Large numbers of viable cells must be promptly available for clinical use. Stem cell technologies, expansion, and banking represent ideal tools to ensure a regular supply. Induced pluripotent stem cell (iPSC) technology could allow a new era in transfusion medicine. The present review summarizes the current state of research on iPSC technology in the field of blood banking, highlighting hurdles, and promises.
Summary
Population aging has imposed cost-effective alternatives to blood donations. Artificial blood is still at the preliminary stages of development, and the need for viable cells seems unsurmountable. Because large numbers of viable cells must be promptly available for clinical use, stem cell technologies, expansion, and banking represent ideal tools to ensure a regular supply. Provided key donors can be identified, induced pluripotent stem cell (iPSC) technology could pave the way to a new era in transfusion medicine, just as it is already doing in many other fields of medicine. The present review summarizes the current state of research on iPSC technology in the field of blood banking, highlighting hurdles, and promises.
Significance
The aging population in Western countries is causing a progressive reduction of blood donors and a constant increase of blood recipients. Because blood is the main therapeutic option to treat acute hemorrhage, cost-effective alternatives to blood donations are being actively investigated. The enormous replication capability of induced pluripotent stem cells and their promising results in many other fields of medicine could be an apt solution to produce the large numbers of viable cells required in transfusion and usher in a new era in transfusion medicine. The present report describes the potentiality, technological hurdles, and promises of induced pluripotent stem cells to generate red blood cells by redifferentiation.
Current and Future Need for Blood Components
Red blood cell (RBC) transfusion is the main therapeutic option for acute hemorrhages. This assumption has led the World Health Organization to include blood within the Model List of Essential Medicines, point 11.1 [1]. In accordance with the World Health Assembly resolution, WHA63.12, the World Health Organization has recognized that achieving self‐sufficiency, unless special circumstances preclude it, in the supply of safe blood components based on voluntary, nonremunerated blood donation, and the security of that supply are important national goals to prevent blood shortages and meet the transfusion requirements of the patient population.
Progressive aging of the population in Westernized countries has two immediate dismal consequences: fewer blood donors and more blood recipients. The Finnish transfusion registry data have demonstrated a marked increase in RBC consumption with increasing age among recipients, beginning at approximately 50 years of age. Those aged 70 to 80 years have an eightfold higher RBC consumption than those aged 20 to 40 years [2].
From Blood Cell Substitutes to Stem Cell-Derived Blood Cells
Artificial oxygen carriers or recombinant hemoglobin-based oxygen carriers tested until now cause vasoconstriction triggered by nitric oxide scavenging and/or oxygen oversupply in the precapillary arterioles. To date, for clinical purposes, one must still rely on whole cells from accurately screened donors. In order to achieve meaningful clinical benefit, a transfusion unit must contain approximatively 1012 RBCs, or 300–600 × 109 platelets. Such a huge number of cells makes transdifferentiation from human adult somatic cell types a scientific exercise with good in vitro results [3] but poor clinical potential.
Currently, advances in genetic engineering have made it possible to knock out the genes of multiple xenoantigens, such as galactose α-1,3-galactose and N-glycolylneuraminic acid. Nevertheless, usage of xenogeneic RBCs from genetically engineered pigs still has major hurdles, leading to rapid clearance from circulation when transfused into nonhuman primates [4]. Thus, only two technologies have been deeply investigated to date for producing universal RBCs.
Modification of Blood Group Antigens Includes Two Basic Methods
First, group O RBCs are safe for transfusion to persons of any ABO blood group, because group O cells bear H chains without terminal A or B monosaccharides. In theory, group O RBCs could be created from cells of another ABO blood group, because the A and B terminal immunodominant sugars (120,000–1,170,000 sites per cell for group A and 610,000–830,000 for group B) could be removed using specific exoglycosidases. This treatment produces enzyme-converted group O RBCs [5]. A successful phase II trial using recombinant enzyme was reported in 2000 [6] but development later stopped. Because of the complexity of group A antigens, conversion of group A RBCs, in particular, A1 RBCs, to group O RBCs is more difficult. However, a new bacterial glycosidase efficiently cleaving antigens on the surface of both A1 and A2 RBCs has been obtained [7].
Second, pegylation camouflages the antigens on the surface of RBCs with nonimmunogenic molecules such as polyethylene glycol (PEG) in a nonspecific manner, to provide O, minor antigen-negative phenotype RBCs. Recent findings that PEG is immunogenic in animals and humans and that PEG antibodies can shorten the survival of PEG-RBCs are disturbing, suggesting that “stealth” RBCs might never become a reality [8]. Neither alternatives formally reduce the need for donors, which is the cornerstone of future problems to come.
Generating Universal RBCs From Progenitor and Stem Cells
Proliferating erythroblasts can be expanded from umbilical cord blood (CB) mononuclear cells ex vivo for 106–107-fold (in ∼50 days) before proliferation arrest. However, the Hayflick number is too low for achieving clinical useful doses, and, unfortunately, the efficiency of terminal differentiation is low [9]. Similarly, human erythroid progenitor cell lines immortalized by transduction of c-MYC and BCL-XL [10] are able to produce enucleated RBCs at low efficiency, and immortalization for autologous use is difficult to achieve [11].
Stem cells are the factor that is really needed [12, 13]. Robust bone marrow (BM) is able to produce a daily count of 2.5 billion RBCs, 7 million platelets, and 850,000 granulocytes per kilogram. Thus, hematopoietic stem cells (HSCs) from peripheral blood [14, 15], BM, or CB [16] were investigated first. The usual protocol was based on inducing massive proliferation of HSCs (up to 1.95 × 106-fold), followed by high-efficiency (95%) terminal erythroid differentiation. However, harvesting HSCs has obvious time and cost limitations when planning autologous use.
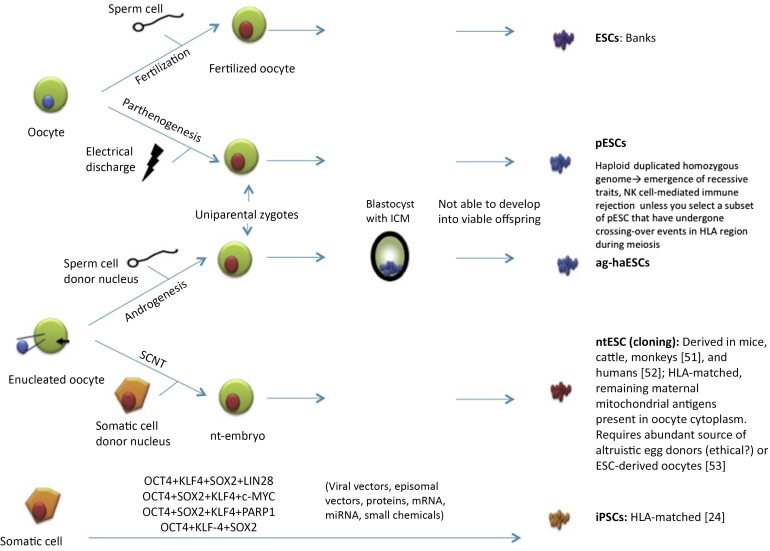
Pluripotent stem cells, able to differentiate into any of the three germ layers, can be isolated from blastocysts (embryonic stem cells [ESCs]) or generated by reprogramming of adult somatic cells (induced pluripotent stem cells [iPSCs]) [17] (Fig. 1). In a leading industrial trial, Ocata Therapeutics, Inc. (Worcester, MA, http://www.ocata.com) first reported that ESCs differentiated into functional oxygen-carrying erythrocytes on a large scale (1010–1011 cells per six-well plate of human ESCs) as a source for clinical grade mass production of RBCs [18]. Similarly, several groups have reported that ESC-derived platelets are functional in vitro and in the microcirculation of living mice [19–21].
Figure 1.
Different strategies to generate pluripotent stem cells [24, 51–53]. Abbreviations: ag-haESC, androgenetic haploid embryonic stem cell; ESC, embryonic stem cell; HLA, human leukocyte antigen; ICM, inner cell mass; iPSC, induced pluripotent stem cell; miRNA, micro-RNA; NK, natural killer; nt, nuclear transfer; pESC, parthenogenetic embryonic stem cell; SCNT, somatic cell nuclear transfer.
Although ESCs represent the most promising type of cells for scientific and clinical applications [22, 23], their use poses a set of ethical concerns. In contrast, iPSCs have great potential to provide an inexhaustible source of progenitors for the generation of large numbers of RBCs and to facilitate the innovative development of allogeneic and rare blood group products for transfusion purposes.
Induced Pluripotent Stem Cells
iPSCs were generated for the first time from murine fibroblasts in 2006 by Takahashi and Yamanaka using ectopic expression of transcription factors Oct4, Klf4, Sox2, and c-Myc (OKSM) [24]. In 2007, Takahashi et al. and Yu et al. successfully reprogrammed primary human fibroblasts using the OKSM cocktail [25] and Klf4, Oct4, Sox2, and LIN28 [26], respectively. Because of transformation concerns, many groups have replaced the use of the proto-oncogene c-Myc [27, 28] with less dangerous genes [29, 30] to increase the safety of delivery approaches and provide tightly reprogramming factors controllable expression systems [31, 32]. Tumorigenicity of undifferentiated iPSCs contaminating the final product is a concern that could be addressed using product irradiation or other clinically approved technologies that kill pathogens and nucleic acid-containing cells [33, 34].
iPSC-derived, pathogen-free, autologous or universal blood cells have the potential to alleviate allogeneic supply shortages. Small-scale bioreactors with disposable kits (e.g., Quantum Cell Expansion System; Terumo BCT, Lakewood, CO, http://www.terumobct.com; or Xuri; General Electric, Stanford, CT, http://www.ge.com) allow for in-hospital expansion of suspension cell cultures [35]. On an industrial size, large-scale bioreactors allow bulk production of iPSCs in the desired numbers and potentially with no Hayflick limit.
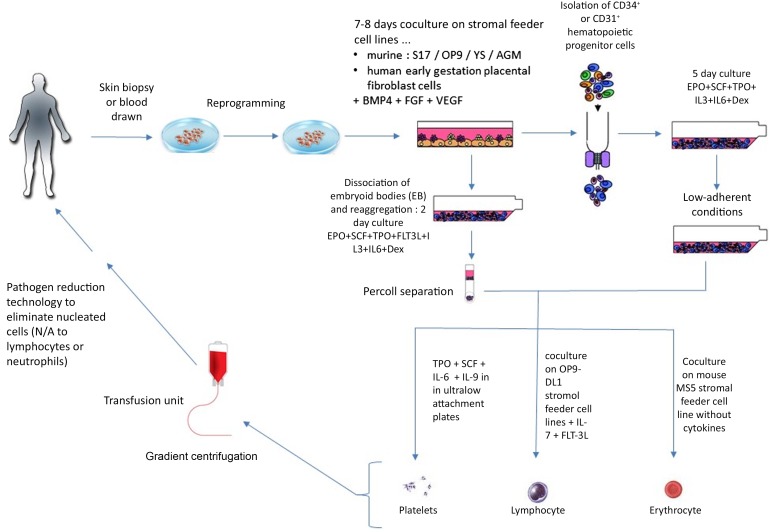
Most methods rely on mouse embryonic fibroblast (MEF) feeders and serum at some point during their culture. Because both MEF and serum can potentially be contaminated with xenogeneic pathogens, their use increases the risk to recipients; hence, serum-free and xeno-free protocols are being developed for generation of iPSCs [36] and redifferentiation to blood cells [37]. Redifferentiation of iPSCs to mature blood cell types seems the most difficult step in blood cell manufacturing from iPSCs [38]. In vitro redifferentiation is based on sequential addition of cytokines at defined concentrations [38] (summarized in Fig. 2).
Figure 2.
Different strategies to redifferentiate iPSCs to RBCs or platelets [35, 39-42, 46]. Abbreviations: BMP4, bone morphogenetic protein 4; Dex, dexamethasone; EPO, erythropoietin; FGF, fibroblast growth factor; FLT3L, FLT3 ligand; iPSCs, induced pluripotent stem cells; IL, interleukin; N/A, not applicable; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
Redifferentiating iPSCs Into RBCs
The major limitations for translating iPSC-derived RBCs into the clinic are (a) inefficient enucleation, (b) difficulty switching to the adult-type (β) globin form, and (c) the possibly insurmountable number of RBCs (1012) needed to generate 1 unit. Transfusing iPSC-derived RBCs is obviously safer (and faster) than transplanting genetically engineered iPSC-derived HSCs but has two major limitations: a short half-life and the need for repeated, lifelong transfusions.
A number of groups [39–41] have reported successful differentiation of iPSCs down the erythroid lineage using a variety of culture systems (stromal feeder-dependent or -independent), generating orthochromatic erythroblasts and reticulocytes (up to 10%), although Kobari et al. [42] have reported up to 26% enucleation for erythroid cells differentiated from iPSCs from an individual with sickle cell disease. The differentiated cells in all reports expressed fetal and embryonic globins, indicating reprogramming of the globin locus from the original parental cell. Erythroid differentiation has been confirmed by morphological analysis and the expression of a very limited number of RBC markers, including glycophorin A and transferrin receptor [40, 41]. Functionally, Kobari et al. [42] have shown that the reticulocytes generated from iPSCs exhibit an oxygen-binding capacity similar to that of CB RBCs, which contain predominantly fetal hemoglobin. Trakarnsanga et al. showed that the proteome of erythroid cells differentiated from iPSC lines is 98% similar to that of normal adult erythroid cells [43].
The iPSC erythroid cells expressed γ- but little β-globin, likely owing, at least in part, to the low level of KLF1 and the absence of BCL11A in these cells, both of which are known to be required for the developmental switch from fetal to adult globin expression [44, 45]. Apart from proteomics and immunophenotype data, studies addressing the function of iPSC-derived RBCs (e.g., the capacity to release oxygen to tissue under normoxia and hypoxia) in vivo are lacking; this should be the focus of investigation in the future.
Redifferentiating iPSCs to Platelets
The production of pathogen-free O donor platelet concentrates with negligible isoagglutinin titers would be the ideal aim. iPSC-derived platelets have been generated [35, 46] and Advanced Cell Technology, Inc. (Worcester, MA) is investing in clinical trials. Feng et al. recently reported a serum and animal feeder-free method that permits differentiation of human iPSCs into megakaryocytes (MKs) and functional platelets in less than 20 days and cryopreservation of MK progenitors, enabling a rapid “surge” capacity when large numbers of platelets are needed. iPSC-derived platelets form aggregates, lamellipodia, and filopodia after activation and circulate in macrophage-depleted animals and incorporate into developing mouse thrombi in a manner identical to human blood platelets. By knocking out the β2-microglobulin gene, platelets that are negative for class I human leukocyte antigen (HLA) have also been generated [37, 47].
Blood Banking and Transfusion
The limited shelf life of primary RBCs and platelets is likely to also remain a problem with iPSC-derived blood cells; however, having a precursor available will allow periodic reconstitution of the master cell bank. Because of the short half-life and absence of a nucleus, investigators and recipients will be spared from concerns regarding reprogramming-induced oncogenicity. Efficient screening to accurately separate all the anucleated cells from the nucleated counterpart appears to be a limiting step for this type of application.
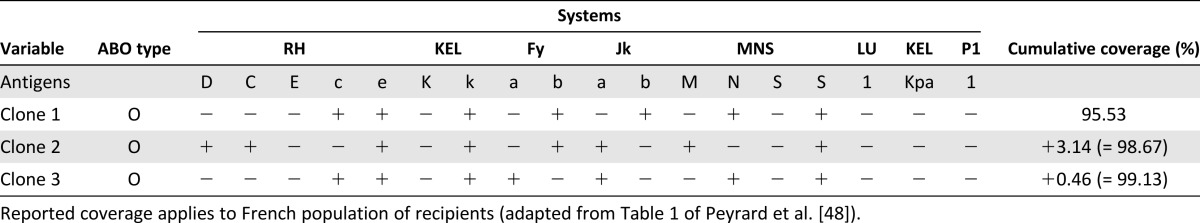
The currently available methods for the production of iPSCs are extremely inefficient and costly, and achieving off-the-shelf universal RBCs from a few key universal donors (homozygous at most relevant loci) would be the preferable approach [48]. The characteristics of the top three most useful universal donors are listed in Table 1 [48].
Table 1.
Phenotype of induced pluripotent stem cell clones that would be theoretically useful for the management of alloimmunized recipients
Although group O RBCs are considered universal, they are still immunogenic for recipients with the Bombay phenotype. The Bombay phenotype is one of the rare phenotypes in the ABO blood group system that fails to express ABH antigens on RBCs and develops natural anti-O isoagglutinins. Nonsense or missense mutations in fucosyltransferase 1 (FUT1) and 2 (FUT2) genes are known to create this phenotype. This blood group is compatible with all other blood groups as a donor, because it does not express the H antigen on RBCs. Jackman et al. reported in 2010 the establishment of human iPSCs from the dermal fibroblasts of a Bombay blood type individual [49].
However, universal ABO compatibility is not the only requirement, because many other different antigenic systems expressed on RBCs are immunogenic. Once alloimmunization occurs, such patients require RBCs from donors with a different blood group antigen combination, making it a challenge to find donors after every successive episode of alloimmunization. Peyrard et al. retrospectively studied a cohort of 16,486 consecutive alloimmunized patients (10-year period), showing 1 to 7 alloantibodies with 361 different antibody combinations [48]. They showed that only three human iPSC clones (those reported in Table 1) would be sufficient to match more than 99% of the 16,486 patients in need of RBC transfusions. The study of the French National Registry of People with a Rare Blood Phenotype/Genotype (10-year period) showed that 15 human iPSC clones would cover 100% of the needs in white patients. In addition, one single human iPSC clone would meet 73% of the needs of alloimmunized patients with sickle cell disease for whom rare cryopreserved RBC units are required. Hence, a very limited number of RBC clones would be able to not only provide for the need for most alloimmunized patients and those with a rare blood group but also to efficiently allow for a policy for alloimmunization prevention in multiply transfused patients [48].
ABO compatibility is also definitively not the only requirement for platelet transfusions. Even with platelet transfusion, other antigen systems (i.e., HLA and human platelet glycoprotein antigen) keep the risk of alloimmunization high. However, in this setting, published studies reporting combined antigen frequencies, which is the prerequisite to identify the coverage achievable with key “universal” donors, are also lacking.
Conclusion
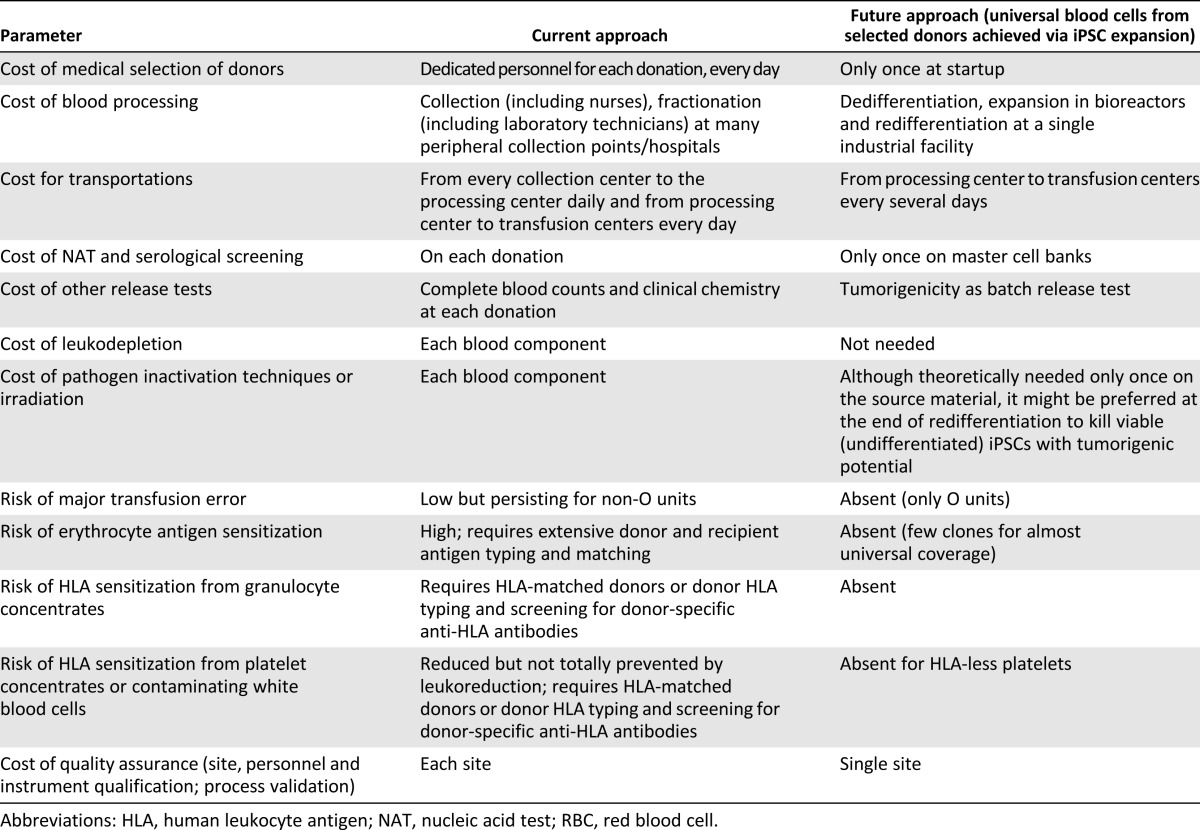
Blood cells are the ideal candidates for clinical trials of iPSC-redifferentiated cell therapies. The proteome of blood cells differentiated from iPSC lines is very similar to that of normal adult blood cells; however, further work to improve the induction of erythroid cells in existing iPSC lines is required before iPSC-derived RBCs are suitable for transfusion therapy. Engineering technology such as bioreactor use might be necessary for successful advances; however, the details and cost will remain the main difficulties. If these can be overcome, we will realize several advantages (summarized in Table 2) in terms of ease of availability and costs to the health care system [50].
Table 2.
Advantages from mass production of universal RBCs from a single donor vs. current approach to blood collection and banking
Acknowledgment
D.F. is grateful to the Italian Society of Hematology and the Italian League against Leukemia for supporting funds.
Author Contributions
D.F.: conception and design, manuscript writing; M.P.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.WHO Model List of Essential Medicines for Adults 19th list (April 2015) Revision August 2015. Available at: http://www.who.int/selection_medicines/committees/expert/20/EML_2015_FINAL_amended_AUG2015.pdf?ua=1. Accessed January 7, 2016.
- 2.Ali A, Auvinen MK, Rautonen J. The aging population poses a global challenge for blood services. Transfusion. 2010;50:584–588. doi: 10.1111/j.1537-2995.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Lu SJ, Lu Y, et al. Transdifferentiation of human hair follicle mesenchymal stem cells into red blood cells by OCT4. Stem Cells Int. 2015;2015:389628. doi: 10.1155/2015/389628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZY, Burlak C, Estrada JL, et al. Erythrocytes from GGTA1/CMAH knockout pigs: Implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21:376–384. doi: 10.1111/xen.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein J. Preparation of transfusable red cells by enzymatic conversion. Prog Clin Biol Res. 1984;165:139–157. [PubMed] [Google Scholar]
- 6.Kruskall MS, AuBuchon JP, Anthony KY, et al. Transfusion to blood group A and O patients of group B RBCs that have been enzymatically converted to group O. Transfusion. 2000;40:1290–1298. doi: 10.1046/j.1537-2995.2000.40111290.x. [DOI] [PubMed] [Google Scholar]
- 7.Olsson ML, Hill CA, de la Vega H, et al. Universal red blood cells—Enzymatic conversion of blood group A and B antigens. Transfus Clin Biol. 2004;11:33–39. doi: 10.1016/j.tracli.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 2008;94:87–95. doi: 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Shah S, Wang J, et al. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol Ther. 2014;22:451–463. doi: 10.1038/mt.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solheim B, Cid J, Osselaer J-C. Pathogen reduction technologies. In: Lonzano MCM, Blajchman M, editors. Global Perspectives in Transfusion Medicine. Bethesda, MD: AABB Press; 2006. pp. 103–148. [Google Scholar]
- 11.Kurita R, Suda N, Sudo K, et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HO. In-vitro stem cell derived red blood cells for transfusion: Are we there yet? Yonsei Med J. 2014;55:304–309. doi: 10.3349/ymj.2014.55.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fast LD, Dileone G, Li J, et al. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46:642–648. doi: 10.1111/j.1537-2995.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 14.Neildez-Nguyen TM, Wajcman H, Marden MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 15.Giarratana MC, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 16.Baek EJ, Kim HS, Kim S, et al. In vitro clinical-grade generation of red blood cells from human umbilical cord blood CD34+ cells. Transfusion. 2008;48:2235–2245. doi: 10.1111/j.1537-2995.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 17.Amabile G, Meissner A. Induced pluripotent stem cells: Current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Lu SJ, Feng Q, Park JS, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu SJ, Li F, Yin H, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21:530–545. doi: 10.1038/cr.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pick M, Azzola L, Osborne E, et al. Generation of megakaryocytic progenitors from human embryonic stem cells in a feeder- and serum-free medium. PLoS One. 2013;8:e55530. doi: 10.1371/journal.pone.0055530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 27.Jincho Y, Araki R, Hoki Y, et al. Generation of genome integration-free induced pluripotent stem cells from fibroblasts of C57BL/6 mice without c-Myc transduction. J Biol Chem. 2010;285:26384–26389. doi: 10.1074/jbc.M110.115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HY, Chien Y, Chen YJ, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011;32:5994–6005. doi: 10.1016/j.biomaterials.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Chiou S-H, Jiang B-H, Yu Y-L, et al. Poly(ADP-ribose) polymerase 1 regulates nuclear reprogramming and promotes iPSC generation without c-Myc. J Exp Med. 2013;210:85–98. doi: 10.1084/jem.20121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doege CA, Inoue K, Yamashita T, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeKelver RC, Choi VM, Moehle EA, et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 2010;20:1133–1142. doi: 10.1101/gr.106773.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayart E, Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther. 2013;13:73–92. doi: 10.2174/1566523211313020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackman RP, Deng X, Bolgiano D, et al. Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion. 2014;54:672–680. doi: 10.1111/trf.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fast LD, Nevola M, Tavares J, et al. Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion. 2013;53:373–381. doi: 10.1111/j.1537-2995.2012.03715.x. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa Y, Nakamura S, Nakajima M, et al. Two differential flows in a bioreactor promoted platelet generation from human pluripotent stem cell-derived megakaryocytes. Exp Hematol. 2013;41:742–748. doi: 10.1016/j.exphem.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Wyles SP, Yamada S, Oommen S, et al. Inhibition of DNA topoisomerase II selectively reduces the threat of tumorigenicity following induced pluripotent stem cell-based myocardial therapy. Stem Cells Dev. 2014;23:2274–2282. doi: 10.1089/scd.2014.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Q, Shabrani N, Thon JN, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep. 2014;3:817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano H, Lee CY, Fox-Talbot K, et al. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation. 2007;84:1174–1182. doi: 10.1097/01.tp.0000287318.94088.d7. [DOI] [PubMed] [Google Scholar]
- 39.Chang CJ, Mitra K, Koya M, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS One. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: Perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobari L, Yates F, Oudrhiri N, et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97:1795–1803. doi: 10.3324/haematol.2011.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trakarnsanga K, Wilson MC, Griffiths RE, et al. Qualitative and quantitative comparison of the proteome of erythroid cells differentiated from human iPSCs and adult erythroid cells by multiplex TMT labelling and nanoLC-MS/MS. PLoS One. 2014;9:e100874. doi: 10.1371/journal.pone.0100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of gamma-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayama N, Nishimura S, Nakamura S, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gras C, Schulze K, Goudeva L, et al. HLA-universal platelet transfusions prevent platelet refractoriness in a mouse model. Hum Gene Ther. 2013;24:1018–1028. doi: 10.1089/hum.2013.074. [DOI] [PubMed] [Google Scholar]
- 48.Peyrard T, Bardiaux L, Krause C, et al. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: Potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011;25:206–216. doi: 10.1016/j.tmrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Jackman RP, Heitman JW, Marschner S, et al. Understanding loss of donor white blood cell immunogenicity after pathogen reduction: Mechanisms of action in ultraviolet illumination and riboflavin treatment. Transfusion. 2009;49:2686–2699. doi: 10.1111/j.1537-2995.2009.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fast LD, DiLeone G, Marschner S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion. 2011;51:1397–1404. doi: 10.1111/j.1537-2995.2010.02984.x. [DOI] [PubMed] [Google Scholar]
- 51.Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 52.Tachibana M, Amato P, Sparman M, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayashi K, Ogushi S, Kurimoto K, et al. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]