Abstract
Background
Prolongation of initial ventricular depolarization on the 12-lead ECG, or delayed intrinsicoid deflection (DID), can indicate left ventricular hypertrophy (LVH). The possibility that this marker could convey distinct risk of sudden cardiac arrest (SCA) has not been evaluated.
Objective
To evaluate the association of DID and SCA in the community.
Methods
In the ongoing prospective, population-based Oregon Sudden Unexpected Death Study (Oregon SUDS, catchment area approx. 1 million), SCA cases were compared to geographic controls with no SCA. Archived ECGs (closest and unrelated to SCA event for cases) were evaluated for the presence of DID defined as ≥0.05 seconds in leads V5 or V6. LV mass and function were evaluated from archived echocardiograms.
Results
SCA cases (n=272, 68.7±14.6 years, 63.6% male) as compared to controls (n=351, 67.6±11.4 years, 63.3% male) were more likely to have DID on ECG (28.3% vs 17.1%, p=0.001). DID was associated with increased SCA odds (OR 1.92; 95% CI 1.31-2.81; p=0.001), but showed poor correlation with LV mass and echocardiographic LVH (kappa 0.13). In multivariate analysis adjusted for clinical and ECG markers, reduced LV ejection fraction and echocardiographic LVH, DID remained an independent predictor of SCA (OR 1.82; 95% CI 1.12-2.97; p=0.016). Additionally, in a sensitivity analysis restricted to narrow QRS, DID and ECG LVH by voltage were each independently associated with SCA risk.
Conclusion
DID was associated with increased SCA risk independent of echocardiographic LVH, ECG LVH and reduced LV ejection fraction, potentially reflecting unique electrical remodeling that warrants further investigation.
Keywords: Sudden cardiac death, intrinsicoid deflection, arrhythmia, left ventricular hypertrophy, electrocardiography
Introduction
Sudden cardiac arrest (SCA) continues to be a leading cause of mortality in the United States causing an estimated 300,000 - 330,000 deaths annually, 2.0 million life years lost for males, and 1.3 million life years lost for females based on recent studies.1,2 The prophylactic implantable cardioverter defibrillator (ICD) is an established method for primary prevention of SCA.3-7 However, current risk stratification processes depend heavily on measurement of left ventricular (LV) ejection fraction (EF), now recognized to be a modest predictor of SCA risk.8 The LV EF as an isolated risk factor has been demonstrated to carry a low short term mortality risk from arrhythmic deaths of less than 5% over a two year span.9 While some additional risk factors beyond LV EF have been reported,10 there is considerable interest in finding novel risk markers for SCA identified using noninvasive and preferably inexpensive clinical tools. The 12-lead electrocardiogram (ECG) is a low-cost, readily available tool for clinical evaluation in the community.
Elevated resting heart rate, and prolongation of the QRS, QTc and JTc intervals have been previously associated with SCA.11-15 Intrinsicoid deflection, or R-wave peak time, represents the early phase of ventricular depolarization, and is defined as the time period from the onset of the QRS complex to the peak of the R wave.16-19 (Figure1) In previous studies, delayed intrinsicoid deflection (DID) ≥0.05 s in lateral precordial leads V5 and V6 has been associated with left ventricular hypertrophy (LVH) and is included in the Romhilt-Estes criteria for ECG diagnosis of LVH.17,19 We and others have previously reported that voltage or electrical LVH vs. anatomic or mass-related LVH may have distinct as well as overlapping effects on risk of atrial and ventricular arrhythmias.20,21 Since DID can be a component of electrical LVH but has not been evaluated in the context of SCA, we hypothesized that it may add value in SCA risk stratification over both echocardiographic LVH and LV ejection fraction. We therefore evaluated the potential association of DID and occurrence of SCA in the community.
Figure 1.
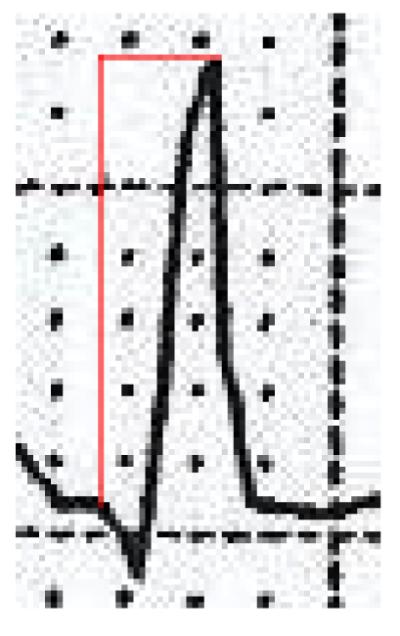
Example of delayed intrinsicoid deflection ≥0.05s in lead V5 identified from an archived electrocardiogram in a patient who eventually suffered SCA.
Methods
Ascertainment of Subjects
We conducted this analysis from the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS). The details of this study have been previously published.13,14,20,22 Cases of SCA in the Portland, Oregon metropolitan area (population approximately one million) are identified via first responders, local hospitals, and the medical examiner’s office. In this study, SCA is defined as a sudden unexpected pulseless state within 1 hour of symptom onset in witnessed cases, and if unwitnessed, within 24 hours of last being seen alive in a normal state of health. Individuals with known terminal illnesses or non-cardiac causes of SCA are excluded. Each case is reviewed in an in-house adjudication process involving three physicians prior to being included in the study. Individuals who survived the episode of SCA (29 of 272 cases) were also included in this study. Of the 200 cases with CAD status known from prior studies or autopsy, presence of significant CAD was established in 92.0%. Autopsy was performed in only a small minority of cases (n=12). Controls were enrolled from the same metropolitan area as the cases. The majority of controls (approx. 90%) were known to have coronary artery disease (CAD), defined as ≥ 50% occlusion of a major coronary artery, previous revascularization, or a history of myocardial infarction. They were recruited from subjects transported by emergency medical system (EMS) for complaints suggesting ongoing ischemia and from patients undergoing coronary angiography or visiting a cardiology outpatient clinic at one of the participating health systems. Subjects with CAD were chosen as controls since it is known from previous studies that CAD is the underlying cause of the large majority of SCA cases. 10
This study was approved by the institutional review boards of Cedars-Sinai Medical Center, Oregon Health and Science University, and all other relevant health systems.
ECG and Echocardiographic Measurements
Individuals with ECG and echocardiographic data available for evaluation from clinical records were included in the study. For cases, the ECG and echocardiogram evaluated were those prior to and closest to their cardiac arrest. For controls, the ECG and echocardiogram were those performed at the time of enrollment in the study or from the most recent clinical encounters. Subjects with history of hypertrophic cardiomyopathy, severe aortic stenosis, and missing ECG data were excluded from the study. Standard 12-lead ECG tracings at 25mm/s paper speed and 10mm/mV amplitude were reviewed by two trained physicians. Measurements including heart rate and QRS duration were entered in the database from the ECG recording. The QT interval was manually measured and corrected for heart rate according to the Bazett’s formula (QTc). JTc intervals were obtained by subtracting the QRS duration from the QTc interval. As JTc durations do not have standardized intervals for prolonged measurements, we established the 75th percentile in controls (355ms) as our upper limit of normal. Intrinsicoid deflection was measured by a trained physician researcher (N.D.) from the onset of QRS to peak of the R wave in lateral precordial leads of V5 and V6; those with duration ≥ 0.05 s were recorded as delayed (Figure 1). A subset of the ECGs was re-measured for DID by an experienced cardiologist (A.A.) to assess interobserver variability (kappa-value 0.89). Subjects were categorized as having ECG LVH if they met either Sokolow-Lyon criteria (RV1 +SV5,6 ≥ 35 mm) or Cornell criteria (RaVL + SV3 > 20 mm in females and > 28 mm in males).23,24
Echocardiographic LVH was defined as LV mass indices greater than 134 g/m2 for men and 110 g/m2 for women.25 LV mass was determined based on the American Society of Echocardiography equation as follows (LV mass = 0.8(1.04([LVIDD+PWTD+IVSTD]3 – [LVIDD]3)) + 0.6 grams) 25 (LVIDD: left ventricular internal diameter in diastole, PWTD: posterior wall thickness in diastole, IVSTD: interventricular septal thickness in diastole). Severe LV dysfunction was defined as LV EF ≤35%.
Statistical Analysis
Case-control comparisons of categorical values were performed using Pearson’s Chi-square tests and quantitative values using independent samples t-tests. We evaluated agreement between echocardiographic LVH and DID values using the Kappa statistic. Multivariable analysis was performed using binary logistic regression models including variables significant in univariate case-control comparisons to yield odds ratio (OR) for association of DID with SCA. In these multivariable analyses, we made adjustments for diabetes mellitus, chronic renal insufficiency (according to physician documentation in the medical records), severe LV dysfunction, resting heart rate, QRS duration, and echocardiographic LVH. We also adjusted for JTc prolongation and QTc prolongation in separate models. Data are presented as n (%) or mean ± SD, and p values ≤ 0.05 were considered statistically significant.
Results
Demographics
From February 1st 2002 to September 10th, 2014, a total of 2321 SCA cases with medical record available (mean age 63.8 ± 18.8 years; 66.5% male) were identified in the Portland, Oregon, metropolitan area. Of these subjects, 682 had an ECG available for analysis. When cases without echocardiogram were excluded, a total of 272 SCA cases and 351 controls were included in the final analysis (Table 1). Cases and controls were similar with respect to age (68.7 ± 14.6 vs. 67.6 ± 11.4 years), and sex (63.6% vs. 63.3% male). Cases were more likely to have a history of diabetes mellitus and chronic renal insufficiency. The traditional marker for risk stratification for ICD implantation, severe LV dysfunction (EF≤ 35%) was more common in cases than controls (25.3% vs. 12.3%, p <0.001). Echocardiographic LVH was more common in cases (40.8% vs. 19.8%, p <0.001).
Table 1.
Patient Characteristics among subjects with Sudden Cardiac Arrest (Cases) and with no episodes of Sudden Cardiac Arrest (Controls).
| All | Cases (n=272) |
Controls (n=351) |
P value |
|---|---|---|---|
| Age | 68.7 ± 14.6 | 67.6 ± 11.4 | 0.312 |
| Male | 173 (63.6%) |
217 (63.3%) | 0.931 |
| Hypertension | 219 (80.5%) |
268 (76.4%) | 0.212 |
| White Non-Hispanic | 216 (79.4%) |
314 (89.5%) | <0.00 1 |
| African American | 33 (12.1%) | 12 (3.4%) | <0.00 1 |
| Diabetes | 134 (49.3%) |
119 (33.9%) | <0.00 1 |
| BMI (kg/m2) | 30.2 ± 9.8 | 30.0 ± 6.8 | 0.823 |
| Cholesterol (mg/dL) | 171.0 ± 48.8 |
172.0 ± 50.0 | 0.748 |
| Current Smoker | 62 (22.8%) | 57 (16.2%) | 0.039 |
| Chronic renal insufficiency | 100 (36.8%) |
57 (16.2%) | <0.00 1 |
| Dialysis | 30 (11.0%) | 2 (0.6%) | <0.00 1 |
| Class I Antiarrhythmic medication | 12 (2.4%) | 12 (4.5%) | 0.153 |
| Class III Antiarrhythmic medication | 24 (9.1%) | 9 (2.7%) | 0.001 |
| Severe LV Dysfunction† | 68 (25.3%) | 43 (12.3%) | <0.00 1 |
| Echocardiographic LVH‡ | 100 (40.8%) |
66 (19.8%) | <0.00 1 |
| QRS Duration (ms) | 105 ± 25 | 100 ± 21 | 0.011 |
| QTc Duration (ms) | 469 ± 48 | 433 ± 36 | <0.00 1 |
| QTc Prolongation§ | 156 (57.4%) |
78 (22.7%) | <0.00 1 |
| Heart Rate (bpm) | 76.9 ± 17.6 | 69.4 ± 15.7 | <0.00 1 |
| JTc Duration (ms) | 364 ± 45.1 | 334 ± 35.2 | <0.00 1 |
| JTc Prolongation (75th %)¶ | 152 (56.3%) |
87 (24.9%) | <0.00 1 |
| Intrinsicoid Deflection ≥ 0.05 s | 77 (28.3%) | 60 (17.1%) | 0.001 |
| Both JTc Prolongation and Intrinsicoid Deflection ≥ 0.05 s |
41 (15.2%) | 14 (4.0%) | <0.00 1 |
Data are presented as n (%) or mean ± SD. LV-Left Ventricular; LVH-Left Ventricular Hypertrophy; BMI-Body Mass Index
Severe LV Dysfunction data available for 618 subjects
Echo LVH data available for 579 subjects
Women ≥ 470 ms, Men ≥ 450 ms. QTc prolongation data available for 615 subjects
JTc interval ≥355ms. JTc prolongation data available for 620 subjects
ECG findings in Cases and Controls
Cases and controls demonstrated significant differences in ECG parameters, as shown in Table 1. Cases had a significantly higher resting heart rate, longer QRS duration, QTc intervals and JTc intervals. QTc prolongation (defined as ≥ 470 ms in women and ≥450 ms in men) was more common among cases as compared to controls. 56.3% of cases had JTc prolongation based on the 75th percentile of controls’ values defining the upper limit of normal (355 ms, p <0.001)
Intrinsicoid Deflection and risk of SCA
DID was observed more commonly in cases than controls (28.3% vs. 17.1%, p = 0.001) (Table 1). To further analyze this ECG finding, we compared all subjects with DID against those without the finding on ECG (Table 2). Subjects with DID were found to have longer QRS durations (119 ms vs. 98 ms, p < 0.001), and QTc intervals (467 ms vs. 444 ms, p < 0.001). The JTc interval, however, was similar between the two groups (348 ms vs. 346 ms, p = n.s.). We also noted a greater adjusted LV mass in those with DID (120.9 vs. 107.3 g/m2, p = 0.001). Subjects with DID were also more likely to have echocardiographic LVH (40.0% vs. 25.6%, p = 0.002) and severe LV dysfunction (28.5% vs. 15.0%, p < 0.001).
Table 2.
Evaluation of ECG/Echo findings in individuals with Delayed Intrinsicoid Deflection as compared to those with normal Intrinsicoid Deflection.
| ECG Finding | Delayed Intrinsicoid Deflection (n=137) |
Normal Intrinsicoid Deflection (n=486) |
value |
|---|---|---|---|
| Severe LV Dysfunction |
39 (28.5%) | 72 (15.0%) | < 0. 0 0 1 |
| Heart Rate (bpm) |
71.5 ± 16.9 | 73.0 ± 17.0 | 0.364 |
| QRS Duration (ms) |
119 ± 28 | 98 ± 19 | <0.001 |
| QTc Duration (ms) |
467 ± 50 | 444 ± 43 | <0.001 |
| JTc Duration (ms) |
348 ± 48 | 346 ± 41 | 0. 6 8 6 |
| LV Mass Index (g/m2) |
120.9 ± 42.0 | 107.3 ± 37.2 | 0. 0 0 1 |
| Echo LVH | 50 (40.0%) | 116 (25.6%) | 0. 0 0 2 |
Data are presented as n (%) or mean ± SD. LV-Left Ventricular; LVH-Left Ventricular Hypertrophy
Univariate analysis showed an association between DID and SCA (OR 1.92; 95% CI 1.31-2.81, p = 0.001). In multivariable analysis that included diabetes, chronic renal insufficiency, severe LV dysfunction, heart rate, QRS duration, echocardiographic LVH and JTc prolongation, DID was associated with increased SCA odds (OR 1.82, 95% CI 1.12-2.97, p = 0.016) (Table 3). Excluding subjects with antiarrhythmic medication did not change the results markedly.
Table 3.
Multivariate adjusted odds ratios for SCA.
| Characteristic | OR (95% CI) | P Value |
|---|---|---|
| Diabetes Mellitus | 1.17 (0.79-1.74) | 0.431 |
| Chronic Renal Insufficiency | 2.14 (1.36-3.38) | 0.001 |
| Severe LV Dysfunction | 1.49 (0.91-2.44) | 0.117 |
| Echo LVH | 1.93 (1.27-2.94) | 0.002 |
| Heart Rate | 1.02 (1.01-1.03) | 0.003 |
| QRS Duration | 1.00 (0.99-1.01) | 0.703 |
| JTc Prolongation (75th %) | 2.70 (1.82-4.01) | <0.001 |
| Intrinsicoid Deflection ≥0.05 s | 1.82 (1.12-2.97) | 0.016 |
Each parameter adjusted for all other variables in the table. LV-Left Ventricular; LVH-Left Ventricular Hypertrophy
Intrinsicoid Deflection vs. other ECG Parameters
The association between DID and SCA was independent of prolonged ventricular repolarization markers (JTc and QTc, Tables 3, 4). The univariate risk for the combination of DID and JTc was greater than either finding alone (OR 4.30, p <0.001). When assessed in a multivariable analysis with diabetes mellitus, chronic renal insufficiency, heart rate, QRS duration, severe LV dysfunction, and echocardiographic LVH, the presence of both DID and JTc prolongation nearly tripled the odds of SCA (OR 2.79; 95% CI 1.40-5.56; p = 0.004). When evaluated in multivariate analysis, QRS duration was not found to be an independent predictor of SCA (Table 3).
Table 4.
The risk of SCA associated with DID in different multivariate models
| OR | 95% CI | P value | |
|---|---|---|---|
| Univariate | 1.92 | 1.31-2.81 | 0.001 |
| Model A† | 1.82 | 1.21-2.74 | 0.004 |
| Model B‡ | 1.85 | 1.14-3.02 | 0.013 |
| Model C§ | 1.82 | 1.12-2.97 | 0.016 |
Adjusted for diabetes mellitus, chronic renal insufficiency, severe LV dysfunction, and heart rate.
Adjusted for diabetes mellitus, chronic renal insufficiency, severe LV dysfunction, heart rate, QRS duration, QTc Prolongation, and Echo LVH.
Adjusted for diabetes mellitus, chronic renal insufficiency, severe LV dysfunction, heart rate, QRS duration, JTc prolongation, and Echo LVH.
Intrinsicoid Deflection and Echocardiographic LVH
We evaluated the relationship between DID and increased left ventricular mass in association with SCA. The agreement between intrinsicoid deflection and anatomical LVH was poor with a Kappa value of 0.13. A total of 49 subjects had both echocardiographic LVH and DID, of which the majority were cases (14.3% were cases vs. 4.2%, were control P < 0.001). As seen in Table 3, DID and echocardiographic LVH were independently associated with SCA. When placed in a multivariable analysis with the aforementioned variables, the presence of both echocardiographic LVH and DID remained significantly associated with SCA (OR 2.55; 95% CI 1.24-5.24; p =0.011).
Intrinsicoid deflection and ECG-LVH sensitivity analysis
We also evaluated the relationship of DID and ECG LVH criteria in association with SCA in a subset of our population with QRS durations ≥ 120 ms excluded. In this analysis, 44 (20.6%) cases and 36 (12.3%) controls were found to have DID (p = 0.012). ECG LVH was also more common in cases than controls (21.5% vs 12.3%, p = 0.006). Of subjects with DID, 23.8% were also found to have ECG LVH. In a multivariate analysis including diabetes mellitus, chronic renal insufficiency, severe LV dysfunction, heart rate, QRS duration, echocardiographic LVH, and ECG LVH, DID remained a significant predictor of SCA (OR 1.84; 95% CI 1.05-3.22; p = 0.032).
Discussion
We report that delayed intrinsicoid deflection, also referred to as R-wave peak time measured on the 12-lead ECG, is associated with SCA. This association remained significant in multivariate analysis after adjusting for increased LV mass as well as severely reduced LV systolic function. Further, in patients with narrow QRS, DID was associated with SCA risk independent of LVH voltage criteria. Prolongation of the early phase of ventricular depolarization, represented by the intrinsicoid deflection, therefore appears to be a form of electrical remodeling that is independently predictive of SCA.
A plethora of research has been published regarding the association of ventricular depolarization and repolarization abnormalities with SCA. QRS duration, QTc interval, JTc interval, LBBB, nonspecific intraventricular conduction delay (IVCD) and ECG-LVH are among the parameters associated with SCA in previous studies including those reported from Oregon SUDS.11-15,20,26,27 Based on our findings, the risk of SCA associated with DID was independent of these markers, thus adding yet another parameter to the growing list of ECG abnormalities associated with SCA. Intrinsicoid deflection is measured from the beginning of the QRS complex to the peak of the R-wave in the lateral precordial leads, consequently, DID mainly reflects slowed impulse conduction during the initial phase of left ventricular depolarization. DID frequently accompanies LVH, and was incorporated into Romhilt-Estes electrocardiographic criteria for diagnosing LVH nearly half a century ago.19 According to a recent study on the prognostic significance of individual components of this LVH criteria, the presence of intrinsicoid deflection ≥0.05s was an independent predictor of mortality when adjusted for the other electrocardiographic components of the score.28
Not surprisingly, DID was associated with increased LV mass, LV dysfunction and prolonged duration of QRS complex and QTc interval in the present study. However, echocardiographic LVH was present only in one-third and electrocardiographic LVH in one-fourth of the subjects with DID, suggesting that DID is not just a marker of increased LV mass or a byproduct of ECG-LVH, but may convey distinctive information regarding myocardial electrical remodeling. The risk of SCA in patients with DID remained nearly two-fold even after adjusting for several variables including QRS duration, JTc interval, and echocardiographic LVH, suggesting that DID adds prognostic information beyond just prolonged depolarization and repolarization, or anatomic LVH.
We and others have previously reported that a diagnosis of LVH based on 12-lead ECG voltage is associated with SCA independent of anatomic LVH, suggesting that in part, electrical remodeling may be a distinct phenomenon from increased LV mass.20 This study provides further insight regarding the clinical manifestations of myocardial electrical remodeling. DID appears to be another ECG marker that contributes to risk of arrhythmogenesis independent of anatomic increase in LV mass. This suggests that while both DID and ECG-LVH are manifestations of electrical remodeling, the pathophysiology may have overlapping as well as distinct aspects. In fact, these findings provide clinical validation for a computer modeling study that simulated how altered conduction velocity together with left ventricular mass and ventricular geometry altered the appearance of the 12-lead ECG. In this model, DID was a product of both increased LV mass and electrical remodeling.29
In the early studies, pathophysiology of DID was postulated to be solely secondary to delayed conduction due to increased LV mass.16,30,31 More recently, several potential mechanisms responsible for slowed impulse conduction and arrhythmogenesis in the context of LVH, have been described. These include myocardial hypertrophy,32 remodeling of interstitial collagen, 33 abnormalities and altered expression of gap junctions 34 and heterogeneity of myocardial repolarization. 35 While all of these abnormalities promote ventricular arrhythmogenesis, the specific mechanisms leading to DID still need to be better evaluated.
There are some limitations of our study, largely related to the inherent properties of community-based studies compared to alternative methodologies. Given that a large percentage of SCA cases have no previous history of cardiac disease, diagnostic studies such as ECG and echocardiogram were not available for review for all study subjects, leading to a potential bias. An additional limitation was that anatomic LVH was determined by the use of transthoracic echocardiographs instead of more advanced and sensitive studies, such as the cardiac MRI. Another potential limitation is the predominantly Caucasian study population, so these data may not necessary directly apply to other racial or ethnic groups. The strengths of this study include the prospective methods used in obtaining subjects from the community to evaluate for SCA. Since CAD is the primary underlying cause of SCA, the majority of controls in this study also had CAD and they were taken from the same geographical area, increasing the likelihood that the differences observed between cases and controls were specific to SCA.
Conclusion
Our findings suggest that DID is a novel 12-lead ECG marker associated with increased risk of SCA. The prognostic value of intrinsicoid deflection appears to extend beyond that of LV function and mass, or other electrocardiographic abnormalities, indicating that DID may be an indicator of adverse myocardial electrical remodeling. This easily obtained and widely available measure warrants further evaluation to evaluate its mechanistic significance and role as a potential predictor of increased SCA risk.
CLINICAL PERSPECTIVES.
Sudden cardiac arrest remains a leading cause of mortality at a global level and more effective methods of prediction and prevention are needed.The 12-lead electrocardiogram (ECG) is a cost-efficient, widely available clinical resource that is being re-investigated with great interest for this purpose. Intrinsicoid deflection represents the early phase of ventricular depolarization, and it is defined as the time from the beginning of the QRS complex to the peak of the R wave. In this community-based study, we report a significant and independent association between delayed intrinsicoid deflection (DID) Z0.05 second in the lateral precordial leads of the 12-lead ECG and sudden cardiac arrest. In this study, DID was associated with sudden cardiac arrest independent of left ventricular (LV) hypertrophy, low LV ejection fraction, and several other ECG markers. These findings suggest that DID may be a novel ECG marker of adverse myocardial electrical remodeling and needs to be investigated further as a potential clinical predictor of sudden cardiac death arrest.
Acknowledgements
Funded in part by National Heart Lung and Blood Institute grant R01HL122492 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Electrophysiology at Cedars-Sinai Medical Center, Los Angeles,California. Dr Aro is funded by grants from the Finnish Cultural Foundation and the Orion Research Foundation.
Abbreviations
- ECG
Electrocardiogram
- SCA
Sudden cardiac arrest
- ICD
Implantable cardioverter defibrillator
- LV
Left ventricular
- EF
Ejection fraction
- CAD
Coronary artery disease
- LVH
Left ventricular hypertrophy
- DID
Delayed intrinsicoid deflection
- OR
Odds ratio
- CI
Confidence interval
Footnotes
Conflicts of Interest: None
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Public health burden of sudden cardiac death in the United States. Circ Arrhythm Electrophysiol. 2014;7:212–7. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N. Engl. J. Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N. Engl. J. Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm. 2008;5:934–55. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll. Cardiol. 2006;47:1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J. Am. Coll. Cardiol. 2007;50:1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 10.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–52. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 11.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 13.Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2001;8:1562–7. doi: 10.1016/j.hrthm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–70. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol. 2011;4:704–10. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 16.Baxley WA, Dodge HT, Sandler H. A quantitative angiocardiographic study of left ventricular hypertrophy and the electrocardiogram. Circulation. 1968;37:509–17. doi: 10.1161/01.cir.37.4.509. [DOI] [PubMed] [Google Scholar]
- 17.Noth PH, Myers GB, Klein HA. The precordial electrocardiogram in left ventricular hypertrophy; a study of autopsied cases. Proc Annu Meet Cent Soc Clin Res U S. 1947;20:54. [PubMed] [Google Scholar]
- 18.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J. 1949;37:161–86. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 19.Romhilt DW, Bove KE, Norris RJ, Conyers E, Conradi S, Rowlands DT, Scott RC. A critical appraisal of the electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Circulation. 1969;40:185–95. doi: 10.1161/01.cir.40.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Chugh H, Gunson K, Jui J, Chugh SS. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11:1040–6. doi: 10.1016/j.hrthm.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Nazarian S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2014;63:2007–13. doi: 10.1016/j.jacc.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J. Am. Coll. Cardiol. 2004;44:1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Sokolow M, Lyon TP. The ventricular complex in right ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J. 1949;38:273–94. doi: 10.1016/0002-8703(49)91335-6. [DOI] [PubMed] [Google Scholar]
- 24.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J. Am. Coll. Cardiol. 1985;6:572–80. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reis G, Alderman MH, Laragh JH. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J. Am. Coll. Cardiol. 1984;4:1222–30. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 26.Zimetbaum PJ, Buxton AE, Batsford W, Fisher JD, Hafley GE, Lee KL, O'Toole MF, Page RL, Reynolds M, Josephson ME. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–9. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 27.Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation. 2003;108:1985–9. doi: 10.1161/01.CIR.0000095027.28753.9D. [DOI] [PubMed] [Google Scholar]
- 28.Estes EH, Zhang ZM, Li Y, Tereschenko LG, Soliman EZ. The Romhilt-Estes left ventricular hypertrophy score and its components predict all-cause mortality in the general population. Am. Heart J. 2015;170:104–9. doi: 10.1016/j.ahj.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacharova L, Szathmary V, Kovalcik M, Mateasik A. Effect of changes in left ventricular anatomy and conduction velocity on the QRS voltage and morphology in left ventricular hypertrophy: a model study. J Electrocardiol. 2010;43:200–8. doi: 10.1016/j.jelectrocard.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Grubschmidt HA, Sokolow M. The reliability of high voltage of the QRS complex as a diagnostic sign of left ventricular hypertrophy in adults. Am. Heart J. 1957;54:689–94. doi: 10.1016/0002-8703(57)90423-4. [DOI] [PubMed] [Google Scholar]
- 31.Grant RP, Dodge HT. Mechanisms of QRS complex prolongation in man; left ventricular conduction disturbances. Am. J. Med. 1956;20:834–52. doi: 10.1016/0002-9343(56)90204-2. [DOI] [PubMed] [Google Scholar]
- 32.Wiegerinck RF, Verkerk AO, Belterman CN, van Veen TA, Baartscheer A, Opthof T, Wilders R, de Bakker JM, Coronel R. Larger cell size in rabbits with heart failure increases myocardial conduction velocity and QRS duration. Circulation. 2006;113:806–13. doi: 10.1161/CIRCULATIONAHA.105.565804. [DOI] [PubMed] [Google Scholar]
- 33.Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2:216–23. doi: 10.1053/eupc.2000.0110. [DOI] [PubMed] [Google Scholar]
- 34.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–75. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 35.Kowey PR, Friechling TD, Sewter J, Wu Y, Sokil A, Paul J, Nocella J. Electrophysiological effects of left ventricular hypertrophy. Effect of calcium and potassium channel blockade. Circulation. 1991;83:2067–75. doi: 10.1161/01.cir.83.6.2067. [DOI] [PubMed] [Google Scholar]


