Left ventricular hypertrophy (LVH) can develop in association with chronic arterial hypertension and other cardiovascular disorders, and is a well-established risk factor for cardiac arrhythmias, cardiovascular events and mortality 1. Over 4 decades ago the Framingham Heart Study reported that electrocardiographic evidence of LVH (ECG LVH) was associated with 3 to 4-fold increase in cardiovascular and all-cause mortality, with a disproportionately high risk of sudden cardiac death (SCD) 2, 3. Subsequently, multiple studies in different population samples have confirmed these associations 4, 5.
In early studies, the 12-lead ECG was the only available method to diagnose LVH in living subjects. In subsequent years, anatomic LVH diagnosed by echocardiography (echo LVH) became the gold standard 6, 7. Despite the development of fairly detailed diagnostic ECG criteria for LVH 8, echocardiographic measurements have virtually replaced the ECG diagnosis of LVH. This shift in clinical practice was driven by the low reported sensitivity (usually <25%), albeit high specificity (up to 95%) of ECG criteria for diagnosis of LVH with echocardiography, magnetic resonance imaging (MRI), or during autopsy 9, 10.
More recently however, we are learning that ECG LVH and echo LVH may be clinically distinct entities. In fact, there are now data to suggest that while these two entities can often overlap, each may provide distinct prognostic and potentially, mechanistic information, especially in the context of cardiac arrhythmias. This review will attempt to put these findings into perspective for the clinical electrophysiologist, by discussing the significance of electrical vs. anatomic LVH for occurrence of atrial fibrillation (AF) and SCD.
Electrical (ECG) vs. Anatomic (echo/MRI) LVH: Evidence in Support of Two Distinct Entities
To diagnose increased left ventricular (LV) mass from the 12-lead ECG, over 30 different ECG criteria have been developed. Most of the commonly used LVH criteria, such as Sokolow and Lyon, and Cornell voltage, rely solely on measuring QRS voltage. However, some also take QRS duration into account (e.g. Cornell voltage-duration product) as well as other ECG abnormalities (Romhilt-Estes point score). There is also significant variation in the echocardiographic definition of LVH. Although most studies have used standardized formulae based on M-mode measurements to determine LV mass with adjustment for body surface area, this was not a uniform practice. Taking the available data into account, the correlation between these LVH ECG criteria and echo LVH is, at best, a moderate one8. Therefore, the ECG was considered to be an insensitive method for diagnosing anatomic LVH, and the increased risk associated with ECG LVH was thought to be directly related to increased left ventricular mass. At the outset we would like to recognize that some of the discordance between ECG and echo LVH could result from extracardiac factors such as age, sex, race or body habitus; or even temporal separation of when the two tests were performed. However, we remain open to the possibility of an alternative explanation for this apparent “low sensitivity” of the ECG. At least in a subgroup of patients, could ECG LVH and echo LVH be distinct entities, one reflecting electrical and the other anatomic remodeling?
It has long been recognized by clinicians that abnormal ECG changes can precede pathological echocardiographic findings in patients with underlying cardiac pathology, such as hypertrophic cardiomyopathy, and that electrical alterations add additional clinical information to the imaging of cardiac structure and function in these patients.11, 12 The first evidence that this may also be relevant for common forms of LVH came from a Swedish study, which demonstrated that ECG LVH and echo LVH carry somewhat different prognostic information (Figure 1, Panel A) 13. In their cohort of 475 men of white European descent investigated at the age of 70 with a 5-year follow-up, both ECG LVH and echo LVH expectedly predicted total and cardiovascular mortality, but, intriguingly, ECG LVH (defined as Cornell product >244 µV·s) was associated with 2.89-fold increase in mortality even after adjustments for echo LVH and several other cardiovascular risk factors. However, given the subjects included in this study, these results could not be generalized to females or other racial/ethnic groups.
Figure 1.
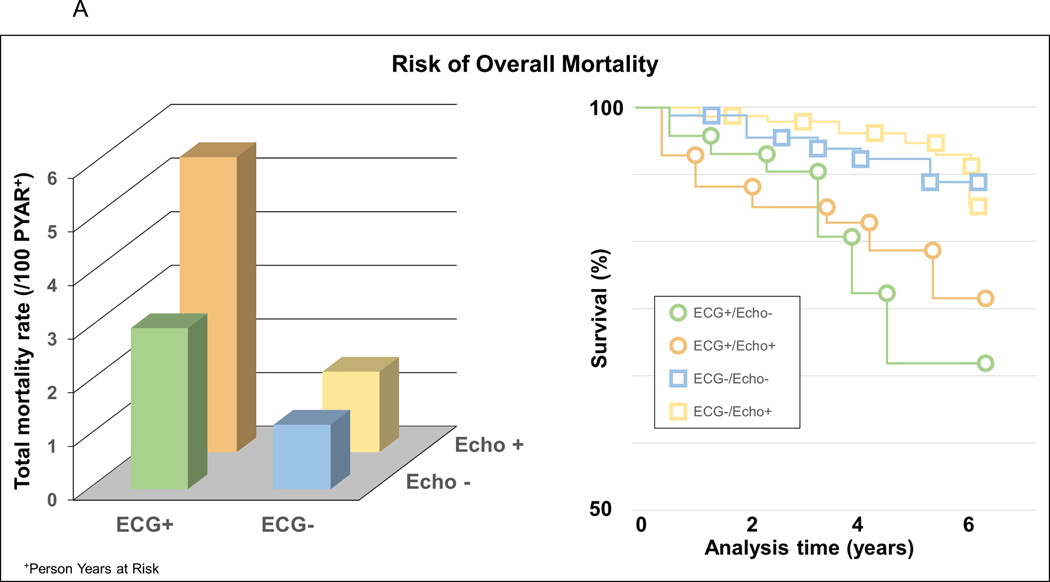
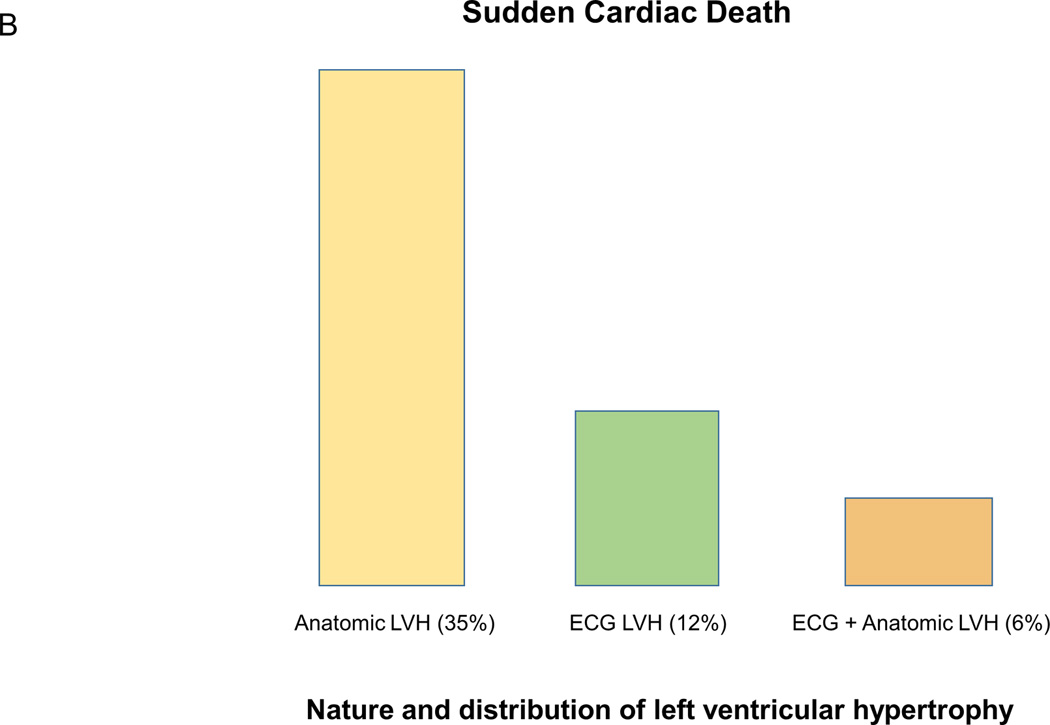
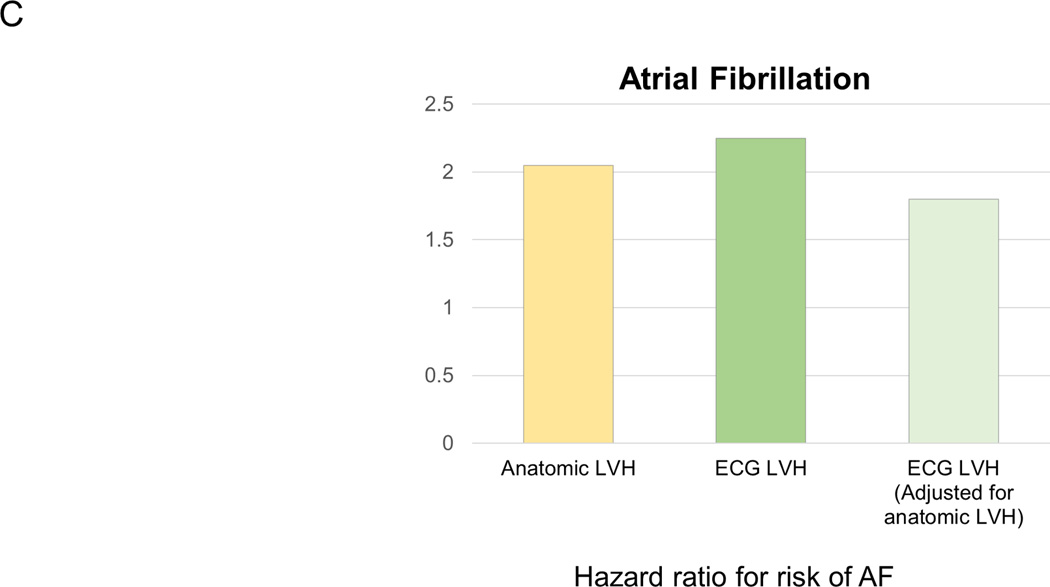
Evidence suggesting that anatomic vs electric left ventricular hypertrophy (LVH) is distinct entities that not only can overlap but also have independent effects on outcomes. A (adapted from Sundström et al13 with permission of the publisher Lippincott Williams & Wilkins. Copyright © 2001), risk of mortality associated with and without ECG LVH (Cornell product criteria, ECG+/ECG−) and echocardiographic LVH (echo+/echo−). B (adapted from Narayanan et al14 with permission of the publisher Elsevier. Copyright © 2001), the relatively small overlap between echo and ECG LVH among cases that suffered sudden cardiac death. C (adapted from Chrispin et al18 with permission of the publisher Elsevier. Copyright © 2001), the independent effects of ECG LVH on increased risk of atrial fibrillation (AF) even when adjusted for anatomic LVH.
A more recent analysis from the Oregon Sudden Unexpected Death Study (Oregon SUDS) has demonstrated that ECG LVH also provides unique prognostic information for increased risk of SCD, even when adjusted for echo LVH 14. Among patients who suffered SCD, there was a relatively low level of agreement between ECG and echocardiography for diagnosis of LVH. 57% of patients with ECG LVH did not have evidence of echo LVH, and conversely, 84% of patients with echo LVH did not have ECG LVH (Figure 1, Panel B). In multivariate analyses, ECG LVH by Sokolow-Lyon criteria was associated with over two-fold increase in the risk of SCD (odds ratio (OR) 2.5, 95% confidence interval (CI) 1.1–6.0), and this risk was not markedly attenuated when adjusted for left ventricular mass (OR 2.4, CI 1.0–6.0). Similar risk of SCD was also associated with echo LVH (OR 2.7, CI 1.5–4.9), but the study lacked power to evaluate the risk of SCD in the subgroup with both ECG and echo LVH. The authors concluded that in some patients ECG LVH may occur in the absence of echo LVH, and that in these patients ECG LVH is potentially a distinct entity with an independent contribution to risk of ventricular arrhythmogenesis and SCD.
Another recent study comparing LVH diagnosed by ECG and cardiac MRI in the Multi-Ethnic Study of Atherosclerosis (MESA) population also showed a discrepancy between diagnoses of LVH by ECG vs. cardiac MRI (MRI LVH) 15. 2.4% of the participants demonstrated LVH by both ECG and MRI, 8.2% had only MRI LVH and 4.4% presented with isolated ECG LVH only. Also, the presence of ECG LVH by Cornell voltage or Sokolow-Lyon criteria was independently predictive of cardiovascular morbidity and mortality to a similar extent as MRI LVH. When LVH was diagnosed on both ECG and MRI, the risk of cardiovascular events was almost threefold compared to subjects without LVH.
It turns out that these observations regarding ECG vs. anatomic LVH have also been made in patients with AF. LVH is often associated with development of atrial arrhythmias such as AF, a risk that seems to correlate alongside with the severity of ECG LVH 16, 17. In the MESA study, LVH detected by cardiac MRI or ECG were both associated with an increased risk of incident AF, but the increased risk associated with ECG LVH using Sokolow-Lyon voltage product persisted even after adjusting for MRI-LVH (Figure 1, Panel C), suggesting that ECG LVH may provide some independent value in AF prediction18. Taken together these four studies do suggest that in a subgroup of patients, ECG LVH can occur in the absence of LVH observed by echo or MRI, and confers independently increased risk of overall mortality, SCD as well as AF. These findings are summarized in Figure 1.
Mechanisms of Arrhythmogenesis in Electrical vs. Anatomic LVH
In order to understand the unique aspects of ECG LVH vs. echo LVH, it may be useful to examine the potential mechanisms of arrhythmogenesis that are operative in either entity, along with the fundamental etiologies of ECG LVH vs. echo LVH. In the big picture, a diagnosis of LVH is independently associated with an increased risk of ventricular arrhythmias 19, 20. In the presence of LVH, ventricular arrhythmias are associated with increased mortality 21. The increased risk of SCD associated with LVH seems to be over and above the risk predicted by clinical factors or left ventricular systolic function 7, 22, 23. The frequency and complexity of these arrhythmias seem to rise with increased left ventricular mass, with two- to threefold increase in the arrhythmias for every 1mm increase in the thickness of left ventricular wall 24. However, from a structural perspective, ECG LVH appears to be distinct from echo LVH (Figure 2). For example, if there is no significant increase in LV mass it seems unlikely that isolated ECG LVH involves myocyte hypertrophy of the extent that is observed in echo LVH. Yet ECG LVH is associated with an increased risk of ventricular and atrial arrhythmias. In hypertensive patients, ECG LVH is associated with increased prevalence of ventricular arrhythmias 25, 26. Findings from the MESA study indicate that ECG LVH has also a role in predicting risk of AF independent of MRI LVH 18. It is thus likely that there is some mechanistic overlap between the two entities but some of the mechanisms may be unique to each form of LVH.
Figure 2.
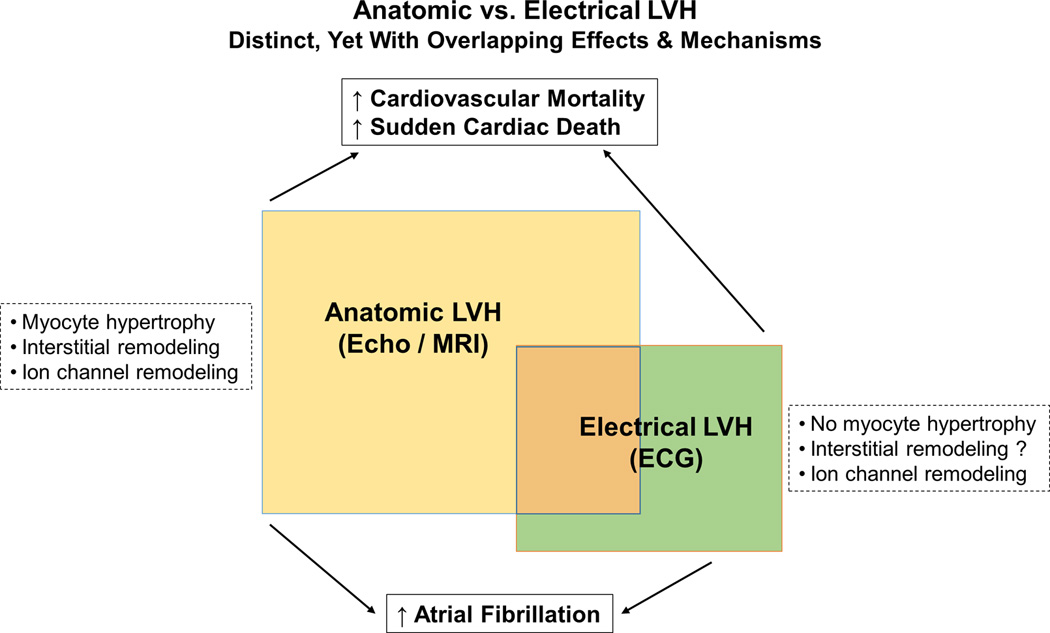
Consequences and mechanisms of anatomic vs electrical LVH.
Myocardial Cellular, Electrical and Interstitial Remodeling
Myocardial hypertrophy involves extensive alterations at the systemic as well as cardiac levels. There are adverse effects on intravascular hemodynamics and neuroendocrine activation, that correlate with myocyte hypertrophy, cell death, myocardial fibrosis and altered electrophysiological properties of the myocardium such as increased action potential duration and decreased conduction velocity (Figure 2). Slowed impulse conduction, a dominant electrophysiological feature of hypertrophied myocardium, can result from increased size of ventricular myocytes, increase in extracellular matrix, reduced cell-to-cell coupling, as well as reduced membrane excitability due to changes in properties of the ion channels 27, 30. LVH is associated with prolongation of ventricular action potentials and increased dispersion of repolarization 27. In animal models, alterations in calcium and Na-Ca exchange currents as well as reductions in potassium currents have been demonstrated in hypertrophied myocardium 31–33. This remodeling of the ion channels is ultimately responsible for prolongation of the action potential, which can be observed as prolonged QT-interval on the surface ECG 34. Prolongation of the action potential duration may predispose to arrhythmias based on early or delayed afterdepolarizations and triggered activity 35, 36, and local differences in the action potential duration may lead to increased dispersion of the repolarization and refractoriness enabling initiation and maintenance of reentrant electrical circuits 37, 38. Increased interstitial fibrosis and collagen deposition in LVH are other prominent features especially in advanced hypertrophy 27, and can create anatomic uncoupling of adjacent myocytes leading to discontinuous and slowed conduction, which may manifest as abnormalities in QRS morphology and duration. This increase in myocardial fibrosis and altered ratios of collagen subtypes has been especially observed in individuals with LVH and SCD 39. Gap junctions are other important determinants of myocardial conduction, and when LVH is present, expression of the predominant ventricular gap junction protein, connexin43 (Cx43), is significantly reduced 40, which has been associated with slowing of impulse propagation and increased susceptibility to ventricular arrhythmias 41. Furthermore, delayed conduction resulting from interstitial remodeling and increased interstitial myocardial fibrosis can result in non-uniform anisotropic cellular coupling, which may be responsible for increased inhomogeneity of myocardial conduction and dispersion of repolarization, creating conditions for micro-reentry and arrhythmogenesis 42.
It is logical that myocyte hypertrophy and extensive interstitial remodeling commonly found in echo LVH, are unlikely to be the dominant mechanisms of arrhythmogenesis in ECG LVH. While the exact mechanisms responsible for increased propensity of ventricular and atrial arrhythmias in ECG LVH still need to be elucidated, it is reasonable to hypothesize ion channel remodeling involving both cell membrane and the gap junctions as the dominant mechanism. The exact nature of true electrical remodeling in LVH has been the subject of active investigation 43, and is likely to largely explain the observed differences between electrical and anatomic LVH. These altered electrophysiological properties of the myocardium seem to be somewhat independent from the morphological transformations in LVH 29, and may serve as a substrate for triggering and maintaining ventricular arrhythmias 44. In some experimental models, reduced Cx43 expression has been associated with diminished QRS amplitudes 41, 45, a phenomenon that along with extracardiac factors may partly explain the common “false negative” results of ECG in diagnosing anatomic LVH. However these factors would not explain the existence of ECG LVH in the absence of anatomic LVH.
Cause of Electric Versus Anatomic LVH
It follows that a clinical history of hypertension, the major and most common factor associated with LVH, may not be a key player in the process of developing ECG LVH. Could there be a special genetic contribution to development of ECG LVH? The greater heritability of ECG LVH compared to anatomic LVH 46 would lend support to this idea. In a genome-wide scan of families with hypertension, a stronger genetic contribution was observed for ECG LVH than echo LVH, and genetic determinants of each of these appeared to be distinct 47. However, these genome-wide association analyses have limitations that will likely be overcome with next-generation sequencing technologies 48, and more work is needed. Finally, in the subset of patients that suffer SCD we should remain open to the possibility that while they manifested with ECG LVH, they may have died prior to developing echo LVH.
Prognostic and Therapeutic Implications
Because both electric and anatomic LVH are associated with increased risk of arrhythmias and overall mortality, 5, 14, prevention and treatment of either form of LVH is likely to make a significant impact on the burden of cardiovascular disease. However, based on the findings discussed in this review, it is likely that separate consideration of these two entities instead of lumping them together is likely to provide more useful prognostic information, for risk of overall cardiovascular mortality, SCD as well as AF. Once the specific form of LVH is established there are potential downstream therapeutic implications. Published reports suggest that antihypertensive therapy can cause regression of anatomic LVH, with reduction of cardiovascular morbidity and mortality, over and above that predicted by lowering blood pressure 51. In animal models, LVH regression has resulted in normalization of electrophysiological cellular abnormalities, such as action potential prolongation and altered repolarization, as well as reduced vulnerability to ventricular fibrillation 52, 53. Reduction of ECG-LVH was first associated with lowered cardiovascular risk in the Framingham Heart Study population over 2 decades ago 54. Since then, studies on pharmacological treatments such as ramipril in the Heart Outcomes Prevention Evaluation (HOPE) trial 55 and losartan in the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) study 56 have shown that reduction of ECG LVH predicts lower risk of cardiovascular mortality, independent of the degree of blood pressure reduction or other clinical factors. Reduction of ECG LVH is associated with reduced risk of SCD, as reported by Wachtell et al 57 from a substudy of the LIFE trial that included patients with hypertension and ECG LVH. Again, since evaluation of LVH was performed only by ECG we do not have the corresponding echo findings that would enable us to understand the role of treating the two different forms of LVH.
Although ECG LVH and echo LVH seem to be distinct entities that do not overlap in a subgroup of patients, there is much that we need to learn about the subgroup that only manifests with ECG LVH. It would be important to put these patients under a microscope to understand how the etiologies and natural history of this condition are different from echo LVH. If there is a larger genetic contribution, are these patients younger and do they have a history of hypertension? Is the increased voltage on the ECG a transient phenomenon triggered by specific factors or is it a permanent phenomenon? A large proportion of patients with echo LVH have diastolic dysfunction. Is this true of subjects with lone ECG LVH? In the LIFE study, wall motion abnormalities were observed in one eighth of the patients with ECG LVH, and these abnormalities were associated with higher values of Cornell voltage-duration product and higher prevalence of ST strain pattern 58. However, these patients were not stratified according to the LV mass, so we are not able to ascribe the findings to those with lone ECG LVH. There is limited evidence that ECG LVH is associated with diastolic dysfunction independent of left ventricular mass 59, but further investigation is needed.
Conclusions
Based on the evidence, we hope to have made the case that the clinical manifestation of LVH takes two forms: electrical LVH observed on the 12-lead ECG and anatomic LVH seen on the echocardiogram. Furthermore, while a large proportion will have both electrical and anatomic LVH, there exist subgroups that will have isolated electrical LVH or isolated anatomic LVH. While absence of LVH by voltage could be attributed to low sensitivity of the 12-lead ECG or other factors, absence of anatomic LVH in a patient with ECG LVH denotes a special subgroup of LVH patients. While there clearly exists overlap between these two conditions, patients with lone ECG LVH or lone echo LVH should be separated from a clinical standpoint. This will enable improved risk stratification of ventricular and atrial arrhythmias, while providing a much-needed opportunity to carefully investigate the potentially divergent etiologies/mechanisms as well as therapeutic implications of these two distinct entities.
Supplementary Material
Acknowledgments
Sources of Funding Dr. Aro is funded by grants from the Finnish Cultural Foundation and the Finnish Foundation for Cardiovascular Research. This work was funded by National Heart Lung and Blood Institute grant R01HL122492 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Electrophysiology at Cedars-Sinai, Los Angeles.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009;52:153–167. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Gordon T, Castelli WP, Margolis JR. Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease. The Framingham study. Ann Intern Med. 1970;72:813–822. doi: 10.7326/0003-4819-72-6-813. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89–105. doi: 10.7326/0003-4819-71-1-89. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Doyle JT, McNamara PM, Quickenton P, Gordon T. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation. 1975;51:606–613. doi: 10.1161/01.cir.51.4.606. [DOI] [PubMed] [Google Scholar]
- 5.Rautaharju PM, Soliman EZ. Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: A critical appraisal. J Electrocardiol. 2014;47:649–654. doi: 10.1016/j.jelectrocard.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 7.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 8.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H American Heart Association E, Arrhythmias Committee CoCC, American College of Cardiology F, Heart Rhythm S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Pewsner D, Juni P, Egger M, Battaglia M, Sundstrom J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: Systematic review. BMJ. 2007;335:711. doi: 10.1136/bmj.39276.636354.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JA, Bluemke DA. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: The Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–658. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregor P, Widimsky P, Cervenka V, Visek V, Hrobonova V. Electrocardiographic changes can precede the development of myocardial hypertrophy in the setting of hypertrophic cardiomyopathy. Int J Cardiol. 1989;23:335–341. doi: 10.1016/0167-5273(89)90193-9. [DOI] [PubMed] [Google Scholar]
- 12.Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, De Luca R, Spataro A, Biffi A, Thiene G, Maron BJ. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358:152–161. doi: 10.1056/NEJMoa060781. [DOI] [PubMed] [Google Scholar]
- 13.Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Chugh H, Gunson K, Jui J, Chugh SS. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11:1040–1046. doi: 10.1016/j.hrthm.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacharova L, Chen H, Estes EH, Mateasik A, Bluemke DA, Lima JA, Burke GL, Soliman EZ. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol. 2015;115:515–522. doi: 10.1016/j.amjcard.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: The losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 17.Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Lindholm LH, Nieminen MS, Edelman JM, Hille DA, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 18.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Nazarian S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:2007–2013. doi: 10.1016/j.jacc.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel WB, Schatzkin A. Sudden death: Lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Nieminen MS, Edelman JM, Dahlof B, Devereux RB, Investigators LS. Prognostic value of changes in the electrocardiographic strain pattern during antihypertensive treatment: The losartan intervention for end-point reduction in hypertension study (LIFE) Circulation. 2009;119:1883–1891. doi: 10.1161/CIRCULATIONAHA.108.812313. [DOI] [PubMed] [Google Scholar]
- 21.Bikkina M, Larson MG, Levy D. Asymptomatic ventricular arrhythmias and mortality risk in subjects with left ventricular hypertrophy. J Am Coll Cardiol. 1993;22:1111–1116. doi: 10.1016/0735-1097(93)90424-y. [DOI] [PubMed] [Google Scholar]
- 22.Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177–1182. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens SM, Reinier K, Chugh SS. Increased left ventricular mass as a predictor of sudden cardiac death: Is it time to put it to the test? Circ Arrhythm Electrophysiol. 2013;6:212–217. doi: 10.1161/CIRCEP.112.974931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17:1277–1282. doi: 10.1016/s0735-1097(10)80135-4. [DOI] [PubMed] [Google Scholar]
- 25.McLenachan JM, Henderson E, Morris KI, Dargie HJ. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317:787–792. doi: 10.1056/NEJM198709243171302. [DOI] [PubMed] [Google Scholar]
- 26.Messerli FH, Ventura HO, Elizardi DJ, Dunn FG, Frohlich ED. Hypertension and sudden death. Increased ventricular ectopic activity in left ventricular hypertrophy. Am J Med. 1984;77:18–22. doi: 10.1016/0002-9343(84)90430-3. [DOI] [PubMed] [Google Scholar]
- 27.Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2:216–223. doi: 10.1053/eupc.2000.0110. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol. 1997;8:887–894. doi: 10.1111/j.1540-8167.1997.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 29.Botchway AN, Turner MA, Sheridan DJ, Flores NA, Fry CH. Electrophysiological effects accompanying regression of left ventricular hypertrophy. Cardiovasc Res. 2003;60:510–517. doi: 10.1016/j.cardiores.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 31.Tomita F, Bassett AL, Myerburg RJ, Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circ Res. 1994;75:296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- 32.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing k+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406–1415. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ, Vos MA. Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: Potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102:2137–2144. doi: 10.1161/01.cir.102.17.2137. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji Y, Opthof T, Yasui K, Inden Y, Takemura H, Niwa N, Lu Z, Lee JK, Honjo H, Kamiya K, Kodama I. Ionic mechanisms of acquired QT prolongation and torsades de pointes in rabbits with chronic complete atrioventricular block. Circulation. 2002;106:2012–2018. doi: 10.1161/01.cir.0000031160.86313.24. [DOI] [PubMed] [Google Scholar]
- 35.Aronson RS. Afterpotentials and triggered activity in hypertrophied myocardium from rats with renal hypertension. Circ Res. 1981;48:720–727. doi: 10.1161/01.res.48.5.720. [DOI] [PubMed] [Google Scholar]
- 36.Ben-David J, Zipes DP, Ayers GM, Pride HP. Canine left ventricular hypertrophy predisposes to ventricular tachycardia induction by phase 2 early afterdepolarizations after administration of BAY K 8644. J Am Coll Cardiol. 1992;20:1576–1584. doi: 10.1016/0735-1097(92)90453-t. [DOI] [PubMed] [Google Scholar]
- 37.Kowey PR, Friechling TD, Sewter J, Wu Y, Sokil A, Paul J, Nocella J. Electrophysiological effects of left ventricular hypertrophy. Effect of calcium and potassium channel blockade. Circulation. 1991;83:2067–2075. doi: 10.1161/01.cir.83.6.2067. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Rials SJ, Wu Y, Salata JJ, Liu T, Bharucha DB, Marinchak RA, Kowey PR. Left ventricular hypertrophy decreases slowly but not rapidly activating delayed rectifier potassium currents of epicardial and endocardial myocytes in rabbits. Circulation. 2001;103:1585–1590. doi: 10.1161/01.cir.103.11.1585. [DOI] [PubMed] [Google Scholar]
- 39.Tamarappoo BK, John BT, Reinier K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Vulnerable myocardial interstitium in patients with isolated left ventricular hypertrophy and sudden cardiac death: A postmortem histological evaluation. J Am Heart Assoc. 2012;1:e001511. doi: 10.1161/JAHA.112.001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 41.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spach MS, Josephson ME. Initiating reentry: The role of nonuniform anisotropy in small circuits. J Cardiovasc Electrophysiol. 1994;5:182–209. doi: 10.1111/j.1540-8167.1994.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Padial L, Bacharova L. Electrical remodeling in left ventricular hypertrophy-Is there a unifying hypothesis for the variety of electrocardiographic criteria for the diagnosis of left ventricular hypertrophy? J Electrocardiol. 2012;45:494–497. doi: 10.1016/j.jelectrocard.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Kamei K, Maehara K, Kimura J, Ishibashi T, Maruyama Y. Comprehensive analyses of arrhythmogenic substrates and vulnerability to ventricular tachycardia in left ventricular hypertrophy in salt-sensitive hypertensive rats. Circ J. 2007;71:390–396. doi: 10.1253/circj.71.390. [DOI] [PubMed] [Google Scholar]
- 45.Bacharova L, Plandorova J, Klimas J, Krenek P, Kyselovic J. Discrepancy between increased left ventricular mass and"normal" QRS voltage is associated with decreased connexin 43 expression in early stage of left ventricular hypertrophy in spontaneously hypertensive rats. J Electrocardiol. 2008;41:730–734. doi: 10.1016/j.jelectrocard.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Mayosi BM, Keavney B, Kardos A, Davies CH, Ratcliffe PJ, Farrall M, Watkins H. Electrocardiographic measures of left ventricular hypertrophy show greater heritability than echocardiographic left ventricular mass. Eur Heart J. 2002;23:1963–1971. doi: 10.1053/euhj.2002.3288. [DOI] [PubMed] [Google Scholar]
- 47.Mayosi BM, Avery PJ, Farrall M, Keavney B, Watkins H. Genome-wide linkage analysis of electrocardiographic and echocardiographic left ventricular hypertrophy in families with hypertension. Eur Heart J. 2008;29:525–530. doi: 10.1093/eurheartj/ehn028. [DOI] [PubMed] [Google Scholar]
- 48.Hong KW, Shin DJ, Lee SH, Son NH, Go MJ, Lim JE, Shin C, Jang Y, Oh B. Common variants in RYR1 are associated with left ventricular hypertrophy assessed by electrocardiogram. Eur Heart J. 2012;33:1250–1256. doi: 10.1093/eurheartj/ehr267. [DOI] [PubMed] [Google Scholar]
- 49.Hypertension detection and follow-up program cooperative group. Five-year findings of the hypertension detection and follow-up program. Prevention and reversal of left ventricular hypertrophy with antihypertensive drug therapy. Hypertension. 1985;7:105–112. [PubMed] [Google Scholar]
- 50.Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The losartan intervention for endpoint reduction in hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 51.Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. doi: 10.1001/jama.292.19.2350. [DOI] [PubMed] [Google Scholar]
- 52.Rials SJ, Wu Y, Ford N, Pauletto FJ, Abramson SV, Rubin AM, Marinchak RA, Kowey PR. Effect of left ventricular hypertrophy and its regression on ventricular electrophysiology and vulnerability to inducible arrhythmia in the feline heart. Circulation. 1995;91:426–430. doi: 10.1161/01.cir.91.2.426. [DOI] [PubMed] [Google Scholar]
- 53.Rials SJ, Wu Y, Xu X, Filart RA, Marinchak RA, Kowey PR. Regression of left ventricular hypertrophy with captopril restores normal ventricular action potential duration, dispersion of refractoriness, and vulnerability to inducible ventricular fibrillation. Circulation. 1997;96:1330–1336. doi: 10.1161/01.cir.96.4.1330. [DOI] [PubMed] [Google Scholar]
- 54.Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 55.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S Heart Outcomes Prevention Evaluation I. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 56.Okin PM, Devereux RB, Jern S, Kjedsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 57.Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: The LIFE study. Circulation. 2007;116:700–705. doi: 10.1161/CIRCULATIONAHA.106.666594. [DOI] [PubMed] [Google Scholar]
- 58.Palmieri V, Okin PM, Bella JN, Gerdts E, Wachtell K, Gardin J, Papademetriou V, Nieminen MS, Dahlof B, Devereux RB Losartan Intervention For End-point reduction in h. Echocardiographic wall motion abnormalities in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. Hypertension. 2003;41:75–82. doi: 10.1161/01.hyp.0000045081.54784.36. [DOI] [PubMed] [Google Scholar]
- 59.Krepp JM, Lin F, Min JK, Devereux RB, Okin PM. Relationship of electrocardiographic left ventricular hypertrophy to the presence of diastolic dysfunction. Ann Noninvasive Electrocardiol. 2014;19:552–560. doi: 10.1111/anec.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


