Abstract
Background
Evidence-based therapies for heart failure (HF) differ significantly according to left ventricular ejection fraction (LVEF). However, few data are available regarding the phenotype and prognosis of HF patients with mid-range LVEF of 40–55%, and the impact of recovered systolic function on the clinical features, functional capacity and outcomes of this population is not known.
Methods and Results
We studied 944 HF patients who underwent clinically indicated cardiopulmonary exercise testing. The study population was categorized according to LVEF as: HFrEF (LVEF<40%; n=620); HFmEF (LVEF was consistently between 40–55%; n=107); HFm-recEF (LVEF=40–55% but previous LVEF<40%; n=170); and HFpEF (LVEF>55%; n=47). HFmEF and HFm-recEF had similar clinical characteristics, which were intermediate between those of HFrEF and HFpEF, and comparable values of predicted peak oxygen consumption and minute-ventilation/carbon dioxide production slope, which were better than HFrEF and similar to HFpEF. After a median of 4.4 [2.9–5.7] years, there were 253 composite events (death, left ventricular assistant device implantation or transplantation). In multivariable Cox-regression analysis, HFm-recEF had lower risk of composite events than HFrEF (HR=0.25; 95%CI=0.13–0.47) and HFmEF (HR=0.31; 95%CI=0.15–0.67), and similar prognosis when compared to HFpEF. In contrast, HFmEF tended to show intermediate risk of outcomes in comparison with HFpEF and HFrEF, albeit not reaching statistical significance in fully adjusted analyses.
Conclusions
HF patients with mid-range LVEF demonstrate a distinct clinical profile from HFpEF and HFrEF patients, with objective measures of functional capacity similar to HFpEF. Within the mid-range LVEF HF population, recovered systolic function is a marker of more favorable prognosis.
Keywords: heart failure, mid-range ejection fraction, recovered systolic function, cardiopulmonary exercise testing
Heart failure (HF) is routinely classified according to left ventricular ejection fraction (LVEF) as HF with reduced LVEF (HFrEF) or HF with preserved LVEF (HFpEF), a distinction driven by the important differences in the evidence base for therapies for HF. Studies of HFrEF have been restricted to patients with LVEF <40%, while diagnostic guidelines for HFpEF typically include patients with LVEF >50–55% (1, 2, 3, 4). As a consequence, few data are available regarding the phenotype, natural history, and prognosis of HF patients with mid-range LVEF of 40–55%. Results from previous reports have suggested that HF patients with mid-range LVEF have clinical features (5, 6, 7) and mortality rates (5) that are intermediate between those of HFrEF and HFpEF. However, substantial heterogeneity may exist within HF patients with mid-range LVEF. In particular, this group may include both patients with de novo HF and HF patients with previously reduced LVEF who have recovered their systolic function (3, 8, 9). This fact is clinically relevant because subjects with recovered LVEF have been reported to have more favorable prognosis among HF patients (9). However, whether mid-range LVEF patients with recovered LVEF have distinct phenotypic and prognostic features compared to those without a previous frankly reduced LVEF is not known. The aim of this study was to compare the clinical features, cardiopulmonary response to exercise, and long-term clinical outcomes in HF patients with mid-range LVEF either without previous frankly reduced LVEF (HFmEF) or with recovery from previous frankly reduced LVEF (HFm-recEF) in relation to each other and to HFrEF and HFpEF patients.
Methods
Study population
This study included 974 patients with a diagnosis of HF who were referred for cardiopulmonary exercise testing (CPET) at the Brigham and Women’s Hospital between July 2007 and June 2013. We excluded participants with missing baseline LVEF data (n=4), who performed arm ergometer exercise testing (n=1), developed tachyarrhythmias during the CPET (n=2), or had ventricular-paced rhythm with decreased or similar peak heart rate when compared to resting heart rate at CPET (n=9), resulting in 958 participants for the analysis. The Partners Human Research Committee approved this study and waived the requirement for informed consent.
Classification of HF patients
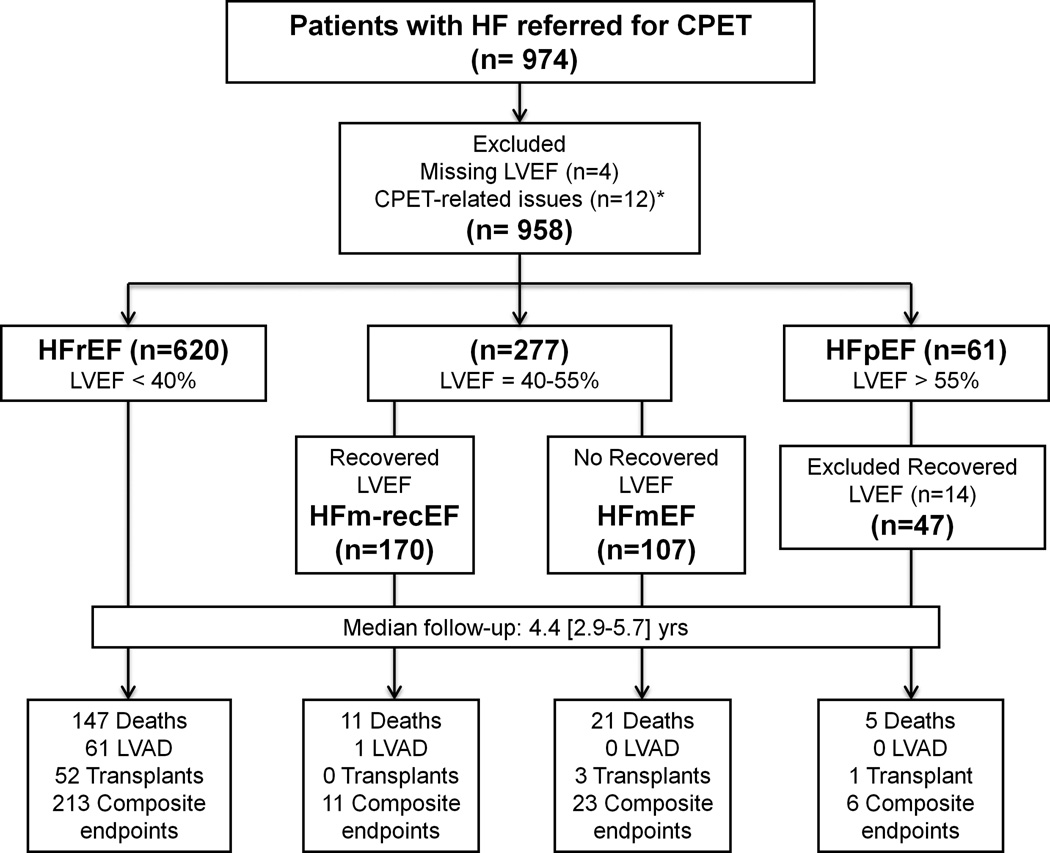
LVEF was assessed clinically at the Brigham and Women’s Hospital by quantitative echocardiography. Baseline LVEF values were obtained from echocardiography exams that were most contemporaneous to the CPET dates (median time difference [25th, 75th percentiles] = 0 [0, 9] days). The study population was categorized based on LVEF as (Figure 1): (1) HFrEF if the LVEF was <40% (n=620); (2) HF with mid-range LVEF if the LVEF was between 40–55% (n=277); and (3) HFpEF if the LVEF was >55% (n=61). Among patients with mid-range LVEF, those who had previously documented LVEF ≥40% (n=100) or a history of recent onset (<3 months) of HF (n=7) were considered to not have recovered systolic function and were labeled as HFmEF (n=107), while those with a documented history of LVEF <40% (n=170) were considered to have recovered mid-range LVEF (HFm-recEF). Sixty-one patients had LVEF >55%, but 17 of them had previously documented LVEF <40%, and were excluded from the analysis, resulting in 47 participants with HFpEF. Among HFpEF patients, 43 subjects had previous echocardiography documentation of no reduced LVEF and 4 subjects had recent onset (<3 months) of HF. The median interval time between previously performed echocardiogram and the echocardiogram that was most contemporaneous to the CPET dates was similar among the studied groups, with median [25th, 75th percentiles] as follows: 2.1 [0.5, 7.3] years for HFm-recEF; 3.4 [0.8, 7.7] years for HFmEF; 4.3 [0.4, 7.8] years for HFpEF; p for between group difference = 0.47, based on Kruskal-Wallis test. The distribution of LVEF according to the studied groups is shown in Supplemental Figure 1.
Figure 1.
Study design. CPET - cardiopulmonary exercise testing; HF – heart failure; HFrEF – HF with reduced LVEF; HFm-recEF – HF with mid-range and recovered LVEF; HFmEF – HF with mid-range and without recovered LVEF; HFpEF – heart failure with preserved LVEF; LVAD – left ventricular assistance device; LVEF – left ventricular ejection fraction. * used arm ergometer (n=1), developed tachyarrhythmias (n=2), and had ventricular-paced rhythm with decreased or similar peak heart rate when compared to resting (n=9) during CPET.
Clinical variables definition
Information on patients’ demographics, current medications, pacemaker, implantable cardioverter-defibrillator or cardiac resynchronization therapy, body mass index, blood pressure, heart rate and gas-exchange variables were collected at the time of CPET. Additional clinical characteristics and laboratory values most contemporaneous to CPET dates were obtained from chart review. In HFmEF and HFm-recEF subjects, LVEF data from the latest echocardiogram exam performed after the CPET test were also collected, except if there was an interval event (myocardial infarction, left ventricular assistant device (LVAD) implantation and heart transplantation) between baseline and follow-up echocardiogram.
Symptomatic HF was defined if patients were NYHA functional class II or greater as determined by the referring physician, or if the patient had a previous history of hospitalization for decompensated HF. Antiarrhythmic medications included amiodarone or digoxin. Glomerular filtration rate was estimated using the CKD-EPI formula (10). Angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers were coded into a single variable (ACEI/ARB). Cardiac resynchronization therapy and implantable cardioverter-defibrillator were coded as a single variable (CRT/ICD).
Exercise protocol
All exercise tests were performed in the Brigham and Women’s Hospital cardiopulmonary exercise laboratory using an upright cycle ergometer (Lode; Groningen, Netherlands; 98% of tests) or a treadmill (General Electric Healthcare; Waukesha, WI; 2% of tests) with the subject breathing room-air. Symptom-limited CPET was performed on all subjects. All pharmacological therapy was continued prior to and through exercise testing. The equipment was calibrated daily in standard fashion using reference gases. Minute ventilation (VE), oxygen consumption (VO2) and carbon dioxide production (VCO2) were acquired breath-by-breath and averaged over a 10-second interval, using a ventilatory expired gas analysis system (MGC Diagnostics, St. Paul MN). Peak VO2 was defined as the highest 10-second averaged VO2 during the last stage of the symptom-limited exercise test. Percent of predicted peak VO2 was determined based on the Wasserman formula (11, 12). VE/VCO2 slope was taken from rest to the gas exchange at peak exercise. Rhythm was monitored with a continuous 12-lead electrocardiogram. Blood pressure was measured using a standard cuff sphygmomanometer. Resting and peak heart rate were obtained from the electrocardiogram. Age-predicted maximal heart rate was estimated by Astrand’s formula (13): 220 – age (years). Chronotropic index was calculated as: (peak heart rate– resting heart rate)/(age-predicted maximal heart rate – resting heart rate) (14).
Outcomes
All-cause death was determined using the National Death Index with complete follow-up through December 31, 2014. The composite endpoint was defined as the composite outcome of LVAD implantation, heart transplantation or all-cause mortality.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed data or median [25th, 75th percentiles] for non-normally distributed data. Categorical variables are expressed as number of subjects and proportion. Comparisons of baseline features among the studied groups were performed using one-way ANOVA followed by Bonferroni test for normally distributed variables and Kruskal-Wallis test followed by Wilcoxon test for non-normally distributed variables. The chi-square test was used to compare categorical variables. Bonferroni correction was also applied for pairwise comparison of non-normal and categorical variables. CPET data are also presented as age-adjusted means ± standard error of the mean with p-values estimated from linear regression among the studied groups.
Univariate and multivariable Cox proportional hazards regression models were used to assess the unadjusted and adjusted association between LVEF categories and all-cause mortality or the composite outcome of LVAD implantation, heart transplantation and all-cause mortality. We tested the proportional hazards assumption for all analyses and no evidence of violation of the proportional-hazards assumption by LVEF categories was observed. Covariates for multivariable Cox-regression models were selected by using a forward stepwise selection procedure (retention p<0.10) including clinical and treatment variables that showed significant differences among the studied groups at baseline as candidate covariates (Supplemental Tables 1 and 2). Two multivariable Cox-regression models were constructed for each outcome: the first model included age, gender and clinical covariates and the second model further included treatment covariates. When all-cause mortality was considered as the outcome, model 1 was adjusted for age, sex, glomerular filtration rate, coronary artery disease, post-chemotherapy, diabetes mellitus, race and hemoglobin, while model 2 was further adjusted for use of pacemaker, diuretics, statins and ACEI/ARB. When the composite endpoint was considered as the outcome, model 1 was adjusted for age, sex, glomerular filtration rate, coronary artery disease and post-chemotherapy, while model 2 was further adjusted for use of diuretics, aldosterone antagonists, anticoagulation and ACEI/ARB.
In patients with HFmEF and HFm-recEF, baseline and follow-up LVEF values were compared using Wilcoxon matched-pairs signed-ranks test. Based on the difference between follow-up and baseline LVEF (ΔLVEF), patients were divided into 3 groups: 1) declining LVEF (ΔLVEF < −7%); 2) stable LVEF (ΔLVEF between −7 and +7%) and 3) increasing LVEF (ΔLVEF > +7%). The threshold of 7% was chosen because this value corresponds to the inter-reader variability reported for quantitative assessment of LVEF by echocardiography (15). Furthermore, we calculated the percentage of HFmEF and HFm-recEF patients who developed frankly reduced LVEF (LVEF<25%), normal LVEF (LVEF>55%) or remained as mid-range LVEF (LVEF of 40–55%) at follow-up.
A two sided p-value <0.05 was considered significant. Statistical analysis was performed using Stata software Version 12.1 (Stata Corp LP, College Station, TX, USA).
Results
Population characteristics
HFmEF had similar clinical features when compared to HFm-recEF, except for higher LVEF (50 [45, 55] vs. 45 [40, 50]% respectively; p=0.003; Table 1). HFmEF and HFm-recEF had average age and prevalence of hypertension that were more similar to those of HFrEF, while the prevalence of men and ischemic cardiomyopathy tended to be more similar to those of HFpEF. Compared to both HFpEF and HFrEF, HFmEF and HFm-recEF were more likely to be previously exposed to chemotherapy and tended to have lower prevalence of symptomatic HF, while HFm-recEF had lower prevalence of diabetes mellitus, and higher estimated glomerular filtration rate.
Table 1.
Baseline clinical and treatment features of study participants.
| Variables | HFrEF n=620 (66%) |
HFm-recEF n=170 (18%) |
HFmEF n=107 (11%) |
HFpEF n=47 (5%) |
|---|---|---|---|---|
| Clinical | ||||
| Age, years | 55.4 ± 13.2 | 52.2 ± 13.0* | 54.4 ± 15.2 | 63.3 ± 15.5*†‡ |
| Male, n (%) | 452 (73) | 104 (61)* | 59 (55)* | 23 (49)* |
| White, n (%) | 507 (82) | 145 (85) | 98 (92)* | 40 (85) |
| Body mass index, kg/m2 | 28.3 ± 5.7 | 28.7 ± 6.1 | 29.4 ± 6.6 | 31.5 ± 8.9* |
| Ischemic cardiomyopathy, n (%) | 188 (30) | 18 (11)* | 12 (12)* | 3 (6)* |
| Post-Chemotherapy, n (%) | 38 (6) | 20 (12) | 17 (16)* | 2 (4) |
| NYHA, n (%) | ||||
| I | 147 (24) | 81 (47)* | 40 (37) | 16 (34) |
| II | 214 (34) | 61 (36) | 35 (33) | 14 (30) |
| III | 210 (34) | 27 (16)* | 30 (28) | 16 (34)† |
| IV | 49 (8) | 1 (1)* | 2 (2) | 1 (2) |
| Previous HF hospitalization, n (%) | 486 (78) | 96 (57)* | 64 (60)* | 41 (87)†‡ |
| Symptomatic HF, n (%) | 560 (90) | 128 (75)* | 77 (72)* | 41 (87) |
| Hypertension, n (%) | 365 (59) | 87 (51) | 62 (58) | 36 (77)† |
| Diabetes mellitus, n (%) | 182 (29) | 27 (16)* | 22 (21) | 15 (32) |
| Coronary artery disease, n (%) | 255 (41) | 36 (21)* | 28 (26)* | 14 (30) |
| Atrial Fibrillation, n (%) | 215 (35) | 46 (27) | 30 (28) | 18 (38) |
| COPD, n (%) | 61 (10) | 16 (10) | 9 (8) | 4 (9) |
| Estimated GFR, mL/min | 72 ± 26 | 82 ± 24* | 75 ± 26 | 67 ± 26† |
| Hemoglobin, g/dL | 13.6 ± 1.7 | 13.5 ± 1.7 | 13.0 ± 2.0* | 12.8 ± 2.0†* |
| LVEF, % | 25 [19, 30] | 45 [40, 50]* | 50 [45, 55]*† | 60 [60, 65]*†‡ |
| Treatment | ||||
| CRT/ICD, n (%) | 335 (54) | 44 (26)* | 15 (14)*† | 1 (2)*† |
| Pacemaker, n (%) | 340 (55) | 47 (28)* | 23 (22)* | 6 (13)* |
| Beta-blockers, n (%) | 555 (90) | 145 (85) | 73 (68)*† | 29 (62)*† |
| ACEI/ARB, n (%) | 510 (82) | 146 (86) | 58 (54)*† | 30 (64)*† |
| Aldosterone antagonists, n (%) | 220 (36) | 38 (22)* | 15 (14)* | 2 (4)*† |
| Diuretics, n (%) | 468 (76) | 80 (47)* | 48 (45)* | 34 (72)†‡ |
| Calcium channel blockers, n (%) | 24 (4) | 13 (8)* | 27 (25)*† | 11 (23)*† |
| Anticoagulation, n (%) | 244 (39) | 43 (25)* | 23 (22)* | 15 (32) |
| Antiplatelets, n (%) | 350 (57) | 68 (40)* | 44 (40)* | 22 (47) |
| Antiarrhythmics, n (%) | 254 (41) | 38 (22)* | 6 (6)*† | 4 (9)* |
| Statins, n (%) | 322 (52) | 72 (43) | 41 (38) | 21 (45) |
p<0.05 compared to HFrEF;
p<0.05 compared to HFm-recEF;
p<0.05 compared to HFmEF.
All p-values are Bonferroni-adjusted. Data are presented as mean ± standard deviation for normally distributed variables and median [25th,75th percentile] for non-normally distributed continuous variables.
ACEI/ARB – angiotensin converting enzyme inhibitor or angiotensin receptor blocker; COPD – chronic obstructive pulmonary disease; CRT/ICD - cardiac resynchronization therapy and/or implantable cardioverter defibrillator; GFR – glomerular filtration rate; HF – heart failure; HFrEF – HF with reduced LVEF; HFm-recEF – HF with mid-range and recovered LVEF; HFmEF – HF with mid-range and without recovered LVEF; HFpEF – heart failure with preserved LVEF; LVEF- left ventricular ejection fraction. NYHA – New York Heart Association Classification.
Regarding medical therapies, HFm-recEF and HFrEF were more likely to use beta-blockers and ACEI/ARB and were less likely to use calcium channel blockers compared to both HFmEF and HFpEF. The prevalence of aldosterone antagonist use and of pacemakers or CRT/ICD in HFm-recEF and HFmEF tended to be intermediate between HFpEF and HFrEF. Both HFm-recEF and HFmEF had a lower prevalence of diuretic use than HFpEF and HFrEF.
Cardiopulmonary Exercise Performance
CPET variables according to LVEF categories are shown in Table 2. Mean peak respiratory exchange ratio (RER), a measure of exercise effort, was >1.1 in all patient groups. HFmEF and HFm-recEF had comparable ventilatory responses to exercise, with a higher peak VO2 (both absolute and percent of predicted) and lower VE/VCO2 slope than HFrEF. HFpEF tended to show lower absolute levels of peak VO2 than HFmEF and HFm-recEF, although the percent of predicted peak VO2 values – which accounts for between group differences in age - were similar between HFpEF and the mid-range LVEF groups. HFmEF and HFm-recEF had similar VE/VCO2 slope when compared to HFpEF. Resting heart rate of HFmEF and HFm-recEF were similar to HFpEF but lower than HFrEF patients. Furthermore, HFm-recEF and HFmEF showed intermediate values of resting systolic blood pressure in comparison with those of HFpEF and HFrEF, but had peak systolic blood pressure values that were similar to HFpEF and higher than HFrEF. Lastly, age-adjusted CPET findings among LVEF categories were concordant to the results obtained in unadjusted analyses (Supplemental Table 3).
Table 2.
Baseline cardiopulmonary exercise testing features of study participants.
| Variables | HFrEF n=620 (66%) |
HFm-recEF n=170 (18%) |
HFmEF n=107 (11%) |
HFpEF n=47 (5%) |
|---|---|---|---|---|
| Ventilatory | ||||
| Peak VO2, mL/min/Kg | 14.4 ± 5.2 | 18.0 ± 6.3* | 17.2 ± 8.8* | 14.6 ± 7.9† |
| % Predicted Peak VO2 | 56.7 ± 18.3 | 70.6 ± 18.4* | 70.0 ± 23.6* | 69.0 ± 20.8* |
| VE/VCO2 Slope | 34.5 ± 9.2 | 28.8 ± 5.8* | 30.6 ± 6.4* | 32.1 ± 7.9 |
| Hemodynamic | ||||
| Resting HR, bpm | 74.2 ± 14.5 | 69.1 ± 12.7* | 69.5 ± 13.7* | 69.9 ± 12.1 |
| Peak HR, bpm | 121.3 ± 28.1 | 131.8 ± 24.8* | 124.4 ± 31.1 | 114.5 ± 27.3† |
| Chronotropic index | 0.52 ± 0.28 | 0.64 ± 0.24* | 0.57 ± 0.28 | 0.51 ± 0.26† |
| Resting SBP, mmHg | 114.1 ± 18.9 | 120.7 ± 19.8* | 120.1 ± 19.2* | 129.2 ± 20.9*†‡ |
| Peak SBP, mmHg | 134.9 ± 26.9 | 151.8 ± 28.0* | 151.6 ± 30.4* | 156.1 ± 33.5* |
| Resting DBP, mmHg | 73.5 ± 11.1 | 75.3 ± 11.9 | 73.8 ± 11.3 | 74.6 ± 9.8 |
| Peak DBP, mmHg | 74.4 ± 12.5 | 77.4 ± 11.8* | 76.0 ± 12.7 | 75.6 ± 12.0 |
| Peak RER | 1.19 ± 0.13 | 1.20 ± 0.12 | 1.20 ± 0.13 | 1.15 ± 0.12† |
p<0.05 compared to HFrEF;
p<0.05 compared to HFm-recEF;
p<0.05 compared to HFmEF.
All p-values are Bonferroni-adjusted. Data are presented as mean ± standard deviation.
HFrEF – HF with reduced LVEF; HFm-recEF – HF with mid-range and recovered LVEF; HFmEF – HF with mid-range and without recovered LVEF; HFpEF – heart failure with preserved LVEF; LVEF- left ventricular ejection fraction; DBP – diastolic blood pressure; HR – heart rate; RER - respiratory exchange ratio; SBP – systolic blood pressure; VE/VCO2 - minute ventilation-carbon dioxide production relationship; VO2 – oxygen consumption.
Outcomes
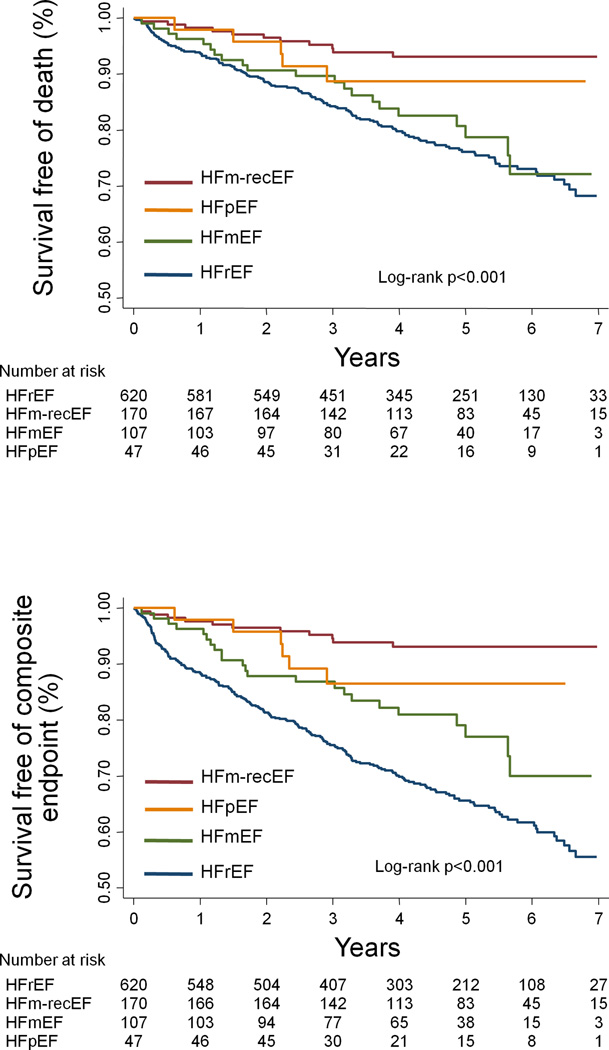
During a median follow-up of 4.4 [2.9–5.7] years, there were 184 (19%) deaths, 62 (7%) LVAD implantations, 56 (6%) transplants, and 253 (27%) composite events. Kaplan-Meier analysis showed that HFrEF had the highest event rates among the studied groups, HFm-recEF had the lowest rate, and HFmEF had event rates intermediate between HFrEF and HFpEF (unadjusted log-rank p <0.001 for the overall difference; Figure 2). Results of univariate and multivariable Cox-regression analysis are shown in Table 3. In fully adjusted analysis, HFm-recEF was associated with lower risk of death [hazard ratio (HR)=0.42; 95% confidence interval (CI)=0.21–0.82; p=0.011] and composite endpoint (HR=0.25; 95%CI=0.13–0.47; p<0.001) than HFrEF, but had similar risk of death and composite endpoint when compared to HFpEF. HFmEF tended to show intermediate risk of outcomes in comparison with HFpEF and HFrEF, albeit not reaching statistical significance in fully adjusted analyses. Compared to HFmEF, HFm-recEF showed a lower risk of composite endpoint (HR=0.31; 95%CI=0.15–0.67; p=0.003) and a trend toward lower risk of death (HR=0.48; 95%CI=0.22–1.05; p=0.067) in analyses adjusted for all potential confounders.
Figure 2.
Kaplan-Meier curves for death and composite endpoint. HFrEF – HF with reduced left ventricular ejection fraction (LVEF); HFm-recEF – HF with mid-range and recovered LVEF; HFmEF – HF with mid-range and without recovered LVEF; HFpEF – heart failure with preserved LVEF. The composite endpoint was defined as the composite outcome of left ventricular assistant device implantation, heart transplantation or all-cause mortality.
Table 3.
Outcomes of study participants.
| Number at Risk |
N of Events |
Rate (95% CI) /100 person-year |
HR (95% CI) (Unadjusted) |
p | HR (95% CI) (Adjusted1) |
p | HR (95% CI) (Adjusted2*) |
p | |
|---|---|---|---|---|---|---|---|---|---|
| Death | |||||||||
| HFrEF | 620 | 147 | 5.6 (4.7–6.5) | Ref | Ref | Ref | |||
| HFm-recEF | 170 | 11 | 1.3 (0.7–2.4) | 0.24 (0.13–0.45) | <0.001† | 0.32 (0.17–0.61) | 0.001* | 0.42 (0.21–0.82) | 0.011 |
| HFmEF | 107 | 21 | 4.6 (3.0–7.1) | 0.83 (0.52–1.30) | 0.41 | 0.74 (0.45–1.21) | 0.23 | 0.87 (0.51–1.46) | 0.59 |
| HFpEF | 47 | 5 | 2.6 (1.1–6.3) | 0.47 (0.19–1.15) | 0.10 | 0.31 (0.11–0.86) | 0.025 | 0.38 (0.14–1.05) | 0.061 |
| Composite endpoint | |||||||||
| HFrEF | 620 | 213 | 8.8 (7.7–10.1) | Ref | Ref | Ref | |||
| HFm-recEF | 170 | 11 | 1.3 (0.7–2.4) | 0.16 (0.08–0.28) | <0.001‡ | 0.19 (0.10–0.36) | <0.001† | 0.25 (0.13–0.47) | <0.001† |
| HFmEF | 107 | 23 | 5.2 (3.5–7.8) | 0.59 (0.39–0.91) | 0.016 | 0.60 (0.38–0.95) | 0.029 | 0.79 (0.49–1.28) | 0.34 |
| HFpEF | 47 | 6 | 3.2 (1.4–7.2) | 0.36 (0.16–0.82) | 0.014 | 0.30 (0.12–0.74) | 0.009 | 0.35 (0.14–0.86) | 0.023 |
p<0.05;
p<0.01;
p<0.001 compared to HFmEF.
The composite endpoint was defined as the composite outcome of left ventricular assistant device implantation, heart transplantation or all-cause mortality.
HFrEF – heart failure with reduced ejection fraction; HFm-recEF – heart failure with recovered mid-range ejection fraction; HFmEF – heart failure with mid-range ejection fraction and no recovered ejection fraction; HFpEF – heart failure with preserved ejection fraction; HR – hazard ratio; CI – Confidence interval.
Adjusted for age, sex, glomerular filtration rate, coronary artery disease, post-chemotherapy, diabetes mellitus, race and hemoglobin with death as outcome and for age, sex, glomerular filtration rate, coronary artery disease and post-chemotherapy with composite endpoint as outcome.
Further adjusted for use of pacemaker, diuretics, statins and angiotensin converting enzyme inhibitors or angiotensin receptor blockers with death as outcome and for use of diuretics, aldosterone antagonists, anticoagulation and angiotensin converting enzyme inhibitors or angiotensin receptor blockers with composite endpoint as outcome.
Temporal change in LVEF among HFmEF and HFm-recEF
To further evaluate the natural history of HFmEF and HFm-recEF, we investigated the temporal change in LVEF in these groups. Follow-up LVEF data were available in 73% (n=78) of HFmEF patients and in 72% (n=123) of HFm-recEF patients. The median time between baseline and follow-up echocardiograms was 2.7 [1.8, 3.7] years. Median values of LVEF showed modest increase in HFmEF (from 50 [44, 55]% to 52 [45, 55]%; p=0.03), but did not change in HFm-recEF (from 48 [40, 55]% to 50 [44, 55]%; p=0.42) at follow-up. Conversely, there was no difference in the proportion of patients who decreased (16% in HFm-recEF and 12% in HFmEF, p=0.77) or increased (20% in HFm-recEF and 27% in HFmEF, p=0.24) LVEF at follow-up in both groups. Older age, higher prevalence of hypertension and lower glomerular filtration rate were associated with reductions in LVEF in patients with HFm-recEF, whereas no studied variable was associated with changes in LVEF in HFmEF (Supplemental Tables 4 and 5). Among HFm-recEF patients, 71% remained with LVEF between 40–55%, while 14% and 15% developed frankly reduced LVEF (values <25%) and normal LVEF (values >55%), respectively, at follow-up. Among HFmEF patients, 66% remained with LVEF between 40–55%, while 13% and 21% of HFmEF developed frankly reduced and normal LVEF values at follow-up, respectively.
Discussion
This study of patients with HF with mid-range LVEF had 3 major novel findings. First, among patients with HF and mid-range LVEF, HFm-recEF had similar clinical characteristics and measures of exercise tolerance, but better prognosis compared to HFmEF. Second, although their clinical characteristics were distinct from HFpEF and HFrEF, HFmEF and HFm-recEF had ventilatory responses to exercise that were better than HFrEF and similar to HFpEF. Third, HFm-recEF had event rates that were lower than HFrEF and similar to HFpEF, while HFmEF had an intermediate risk of outcomes in comparison with HFpEF and HFrEF. These findings suggest that, among HF patients with mid-range LVEF, recovered systolic function is a marker of more favorable prognosis despite similar clinical characteristics and cardiopulmonary response to exercise.
In the present study, HFm-recEF had lower risk of death, LVAD implantation and heart transplantation in comparison with HFmEF and HFrEF. These findings provide further support to the idea that HF patients with recovered LVEF have better prognosis when compared to patients with lower LVEF but also to patients with similar LVEF levels but without previously reduced systolic function (9). In contrast, HFm-recEF showed similar prognosis as HFpEF, indicating that HF with mid-range LVEF and recovered systolic function still carries a significant risk of adverse outcomes. These latter findings are clinically relevant because they provide additional basis for maintaining subjects with recovered LVEF on background medical and device therapy, as previously recommended (8, 9, 16). The differences in prognosis between HFmEF and HFm-recEF may also help explain some reported discrepancies regarding prognosis in the mid-range LVEF HF population. In the Cardiovascular Health Study, survival of mid-range LVEF was intermediate between HFpEF and HFrEF (5), while in the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program, those with mid-range LVEF had mortality rates more similar to HFpEF (6). In the light of our findings, it can be speculated that mid-range LVEF HF populations with higher prevalence of recovered LVEF would have prognosis more similar to HFpEF while those with lower prevalence of recovered systolic function would tend to have an intermediate risk of outcomes in comparison with HFpEF and HFrEF. However, further studies are needed to confirm this hypothesis.
Consistent with previous studies in HF, our mid-range LVEF groups had intermediate clinical characteristics (Supplemental Tables 6 and 7) (1, 5, 6, 7, 17, 18, 19) and tended to have less clinical manifestations of HF when compared to HFrEF and HFpEF (4, 6). HFmEF and HFm-recEF were also more likely to have a history of prior exposure to chemotherapy, pointing toward chemotherapy cardiotoxicity as a potential risk factor for the development of mid-range LVEF. Furthermore, our finding that most of HFmEF and HFm-recEF patients remained with LVEF between 40–55% after a median of 2.8 years of follow-up indicates that HF with mid-range LVEF is not necessarily a transition step of the progression from normal LVEF to HFrEF or vice-versa. It was noteworthy that a high proportion (61%) of our mid-range LVEF sample had recovered LVEF. Likewise, high rates of improved systolic function were also reported among HF populations with LVEF>40% or LVEF>50% (9, 20), which implies that the prevalence of recovered LVEF among HF with mid-range LVEF is substantial.
The reasons for the difference in prognosis between HFmEF and HFm-recEF are not clear. Exercise performance measures with validated prognostic value, such as peak VO2, percent of predicted peak VO2 and VE/VCO2 slope (21, 22), had comparable values in both mid-range LVEF groups, making differences in cardiopulmonary capacity unlikely as a cause. Additionally, the fact that HFm-recEF had lower baseline LVEF levels than HFmEF suggests that the more favorable prognosis in HFm-recEF is not explained by superior LV systolic performance of this group. HFm-recEF patients were more likely to use beta-blockers, ACEI/ARB and CRT/ICD than HFmEF patients. Although we were unable to estimate changes in medical therapy over time, these findings suggest that differences in treatment regimens may partially account for the differences in outcomes. However, further studies are necessary to understand the precise mechanisms by which HFmEF and HFm-recEF had different outcomes.
Several limitations of this study should be acknowledged. This is an observational study and therefore unmeasured confounding factors may influence the observed associations. As noted above, HFm-recEF patients were more likely to use beta-blockers and ACEI/ARB than HFmEF patients, which may be related to differences in tolerability of these medications and may have influenced differences in outcomes. Furthermore, we were unable to evaluate the influence of changes in medical therapy over time on measured outcomes. Skeletal muscle function and CPET performance can be influenced by duration of HF and the presence of cachexia, but these data were not available in our study. Similarly, biomarkers such as NT-proBNP are prognostically relevant in HF but were not uniformly assessed or available in our study population. Our sample included patients referred for CPET from a tertiary medical center and therefore might not be representative of the overall HF population, potentially limiting the generalizability of our findings. Since physicians who ordered CPET did not follow any standardized protocol, it is possible that our findings were influenced by indication bias. However, we tried to overcome this potential limitation by adjusting our Cox-regression models for important clinical characteristics. LVAD and heart transplantation were only obtained by clinical charts of Brigham and Women’s Hospital, which might have led to underestimation of these outcomes. Nevertheless, the frequency that HF patients get these treatments at a referral institution different from where they are being longitudinally followed is usually low.
Conclusion
HF patients with mid-range LVEF demonstrate a distinct clinical profile from HFpEF and HFrEF patients, with objective measures of functional capacity similar to HFpEF. Within the mid-range LVEF HF population, recovered systolic function is a marker of more favorable prognosis, suggesting that identification of recovered LVEF status is important in prognostication and should be systematically assessed.
Supplementary Material
Clinical Perspective.
Few data are available regarding the phenotype, natural history, and prognosis of heart failure (HF) patients with mid-range ejection fraction (LVEF) of 40–55%. Our study provides novel evidence that, among HF patients with mid-range LVEF, recovered systolic function is associated with more favorable prognosis than mid-range without prior reduced EF, despite similar clinical characteristics and cardiopulmonary response to exercise. Furthermore, our data suggest that HF patients with mid-range LVEF and systolic function have similar prognosis as compared to patients with preserved LVEF, indicating that HF with mid-range LVEF and recovered systolic function still carries a significant risk of adverse outcomes.
Acknowledgments
Sources of Funding
The work for this manuscript was supported by NHLBI grant 1K08HL116792-01A1 (A.M.S.), AHA grant 14CRP20380422 (A.M.S.) and the Brazilian National Council for Scientific and Technological Development grant 249481/2013-8 (W.N.J.).
Disclosures
Dr Shah reports receiving research support from Novartis, Gilead, and Actelion.
References
- 1.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (>or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%) Eur J Heart Fail. 2014;16:1049–1055. doi: 10.1002/ejhf.159. [DOI] [PubMed] [Google Scholar]
- 5.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 7.He KL, Burkhoff D, Leng WX, Liang ZR, Fan L, Wang J, Maurer MS. Comparison of ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40% to 55% versus <40% Am J Cardiol. 2009;103:845–851. doi: 10.1016/j.amjcard.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groote P, Fertin M, Duva Pentiah A, Goéminne C, Lamblin N, Bauters C. Long-term functional and clinical follow-up of patients with heart failure with recovered left ventricular ejection fraction after β-blocker therapy. Circ Heart Fail. 2014;7:434–439. doi: 10.1161/CIRCHEARTFAILURE.113.000813. [DOI] [PubMed] [Google Scholar]
- 9.Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–2387. doi: 10.1161/CIRCULATIONAHA.113.006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 12.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Moore B, Kitzman D, Peberdy MA, Bensimhon D, Chase P, Forman D, West E, Guazzi M. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ Heart Fail. 2009;2:113–120. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 14.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Himelman RB, Cassidy MM, Landzberg JS, Schiller NB. Reproducibility of quantitative two-dimensional echocardiography. Am Heart J. 1988;115:425–431. doi: 10.1016/0002-8703(88)90491-7. [DOI] [PubMed] [Google Scholar]
- 16.Moon J, Ko YG, Chung N, Ha JW, Kang SM, Choi EY, Rim SJ. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25:e147–e150. doi: 10.1016/s0828-282x(09)70497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young JB, Dunlap ME, Pfeffer MA, Probstfield JL, Cohen-Solal A, Dietz R, Granger CB, Hradec J, Kuch J, McKelvie RS, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Held P, Solomon SD, Yusuf S, Swedberg K Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) Investigators and Committees. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 2004;110:2618–2626. doi: 10.1161/01.CIR.0000146819.43235.A9. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 19.Toma M, Ezekowitz JA, Bakal JA, O'Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF Trial. Eur J Heart Fail. 2014;16:334–341. doi: 10.1002/ejhf.19. [DOI] [PubMed] [Google Scholar]
- 20.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17:527–532. doi: 10.1016/j.cardfail.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 22.Gargiulo P, Olla S, Boiti C, Contini M, Perrone-Filardi P, Agostoni P. Predicted values of exercise capacity in heart failure: where we are, where to go. Heart Fail Rev. 2014;19:645–653. doi: 10.1007/s10741-013-9403-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.