Abstract
Certain forms of heart disease involve gross morphological changes to the myocardium that alter its hemodynamic loading conditions. These changes can ultimately lead to the increased deposition of extracellular matrix (ECM) proteins, such as collagen and fibronectin, which together work to pathologically alter the myocardium’s bulk tissue mechanics. In addition to changing the mechanical properties of the heart, this maladaptive remodeling gives rise to changes in myocardium electrical conductivity and synchrony since the tissue’s mechanical properties are intimately tied to its electrical characteristics. This phenomenon, called mechanoelectrical coupling (MEC), can render individuals affected by heart disease arrhythmogenic and susceptible to sudden cardiac death (SCD). The underlying mechanisms of MEC have been attributed to various processes, including the action of stretch activated channels and changes in troponin C-Ca2+ binding affinity. However, changes in the heart post infarction or due to congenital myopathies are also accompanied by shifts in the expression of various molecular components of cardiomyocytes, including the mechanosensitive family of integrin proteins. As transmembrane proteins, integrins mechanically couple the ECM with the intracellular cytoskeleton and have been implicated in mediating ion homeostasis in various cell types, including neurons and smooth muscle. Given evidence of altered integrin expression in the setting of heart disease coupled with the associated increased risk for arrhythmia, we argue in this review that integrin signaling contributes to MEC. In light of the significant mortality associated with arrhythmia and SCD, close examination of all culpable mechanisms, including integrin-mediated MEC, is necessary.
Keywords: mechanoelectrical coupling, integrin, Sudden Cardiac Death, hypertrophic cardiomyopathy, costamere
1. Introduction
The predominant means of coordinating the heart’s pumping activity is the spread of an excitation wavefront through gap junctions in the cardiac musculature. Interruption of the ordered propagation of the wavefront is associated with various cardiomyopathies or attributed to traumatic interruptions because of exogenous mechanical forces, referred to as commotio cordis (Maron et al., 1995). Mortality due to Sudden Cardiac Death (SCD) is directly correlated to the incidence of Hypertrophic Cardiomyopathy (HCM) (Frey et al., 2011), and to maladaptive remodeling of an infarcted region of the heart (Zipes and Wellens, 1998). In both conditions, localized remodeling of the cellular microenvironment results in fibrosis, changes in cell morphology, cytoskeletal alterations, and changes in the bulk mechanical properties of the tissue (Chien et al., 2008; Maron, 2002; Parker and Ingber, 2007; van den Borne et al., 2009). These changes in the mechanical properties of the heart ultimately lead to alterations in the myocardium’s electrical properties that can be arrhythmogenic. This feedback, referred to as mechanoelectrical coupling (MEC), represents a range of events that span wide temporal and spatial scales. Previous studies of MEC at the tissue and whole organ levels have proposed that acute mechanisms such as stretch-activated ion channels (Riemer and Tung, 2003), changes in the binding affinity of troponin C to calcium ions (Tavi et al., 1998), and interactions between cardiomyocytes and mechano-sensitive cells are involved (Kamkin et al., 2005). However, the underlying cellular mechanism of MEC is still unclear and it is important to determine specific pathways by which mechanical forces are transduced to chemical and electrical signaling within myocytes.
Concomitant with changes in the extracellular matrix (ECM) network during fibrosis, integrin expression also changes in cardiac myocytes (Bujak and Frangogiannis, 2007; Dullens et al., 2011; Ross and Borg, 2001; Sheehy et al., 2009). Integrins, and the focal adhesion proteins that anchor them, propagate mechanical forces from the extracellular space to the intracellular space by providing mechanical coupling between the extracellular matrix and cytoskeleton (Alenghat and Ingber, 2002; Wang et al., 1993). Integrins, which are made up of α and β subunits that together form heterodimers spanning the cell membrane, are the primary sensors of mechanical forces propagated through the ECM network and have been implicated in a myriad of biological processes, ranging from the immune response to key roles in development (reviewed in (Hynes, 2002)). In physiological conditions, these forces encode information that cells transduce for homeostasis and adaptive responses. In pathological conditions, these forces can maladaptively activate the same signaling pathways for a negative result. In the healthy myocyte, integrins are commonly localized within the costameres, anchoring the sarcomeric z-discs in the outer most regions of the myofibrillar array (Borg et al., 2000; Ross and Borg, 2001; Samarel, 2005) (Fig. 1). This structured colocalization of integrins with costameres is subsequently lost under pathological conditions, where integrin proteins alone localize to cardiomyocyte termini bordering regions of cardiac scarring (Matsushita et al., 1999). While whole organ and tissue stretch models have highlighted the role of stretch activated channels (Hansen et al., 1991; Stacy Jr et al., 1992) in MEC, data regarding how cytoskeleton altering interventions can affect incidence of arrhythmias (Madias et al., 2008; Parker et al., 2001) suggest that the traditional view of membrane stretch-induced opening of stretch-activated ion channels cannot solely describe the mechanisms underlying MEC. In this report, we suggest that integrin signaling may contribute to electrical synchrony and MEC in the heart and review the literature in support of this argument.
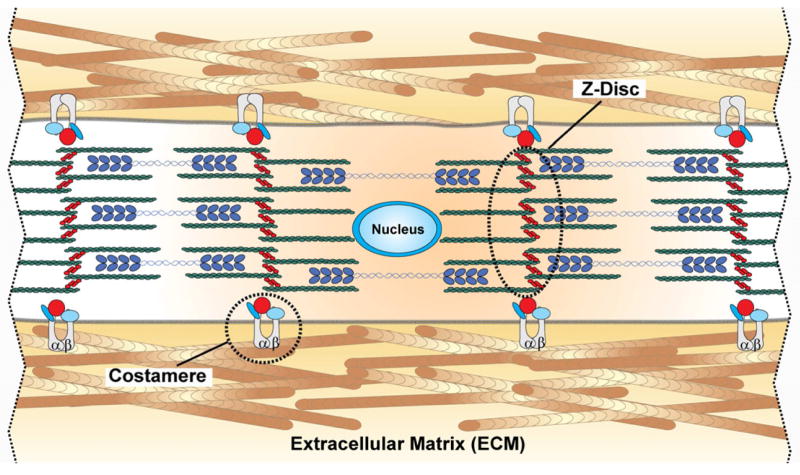
Figure 1.
Schematic depicting cardiac myofibril organization in vivo. Integrins colocalized to the costamere help mechanically couple the intracellular z-disc to the ECM. Z-discs anchor the contractile machinery of the myocyte, namely actin (green) and myosin (blue). Adjacent myocytes are coupled to each other via adherens junctions.
2. Hypertrophic cardiomyopathy, fibrosis, integrin expression, and incidence of SCD
HCM is a disease commonly attributed to autosomally dominant mutations in sarcomeric protein-encoding genes (reviewed in (Frey et al., 2011)). Cardiac hypertrophy is characterized by gross morphological changes in the heart and in the case of maladaptive hypertrophy, reduced cardiac output. Fibrosis is often considered a contributor to the increased propensity of these patients for arrhythmias (Varnava et al., 2001a; Varnava et al., 2001b) and increased interstitial collagen deposition (Factor et al., 1991; Shirani et al., 2000) contributes to changes in cellular architecture within the ventricle (Ferrans et al., 1972; Maron et al., 1979; St John Sutton et al., 1980). Changes in myocyte shape serve to distort the alignment of anisotropic laminar muscle, contributing to reduced ejection fraction and arrhythmogenesis (Maron et al., 1981; Teare, 1958). The remodeled myocardium is also marked by changes in both integrin expression and localization. For instance increases in α1 integrin expression have been noted along with the shedding of β1 integrin into the extracellular matrix surrounding cardiomyocytes (Ding et al., 2000; Hauselmann et al., 2011; Terracio et al., 1991). It should be noted that these changes are not typically uniform, and usually concentrate in the left ventricle (Klues et al., 1995; Shapiro and McKenna, 1983; Spirito et al., 2000). Moreover, the left ventricle may not be diffusely hypertrophied and reports of localized hypertrophy within the ventricles are common (Louie and Maron, 1987; Spirito et al., 1986; Webb et al., 1990). The spatial heterogeneity of hypertrophy also increases the risks for arrhythmias, especially ventricular tachycardia and ventricular fibrillation (Elliott et al., 1999; Nicod et al., 1988; Silka et al., 1993). Arrhythmias occur in 20% of patients with HCM (Maron et al., 2007), and patients with dilated and hypertrophic cardiomyopathy account for 10–15% of SCD cases (Huikuri et al., 2001). Therefore, HCM is characterized by several changes within the cardiac tissue microenvironment that include altered integrin expression, cell shape, cell-cell coupling, and cell-ECM coupling that may contribute to fatal arrhythmias.
3. The infarct scar, integrin expression, and irregular cardiac rhythms
The post-infarcted heart stabilizes the necrotic region by a variety of means, including changes in tissue form, cellular demographics, and alterations in the ECM network in a process collectively referred to as scarring (Sun and Weber, 2000). The scarring process is a complex result of the adaptive and maladaptive actions of a heterogeneous cell population within the heart. Scarring is often considered an obstacle to action potential conduction and facilitates reentry that leads to electrical dyssynchrony with the scar or border zone as the foci of arrhythmogenesis. However, other concurrent and coupled processes may be contributors to post-infarction arrhythmias. ECM protein deposition, predominantly of collagen type III along with crosslinking of collagen type I fibers confers added strength to the scar (Cleutjens et al., 1999; Smith-Mungo and Kagan, 1998). The fibronectin content of the ECM also increases, as it is deposited and integrated into the network structure (Knowlton et al., 1992; Ratajska and Campbell, 1995). Concurrent changes in cellular integrin expression mirror changes in the ECM network architecture, as the expression levels of α5, β1, and β3 integrins in both peri-infarcted and noninfarcted regions increase (Bouzeghrane et al., 2004; Nawata et al., 1999; Sun et al., 2003). Furthermore, targeted excision of the β1 integrin gene disrupted myocyte membrane integrity and caused postnatal cardiac fibrosis (Shai et al., 2002). In addition, it reduced integrin-linked kinase activity during control conditions and under hemodynamic stress (Li et al., 2012). Thus, in this case, the way myocytes sense mechanical forces and the networks that condition, filter, and propagate these forces in the extracellular space are changing concurrently, but not necessarily coupled in an adaptive fashion.
The microscopic remodeling of the myocardium following infarction can be measured functionally in the heart’s electrical properties and help predict fatal events, which include arrhythmias and SCD (Davies and Thomas, 1984; de Luna et al., 1989; Naghavi et al., 2003). For instance, altered integrin expression itself may affect cardiac rhythm since the over-expression of an active form of α5 integrin in mice is arrhythmogenic (Valencik and McDonald, 2001). Moreover, reports detailing in vivo electroanatomic mapping of the heart, via contrast-enhanced cardiac MRI combined with electrogram mapping of cardiac electrical properties, suggest correlative changes in the electrophysiology of scarred hearts, including increased propensity for ventricular tachycardia following electrical stimulus (Nakahara et al., 2011; Schmidt et al., 2007). In the absence of post-infarction scarring, reports have revealed a lower propensity for arrhythmia, as suggested by the QRS complex score (Strauss et al., 2011; Strauss et al., 2008). The aggregate of clinical observations of the incidence of arrhythmia and post-mortem evaluation of fibrosis in HCM or fibrotic scar formation following myocardial infarction suggest that architectural and micromechanical signaling within the cardiac microenvironment may be a potential contributor to cardiac dysrhythmia. Although causality between integrin signaling and arrhythmogenesis has not been reported, alterations in integrin and ECM expression in the infarct scar, and the associated increase in electrical disturbances in scarred regions, suggest a heretofore unexplored relationship.
4. Integrins, ECM, and the Costamere
Cardiomyocytes couple each other axially via adherens junctions (Hoshijima, 2006). Myocytes are also coupled via integrin attachment to the ECM. Contracting cardiac muscle cells use integrin attachments to pull against the ECM as they shorten during systole and during diastole, recoil is facilitated by the elasticity of the ECM network. This connectivity is mediated by a protein ensemble, the costamere, which anchors sarcomeric z-disks to the ECM via the integrin cluster (reviewed in (Samarel, 2005)) (see Fig. 1). In vitro studies of myocytes adhered to flexible substrata revealed contraction-induced wrinkling patterns on flexible substrates with spacing that registered with intracellular z-discs (Danowski et al., 1992). These integrin-based adhesion sites contain proteins commonly associated with focal adhesions in non-myocytes, such as vinculin (Terracio et al., 1990), and depend on integrin signaling for proper function (Li et al., 2012; Sharp et al., 1997; Wu et al., 2010). Given their role in the costamere, integrins have been the target of several studies of structural remodeling of the heart during development and disease (reviewed in (Ross and Borg, 2001)). Integrins can effect responses through a variety of downstream signaling pathways, including focal adhesion kinase (FAK), integrin-linked kinase (ILK), Src kinase, tyrosine phosphatase, and small GTPases such as Rho (Tirziu et al., 2010). The triggering of these downstream effectors is mediated by mechanically-induced conformational changes in the integrin proteins which change the binding affinity of associated focal adhesion proteins on the cytoplasmic side of the cell membrane (Askari, et al., 2009; Campbell and Humphries, 2011).
Direct evidence of the role of integrin mediated modulation of ion channel activity in cardiac myocytes is reported by several investigators. A common technique to investigate integrin signaling is to use micron-scale beads coated with antibodies against the integrin of interest, or extracellular matrix proteins. Glass beads are often used to examine integrin binding, but in mechanotransduction experiments, paramagnetic beads and a magnetic tweezer (Hemphill, et al., 2011), or magnetic twisting device (Tagawa, et al., 1997), are used to induce mechanical displacement of the bead in the transiently applied magnetic field. Mechanical displacement of β1 integrin bound paramagnetic beads in isolated rabbit ventricular myocytes during patch clamp recording increased outward rectifying chloride current (Browe and Baumgarten, 2003). The relationship between calcium signaling and integrin binding was explored in a study where collagen I-coated 10 μm glass spheres bound to the apical surface of isolated neonatal rat ventricular myocytes slowed calcium transient decay relative to controls (Barac et al., 2008). Direct mechanical stretch of single cardiomyocytes with a glass stylus during simultaneous patch clamp recording, resulting in transient, measureable sarcomere elongation, highlight the technical difficulty of integrin MEC signaling measurements, while demonstrating the stretch-sensitivity of non-selective cation currents, TRPC6, and inwardly rectifying K+-selective currents, IK1 (Dyachenko et al., 2008). Increases in these currents during stretch protocols suggests two more stretch-sensitive ion channels amongst a growing list of channels with kinetics that are modulated by a variety of mechano-control mechanisms.
By what mechanisms might integrins modulate contraction? In addition to the aforementioned studies of integrin-mediated modulation of ion channel currents, other studies suggest that integrin activation in cardiomyocytes modulated ionic currents via downstream effects on the Angiotensin II Type 1 receptor (Browe and Baumgarten, 2004; Dyachenko et al., 2009). Furthermore, addition of the integrin ligand, Arginine-Glycine-Aspartic acid (RGD), to neonatal rat cardiomyocytes activated integrins and induced NO-mediated Ca2+ release from the RyR2 in the sarcoplasmic reticulum (van der Wees et al., 2006). Transgenic mice models that conditionally overexpressed α5 integrins displayed muted QRS complex and reduced tissue conduction anisotropy, suggesting effects on cell-cell coupling, presumably due to altered effects on gap junction coupling (Valencik et al., 2006). These studies suggest a series of pathways and secondary messengers that transduce integrin signaling to ion channel kinetics on the cell membrane and in the sarcoplasmic reticulum. The temporal dynamics of these studies suggest that integrin signaling may offer real-time control of beat to beat excitation thresholds, ionic currents and action potential morphology, and contraction dynamics.
5. Integrin signaling in ionic homeostasis in nonmyocytes
Integrin-mediated effects on ion channel activity have been noted in several other cell types, suggesting a biologically conserved mechanism for integrin-mediated MEC signaling. The role of integrins in cellular mechanotransduction in mammalian cells has been studied extensively in noncardiac cells (Alenghat and Ingber, 2002; Parker et al., 2002; Wang et al., 1993). In neurons, activation of α5β1 integrin raised intracellular calcium concentration, an important component of synapse maturation and long-term memory potentiation (Lin et al., 2008). Integrin dependent ion channel activity is also implicated in c-Src phosphorylation-mediated modulation of calcium channel activity in vascular smooth muscle cells (Gui et al., 2006; Yang et al., 2010). In addition to mediating influx of extracellular Ca2+, intracellular calcium release, via ryanodine receptors, may also be regulated by α5β1 activation, as evidenced by data from pulmonary arterial smooth muscle cells (Umesh et al., 2006). Calcium influx via the Transient Receptor Potential Vanilloid-4 (TRPV4) channel is also initiated by forces applied to β1 integrins in bovine capillary endothelial cells (Matthews et al., 2010). Meanwhile, treatment of rat forebrain neurons and cremastor muscle arteriole smooth muscle cells with anti α5β1 antibody-coated beads resulted in increased L-type calcium channel current, which was inhibited by FAK and c-Src inhibitors (Gui et al., 2006). The same current was reported to be inhibited if ανβ3 specific ligands were used (Wu et al., 1998). In addition to modulating Ca2+ currents, integrins may affect K+ activity. In bovine pulmonary artery endothelial cells, treatment of cells with soluble vitronectin was shown to induce K+ currents via ανβ3 integrin activation. However, ανβ3 activation regulates Ca2+-dependent K+ current in bovine capillary endothelial cells (Kawasaki et al., 2004). The importance of integrin subtype specificity on ion channel conductance as a function of cell type suggests a robust system of ionic regulation via integrins. These effects may be rapid as well, as evidenced by the activation of voltage-gated Ca2+ channels in neurons that occur on the order of minutes (Wildering et al., 2002). This is not surprising, since these integrin-mediated pathways have been shown to involve the phosphorylation cascades associated with FAK or Src kinases (Wu et al., 2001). Integrin-mediated ion channel effects can be found in additional cell types, ranging from osteoclasts, where activation of β1 and β3 integrins upregulated cytosolic Ca2+ (Chenu et al., 1994; Tanabe et al., 2011), to monocytes, where K+ currents are modulated by VLA-4 (α4β1) integrins (Colden-Stanfield, 2002; Colden-Stanfield and Scanlon, 2000). Thus, a wide variety of integrin-mediated ion channel effects have been described in multiple cell types, suggesting that they may also play a role in cardiomyocyte electrical homeostasis.
6. Mechanoelectrical coupling in maladaptive remodeling of the heart?
Integrin signaling may contribute to the propensity of heart failure patients to suffer from arrhythmias (Fig. 2). Patients diagnosed with congestive heart failure exhibit high rates of ventricular arrhythmia and SCD (Gradman et al., 1989; Holmes et al., 1985). Half of patients presenting with left ventricular hypertrophy are afflicted with premature ventricular complexes (Ghali et al., 1991). Heart disease patients often present with ventricular tachycardia that progresses to ventricular fibrillation in the setting of myocardial infarction (MI) (Bayes de Luna et al., 1989; Huikuri et al., 2001; Mehta et al., 1997; Wit and Janse, 1992). These diseases are characterized by macroscopically remodeled myocardium, typified by increased left ventricular mass due to molecular changes in both the extracellular matrix composition and cellular changes, including altered fiber orientation (reviewed in (Chien et al., 2008; Zipes and Wellens, 1998)). Concurrent with these gross morphological changes, and perhaps a contributor, are changes in integrin expression profiles and fibrosis (Bujak and Frangogiannis, 2007; Dullens et al., 2011; Sheehy et al., 2009). The summary of these observations suggest integrin mediated MEC signaling as a possible mechanism that couples the anatomical changes in the heart with the increased likelihood of cardiac arrhythmia and decreased cardiac output.
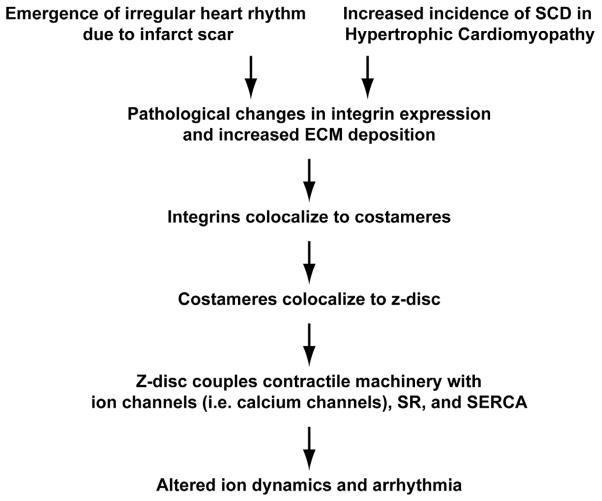
Figure 2.
Progression of the integrin-mediated arrhythmia hypothesis. Arrhythmias following scarring due to cardiac ischemia and the concomitant increase in sudden cardiac death (SCD) in hypertrophic cardiomyopathy (HCM) are due to changes in integrin expression. Given the role of integrins in mediating z-disc mechanical continuity with the ECM as part of the costamere, changes in their expression can affect a host of other processes associated with this structure, including ion homeostasis.
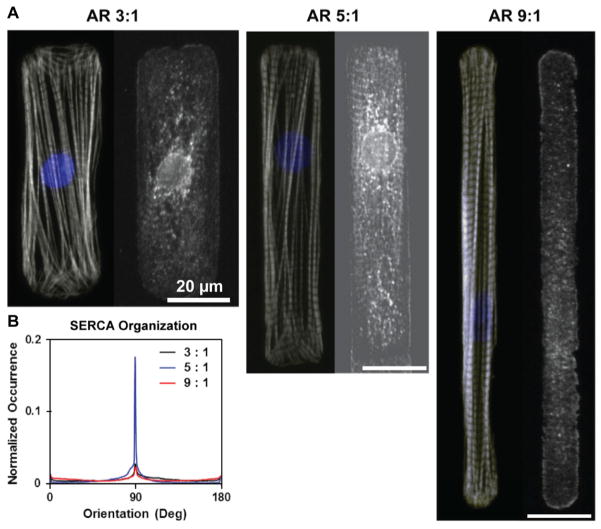
How might changes in integrin binding to ECM at the costamere affect ionic currents during the cardiac cycle? Pathological cardiac tissue remodeling is manifested microscopically by measurable disturbances in cell shape and excess ECM deposition. Increased deposition of fibrillar ECM proteins, including collagen, is an important part of the pathological hypertrophic response (Weber and Brilla, 1991). In vitro studies by our laboratory have demonstrated how cardiac myocytes remodel their shape and myofibrils with respect to geometric cues in the ECM (Bray et al., 2010; Bray et al., 2008; Geisse et al., 2009; Parker et al., 2008). Within the sarcomere, the z-disc spatially organizes signaling proteins and organelles that temporally coordinate the contractile cycle within the myocyte (Frank and Frey, 2011). Disruption of the organization of this multiplexer may be one potential contributor to pathological MEC feedback in the diseased heart. Modulation of cell shape by integrin attachment to the extracellular matrix illustrates how integrin binding of the ECM and myocyte shape may play an important role in the spatiotemporal organization of ionic signaling in the myocytes. Culturing neonatal rat myocytes on micropatterned islands results in myocytes whose shape reflects the island shape and induces the shape-sensitive alignment of the myofibrillar array (Bray, et al., 2008; Grosberg, et al., 2011). This is apparent when myocytes are cultured on rectangular islands of fibronectin with uniform surface area, but changing aspect ratio (length:width) (Fig. 3A). Immunostaining of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) indicated that the sarcoplasmic reticulum had the highest propensity for colocalization at the sarcomere z-line in myocytes with a 5:1 aspect ratio. In myocytes engineered to resemble the length and width dimensions of a hypertrophic myocyte (3:1 aspect ratio), some colocalization is observed amidst diffuse staining. A similar observation was made in myocytes approaching the dimensions of those typically observed in dilated cardiomyopathy in vivo (9:1 aspect ratio) (Gerdes and Capasso, 1995). The overall alignment of SERCA was quantitatively captured using methods utilized in (Grosberg et al., 2011) and indicated a higher degree of SERCA organization in the 5:1 aspect ratio myocyte (Fig. 3B). Thus, integrin attachment to the extracellular matrix mediates cytoskeletal architecture and may influence the spatial organization of Ca2+ stores within the sarcoplasmic reticulum and Ca2+ signaling. Our group has previously reported a supportive finding, where myocytes attached by integrin connection to micropatterned fibronectin organized into 2D tissues, self-assembled their contractile apparatuses with respect to the cellular alignment, and displayed Ca2+ transients that increased with myofibrillar alignment (Pong, et al., 2011). The aggregate of these reports suggest that integrin mediated cell shape and architecture regulate ionic signaling in cardiac myocytes.
Figure 3.
Cell shape effects on Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) organization. (A) SERCA patterning for different cell aspect ratios (AR) (right panels) along with actin (gray) and overlaid DAPI (blue) (left panels). Hypertrophied myocytes (AR 3:1) and elongated myocytes (AR 9:1) contained diffuse SERCA staining while healthy myocytes (AR 5:1) revealed a distinct SERCA pattern that aligned with z-discs. (B) Quantification of SERCA alignment as a function of cell aspect ratio.
In addition to changes in cell shape in the failing heart, ECM deposition during scarring or cardiac hypertrophy affects the orientation and shape of myocytes within the laminar muscle of the ventricles. These changes, as indicated by Diffuse Tensor MRI imaging in infarcted rat hearts (Chen et al., 2003), suggest that mechanical synchrony is important for heart function, and its dysfunction during heart disease may be arrhythmogenic (Choi et al., 2003). Despite the study of organ-level cardiac dysfunction in the setting of scarring, a thorough understanding of the undermined mechanical relationship (via the ECM) between individual cardiomyocytes is lacking. The mechanical influence of contractile cells on one another has been examined in C2C12 skeletal myoblasts cultured on polyacrylamide (PA) gels, where myoblasts coaligned due to traction forces conveyed through the substrate between 5 and 10 cell widths apart (Engler et al., 2004). Moreover, the theoretical basis of this observation has been stipulated by work which suggests that mechanical interactions via the ECM are a function of intercellular distance and cellular orientation (Schwarz and Sanfran, 2002; Bischofs and Schwarz, 2003; Bischofs, et al., 2004). Given these findings and the noted changes in diseased hearts, we hypothesize that the ability of cardiomyocytes to mechanically ‘feel’ one another is an important component of contraction synchrony in the heart and is disrupted in the setting of heart disease. For instance, study of chick embryonic cardiomyocytes has revealed that only 17% of cardiomyocytes up to 10μm apart on 47 kPa PA gels beat together while 70% of cardiomyocytes within the same range beat together on softer 1 kPa PA gels (Tang et al., 2011). Myocyte pairs cultured on the stiffer gel simulate pathological increases in ECM stiffness, which stymies the ability of neighboring myocytes to mechanically regulate one another. While these studies implicate a role for mechanical signaling via the ECM in synchronous myocyte contraction, further studies that elucidate the specific mechanisms that transduce these mechanical signals are required. While stretch activated channels are an apparent candidate, the aforementioned mechanical sensors present at the z-disc may also play an important role. These structures provide mechanical continuity between the substrate and the sarcomere and allow extracellular mechanical stimuli to gain deep intracellular access. Such stimuli may also elicit strain dependent responses such as the Frank-Starling relationship, which regulates strength of contraction, and whose molecular mechanism remains to be fully described (reviewed in (Campbell, 2011)). By utilizing controlled in vitro techniques (i.e. microcontact printing) cardiomyocyte orientation and intercellular distance are controlled and specific parameters that affect cardiomyocyte beating synchrony and strength of contraction can be readily determined.
7. Conclusion
Cardiac remodeling following an infarct or as a result of Hypertrophic Cardiomyopathy leads to pathological alterations in myocardium tissue architecture. The gross changes, which comprise primarily of increased ECM deposition that adversely affects tissue mechanical properties, also correspond with shifts in myocyte integrin expression. As proteins that mechanically link the ECM to the intracellular cytoskeleton, integrins play an important role in transducing mechanical stimuli into chemical cues. As such, integrins play a central role in mediating the response of surviving myocytes to changes in tissue properties of the diseased heart. Given the high rate of arrhythmias and SCD in the setting of pathological cardiac remodeling, modified integrin expression must be considered as a mechanism that underlies pathological MEC in the context of the diseased heart.
The highly organized structure of the myocyte’s contractile machinery at the costamere directly couples prominent organelles to the ECM via integrins. For example, interactions between many myosin and actin filaments are bounded by the z-disc that in turn terminates at the costamere. Other constituents of this region that demarcate z-disc interactions with the costamere include ion channels, the SR, and SERCA. This spatial organization establishes linkages across multiple spatial scales, ranging from gross ECM to integrins, which ultimately transmit mechanical stimuli directly to the costamere and its constituents. These interactions can subsequently alter local ion channel dynamics, leading to electrical instabilities that may prove to be arrhythmogenic (see Fig. 2). Thus the intimate relationship between myocyte mechanical sensors and ion metabolism regulators underscores the possibility of integrin-mediated MEC.
Isolating the specific contributions of integrin signaling to MEC poses various experimental challenges. While in vivo models have proved to be important in elucidating the contributions of stretch activated channels to MEC, they are limited in isolating effects of integrins alone. Moreover, animal integrin knockout models are challenging given the importance of integrin signaling during heart development not to mention that applying specific localized mechanical stimulus to integrin proteins in vivo is also a daunting task. While in vitro systems lack the three dimensional structure granted in in vivo models, they provide a mechanism by which investigators can probe specific integrin signaling cascades at the cellular level while controlling for other mediators of MEC. Although in vitro experiments provide an avenue for assessing integrin-mediated MEC, they present their own set of challenges. Successful isolation, culture, and control of spatial organization of primary harvest cardiomyocytes add layers of complexity to already challenging experiments. In addition, study of electrical dynamics of cultured cardiac tissues in vitro may be marred by tissue imperfections that are due to unnatural culture conditions. However, important mechanistic details underlying integrin-mediated MEC can still be gleaned in vitro and may ultimately be applied to in vivo models. In conclusion, this review argues for the role of integrin signaling in MEC coupling in the heart and suggests a hypothesis that does not exclude the role of stretch-activated channels, but rather offers an alternative view of a signaling pathway with broader regulatory control over cellular function than the ion channel itself.
Acknowledgments
We would like to acknowledge comments by Ashutosh Agarwal, Francesco S. Pasqualini, and Sean Sheehy of the Disease Biophysics Group on the manuscript. This work was supported by NIH R01 HL079126.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002:1–4. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–70. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barac D, Reisner Y, Silberman M, Zeevi-Levin N, Danon A, Salomon O, Shoham M, Shilkrut M, Kostin S, Schaper J. Mechanical load induced by glass microspheres releases angiogenic factors from neonatal rat ventricular myocytes cultures and causes arrhythmias. J Cell Mol Med. 2008;12:2037–2051. doi: 10.1111/j.1582-4934.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–9. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- Bischofs IB, Safran SA, Schwarz US. Elastic interactions of active cells with soft materials. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:021911–17. doi: 10.1103/PhysRevE.69.021911. [DOI] [PubMed] [Google Scholar]
- Bischofs IB, Schwarz US. Cell organization in soft media due to active mechanosensing. Proc Natl Acad Sci U S A. 2003;100:9274–9. doi: 10.1073/pnas.1233544100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg TK, Goldsmith EC, Price R, Carver W, Terracio L, Samarel AM. Specialization at the Z line of cardiac myocytes. Cardiovasc Res. 2000;46:277–285. doi: 10.1016/s0008-6363(99)00433-2. [DOI] [PubMed] [Google Scholar]
- Bouzeghrane F, Mercure C, Reudelhuber T, Thibault G. [alpha] 8 [beta] 1 integrin is upregulated in myofibroblasts of fibrotic and scarring myocardium. J Mol Cell Cardiol. 2004;36:343–353. doi: 10.1016/j.yjmcc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Bray MA, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials. 2010;31:5143–50. doi: 10.1016/j.biomaterials.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65:641–651. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe DM, Baumgarten CM. Stretch of β1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe DM, Baumgarten CM. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl− current elicited by β1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol. 2004;124:273–287. doi: 10.1085/jgp.200409040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KS. Impact of myocyte strain on cardiac myofilament activation. Pflugers Arch. 2011;462:3–14. doi: 10.1007/s00424-011-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Song SK, Liu W, McLean M, Allen JS, Tan J, Wickline SA, Yu X. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am J Physiol Heart Circ Physiol. 2003;285:H946–54. doi: 10.1152/ajpheart.00889.2002. [DOI] [PubMed] [Google Scholar]
- Chenu C, Colucci S, Grano M, Zigrino P, Barattolo R, Zambonin G, Baldini N, Vergnaud P, Delmas P, Zallone A. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994;127:1149–1158. doi: 10.1083/jcb.127.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–7. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- Choi BR, Liu T, Lavasani M, Salama G. Fiber orientation and cell-cell coupling influence ventricular fibrillation dynamics. J Cardiovasc Electrophysiol. 2003;14:851–860. doi: 10.1046/j.1540-8167.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- Cleutjens JP, Blankesteijn WM, Daemen MJ, Smits JF. The infarcted myocardium: simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc Res. 1999;44:232–41. doi: 10.1016/s0008-6363(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M. Clustering of very late antigen-4 integrins modulates K+ currents to alter Ca2+-mediated monocyte function. Am J Physiol Cell Physiol. 2002;283:C990–C1000. doi: 10.1152/ajpcell.00481.2001. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M, Scanlon M. VCAM-1-induced inwardly rectifying K+ current enhances Ca2+ entry in human THP-1 monocytes. Am J Physiol Cell Physiol. 2000;279:C488–C494. doi: 10.1152/ajpcell.2000.279.2.C488. [DOI] [PubMed] [Google Scholar]
- Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–20. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- de Luna AB, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117:151–9. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- Ding B, Price RL, Goldsmith EC, Borg TK, Yan X, Douglas PS, Weinberg EO, Bartunek J, Thielen T, Didenko VV, Lorell BH. Left ventricular hypertrophy in ascending aortic stenosis mice: anoikis and the progression to early failure. Circulation. 2000;101:2854–62. doi: 10.1161/01.cir.101.24.2854. [DOI] [PubMed] [Google Scholar]
- Dullens HF, Schipper ME, van Kuik J, Sohns W, Scheenstra M, van Wichen DF, Van Oosterhout MF, de Jonge N, de Weger RA. Integrin expression during reverse remodeling in the myocardium of heart failure patients. Cardiovasc Pathol. 2011 doi: 10.1016/j.carpath.2011.09.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dyachenko V, Christ A, Gubanov R, Isenberg G. Bending of z-lines by mechanical stimuli: an input signal for integrin dependent modulation of ion channels? Prog Biophys Mol Biol. 2008;97:196–216. doi: 10.1016/j.pbiomolbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Dyachenko V, Rueckschloss U, Isenberg G. Modulation of cardiac mechanosensitive ion channels involves superoxide, nitric oxide and peroxynitrite. Cell Calcium. 2009;45:55–64. doi: 10.1016/j.ceca.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;33:1596–1601. doi: 10.1016/s0735-1097(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SM, Butany J, Sole MJ, Wigle ED, Williams WC, Rojkind M. Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1991;17:1343–51. doi: 10.1016/s0735-1097(10)80145-7. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ, Morrow AG, Roberts WC. Myocardial ultrastructure in idiopathic hypertrophic subaortic stenosis. A study of operatively excised left ventricular outflow tract muscle in 14 patients. Circulation. 1972;45:769–92. doi: 10.1161/01.cir.45.4.769. [DOI] [PubMed] [Google Scholar]
- Frank D, Frey N. Cardiac Z-disc signaling network. J Biol Chem. 2011;286:9897–904. doi: 10.1074/jbc.R110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol. 2011;2:91–100. doi: 10.1038/nrcardio.2011.159. [DOI] [PubMed] [Google Scholar]
- Geisse NA, Sheehy SP, Parker KK. Control of myocyte remodeling in vitro with engineered substrates. In Vitro Cell Dev Biol Anim. 2009;45:343–350. doi: 10.1007/s11626-009-9182-9. [DOI] [PubMed] [Google Scholar]
- Gerdes AM, Capasso JM. Structural remodeling and mechanical dysfunction of cardiac myocytes in heart failure. J Mol Cell Cardiol. 1995;27:849–56. doi: 10.1016/0022-2828(95)90000-4. [DOI] [PubMed] [Google Scholar]
- Ghali JK, Kadakia S, Cooper RS, Liao Y. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17:1277–1282. doi: 10.1016/s0735-1097(10)80135-4. [DOI] [PubMed] [Google Scholar]
- Gradman A, Deedwania P, Cody R, Massie B, Packer M, Pitt B, Goldstein S. Predictors of total mortality and sudden death in mild to moderate heart failure. J Am Coll Cardiol. 1989;14:564–570. doi: 10.1016/0735-1097(89)90093-4. [DOI] [PubMed] [Google Scholar]
- Grosberg A, Kuo PL, Guo CL, Geisse NA, Bray MA, Adams WJ, Sheehy SP, Parker KK. Self-organization of muscle cell structure and function. PLoS Comput Biol. 2011;7:e1001088. doi: 10.1371/journal.pcbi.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1. 2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–25. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- Hansen DE, Borganelli M, Stacy G, Taylor LK. Dose-dependent inhibition of stretch-induced arrhythmias by gadolinium in isolated canine ventricles. Evidence for a unique mode of antiarrhythmic action. Circ Res. 1991;69:820–831. doi: 10.1161/01.res.69.3.820. [DOI] [PubMed] [Google Scholar]
- Hauselmann SP, Rosc-Schluter BI, Lorenz V, Plaisance I, Brink M, Pfister O, Kuster GM. beta1-Integrin is up-regulated via Rac1-dependent reactive oxygen species as part of the hypertrophic cardiomyocyte response. Free Radic Biol Med. 2011;51:609–18. doi: 10.1016/j.freeradbiomed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Hemphill MA, Dabiri BE, Gabriele S, Kerscher L, Franck C, Goss JA, Alford PW, Parker KK. A possible role for integrin signaling in diffuse axonal injury. PLoS One. 2011;6:e22899. doi: 10.1371/journal.pone.0022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JR, Kubo SH, Cody RJ, Kligfield P. Milrinone in congestive heart failure: observations on ambulatory ventricular arrhythmias. Am Heart J. 1985;110:800–806. doi: 10.1016/0002-8703(85)90460-0. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–25. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Kamkin A, Kiseleva I, Lozinsky I, Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005;100:337–45. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- Kawasaki J, Davis GE, Davis MJ. Regulation of Ca2+-dependent K+ current by αvβ3 integrin engagement in vascular endothelium. J Biol Chem. 2004;279:12959–66. doi: 10.1074/jbc.M313791200. [DOI] [PubMed] [Google Scholar]
- Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26:1699–708. doi: 10.1016/0735-1097(95)00390-8. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest. 1992;89:1060–8. doi: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wu Y, Manso AM, Gu Y, Liao P, Israeli S, Yajima T, Nguyen U, Huang MS, Dalton ND, Peterson KL, Ross RS. beta1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses. Am J Pathol. 2012;180:952–62. doi: 10.1016/j.ajpath.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Hilgenberg L, Smith M, Lynch G, Gall C. Integrin regulation of cytoplasmic calcium in excitatory neurons depends upon glutamate receptors and release from intracellular stores. Mol Cell Neurosci. 2008;37:770–780. doi: 10.1016/j.mcn.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie EK, Maron BJ. Apical hypertrophic cardiomyopathy: clinical and two-dimensional echocardiographic assessment. Ann Intern Med. 1987;106:663–70. doi: 10.7326/0003-4819-106-5-663. [DOI] [PubMed] [Google Scholar]
- Madias C, Maron BJ, Supron S, Estes N, III, Link MS. Cell Membrane Stretch and Chest Blow-Induced Ventricular Fibrillation: Commotio Cordis. J Cardiovasc Electrophysiol. 2008;19:1304–1309. doi: 10.1111/j.1540-8167.2008.01267.x. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Hypertrophic cardiomyopathy. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Anan TJ, Roberts WC. Quantitative analysis of the distribution of cardiac muscle cell disorganization in the left ventricular wall of patients with hypertrophic cardiomyopathy. Circulation. 1981;63:882–94. doi: 10.1161/01.cir.63.4.882. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Poliac LC, Kaplan JA, Mueller FO. Blunt impact to the chest leading to sudden death from cardiac arrest during sports activities. N Engl J Med. 1995;333:337–42. doi: 10.1056/NEJM199508103330602. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Sato N, Roberts WC, Edwards JE, Chandra RS. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum. Comparison of fetuses and infants with and without congenital heart disease and patients with hypertrophic cardiomyopathy. Circulation. 1979;60:685–96. doi: 10.1161/01.cir.60.3.685. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–12. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999;85:1046–55. doi: 10.1161/01.res.85.11.1046. [DOI] [PubMed] [Google Scholar]
- Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface β1 integrins. Integr Biol. 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Curwin J, Gomes JA, Fuster V. Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–23. doi: 10.1161/01.cir.96.9.3215. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KEJ, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient - A call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Vaseghi M, Ramirez RJ, Fonseca CG, Lai CK, Finn JP, Mahajan A, Boyle NG, Shivkumar K. Characterization of Myocardial Scars: Electrophysiological-Imaging Correlates in a Porcine Infarct Model. Heart Rhythm. 2011;7:1060–7. doi: 10.1016/j.hrthm.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Nawata J, Ohno I, Isoyama S, Suzuki J, Miura S, Ikeda J, Shirato K. Differential expression of α1, α3 and α5 integrin subunits in acute and chronic stages of myocardial infarction in rats. Cardiovasc Res. 1999;43:371–81. doi: 10.1016/s0008-6363(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Nicod P, Polikar R, Peterson KL. Hypertrophic cardiomyopathy and sudden death. N Engl J Med. 1988;318:1255–7. doi: 10.1056/NEJM198805123181907. [DOI] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16:1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1267–79. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103:340–2. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KK, Taylor LK, Atkinson B, Hansen DE, Wikswo JP. The effects of tubulin-binding agents on stretch-induced ventricular arrhythmias. Eur J Pharmacol. 2001;417:131–40. doi: 10.1016/s0014-2999(01)00856-1. [DOI] [PubMed] [Google Scholar]
- Pong T, Adams WJ, Bray MA, Feinberg AW, Sheehy SP, Werdich AA, Parker KK. Hierarchical architecture influences calcium dynamics in engineered cardiac muscle. Exp Biol Med (Maywood) 2011;236:366–73. doi: 10.1258/ebm.2010.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajska A, Campbell SE. Fibronectin accumulation within cardiac myocytes in rats with elevated plasma angiotensin II. Cardiovasc Pathol. 1995;4:57–67. doi: 10.1016/1054-8807(94)00042-p. [DOI] [PubMed] [Google Scholar]
- Riemer TL, Tung L. Stretch-induced excitation and action potential changes of single cardiac cells. Prog Biophys Mol Biol. 2003;82:97–110. doi: 10.1016/s0079-6107(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–9. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marbán E, Tomaselli GF. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz US, Safran SA. Elastic interactions of cells. Phys Rev Lett. 2002;88:048102. doi: 10.1103/PhysRevLett.88.048102. [DOI] [PubMed] [Google Scholar]
- Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–64. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- Shapiro LM, McKenna WJ. Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: a two-dimensional echocardiographic study. J Am Coll Cardiol. 1983;2:437–44. doi: 10.1016/s0735-1097(83)80269-1. [DOI] [PubMed] [Google Scholar]
- Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol Cell Physiol. 1997;273:H546–56. doi: 10.1152/ajpheart.1997.273.2.H546. [DOI] [PubMed] [Google Scholar]
- Sheehy SP, Huang S, Parker KK. Time-warped comparison of gene expression in adaptive and maladaptive cardiac hypertrophy. Circ Cardiovasc Genet. 2009;2:116–24. doi: 10.1161/CIRCGENETICS.108.806935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/s0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- Silka MJ, Kron J, Dunnigan A, Dick M. Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. Circulation. 1993;87:800–7. doi: 10.1161/01.cir.87.3.800. [DOI] [PubMed] [Google Scholar]
- Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–98. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- Spirito P, Bellone P, Harris KM, Bernabò P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–85. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- Spirito P, Maron BJ, Bonow RO, Epstein SE. Severe functional limitation in patients with hypertrophic cardiomyopathy and only mild localized left ventricular hypertrophy. J Am Coll Cardiol. 1986;8:537–44. doi: 10.1016/s0735-1097(86)80180-2. [DOI] [PubMed] [Google Scholar]
- St John Sutton MG, Lie JT, Anderson KR, O’Brien PC, Frye RL. Histopathological specificity of hypertrophic obstructive cardiomyopathy. Myocardial fibre disarray and myocardial fibrosis. Br Heart J. 1980;44:433–43. doi: 10.1136/hrt.44.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy GP, Jr, Jobe R, Taylor LK, Hansen DE. Stretch-induced depolarizations as a trigger of arrhythmias in isolated canine left ventricles. Am J Physiol. 1992;263:H613–21. doi: 10.1152/ajpheart.1992.263.2.H613. [DOI] [PubMed] [Google Scholar]
- Strauss DG, Poole JE, Wagner GS, Selvester RH, Miller JM, Anderson J, Johnson G, McNulty SE, Mark DB, Lee KL. An ECG index of myocardial scar enhances prediction of defibrillator shocks: An analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2011;8:38–45. doi: 10.1016/j.hrthm.2010.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss DG, Selvester RH, Lima JAC, Arheden H, Miller JM, Gerstenblith G, Marbán E, Weiss RG, Tomaselli GF, Wagner GS. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–36. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Opavsky MA, Stewart DJ, Rabinovitch M, Dawood F, Wen WH, Liu PP. Temporal Response and Localization of Integrins β1 and β3 in the Heart After Myocardial Infarction. Circulation. 2003;107:1046–52. doi: 10.1161/01.cir.0000051363.86009.3c. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–6. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G., 4th Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res. 1997;80:281–9. doi: 10.1161/01.res.80.2.281. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Wheal BD, Kwon J, Chen HH, Shugg RP, Sims SM, Goldberg HA, Dixon SJ. Osteopontin signals through Calcium-NFAT in osteoclasts: A novel RGD-dependent pathway promoting cell survival. J Biol Chem. 2011;46:39871–81. doi: 10.1074/jbc.M111.295048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Bajaj P, Bashir R, Saif TA. How far cardiac cells can see each other mechanically. Soft Matter. 2011;7:6151–8. [Google Scholar]
- Tavi P, Han C, Weckström M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: role of increased troponin C affinity and stretch-activated ion channels. Circ Res. 1998;83:1165–77. doi: 10.1161/01.res.83.11.1165. [DOI] [PubMed] [Google Scholar]
- Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958;20:1–8. doi: 10.1136/hrt.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg T. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res. 1991;68:734–44. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- Terracio L, Simpson DG, Hilenski L, Carver W, Decker RS, Vinson N, Borg TK. Distribution of vinculin in the Z-disk of striated muscle: Analysis by laser scanning confocal microscopy. J Cell Physiol. 1990;145:78–87. doi: 10.1002/jcp.1041450112. [DOI] [PubMed] [Google Scholar]
- Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–37. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh A, Thompson MA, Chini EN, Yip KP, Sham JSK. Integrin ligands mobilize Ca2+ from ryanodine receptor-gated stores and lysosome-related acidic organelles in pulmonary arterial smooth muscle cells. J Biol Chem. 2006;281:34312–23. doi: 10.1074/jbc.M606765200. [DOI] [PubMed] [Google Scholar]
- Valencik ML, McDonald JA. Cardiac expression of a gain-of-function α5-integrin results in perinatal lethality. Am J Physiol Heart Circ Physiol. 2001;280:H361–7. doi: 10.1152/ajpheart.2001.280.1.H361. [DOI] [PubMed] [Google Scholar]
- Valencik ML, Zhang D, Punske B, Hu P, McDonald JA, Litwin SE. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ Res. 2006;99:1403–10. doi: 10.1161/01.RES.0000252291.88540.ac. [DOI] [PubMed] [Google Scholar]
- van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2009;7:30–7. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- van der Wees CG, Bax WH, van der Valk EJ, van der Laarse A. Integrin stimulation induces calcium signalling in rat cardiomyocytes by a NO-dependent mechanism. Pflugers Arch. 2006;451:588–95. doi: 10.1007/s00424-005-1402-x. [DOI] [PubMed] [Google Scholar]
- Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001a;104:1380–4. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001b;88:275–9. doi: 10.1016/s0002-9149(01)01640-x. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Webb JG, Sasson Z, Rakowski H, Liu P, Douglas Wigle E. Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol. 1990;15:83–90. doi: 10.1016/0735-1097(90)90180-w. [DOI] [PubMed] [Google Scholar]
- Weber K, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–65. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AGM. Rapid neuromodulatory actions of integrin ligands. J Neurosci. 2002;22:2419–26. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit AL, Janse MJ. Experimental models of ventricular tachycardia and fibrillation caused by ischemia and infarction. Circulation. 1992;85:I32–42. [PubMed] [Google Scholar]
- Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–92. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by αvβ3 and α5β1 integrin ligands. J Cell Biol. 1998;143:241–52. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Sun Z, Foskett A, Trzeciakowski JP, Meininger GA, Muthuchamy M. Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions. Am J Physiol Heart Circ Physiol. 2010;298:H2071–81. doi: 10.1152/ajpheart.01156.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu X, Gui P, Wu J, Sheng JZ, Ling S, Braun AP, Davis GE, Davis MJ. α5β1 Integrin Engagement Increases Large Conductance, Ca2+-activated K+ Channel Current and Ca2+ Sensitivity through c-src-mediated Channel Phosphorylation. J Biol Chem. 2010;285:131–41. doi: 10.1074/jbc.M109.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]