Abstract
Background: Chromosome 9p21 variants are among the most robust genetic markers for coronary artery disease (CAD), and previous studies have suggested that genetic effects of this locus might be modified by dietary factors. Intake of sugar-sweetened beverages (SSBs), which are the main dietary source of added sugar, has been shown to interact with genetic factors in affecting CAD risk factors such as obesity.
Objective: We aimed to test whether SSB intake modified the association between chromosome 9p21 variants and CAD risk in Hispanics living in Costa Rica.
Design: The current study included 1560 incident cases of nonfatal myocardial infarction (MI) and 1751 population-based controls. Three independent single nucleotide polymorphisms (SNPs) at the chromosome 9p21 locus were genotyped. SSB intake was assessed with the use of a food-frequency questionnaire and was defined as the frequency of intake of daily servings of sweetened beverages and fruit juice.
Results: We showed a significant interaction between SSB intake and one of the 3 variants (i.e., rs4977574) on MI risk. The per–risk allele OR (95% CI) of rs4977574 for MI was 1.44 (1.19, 1.74) in participants with higher SSB consumption (>2 servings/d), 1.21 (1.00, 1.47) in those with average consumption (1–2 servings/d), and 0.97 (0.81, 1.16) in subjects with lower consumption (<1 serving/d; P-interaction = 0.005). A genetic risk score derived from the sum of risk alleles of the 3 SNPs also showed a significant interaction with SSB intake on MI risk (P-interaction = 0.03).
Conclusion: Our data suggest that unhealthy dietary habits such as higher intake of SSBs could exacerbate the effects of chromosome 9p21 variants on CAD.
Keywords: gene-diet interaction, Hispanics, myocardial infarction, sugar-sweetened beverage, genetics
INTRODUCTION
Coronary artery disease (CAD)5 is one of the largest killers and leading causes of disability worldwide. Chromosome 9p21 represents one of the strongest CAD-associated loci identified to date, and its association with CAD persists in various populations with different ethnicities (1). In Hispanics living in Costa Rica, we have reported that the chromosome 9p21 variants were related to risk of myocardial infarction (MI) (2). The risk alleles of the CAD-associated 9p21 variants are generally common and associated with 20% to 30% increased risk/copy (1). It has been reported that the effect of chromosome 9p21 variants on CAD may be modified by dietary factors such as dietary patterns in multiple ethnic groups including Caucasians, Asians, and Hispanics, and such interactions were replicated in an independent large cohort (3).
Hispanics living in the United States and Latin America are affected by excessive rates of cardiovascular disease risk factors (4); e.g., ∼80% of adult Hispanics in the United States are overweight or obese (5). However, their CAD prevalence (6.1%) (6) appears to be relatively low (7). It has been proposed that the specific genetic makeup of this admixed population and gene-environment interactions may partly explain the paradox (8). Sugar sweetened beverages (SSBs) are the main dietary source of added sugar in Hispanics living in the United States and Latin America. In a previous study, we showed that the consumption of SSBs, which are products that contain added, naturally derived caloric sweeteners such as sucrose (table sugar), high-fructose corn syrup, or fruit-juice concentrates, significantly interacted with genetic variants in affecting obesity, which is a major cardiovascular disease risk factor (9).
Therefore, we hypothesized that habitual SSB consumption might also modify the genetic effects on MI risk. In the current study, we examined whether SSB intake modified the association of the chromosome 9p21 common variants with risk of nonfatal acute MI in Hispanics living in Costa Rica (2).
METHODS
Study population
The Hispanic study population used in the current study has been described in details previously (10, 11). Briefly, the participants, who were from 34 counties in the Central Valley of Costa Rica, covered a full range of socioeconomic levels as well as urban, periurban, and rural lifestyles. Eligible case subjects were adult residents who were diagnosed as survivors of a first acute MI by 2 independent cardiologists at any of the 6 recruiting hospitals in the catchment area between 1994 and 2004. All cases met the WHO criteria for MI, which require typical symptoms plus either elevations in cardiac enzyme concentrations or diagnostic changes in an electrocardiograph. One free-living control subject for each case, who was matched for age (±5 y), sex, and area of residence (county), was randomly selected with the use of information available at the National Census and Statistics Bureau of Costa Rica. Because of the comprehensive social services provided in Costa Rica, all persons had access to medical care without regard to income. Therefore, control subjects came from the source population who gave rise to the cases and were not likely to have had CAD that was not diagnosed because of poor access to medical care. In total, 1560 incident MI cases and 1751 population-based controls with genotyping data, SSB measurements, and covariate information were included in the current study. All subjects gave informed consent on documents approved by the Human Subjects Committee of the Harvard T. H. Chan School of Public Health and the University of Costa Rica.
Assessment of SSB intakes
Dietary intake was assessed with the use of a semiquantitative food-frequency questionnaire (FFQ) that was developed and validated specifically for the Costa Rican population (12–15). The following sweetened-beverage items (serving sizes) were assessed (16): Coke (The Coca-Cola Company), Pepsi (PepsiCo Inc.), and other sodas (1 can; 355 mL); Caffeine-Free Coke, Pepsi and other sodas (1 can; 355 mL); orange juice (1 glass; 237 mL); other fruit juices (1 glass; 237 mL); commercially available sweetened beverages (1 serving; 237 mL); and fresco (1 glass; 237 mL). Fresco is a popular traditional homemade beverage in Latin America that is often made by blending together fresh fruit, sugar, and water. Commercially available and homemade sweetened beverages as well as all sugar-sweetened soda beverages, which mostly consisted of regular Coke, Pepsi, and other colas (84%) were combined into the category SSBs except fruit juice. Natural homemade juices that were squeezed from various fruit [mainly orange juice (76%)] were combined into the category fruit juice. In current study, SSB intake was defined as the sum of the frequencies (daily servings) of consumption of “SSBs except fruit juice” and “fruit juice.”
Assessment of covariates
All study participants were visited in their homes for the collection of data. Information on sociodemographic characteristics, smoking, physical activity, and medical history was collected through an interview with the use of closed-ended questionnaires. All anthropometric measurements were taken on subjects who were wearing light clothing and no shoes, were collected in duplicate, and were averaged out for analyses. Alcohol consumption was assessed with the use of a validated FFQ.
Single nucleotide polymorphism selection and genotype determination
Genomic DNA was extracted from the buffy coat fraction of centrifuged blood with the use of a QIAmp Blood Kit (Qiagen). For the current study, we selected single nucleotide polymorphisms (SNPs) in the chromosome 9p21 region that have previously been associated with coronary artery disease or MI from genome wide association studies (17–21). To increase the a priori likelihood of detecting associations in Costa Ricans, we selected SNPs that were associated with coronary artery disease or MI in ≥2 studies and had r2 values of <0.8 in the white, African, and Asian populations from the HapMap project for each respective locus. As a result, the following 4 chromosome 9p21 SNPs were genotyped: rs10757274, rs4977574, rs2383206, and rs1333049. Because rs10757274 and rs4977574 are in nearly perfect linkage disequilibrium (r2 = 0.99) (2), we included only rs4977574 in the current study. The pairwise r2 values of the other SNPs were all <0.07. Genotyping was performed with the TaqMan Allelic Discrimination System (Applied Biosystems Inc.) (22, 23) with the use of custom genotyping assays from the Assays-by-Design Service of Applied Biosystems Inc. Replicate quality-control samples yielded >99% concordance, and the overall call rate was >95%.
Statistical analyses
Chi-square tests were used to assess whether the SNPs were in Hardy-Weinberg equilibrium and to determine differences in genotype frequencies between MI cases and controls. Because some matched case-control pairs were broken as a result of missing genotyping, an unconditional logistic regression was used to calculate ORs of MI. Three hierarchical models were used. Model 1 was adjusted for age (y) and sex. Model 2 further adjusted for alcohol-drinker status (never, past, or current), current smoking status (yes or no), prevalent disease status of diabetes, hypertension, and dyslipidemia (yes or no for all), household income ($/mo), area of residence (urban or other), family history of MI (yes or no), physical activity (total metabolic equivalent task hours/d), regular aspirin intake (defined as ≥1 time/wk; yes or no), caloric intake (kcal/d), and genetic admixture (proportion of Amerindian and West African ancestral ethnicities) (24, 25). Our study population was derived from the admixture of founders of Southern European, Amerindian, and West African origins (25). Model 3 further included BMI (in kg/m2) with consideration that BMI might act as an important mediator of the gene-SSB interaction on CAD (9).
A genetic risk score (GRS) was calculated with the use of the 3 chromosome 9p21 SNPs. For this purpose, we assumed that each SNP was independently associated with MI risk according to an additive genetic model (26). We primarily analyzed unweighted GRSs with an assumption that each SNP contributed equally to risk of MI and calculated the GRS by summing the number of risk alleles at each locus. In a sensitivity analysis, we also used a weighted GRS with each SNP weighted according to its relative effect in Hispanics (2), and the results were similar to those with the use of the unweighted GRS.
Individual genotype-SSB– and GRS-SSB–interaction multiplicative terms were included in the 3 models to test for potential gene-diet interactions. All statistical analyses were performed with SAS software (version 9.4; SAS Institute). In analyses of individual SNPs, a 2-sided P value <0.02 (0.05 ÷ 3 = 0.02) was considered to be significant because 3 independent SNPs were analyzed; in all other analyses, a 2-sided P value <0.05 was considered to be significant.
RESULTS
Table 1 shows the characteristics of participants by MI status. In comparison with controls, MI cases had higher daily alcohol, SSB, and energy intakes; had slightly lower BMI and household income; and were more likely to be current smokers, to be diabetic, to be hypertensive, and to take aspirin regularly.
TABLE 1.
Characteristics of participants by MI status1
| MI cases | Non-MI controls | P2 | |
| Participants, n | 1560 | 1751 | — |
| Age, y | 57.7 ± 11.03 | 57.6 ± 11.2 | 0.85 |
| Women, n (%) | 378 (24.2) | 435 (24.8) | 0.68 |
| BMI, kg/m2 | 26.0 ± 3.9 | 26.4 ± 4.3 | 0.0006 |
| Physical activity, total MET-h/d | 34.8 ± 16.0 | 35.6 ± 15.8 | 0.13 |
| Alcohol consumption, g/d | 7.7 ± 20.1 | 6.2 ± 14.8 | 0.01 |
| Total calories, kcal/d | 2744.2 ± 961.9 | 2467.6 ± 773.0 | <0.0001 |
| Current smoker, n (%) | 640 (41.0) | 385 (22.0) | <0.0001 |
| Family history of myocardial infarction, n (%) | 186 (11.9) | 138 (7.9) | 0.0001 |
| Aspirin intake ≥1/wk, n (%) | 314 (20.1) | 269 (15.4) | 0.0004 |
| Individual European admixture proportion, % | 57.5 ± 8.1 | 57.9 ± 7.9 | 0.09 |
| Prevalent diabetes, n (%) | 362 (23.2) | 238 (13.6) | <0.0001 |
| Prevalent hypertension, n (%) | 582 (37.3) | 510 (29.1) | <0.0001 |
| Prevalent hypercholesterolemia, n (%) | 474 (30.4) | 481 (27.5) | 0.06 |
| Household income, $/mo | 510.8 ± 391.5 | 580.3 ± 426.7 | <0.0001 |
| Urban area of residence, n (%) | 600 (38.5) | 708 (40.4) | 0.25 |
| Sugar-sweetened beverages, servings/d | 1.9 ± 1.6 | 1.7 ± 1.3 | 0.01 |
| rs4977574 (risk allele G), n (%) | 0.002 | ||
| AA | 422 (27.1) | 540 (30.8) | |
| AG | 795 (51.0) | 891 (50.9) | |
| GG | 343 (22.0) | 320 (18.3) | |
| rs2383206 (risk allele G), n (%) | 0.02 | ||
| AA | 245 (15.7) | 307 (17.5) | |
| AG | 750 (48.1) | 887 (50.7) | |
| GG | 565 (36.2) | 557 (31.8) | |
| rs1333049 (risk allele C), n (%) | 0.03 | ||
| GG | 338 (21.7) | 435 (24.8) | |
| GC | 821 (52.6) | 922 (52.7) | |
| CC | 401 (25.7) | 394 (22.5) | |
| Genetic risk score, risk allele | 3.2 ± 1.9 | 3.0 ± 1.9 | 0.003 |
MET-h, metabolic equivalent task hours; MI, myocardial infarction.
For continuous variables, a general linear model was used for comparisons; for categorical variables, the chi-square test was used for comparisons.
Mean ± SD (all such values of continuous variables).
All the 3 chromosome 9p21 SNPs were commonly distributed in the current study participants, with minor allele frequencies that ranged from 0.41 to 0.50, none of which displayed significant deviations from Hardy-Weinberg equilibrium. All SNPs showed significantly different distributions between MI cases and controls (Table 1). We previously reported that the ORs for MI per risk allele ranged from 1.12 to 1.16 for the current 3 SNPs in the Hispanic population (2). The GRS on the basis of the 3 SNPs ranged from 0 to 6, and the mean score was higher in MI cases than in controls (Table 1). The increment of one daily serving of SSBs was related to 6% higher odds of having MI in the current population (age- and sex-adjusted OR: 1.06; 95% CI: 1.01, 1.12).
We explored the association of a genetic predisposition with MI according to categories of SSB intake and further examined whether SSBs modified the genetic effect of the 3 chromosome 9p21 SNPs on MI in Hispanics. The genetic association with MI showed a trend to be stronger in participants with higher intakes of SSBs than in those with lower intakes, which was consistent across the 3 SNPs (Table 2). We showed SSBs significantly modified the effect of SNP rs4977574 on MI in models 2 and 3 (both P-interaction = 0.005). In model 3, the OR of MI for the increment of one risk allele (G allele) of rs4977574 was 48% higher [(1.44 – 0.97) ÷ 0.97] in participants with SSB intake >2 servings/d than in those with intake of <1 serving/d. No significant interactions were detected between SSBs and the other 2 SNPs rs2383206 and rs1333049 on MI (all P-interaction > 0.02). Adjustment for BMI did not change the result materially.
TABLE 2.
OR (95% CIs) of myocardial infarction cases per plus one risk allele according to intake of sugar-sweetened beverages (n = 3311)1
| Sugar-sweetened beverages, servings/d |
||||
| <1 | 1–2 | >2 | P-interaction | |
| rs4977574 | ||||
| Model 1 | 1.02 (0.87, 1.20) | 1.24 (1.04, 1.48) | 1.28 (1.07, 1.52) | 0.06 |
| Model 2 | 0.97 (0.81, 1.16) | 1.22 (1.01, 1.48) | 1.45 (1.20, 1.75) | 0.005 |
| Model 3 | 0.97 (0.81, 1.16) | 1.21 (1.00, 1.47) | 1.44 (1.19, 1.74) | 0.005 |
| rs2383206 | ||||
| Model 1 | 1.04 (0.88, 1.23) | 1.20 (1.01, 1.43) | 1.19 (1.00, 1.42) | 0.27 |
| Model 2 | 1.00 (0.83, 1.19) | 1.21 (1.00, 1.46) | 1.28 (1.06, 1.55) | 0.10 |
| Model 3 | 1.00 (0.83, 1.20) | 1.20 (0.99, 1.46) | 1.28 (1.06, 1.55) | 0.09 |
| rs1333049 | ||||
| Model 1 | 1.07 (0.90, 1.26) | 1.12 (0.94, 1.34) | 1.25 (1.05, 1.48) | 0.19 |
| Model 2 | 1.05 (0.87, 1.26) | 1.10 (0.91, 1.34) | 1.33 (1.11, 1.60) | 0.11 |
| Model 3 | 1.05 (0.87, 1.26) | 1.09 (0.90, 1.33) | 1.34 (1.11, 1.61) | 0.10 |
Unconditional logistic regression was used to calculate ORs of myocardial infarction. In model 1, adjustments included age and sex; in model 2, adjustments further included genetic admixture (proportion of Amerindian and West African ancestral ethnicities), alcohol intake, cigarette smoking, prevalent disease status (diabetes, hypertension, and dyslipidemia), household income, area of residence, family history of myocardial infarction, physical activity, regular aspirin intake, and caloric intake; in model 3, adjustments further included BMI.
Although fruit juices do not have added sugars and are widely marketed as healthy, they contain similar caloric contents as other SSBs and have been associated with increased risk of weight gain (27). We further performed analyses to test interactions of SNP rs4977574 with SSBs except fruit juice and fruit juice separately. The OR (95% CI) of MI for an increment of each risk allele (G allele) in rs4977574 was 1.03 (0.89, 1.20) in participants with intake of <1 serving/d SSBs except fruit juice, 1.19 (0.95, 1.49) in participants with intake of 1–2 servings/d, and 1.51 (1.22, 1.87) in participants with intake of >2 servings/d (Table 3) (P-interaction = 0.009 in the full model). No significant interactions were shown between the rs4977574 genotype and fruit juice on MI (Table 3).
TABLE 3.
ORs (95% CIs) of myocardial infarction cases per plus one risk allele of rs4977574 according to intake of sugar-sweetened beverages (besides fruit juice) and fruit juice (n = 3311)1
| Sugar-sweetened beverages except fruit juice, servings/d |
Fruit juice only, servings/d |
||||||
| <1 | 1–2 | >2 | P-interaction | <1 | ≥1 | P-interaction | |
| Model 1 | 1.11 (0.96, 1.27) | 1.11 (0.91, 1.36) | 1.35 (1.12, 1.64) | 0.13 | 1.06 (0.91, 1.22) | 1.27(1.11, 1.45) | 0.07 |
| Model 2 | 1.04 (0.89, 1.21) | 1.19 (0.95, 1.49) | 1.52 (1.23, 1.88) | 0.009 | 1.05 (0.89, 1.22) | 1.30(1.12, 1.50) | 0.08 |
| Model 3 | 1.03 (0.89, 1.20) | 1.19 (0.95, 1.49) | 1.51 (1.22, 1.87) | 0.009 | 1.04 (0.89, 1.22) | 1.30(1.12, 1.50) | 0.08 |
Unconditional logistic regression was used to calculate ORs of myocardial infarction. In model 1, adjustments included age and sex; in model 2, adjustments further included genetic admixture (proportion of Amerindian and West African ancestral ethnicities), alcohol intake, cigarette smoking, prevalent disease status (diabetes, hypertension, and dyslipidemia), household income, area of residence, family history of myocardial infarction, physical activity, regular aspirin intake, and caloric intake; in model 3, adjustments further included BMI.
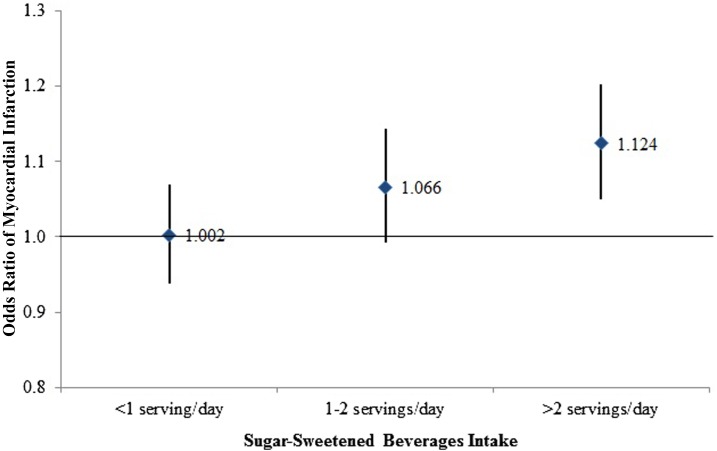
We further assessed the interactions between the composite GRS and SSBs. The OR (95% CI) of MI for the increment of each risk allele of GRS was 1.00 (0.94, 1.07) in participants with SSB intake of <1 serving/d, 1.07 (0.99, 1.14) in participants with intake of 1–2 servings/d, and 1.12 (1.05, 1.20) in participants with intake of >2 servings/d (P-interaction = 0.03) (Figure 1). Adjustment for BMI did not change the result materially.
FIGURE 1.
ORs (95% CIs) of myocardial infarction per risk allele of genetic risk score by sugar-sweetened beverage intake (n = 3311). An unconditional logistic regression was used to calculate ORs of myocardial infarction. Adjustments include age, sex, genetic admixture (proportion of Amerindian and West African ancestral ethnicities), alcohol intake, cigarette smoking, prevalent disease status (diabetes, hypertension, and dyslipidemia), household income, area of residence, family history of myocardial infarction, physical activity, regular aspirin intake, caloric intake, and BMI. P-interaction = 0.03.
DISCUSSION
In a Hispanic population living in Costa Rica, we reported that SSB intake modified the association between chromosome 9p21 SNP rs4977574 and risk of MI, which appeared to be stronger in participants who had a higher intake of SSBs than in those who had a lower SSB intake. For the increment of each risk allele in SNP rs4977574, we observed a 44% significant increase in the odds of MI in participants with an SSB intake of >2 servings/d and a nonsignificant near-null odd in subjects with <1 serving/d.
Our findings are in line with a previous study that showed that the genetic associations of the chromosome 9p21 variants with CAD were modified by dietary factors. Do et al. (3) reported a significant interaction between chromosome 9p21 SNP rs2383206/rs4977574 and a factor-analysis-derived “prudent” diet pattern score on MI in multiple ethnicities including Latin American (3). The prudent diet pattern was characterized by high intakes of raw vegetables, fruit, green leafy vegetables, nuts, desserts, and dairy products (3, 28) but did not include SSBs. In general, our findings were consistent with the implications from this study (i.e., a healthy diet may diminish, whereas an unhealthy diet may exacerbate, the adverse effect of chromosome 9p21 high-risk genotypes on CAD risk). Besides dietary factors, a few studies have indicated that risk associated with chromosome 9p21 might be magnified in the presence of abdominal obesity (29), type 2 diabetes (30), or poor glycemic control in diabetic patients (31). Because SSB intake is highly correlated with these conditions, we speculate that similar mechanisms may be involved in the observed interactions. Future investigations are warranted to test the hypotheses.
Our study indicated that different types of SSBs might interact with chromosome 9p21 genotype distinctly. We showed that fruit juice did not interact with the chromosome 9p21 genotype on CAD risk, whereas other types of SSBs showed significant interactions. The exact mechanism of such a difference is unknown. However, in Costa Ricans, the associations with adiposity were only significant for other types of SSBs but not for fruit juice (16). These observations suggest that fruit juice and other types of SSBs might play dissimilar roles in the driving gene-diet interaction on MI risk.
We found that only one of the 3 chromosome 9p21 SNPs showed a significant interaction with SSBs on MI risk although all of these SNPs were independently related to CAD risk. The 3 SNPs were not in linkage disequilibrium with each other. These data suggest different mechanisms regarding the gene-diet interaction on MI risk of these SNPs.
A “Hispanic Paradox” in the US population (e.g., a less favorable cardiovascular disease risk-factor profile but a paradoxically lower CAD prevalence and mortality) has been observed for decades, although the causes are controversial (32). Besides diverse residual confounding because of, e.g., ethnic differences in the genetic structure, a modification of the genetic effects by environmental factors such as dietary and behavioral habits may also partly have accounted for such paradoxical observations (33). In a previous study, a stronger interaction between chromosome 9p21 SNPs and the dietary pattern was shown in Latin Americans than in Europeans (3). It remains to be validated whether the interaction between chromosome 9p21 genotypes and SSBs persists in other populations. These data, taken together, may provide a potential preliminary clue for the mechanisms of such population-specific phenomenon from a gene-diet–interaction aspect.
Strengths of the current study include a large sample of an Hispanic population with well-characterized clinical profiles. The study also benefited from the application of a detailed dietary assessment with the use of the standardized FFQ that was designed and validated specifically for Costa Ricans (16). This FFQ enabled us to separately analyze different types of sugary beverages. We acknowledge that recall bias might have affected the observed gene-diet interactions. In our study, cases and controls were carefully matched, and such a design might have minimized the influence of recall bias (34). Furthermore, the causal effects of chromosome 9p21 and SSBs on MI have been well established, and reverse causality is biologically impossible. Our finding might be generalizable to US Costa Ricans or other Hispanic populations who share similar life styles and, to a lesser extent, genomic makeups. We acknowledge that additional replications in other Hispanic populations are warranted to verify our findings.
In conclusion, we observed significant interactions between habitual SSB intake and the chromosome 9p21 variant on MI in Hispanics. Higher intake of SSBs exacerbates genetic risk. To our knowledge, our findings lend novel support to potential interplays between genetic and dietary factors in the cause of cardiovascular disease and may provide clues to understand the potential mechanisms that underlie the effects of chromosome 9p21 genotypes on CAD.
Acknowledgments
The authors’ responsibilities were as follows—YZ and LQ: contributed to the study concept and design, data analysis and interpretation, and drafting and critical revision of the manuscript; YL, TH, and H-LC: critically revised the manuscript; HC and LQ: contributed to the study concept, acquisition of data, design and funding of the initial project, and critical revision of the manuscript; and LQ: was the guarantor of the work and, as such, had full access to all data in the study and took responsibility for the integrity of data and accuracy of the data analysis. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CAD, coronary artery disease; FFQ, food-frequency questionnaire; GRS, genetic risk score; MI, myocardial infarction; SNP, single nucleotide polymorphism; SSB, sugar-sweetened beverage.
REFERENCES
- 1.Samani NJ, Schunkert H. Chromosome 9p21 and cardiovascular disease: the story unfolds. Circ Cardiovasc Genet 2008;1:81–4. [DOI] [PubMed] [Google Scholar]
- 2.Qi L, Ma J, Qi Q, Hartiala J, Allayee H, Campos H. Genetic risk score and risk of myocardial infarction in Hispanics. Circulation 2011;123:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do R, Xie C, Zhang X, Mannisto S, Harald K, Islam S, Bailey SD, Rangarajan S, McQueen MJ, Diaz R, et al. The effect of chromosome 9p21 variants on cardiovascular disease may be modified by dietary intake: evidence from a case/control and a prospective study. PLoS Med 2011;8:e1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:e21–181. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevalence of coronary heart disease—United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2011;60:1377–81. [PubMed] [Google Scholar]
- 7.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi L, Campos H. Genetic predictors for cardiovascular disease in hispanics. Trends Cardiovasc Med 2011;21:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, Ridker PM, Hunter DJ, Willett WC, Rimm EB, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Narváez EA, Sacks FM, Campos H. Abdominal obesity and hyperglycemia mask the effect of a common APOC3 haplotype on the risk of myocardial infarction. Am J Clin Nutr 2008;87:1932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation 2008;118:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylin A, Kabagambe EK, Siles X, Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 2002;76:750–7. [DOI] [PubMed] [Google Scholar]
- 13.El-Sohemy A, Baylin A, Ascherio A, Kabagambe E, Spiegelman D, Campos H. Population-based study of α- and γ-tocopherol in plasma and adipose tissue as biomarkers of intake in Costa Rican adults. Am J Clin Nutr 2001;74:356–63. [DOI] [PubMed] [Google Scholar]
- 14.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr 2002;76:172–9. [DOI] [PubMed] [Google Scholar]
- 15.Kabagambe EK, Baylin A, Siles X, Campos H. Individual saturated fatty acids and nonfatal acute myocardial infarction in Costa Rica. Eur J Clin Nutr 2003;57:1447–57. [DOI] [PubMed] [Google Scholar]
- 16.Rhee JJ, Mattei J, Campos H. Association between commercial and traditional sugar-sweetened beverages and measures of adiposity in Costa Rica. Public Health Nutr 2012;15:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, et al. Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 2007;316:1491–3. [DOI] [PubMed] [Google Scholar]
- 19.Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, Linsel-Nitschke P, Kathiresan S, Wright B, Trégouët DA, et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet 2009;41:280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 2009;41:334–41. Erratum in: Nat Genet. 2009;41:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trégouët DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet 2009;41:283–5. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ. SNP genotyping by the 5′-nuclease reaction. Methods Mol Biol 2003;212:129–47. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 1999;14:143–9. [DOI] [PubMed] [Google Scholar]
- 24.Bare LA, Ruiz-Narváez EA, Tong CH, Arellano AR, Rowland CM, Catanese JJ, Sacks FM, Devlin JJ, Campos H. Investigation of KIF6 Trp719Arg in a case-control study of myocardial infarction: a Costa Rican population. PLoS One 2010;5;e13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Narváez EA, Bare L, Arellano A, Catanese J, Campos H. West African and Amerindian ancestry and risk of myocardial infarction and metabolic syndrome in the Central Valley population of Costa Rica. Hum Genet 2010;127:629–38. [DOI] [PubMed] [Google Scholar]
- 26.Balding DJ. A tutorial on statistical methods for population association studies. Nat Rev Genet 2006;7:781–91. [DOI] [PubMed] [Google Scholar]
- 27.Faith MS, Dennison BA, Edmunds LS, Stratton HH. Fruit juice intake predicts increased adiposity gain in children from low-income families: weight status-by-environment interaction. Pediatrics 2006;118:2066–75. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929–37. [DOI] [PubMed] [Google Scholar]
- 29.Ye S, Willeit J, Kronenberg F, Xu Q, Kiechl S. Association of genetic variation on chromosome 9p21 with susceptibility and progression of atherosclerosis: a population-based, prospective study. J Am Coll Cardiol 2008;52:378–84. [DOI] [PubMed] [Google Scholar]
- 30.Rivera NV, Carreras-Torres R, Roncarati R, Viviani-Anselmi C, De Micco F, Mezzelani A, Koch W, Hoppmann P, Kastrati A, Stewart AF, et al. Assessment of the 9p21.3 locus in severity of coronary artery disease in the presence and absence of type 2 diabetes. BMC Med Genet 2013;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doria A, Wojcik J, Xu R, Gervino EV, Hauser TH, Johnstone MT, Nolan D, Hu FB, Warram JH. Interaction between poor glycemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA 2008;300:2389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman-Garber I, Villa AR, Caballero E. Diabetes and cardiovascular disease. Is there a true Hispanic paradox? Rev Invest Clin 2004;56:282–96. [PubMed] [Google Scholar]
- 33.Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: a focus on physical activity and lifestyle changes. Curr Mol Med 2008;8:519–32. [DOI] [PubMed] [Google Scholar]
- 34.Friedenreich CM, Howe GR, Miller AB. Recall bias in the association of micronutrient intake and breast cancer. J Clin Epidemiol 1993;46:1009–17. [DOI] [PubMed] [Google Scholar]