Abstract
Early-onset familial Alzheimer’s disease (EOFAD) and late-onset sporadic AD (LOSAD) both follow a similar pathological and biochemical course that includes: neuron and synapse loss and dysfunction, microvascular damage, microgliosis, extracellular amyloid-β deposition (Aβ), and the deposition of phosphorylated tau protein in the form of intracellular neurofibrillary tangles in affected brain regions. Any mechanistic explanation of AD must accommodate these biochemical and neuropathological features for both forms of the disease. Cell cycle abnormalities represent another major biochemical and neuropathological feature common to both EOFAD and LOSAD, and 1) appear very early in the disease process, prior to the appearance of plaques and tangles, and 2) explain the biochemical (e.g., tau phosphorylation), neuropathological (e.g., neuron hypertrophy) and cognitive changes observed in EOFAD and LOSAD. Since neurogenesis after the formation of a memory is sufficient to induce forgetting, any stimulus that promotes cell cycle re-entry will be a negative event for memory. In this insight paper, we propose that aberrant re-entry of terminally differentiated, post-mitotic neurons into the cell cycle is a common pathway that explains both early and late-onset forms of AD. In the case of EOFAD, mutations in APP, PSEN1, and PSEN2 that alter AβPP and Notch processing drive reactivation of the cell cycle, while in LOSAD, age-related reproductive endocrine dyscrasia that upregulates mitogenic TNF signaling, AβPP processing toward the amyloidogenic pathway and tau phosphorylation drives reactivation of the cell cycle. Inhibition of cell cycle reentry of post-mitotic neurons may be a useful therapeutic strategy to prevent or halt disease progression.
Keywords: Alzheimer’s disease, amyloid-β protein precursor, Cdk-5, cell cycle reentry, cognition, endocrine dyscrasia, hypothalamic-pituitary-gonadal axis, luteinizing hormone, presenilin, tau
The neurodegenerative disorder of Alzheimer’s disease (AD) accounts for ~70% of all dementia cases [1, 2] and is characterized neurologically by progressive memory loss, impairments in behavior, language, and visuo-spatial skills ultimately leading to death [3]. AD is usually divided into two forms: (1) familial cases with Mendelian inheritance of predominantly early-onset (<60 years, early-onset familial AD [EOFAD]), and (2) late-onset cases with undefined genetics (≥60 years, late-onset AD [LOSAD]) [4]. The three clinically indistinguishable subtypes of EOFAD based on underlying genetics are: Alzheimer disease type 1 (AD1), caused by mutation of APP (10%–15% of EOFAD); Alzheimer disease type 3 (AD3), caused by mutation of PSEN1, (30%–70% of EOFAD); and Alzheimer disease type 4 (AD4), caused by mutation of PSEN2 (<5% of EOFAD). EOFAD comprises ~1–5% of the total AD population [5] and follows a more aggressive course with shorter relative survival time [6]. Neuropathologically, both EOFAD and LOSAD are characterized by neuron and synapse loss and dysfunction, microvascular damage, microgliosis (inflammation), the deposition of amyloid-β (Aβ) in extracellular amyloid (neuritic) plaques, and the deposition of phosphorylated tau protein in the form of intracellular neurofibrillary tangles (NFTs) in affected brain regions. EOFAD may have a more variable presentation, including posterior cortical atrophy, frontal variants, and linguistic presentations [7]. Furthermore, the pathology in EOFAD may be more severe with prominent synaptic fallout and neuronal loss [8]. EOFAD is also characterized by more severe perfusion and metabolic defects [9]. Although amnesic presentation is observed, it is less common [7].
Research over the last decade has suggested that cell cycle abnormalities also represent a major neuropathological feature for both EOFAD [10, 11] and LOSAD (e.g., [12–15]). These abnormalities appear very early in the disease process, prior to the appearance of plaques and tangles and can explain many of the biochemical and neuropathological changes observed [16]. Thus, neuronal cell cycle regulatory failure may be a significant component of the pathogenesis of AD [17].
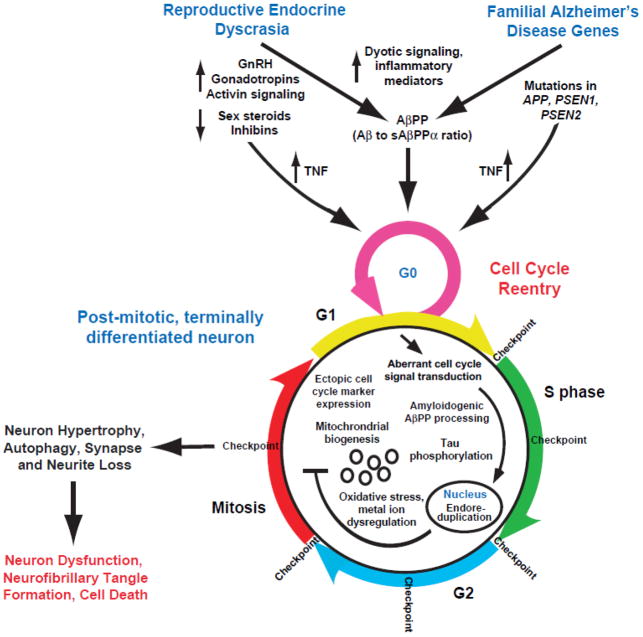
Although there are differences, EOFAD and LOSAD follow a similar pathological and biochemical disease course. Any mechanistic explanation of AD pathogenesis must therefore accommodate both forms of the disease. In this insight paper, we propose that aberrant reentry of post-mitotic neurons into the cell cycle explains both early and late-onset forms of AD; with aberrant cell cycle signaling being driven in EOFAD by mutations in APP, PSEN1, and PSEN2 and in LOSAD by age-related endocrine dyscrasia (Fig. 1).
Figure 1.
Endocrine dyscrasia and AD-related genetic mutations intersect at the cell cycle to drive neurodegeneration and cognitive decline in AD. Model of aberrant cell cycle reentry initiated by 1) aging-related endocrine dyscrasia leading to LOSAD and 2) genetic mutations in APP, PS1, and PS2 leading to EOFAD. Endocrine dyscrasia and genetic mutations of APP, PS1, and PS2 alter TNF and AβPP metabolism to reactivate the cell cycle in a post-mitotic, terminally differentiated neuron. This abortive cell cycle reentry drives neuron hypertrophy, autophagy, synapse and neuron loss, neuron dysfunction, amyloid deposition, neurofibrillary tangle formation, and ultimately cell death.
EVIDENCE FOR CELL CYCLE REENTRY OF POST-MITOTIC NEURONS IN THE ALZHEIMER’S DISEASE BRAIN
In this section we focus on the re-entry of post-mitotic, terminally differentiated neurons into a cell cycle. While neurogenesis is well known to proceed within the subventricular zone and dentate gyrus [18, 19] of the adult brain for the maintenance of brain structure and function, the evidence presented here is for terminally differentiated neurons resident in the hippocampus and cortical regions of the brain.
Aberrant cell cycle signaling in AD
Although numerous hypotheses have been postulated to explain AD, much evidence now exists that AD is a disease of aberrant, albeit unsuccessful, reentry of neurons into the cell cycle resulting in synapse and neurite contraction and neuron death ([14, 16, 20–41]; see [12–15] for reviews). The unscheduled initiation of a cell division cycle in a mature, normally post-mitotic neuron has been demonstrated to lead to an abortive re-activation of a variety of cell cycle components and ultimately the demise of the cell (Fig. 1). Neuronal changes supporting the involvement of cell cycle related events in the etiology of AD include:
Cell cycle markers
The ectopic expression of a number of cell cycle proteins have been reported in those regions of the brain affected by AD (e.g., cyclin B1, cdc2 kinase, PCNA, cdk4, p16, but not cyclins A and D), but not in areas unaffected by AD pathology or in control brains [20, 21, 42]. As might be expected of a process that is central to the etiology of neurodegeneration, changes in cell cycle markers have been found early in the disease process in those individuals with mild cognitive impairment (MCI) [42]. These researchers found markedly increased numbers of neurons immunopositive for cyclin D (5.2% and 6.3%) and PCNA (7.0% and 7.1%) in MCI and AD, respectively, compared with controls (0.4% and 0.4%, respectively). A number of the cell cycle regulators have been detected in vulnerable neurons before lesion formation [43, 44]. Progression into the cell cycle as assessed by the expression of different cell cycle markers appears to be dependent upon AD severity; neurons positive for NFTs stain strongly for cdc2 kinase (cdk1) and its associated cyclin B1 in hippocampal regions of the AD brain, suggesting that in some cases the G1/S checkpoint has been bypassed and that the cell cycle is arrested at G2. These findings led Herrup and colleagues [42] to suggest that both the mechanism of cell loss (a cell cycle-induced death) and the rate of cell loss (a slow atrophy over months) are identical at all stages of the disease process.
Chromosome replication (endoreduplication)
The most compelling evidence that differentiated neurons reenter the cell cycle comes from Yang, Herrup, and colleagues [22] who demonstrated that a significant number of neurons in affected regions of AD brain (hippocampal pyramidal and basal forebrain neurons) have undergone full or partial DNA replication, suggesting certain neurons have completed S phase. Cells in unaffected regions of the AD brain or in the hippocampus of nondemented age-matched controls show no such anomalies. Therefore, AD neurons appear to complete a nearly full S phase, but because mitosis is not initiated, the cells remain polyploid. This genetic imbalance seems to persist for many months before the neurons die [22, 42] and this genomic replication without cytokinesis (endoreduplication) will have dramatic implications for the overexpression of neuronal proteins such as amyloid-β protein precursor (AβPP), as is the case with Down syndrome [45] and AD (see below). Endoreduplication in plants is a well-described phenomenon that allows for sufficient protein synthesis [46]. However, such overexpression in a normally differentiated cell population appears to promote neuron death.
Neuronal hypertrophy
DNA content is almost invariably associated with the size of a cell [47]. In this respect, hypertrophy of neuronal cell bodies, nuclei, and nucleoli of CA1 of hippocampus and anterior cingulate gyrus neurons has been reported in asymptomatic AD and MCI subjects [48–51].
Mitochondrial alterations
Changes suggestive of mitochondria replication have been reported in those neurons vulnerable to AD neuropathology [23]. Pyramidal neurons of the AD brain contain 3-fold elevated levels of cytoplasmic mitochondrial DNA and increased Cox-1 expression, indicative of de novo mitochondrion synthesis that would be expected during cell division to meet the energy demands of the newly created daughter cells [24]. Unlike division competent neurons, it remains to be determined if such alterations in mitochondrial metabolism in differentiated neurons are responsible for an imbalance in energy metabolism observed in the AD brain.
Tau phosphorylation and NFT formation
Phosphorylation of the microtubule-associated protein tau occurs during metaphase of neuronal division, and during differentiation [25, 26], hyperphosphorylated tau is observed in neurons of the fetal brain [25]. Disassembly of the rigid microtubule structure of neurons for neuronal division is accomplished by removing the microtubule stabilizing protein tau, by its phosphorylation. Therefore, it is interesting that hyperphosphorylation of the microtubule-associated protein tau, as detected in NFT, is one of the major pathological features of neuronal degeneration in AD [52], and indicates attempted division of pyramidal neurons in AD.
Mitotic signal transduction pathways
Signal transduction pathways, regulated by a variety of mitogens and growth factors, are upregulated in the AD brain [27]. Mitogen-activated protein kinases such as ERK and other signal transduction and transcription activators, such as Janus kinase and phosphoinositol 3-kinase/Akt [28], play a major role in the entry of cells into the cell cycle, as well as controlling their progression throughout the various stages [29]. These pathways are associated with AD neuropathology [30].
AD neurons proceed to metaphase and then arrest?
The above data suggest that most all the biochemical and pathological changes associated with AD can be explained by the aberrant reentry of terminally differentiated neurons into the cell cycle (e.g., chromosomal replication leading to polyploidy, upregulation of cell cycle markers, tau phosphorylation, AβPP metabolism and Aβ deposition, neuronal hypertrophy, oxidative stress, increased mitochondrial DNA and Cox-1 expression, upregulated growth factor signaling pathways, synapse loss, and death of differentiated neurons) [12, 53]. Two studies support this claim. Forced cell cycle activation in terminally differentiated neurons via conditional expression of the simian virus 40 large T antigen (oncogene) forms Aβ deposits and tau pathology in the mouse cortex [54]. Similarly, forced cell cycle activation in primary neurons is accompanied by tau phosphorylation [55]. Not surprisingly, the AD brain displays many of the neuropathological and biochemical changes observed in the rapidly growing and differentiating fetal brain, namely the presence of Aβ [56], hyperphosphorylated tau [25], and presenilin expression [57]. It is unclear whether neurons in the AD brain are proceeding via the normal cell cycle division pathway, or an aberrant, uncoordinated, pathway. In this connection, the failure of microtubules to form spindle fibers to attach to kinetochores [58, 59] has been shown to arrest the cell in metaphase (M checkpoint). Likewise, improper alignment of the spindle will block cytokinesis; either of these processes if irreparable triggers neuron death.
EVIDENCE THAT CELL CYCLE REENTRY INDUCES MEMORY LOSS
It has been demonstrated that neurogenesis in the adult brain is linked to memory loss [60]. Increasing neurogenesis after the formation of a memory was sufficient to induce forgetting in adult mice. However, in contrast, during infancy, when hippocampal neurogenesis levels are high and freshly generated memories tend to be rapidly forgotten (infantile amnesia), decreasing neurogenesis after memory formation mitigated forgetting. This is supported by studies in precocial species, including guinea pigs and degus, where most granule cells are generated prenatally [60]. Consistent with reduced levels of postnatal hippocampal neurogenesis, infant guinea pigs and degus do not exhibit forgetting. However, increasing neurogenesis after memory formation induced infantile amnesia in these species [60].
EVIDENCE THAT THE DIFFERENTIAL REGULATION OF AβPP PROCESSING REGULATES CELL CYCLE REENTRY AND NEURODEGENERATION
Mutations associated with EOFAD drive AβPP processing and cell cycle reentry
AβPP, PS1, and PS2 are developmental proteins [57, 61–63]. It is therefore not surprising that mutations in these genes might impact cell cycle dynamics.
The overexpression of FAD mutant AβPP, which promotes Aβ generation [64], has been shown to promote the aberrant reentry of primary neurons into the cell cycle, as demonstrated by the initial induction of DNA synthesis and cell cycle marker expression, followed by apoptotic cell death [65]. Recently it has been shown that Aβ signaling through tau is necessary to drive ectopic neuronal cell cycle reentry in mouse primary neurons and in an AβPP-transgenic (hAPPJ20) mouse [66]. Overexpression of FAD mutants of AβPP [67–69] has been shown to induce apoptosis in both primary neurons and cell lines. The expression of the Swedish double mutations of AβPP or the AβPP intracellular domain, into nerve growth factor differentiated PC12 cells or rat primary cortical neurons reactivates the cell cycle by upregulating cyclins D1 and B1 [70]. Elevations in cyclins D1 and B1 expression are observed in the brains of Tg2576 mice harboring the Swe-AβPP (AβPPsw+) mutations.
Like FAD mutant AβPP, presenilin-1 FAD mutations also increase Aβ production [71] and induce cell cycle abnormalities in mitotically competent cells [72–75]. Mice expressing the knock-in presenilin-1 mutation M146V (presenilin-1 KIM146V) display accelerated entry of cortical neurons into the cell cycle as determined by accumulation of cyclin D1 and phosphoretinoblastoma proteins, and by an increase in BrdU incorporation rates [76]. These neurons become arrested at S phase or underwent apoptosis, a response that was blocked by downregulating cyclin D1 or inhibiting the cell cycle with quercetin, but not by γ-secretase inhibition. The results of γ-secretase inhibition in this study are difficult to interpret given the non-specificity of action of such inhibitors. In this regard, presenilin-1 mutations have been proposed to affect γ-secretase processing of Notch, accounting for impairments in self-renewal and altered differentiation toward neuronal lineages in subventricular zone neural progenitor cells expressing the FAD-linked presenilin-1 DeltaE9 variant [75]. Given that presenilin-1 is one component of the γ-secretase complex that processes AβPP and Notch, and that presenilin-1 mutations promote Aβ production [77], these results suggest that presenilin-1 mutations also may induce cell cycle abnormalities via alterations in the processing of AβPP or Notch.
Overexpression of PS1 and PS2, mutations of these proteins: PS1(P117L), PS1(P267S), PS1(E280A), PS2(N141I), and the carboxyl-terminally deleted PS2 construct PS2(166aa) in HeLa cells arrests the cells in the G1 phase of the cell cycle [78, 79]. The highly pathogenic AD PS1 (P117R) mutation, but not other PS1 mutations, causes a specific increase in key G1/S phase regulatory proteins, p53, and its effector p21, causing G1 phase prolongation with simultaneous S phase shortening, and lowering basal apoptosis in human lymphocytes [10]. Lymphocytes from AD patients have been demonstrated to show an enhanced rate of proliferation and increased phosphorylation of the retinoblastoma protein and other members of the family of pocket proteins compared with cell lines derived from normal age-matched controls [80]. Changes in these cell cycle proteins in lymphocytes have been proposed as a potential biomarker for the diagnosis of AD [10, 80–84].
That AβPP is directly involved in cell cycle signaling and neurogenesis has recently been reviewed (Atwood and Bowen, Hormones and Behavior, in submission). Briefly, it has been shown that the differential processing of AβPP regulates the proliferation and differentiation of human embryonic stem cells (hESC); Aβ promotes hESC proliferation while sAβPPα drives hESCs toward a neuronal precursor cell phenotype [85, 86]. In differentiated neurons, Aβ1–42 promotes neurogenesis of subventricular zone precursor cells derived from developing or young adult animals [26, 87–92]. In vivo, an increase in hippocampal neurogenesis and/or proliferation has been reported in younger transgenic mouse models overexpressing AβPP mutations [88, 93–97]. The impact of other AβPP mutations that lead to cerebral amyloid angiopathy and stroke (e.g., Dutch and Iowa mutations) on cell cycle reentry has not been explored.
Age-related endocrine dyscrasia drives AβPP processing and cell cycle reentry
We and others have demonstrated that the endocrine dyscrasia following menopause and during andropause (suppressed sex steroid and inhibin signaling, elevated gonadotropin-releasing hormone 1 (GnRH1), luteinizing hormone (LH), follicle-stimulating hormone, and activin signaling) regulates AβPP processing pathways both in vivo and in vitro [98]. LH/human chorionic gonadotropin (hCG), when in balance with other hormones of the HPG axis, is a well-known mitogenic signal that drives normal cell proliferation and differentiation (reviewed in [18, 85, 99, 100]). However, when the ratio of LH:sex steroids increases, the differentiation signal is lost as AβPP processing toward the amyloidgenic pathway drives cell cycle reentry without the requisite differentiation signal to allow for completion of the cell cycle [101–108]. The increase in circulating LH at menopause and during andropause corresponds to the time when increases in Aβ [109] and cell cycle reactivation are observed during MCI [110]. In human studies, multiple linear regression analysis reveals that serum LH concentration, but not testosterone, significantly correlates with plasma Aβ levels in men [111, 112], suggesting that increased serum LH concentration is associated with the accumulation of Aβ in plasma.
Reproductive endocrine dyscrasia may act via the regulation of inflammatory cytokines. Gonadotropins likely regulate AβPP processing and Aβ generation and deposition via tumor necrosis factor (TNF) (see [113, 114] for reviews), a master inflammatory cytokine, and which is known to alter cell cycle dynamics [115]. LH, which is elevated in men with AD, is positively correlated with TNF [116].
LH also has been shown to regulate tau phosphorylation in vitro [117] and in vivo [108]. Genetic ablation of LHCGR in APPsw+ mice decreased tau phosphorylation by ~50% that induced by AβPP overexpression in these mice [108]. The residue-specific phosphorylation of tau in mitotically active neurons is driven by cyclin-dependent kinases (Cdk; [25, 118–121]. Several Cdks are associated with phosphorylated tau in AD and in vitro phosphorylate tau in a manner similar to that found in AD [122–125]. A number of other kinases such as glycogen synthase kinase-3β (GSK3β) also are pivotal in tau phosphorylation (e.g., [126]). Compelling evidence that reactivation of the cell cycle induces tau phosphorylation is provided by two studies: McShea and colleagues [55] demonstrate that cell cycle induction in vitro induces tau phosphorylation, while Park and colleagues [54] demonstrate that cell cycle induction in vivo induces NFT and amyloid deposits. More recently, it has been demonstrated that specific phosphorylation of tau (Thr231) can promote MAPK activation in PC12 cells, which in turn could (further) activate the cell cycle reentry mechanisms in neurons [127, 128].
In reproductive tissues, LH has been shown to increase both the expression and kinase activity of Cdk5 in Leydig TM3 cells [129]. Supporting LH-induced Cdk5 metabolism, a significant decrease in Cdk5 expression and activity has been noted in rat testis after hypophysectomy [129]. These LH-mediated changes in Cdk5 metabolism are important in the context of reports that Cdk5 is a potent cell cycle suppressor [130, 131]. In particular, although Cdk5 is normally located in both nucleus and cytoplasm [131–135], the loss of nuclear Cdk5 leads to a failure of cell cycle suppression both in vivo and in vitro. Cell cycle activity detected in Cdk5−/− neurons includes the abnormal expression of cell cycle proteins such as cyclin D, cyclin A, and PCNA (proliferating cell nuclear antigen) as well as 5-bromo-2-deoxyuridine incorporation [130]. Similar cell cycle events are found in neurons at risk for death in AD [43, 136]. In post-mitotic neurons in culture, Cdk5 nuclear export is required for cell cycle reentry [137]. Cell cycle suppression by Cdk5 requires its binding to the p35 activator protein and E2F1. Formation of this complex excludes the E2F1 cofactor, DP1, thus inhibiting E2F1 binding to the promoters of various cell cycle genes. In this way, the formation of the E2F1–Cdk5–p35 complex in the nucleus prevents the advance of the cell cycle and appears to be a neuroprotective function of Cdk5 [138]. The Cdk5 activator protein p25, however, preferentially binds GSK3β which leads to enhanced phosphorylation of tau, but decreased phosphorylation of β-catenin. Coexpression of GSK3β and p25 in cultured neurons results in a neurodegeneration phenotype [139]. LH/hCG also regulates the phosphorylation of microtubule-associated proteins and GSK3β phosphorylation [140].
LH induced changes in both AβPP metabolism and Cdk5 metabolism (and concurrent tau phosphorylation) may be required for cell cycle reentry. While both Aβ and P301L tau expression independently affect the regulation of cell proliferation and synaptic elements, cell cycle reentry as assessed by DNA synthesis is only observed when SH-SY5Y cells overexpressing human wild-type or P301L tau were incubated with Aβ [141]. Similarly, studies using differentiated neurons exposed to Aβ exhibit Cdk5-mediated tau hyperphosphorylation, cell cycle reentry, and neuronal loss [66, 142, 143]. Inhibition of Cdk5 activity or tau phosphorylation (reviewed in [26]) prevents Aβ-mediated cell death. In vivo, icv-injection of mice with Aβ activates Cdk5, promoting tau phosphorylation, cell cycle induction, synaptotoxicity, and death of post-mitotic neurons [143–145]. The sex hormone-mediated changes in AβPP and Cdk5 metabolism (see above) may therefore be responsible for inducing cell cycle reactivation and apoptotic death of post-mitotic neurons. Similar cell cycle changes were not observed in the 3xTg-AD model [146], although the hormonal status of these young mice is different to the hormonal status of older mice.
Advanced glycation end-products also have been demonstrated to be mitogenic signals that trigger cell cycle reentry of neurons in a mouse model of neurodegeneration [147]. Sex hormones are known to regulate advanced glycation end-product levels [148, 149], although whether age-related endocrine dyscrasia alters advanced glycation end-product formation/degradation remains to be determined. Likewise, it is not known whether advanced glycation end-product-induced cell cycle reentry is mediated via Aβ and tau metabolism.
EVIDENCE THAT EOFAD MUTATIONS AND ENDOCRINE DYSCRASIA INDUCE COGNITIVE DECLINE
Mutations in APP, PSEN1, and PSEN2 induce EOFAD (Fig. 1) [150–154]. Similarly, epidemiological and clinical evidence supports endocrine dyscrasia (particularly high LH:sex steroids) as regulating cognitive health and AD (Fig. 1), and includes: 1) the abrupt cognitive deficits observed in premenopausal women following chemical castration (with GnRH agonists), deficits that are reversible with simultaneous administration of 17β-estradiol [155]; 2) the increased risk of dementia in premenopausal women who have had a bilateral oophorectomy [156]; 3) improvement in cognitive performance of cognitively healthy postmenopausal women taking 17β-estradiol in 12 separate trials [157]; 4) the increased prevalence of cognitive disease in women, which correlates with the abrupt earlier loss of gonadal function [158–163]; 5) the negative correlation between serum 17β-estradiol in women [164] and testosterone in men [165, 166] with AD, but positive correlation between serum gonadotropins in men and women with AD [116, 165–171]; 6) the 50% decrease in the prevalence of AD following treatment with GnRH agonists (gonadotropin-lowering drug Lupron Depot®) [172, 173] (Beaird, Bowen, Perry, Atwood et al., unpublished data); 7) the significant improvement in memory in men with prostate cancer treated with GnRH1 agonists after 6 to 12 months [174], and 8) the stabilization of cognitive performance in women with mild to moderate AD taking acetylcholine esterase inhibitors and a GnRH1 agonist over a 48-week period [175]. The loss of cell cycle control also has been demonstrated in another neurodegenerative condition, ataxia-telangiectasia, and likely represents a common disease mechanism that underlies various (neurodegenerative) diseases and conditions in humans and animals [176–178].
Therapeutic strategies targeting the cell cycle
Therapeutics that target aberrant cell cycle reentry are therefore most likely to succeed in preventing or reversing the neurodegeneration associated with EOFD and LOSAD. One approach has involved the rebalancing of reproductive hormones after menopause and during andropause. Although hormone replacement therapy with either 17β-estradiol or testosterone only elicits partial rebalancing of the HPG axis, these physiologically relevant sex steroids have been shown to decrease the incidence and delay the onset of cognitive decline among elderly women and men (reviewed in [157, 179, 180]). Indeed, an improvement in cognition has been reported in women with AD treated with 17β-estradiol in three controlled [181–183] intervention studies, and in men with AD treated with testosterone in two controlled intervention studies [179, 180]. Moreover, 17β-estradiol has been shown to improve the cognition of cognitively normal post-menopausal women in 12 of 15 studies (three studies indicated no difference) [157].
Another strategy to rebalancing the HPG axis to prevent aberrant cell cycle activation has involved the use of the GnRH superagonist leuprolide acetate (Lupon Depot). Leuprolide acetate acts to suppress gonadotropins and sex steroids, thereby rebalancing the ratios of these hormones, albeit at lower concentrations. Leuprolide acetate also only partially rebalances the axis as it does not greatly impact other hormones of the HPG axis. A Phase II dose ranging study performed in 2003/2004, although only recently published [175], demonstrated that the combination of leuprolide acetate and an acetylcholinesterase inhibitor (AChEI) was both safe and efficacious in the treatment of women with mild to moderate AD. Sub-group analysis of cognitive performance in women with mild to moderate AD taking AChEIs and implanted subcutaneously at 0, 12, 24, and 36 weeks with high-dose (22.5 mg) leuprolide acetate showed a stabilization in cognitive decline (ADAS-Cog, ADCS-CGIC) and activities of daily living (ADCS-ADL) over a 48 week period. A similar effect was not observed in the low dose Lupron group taking AChEIs, or in the placebo group taking AChEIs, or when patients were treated with Lupron alone. A possible mechanism of action for this combination therapy is that Lupron acts to halt any further cell cycle-related neurodegeneration and suppress neuroinflammation thereby allowing AChEIs to act on remaining neurons to maintain cholinergic function [175]. A small study has confirmed these results, demonstrating that suppression of androgens and gonadotropins with GnRH1 agonists in men with prostate cancer significantly improved visual-memory (Rey test) 6 and 12 months, and significantly improved inversed number-memory test (WAIS) after 6 months [174]. Thus, global cognitive performances were preserved after 12 months of androgen and gonadotropin suppression.
Preclinical studies have demonstrated the efficacy of increasing sex steroids and suppressing gonadotropins in reversing neurodegeneration and cognitive decline in animal models of AD (reviewed in [98, 184]). Preclinical studies in mouse models of AD also have demonstrated that specific inhibitors of the cell cycle, such as the oral administration of the synthetic retinoid X receptor-selective retinoid bexarotene, stimulates the rapid reversal of cognitive, social, and olfactory deficits, improves neural circuit function and enhances the clearance of soluble Aβ within hours in an apolipoprotein E-dependent manner [185, 186].
Non-steroidal anti-inflammatory drugs (NSAIDs) also have been shown to prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of AD [187]. Inflammation in AD is likely driven by the neurodegeneration induced by excessive Aβ deposition and cell cycle reentry in the case of EOFAD, and by the neurodegeneration induced by excessive Aβ deposition and cell cycle reentry driven by reproductive endocrine dyscrasia in the case of LOSAD [113, 114]. In the study of Herrup and colleagues, inflammation promoted by LPS at young ages in R1.40 mice induced the early appearance of cell cycle reactivation, whereas treatment with two different NSAIDs blocked neuronal cell cycle activation and alterations in brain microglia without altering AβPP processing and steady-state Aβ levels [187]. Since retrospective human epidemiological studies, but not prospective clinical trials, have identified long-term use of NSAIDs as protective against AD, these authors have suggested that NSAID use in human AD may need to be initiated as early as possible to prevent disease progression.
SUMMARY
Aberrant cell cycle reactivation in neurons is a common pathway that can explain familial AD, late-onset AD, Down syndrome, and other neurological diseases. In the case of EOFAD, mutations in APP, PSEN1, and PSEN2 that alter AβPP and Notch processing drive reactivation of the cell cycle [11, 66], while in LOSAD, endocrine dyscrasia that drives AβPP processing towards the amyloidogenic pathway and tau phosphorylation (via TNF) [113, 114] drive reactivation of the cell cycle in mature post-mitotic neurons [101]. Since neurogenesis after the formation of a memory has been shown to be sufficient to induce forgetting [60], any stimulus that promotes cell cycle reentry of post-mitotic neurons appears to be a negative event for memory. In this connection, since hormones of the HPG axis regulate fetal and adult neurogenesis, any disruption in their signaling such as occurs following menopause and during andropause leads to dyotic signaling that drives aberrant reentry of neurons into the cell cycle would therefore result in the loss of previously encoded memories and prevent the retention of new information as memories. Similarly, EOFAD mutations that drive cell proliferation are going to limit the capacity of the brain to store memories. The relationship between these mutations and endocrine dyscrasia with aging between 30 and 60 warrants exploration.
It is hoped that this understanding of the mechanisms driving cell cycle reentry of neurons can be used to develop appropriate therapies for this devastating disease. Upstream targets are more likely to hold promise in preventing or halting the progression of AD. This is becoming evident from the failure of numerous clinical trials aimed at decreasing brain Aβ concentration and aggregation, since downstream targets such as Aβ have multiple functions [188] and the non-discrepant removal of all forms of Aβ results in serious side-effects. This is becoming more evident from our increased understanding of the roles of AβPP in cell cycle events, which indicates that the appropriate regulation of AβPP cleavage products is required for normal neuron survival. As a result, drugs that prevent cell cycle reentry of vulnerable post-mitotic neurons in the brain may not be effective unless they are specific. The inhibition of cell cycle reentry of neurons is a therapeutic target that could be achieved by the rebalancing of the reproductive and stress hormone axes in the case of LOSAD, and by clustered, regularly interspaced, short palindromic repeat (CRISPR) genome editing in the case of EOFAD, particularly in conjunction with in vitro fertilization techniques, for those individuals carrying AβPP, PS1, or PS2 mutations.
Acknowledgments
This material is the result of work supported with resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The opinions expressed herein are those of the authors. The contents do not represent the views of the Department of Veterans Affairs or the US government. This article is Geriatrics Research, Education and Clinical Center VA paper 2015–011.
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/14-3210r2).
References
- 1.Cotter VT. The burden of dementia. Am J Manag Care. 2007;13(Suppl 8):S193–197. [PubMed] [Google Scholar]
- 2.Alzheimer’s-Association. Alzheimer’s Disease Facts and Figures 2007. 2007. [Google Scholar]
- 3.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 4.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Bird TD. Early-Onset Familial Alzheimer Disease. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 6.Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol. 1983;40:143–146. doi: 10.1001/archneur.1983.04050030037006. [DOI] [PubMed] [Google Scholar]
- 7.Balasa M, Gelpi E, Antonell A, Rey MJ, Sanchez-Valle R, Molinuevo JL, Llado A. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76:1720–1725. doi: 10.1212/WNL.0b013e31821a44dd. [DOI] [PubMed] [Google Scholar]
- 8.Nochlin D, van Belle G, Bird TD, Sumi SM. Comparison of the severity of neuropathologic changes in familial and sporadic Alzheimer’s disease. Alz Dis Assoc Dis. 1993;7:212–222. [PubMed] [Google Scholar]
- 9.Yasuno F, Imamura T, Hirono N, Ishii K, Sasaki M, Ikejiri Y, Hashimoto M, Shimomura T, Yamashita H, Mori E. Age at onset and regional cerebral glucose metabolism in Alzheimer’s disease. Dem Ger Cogn Dis. 1998;9:63–67. doi: 10.1159/000017024. [DOI] [PubMed] [Google Scholar]
- 10.Bialopiotrowicz E, Szybinska A, Kuzniewska B, Buizza L, Uberti D, Kuznicki J, Wojda U. Highly pathogenic Alzheimer’s disease presenilin 1 P117R mutation causes a specific increase in p53 and p21 protein levels and cell cycle dysregulation in human lymphocytes. J Alzheimers Dis. 2012;32:397–415. doi: 10.3233/JAD-2012-121129. [DOI] [PubMed] [Google Scholar]
- 11.McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, Kozlowski MR, Neve KA, Neve RL. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. Journal Neurosci. 2003;23:6914–6927. doi: 10.1523/JNEUROSCI.23-17-06914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- 13.Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J Neurosci Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Bu B, Xie M, Zhang M, Yu Z, Tao D. Neural cell cycle dysregulation and central nervous system diseases. Prog Neurobiol. 2009;89:1–17. doi: 10.1016/j.pneurobio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neve RL, McPhie DL. Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis. Biochim Biophys Acta. 2007;1772:430–437. doi: 10.1016/j.bbadis.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nature neuroscience. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 19.Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod. 2009;80:1146–1151. doi: 10.1095/biolreprod.108.075341. [DOI] [PubMed] [Google Scholar]
- 20.Smith CJ, Anderton BH, Davis DR, Gallo JM. Tau isoform expression and phosphorylation state during differentiation of cultured neuronal cells. FEBS Lett. 1995;375:243–248. doi: 10.1016/0014-5793(95)01221-y. [DOI] [PubMed] [Google Scholar]
- 21.Vincent TS, Hazen-Martin DJ, Garvin AJ. Inhibition of insulin like growth factor II autocrine growth of Wilms’ tumor by suramin in vitro and in vivo. Cancer Lett. 1996;103:49–56. doi: 10.1016/0304-3835(96)04186-9. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropath Exper Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 25.Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Perry G, Chan HW, Verdile G, Martins RN, Smith MA, Atwood CS. Amyloid-beta-induced toxicity of primary neurons is dependent upon differentiation-associated increases in tau and cyclin-dependent kinase 5 expression. J Neurochem. 2004;88:554–563. doi: 10.1046/j.1471-4159.2003.02196.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu XF, Liu ZC, Xie BF, Li ZM, Feng GK, Yang D, Zeng YX. EGFR tyrosine kinase inhibitor AG1478 inhibits cell proliferation and arrests cell cycle in nasopharyngeal carcinoma cells. Cancer Lett. 2001;169:27–32. doi: 10.1016/s0304-3835(01)00547-x. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJ. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 29.Rubinfeld H, Seger R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol Biol. 2004;250:1–28. doi: 10.1385/1-59259-671-1:1. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Raina AK, Boux H, Simmons ZL, Takeda A, Smith MA. Activation of oncogenic pathways in degenerating neurons in Alzheimer disease. Int J Dev Neurosci. 2000;18:433–437. doi: 10.1016/s0736-5748(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 31.Su B, Wang X, Bonda D, Perry G, Smith M, Zhu X. Abnormal Mitochondrial Dynamics-A Novel Therapeutic Target for Alzheimer’s Disease? Mol Neurobiol. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonda DJ, Evans TA, Santocanale C, Llosa JC, Vina J, Bajic VP, Castellani RJ, Siedlak SL, Perry G, Smith MA, Lee HG. Evidence for the Progression through S-phase in the Ectopic Cell Cycle Re-entry of Neurons in Alzheimer Disease. Aging. 2009;1:382–388. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakur A, Siedlak SL, James SL, Bonda DJ, Rao A, Webber KM, Camins A, Pallas M, Casadesus G, Lee HG, Bowser R, Raina AK, Perry G, Smith MA, Zhu X. Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. Int J Clin Exp Pathol. 2008;1:134–146. [PMC free article] [PubMed] [Google Scholar]
- 34.Granic A, Padmanabhan J, Norden M, Potter H. Alzheimer A{beta} Peptide Induces Chromosome Mis-Segregation and Aneuploidy, Including Trisomy 21; Requirement for Tau and APP. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boeras DI, Granic A, Padmanabhan J, Crespo NC, Rojiani AM, Potter H. Alzheimer’s presenilin 1 causes chromosome missegregation and aneuploidy. Neurobiol Aging. 2008;29:319–328. doi: 10.1016/j.neurobiolaging.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geller LN, Potter H. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer’s disease. Neurobiol Dis. 1999;6:167–179. doi: 10.1006/nbdi.1999.0236. [DOI] [PubMed] [Google Scholar]
- 37.Arendt T. Dysregulation of neuronal differentiation and cell cycle control in Alzheimer’s disease. J Neural Transm Suppl. 2002:77–85. doi: 10.1007/978-3-7091-6139-5_8. [DOI] [PubMed] [Google Scholar]
- 38.Bowser R, Smith MA. Cell cycle proteins in Alzheimer’s disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–254. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- 39.Bonda DJ, Bajic VP, Spremo-Potparevic B, Casadesus G, Zhu X, Smith MA, Lee HG. Cell Cycle Aberrations and Neurodegeneration: A Review. Neuropathol Appl Neurobiol. doi: 10.1111/j.1365-2990.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Abeta oligomers induce neuronal cell cycle events in Alzheimer’s disease. J Neurosci. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy Z. The dysregulation of the cell cycle and the diagnosis of Alzheimer’s disease. Biochim Biophys Acta. 2007;1772:402–408. doi: 10.1016/j.bbadis.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging. 1998;19:287–296. doi: 10.1016/s0197-4580(98)00071-2. [DOI] [PubMed] [Google Scholar]
- 45.Beyreuther K, Pollwein P, Multhaup G, Monning U, Konig G, Dyrks T, Schubert W, Masters CL. Regulation and expression of the Alzheimer’s beta/A4 amyloid protein precursor in health, disease, and Down’s syndrome. Ann N Y Acad Sci. 1993;695:91–102. doi: 10.1111/j.1749-6632.1993.tb23035.x. [DOI] [PubMed] [Google Scholar]
- 46.Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo YM, Liu Y. Investigating the hows and whys of DNA endoreduplication. J Exp Bot. 2001;52:183–192. [PubMed] [Google Scholar]
- 47.Olmo E. Nucleotype and cell size in vertebrates: a review. Basic Appl Histochem. 1983;27:227–256. [PubMed] [Google Scholar]
- 48.Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exper Neurol. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73:665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alz Dis. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iqbal K, del Alonso AC, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Vadakkadath Meethal S, Atwood CS. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62:257–270. doi: 10.1007/s00018-004-4381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci. 2007;27:2969–2978. doi: 10.1523/JNEUROSCI.0186-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Takashima S, Kuruta H, Mito T, Nishizawa M, Kunishita T, Tabira T. Developmental and aging changes in the expression patterns of beta-amyloid in the brains of normal and Down syndrome cases. Brain Dev. 1990;12:367–371. doi: 10.1016/s0387-7604(12)80066-0. [DOI] [PubMed] [Google Scholar]
- 57.Berezovska O, Xia MQ, Page K, Wasco W, Tanzi RE, Hyman BT. Developmental regulation of presenilin mRNA expression parallels notch expression. J Neuropathol Exp Neurol. 1997;56:40–44. doi: 10.1097/00005072-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- 59.Chan GK, Yen TJ. The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog Cell Cycle Res. 2003;5:431–439. [PubMed] [Google Scholar]
- 60.Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 61.Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS. Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. BBRC. 2007;364:522–527. doi: 10.1016/j.bbrc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. J Biol Chem. 2011;286:24264–24274. doi: 10.1074/jbc.M111.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 65.McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, Kozlowski MR, Neve KA, Neve RL. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J Neurosci. 2003;23:6914–6927. doi: 10.1523/JNEUROSCI.23-17-06914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, Roberson ED, Bloom GS. Amyloid-beta signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J Cell Sci. 2013;126:1278–1286. doi: 10.1242/jcs.1125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McPhie DL, Golde T, Eckman CB, Yager D, Brant JB, Neve RL. beta-Secretase cleavage of the amyloid precursor protein mediates neuronal apoptosis caused by familial Alzheimer’s disease mutations. Brain Res Mol Brain Res. 2001;97:103–113. doi: 10.1016/s0169-328x(01)00294-7. [DOI] [PubMed] [Google Scholar]
- 68.Yamatsuji T, Matsui T, Okamoto T, Komatsuzaki K, Takeda S, Fukumoto H, Iwatsubo T, Suzuki N, Asami-Odaka A, Ireland S, Kinane TB, Giambarella U, Nishimoto I. G protein-mediated neuronal DNA fragmentation induced by familial Alzheimer’s disease-associated mutants of APP. Science. 1996;272:1349–1352. doi: 10.1126/science.272.5266.1349. [DOI] [PubMed] [Google Scholar]
- 69.Yamatsuji T, Okamoto T, Takeda S, Murayama Y, Tanaka N, Nishimoto I. Expression of V642 APP mutant causes cellular apoptosis as Alzheimer trait-linked phenotype. Embo J. 1996;15:498–509. [PMC free article] [PubMed] [Google Scholar]
- 70.Ahn KW, Joo Y, Choi Y, Kim M, Lee SH, Cha SH, Suh YH, Kim HS. Swedish amyloid precursor protein mutation increases cell cycle-related proteins in vitro and in vivo. J Neurosci Res. 2008;86:2476–2487. doi: 10.1002/jnr.21690. [DOI] [PubMed] [Google Scholar]
- 71.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 72.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer’s disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veeraraghavalu K, Choi SH, Zhang X, Sisodia SS. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J Neurosci. 30:6903–6915. doi: 10.1523/JNEUROSCI.0527-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malik B, Currais A, Andres A, Towlson C, Pitsi D, Nunes A, Niblock M, Cooper J, Hortobagyi T, Soriano S. Loss of neuronal cell cycle control as a mechanism of neurodegeneration in the presenilin-1 Alzheimer’s disease brain. Cell Cycle. 2008;7:637–646. doi: 10.4161/cc.7.5.5427. [DOI] [PubMed] [Google Scholar]
- 77.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 78.Janicki SM, Monteiro MJ. Presenilin overexpression arrests cells in the G1 phase of the cell cycle. Arrest potentiated by the Alzheimer’s disease PS2(N141I)mutant. The Am J Path. 1999;155:135–144. doi: 10.1016/S0002-9440(10)65108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janicki SM, Stabler SM, Monteiro MJ. Familial Alzheimer’s disease presenilin-1 mutants potentiate cell cycle arrest. Neurobiol Aging. 2000;21:829–836. doi: 10.1016/s0197-4580(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 80.de las Cuevas N, Urcelay E, Hermida OG, Saiz-Diaz RA, Bermejo F, Ayuso MS, Martin-Requero A. Ca2+/calmodulin-dependent modulation of cell cycle elements pRb and p27kip1 involved in the enhanced proliferation of lymphoblasts from patients with Alzheimer dementia. Neurobiol Dis. 2003;13:254–263. doi: 10.1016/s0969-9961(03)00040-8. [DOI] [PubMed] [Google Scholar]
- 81.Bialopiotrowicz E, Kuzniewska B, Kachamakova-Trojanowska N, Barcikowska M, Kuznicki J, Wojda U. Cell cycle regulation distinguishes lymphocytes from sporadic and familial Alzheimer’s disease patients. Neurobiol Aging. 2011;32:2319, e2313–2326. doi: 10.1016/j.neurobiolaging.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 82.Tan M, Wang S, Song J, Jia J. Combination of p53(ser15) and p21/p21(thr145) in peripheral blood lymphocytes as potential Alzheimer’s disease biomarkers. Neurosci Let. 2012;516:226–231. doi: 10.1016/j.neulet.2012.03.093. [DOI] [PubMed] [Google Scholar]
- 83.Zhou X, Jia J. P53-mediated G(1)/S checkpoint dysfunction in lymphocytes from Alzheimer’s disease patients. Neurosci Let. 2010;468:320–325. doi: 10.1016/j.neulet.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 84.Lanni C, Racchi M, Uberti D, Mazzini G, Stanga S, Sinforiani E, Memo M, Govoni S. Pharmacogenetics and pharmagenomics, trends in normal and pathological aging studies: focus on p53. Curr Pharm Design. 2008;14:2665–2671. doi: 10.2174/138161208786264133. [DOI] [PubMed] [Google Scholar]
- 85.Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS. Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. Biochem Biophys Res Commun. 2007;364:522–527. doi: 10.1016/j.bbrc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calafiore M, Battaglia G, Zappala A, Trovato-Salinaro E, Caraci F, Caruso M, Vancheri C, Sortino MA, Nicoletti F, Copani A. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to beta-amyloid. Neurobiol Aging. 2006;27:606–613. doi: 10.1016/j.neurobiolaging.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 91.Heo C, Chang KA, Choi HS, Kim HS, Kim S, Liew H, Kim JA, Yu E, Ma J, Suh YH. Effects of the monomeric, oligomeric, and fibrillar Abeta42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem. 2007;102:493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- 92.Sotthibundhu A, Li QX, Thangnipon W, Coulson EJ. Abeta(1-42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30:1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004;164:975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 95.Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw, Ind) mice. Neurobiol Dis. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez-Toledano MA, Shelanski ML. Increased Neurogenesis in Young Transgenic Mice Overexpressing Human APP_{Sw, Ind} J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- 97.Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172:1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- 99.Cole LA. Human chorionic gonadotropin and associated molecules. Exp Rev Mol Diagnos. 2009;9:51–73. doi: 10.1586/14737159.9.1.51. [DOI] [PubMed] [Google Scholar]
- 100.Cole LA. HCG variants, the growth factors which drive human malignancies. Am J Cancer Res. 2012;2:22–35. [PMC free article] [PubMed] [Google Scholar]
- 101.Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 102.Saberi S, Du YP, Christie M, Goldsbury C. Human chorionic gonadotropin increases beta-cleavage of amyloid precursor protein in SH-SY5Y cells. Cell and Mol Neurobiol. 2013;33:747–751. doi: 10.1007/s10571-013-9954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 104.Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wahjoepramono EJ, Wijaya LK, Taddei K, Bates KA, Howard M, Martins G, deRuyck K, Matthews PM, Verdile G, Martins RN. Direct exposure of guinea pig CNS to human luteinizing hormone increases cerebrospinal fluid and cerebral beta amyloid levels. Neuroendocrinol. 2011;94:313–322. doi: 10.1159/000330812. [DOI] [PubMed] [Google Scholar]
- 108.Lin J, Li X, Yuan F, Lin L, Cook CL, Rao Ch V, Lei Z. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:253–261. doi: 10.1097/NEN.0b013e3181d072cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, Wallin A, Minthon L, Blennow K. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 110.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neuosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verdile G, Yeap BB, Clarnette RM, Dhaliwal S, Burkhardt MS, Chubb SA, De Ruyck K, Rodrigues M, Mehta PD, Foster JK, Bruce DG, Martins RN. Luteinizing hormone levels are positively correlated with plasma amyloid-beta protein levels in elderly men. J Alzheimers Dis. 2008;14:201–208. doi: 10.3233/jad-2008-14208. [DOI] [PubMed] [Google Scholar]
- 112.Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, Martins RN. Associations between gonadotropins, testosterone and beta amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry. 2014;19:69–75. doi: 10.1038/mp.2012.147. [DOI] [PubMed] [Google Scholar]
- 113.Clark I, Atwood C, Bowen R, Paz-Filho G, Vissel B. Tumor necrosis factor-induced cerebral insulin resistance in Alzheimer’s disease links numerous treatment rationales. PharmReviews. 2012;64:1004–1026. doi: 10.1124/pr.112.005850. [DOI] [PubMed] [Google Scholar]
- 114.Clark IA, Atwood CS. Is TNF a link between aging-related reproductive endocrine dyscrasia and Alzheimer’s disease? J Alzheimers Dis. 2011;27:691–699. doi: 10.3233/JAD-2011-110887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Darzynkiewicz Z, Williamson B, Carswell EA, Old LJ. Cell cycle-specific effects of tumor necrosis factor. Cancer Res. 1984;44:83–90. [PubMed] [Google Scholar]
- 116.Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alz Dis Assoc Dis. 2013;27:153–156. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- 117.Casadesus G, Webber K, Atwood C, Bowen R, Perry G, Smith M. 10th Int Conf Alz Dis and Related Disorders.2006. [Google Scholar]
- 118.Brion JP, Couck AM. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol. 1995;147:1465–1476. [PMC free article] [PubMed] [Google Scholar]
- 119.Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- 120.Kanemaru K, Takio K, Miura R, Titani K, Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1992;58:1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- 121.Pope WB, Lambert MP, Leypold B, Seupaul R, Sletten L, Krafft G, Klein WL. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp Neurol. 1994;126:185–194. doi: 10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- 122.Arendt T, Holzer M, Grossmann A, Zedlick D, Bruckner MK. Increased expression and subcellular translocation of the mitogen activated protein kinase kinase and mitogen-activated protein kinase in Alzheimer’s disease. Neuroscience. 1995;68:5–18. doi: 10.1016/0306-4522(95)00146-a. [DOI] [PubMed] [Google Scholar]
- 123.Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- 124.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagy Z, Esiri MM, Cato AM, Smith AD. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 126.Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 2004;6:659–671. doi: 10.3233/jad-2004-6610. discussion 673–681. [DOI] [PubMed] [Google Scholar]
- 127.Leugers CJ, Koh JY, Hong W, Lee G. Tau in MAPK activation. Frontiers Neurol. 2013;4:161. doi: 10.3389/fneur.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leugers CJ, Lee G. Tau potentiates nerve growth factor-induced mitogen-activated protein kinase signaling and neurite initiation without a requirement for microtubule binding. J Biol Chem. 2010;285:19125–19134. doi: 10.1074/jbc.M110.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Musa FR, Takenaka I, Konishi R, Tokuda M. Effects of luteinizing hormone, follicle-stimulating hormone, and epidermal growth factor on expression and kinase activity of cyclin-dependent kinase 5 in Leydig TM3 and Sertoli TM4 cell lines. J Androl. 2000;21:392–402. [PubMed] [Google Scholar]
- 130.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7:3487–3490. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 133.Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle. 2011;10:1208–1214. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 2010;285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chow HM, Guo D, Zhou JC, Zhang GY, Li HF, Herrup K, Zhang J. CDK5 activator protein p25 preferentially binds and activates GSK3beta. PNAS USA. 2014;111:E4887–4895. doi: 10.1073/pnas.1402627111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Flynn MP, Maizels ET, Karlsson AB, McAvoy T, Ahn JH, Nairn AC, Hunzicker-Dunn M. Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A-dependent dephosphorylation of microtubule-associated protein 2D. Mol Endocrinol. 2008;22:1695–1710. doi: 10.1210/me.2007-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoerndli FJ, Pelech S, Papassotiropoulos A, Gotz J. Abeta treatment and P301L tau expression in an Alzheimer’s disease tissue culture model act synergistically to promote aberrant cell cycle re-entry. Eur J Neurosci. 2007;26:60–72. doi: 10.1111/j.1460-9568.2007.05618.x. [DOI] [PubMed] [Google Scholar]
- 142.Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer’s disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lopes JP, Oliveira CR, Agostinho P. Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-beta and prion peptides. Cell Cycle. 2009;8:97–104. doi: 10.4161/cc.8.1.7506. [DOI] [PubMed] [Google Scholar]
- 144.Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer’s disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lopes JP, Oliveira CR, Agostinho P. Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: the role of Cdk5. Aging Cell. 9:64–77. doi: 10.1111/j.1474-9726.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- 146.Lopes JP, Blurton-Jones M, Yamasaki TR, Agostinho P, LaFerla FM. Activation of cell cycle proteins in transgenic mice in response to neuronal loss but not amyloid-beta and tau pathology. J Alzheimers Dis. 2009;16:541–549. doi: 10.3233/JAD-2009-0993. [DOI] [PubMed] [Google Scholar]
- 147.Kuhla A, Ludwig SC, Kuhla B, Munch G, Vollmar B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 148.Diamanti-Kandarakis E, Lambrinoudaki I, Economou F, Christou M, Piperi C, Papavassiliou AG, Creatsas G. Androgens associated with advanced glycation end-products in postmenopausal women. Menopause. 2010;17:1182–1187. doi: 10.1097/gme.0b013e3181e170af. [DOI] [PubMed] [Google Scholar]
- 149.Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol. 2005;62:37–43. doi: 10.1111/j.1365-2265.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- 150.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 151.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 152.Van Broeckhoven C, Haan J, Bakker E, Hardy JA, Van Hul W, Wehnert A, Vegter-Van der Vlis M, Roos RA. Amyloid beta protein precursor gene and hereditary cerebral hemorrhage with amyloidosis (Dutch) Science. 1990;248:1120–1122. doi: 10.1126/science.1971458. [DOI] [PubMed] [Google Scholar]
- 153.Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 154.Lannfelt L, Bogdanovic N, Appelgren H, Axelman K, Lilius L, Hansson G, Schenk D, Hardy J, Winblad B. Amyloid precursor protein mutation causes Alzheimer’s disease in a Swedish family. Neuroscience Lett. 1994;168:254–256. doi: 10.1016/0304-3940(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 155.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 156.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 157.Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005;62:299–312. doi: 10.1007/s00018-004-4385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 159.McGonigal G, Thomas B, McQuade C, Starr JM, MacLennan WJ, Whalley LJ. Epidemiology of Alzheimer’s presenile dementia in Scotland, 1974–88. BMJ (Clinical research ed) 1993;306:680–683. doi: 10.1136/bmj.306.6879.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 162.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 163.Hy LX, Keller DM. Prevalence of AD among whites: a summary by levels of severity. Neurology. 2000;55:198–204. doi: 10.1212/wnl.55.2.198. [DOI] [PubMed] [Google Scholar]
- 164.Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- 165.Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 166.Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 167.Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- 168.Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- 169.Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro Endocrinol Lett. 2003;24:203–208. [PubMed] [Google Scholar]
- 170.Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E, Prince R, Devine A, Mehta P, Beilby J, Atwood CS, Martins RN. Gonadotropins and cognition in older women. J Alzheimers Dis. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- 171.Hyde Z, Flicker L, Almeida OP, McCaul KA, Jamrozik K, Hankey GJ, Chubb SA, Yeap BB. Higher luteinizing hormone is associated with poor memory recall: the health in men study. J Alzheimers Dis. 2010;19:943–951. doi: 10.3233/JAD-2010-1342. [DOI] [PubMed] [Google Scholar]
- 172.D’Amico AV, Braccioforte MH, Moran BJ, Chen MH. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer Dis Assoc Dis. 2010;24:85–89. doi: 10.1097/wad.0b013e31819cb8f4. [DOI] [PubMed] [Google Scholar]
- 173.Bowen RL, Beaird H, Atwood CS, Smith MA, Rimm AA. Men treated for prostate cancer have a decreased incidence of dementia. 9th Int Cong Alzheimer Dis 2004 [Google Scholar]
- 174.Nedelec C, Ragot S, Irani J, Pires C, Gil R, Dore B. Effects by androgen suppression with luteinizing hormone on cognitive functions in men treated for cancer of prostate. Prog Urol. 2009;19:47–53. doi: 10.1016/j.purol.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 175.Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of lupron depot in the treatment of women with Alzheimer’s disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. J Alzheimers Dis. 2015;44:549–560. doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- 176.Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- 177.Yang Y, Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: a unified disease mechanism. J Neurosci. 2005;25:2522–2529. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Atwood CS, Barzilai N, Bowen RL, Brown-Borg HM, Jarrard DF, Fu VX, Heilbronn LK, Ingram DK, Ravussin E, Schwartz RS, Weindruch R. Pennington scientific symposium on mechanisms and retardation of aging. Exp Gerontol. 2003;38:1217–1226. doi: 10.1016/j.exger.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 179.Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- 180.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 181.Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 182.Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- 183.Wharton W, Baker LD, Gleason CE, Dowling M, Barnet JH, Johnson S, Carlsson C, Craft S, Asthana S. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. J Alzheimers Dis. 2011;26:495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Atwood C, Bowen R. The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers cell cycle reentry of post-mitotic neurons, neurodegeneration and cognitive disease. Hor Behav. 2015 doi: 10.1016/j.yhbeh.2015.06.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Landreth GE, Cramer PE, Lakner MM, Cirrito JR, Wesson DW, Brunden KR, Wilson DA. Response to comments on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924-g. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Varvel NH, Bhaskar K, Kounnas MZ, Wagner SL, Yang Y, Lamb BT, Herrup K. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J Clin Invest. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Atwood C. Amyloid-β aggregation as a protective acute-phase response to injury/neurodegeneration: A barrier function for amyloid-β deposits. In: Rigacci S, Bucciantini M, editors. Functional Amyloid Aggregation. Research Signpost; Kerala, India: 2010. pp. 115–134. [Google Scholar]