Abstract
The Colorado potato beetle, Leptinotarsa decemlineata, has developed resistance to most registered pesticides and has become one of the most difficult insect pests to control. Development of new biopesticides targeting this pest might solve the resistance problem and contribute to sustainable crop production. Laboratory experiments were conducted to assess the efficacy of Isaria fumosorosea (syn. Paecilomyces fumosoroseus) strain CCM 8367 against L. decemlineata when applied alone or combined with the entomopathogenic nematode Steinernema feltiae. The last-instar larvae of the Colorado potato beetle showed the highest susceptibility to I. fumosorosea followed by pre-pupae and pupae. The median lethal concentration (LC50) was estimated to be 1.03×106 blastospores/ml. The strain CCM 8367 was more virulent, causing 92.6% mortality of larvae (LT50 = 5.0 days) compared to the reference strain Apopka 97, which caused 54.5% mortality (LT50 = 7.0 days). The combined application of the fungus with the nematodes increased the mortality up to 98.0%. The best results were obtained when S. feltiae was applied simultaneously with I. fumosorosea (LT50 = 2.0 days); later application negatively affected both the penetration rate and the development of the nematodes. We can conclude that the strain CCM 8367 of I. fumosorosea is a prospective biocontrol agent against immature stages of L. decemlineata. For higher efficacy, application together with an entomopathogenic nematode is recommended.
Introduction
The Colorado potato beetle (CPB), Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), is one of the most economically damaging insect pests of potatoes (Solanum tuberosum L.) in the USA and through much of Europe [1]. The first European population was established in France in 1922. By the end of the 20th century, the pest had become a problem all over Europe, in Asia Minor, Iran, Central Asia, and western China [2,3]. In warm and dry regions, the population of CPB regularly occurs in high abundances and develops two complete generations. Adults and larvae feed on leaves; a single insect can eat at least 100 cm2 of potato foliage in its lifetime. Without control measures, the beetle can cause severe reductions in tuber yield or quality (tuber size). Because of warm weather conditions during the 1990s, severe losses occurred in Germany and Poland. Consequently, insecticide use increased considerably. On average, 2–3 treatments per year were performed [4]. The CBP has developed resistance to most registered pesticides [5–8] and become one of the most difficult insect pests to control.
One possibility for regulating the pest is to use genetically modified (GM) potatoes expressing Bacillus thuringiensis delta-endotoxin that is toxic to CPB. GM potatoes have been registered and sold in the USA from 1995–2000 but were discontinued in response to consumer concerns about genetically modified crops [9].
A prospective solution to the CPB resistance problem could be to develop microbial biopesticides targeted against CPB as alternatives to broad-spectrum chemical insecticides. A substantial number of mycoinsecticides and mycoacaricides have been developed worldwide since the 1960s. Products based on Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) (33.9%), Metarhizium anisopliae (Metsch.) Sorokin, (Hypocreales: Clavicipitaceae) (33.9%), Isaria fumosorosea (WIZE) Brown & Smith (Hypocreales: Cordycipitaceae) (5.8%), and B. brongniartii (Saccardo) (Hypocreales: Cordycipitaceae) (4.1%) are the most common among the 171 products available [10]. The targets comprise insects in the orders Hemiptera, Coleoptera, Lepidoptera, Thysanoptera, and Orthoptera, distributed among at least 48 families. A broader appreciation for the attributes of entomopathogens is envisioned, and synergistic combinations of microbial control agents with other technologies is expected to occur in the future [11].
Isaria fumosorosea was known as Paecilomyces fumosoroseus for more than 30 years and was recently transferred to the genus Isaria [12]. Genetic analysis demonstrated that there are at least three monophyletic groups of I. fumosorosea [13–15]. Because of the high level of genetic diversity along with the difficulties of exact identification, I. fumosorosea must be seen as a species complex, and its taxonomic revision is urgently needed [12]. It is commonly found in the soil [16] but has been reported on plants, in water, and less commonly, in air on every continent except Antarctica [12]. It has been isolated from over 40 species of arthropods, representing 10 orders. Some of the more commonly known susceptible organisms include weevils, ground beetles, plant beetles, aphids, whiteflies, psyllids, wasps, termites, thrips, and a wide variety of butterflies and moths [17–19]. It therefore has received significant attention as a possible biological control agent for several economically important insect pests of agricultural crops [20]. Like most entomopathogenic fungi, it infects its host by breaching the cuticle [21]. Various metabolites allow the pathogen to physically penetrate the host and inhibit its regulatory system. For I. fumosorosea, these include proteases, chitinases, chitosanase, and lipase [22]. These enzymes allow the fungus to breach the insect cuticle and disperse through the hemocoel. Isaria fumosorosea and other species within the genus also produce beauvericin [23], a compound that appears to paralyze host cells [21]. Susceptible insects exposed to blastospores and conidia of I. fumosorosea show declined growth and high levels of mortality [18]. Various strains of I. fumosorosea are successfully used in the biocontrol of many pest insects and mites, and several commercially produced mycopesticides based either on I. fumosorosea alone or in combination with other entomopathogenic species have been developed in America, Europe or Asia [12]. To our knowledge, no research on the control of CPB by this species of entomopathogenic fungus has been published.
Entomopathogenic nematodes (EPNs) in the families Steinernematidae and Heterorhabditidae (Rhabditida, Nematoda) are obligate pathogens of insects [24] and are associated with specific symbiotic bacteria of the genera Xenorhabdus and Photorhabdus, respectively [25]. Because of their ability to infect various insects [26], the possibility of mass production by industrial techniques [27] and the relative safety to non-target organisms [28] and the environment [29], EPNs represent an attractive agent for the inundative biological control of many insect pests [30]. EPNs have been shown to infect and kill the CPB [31,32]; however, their efficiency against the CPB in the field is limited by various factors, including depth of beetle pupation, beetle migration and insensitivity of the adult beetles to nematode infection [33].
Biocontrol can be improved by using combinations of different biocontrol agents [34–36]. EPNs and entomopathogenic fungi together performed more efficiently than when applied alone [37,38]. It is thus expected that simultaneous application of the fungus and nematodes could improve their performance against CPB.
The aim of our work was to assess the efficiency of a new strain of the entomopathogenic fungus I. fumosorosea isolated in the Czech Republic [39] and the entomopathogenic nematode Steinernema feltiae (Filipjev) (Rhabditida: Steinernematidae) strain Ustinov against L. decemlineata either applied alone or in combination.
Materials and Methods
Ethics Statement
This study did not involve endangered or protected species. Leptinotarsa decemlineata is a common pest occurring in most of potato fields so no specific permissions were required for its collection. The insects were collected from private land and the owner gave permission to conduct the study on this site.
Plants
Potato plants, Solanum tuberosum L. (Solanaceae) cv. Désirée and Superior obtained from the bank of potato genetic resources at the Potato Research Institute Havlíčkův Brod, Czech Republic, were used for insect rearing and bioassays with CPB larvae. The data on the cultivars are accessible on the website http://europotato.org. The plants were grown in large pots (20 cm diameter, 18.5 cm height) containing universal horticultural substrate B (Rašelina Soběslav, Czech Republic) including minerals and fertilizers. The greenhouse was air conditioned, and maintained at a temperature of 23–25°C. No other fertilizers or pesticides were applied to the plants.
Insects
Laboratory culture of CPB maintained at the Institute of Entomology, České Budějovice, was established from several hundred adult individuals collected from potato fields in the vicinity of České Budějovice (South Bohemia, Czech Republic, 49°N) in 2004. Larvae and adults were reared on potato plants in a greenhouse under controlled conditions (temperature 24±2°C, photoperiod 16L:8D). Every year, the culture of CPB beetles is supplemented by around 500 fresh adults collected in the field to prevent the genetic shift (degenerations of culture). Wax moth larvae, Galleria mellonella L. (Lepidoptera: Pyralidae) for nematode multiplication were reared in the dark at 30°C on artificial diet [40].
Entomopathogenic fungi
The I. fumosorosea isolate originated from the horse chestnut leaf miner, Cameraria ohridella Decka and Dimic (Lepidoptera: Gracillariidae). The strain is deposited under number CCM 8367 as a patent culture in the Czech Collection of Microorganisms in Brno [39]. As a reference strain, we used an isolate cultured from the commercial product PreFeRal® WG (Biobest, Belgium; I. fumosorosea strain Apopka 97 as an active ingredient). Blastospores of both strains were obtained after 120 hours of submerged cultivation in growth media (glucose, maltose, starch and peptone) using an orbital shaker at 140 cycles per minute at 23°C. The number of blastospores in suspension was counted with a Bürker counting chamber (Brand, Wertheim, Germany) and adjusted to required concentration. The soaking agent Tween 80 (Sigma-Aldrich) was added to the suspension at a concentration of 0.02% (v/v).
Entomopathogenic nematodes
Steinernema feltiae, strain Ustinov, originating from Izhevsk (Russia), was reared for more than 25 years under laboratory conditions using the last larval instar of G. mellonella as a host. The emerging infective juveniles (IJ) were harvested from White traps [41] and subsequently stored in water at 10°C for 10–21 days [27]. The viability of IJs was checked under a microscope before use in the experiments. The species identity of the nematode was confirmed by sequencing the ITS region of the rDNA (Genbank accession number: KT809344).
Bioassays
General conditions
The experiments were conducted using polystyrene Petri dishes (9 cm in diameter, Gosselin SAS, France) lined with moist filter paper (KA 2, Amersil–FILPAP, Ltd., Czech Republic) over a period of two years. Each test described below was repeated twice or thrice; each replication was from different generation of CPB and tested 20–40 insect individuals. All of the Petri dishes containing inoculated/control individuals were placed in an incubator under controlled conditions (23±1˚C and 16L:8D photoperiod). The treated and control insects were monitored at 24-h intervals to record daily mortality for a period of seven days.
The efficacy of strain CCM 8367 against immature stages of L. decemlineata
Last-instar larvae, pre-pupae and pupae of CPB in the treated group and in the control group were individually placed into Petri dishes. Treated groups: each specimen was immersed in the suspension of blastospores of strain CCM 8367 at concentration 5×107 spores/ml before it was placed in the Petri dish, and additionally, one milliliter of the same suspension was applied topically to the specimen. Control groups: all specimens were treated identically as described above by using distilled water and Tween 80 at a 0.02% concentration. For the last larval instar, fresh potato leaves were placed into each dish and replaced daily. Filter paper and a piece of cotton wool were moistened by distilled water daily to maintain optimal humidity inside the Petri dishes.
Dose-response of CPB larvae to I. fumosorosea CCM 8367 and efficacy comparison with the Apopka 97 strain
Lethal concentrations (LC50 and LC90) of CCM 8367 blastospores were estimated from cumulative mortality of the last-instar-larvae of CPB at four concentrations ranging from 5×104 to 5×107 spores/ml of suspension. The efficacy of CCM 8367 strain was compared with commercial Apopka 97 strain applied to the larvae at concentration 5×107 spores/ml of suspension. Control was treated by distilled water and Tween 80 at a 0.02% concentration. The bioassay was performed as described above.
The efficacy of S. feltiae against immature stages of L. decemlineata
Last-instar larvae, pre-pupae and pupae of CPB were individually placed into the Petri dishes, and then 500 IJ in distilled water were added to each dish. The control group was treated with distilled water only. Dead individuals were collected daily and cadavers were incubated for 2–3 days at the same conditions to allow nematodes to develop into adults. Cadavers were then rinsed in water to remove nematodes from the surface and then dissected in a sterile Petri dish. The number of nematodes inside each cadaver was recorded to evaluate a penetration rate.
The efficacy of the combined application of I. fumosorosea CCM 8367 and S. feltiae against L. decemlineata larvae
CPB last-instar larvae were immersed into suspension of CCM 8367 blastospores and individually placed into Petri dishes as described above, and 1 ml of the CCM 8367 blastospore suspension was applied topically to the larvae. Then, 500 IJ of S. feltiae in distilled water were added in the middle of each Petri dish either immediately, 24, 48 or 72 hours after fungus application. Control was treated by distilled water and Tween 80 at a 0.02% concentration. Infection symptoms and the number of dead larvae in each treatment and control were recorded daily.
The effect of the CCM 8367 strain on the penetration rate and the size of S. feltiae that developed inside L. decemlineata larvae
Dead CPB larvae from the combined application bioassays were incubated for 2–3 days and the S. feltiae penetration rate was evaluated as described above. Next, the adults of the first generation of nematodes that developed inside cadavers were randomly selected, collected, rinsed in water and their body size (body length and maximal body width) was measured using a Carl Zeiss (Jena, Germany) light microscope at 40×. In addition, the body size of S. feltiae developed in the last-instar CPB larvae inoculated only with nematodes was also measured.
Histopathology
Development and growth of I. fumosorosea CCM 8367 and S. feltiae in CPB tissue were investigated using histopathological techniques. Ten dead larvae from the experiment in which nematodes were applied simultaneously with fungus, were collected 48 and 72 hours after the treatment and put into alcoholic Bouin’s fixative solution, in a graded series of ethyl alcohol, cleared in methyl benzoate and embedded in paraffin with a high melting point. Next, 10 μm thick longitudinal sections of the larvae were cut with a Leica RM 2165 (Leica Biosystems, Nussloch, Germany) rotator microtome. Sections were then put on microscope slides and prepared according to Mallory’s procedure [42,43]. Stained sections were mounted in Canada balsam (Permount Fisher synthetic medium) and dried at room temperature [42,43]. Development of both the fungus and nematodes was investigated using an Olympus BX51 (Tokio, Japan) light microscope, and micrographs were taken with an Olympus DP50 (Tokio, Japan) digital camera attached to the microscope.
Data presentation and statistical analysis
The obtained mortality data were corrected for mortality in the control group using the Abbott equation [44]. The Kaplan–Meier product limit estimate calculated in the LIFETEST procedure in SAS/STAT [45] was used to determine both the mean and the median time to death (LT50, the number of days until 50% of insects were dead) for each treatment. Wilcoxon and log-rank test statistics (PROC LIFETEST [45]) were used to test the global hypothesis that mortality (time to death) differed between treatments. Significance was set at α⩽0.05, and where multiple comparisons were performed, the Holm-Bonferroni correction [46] was applied. Data from dose-response experiment were analysed using Probit analysis (PROC PROBIT [45]) to estimate lethal concentrations (LC50 amd LC90). The penetration rate of S. feltiae was calculated using the equation P = N*100/T, in which P is a percentage of penetration, N is a number of nematodes counted in a cadaver, and T is an original number of nematodes used in the treatment. Since percentage data are not normally distributed, the effects of both the CPB developmental stage and the delay of S. feltiae application on the S. feltiae penetration rate were analyzed using a Kruskal-Wallis test [47] followed by Dunn's multiple comparison post test (PROC NPAR1WAY [45] and SAS macro [48]). Multivariate analysis of variance (MANOVA) using the Generalized Linear Models procedure (PROC GLM) in SAS [45] was used to analyze data on the body size of adult S. feltiae developed in I. fumosorosea-infected larvae. In addition, a correlation analysis of the body size data was conducted, and a model describing the relationship between the delay of nematode application and the length of S. feltiae was fitted by linear regression.
Results
The efficacy of I. fumosorosea CCM 8367 against immature stages of L. decemlineata
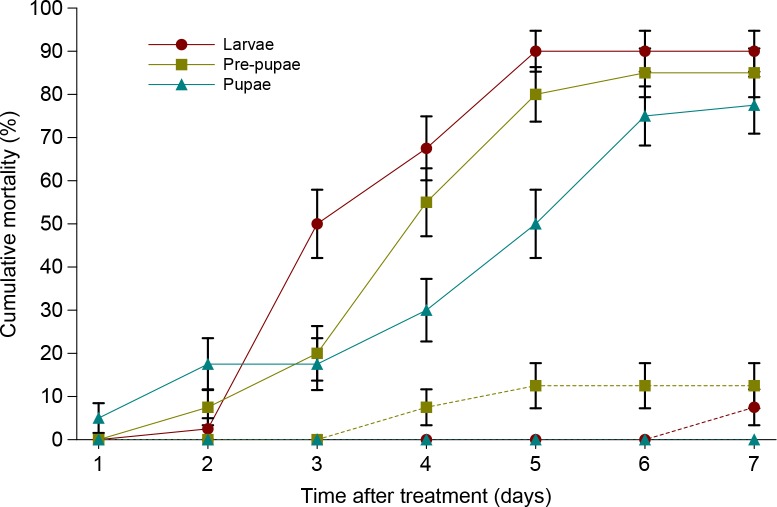
The last-instar larvae of CPB showed the highest susceptibility to I. fumosorosea strain CCM 8367 followed by pre-pupae and pupae. Cumulative mortality at the end of experiment reached 90.0, 85.0 and 77.1% in last-instar larvae, pre-pupae and pupae, respectively (Fig 1). Survival analysis revealed a significant effect of developmental stage on susceptibility to the fungus (Wilcoxon test, χ2 = 11.07, P = 0.0039; log-rank test, χ2 = 9.38, P = 0.0092). Table 1 shows corrected mortality, the mean and median survival times and the associated statistical multiple comparison. All dead individuals showed symptoms of I. fumosorosea infection by mycelia growing on a cadaver usually four to five days after inoculation.
Fig 1. Cumulative mortality of last-instar larvae, pre-pupae and pupae of L. decemlineata treated by I. fumosorosea CCM 8367.
Dashed lines indicate mortality in controls. Vertical bars indicate standard error.
Table 1. Corrected mortality (%), mean survival time and LT50 (days) of L. decemlineata immature stages treated by suspensions of 5×107 blastospores/ml of I. fumosorosea CCM 8367.
| Treated Stage | Mortalitya | Mean survival time ± SEb | LT50 (95% CI) | Nc | Wilcoxon testd |
|---|---|---|---|---|---|
| Last-instar larva | 89.2 | 3.80±0.15 | 3.5 (3.0–4.0) | 40 | a |
| Pre-pupa | 82.9 | 4.38±0.19 | 4.0 (4.0–5.0) | 40 | ab |
| Pupa | 77.5 | 4.80±0.27 | 5.0 (5.0–6.0) | 40 | b |
a Percent of dead individuals at the end of experiment corrected for mortality in control using Abbott equation [44].
b The mean survival time and its standard error were underestimated because the largest observation was censored.
c Total number of individuals in bioassay.
d Identical lowercase letters within a column indicates no significant differences at α = 0.05 adjusted according to the Holm-Bonferroni method for multiple comparisons [46].
Dose-response of CPB larvae to I. fumosorosea CCM 8367 and efficacy comparison with the Apopka 97 strain
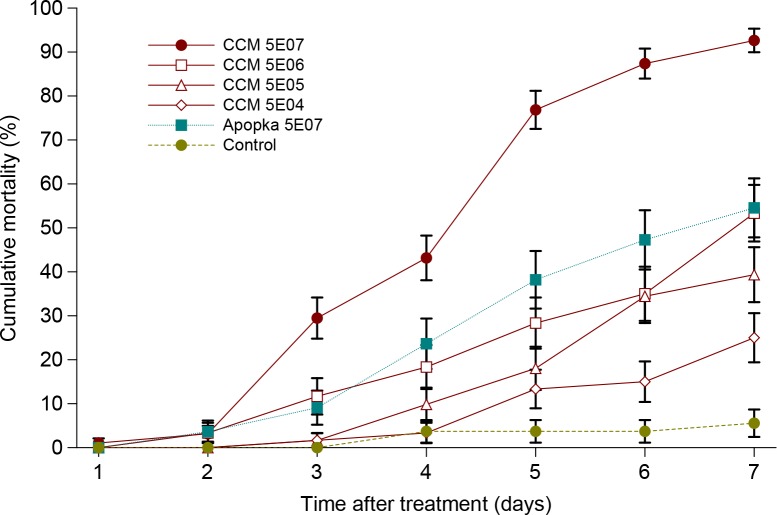
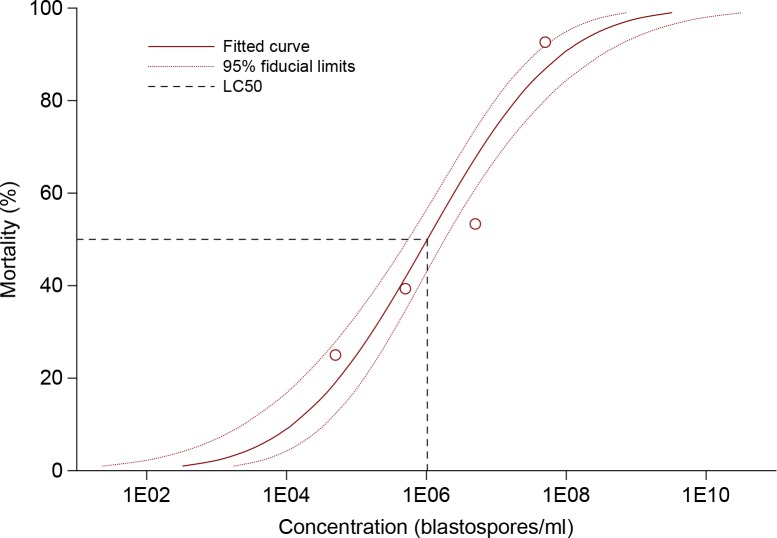
Cumulative mortality of CPB larvae at the end of experiment reached 25.0, 39.4, 53.3 and 92.6 when treated by CCM 8367 strain at concentration of 5×104, 5×105, 5×106 and 5×107 blastospores/ml, respectively (Fig 2). The log-probit regression line describing relationship between concentration and mortality has a form y = -4.002 + 0.666x (Fig 3). The estimated values of LC50 and LC90 were 1.03×106 and 8.67×107, respectively. In Apopka 97 strain applied at concentration of 5×107, cumulative mortality reached 54.5% (Fig 2) with LT50 1.4 fold higher compared to CCM 8367 (Table 2). Survival analysis revealed highly significant differences in mortality rates between the two strains of I. fumosorosea (Wilcoxon test, χ2 = 21.85, P < 0.0001; log-rank test, χ2 = 27.97, P < 0.0001).
Fig 2. Cumulative mortality of L. decemlineata last-instar larvae treated by CCM 8367 and Apopka 97 strains of I. fumosorosea.
Vertical bars indicate standard error.
Fig 3. Concentration-mortality response of CPB larvae to I. fumosorosea strain CCM 8367.
Table 2. Corrected mortality (%), mean survival time and LT50 (days) of L. decemlineata last-instar larvae treated by CCM 8367 and Apopka 97 strains of I. fumosorosea.
| Strain | Dose | Mortalitya | Mean survival time ± SEb | LT50 (95% CI) | Nc |
|---|---|---|---|---|---|
| CCM 8367 | 5×104 | 20.6 | 6.67±0.11 | NA | 60 |
| 5×105 | 35.8 | 6.36±0.14 | NA | 61 | |
| 5×106 | 50.6 | 6.03±0.20 | 7.0 (7.0-NA) | 60 | |
| 5×107 | 92.2 | 4.59±0.15 | 5.0 (4.0–5.0) | 95 | |
| Apopka 97 | 5×107 | 51.9 | 5.78±0.21 | 7.0 (5.0-NA) | 55 |
a, b, c For explanations see Table 1.
The efficacy of S. feltiae against immature stages of L. decemlineata
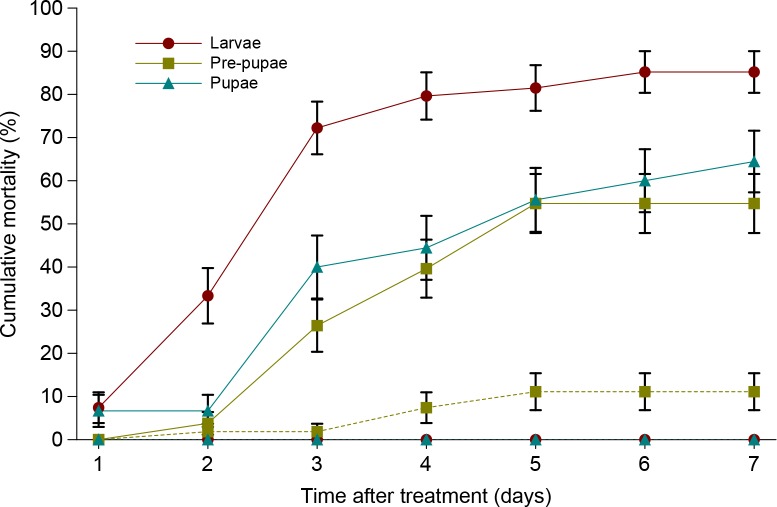
S. feltiae was able to invade, develop and cause high mortality in all tested developmental stages of CPB (Fig 4). The most effective was against the last larval instar of CPB when the percentage of uncorrected mortality was 85.2%, whereas mortality of pre-pupae and pupae was 54.7.7% and 64.4%, respectively. Survival analysis revealed a significant effect of developmental stage on susceptibility to nematodes (Wilcoxon test, χ2 = 29.40, P < 0.0001; log-rank test, χ2 = 24.64, P < 0.0001) indicating differences in survival times (Table 3).
Fig 4. Cumulative mortality of last-instar larvae, pre-pupae and pupae of L. decemlineata treated by S. feltiae.
Dashed lines indicate mortality in controls. Vertical bars indicate standard error.
Table 3. Corrected mortality (%), mean survival time and LT50 (days) of L. decemlineata immature stages inoculated with 500 IJs of S. feltiae.
| Treated Stage | Mortalitya | Mean survival time ± SEb | LT50 (95% CI) | Nc | Wilcoxon testd |
|---|---|---|---|---|---|
| Last-instar larva | 85.2 | 3.26±0.21 | 3.0 (NA-NA) | 54 | a |
| Pre-pupa | 49.1 | 4.30±0.13 | 5.0 (4.0-NA) | 53 | b |
| Pupa | 64.4 | 4.87±0.31 | 5.0 (3.0–7.0) | 45 | b |
a, b, c, d For explanations see Table 1.
The mean number of nematodes that successfully invaded the host and developed into adults inside cadavers and the calculated penetration rates are shown in Table 4. The highest percentage of invaded IJ of S. feltiae was found in last-instar larvae. Besides normal adults, also fertilised females and first stage juveniles of the second generations were observed in some cadavers. The Kruskal-Wallis test revealed a highly significant effect of developmental stage of CPB on the penetration rate of IJ (χ2 = 44.70, DF = 2, P<0.0001).
Table 4. Mean number of S. feltiae found in cadavers of L. decemlineata immature stages inoculated with 500 IJs per dish and calculated penetration rates.
| Treated stage | Number of S. feltiae | Sex ratioa | Penetration rate (%) | Nc |
|---|---|---|---|---|
| mean ± SE | mean ± SEb | |||
| Last-instar larva | 133.39 ± 7.66 | 54.94 | 26.68 ± 1.53a | 46 |
| Pre-pupa | 68.39 ± 6.06 | 62.36 | 13.68 ± 1.21b | 31 |
| Pupa | 68.31 ± 6.64 | 61.99 | 13.66 ± 1.33b | 42 |
a Percentage of females out of all adults.
b Values followed by identical lowercase letters within a column are not significantly different at α = 0.05 (Dunn's multiple comparison test).
c Number of CPB cadavers dissected.
The efficacy of a combined application of I. fumosorosea CCM 8367 and S. feltiae against L. decemlineata larvae
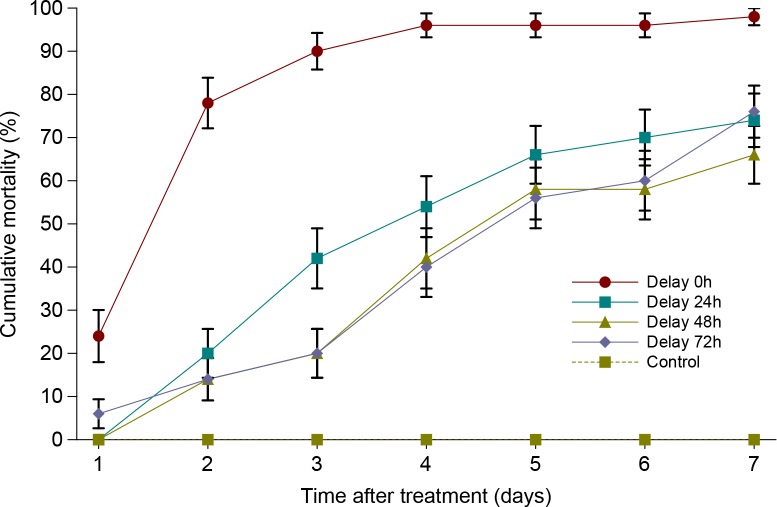
The results of the trials in which S. feltiae inoculation was delayed 0–72 hours after I. fumosorosea CCM 8367 application are shown in Fig 5. The percentage of mortality of the last-instar CPB larvae treated simultaneously by the nematodes and the fungus reached 78% 48 hours after the treatment and increased to 98% on the seventh day. Only symptoms of the nematode infection appeared on dead larvae in this treatment. When nematodes were applied 24 hours after the fungus, the increase of mortality was slower and total cumulative percentage of mortality reached 70% seven days after the treatment. The mortality of larvae treated by nematodes 48 hours after fungus application reached 66%. When nematodes were applied 72 hours after the fungus, larval mortality was 76% at the end of experiment. Survival analysis revealed a highly significant effect of delay between both agents’ applications on mortality of CPB larvae (Wilcoxon test, χ2 = 90.68, P < 0.0001; log-rank test, χ2 = 84.04, P < 0.0001). Multiple comparisons revealed differences between simultaneous application and delayed applications (Table 5).
Fig 5. Cumulative mortality of L. decemlineata last-instar larvae treated by I. fumosorosea CCM 8367 in combination with S. feltiae.
Vertical bars indicate standard error.
Table 5. Corrected mortality (%), mean survival time and LT50 (days) of L. decemlineata last-instar larvae treated by I. fumosorosea CCM 8367 in combination with S. feltiae.
| Delay of S. feltiae application (hrs) | Mortalitya | Mean survival time ± SEb | LT50 (95% CI) | Nc | Wilcoxon testd |
|---|---|---|---|---|---|
| 0 | 98.0 | 2.20±0.18 | 2.0 (NA-NA) | 50 | a |
| 24 | 74.0 | 4.48±0.28 | 4.0 (3.0–5.0) | 50 | b |
| 48 | 66.0 | 5.08±0.26 | 5.0 (4.0–7.0) | 50 | b |
| 72 | 76.0 | 5.04±0.28 | 5.0 (4.0–7.0) | 50 | b |
a, b, c, d For explanations see Table 1.
The effect of the CCM 8367 strain on the penetration rate and the size of S. feltiae that developed inside L. decemlineata larvae
Dissection of dead CPB larvae revealed lower number of S. feltiae per cadaver (Table 6) compared to a single-agent bioassay (Table 4). This was obvious mainly when nematodes were applied 24 hours and later after fungus inoculation where IJs failed to develop to adult stages in part of the cadavers. The effect of S. feltiae application delay on the penetration rate of IJ was statistically highly significant (Kruskal-Wallis, χ2 = 46.00, DF = 3, P<0.0001). Similarly, the percentage of cadavers where only dead nematode adults were found increased with delay in nematode application after fungus. Sex ratio of S. feltiae in cadavers, however, was not affected (Table 6).
Table 6. Mean number of S. feltiae found in cadavers of L. decemlineata last-instar larvae inoculated with 500 IJs per dish in different times after the fungus application, calculated penetration rates and number of cadavers with either alive or dead nematodes.
| Delay of S. feltiae | Number of S. feltiae | Sex ratioa | Penetration rate (%) | Nc | Nsf d | |
|---|---|---|---|---|---|---|
| application (hrs) | mean ± SE | mean ± SEb | Alive | Dead | ||
| 0 | 59.60 ± 8.05 | 51.07 | 11.92 ± 1.61a | 25 | 25 | 0 |
| 24 | 18.91 ± 4.08 | 46.75 | 3.78 ± 0.82b | 23 | 7 | 1 |
| 48 | 7.09 ± 1.72 | 50.00 | 1.42 ± 0.34b | 23 | 1 | 4 |
| 72 | 16.43 ± 5.08 | 49.46 | 3.29 ± 1.0b | 23 | 2 | 3 |
a, b, c For explanations see Table 4.
d Number of cadavers with S. feltiae adults either alive or dead.
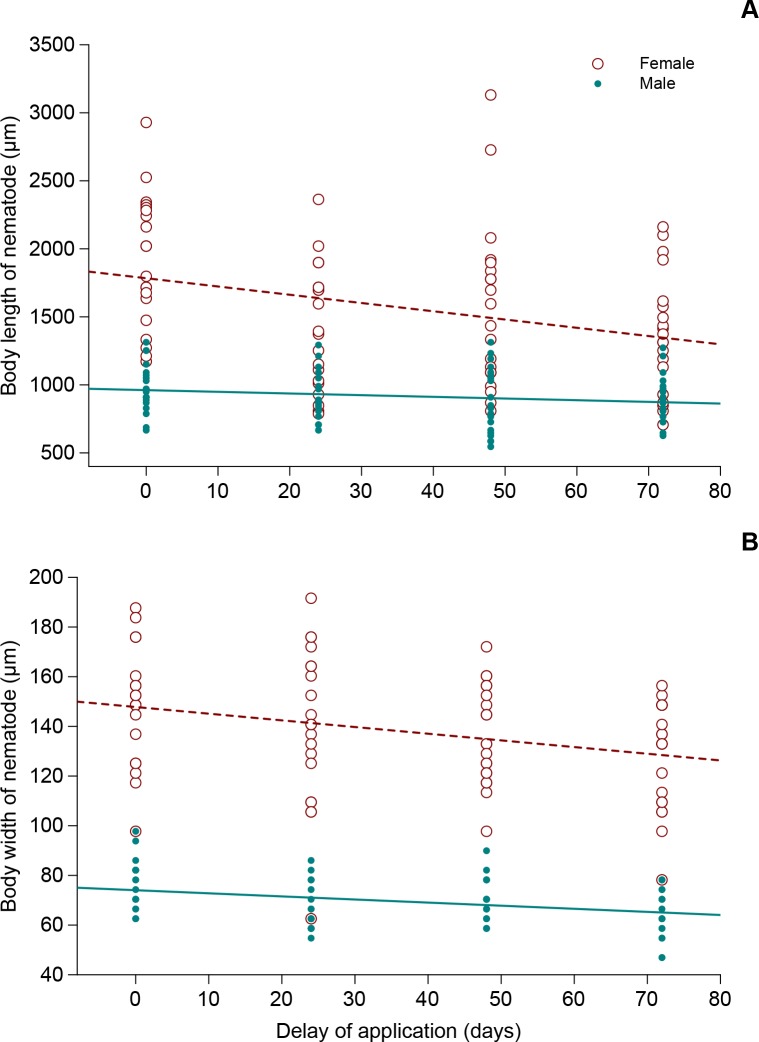
The size of first-generation S. feltiae adults recovered from CPB larvae treated simultaneously with both the nematode and I. fumosorosea was similar to the control, i.e., without fungus (Table 7). In other trials, however, nematode size decreased with the increasing time between fungus and nematode application. Results of the MANOVA indicate that there was a highly significant overall delay effect (Wilk’s λ = 0.881, F6,308 = 3.36, P = 0.0032) and a highly significant overall effect of sex (Wilk’s λ = 0.205, F2,154 = 298.76, P < 0.0001). The effect of application delay on body length and width of S. feltiae males and females inside of I. fumosorosea-treated CPB larvae is shown in Fig 6. A linear correlation analysis showed that the body length of S. feltiae females was negatively correlated with the nematode application delay (R2 = 0.0941; N = 80; P = 0.0056). The relationship can be described by model y = 1783.86–6.07x. A similar trend was found in the female body width (R2 = 0.0846; N = 80; P = 0.0089; y = 147.80–0.27x) and male body width (R2 = 0.1101; N = 80; P = 0.0026; y = 74.04–0.12x). No significant correlation was found in body length of males (R2 = 0.0302; N = 80; P = 0.1231).
Table 7. Mean body length and body width (μm±SE) of S. feltiae adults recovered from cadavers of L. decemlineata last-instar larvae infected by I. fumosorosea CCM 8367 and inoculated with 500 IJs per dish in different times after the fungus application.
| Delay of S. feltiae application (hrs) | Females | Males | ||||
|---|---|---|---|---|---|---|
| Length | Width | N | Length | Width | N | |
| 0 | 1907.89±108.13 | 148.19±5.16 | 20 | 977.68±39.66 | 76.05±2.26 | 20 |
| 24 | 1415.01±101.68 | 139.98±7.01 | 20 | 917.08±37.60 | 67.06±2.07 | 20 |
| 48 | 1566.51±136.73 | 136.46±4.38 | 20 | 881.73±53.87 | 69.99±1.92 | 20 |
| 72 | 1371.58±96.52 | 127.86±4.82 | 20 | 890.82±38.01 | 65.10±2.17 | 20 |
| Controla | 1671.55±37.31 | 172.04±2.89 | 12 | 1026.83±28.00 | 94.17±2.87 | 12 |
a S. feltiae from larvae not treated by I. fumosorosea.
Fig 6. Linear regressions between the body size parameters and nematode inoculation delay.
Body length (A) and body width (B) of S. feltiae adults developed inside cadavers of L. decemlineata last-instar larvae and following I. fumosorosea CCM 8367 application.
Histopathology
Longitudinal sections of CPB last-instar larvae showed the development of infection by I. fumosorosea CCM 8367 inside larvae 48 hours after the treatment (Fig 7A). Twenty-four hours later the fungus invaded all insect tissues (Fig 7B) whereas the development of S. feltiae was not affected by I. fumosorosea infection in CPB cadavers (Fig 7C).
Fig 7. Longitudinal sections of L. decemlineata last-instar larvae infected with both I. fumosorosea and S. feltiae simultaneously.
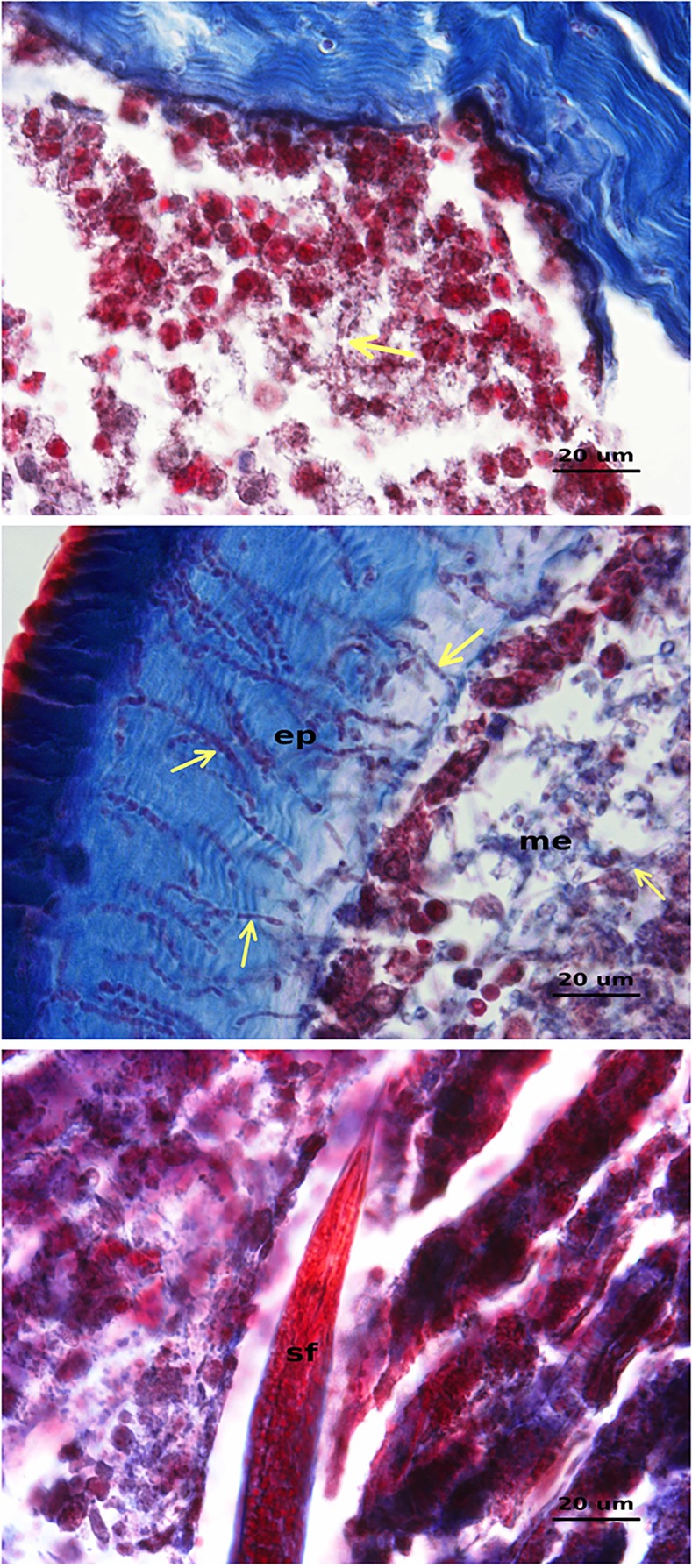
(A) Insect tissues with a few hyphal bodies of I. fumosorosea (yellow arrows) 48 hours after the treatment. (B) Hyphal bodies of I. fumosorosea (yellow arrows) penetrating epidermis (ep) and mesoderm tissues (me) 72 hours after the treatment. (C) Front part of a fertile adult female of S. feltiae (sf) full of eggs inside of tissue thoroughly invaded by hyphae of I. fumosorosea 72 hours after the treatment.
Discussion
The efficacy of I. fumosorosea against L. decemlineata
Numerous trials have been conducted that use entomopathogenic fungi against the CPB. For example, the effect of B. bassiana on foliage consumption by fourth-instar CPB was studied by Fargues et al. [49]. The treated larvae consumed significantly less than control larvae, and increasing the fungus dose reduced the feeding period. The highest reduction in total food consumption per larva caused by B. bassiana was 76.2% at a dose 105 conidia/cm2. Wraight and Ramos [50] evaluated the effects of various spray-application parameters on the efficacy of B. bassiana foliar treatments against CPB larvae during three field seasons. All treatments applied against late instars, including sprays at weekly intervals and from above the canopy, resulted in significant reductions (53–84%) of first-generation adult beetle populations. Another species of entomopathogenic fungus successfully tested against CPB was I. farinosa. In various experiments, including field trials with this species alone and in combination with other fungi performed in Poland [51,52], the Czech Republic [53] and in Austria [54], high efficacy of fungus treatment was reported.
The present research studied the efficacy of I. fumosorosea against the immature stages of CPB. The species has a worldwide distribution, and its natural occurrence in soil samples was reported from many countries. For example, in the Czech Republic, 16 strains of I. fumosorosea were isolated from various soils by Landa et al. [55]. In another soil-sample survey [56], I. fumosorosea was the dominant species (77.6%) whereas I. farinosa occurred rarely (1.7%). The species has a relatively wide host range across several insect orders, including Acari [12]. It was also reported to infect L. decemlineata [57].
The strain CCM 8367 used in this study was isolated from C. ohridella, an invasive pest of horse chestnut in Europe [58]. The first record of I. fumosorosea infecting pupae of this host was reported by Zemek et al. [59]. Because this strain showed high virulence against C. ohridella and other pests [60–62], it was considered to have an application potential and therefore patented [39].
Our study demonstrates a high efficacy of the CCM 8367 strain of I. fumosorosea against all three immature stages of CPB tested. The most virulent was against the CPB last-instar larvae when it caused nearly 93% mortality 7 days after the treatment by suspension of blastospores with a concentration of 5×107 spores/ml. Strain Apopka 97 at the same concentration caused significantly lower mortality of CPB larvae. This strain originates from a mealybug Phenacoccus sp. in Apopka, Florida, USA [63], and several studies have demonstrated its high efficacy against whiteflies [64,65], psyllids [66,67] and thrips [68]. Presently, it is readily available as a commercial product in the USA (PFR 97TM, Certis Columbia, MD) and Europe (PreFeRal® WG, Biobest, Belgium).
The efficacy of S. feltiae against L. decemlineata
The application of EPNs in biological control was traditionally used to control soil pests [69]. Research from the last two decades also indicates their potential against foliar pests, but only under special conditions [70,71]. The results of the present study show that S. feltiae caused medium to high mortality in immature stages of CPB. The highest efficacy (85.2%) was found when nematodes were applied to last-instar larvae. This is consistent with the well-known fact that EPNs are most effective in controlling younger developmental stages because entering the host is much easier [72,73]. Steinernema carpocapsae (Weiser), S. feltiae and Heterorhabditis bacteriophora Poinar strains applied at a dose of 164.6 nematodes/cm2 of soil were able to kill 100% of CPB pre-pupae under laboratory conditions [74]. Other laboratory experiments showed that adults of L. decemlineata are also sensitive to EPNs [32,73]. The efficacy of two strains of S. feltiae against CPB was tested in a field experiment by Laznik et al. [75]. Both strains significantly decreased the number of larvae, whereas no effect on CPB eggs and adults was observed.
The percentage of nematodes invading CPB immature stages (penetration rate) was low, ranging from 13.7% to 26.7%. These results are similar to the findings of Epsky and Capinera [76] who observed that 10–50% of applied S. carpocapsae successfully infected the host. The percentage of invading nematodes of S. feltiae and H. bacteriophora to CPB under laboratory and greenhouse conditions ranged between 10–50% [77]. The study performed by Armer et al. [78] showed that although Heterorhabditis marelatus Liu and Berry is capable of successfully attacking and killing CPB, the nematode is incapable of completing its life cycle in the beetle. This phenomenon was later attributed to stress on the nematode symbiont Photorhabdus temperata Fischer-Le Saux, Viallard, Brunel, Normand and Boemare and potential interference from the enteric bacteria of the beetle [79]. Similarly, Campos-Herrera and Gutierrez [80] reported that neither S. feltiae was able to reproduce in CPB. In our experiments, we did not study the ability of S. feltiae to reproduce in CPB, however during the dissections, we did not observe any negative effect on the first generation adults.
The efficacy of the combined application of I. fumosorosea CCM 8367 and S. feltiae against L. decemlineata larvae
The combination of I. fumosorosea CCM 8367 with nematodes increased the application efficiency compared to single biocontrol agent application. The best results were obtained when S. feltiae was applied simultaneously with I. fumosorosea. A synergistic effect was reported, e.g., when entomopathogenic nematodes were applied together with Paenibacillus popilliae [81,82], or with Bacillus thuringiensis Berliner subspecies japonensis against Cyclocephala spp. [83,84]. Another study [85] demonstrated that the application of B. bassiana with H. bacteriophora resulted in higher total mortality of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) in soil than when either nematodes or fungi were separately applied. However, when the same fungus species was combined with S. carpocapsae, insect mortality was not significantly different compared with S. carpocapsae alone [85]. By contrast, Shapiro-Ilan et al. [86] found that when pairs of nematode and fungal pathogens attacked the larvae of the weevil Curculio caryae, most pairings were less effective than a single highly effective entomopathogenic species. This antagonism may have resulted from negative interactions between microbes or their toxins before or during the infection process.
Our results demonstrate a normal development of nematodes when they were applied simultaneously with the fungus. Similar conclusions were reported in other studies showing that when B. bassiana and S. carpocapsae or H. bacteriophora were applied simultaneously to a host, the nematodes developed normally and produced progeny [87,88]. When, however, S. feltiae was applied more than 24 hours after I. fumosorosea CCM 8367 treatment, its development was negatively affected. Only a part of the nematodes developed to adults, and in some cadavers, only dead adults were observed. Furthermore, adult body size expressed as body length and width of nematodes developing inside the cadavers in these treatments was lower in comparison to control. This could be because of the anti-bacterial activity of some metabolites/toxins produced by I. fumosorosea that negatively affect both the developing nematodes and their symbiotic bacteria, Xenorhabdus bovienii. A similar negative effect was observed in the interactions between the fungi M. anisopliae and S. glaseri [89] and H. bacteriophora [90] and between B. bassiana and S. ichnusae [91]; however, this was not explored in our study.
Conclusions
We found that (1) both I. fumosorosea CCM 8367 and S. feltiae Ustinov showed high virulence against L. decemlineata, (2) the most sensitive stage of CPB is the last-instar larva, (3) simultaneous application of both biocontrol agents increases their efficacy compared to single species application and (4) later application of S. feltiae has a negative effect on both the penetration rate and the development of nematodes inside a CPB host. Further research, including soil experiments in greenhouses and in the field, is necessary before these strains can be recommended for application as biopesticides.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
Plant material was kindly provided by Potato Research Institute Havlíčkův Brod, Ltd., Czech Republic. The authors thank Prof. Zdeněk Landa for providing sample of PreFeRal WG, Dr. Eva Prenerová and Dr. Katka Šimáčková for preparation of blastospores of both I. fumosorosea strains and Dr. Manal M. Adel for helpful ideas and discussion. Mrs. Jana Jabůrková, Mrs. Lenka Kropáčková, Mrs. Radka Tanzer Fabianová, Mrs. Zdenka Svobodová and Mrs. Barbora Kozelková are thanked for their technical assistance. This manuscript was edited for English language by American Journal Experts (AJE).
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was conducted with institutional support RVO:60077344 (www.cas.cz) (HMH OH VP RZ) and co-financed by the European Social Fund and National budget of the Czech Republic (projects TTM, reg. No. CZ.1.07/2.4.00/12.0082 and Postdok BIOGLOBE, reg. No. CZ.1.07/2.3.00/30.0032) (www.msmt.cz) (RZ) and Ministry of Agriculture, reg. No. 206553/2011-MZE-17253 (eagri.cz/public/web/en/mze/ministry/) (OH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hare JD. Ecology and management of the Colorado potato beetle. Annu Rev Entomol. 1990;35: 81–100. 10.1146/annurev.en.35.010190.000501 [DOI] [Google Scholar]
- 2.Jolivet P. Le doryphore menace l’Asie Leptinotarsa decemlineata Say 1824 (Col. Chrysomelidae). Entomol Paris. 1991;47: 29–48. [Google Scholar]
- 3.Weber D. Colorado beetle: pest on the move. Pestic Outlook. 2003;14: 256–259. 10.1039/B314847P [DOI] [Google Scholar]
- 4.Jörg E, Beck W. Schadwirkung und Bekämpfung des Kartoffelkäfers. Kartoffelbau. 51: 202–204. [Google Scholar]
- 5.Mota-Sanchez D, Hollingworth RM, Grafius EJ, Moyer DD. Resistance and cross-resistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pest Manag Sci. 2006;62: 30–37. 10.1002/ps.1120 [DOI] [PubMed] [Google Scholar]
- 6.Alyokhin A, Dively G, Patterson M, Castaldo C, Rogers D, Mahoney M, et al. Resistance and cross-resistance to imidacloprid and thiamethoxam in the Colorado potato beetle Leptinotarsa decemlineata. Pest Manag Sci. 2007;63: 32–41. 10.1002/ps.1305 [DOI] [PubMed] [Google Scholar]
- 7.Zichova T, Kocourek F, Salava J, Nad’ova K, Stara J. Detection of organophosphate and pyrethroid resistance alleles in Czech Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) populations by molecular methods. Pest Manag Sci. 2010;66: 853–860. 10.1002/ps.1952 [DOI] [PubMed] [Google Scholar]
- 8.Szendrei Z, Grafius E, Byrne A, Ziegler A. Resistance to neonicotinoid insecticides in field populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Pest Manag Sci. 2012;68: 941–946. 10.1002/ps.3258 [DOI] [PubMed] [Google Scholar]
- 9.Grafius EJ, Douches DS. The present and future role of insect-resistant genetically modified potato cultivars in IPM In: Romeis J, Shelton AM, Kennedy GG, editors. Integration of Insect-Resistant Genetically Modified Crops within IPM Programs. Grafius Edward J.; Michigan State Univ, Dept Entomol, E Lansing, MI 48824 USA: Springer, Po Box 17, 3300 Aa Dordrecht, Netherlands; 2008. pp. 195–221. [Google Scholar]
- 10.Faria MR de, Wraight SP. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43: 237–256. 10.1016/j.biocontrol.2007.08.001 [DOI] [Google Scholar]
- 11.Lacey L., Frutos R, Kaya H., Vail P. Insect pathogens as biological control agents: Do they have a future? Biol Control. 2001;21: 230–248. 10.1006/bcon.2001.0938 [DOI] [Google Scholar]
- 12.Zimmermann G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Sci Technol. 2008;18: 865–901. 10.1080/09583150802471812 [DOI] [Google Scholar]
- 13.Fargues J, Bon MC, Manguin S, Couteaudier Y. Genetic variability among Paecilomyces fumosoroseus isolates from various geographical and host insect origins based on the rDNA-ITS regions. Mycol Res. 2002;106: 1066–1074. 10.1017/S0953756202006408 [DOI] [Google Scholar]
- 14.Dalleau-Clouet C, Gauthier N, Risterucci AM, Bon MC, Fargues J. Isolation and characterization of microsatellite loci from the entomopathogenic hyphomycete, Paecilomyces fumosoroseus. Mol Ecol Notes. 2005;5: 496–498. 10.1111/j.1471-8286.2005.00968.x [DOI] [Google Scholar]
- 15.Gauthier N, Dalleau-Clouet C, Fargues J. Microsatellite variability in the entomopathogenic fungus Paecilomyces fumosoroseus: genetic diversity and population structure. Mycologia. 2007;99: 693–704. 10.3852/mycologia.99.5.693 [DOI] [PubMed] [Google Scholar]
- 16.Cantone FA, Vandenberg JD. Intraspecific diversity in Paecilomyces fumosoroseus. Mycol Res. 1998;102: 209–215. 10.1017/S0953756297004590 [DOI] [Google Scholar]
- 17.Smith P. Control of Bemisia tabaci and the potential of Paecilomyces fumosoroseus as a biopesticide. Biocontrol News Inf. 1993;14: 71N–78N. [Google Scholar]
- 18.Dunlap CA, Jackson MA, Wright MS. A foam formulation of Palecilomyces fumosoroseus, an entomopathogenic biocontrol agent. Biocontrol Sci Technol. 2007;17: 513–523. 10.1080/09583150701311614 [DOI] [Google Scholar]
- 19.Hoy MA, Singh R, Rogers ME. Evaluations of a novel isolate of Isaria fumosorosea for control of the asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla Entomol. 2010;93: 24–32. [Google Scholar]
- 20.Kim JS, Je YH, Roh JY. Production of thermotolerant entomopathogenic Isaria fumosorosea SFP-198 conidia in corn-corn oil mixture. J Ind Microbiol Biotechnol. 2010;37: 419–423. 10.1007/s10295-010-0692-y [DOI] [PubMed] [Google Scholar]
- 21.Hajek AE, St Leger RJ. Interactions between fungal pathogens and insect hosts In: Mittler TE, Radovsky FJ, Resh VH, editors. Annual Review of Entomology. Boyce Thompson Inst. Plant Res., Ithaca, NY 14853–1801, USA: Annual Reviews Inc., P.O. Box 10139, 4139 El Camino Way, Palo Alto, California 94306, USA; 1994. pp. 293–322. [Google Scholar]
- 22.Ali S, Huang Z, Ren S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J Pest Sci. 2010;83: 361–370. 10.1007/s10340-010-0305-6 [DOI] [Google Scholar]
- 23.Luangsa-ard JJ, Berkaew P, Ridkaew R, Hywel-Jones NL, Isaka M. A beauvericin hot spot in the genus Isaria. Mycol Res. 2009;113: 1389–1395. 10.1016/j.mycres.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 24.Poinar GO. Nematodes for biological control of insects CRC Press Inc., Florida; 1979. [Google Scholar]
- 25.Boemare N, Akhurst R, Mourant R. Dna relatedness between Xenorhabdus spp (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen-nov. Int J Syst Bacteriol. 1993;43: 249–255. [Google Scholar]
- 26.Laumond C, Mauléon H, Kermarrec A. Données nouvelles sur le spectre d’hôtes et le parasitisme du nématode entomophage Neoaplectana carpocapsae. Entomophaga. 1979;24: 13–27. 10.1007/BF02377505 [DOI] [Google Scholar]
- 27.Woodring JL, Kaya HK. Steinernematid and Heterorhabditid Nematodes: A Handbook of Biology and Techniques [Internet]. Arkansas Agricultural Experiment Station; 1988. Available: http://books.google.cz/books?id=-T5AkgAACAAJ [Google Scholar]
- 28.Bathon H. Impact of entomopathogenic nematodes on non-target hosts. Biocontrol Sci Technol. 1996;6: 421–434. 10.1080/09583159631398 [DOI] [Google Scholar]
- 29.Ehlers RU, Hokkanen HMT. Insect biocontrol with non-endemic entomopathogenic nematodes (Steinernema and Heterorhabditis spp): Conclusions and recommendations of a combined OECD and COST Workshop on Scientific and Regulatory Policy Issues. Biocontrol Sci Technol. 1996;6: 295–302. 10.1080/09583159631280 [DOI] [Google Scholar]
- 30.Georgis R. The Biosys experiment: an insider’s perspective In: Gaugler R, editor. Entomopathogenic nematology. Wallingford: CABI; 2002. pp. 357–372. Available: http://www.cabi.org/cabebooks/ebook/20023023459 [Google Scholar]
- 31.Welch HE, Briand LJ. Tests of the nematode DD 136 and an associated bacterium for control of the Colorado potato beetle, Leptinotarsa decemlineata (Say). Can Entomol. 1961;93: 759–763. 10.4039/Ent93759-9 [DOI] [Google Scholar]
- 32.Stewart JG, Boiteau G, Kimpinski J. Management of late-season adults of the Colorado potato beetle (Coleoptera: Chrysomelidae) with entomopathogenic nematodes. Can Entomol. 1998;130: 509–514. [Google Scholar]
- 33.Belair G, Wright DJ, Curto G. Vegetable and tuber crop applications In: Grewal PS, Ehlers RU, ShapiroIlan DI, editors. Nematodes as Biocontrol Agents. Oxfordshire, UK: CABI Publishing; 2005. pp. 255–264. [Google Scholar]
- 34.Chang GC. Comparison of single versus multiple species of generalist predators for biological control. Environ Entomol. 1996;25: 207–212. [Google Scholar]
- 35.Guetsky R, Shtienberg D, Elad Y, Dinoor A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology. 2001;91: 621–627. 10.1094/PHYTO.2001.91.7.621 [DOI] [PubMed] [Google Scholar]
- 36.Otsuki H, Yano S. Functionally different predators break down antipredator defenses of spider mites. Entomol Exp Appl. 2014;151: 27–33. 10.1111/eea.12164 [DOI] [Google Scholar]
- 37.Ansari MA, Shah FA, Butt TM. Combined use of entomopathogenic nematodes and Metarhizium anisopliae as a new approach for black vine weevil, Otiorhynchus sulcatus, control. Entomol Exp Appl. 2008;129: 340–347. 10.1111/j.1570-7458.2008.00783.x [DOI] [Google Scholar]
- 38.Ansari MA, Shah FA, Butt TM. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Sci Technol. 2010;20: 99–105. 10.1080/09583150903420031 [DOI] [Google Scholar]
- 39.Prenerova E, Zemek R, Volter L, Weyda F. Strain of entomopathogenic fungus Isaria fumosorosea CCM 8367 (CCEFO.011.PFR) and the method for controlling insect and mite pests. Patent US 08574566, 2013.
- 40.Haydak M. H. A food for rearing laboratory insects. J Econom Entomol. 1936;9: 1026. [Google Scholar]
- 41.White GF. A method for obtaining infective nematode larvae from cultures. Science. 1927;66: 302–303. 10.1126/science.66.1709.302-a [DOI] [PubMed] [Google Scholar]
- 42.Wolf J. Mikroskopická technika Praha: SZN; 1954. [Google Scholar]
- 43.Vacek Z. Histológia a histologická technika Martin, Slovakia: Osveta; 1974. [Google Scholar]
- 44.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18: 265–267. 10.1093/jee/18.2.265a [DOI] [Google Scholar]
- 45.SAS Institute. The SAS System for Linux, Release 8.2 SAS Online doc. Version 8. Cary, North Carolina: SAS Institute; 2000. [Google Scholar]
- 46.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6: 65–70. [Google Scholar]
- 47.Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences 2. ed., [reprinted]. Boston, Mass.: McGraw-Hill; 2003. [Google Scholar]
- 48.Elliott AC, Hynan LS. A SAS (R) macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Programs Biomed. 2011;102: 75–80. 10.1016/j.cmpb.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 49.Fargues J, Delmas JC, Lebrun RA. Leaf consumption by larvae of the Colorado potato beetle (Coleoptera: Chrysomelidae) infected with the entomopathogen, Beauveria bassiana. J Econ Entomol. 1994;87: 67–71. [Google Scholar]
- 50.Wraight S., Ramos M. Application parameters affecting field efficacy of Beauveria bassiana foliar treatments against Colorado potato beetle Leptinotarsa decemlineata. Biol Control. 2002;23: 164–178. 10.1006/bcon.2001.1004 [DOI] [Google Scholar]
- 51.Bajan C, Kmitowa K. The effect of entomogenous fungi Paecilomyces farinosus Dicks. Brown et Smith and Beauveria bassiana Bals. Vuill. on the oviposition by Leptinotarsa decemlineata Say females, and on the survival of larvae. Ekol Pol. 1972;20: 423–432. [Google Scholar]
- 52.Bajan C, Fedorko A, Kmitowa K, Wojciechowska M. Utilization of parasitic microorganisms to decrease Colorado beetle quantity. Bull Acad Pol Sci [Biol]. 1978;26: 715–717. [Google Scholar]
- 53.Samsinakova A. Effects of fungic preparations on larvae of colorado beetle, Leptinotarsa decemlineata. Acta Entomol Bohemoslov. 1977;74: 76–80. [Google Scholar]
- 54.Ramisch I. Paecilomyces farinosus Dicks. ex Fr. als parasit des Kartoffelkafers Leptinotarsa decemlineata Say. Nova Hedwig. 1976;271: 199–214. [Google Scholar]
- 55.Landa Z, Hornák P, Charvátová H, Osborne LS. Distribution, occurrence and potential use of entomopathogenic fungi in arable soils in Czech republic. Proceedings of International Conference ISTRO “Current Trends in the Research of Soil Environment.” Brno, Czech Republic: Czech Branch of ISTRO; 2002. pp. 195–201.
- 56.Prenerová E, Zemek R, Weyda F, Volter L. Entomopathogenic fungi isolated from soil in the vicinity of Cameraria ohridella infested horse chestnut trees. In: Ehlers RU, Crickmore N, Enkerli J, Glazer I, Lopez-Ferber M, Tkaczuk C, editors. IOBC/WPRS Bulletin. 2009. pp. 321–324.
- 57.Bajan C. Paecilomyces fumosoroseus (Wize)–pathogenic agent of the Colorado beetle (Leptinotarsa decemlineata Say). Ekol Pol. 1973;21: 705–713. [Google Scholar]
- 58.Sefrova H, Lastuvka Z. Dispersal of the horse-chestnut leafminer, Cameraria ohridella Deschka & Dimic, 1986, in Europe: its course, ways and causes (Lepidoptera: Gracillariidae). Entomol Z. 2001;111: 194–198. [Google Scholar]
- 59.Zemek R, Prenerova E, Weyda F. The first record of entomopathogenic fungus Paecilomyces fumosoroseus (Deuteromycota: Hyphomycetes) on the hibernating pupae of Cameraria ohridella (Lepidoptera: Gracillariidae). Entomol Res. 2007;37: A135–A136. [Google Scholar]
- 60.Zemek R, Hussein HM, Prenerová E. Laboratory evaluation of Isaria fumosorosea against Spodoptera littoralis. Commun Agric Appl Biol Sci. 2012;77: 685–689. [PubMed] [Google Scholar]
- 61.Zemek R, Prenerova E, Awad M, Hussein HM. Potential of the strain of entomopathogenic fungus Isaria fumosorosea CCM 8367 as a biological control agent against Cameraria ohridella and other pests. Acta Fytotech Zootech. 2012;15: 79–80. [Google Scholar]
- 62.Hussein HM, Zemek R, Habuštová SO, Prenerová E, Adel MM. Laboratory evaluation of a new strain CCM 8367 of Isaria fumosorosea (syn. Paecilomyces fumosoroseus) on Spodoptera littoralis (Boisd.). Arch Phytopathol Plant Prot. 2013;46: 1307–1319. 10.1080/03235408.2013.765677 [DOI] [Google Scholar]
- 63.Vidal C, Osborne LS, Lacey LA, Fargues J. Effect of host plant on the potential of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) for controlling the silverleaf whitefly, Bemisia argentifolii (Homoptera: Aleyrodidae) in greenhouses. Biol Control. 1998;12: 191–199. 10.1006/bcon.1998.0625 [DOI] [Google Scholar]
- 64.Osborne LS. Biological control of whiteflies and other pests with a fungal pathogen [Internet]. WO_1990_010388_A1, 1993. Available: http://www.google.com/patents/CA1318272C?cl=en
- 65.Bolckmans K, Sterk G, Eyal J, Sels B, Stepman W. PreFeRal, (Paecilomyces fumosoroseus strain Apopka 97), A new microbial insecticide for the biological control of whiteflies in greenhouses. Meded Fac Landbouwkd En Toegepaste Biol Wet Univ Gent. 1995;60: 707–711. [Google Scholar]
- 66.Avery PB, Wekesa VW, Hunter WB, Hall DG, McKenzie CL, Osborne LS, et al. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Technol. 2011;21: 1065–1078. 10.1080/09583157.2011.596927 [DOI] [Google Scholar]
- 67.Stauderman K, Avery P, Aristizabal L, Arthurs S. Evaluation of Isaria fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Technol. 2012;22: 747–761. 10.1080/09583157.2012.686599 [DOI] [Google Scholar]
- 68.Arthurs SP, Aristizabal LF, Avery PB. Evaluation of entomopathogenic fungi against chilli thrips, Scirtothrips dorsalis. J Insect Sci Tucson. 2013;13: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishibashi N, Choi D. Biological control of soil pests by mixed application of entomopathogenic and fungivorous nematodes. J Nematol. 1991;23: 175–181. [PMC free article] [PubMed] [Google Scholar]
- 70.Arthurs S, Heinz KM, Prasifka JR. An analysis of using entomopathogenic nematodes against above-ground pests. Bull Entomol Res. 2004;94: 297–306. 10.1079/BER2003309 [DOI] [PubMed] [Google Scholar]
- 71.Lacey LA, Georgis R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol. 2012;44: 218–225. [PMC free article] [PubMed] [Google Scholar]
- 72.Lebeck L, Gaugler R, Kaya H, Hara A, Johnson M. Host stage suitability of the leafminer Liriomyza trifolii (Diptera, Agromyzidae) to the entomopathogenic nematode Steinernema carposapsae (Rhabditida, Steinernematidae). J Invertebr Pathol. 1993;62: 58–63. 10.1006/jipa.1993.1074 [DOI] [Google Scholar]
- 73.Trdan S, Vidrih M, Andjus L, Laznik Z. Activity of four entomopathogenic nematode species against different developmental stages of Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera, Chrysomelidae). Helminthologia. 2009;46: 14–20. 10.2478/s11687-009-0003-1 [DOI] [Google Scholar]
- 74.Cantelo W, Nickle W. Susceptibility of prepupae of the Colorado potato beetle (Coleoptera, Chrysomelidae) to entomopathogenic nematodes (Rhabditida, Steinernematidae, Heterorhabditidae). J Entomol Sci. 1992;27: 37–43. [Google Scholar]
- 75.Laznik Z, Toth T, Lakatos T, Vidrih M, Trdan S. Control of the Colorado potato beetle (Leptinotarsa decemlineata [Say]) on potato under field conditions: a comparison of the efficacy of foliar application of two strains of Steinernema feltiae (Filipjev) and spraying with thiametoxam. J Plant Dis Prot. 2010;117: 129–135. [Google Scholar]
- 76.Epsky N, Capinera J. Quantification of invasion of 2 strains of Steinernema carpocapsae (Weiser) into 3 lepidopteran larvae. J Nematol. 1993;25: 173–180. [PMC free article] [PubMed] [Google Scholar]
- 77.Adel MM, Hussein HM. Effectiveness of entomopathogenic nematodes Steinernema feltiae and Heterorhabditis bacteriophora on the Colorado potato beetle Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) under laboratory and greenhouse conditions. Arch Phytopathol Plant Prot. 2010;43: 1485–1494. 10.1080/03235400802538473 [DOI] [Google Scholar]
- 78.Armer CA, Berry RE, Reed GL, Jepsen SJ. Colorado potato beetle control by application of the entomopathogenic nematode Heterorhabditis marelata and potato plant alkaloid manipulation. Entomol Exp Appl. 2004;111: 47–58. 10.1111/j.0013-8703.2004.00152.x [DOI] [Google Scholar]
- 79.Blackburn MB, Farrar RR, Gundersen-Rindal DE, Lawrence SD, Martin PAW. Reproductive failure of Heterorhabditis marelatus in the Colorado potato beetle: Evidence of stress on the nematode symbiont Photorhabdus temperata and potential interference from the enteric bacteria of the beetle. Biol Control. 2007;42: 207–215. 10.1016/j.biocontrol.2007.04.008 [DOI] [Google Scholar]
- 80.Campos-Herrera R, Gutiérrez C. A laboratory study on the activity of Steinernema feltiae (Rhabditida: Steinernematidae) Rioja strain against horticultural insect pests. J Pest Sci. 2009;82: 305–309. 10.1007/s10340-009-0247-z [DOI] [Google Scholar]
- 81.Thurston G, Kaya H, Burlando T, Harrison R. Milky disease bacterium as a stressor to increase susceptibility of scarabaeid larvae to an entomopathogenic nematode. J Invertebr Pathol. 1993;61: 167–172. 10.1006/jipa.1993.1030 [DOI] [Google Scholar]
- 82.Thurston GS, Kaya HK, Gaugler R. Characterizing the enhanced susceptibility of milky disease-infected scarabaeid grubs to entomopathogenic nematodes. Biol Control. 1994;4: 67–73. 10.1006/bcon.1994.1012 [DOI] [Google Scholar]
- 83.Koppenhöfer AM, Kaya HK. Additive and synergistic Interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol Control. 1997;8: 131–137. 10.1006/bcon.1996.0498 [DOI] [Google Scholar]
- 84.Koppenhofer AM, Choo HY, Kaya HK, Lee DW, Gelernter WD. Increased field and greenhouse efficacy against scarab grubs with a combination of an entomopathogenic nematode and Bacillus thuringiensis. Biol Control. 1999;14: 37–44. 10.1006/bcon.1998.0663 [DOI] [Google Scholar]
- 85.Barbercheck M, Kaya H. Competitive interactions between entomopathogenic nematodes and Beuveria-bassiana (Deuteromycotina, Hyphomycetes) in soilborne larvae. Environ Entomol. 1991;20: 707–712. [Google Scholar]
- 86.Shapiro-Ilan DI, Jackson M, Reilly CC, Hotchkiss MW. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae). Biol Control. 2004;30: 119–126. 10.1016/j.biocontrol.2003.09.014 [DOI] [Google Scholar]
- 87.Barberchek ME, Kaya HK. Interactions between Beauveria bassiana and the entomogenous nematodes, Steinernema feltiae and Heterorhabditis heliothidis. J Invertebr Pathol. 1990;55: 225–234. 10.1016/0022-2011(90)90058-E [DOI] [Google Scholar]
- 88.Kaya HK, Koppenhofer AM. Effects of microbial and other antagonistic organism and competition on entomopathogenic nematodes. Biocontrol Sci Technol. 1996;6: 357–371. 10.1080/09583159631334 [DOI] [Google Scholar]
- 89.Ansari MA, Tirry L, Moens M. Interaction between Metarhizium anisopliae CLO 53 and entomopathogenic nematodes for the control of Hoplia philanthus. Biol Control. 2004;31: 172–180. 10.1016/j.biocontrol.2004.04.002 [DOI] [Google Scholar]
- 90.Acevedo JPM, Samuels RI, Machado IR, Dolinski C. Interactions between isolates of the entomopathogenic fungus Metarhizium anisopliae and the entomopathogenic nematode Heterorhabditis bacteriophora JPM4 during infection of the sugar cane borer Diatraea saccharalis (Lepidoptera: Pyralidae). J Invertebr Pathol. 2007;96: 187–192. [DOI] [PubMed] [Google Scholar]
- 91.Tarasco E, Santiago Alvarez C, Triggiani O, Quesada Moraga E. Laboratory studies on the competition for insect haemocoel between Beauveria bassiana and Steinernema ichnusae recovered in the same ecological niche. Biocontrol Sci Technol. 2011;21: 693–704. 10.1080/09583157.2011.570428 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.