Abstract
Stable triarylmethyl radicals are ideal spin labels used for biomedical electron paramagnetic resonance applications. Previously reported structures exhibit polar charged functions for water solubilization preventing them from crossing the cell membrane. We report the synthesis of a triarylmethyl radical conjugated to poly-arginine peptide allowing intracellular delivery the paramagnetic label.
Graphical Abstract

Stable radicals of tetrathiatriarylmethyl represent soluble spin labels and probes widely used for biomedical electron paramagnetic resonance (EPR) spectroscopy and imaging[1]. They combine excellent biological stability and extraordinary narrow EPR line width as a consequence of their long relaxation times, therefore resulting in a high signal to noise ratio. The six thioacetonide moieties provide stability to the radical by electronic and steric effects while the para-positions are used to provide water solubility through substitution with polar charged functions such as carboxylic acids (e.g., see Figure 1 for the structure of CT-03 derivative also known as Finland trityl). Bimolecular collisions between the paramagnetic probe and molecular oxygen (3O2) induce a line broadening of the EPR line of the trityl due to pair-wise Heisenberg spin exchange providing a noninvasive, sensitive and quantitative way to measure oxygen in a biological media in vitro and in vivo[2, 3]. Depending on the particular structure of the trityl probe other biological relevant parameters can be measured. For example the phosphonated derivative allows simultaneous measurement of dissolved oxygen, pH and phosphate concentration in the physiological range in living tissues [4, 5]. Tetrathiatriarylmethyl disulfide biradicals allow for EPR assessment of biologically relevant thiols such as glutathione (GSH) [6] while the nitroxide-trityl biradical allows for assessment of tissue redox [7].
Figure 1.
Chemical structure of CT-03 and cell permeable AMT-02.
Trityl radicals with charged para-functions such as CT-03 are cell impermeable therefore allowing assessment of important physiological parameters in the extracellular environment [5, 8]. However, intracellular pH, redox status and GSH level provide complementary information of particular importance to physiology and pathology of various diseases such as cancer[9]. Recently, we synthesized a cell-permeable trityl radical AMT-02 (Figure 1) which contain biodegradable acetoxymethyl esters. After crossing the membranes, the esters are hydrolyzed to CT-03 radical. However, this approach is limited by the low water solubility of AMT-02, the slow intra-cellular hydrolysis leading to CT-03 formation and its subsequent process of excretion from the cells tested [10]. Due to the lipophilic nature of the thioacetonide moieties CT-03 binds to albumin in its sub-domain IIA (Sudlow’s site I) and presumably to other biomolecules leading to an increase in the EPR line width. This line broadening leads to a decrease of the signal intensity by a factor of five when excess of bovine serum albumin (BSA) has been used [11]. The above factors make application of AMT-02 probe for the measurement of oxygen complex [10].
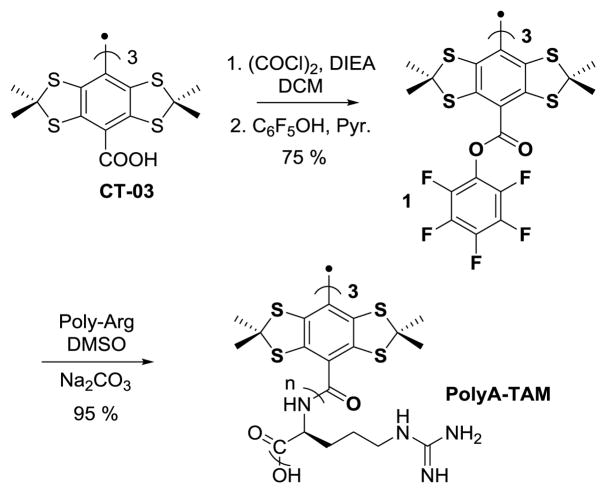
Small poly-arginine peptides are known for their ability to cross cell membranes and have been used to deliver nanosized cargo or small molecules inside cells [12]. In this report, we explore poly-arginine peptides as a carrier for intracellular delivery of a trityl spin label. The activated tri-pentafluorophenol esters 1 (Scheme 1) have been proven to be an excellent intermediate to bind a small peptide to the trityl in a good yield [13]. Following this strategy, the poly-arginine conjugated trityl PolyA-TAM was synthesized. In the first step CT-03 is activated by oxalyl chloride to lead to the formation of the trityl acyl chloride which is directly engaged in reaction with pentafluorophenol yielding the pentafluorophenol activated ester of CT-03. The pure ester is obtained in 75% yield after flash chromatography. Finally coupling the pentafluorophenol ester with commercially available poly-arginine chains (8≤n≤12, MW=1900 Almenda polymer) in DMSO led to the desired polyA-TAM. After purification by dialysis using 5,000 cutoff membrane, the pure radical was obtained in 95% yield as a green solid. The radical was stable, no degradation was observed in water for 24 h at room temperature in oxygenated water.
Scheme 1.
Synthesis of PolyA-TAM from CT-03.
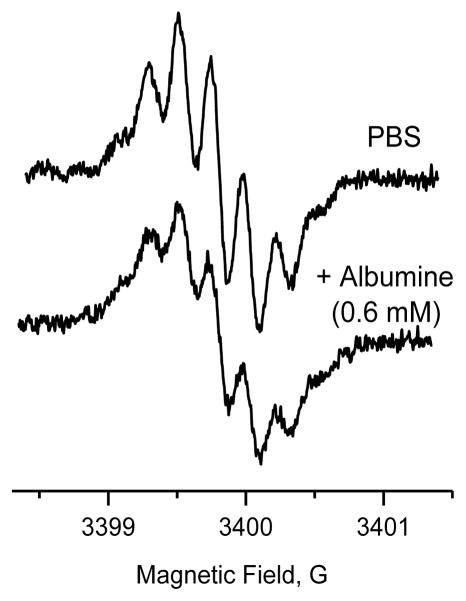
The EPR spectrum of PolyA-TAM in aqueous solution shown in Figure 2. According to the presence of three amide functions, the spectral pattern shows seven partially overlapped lines (1:3:6:7:6:3:1) as a result of hyperfine couplings with three equivalent nitrogen nuclei (14N, I=1) with an hyperfine splitting constant (hfc) of 220 mG, in agreement with the previously reported para-amide derivatives of trityl radicals [14]. This spectral assignment supports the presence of three poly-arginine chains bound to the trityl. We anticipated that the charge nature of the poly-arginine chains associated with the bulkiness of our newly synthesized EPR spin label will prevent binding to albumin. In agreement with this hypothesis addition of 600 μM of BSA did not affect EPR spectrum of PolyA-TAM except for a small linewidth broadening of 10 mG (See Supplementary Data for simulated spectra) apparently due to a change of viscosity of the media.
Figure 2.
X-band EPR spectra of 100 μM PolyA-TAM solution in deoxygenated 10 mM PBS buffer (pH=7.4, room temperature) before and after addition of BSA. Acquisition parameters were as follows: sweep width, 3G; power, 0.2mW; modulation frequency, 100 KHz; modulation amplitude, 0.05G; conversion time, 80 ms; time constant, 163.84 ms; resolution, 1024 pt.
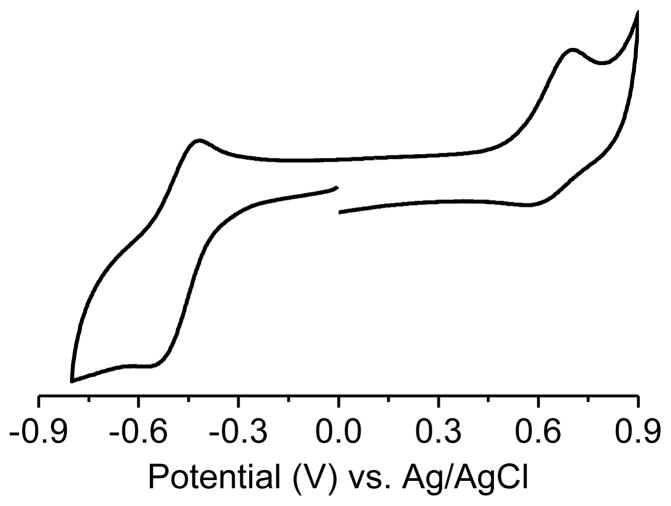
Recent study of the metabolism of CT-03 has shown that the EPR signal can decay due to reduction of the radical under anaerobic conditions [15] by liver microsomes followed by protonation of the anion or by oxidation to cation by some peroxidases or related hemeproteins [16]. The resulting cation can subsequently react with C-, S-, -P or N- nucleophiles [17, 18]. In addition, oxidative decarboxylation of CT-03 by superoxide radical or peroxyl radicals lead to a diamagnetic quinone-methide product[19]. Substitution of the carboxylic acid by amide functions protect against the latter reaction. In order to evaluate the sensitivity toward oxidation and reduction, the redox potentials were measured by cyclic voltammetry. In phosphate buffer, pH = 7.4, the votammogram shows a reduction of the radical to the anion. The half wave of reduction reaches −0.475 V vs Ag/AgCl indicating that polyA-TAM is easier to reduce than CT-03 in its carboxylate form (see Table 1). The oxidation of the radical to the cation was observed with a half wave of E1/2 = +0.666 vs Ag/AgCl indicating that the polyA-TAM is more difficult to oxidize than CT-03 (Table 1). These results are consistent with a higher electron withdrawing effect of amide function compared with the carboxylate ones.
Table 1.
Oxidation and reduction half wave potentials for PolyA-TAM and CT-03
| Compound | Solvent | E1/2 oxa | E1/2 reda |
|---|---|---|---|
| CT-03[13] | PBS pH=7.4 | +0.434 | −0.642 |
|
|
|||
| PolyA-TAM | Water pH=7.4 | +0.666 | −0.475 |
Calculated according to E1/2 = (Epa + Epc)/2
To investigate the ability of PolyA-TAM probe to permeate the cellular membrane and accumulate in intracellular space, MDA-MB-231 cells were incubated in the presence of 3 μM of PolyA-TAM probe for different times (30 min, 1h, 2h). Then cells were washed three times with 20 ml of PBS, trypsinized, collected and centrifuged. Pellet was dispersed in PBS, loaded into glass capillary followed by EPR spectra acquisition. The observed EPR signal intensity (see Figure 4) corresponds to about 3.5 μM of PolyA-TAM in the sample of 7.5×107 cell/ml while no EPR signal was found in the supernatant. This corresponds to a 200 μM intracellular concentration of PolyA-TAM assuming that the volume of single MDA-MB-231 cell equals is 2000 μm3. No significant change of the signal intensity was observed after 30 min of incubation indicating that the penetration of the PolyA-TAM into the cell is comparatively fast. The total spectral line widths are similar for PolyA-TAM measured in buffer and inside the cells (cf. Figures 2 and 4). The disappearance of additional hyperfine splitting in cellular sample might arise from broadening of individual components due to higher intracellular viscosity.
Figure 4.
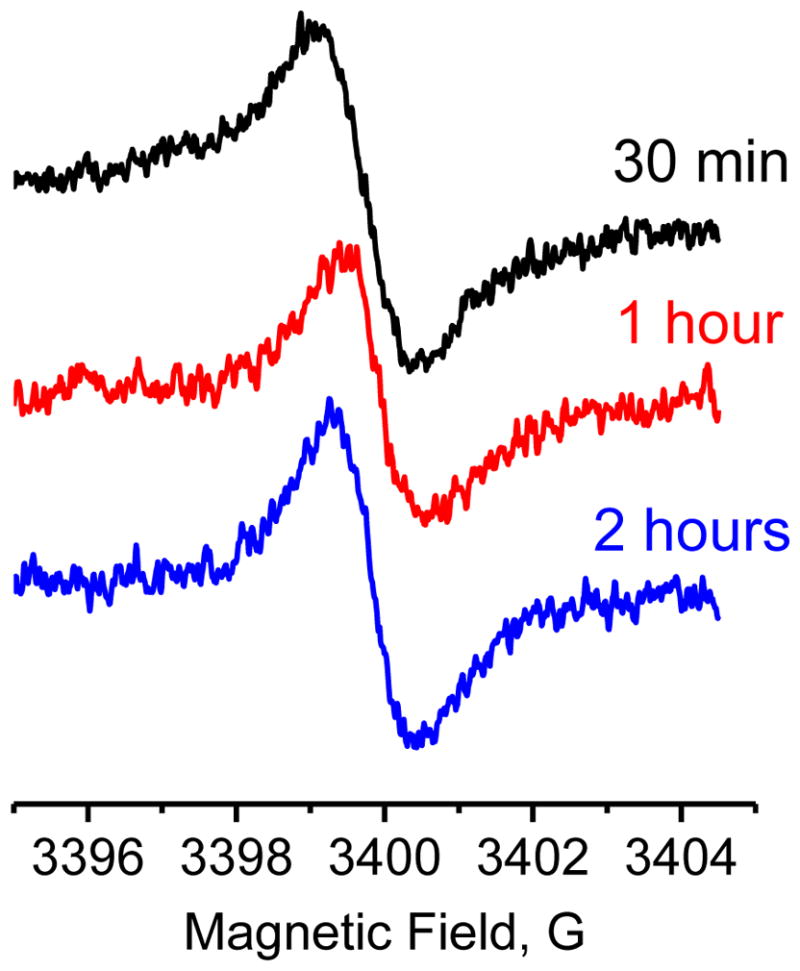
X-band EPR spectra of internalized PolyA-TAM measured after different incubation times at 37 °C. Acquisition parameters were as following: sweep width, 10 G; power, 0.63 mW; modulation frequency, 100 KHz; modulation amplitude, 0.1 G; conversion time, 80 ms; time constant, 367.28 ms; resolution, 1024 pt.
In conclusion, we have synthesized a new trityl spin label linked to three poly-arginine peptides. Due to coupling with three nitrogen nuclei of the amide functions, the spectrum shows a septet pattern limiting its application as oximetric EPR probe and decreasing the signal to noise ratio. No binding to albumin was observed validating our hypothesis that charged nature of the poly-arginine and the bulkiness of the molecule prevent this interaction. The polyA-TAM is able to penetrate across the cellular membrane and was found to be stable inside the cells for hours. Further improvement of the probe structure can be achieved by introduction of a linker between the parent trityl and the poly-arginine chains therefore eliminating hyperfine splitting-induced line broadening and improving signal-to-noise ratio. This strategy was previously reported to bind CT-03 to polyethylene glycol moieties through using small deuterated ester linker providing a narrow single line EPR spectrum.[20]
Supplementary Material
Figure 3.
Cyclic voltammogram of PolyA-TAM recorded in water at pH = 7.4, scan rate = 0.2 V/s.
Acknowledgments
This work was partially supported by NIH grants: EB016096, EB014542, CA194013, CA192064, U54GM104942 and F.S.R Universite catholique de Louvain Fellowship awarded to B.D. The WVCTSI is acknowledged for start-up to T.D.U, V.V.K, A.A.B
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Supplementary Data is available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khramtsov VV, Zweier JL. Functional in vivo EPR Spectroscopy and Imaging Using Nitroxide and Trityl Radicals. In: Robin Hicks NJWS, editor. Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. New York: Ltd; 2010. p. 537. [Google Scholar]
- 2.Elas M, Ahn KH, Parasca A, Barth ED, Lee D, Haney C, Halpern HJ. Clin Cancer Res. 2006;12:4209. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad R, Kuppusamy P. Chem Rev. 2010;110:3212. doi: 10.1021/cr900396q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhimitruka I, Bobko AA, Eubank TD, Komarov DA, Khramtsov VV. J Am Chem Soc. 2013;135:5904. doi: 10.1021/ja401572r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. Angew Chem Int Ed. 2014;53:2735. doi: 10.1002/anie.201310841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Song Y, Rockenbauer A, Sun J, Hemann C, Villamena FA, Zweier JL. J Org Chem. 2011;76:3853. doi: 10.1021/jo200265u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Villamena FA, Song Y, Sun J, Rockenbauer A, Zweier JL. J Org Chem. 2010;75:7796. doi: 10.1021/jo1016844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams BB, al Hallaq H, Chandramouli GVR, Barth ED, Rivers JN, Lewis M, Galtsev VE, Karczmar GS, Halpern HJ. Magn Reson Med. 2002;47:634. doi: 10.1002/mrm.10089. [DOI] [PubMed] [Google Scholar]
- 9.Khramtsov VV, Gillies RJ. Antioxid Redox Sign. 2014;21:723. doi: 10.1089/ars.2014.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Villamena FA, Sun J, Wang T-y, Zweier JL. Free Radical Biology and Medicine. 2009;46:876. doi: 10.1016/j.freeradbiomed.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Liu Y, Liu W, Villamena FA, Zweier JL. RSC Adv. 2014;4:47649. doi: 10.1039/C4RA04616A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin MC, Zhang J, Min KA, Lee K, Byun Y, David AE, He H, Yang VC. J Biomed Mater Res A. 2014;102:575. doi: 10.1002/jbm.a.34859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driesschaert B, Levêque P, Gallez B, Marchand-Brynaert J. Tet Lett. 2013;54:5924. [Google Scholar]
- 14.Driesschaert B, Levêque P, Gallez B, Marchand-Brynaert J. Eur J Org Chem. 2014;2014:8077. [Google Scholar]
- 15.Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Chem Res Tox. 2009;22:1342. doi: 10.1021/tx9001379. [DOI] [PubMed] [Google Scholar]
- 16.Decroos C, Li Y, Soltani A, Frapart Y, Mansuy D, Boucher JL. Arch Biochem Biophys. 2010;502:74. doi: 10.1016/j.abb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Decroos C, Boucher JL, Mansuy D, Xu-Li Y. Chem Res Tox. 2014;27:627. doi: 10.1021/tx400467p. [DOI] [PubMed] [Google Scholar]
- 18.Decroos C, Prange T, Mansuy D, Boucher JL, Li Y. Chem Commun. 2011;47:4805. doi: 10.1039/c1cc10426h. [DOI] [PubMed] [Google Scholar]
- 19.Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Chem Commun. 2009:1416. doi: 10.1039/b819259f. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Liu Y, Hemmann C, Villamena F-A, Zweier J-L. J Org Chem. 2013;78:1371. doi: 10.1021/jo301849k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.