose) polymerase (PARP) or cleaved caspase-3 was noticeable in either cell line. However, when the cells were treated with TAPI-2 and low-dose 5-FU together, a dramatic increase in both cleaved PARP and cleaved caspase-3 was detected. No change was detected in the cells treated with TAPI-2 alone, suggesting that blocking ADAM17 alone was not sufficient to induce apoptosis in CRC cells.
Figure 3.
ADAM17 inhibitor TAPI-2 sensitized colorectal cancer (CRC) cells to chemotherapy. CRC cells were treated without (- and Ctrl) or with (+ and TAPI-2) inhibitors and 5-fluorouracil. (A, B): Decreased cell viability was determined by the MTT assay and is presented as a percentage of the control. Horizontal dashed lines represent 50% viable cells. (C, D): Decreased medium lethal doses of 5-FU. (E): Western blotting showed increased protein levels of apoptotic markers. α-Tubulin was used as the loading control. Data are presented as mean ± SEM; *, p < .05, Student's t test. Abbreviations: 5-FU, 5-fluorouracil; ADAM17, A disintegrin and metalloproteinase domain 17; Ctrl, control; PARP, poly(ADP-ribose) polymerase.
ADAM17 Cleaved Notch1 Receptor and Its Ligands Jagged-1 and -2
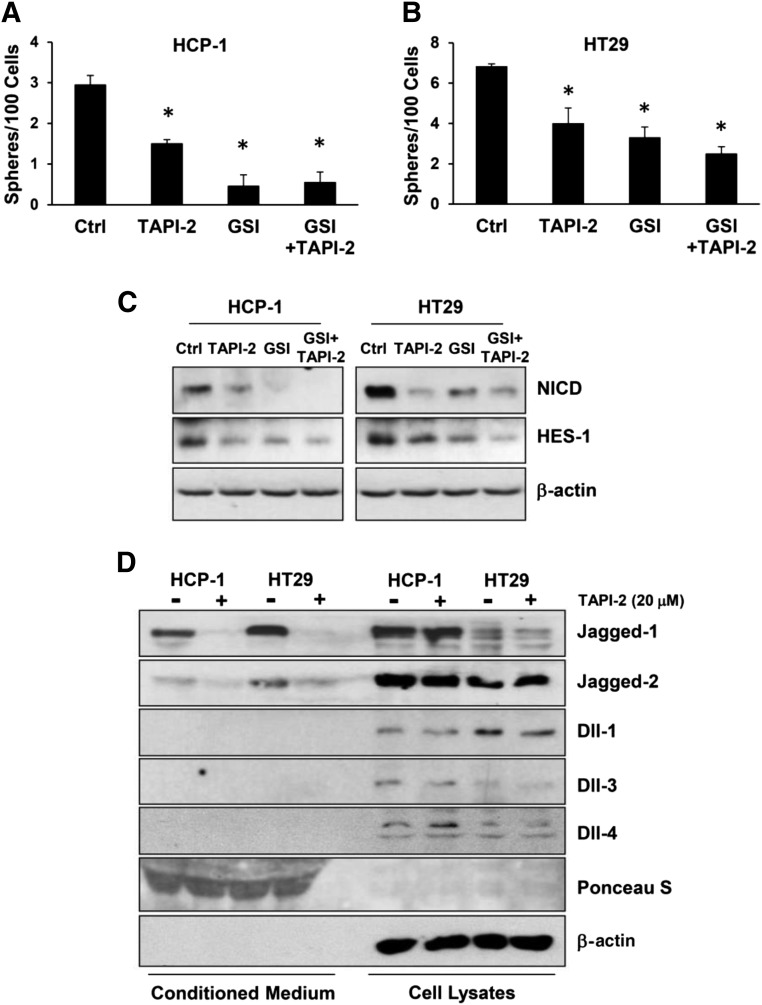
To elucidate the mechanisms by which ADAM17 mediates the CSC phenotype in CRC cells, we first confirmed that cleavage of Notch1 was essential in the process. We used the ADAM17 inhibitor TAPI-2 and the γ-secretase inhibitor separately and demonstrated a decrease in the number of spheres formed by CRC cells (Fig. 4A, 4B). Moreover, when the cells were treated with both inhibitors, TAPI-2 did not cause an additional decrease in the number of spheres compared with GSI alone. This suggests that the effect of blocking ADAM17 on the CSC phenotype was mediated by the downstream cleavage of Notch1 by γ-secretase. The downregulation of NICD and HES-1 by TAPI-2 and GSI were confirmed at protein levels by Western blotting (Fig. 4C). Previously, our laboratory described a new role for ADAM17 in cleaving the Notch ligand Jagged-1 in endothelial cells for Notch1 activation in surrounding CRC cells [8]. Therefore, we investigated the role of ADAM17 in cleaving Notch ligands in CRC cells. Examining equal portions of CM and cell lysates by Western blotting, we showed that abundant soluble Jagged-1 is present in the CM of CRC cells and that TAPI-2 dramatically decreased the level of soluble Jagged-1 released by both CRC cells (Fig. 4D; supplemental online Fig. 2C). In HCP-1 cells, similar amounts of soluble Jagged-1 were released in CM compared with membrane-bound Jagged-1 in cell lysates. In HT29 cells, most Jagged-1 proteins were released in CM, with very low levels detected in the cell lysates. The same effects were found when knocking down ADAM17 expression using siRNAs (supplemental online Fig. 4). Jagged-2 was also released by CRC cells, to a lesser extent compared with Jagged-1, and TAPI-2 also blocked the secretion without affecting the total Jagged-2 protein levels in the cells. In contrast, Dll1, Dll3, and Dll4 were mostly detected in the cell lysates, and TAPI-2 did not change the protein levels of these ligands. Similar results were found in SW620 and SW1222 CRC cells. Treatment of TAPI-2 in these cells caused a decrease in sphere formation, a decrease in soluble Jagged-1 and -2 in the conditioned medium, a decrease in protein levels of NICD and HES-1, and an increase in chemosensitivity (supplemental online Fig. 5).
Figure 4.
ADAM17 cleaved Notch1 receptor and its ligands Jagged-1 and Jagged-2. Colorectal cancer cells were treated without (Ctrl) or with ADAM17 inhibitor (TAPI-2) or γ-secretase inhibitor. (A, B): Decreased sphere formation assay per 100 cells per well. (C): Western blotting revealed decreased protein levels of NICD and HES-1. (D): Western blotting showed protein levels of Notch ligands in conditioned medium and cell lysates. Ponceau S and β-actin were used as loading controls. Data are presented as mean ± SEM; ∗, p < .05, Student's t test. Abbreviations: ADAM17, A disintegrin and metalloproteinase domain 17; Ctrl, control; GSI, γ-secretase inhibitor; NICD, Notch1 intracellular domain.
Discussion
Although the results of many preclinical studies have suggested that targeting CSCs might be beneficial in the clinic, to date, no CSC-targeting therapy has been shown to significantly benefit patients in clinical studies [26]. One of the major challenges in targeting CSCs is the lack of an appropriate in vitro model system for the reliable identification of CSCs. Recently, our laboratory demonstrated the importance of using early passage of primary cancer cells for studying CSCs in CRC cells [27]. Moreover, although many markers have been proposed as CSC markers [28], our laboratory [27] and another group [29] independently demonstrated that many of those markers are not reliable for detecting the CSC phenotype of CRC cells in vitro. We found that, in addition to the sphere formation, a recognized surrogate for the CSC phenotype, the ALDEFLUOR assay is another reliable quantitative assay to assess the CSC phenotype in early passages of newly established cell lines. In the present study, we used the HCP-1 human CRC primary cell line, as well as several established CRC cell lines, to demonstrate that ADAM17 mediates the CSC phenotype in CRC cells via the Notch1 signaling pathway and that inhibition of ADAM17 sensitized CRC cells to 5-FU.
ADAM17 regulation of the CSC phenotype has been studied in various cancers [21, 22, 30]. Our study has shown for the first time that inhibiting ADAM17 in CRC cells, by knocking down gene expression with siRNAs or by an inhibitor, induces a significant decrease in the CSC phenotype, as detected by the sphere formation and ALDEFLUOR assays. We used the ADAM17 inhibitor TAPI-2 at a dose close to the half maximal inhibitory concentration to minimize the off-target effects on other proteinases (e.g., ADAM10 and matrix metalloproteinases). The dose-dependent effects of TAPI-2 on CRC cells, together with the finding that TAPI-2 and ADAM17-specific siRNAs induced similar levels of inhibition in the CSC phenotype, suggest that the TAPI-2 used in the present study primarily inhibited ADAM17. In agreement with previous studies [12], we showed that Notch1 is cleaved and activated by ADAM17. Although ADAM17 regulation of other CSC-associated pathways has been shown in breast cancer [31], we did not observe ADAM17 mediating the other CSC-associated pathways we examined (Nanog, Sonic Hedgehog, and Wnt/β-catenin). Moreover, when the downstream S3 cleavage of Notch1 was blocked by GSI, TAPI-2 did not induce additional inhibition in the CSC phenotype or levels of cleaved NICD. Together, the data suggest that ADAM17 regulation of the CSC phenotype in CRC cells is mediated by the Notch1 pathway.
Studies in different cancers (including CRCs) have assessed the strategy of blocking cancer cell growth by inhibiting ADAM17 alone or combined with inhibiting members of the epidermal growth factor receptor family (EGFRs and HERs) [15, 32–34]. In our study, ADAM17 regulation of the CSC phenotype demonstrated that ADAM17 plays a role in mediating the CRC cell response to chemotherapy. Therefore, we examined the effects of blocking ADAM17 on the CRC cell response to chemotherapy. Our data showed that TAPI-2 sensitized CRC cells to 5-FU and SN38, but not oxaliplatin. This discrepancy can be explained because these chemotherapy agents induce cytotoxic effects by different mechanisms. However, because oxaliplatin is rarely used as a single agent in the clinic to treat patients with CRC, this negative result does not affect the clinical relevance of our findings. In contrast, 5-FU and irinotecan are used as single agents to treat patients with mCRC. The finding of ADAM17 inhibition leading to decreased cell viability and increased apoptosis compared with single-agent therapy was consistent with those from a previous study, which also showed that blocking ADAM17 sensitized CRC cells to chemotherapy [35]. In that study, the investigators suggested that EGFR was involved in ADAM17-mediated chemoresistance. However, owing to the large number of downstream targets regulated by ADAM17 [13, 36, 37], it is unlikely that a single pathway is responsible for ADAM17-mediated chemoresistance. A very recent study using a new ADAM17 antibody demonstrated EGFR-dependent and -independent effects of tumor growth inhibition by blocking ADAM17 in vivo [38]. The focus of our study was to demonstrate the role of CRC-derived ADAM17 in the regulation of the CSC phenotype that might mediate chemoresistance in CRC cells. Our results suggest that inhibiting ADAM17, concomitant with standard chemotherapy, increases drug efficacy in CRC cells.
The present study has shown for the first time that, unlike the canonical juxtacrine activation of Notch1 by membrane-bound ligands [39], the Notch ligands Jagged-1 and -2 can be released in soluble forms in CRC cells by ADAM17. We recently demonstrated a similar mechanism by which endothelial-derived ADAM17 cleaved Jagged-1 from endothelial cells and activated the Notch1 pathway in surrounding CRC cells [8]. Data from our study suggest that in addition to endothelial cells, CRC cells are capable of releasing Jagged-1 and -2 from the cell membrane by ADAM17. The higher levels of soluble Jagged-1 and -2 released by HT29 cells compared with HCP-1 cells support our finding that HT29 cells have a higher capacity of the CSC phenotype than HCP-1 cells. Soluble Jagged-1 and -2 driven Notch1 activation might be the main CSC phenotype-driven pathway in CRC cells. In contrast, we did not detect ADAM17-dependent cleavage of Delta-like ligands, which have been reported to be cleaved by ADAM proteins [40, 41]. In those studies, cleavage of the Delta-like ligands was shown to block the Notch pathway. The absence of ADAM17-mediated Delta-like cleavage fits our model that ADAM17 activates Notch1, possibly by releasing Jagged-1 and -2, and promotes the CSC phenotype in CRC cells. Moreover, a large portion of soluble Jagged-1 in the CM compared with membrane-bound Jagged-1 in the cell lysates suggest an important role for soluble Jagged-1 in regulating the Notch1 pathway in CRC cells. Overexpression of ADAM17 in CRC cells did not cause any increase in protein levels of NICD, HES-1, or soluble Jagged-1, neither did we detect significant increase in the sphere formation (data not shown). This is possibly because ADAM17 is already overexpressed in CRC cells compared with normal mucosa and overexpressing a highly expressed gene in CRC cells did not cause additional effects on downstream targets.
Conclusion
ADAM17 is a potential target for treating patients with CRC and perhaps other cancers [16, 22, 32, 33, 38]. In other studies, ADAM17 was shown to be downstream of K-Ras activation [17], suggesting a potential for using ADAM17-targeted therapy in CRC patients with K-Ras mutations. The present study elucidated the role of ADAM17 in regulating the CSC phenotype and chemoresistance in CRC cells. Our findings suggest that the combination of chemotherapy and ADAM17 inhibition can lead to better treatment outcomes for patients with CRC compared with standard regimens. Moreover, we have demonstrated a new role for ADAM17 in cleaving the Notch ligands Jagged-1 and -2 in CRC cells. However, owing to the large number of downstream targets of ADAM17, the overall effects of blocking ADAM17 in patients with CRC need to be fully elucidated in future studies.
Supplementary Material
Acknowledgments
This project was supported by NIH Grant R01CA157880 (L.M.E) and the William C. Liedtke, Jr., Chair in Cancer Research (L.M.E).
Author Contributions
R.W.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; X.Y., R.B., and F.F.: data analysis and interpretation; D.R.B.: data analysis and interpretation, manuscript writing; L.X.: general technical support; L.M.E.: financial support, conception and design, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Fakih MG. Metastatic colorectal cancer: Current state and future directions. J Clin Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 2.Prenen H, Vecchione L, Van Cutsem E. Role of targeted agents in metastatic colorectal cancer. Target Oncol. 2013;8:83–96. doi: 10.1007/s11523-013-0281-x. [DOI] [PubMed] [Google Scholar]
- 3.Davies JM, Goldberg RM. Treatment of metastatic colorectal cancer. Semin Oncol. 2011;38:552–560. doi: 10.1053/j.seminoncol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Flemming A. Cancer stem cells: Targeting the root of cancer relapse. Nat Rev Drug Discov. 2015;14:165. doi: 10.1038/nrd4560. [DOI] [PubMed] [Google Scholar]
- 5.Botchkina G. Colon cancer stem cells—From basic to clinical application. Cancer Lett. 2013;338:127–140. doi: 10.1016/j.canlet.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Todaro M, Francipane MG, Medema JP, et al. Colon cancer stem cells: Promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 7.Takebe N, Harris PJ, Warren RQ, et al. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Ye X, Fan F, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–185. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das I, Craig C, Funahashi Y, et al. Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J Biol Chem. 2004;279:30771–30780. doi: 10.1074/jbc.M309252200. [DOI] [PubMed] [Google Scholar]
- 10.Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 11.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 12.Mumm JS, Schroeter EH, Saxena MT, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 13.Gooz M. ADAM-17: The enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15:2319–2335. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 15.Merchant NB, Voskresensky I, Rogers CM, et al. TACE/ADAM-17: A component of the epidermal growth factor receptor axis and a promising therapeutic target in colorectal cancer. Clin Cancer Res. 2008;14:1182–1191. doi: 10.1158/1078-0432.CCR-07-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards FM, Tape CJ, Jodrell DI, et al. Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS One. 2012;7:e40597. doi: 10.1371/journal.pone.0040597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Schaeybroeck S, Kyula JN, Fenton A, et al. Oncogenic Kras promotes chemotherapy-induced growth factor shedding via ADAM17. Cancer Res. 2011;71:1071–1080. doi: 10.1158/0008-5472.CAN-10-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgart A, Seidl S, Vlachou P, et al. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70:5368–5378. doi: 10.1158/0008-5472.CAN-09-3763. [DOI] [PubMed] [Google Scholar]
- 19.McGowan PM, Ryan BM, Hill AD, et al. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res. 2007;13:2335–2343. doi: 10.1158/1078-0432.CCR-06-2092. [DOI] [PubMed] [Google Scholar]
- 20.Zhou BB, Peyton M, He B, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamarajan P, Shin JM, Qian X, et al. ADAM17-mediated CD44 cleavage promotes orasphere formation or stemness and tumorigenesis in HNSCC. Cancer Med. 2013;2:793–802. doi: 10.1002/cam4.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Chen L, Zhang R, et al. ADAM17 regulates self-renewal and differentiation of U87 glioblastoma stem cells. Neurosci Lett. 2013;537:44–49. doi: 10.1016/j.neulet.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Kwon IK, Singh N, et al. Type 2 cGMP-dependent protein kinase regulates homeostasis by blocking c-Jun N-terminal kinase in the colon epithelium. Cell Death Differ. 2014;21:427–437. doi: 10.1038/cdd.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: Results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 26.Vidal SJ, Rodriguez-Bravo V, Galsky M, et al. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2014;33:4451–4463. doi: 10.1038/onc.2013.411. [DOI] [PubMed] [Google Scholar]
- 27.Fan F, Bellister S, Lu J, et al. The requirement for freshly isolated human colorectal cancer (CRC) cells in isolating CRC stem cells. Br J Cancer. 2015;112:539–546. doi: 10.1038/bjc.2014.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema JP. Cancer stem cells: The challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 29.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu CC, Tsai LL, Wang ML, et al. miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013;73:3425–3440. doi: 10.1158/0008-5472.CAN-12-3840. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Liao D, Chen C, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2013;31:248–258. doi: 10.1002/stem.1281. [DOI] [PubMed] [Google Scholar]
- 32.Caiazza F, McGowan PM, Mullooly M, et al. Targeting ADAM-17 with an inhibitory monoclonal antibody has antitumour effects in triple-negative breast cancer cells. Br J Cancer. 2015;112:1895–1903. doi: 10.1038/bjc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Benaich N, Tape C, et al. Targeting the sheddase activity of ADAM17 by an anti-ADAM17 antibody D1(A12) inhibits head and neck squamous cell carcinoma cell proliferation and motility via blockage of bradykinin induced HERs transactivation. Int J Biol Sci. 2014;10:702–714. doi: 10.7150/ijbs.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGowan PM, Mullooly M, Caiazza F, et al. ADAM-17: A novel therapeutic target for triple negative breast cancer. Ann Oncol. 2013;24:362–369. doi: 10.1093/annonc/mds279. [DOI] [PubMed] [Google Scholar]
- 35.Kyula JN, Van Schaeybroeck S, Doherty J, et al. Chemotherapy-induced activation of ADAM-17: A novel mechanism of drug resistance in colorectal cancer. Clin Cancer Res. 2010;16:3378–3389. doi: 10.1158/1078-0432.CCR-10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Effenberger T, von der Heyde J, Bartsch K, et al. Senescence-associated release of transmembrane proteins involves proteolytic processing by ADAM17 and microvesicle shedding. FASEB J. 2014;28:4847–4856. doi: 10.1096/fj.14-254565. [DOI] [PubMed] [Google Scholar]
- 37.Sukor S, McGowan PM, Mullooly M, et al. ADAM17 as a novel therapeutic target for HER2-positive breast cancer. J Clin Oncol. 2012;30(suppl):622a. [Google Scholar]
- 38.Rios-Doria J, Sabol D, Chesebrough J, et al. A monoclonal antibody to ADAM17 inhibits tumor growth by inhibiting EGFR and non-EGFR mediated pathways. Mol Cancer Ther. 2015;14:1637–1649. doi: 10.1158/1535-7163.MCT-14-1040. [DOI] [PubMed] [Google Scholar]
- 39.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 40.Sun D, Li H, Zolkiewska A. The role of Delta-like 1 shedding in muscle cell self-renewal and differentiation. J Cell Sci. 2008;121:3815–3823. doi: 10.1242/jcs.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muraguchi T, Takegami Y, Ohtsuka T, et al. RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat Neurosci. 2007;10:838–845. doi: 10.1038/nn1922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.