Abstract
Sex hormones are the physiological factors that regulate neurogenesis during embryogenesis and continuing through adulthood. These hormones support the formation of brain structures such as dendritic spines, axons and synapses required for the capture of information (memories). Intriguingly, a recent animal study has demonstrated that induction of neurogenesis results in the loss of previously encoded memories in animals (e.g. infantile amnesia). In this connection, much evidence now indicates that Alzheimer’s disease (AD) also involves aberrant re-entry of post-mitotic neurons into the cell cycle. Cell cycle abnormalities appear very early in the disease, prior to the appearance of plaques and tangles, and explain the biochemical, neuropathological and cognitive changes observed with disease progression. Since sex hormones control when and how neurons proliferate and differentiate, the endocrine dyscrasia that accompanies menopause and andropause is a key signaling event that impacts neurogenesis and the acquisition, processing, storage and recall of memories. Here we review the biochemical, epidemiological and clinical evidence that alterations in endocrine signaling with menopause and andropause drive the aberrant re-entry of post-mitotic neurons into an abortive cell cycle with neurite retraction that leads to neuron dysfunction and death. When the reproductive axis is in balance, luteinizing hormone (LH), and its fetal homolog, human chorionic gonadotropin (hCG), promote pluripotent human and totipotent murine embryonic stem cell and neuron proliferation. However, strong evidence supports menopausal/andropausal elevations in the ratio of LH:sex steroids as driving aberrant mitotic events mediated by the upregulation of tumor necrosis factor, amyloid-β precursor protein processing towards the production of mitogenic Aβ, and the activation of Cdk5, a key regulator of cell cycle progression and tau phosphorylation (a cardinal feature of both neurogenesis and neurodegeneration). Cognitive studies also demonstrate the negative consequences of a high LH:sex steroid ratio on human cognitive performance. Prospective epidemiological and clinical evidence in humans supports lowering the ratio of circulating gonadotropins-GnRH to sex steroids in reducing the incidence of AD and halting cognitive decline. Together, these data support endocrine dyscrasia and the subsequent loss of cell cycle control as an important etiological event in the development of neurodegenerative diseases including AD, stroke and Parkinson’s disease.
Keywords: endocrine dyscrasia, luteinizing hormone, gonadotropin-releasing hormone, sex steroids, dyotic signaling, Alzheimer’s disease, stroke, blood-brain barrier, Parkinson’s disease, cognition, amyloid-beta precursor protein, tau, Cdk-5, cell cycle re-entry, hypothalamic-pituitary-gonadal axis, menopause, andropause, senescence, hormone replacement therapy
1. Introduction
This review summarizes data collected over the last 15 years supporting age-related endocrine dyscrasia as the etiological event driving age-related neurodegeneration. Endocrine dyscrasia associated with menopause and andropause, i.e. suppressed sex steroid and inhibin signaling, elevated gonadotropin-releasing hormone 1 (GnRH1), luteinizing hormone (LH), follicle-stimulating hormone and activin signaling - has been postulated to lead to aberrant mitogenic/differentiative (dyotic) signaling that drives the re-entry of neurons into an abortive cell cycle that leads to cell death (Atwood, et al. 2005). New data supports endocrine dyscrasia and the subsequent alterations in downstream cell cycle signaling as causative of Alzheimer’s disease (AD), vascular dementia, stroke, Parkinson’s disease (PD) and other age-related neurological diseases. The elucidation of the non-gonadal functions of gonadotropins in the brain has revealed the importance of these hormones in regulating the early development, adult maintenance as well as the senescent decline of brain structure and function (Vadakkadath Meethal, et al. 2010).
2. Endocrine Dyscrasia
The decline in gonadal production of sex steroids and inhibins following menopause and during andropause leads to a loss of hypothalamic feedback inhibition that stimulates GnRH1 and gonadotropin production (Larson, et al. 2003). In addition, the decrease in gonadal inhibin production at this time (Reichlin 1998) results in decreased activin receptor inhibition, and together with the increase in bioavailable activin (Gray, et al. 2002) leads to a further increase in the secretion of GnRH1 and gonadotropins (MacConell, et al. 1999; Schwall, et al. 1988; Weiss, et al. 1993). Thus, the lack of negative feedback from the ovary/testis (progesterone (P4), 17β-estradiol (E2), testosterone and inhibin) is responsible for the unopposed and marked elevations in the secretion of GnRH1 and gonadotropins that is associated with ovarian and testicular senescence (Atwood et al. 2005; Chakravarti, et al. 1976; Neaves, et al. 1984; Reame, et al. 1996; Schmidt, et al. 1996). Although post-reproductive elevations in gonadotropins have been recognized for decades (Chakravarti et al. 1976; Couzinet and Schaison 1993; Neaves et al. 1984), their implications to the health of the brain only began to be elucidated in the early 2000’s (Bowen, et al. 2000; Bowen, et al. 2002a; Bowen, et al. 2004b). These discoveries led to the development of a new theory of aging (The Reproductive-Cell Cycle Theory of Aging) that proposed reproductive hormones in balance promote brain growth and development but that the endocrine dyscrasia following menopause and during andropause induces the brain senescent phenotype via alterations in cell cycle signaling (Atwood and Bowen 2011; Bowen and Atwood 2004). This mechanism is relevant to all reproductive species. An important basis for the development of this theory was that receptors for hypothalamic-pituitary-gonadal (HPG) axis hormones are present not only in the brain, but in other tissues of the body (reviewed in (Bowen and Atwood 2004; Vadakkadath Meethal and Atwood 2005)). Moreover, the elevations in circulating hCG early in life required for embryogenesis (Gallego, et al. 2008; Vadakkadath Meethal et al. 2010), and of the elevation in LH, a hCG homolog, post-menopause and during andropause (Chakravarti et al. 1976; Couzinet and Schaison 1993; Neaves et al. 1984), indicated the cell cycle regulatory properties for these hormones may dictate both developmental and senescent pathways in the brain (hCG and LH share 83 % amino acid sequence homology and bind a common receptor with similar affinity (Fiddes and Talmadge 1984)).
3. Gonadotropins, GnRH1 and Developmental Neurogenesis
3.1 Neurogenic Properties of Gonadotropins and GnRH1
Gonadotropin signaling is a crucial early signal for embryonic and neural development. hCG signaling via its human embryonic stem cell (hESC) receptor is essential for the proliferation of pluripotent hESC; inhibition of LH/hCG receptor (LHCGR) signaling with P-antisense oligonucleotides suppressed hESC proliferation, as did a specific blocking antibody against the extracellular activation site of LHCGR, an effect that was reversed by treatment with hCG (Gallego, et al. 2010). hESCs express mRNA and protein for the full-length mature LH/hCG receptor (LHCGR; 92 kDa) (Gallego et al. 2010) and expression does not alter upon differentiation into embryoid bodies (EBs; structures that resemble early post-implantation embryos containing all three germ layers) (O’Shea 1999), or into neuroectodermal rosettes, which consist of >90% columnar neural precursor cells (NPC) and are the in vitro equivalent of a rudimentary neural tube (Gallego et al. 2010; Li and Zhang 2006). The immediate production of hCG following conception is therefore likely required to signal the proliferation of hESC during early embryogenesis. These data are supported by the known proliferative properties of (hyperglycosylated) hCG, which has been demonstrated to act as an autocrine factor on extravillous invasive cytotrophoblast cells to initiate and control invasion as occurs 1) at implantation of pregnancy and the establishment of hemochorial placentation, and 2) during malignancy such as with invasive hydatidiform mole and choriocarcinoma (Cole 2009). The neurogenic functions of hCG/LH may be mediated (or coordinated) via the upregulation in the synthesis of P4 or other sex steroids, as P4 has been found to be essential for the specification of pluripotent stem cells into a neuronal phenotype (Gallego et al. 2010; Gallego, et al. 2009). The requirement for progestagens and estrogens for the growth, development and day-to-day maintenance and connectivity of neurons is well described (Liu and Diaz Brinto 2011).
Hippocampal neurogenesis persists in adult mammals, but its rate declines dramatically with age (Tan, et al. 2010). Continued adult neurogenesis appears to be important for the normal functioning of the adult brain since the experimentally-induced decline in neurogenesis produces severe impairments in performance on some, although not all, memory tasks (Deng, et al. 2010). It has been shown that the age-dependent decline in neurogenesis is reversible in rodents (Tan et al. 2010). Adult neurogenesis may be regulated by HPG hormones via their receptors. LHCGR is expressed throughout all regions of the mammalian brain (reviewed in (Liu, et al. 2007a)), with the highest density of receptors being found in neurons within the hippocampus followed by the hypothalamus, cerebellum, choroid plexus, ependymal tanycytes of third, fourth, and lateral ventricles, cortex, brain stem, and anterior pituitary (al-Hader, et al. 1997a; al-Hader, et al. 1997b; Bukovsky, et al. 2003; Lei, et al. 1993). Subcutaneous administration of LH has been shown to induce neurogenesis in the hippocampus of the adult mouse (Mak, et al. 2007). Likewise, GnRH receptor 1 (GnRHR1) is localized to extrapituitary cells in the mammalian brain including the hippocampus, amygdala, entorhinal cortex and subiculum, with lower levels in the septum and frontal cortex (reviewed in (Vadakkadath Meethal and Atwood 2005; Wilson, et al. 2006b)), and in sheep there is evidence that GnRH1 directly, or indirectly via LH, induces neurogenesis in the hippocampus (Hawken, et al. 2009). The localization and relative expression of these hormones and their receptors relative to AD neuropathology requires further elucidation.
LH and hCG have powerful mitogenic properties (Berndt, et al. 2009; Berndt, et al. 2006; Davies, et al. 1999; Harris, et al. 2002; Horiuchi, et al. 2000; Sriraman, et al. 2001; Webber and Sokoloff 1981; Zygmunt, et al. 2002). LH secretion has been associated with increased proliferation of granulosa cells and activation of MAPKs such as ERK (Cameron, et al. 1996; Sasson, et al. 2004; Srisuparp, et al. 2003), and other signal transduction and transcription activators (Carvalho, et al. 2003); all of which play a major role in cell cycle events (Rubinfeld and Seger 2004) and, importantly, are associated with AD pathology (Perry, et al. 1999; Zhu and Watt 1999; Zhu, et al. 2002; Zhu, et al. 2000; Zhu, et al. 2001). LH levels also are associated with increased T-cell proliferation and activation (Athreya, et al. 1993b; Sabharwal, et al. 1992b) and peripheral blood lymphocyte proliferation (Costa, et al. 1990).
Further evidence for a role of LH/hCG in cell proliferation is demonstrated by the expression of membrane-associated LH, hCG and their subunits and fragments by tumor cells; hCG expression is considered a characteristic of certain cancer cells (Krichevsky, et al. 1995; Whitfield and Kourides 1985; Yokotani, et al. 1997) and is a common serum marker used to determine the progression or regression of cancer following chemotherapy (Stenman, et al. 2004). Both transformed neurons (M17 neuroblastoma cells) and primary neurons express LH (Wilson et al. 2006b). Thus, the intracellular accumulation of LH in pyramidal neurons in the AD compared with age-matched control brain (Bowen et al. 2002a) is suggestive of a cellular transformation from a differentiated to a mitotic phenotype.
3.2 Gonadotropins and Mitochondrial Biogenesis
Changes suggestive of mitochondrial replication have been reported in pyramidal neurons, those vulnerable to AD neuropathology (Hirai, et al. 2001). Pyramidal neurons of the AD brain contain 3-fold elevated levels of cytoplasmic mitochondrial DNA and increased Cox-1 expression, indicative of de novo mitochondrion synthesis that would be expected during cell division to meet the energy demands of the newly created daughter cells (Nunomura, et al. 2001). Interestingly, COX2 expression and prostaglandins synthesis in granulosa cells are upregulated by LH/hCG (Duffy and Stouffer 2001), suggesting a role for gonadotropins in mitochondrial biogenesis along with their well-known role in mitochondrial steroidogenesis.
4. Gonadotropins, GnRH1 and Cell Cycle Abnormalities in Alzheimer’s Disease
The neurodegenerative disorder AD accounts for ~ 70% of all dementia cases (Alzheimer’s-Association 2007; Cotter 2007) and is characterized neurologically by progressive memory loss, impairments in behavior, language, and visuo-spatial skills ultimately leading to death (McKhann, et al. 1984). Pathologically, the disease is characterized by neuron and synapse loss and dysfunction, microgliosis and the extracellular deposition of amyloid-β (Aβ) in amyloid plaques and the intracellular deposition of phosphorylated tau protein in neurofibrillary tangles. In addition, research over the last decade has revealed that cell cycle abnormalities represent a major neuropathological feature of AD. These abnormalities appear very early in the disease process, prior to the appearance of plaques and tangles and can explain many of the biochemical and neuropathological changes observed (Lee, et al. 2009). Thus, neuronal cell cycle regulatory failure may be a significant component of the pathogenesis of AD (Neve and McPhie 2007).
4.1 Aberrant Cell Cycle Signaling in AD
Although numerous hypotheses have been postulated to explain AD, much evidence now exists that AD is a disease of aberrant, albeit unsuccessful, re-entry of neurons into the cell cycle resulting in synapse and neurite contraction and neuron death (see (Atwood et al. 2005; Herrup, et al. 2004; Raina, et al. 2000; Wang, et al. 2009) for reviews (Arendt 2002; Boeras, et al. 2008; Bonda, et al.; Bonda, et al. 2009; Bowser and Smith 2002; Carvalho et al. 2003; Geller and Potter 1999; Goedert, et al. 1993; Granic, et al. 2009; Herrup et al. 2004; Hirai et al. 2001; Lee et al. 2009; Liu, et al. 2004; Nunomura et al. 2001; Rubinfeld and Seger 2004; Smith, et al. 1995; Su, et al.; Thakur, et al. 2008; Varvel, et al. 2008; Vincent, et al. 1996b; Yang, et al. 2001; Zhu et al. 2000; Zhu et al. 2001)). The unscheduled initiation of a cell division cycle in a mature, normally post-mitotic neuron has been demonstrated to lead to an abortive re-activation of a variety of cell cycle components and ultimately the demise of the cell. Neuronal changes supporting the involvement of cell cycle related events in the etiology of AD include:
Cell Cycle Markers
The ectopic expression of a number of cell cycle proteins have been reported in those regions of the brain affected by AD (e.g. cyclin B1, cdc2 kinase, PCNA, cdk4, p16, but not cyclins A and D), but not in areas unaffected by AD pathology or in control brains (Smith et al. 1995; Vincent et al. 1996b). A number of the cell cycle regulators have been detected in vulnerable neurons before lesion formation (Busser, et al. 1998; Vincent, et al. 1998). Progression into the cell cycle as assessed by the expression of different cell cycle markers appears to be dependent upon AD severity; neurons positive for NFT’s stain strongly for cdc2 kinase (cdk1) and its associated cyclin B1 in hippocampal regions of the AD brain, suggesting that in some cases the G1/S checkpoint has been bypassed and that the cell cycle is arrested at G2. Changes in cell cycle markers have been found for mild cognitive impairment leading Herrup et al. (Yang, et al. 2003) to suggest that both the mechanism of cell loss (a cell cycle-induced death) and the rate of cell loss (a slow atrophy over months) are identical at all stages of the disease process.
Chromosome Replication (Endoreduplication)
The most compelling evidence that differentiated neurons re-enter the cell cycle comes from Yang, Herrup and colleagues (Yang et al. 2001) who demonstrated that a significant number of neurons in affected regions of AD brain (hippocampal pyramidal and basal forebrain neurons) have undergone full or partial DNA replication, indicating certain neurons have completed S phase. Cells in unaffected regions of the AD brain or in the hippocampus of nondemented age-matched controls show no such anomalies. Therefore, AD neurons appear to complete a nearly full S phase, but because mitosis is not initiated, the cells remain polyploid. This genetic imbalance seems to persist for many months before the neurons die (Yang et al. 2001; Yang et al. 2003) and this genomic replication without cytokinesis (endoreduplication) will have dramatic implications for the overexpression of neuronal proteins such as AβPP, as is the case with Down syndrome (Beyreuther, et al. 1993) and AD (see below). Endoreduplication in plants is a well-described phenomenon that allows for sufficient protein synthesis (Larkins, et al. 2001). However, such overexpression in a normally differentiated cell population appears to promote neuron death.
Neuronal Hypertrophy
DNA content is almost invariably associated with the size of a cell. Hypertrophy of the neuronal cell bodies, nuclei, and nucleoli of CA1 of hippocampus and anterior cingulate gyrus neurons has been reported in asymptomatic AD and MCI subjects (Iacono, et al. 2009; Iacono, et al. 2008; O’Brien, et al. 2009; Riudavets, et al. 2007).
Mitochondrial Alterations
Changes suggestive of mitochondria replication have been reported in those neurons vulnerable to AD neuropathology (Hirai et al. 2001). Pyramidal neurons of the AD brain contain 3-fold elevated levels of cytoplasmic mitochondrial DNA and increased Cox-1 expression, indicative of de novo mitochondrion synthesis that would be expected during cell division to meet the energy demands of the newly created daughter cells (Nunomura et al. 2001). Unlike division competent neurons, it remains to be determined if such alterations in mitochondrial metabolism in differentiated neurons are responsible for an imbalance in energy metabolism observed in the AD brain.
Tau Phosphorylation and Neurofibrillary Tangle Formation
Phosphorylation of the microtubule-associated protein tau occurs during metaphase of neuronal division and during differentiation (Goedert et al. 1993; Liu et al. 2004) hyperphosphorylated tau is observed in neurons of the fetal brain (Goedert et al. 1993). Disassembly of the rigid microtubule structure of neurons for neuronal division is accomplished by removing the microtubule stabilizing protein tau, by its phosphorylation. Therefore, it is interesting that hyperphosphorylation of the microtubule-associated protein tau, as detected in neurofibrillary tangles, is one of the major pathological features of neuronal degeneration in AD (Iqbal, et al. 2005), and indicates attempted division of pyramidal neurons in AD.
Mitotic Signal Transduction Pathways
Signal transduction pathways, regulated by a variety of mitogens and growth factors, are upregulated in the AD brain (Zhu et al. 2001). Mitogen-activated protein kinases such as ERK and other signal transduction and transcription activators such as Janus kinase and phosphoinositol 3-kinase/Akt (Carvalho et al. 2003) play a major role in the entry of cells into the cell cycle, as well as controlling their progression throughout the various stages (Rubinfeld and Seger 2004). These pathways are associated with AD neuropathology (Zhu et al. 2000).
AD Neurons Proceed to Metaphase and then Arrest?
The above data suggest that most all the biochemical and pathological changes associated with AD can be explained by the aberrant reentry of fully differentiated neurons into the cell cycle (e.g. chromosomal replication leading to polyploidy, upregulation of cell cycle markers, tau phosphorylation, AβPP metabolism and Aβ deposition (see below), oxidative stress, increased mitochondrial DNA and Cox-1 expression, upregulated growth factor signaling pathways, synapse loss and death of differentiated neurons). Indeed, the AD brain displays many of the neuropathological and biochemical changes observed in the fetal brain, namely the presence of Aβ (Takashima, et al. 1990), hyper-phosphorylated tau (Goedert et al. 1993) and presenilin expression (Berezovska, et al. 1997). Therefore, Aβ appears to be involved with neuron death/remodeling, just as tau phosphorylation is known to destabilize microtubules in order for neuron division to occur during development (Goedert et al. 1993; Liu et al. 2004). It is unclear whether neurons are proceeding via the normal cell cycle division pathway, or an aberrant, uncoordinated, pathway. The failure of microtubules to form spindle fibers to attach to kinetochores (Chan and Yen 2003; Glotzer 1997) has been shown to arrest the cell in metaphase (M checkpoint). Likewise, improper alignment of the spindle will block cytokinesis; either of these processes if irreparable triggers neuron death.
There are a number of morphological and biochemical similarities between the aging brain and the fetal brain including the presence of Aβ and AβPP (Arai, et al. 1997; Takashima et al. 1990), hyper-phosphorylated tau (Goedert et al. 1993) and presenilin-1 expression (Berezovska et al. 1997). This increased developmental protein expression in the AD brain suggests reactivation of the cell cycle in differentiated neurons of the AD brain (Herrup and Yang 2007) and explains the majority of the biochemical and pathological features associated with the disease (Atwood et al. 2005; Meethal, et al. 2005). Two studies support this claim. Forced cell cycle activation in terminally differentiated neurons via conditional expression of the simian virus 40 large T antigen (oncogene) forms Aβ deposits and tau pathology in the mouse cortex (Park, et al. 2007). Similarly, forced cell cycle activation in primary neurons is accompanied by tau phosphorylation (McShea, et al. 2007).
Most recently, it has been demonstrated that neurogenesis in the adult brain is linked to memory loss (Akers, et al. 2014) (see next Section on ‘Neurodegenerative Diseases’). Increasing neurogenesis after the formation of a memory was sufficient to induce forgetting in adult mice. However, in contrast, during infancy, when hippocampal neurogenesis levels are high and freshly generated memories tend to be rapidly forgotten (infantile amnesia), decreasing neurogenesis after memory formation mitigated forgetting. Since hormones of the HPG axis regulate fetal and adult neurogenesis, any disruption in their signaling such as following menopause and during andropause whereby dyotic signaling drives aberrant re-entry of neurons into the cell cycle would therefore result in the loss of previously encoded memories and prevent the retention of new information as memories. This is supported by studies in precocial species, including guinea pigs and degus, where most granule cells are generated prenatally (Akers et al. 2014). Consistent with reduced levels of postnatal hippocampal neurogenesis, infant guinea pigs and degus do not exhibit forgetting. However, increasing neurogenesis after memory formation induced infantile amnesia in these species (Akers et al. 2014).
Based on this overwhelming evidence indicating that post-mitotic neurons re-enter the cell cycle in the AD brain, in 2000 we asked the question: “What are the physiological signaling factors driving the aberrant reactivation of the cell cycle in the late-onset AD brain”.
4.2 Gonadotropins Mediate Cell Cycle Signaling via AβPP and Cdk5
4.2.1 AβPP Modulation of Cell Cycle Signaling
Numerous reports now indicate that amyloidogenic pathways are involved in cell cycle signaling and neurogenesis. In vitro studies indicate that Aβ1–42 promotes neurogenesis of subventricular zone precursor (SVZ) cells derived from developing or young adult animals (Calafiore, et al. 2006; Heo, et al. 2007; Jin, et al. 2004a; Liu et al. 2004; Lopez-Toledano and Shelanski 2004; Plant, et al. 2003; Sotthibundhu, et al. 2009). Plant and colleagues (2003) demonstrated that the production of Aβ was a critical requirement for the viability of central neurons. Inhibition of secretase activity or sequestering of Aβ with antibodies decreased the viability of rat cortical neurons, rat cerebellar granule neurons and human SH-SY5Y cells, but secretase inhibitors did not alter the viability of rat astrocytes and a number of non-neuronal cell lines (HEK293, DDT1-FM2, and human teratorhabdoid tumor cells). In vivo, an increase in hippocampal neurogenesis and/or proliferation has been reported in younger AD transgenic mouse models (Donovan, et al. 2006; Ermini, et al. 2008; Gan, et al. 2008; Jin et al. 2004a; Jin, et al. 2004b; Lopez-Toledano and Shelanski 2007), although hippocampal neurogenesis is decreased in a variety of AD mouse models displaying a ‘pathological’ plaque burden (Dong, et al. 2004; Donovan et al. 2006; Gan et al. 2008; Zhang, et al. 2007). Sotthibundhu et al., (Sotthibundhu et al. 2009) have shown that endogenous generation of Aβ in C100 and APP/presenilin-1 transgenic models of AD stimulates neurogenesis of young adult SVZ precursors. Overexpression of AβPPsw+ and AβPPwt protein in transgenic mice induces hypertrophy of cortical neurons (Oh, et al. 2009). Since cell size is strongly correlated with DNA content both between and within species (Gregory 2001, 2002), these results imply a central role for AβPP and/or Aβ in driving post-mitotic neurons back into the (S-phase of the) cell cycle. Interestingly, injection of a lentiviral-Aβ42 vector into the primary motor cortex of rats also results in astrogliosis (Rebeck, et al. 2010).
Supporting the neurotrophic/mitogenic properties of Aβ, we reported that hESCs express AβPP and truncated variants, together with ADAM-10 (α-secretase), BACE-1 (β-secretase), and five known components of the γ-secretase complex: presenilin-1, nicastrin, APH-1, PEN-2, and CD147 (Porayette, et al. 2009; Porayette, et al. 2007). These secretases were demonstrated to be functional with the differential processing of AβPP via these enzymes regulating the proliferation and differentiation of hESCs. hESCs endogenously produce Aβ1–40 and to a lesser extent, Aβ1–42. When added exogenously in soluble and fibrillar forms Aβ1–42 markedly increases hESC proliferation (Porayette et al. 2009), but does not induce cell differentiation into NPC. Chen and Dong (Chen and Dong 2009) reported soluble Aβ promotes neuronal growth (entry into the S-phase) as well as differentiation (at the end of the S-phase) in primary neural progenitor cells; Aβ1–40 increased neuronal markers, while Aβ1–42 induced astrocyte markers in neural progenitor cells.
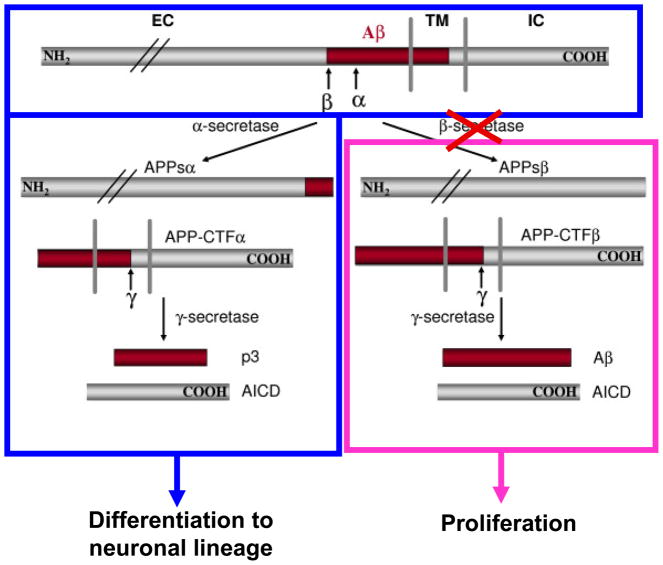
These reports suggest a normal physiological function for Aβ in cell cycle signaling. However, the overexpression of wild-type AβPP, which promotes Aβ generation (Citron, et al. 1997), has been shown to promote the aberrant re-entry of primary neurons into the cell cycle, as demonstrated by the initial induction of DNA synthesis and expression of cell cycle markers, followed by apoptotic cell death (McPhie, et al. 2003). Reactivation of the cell cycle following increased AβPP expression or processing involves the upregulation of p21-activated kinase-3 (McPhie et al. 2003), cyclins D1 and B1, and Rb (Ahn, et al. 2008; Malik, et al. 2008). Overexpression of wild-type AβPP (Bursztajn, et al. 1998; McPhie, et al. 2001; Nishimura, et al. 1998) and familial AD (FAD) mutant AβPP (McPhie et al. 2001; Yamatsuji, et al. 1996) has been shown to induce apoptosis in both primary neurons and cell lines. Therefore, the regulated processing of AβPP appears necessary for maintaining normal cell cycle dynamics. In this regard, we reported for hESC that Aβ promotes cell proliferation, while secreted AβPPα treatment suppresses hESC proliferation and promotes the differentiation of pluripotent hESC into a neuronal phenotype (NPCs) (Porayette et al. 2009), a finding supported by LaFerla and colleagues (Freude, et al. 2011) (Fig. 1). Likewise, inhibition of AβPP cleavage by β-secretase inhibitors significantly suppressed hESC proliferation and promoted nestin expression, an early marker of neural precursor cell formation. These data are consistent with numerous reports demonstrating the involvement of non-amyloidogenic fragments of AβPP in neuronal differentiation (Allinquant, et al. 1995; Clarris, et al. 1995; LeBlanc, et al. 1992; Majocha, et al. 1994; Milward, et al. 1992; Ohsawa, et al. 1997; Perez, et al. 1997; Qiu, et al. 1995; Small, et al. 1994); reviewed in (Gralle and Ferreira 2007). Recently, it has been shown that Aβ signaling through tau is necessary to drive ectopic neuronal cell cycle re-entry in mouse primary neurons and in an APP-transgenic (hAPPJ20) mouse (Seward, et al. 2013).
Figure 1. Model of AβPP Differential Processing During hESC Differentiation.
The processing of AβPP towards the amyloidogenic pathway promotes hESC proliferation (pink box) whereas non-amyloidogenic processing induces hESC differentiation into NPCs (blue box). Inhibition of β-secretase cleavage of AβPP (red cross) significantly suppresses hESC proliferation and promotes nestin expression and NPC formation. Addition of secreted AβPPα suppresses hESC proliferation and promotes the formation of NPCs.
The above studies indicate that while the early expression and differential processing of AβPP are normal processes important for neurogenesis in the embryo and adult brain, alterations in AβPP expression or processing resulting in Aβ production lead to aberrant cell cycle re-entry. Since AβPP is involved in cell cycle signaling, whatever regulates AβPP processing and expression is therefore directly implicated in cell cycle dynamics in the brain.
4.2.2 LH/hCG Modulates AβPP Processing and Expression
We discovered in 2004 that LH/hCG promotes the processing of AβPP towards the amyloidogenic pathway in vitro and in vivo (Bowen et al. 2004b). LH induced an increase in the generation and secretion of Aβ, coupled with decreased secretion of AβPP and increased AβPPCT100 production (sAβPPβ) in human M17 neuroblastoma cells (Bowen et al. 2004b). An increase in sAβPPβ levels has also been observed in the SH-SY5Y neuroblastoma cell line following treatment with 30 mIU but not 10 mIU/ml of hCG, supporting the notion that menopausal concentrations of LH contributes to elevated Aβ levels at least in part by increasing β-cleavage of APP by β-site APP cleaving enzyme (Saberi, et al. 2013). We also have determined using a developmental system of neurogenesis that hCG induces the expression of AβPP in hESC (Porayette et al. 2007).
The role of LH/hCG in regulating the expression and processing of AβPP was further supported by our studies utilizing GnRH1 agonists to suppress gonadotropin production. Treatment of C57/Bl6 mice with the GnRH1 agonist leuprolide acetate, which suppresses the serum concentrations of both sex steroids and gonadotropin resulted in a 3.5-fold and a 1.5-fold reduction in total brain Aβ1–42 and Aβ1–40 concentrations, respectively, after 2 months of treatment (Bowen et al. 2004b). Given that ovariectomy suppresses circulating sex steroids but elevates gonadotropins (Liu, et al. 2007b) and generally increases brain Aβ (Gouras, et al. 2000; Pike, et al. 2009; Xu, et al. 1998), our results suggested that AβPP processing towards the amyloidogenic pathway was due to the suppression of LH (Bowen et al. 2004b) and not the suppression of sex steroids (Gouras et al. 2000; Xu et al. 1998). These data were confirmed by Thornton and colleagues who demonstrated that hCG treatment of E2-implanted female rats significantly increased soluble Aβ40 and Aβ42 levels in whole brains and hippocampi (Berry, et al. 2008). Likewise, Wahjoepramono and colleagues demonstrated that LH implanted in slow-release pellets on the cortex (interhemispheric subdural space of the frontal lobe) of gonadectomized male guinea pigs (which express the human form of Aβ) significantly increased cerebrospinal fluid, hippocampal and frontal cortex concentrations of Aβ40 (Wahjoepramono, et al. 2011). AβPP and its C-terminal fragments (AβPP-CTF) were significantly increased in the brain in response to LH exposure. These workers also demonstrated that ovariectomy increases cerebrospinal fluid and hippocampal, but not frontal cortex, concentrations of Aβ40.
To date, studies of non-transgenic female animals (i.e. wild-type) have been consistent, with rats (Berry et al. 2008) and guinea pigs (Wahjoepramono et al. 2011) showing LH elevates Aβ production. Suppression of gonadotropins with leuprolide acetate also has been demonstrated to decrease Aβ load in wild-type mice (Bowen et al. 2004b), and, albeit modestly, in aged transgenic mice carrying AβPP Swedish (APPsw+) mutations (Casadesus, et al. 2006b). Together, these results indicate that LH/hCG is a physiologically relevant signal that regulates neuronal AβPP metabolism. This is supported by evidence from human studies. Multiple linear regression analysis reveals that serum LH concentration, but not testosterone, significantly correlates with plasma Aβ levels in men (Verdile, et al. 2014; Verdile, et al. 2008), suggesting that increased serum LH concentration, rather than lower serum free testosterone, is associated with the accumulation of Aβ in plasma. Contrary to these findings, a decrease in brain Aβ and improvement in cognition following leuprolide acetate treatment was not observed in the overexpressing APPsw+, PS1(M146V), and tau(P301L) (triple) transgenic male mouse (Rosario, et al. 2012). Whether the data reported by Rosario and colleagues (Rosario et al. 2012) is an artifact of the 3xTg mouse as suggested by a recent report, or a result of gender is not clear and warrants further investigation (Palm, et al. 2014).
The role of LH in mediating AβPP processing was confirmed in a bigenic mouse model that expresses APPsw+ in the background of a LH receptor (Lhr) knockout mouse (APPsw+/Lhr−−/; (Lin, et al. 2010). Despite the ~10-fold elevation in AβPP/Aβ production by APPsw+ mice (Hsiao, et al. 1996), genetic ablation of Lhr in either males or females resulted in a significant reduction in amyloid load and total number of Aβ plaques in the hippocampus and cerebral cortex of male and female mice. These results not only demonstrate that LH signaling is required for AβPP processing, but that normally endogenous LH is a major signaling factor in the processing of AβPP to Aβ in all transgenic mouse models. Elevations in circulating LH post-reproduction in the mouse (Wilson, et al. 2008) would explain the aging requirement for the deposition of Aβ in these APPsw+ overexpressing animals, while the induction of the estrus cycle (and the LH surge) at puberty may be a sufficient signal for the earlier amyloidosis (6–9 months) in mice overexpressing both AβPP and presenilin-1 (The Jackson Laboratories; (Borchelt, et al. 1996)). The influence of mutations in AβPP and presenilin-1 must also be taken into account in the generation and deposition of Aβ, nonetheless, the fact that these models overexpressing AβPP and presenilin-1 mutations do not develop Aβ immediately indicates the importance of LH signaling in Aβ generation and amyloidosis. LH-induced amyloidosis also would explain the female predisposition for amyloidosis in these transgenic models (Lee, et al. 2002; Wang, et al. 2003); female mice become post-reproductive earlier than male mice (i.e. they experience endocrine dyscrasia earlier than male mice).
Several other Aβ deposition-related neuropathologic features and functionally relevant molecules are markedly improved with Lhr ablation in APPsw+ mice, including decreased astrogliosis, reductions of elevated phosphorylated tau, c-fos, α7-nicotinic acetylcholine receptor, and restoration of the altered neuropeptide Y receptors Y1 and Y2. Together with the data presented in Bowen et al., (Bowen et al. 2004b) and Berry et al., (Berry et al. 2008), these data suggest that LH rather than sex steroids regulate AβPP processing (it should be noted that in Lhr−−/− mice, while females have suppressed serum E2 (~60 %) compared with Lhr−+/+ mice, supporting LH regulation of AβPP processing, male Lhr−−/− mice have 3-fold elevated E2 levels compared with Lhr−+/+ mice; (Lei, et al. 2001)). A study provides support for testosterone independently regulating AβPP metabolism. Li and colleagues (McAllister, et al. 2010) using male aromatase knock-out mice (decreased E2, but elevated testosterone and LH - at least in females; (Liew, et al. 2010) crossed with an AβPP transgenic mouse (APP23/Ar+/−) demonstrated a suppression of β-secretase cleavage of AβPP and decreased deposition of Aβ. Hormone levels in these AβPP transgenic crosses were not assessed, making it difficult to determine the contribution of LH and sex steroids to amyloidosis. These results do however indicate that the ratio of sex steroids:LH may be more important in determining the direction of AβPP processing. In this respect, the role of continuous versus cyclic P4 on AβPP processing also needs to be addressed (Carroll, et al. 2010).
Corticotropin-releasing hormone, released following stress, appears to decrease amyloidogenic processing of AβPP (Lezoualc’h, et al. 2000), and is consistent with the suppression of gonadotropins following CRH treatment (Barbarino, et al. 1989a; Barbarino, et al. 1989b).
4.2.3 LH Modulates Tau Phosphorylation
The phosphorylation of tau is another mitogenic-associated event that normally occurs during metaphase of neuronal division, and is observed during differentiation of neurons in the fetal brain (Goedert et al. 1993; Liu et al. 2004). Tau is a major protein component of the neurofibrillary tangle (NFT), the major intracellular pathology of AD, and is composed of highly phosphorylated form of the microtubule-associated protein tau (Grundke-Iqbal, et al. 1986; Ihara, et al. 1986; Iqbal et al. 2005; Iqbal, et al. 1984). Tau’s major cellular role is thought to involve regulation of neuronal microtubule assembly and stabilization of microtubules against depolymerization in vivo (Drubin, et al. 1986; Drubin and Kirschner 1986; Kanai, et al. 1989). Disassembly of the rigid microtubule structure of neurons for neuronal division is accomplished by removing the microtubule stabilizing protein tau via its phosphorylation. The residue-specific phosphorylation of tau in mitotically active neurons is driven by cyclin-dependent kinases (Cdk; (Brion and Couck 1995; Brion, et al. 1994; Goedert et al. 1993; Kanemaru, et al. 1992; Pope, et al. 1994). Several Cdk’s are associated with phosphorylated tau in AD and in vitro phosphorylate tau in a manner similar to that found in AD (Arendt, et al. 1995; Arendt, et al. 1996; Nagy, et al. 1997; Vincent, et al. 1996a). A number of other kinases such as glycogen synthase kinase-3β (GSK3β) also are pivotal in tau phosphorylation (e.g. (Lovell, et al. 2004). Compelling evidence that reactivation of the cell cycle induces tau phosphorylation is provided by two studies: McShea and colleagues (McShea et al. 2007) demonstrate that cell cycle induction in vitro induces tau phosphorylation while Park and colleagues (Park et al. 2007) demonstrate that cell cycle induction in vivo induces NFT and amyloid deposits. More recently, it has been demonstrated that specific phosphorylation of tau (Thr231) can promote MAPK activation in PC12 cells, which in turn could (further) activate the cell cycle re-entry mechanisms in neurons (Leugers, et al. 2013; Leugers and Lee 2010).
LH has been reported to regulate tau phosphorylation in vitro (Casadesus, et al. 2006a) and in vivo (Lin et al. 2010). Genetic ablation of Lhr in APPsw+ mice decreased tau phosphorylation by ~50% that induced by AβPP overexpression in these mice (Lin et al. 2010). LH has been shown to modulate the expression of numerous genes involved in cytoskeletal organization (Sasson et al. 2004). In this respect, we have observed concurrent increases in both LH expression (Liu et al. 2007b) and tau phosphorylation (Goedert et al. 1993; Liu et al. 2004) by rat embryonic neurons during in vitro differentiation. In reproductive tissues, LH has been shown to increase both the expression and kinase activity of Cdk5 in Leydig TM3 cells (Musa, et al. 2000). Supporting LH-induced Cdk5 expression, a significant decrease in Cdk5 expression and activity has been noted in rat testis after hypophysectomy (Musa et al. 2000).
These LH-mediated changes in Cdk5 metabolism are important in the context of reports that Cdk5 is a potent cell cycle suppressor (Cicero and Herrup 2005; Zhang and Herrup 2008). In particular, although Cdk5 is normally located in both nucleus and cytoplasm (Nikolic, et al. 1996; Zhang and Herrup 2008, 2011; Zhang, et al. 2010a; Zhang, et al. 2010b), the loss of nuclear Cdk5 leads to a failure of cell cycle suppression both in vivo and in vitro. Cell cycle activity detected in Cdk5−/− neurons includes the abnormal expression of cell cycle proteins such as cyclin D, cyclin A, and PCNA (proliferating cell nuclear antigen) as well as 5-bromo-2-deoxyuridine incorporation (Cicero and Herrup 2005). Similar cell cycle events are found in neurons at risk for death in AD (Busser et al. 1998; O’Hare, et al. 2005). In post-mitotic neurons in culture, Cdk5 nuclear export is required for cell cycle re-entry (Zhang, et al.). Cell cycle suppression by Cdk5 requires its binding to the p35 activator protein and E2F1. Formation of this complex excludes the E2F1 cofactor, DP1, thus inhibiting E2F1 binding to the promoters of various cell cycle genes. In this way, the formation of the E2F1–Cdk5–p35 complex in the nucleus prevents the advance of the cell cycle and appears to be a neuroprotective function of Cdk5 (Zhang, et al.).
4.2.4 Endocrine Dyscrasia Regulates Both Aβ and Tau Metabolism in the Reactivation of the Cell Cycle
LH-induced changes in both AβPP metabolism and Cdk5 metabolism (and concurrent tau phosphorylation) may be required for cell cycle re-entry. While both Aβ and P301L tau expression independently affect the regulation of cell proliferation and synaptic elements, cell cycle re-entry as assessed by DNA synthesis is only observed when SH-SY5Y cells overexpressing human wild-type or P301L tau were incubated with Aβ (Hoerndli, et al. 2007). Similarly, in vitro studies using differentiated neurons exposed to Aβ exhibit Cdk5-mediated tau hyperphosphorylation, cell cycle re-entry and neuronal loss (Lopes, et al. 2007a, 2009b; Seward et al. 2013). Inhibition of Cdk5 activity or tau phosphorylation (reviewed in (Liu et al. 2004) prevents Aβ-mediated cell death. In vivo, icv-injection of mice with Aβ activates Cdk5, promoting tau phosphorylation, cell cycle induction, synaptotoxicity, and death of post-mitotic neurons (Lopes, et al., 2007b; Lopes et al. 2009b). The sex hormone mediated changes in AβPP and Cdk5 metabolism (see above) may therefore be responsible for inducing cell cycle reactivation and death of post-mitotic neurons. Similar cell cycle changes were not observed in the 3xTg-AD model (Lopes, et al. 2009a), although the hormonal status of these young mice is different to the hormonal status of older mice.
The role of gonadotropins in regulating Aβ clearance in the regulation of this mitotic signal has not been explored. Gonadectomy decreases the expression/activity of the Aβ cleavage enzymes neprilysin and insulin-degrading enzymes in normal and transgenic rodents, an effect that is reversed by exogenous E2 administration (Huang, et al. 2004; Jayaraman, et al. 2012; Yue, et al. 2005; Zhao, et al. 2011). Although E2 and P4 increase IDE expression in vitro, like the in vivo results it awaits to be determined if the ratio of goandotropins:sex steroids is important in the regulation of Aβ cleavage enzymes.
Endocrine dyscrasia also may be responsible for altered AβPP processing and Aβ generation and deposition in the brains of semelparous spawning kokanee salmon (Oncorhynchus nerka kennerlyi; (Maldonado, et al. 2000). Aβ immunoreactivity is not found in immature (somatically mature but sexually immature) fish, and while first appearing in maturing or sexually mature fish, extracellular Aβ deposition dramatically increases between sexually mature and spawning fish (1–2 weeks in all fish) in an extremely rapid and synchronized process (Maldonado, et al. 2002). Since spawning in salmon is well recognized to involve major endocrine changes (Ando and Urano 2005) and somatic senescence, these results suggest endocrine mechanisms mediating Aβ deposition as a late event in the life cycle of neurons.
Advanced glycation end-products also have been demonstrated to be mitogenic signals that trigger cell cycle reentry of neurons in a mouse model of neurodegeneration (Kuhla, et al. 2014). Sex hormones are known to regulate advanced glycation end-product levels (Diamanti-Kandarakis, et al. 2010; Diamanti-Kandarakis, et al. 2005), although whether age-related endocrine dyscrasia alters advanced glycation end-product formation/degradation remains to be determined. Likewise, it is not known whether advanced glycation end-product-induced cell cycle reentry is mediated via Aβ and tau metabolism.
4.2.5 Endocrine Dyscrasia Regulates Inflammatory Cytokines, Neuroinflammation and Microgliosis
LH, which is elevated in men with AD, is positively correlated with tumor necrosis factor (TNF) (Butchart, et al. 2013). Gonadotropins are known to regulate AβPP processing and Aβ generation and deposition via TNF (see (Clark, et al. 2012; Clark and Atwood 2011) for reviews), a master inflammatory cytokine, which is also known to alter cell cycle dynamics (Darzynkiewicz et al., 1984). FSH has been reported to induce TNF in vitro (Iqbal et al., 2006), while leuprolide acetate has been reported to reduce a number of inflammatory cytokines, namely IL-1β (Meresman et al., 2003), IL-6 (Ferreira et al., 2010; Ficicioglu et al., 2010), and MCP-1(Khan et al., 2010), all of which are induced by TNF (Shalaby et al., 1989; Mueller et al., 2010; Charles et al., 1999) and reduced by anti-TNF treatment (Redl et al., 1996; Brennan et al., 1989; Charles et al., 1999). Insulin resistance, commonly a TNF-induced state, is routinely seen in late pregnancy (Ryan et al., 1985), a time when hCG is markedly elevated. Late pregnancy is also a time of physiological low-grade inflammation (de Castro et al., 2011) that is plausibly regulated by the interactions of gonadotropins and TNF. A role for gonadotropins in the regulation of TNF production is supported by the capacity of both E2 and P4 to reduce TNF expression in astrocytes (Kipp et al., 2007).
The expression and processing of AβPP is regulated by inflammatory cytokines, including TNF and IL-1. These cytokines regulate the promoter region of the AβPP gene (Ge and Lahiri, 2002), with its induction by these inflammatory cytokines reported in endothelial cells (Goldgaber et al., 1989), skeletal muscle (Schmidt et al., 2008), and 3T3 L1 adipocytes (So mmer et al., 2009) as well as brain (Brugg et al., 1995; Buxbaum et al., 1998). Regarding AβPP cleavage, IFN-γ, IL-1β and TNF specifically stimulate α-secretase activity, with an accompanying increased production of Aβ (Liao et al., 2004). IFN-γ and TNF were subsequently shown to enhance Aβ production from AβPP-expressing astrocytes and cortical neurons, and the numbers of astrocytes expressing IFN-γ to have increased (Yamamoto et al., 2007). This group also showed that TNF directly stimulates β-site AβPP-cleaving enzyme (BACE-1, or β-secretase) expression and thus enhance β-site processing of AβPP in astrocytes, and that TNFR1 depletion reduced BACE-1 activity (Yamamoto et al., 2007). Inhibition of soluble TNF signaling in the 3 x Tg mouse model of AD prevents the LPS-induced accumulation of 6E10-immunoreactive protein in hippocampus, cortex, and amygdala (McAlpine, et al. 2009). TNF also regulates Aβ-mediated alterations in synaptic transmission and plasticity (reviewed in (Clark et al. 2012; Clark and Atwood 2011)). Indeed, the release of pro-inflammatory cytokines from astrocytes is necessary for Aβ to be neurotoxic and for tau phosphorylation to be initiated (Garwood et al., 2011).
The elevation in circulating LH and FSH with aging also may explain the increased microgliosis associated with the aging and AD brain. Microgliosis is elevated in the FSH receptor knockout (FORKO) mouse that also carries expresses the amyloid-β precursor protein (APPsw+, Swedish mutation) gene and the presenilin-1 lacking exon 9 (PS1Δ9) gene compared with background APPsw+/PS1Δ9 mice (Prat, et al. 2011). FORKO mice have markedly elevated circulating LH and FSH concentrations (Danilovich, et al. 2001). Intriguingly, by 12 months of age more than 92% of FORKO animals develop various kinds of ovarian pathology, including neoplasms of sex cord-stromal type as well as cysts (Danilovich et al. 2001).
GnRH1 agonists regulate pro-inflammatory cytokine expression; high levels of agonists increase cytokine expression and low levels of agonists decrease cytokine expression ((Meresman, et al. 2003) (Raga, et al. 2008). Whether this effect is mediated directly through GnRHR1, or via indirect effects of gonadotropins or sex steroids is unclear.
4.2.6 Endocrine Dyscrasia and Cell Cycle Abnormalities in Peripheral Tissues in Alzheimer’s Disease
That endocrine dyscrasia promotes altered cell cycle signaling in AD is supported by studies in peripheral tissues demonstrating that LH induces lymphocyte proliferation (Athreya, et al. 1993a; Sabharwal, et al. 1992a) via LHCGR (Athreya et al. 1993a; Lin, et al. 1995), and that there are numerous cell cycle abnormalities in peripheral blood lymphocytes from those with AD (for example (Bajic, et al. 2008; Bialopiotrowicz, et al. 2011; Esteras, et al. 2012; Fischman, et al. 1984; Munoz, et al. 2008; Nagy, et al. 2002; Song, et al. 2012a; Song, et al. 2012b; Spremo-Potparevic, et al. 2004; Stieler, et al. 2012; Stieler, et al. 2001; Urcelay, et al. 2001; Yoon, et al. 2010; Zhou and Jia 2010). The contribution of direct hormonal changes versus hormonal driven epigenetic changes induced by the reproductive endocrine dyscrasia milieu on peripheral lymphocytes requires investigation.
4.2.7 Gonadotropins, GnRH1 and Cognition
The LH/hCG-mediated biochemical events described above are a portent to cognition changes. High concentrations of LH/hCG have been attributed to cognitive decline in LHβ-transgenic mice (Casadesus, et al. 2007), ovariectomized rats (Berry et al. 2008), ovariectomized C57/BL6 mice (Bryan, et al. 2010) and rats (Ziegler and Thornton 2010), castrated male rats (McConnell, et al. 2012), and presenilin 1 knock-in mice (Barron, et al. 2010). Conversely, suppression of gonadotropins with leuprolide acetate has been shown to improve cognitive performance in aged transgenic mice carrying the AβPP with the Swedish mutation (Casadesus et al. 2006b). As was the case for Aβ deposition, cognitive decline can be attributed to the increase in LH and not the loss of E2 (Berry et al. 2008; McConnell et al. 2012). In an object location memory task, ovariectomized rats treated with E2 and either a single high dose (400 IU/kg) or a lower repeated dose of hCG (75 IU/kg hourly for 8 h) showed spatial memory disruption compared to ovariectomized rats treated with E2 alone. Tests on another spatial memory task, the Barnes maze, confirmed that hCG (400 IU/kg) impaired memory: although E2 treated animals made significantly fewer hole errors across time, E2+hCG-treated animals did not. Strong support for this mechanism also is provided by Casadesus and colleagues (Casadesus et al. 2007) who showed that mice overexpressing LHβ, but not Lhr−/− mice (high LH but no LH signaling), show decreased hippocampal-associated cognitive performance, as measured with the Y-maze task, when compared to aged-matched wild-type animals. This group has also shown that suppression of ovariectomy-induced elevations in LH using the GnRH1 super-analogue, leuprolide acetate, improves cognitive function in the Morris water maze and Y-maze tests in the absence of E2 (Bryan et al. 2010). These effects appeared to be independent of the modulation of estrogen receptors α and β, or activation of CYP19 and StAR associated with the production of endogenous E2. However, pathways associated with improved cognition such as CaMKII and GluR1-Ser831 were up-regulated by leuprolide treatment but not by chronic long-term E2 replacement, suggestive of independent cognition-modulating properties by gonadotropins and sex steroids. Contrary to these findings, an improvement in cognition following leuprolide acetate treatment was not observed in the male overexpressing APPsw+, PS1(M146V), and tau(P301L) (triple) transgenic mice (Rosario et al. 2012). However, leuprolide acetate did improve spatial memory in ovariectomized 21-month old female triple transgenic mice (Palm et al. 2014). As mentioned earlier for brain Aβ, whether the data is an artifact of the 3xTg mouse, and/or a result of gender is not clear.
In humans, epidemiological studies also support elevated LH as an etiological factor in cognitive performance and AD. Compared to age-matched controls, circulating levels of gonadotropins have been shown to increase in subjects with AD (Bowen et al. 2000; Butchart et al. 2013; Hogervorst, et al. 2004; Hogervorst, et al. 2003; Hogervorst, et al. 2001; Short, et al. 2001). High circulating LH levels also have been associated with lower cognitive score in older post-menopausal women and in those post-menopausal women that are depressed (Rodrigues, et al. 2008). Similarly, in elderly men, higher serum LH has been associated with poor performance of immediate recall in healthy elderly men (as assessed with the California Verbal Learning Test Second Edition), independent of total and free testosterone concentrations (Hyde, et al. 2010), suggesting increased serum LH concentration, rather than lower serum testosterone, is associated with the poor memory recall. In contrast, these workers found that only total and free testosterone levels were associated with Standardized Mini-Mental State Examination score, suggesting independent actions of gonadotropins and androgens in differing cognitive domains. That LH signaling is involved in cognitive loss associated with AD is supported by the reversal of risk of AD in APOE e4 males carrying one or two C-alleles at lhcgr2 in the LHCGR (Haasl, et al. 2008). The elevations in circulating gonadotropins in AD also implicate elevations in circulating and tissue GnRH1 concentrations in the etiology of the disease, despite its short half-life in the serum (Fauconnier, et al. 1978; Redding, et al. 1973). Massive elevations in hypothalamic GnRH1 production have been noted in rhesus monkeys post-menopause (Gore, et al. 2004). GnRH1 signaling via GnRH1 receptor in extrapituitary tissues (Wilson et al. 2006b) of the brain can induce a long-lasting enhancement of synaptic transmission mediated by ionotropic glutamate receptors in CA1 pyramidal neurons of rat hippocampal slices (He, et al. 1999; Lu, et al. 1999). GnRH1 upregulates hippocampal spinophilin, a dendritic spine marker (Prange-Kiel, et al. 2008). Determination of brain and circulating GnRH concentrations in AD and their impact on neuron metabolism are required.
Elevations in gonadotropin levels in Klinefelter syndrome, a supernumerary X chromosome syndrome (XXY) that results in hypergonadotropic hypogonadism, also have been associated with cognitive performance. Aside from physical changes and infertility, those with Klinefelter syndrome have characteristic cognitive and language deficits of varying severity (Boada, et al. 2009), although examination of memory performance in older men has not been well characterized. Klinefelter men seem less accurate in perception of socio-emotional cues such as angry facial expressions, they are less able to identify and verbalize their emotions, but experience increased levels of emotional arousal, in comparison to the general population (van Rijn, et al. 2006). A mouse model of Klinefelter syndrome that are hypergonadotropic for serum and pituitary LH and serum FSH, with a trend towards lower serum testosterone, did not differ from their wild type littermates with respect to locomotion, exploration and anxiety-related behavior, but did display poorer recognition memory compared with controls (Lewejohann, et al. 2009).
Support for gonadotropins as mediating cognitive performance is found in studies on individuals with Down syndrome who have elevated serum concentrations of gonadotropins throughout life and develop AD-like neuropathology if they live into the fourth decade (Oliver and Holland 1986). In sharp contrast to the general population, males with Down syndrome develop these neuropathological changes earlier and more often than their female counterparts. The reversal in gender predilection cannot be explained on the basis of sex steroid concentrations since there is no difference between Down syndrome individuals and the general population. However, the increase in gonadotropin concentrations is more pronounced and occurs at an earlier age in Down syndrome males than in Down syndrome females (Schupf, et al. 1998).
Further evidence for endocrine dyscrasia as mediating cognitive decline is provided by two important epidemiological studies indicating that suppression of gonadotropins is associated with a decreased risk of developing AD. In the first of these studies, we addressed the risk of AD in men treated for prostate cancer since it is estimated that approximately 40% of prostatectomy patients will experience disease recurrence and undergo GnRH1 agonist therapy (Bowen, et al. 2004a). All Medicare hospitalization claims for men aged 67 to 75 during the years 1984 through 1997 were used to compare the incidence of dementia in men who underwent prostatectomy for prostate cancer (n=139,414) compared to three selected aged-matched control groups in which men underwent cholecystectomy for gallbladder disease (n=255,493), herniorrhaphy for inguinal hernia (n=162,115), and transurethral prostatectomy for benign prostatic hyperplasia (n=635,615). Since the only indication for GnRH1 agonist therapy in men is prostate cancer, none of the men in the control groups would be expected to have received GnRH1 therapy. At five years from the procedure date the relative risk of dementia for the control groups compared to the prostate cancer group was significantly increased 1.8 to 2.92-fold (Bowen et al. 2004a). In a second study, D’Amico and colleagues (D’Amico, et al. 2010)confirmed that in 6,647 men treated with brachytherapy for prostate cancer with (N = 1,700) or without (N = 4,947) GnRH1 agonist therapy between 1997 and 2007, that there was a significant reduction in the risk of death from AD in men who were treated with a GnRH1 agonist for a median of 4.0 months as compared with those who were not [adjusted hazard ratio: 0.45 (95% confidence interval, 0.25–0.83); P = 0.01]. These finding support the premise that GnRH1 agonists are associated with a decreased risk of death from AD.
In addition to GnRH1 agonists, it has been reported that the decapeptide Cetrorelix, an GnRH1 antagonist that inhibits gonadotropin and sex steroid secretion, has multiple effects on brain function (Telegdy, et al. 2009). Cetrorelix administration into the lateral ventricle of the mouse brain was able to correct the impairment of the memory consolidation caused by Aβ25–35. Cetrorelix also elicits anxiolytic and antidepressive action, but it did not influence the open-field behavior. Similar results were observed in a subsequent study using rats (Telegdy, et al. 2010). The effects of GnRH1 agonists and antagonist may be mediated via suppression of gonadotropin and sex steroid concentration, and/or directly via GnRHR1 previously identified in rodent and human brain (reviewed in (Vadakkadath Meethal and Atwood 2005; Wilson et al. 2008). Further studies are required to tease out the exact mechanisms by which GnRH1 signaling modulators regulate cognitive function.
5. Other Neurodegenerative Diseases
5.1 Blood-Brain Barrier Failure - Stroke, Meningitis and Encephalitis
The localization of LH/CGR to endothelial cells and smooth muscle cells of the vasculature (Berndt et al. 2006; Lei, et al. 1992; Toth, et al. 2001) suggests a role for LH/hCG in maintaining the dynamic structure of the blood-brain barrier and the vasculature. Indeed, hCG promotes angiogenesis by inducing vascular endothelial growth factor up-regulation (Berndt et al. 2006; Licht, et al. 2002; Zygmunt et al. 2002). Recent data demonstrate that physiological concentrations of hCG (10–400 IU/ml) significantly enhance pericyte sprouting and migration and give rise to the maturation and coverage of endothelial capillaries (Berndt et al. 2009). In a three-dimensional coculture model of endothelial and perivascular cells, hCG enhances vessel tube formation and endothelial/mural cell adhesion. In addition, hCG was shown to stimulate the proliferation of human umbilical vein endothelial cells and smooth muscle cells. hCG appears to mediate proliferative effects via protein kinase A and phospholipase C/protein kinase C pathways, while only phospholipase C/protein kinase C pathways were required for migrative processes. Interestingly, in vivo administration of hCG reduces vascular resistance in human uterus and decreases in vitro the formation of vasoconstrictor eicosanoids of the vascular wall (Toth et al. 2001; Toth, et al. 1994).
These observations have significant ramifications for hCG/LH-induced vascularization in the adult brain, since any dysregulation of LH and/or sex steroid signaling might be expected to alter cell cycle dynamics and vascular properties. In this respect, well characterized changes have been reported for the cerebrovasculature in the aged brain (and more so in the AD brain) and include decreased microvascular density, loss of endothelium, increased tortuosity, twisted/string vessels, fragmentation of the microvasculature, loss of the fine perivascular neural plexus, and lumpy vessels (de la Torre and Hachinski 1997). Our studies suggest that alterations in the permeability of the blood-brain barrier, and therefore vascular structure, may be mediated by the endocrine dyscrasia associated with menopause and andropause (Wilson et al. 2008). Such changes also might alter the selective permeability of the blood-brain barrier leading to stroke resulting from vascular hemorrhage; encephalitis as a result of serum components leaking into the brain; or meningitis due to foreign pathogens entering the brain.
The blood-brain barrier maintains brain homeostasis by limiting entry of substances to the central nervous system (Elrich 1885) through interaction of transmembrane and intracellular proteins that make up endothelial cell tight-junctions and gap-junctions. Ovariectomy was found to induce an increase in Evan’s blue dye extravasation into the brain of mice and a redistribution of the gap-junction protein connexin-43 along the extracellular microvascular endothelium (Wilson et al. 2008). Although, sex steroids have has been implicated in the modulation of blood-brain barrier permeability and vascularization (Bake and Sohrabji 2004), changes in the selective permeability of the blood-brain barrier following menopause is likely due to elevations in LH, (or at least an increase in the ratio of LH:sex steroids) since: 1) an increase in connexin-43 expression in the mouse brain following ovariectomy was suppressed in ovariectomized animals treated with leuprolide acetate (Wilson et al. 2008), 2) hCG regulates angiogenesis-vessel maturation by stimulating perivascular cell recruitment, migration, and proliferation, and by inference must be involved in vascular compliance (Berndt et al. 2009), and 3) hCG regulates tissue remodeling by increasing matrix metalloproteinase-9 production (Berndt et al. 2006; Licht et al. 2002). In this context, LH, which becomes markedly elevated with reproductive senescence, has been demonstrated to potently down-regulate, via PKA/MAPK signaling pathways, the expression of connexin-43 in large preovulatory follicles, theca cells and follicles undergoing atresia (Granot and Dekel 1994, 1997; Kalma, et al. 2004; Wehrenberg and Rune 2000; Wiesen and Midgley 1993). Likewise, hCG also potently decreases connexin-43 expression and morphological gap junctions in human myometrial smooth muscle cells (Ambrus and Rao 1994). In addition, LH decreases connexin-43 expression and endometrial thickness in the human endometrium (Granot, et al. 2000), promotes germinal vesicle breakdown possibly through a reduction of connexin-43 in cumulus cells (Shimada and Terada 2002) and induces a decrease in the integrity of gap junctions in apoptotic human granulosa cells (Sasson and Amsterdam 2002). LH has been shown to induce oocyte maturation via the interruption of cell-to-cell communication within the ovarian follicle, a process possibly mediated by the phosphorylation of connexin-43 (Sela-Abramovich, et al. 2005). Recently, hCG administration has been associated with heart complication including spontaneous coronary artery dissection (Lempereur, et al. 2014) and patent foramen ovale (Pektezel, et al. 2014).
Together, these results suggest that gonadotropins can regulate tissue-barrier function and that the elevation in circulating gonadotropins and decrease in circulating sex steroids following reproductive senescence may be responsible for the loss of selective permeability of the blood-brain barrier. The changes in vascular structure induced by age-related endocrine dyscrasia are likely to promote amyloidosis, since amyloid has barrier functions (reviewed in Atwood, (Atwood, et al. 2002; Atwood, et al. 2003). Paradoxically, elevated LH promotes Aβ production at a time when it also promotes changes to the selective permeability of the blood-brain barrier. Thus, the endocrine dyscrasia associated with aging likely plays a pivotal role in the alterations in vascular structure and function observed in the aging brain, stroke, vascular dementia and the development of AD.
5.2 Parkinson’s Disease
Differences in the neuroendocrine regulation of LH secretion in post-menopausal women with PD has been reported (Cagnacci, et al. 1991). Although plasma FSH was unchanged between PD and controls, plasma LH levels are significantly lower in Parkinsonian than in control women (Bonuccelli, et al. 1990; Cagnacci et al. 1991). While estrogens significantly blunted plasma FSH levels, plasma LH levels were reduced only in controls, but not in women with PD. In women with PD, estrogens also failed to restore the LH response to naloxone, suggesting reduced endogenous opioid control of the HPG axis in PD (Cagnacci et al. 1991). Other hypophyseal hormones and gonadal steroids are unchanged, suggesting a selective alteration of hypothalamic dopaminergic mechanisms which regulate LH secretion (Bonuccelli et al. 1990). Subsequently, it was found that dopaminergic therapy could restore LH (and adrenocorticotropic hormone/cortisol) responses to naloxone in Parkinsonian patients, indicating that Parkinson’s-related dopaminergic alterations may underlie the defective endogenous opioid control of LH (and adrenocorticotropic hormone/cortisol) secretion (Volpi, et al. 1994). Given that the neurotransmitter mechanisms which regulate LH secretion are altered, and that such changes may alter brain (i.e. hippocampal, cortical) LH production and subsequently neurosteroid production (Bowen et al. 2002a; Liu et al. 2007b; Meethal, et al. 2009a), endocrine dyscrasia in PD might also alter cell cycle dynamics in the substantia nigra.
The age-related course of PD supports endocrine mechanisms as one component in the etiology of PD (average age at onset ~50–55; (Haaxma, et al. 2007). Women develop PD at an average age of around 53 (~2 years post-menopause after onset of endocrine dyscrasia), an age of onset that correlates positively with parity, age at menopause and fertile life span. Men have a slightly earlier onset of PD (~51 years of age) that may relate to earlier endocrine dyscrasia (HPG hormone changes commence in men at ~30 years of age) (Belanger, et al. 1994; Hammar 1985; Muta, et al. 1983; Neaves et al. 1984; Takahashi, et al. 1983).
6. Endocrine Dyscrasia, Loss of Cell Cycle Regulation and Cognitive Consequences
The dyotic signaling that occurs following menopause at around 51 years of age in women, and throughout andropause that commences around 30 years of age in men, would be expected to increase amyloidogenic processing of AβPP and alter Cdk5/tau metabolism due to the increased LH/sex steroid ratio as outlined in the previous sections. In the brain, such signaling would drive the reactivation of the cell cycle in post-mitotic neurons, leading to their demise/dysfunction and a decline in cognitive performance (Akers et al. 2014). A decline in cognition might be expected to result from both the dysfunction of neurons attempting to divide (decreased neural transmission and synapses) together with the accumulative loss of neurons and their stored information. Indeed, increasing neurogenesis after the formation of a memory has been recently shown to be sufficient to induce forgetting in adult mice (Akers et al. 2014). By contrast, these authors also showed that during infancy, when hippocampal neurogenesis levels are high and freshly generated memories tend to be rapidly forgotten (infantile amnesia), decreasing neurogenesis after memory formation mitigated forgetting. Importantly, the infant-related increase in circulating LH and sex steroids (and activins/inhibins) during the first few months post-partum correlate with both the rapid growth of the brain and infantile amnesia at this time; the infant doubles in size during the first year of life (see (Bowen and Atwood 2004) for review).
In the vasculature, such dyotic signaling also promotes cell cycle re-entry, leading to cerebrovascular pathology, a compromised blood-brain barrier and eventually stroke.
That LH is available to the brain for such signaling is evidenced by findings that the highest density of LH receptors in the brain is found within the hippocampus (al-Hader et al. 1997a; al-Hader et al. 1997b; Lei et al. 1993), that LH crosses the blood-brain barrier (Lukacs, et al. 1995), and that LH accumulates intracellularly in the pyramidal neurons of AD compared with age-matched control brains (Bowen et al. 2002a).
Although the above sections describe LH as ‘the villain’, it is not only the elevation in gonadotropins that allow for cell cycle re-entry in post-mitotic cells, but the loss of differentiative sex steroids (Bowen and Atwood 2004). Thus, the ratio of gonadotropins:sex steroids is the real indicator of dyotic signaling leading to aberrant cell cycle re-entry. The loss of sex steroids as a component of decreasing cognitive function has been discussed in length elsewhere (Atwood et al. 2005), but is also clearly demonstrated by the decrement in cognitive performance associated with the sudden suppression of serum sex steroids to castrate levels in pre-menopausal women following chemical castration (using GnRH1 superagonists), and by the amelioration of these effects with administration of E2 (Newton, et al. 1996; Sherwin and Tulandi 1996; Varney, et al. 1993).
Dyotic signaling can therefore explain cognitive decline after menopause and at later stages of andropause. As such, decreasing LH signaling, and/or increasing sex steroid signaling (which has the added benefit of partially suppressing LH signaling; (Abdel-sayed, et al. 1989), may prove to be useful therapeutic strategies for cognitive decline associated with aging and age-related neurodegenerative diseases. Better still, complete rebalancing of all the hormones in the HPG axis following menopause and during andropause would be expected to ultimately offset neurodegeneration initiated by age-related endocrine dyscrasia.
7. Therapeutic Implications Based on Dyotic Signaling Mechanisms
Strategies to reverse dyotic signaling have been previously described (Atwood et al. 2005). In short, they consist of reestablishing circulating concentrations of HPG axis hormones to that of the young adult in terms of both concentration and cyclicity. This could be achieved pharmacologically (e.g. with HRT), or with cellular replacement-regeneration technologies.
Suppression of LH and GnRH1 signaling is one therapeutic option for the treatment of AD. This could be achieved using GnRH1 agonists or antagonists, or via supplementation with sex steroids. A small study has demonstrated that suppression of androgens and gonadotropins with GnRH1 agonists in men with prostate cancer significantly improved visual-memory (Rey test) 6 and 12 months, and significantly improved inversed number-memory test (WAIS) after 6 months (Nedelec, et al. 2009). Thus, global cognitive performances were preserved after 12 months of androgen and gonadotropin suppression. These data are consistent with an earlier study that tested leuprolide acetate (Lupron Depot) for the treatment of AD in a Phase II dose ranging study, although only recently published (Bowen, et al. 2014). Sub-group analysis of cognitive performance in women with mild to moderate AD taking acetylcholine esterase inhibitors (AChEI’s) and implanted subcutaneously at 0, 12, 24 and 36 weeks with high-dose (22.5 mg) leuprolide acetate showed a stabilization in cognitive decline (ADAS-Cog, ADCS-CGIC) and activities of daily living (ADCS-ADL) over a 48 week period. A similar effect was not observed in the low dose Lupron group taking AChEI’s, or in the placebo group taking AChEI’s, or when patients were treated with Lupron alone. A possible mechanism of action for this combination therapy is that Lupron acts to halt any further neurodegeneration thereby allowing AChEI’s to act on remaining neurons to maintain cholinergic function (Bowen et al. 2014). The dose effect seen in the study suggests that Lupron’s action is not solely due to its suppression of peripheral circulating concentrations of gonadotropins, which were similarly suppressed in low-dose and high-dose groups. Therefore, Lupron’s actions also might be due to its direct effect on GnRHR1 signaling within the brain (Wilson, et al. 2006a). GnRHR1s are expressed throughout the brain and their expression correlates to those areas with AD neuropathology (Wilson et al. 2006a). In this connection, we recently identified the existence of autocrine/paracrine feedback loops within the brain, in essence a feedback loop similar to the HPG axis that regulates neurohormone production (Meethal, et al. 2009b). Since GnRHR1 mediates neuronal LH expression and LH receptor signaling, high doses of Lupron might suppress the neuroautocrine production of LH, which we have previously demonstrated is elevated in expression and colocalizes with AD neuropathology (Bowen, et al. 2002b) while low doses might stimulate LH production. These findings together with biological and epidemiological evidence presented earlier suggest that the effects seen with Lupron are one of potential disease modification rather than symptomatic improvement (Atwood and Bowen 2011; Atwood et al. 2005; Berry et al. 2008; Bowen and Atwood 2004; Bowen et al. 2004a; Bowen et al. 2000; Bowen et al. 2002a; Bowen, et al. 2004a; Bryan et al. 2010; Casadesus et al. 2007; Casadesus et al. 2006b; D’Amico, et al. ; Lin et al. 2010; Short et al. 2001). Based on these clinical and epidemiological studies (Bowen et al. 2004a; D’Amico et al. 2010), further clinical studies utilizing strategies to rebalance gonadotropins and sex steroids are warranted.
These studies indicate that it is not so much the absolute concentrations of sex steroids and gonadotropins (which are both low following GnRH1 agonist treatment), but their ratio, that is important in determining whether post-mitotic cells (neurons) are driven back into the cell cycle. This is supported by HRT studies where administered sex steroids not only increase circulating sex steroids, but suppress LH and GnRH1 concentrations. Pharmacological HRT’s consisting of physiologically relevant sex steroids (i.e. E2 or testosterone) have been shown to decrease the incidence and delay the onset of cognitive decline among elderly women and men (reviewed in (Cherrier, et al. 2005; Gleason, et al. 2005; Tan and Pu 2003)). Indeed, an improvement in cognition has been reported in women with AD treated with E2 in 3 controlled (Asthana, et al. 2001; Asthana, et al. 1999; Wharton, et al. 2011) and 5 uncontrolled (Fillit, et al. 1986; Honjo, et al. 1989; Ohkura, et al. 1994a, 1995; Ohkura, et al. 1994b) intervention studies. Similarly, an improvement in cognition has been reported in two case-controlled intervention study of men with AD administered testosterone (Cherrier et al. 2005; Tan and Pu 2003). Moreover, E2 has been shown to improve the cognition of cognitively normal post-menopausal women in 12 of 15 studies (3 studies indicated no difference) (Gleason et al. 2005).
Intriguing, but largely ignored studies of the neuroregenerative properties of hCG on the transected spinal cord clearly demonstrate the neurotropic properties of HPG axis hormones. hCG improves the restoration of physiological continuity of the completely transected spinal cord in rats (Patil, et al. 1990) while LH induces neurogenesis in the adult mouse hippocampus (Mak et al. 2007). Whether these properties of hCG/LH in the adult brain are mediated directly, via P4 as we have demonstrated for embryonic neurulation (Gallego et al. 2008; Gallego et al. 2009), or E2, remains to be determined, although progestagens have been shown to significantly increase rat neuroprogenitor cell and human neural stem cell proliferation (Gould, et al. 2000; Wang, et al. 2005), and promote neurite development and migration that lead to changes in synaptogenesis (Leranth, et al. 2002; Masumoto, et al. 1991; McEwan, et al. 1996; Simerly 2002). In addition, P4 enhances learning and memory in tasks mediated by the prefrontal cortex and/or hippocampus of aged mice (Frye, et al. ; Frye and Walf, 2008a, 2009) and ovariectomized mice (Frye and Walf 2008b). It remains an enigma as to why P4 has not been tested clinically for the treatment of age-related neurodegenerative diseases.
8. Conclusion
While most attention in the field of neurodegenerative diseases has focused on sex steroids, the endocrine dyscrasia that accompanies menopause and andropause involves all hormones of the axis, such that the decline in circulating gonadal sex steroids and inhibins results in elevations in circulating gonadotropins and gonadotropin-releasing hormone. Evidence continues to build that this dyotic hormonal milieu drives post-mitotic neurons into an abortive cell cycle leading to the biochemical and neuropathological features of the disease with resultant neuronal dysfunction, cell death and cognitive decline. If overwhelming mitogenic signals in the form of gonadotropins and GnRH are the crux of the age-related neurodegeneration in AD then strategies to rebalance these hormones provide an obvious path to preventing and treating cognitive diseases.
Acknowledgments
This research was funded by the Alzheimer’s Association. This material is the result of work supported with resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The opinions expressed herein are those of the authors. The contents do not represent the views of the Department of Veterans Affairs or the US government. This article is Geriatrics Research, Education and Clinical Center VA paper 2014–xx.
Footnotes
Disclosure Statement
There are no actual or potential conflicts of interest.
References
- Abdel-sayed WS, Toppozada HK, Said SA, El-sayed OK. Some metabolic and hormonal changes in women using long acting injectable contraceptives. Alex J Pharm Sci. 1989;3:29–32. [PubMed] [Google Scholar]
- Ahn KW, Joo Y, Choi Y, Kim M, Lee SH, Cha SH, Suh YH, Kim HS. Swedish amyloid precursor protein mutation increases cell cycle-related proteins in vitro and in vivo. J Neurosci Res. 2008;86:2476–2487. doi: 10.1002/jnr.21690. [DOI] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- al-Hader AA, Lei ZM, Rao CV. Neurons from fetal rat brains contain functional luteinizing hormone/chorionic gonadotropin receptors. Biol Reprod. 1997a;56:1071–1076. doi: 10.1095/biolreprod56.5.1071. [DOI] [PubMed] [Google Scholar]
- al-Hader AA, Tao YX, Lei ZM, Rao CV. Fetal rat brains contain luteinizing hormone/human chorionic gonadotropin receptors. Early pregnancy : biology and medicine : the official journal of the Society for the Investigation of Early Pregnancy. 1997b;3:323–329. [PubMed] [Google Scholar]
- Allinquant B, Hantraye P, Mailleux P, Moya K, Bouillot C, Prochiantz A. Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol. 1995;128:919–927. doi: 10.1083/jcb.128.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s-Association. Alzheimer’s Disease Facts and Figures 2007. 2007. [Google Scholar]
- Ambrus G, Rao CV. Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology. 1994;135:2772–2779. doi: 10.1210/endo.135.6.7988470. [DOI] [PubMed] [Google Scholar]
- Ando H, Urano A. Molecular regulation of gonadotropin secretion by gonadotropin-releasing hormone in salmonid fishes. Zoolog Sci. 2005;22:379–389. doi: 10.2108/zsj.22.379. [DOI] [PubMed] [Google Scholar]
- Arai Y, Suzuki A, Mizuguchi M, Takashima S. Developmental and aging changes in the expression of amyloid precursor protein in Down syndrome brains. Brain Dev. 1997;19:290–294. doi: 10.1016/s0387-7604(97)00559-7. [DOI] [PubMed] [Google Scholar]
- Arendt T. Dysregulation of neuronal differentiation and cell cycle control in Alzheimer’s disease. Journal of neural transmission. Supplementum. 2002:77–85. doi: 10.1007/978-3-7091-6139-5_8. [DOI] [PubMed] [Google Scholar]
- Arendt T, Holzer M, Grossmann A, Zedlick D, Bruckner MK. Increased expression and subcellular translocation of the mitogen activated protein kinase kinase and mitogen-activated protein kinase in Alzheimer’s disease. Neuroscience. 1995;68:5–18. doi: 10.1016/0306-4522(95)00146-a. [DOI] [PubMed] [Google Scholar]
- Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuroreport. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Athreya BH, Pletcher J, Zulian F, Weiner DB, Williams WV. Subset-specific effects of sex hormones and pituitary gonadotropins on human lymphocyte proliferation in vitro. Clinical immunology and immunopathology. 1993a;66:201–211. doi: 10.1006/clin.1993.1026. [DOI] [PubMed] [Google Scholar]
- Athreya BH, Pletcher J, Zulian F, Weiner DB, Williams WV. Subset-specific effects of sex hormones and pituitary gonadotropins on human lymphocyte proliferation in vitro. Clinical immunology and immunopathology. 1993b;66:201–211. doi: 10.1006/clin.1993.1026. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bishop GM, Perry G, Smith MA. Amyloid-beta: a vascular sealant that protects against hemorrhage? Journal of neuroscience research. 2002;70:356. doi: 10.1002/jnr.10388. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bowen RL. The reproductive-cell cycle theory of aging: an update. Experimental Gerontology. 2011;46:100–107. doi: 10.1016/j.exger.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bowen RL, Smith MA, Perry G. Cerebrovascular requirement for sealant, anticoagulant and remodeling molecules that allow for the maintenance of vascular integrity and blood supply. Brain research. Brain research reviews. 2003;43:164–178. doi: 10.1016/s0165-0173(03)00206-6. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- Bajic VP, Spremo-Potparevic B, Zivkovic L, Djelic N, Smith MA. Is the time dimension of the cell cycle re-entry in AD regulated by centromere cohesion dynamics? Bioscience hypotheses. 2008;1:156–161. doi: 10.1016/j.bihy.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Barbarino A, De Marinis L, Folli G, Tofani A, Della Casa S, D’Amico C, Mancini A, Corsello SM, Sambo P, Barini A. Corticotropin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism: clinical and experimental. 1989a;38:504–506. doi: 10.1016/0026-0495(89)90208-4. [DOI] [PubMed] [Google Scholar]
- Barbarino A, De Marinis L, Tofani A, Della Casa S, D’Amico C, Mancini A, Corsello SM, Sciuto R, Barini A. Corticotropin-releasing hormone inhibition of gonadotropin release and the effect of opioid blockade. The Journal of clinical endocrinology and metabolism. 1989b;68:523–528. doi: 10.1210/jcem-68-3-523. [DOI] [PubMed] [Google Scholar]
- Barron AM, Verdile G, Taddei K, Bates KA, Martins RN. Effect of chronic hCG administration on Alzheimer’s-related cognition and A beta accumulation in PS1KI mice. Endocrinology. 2010;151:5380–5388. doi: 10.1210/en.2009-1168. [DOI] [PubMed] [Google Scholar]
- Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- Berezovska O, Xia MQ, Page K, Wasco W, Tanzi RE, Hyman BT. Developmental regulation of presenilin mRNA expression parallels notch expression. J Neuropathol Exp Neurol. 1997;56:40–44. doi: 10.1097/00005072-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Berndt S, Blacher S, Perrier d’Hauterive S, Thiry M, Tsampalas M, Cruz A, Pequeux C, Lorquet S, Munaut C, Noel A, et al. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. 2009;94:4567–4574. doi: 10.1210/jc.2009-0443. [DOI] [PubMed] [Google Scholar]
- Berndt S, Perrier d’Hauterive S, Blacher S, Pequeux C, Lorquet S, Munaut C, Applanat M, Herve MA, Lamande N, Corvol P, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. Faseb J. 2006;20:2630–2632. doi: 10.1096/fj.06-5885fje. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K, Pollwein P, Multhaup G, Monning U, Konig G, Dyrks T, Schubert W, Masters CL. Regulation and expression of the Alzheimer’s beta/A4 amyloid protein precursor in health, disease, and Down’s syndrome. Ann N Y Acad Sci. 1993;695:91–102. doi: 10.1111/j.1749-6632.1993.tb23035.x. [DOI] [PubMed] [Google Scholar]
- Bialopiotrowicz E, Kuzniewska B, Kachamakova-Trojanowska N, Barcikowska M, Kuznicki J, Wojda U. Cell cycle regulation distinguishes lymphocytes from sporadic and familial Alzheimer’s disease patients. Neurobiology of Aging. 2011;32:2319, e2313–2326. doi: 10.1016/j.neurobiolaging.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev. 2009;15:284–294. doi: 10.1002/ddrr.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeras DI, Granic A, Padmanabhan J, Crespo NC, Rojiani AM, Potter H. Alzheimer’s presenilin 1 causes chromosome missegregation and aneuploidy. Neurobiol Aging. 2008;29:319–328. doi: 10.1016/j.neurobiolaging.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Bajic VP, Spremo-Potparevic B, Casadesus G, Zhu X, Smith MA, Lee HG. Cell Cycle Aberrations and Neurodegeneration: A Review. Neuropathol Appl Neurobiol. doi: 10.1111/j.1365-2990.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda DJ, Evans TA, Santocanale C, Llosa JC, Vina J, Bajic VP, Castellani RJ, Siedlak SL, Perry G, Smith MA, et al. Evidence for the Progression through S-phase in the Ectopic Cell Cycle Reentry of Neurons in Alzheimer Disease. Aging. 2009;1:382–388. doi: 10.18632/aging.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli U, Piccini P, Napolitano A, Cagnacci A, Paoletti AM, Melis GB, Muratorio A. Reduced luteinizing hormone secretion in women with Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1990;2:225–231. doi: 10.1007/BF02257653. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Beaird H, Atwood CS, Smith MA, Rimm AA. Men treated for prostate cancer have a decreased incidence of dementia. 9th International Congress on AD.2004a. [Google Scholar]
- Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A Clinical Study of Lupron Depot in the Treatment of Women with Alzheimer’s Disease: Preservation of Cognitive Function in Patients Taking an Acetylcholinesterase Inhibitor and Treated with High Dose Lupron Over 48 Weeks. Journal of Alzheimer’s disease : JAD. 2014 doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Smith MA, Harris PL, Kubat Z, Martins RN, Castellani RJ, Perry G, Atwood CS. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. J Neurosci Res. 2002a;70:514–518. doi: 10.1002/jnr.10452. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Smith MA, Harris PL, Kubat Z, Martins RN, Castellani RJ, Perry G, Atwood CS. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. Journal of neuroscience research. 2002b;70:514–518. doi: 10.1002/jnr.10452. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. The Journal of biological chemistry. 2004a;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004b;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Bowser R, Smith MA. Cell cycle proteins in Alzheimer’s disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–254. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- Brion JP, Couck AM. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol. 1995;147:1465–1476. [PMC free article] [PubMed] [Google Scholar]
- Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu X, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. Journal of neurochemistry. 2010;112:870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukovsky A, Indrapichate K, Fujiwara H, Cekanova M, Ayala ME, Dominguez R, Caudle MR, Wimalsena J, Elder RF, Copas P, et al. Multiple luteinizing hormone receptor (LHR) protein variants, interspecies reactivity of anti-LHR mAb clone 3B5, subcellular localization of LHR in human placenta, pelvic floor and brain, and possible role for LHR in the development of abnormal pregnancy, pelvic floor disorders and Alzheimer’s disease. Reprod Biol Endocrinol. 2003;1:46. doi: 10.1186/1477-7827-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursztajn S, DeSouza R, McPhie DL, Berman SA, Shioi J, Robakis NK, Neve RL. Overexpression in neurons of human presenilin-1 or a presenilin-1 familial Alzheimer disease mutant does not enhance apoptosis. J Neurosci. 1998;18:9790–9799. doi: 10.1523/JNEUROSCI.18-23-09790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer disease and associated disorders. 2013;27:153–156. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Melis GB, Soldani R, Bonuccelli U, Piccini P, Napolitano A, Muratorio A, Fioretti P. Altered neuroendocrine regulation of luteinizing hormone secretion in postmenopausal women with Parkinson’s disease. Neuroendocrinology. 1991;53:549–555. doi: 10.1159/000125773. [DOI] [PubMed] [Google Scholar]
- Calafiore M, Battaglia G, Zappala A, Trovato-Salinaro E, Caraci F, Caruso M, Vancheri C, Sortino MA, Nicoletti F, Copani A. Progenitor cells from the adult mouse brain acquire a neuronal phenotype in response to beta-amyloid. Neurobiol Aging. 2006;27:606–613. doi: 10.1016/j.neurobiolaging.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5’-monophosphates in porcine granulosa cells. Biol Reprod. 1996;55:111–119. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer’s disease mice. Endocrinology. 2010;151:2713–2722. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, et al. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber K, Atwood C, Bowen R, Perry G, Smith M. Luteinizing hormone mediates Alzheimer-type changes in neurons. 10th International Conference on Alzheimer’s Disease and Related Disorders.2006a. [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006b;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JW. Hormonal profiles after the menopause. Br Med J. 1976;2:784–787. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Yen TJ. The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Progress in cell cycle research. 2003;5:431–439. [PubMed] [Google Scholar]
- Chen Y, Dong C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 2009;16:386–394. doi: 10.1038/cdd.2008.94. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Clark I, Atwood C, Bowen R, Paz-Filho G, Vissel B. Tumor necrosis factor-induced cerebral insulin resistance in Alzheimer’s disease links numerous treatment rationales. Pharmacological reviews. 2012;64:1004–1026. doi: 10.1124/pr.112.005850. [DOI] [PubMed] [Google Scholar]
- Clark IA, Atwood CS. Is TNF a link between aging-related reproductive endocrine dyscrasia and Alzheimer’s disease? Journal of Alzheimer’s disease : JAD. 2011;27:691–699. doi: 10.3233/JAD-2011-110887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarris HJ, Key B, Beyreuther K, Masters CL, Small DH. Expression of the amyloid protein precursor of Alzheimer’s disease in the developing rat olfactory system. Brain Res Dev Brain Res. 1995;88:87–95. doi: 10.1016/0165-3806(95)00083-p. [DOI] [PubMed] [Google Scholar]
- Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Kaylor G, Murphy SD. In vitro and in vivo modulation of cholinergic muscarinic receptors in rat lymphocytes and brain by cholinergic agents. International journal of immunopharmacology. 1990;12:67–75. doi: 10.1016/0192-0561(90)90069-y. [DOI] [PubMed] [Google Scholar]
- Cotter VT. The burden of dementia. Am J Manag Care. 2007;13(Suppl 8):S193–197. [PubMed] [Google Scholar]
- Couzinet B, Schaison G. The control of gonadotrophin secretion by ovarian steroids. Hum Reprod Suppl. 1993;2:97–101. doi: 10.1093/humrep/8.suppl_2.97. [DOI] [PubMed] [Google Scholar]
- D’Amico AV, Braccioforte MH, Moran BJ, Chen MH. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer Dis Assoc Disord. 24:85–89. doi: 10.1097/wad.0b013e31819cb8f4. [DOI] [PubMed] [Google Scholar]
- D’Amico AV, Braccioforte MH, Moran BJ, Chen MH. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer disease and associated disorders. 2010;24:85–89. doi: 10.1097/wad.0b013e31819cb8f4. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Roy I, Sairam MR. Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice. Endocrinology. 2001;142:3673–3684. doi: 10.1210/endo.142.8.8320. [DOI] [PubMed] [Google Scholar]
- Davies BR, Finnigan DS, Smith SK, Ponder BA, Aogsponmose GE. Administration of gonadotropins stimulates proliferation of normal mouse ovarian surface epithelium. Gynecol Endocrinol. 1999;13:75–81. doi: 10.3109/09513599909167536. [DOI] [PubMed] [Google Scholar]
- de la Torre J, Hachinski V. Cerebrovascular pathology in Alzheimer’s disease. New York: The New York Academy of Sciences; 1997. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews. Neuroscience. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Lambrinoudaki I, Economou F, Christou M, Piperi C, Papavassiliou AG, Creatsas G. Androgens associated with advanced glycation end-products in postmenopausal women. Menopause. 2010;17:1182–1187. doi: 10.1097/gme.0b013e3181e170af. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clinical endocrinology. 2005;62:37–43. doi: 10.1111/j.1365-2265.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Drubin D, Kobayashi S, Kirschner M. Association of tau protein with microtubules in living cells. Ann N Y Acad Sci. 1986;466:257–268. doi: 10.1111/j.1749-6632.1986.tb38398.x. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod. 2001;7:731–739. doi: 10.1093/molehr/7.8.731. [DOI] [PubMed] [Google Scholar]
- Elrich P. Das Sauerstoffbeduerfnis des organismus. Einus farbenanalytiche studie. 1885;8:167. [Google Scholar]
- Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172:1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteras N, Bartolome F, Alquezar C, Antequera D, Munoz U, Carro E, Martin-Requero A. Altered cell cycle-related gene expression in brain and lymphocytes from a transgenic mouse model of Alzheimer’s disease [amyloid precursor protein/presenilin 1 (PS1)] The European journal of neuroscience. 2012;36:2609–2618. doi: 10.1111/j.1460-9568.2012.08178.x. [DOI] [PubMed] [Google Scholar]
- Fauconnier JP, Teuwissen B, Thomas K. Rate of disappearance in plasma of synthetic LH-RH intravenously injected in man. Gynecol Obstet Invest. 1978;9:229–237. doi: 10.1159/000300989. [DOI] [PubMed] [Google Scholar]
- Fiddes JC, Talmadge K. Structure, expression, and evolution of the genes for the human glycoprotein hormones. Recent Prog Horm Res. 1984;40:43–78. doi: 10.1016/b978-0-12-571140-1.50006-2. [DOI] [PubMed] [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Fischman HK, Reisberg B, Albu P, Ferris SH, Rainer JD. Sister chromatid exchanges and cell cycle kinetics in Alzheimer’s disease. Biological Psychiatry. 1984;19:319–327. [PubMed] [Google Scholar]
- Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. The Journal of biological chemistry. 2011;286:24264–24274. doi: 10.1074/jbc.M111.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3alpha,5alpha-THP. Neuroreport. 21:590–595. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone enhances learning and memory of aged wildtype and progestin receptor knockout mice. Neurosci Lett. 472:38–42. doi: 10.1016/j.neulet.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett. 2008a;437:116–120. doi: 10.1016/j.neulet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008b;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone reduces depression-like behavior in a murine model of Alzheimer’s Disease. Age (Dordr) 2009;31:143–153. doi: 10.1007/s11357-009-9091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego MJ, Porayette P, Kaltcheva M, Bowen RL, Vadakkadath Meethal S, Atwood CS. Trophoblastic hormones direct early human embryogenesis. 2008 Available from Nature Precedings http://hdl.handle.net/10101/npre.2008.2671.1.
- Gallego MJ, Porayette P, Kaltcheva M, Bowen RL, Vadakkadath Meethal S, Atwood CS. The pregnancy hormones hCG and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Research and Therapy. 2010;1:28. doi: 10.1186/scrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego MJ, Porayette P, Kaltcheva MM, Meethal SV, Atwood CS. Opioid and progesterone signaling is obligatory for early human embryogenesis. Stem Cells Dev. 2009;18:737–740. doi: 10.1089/scd.2008.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw, Ind) mice. Neurobiol Dis. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller LN, Potter H. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer’s disease. Neurobiol Dis. 1999;6:167–179. doi: 10.1006/nbdi.1999.0236. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005;62:299–312. doi: 10.1007/s00018-004-4385-z. [DOI] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Crowther RA, Six J, Lubke U, Vandermeeren M, Cras P, Trojanowski JQ, Lee VM. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82:11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Granic A, Padmanabhan J, Norden M, Potter H. Alzheimer A{beta} Peptide Induces Chromosome Mis-Segregation and Aneuploidy, Including Trisomy 21; Requirement for Tau and APP. Mol Biol Cell. 2009 doi: 10.1091/mbc.E09-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot I, Dekel N. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem. 1994;269:30502–30509. [PubMed] [Google Scholar]
- Granot I, Dekel N. Developmental expression and regulation of the gap junction protein and transcript in rat ovaries. Molecular reproduction and development. 1997;47:231–239. doi: 10.1002/(SICI)1098-2795(199707)47:3<231::AID-MRD1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N, Bechor E, Segal I, Fieldust S, Barash A. Temporal analysis of connexin43 protein and gene expression throughout the menstrual cycle in human endometrium. Fertil Steril. 2000;73:381–386. doi: 10.1016/s0015-0282(99)00531-2. [DOI] [PubMed] [Google Scholar]
- Gray PC, Bilizikjian LM, Vale W. Antagonism of activin by inhibin and inhibin receptors: a functional role for betaglycan. Mol Cell Endocrinol. 2002;188:254–260. doi: 10.1016/s0303-7207(02)00037-0. [DOI] [PubMed] [Google Scholar]
- Gregory TR. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biological reviews of the Cambridge Philosophical Society. 2001;76:65–101. doi: 10.1017/s1464793100005595. [DOI] [PubMed] [Google Scholar]
- Gregory TR. Genome size and developmental complexity. Genetica. 2002;115:131–146. doi: 10.1023/a:1016032400147. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- Haasl RJ, Ahmadi MR, Meethal SV, Gleason CE, Johnson SC, Asthana S, Bowen RL, Atwood CS. A luteinizing hormone receptor intronic variant is significantly associated with decreased risk of Alzheimer’s disease in males carrying an apolipoprotein E epsilon4 allele. BMC medical genetics. 2008;9:37. doi: 10.1186/1471-2350-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar M. Impaired in vitro testicular endocrine function in elderly men. Andrologia. 1985;17:444–449. doi: 10.1111/j.1439-0272.1985.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHbeta-subunit promoter. Endocrinology. 2002;143:1018–1025. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod. 2009;80:1146–1151. doi: 10.1095/biolreprod.108.075341. [DOI] [PubMed] [Google Scholar]
- He D, Funabashi T, Sano A, Uemura T, Minaguchi H, Kimura F. Effects of glucose and related substrates on the recovery of the electrical activity of gonadotropin-releasing hormone pulse generator which is decreased by insulin-induced hypoglycemia in the estrogen-primed ovariectomized rat. Brain Res. 1999;820:71–76. doi: 10.1016/s0006-8993(98)01358-4. [DOI] [PubMed] [Google Scholar]
- Heo C, Chang KA, Choi HS, Kim HS, Kim S, Liew H, Kim JA, Yu E, Ma J, Suh YH. Effects of the monomeric, oligomeric, and fibrillar Abeta42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem. 2007;102:493–500. doi: 10.1111/j.1471-4159.2007.04499.x. [DOI] [PubMed] [Google Scholar]
- Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature reviews. Neuroscience. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Pelech S, Papassotiropoulos A, Gotz J. Abeta treatment and P301L tau expression in an Alzheimer’s disease tissue culture model act synergistically to promote aberrant cell cycle re-entry. Eur J Neurosci. 2007;26:60–72. doi: 10.1111/j.1460-9568.2007.05618.x. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro endocrinology letters. 2003;24:203–208. [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Honjo H, Ogino Y, Naitoh K. In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type) J Steroid Biochem. 1989;34:521–525. doi: 10.1016/0022-4731(89)90137-4. [DOI] [PubMed] [Google Scholar]
- Horiuchi A, Nikaido T, Yoshizawa T, Itoh K, Kobayashi Y, Toki T, Konishi I, Fujii S. HCG promotes proliferation of uterine leiomyomal cells more strongly than that of myometrial smooth muscle cells in vitro. Mol Hum Reprod. 2000;6:523–528. doi: 10.1093/molehr/6.6.523. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Huang J, Guan H, Booze RM, Eckman CB, Hersh LB. Estrogen regulates neprilysin activity in rat brain. Neuroscience Letters. 2004;367:85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- Hyde Z, Flicker L, Almeida OP, McCaul KA, Jamrozik K, Hankey GJ, Chubb SA, Yeap BB. Higher luteinizing hormone is associated with poor memory recall: the health in men study. Journal of Alzheimer’s disease : JAD. 2010;19:943–951. doi: 10.3233/JAD-2010-1342. [DOI] [PubMed] [Google Scholar]
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, Troncoso JC. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73:665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. Journal of neuropathology and experimental neurology. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. Journal of biochemistry. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Iqbal K, del Alonso AC, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Zaidi T, Thompson CH, Merz PA, Wisniewski HM. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol (Berl) 1984;62:167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, Murphy MP, Beckett TL, Finch CE, Brinton RD, et al. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–5479. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proc Natl Acad Sci U S A. 2004a;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004b;164:975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalma Y, Granot I, Galiani D, Barash A, Dekel N. Luteinizing hormone-induced connexin 43 down-regulation: inhibition of translation. Endocrinology. 2004;145:1617–1624. doi: 10.1210/en.2003-1051. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisawa M, Masaki T, Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989;109:1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K, Takio K, Miura R, Titani K, Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1992;58:1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- Krichevsky A, Campbell-Acevedo EA, Tong JY, Acevedo HF. Immunological detection of membrane-associated human luteinizing hormone correlates with gene expression in cultured human cancer and fetal cells. Endocrinology. 1995;136:1034–1039. doi: 10.1210/endo.136.3.7867557. [DOI] [PubMed] [Google Scholar]
- Kuhla A, Ludwig SC, Kuhla B, Munch G, Vollmar B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer’s disease brain. Neurobiology of Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo YM, Liu Y. Investigating the hows and whys of DNA endoreduplication. J Exp Bot. 2001;52:183–192. [PubMed] [Google Scholar]
- Larson P, Kronenberg H, Melmed S, Polonsky K. Williams Textbook of Endocrinology. Philadelphia, PA: Saunders; 2003. [Google Scholar]
- LeBlanc AC, Kovacs DM, Chen HY, Villare F, Tykocinski M, Autilio-Gambetti L, Gambetti P. Role of amyloid precursor protein (APP): study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992;31:635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Molecular endocrinology. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Reshef E, Rao V. The expression of human chorionic gonadotropin/luteinizing hormone receptors in human endometrial and myometrial blood vessels. J Clin Endocrinol Metab. 1992;75:651–659. doi: 10.1210/jcem.75.2.1379262. [DOI] [PubMed] [Google Scholar]
- Lempereur M, Grewal J, Saw J. Spontaneous coronary artery dissection associated with beta-HCG injections and fibromuscular dysplasia. The Canadian journal of cardiology. 2014;30:464 e461–463. doi: 10.1016/j.cjca.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Leugers CJ, Koh JY, Hong W, Lee G. Tau in MAPK activation. Frontiers in neurology. 2013;4:161. doi: 10.3389/fneur.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leugers CJ, Lee G. Tau potentiates nerve growth factor-induced mitogen-activated protein kinase signaling and neurite initiation without a requirement for microtubule binding. The Journal of biological chemistry. 2010;285:19125–19134. doi: 10.1074/jbc.M110.105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewejohann L, Damm OS, Luetjens CM, Hamalainen T, Simoni M, Nieschlag E, Gromoll J, Wistuba J. Impaired recognition memory in male mice with a supernumerary X chromosome. Physiol Behav. 2009;96:23–29. doi: 10.1016/j.physbeh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Lezoualc’h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid beta precursor protein and with the suppression of nuclear factor-kappaB. Molecular endocrinology. 2000;14:147–159. doi: 10.1210/mend.14.1.0403. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S. In vitro differentiation of neural precursors from human embryonic stem cells. Methods in Molecular Biology. 2006;331:169–177. doi: 10.1385/1-59745-046-4:168. [DOI] [PubMed] [Google Scholar]
- Licht P, Russu V, Lehmeyer S, Moll J, Siebzehnrubl E, Wildt L. Intrauterine microdialysis reveals cycle-dependent regulation of endometrial insulin-like growth factor binding protein-1 secretion by human chorionic gonadotropin. Fertil Steril. 2002;78:252–258. doi: 10.1016/s0015-0282(02)03226-0. [DOI] [PubMed] [Google Scholar]
- Liew SH, Drummond AE, Jones ME, Findlay JK. The lack of estrogen and excess luteinizing hormone are responsible for the female ArKO mouse phenotype. Molecular and cellular endocrinology. 2010;327:56–64. doi: 10.1016/j.mce.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Lin J, Li X, Yuan F, Lin L, Cook CL, Rao Ch V, Lei Z. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. Journal of neuropathology and experimental neurology. 2010;69:253–261. doi: 10.1097/NEN.0b013e3181d072cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Lojun S, Lei ZM, Wu WX, Peiner SC, Rao CV. Lymphocytes from pregnant women express human chorionic gonadotropin/luteinizing hormone receptor gene. Molecular and cellular endocrinology. 1995;111:R13–17. doi: 10.1016/0303-7207(95)03565-o. [DOI] [PubMed] [Google Scholar]
- Liu L, Diaz Brinto D. Gonadal Hormones, Neurosteroids, and Clinical Progestins as Neurogenic Regenerative Agents: Therapeutic Implications. In: Mellon AGaS., editor. Hormones in Neurodegeneration, Neuroprotection and Neurogenesis. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co; 2011. pp. 281–303. [Google Scholar]
- Liu T, Perry G, Chan HW, Verdile G, Martins RN, Smith MA, Atwood CS. Amyloid-beta-induced toxicity of primary neurons is dependent upon differentiation-associated increases in tau and cyclin-dependent kinase 5 expression. J Neurochem. 2004;88:554–563. doi: 10.1046/j.1471-4159.2003.02196.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Wimalasena J, Bowen RL, Atwood CS. Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. Journal of neurochemistry. 2007a;100:1329–1339. doi: 10.1111/j.1471-4159.2006.04307.x. [DOI] [PubMed] [Google Scholar]
- Liu T, Wimalasena J, Bowen RL, Atwood CS. Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. J Neurochem. 2007b;100:1329–1339. doi: 10.1111/j.1471-4159.2006.04307.x. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Blurton-Jones M, Yamasaki TR, Agostinho P, LaFerla FM. Activation of cell cycle proteins in transgenic mice in response to neuronal loss but not amyloid-beta and tau pathology. J Alzheimers Dis. 2009a;16:541–549. doi: 10.3233/JAD-2009-0993. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Oliveira CR, Agostinho P. Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: the role of Cdk5. Aging Cell. 9:64–77. doi: 10.1111/j.1474-9726.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer’s disease and prion-related encephalopathies. Cell Mol Neurobiol. 2007a;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Oliveira CR, Agostinho P. Role of cyclin-dependent kinase 5 in the neurodegenerative process triggered by amyloid-Beta and prion peptides: implications for Alzheimer’s disease and prion-related encephalopathies. Cellular and molecular neurobiology. 2007b;27:943–957. doi: 10.1007/s10571-007-9224-3. [DOI] [PubMed] [Google Scholar]
- Lopes JP, Oliveira CR, Agostinho P. Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-beta and prion peptides. Cell Cycle. 2009b;8:97–104. doi: 10.4161/cc.8.1.7506. [DOI] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24:5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Increased Neurogenesis in Young Transgenic Mice Overexpressing Human APP_{Sw, Ind} J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 2004;6:659–671. doi: 10.3233/jad-2004-6610. discussion 673–681. [DOI] [PubMed] [Google Scholar]
- Lu F, Yang JM, Wu JN, Chen YC, Kao YH, Tung CS, Yang SN. Activation of gonadotropin-releasing hormone receptors produces neuronal excitation in the rat hippocampus. The Chinese journal of physiology. 1999;42:67–71. [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- MacConell LA, Lawson MA, Mellon PL, Roberts VJ. Activin A regulation of gonadotropin-releasing hormone synthesis and release in vitro. Neuroendocrinology. 1999;70:246–254. doi: 10.1159/000054483. [DOI] [PubMed] [Google Scholar]
- Majocha RE, Agrawal S, Tang JY, Humke EW, Marotta CA. Modulation of the PC12 cell response to nerve growth factor by antisense oligonucleotide to amyloid precursor protein. Cell Mol Neurobiol. 1994;14:425–437. doi: 10.1007/BF02088829. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Maldonado TA, Jones RE, Norris DO. Distribution of beta-amyloid and amyloid precursor protein in the brain of spawning (senescent) salmon: a natural, brain-aging model. Brain Res. 2000;858:237–251. doi: 10.1016/s0006-8993(99)02328-8. [DOI] [PubMed] [Google Scholar]
- Maldonado TA, Jones RE, Norris DO. Timing of neurodegeneration and beta-amyloid (Abeta) peptide deposition in the brain of aging kokanee salmon. Journal of neurobiology. 2002;53:21–35. doi: 10.1002/neu.10090. [DOI] [PubMed] [Google Scholar]
- Malik B, Currais A, Andres A, Towlson C, Pitsi D, Nunes A, Niblock M, Cooper J, Hortobagyi T, Soriano S. Loss of neuronal cell cycle control as a mechanism of neurodegeneration in the presenilin-1 Alzheimer’s disease brain. Cell Cycle. 2008;7:637–646. doi: 10.4161/cc.7.5.5427. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Natori S, Iwamoto H, Uchida E, Ohashi M, Sakamoto S, Nawata H. Effect of insulin, glucagon or dexamethasone on the production of insulin-like growth factor I in cultured rat hepatocytes. Fukuoka igaku zasshi = Hukuoka acta medica. 1991;82:136–141. [PubMed] [Google Scholar]
- McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiology of Disease. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Hormones and behavior. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- McEwan PE, Lindop GB, Kenyon CJ. Control of cell proliferation in the rat adrenal gland in vivo by the renin-angiotensin system. Am J Physiol. 1996;271:E192–198. doi: 10.1152/ajpendo.1996.271.1.E192. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McPhie DL, Coopersmith R, Hines-Peralta A, Chen Y, Ivins KJ, Manly SP, Kozlowski MR, Neve KA, Neve RL. DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J Neurosci. 2003;23:6914–6927. doi: 10.1523/JNEUROSCI.23-17-06914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie DL, Golde T, Eckman CB, Yager D, Brant JB, Neve RL. beta-Secretase cleavage of the amyloid precursor protein mediates neuronal apoptosis caused by familial Alzheimer’s disease mutations. Brain Res Mol Brain Res. 2001;97:103–113. doi: 10.1016/s0169-328x(01)00294-7. [DOI] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS. Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. J Neurochem. 2009a;110:1014–1027. doi: 10.1111/j.1471-4159.2009.06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS. Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors. Journal of neurochemistry. 2009b;110:1014–1027. doi: 10.1111/j.1471-4159.2009.06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meethal SV, Smith MA, Bowen RL, Atwood CS. The gonadotropin connection in Alzheimer’s disease. Endocrine. 2005;26:317–326. doi: 10.1385/ENDO:26:3:317. [DOI] [PubMed] [Google Scholar]
- Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Baranao RI. Effect of GnRH analogues on apoptosis and release of interleukin-1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Human reproduction. 2003;18:1767–1771. doi: 10.1093/humrep/deg356. [DOI] [PubMed] [Google Scholar]
- Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Munoz U, Bartolome F, Bermejo F, Martin-Requero A. Enhanced proteasome-dependent degradation of the CDK inhibitor p27(kip1) in immortalized lymphocytes from Alzheimer’s dementia patients. Neurobiology of Aging. 2008;29:1474–1484. doi: 10.1016/j.neurobiolaging.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Musa FR, Takenaka I, Konishi R, Tokuda M. Effects of luteinizing hormone, follicle-stimulating hormone, and epidermal growth factor on expression and kinase activity of cyclin-dependent kinase 5 in Leydig TM3 and Sertoli TM4 cell lines. J Androl. 2000;21:392–402. [PubMed] [Google Scholar]
- Muta K, Maki T, Kato K, Ibayashi H. The age-related changes in serum 17-hydroxyprogesterone secretion in men. Nippon Naibunpi Gakkai Zasshi. 1983;59:20–30. doi: 10.1507/endocrine1927.59.1_20. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Combrinck M, Budge M, McShane R. Cell cycle kinesis in lymphocytes in the diagnosis of Alzheimer’s disease. Neuroscience Letters. 2002;317:81–84. doi: 10.1016/s0304-3940(01)02442-9. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Cato AM, Smith AD. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol (Berl) 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- Neaves WB, Johnson L, Porter JC, Parker CR, Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–763. doi: 10.1210/jcem-59-4-756. [DOI] [PubMed] [Google Scholar]
- Nedelec C, Ragot S, Irani J, Pires C, Gil R, Dore B. Effects by androgen suppression with luteinizing hormone on cognitive functions in men treated for cancer of prostate. Prog Urol. 2009;19:47–53. doi: 10.1016/j.purol.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Neve RL, McPhie DL. Dysfunction of amyloid precursor protein signaling in neurons leads to DNA synthesis and apoptosis. Biochim Biophys Acta. 2007;1772:430–437. doi: 10.1016/j.bbadis.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C, Slota D, Yuzpe AA, Tummon IS. Memory complaints associated with the use of gonadotropin-releasing hormone agonists: a preliminary study. Fertil Steril. 1996;65:1253–1255. doi: 10.1016/s0015-0282(16)58351-4. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) Journal of Alzheimer’s disease : JAD. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea KS. Embryonic stem cell models of development. The Anatomical record. 1999;257:32–41. doi: 10.1002/(SICI)1097-0185(19990215)257:1<32::AID-AR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Oh ES, Savonenko AV, King JF, Fangmark Tucker SM, Rudow GL, Xu G, Borchelt DR, Troncoso JC. Amyloid precursor protein increases cortical neuron size in transgenic mice. Neurobiology of Aging. 2009;30:1238–1244. doi: 10.1016/j.neurobiolaging.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J. 1994a;41:361–371. doi: 10.1507/endocrj.41.361. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7: case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Watabe H, Segawa Y, Mitsuya K, Enomoto H, Hayashi M, Yaoi Y. A clinical study on memory function in climacteric and periclimacteric women. Nippon Sanka Fujinka Gakkai Zasshi. 1994b;46:271–276. [PubMed] [Google Scholar]
- Ohsawa I, Takamura C, Kohsaka S. The amino-terminal region of amyloid precursor protein is responsible for neurite outgrowth in rat neocortical explant culture. Biochem Biophys Res Commun. 1997;236:59–65. doi: 10.1006/bbrc.1997.6903. [DOI] [PubMed] [Google Scholar]
- Oliver C, Holland AJ. Down’s syndrome and Alzheimer’s disease: a review. Psychological medicine. 1986;16:307–322. doi: 10.1017/s0033291700009120. [DOI] [PubMed] [Google Scholar]
- Palm R, Chang J, Blair J, Garcia-Mesa Y, Lee HG, Castellani RJ, Smith MA, Zhu X, Casadesus G. Down-regulation of serum gonadotropins but not estrogen replacement improves cognition in aged-ovariectomized 3xTg AD female mice. Journal of neurochemistry. 2014;130:115–125. doi: 10.1111/jnc.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci. 2007;27:2969–2978. doi: 10.1523/JNEUROSCI.0186-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AA, Filmore K, Hill D. The effect of human chorionic gonadotropin (HCG) on restoration of physiological continuity of the spinal cord. A preliminary report. International surgery. 1990;75:54–57. [PubMed] [Google Scholar]
- Pektezel MY, Bas DF, Topcuoglu MA, Arsava EM. Paradoxical Consequence of Human Chorionic Gonadotropin Misuse. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014 doi: 10.1016/j.jstrokecerebrovasdis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Perez RG, Zheng H, Van der Ploeg LH, Koo EH. The beta-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. J Neurosci. 1997;17:9407–9414. doi: 10.1523/JNEUROSCI.17-24-09407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SS, Kim M, Spangrude GJ. Direct effects of cyclosporin A on proliferation of hematopoietic stem and progenitor cells. Cell transplantation. 1999;8:339–344. doi: 10.1177/096368979900800401. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant LD, Boyle JP, Smith IF, Peers C, Pearson HA. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope WB, Lambert MP, Leypold B, Seupaul R, Sletten L, Krafft G, Klein WL. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp Neurol. 1994;126:185–194. doi: 10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porayette P, Gallego MJ, Kaltcheva MM, Meethal SV, Atwood CS. Amyloid-beta precursor protein expression and modulation in human embryonic stem cells: a novel role for human chorionic gonadotropin. Biochem Biophys Res Commun. 2007;364:522–527. doi: 10.1016/j.bbrc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Biol. 2008;180:417–426. doi: 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A, Behrendt M, Marcinkiewicz E, Boridy S, Sairam RM, Seidah NG, Maysinger D. A novel mouse model of Alzheimer’s disease with chronic estrogen deficiency leads to glial cell activation and hypertrophy. Journal of aging research. 2011;2011:251517. doi: 10.4061/2011/251517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raga F, Casan EM, Bonilla-Musoles F. Gonadotropin-releasing hormone (GnRH)-I regulation of interleukin (IL)-1b and IL-1 receptor antagonist expression in cultured human endometrial stromal cells. The journal of obstetrics and gynaecology research. 2008;34:464–472. doi: 10.1111/j.1447-0756.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J Neurosci Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Reame NE, Kelche RP, Beitins IZ, Yu MY, Zawacki CM, Padmanabhan V. Age effects of follicle-stimulating hormone and pulsatile luteinizing hormone secretion across the menstrual cycle of premenopausal women. J Clin Endocrinol Metab. 1996;81:1512–1518. doi: 10.1210/jcem.81.4.8636360. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Hoe HS, Moussa CE. Beta-amyloid1–42 gene transfer model exhibits intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss. The Journal of biological chemistry. 2010;285:7440–7446. doi: 10.1074/jbc.M109.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding TW, Kastin AJ, Gonzales-Barcena D, Coy DH, Coy EJ, Schalch DS, Schally AV. The half-life, metabolism and excretion of tritiated luteinizing hormone-releasing hormone (LH-RH) in man. J Clin Endocrinol Metab. 1973;37:626–631. doi: 10.1210/jcem-37-4-626. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Williams Textbook of Endocrinology. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. 10. Philadelphia PA: WB Saunders Co; 1998. pp. 165–248. [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiology of Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Verdile G, Foster JK, Hogervorst E, Joesbury K, Dhaliwal S, Corder EH, Laws SM, Hone E, Prince R, et al. Gonadotropins and cognition in older women. J Alzheimers Dis. 2008;13:267–274. doi: 10.3233/jad-2008-13304. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Pike CJ. Evaluation of the effects of testosterone and luteinizing hormone on regulation of beta-amyloid in male 3xTg-AD mice. Brain Research. 2012;1466:137–145. doi: 10.1016/j.brainres.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol Biol. 2004;250:1–28. doi: 10.1385/1-59259-671-1:1. [DOI] [PubMed] [Google Scholar]
- Saberi S, Du YP, Christie M, Goldsbury C. Human chorionic gonadotropin increases beta-cleavage of amyloid precursor protein in SH-SY5Y cells. Cellular and molecular neurobiology. 2013;33:747–751. doi: 10.1007/s10571-013-9954-3. [DOI] [PubMed] [Google Scholar]
- Sabharwal P, Varma S, Malarkey WB. Human thymocytes secrete luteinizing hormone: an autocrine regulator of T-cell proliferation. Biochemical and biophysical research communications. 1992a;187:1187–1192. doi: 10.1016/0006-291x(92)91322-h. [DOI] [PubMed] [Google Scholar]
- Sabharwal P, Varma S, Malarkey WB. Human thymocytes secrete luteinizing hormone: an autocrine regulator of T-cell proliferation. Biochem Biophys Res Commun. 1992b;187:1187–1192. doi: 10.1016/0006-291x(92)91322-h. [DOI] [PubMed] [Google Scholar]
- Sasson R, Amsterdam A. Stimulation of apoptosis in human granulosa cells from in vitro fertilization patients and its prevention by dexamethasone: involvement of cell contact and bcl-2 expression. J Clin Endocrinol Metab. 2002;87:3441–3451. doi: 10.1210/jcem.87.7.8676. [DOI] [PubMed] [Google Scholar]
- Sasson R, Rimon E, Dantes A, Cohen T, Shinder V, Land-Bracha A, Amsterdam A. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol Hum Reprod. 2004;10:299–311. doi: 10.1093/molehr/gah041. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Gindoff PR, Baron DA, Rubinow DR. Basal and stimulated gonadotropin levels in the perimenopause. Am J Obstet Gynecol. 1996;175:643–650. doi: 10.1053/ob.1996.v175.a74255. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Nightingale B, Rodriguez A, Tycko B, Mayeux R. Earlier onset of Alzheimer’s disease in men with Down syndrome. Neurology. 1998;50:991–995. doi: 10.1212/wnl.50.4.991. [DOI] [PubMed] [Google Scholar]
- Schwall RH, Szonyi E, Mason AJ, Nikolics K. Activin stimulates secretion of follicle-stimulating hormone from pituitary cells desensitized to gonadotropin-releasing hormone. Biochem Biophys Res Commun. 1988;151:1099–1104. doi: 10.1016/s0006-291x(88)80479-0. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, Roberson ED, Bloom GS. Amyloid-beta signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. Journal of cell science. 2013;126:1278–1286. doi: 10.1242/jcs.1125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod. 2002;8:612–618. doi: 10.1093/molehr/8.7.612. [DOI] [PubMed] [Google Scholar]
- Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clinic proceedings. Mayo Clinic. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Small DH, Nurcombe V, Reed G, Clarris H, Moir R, Beyreuther K, Masters CL. A heparin-binding domain in the amyloid protein precursor of Alzheimer’s disease is involved in the regulation of neurite outgrowth. J Neurosci. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Anderton BH, Davis DR, Gallo JM. Tau isoform expression and phosphorylation state during differentiation of cultured neuronal cells. FEBS letters. 1995;375:243–248. doi: 10.1016/0014-5793(95)01221-y. [DOI] [PubMed] [Google Scholar]
- Song J, Wang S, Tan M, Jia J. G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer’s disease. Neuroscience Letters. 2012a;526:144–149. doi: 10.1016/j.neulet.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Song M, Kwon YA, Lee Y, Kim H, Yun JH, Kim S, Kim DK. G1/S cell cycle checkpoint defect in lymphocytes from patients with Alzheimer’s disease. Psychiatry investigation. 2012b;9:413–417. doi: 10.4306/pi.2012.9.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotthibundhu A, Li QX, Thangnipon W, Coulson EJ. Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30:1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Spremo-Potparevic B, Zivkovic L, Djelic N, Bajic V. Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through the cell cycle. Experimental Gerontology. 2004;39:849–854. doi: 10.1016/j.exger.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Rao VS, Rajesh N, Vasan SS, Rao AJ. A preliminary study on the possible role for luteinizing hormone in androgen independent growth of prostate. Reproductive biomedicine online. 2001;3:6–13. doi: 10.1016/s1472-6483(10)61956-6. [DOI] [PubMed] [Google Scholar]
- Srisuparp S, Strakova Z, Brudney A, Mukherjee S, Reierstad S, Hunzicker-Dunn M, Fazleabas AT. Signal transduction pathways activated by chorionic gonadotropin in the primate endometrial epithelial cells. Biol Reprod. 2003;68:457–464. doi: 10.1095/biolreprod.102.007625. [DOI] [PubMed] [Google Scholar]
- Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clinical biochemistry. 2004;37:549–561. doi: 10.1016/j.clinbiochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Stieler J, Grimes R, Weber D, Gartner W, Sabbagh M, Arendt T. Multivariate analysis of differential lymphocyte cell cycle activity in Alzheimer’s disease. Neurobiology of Aging. 2012;33:234–241. doi: 10.1016/j.neurobiolaging.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieler JT, Lederer C, Bruckner MK, Wolf H, Holzer M, Gertz HJ, Arendt T. Impairment of mitogenic activation of peripheral blood lymphocytes in Alzheimer’s disease. Neuroreport. 2001;12:3969–3972. doi: 10.1097/00001756-200112210-00023. [DOI] [PubMed] [Google Scholar]
- Su B, Wang X, Bonda D, Perry G, Smith M, Zhu X. Abnormal Mitochondrial Dynamics-A Novel Therapeutic Target for Alzheimer’s Disease? Mol Neurobiol. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Higashi Y, LaNasa JA, Yoshida K, Winters SJ, Oshima H, Troen P. Studies of the human testis. XVIII. Simultaneous measurement of nine intratesticular steroids: evidence for reduced mitochondrial function in testis of elderly men. J Clin Endocrinol Metab. 1983;56:1178–1187. doi: 10.1210/jcem-56-6-1178. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kuruta H, Mito T, Nishizawa M, Kunishita T, Tabira T. Developmental and aging changes in the expression patterns of beta-amyloid in the brains of normal and Down syndrome cases. Brain Dev. 1990;12:367–371. doi: 10.1016/s0387-7604(12)80066-0. [DOI] [PubMed] [Google Scholar]
- Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. The aging male : the official journal of the International Society for the Study of the Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- Tan YF, Preston E, Wojtowicz JM. Enhanced post-ischemic neurogenesis in aging rats. Frontiers in neuroscience. 2010;4 doi: 10.3389/fnins.2010.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Adamik A, Tanaka M, Schally AV. Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regulatory peptides. 2010;159:142–147. doi: 10.1016/j.regpep.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Telegdy G, Tanaka M, Schally AV. Effects of the LHRH antagonist Cetrorelix on the brain function in mice. Neuropeptides. 2009;43:229–234. doi: 10.1016/j.npep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Thakur A, Siedlak SL, James SL, Bonda DJ, Rao A, Webber KM, Camins A, Pallas M, Casadesus G, Lee HG, et al. Retinoblastoma protein phosphorylation at multiple sites is associated with neurofibrillary pathology in Alzheimer disease. Int J Clin Exp Pathol. 2008;1:134–146. [PMC free article] [PubMed] [Google Scholar]
- Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors--a review. Reproductive biology. 2001;1:5–11. [PubMed] [Google Scholar]
- Toth P, Lukacs H, Hiatt ES, Reid KH, Iyer V, Rao CV. Administration of human chorionic gonadotropin affects sleep-wake phases and other associated behaviors in cycling female rats. Brain Res. 1994;654:181–190. doi: 10.1016/0006-8993(94)90478-2. [DOI] [PubMed] [Google Scholar]
- Urcelay E, Ibarreta D, Parrilla R, Ayuso MS, Martin-Requero A. Enhanced proliferation of lymphoblasts from patients with Alzheimer dementia associated with calmodulin-dependent activation of the na+/H+ exchanger. Neurobiology of Disease. 2001;8:289–298. doi: 10.1006/nbdi.2000.0381. [DOI] [PubMed] [Google Scholar]
- Vadakkadath Meethal S, Atwood CS. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62:257–270. doi: 10.1007/s00018-004-4381-3. [DOI] [PubMed] [Google Scholar]
- Vadakkadath Meethal S, Porayette P, Wilson AC, Basson JJ, Liu T, Atwood CS. Functional Consequences of Gonadotropin Signaling in the Brain During Development, Adulthood and Senescence. In: Kumar A, Rao CV, Chaturvedi PK, editors. Gonadal and Nongonadal Actions of Gonadotropins. New Delhi: Narosa Publishing House; 2010. pp. 249–274. [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Kahn R. Klinefelter’s syndrome (karyotype 47, XXY) and schizophrenia-spectrum pathology. Br J Psychiatry. 2006;189:459–460. doi: 10.1192/bjp.bp.105.008961. [DOI] [PubMed] [Google Scholar]
- Varney NR, Syrop C, Kubu CS, Struchen M, Hahn S, Franzen K. Neuropsychologic dysfunction in women following leuprolide acetate induction of hypoestrogenism. Journal of assisted reproduction and genetics. 1993;10:53–57. doi: 10.1007/BF01204441. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Abeta oligomers induce neuronal cell cycle events in Alzheimer’s disease. J Neurosci. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, et al. Associations between gonadotropins, testosterone and beta amyloid in men at risk of Alzheimer’s disease. Molecular psychiatry. 2014;19:69–75. doi: 10.1038/mp.2012.147. [DOI] [PubMed] [Google Scholar]
- Verdile G, Yeap BB, Clarnette RM, Dhaliwal S, Burkhardt MS, Chubb SA, De Ruyck K, Rodrigues M, Mehta PD, Foster JK, et al. Luteinizing hormone levels are positively correlated with plasma amyloid-beta protein levels in elderly men. J Alzheimers Dis. 2008;14:201–208. doi: 10.3233/jad-2008-14208. [DOI] [PubMed] [Google Scholar]
- Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996a;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging. 1998;19:287–296. doi: 10.1016/s0197-4580(98)00071-2. [DOI] [PubMed] [Google Scholar]
- Vincent TS, Hazen-Martin DJ, Garvin AJ. Inhibition of insulin like growth factor II autocrine growth of Wilms’ tumor by suramin in vitro and in vivo. Cancer Lett. 1996b;103:49–56. doi: 10.1016/0304-3835(96)04186-9. [DOI] [PubMed] [Google Scholar]
- Volpi R, Caffarra P, Scaglioni A, Maestri D, Chiodera P, Coiro V. Restoration of ACTH/cortisol and LH responses to naloxone by chronic dopaminergic treatment in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1994;7:1–11. doi: 10.1007/BF02252658. [DOI] [PubMed] [Google Scholar]
- Wahjoepramono EJ, Wijaya LK, Taddei K, Bates KA, Howard M, Martins G, deRuyck K, Matthews PM, Verdile G, Martins RN. Direct exposure of guinea pig CNS to human luteinizing hormone increases cerebrospinal fluid and cerebral beta amyloid levels. Neuroendocrinology. 2011;94:313–322. doi: 10.1159/000330812. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bu B, Xie M, Zhang M, Yu Z, Tao D. Neural cell cycle dysregulation and central nervous system diseases. Prog Neurobiol. 2009;89:1–17. doi: 10.1016/j.pneurobio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Webber RJ, Sokoloff L. In vitro culture of rabbit growth plate chondrocytes. 1. Age-dependence of response to fibroblast growth factor and “chondrocyte growth factor”. Growth. 1981;45:252–268. [PubMed] [Google Scholar]
- Wehrenberg U, Rune GM. Spontaneous luteinization of antral marmoset follicles in vitro. Mol Hum Reprod. 2000;6:504–509. doi: 10.1093/molehr/6.6.504. [DOI] [PubMed] [Google Scholar]
- Weiss J, Crowley WF, Jr, Halvorson LM, Jameson JL. Perifusion of rat pituitary cells with gonadotropin-releasing hormone, activin, and inhibin reveals distinct effects on gonadotropin gene expression and secretion. Endocrinology. 1993;132:2307–2311. doi: 10.1210/endo.132.6.8504735. [DOI] [PubMed] [Google Scholar]
- Wharton W, Baker LD, Gleason CE, Dowling M, Barnet JH, Johnson S, Carlsson C, Craft S, Asthana S. Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. Journal of Alzheimer’s disease : JAD. 2011;26:495–505. doi: 10.3233/JAD-2011-110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield GK, Kourides IA. Expression of chorionic gonadotropin alpha- and beta-genes in normal and neoplastic human tissues: relationship to deoxyribonucleic acid structure. Endocrinology. 1985;117:231–236. doi: 10.1210/endo-117-1-231. [DOI] [PubMed] [Google Scholar]
- Wiesen JF, Midgley AR., Jr Changes in expression of connexin 43 gap junction messenger ribonucleic acid and protein during ovarian follicular growth. Endocrinology. 1993;133:741–746. doi: 10.1210/endo.133.2.8393773. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Clemente L, Liu T, Bowen RL, Meethal SV, Atwood CS. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim Biophys Acta. 2008;1782:401–407. doi: 10.1016/j.bbadis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Salamat MS, Haasl RJ, Roche KM, Karande A, Meethal SV, Terasawa E, Bowen RL, Atwood CS. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. The Journal of endocrinology. 2006a;191:651–663. doi: 10.1677/joe.1.07047. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Salamat MS, Haasl RJ, Roche KM, Karande A, Meethal SV, Terasawa E, Bowen RL, Atwood CS. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. The Journal of endocrinology. 2006b;191:651–663. doi: 10.1677/joe.1.07047. [DOI] [PubMed] [Google Scholar]
- Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- Yamatsuji T, Okamoto T, Takeda S, Murayama Y, Tanaka N, Nishimoto I. Expression of V642 APP mutant causes cellular apoptosis as Alzheimer trait-linked phenotype. Embo J. 1996;15:498–509. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani T, Koizumi T, Taniguchi R, Nakagawa T, Isobe T, Yoshimura M, Tsubota N, Hasegawa K, Ohsawa N, Baba S, et al. Expression of alpha and beta genes of human chorionic gonadotropin in lung cancer. Int J Cancer. 1997;71:539–544. doi: 10.1002/(sici)1097-0215(19970516)71:4<539::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Yoon SC, Kwon YA, Kim H, Kim S, Ahn Jo S, Kim DK. Altered cell viability and proliferation activity of peripheral lymphocytes in patients with Alzheimer’s disease. Psychiatry investigation. 2010;7:68–71. doi: 10.4306/pi.2010.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle. 2008;7:3487–3490. doi: 10.4161/cc.7.22.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle. 2011;10:1208–1214. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem. 285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Herrup K. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. The Journal of biological chemistry. 2010a;285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010b;30:5219–5228. doi: 10.1523/JNEUROSCI.5628-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiology of Aging. 2011;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jia J. P53-mediated G(1)/S checkpoint dysfunction in lymphocytes from Alzheimer’s disease patients. Neuroscience Letters. 2010;468:320–325. doi: 10.1016/j.neulet.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neuro-Signals. 2002;11:270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Boux H, Simmons ZL, Takeda A, Smith MA. Activation of oncogenic pathways in degenerating neurons in Alzheimer disease. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18:433–437. doi: 10.1016/s0736-5748(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Zhu XF, Liu ZC, Xie BF, Li ZM, Feng GK, Yang D, Zeng YX. EGFR tyrosine kinase inhibitor AG1478 inhibits cell proliferation and arrests cell cycle in nasopharyngeal carcinoma cells. Cancer Lett. 2001;169:27–32. doi: 10.1016/s0304-3835(01)00547-x. [DOI] [PubMed] [Google Scholar]
- Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Hormones and behavior. 2010;58:705–713. doi: 10.1016/j.yhbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]