Abstract
Serum alpha-fetoprotein (AFP) has been increasingly recognized as a marker for a poor prognosis after liver transplantation (LT) for hepatocellular carcinoma (HCC). Many published reports, however, have included a large proportion of patients with HCC beyond the Milan criteria, and the effects of incorporating AFP as an exclusion criterion for LT remain unclear. We studied 211 consecutive patients undergoing LT for HCC within the Milan criteria according to imaging under the Model for End-Stage Liver Disease organ allocation system between June 2002 and January 2009. The majority (93.4%) had locoregional therapy before LT. The median follow-up was 4.5 years (minimum = 2 years). The Kaplan-Meier 1- and 5-year patient survival rates were 94.3% and 83.4%, respectively. In a univariate analysis, significant predictors of HCC recurrence included vascular invasion [hazard ratio (HR) = 10, 95% confidence interval (CI) = 3.9–26, P < 0.001], a pathological tumor stage beyond the University of California San Francisco criteria (HR = 4.1, 95% CI = 1.36–12.6, P = 0.01), an AFP level > 1000 ng/mL (HR = 4.5, 95% CI = 1.3–15.3, P = 0.02), and an AFP level > 500 ng/mL (HR = 3.1, 95% CI = 1.04–9.4, P = 0.04). In a multivariate analysis, vascular invasion was the only significant predictor of tumor recurrence (HR = 5.6, 95% CI = 1.9–19, P = 0.02). An AFP level > 1000 ng/mL was the strongest pretransplant variable predicting vascular invasion (odds ratio = 6.8, 95% CI = 1.6–19.1, P = 0.006). The 1- and 5-year rates of survival without recurrence were 90% and 52.7%, respectively, for patients with an AFP level ≤ 1000 ng/mL and 95% and 80.3%, respectively, for patients with an AFP level 1000 ng/mL (P = 0.026). Applying an AFP level > 1000 ng/mL as a cutoff would have resulted in the exclusion of 4.7% of the patients from LT and a 20% reduction in HCC recurrence. In conclusion, an AFP level > 1000 ng/mL may be a surrogate for vascular invasion and may be used to predict posttransplant HCC recurrence. Incorporating an AFP level > 1000 ng/mL as an exclusion criterion for LT within the Milan criteria may further improve posttransplant outcomes.
Hepatocellular carcinoma (HCC) is the seventh most common cancer worldwide and the cause of more than a million deaths annually.1,2 The incidence of HCC has been rising in the United States for more than 2 decades.1 There is also a growing demand for liver transplantation (LT) as a treatment for early-stage HCC because it offers the best chance for a long-term cure.3 After the Model for End-Stage Liver Disease (MELD) system of organ allocation for HCC was implemented in 2002, the proportion of patients receiving priority listing with an HCC MELD exception increased from 10.5% in 2002 to 15.5% in 2008.4 It has been recommended at a recent international consensus conference5 that deceased donor LT for HCC should achieve the same posttransplant survival achieved by deceased donor LT for nonmalignant indications in the current era of severe organ shortages and that the Milan criteria6 should remain the benchmark for the selection of candidates for LT. Although many single-center studies have shown excellent post-transplant outcomes for patients with HCC within the Milan criteria or modestly expanded criteria for patient selection,7–9 registry data that reflect a more global experience with LT have continued to show inferior results for HCC versus non-HCC indications despite adherence to the Milan criteria.10 It has been shown that 10% to 15% of patients with HCC meeting the Milan criteria before LT develop posttransplant HCC recurrence.3,6–8 There is also growing evidence that factors beyond tumor size and number are associated with a more aggressive tumor biology, and this results in a greater risk for HCC recurrence after LT.11–15
Although the value of serum alpha-fetoprotein (AFP) in HCC screening has been questioned,11 the AFP level has been increasingly recognized as a prognostic marker for HCC. High AFP levels have been consistently shown to predict poor outcomes after LT for HCC.7,10,13–23 The AFP levels associated with a greater risk of HCC recurrence or worse survival after LT have ranged from a low of 20 ng/mL to >1000 ng/mL.16 These published reports, however, have been heterogeneous with respect to LT selection criteria as well as the use of local regional therapy (LRT). The largest of these studies have used registry data and have relied only on posttransplant mortality rather than HCC recurrence as the primary outcome measure.10,17–20 Furthermore, the level at which AFP should be applied as an exclusion criterion for LT and the effects on posttransplant outcomes have not been fully elucidated.
The primary objectives of this study were to further examine the association between the AFP level at the time of LT and posttransplant outcomes and to identify the optimal AFP cutoff for predicting HCC recurrence and serving as an exclusion criterion for LT in patients with pretransplant HCC at a stage within the Milan criteria. We also sought to evaluate the correlation between AFP and microvascular invasion as well as other potential explant histological markers of tumor recurrence after LT.
PATIENTS AND METHODS
Patient Population
The study population included 211 consecutive patients who were 18 years old or older, had pretransplant HCC meeting T2 criteria (1 lesion of 2–5 cm or 2–3 lesions, each up to 3 cm) according to imaging, and had received a MELD exception for LT between June 1, 2002 and January 31, 2009 at our center. Only patients meeting T2 criteria were considered, whereas additional patients who underwent LT after HCC down-staging and patients who underwent transplantation according to expanded criteria beyond the Milan criteria during this period were excluded from the analysis. The minimal duration of follow-up after LT was 2 years so that we could capture most cases of HCC recurrence after LT. This study protocol was approved by our institution review board.
At the start of this study, decisions regarding LRT for patients with HCC awaiting LT were made on a case-by-case basis and depended on liver function and tumor size and location. Since September 2004, all decisions regarding LRT have been made at a weekly multidisciplinary liver tumor board attended by transplant hepatologists, transplant surgeons, oncologists, interventional radiologists, and radiologists with expertise in diagnostic abdominal imaging. The diagnosis of HCC was based on radiographic characteristics, including arterial phase enhancement and portal venous phase washout for lesions measuring at least 1 cm. Nodules not showing these characteristics were considered indeterminate for HCC and were followed by computed tomography or magnetic resonance imaging every 3 months so that interval growth could be observed and the review by the tumor board could be repeated. Percutaneous biopsy was not routinely performed for the purpose of diagnosing HCC. Nodules < 1 cm were not counted as HCC.
Histopathological Analysis
Explant histopathological features evaluated in this study included the tumor size, the number of tumor nodules, the histological grade of differentiation based on the Edmondson and Steiner criteria24 (grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated), and the presence or absence of microvascular or macrovascular invasion. Pathological tumor staging was based on the United Network for Organ Sharing tumor-node-metastasis staging system.3 Explant tumor staging in this study was based on the size and number of only viable tumors.
Statistical Analysis
The primary outcomes of this study were posttransplant HCC recurrence and patient survival 5 years after LT. Patient characteristics were summarized as medians and ranges for continuous variables and as proportions for categorical variables. The Kaplan-Meier method was used to estimate the probabilities of survival and tumor recurrence. Logistic regression was used to evaluate the association between AFP and explant histological features predicting HCC recurrence. Cox proportional hazards models were used to evaluate predictors of HCC recurrence. Predictors of HCC recurrence with a univariate P value < 0.1 were evaluated in the multivariate analysis, with the final model selected by backward elimination (P for removal > 0.05). Statistical analyses were performed with Stata 12 (StataCorp, College Station, TX).
RESULTS
Baseline Patient Characteristics
The baseline demographic and clinical characteristics of the 211 patients composing the study population are summarized in Table 1. The median age of the patients was 62.7 years, 162 (76.8%) were men, and 70 (33.2%) were Asian. In terms of the etiology of liver disease, patients with hepatitis C and hepatitis B made up 88% of the study population. At the time of LT, the median calculated MELD score was 11. As for tumor characteristics, the distribution of the AFP levels for the cohort (all obtained within the 3 months before LT) is summarized in Table 1. The median AFP level was 11 ng/mL, and 4.7% had an AFP level > 1000 ng/mL. The majority of the patients (93.4%) underwent LRT, and 39.8% of the patients had a complete response to LRT according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).25,26 Only 4 patients underwent living donor LT.
TABLE 1.
Baseline Characteristics of the Study Population (n = 211)
| Characteristic | Value |
|---|---|
| Age (years)* | 62.7 (21–78) |
| Male [n (%)] | 162 (76.8) |
| Asian [n (%)] | 70 (33.2) |
| Etiology of liver disease [n (%)] | |
| Hepatitis C virus | 132 (62.6) |
| Hepatitis B virus | 53 (25.1) |
| Other | 26 (12.3) |
| MELD score* | 11 (6–27) |
| Child-Pugh class [n (%)] | |
| A | 128 (60.7) |
| B | 52 (24.6) |
| C | 31 (14.7) |
| AFP level (ng/mL)*† | 11 (1.6–6990) |
| AFP categories [n (%)] | |
| <20 ng/mL | 125 (59.2) |
| 21–100 ng/mL | 49 (23.2) |
| 101–500 ng/mL | 19 (9.0) |
| 501–1000 ng/mL | 8 (3.8) |
| >1000 ng/mL | 10 (4.7) |
| LRT [n (%)] | 197 (93.4) |
| TACE only‡ | 78 (37.0) |
| Ablation only§ | 57 (27.0) |
| RFA | 50 (23.7) |
| PEI | 7 (3.3) |
| Combination‖ | 62 (29.4) |
| TACE 1 PEI | 14 (6.6) |
| TACE 1 RFA | 46 (21.8) |
| Other | 2 (0.95) |
The data are presented as medians and ranges.
Last AFP level obtained within the 3 months before LT.
Seventy-eight patients underwent 101 elective TACE treatments (not including TACE performed within the 24 hours before orthotopic LT).
Fifty patients received a total of 52 RFA treatments, and the 7 patients treated with PEI had a total of 18 treatment sessions.
Sixty-two patients received a combination of 84 treatments (most commonly TACE and RFA). The 2 patients in the other category included 1 patient treated with TACE and radioembolization and 1 patient treated with RFA and PEI.
Explant Histological Characteristics
Explant histopathological characteristics are summarized in Table 2. Fifteen of the 211 patients (7.1%) had vascular invasion on explant. Complete necrosis of the tumor was seen in 88 patients (41.7%) in response to pretransplant locoregional therapy (LRT). There was a good correlation between a pretransplant radiographic assessment of a complete response and complete tumor necrosis in the explant. Overall, 79 of the 84 patients (94%) with a complete response according to a radiographic assessment had no residual tumor in the explant. Another 9 patients with residual tumor according to pretransplant imaging had no tumor in the explant. Understaging of tumors beyond the Milan criteria (T1 and T2) was observed for 18.5%, including 10.4% with T3A tumors [within the University of California San Francisco (UCSF) criteria]7 and 8.1% with T3B or T4 tumors (beyond the UCSF criteria). The majority had well differentiated or moderately differentiated tumors, and only 4.3% had poorly differentiated tumors.
TABLE 2.
Explant Histological Tumor Characteristics (n = 211)
| Characteristic | Value |
|---|---|
| Vascular invasion [n (%)] | |
| Yes | 15 (7.1) |
| No | 196 (92.9) |
| Pathological tumor stage [n (%)]* | |
| No viable tumor (complete necrosis) | 88 (41.7) |
| T1-T2 | 84 (39.8) |
| T3A (beyond Milan criteria but within UCSF criteria) | 22 (10.4) |
| T3B-T4 (beyond UCSF criteria) | 17 (8.1) |
| Histological tumor grade [n (%)] | |
| Complete necrosis | 88 (41.7) |
| Well differentiated | 60 (28.4) |
| Moderately differentiated | 54 (25.6) |
| Poorly differentiated | 9 (4.3) |
The UCSF criteria are as follows: 1 lesion ≤6.5 cm or 2 to 3 lesions, each ≤4.5 cm, with a total tumor diameter ≤8 cm.
Posttransplant Outcomes
The median posttransplant follow-up was 4.5 years, and there was a minimum follow-up of 2 years among survivors. HCC recurrence developed in 19 patients (9%) and accounted for 61.3% of all deaths in the first 5 years after LT. The majority of HCC recurrences (73.7%) occurred within the first 2 years after LT. HCC was the cause of 11 of 31 deaths (35.5%) within the first 5 years after LT. The Kaplan-Meier 1- and 5-year patient survival rates were 94.3% and 83.4%, respectively. The 1- and 5-year recurrence-free probabilities were 97.1% and 90.4%, respectively.
Predictors of HCC Recurrence
In the univariate analysis, the significant predictors of HCC recurrence were vascular invasion [hazard ratio (HR) = 10, 95% confidence interval (CI) = 3.9–26, P < 0.001], a pathological tumor stage exceeding the UCSF criteria (HR = 4.1, 95% CI = 1.36–12.6, P = 0.01), an AFP level > 1000 ng/mL (HR = 4.5, 95% CI = 1.3–15.3, P = 0.02), and an AFP level > 500 ng/mL (HR = 3.1, 95% CI = 1.04–9.4, P = 0.043; Table 3). Other factors, including an AFP level > 300 ng/mL, an AFP level > 400 ng/mL, a diagnosis of liver disease, a complete response to LRT according to the mRECIST criteria, and the histological grade of differentiation, were not significant predictors of HCC recurrence. In the multivariate model, vascular invasion (HR = 5.6, 95% CI = 1.6–19, P = 0.006) was the only significant predictor of tumor recurrence. An AFP level > 1000 ng/mL was only marginally significant as a predictor of HCC recurrence (Table 4). The sensitivity and specificity of an AFP level > 1000 ng/mL for predicting HCC recurrence were 0.14 and 0.96, respectively.
TABLE 3.
Univariate Analysis of Predictors of HCC Recurrence
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Vascular invasion | 10 | 3.9–26 | <0.001 |
| Pathological stage beyond UCSF criteria* | 4.1 | 1.36–12.6 | 0.01 |
| AFP level > 1000 ng/mL | 4.5 | 1.3–15.3 | 0.02 |
| AFP level > 500 ng/mL | 3.1 | 1.04–9.4 | 0.04 |
| AFP level > 400 ng/mL | 2.9 | 0.97–8.8 | 0.06 |
| AFP level > 300 ng/mL | 2.9 | 0.95–8.6 | 0.06 |
The UCSF criteria are as follows: 1 lesion ≤6.5 cm or 2 to 3 lesions, each ≤4.5 cm, with a total tumor diameter ≤8 cm.
TABLE 4.
Multivariate Analysis of Predictors of HCC Recurrence
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Vascular invasion | 5.6 | 1.6–19 | 0.0063 |
| AFP level > 1000 ng/mL | 1.54 | 0.36–6.5 | 0.056 |
| Pathological stage | 2.2 | 0.6–7.6 | 0.23 |
| beyond UCSF criteria* |
The UCSF criteria are as follows: 1 lesion ≤6.5 cm or 2 to 3 lesions, each ≤4.5 cm, with a total tumor diameter ≤8 cm.
Association Between AFP and Vascular Invasion
Vascular invasion was the only factor predicting post-transplant HCC recurrence, but this is unknown before LT. We, therefore, evaluated the association between pretransplant factors and the presence of vascular invasion. We found a significant association between AFP levels and the risk for vascular invasion starting at an AFP level > 300 ng/mL (Table 5). Within the AFP range of >300 to 1000 ng/dL, the higher the AFP level was, the greater the odds ratio was for microvascular invasion. An AFP level > 1000 ng/mL was the strongest predictor of vascular invasion with an odds ratio of 6.8 (95% CI = 1.6–19.1, P = 0.006). We also performed an analysis of the sensitivity and specificity of AFP in predicting vascular invasion within the same range of >300 to >1000 ng/dL (Table 5). The sensitivity was low at all AFP cutoffs, whereas an AFP level > 1000 ng/dL had the highest specificity (0.96) for predicting vascular invasion.
TABLE 5.
Association Between AFP and Vascular Invasion
| Variable | Odd Ratio | 95% CI | P Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| AFP >1000 ng/mL | 6.8 | 1.6–19.1 | 0.006 | 0.20 | 0.96 |
| AFP >500 ng/mL | 4.7 | 1.3–16.8 | 0.02 | 0.27 | 0.93 |
| AFP >400 ng/mL | 4.4 | 1.2–15.5 | 0.02 | 0.27 | 0.92 |
| AFP >300 ng/mL | 4.1 | 1.2–14.3 | 0.03 | 0.27 | 0.92 |
AFP as an Exclusion Criterion for LT
An AFP level > 1000 ng/mL was the strongest pre-transplant variable predicting HCC recurrence as well as vascular invasion. The 1- and 5-year rates of survival without HCC recurrence were 90% and 52.7%, respectively, for patients with a preoperative AFP level > 1000 ng/mL and 95% and 80.3%, respectively, for patients with an AFP level ≤1000 ng/mL (P = 0.026; Fig. 1). Applying an AFP level > 1000 ng/mL as a cutoff would have resulted in the exclusion of 4.7% of the patients from LT and a 20% reduction in the rate of HCC recurrence. At a lower AFP cutoff of 400 ng/mL, approximately twice the number of patients (9%) would have been excluded to reduce the rate of HCC recurrence by an additional 6% in comparison with an AFP level > 1000 ng/mL. In comparison with lower AFP cutoffs, AFP > 1000 ng/mL also had the highest specificity of 96% for predicting HCC recurrence.
Figure 1.
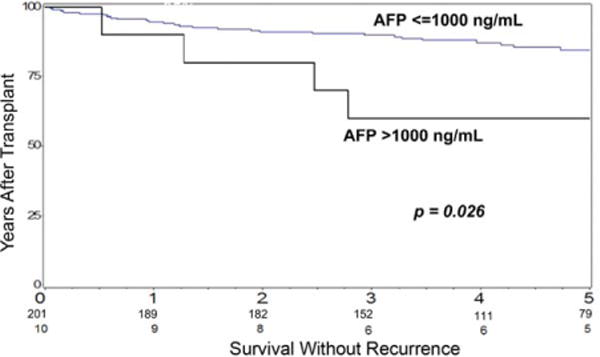
Kaplan-Meier probabilities of survival without tumor recurrence after LT for the subgroup with an AFP level > 1000 ng/mL and the subgroup with an AFP level ≤1000 ng/mL. The difference was statistically significant according to a log-rank test.
AFP Level > 1000 ng/mL and Effects of LRT
Six of the 10 patients whose last AFP levels before LT were >1000 ng/mL underwent a total of 12 elective LRTs [transarterial chemoembolization (TACE), 6; laparoscopic or percutaneous radiofrequency ablation (RFA), 5; and percutaneous ethanol injection (PEI), 1]. They had persistently elevated AFP levels > 1000 ng/mL despite LRT, and 3 of these patients suffered from HCC recurrence after LT. Only 1 of these patients had an initial decrease in the AFP level to <1000 ng/mL, but there was a subsequent rise in the AFP level to >1000 ng/mL before LT, and the patient developed HCC recurrence. Three of the 10 patients underwent LT in the early period in 2002 and did not receive elective LRT but underwent only TACE within the 24 hours before LT; thus, the effect of LRT on AFP could not be assessed. One patient received no treatment because of the short wait-list time for blood type AB. None of these 4 patients experienced HCC recurrence.
In addition to the 10 patients with an AFP level > 1000 ng/mL at the time of listing and at LT as described previously, there were 12 other patients in this cohort with an initial AFP level > 1000 ng/mL at listing who showed decreases in AFP to <1000 ng/mL by the time of LT as a result of LRT. Only 1 of these 12 patients developed HCC recurrence after LT. The remaining 11 patients were still alive after a mean post-LT follow-up of 5.9 years. Another 16 patients had an initial AFP level > 1000 ng/mL at the time of listing but did not undergo LT because of tumor progression (dropout) or other reasons for LT exclusion.
Overall, 38 patients presented with an AFP level > 1000 ng/mL at the time of listing: 16 patients (42.1%) did not undergo LT because of tumor progression (dropout) or other reasons despite LRT, 10 patients underwent LT with an AFP level still > 1000 ng/mL at the time of LT, and 12 patients showed decreases in the AFP level to <1000 ng/mL at the time of LT as a result of LRT.
DISCUSSION
Data suggesting a high AFP level is an important marker of prognosis after LT have continued to emerge. The present study was aimed at establishing an AFP threshold that could serve as an exclusion criterion for LT within a homogeneous population of patients with HCC meeting T2 criteria according to imaging. Our analysis was based on the last AFP level within the 3 months before LT. More than 93% of the patients in our cohort underwent 1 or more LRTs before LT. We found that an AFP level > 1000 ng/mL was the strongest pretransplant variable predicting HCC recurrence after LT and was associated with an almost 5-fold increase in risk. The 5-year survival rate was only 53% without recurrence for patients with a preoperative AFP level > 1000 ng/mL, whereas it was 80% for those with an AFP level ≤1000 ng/mL. A key question that has not been addressed in most other published reports is how many patients would have to be excluded from LT at a specific AFP threshold to prevent 1 HCC recurrence. In this study, we found that applying an AFP level > 1000 ng/mL as the cutoff would have resulted in the exclusion of approximately 5% of patients from LT and a 20% reduction in the rate of HCC recurrence. Applying a lower AFP threshold would have resulted in a greater reduction in the recurrence rate but at the expense of excluding many more patients from LT who did not have tumor recurrence and would have benefited from LT. We also found that patients who had an initial AFP level > 1000 ng/mL but showed a decrease in the AFP level to <1000 ng/mL after LRT had a more favorable prognosis; only 1 of 12 had HCC recurrence after LT.
Our findings are consistent with several studies that have focused specifically on the effect of AFP on posttransplant outcomes.14,22,23 The Liver Transplantation French Study Group14 recently developed and validated a prognostic model combining AFP with the tumor size and number that was significantly better than the Milan criteria alone in predicting HCC recurrence and overall survival after LT. Similarly to us, they found an AFP level > 1000 ng/mL to be associated with a significantly higher risk of HCC recurrence and worse posttransplant survival among patients with HCC within the Milan criteria. A subset of their patients with HCC exceeding the Milan criteria had 5-year survival rates close to 70% as long as their AFP levels were <100 ng/mL. In contrast to single or multicenter studies, a number of other studies using large transplant registry data have reported lower AFP thresholds ranging from 67 to 667 ng/mL for predicting posttransplant survival.10,17–20 These results have been based on very large numbers of patients, but they are limited by missing information on HCC recurrence and a reliance on overall survival as the endpoint. Interestingly, an AFP level > 1000 ng/mL was identified as the only factor predicting treatment failure in our experience with tumor down-staging before LT.27
Although microvascular invasion is well established as a poor prognostic marker for LT for HCC,3 the presence of microvascular invasion cannot be determined before LT.28 In the present study, microvascular invasion was the only significant predictor of HCC recurrence in the multivariate analysis. We observed a strong correlation between AFP and the presence of microvascular invasion in the liver explant. Within the AFP range of > 300 to 1000 ng/dL, the higher the AFP level was, the greater the likelihood was for microvascular invasion (Table 5). Very few prior studies have reported a correlation between AFP and vascular invasion. In a study by Fujiki et al.,21 an AFP level > 800 ng/mL was associated with a significantly increased risk of microvascular invasion as well as a poorly differentiated grade in comparison with an AFP level < 200 ng/mL. In the multicenter French study,14 both an AFP level > 100 ng/mL and an AFP level > 1000 ng/mL were found to be associated with microvascular invasion as well as a poorly differentiated tumor grade. Our results suggest that a very high AFP level > 1000 ng/mL despite LRT may serve as a surrogate for microvascular invasion and help to further refine selection criteria for LT.
Several other studies have focused on dynamic changes in the AFP level after LRT before LT for predicting posttransplant outcomes.13,19,29,30 Merani et al.18 showed that a reduction in the AFP level from >400 ng/mL at LT listing to 400 ng/mL before LT as a result of LRT was associated with significantly better posttransplant survival in comparison with persistently elevated AFP levels > 400 ng/mL (81% versus 48% at 3 years). Lai et al.13 and Vibert et al.29 proposed the use of a positive AFP slope > 15 ng/mL/month for discriminating prognoses. These studies relied on only 2 data points for calculating the AFP slope, and they did not sufficiently account for the random fluctuations in the AFP levels frequently seen in chronic liver disease. The use of the initial AFP level at the time of listing in these studies also did not account for the initial effects of LRT on AFP. Further investigations are, therefore, needed to determine whether a rising AFP level (or a positive AFP slope) is better than specific pretransplant AFP levels in discriminating prognoses after LT.
Our study has several limitations—most notably the retrospective study design and the small number of patients with an AFP level > 1000 ng/mL before LT. Because our center is within a region with a prolonged wait-list time, many patients with high AFP levels would have been removed from our waiting list (dropout) but may have undergone transplantation at other centers with short wait-list times. Indeed, more than 40% of our patients with an initial AFP level > 1000 ng/mL were removed from our waiting list during the study period. A recent analysis from our center has also demonstrated a strong association between a higher AFP level and a greater risk of dropout from the waiting list for LT.31 The very small number of patients in our study with a poorly differentiated tumor grade (4.3%) may be the result of this selection process, and this may also explain why the grade of tumor differentiation was not predictive of HCC recurrence in our study. It is interesting to point out that a lack of a complete response to LRT according to mRECIST was not associated with a higher rate of HCC recurrence in comparison with patients with a complete response in our study. In contrast, a recent multicenter European study by Lai et al.13 suggests that patients with progressive disease according to mRECIST after LRT have worse outcomes after LT. It is important to consider the effects of wait-list dynamics on the outcomes of patients exhibiting progressive disease after LRT. At our center, patients with progressive disease after the first LRT had an 85% 1-year cumulative incidence of wait-list dropout.31 Because we have demonstrated that a persistently high AFP level > 1000 ng/mL despite LRT is a marker of a poor tumor biology or vascular invasion and predicts an increased risk of post-LT HCC recurrence, a response to LRT based on a decrease in AFP during the observation period before LT should be considered an important endpoint under the ablate-and-wait principle in the selection of candidates with HCC for LT.32
In summary, the present study suggests that applying an AFP level > 1000 ng/mL as an exclusion criterion may further improve posttransplant outcomes for patients with pretransplant HCC at a stage meeting the Milan criteria. This approach would result in the exclusion of approximately 5% of candidates from LT and a reduction in the rate of HCC recurrence of 20%. At our institution, we have now incorporated an AFP level > 1000 ng/mL as an exclusion criterion for LT for patients within the Milan criteria as well as patients undergoing down-staging before LT.27 Those with an AFP level > 1000 ng/mL are required to show a response to LRT with a decrease in the AFP level to <500 ng/mL before LT. The impact of this change in the selection criteria on posttransplant outcomes is being prospectively evaluated.
Acknowledgments
The work was presented during an oral session at the annual meeting of the American Association for the Study of Liver Diseases in Boston, MA, on November 10, 2011, and it was supported by a grant from the National Institutes of Health to the Liver Center at the University of California San Francisco (PO1-DK26743).
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- LRT
locoregional therapy
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- PEI
percutaneous ethanol injection
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- UCSF
University of California San Francisco
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 4.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. for OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 8.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8:1982–1989. doi: 10.1111/j.1600-6143.2008.02351.x. [DOI] [PubMed] [Google Scholar]
- 10.Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 12.Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]
- 13.Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, et al. for European Hepatocellular Cancer Liver Transplant Study Group Alpha-fetoprotein and modified Response Evaluation Criteria in Solid Tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108–1118. doi: 10.1002/lt.23706. [DOI] [PubMed] [Google Scholar]
- 14.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. for Liver Transplantation French Study Group Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–984. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N, Yao FY. Moving past “one size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19:1055–1058. doi: 10.1002/lt.23730. [DOI] [PubMed] [Google Scholar]
- 16.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplant for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 17.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634–645. doi: 10.1002/lt.23652. [DOI] [PubMed] [Google Scholar]
- 18.Merani S, Majno P, Kneteman NM, Berney T, Morel P, Mentha G, Toso C. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol. 2011;55:814–819. doi: 10.1016/j.jhep.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Mailey B, Artinyan A, Khalili J, Denitz J, Sanchez-Luege N, Sun CL, et al. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg. 2011;146:26–33. doi: 10.1001/archsurg.2010.295. [DOI] [PubMed] [Google Scholar]
- 20.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 21.Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, Uemoto S. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009;9:2362–2371. doi: 10.1111/j.1600-6143.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 22.Todo S, Furukawa H, Tada M, for Japanese Liver Transplantation Study Group Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2007;13(suppl 2):S48–S54. doi: 10.1002/lt.21334. [DOI] [PubMed] [Google Scholar]
- 23.Sotiropoulos GC, Lang H, Nadalin S, Neuhäuser M, Molmenti EP, Baba HA, et al. Liver transplantation for hepatocellular carcinoma: University Hospital Essen experience and metaanalysis of prognostic factors. J Am Coll Surg. 2007;205:661–675. doi: 10.1016/j.jamcollsurg.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M, for American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao FY, Kerlan RK, Jr, Hirose R, Davern TJ, III, Bass NM, Feng S, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(suppl 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 29.Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 30.Dumitra TC, Dumitra S, Metrakos PP, Barkun JS, Chaudhury P, Deschênes M, et al. Pretransplantation α-fetoprotein slope and Milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation. 2013;95:228–233. doi: 10.1097/TP.0b013e31827743d7. [DOI] [PubMed] [Google Scholar]
- 31.Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343–1353. doi: 10.1002/lt.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl. 2010;16:925–929. doi: 10.1002/lt.22103. [DOI] [PubMed] [Google Scholar]


