This study indicated that activated skin-derived mesenchymal stem cells (S-MSCs) produced high amounts of soluble tumor necrosis factor (TNF) receptor 1 (sTNFR1), which neutralized TNF-α and inhibited Th17 cell polarization. The results identified S-MSC-secreted sTNFR1 and its target TNF-α as essential regulators for Th17 cell differentiation and revealed a novel mechanism underlying MSC-mediated immunomodulatory function in autoimmunity.
Keywords: T cells, Autoimmune disease, Differentiation, Tissue-specific stem cells
Abstract
T helper 17 (Th17) cells play an important role in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). Th17 cell differentiation from naïve T cells can be induced in vitro by the cytokines transforming growth factor β1 and interleukin-6. However, it remains unclear whether other regulatory factors control the differentiation of Th17 cells. Mesenchymal stem cells (MSCs) have emerged as a promising candidate for inhibiting Th17 cell differentiation and autoimmune diseases. Despite the fact that several molecules have been linked to the immunomodulatory function of MSCs, many other key MSC-secreted regulators that are involved in inhibiting Th17 cell polarization are ill-defined. In this study, we demonstrated that the intraperitoneal administration of skin-derived MSCs (S-MSCs) substantially ameliorated the development of EAE in mice. We found that the proinflammatory cytokine tumor necrosis factor (TNF)-α, a key mediator in the pathophysiology of MS and EAE, was capable of promoting Th17 cell differentiation. Moreover, under inflammatory conditions, we demonstrated that S-MSCs produced high amounts of soluble TNF receptor 1 (sTNFR1), which binds TNF-α and antagonizes its function. Knockdown of sTNFR1 in S-MSCs decreased their inhibitory effect on Th17 cell differentiation ex vivo and in vivo. Thus, our data identified sTNFR1 and its target TNF-α as critical regulators for Th17 cell differentiation, suggesting a previously unrecognized mechanism for MSC therapy in Th17-mediated autoimmune diseases.
Significance
This study showed that administration of skin-derived mesenchymal stem cells (S-MSCs) was able to alleviate the clinical score of experimental autoimmune encephalomyelitis by inhibiting the differentiation of T helper 17 (Th17) cells. Tumor necrosis factor (TNF)-α is a critical cytokine for promoting Th17 cell differentiation. It was discovered that activated S-MSCs produced high amount of soluble TNF receptor 1 (sTNFR1), which neutralized TNF-α and inhibited Th17 cell polarization. The data identified S-MSC-secreted sTNFR1 and its target TNF-α as essential regulators for Th17 cell differentiation and revealed a novel mechanism underlying MSC-mediated immunomodulatory function in autoimmunity.
Introduction
Mesenchymal stem cells (MSCs), also known as multipotent mesenchymal stromal cells, are a heterogeneous population of cells with fibroblast-like morphology originally observed in bone marrow that proliferate in vitro as plastic-adherent cells [1]. In addition to bone marrow, cells with the phenotypic and functional characteristics of MSCs have been identified in various connective tissues [2]. However, MSCs isolated from these tissues are possibly limited by availability, extractive invasiveness, or limited proliferative capacity. Of particular significance, skin, as the largest organ, provides an easily accessible and ideal source of tissue for the isolation of stem cells, including MSCs [3].
In addition to their multilineage differentiation potential, MSCs exert a broad spectrum of immunoregulatory effects on cells of both innate and adaptive immunity [4]. When interacting with T cells, MSCs suppress T-cell proliferation and induce T-cell anergy in a non-major histocompatibility complex-restricted and partially contact-dependent manner [5, 6]. The inhibition of T cell proliferation by MSCs is thought to be mediated through lymphocyte arrest in the G0/G1 phase of the cell cycle [7]. Furthermore, accumulated evidence has demonstrated that the cytokine profile of T-cell subsets is influenced by MSCs, because their addition to an in vitro activated T-cell culture results in a decreased release of proinflammatory cytokines, such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-6 and IL-17, and increased levels of anti-inflammatory cytokines, such as IL-4 and IL-10 [8, 9]. Additionally, MSCs have been reported to enhance the proportion of regulatory T (Treg) cells in in vitro coculture [8] and to favor the induction of Th2 (T helper 2) cells [9]. Furthermore, MSCs are capable of regulating the function of B cells, dendritic cells, natural killer cells, and macrophages either directly through cell-cell contact or indirectly via the production of soluble mediators [8, 10–12].
Recurrent data suggest that various soluble factors secreted from MSCs play a critical role in the suppressive function of MSCs. For example, neutralization of either IL-10 or indoleamine 2,3-dioxygenase, an enzyme catabolizing the essential amino acid tryptophan to kynurenine, in a coculture of MSCs and activated peripheral blood mononuclear cells resulted in abrogation of the proliferation inhibitory effect exerted by the MSCs [13]. Other soluble factors that have been recently reported in the context of MSC-mediated immunoregulation include hepatocyte growth factor, transforming growth factor β1 (TGF-β1), prostaglandin E2 (PGE2), inducible nitric-oxide synthase, soluble HLA-G5, and galectin-3 [5, 8, 12, 14, 15].
T helper 17 (Th17) cells are a novel class of helper CD4+ T cells described in 2005 that secrete IL-17A, IL-17F, IL-21, and IL-22 [16–18]. Th17 cells protect the host against extracellular pathogens encountered at mucosal surfaces and play a detrimental role in experimental murine models of autoimmune disease, such as rheumatoid arthritis, psoriasis, and multiple sclerosis [16, 19–21]. Experimental autoimmune encephalomyelitis (EAE) is an autoimmune disease with pathological and clinical similarities to the major human demyelinating disease multiple sclerosis (MS). Langrish et al. [17] have revealed that Th17 cells are critical for the development of EAE, Consistently, therapeutic neutralization of IL-17 could ameliorate clinical symptoms of EAE [22]. IL-17-deficient mice exhibited delayed onset, reduced disease severity, and early recovery in EAE [20]. Thus, Th17 cells are a major contributor of EAE, and inhibiting the Th17 cell differentiation may be a potential therapeutic strategy for treatment of EAE.
TNF-α is a crucial proinflammatory cytokine in the pathogenesis of both EAE and MS. TNF-α has been detected in inflammatory foci in both diseases [23–25]. In addition, increased production of TNF-α by blood cells of MS patients has been shown to occur and correlate with disease activity [26, 27]. Recently, TNF-α was shown to drive the production of IL-17 with the ability to differentiate T cells toward a Th17 phenotype [28, 29]. TNF-α action may be limited during physiological processes by naturally occurring TNF-α inhibitors comprised of extracellular domains of the TNF-α cellular receptors whose ectodomains are released from cell membranes by proteolysis [30–32]. Yagi et al. [33] showed that soluble TNF receptor 1 (sTNFR1) derived from reactive MSCs could attenuate systemic inflammation. However, there is no evidence implicating the immunoregulatory role of S-MSC-derived sTNFR1 on Th17 cell differentiation.
Here, we showed that administration of S-MSCs was able to alleviate the clinical score of EAE by inhibiting the differentiation of Th17 cells. TNF-α is a critical cytokine for promoting Th17 cell differentiation. We further discovered that activated S-MSCs produced high amount of sTNFR1, which neutralized TNF-α and inhibited Th17 cell polarization. Our data identified S-MSC-secreted sTNFR1 and its target TNF-α as essential regulators for Th17 cell differentiation and revealed a novel mechanism underlying MSC-mediated immunomodulatory function in autoimmunity.
Materials and Methods
Mice
C57BL/6J mice (stock number 000664) and B6.Cg-FoxP3tm2tch/J mice (stock number 006772, designated as FoxP3EGFP) were purchased from the Jackson Laboratory (Bar Harbor, ME, http://www.jax.org). B6.129S6-Rag2tm1Fwa N12 (RAG2−/−) mice were purchased from Taconic Laboratories (Hudson, NY, http://www.taconic.com). The mice were kept under specific pathogen-free conditions in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with the approval (SYXK-2003-0026) of the Scientific Investigation Board of Shanghai Jiao Tong University School of Medicine (Shanghai, China). To ameliorate any suffering of mice observed throughout these experimental studies, the mice were euthanized by CO2 inhalation.
Cells
S-MSCs were generated from skin of neonatal mice as previously reported [3]. Briefly, the dermis from neonatal mice (1–3 days old) were digested with collagenase I (Gibco, Grand Island, NY, http://www.invitrogen.com), and then cells were washed with Hanks’ balanced saline solution three times. Subsequently cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Six hours later, nonadherent cells were removed. After 2 days, the cells were subcultured and reseeded. The cells were used for the 4th to 10th passages. The supernatant was harvested at 48 hours from S-MSCs cultures at a cell density of 5 × 105 cells/ml. Splenocytes were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol.
Flow Cytometry
To detect intracellular expression of IL-17A in CD4+ T cells, splenocytes or Percoll-purified cell infiltrates of central nervous system (CNS) were first treated with 1 μg/ml inomycine (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 50 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich) and GolgiPlug (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) for 4–6 hours at 37°C. The cells then were stained with cell surface antigen antibodies. After fixation and permeabilization (BD Biosciences), the cells were stained with anti-IL-17A, anti-IFN-γ, and anti-TNF-α mAb. Stained cells were assayed with a FACSCanto II (BD Biosciences), and the data were analyzed with FlowJo software. For flow cytometry, monoclonal antibodies against CD4 (clone GK1.5), CD25 (clone PC61.5), CD62L (clone MEL-14), IL-17A (clone ebio17b7), and IFN-γ (clone XMG1.2) were purchased from eBioscience Inc. (San Diego, CA, http://www.ebioscience.com). Anti-TNF-α (clone MP6-XT22) antibody was purchased from BioLegend (San Diego, CA, http://www.biolegend.com).
T-Cell Subset Differentiation In Vitro
T cells were obtained from spleen and lymph nodes of 6-week-old mice. Naïve CD4+ T cells (CD4+CD25−CD62Lhigh) were sorted by FACSAria III (BD Biosciences) after enrichment of CD4+ T cells by the mouse CD4+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). Cell purity was >94% as determined by flow cytometry. T cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, and 50 μM β-mercaptoethanol. The naïve T cells were activated with 5 μg/ml plate-bound anti-CD3 (1452C11; BD Biosciences) plus 2 μg/ml soluble anti-CD28 antibodies (37.51; BD Biosciences). The Th17 cell differentiation conditions included 10 ng/ml recombinant murine (rm)IL-6 (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), 2 ng/ml rmTGF-β1 (R&D Systems), 10 μg/ml anti-IL-4 (11B11; Biolegend), and 10 μg/ml anti-IFN-γ (XMG1.2; eBioscience). The Th1 cell differentiation conditions included 10 ng/ml rmIL-12 (R&D Systems), 10 ng/ml rmIL-2 (R&D Systems), and 10 μg/ml anti-IL-4 antibody (11B11; Biolegend). Splenocytes from RAG2−/− mice were regarded as antigen-presenting cells (APCs). APCs were irradiated at 3,000 rad before TNF-α treatment or the coculture using Th17 polarized cells.
Gene Expression Analysis
Total RNA was extracted from frozen tissue or cultured cells with TRIzol reagent (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Reverse transcription was performed using the PrimeScript RT reagent kit (Takara, Otsu, Japan, http://www.takara.co.jp). Quantitative polymerase chain reaction (PCR) was carried out using the SYBR Green PCR Master Mix (TaKaRa) in an ABI Prism 7500 thermal cycler (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). All gene expression results were normalized to the expression of housekeeping gene β-actin and determined by the equation 2−ΔCT. The primer sequences are listed in supplemental online Table 1.
ELISA
Quantitative analysis of IL-17A, INF-γ, TNF-α, and sTNFR1 protein levels was performed by enzyme-linked immunosorbent assay (ELISA), using commercially available kits (RayBiotech, Norcross, GA, http://www.raybiotech.com). Serum was obtained from EAE mice transplanted with S-MSCs or phosphate-buffered saline (PBS). In other experiments, supernatants from splenocytes were analyzed after 48 hours of culturing. The supernatants were analyzed according to the manufacturer’s instructions. Standard, control, and test samples were added to each well and incubated for an appropriate time with adequate primary antibody and conjugated. After washing, substrate solution was added to each well for 30 minutes at room temperature in the dark. Finally, the stop solution was added, and the optical density of each well was determined within 30 minutes using a microplate reader (wavelength 450 nm).
Induction, Clinical Evaluation, and Treatment of EAE
EAE was induced in 6- to 8-week-old mice by subcutaneous immunization with 300 μg of myelin oligodendrocyte glycoprotein peptide35–55 (MOG35–55) in CFA (Sigma-Aldrich) comprising 500 μg of killed Mycobacterium tuberculosis (Sigma-Aldrich). Additionally, 200 ng of pertussis toxin (Sigma-Aldrich), dissolved in 200 μl of PBS, was administrated intravenously at the day of immunization and again 2 days thereafter. The clinical EAE score was evaluated daily on a 0–5 scale (0, healthy; 1, limp tail; 2, ataxia; 3, paralysis of hind limbs and/or paresis of fore limbs; 4, tetraparalysis; 5, moribund or dead). The mice were observed for 30 days. For a preventive protocol, 1 × 106 S-MSCs were administrated i.p. into C57BL/6J mice 3 days before immunization. For a therapeutic protocol, 1 × 106 S-MSCs were injected i.p. into EAE mice on day 8 postimmunization.
Histology and Immunofluorescence
The spinal cord was fixed in 4% paraformaldehyde and paraffin-embedded. We stained 5-μm sections with Luxol fast blue or H&E. For immunofluorescence staining, we stained spinal cord frozen sections with rat antibody to mouse CD4 (H129.19; BD Biosciences), F4/80 (BM8; eBioscience), and Alexa 555-conjugated donkey anti-rat IgG1 (705-166-147; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com), Alexa 555-conjugated goat anti-rat IgG1, or IgG2as secondary antibodies. 4′,6-Diamidino-2-phenylindole (Sigma-Aldrich) was used for nuclear staining.
Transwell Experiments
Transwell experiments were performed in 24-well plates. Splenocytes (5 × 105) derived from EAE mice 18 days postimmunization were placed in upper chamber (Corning Enterprises, Corning, NY, http://www.corning.com) in the presence of MOG35–55 (20 μg/ml). S-MSCs (5 × 104) were plated in the lower chamber. The splenocytes were harvested after 24 hours.
Transfection of S-MSCs With siRNA
RNA interference technology was used to generate specific knockdown of TNFR1-mRNA transcription in S-MSCs. Sense and antisense oligonucleotides were synthesized by GenePharma (Shanghai, China, http://www.genepharma.com). Nonspecific small interfering RNA (siRNA), which has no target in the mouse transcriptome, was used as a negative control and was also purchased from GenePharma. The sequences were as follows: TNFR1-si637 sense, 5′-GGAGAUCUCUCCUUGCCAATT-3′, and antisense, 5′-UUGGCAAGGAGAGAUCUCCTT-3′; TNFR1-si894 sense, 5′-CCGCUUGCAAAUGUCACAATT-3′, and antisense, 5′-UUGUGACAUUUGCAAGCGGTT-3′; TNFR1-si1024 sense, 5′-CCGAAGUCUACUCCAUCAUTT-3′, and antisense, 5′-AUGAUGGAGUAGACUUCGGTT-3′; and control siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′, and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′. S-MSCs were plated into 24-well plates 1 day before transfection and allowed to reach approximately 50% confluence after 24 hours of incubation. siRNA at a final concentration of 15 nM was combined with 1.5 μl of Superfectin II in vitro siRNA transfection reagent (Pufei Biotech, Shanghai, China, http://www.pufei.com) in a total volume of 50 μl of transfection buffer and allowed to incubate for 15 minutes at room temperature. The transfection mixture was then applied to S-MSCs and incubated for 24 hours at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, S-MSCs were used in experiments.
Lentivirus Production and Stable Transfection of S-MSCs
A third-generation lentiviral vector pLVXshRNA2 was used to express small hairpin RNAs (shRNAs) from the U6 promoter. In addition to expressing shRNAs, pLVX-shRNA2 also expresses the fluorescent protein ZsGreen1, a human codon-optimized variant of the reef coral Zoanthus sp green fluorescent protein (ZsGreen). For knockdown of TNFR1 expression, four target sequences were designed. The target sequences used were as follows: sh1, 5′-GGTTATCTTGCTAGGTCTTTG-3′; sh2, 5′-CCCGAAGTCTACTCCATCATT-3′; sh3, 5′-CCTCGTGCTTTCCAAGATGAA-3′; sh4, 5′-CCTTAGGAAGAGGGACTTGAA-3′; and scrambled control, 5′-TGTTCGCATTATCCGAACCAT-3′. Briefly, the complementary DNA oligonucleotides that consisted of a sequence-specific 21 nucleotide followed by the loop sequence (CTCGAG) and finally the reverse complement of the targeting sequence were synthesized, annealed, and inserted in pLVX-shRNA2 vector (Clontech, Mountain View, CA, http://www.clontech.com) between BamHI and EcoRI sites downstream of the U6 promoter.
For lentivirus generation, 4 × 106 293T cells were plated in a 100-mm dish 1 day before transfection. The shRNA-expressing lentiviral plasmid was cotransfected with plasmids pMD2.G and pSPAX2 into 293T cells by calcium phosphate precipitation. Two days post-transfection, the purity of ZsGFP+ cells was above 99% as determined by FACS analysis. Viral containing media were collected, filtered, and concentrated by ultracentrifugation. Viral titers were measured by serial dilution on 293T cells followed by flow cytometric analysis after 48 hours. For viral transduction, lentiviral vectors at a multiplicity of infection of 20 were added to cultured S-MSCs. The day after transduction, viral supernatant was replaced with fresh medium. Clonal lines were screened for TNFR1 by quantitative PCR. One stable transfected S-MSC line showing 95% decreased TNFR1 at RNA levels was passaged and used for experiments.
Statistical Analysis
The data are presented as the means ± SEM and analyzed with GraphPad Prism 5. For pairwise comparisons of parametric and nonparametric data, the Student’s t test, and Mann-Whitney-Wilcoxon rank-sum test were used, respectively. Probability values of < .05 were considered significant, and tests were performed two-sided.
Results
S-MSCs Ameliorated Autoimmune Disease
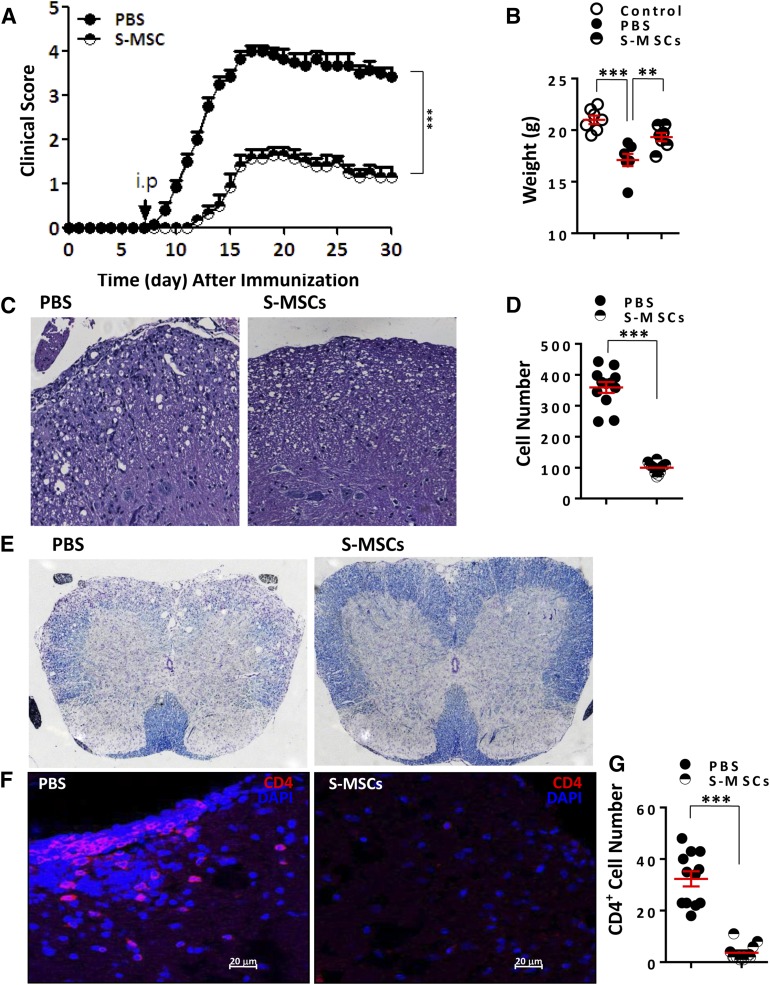
We investigated the potential therapeutic effects of S-MSCs in autoimmunity using EAE, a mouse model for MS. S-MSCs were injected i.p. into mice at day 8 postimmunization. Notably, the administration of S-MSCs substantially delayed the onset of disease and markedly reduced EAE severity accompanied by a significant prevention of body weight loss in EAE mice compared with PBS-treated mice with established EAE (Fig. 1A, 1B). In contrast to PBS, the injection of S-MSCs resulted in decreased inflammatory cell infiltrates and demyelination in the affected spinal cord of EAE mice at day 18 after immunization (Fig. 1C–1E). Consistently, S-MSC treatment reduced the numbers of CD4+ T cells (Fig. 1F, 1G). Thus, our results unequivocally demonstrate that S-MSCs derived from skin are capable of ameliorating the development of autoimmune disease.
Figure 1.
Amelioration of experimental autoimmune encephalomyelitis (EAE) by S-MSCs. (A): Clinical scores (means ± SEM) of mice with established EAE treated with PBS or S-MSCs at indicated time points (n = 10 mice per group). (B): Body weight of mice treated with PBS and S-MSCs or untreated control mice at day 30 after immunization (n = 7). (C): Spinal cord sections derived from PBS- or S-MSC-treated mice at day 18 after immunization were examined by H&E staining for infiltrating lymphocytes. (D): Infiltrating lymphocytes in the spinal cord of EAE mice treated with PBS or S-MSCs were counted. (E): Luxol fast blue staining of spinal cord sections from PBS- or S-MSC-treated mice at day 18 after immunization for visualizing demyelination. (F): Infiltrating CD4+ T cells of spinal cords from PBS- or S-MSC-treated EAE mice were analyzed by immunofluorescence staining. (G): The positively stained CD4+ T cells. For all measurements, the median of specifically stained cells counted in 12 high power fields is presented, and three separate cryosections of each mouse spinal cord were examined (four mice for each group). ∗∗, p < .01; ∗∗∗, p < .001. Representative data are shown that have been reproduced in two or three independent experiments. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PBS, phosphate-buffered saline; S-MSC, skin-derived mesenchymal stem cell.
S-MSCs Inhibited the Th17 Cell Differentiation In Vivo
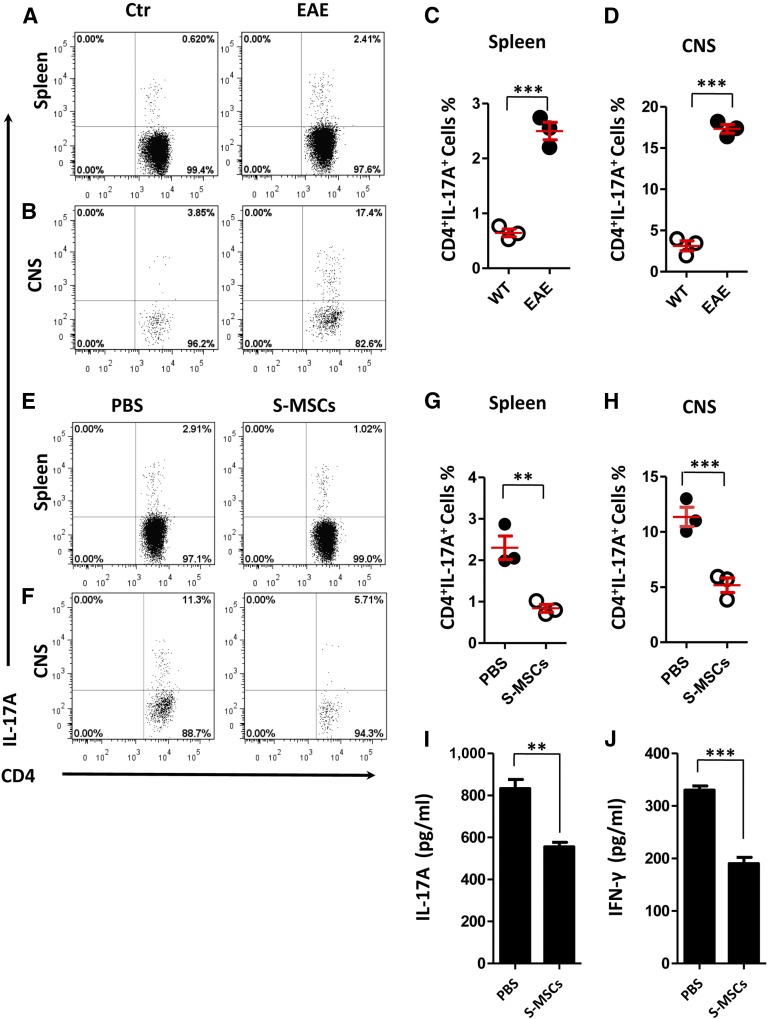
It has been reported that Th17 cells are critical for the development of EAE [17]. To elucidate the mechanism of therapeutic properties of S-MSCs in EAE, we investigated the inhibitory effect of S-MSCs on Th17 cell induction in vivo. We first induced EAE and assessed the frequency of Th17 cells in inflamed spleen and CNS derived from untreated controls and EAE mice at day 18 postimmunization. We found that the number of Th17 cells was significantly higher in the spleens and CNSs of EAE mice compared with healthy controls (Fig. 2A–2D). Therefore, these data support the notion that Th17 cells are important for the development of EAE. We next induced EAE and administrated i.p. S-MSCs into mice at day 8 postimmunization. Splenocytes and CNS infiltrates underwent flow cytometric analysis of Th17 cells 10 days after S-MSC treatment. We showed that intraperitoneal injection of S-MSCs resulted in marked inhibition of Th17 cell induction in both spleen and CNS (Fig. 2E–2H). Consistently, serum levels of IL-17A were significantly decreased following S-MSC treatment in EAE mice (Fig. 2I). In agreement with published data [9], we also observed that S-MSC treatment was capable of reducing the levels of IFN-γ in serum (Fig. 2J). Moreover, the expression levels of Th2- and Treg-associated genes in splenocytes were remarkably increased (supplemental online Fig. 1A–1C). Consistently, numbers of FoxP3EGFP+ Treg cells were significantly increased after S-MSC treatment (supplemental online Fig. 1D–1G). Together, our data suggest that S-MSCs are capable of inhibiting Th1 and Th17 cell differentiation and inducing Th2 and Treg cell subsets in vivo. Given that the Th17 cell polarization is critical for the pathogenesis of EAE, our results indicate that S-MSCs might ameliorate EAE, at least partially by inhibiting Th17 cell differentiation.
Figure 2.
S-MSCs inhibited the Th17 cell differentiation in vivo. (A–D): Flow cytometric analyses of IL-17A+ CD4+ T cells derived from the spleen (A, C) and CNS (B, D) of healthy controls or EAE mice 18 days postimmunization (n = 3 mice per group). (E–H): Intracellular staining of IL-17A in CD4+T cells for splenocytes (E, G) and CNS (F, H) derived from EAE mice treated with PBS or S-MSCs (n = 3 mice per group, day 18 after immunization). (I, G): Protein levels of IL-17A and INF-γ in serum derived from EAE mice treated with PBS or S-MSCs were analyzed by ELISA (n = 7). ∗∗, p < .01; ∗∗∗, p < .001. The data are representative of three independent experiments. Abbreviations: CNS, central nervous system; Ctr, control; EAE, experimental autoimmune encephalomyelitis; IL, interleukin; PBS, phosphate-buffered saline; S-MSC, skin-derived mesenchymal stem cell; WT, wild type.
Secreted Factors From S-MSCs Inhibited Th17 Cell Differentiation Ex Vivo
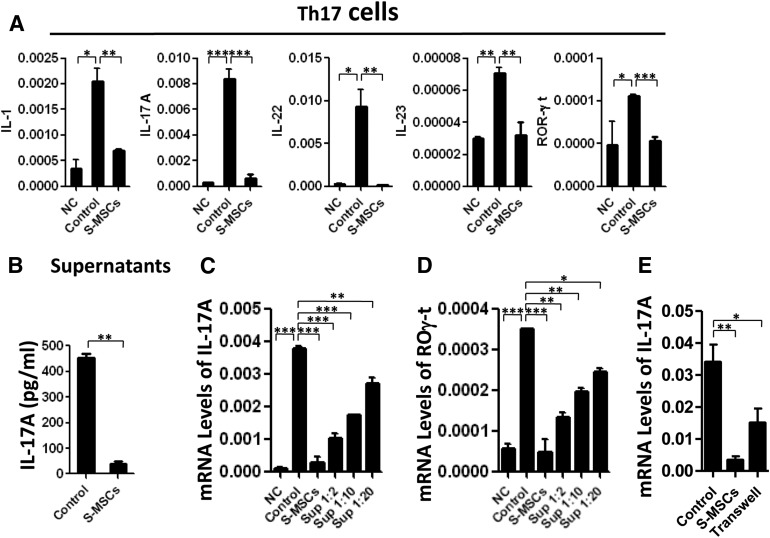
Because S-MSCs clearly impact Th17 cell differentiation in EAE in vivo, we sought to analyze cytokines, as well as T cell subset-specific transcription factors expressed by Th17 cells in the coculture of MOG35–55-stimulated myelin-sensitized lymphocytes with or without S-MSCs. We observed that the expression of Th17 genes (IL-1, IL-17A, IL-22, and IL-23), particularly their transcription factor retinoic acid receptor-related orphan receptor (ROR)-γt, were significantly suppressed by S-MSCs compared with the untreated controls (Fig. 3A). Correspondingly, a pronounced decrease in IL-17A released to the supernatant was noted in the presence of S-MSCs (Fig. 3B). We next compared the inhibitory effect of S-MSCs supernatant with S-MSCs on the induction of Th17 cells in splenocytes stimulated with MOG35–55 ex vivo. The addition of S-MSC supernatant significantly suppressed the induction of Th17 cells in a dose-dependent manner, whereas the direct suppressive effect of S-MSCs was more remarkable than the S-MSC supernatant (Fig. 3C, 3D). A Transwell experiment further confirmed that both cell-cell contact and soluble factors released by S-MSCs collaborated in their immunomodulatory properties (Fig. 3E). These findings strongly support a mechanism underlying S-MSC therapy that acts partially through secreted factor(s) to selectively inhibit excessive Th17 cell differentiation, leading to delayed onset and reduced severity of clinical EAE.
Figure 3.
Secreted factors from S-MSCs inhibited Th17 cell differentiation ex vivo. Splenocytes (1 × 106) isolated from experimental autoimmune encephalomyelitis mice 18 days postimmunization were restimulated with MOG35–55 (20 μg/ml) for 2 days in the presence or absence of S-MSCs (2 × 105). (A): mRNA levels of Th17 cell factors (IL-1, IL-17A, IL-22, IL-23, and ROR-γt) were analyzed by quantitative polymerase chain reaction (qPCR). (B): Protein levels of IL-17A in supernatant were detected by enzyme-linked immunosorbent assay. (C, D): mRNA levels of Th17 cell factors (IL-17A and ROR-γt) were analyzed by qPCR. (E): mRNA levels of IL-17A were analyzed by qPCR when S-MSCs cocultured with splenocytes contact directly or separation by Transwell. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. The data are representative of at least three independent experiments. Abbreviations: Control, restimulated splenocytes; IL, interleukin; NC, nonstimulated splenocytes; ROR-γt, retinoic acid receptor-related orphan receptor γt; S-MSC, restimulated splenocytes cocultured with skin-derived mesenchymal stem cells; Sup, restimulated splenocytes cultured in the presence of supernatant of S-MSCs at the indicated dilutions.
High Levels of sTNFR1 Were Secreted by S-MSCs
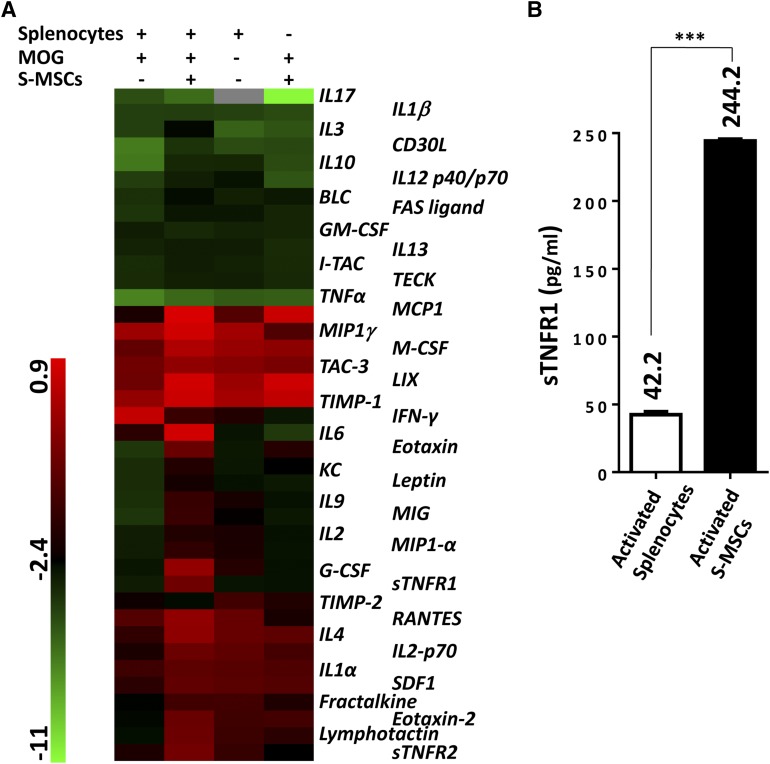
We then focused on identifying the secreted factor(s) responsible for the observed effects of S-MSCs. We used protein array technology and investigated 40 pro- and anti-inflammatory factors in the coculture of S-MSCs and myelin-sensitized splenocytes stimulated with MOG35–55. Of significance, we detected that sTNFR1 and sTNFR2 were remarkably increased in cocultures (Fig. 4A). sTNFR1 was 3.8-fold higher, and sTNFR2 was 2.0-fold higher in the cocultures of S-MSCs than in splenocytes only stimulated with MOG35–55 (data not show). Our observation is inconsistent with a previous report in which sTNFR1 was found to be produced by human MSCs under inflammatory stimulation [33]. Thus, we focused on the role of sTNFR1 released by activated S-MSCs in the Th17 cell polarization and autoimmune disease. We measured the expression levels of sTNFR1 in the supernatant by ELISA. Our data revealed that increased sTNFR1 was abundantly secreted from activated S-MSCs but not splenocytes (Fig. 4B). Thus, these data suggest that S-MSCs are capable of producing high amounts of sTNFR1 upon their activation.
Figure 4.
High levels of sTNFR1 were secreted by S-MSCs. (A): Mouse cytokine antibody array screening of supernatant derived from cultured cells indicated on the top of the figure (six mice were used for each group). (B): Splenocytes restimulated with MOG35–55 in the presence of S-MSCs for 24 hours and then splenocytes and S-MSCs were separated and subsequently cultured for another 24 hours. The supernatants were harvested, and the expression of sTNFR1 was examined by enzyme-linked immunosorbent assay (n = 4). The amounts of secreted sTNFR1 were listed. ∗∗∗, p < .001. The data in (B) are representative of at least two independent experiments. Abbreviations: MOG, myelin oligodendrocyte glycoprotein; S-MSC, skin-derived mesenchymal stem cell; sTNFR1, soluble tumor necrosis factor receptor 1.
TNF-α Promoted Th17 Cell Differentiation
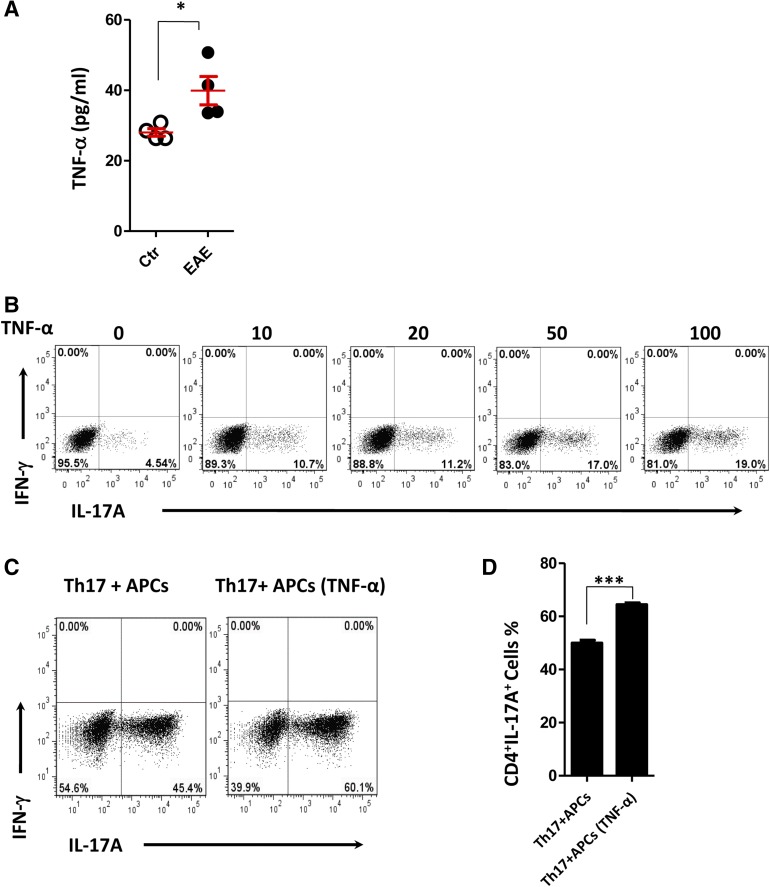
sTNFR1 with highly specific neutralizing activity of TNF-α in vitro and in vivo has been reported [30, 34, 35]. Thus, we investigated the role of TNF-α in Th17 cell differentiation. We first measured the expression levels of TNF-α in serum in EAE mice by ELISA. In contrast with nonimmunized controls, TNF-α expression was significantly increased in EAE mice (Fig. 5A).To date, the functional analysis of TNF-α in CD4+ T cell lineage differentiation remains unclear. We differentiated naïve T cells in vitro under polarizing conditions for the generation of Th17 cells in the absence or presence of TNF-α with various concentrations. In this setting, TNF-α improved the differentiation of Th17 cells in a dose-dependent manner (Fig. 5B) but did not significantly affect the differentiation of Th1 cells (supplemental online Fig. 2A). On the other hand, cytokines primarily produced by APCs are involved in naïve CD4+ T cell differentiation into Th17 cells. It is possible that TNF-α acts on APCs, which may release inflammatory factors that promote Th17 cell differentiation. To test this hypothesis, we stimulated APCs derived from Rag2−/− mice with various concentrations of TNF-α. We found that TNF-α strongly induces the production of IL-1β and IL-6 from APCs, but not IL-23 (supplemental online Fig. 2B–2D). We next determined whether APCs after treatment with TNF-α could enhance Th17 cell differentiation. As expected, the percentage of Th17 cells was significantly increased when APCs were treated with TNF-α before the coculture (Fig. 5C, 5D). Taken together, our data suggested that TNF-α promotes Th17 cell polarization both directly and indirectly.
Figure 5.
TNF-α promoted Th17 cell differentiation. (A): TNF-α expression in serum derived from healthy controls and EAE mice 18 days postimmunization was detected by enzyme-linked immunosorbent assay (n = 4). (B): Representative flow cytometry of IL-17A expression in Th17 cell differentiation in the presence of TNF-α with various concentrations for 3 days. (C, D): Flow cytometry of IL-17A expression in Th17 cell differentiation, and APCs treated with or without TNF-α (10 ng/ml) before coculture with Th17 polarized cells. Numbers adjacent to outlined areas indicate the percentage of cells in each. ∗, p < .05; ∗∗∗, p < .001. The data are representative of three independent experiments (means ± SEM). Abbreviations: APC, antigen presenting cell; Ctr, control; EAE, experimental autoimmune encephalomyelitis; IFN, interferon; IL, interleukin; TNF-α, tumor necrosis factor α.
S-MSCs Inhibited Th17 Cell Polarization Through Releasing sTNFR1
In order to directly identify the role of sTNFR1 in Th17 cell polarization, we differentiated naïve CD4+T cells in vitro under polarizing conditions for the generation of Th17 cells in presence of sTNFR1. We found that the percentage of Th17 cells was significantly decreased when sTNFR1 existed and rescued when exogenous TNF-α were added (Fig. 6A, 6B). Therefore, we propose that autocrine TNF-α derived from T cells may facilitate Th17 cell differentiation in vitro. To confirm this, we measured the time-dependent appearance of IL-17 and TNF-α during the process of Th17 cell differentiation. Our data showed that IL-17 abundance was gradually increased during Th17 cell differentiation, and correspondingly TNF-α levels were significantly induced (supplemental online Fig. 3A, 3B). Collectively these results indicate that autocrine TNF-α enhanced Th17 cell induction in vitro. To directly investigate the role of sTNFR1 released by S-MSCs on Th17 cell differentiation, we silenced the expression of sTNFR1 with specific siRNAs in S-MSCs. We found that siRNA-mediated knockdown resulted in greater than 74% inhibition of sTNFR1 expression at protein levels (supplemental online Fig. 3C). We next cultured inflamed splenocytes from EAE mice together with S-MSCs and found that silencing sTNFR1 in S-MSCs led to an abrogated inhibition of IL-17 expression ex vivo (Fig. 6C). Consistently, we demonstrated significant low levels of sTNFR1 in the cocultures when sTNFR1 was silenced in S-MSCs (Fig. 6D). To further investigate the role of sTNFR1 in the suppressive function of S-MSCs on the Th17 cell differentiation in vivo system, we generated S-MSCs stably expressing TNFR1-targeted shRNA. shRNA-mediated knockdown resulted in approximately 95% inhibition of TNFR1 expression at RNA levels (supplemental online Fig. 3D). We then injected i.p. S-MSCs (shTNFR1) into EAE mice and found that the administration of S-MSCs (shTNFR1) partially abrogates their inhibition (Fig. 6E), suggesting that some other factors contribute to the inhibitory effects mediated by S-MSCs. This phenomenon was further supported by the fact that S-MSCs at a ratio of 1:4 led to 10-fold reduction of Th17 cell differentiation (Fig. 6F, 6G). By contrast, the addition of sTNFR1 only resulted in twofold reduction of Th17 cell differentiation (Fig. 6A, 6B). Taken together, our data indicate that S-MSCs inhibit Th17 cell differentiation, partially via releasing sTNFR1.
Figure 6.
S-MSCs inhibited Th17 cell polarization through releasing sTNFR1. (A, B): Flow cytometry of IL-17A expression in Th17 cell differentiation in the absence or presence of sTNFR1 (100 ng/ml) or TNF-α (10 ng/ml) for 3 days. (C, D): The levels of IL-17A and sTNFR1 were evaluated by enzyme-linked immunosorbent assay. The supernatant derived from splenocytes restimulated with MOG35–55 in the presence of S-MSCs transfected with siNC or si894 (n = 4). (E): Experimental autoimmune encephalomyelitis clinical scores (means ± SEM) of mice treated with S-MSCs (shTNFR1) or S-MSCs (shNC) or PBS (n = 6). (F, G): Flow cytometry of IL-17A expression in Th17 cell polarization in the absence or presence of S-MSCs for 3 days. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. The data in (E) are representative of two independent experiments. Abbreviations: IL, interleukin; ns, not significant; PBS, phosphate-buffered saline; sh, small hairpin; si, small interfering; S-MSC, skin-derived mesenchymal stem cell; sTNFR1, soluble tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor α.
Discussion
One of the important characteristics of MSCs is their immunoregulatory function, which makes these cells promising for treatment of autoimmune diseases, but their mechanisms of action remain incompletely understood. In the present study, we have addressed such issues with a focus on the capacity of S-MSCs derived from skin to inhibit the differentiation of Th17 cells, a population of CD4+ T cells that plays an important role in the pathogenesis of autoimmune diseases [36, 37].
Several studies have shown that MSCs can regulate the polarization of Th17 cells. Rafei et al. [38] demonstrated that MSCs could inhibit Th17 cells via MSCs-derived CCL2 secretion. Duffy et al. [39] showed that Th17 cell differentiation was suppressed by PGE2 derived from MSCs. MSCs-derived IL-10 was also demonstrated to inhibit Th17 cell differentiation [40]. On the contrary, Guo et al. [41] showed that MSCs promoted the expansion of Th17 cells via IL-6. Thus, there is a contradiction regarding to the immunoregulatory role of MSCs on Th17 cells, and the underlying mechanisms need further investigation. In our study, we demonstrate that S-MSCs are capable of suppressing Th17 cell polarization through a mechanism that is mediated by sTNFR1 neutralizing the activity of TNF-α.
MS is a devastating neurological disorder frequently affecting young adults. The pathological changes within the CNSs of MS patients combine demyelination and inflammatory infiltrates. Although the precise mechanism leading to myelin damage is not well-known, these pathological changes are believed to be involving cytotoxic cytokine, TNF-α [42]. Multiple lines of evidence implicate the proinflammatory cytokine TNF-α in the pathogenesis of both EAE and MS. TNF-α has been detected in inflammatory foci in both diseases [23–25]. In addition, increased production of TNF-α by blood cells of MS patients has been shown to occur and correlate with disease activity [26, 27]. In MS lesions, immunoreactivity with TNF-α has been revealed in inflammatory cells, as well as in astrocytes and microglia [23, 24]. Moreover, disease progression in MS patients has been correlated with high levels of TNF-α in cerebrospinal fluid (CSF) [43]. Surprisingly, clinical trials with nonselective TNF inhibitors in MS patients resulted in disease exacerbation [44, 45]. Moreover, it was reported that some patients with rheumatoid arthritis treated with anti-TNF therapy developed neurological symptoms [46, 47]. TNF is expressed as a transmembrane protein (mTNF) that can be processed into a soluble form (sTNF) and exerts their functions via two receptors, TNFR1 and TNFR2 [48, 49]. TNFR1 is predominantly activated by sTNF [50] and has proinflammatory effects [51]. In contrast, TNFR2 is preferentially activated by mTNF [50] and implicated in neuroprotection and remyelination [52]. Thus, it is becoming clear that TNF mediates specific and often opposing effects via two different receptors. The distinct roles of these receptors may provide a mechanistic explanation for the failure of TNF-neutralizing therapy in MS. The sTNFR1 secreted from S-MSCs could selectively neutralize sTNF to inhibit the proinflammatory effects but retain the neuroprotection and remyelination effects.
Recently, a high percentage of Th17 cells were also found in the EAE mice [17]. Microarray analysis demonstrated that IL-17 transcript is upregulated in MS lesion [53]. IL-17 concentrations in CSF are significantly higher in MS patients than healthy subjects [54]. The expression levels of IL-23, a critical cytokine for Th17 cells, in monocyte-derived dendritic cells are higher in MS patients than those in healthy controls [55]. Furthermore, memory T cells producing IL-17 and IL-22 infiltrate into MS lesions [56]. These results convincingly suggest that Th17 cells may play important roles in the pathology of MS and that the inhibition of Th17 cell differentiation may be a therapeutic strategy in MS. Despite the fact that both TNF-α and IL-17 play crucial roles in the progression of MS and EAE, to our knowledge little is known about how TNF-α regulates Th17 cell differentiation in autoimmune diseases. In our study, we showed that serum from EAE mice contained abundant amounts of TNF-α, whereas serum from control mice did not. We demonstrated that naïve CD4+ T cells exposed to recombinant TNF-α promoted Th17 cell differentiation in vitro but had a minor effect on Th1 cell differentiation. Moreover, we found that TNF-α activated APCs, which were also able to produce IL-1β and IL-6, which are critical cytokines involved in promoting Th17 cell differentiation. Thus, the observed effect of TNF-α on Th17 cell differentiation is not only directed toward Th17 cells but also indirectly mediated through APCs. Our data indicate that inhibition of Th17 differentiation by blocking TNF-α activity may provide a new therapeutic approach for MS and EAE, as well as other Th17-mediated autoimmune diseases.
Here, we used MSCs derived from dermis and attempted to uncover a mechanism through which S-MSCs affect autoimmune disease. We showed that S-MSCs improved the clinical outcome of EAE partially by inhibiting differentiation of Th17 cells, as well as the expression of the transcription factors ROR-γt. A cytokine protein array-based analysis helped us to identify sTNFR1, which could block TNF-α signaling, as a critical mediator released by S-MSCs when exposed to inflammatory microenviroment. sTNFR1 (55 kDa) is one of the proteolytic shedding soluble extracellular domains of the TNFR1 modulating the activity of TNF-α [57]. Administration of TNF-α or LPS to human MSCs increases the concentration of soluble TNF receptors in the media, suggesting that soluble receptors may be part of a negative feedback mechanism to inhibit the biological effects of TNF-α [58].
sTNFR1 has been shown to inhibit the development of EAE induced by adoptive transfer of MBP-sensitized T lymphocytes [32]. sTNFR1 was reported to have highly specific TNF-α neutralizing activity [30]. sTNFR1 has advantages over the use of anti-TNF-α antibodies; for example, sTNFR1 of autologous origin may lack immunogenicity, a serious problem in antibody-based immunotherapy. Moreover, sTNFR1, being of low molecular mass, could penetrate better through the blood-brain barrier to reach higher concentration in the CNSs of MS patients [32]. We here present several lines of evidence to support that sTNFR1 released from S-MSCs is one of the primary mediators to inhibit Th17 cell differentiation. First, combining protein array technology and ELISA, we identified that sTNFR1 released by S-MSCs was remarkably increased in supernatants in coculture of S-MSCs and myelin-sensitized splenocytes stimulated with MOG35–55. Second, we demonstrated that Th17 cell differentiation was disrupted when sTNFR1 is present in culture and that the addition of exogenous TNF-α rescued Th17 cell differentiation. Third, administration of S-MSCs (shTNFR1), which have reduced levels of sTNFR1, partially abrogated their inhibition of clinical symptoms in mice with EAE. MSCs secreted some other antioxidative growth factors, and neutrophic factors may in part involve in the improving effects of S-MSCs on EAE [59–61].
Conclusion
We have provided direct evidence that skin-derived MSCs possess great therapeutic potential in autoimmune disease. TNF-α not only damages oligodendrocytes and myelin sheaths [62] but also promotes Th17 cell differentiation in EAE. Our results reveal that S-MSCs secret sTNFR1, which has highly specific neutralizing activity of TNF-α, and silencing TNFR1 gene expression in S-MSCs resulted in defective suppressive properties on the Th17 cell differentiation in ex vivo and in vivo settings. Together, the present study uncovers a mechanism through which S-MSCs improve clinical outcomes in autoimmune diseases and lend strong support to the clinical utility of S-MSCs in the treatment of various autoimmune disorders.
Supplementary Material
Acknowledgments
This work is supported by the 973 Program and National Natural Science Foundation of China (Grants 31330026, 31500730, 2012CB917100, 2014CB541905, 81202304, and 91029730) and by the Leading Academic Discipline Project of the Shanghai Municipal Education Commission (Grants J50208 and J50207).
Author Contributions
F.K. and Honglin Wang: conception and design, collection and/or assembly of data, manuscript writing, final approval of manuscript; L.Z., Z.L., S.Y., Z.X., J.B., H.Z., F.L., W.C., Y.S., Y.G., and Hong Wang: collection and/or assembly of data.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 2.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 3.Ke F, Zhang L, Liu Z, et al. Autocrine interleukin-6 drives skin-derived mesenchymal stem cell trafficking via regulating voltage-gated Ca(2+) channels. Stem Cells. 2014;32:2799–2810. doi: 10.1002/stem.1763. [DOI] [PubMed] [Google Scholar]
- 4.Sioud M, Mobergslien A, Boudabous A, et al. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71:267–274. doi: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 5.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 7.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 9.Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91. doi: 10.1016/j.jneuroim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 15.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 16.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 20.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 21.Nakae S, Nambu A, Sudo K, et al. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 22.Hofstetter HH, Ibrahim SM, Koczan D, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Hofman FM, Hinton DR, Johnson K, et al. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selmaj K, Raine CS, Cannella B, et al. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issazadeh S, Ljungdahl A, Höjeberg B, et al. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: Dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J Neuroimmunol. 1995;61:205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- 26.Chofflon M, Juillard C, Juillard P, et al. Tumor necrosis factor alpha production as a possible predictor of relapse in patients with multiple sclerosis. Eur Cytokine Netw. 1992;3:523–531. [PubMed] [Google Scholar]
- 27.Głabiński A, Mirecka M, Pokoca L. Tumor necrosis factor alpha but not lymphotoxin is overproduced by blood mononuclear cells in multiple sclerosis. Acta Neurol Scand. 1995;91:276–279. doi: 10.1111/j.1600-0404.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto S, Iwai S, Tsujiyama K, et al. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol. 2007;179:1449–1457. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 29.Sugita S, Kawazoe Y, Imai A, et al. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behçet’s disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Zee KJ, Kohno T, Fischer E, et al. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohler KM, Torrance DS, Smith CA, et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 32.Selmaj K, Papierz W, Glabiński A, et al. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol. 1995;56:135–141. doi: 10.1016/0165-5728(94)00139-f. [DOI] [PubMed] [Google Scholar]
- 33.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–1864. doi: 10.1038/mt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loetscher H, Pan YC, Lahm HW, et al. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- 35.Kohno T, Brewer MT, Baker SL, et al. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci USA. 1990;87:8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettelli E, Oukka M, Kuchroo VKT. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 37.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 38.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 39.Duffy MM, Pindjakova J, Hanley SA, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 40.Qu X, Liu X, Cheng K, et al. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol. 2012;40:761–770. doi: 10.1016/j.exphem.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z, Zheng C, Chen Z, et al. Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol. 2009;39:2840–2849. doi: 10.1002/eji.200839070. [DOI] [PubMed] [Google Scholar]
- 42.Waksman B. Mechanisms in multiple sclerosis. Nature. 1985;318:104–105. doi: 10.1038/318104a0. [DOI] [PubMed] [Google Scholar]
- 43.Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 44.van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 45.The Lenercept Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–465. [PubMed] [Google Scholar]
- 46.Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885–1888. doi: 10.1212/wnl.57.10.1885. [DOI] [PubMed] [Google Scholar]
- 47.Richez C, Blanco P, Lagueny A, et al. Neuropathy resembling CIDP in patients receiving tumor necrosis factor-alpha blockers. Neurology. 2005;64:1468–1470. doi: 10.1212/01.WNL.0000158681.29117.8B. [DOI] [PubMed] [Google Scholar]
- 48.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 49.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 50.Grell M, Douni E, Wajant H, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 51.Akassoglou K, Bauer J, Kassiotis G, et al. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: Models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontaine V, Mohand-Said S, Hanoteau N, et al. Neurodegenerative and neuroprotective effects of tumor Necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 54.Ishizu T, Osoegawa M, Mei FJ, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- 55.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 56.Kebir H, Kreymborg K, Ifergan I, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hale KK, Smith CG, Baker SL, et al. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine. 1995;7:26–38. doi: 10.1006/cyto.1995.1004. [DOI] [PubMed] [Google Scholar]
- 58.Lantz M, Malik S, Slevin ML, et al. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine. 1990;2:402–406. doi: 10.1016/1043-4666(90)90048-x. [DOI] [PubMed] [Google Scholar]
- 59.Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res (Amst) 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Crigler L, Robey RC, Asawachaicharn A, et al. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 61.Kemp K, Hares K, Mallam E, et al. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010;114:1569–1580. doi: 10.1111/j.1471-4159.2009.06553.x. [DOI] [PubMed] [Google Scholar]
- 62.Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.