Abstract
Background: Measurements of maternal fat mass (FM) are important for studies of maternal and fetal health. Common methods of estimating FM have not been previously compared in pregnancy with measurements using more complete body composition models.
Objectives: The goal of this pilot study was to compare multiple methods that estimate FM, including 2-, 3- and 4-compartment models in pregnant women at term, and to determine how these measures compare with FM by dual-energy X-ray absorptiometry (DXA) 2 wk postpartum.
Design: Forty-one healthy pregnant women with prepregnancy body mass index (in kg/m2) 19 to 46 underwent skinfold thickness (SFT), bioelectrical impedance analysis (BIA), body density (Db) via air displacement plethysmography (ADP), and deuterium dilution of total body water (TBW) with and without adjustments for gestational age using van Raaij (VRJ) equations at 37–38 wk of gestation and 2 wk postpartum to derive 8 estimates of maternal FM. Deming regression analysis and Bland-Altman plots were used to compare methods of FM assessment.
Results: Systematic differences in FM estimates were found. Methods for FM estimates from lowest to highest were 4-compartment, DXA, TBW(VRJ), 3-compartment, Db(VRJ), BIA, air displacement plethysmography body density, and SFT ranging from a mean ± SD of 29.5 ± 13.2 kg via 4-compartment to 39.1 ± 11.7 kg via SFT. Compared with postpartum DXA values, Deming regressions revealed no substantial departures from trend lines in maternal FM in late pregnancy for any of the methods. The 4-compartment method showed substantial negative (underestimating) constant bias, and the air displacement plethysmography body density and SFT methods showed positive (overestimating) constant bias. ADP via Db(VRJ) and 3-compartment methods had the highest precision; BIA had the lowest.
Conclusions: ADP that uses gestational age-specific equations may provide a reasonable and practical measurement of maternal FM across a spectrum of body weights in late pregnancy. SFT would be acceptable for use in larger studies. This trial was registered at clinicaltrials.gov as NCT02586714.
Keywords: air displacement plethysmography, dual-energy X-ray absorptiometry, maternal body composition, maternal fat mass, maternal obesity, pregnancy, skinfold thickness, total body water
INTRODUCTION
Obesity rates in the United States have risen dramatically over the past several decades (1), including among reproductive aged women. In 2009, it was estimated that 1 in 4 pregnant women were obese before pregnancy with a BMI (in kg/m2) ≥30 (2). Obese women are more likely to have babies who experience excessive fetal growth (3) and to have pregnancies complicated by preeclampsia, gestational diabetes, and the need for cesarean delivery (4, 5). Understanding the physical and metabolic abnormalities experienced by obese women and their babies, especially as they relate to increased maternal body fatness, is a high priority in nutrition research.
The BMI classification of obesity has been useful for epidemiologic studies, for public health evaluations, and in clinical practice because it is simple to calculate by using readily available information (6–8). However, using BMI as a proxy for fat mass (FM)6 does not account for potential confounding effects of high or low lean (or nonfat) mass on health outcomes. In pregnant women, BMI has been shown to significantly correlate with percent body fat, although this relation weakens in late gestation (9, 10). Thus, BMI is a poor indicator of variations in maternal fat and lean mass later in pregnancy that may regulate fetal and neonatal growth.
Body composition assessment during pregnancy is challenging because of the addition of the uterine contents as well as marked increases in FM and total body water (TBW), which may systematically alter results of common techniques for body composition measurement such as skinfold thickness (SFT) and bioelectrical impedance analysis (BIA) (8). Various studies have used 3-compartment and 4-compartment modeling to quantify whole body fat, water, and fat-free mass (FFM) (consisting primarily of proteins and minerals) and overcome challenges associated with these measurements in pregnancy (11–16). In those studies, body density (Db) was typically determined with underwater weighing and TBW by isotope dilution (11–13, 17–19). The expense, time, and specialty equipment required to perform these techniques have prohibited their use in large-scale clinical studies. A simpler technique to estimate Db, air displacement plethysmography (ADP), has become more widely available for research use (20). However, this technique has not been compared with other methods of body composition measurement in pregnancy.
We sought to answer a question that frequently arises among scientists who study body composition changes during pregnancy: how do the aforementioned methods of measuring maternal FM compare, and which is the most appropriate for specific clinical and research applications? We also set out to determine the validity of BMI categories of lean, overweight, and obese, which are commonly used clinically and in research as surrogates for FM during pregnancy.
METHODS
Study design
This was a planned pilot feasibility study in support of a larger project to prospectively study the effects of body composition in pregnancy on placental function. Forty-one healthy pregnant women in their third trimester with a singleton gestation of varying prepregnancy BMIs (n = 11 normal weight, BMI 18.5–24.9; n = 15 overweight, BMI 25–29.9; and n = 15 obese, BMI ≥30) were recruited from Oregon Health & Science University obstetric clinics from July 2012 to August 2013. Exclusion criteria included active maternal infection, documented fetal congenital anomalies, substance abuse, chronic illness requiring regular medication use, maternal diabetes, chorioamnionitis, significant medical conditions (active cancers or cardiac, renal, hepatic, or pulmonary diseases), or an abnormal 2-h 75-g glucose tolerance test. The Oregon Health & Science University institutional review board approved the study protocol, and each subject provided signed informed consent before enrollment.
Subjects presented for their study visits in the morning after an overnight fast. During the first visit, at 37–38 wk of gestation, they underwent 4 measures—1) ADP, 2) TBW volume by deuterium dilution (2H2O), 3) SFT, and 4) BIA—to generate 8 estimates of fat mass (see below and Table 1). To determine maternal FM without the presence of the fetus or placenta and to avoid radiation exposure to the fetus, subjects returned for a second study visit 2 wk postpartum for repeat measurements by the above methods plus a dual-energy X-ray absorptiometry scan (DXA) scan.
TABLE 1.
Equations used for maternal fat mass calculations1
| Anatomic region | Equation | Reference | |
| Anthropometric equations (SFT) | Db | 1.1581 – 0.0720 (log of the sum of TSF + BSF + SSSF + SISF) | (21) |
| Fat, % | [(4.95/Db) – 4.5] × 100 | (22) | |
| Fat mass, kg | Fat (%) × wt | ||
| BIA | Fat mass, kg | Fat (%) × wt | |
| Db(VRJ) for 37 wk of gestation | Fat mass, kg | (Wt/100) × [(519/Db) − 476] | (16) |
| TBW(VRJ) for 37 wk of gestation | Fat mass, kg | Wt – (TBW/0.747) | (16) |
| 3-C model | Fat mass, kg | Wt (2.118/Db) – 0.78 (TBW/wt) – 1.354 | (23) |
| 4-C model | Fat mass, kg | 2.747 Bvol – 0.71 TBW + 1.46 BMC – 2.05 wt | (24) |
BIA, bioelectrical impedance analysis; BMC, bone mineral content (kg); BSF, biceps skinfold (mm); Bvol, body volume (m3); Db, body density (kg/m3); Db(VRJ), van Raaij body density; SFT, skinfold thickness (mm); SISF, suprailiac skinfold (mm); SSSF, subscapular skinfold (mm); TBW, total body water (kg); TBW(VRJ), van Raaij total body water; TSF, triceps skinfold (mm); wt, weight (kg); 3-C, 3-compartment; 4-C, 4-compartment.
Impact of pregnancy on techniques for measuring body composition
Many of the specific assumptions that underlie methods of body composition assessment, particularly hydration and density of FFM, are affected by pregnancy, as reviewed by Widen and Gallagher (25) and Lederman (26). The classic body composition model predicts FFM from TBW by the equation FFM = TBW/0.732, on the assumption that water accounts for 73.2% of FFM. However, FFM hydration increases throughout pregnancy in an amount that varies between individual women (26). Thus, changes in FFM hydration will affect FM calculated from TBW by 2H2O dilution and as assessed by ADP. Using the estimate of 73% FFM hydration in ADP calculations will result in an underestimation of TBW and hence an overestimation of FM.
FFM density is essential for the determination of overall Db used to calculate FM by SFT, 3-compartment, 4-compartment, and ADP methods. Although assumed to be 1.10 g/cm3 in nonpregnant women, it is thought to decrease during pregnancy (25). Use of the standard FFM density would therefore result in an underestimation of FM. In an effort to correct for these changes in FFM hydration and density during pregnancy, van Raaij (VRJ) et al. (16) generated gestational age-specific equations for Db and TBW, which we have used in our calculations [Db(VRJ) and TBW(VRJ)].
SFT measurements have been shown to significantly change shortly after delivery despite suspected minimal changes in edema or FM (25). This suggests a potential alteration in the distribution of body fat and water during pregnancy rather than a quantitative change (26).
Because of low-level radiation use, DXA is contraindicated during pregnancy and was only used postpartum in the present study. DXA determines soft tissue composition, including FM, by analyzing the proportion of lean and fat in areas lacking bone as determined by differential photon attenuation coefficients in each pixel (27, 28). As such, DXA is limited in its ability to assess lean and fat in soft tissue areas that overlie bone and may also be affected by tissue depth, especially in morbidly obese individuals (29). Although DXA is less reliant than other methods on assumptions regarding constant values for density and hydration of FFM (28), concern remains that overhydration may result in small but systematic errors in FM estimation with DXA as well (30).
Body composition measurements
ADP
Whole-body composition was measured by ADP by using a BodPod (Life Measurement Inc.). The subject changed into a bathing suit or spandex clothing, including a swimming cap, and body weight and height were measured. The subject sat inside the BodPod while the air displaced by the body was measured. Results included percent body fat, FM, lean mass, body volume, and Db [Db(BodPod)]. Residual lung volume was directly measured in 35 of 41 subjects. Six subjects were unable to successfully perform direct lung volume measurement, and therefore the standardized volume was substituted, which has been shown in pregnancy to have a very slight overestimation on body fat assessment (31).
TBW by 2H2O
After an overnight fast, a baseline saliva sample (at least 1 mL) was obtained with Sarstedt salivettes. After collection, the salivette was spun at 1500 × g at room temperature for 10 min. The liquid was transferred to a 1.2-mL internally threaded cryogenic vial tightly capped and sealed to avoid dead space and frozen at −80°C. The 99.8% enriched 2H2O (Cambridge Isotope Laboratories) was diluted with deionized water and divided into aliquots of ∼5-g individual doses, which the subjects were then given to drink. The bottles were weighed to the nearest 0.1 mg before and after each dose administration to determine the exact amount of 2H2O ingested. Subjects were instructed not to eat or drink anything after the dose. It has been suggested that full isotopic equilibration with the TBW pool may require up to 4 h or more in late gestation (32). However, in practical terms, a prolonged overnight fast in late gestation is quite difficult, and thus the postdose sample was collected 3 h after 2H2O ingestion as per Lederman et al. (17). If isotopic equilibration was not reached by 3 h, our results would overestimate TBW and underestimate FM. Saliva samples were frozen for later batch analysis. The samples were processed in duplicate for measurement of deuterium enrichment by wavelength-scanned cavity ring-down spectroscopy (Isotopic Water Analyzer, L2120-I; Picarro), and TBW was calculated as previously described (33).
SFT
Maternal anthropometric measurements were performed by the same trained examiner during both visits by using a method validated in nonpregnant populations (34). Midarm circumference was measured with the right arm hanging relaxed at the patient’s side, at the midpoint between the acromion and olecranon process. Measurements were performed with a calibrated Lange caliper that applied 10 g/mm2 pressure at 4 sites: 1) triceps skinfold—a vertical fold at the same level as the midarm circumference measurement, on the posterior aspect of the arm, with the right arm held vertically; 2) biceps skinfold—a vertical fold at the same level as the midarm circumference, over the short head of the biceps, with the arm placed supinated on the patient’s thigh; 3) subscapular skinfold—just below the tip of the inferior angle of the scapula, at an angle of 45° to the vertical; and 4) suprailiac skinfold—immediately above the iliac crest, in the midaxillary line.
BIA
Whole-body composition was measured with the Body Composition Analyzer TBF-410 (Tanita Corporation of America Inc.). The electrical impedance was measured when a low-level electric current (500 μA) was applied between the feet, placed on pressure contact stainless steel food pads. Weight and bioelectrical impedance were measured and, with input of age and height, a report generated for percent body fat. To account for the weight of clothing worn, 0.8 kg was subtracted from all weights.
DXA
Whole-body composition was assessed by using DXA scans at 2 wk postpartum only. Bone mineral content (BMC) and FM were attained with a QDR Discovery A Densitometer (Hologic Inc.).
Calculations of FM
Most of our FM estimates were based on 2-compartment models. Equations for the calculation of FM by SFT and BIA are shown in Table 1. FM by DXA was generated by the equipment software. FM from ADP was measured in 2 ways. FM Db(BodPod) was generated by the equipment software. FM Db(VRJ) was calculated from Db(BodPod) by using an equation to account for alterations in FFM hydration and density at 37 wk of gestational age as described by van Raaij et al. (16) (Table 1). Similarly, calculation of FM from TBW measured with 2H2O dilution was done by using equations modified for pregnancy (16) (Table 1).
The 3-compartment body composition model, as described by Forbes (23) and explained in Hopkinson et al. (12), was used to calculate FM with the Db determined from ADP and TBW from 2H2O (Table 1). A 4-compartment model used BMC obtained by DXA in addition to Db from ADP and TBW from 2H2O to calculate FM (24) (Table 1).
Statistical analysis
Statistical analysis was performed by using the R package Method Comparison Regression, version 1.2.1 (https://cran.r-project.org/web/packages/mcr/index.html). The postpartum DXA FM was elected as the comparison method to show the variation between methods because it is an independent assessment technique that, unlike the 4-compartment model, does not depend on multiple other methods (TBW, Db via ADP, and BMC via DXA), each of which is subject to its own limitations in pregnancy that may result in propagation of error and the potential bias of including the same numeric result in a comparison calculation [e.g., 4-compartment method includes the same Db value as Db(BodPod), Db(VRJ), and the 3-compartment method] (28). Estimates of mean FM were compared by using ANOVA. The bias and 95% ± SD limits of agreement for comparison of the estimates of body FM were established as per Fuller et al. (24). The line of calibration for FM as estimated by Db(VRJ), Db(BodPod), TBW(VRJ), 3-compartment model [Db(BodPod) and 2H2O], 4-compartment model [Db(BodPod), 2H2O, and BMC via DXA), SFT, BIA, and DXA was determined by Deming regression analysis (35) rather than simple linear regression to account for expected errors within each set of measurements. The precision of the Deming regression model was assessed by bootstrap resampling to construct a 95% CI around the coefficient for the slope. If the slope is far from 1, this suggests the alternative measure differs from the comparison according to a systematic trend (i.e., more than just a constant). Bland-Altman plots (36) were subsequently created to analyze the agreement between FM estimates obtained with each method compared with each of the 7 other methods.
RESULTS
There were no differences in gestational age at delivery, infant birth weight, or cesarean delivery between the normal-weight, overweight, and obese groups of women (Table 2). Obese women were more likely to be multiparous (66%) compared with overweight (33.3%) and normal-weight (9.1%) women.
TABLE 2.
Maternal demographic characteristics
| Normal weight (n = 11) | Overweight (n = 15) | Obese (n = 15) | |
| Age, y | 31.5 ± 4.91 | 31.6 ± 6.5 | 27.2 ± 10.0 |
| Parity, n (%) | |||
| 0 | 10 (90.9) | 10 (66.7) | 5 (33.3) |
| 1 | 1 (9.1) | 5 (33.3) | 8 (53.3) |
| 2 | 0 | 0 | 2 (13.3) |
| Race, n (%) | |||
| American Indian/Alaskan native | 0 | 0 | 1 (6.7) |
| Asian American | 1 (9.1) | 1 (6.7) | 1 (6.7) |
| Black/African American | 0 | 1 (6.7) | 1 (6.7) |
| Pacific Islander | 0 | 1 (6.7) | 0 |
| White/Caucasian | 10 (90.9) | 11 (73.3) | 10 (66.7) |
| Unknown | 0 | 1 (6.7) | 2 (13.3) |
| Ethnicity, n (%) | |||
| Hispanic | 0 | 1 (6.7) | 4 (26.7) |
| Non-Hispanic | 11 (100) | 14 (93.3) | 10 (66.7) |
| Unknown | 0 | 0 | 1 (6.7) |
| Height, cm | 167.4 ± 11.3 | 164.4 ± 6.8 | 165.7 ± 6.9 |
| Prepregnancy BMI, kg/m2 | 22.2 ± 2.0 | 27.4 ± 1.5 | 37.5 ± 5.1 |
| BMI at study enrollment, kg/m2 | 26.3 ± 2.8 | 32.4 ± 2.6 | 37.3 ± 11.2 |
| Gestational age at visit 1, wk | 37.9 ± 0.7 | 37.7 ± 0.5 | 37.6 ± 0.6 |
| Gestational age at delivery, wk | 40.2 ± 1.1 | 40.0 ± 1.1 | 40.1 ± 1.1 |
| Infant birth weight, kg | 3.5 ± 0.5 | 3.5 ± 0.3 | 3.6 ± 0.5 |
| Cesarean deliveries, n (%) | 3 (27.3) | 4 (26.7) | 4 (28.6) |
Mean ± SD (all such values).
All 41 subjects presented for the first study visit at 37–38 wk of gestation and have complete data for SFT, BIA, Db(BodPod), and Db(VRJ). The second visit took place a mean ± SD of 13 ± 4.4 d postpartum. The duration between the visits was a mean ± SD of 4.1 ± 1.4 wk. One subject delivered at an outside hospital and did not present for the second study visit. Two other subjects did not present for the second study visit and were lost to follow-up; therefore, DXA results are available for 38 women. During the first study visit, 7 women appeared to have drunk fluids between 2H2O administration and saliva collection, resulting in very dilute saliva enrichments, which led to calculated TBW greater than total body weight. Thus, 2H2O data were excluded for these 7 women and TBW(VRJ) and the 3-compartment method were not calculated, leaving data from 34 subjects available for assessment by these methods. Four-compartment modeling relies on both TBW via 2H2O and BMC via DXA, and hence 4-compartment data were determined for 33 women. There are complete data for both visits for 33 women.
Body weight ranged from 64.2–134.5 kg at 37 wk of gestation to 55.2–128.1 kg at 2 wk postpartum. The mean ± SD change in body weight between visits was 7.6 ± 2.3 kg. At the 37-wk study visit, there was more than a 10-kg difference between estimates of FM, with the highest estimate seen with SFT (mean ± SD: 39.1 ± 11.7 kg) and the lowest estimate with the 4-compartment method (mean ± SD: 29.5 ± 13.2 kg) (Table 3). The mean ± SD TBW by 2H2O was 42.0 ± 6.4 kg, and the mean ± SD hydration constant for FFM was 0.767 ± 0.08 with FM as determined by Db(VRJ). At the 2-wk postpartum visit, the estimated mean ± SD body fat ranged from 28.9 ± 13.6 kg via the 4-compartment method to 35.4 ± 11.7 kg via SFT. The mean ± SD TBW by 2H2O was 35.7 ± 5.4 kg, and the mean ± SD hydration constant for FFM was 0.739 ± 0.05 with FM as determined by Db(BodPod). At 2 wk postpartum, there was a reduction in FM with all measurements. The greatest reduction (3.7 kg) was seen with the SFT method, whereas the smallest change was seen with the 3-compartment and 4-compartment methods (0.8 kg and 0.6 kg, respectively). If one compares what in theory are the best ADP-based methods before delivery and postpartum, Db(VRJ) at 37 wk and Db(BodPod) 2 wk postpartum, the difference by those methods was similar to the 3-compartment and 4-compartment models at 0.9 kg.
TABLE 3.
Fat mass estimate by method for all subjects with complete data (n = 33)1
| Model | 37 wk of gestation fat mass, kg | 2 wk postpartum fat mass, kg | Change in fat mass, kg |
| SFT2 | 39.1 ± 11.7 | 35.4 ± 11.7 | −3.7 ± 16.6 |
| BIA2 | 36.2 ± 14.5 | 32.8 ± 14.4 | −3.4 ± 20.4 |
| Postpartum DXA3,5 | — | 33.6 ± 13.1 | — |
| Db(BodPod)2 | 37.9 ± 13.2 | 35.0 ± 14.0 | −2.9 ± 19.2 |
| Db(VRJ)3,4 | 35.9 ± 13.0 | — | — |
| TBW(VRJ)3,4 | 34.9 ± 13.5 | — | — |
| 3-C2 | 35.5 ± 13.3 | 34.7 ± 13.3 | −0.8 ± 18.8 |
| 4-C2,5 | 29.5 ± 13.2 | 28.9 ± 13.6 | −0.6 ± 19.1 |
All values are means ± SDs. BIA, bioelectrical impedance analysis; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; SFT, skinfold thickness; TBW(VRJ), van Raaij total body water; 3-C, 3-compartment; 4-C, 4-compartment.
Maternal-fetal fat mass.
Maternal fat mass.
Calculated during pregnancy only.
DXA performed postpartum.
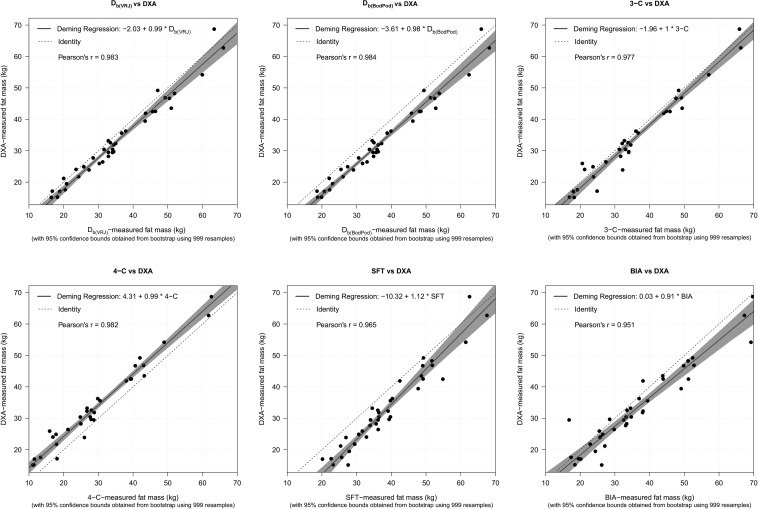
Complete values for all Deming regression analyses for comparison of FM between methods at 37 wk of gestation are shown in Table 4. Comparisons of FM measurements by Deming regressions revealed no major departures from DXA trend lines in maternal FM at 37 wk of gestation for any of the methods (Figure 1). As FM increased, BIA showed increased bias (overestimation) and SFT showed decreased bias (overestimation at lower fat mass) away from DXA compared with the other methods. Substantial constant bias was seen in the 4-compartment, Db(BodPod), and SFT methods, with the 4-compartment method showing negative (underestimating) and Db(BodPod) and SFT showing positive (overestimating) constant bias. The tightest precision was obtained via use of ADP determination of Db by both Db(VRJ) and Db(BodPod) with Pearson’s r = 0.983 and 0.984, respectively, followed by the 4-compartment method with Pearson’s r = 0.982.
TABLE 4.
Pairwise comparison of Deming regression estimates between methods at 37 wk of gestation for maternal fat mass (kg)1
| Method | BIA | PP DXA | Db(BodPod) | Db(VRJ) | TBW(VRJ) | 3-C | 4-C |
| SFT (n = 41) | |||||||
| Intercept | 9.2 (5.6, 12.5) | 9.2 (5.8, 12.4) | 6.2 (3.6, 8.5) | 7.6 (5.3, 10.1) | 10.6 (4.3, 16.1) | 7.3 (1.9, 11.2) | 13.2 (9.4, 16.3) |
| Slope | 0.8 (0.8, 0.9) | 0.9 (0.8, 1.0) | 0.9 (0.8, 0.9) | 0.9 (0.8, 0.9) | 0.8 (0.7, 1.0) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.0) |
| BIA (n = 41) | |||||||
| Intercept | −0.03 (−6.2, 3.9) | −3.6 (−9.2, 0.8) | −1.9 (−7.4, 2.7) | −1.0 (−12.4, 5.4) | −4.1 (−14, 1.8) | 3.8 (−3.1, 8.4) | |
| Slope | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (0.9, 1.5) | 1.2 (1.0, 1.4) | 1.1 (1.0, 1.3) | |
| PP DXA (n = 38) | |||||||
| Intercept | −3.6 (−6.7, −0.8) | −2.0 (−5.0, 0.8) | −0.32 (−5.2, 4.5) | −2.0 (−5.2, 1.2) | 4.3 (2.0, 6.5) | ||
| Slope | 1.0 (0.1, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | ||
| Db(BodPod) (n = 41) | |||||||
| Intercept | 4.9 (−1.0, 11.0) | 4.9 (−1.0, 11.0) | 1.7 (−3.5, 5.0) | 8.5 (5.0, 11.2) | |||
| Slope | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.0 (1.0, 1.2) | 1.0 (0.9, 1.1) | |||
| Db(VRJ) (n = 41) | |||||||
| Intercept | 3.5 (−3.7, 9.3) | 0.2 (−5.3, 3.7) | 6.9 (3.8, 9.6) | ||||
| Slope | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.1) | ||||
| TBW(VRJ) (n = 34) | |||||||
| Intercept | −3.2 (−7.7, −1.6) | 4.7 (2.0, 7.0) | |||||
| Slope | 1.1 (1.0, 1.2) | 1.0 (1.0, 1.1) | |||||
| 3-C (n = 34) | |||||||
| Intercept | 6.2 (5.0, 7.0) | ||||||
| Slope | 1.0 (1.0, 1.0) |
All values are coefficients; 95% CIs in parentheses. BIA, bioelectrical impedance analysis; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; PP, postpartum; SFT, skinfold thickness; TBW(VRJ), van Raaij total body water; 3-C, 3-compartment; 4-C, 4-compartment.
FIGURE 1.
Deming regressions showing relation between fat mass (kg) estimated with DXA scan (n = 38) compared with Db(VRJ) (n = 41), Db(BodPod) (n = 41), 3-C method (n = 34), 4-C method (n = 33), SFT (n = 41), and BIA (n = 41). BIA, bioelectrical impedance analysis; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; SFT, skinfold thickness; 3-C, 3-compartment; 4-C, 4-compartment.
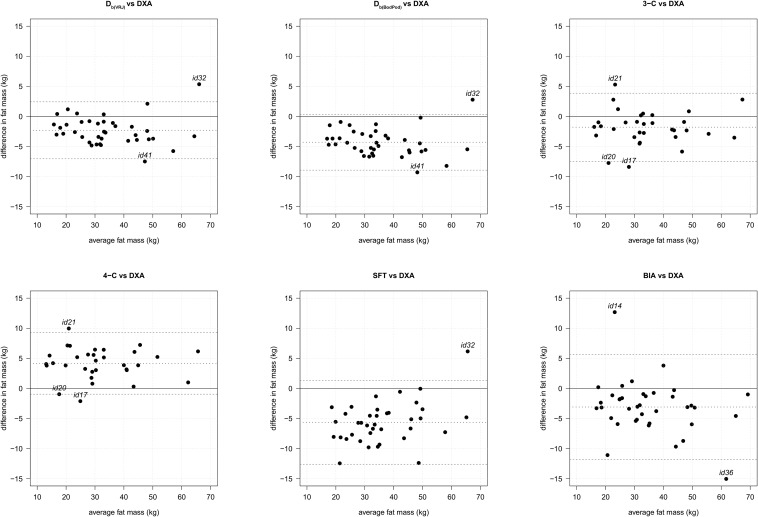
Complete values for all Bland-Altman analyses for comparison of FM between methods at 37 wk of gestation are shown in Table 5. After accounting for agreement between DXA and each method via Bland-Altman analysis (Figure 2), the most precise measurement with the tightest 95% CI for the difference was Db(VRJ) with a mean ± SD FM difference of −2.31 ± 2.37 kg, followed by the 3-compartment method with a mean ± SD FM difference of −1.35 ± 2.83 kg. Careful review of the outliers identified on the Bland-Altman analyses shows no common trends, including BMI category, between individuals that could explain the variations in measurement with DXA values. The complete Deming regression and Bland-Altman analyses for comparison of FM between methods at the 2-wk postpartum visit are available in Supplemental Tables 1 and 2.
TABLE 5.
Bland-Altman mean difference in maternal fat mass (kg) between methods at 37 wk of gestation1
| BIA | PP DXA | Db(BodPod) | Db(VRJ) | TBW(VRJ) | 3-C | 4-C | |
| SFT (n = 41) | 2.40 ± 4.45 | −5.64 ± 3.48 | −1.40 ± 3.11 | −3.40 ± 3.12 | −5.14 ± 8.40 | −4.27 ± 5.54 | −9.70 ± 4.15 |
| BIA (n = 41) | −3.09 ± 4.37 | 1.0 ± 4.62 | −1.0 ± 4.78 | −2.49 ± 9.52 | −1.65 ± 6.98 | −6.83 ± 5.49 | |
| PP DXA (n = 38) | −4.29 ± 2.31 | −2.31 ± 2.37 | −1.35 ± 4.38 | −1.79 ± 2.83 | 4.16 ± 2.55 | ||
| Db(BodPod) (n = 41) | −2.0 ± 0.30 | 4.02 ± 7.98 | 3.13 ± 4.76 | 8.44 ± 2.89 | |||
| Db(VRJ) (n = 41) | 2.04 ± 7.97 | 1.14 ± 4.74 | 6.48 ± 2.95 | ||||
| TBW(VRJ) (n = 34) | −0.93 ± 3.35 | 5.44 ± 2.31 | |||||
| 3-C (n = 34) | 5.94 ± 0.89 |
All values are means ± SDs. BIA, bioelectrical impedance analysis; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; PP, postpartum; SFT, skinfold thickness; TBW(VRJ), van Raaij total body water; 3-C, 3-compartment; 4-C, 4-compartment.
FIGURE 2.
Bland-Altman plots of fat mass estimated from DXA scan (n = 38) compared with Db(VRJ) (n = 41), Db(BodPod) (n = 41), 3-C method (n = 34), 4-C method (n = 33), SFT (n = 41), and BIA (n = 41). Middle dotted line indicates mean difference. Dashed lines indicate ±2 SD. Outliers identified by study identification number. BIA, bioelectrical impedance analysis; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; SFT, skinfold thickness; 3-C, 3-compartment; 4-C, 4-compartment.
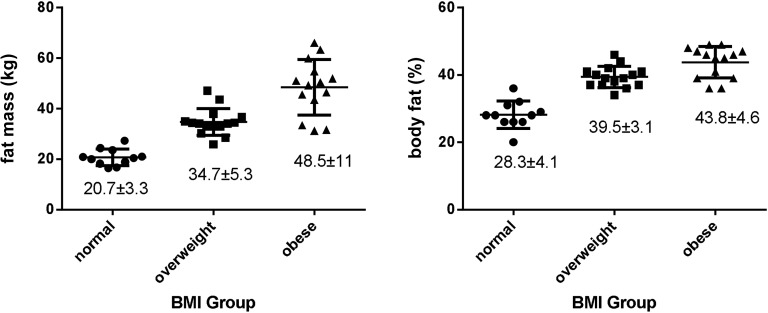
Based on the Db(VRJ) estimation, mean maternal FM and percent body fat (Figure 3) increased with increasing BMI category. Although normal-weight women consistently had lower FM and percent body fat than did overweight and obese women, there was substantial overlap in values between overweight and obese women, including 3 obese women with FM <75th percentile for overweight women.
FIGURE 3.
Maternal fat mass (left graph) and percent fat (right graph) by BMI group (n = 41). Values are given as means ± SDs.
DISCUSSION
The present report was undertaken to compare multiple methods of maternal FM assessment in late pregnancy in women with a wide range of BMIs. Assessment of maternal FM in pregnancy is particularly challenging due to the inability to separate the uterine contents, including fetus, placenta, and amniotic fluid, from the maternal tissues. Some authors have attempted to correct for this by using gestational age–specific equations (16), but these methods, including SFT, BIA, Db(BodPod), and 3-compartment and 4-compartment methods, still cannot disentangle the maternal-fetal unit.
Choosing a reference or “gold-standard” method in this population is challenging. Imaging methods, such MRI and DXA scans, are currently considered the most accurate to quantify fat and lean mass. However, MRIs are labor intensive to analyze and cost-prohibitive for use in larger scale studies. DXA scans are less expensive and simpler to analyze but are contraindicated during pregnancy due to radiation exposure. Some studies use a 4-compartment model as the reference comparison (12). However, this 4-compartment model relies on the same numerical values that are individually subject to their own limitations in pregnancy (TBW, Db via ADP) as previously described and BMC via DXA, which can only be measured in the postpartum state. Thus, the 4-compartment model will result in the propagation of any error generated by those other methods. Ultimately, we reasoned that a DXA scan 2 wk after delivery would reflect FM in the late third trimester, without the confounding factors contributed by the fetus, amniotic fluid, placenta, and increased TBW. However, DXA may be affected by changes in tissue depth and soft tissue hydration after delivery (29, 30); thus, although DXA is validated for FM assessment in other populations (27), including at 12 wk postpartum (28), it has not been validated at the 2-wk postpartum time point used in this study.
Previous studies have typically evaluated body FM measurements in pregnancy by using one or 2 methods, most commonly SFT and underwater weighing (13, 17–19, 37–40); some have used their data to generate new anthropometric equations specifically for pregnant women (11, 16, 18). SFT is likely to vary throughout pregnancy, especially later in gestation due to increased water retention (41, 42), and may not accurately reflect unique changes in maternal fat deposition and distribution that occur as gestation progresses (26). Our study confirmed prior investigations showing a bias toward higher FM values by SFT compared with other methods (18), suggesting that the increase in TBW in late gestation may contribute to greater SFT values, which, in turn, decrease Db calculations and overestimate total FM, especially in normal-weight women. Despite these potential limitations, we found that the Deming regression for FM between DXA and 37-wk SFT was reasonably close (slope of 1.1; 95% CI: 0.99, 1.3), suggesting that SFT may be acceptable for use in field studies including large numbers of pregnant women. On the other hand, the wide range of values of percent body fat obtained with BIA, despite its ease of use, raises significant concerns regarding the usefulness of this method in late pregnancy.
We found that use of ADP for determination of Db was well tolerated in the third trimester of pregnancy and, with the use of the VRJ equation [Db(VRJ)] based on gestational age (16), provided the most precise estimate of maternal FM compared with postpartum DXA. Using the pregnancy-specific calculation [Db(VRJ)] resulted in a tighter correlation with DXA values for FM and also a narrower 95% CI for the difference in the Bland-Altman plots compared with the standard Db(BodPod) calculation. Our 3-compartment data showing a mean ± SD difference in FM of −1.79 ± 2.83 kg from DXA supports the use of this model for late-gestation maternal FM evaluation as well. However, we found obtaining reliable TBW measures for use in the 3-compartment model to be challenging in this population (see below), and given the slightly tighter precision of the Db(VRJ) method, this latter technique was judged to be a superior tool for FM measurement.
Our data can be used to highlight the variations in body fat (kg) and percent body fat in pregnant women within current BMI categories for overweight and obese. For example, 2 women with class 1 obesity (BMI 31 compared with 33) had a 15% difference in percent body fat (24.7% compared with 39.2%), which corresponds to a 22-kg difference in FM (21.2 kg compared with 43.0 kg). Even greater overlap between the overweight and obese groups was found for percent body fat measurements. These discrepancies may prevent the proper categorization of distinct physiologic and metabolic characteristics important for perinatal complications based on the patients’ degree of adiposity and may contribute to the significant variations in results from perinatal epidemiologic studies using BMI as a surrogate for body fatness.
This study has several limitations. We could not obtain DXA scan data at the 37-wk study visit for use in the 4-compartment model and, instead, relied on a 2-wk postpartum measurement. Previous studies have found minimal change in BMC immediately after delivery (43), and 2 wk was believed to allow for resolution of delivery-related fluid shifts before significant changes in FM would be expected to occur. As anticipated, our FM values determined from the 2-wk postpartum DXA scan were lower than the 37-wk fat FM assessments, likely due to the loss of the uterine contents. Although we cannot rule out changes in FM between visits, the precision and minimal bias in FM between DXA and Db(VRJ) are reassuring that the accuracy did not vary based on obesity status. Therefore, we felt it was reasonable to use a postpartum DXA both to measure BMC for use in the 4-compartment calculation and to become our comparison value for maternal FM measured at 37 wk of gestation. We acknowledge that researchers may have other preferences for a comparison standard and have provided our raw data for Deming regression and Bland-Altman analyses between each method to allow for additional comparisons (Tables 4 and 5, Supplemental Tables 1 and 2). In addition, despite strict instructions and close monitoring, 7 subjects appeared to have consumed fluids during the 2H2O salivary equilibration period, which can alter the TBW determination. We found that the required overnight fast, followed by an additional 3 h with no water or food intake and minimized activity during the salivary collection period, is challenging for women during the third trimester of pregnancy and likely contributed to variation in the TBW measurements determined by 2H2O in this study. These experiences highlight the challenges associated with attempting to accurately measure body composition in pregnancy. This study was completed in a midsized metropolitan area among subjects in late pregnancy with a range of BMIs from normal to obese and included 18% non-Caucasians but may not be generalizable to other populations or other time points in pregnancy.
In summary, this study compared multiple body composition measurement methods for maternal FM used in both clinical and research settings of women in late pregnancy. We specifically examined women of various prepregnancy BMI weight categories to help determine the most effective tool for studies of obesity-related comorbidities. For larger field studies and studies with limited resources, SFT can reasonably estimate maternal FM, whereas BIA measures of FM appear to be less reliable. Where available, the use of ADP with pregnancy-specific formulas is perhaps the preferred method for assessing maternal FM in late pregnancy. It is well tolerated by subjects and less prone to measurement error than obtaining salivary collection samples for 2H2O for use in 3-compartment equations.
Acknowledgments
The authors’ responsibilities were as follows—NEM, EJM, KLT, and JQP: designed the research; NEM, EJM, and EKH: conducted the research; NEM, EJM, JCK, JYL, JW, KLT, and JQP: analyzed the data and wrote the article; NEM: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: ADP, air displacement plethysmography; BIA, bioelectrical impedance analysis; BMC, bone mineral content; Db, body density; Db(BodPod), air displacement plethysmography body density; Db(VRJ), van Raaij body density; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; FM, fat mass; SFT, skinfold thickness; TBW, total body water; TBW(VRJ), van Raaij total body water; VRJ, van Raaij; 2H2O, deuterium dilution.
REFERENCES
- 1.Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 2013;37:889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins CL, Zapata LB, Farr SL, Kroelinger CD, Morrow B, Ahluwalia I, D’Angelo DV, Barradas D, Cox S, Goodman D, et al. Core state preconception health indicators—pregnancy risk assessment monitoring system and behavioral risk factor surveillance system, 2009. MMWR Surveill Summ 2014;63:1–62. [PubMed] [Google Scholar]
- 3.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol 2012;206:417.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mission JF, Marshall NE, Caughey AB. Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv 2013;68:389–99. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 105: bariatric surgery and pregnancy. Obstet Gynecol 2009;113:1405–13. [DOI] [PubMed] [Google Scholar]
- 6.Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes 1985;9:147–53. [PubMed] [Google Scholar]
- 7.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr 2002;75:978–85. [DOI] [PubMed] [Google Scholar]
- 8.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452. [PubMed] [Google Scholar]
- 9.Lindsay CA, Huston L, Amini SB, Catalano PM. Longitudinal changes in the relationship between body mass index and percent body fat in pregnancy. Obstet Gynecol 1997;89:377–82. [DOI] [PubMed] [Google Scholar]
- 10.Sewell MF, Huston-Presley L, Amini SB, Catalano PM. Body mass index: a true indicator of body fat in obese gravidas. J Reprod Med 2007;52:907–11. [PubMed] [Google Scholar]
- 11.Catalano PM, Wong WW, Drago NM, Amini SB. Estimating body composition in late gestation: a new hydration constant for body density and total body water. Am J Physiol 1995;268:E153–8. [DOI] [PubMed] [Google Scholar]
- 12.Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith EO. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. Am J Clin Nutr 1997;65:432–8. [DOI] [PubMed] [Google Scholar]
- 13.Huston Presley L, Wong WW, Roman NM, Amini SB, Catalano PM. Anthropometric estimation of maternal body composition in late gestation. Obstet Gynecol 2000;96:33–7. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy EA, Strauss BJ, Walker SP, Permezel M. Determination of maternal body composition in pregnancy and its relevance to perinatal outcomes. Obstet Gynecol Surv 2004;59:731,42; quiz 745–6. [DOI] [PubMed] [Google Scholar]
- 15.Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol 1979;86:929–40. [DOI] [PubMed] [Google Scholar]
- 16.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr 1988;48:24–9. [DOI] [PubMed] [Google Scholar]
- 17.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN Jr. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol 1997;90:483–8. [DOI] [PubMed] [Google Scholar]
- 18.Paxton A, Lederman SA, Heymsfield SB, Wang J, Thornton JC, Pierson RN Jr. Anthropometric equations for studying body fat in pregnant women. Am J Clin Nutr 1998;67:104–10. [DOI] [PubMed] [Google Scholar]
- 19.Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol 1999;87:196–202. [DOI] [PubMed] [Google Scholar]
- 20.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr 2002;75:453–67. [DOI] [PubMed] [Google Scholar]
- 21.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr 1967;21:681–9. [DOI] [PubMed] [Google Scholar]
- 22.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 1993;9:480–91; discussion 480, 492. [PubMed]
- 23.Forbes G. Techniques for estimating body composition In: Fobes G, editor. Human body composition New York: Springer-Verlag; 1987. p. 5–100. [Google Scholar]
- 24.Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci (Lond) 1992;82:687–93. [DOI] [PubMed] [Google Scholar]
- 25.Widen EM, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 2014;68:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lederman SA. Pregnancy. In: Heymsfield SB, Lohman TG, Wang Z and Going TG, editors. Human body composition. Champaign (IL): Human Kinetics, 2005.
- 27.Lohman TG, Chen Z. Dual-energy X-ray absorptiometry. In: Heymsfield SB, Lohman TG, Wang Z, Going TG, editors. Human body composition. Champaign (IL): Human Kinetics; 2005. p. 63–77.
- 28.Butte NF, Hopkinson JM, Ellis KJ, Wong WW, Smith EO. Changes in fat-free mass and fat mass in postpartum women: a comparison of body composition models. Int J Obes Relat Metab Disord 1997;21:874–80. [DOI] [PubMed] [Google Scholar]
- 29.Jebb SA, Goldberg GR, Elia M. DXA measurements of fat and bone mineral density in relation to depth and adiposity. Basic Life Sci 1993;60:115–9. [DOI] [PubMed] [Google Scholar]
- 30.Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol 1998;274:E808–16. [DOI] [PubMed] [Google Scholar]
- 31.Henriksson P, Lof M, Forsum E. Assessment and prediction of thoracic gas volume in pregnant women: an evaluation in relation to body composition assessment using air displacement plethysmography. Br J Nutr 2013;109:111–7. [DOI] [PubMed] [Google Scholar]
- 32.Denne SC, Patel D, Kalhan SC. Total body water measurement in normal and diabetic pregnancy: evidence for maternal and amniotic fluid equilibrium. Biol Neonate 1990;57:284–91. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JP, Mulligan K, Fan B, Sherman JL, Murphy EJ, Tai VW, Powers CL, Marquez L, Ruiz-Barros V, Shepherd JA. Dual-energy X-ray absorptiometry–based body volume measurement for 4-compartment body composition. Am J Clin Nutr 2012;95:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr 1982;36:680–90. [DOI] [PubMed] [Google Scholar]
- 35.Linnet K. Evaluation of regression procedures for methods comparison studies. Clin Chem 1993;39:424–32. [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10. [PubMed] [Google Scholar]
- 37.Forsum E, Henriksson P, Lof M. The two-component model for calculating total body fat from body density: an evaluation in healthy women before, during and after pregnancy. Nutrients 2014;6:5888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidebottom AC, Brown JE, Jacobs DR Jr. Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reprod Biol 2001;94:216–23. [DOI] [PubMed] [Google Scholar]
- 39.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr 2000;84:95–101. [DOI] [PubMed] [Google Scholar]
- 40.Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr 1967;21:439–51. [DOI] [PubMed] [Google Scholar]
- 41.Fidanza F. The density of fat-free body mass during pregnancy. Int J Vitam Nutr Res 1987;57:104. [PubMed] [Google Scholar]
- 42.Lof M, Forsum E. Hydration of fat-free mass in healthy women with special reference to the effect of pregnancy. Am J Clin Nutr 2004;80:960–5. [DOI] [PubMed] [Google Scholar]
- 43.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr 1995;61:514–23. [DOI] [PubMed] [Google Scholar]