SUMMARY
The human naive pluripotent stem cell (PSC) state, corresponding to a pre-implantation stage of development, has been difficult to capture and sustain in vitro. We report that the Hippo pathway effector YAP is nuclearly localized in the inner cell mass of human blastocysts. Overexpression of YAP in human embryonic stem cells (ESCs) and induced PSCs (iPSCs) promotes the generation of naive PSCs. Lysophosphatidic acid (LPA) can partially substitute for YAP to generate transgene-free human naive PSCs. YAP- or LPA-induced naive PSCs have a rapid clonal growth rate, a normal karyotype, the ability to form teratomas, transcriptional similarities to human pre-implantation embryos, reduced heterochromatin levels, and other hallmarks of the naive state. YAP/LPA act in part by suppressing differentiation-inducing effects of GSK3 inhibition. CRISPR/Cas9-generated YAP−/− cells have an impaired ability to form colonies in naive but not primed conditions. These results uncover an unexpected role for YAP in the human naive state, with implications for early human embryology.
Graphical abstract
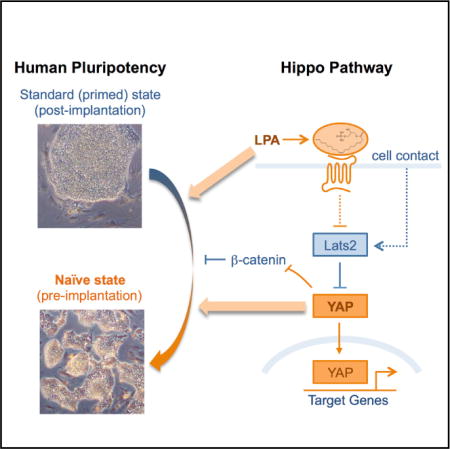
INTRODUCTION
Human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can be propagated almost indefinitely and can give rise to all cell types of the body (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Thomson et al., 1998; Yu et al., 2007). Because of these properties, human PSCs areanexcellent systemto study cellular differentiation in normal and diseased states, with potential far-reaching implications in regenerative medicine. Unlike mouse ESCs (Evans and Kaufman, 1981; Martin, 1981), which are isolated from the inner cell mass (ICM) of blastocysts and can be preserved in vitro in a naive ICM-like state, human ESCs and iPSCs are in a so-called “primed” pluripotent state that resembles the post-implantation epiblast (Nichols and Smith, 2009). The derivation and characterization of human naive PSCs is of significant interest for the following reasons: (1) they are expected to have an elevated growth rate and efficient clonal growth from single cells, relative to human primed PSCs; (2) this higher ease of culture is anticipated to facilitate genome engineering using homologous recombination, transcription activator-like effector nucleases (TALENs), or clustered regularly interspaced short palindromic repeat (CRISPR) approaches (Hsu et al., 2014); and (3) naive PSCs provide an opportunity to dissect the regulation of otherwise inaccessible stages of human embryology.
It has been suggested that the ability to maintain mouse cells in a naive state may be possible because mouse blastocysts can enter a dormant state called diapause, where development is suspended for days to weeks while the uterine environment is unfavorable (Nichols et al., 2009). Given that human embryos do not undergo diapause, it remained unclear whether the transcriptional circuitry to sustain the naive state is present in human cells. However, as early as 2009, several groups reported conditions that allowed the derivation of human cells in alternative pluripotent states with some features of the mouse naive state, although the cells carried transgenes and could not be maintained long term (Buecker et al., 2010; Hanna et al., 2010; Li et al., 2009). Subsequently, different panels of growth factors and small molecules that could promote maintenance of transgene-free human naive-like PSCs were described (Chan et al., 2013; Gafni et al., 2013; Ware et al., 2014). These methods were often not straightforward to implement in different human PSCs, and subsequent studies questioned the extent to which the cells derived approached the naive state (Takashima et al., 2014; Theunissen et al., 2014). Theunissen et al. (2014) used a systematic approach to identify small molecules that support maintenance of naive-like human ESCs induced by overexpression of NANOG and KLF2. The authors successfully identified a combination that can induce and maintain OCT4 distal enhancer (DE) activity, a key criterion of mouse naive pluripotency, even in the absence of transgene induction. However, these transgene-free cells have a slow growth rate, and their long-term karyotypic stability was not assessed. Takashima et al. (2014) also used overexpression of NANOG and KLF2 to trigger the transcriptional circuitry of naive pluripotency in human cells, and they observed that optimal cell growth requires transgene induction. These recent studies lend encouragement to the notion that the naive pluripotent state may indeed be achieved in human cells, although it may be less stable than in mouse cells, and they pave the way for the identification of novel factors and culture conditions that may contribute to stabilization of human naive PSCs.
The Hippo pathway is emerging as a major regulator of stem cells and organ growth, and its dysfunction is common in several types of cancer (Mo et al., 2014). We previously found that Yes-associated protein (YAP), a signaling and transcriptional effector that is inhibited by the Hippo pathway, is upregulated in multiple mouse stem and progenitor cells, including ESCs (Ramalho-Santosetal., 2002). Recent studies from our lab in human iPSCs, including an analysis of Hippo signaling (Qin et al., 2012) and a genome-wide RNAi screen (Qin et al., 2014), revealed that the Hippo pathway is a barrier to reprogramming to pluripotency. The divergent roles of the Hippo pathway in mouse versus human PSCs suggested to us that YAP may regulate human naive pluripotency (Qin et al., 2012).
We report here that YAP overexpression in human ESCs and iPSCs promotes the generation of naive PSCs. YAP-induced naive PSCs (Yin-PSCs) are capable of rapid growth long term with a stable karyotype; have a transcriptional profile, heterochromatin levels, and the activity of the OCT4 DE typical of the naive state; and form teratomas containing tissues from all three germ layers. YAP overexpression can be mimicked by supplementing the culture medium with lysophosphatidic acid (LPA), a small molecule inhibitor of the Hippo pathway and an activator of YAP (Yu et al., 2012). Our data suggest that YAP and LPA contribute to maintenance of naive pluripotency in part by suppressing differentiation-inducing effects of GSK3 inhibition. Moreover, YAP−/− human ESCs have an impaired ability to form naive colonies.
RESULTS
YAP Overexpression in Human ESCs and iPSCs Promotes a Naive State
Our previous work (Qin et al., 2012) raised the possibility that YAP might regulate human naive pluripotency, but available data from mouse studies do not support such a role. In mice, Yap has been reported to be excluded from the nucleus of ICM cells and has been implicated in trophectoderm development (Nishioka et al., 2009). We therefore analyzed YAP expression in human blastocysts by single-cell qRT-PCR and immunofluorescence. Interestingly, we found that YAP mRNA is upregulated in the epiblast of the blastocyst and that YAP protein is localized to the nucleus in both the ICM and the trophectoderm (Figures 1A, S1A, and S1B). The nuclear distribution of YAP is distinct in these two cell populations, with a diffuse pattern in the ICM and a more heterogeneous pattern in the trophectoderm (Figures S1A and S1B). These data suggest that YAP may play an unanticipated role in human naive pluripotency in vivo.
Figure 1. YAP Overexpression Promotes the Generation of Human Naive ESCs.
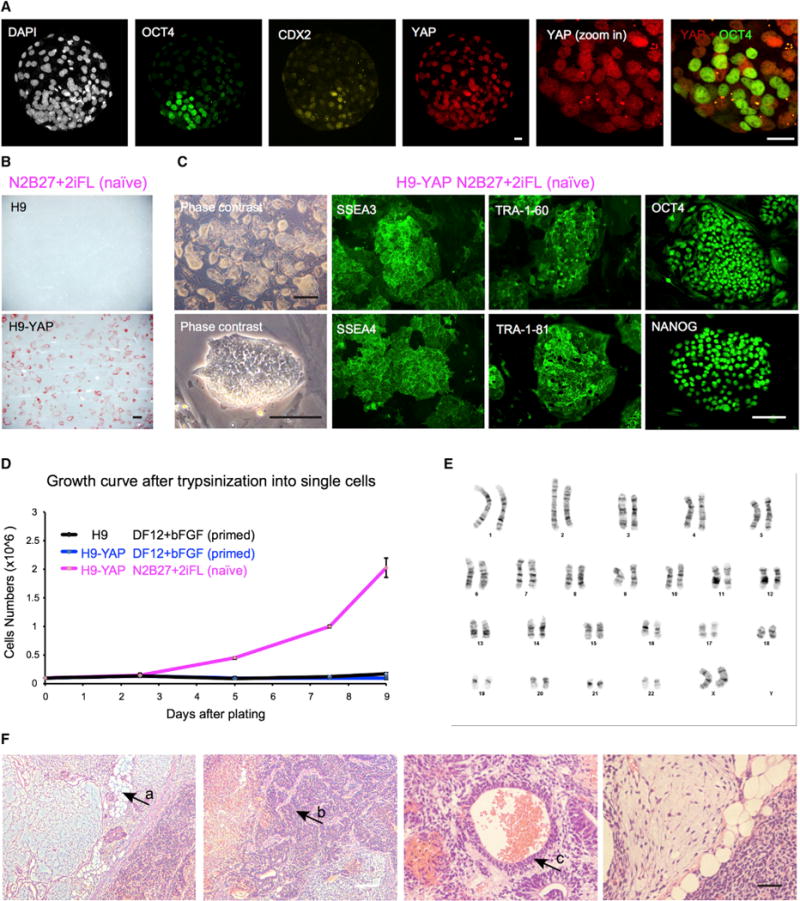
(A) YAP protein is localized to the nucleus in both the trophectoderm and the ICM of human blastocysts, as shown by immunofluorescence. White, DAPI; green, OCT4 (ICM); yellow, CDX2 (trophectoderm); red, YAP. Representative image of n = 16 human blastocysts. Scale bar, 20 μm.
(B) In naive medium N2B27+2iFL, human H9 ESCs with YAP overexpression (H9-YAP) maintain expression of the pluripotency marker AP, while control H9 cells differentiate and do not express AP. Scale bar, 500 μm.
(C) H9-YAP cells in N2B27+2iFL have a naive-specific dome-like colony morphology and show strong positive immunostaining for pluripotency markers SSEA3, SSEA4, TRA-1-60, TRA-1-81, OCT4, and NANOG. Black scale bar, 500 mm; white scale bar, 150 μm.
(D) Growth curves of H9 in DF12+bFGF primed medium, H9-YAP in DF12+bFGF primed medium, and H9-YAP in N2B27+2iFL naive medium after trypsinization to single cells. Only H9-YAP cells in naive medium have a high proliferate rate. Error bars represent SD.
(E) H9-YAP cells in N2B27+2iFL have a normal female karyotype (46, XX), as evaluated after 20 passages in naive culture conditions.
(F) H9-YAP cells in N2B27+2iFL are able to form teratomas comprising tissues derived from all three germ layers. (a) Adipocytes (mesoderm). (b) Neural tissue (ectoderm). (c) Epithelium (endoderm). White scale bar, 200 μm; black scale bar, 50 μm.
We set out to test the role of YAP in human naive pluripotency. In our initial attempts to generate putative human naive PSCs, we transferred regular primed H9 ESCs, routinely cultured in DF12+bFGF, into serum-free N2B27-based medium supplemented with ERK inhibitor (PD0325901), GSK3 inhibitor (CHIR99021), Forskolin and human LIF (N2B27+2iFL). This is essentially the 2i medium used for culture of mouse naive PSCs (Ying et al., 2008), with the addition of the adenylyl cyclase activator Forskolin, because both our unpublished results and other published findings (Hanna et al., 2010) suggested that the cyclic AMP pathway may facilitate survival of naive-like cells. Under these culture conditions, however, H9 cells rapidly differentiate and completely lose expression of the pluripotency marker alkaline phosphatase (AP) within four passages (Figure 1B). We next investigated the ability of YAP to induce the naive state. We generated H9 cells overexpressing YAP in primed conditions and tested their ability to grow in the N2B27+2iFL medium, with trypsinization to single cells at every passage. Control empty-vector lentiviral infection has no effect on the transition of H9 cells to naive conditions (data not shown). Strikingly, YAP overexpression abolishes the differentiationinducing effect of the N2B27+2iFL medium, and the cells retain strong expression of AP (Figure 1B). After three passages in N2B27+2iFL, the cells begin to show an elevated clonal growth rate, and they propagate readily in culture with a naive-specific dome-like colony morphology for more than 70 passages (Figure 1C). Moreover, they express pluripotency markers including SSEA3, SSEA4, TRA-1-60, TRA-1-81, OCT4, and NANOG (Figures 1C and S1C). Compared with H9 and H9-YAP cells cultured in primed conditions, H9-YAP cells in N2B27+2iFL have drastically increased clonal growth after trypsinization to single cells (Figure 1D), with split ratios of about 1:50 every 3 to 5 days. These cells, which we call Yin-PSCs, maintain a stable karyotype (Figure 1E) and form teratomas containing tissues from all three germ layers after injection into immune-deficient mice (Figure 1F).
We next investigated whether Yin-PSCs can be derived using ESC lines other than H9, which is female, or using iPSCs. A male human ESC line (H1) and a female iPSC line derived from lung fibroblasts (IMR-90) were used for further studies. Similar to the procedure described earlier, we transferred H1-YAP and IMR-90 iPSC-YAP cells into the N2B27+2iFL medium and trypsinized them to single cells at each passage. Unlike H9-YAP, however, H1-YAP and IMR-90 iPSC-YAP cells in N2B27+2iFL differentiated after several passages (data not shown). These results indicate that different PSCs respond differently to the transition to naive conditions and that H9 cells may be particularly capable of making this transition. Consequently, we explored alternatives to the N2B27+2iFL medium, which was optimized for mouse naive PSCs. The mTeSR medium was specifically developed for culture of human PSCs (Ludwig et al., 2006) and has been widely used. We therefore tested mTeSR as the base medium, instead of N2B27. The mTeSR+2iFL medium allowed for the expansion of H1-YAP and IMR-90 iPSC-YAP cells with naive morphology and growth rates for at least passage 17 (H1-YAP) and 22 (IMR-90 iPSC-YAP) to date. Similar to H9-YAP in N2B27+2iFL, H1-YAP and IMR-90 iPSC-YAP cells grown in mTeSR+2iFL express pluripotency markers including SSEA3, SSEA4, and TRA-1-81 (Figures S1D and S1E); have normal karyotypes (Figures S1F and S1G); and form teratomas containing tissues from all three germ layers (Figures S1H and S1I). Moreover, mTeSR+2iFL medium also allows the generation of Yin-PSCs from H9 ESCs (see Figure 3), WIBR3 female ESCs (see Figure 4), and iPSCs derived from male BJ fibroblasts (data not shown). Thus, YAP overexpression promotes the generation of naive PSCs from multiple male and female human ESC and iPSC lines.
LPA Activates YAP and Promotes Generation of Transgene-free Human Naive PSCs
We next sought small molecules that would have effects similar to YAP overexpression to avoid the need for transgenes in the induction of the naive state. Toward this end, we explored existing data on the regulation of the Hippo pathway in other cellular contexts. The Hippo pathway kinases LATS1 and LATS2 phosphorylate YAP and lead to its degradation by the proteasome (Mo et al., 2014). Serum-borne LPA has been shown to act via Gα12/13-coupled receptors to inhibit LATS1/2, thereby leading to the activation of YAP in human transformed cell lines (Yu et al., 2012). In agreement with these findings, we found that adding LPA to H9 cells leads to an increase in the levels of YAP (Figures 2A and 2B), although these levels are much lower compared with lentiviral YAP overexpression (Figure S2A). We transferred H9 cells to mTeSR+2iFL naive medium containing LPA and trypsinized them to single cells at each passage. In the presence of LPA, H9 cells adopt a domed-colony morphology similar to Yin-PSCs (Figure 2C), express pluripotency markers (Figure 2C), maintain a stable karyotype (Figure S2B), and are capable of giving rise to teratomas containing tissues from all three germ layers (Figure 2D). LPA-induced naive PSCs (Lin-PSCs) have an elevated expansion rate, albeit lower than that of Yin-PSCs: they can be passaged at a 1:10 split ratio every 3 to 5 days for at least 20 passages to date. We were also able to derive Lin-PSCs from WIBR3 human ESCs (see Figure 3). In summary, transgene-free human naive PSCs can be generated using LPA to activate YAP.
Figure 2. LPA Can Activate YAP and Promotes a Naive State of Pluripotency.
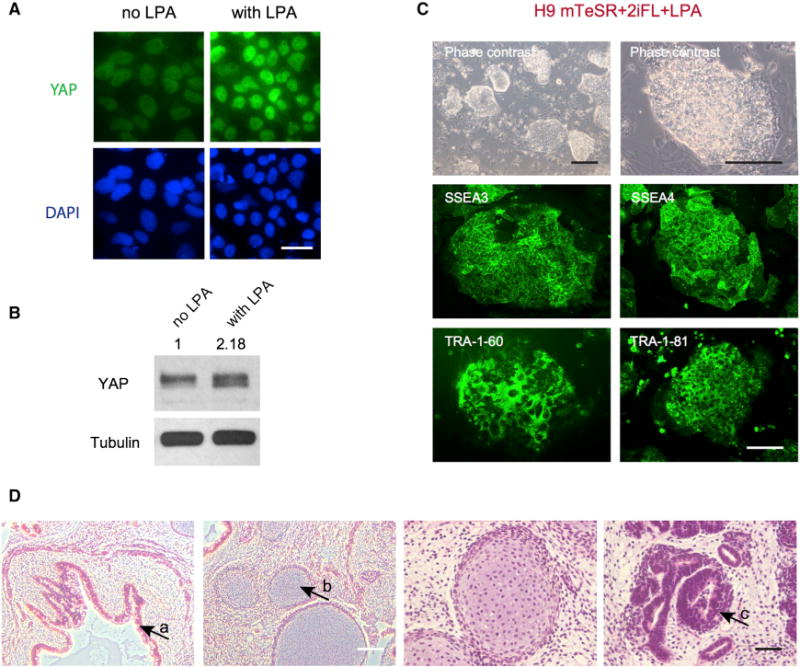
(A) LPA increases the level of YAP in human H9 ESCs, as shown by immunofluorescence. Immunostaining was performed 2 days after adding LPA. Green, YAP; blue, DAPI. Scale bar, 50 μm.
(B) Western blotting confirming that LPA increases YAP protein levels. Tubulin was used as loading control.
(C) H9 cells in N2B27+2iFL+LPA naive medium have a naive-specific dome-like colony morphology and show strong positive immunostaining for pluripotency markers SSEA3, SSEA4, TRA-1-60, and TRA-1-81. Black scale bar, 500 μm; white scale bar, 150 μm.
(D) H9-YAP cells in N2B27+2iFL are able to form teratomas comprising tissues derived from all three germ layers. (a) Gut-like epithelium (endoderm). (b) Cartilage (mesoderm). (c) Neural tissue (ectoderm). White scale bar, 200 μm; black scale bar, 50 μm.
Yin-PSCs and Lin-PSCs Have a Naive-Associated Transcriptional Profile Distinct from that of Primed PSCs
We characterized the transcriptional profiles of Yin-PSCs and Lin-PSCs and compared them to those of primed PSCs using Illumina microarrays. We analyzed H9 ESCs, H1 ESCs, and IMR-90 iPSCs cultured in primed (DF12+bFGF) medium; H9-YAP, H1-YAP, and IMR-90 iPSC-YAP cells cultured in primed medium; H9-YAP cultured in N2B27+2iFL naive medium; H9-YAP, H1-YAP, and IMR-90 iPSC-YAP cells cultured in mTeSR+2iFL naive medium; and H9 and WIBR3 cultured in mTeSR+2iFL+LPA. Pearson correlation analysis revealed that naive and primed PSCs represent two groups with distinct transcriptional profiles (Figure 3A). Principal-component analysis confirmed that all six naive PSCs cluster together and are separated from the other six primed cell lines along PC1 (Figure S3A).
Figure 3. Yin-PSCs and Lin-PSCs Have a Naive-like Transcriptional Profile Distinct from that of Primed PSCs.
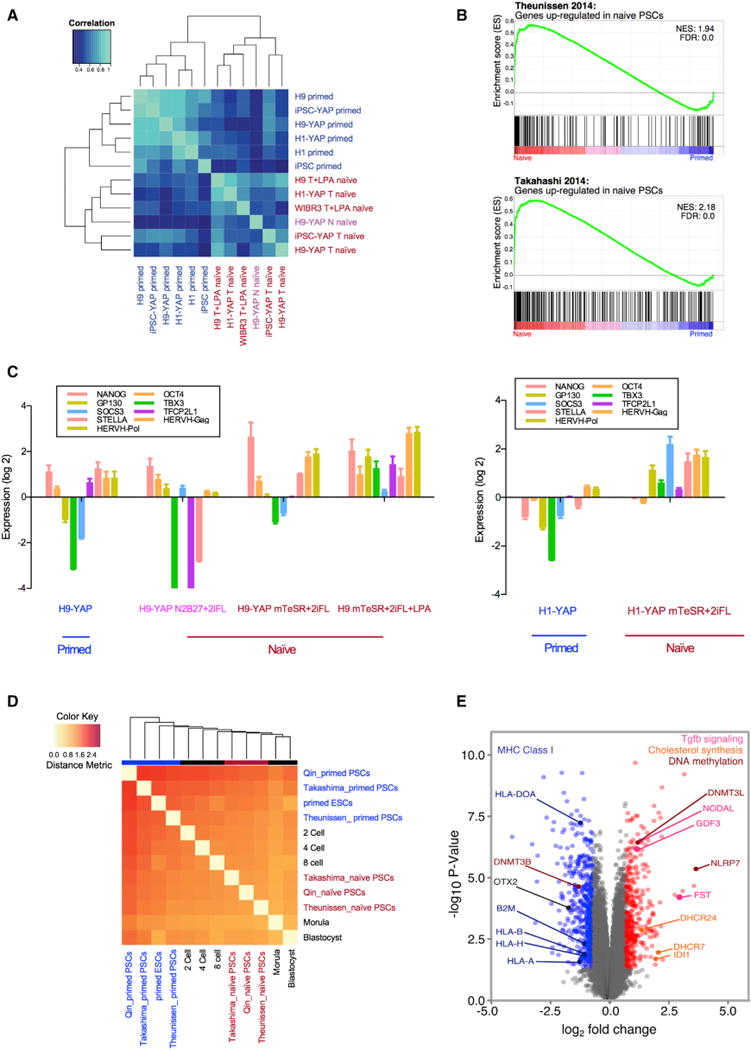
(A) Primed PSCs and Yin- and Lin-PSCs have distinct gene expression profiles, as shown by unsupervised hierarchical clustering. The top 1,000 genes with the highest coefficient of variation were used to cluster samples using Pearson correlation coefficients. Six human primed PSC samples include H9, H1, IMR-90 iPSCs, H9-YAP, H1-YAP, and IMR-90 iPSC-YAP cells. Six human naive PSC samples include H9-YAP in N2B27+2iFL (H9-YAP N naive), H9-YAP in mTeSR+2iFL (H9-YAP T naive), H1-YAP in mTeSR+2iFL (H1-YAP T naive), IMR-90 iPSC-YAP cells in mTeSR+2iFL (iPSC-YAP T naive), H9 in mTeSR+2iFL+LPA (H9 T+LPA naive), and WIBR3 in mTeSR+2iFL+LPA (WIBR3 T+LPA naive). Primed cells are indicated in blue. H9-YAP in N2B27+2iFL naive medium is in pink, and all other naive cells are in red.
(B) GSEA reveals that Yin-PSCs and Lin-PSCs have gene expression profiles concordant with naive PSCs from other studies. The upper panel is the enrichment plot for the gene set of Theunissen et al. (2014) upregulated in naive by Log2 FC > 3.0. The lower panel is the enrichment plot for the gene set of Takahashi et al. (2014) upregulated in naive by Log2 FC > 2.0. Vertical black bars represent the position of genes upregulated in naive cells in the Takashima et al. (2014) or Theunissen et al. (2014) studies, distributed along the differential expression values for the entire transcriptome in this study, and ranked from upregulated in Yin-PSCs and Lin-PSCs (red, left) to upregulated in parental primed PSCs (blue, right).
(C) Yin-PSCs and Lin-PSCs express markers specific for naive pluripotency, as confirmed by qRT-PCR. Values were normalized to GAPDH and UBB and then compared to H9 in primed medium (left panel) or H1 in primed medium (right panel). Data are averages of triplicate PCR reactions, and error bars represent SD.
(D) Hierarchical clustering shows that naive Yin-PSCs and Lin-PSCs are similar to naive cells from two other studies (Takashima et al., 2014; Theunissen et al., 2014) and to human pre-implantation embryos. In vivo states at various developmental stages (Yan et al., 2013) are included in the clustering.
(E) Volcano plot of significantly differentially expressed genes among the six human naive PSC samples and the six human primed PSC samples. Highlighted in red are genes with aLog2 FC > 0.7 in naive and p < 0.05. In blue are genes with a Log2 FC < −0.7 and p < 0.05 in naive. See text for a description of the specific genes indicated.
We next sought to identify the sets of genes that account for the distinct profiles of naive and primed PSCs. In Yin-PSCs and Lin-PSCs, 350 genes are upregulated with Log2 fold change (FC) > 0.7 and p < 0.05 (Data S1). These genes are enriched for functions in gastrulation, embryonic morphogenesis, formation of primary germ layers, and transforming growth factor β (TGF-β) signaling (Figure S3B; Data S2). Surprisingly, genes related to sterol and cholesterol biosynthesis are also highly enriched in this gene set (Figure S3B; Data S2; also see below). Conversely, 510 genes are downregulated in naive cells (Data S1). These genes are associated with functions in plasma membrane, cell motion, and positive regulation of differentiation (Figure S3C; Data S3).
A recent study (Huang et al., 2014) documents that among several alternate human PSCs, two studies (Takashima et al., 2014; Theunissen et al., 2014) stand out as representing naive cells with similarities to human pre-implantation embryos. To directly compare the overall transcriptional profiles of Yin-PSCs and Lin-PSCs to those two studies, we used gene set enrichment analysis (GSEA). This analysis revealed that genes upregulated in naive PSCs derived by the Jaenisch group (Theunissen et al., 2014) or the Smith group (Takashima et al., 2014) are also coordinately upregulated in Yin-PSCs and Lin-PSCs (Figure 3B). The same pattern of similarity to the other two studies is observed for genes downregulated in naive PSCs (Figure S3D).
We next used qRT-PCR to assess the expression levels of specific markers associated with naive pluripotency. We chose to test the expression of NANOG, OCT4, and REX1, which are typically associated with the self-renewal and pluripotency; TFCP2L1, the knockdown of which inhibits resetting of primed cells toward naive pluripotency (Takashima et al., 2014); GP130, SOCS3, and TBX3, which are related to the LIF-STAT3 pathway; and HERVH, a primate-specific endogenous retrovirus reported as a hallmark of naive-like ESCs (Wang et al., 2014). In all three cell lines examined (H9, H1, and IMR-90 iPSCs), these markers tend to be upregulated in naive cells relative to primed cells (Figures 3C and S3E). The upregulation of these makers is more consistent in mTeSR+2iFL or mTeSR+2iFL+LPA than in N2B27+2iFL (Figure 3C), supporting our previous observation that the mTeSR base medium is a better choice for induction and maintenance of the human naive PSC state. Taken together, these data demonstrate that Yin-PSCs and Lin-PSCs have a coordinately distinct transcriptional profile that is associated with naive pluripotency.
We then compared the transcriptional profiles of naive Yin-PSCs and Lin-PSCs and primed PSCs with those of pre-implantation stages of human development (Yan et al., 2013). We used a weighted gene co-expression network analysis (WGCNA) (Zhang and Horvath, 2005), and a rigorous information theoretic metric called variation of information (Meilă, 2007) to measure the distance between the co-expression networks of two cell types. A major advantage of this information theoretic method lies in its ability to compare the co-expression networks as a whole without selecting a single module, which may be biased and represent only a small portion of complex interactions. Compared with other previously used methods (Huang et al., 2014), our approach (described in Supplemental Experimental Procedures) captures a more global and unbiased view of similarity between cell types. We found that transcriptionally, Yin-PSCs and Lin-PSCs resemble human pre-implantation embryos and show high similarities with the naive PSC datasets by Takashima et al. (2014) and Theunissen et al. (2014). In contrast, primed PSCs from all three labs exhibit larger variability and form an outlier group with lower similarities to pre-implantation embryos (Figure 3D). Thus, treatment of human primed PSCs with YAP or LPA resets them to a naive state similar to human pre-implantation embryos.
We next sought to extract further biological insights from these expression datasets. We queried the data for genes that have high specificity of expression at the blastocyst stage and are upregulated in naive Yin-PSCs and Lin-PSCs relative to primed cells (Figure S3F). Similar to the previous gene ontology analysis (Figure S3B; Data S2), we found many of these genes to be involved in TGF-β signaling (GDF3, NODAL, FST), DNA methylation (DNMT3L, NLRP7), and cholesterol biosynthesis (DHCR24, DHCR7, IDI1) (Figure 3E). DNMT3L is an epigenetic regulator previously found to be highly upregulated in naive human PSCs (Theunissen et al., 2014). The observation that cholesterol biosynthesis genes are highly upregulated in both naive PSCs (Figures 3E and S3B; Data S2) and blastocysts (Figure S3F) suggests a potential role for this pathway in the naive state and early human development. Conversely, OTX2, DNMT3B, B2M, HLA-A, HLA-B, HLA-H, and HLA-DOA are strongly downregulated in naive cells (Figure 3E). OTX2 is a pioneer transcription factor that drives the transition from the naive to the primed state in mouse (Buecker et al., 2014) and was previously shown to be sharply downregulated in human naive PSCs (Theunissen et al., 2014). Downregulations of B2M and HLA genes in naive cells has, to our knowledge, not been previously reported and suggests that from an immunological point of view, naive cells are more undifferentiated and less immunogenic than primed cells (Chen et al., 2015; Drukker et al., 2002).
Further Characterization of Naive Pluripotency in Yin-PSCs and Lin-PSCs
In addition to having a distinct transcriptional program, naive pluripotency is associated with unique features of the chromatin landscape and enhancer activity. Although X chromosome reactivation is a hallmark of female naive PSCs in mouse, the available data are contradictory in human cells (Takashima et al., 2014; Theunissen et al., 2014). We nevertheless explored the X chromosome status by staining for histone 3 lysine 27 trimethylation, a reliable marker of X chromosome inactivation. We found that low-passage primed female H9 ESCs or IMR-90 iPSCs already display significant levels of X chromosome reactivation, precluding the use of this marker as a criterion for the naive state (data not shown). We did find that primed IMR-90 iPSC-YAP have mosaic X inactivation and that this is lost when the cells are converted to the naive state (data not shown). On the whole, the existing data and our analysis suggest that X chromosome reactivation may not unequivocally distinguish the naive state from the primed state in human cells (Takashima et al., 2014; Theunissen et al., 2014; Vallot et al., 2015).
Histone 3 lysine 9 trimethylation (H3K9me3) is a repressive chromatin mark highly enriched at heterochromatin, and H3K9me3 foci have been shown to be depleted in naive PSCs (Takashima et al., 2014). In agreement with this finding, the condensed H3K9me3 foci observed in primed PSCs almost disappear in Yin-PSCs and Lin-PSCs (Figures 4A and S4A). Western blotting confirmed a reduction in the total levels of H3K9me3 in Yin-PSCs and Lin-PSCs (Figure 4B).
Figure 4. YAP Regulates the Human Naive State and Acts in Part by Modulating Wnt Signaling.
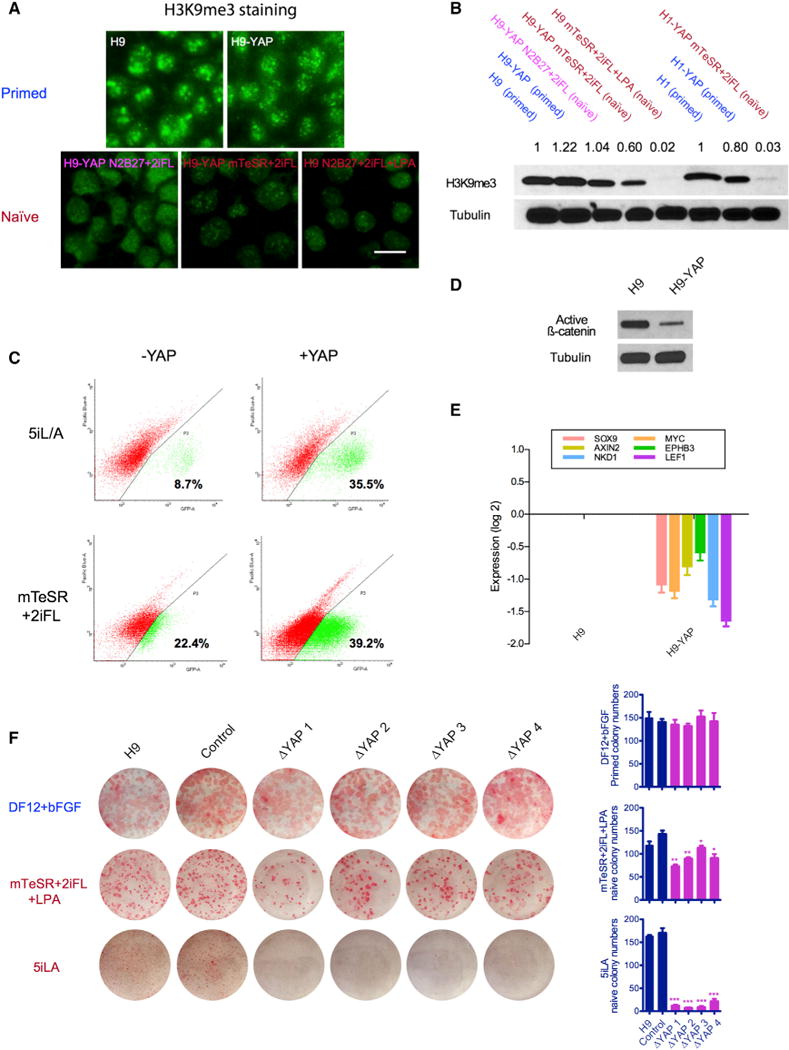
(A) H3K9me3 is strongly reduced in naive H9 ESCs, as seen by immunofluorescence. Upper panel: H9 and H9-YAP in DF12+bFGF primed medium. Lower panel: H9-YAP in N2B27+2iFL naive medium, H9-YAP in mTeSR+2iFL naive medium, and H9 in mTeSR+2iFL+LPA naive medium. Scale bar, 20 μm.
(B) Decreased total amount of H3K9me3 in Yin-PSCs and Lin-PSCs was confirmed by western blotting. Tubulin was used as loading control. Values indicate densitometry analysis of the H3K9me3 level normalized to tubulin.
(C) Flow cytometric analysis of the proportion of OCT4-ΔPE-GFP+ WIBR3 cells with or without YAP overexpression in 5i/L/A (upper panels) or mTeSR+2iFL (lower panels) media. Cells were expanded in bulk and analyzed at passage 3, in the absence of colony picking. YAP overexpression increases the ratio of OCT4-ΔPE-GFP + cells in both media.
(D) YAP overexpression decreases levels of unphosphorylated (active) β-catenin, as shown by western blotting. Tubulin was used as loading control.
(E) YAP overexpression decreases the expression of Wnt target genes, as shown by qRT-PCR. Values were normalized to GAPDH and UBB and then compared to H9. Data are averages of triplicate PCR reactions, and error bars represent SD.
(F) YAP knockout impairs the ability of ESCs to form naive colonies. H9 controls and four clones of CRISPR/Cas9-generated YAP−/− cells cultured in DF12+bFGF (primed), mTeSR+2iFL+LPA (naive), and 5i/L/A (naive, Theunissen et al., 2014) were trypsinized to single cells, counted, and plated onto MEFs in the presence of ROCK inhibitor. Seven days later, AP staining was performed and colony numbers were counted. Error bars represent SD. *p < 0.05; **p < 0.01; ***p < 0.001.
A further signature of naive pluripotency is the activation of the endogenous DE of OCT4. In mouse, the DE drives Oct4 expression in ESCs, the ICM, and primordial germ cells (Yeom et al., 1996), while the proximal enhancer (PE) activates Oct4 expression in primed epiblast stem cells (EpiSCs) and epiblast cells (Tesar et al., 2007). Theunissen et al. (2014) established a reporter for OCT4 DE activity in the human ESC line WIBR3 by deleting the PE in an OCT4-GFP knockin allele. These OCT4-ΔPE-GFP ESCs allowed the authors to identify a cocktail of inhibitors and growth factors (termed 5i/L/A) that promotes maintenance of naive human ESCs with OCT4-ΔPE activity. Using this cell line, we found that YAP overexpression dramatically increases endogenous OCT4 DE activity in both 5i/L/A and mTeSR+2iFL media, indicating that YAP promotes naive pluripotency in multiple culture conditions (Figure 4C). Taken together, our data demonstrate that Yin-PSCs and Lin-PSCs meet the key criteria of naive pluripotency.
YAP Regulates the Human Naive State and Acts in Part by Modulating Wnt Signaling
We sought to gain insights into the mechanisms by which YAP and LPA promote naive pluripotency. As mentioned earlier, H9 ESCs in the absence of YAP overexpression or LPA undergo rapid morphological differentiation when cultured in 2i medium (PD0325901+CHIR99021) after three passages (Figure S4B). We asked whether only one inhibitor or the combination of both induces differentiation. Short-term treatment of H9 cells with PD0325901 (ERK inhibitor) or CHIR99021 (GSK3 inhibitor) separately revealed that CHIR99021 is the major contributor to this effect (Figure S4B). qRT-PCR shows that CHIR99021 leads to an increase in the expression level of differentiation markers, most notably CDX2 (trophectoderm) but also T (mesoderm), SOX17 (endoderm), and SOX1 (ectoderm) (Figure S4C). We found that LPA suppresses the differentiation-inducing effects of CHIR99021 and strongly downregulates SOX17 (Figure S4C), which in addition to being an endoderm regulator, is a marker of the primed state (Tesar et al., 2007). In agreement with previous data in other systems (Gregorieff et al., 2015; Park et al., 2015), YAP overexpressing cells display lower levels of active β-catenin (Figure 4D) and Wnt target genes (Figure 4E). These data suggest that YAP promotes naive pluripotency in part by suppressing differentiation-inducing effects of GSK3 inhibition.
Finally, we tested whether endogenous YAP regulates human naive pluripotency in the absence of transgene induction. We used CRISPR/Cas9 to knockout the expression of YAP in primed H9 ESCs. Western blotting confirmed the loss of YAP protein in four independent mutant clones (Figure S4D) that carry frame-shift mutations at the CRISPR/Cas9 target sites (Figure S4E). We then compared self-renewal in standard primed medium, our mTeSR 2iFL+LPA medium, and the 5i/L/A formulation of Theunissen et al. (2014) but not the culture conditions from the protocol of Takashima et al. (2014), because it requires overexpression of NANOG and KLF2. We found that YAP is not required for self-renewal of primed ESCs (Figure 4F). However, the ability to form naive colonies after transfer to naive media is impaired in YAP−/− cells, most strikingly in the 5i/L/A medium (Figure 4F) (Theunissen et al., 2014). These data lend strong support to the conclusion that YAP promotes naive pluripotency in human cells.
DISCUSSION
We show here that YAP or its activator LPA promote the transition to the naive PSC state in multiple human ESC and iPSC lines. Yin-PSCs and Lin-PSCs can be maintained long term with robust clonal growth and fulfill the key criteria of naive pluripotency. YAP therefore represents an unexpected regulator of the human naive state, with potential implications for our understanding of early human embryology. Moreover, observations made during the course of this work shed light on the conditions that underlie the successful derivation and maintenance of human naive PSCs.
First, our results indicate that different human PSCs lines can have distinct abilities to maintain naive pluripotency: (1) in N2B27+2iFL naive medium, Yin-PSCs can only be derived from H9-YAP but not H1-YAP, IMR-90 iPSC-YAP, or other cell lines (Figure 1); (2) in mTeSR+2iFL+LPA naive medium, Lin-PSCs can be generated from H9 and WIBR3 (Figure 2), but in the same conditions, H1 and IMR-90 iPSCs begin to differentiate after several passages (data not shown); and (3) in 5i/L/A medium, naive cells can be readily derived from H9 and WIBR3 (Theunissen et al., 2014) but not from H1 or IMR-90 iPSCs in our hands (data not shown). Importantly, YAP overexpression in mTeSR-based medium can convert all PSC lines tested to the naive state. However, in the absence of YAP overexpression or in sub-optimal culture media, H9 and WIBR3 cells are more easily induced to naive pluripotency compared with other cells lines tested. In mice, it is well known that the ability to derive ESCs is strongly dependent on the genetic strain. The 129 strain, or hybrids thereof, yields ESCs efficiently, and this may be related to the high incidence of spontaneous germ cell tumors in these mice (Blair et al., 2011; Bustamante-Marín et al., 2013). Some other strains, for example, non-obese diabetic mice, are considered non-permissive for ESC derivation under canonical culture conditions and require further modifications of the media (Hanna et al., 2009). It will be interesting to determine whether there is a genetic or epigenetic predisposition to achieve ICM-like naive pluripotency in different human PSCs. Both H9 and WIBR3 are female cells, and the additional X chromosome could potentially contribute factors that facilitate maintenance of the naive state. Mouse female ESCs have both X chromosomes active, but in human cells there does not seem to be a strict correlation between XaXa status and naive pluripotency (data not shown) (Takashima et al., 2014; Theunissen et al., 2014; Vallot et al., 2015). Further research using a large panel of PSCs will be needed to explore the differential propensities of human primed PSCs to achieve a stable naive state.
Second, our work underscores the importance of the culture medium formulation in the derivation of naive PSCs, including the base medium. Yin-PSCs could not be derived in N2B27 base medium for several of the lines tested, and H9 Yin-PSCs grown in N2B27 only partially express naive state markers (Figure 3C) and have intermediate levels of H3K9me3 (Figures 4A and 4B). The mTeSR medium was superior in inducing the naive state of H9 Yin-PSCs (Figures 3C, 4A, and 4B) and allowed the derivation of Yin-PSCs from multiple ESCs and iPSCs resistant to conversion in N2B27. Unlike N2B27, mTeSR is supplemented with basic fibroblast growth factor (bFGF) and TGF-β. In mouse ESCs, bFGF and TGF-β are considered critical for primed EpiSCs but not for naive pluripotency (Blair et al., 2011). However, genes related to TGF-β signaling pathway, including GDF3, NODAL, LEFTY2, SPP1, and CER1, are upregulated in Yin-PSCs and Lin-PSCs (Figure S3B; Data S2). In agreement with these findings, other groups included bFGF and TGF-β/activin in their medium to culture human naive PSCs (Gafni et al., 2013; Theunissen et al., 2014). Unlike the case in mice, it appears that stable maintenance of the human naive PSC state requires growth factors in addition to LIF, possibly including bFGF and TGF-β. Previous studies used the mTeSR medium but failed to derive human naive PSCs with similarities to human pre-implantation embryos (Chan et al., 2013; Huang et al., 2014; Ware et al., 2014). We believe that this is due to variations in media composition and the use of different small molecules; in addition, they did not use mTeSR with YAP overexpression or LPA. Our data show that in the presence of exogenous YAP or LPA, mTeSR supports an authentic naive transcription profile resembling human pre-implantation embryos (Figure 3D). The quality of our mTeSR+2iFL+LPA formulation for culturing naive cells is further highlighted by its increased resilience to YAP loss compared with 5i/L/A (Theunissen et al., 2014) (Figure 4F). LPA may induce downstream effects, in addition to activating YAP, such as activation of the closely related protein TAZ (Yu et al., 2012). However, YAP overexpression induces a naive state with a faster growth rate and in more cell lines than exposure to LPA, likely because transgene overexpression achieves significantly higher levels of YAP than does LPA treatment (Figure S2A versus Figure 2B). It will be interesting to explore other methods to induce high levels of YAP in a transgene-free manner.
Third, this work reveals that YAP is an unexpected regulator of the human naive state. YAP overexpression in primed culture conditions alone does not change colony morphology, sensitivity to trypsin (Figure 1D), expression of naive markers (Figures 3C and S3E), or levels of H3K9me3 (Figures 4A, 4B, and S4A). Moreover, the transcriptional profile is not significantly altered between PSCs and PSC-YAP cells in primed medium (Figure 3A). These data suggest that YAP acts in conjunction with the modulation of signaling pathways that are induced by the naive medium. In support of this notion, we found that LPA suppresses differentiation-inducing effects of GSK3 inhibition by CHIR99021 (Figures S4B and S4C). GSK3 inhibition enhances cell growth capacity and viability of mouse naive PSCs (Ying et al., 2008). However, we found that in human ESCs, GSK3 inhibition has a dual effect: it promotes survival and proliferation but also induces differentiation (Figure S4C). It has been reported that lowering the concentration of CHIR99021 is beneficial for maintenance of human naive pluripotency (Takashima et al., 2014; Theunissen et al., 2014). Taken together, these results indicate that additional modulation of signaling is required to balance the effects of GSK3 inhibition on the naive state, and our work reveals that activation of YAP provides such balance. One effect of GSK3 inhibition is the stabilization of β-catenin and activation of the canonical Wnt pathway, both of which are countered by YAP overexpression (Figures 4D and 4E), in agreement with studies in other systems (Azzolin et al., 2014; Gregorieff et al., 2015; Park et al., 2015). These results suggest that the combination of GSK3 inhibition with YAP activation provides the appropriate balance of Wnt signaling to sustain the naive state. Our findings parallel a report that YAP reprograms intestinal stem cells toward regeneration by antagonizing a differentiation-inducing effect promoted by high levels of Wnt signaling (Gregorieff et al., 2015). It will be interesting to further explore the potential interactions between YAP and Wnt signaling in human naive PSCs. Moreover, YAP may in parallel cooperate with other factors downstream of 2iFL, TGF-β, or bFGF to implement the transcriptional program of the naive state. Lin-PSCs provide a platform to functionally dissect the interactions between YAP and other cellular pathways, as well as to identify other small molecules that contribute to stabilizing the human naive state.
Finally, this work raises interesting questions regarding the regulation of naive pluripotency in early human and mouse embryology. In human blastocysts, YAP protein is nuclearly localized in both the trophectoderm and the ICM (Figures 1A and S1B). However, in mice, Yap has been reported to be excluded from the nucleus of ICM cells and has been implicated in trophectoderm development (Nishioka et al., 2009). These findings could be interpreted as Yap having no role in the development of mouse pluripotent cells. However, cytoplasmic Yap may still contribute to the regulation of the ICM, for example, by interacting with other pathways, including Wnt signaling (Azzolinetal.,2014). Moreover, Yap−/− mice do not have implantation defects, which would be indicative of abnormal trophectoderm development (Morin-Kensicki et al., 2006). Yap and Taz may act redundantly, because both are present in the nucleus and cytoplasm during early cleavage stages, and Yap/Taz double mutant embryos arrest before the morula stage (Nishioka et al., 2009). Thus, an alternative possibility is that YAP activation reverts human PSCs to a pluripotent state with similarities to cleavage-stage embryos, a possibility supported by our transcriptional analysis (Figure 3D). Our findings warrant further study of the role of the Hippo pathway in early mouse and human development and provide a platform for chemically and genetically dissecting human naive pluripotency.
EXPERIMENTAL PROCEDURES
Culture of Human Primed PSCs
All research described here was done with the approval of the Stanford University Institutional Review Board–RENEW Biobank and the UCSF Human Gamete, Embryo and Stem Cell Research Committee. H1 (P27) and H9 (P28) human ESCs were obtained from the WiCell Research Institute. WIBR3 human ESCs with OCT4-ΔPE-GFP reporter were provided by Dr. Rudolf Jaenisch. Human iPSCs were generated from BJ and IMR-90 fibroblasts (ATCC) using retroviruses (Harvard Gene Therapy Initiative) leading to the overexpression of OCT4, SOX2, KLF4, and c-MYC as described earlier (Qin et al., 2014). Human primed ESCs and iPSCs were cultured on irradiated mouse embryonic fibroblasts (MEFs) in primed medium (DF12+bFGF) consisting of 80% DMEM/F 12, 20% knockout serum replacement, 1 mM L-glutamax, 1% non-essential amino acids, 0.1 mM beta-mercaptoethanol, 1% penicillinstreptomycin, and 8 ng/ml bFGF (all from Invitrogen). Cells were passaged with collagenase IV (Invitrogen) every 7 days. For YAP overexpression, YAP cDNA was cloned into the lentiviral vector pGAMA downstream of an EF1 α promoter (EF1 α-YAP-T2A-mCherry). Lentiviruses were produced and human PSCs were infected as described earlier (Qin et al., 2014). YAP overexpressing cells were trypsinized, isolated by fluorescence-activated cell sorting for mCherry-positive cells, plated in the presence of ROCK inhibitor Y-27632 (Stemgent, 5 μM), and expanded in standard primed conditions.
Culture of Human Naive PSCs
To derive human naive PSCs, primed cells were switched to naive media based on N2B27 or mTeSR. N2B27 medium consisted of a 1:1 mixture of DMEM/F12 and Neurobasal medium, with 1% N2 supplement, 2% B27 supplement, 1 mM L-glutamax, 0.1 mM beta-mercaptoethanol, 1× non-essential amino acids, and 1× penicillin-streptomycin (all from Invitrogen). The mTeSR1 medium was purchased from STEMCELL. N2B27 and mTeSR base media were supplemented with 2iFL: PD0325901 (Stemgent, 0.5 μM), CHIR99021 (Selleck Chemicals, 3 μM), Forskolin (Sigma, 10 μM) and recombinant human LIF(EMD Millipore, 10 ng/ml). LPA (Sigma, 10 μM) was added to naive media when indicated. The ROCK inhibitor Y-27632 (Stemgent, 5 μM) was used for the first three passages. Human naive PSCs were maintained on irradiated MEFs. To passage naive PSCs, cells were briefly washed with PBS, dissociated to single cells using TrypLE (Invitrogen), centrifuged in fibroblast medium (DMEM with 10% fetal bovine serum, 1 mM glutamax, 1× non-essential amino acids, 1× sodium pyruvate, 1× penicillin/streptomycin, and 0.06 mM β-mercaptoethanol, all from Invitrogen), and seeded onto irradiated MEFs in naive medium. The 5i/L/A medium was prepared as described earlier (Theunissen et al., 2014).
Supplementary Material
Highlights.
YAP overexpression promotes a naive state in human PSCs
LPA partially substitutes for YAP in the derivation of transgene-free naive PSCs
YAP regulates the human naive state by modulating Wnt signaling
YAP is required for efficient self-renewal of human naive PSCs
Acknowledgments
We are grateful to Robert Blelloch, Marco Conti, and Susan Fisher for input during the course of this work; to Alex Dunn for antibodies and discussions; and to members of the M.R.-S. lab for discussions and critical reading of the manuscript. We thank Rudolf Jaenisch for the OCT4-ΔPE-GFP WIBR3 human ESCs, Fong Ming Koh for providing the pGAMA vector, and Kathryn Blaschke and Yuki Ohi for initial cloning of the YAP cDNA. This work was partially supported by a Siebel Stem Cells Institute Scholarship and a Stinehart Reed Seed Grant to V.S., NIH R01 CA163336 to J.S.S., and CIRM grant RB4-06028 and NIH R01 OD012204to M.R.-S.
Footnotes
ACCESSION NUMBERS
The accession number for the microarray data reported in this paper is GEO: GSE69200.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and three data files and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.02.036.
AUTHOR CONTRIBUTIONS
H.Q. and M.R.-S. conceived the project and developed the experimental design. H.Q. carried out most of the bench experiments with technical assistance from LB. and P.W. M.H., M.P., and J.S.S. performed bioinformatic analyses; Y.L, H.Q., and L.S.Q. generated YAP−/− ESCs; M.W., J.D.-D., and V.S. analyzed YAP expression in human blastocysts; and Z.Q. performed karyotyping analyses under the supervision of J.Y. H.Q. and M.R.-S. wrote the manuscript, with input from all authors.
References
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Blair K, Wray J, Smith A. The liberation of embryonic stem cells. PLoS Genet. 2011;7:e100. doi: 10.1371/journal.pgen.1002019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buecker C, Chen HH, Polo JM, Daheron L, Bu L, Barakat TS, Okwieka P, Porter A, Gribnau J, Hochedlinger K, Geijsen N. A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell. 2010;6:535–546. doi: 10.1016/j.stem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buecker C, Srinivasan R, Wu Z, Calo E, Acampora D, Faial T, Simeone A, Tan M, Swigut T, Wysocka J. Reorganization of enhancer patterns in transition from naive to primed pluripotency. Cell Stem Cell. 2014;14:838–853. doi: 10.1016/j.stem.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marín X, Garness JA, Capel B. Testicular teratomas: an intersection of pluripotency, differentiation and cancer biology. Int J Dev Biol. 2013;57:201–210. doi: 10.1387/ijdb.130136bc. [DOI] [PubMed] [Google Scholar]
- Chan YS, Göke J, Ng JH, Lu X, Gonzales KAU, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Chen HF, Yu CY, Chen MJ, Chou SH, Chiang MS, Chou WH, Ko BS, Huang HP, Kuo HC, Ho HN. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant. 2015;24:845–864. doi: 10.3727/096368913X674639. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Mitalipova M, Cheng AW, Cassady JP, Staerk J, Carey BW, Lengner CJ, Foreman R, Love J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Maruyama T, Fan G. The naive state of human pluripotent stem cells: a synthesis of stem cell and preimplantation embryo transcriptome analyses. Cell Stem Cell. 2014;15:410–415. doi: 10.1016/j.stem.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilă M. Comparing clusterings—an information based distance. J Multivar Anal. 2007;98:873–895. [Google Scholar]
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O’Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Blaschke K, Wei G, Ohi Y, Blouin L, Qi Z, Yu J, Yeh RF, Hebrok M, Ramalho-Santos M. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Hum Mol Genet. 2012;21:2054–2067. doi: 10.1093/hmg/dds023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diaz A, Blouin L, Lebbink RJ, Patena W, Tanbun P, LeProust EM, McManus MT, Song JS, Ramalho-Santos M. Systematic identification of barriers to human iPSC generation. Cell. 2014;158:449–461. doi: 10.1016/j.cell.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vallot C, Ouimette JF, Makhlouf M, Féraud O, Pontis J, Côme J, Martinat C, Bennaceur-Griscelli A, Lalande M, Rougeulle C. Erosion of X chromosome inactivation in human pluripotent cells initiates with XACT coating and depends on a specific heterochromatin landscape. Cell Stem Cell. 2015;16:533–546. doi: 10.1016/j.stem.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Wang J, Xie G, Singh M, Ghanbarian AT, Raskó T, Szvetnik A, Cai H, Besser D, Prigione A, Fuchs NV, et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–409. doi: 10.1038/nature13804. [DOI] [PubMed] [Google Scholar]
- Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Yang M, Guo H, Yang L, Wu J, Li R, Liu P, Lian Y, Zheng X, Yan J, et al. Single-cell RNA-seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hübner K, Schöler HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


