Abstract
Background: Studies have suggested that the fat mass and obesity–associated (FTO) genotype is associated with individual variability in weight loss in response to diet/lifestyle interventions, but results are inconsistent.
Objective: We aimed to provide a summary of the literature evaluating the relation between the FTO genotype and weight loss in response to diet/lifestyle interventions.
Design: A search of English-language articles in the PubMed and Embase databases (through 30 April 2015) was performed. Eligible studies were diet/lifestyle weight-loss intervention studies conducted in adults that reported changes in body weight or body mass index (BMI) by the FTO variant rs9939609 (or its proxy). Differences in weight loss between FTO genotypes across studies were pooled with the use of fixed-effect models.
Results: A meta-analysis of 10 studies (comprising 6951 participants) that reported the results of additive genetic models showed that individuals with the FTO TA genotype and AA genotype (those with the obesity-predisposing A allele) had 0.18-kg (95% CI: −0.09-, 0.45-kg; P = 0.19; NS) and 0.44-kg (95% CI: 0.09-, 0.79-kg; P = 0.015) greater weight loss, respectively, than those with the TT genotype. A meta-analysis of 14 studies (comprising 7700 participants) that reported the results of dominant genetic models indicated a 0.20-kg (−0.43-, 0.04-kg) greater weight loss in the TA/AA genotype than in the TT genotype (P = 0.10). In addition, differences in weight loss between the AA genotype and TT genotype were significant in studies with a diet intervention only, adjustment for baseline BMI or body weight, and several other subgroups. However, the relatively small number of studies limited these stratified analyses, and there was no statistically significant difference between subgroups.
Conclusions: This meta-analysis suggests that individuals carrying the homozygous FTO obesity-predisposing allele may lose more weight through diet/lifestyle interventions than noncarriers. Our data provide evidence for genetic variability in response to diet/lifestyle interventions on weight loss, although clinical applications of these findings need further investigations.
Keywords: FTO genotype, lifestyle intervention, weight loss, meta-analysis, diet
See corresponding editorial on page 963.
INTRODUCTION
Obesity and its comorbidity have become major public health problems throughout the world (1). It is well established that diet/lifestyle interventions can achieve weight loss (2). However, individual variability in response to interventions has long been noted in weight-loss trials (3, 4). Besides behavioral and psychological characteristics, genetic factors may explain why diet/lifestyle interventions are more effective for some individuals than for others (2, 5). Thus, a better understanding of the modification effects of genetic variation on weight loss in response to diet/lifestyle interventions may help to develop more effective strategies for weight loss, such as individualized interventions based on one’s genetic background (5, 6).
With the advent of genome-wide association studies, many genetic loci have been identified as associated with obesity and related traits (7). Given its strong effect on obesity and possible biological function in regulating energy balance (8), there is great interest in the fat mass and obesity–associated (FTO) gene. Recent large-scale analyses found that the obesity-risk allele (A allele) of the FTO variant is associated with increased food intake (9, 10), and previous studies also reported that the FTO obesity-risk allele was associated with a reduced response in hunger and satiety after the meal in adults and children (11, 12). A number of studies have examined whether diet/lifestyle–induced weight loss differs between the FTO genotype groups (13–21). However, results from these previous studies remain contradictory, and discrepancies might be due to small sample size, moderate genetic effect, types of interventions, variation in study duration, and other characteristics. Therefore, to increase statistical power and achieve a more precise estimation of effects, we conducted a systematic review and meta-analysis of randomized weight-loss trials in adults to provide a summary of the literature evaluating the relation between FTO genotype and weight loss in response to diet/lifestyle interventions.
METHODS
Literature search
This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (22, 23). We searched the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and Embase (http://www.embase.com) databases through 30 April 2015 for diet/lifestyle intervention studies examining the relation between FTO genotype and weight loss in adults.
Two search themes are specified. The first theme identified relevant terms for FTO, and combined exploded versions of the MeSH terms “fat mass and obesity–associated genes” and “FTO.” The second theme identified relevant terms for weight and BMI, and combined exploded versions of the MeSH terms “body weight” and “ body mass index.” Two search terms were combined with the use of the Boolean operator “and.” Additional articles were identified from reference lists of selected studies. Our search strategy included terms for BMI because some of these studies also report change in BMI. Details regarding search terms are shown in Supplemental Table 1.
Study selection
Articles were included if they met the following criteria: 1) they were diet/lifestyle weight-loss intervention studies; 2) they were conducted in adults aged ≥18 y; 3) they did not involve medication interventions; 4) they reported changes in body weight or BMI by FTO genotypes; 5) they included peer-reviewed publications with sufficient information for the analysis; and 6) they were in the English language. Two investigators independently screened all of the studies by title or abstract and then by a full-text review. Discrepancies of screening results between the 2 investigators were solved by discussing with a senior investigator.
Data extraction
We extracted the following information from each identified article: basic information from studies (authors, publication year, study duration, number of participants, and FTO variant and its minor allele frequency), demographics of participants (mean age, sex ratio, mean BMI, race, and ethnicity), intervention methods, analysis strategy (statistical models, with covariates included in the models), and mean weight changes and their corresponding SDs. SDs were calculated with the use of SEs or 95% CIs when necessary. For articles with missing SDs for measurement of change (20, 24–26), change-from-baseline SDs were imputed by using the correlation coefficient method presented in the Cochrane Handbook for Systematic Reviews of Interventions (27). We used a correlation coefficient of 0.9 between baseline and follow-up weight because the correlation between body weights at the 2 time points was assumed to be very high. One study presented the results (mean weight changes) in a figure (24), and we extracted the estimates carefully from the given figure. For studies with an initial weight-loss phase followed by a weight-maintenance phase (16, 17, 24), we used data on long-term weight loss. For studies that reported results of weight change without exactable data (14, 28, 29), we contacted the first or corresponding authors to request detailed data; 2 authors replied with the requested data (14, 28). We also contacted the authors of the studies that only reported data in dominant genetic models and requested data on additive genetic models (13, 15, 25, 26), although none of them replied to our request. Thus, we did not include these 4 studies in our primary meta-analysis of studies reporting data in additive genetic models.
Data synthesis and statistical analysis
The difference in weight loss (in kg) between the FTO genotype groups in response to diet/lifestyle interventions was designated as our principal effect. In this meta-analysis, each selected study was considered to be a single study unit, and mean weight loss by the FTO genotype groups in the overall study sample, regardless of intervention differences, was taken into account. For studies that reported weight loss for intervention groups separately (15, 18, 27), we combined the results of different groups with the use of the combining method recommended by the Cochrane Handbook for Systematic Reviews of Interventions (30). We used the same method to combine results of the FTO TA and AA genotype groups for studies that provided results of additive genetic models (16–21, 24, 31). All of the data synthesis was conducted after the data collecting and data requesting process.
A heterogeneity test was conducted with the use of 2 different methods, the Cochran’s Q test and the I2 statistic (32, 33). A P value <0.1 and I2 >50% were defined to indicate statistically significant heterogeneity in meta-analysis, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. Because no significant heterogeneity was detected in our analysis, results were presented in the fixed-effect model. In addition, the possibility of publication bias was evaluated by using a Begg’s test and funnel plots (34, 35). Moreover, stratified analyses were performed to evaluate the influence of study characteristics on results. STATA software (version 12.0) was used to perform the meta-analysis.
RESULTS
Results of literature search
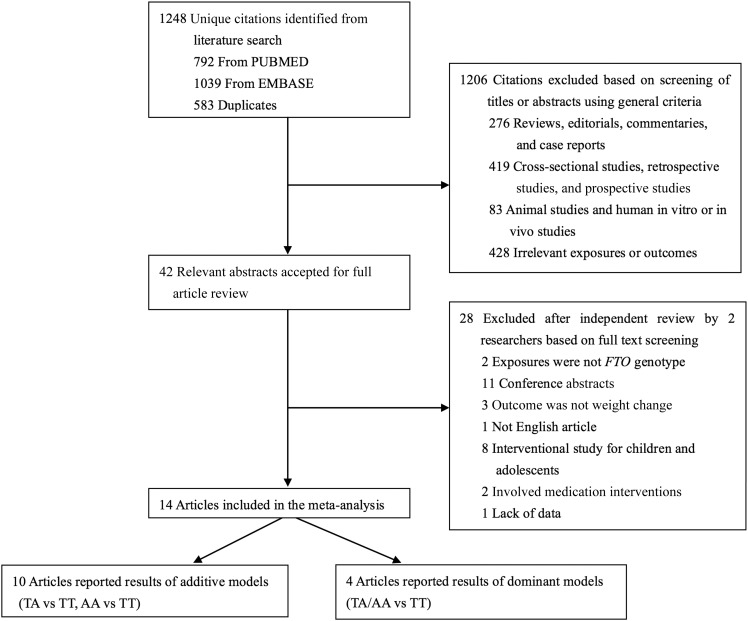
A total of 1248 unique citations were identified by our search strategy (792 from PubMed and 1039 from Embase, with 583 duplicates), of which 42 were accepted for full text review after screening by titles and abstracts. From the remaining 42 articles, we excluded papers with results stratified by genotypes other than FTO (n = 2) (36, 37), conference abstracts (n = 11) (38–48), studies in children (n = 8) (49–56), those that did not have weight or BMI change as the main outcome (n = 3) (57–59), those with data not available (n = 1) (29), and studies that were not in the English language (n = 1) (60). Ultimately, 14 articles were eligible and included in our meta-analysis (13–21, 24–26, 28, 31). A detailed screening flow is shown in Figure 1. Of the 14 selected articles, 10 reported the results of an additive genetic model (TA compared with TT, AA compared with TT) (6, 7, 11, 12, 16–21), whereas the other 4 articles reported the results of a dominant genetic model (TA/AA compared with TT) (13, 15, 25, 26), which was mainly the result of limited sample size in these 4 studies. Given the known additive genetic effect of FTO variant on BMI and obesity risk, our primary analyses focused on the 10 studies that reported additive genetic model results.
FIGURE 1.
Literature search for the meta-analysis. FTO, fat mass and obesity–associated.
Study characteristics
The primary characteristics of the 14 studies included in our meta-analysis are shown in Table 1. Overall, 7700 participants were included in this meta-analysis, with 33.1% (n = 2547) having the TT genotype (reference group). The sample size varied from 75 to 3756 in the 14 studies. Nine studies were conducted in European countries, 3 studies were conducted in the United States, 1 study was conducted in Brazil, and 1 study was conducted in Japan. The age of participants ranged from 18 to 80 y, and the mean baseline BMI (in kg/m2) of each study ranged from 28.5 to 41.8. Three studies recruited female participants exclusively (20, 24, 31), and the remaining 11 studies recruited both sexes. Thirteen studies investigated the FTO single-nucleotide polymorphism rs9939609, and one study examined a perfect proxy single-nucleotide polymorphism (rs8050136; r2 = 1) (20).
TABLE 1.
Characteristics of studies included in the meta-analysis1
| Study first author and year (ref) | Enrollment or completers, n | Country or region | Age, y | Male, % | BMI, kg/m2 | Intervention | Energy restricted | Duration | FTO SNP | MAF | Adjustment |
| Curti 2013 (26)2 | 134 | Brazil | 56.6 (18–80)3 | 34 | 30.4 (NA) | Diet and exercise | Yes | 9 mo | rs9939609 | 0.42 | NA |
| de Luis 2012 (15)2 | 305 | Spain | 43.5 (NA) | 26 | 36.6 (>30) | Diet and exercise | Yes | 3 mo | rs9939609 | 0.44 | NA |
| de Luis 2013 (13)2 | 106 | Spain | 49.5 (NA) | 34 | 34.8 (>30) | Low-fat hypocaloric diet | Yes | 3 mo | rs9939609 | 0.45 | NA |
| Grau 2009 (18) | 618 | Europe | NA (20–50) | 25 | 35.5 (≥30) | Two low-energy diets with either low or high fat content | Yes | 2 y | rs9939609 | 0.40 | Age, sex, baseline weight, fat-free mass, fat mass, WC, fat oxidation |
| Haupt 2008 (25)2 | 204 | Germany | 45.9 (NA) | 40 | 29.1 (>27) | Diet and exercise | Yes | 9 mo | rs8050136 | NA | Unadjusted |
| Lappalainen 2009 (28) | 412 | Finland | 55.3 (40–56) | 33 | 31.2 (≥25) | Diet and exercise | Yes | 4 y | rs9939609 | 0.42 | Age, sex, baseline BMI |
| Matsuo 2012 (31) | 204 | Japan | 51.9 (24–66) | 0 | 28.5 (≥25) | Dietary lectures | No | 14 wk | rs9939609 | 0.26 | Age, menstrual status, baseline values |
| McCaffery 2013 (14) | 3756 | United States | 59.0 (45–76) | 44 | 36.2 (>25) | Diet and exercise | Yes | 1 y | rs9939609 | 0.49 | Age, sex, study site, ancestry informative markers |
| Mitchell 2010 (20) | 234 | United States | 58.1 (45–75) | 0 | 31.6 (25–43) | Three groups of different exercise intensity | No | 6 mo | rs8050136 | 0.43 | Exercise assignment |
| Rauhio 2013 (24) | 75 | Finland | 39.6 (25–45) | 0 | 34.0 (>30) | Very low–energy diet followed by weight maintenance | Yes | 1 y | rs9939609 | 0.45 | Age, sex |
| Razquin 2010 (19) | 776 | Spain | 67.8 (55–80) | 45 | 29.2 (NA) | Two Mediterranean diets and one conventional low-fat diet | Yes | 3 y | rs9939609 | 0.45 | Age, sex, baseline BMI, diabetes status |
| Verhoef 2014 (17) | 148 | Netherlands | NA (20–50) | 35 | 32 (27–38) | Very low–energy diet followed by weight maintenance | Yes | 5 mo | rs9939609 | 0.39 | Age, sex, baseline weight, short-term weight loss |
| Woehning 2013 (16) | 125 | Germany | 44.6 (18–72) | 33 | 41.8 (≥30) | Very low–energy diet followed by weight maintenance | Yes | 52 wk | rs9939609 | 0.48 | NA |
| Zhang 2012 (21) | 603 | United States | 51 (30–70) | 40 | 33 (25–40) | Four low-energy diets with different compositions of macronutrients | Yes | 2 y | rs1558902 | 0.40 | Age, sex, race, baseline BMI, intervention group |
FTO, fat mass and obesity–associated; MAF, minor allele frequency; NA, not available; ref, reference; SNP, single-nucleotide polymorphism; WC, waist circumference.
Not included in the primary meta-analysis of studies that reported results of additive genetic models because they reported only results of dominant genetic models.
Mean; range in parentheses (all such values).
The intervention methods were diverse. Diet modification was used in 12 trials (13–19, 21, 24–26, 28), and 4 of them combined interventions on diet with physical activity (14, 25, 26, 28). One study only used physical activity modulation (20), and the other study involved only nutritional education to encourage people to consume a balanced and healthy diet (31). The interventions varied in length from 3 mo to 4 y, with a median duration of 9 mo.
In 9 studies, changes in body weight were calculated from multivariable-adjusted models (14, 17–21, 24, 28, 31). One study explicitly mentioned that the model was unadjusted (25). For the other 4 studies (13, 15, 16, 18), no information was provided regarding the statistical adjustment.
FTO genotype and weight loss
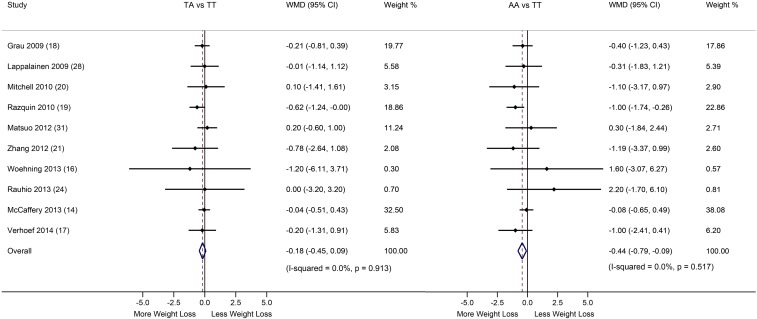
We first conducted a meta-analysis of 10 studies (including 6951 subjects) with data from additive genetic models to compare weight loss across the FTO genotype groups. Greater weight loss induced by diet/lifestyle interventions was observed in the FTO TA genotype [−0.18 kg (95% CI: −0.45, 0.09 kg); P = 0.19; NS] and AA genotype [−0.44 kg (95% CI: −0.09, −0.79 kg); P = 0.015] groups than in the TT genotype group (Q Qi, unpublished data, 2014) (Figure 2). No heterogeneity was observed in either comparison (AA compared with TT: I2 = 0.0%; TA compared with TT: I2 = 0.0%).
FIGURE 2.
Differences in weight loss in the FTO TA and AA genotype groups compared with the TT genotype group. Data are WMDs and 95% CIs in weight loss between the FTO genotype groups across studies, and pooled results were calculated with the use of fixed-effect models. A Cochran’s Q test and the I2 statistic were used to test the heterogeneity between studies. Horizontal lines denote the 95% CIs; solid diamonds represent the point estimated of each study. The open diamond represents the pooled estimate of the lifestyle intervention effect. The dashed lines indicated the point estimate of the pooled result. FTO, fat mass and obesity–associated; WMD, weighted mean difference.
When carrying out a meta-analysis of 14 studies that reported dominant genetic model results as a secondary analysis, a tendency for greater weight loss was observed in the TA/AA genotype with TT genotype groups [−0.20 kg (95% CI: −0.43, 0.04 kg), P = 0.10; I2 = 0.0%] (Supplemental Figure 1).
Stratified analysis
Because studies included in the meta-analysis varied in intervention methods and many other characteristics, we then performed subgroup analyses to investigate the influences of study characteristics on pooled results. Studies were classified according to age (mean age <50 or ≥50 y), sex (mixed or women only), BMI (mean baseline BMI <35 or ≥35), baseline adjustments (adjusted for baseline BMI or body weight, or not adjusted for baseline BMI or body weight), regions in which studies were conducted (America, Europe, or Asia), methods of intervention (diet only or other interventions), and study duration (<1 y or ≥1 y).
A total of 10 studies with 6951 participants were included in the stratified analyses. No statistically significant difference between subgroups was observed for any of these stratified analyses (Table 2), although some significant results were observed in several subgroups. Specifically, a significantly greater weight change was observed in those with the TA genotype [−0.40 kg (95% CI: −0.79, −0.01 kg); P = 0.04] and AA genotype [−0.72 kg (95% CI: −1.21, −0.23 kg); P = 0.004] than in those with the TT genotype group in studies that used diet intervention only (all energy-restricted). In studies with adjustment for baseline BMI or body weight, we also found significantly greater weight loss in the AA genotype group than in the TT genotype group [−0.70 kg (95% CI: −1.16, −0.23 kg); P = 0.003]. In addition, significantly greater weight change was observed in the AA genotype group than in the TT genotype group in studies with participants who were ≥50 y old [−0.44 kg (95% CI: −1.12, −0.28 kg); P = 0.033], those with participants who had a baseline mean BMI <35 [−0.70 kg (95% CI: −1.16, −0.23 kg); P = 0.003], studies with men and women combined (mixed) [−0.46 kg (95% CI: −0.83, 0.10 kg); P = 0.013], and studies conducted in Europe [−0.42 kg (95% CI: −0.79, −0.05 kg), P = 0.026].
TABLE 2.
Stratified analysis of FTO genotype and weight-loss according to study characteristics1
| TA vs. TT |
AA vs. TT |
|||||||||
| Subgroups | Sample size, n | Studies, n | WMD | 95% CI | I2 value | P between groups | WMD | 95% CI | I2 value | P between groups |
| Mean age | 0.88 | 0.95 | ||||||||
| <50 | 966 | 4 | −0.21 | (−0.73, 0.31) | 0.0 | −0.42 | (−1.12, 0.28) | 3.2 | ||
| ≥50 | 5985 | 6 | −0.17 | (−0.48, 0.15) | 0.0 | −0.44 | (−0.85, −0.04) | 1.4 | ||
| Mean BMI at baseline | 0.61 | 0.08 | ||||||||
| <35 | 2452 | 7 | −0.25 | (−0.64, 0.14) | 0.0 | −0.79 | (−1.33, −0.26) | 0.0 | ||
| ≥35 | 4499 | 3 | −0.11 | (−0.48, 0.26) | 0.0 | −0.17 | (−0.63, 0.30) | 0.0 | ||
| Duration | 0.51 | 0.74 | ||||||||
| <1 y | 1204 | 4 | −0.07 | (−0.49, 0.35) | 0.0 | −0.53 | (−1.18, 0.12) | 0.0 | ||
| ≥1 y | 5747 | 6 | −0.25 | (−0.60, 0.09) | 0.0 | −0.40 | (−0.82, 0.02) | 25.1 | ||
| Intervention | 0.12 | 0.11 | ||||||||
| Diet intervention only | 2345 | 6 | −0.40 | (−0.79, −0.01) | 0.0 | −0.72 | (−1.21, −0.23) | 0.0 | ||
| Other2 | 4606 | 4 | 0.02 | (−0.35, 0.39) | 0.0 | −0.15 | (−0.65, 0.49) | 0.0 | ||
| Sex | 0.28 | 0.61 | ||||||||
| Mixed | 6438 | 7 | −0.24 | (−0.53, 0.05) | 0.0 | −0.46 | (−0.83, −0.10) | 0.0 | ||
| Female | 513 | 3 | 0.17 | (−0.52, 0.86) | 0.0 | −0.09 | (−1.48, 1.30) | 15.5 | ||
| Region | 0.40 | 0.38 | ||||||||
| America | 4593 | 3 | −0.07 | (−0.51, 0.37) | 0.0 | −0.22 | (−0.75, 0.32) | 0.0 | ||
| Europe | 2154 | 6 | −0.34 | (−0.72, 0.03) | 0.0 | −0.66 | (−1.14, −0.18) | 0.0 | ||
| Asia | 204 | 1 | 0.20 | (−0.60, 1.00) | NA | 0.30 | (−0.79, −0.09) | NA | ||
| Adjusted for baseline BMI or body weight | 0.66 | 0.19 | ||||||||
| Yes | 2761 | 6 | −0.26 | (−0.60, 0.08) | 0.0 | −0.70 | (−1.16, −0.23) | 0.0 | ||
| No | 4190 | 4 | −0.04 | (−0.51, 0.43) | 0.0 | −0.09 | (−0.63, 0.46) | 0.0 | ||
Differences in weight loss after intervention between FTO genotypes across studies were pooled with the use of fixed-effect models. The heterogeneity between studies for each group was tested by I2. P values between groups were tested with the use of Cochran’s Q test. FTO, fat mass and obesity–associated; NA, not available; WMD, weighted mean difference.
Includes studies with exercise interventions, both exercise and diet interventions, or nutritional education.
Sensitivity analysis
We conducted a sensitivity analysis in the 10 primary studies by excluding one study at a time to examine the individual effect of each study on the overall results. The estimates (differences in weight loss) comparing the FTO AA and TT genotypes ranged from −0.66 kg to −027 kg, with the biggest influence coming from the study by McCaffery et al. (14) (Supplemental Figure 2). The results became even more significant after the exclusion of this study [AA compared with TT, −0.66 kg (95% CI: −1.10, −0.21 kg); P = 0.004].
In addition, we repeated the meta-analysis by excluding studies without reporting SDs for measurements of weight change, and the results were similar [TA compared with TT: −0.19 kg (95% CI: −0.46, 0.08 kg); P = 0.19. AA compared with TT: −0.44 kg (95% CI: −0.80, −0.08 kg); P = 0.016].
Publication bias
On the basis of funnel plots (Supplemental Figure 3) and Egger’s tests, no significant publication bias was observed in this meta-analysis (TA compared with TT: P = 0.78; AA compared with TT: P = 0.75).
DISCUSSION
In this meta-analysis of weight-loss trials, we found that individuals carrying the homozygous FTO obesity-predisposing allele (AA genotype) had greater weight loss than did noncarriers (TT genotype) after diet/lifestyle interventions. Furthermore, differences in weight loss between the FTO AA and TT genotype groups became more significant in several subgroups stratified by various study and participant characteristics.
To the best of our knowledge, no previous meta-analysis has been conducted to investigate the effect of the FTO variant on weight loss in response to diet/lifestyle interventions. All previous meta-analyses were based on observational studies investigating interrelations between FTO variant, diet/lifestyle factors, and obesity. For example, a large meta-analysis suggested that greater physical activity attenuates the association of FTO genotype and obesity in adults (61). Moreover, our prior meta-analysis indicated that the obesity-predisposing allele of FTO is associated with higher total energy intake in adults and higher protein intake in children (9, 10), although whether there is an interaction between FTO genotype and dietary intake on obesity remains controversial. In the current meta-analysis, we examined the FTO genetic effect on weight loss in randomized intervention trials, which provided more reliable evidence because the study conditions were prescribed and the confounding effects were maximally reduced. Taken together, these findings support the interactive roles of the FTO gene and diet/lifestyle factors in the regulation of body weight.
It is not surprising that the observed difference in weight loss between the FTO genotype groups is modest, which is consistent with the modest effect of the FTO variant on BMI (∼0.35/allele) (61). In addition, although the result was not significant when comparing the TA and TT genotype groups, the trend of weight loss increased across the 3 genotype groups, which is in line with the additivity of the FTO genetic effect. Admittedly, the observed difference in weight loss between the FTO genotype groups may not translate into a clinically important benefit; however, the true effect might be underestimated because of heterogeneity of intervention modality. For example, the effect size is greater with diet interventions [−0.72 kg (95% CI: −1.21, −0.22 kg) between the AA and TT genotypes] than with other interventions [−0.15 kg (95% CI: −0.65, −0.49 kg)], although there is no statistically significant difference between subgroups, which might be due to the limited number of studies and participants in this stratified analysis. Thus, more studies are needed to examine whether intervention modality may influence the FTO genetic effect on weight loss, and the clinical relevance of our findings needs further investigations.
It is interesting that the homozygous FTO obesity-predisposing genotype is associated with greater weight loss induced by diet/lifestyle interventions. Several other previous studies also reported that individuals carrying risk alleles exhibited greater improvement of respective traits than did noncarriers after diet interventions (62–65). One may argue that this may reflect baseline weight difference for different genotypes: heavier people (the risk allele carriers) tend to lose more weight. However, the significant FTO genotype effect on weight loss was observed in the meta-analysis of 6 studies with adjustment for baseline BMI or body weight [−0.70 kg (95% CI: −1.16, −0.23 kg) between the AA and TT genotypes], and there was no significant difference between groups with and without adjustment for baseline BMI or body weight. In addition, it is possible that individuals carrying the FTO obesity genotype may have attempted more frequently to lose body weight and are therefore more successful in weight loss, at least in the short term. However, in the long term, their genetic predisposition to obesity could result in a cycle of weight loss and regain. This might explain a previously reported association between the FTO genotype and variation in BMI (66). Nevertheless, further studies are needed to investigate whether the observed genetic influence on diet/lifestyle–induced weight loss could be maintained in the long term.
The mechanisms underlying our findings are unknown but might be related to the potential role of FTO in regulating energy homeostasis. FTO expression in the hypothalamus is regulated by feeding, fasting, and energy restriction (67–73). Both animal and human studies support the association between FTO and food intake (9, 10, 74), and FTO also has been linked to habitual appetitive behaviors (11, 12) and appetite-related hormones (ghrelin and leptin) (75, 76). Moreover, a recent study reported that the obesity-predisposing allele of the FTO variant was associated with a reduction in food cravings and appetite during an intervention with hypocaloric weight-loss diets (44). Consistently, our subgroup analysis also indicates a stronger effect of FTO on weight loss in response to energy-restricted diet interventions than with other interventions. In addition, it should be noted that the FTO variants may affect the functions of other genes rather than the FTO itself. A recent study reported that the FTO obesity-associated variants are associated with expression of the homeobox gene Iroquois-class homeobox protein 2 (IRX3) (77).
Several limitations of this meta-analysis should be acknowledged. First, we were unable to include 4 studies (13, 15, 25, 26) in our primary analysis because of difficulty in obtaining additive genetic model results. However, a secondary meta-analysis of 14 studies based on the dominant genetic model showed similar but nonsignificant results. Second, studies included in our meta-analysis varied in intervention methods and duration, sample size, study setting, race/ethnicity, and other participant characteristics, although there was no significant heterogeneity in results across individual studies or subgroups. We had a limited sample size in the stratified analyses, and more studies are needed. Third, we examined the FTO genetic effect on weight loss regardless of the various diets and other interventions applied in the individual trials, although some specific FTO–diet intervention interactions have been reported (19, 21). However, there are insufficient numbers of published studies with a similar design for the meta-analysis. Moreover, we only included papers published in the English language, and may have missed some eligible studies published in other languages.
In conclusion, this study provides some evidence from a meta-analysis that weight loss varies between the FTO genotypes in response to diet/lifestyle interventions. Our findings provide some support for considering genetic variability in response to diet/lifestyle interventions in the development of more effective strategies for weight loss. Nevertheless, more studies are needed to explore which types of diet/lifestyle interventions most powerfully facilitate the FTO genetic effect on weight loss.
Acknowledgments
We thank Jeanne McCaffery, from the Miriam Hospital and the Warren Alpert School of Medicine at Brown University, and Tiina Jääskeläinen, from the Department of Clinical Nutrition, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, for providing unpublished data.
The authors’ responsibilities were as follows—LX and QQ: conceived of and designed the research, performed the statistical analysis, and wrote the manuscript; LX, BP, and QQ: conducted the literature review and data collection; and all authors: revised the manuscript for intellectual content and read and approved the final version of the manuscript. None of the authors reported a conflict of interest related to the study.
REFERENCES
- 1.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–6. [DOI] [PubMed] [Google Scholar]
- 2.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espeland MA, Bray GA, Neiberg R, Rejeski WJ, Knowler WC, Lang W, Cheskin LJ, Williamson D, Lewis CB, Wing R, et al. Describing patterns of weight changes using principal components analysis: results from the Action for Health in Diabetes (Look AHEAD) research group. Ann Epidemiol 2009;19:701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA, Wadden TA. Improving long-term weight loss maintenance: can we do it? Obesity (Silver Spring) 2015;23:2–3. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Wylie-Rosett J, Qi Q. Dietary interventions for weight loss and maintenance: preference or genetic personalization? Curr Nutr Rep 2013;2:189–98. [Google Scholar]
- 7.Lu Y, Loos RJ. Obesity genomics: assessing the transferability of susceptibility loci across diverse populations. Genome Med 2013;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos RJ, Yeo GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 2014;10:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Q, Kilpelainen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, Sonestedt E, Chu AY, Renstrom F, Lin X, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet 2014;23:6961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Q, Downer MK, Kilpelainen TO, Taal HR, Barton SJ, Ntalla I, Standl M, Boraska V, Huikari V, Kiefte-de Jong JC, et al. Dietary intake, FTO genetic variants, and adiposity: a combined analysis of over 16,000 children and adolescents. Diabetes 2015;64:2467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Hoed M, Westerterp-Plantenga MS, Bouwman FG, Mariman EC, Westerterp KR. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr 2009;90:1426–32. [DOI] [PubMed] [Google Scholar]
- 12.Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 2008;93:3640–3. [DOI] [PubMed] [Google Scholar]
- 13.de Luis DA, Aller R, Conde R, Izaola O, Sagrado MG, Sanz JC. The rs9939609 gene variant in FTO modified the metabolic response of weight loss after a 3-month intervention with a hypocaloric diet. J Investig Med 2013;61:22–6. [DOI] [PubMed] [Google Scholar]
- 14.McCaffery JM, Papandonatos GD, Huggins GS, Peter I, Kahn SE, Knowler WC, Hudnall GE, Lipkin EW, Kitabchi AE, Wagenknecht LE, et al. FTO predicts weight regain in the Look AHEAD clinical trial. Int J Obes (Lond) 2013;37(12):1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Luis DA, Aller R, Izaola O, de la Fuente B, Conde R, Sagrado MG, Primo D. Evaluation of weight loss and adipocytokines levels after two hypocaloric diets with different macronutrient distribution in obese subjects with rs9939609 gene variant. Diabetes Metab Res Rev 2012;28:663–8. [DOI] [PubMed] [Google Scholar]
- 16.Woehning A, Schultz JH, Roeder E, Moeltner A, Isermann B, Nawroth PP, Wolfrum C, Rudofsky G. The A-allele of the common FTO gene variant rs9939609 complicates weight maintenance in severe obese patients. Int J Obes (Lond) 2013;37(1):135–9. [DOI] [PubMed] [Google Scholar]
- 17.Verhoef SP, Camps SG, Bouwman FG, Mariman EC, Westerterp KR. Genetic predisposition, dietary restraint and disinhibition in relation to short and long-term weight loss. Physiol Behav 2014;128:247–51. [DOI] [PubMed] [Google Scholar]
- 18.Grau K, Hansen T, Holst C, Astrup A, Saris WHM, Arner P, Rossner S, MacDonald I, Polak J, Oppert JM, et al. Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. Int J Obes (Lond) 2009;33:1227–34. [DOI] [PubMed] [Google Scholar]
- 19.Razquin C, Martinez JA, Martinez-Gonzalez MA, Bes-Rastrollo M, Fernandez-Crehuet J, Marti A. A 3-year intervention with a Mediterranean diet modified the association between the rs9939609 gene variant in FTO and body weight changes. Int J Obes (Lond) 2010;34(2):266–72. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JA, Church TS, Rankinen T, Earnest CP, Sui X, Blair SN. FTO genotype and the weight loss benefits of moderate intensity exercise. Obesity (Silver Spring) 2010;18:641–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Qi Q, Zhang C, Smith SR, Hu FB, Sacks FM, Bray GA, Qi L. FTO genotype and 2-year change in body composition and fat distribution in response to weight-loss diets: The pounds lost trial. Diabetes 2012;61:3005–11. Correction published in: Diabetes 2013;62:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 24.Rauhio A, Uusi-Rasi K, Nikkari ST, Kannus P, Sievanen H, Kunnas T. Association of the FTO and ADRB2 genes with body composition and fat distribution in obese women. Maturitas 2013;76:165–71. [DOI] [PubMed] [Google Scholar]
- 25.Haupt A, Thamer C, Machann J, Kirchhoff K, Stefan N, Tschritter O, Machicao F, Schick F, Haring HU, Fritsche A. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008;16:1969–72. [DOI] [PubMed] [Google Scholar]
- 26.Curti MLR, Rogero MM, Baltar VT, Barros CR, Siqueira-Catania A, Ferreira SRG. FTO T/A and peroxisome proliferator-activated receptor-(gamma) Pro12Ala polymorphisms but not ApoA1 -75 are associated with better response to lifestyle intervention in Brazilians at high cardiometabolic risk. Metab Syndr Relat Disord 2013;11:169–76. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. Chichester (United Kingdom); Hoboken (NJ): Wiley-Blackwell; 2011.
- 28.Lappalainen TJ, Tolppanen AM, Kolehmainen M, Schwab U, Lindstrom J, Tuomilehto J, Pulkkinen L, Eriksson JG, Laakso M, Gylling H, et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study. Obesity (Silver Spring) 2009;17:832–6. [DOI] [PubMed] [Google Scholar]
- 29.Dlouha D, Suchanek P, Lanska V, Hubacek JA. Body mass index change in females after short-time life style intervention is not dependent on the FTO polymorphisms. Physiol Res 2011;60(1):199–202. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;25:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuo T, Nakata Y, Murotake Y, Hotta K, Tanaka K. Effects of FTO genotype on weight loss and metabolic risk factors in response to calorie restriction among Japanese women. Obesity (Silver Spring) 2012;20:1122–6. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stofan JR, Osterberg KL, Horswill CA, Lacambra M, Eichner ER, Anderson SA, Murray R. Daily fluid turnover during preseason training in U.S. college football. Int J Sport Nutr Exerc Metab 2007;17:340–51. [DOI] [PubMed] [Google Scholar]
- 37.Cha S, Koo I, Park BL, Jeong S, Choi SM, Kim KS, Shin HD, Kim JY. Genetic effects of FTO and MC4R polymorphisms on body mass in constitutional types. Evid Based Complement Alternat Med 2011;2011:106390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari HK, Patki A, Lieberman J, Stroup TS, Allison DB, Leibel RL, Chung WK. Association of allelic variation in genes mediating aspects of energy homeostasis with weight gain during administration of antipsychotic drugs (CATIE study). Front Genet 2011;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehmi J, Rai BK, Chambers JC, Kooner JK. Weight change following dietary intervention: the role of common genetic variants in FTO and MC4R. Eur Heart J 2009;30:953–4. [Google Scholar]
- 40.Moreles A, Rendo T, Campoy C, Zapatera B, Moreno L, Garagorri J, Marcos A, Martinez JA, Azcona-Sanjulian MC, Marti A. Influence of eight obesity-related SNPs with body mass index and weight loss in Spanish adolescents after a lifestyle intervention. Obes Rev 2011;12:43–4. [Google Scholar]
- 41.Moraes TI, Oliveira R, Kiyokawa RK, Sousa MC, Cerda A, Hirata MH, Fajardo CM, Dorea EL, Damasceno NRT, Jorge MIE, et al. The relationship between FTO polymorphisms and anthropometric and metabolic profile in individuals with ms enrolled in a nutritional orientation program. J Nutrigenet Nutrigenomics 2012;5:287. [Google Scholar]
- 42.Luglio HF, Aller E, Bowman F, Mariman E, Van Baak M. Genetic risk score as a predictor of weight loss during a lifestyle intervention in obese adults. J Nutrigenet Nutrigenomics 2012;5:233–4. [Google Scholar]
- 43.Hubacek J, Ceska R, Dlouha D, Zlatohlavek L. Effect of fto and MC4R variants on BMI decrease in children. Obes Facts 2012;5:172. [Google Scholar]
- 44.Huang T, Qi Q, Li Y, Hu FB, Bray GA, Sacks FM, Williamson DA, Qi L. FTO genotype, dietary protein, and change in appetite: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr 2014;99:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbron J, Zaahl M, Kotze M, Van Der Merwe L, Senekal M. Effect of eight polymorphisms on weight loss outcomes in overweight/obese Caucasian adults. J Nutrigenet Nutrigenomics 2010;3:120. [Google Scholar]
- 46.Ehehalt S, Schum J, Blumenstock G, Weber K, Schweizer R, Ranke MB, Binder G. FTO-risk alleles had no impact on body composition and parameters of metabolism before and after a lifestyle intervention programme in obese children and adolescents. Horm Res Paediatr 2011;76:266. [Google Scholar]
- 47.Dusatkova L, Zamrazilova H, Sedlackova B, Vcelak J, Hlavaty P, Bendlova B, Kunesova M, Hainer V. BDNF and FTO gene variants modified the response to short-term weight management in overweight and obese adolescents. Horm Res Paediatr 2013;80:104–5. [Google Scholar]
- 48.Curti MLR, Rogero MM, Barros CR, Siqueira-Catani A, Baltar VT, Ferreira SRG. Indicated for the young investigator award FTO T/A, PPARgamma PRO12ALA but not APOA1-75 polymorphisms are associated with better response to lifestyle intervention in Brazilians at high cardiometabolic risk. J Nutrigenet Nutrigenomics 2012;5:252. [Google Scholar]
- 49.Lauria F, Siani A, Bammann K, Foraita R, Huybrechts I, Iacoviello L, Koni AC, Kourides Y, Marild S, Molnar D, et al. Prospective analysis of the association of a common variant of FTO (rs9939609) with adiposity in children: results of the IDEFICS study. PLoS One 2012;7:e48876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller TD, Hinney A, Scherag A, Nguyen TT, Schreiner F, Schafer H, Hebebrand J, Roth CL, Reinehr T. ‘Fat mass and obesity associated’ gene (FTO): no significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Med Genet 2008;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinehr T, Hinney A, Toschke AM, Hebebrand J. Aggravating effect of INSIG2 and FTO on overweight reduction in a one-year lifestyle intervention. Arch Dis Child 2009;94:965–7. [DOI] [PubMed] [Google Scholar]
- 52.Scherag A, Kleber M, Boes T, Kolbe AL, Ruth A, Grallert H, Illig T, Heid IM, Toschke AM, Grau K, et al. SDCCAG8 obesity alleles and reduced weight loss after a lifestyle intervention in overweight children and adolescents. Obesity (Silver Spring) 2012;20:466–70. [DOI] [PubMed] [Google Scholar]
- 53.Zlatohlavek L, Vrablik M, Motykova E, Ceska R, Vasickova L, Dlouha D, Hubacek JA. FTO and MC4R gene variants determine BMI changes in children after intensive lifestyle intervention. Clin Biochem 2013;46:313–6. [DOI] [PubMed] [Google Scholar]
- 54.Moleres A, Rendo-Urteaga T, Zulet MA, Marcos A, Campoy C, Garagorri JM, Martinez JA, Azcona-Sanjulian MC, Marti A. Obesity susceptibility loci on body mass index and weight loss in Spanish adolescents after a lifestyle intervention. J Pediatr 2012;161:466–70.e2. [DOI] [PubMed] [Google Scholar]
- 55.Schum J, Blumenstock G, Weber K, Schweizer R, Pfaff C, Schurr N, Ranke MB, Binder G, Ehehalt S. Variants of the FTO gene in obese children and their impact on body composition and metabolism before and after lifestyle intervention. Exp Clin Endocrinol Diabetes 2012;120:128–31. [DOI] [PubMed] [Google Scholar]
- 56.Hinney A, Wolters B, Putter C, Grallert H, Illig T, Hebebrand J, Reinehr T. No impact of obesity susceptibility loci on weight regain after a lifestyle intervention in overweight children. Journal of pediatric endocrinology & metabolism. J Pediatr Endocrinol Metab 2013;26:1209–13. [DOI] [PubMed] [Google Scholar]
- 57.Matsuo T, Nakata Y, Hotta K, Tanaka K. The FTO genotype as a useful predictor of body weight maintenance: Initial data from a 5-year follow-up study. Metabolism 2014;63:912–7. [DOI] [PubMed] [Google Scholar]
- 58.Rankinen T, Rice T, Teran-Garcia M, Rao DC, Bouchard C. FTO genotype is associated with exercise training-induced changes in body composition. Obesity (Silver Spring) 2010;18:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang T, Qi Q, Li Y, Hu F, Bray GA, Sacks FM, Qi L. FTO genotype, dietary protein, and change in appetite: The pounds lost trial. Am J Clin Nutr 2014;99:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinehr T, Hinney A, Friedel S, Muller T, Hebebrand J. Impact of genetic markers on overweight reduction in a lifestyle intervention. Aktuel Ernahrungsmed 2008;33:231–6. [Google Scholar]
- 61.Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, Ahmad T, Mora S, Kaakinen M, Sandholt CH, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 2011;8:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi Q, Durst R, Schwarzfuchs D, Leitersdorf E, Shpitzen S, Li Y, Wu H, Champagne CM, Hu FB, Stampfer MJ, et al. CETP genotype and changes in lipid levels in response to weight-loss diet intervention in the POUNDS LOST and DIRECT randomized trials. J Lipid Res 2015;56:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. APOA5 genotype modulates 2-y changes in lipid profile in response to weight-loss diet intervention: the Pounds Lost Trial. Am J Clin Nutr 2012;96:917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marín C, Perez-Martinez P, Delgado-Lista J, Gomez P, Rodriguez F, Yubero-Serrano EM, Garcia-Rios A, Camargo A, Perez-Jimenez F, Lopez-Miranda J. The insulin sensitivity response is determined by the interaction between the G972R polymorphism of the insulin receptor substrate 1 gene and dietary fat. Mol Nutr Food Res 2011;55:328–35. [DOI] [PubMed] [Google Scholar]
- 66.Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, Rose LM, Thorleifsson G, Steinthorsdottir V, Magi R, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 2012;490:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schioth HB. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008;149:2062–71. [DOI] [PubMed] [Google Scholar]
- 70.Olszewski PK, Fredriksson R, Olszewska AM, Stephansson O, Alsio J, Radomska KJ, Levine AS, Schioth HB. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci 2009;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang P, Yang FJ, Du H, Guan YF, Xu TY, Xu XW, Su DF, Miao CY. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol Med 2011;17:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boender AJ, van Rozen AJ, Adan RA. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity (Silver Spring) 2012;20:2420–5. [DOI] [PubMed] [Google Scholar]
- 73.Tung YC, Ayuso E, Shan X, Bosch F, O’Rahilly S, Coll AP, Yeo GS. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS One 2010;5:e8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Bruning JC, Nolan PM, Ashcroft FM, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010;42:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karra E, O’Daly OG, Choudhury AI, Yousseif A, Millership S, Neary MT, Scott WR, Chandarana K, Manning S, Hess ME, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 2013;123:3539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benedict C, Axelsson T, Soderberg S, Larsson A, Ingelsson E, Lind L, Schioth HB. Fat mass and obesity-associated gene (FTO) is linked to higher plasma levels of the hunger hormone ghrelin and lower serum levels of the satiety hormone leptin in older adults. Diabetes 2014;63:3955–9. [DOI] [PubMed] [Google Scholar]
- 77.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 2014;507:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]