Abstract
Phosphopantetheinyl transferase (E.C. 2.7.8.-) activates biosynthetic pathways that synthesize both primary and secondary metabolites in bacteria. Inhibitors of these enzymes have the potential to serve as antibiotic compounds that function through a unique mode of action and possess clinical utility. Here we report a direct and continuous assay for this enzyme class based upon monitoring polarization of a fluorescent phosphopantetheine analog as it is transferred from a low molecular weight coenzyme A substrate to higher molecular weight protein acceptor. We demonstrate the utility of this method for the biochemical characterization of phosphopantetheinyl transferase Sfp, a canonical representative from this class. We also establish the portability of this technique to other homologs by adapting the assay to function with the human phosphopantetheinyl transferase, a target for which a microplate detection method does not currently exist. Comparison of these targets provides a basis to predict therapeutic index of inhibitor candidates and offers a valuable characterization of enzyme activity.
Introduction
Antibiotic resistance is a persistent threat to modern medicine that contributes to increasing mortality statistics in both developed and underdeveloped countries. The top threats currently challenging worldwide healthcare efforts include multidrug resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis1 and methicillin resistant Staphlyococcus aureus (MRSA).2, 3 In addition to these noxious agents, Vibrio cholera,4 Pseudomonas aeruginosa,5 Staphylococcus pneumonia,6 and bacteria responsible for meningococcal meningitis7 have recently shown an increased capacity to resist current drug regimens. While industry has found some success though augmentation of known pharmacophores to circumvent resistance mechanisms, the development of new chemical entities that act on novel drug targets opens new avenues to antimicrobial development and extends the efficacy of existing therapeutics.
Among bacterial and fungal targets that are currently unaddressed in the clinic, Sfp-type phosphopantetheinyl transferases (Sfp-PPTases) have received much attention recently for their involvement in the virulence of many pathogenic bacteria8-11 and fungi.12, 13 Representative virulence factors from these organisms include mycolic acid and mycobactin in M. tuberculosis,14, 15 pyoverdine in P. aeruginosa,16 mycolactone in M. ulcerans,17 vibriobactin in V. cholera,18 and yersiniabactin in Y. pestis.19 In addition to the manufacture of these compounds, Sfp-PPTases possess the additional capability to activate primary metabolism if the AcpS-PPTase gene becomes lost or inactivated, an event illustrated by genetic knockout Streptomyces coelicolor and Bacillus subtilis case studies20, 21 and observed naturally in Pseudomonas spp. 10, 22
To test hypotheses concerning the association of Sfp-PPTases with bacterial virulence, we have begun a campaign to identify inhibitors of Sfp from B. subtilis, the canonical representative and namesake of this enzyme class. During this work, we have found it necessary to assess compound cross-reactivity with the human PPTase (hPPTase),23 where inhibitory action could induce mitochondrial damage and present a liability of mechanism-based toxicity. Herein, we detail the challenges encountered when adapting our established screening assay24 for use with the human PPTase. To address this issue, we have developed a novel fluorescent polarization assay that monitors the complete process of the PPTase reaction. This new technique directly detects the incorporation of a fluorescent phosphopantetheinyl appendage onto a whole-protein product. We demonstrate the ability of this assay to biochemically characterize Sfp-PPTase, adapt the detection format to function with the human enzyme, and demonstrate that this methodology can reliably report the inhibitory activity of test compounds toward this critical anti-target in order to predict therapeutic index of inhibitor candidates.
Materials & Methods
General
Glassware and stir bars used in synthetic techniques were oven dried. General purpose solvents were ACS grade unless otherwise noted. Solvents used for HPLC were HPLC-grade only. Tandem HPLC/ESI-MS analysis was performed on a Waters HPLC (Waters Corporation, Milford, MA) using an XBridge C18 2.5 μm 3.0 × 50 mm (Waters Corporation, Milford, MA) column, running on a gradient method using acetonitrile and water with 0.1% formic acid. A MicroMass ZMD (Waters Corporation, Milford, MA) electron spray injection mass spectrometer was in line with the HPLC. All protein concentrations were determined using UV absorbance. Rhodamine-CoA concentration was quantified through serial dilution and comparison of fluorescence (485 nm / 595 nm excitation / emission) to a 100 μM rhodamine-CoA standard stock using a HTS 7000 plus Bioassay Reader (Perkin Elmer, Waltham, MA). Rhodamine-CoA was prepared as previously described25 and subjected to LC/MS analysis for product confirmation (Figure S1).
Recombinant protein expression
Sfp in pET29b (Novagen, La Jolla, CA),26 VibB in pET24b (Novagen, La Jolla, CA),27 ActACP in pET24b,28 and the 90 amino acid ACP (hACP) from human fatty acid synthase (EC 2.3.1.85) synthesized in a pJexpress401vector (DNA2.0, Menlo Park, CA) were transformed, expressed and purified using BL-21(DE3) E. coli cultured with rotary shaking at 37°C in lysogeny broth containing kanamycin (50 μg/mL). Native Sfp was prepared as previously described.29 When the optical density (OD) reached 0.6, expression was induced by the addition of Isopropyl-β-D-1-thiogalactoside (IPTG) to a concentration of 1 mM, for a period of 4 hours at 37°C for Sfp, ActACP, hACP-containing cells and overnight at 16°C for VibB. hPPTase was cultured at 37°C in LB with 25 μg/mL chloramphenicol in TOP10 E. coli (Life Technologies, Grand Island, NY). Upon reaching OD of 0.6, 1 mM IPTG was added to induce hPPTase protein expression overnight at 16°C with shaking. Recombinant cell cultures were centrifuged at 2000 rpm for 30 minutes, with cell pellets frozen at −20°C until use.
Protein purification
Cells thawed on ice were re-suspended in lysis buffer [50 mM TrisCl pH 8.0, 500 mM NaCl, 1 mM DTT, 5 μg/mL DNase I & 5 μg/mL RNAse A, 0.1 mg/mL lysozyme and lysed with either French pressure (Thermo Electron Corp/Thermo Scientific, Waltham, MA) or Model#110 F microfluidizer (Microfluidics Corporation, Newton, MA). Cell debris was cleared by centrifugation for 45 minutes at 10,000 g at 4°C. Soluble protein extracts were decanted and subjected to IMAC using Ni-NTA resin. Fractions containing the purified target proteins were dialyzed against 50 mM TrisCl pH 8.0, 500 mM NaCl, 1 mM DTT, the dialysate concentrated using Amicon-Ultra centrifugal filters (Millipore, Billerica, MA) appropriate for the target protein's molecular weight. Samples were flash frozen and stored at −80°C until use. Protein samples were evaluated for purity by SDS-PAGE (Figure S2) and concentrations were determined using UV spectroscopy at 280 nm with calculated extinction coefficients30: Sfp = 28880 M−1cm−1, hPPTase = 56950 M−1cm−1, VibB = 48930 M−1cm−1, hACP = 1490 M−1cm−1, ActACP = 2980 M−1cm−1.
FRET-based PPTase assay
PPTase labeling activity of FITC-YbbR was determined using previously reported procedures.24 The final buffer composition includes 50 mM HEPES pH 7.6, 10 mM MgCl2, 1 mg/mL BSA, 0.01% Tween-20, and 10% DMSO. Briefly, 10 μL of 25 μM FITC-Ybbr & 25 μM rhodamine-CoA was added to 5 μL DMSO in a 96-well Costar cat no. 3694 black 96-well plate (Corning, Lowell, MA), and the reaction initiated by the addition of 35 μL enzyme solution (buffer blank, 37 nM Sfp, 143 nM hPPTase). Raw fluorescence units were acquired at 492 nm excitation and 535 nm emission using a HTS 7000 plus Bioassay Reader (Perkin Elmer, Waltham, MA).
Crypto- VibB, hACP, & ActACP preparation
A crypto-VibB standard was prepared via reaction of 50 μM (30 nmol) apo-VibB with 220 μM (110 nmol) rhodamine-CoA in 0.5 mL in 50 mM HEPES pH 7.5, 10 mM MgCl2 and 20% glycerol with 200 nM native Sfp at 37°C for 24 hours. Crypto-VibB was re-purified using Ni-NTA chromatography, desalted with PD-10 column (GE Healthcare BioSciences, Pittsburgh, PA) into desalt buffer [50 mM TrisCl pH 7.5, 250 mM NaCl, 10% glycerol], and spin-concentrated with a 0.5 mL 3 kDa MWCO Amicon-Ultra filters.
Crypto-hACP standard was prepared via 16 hour reaction at 37°C of 130 μM (33 nmol) apo-hACP with 270 μM (66 nmol) rhodamine-CoA in 0.25 mL in 50 mM HEPES pH 7.5, 10, MgCl2, with 1 mM DTT and 200 nM native Sfp. Crypto- hACP was repurified using Ni-NTA chromatography, desalted into desalt buffer with 0.1 mM additional DTT, and spin-concentrated with a 0.5 mL 3 kDa MWCO Amicon-Ultra filter.
Crypto-ActACP was prepared via 16 hour reaction at 37°C of 150 μM (60 nmol) apo-hACP with 300 μM (120 nmol) rhodamine-CoA in 0.4 mL in 50 mM HEPES pH 7.5, 10, MgCl2, and 200 nM native Sfp. Crypto-ActACP was repurified using Ni-NTA chromatography, desalted into desalt buffer, and spin-concentrated with a 0.5 mL 3 kDa MWCO Amicon-Ultra filter.
SDS-PAGE confirmed rhodamine-labeling of carrier proteins (Figure S3a). Quantification of the concentrated crypto- carrier proteins was performed through serial dilution and comparison of fluorescence (485 nm excitation & 595 nm emission) to a 100 μM rhodamine-CoA standard stock on a HTS 7000 plus Bioassay Reader (Perkin Elmer, Waltham, MA).
Linear dilution of crypto- carrier proteins analyzed by fluorescence polarization
Crypto- VibB and ActACP were prepared in 50 mM TrisCl pH 8 at 10 μM with and without 1 mg/mL BSA for 100% labeled sample. Apo- VibB and ActACP at 10 μM with and without 1 mg/mL BSA were prepared with 10 μM free rhodamine-CoA as the 0% labeled sample. An 11-point linear dilution was prepared by combining the crypto- and apo- carrier protein mixtures in a 50 μL volume in Costar cat. no 3694 black 96-well plates (Corning, Lowell, MA) to give 0.5 μM increments of crypto from 0-10 μM.
Removal of coenzyme A from Sfp enzyme preparation
One aliquot of Sfp preparation was treated with calf intestinal phosphatase (CIP) (Worthington Biochemicals, Lakewood, NJ) to remove pre-bound CoA for comparison to untreated PPTase dissociation constants. CIP (100 U at 3800 U/mL) was added to Sfp (10 mg) in 5 mL 50 mM TrisCl pH 8, 250 mM NaCl, 10% glycerol, 10 mM MgCl2, along with a sufficient quantity of nickel resin (1.5 mL bed volume) for binding. Incubation of the mixture proceeded with gentle rocking at room temperature. Nickel resin was washed with 50 mM MES pH 6.2, 500 mM NaCl, 10% glycerol, 1 mM CaCl2 and then eluted with 300 mM imidazole in the same MES buffer as the wash. The resultant apo-Sfp was buffer exchanged with a centrifugal filter prior to use by concentrating the protein to 0.5 mL with a 10 kDa MWCO spin filter (EMD Millipore, Billerica, MA), and adding previous MES buffer without imidazole to 5 mL, mixing, and concentrating to 0.5 mL again. This cycle was repeated 3 times to reduce imidazole to a trivial concentration. Incubation of CIP-treated and untreated Sfp with apo-ACP from E. coli demonstrates effective re-purification (Figure S4a) and removal of coenzyme A from Sfp preparation (Figure S4b).
Determination of PPTase dissociation constant, KD
The dissociation constant that the PPTase enzymes exhibit for Rhodamine-CoA was performed similar to existing methods.31 Briefly, a 1:2 serial dilution of PPTase in 50 mM HEPES pH 7.6, 0.01% Tween-20, 10 mM MgCl2 was conducted across 11-points with a blank lacking PPTase. This enzyme serial dilution was pipetted in triplicate volumes of 25 μL into three rows of a 96-well plate containing 25 μL of 50 nM Rhodamine-CoA in the same buffer to give a range of 100 – 0.10 μM top final [hPPTase] and 86 – 0.08 μM top final [Sfp]. After mixing, plates were incubated for 20 minutes at room temperature, centrifuged 2 minutes at 2000 rpm, and measured using described fluorescence polarization parameters.
Data analysis
Dissociation constant (KD) calculations for PPTases were performed by converting polarization values into anisotropy, and using JMP 10 (SAS Institute Inc., Cary, NC) software to obtain nonlinear fits with equations 1 and 2 used previously.31
| (1) |
| (2) |
Raw data points and nonlinear regression were then plotted in GraphPad Prism 5.00 (GraphPad Software, La Jolla, CA). For IC50 determination, raw data were normalized by setting 0% activity to sample wells lacking PPTase, and 100% activity to sample wells that received control vehicle. Resulting percent activities were fitted to the 4-parameter Hill equation in GraphPad Prism. Dose-response curves that exhibited prominent activation or no response were labeled as inactive for the purposes of discussion.
Fluorescence-polarization-based phosphopantetheinylation assay
Activity assays were performed in Costar cat. no 3694 black 96-well plates (Corning, Lowell, MA) in a buffer containing 50 mM Sodium-HEPES, 10 mM MgCl2, 1 mg/mL BSA, 10% DMSO, 0.01% Tween-20. PPTase in the assay is used at concentrations of 100 nM while measuring inhibitor response, and 5 nM for kinetic evaluation at various pH conditions. Substrates rhodamine-CoA and carrier protein (VibB or hACP) are present for all analysis at concentrations of 5 μM and 10 μM, respectively. Microwell reaction final volumes are 50 μL, evaluated at 45 minute time points for Sfp and hPPTase.
Evaluation of PPTase dilution labeling with fluorescence polarization
Sfp and human PPTase were diluted to 1.43 μM in 1.43X reaction buffer (72 mM Sodium-HEPES, 14.3 mM MgCl2, 1.43 mg/mL BSA, 0.0143% Tween-20), and subjected to an 11-point 1:2 serial dilution into 1.43X reaction buffer. PPTase serial dilution (1430-1.4 nM, 35 μL) was then added to 5 μL DMSO blank and incubated at room temperature for 10-15 minutes. Reactions were initiated with the addition of 10 μL substrate mix (25 μM rhodamine-CoA & 50 μM VibB or hACP), centrifuged 2 minutes at 2000 rpm, and analyzed in kinetic mode for 70 minutes with previously mentioned fluorescence polarization setup (Figures S5 & S6).
Detergent and DMSO evaluation with fluorescent polarization
Sfp and human PPTase were diluted to 143 nM in 1.43X reaction buffer (72 mM Sodium-HEPES, 14.3 mM MgCl2, 1.43 mg/mL BSA) with 0.0143% Tween-20, Triton X-100, NP-40, or no detergent. PPTase (143 nM, 35 μL) was then added to 5 uL DMSO or milliQ water and incubated at room temperature for 10-15 minutes. Reactions were initiated with the addition of 10 μL substrate mix (25 μM rhodamine-CoA & 50 μM VibB or hACP), centrifuged 2 minutes at 2000 rpm, and analyzed in kinetic mode for 70 minutes with previously mentioned fluorescence polarization setup (Figures S7 & S8).
PPTase pH-dependence with fluorescence polarization
Kinetic analysis of Sfp and hPPTase activity at various pH conditions was carried out similarly to the regular fluorescence polarization reaction conditions, but with lower final PPTase concentrations of 5 nM and various buffers. Buffers were prepared as 10X solutions at 500 mM with 100 mM MgCl2, adjusted to pH with HCl or NaOH, and diluted with milliQ water. Sodium acetate (anhydrous) served as the pH 4.5 and 5.0 buffers. MES (free acid) served as the pH 5.5, 6.0, and 6.5 buffers. HEPES (sodium salt) served as the pH 7.0 and 7.5 buffers. Tris (base) served as the pH 8.0, 8.5, and 9.0 buffers. The 10X buffers were otherwise prepared and implemented as fluorescence polarization assay conditions describe. Samples were evaluated with 10% DMSO and the same substrate concentrations as the described fluorescence polarization assay conditions. Data analysis utilized the crypto-VibB (Figure S3b) and hACP standards (Figure S3c) prepared at 5, 2.5, 1.25, and 0 μM crypto- carrier protein concentrations to calculate activity from raw polarization over a 70 minute period. Linear fits from crypto- standards allowed calculation of activity from the earliest time-points to provide non-negative activities, typically ranging from 5-10% substrate consumption.
Statistical analysis
Statistical values including Z’ value were calculated for the fluorescence polarization assay using triplicate samples (n=3) to gauge its suitability for high-throughput screening applications, in which the standard deviation (σ) and the signal (μ) in milli-polarization or mP units for both bound (covalently attached rhodamine-VibB product) and unbound (mixture of unreacted rhodamine-CoA and apo-VibB carrier protein).
| (3) |
SDS-PAGE vs. fluorescence polarization signal comparison
Crypto-VibB standard linear dilutions were prepared at 50 μL volumes in Costar 3694 96-well plate in 50 mM TrisCl and 100 mM EDTA pH 7.0 in 0.5 μM increments from 0 μM to 5 μM, and supplemented with free rhodamine-CoA to a total crypto-VibB + rhodamine-CoA concentration of 5 μM to represent product completion in 10% increments. Fluorescence polarization was evaluated in the GenioS Pro plate reader (Tecan Systems Inc, San Jose, CA) at 535 nm polarized excitation and 580 nm polarized emission filters with a gain of 40 and g-factor of 0.76. These samples were then removed from the microtiter plate, diluted 1:10 in 50 mM TrisCl and 100 mM EDTA, an equal volume of 2X SDS-PAGE loading dye added, and heated to 70°C for 5 minutes. 10 μL of each sample loaded onto each lane of a 12% SDS-PAGE gel. The resulting gels were fixed 30 minutes in 40% methanol & 10% acetic acid, washed in warm milliQ water, and visualized on a Typhoon TRIO Variable Mode Imager (GE Healthcare BioSciences, Pittsburgh, PA) at 50-μm resolution with 532 nm green laser excitation and 580BP30 nm emission filter and a photomultiplier tube setting of 500. Gel image data was collected as a .gel file format and crypto-VibB protein bands were quantified using ImageJ software (NIH, Bethesda, MD), utilizing integrated pixel density, background subtraction from fluorescent protein bands and fixed scan regions. Signal data collected allowed comparison of FP data to gel data over increasing percent carrier protein labeling, as well as calculating a ratio of integrated pixel density to polarization units. Gel staining was also performed for validation of the fluorescent gel data.
Synthesis of 2’-deoxyadenosine-3,5-bisphosphate (2’-deoxy-PAP)
2’-deoxyadenosine-3,5-bisphosphate was prepared according to existing methodology.32 Briefly, 2’-deoxyadenosine (270 mg, 1 mmol, Spectrum, Gardena, CA) and proton sponge (1 g, 5 mmol, Sigma, St. Louis, MO) were dried overnight over phosphorus pentoxide and subsequently transferred to a 100 mL round bottom flask with stir bar. Trimethyl phosphate (20 mL, 170 mmol) was added, and sealed with a septum under argon, stirring 5 minutes. The reaction was initiated with addition of phosphorus oxychloride (380 μL, 4 mmol, EMD Millipore, Billerica, MA) and was stirred on ice for 2 hours. The reaction was quenched by addition of triethyl ammonium bicarbonate (20 mL, 125 mmol, pH 7.5) and 30 mL milliQ water. Purification was performed with DEAE A-25 Sephadex (Amersham, Piscataway, NJ) rinsed with ethanol and swollen overnight in milliQ water. Resin was poured into a 30 × 2.5 cm column and equilibrated to the starting mobile phase at room temperature. Reaction mixture was loaded onto the gravity-fed column and separated using a gradient maker producing a gradient of approximately 0.01-0.5 M ammonium bicarbonate pH ~ 7.5. Fractions containing doubly-phosphorylated compound were discerned via normal phase TLC by lack of migration from baseline with 1:2:1 water:butanol:acetic acid mobile phase. Fractions containing the desired compound were combined, frozen, and lyophilized. Incomplete removal of solvent was followed by further concentration under centrifugation with vacuum. Final product was analyzed by ESI-MS (positive mode HR-ESI-FT-MS) to confirm desired molecular weight (Figure S9).
Fluorescence polarization inhibitor screen
All inhibitors were serially diluted in DMSO and transferred in triplicate to a 96-well plate prior to enzyme addition. Inhibitors of Sfp from previous LOPAC screen33 were serially diluted and utilized at final top concentrations of 100 μM except SCH-202676 which was used at final top concentration of 50 μM. 2’-deoxy-PAP and PAP were implemented at a top final concentration of 500 μM for both Sfp and hPPTase. Unmodified coenzyme A was used at a top final concentration of 500 μM and 100 μM for Sfp and hPPTase, respectively. Inhibitor in 5 μL DMSO was added to each well, followed by 35 μL of 143 nM Sfp in PPTase reaction buffer (72 mM HEPES pH 7.6, 1.43 mg/mL BSA, 0.0143 % Tween-20), mixed by pipetting. Plates were incubated 10 minutes at room temperature, followed by addition of 10 μL substrate mix (50 μM VibB, 25 μM rhodamine-CoA diluted into 10 mM Tris pH 8.0). Plates were centrifuged 2 minutes at 2000 rpm, and acquisition began in kinetic mode for 1 hour on GenioS Pro plate reader (Tecan Systems Inc, San Jose, CA). 45 minute time points (post-addition) were used for IC50 calculations of test inhibitors. Acquisition utilized the Tecan plate reader using 535nm excitation and 580 nm emission filters.
SCH-202676 inhibition and inactivation
Methods for evaluating SCH via gel-based methods utilized the following buffer conditions to emulate the FP assay: 50 mM Sodium-HEPES, 10 mM MgCl2, 1 mg/mL BSA, 10% DMSO, 0.01% Tween-20. Sfp and hPPTase were used at top final concentrations of 50 nM, and top final SCH-202676 at 100 μM. PPTase samples were prepared in 1.43X reaction buffer with or without 1 mM DTT, and 14 uL of PPTase mixture was added to 2 μL SCH in DMSO or a DMSO blank. Samples were incubated 10 minutes at room temperature, followed by addition of 4 μL substrate mix (25 μM rhodamine-CoA & 50 μM carrier protein) to initiate the reaction. PPTases were also evaluated following desalting to removal residual DTT from protein purification by desalting into respective lysis buffers without DTT/DNAse/RNase/lysozyme using a PD-10 desalting column (GE Healthcare BioSciences, Pittsburgh, PA). Reactions were incubated 30 minute at room temperature, then prepared immediately for electrophoresis with Urea-PAGE (20% polyacrylamide) by the addition of 5 μL of 5X loading dye and loading 12.5 μL of the mixture and running the gel for 2 hours at 160V. Gels were imaged directly after electrophoresis without fixing for all BSA-containing VibB (Figure S10) and ActACP (Figure S11), and after fixing for reactions without BSA containing both VibB and ActACP (Figure S12).
Possible thiol-reactivity was also evaluated for SCH-202676 by HPLC coupled with ESI-MS analysis. SCH-202676 was prepared at a volume of 20 μL at 200 μM in DMSO discretely, as well as with either 200 μM L-cysteine or 200 μM DTT. Samples were diluted with milliQ water to give the desired compound concentrations, as well as a final DMSO concentration of 30%. Samples were incubated at room temperature for 3 hours, subjected to HPLC/ESI-MS analysis (Figure S13), and compared to DTT and L-cys controls (Figure S14).
Results & Discussion
Production and qualification of PPTases
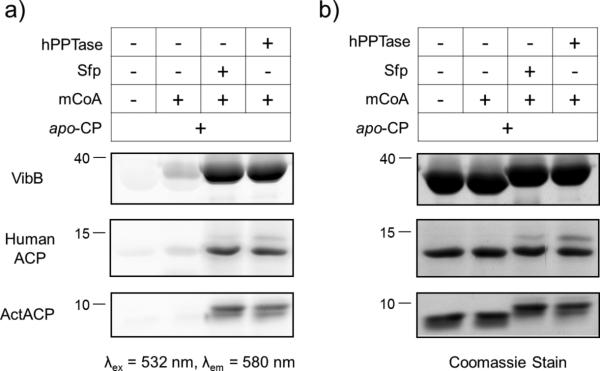
Following the expression and purification of the proteins used in these studies, we initially sought to confirm the function of the hPPTase with a qualitative gel-based phosphopantetheinylation assay.34 In this experiment, we tested the ability of PPTase enzymes to catalyze the transfer of a fluorescent phosphopantetheinyl appendage to a diverse set of carrier protein (CP) domains from a rhodamine-modified coenzyme A substrate. The results of these experiments clearly demonstrated function of the recombinant protein, and revealed relaxed substrate specificity with respect to the identity of the CP substrate as well as the modified CoA (Figure 1). Both hPPTase and Sfp modified the ACP domains of S. coelicolor polyketide synthase (PKS) ActACP, human fatty acid synthase (FAS) ACP, and V. cholera vibriobactin peptidyl carrier protein (PCP) VibB.
Figure 1. Gel-based fluorescent carrier protein labeling.
We used SDS-PAGE gel analysis prior to implementing fluorescence polarization techniques to verify that both Sfp and human PPTase (hPPTase) can append rhodamine-CoA (mCoA) to three types of apo carrier proteins indicated by fluorescent (a) and coomassie (b) visualization. VibB represents the peptidyl carrier protein (PCP) from NRPS biosynthesis, human ACP represents fatty acid synthesis (FAS) pathways, and ActACP represents polyketide synthase (PKS) pathways
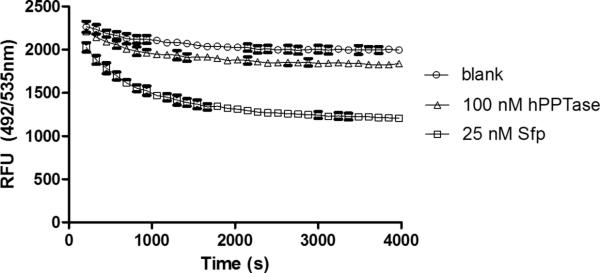
Next, we sought to implement the FRET screen that we previously described for both Sfp and AcpS PPTase,24 with the idea that the assay may be implemented for hPPTase by simple substitution of the enzyme. Unfortunately, we observed very low significant change in the assay signal after an hour at room temperature, even with 100 nM hPPTase compared to 25 nM Sfp relative to a buffer control (Figure 2). We can speculate that the inability of hPPTase to transfer easily into the FRET screen protocol may arise from required sequence specificity not matching the Sfp peptide substrate, a result we have observed for other PPTase enzymes (data not shown). Thus, we sought another method to monitor enzymatic activity.
Figure 2. Human PPTase tested in FRET assay format.
Fluorescein (FITC) conjugated to the minimal peptide Ybbr was subjected to PPTase activity evaluation using standard PPTase reaction conditions, including rhodamine-CoA. Reduction in fluorescent signal for fluorescein indicates rhodamine-pantetheine conjugation, and thus PPTase activity. Human PPTase (hPPTase) possesses poor labeling activity compared to Sfp under these conditions.
Design of the activity-based fluorescence polarization assay
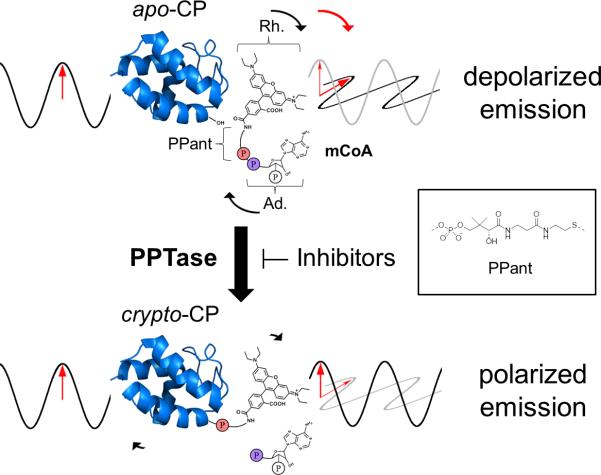
We reviewed the literature for methods to monitor PPTase activity; as the enzyme class has seen been a target of interest recently in the infectious disease research community due to implication in virulence.8-10 While we found numerous methods, we desired a homogenous format free of wash steps that directly monitors the complete landscape of the phosphopantetheinylation reaction without relying on readouts from coupled enzyme systems. These features would simplify the implementation of the method in an automated setting, focus on the bisubstrate phosphopantetheinylation reaction instead of individual substrate binding, and minimize the potential for false positive hits arising through inhibition of coupling partners; characteristics that we required from a finalized protocol. As such, we found the existing methods unsuitable, and envisioned the application of fluorescence polarization (FP) as a technique that could exploit the size difference between the species that phosphopantetheine is attached to in the CoA substrate and holo-CP product (Figure 3); a feature we have utilized routinely in gel-based PPTase activity detection.20
Figure 3. Measuring PPTase Activity with Fluorescence Polarization.
Free rhodamine-CoA (mCoA) containing rhodamine (Rh.), phophopantetheine (PPant) and adenosine (Ad.) components has its rhodamine-phosphopantetheine moiety transferred from mCoA to the apo carrier protein by the PPTase, generating the modified crypto carrier protein. The difference in tumbling rates of the unreacted rhodamine-CoA, 1.3 kDa versus bound crypto-CP (34 kDa in the case of VibB) due to total complex size creates a stronger polarized signal for bound reaction fluorophore.
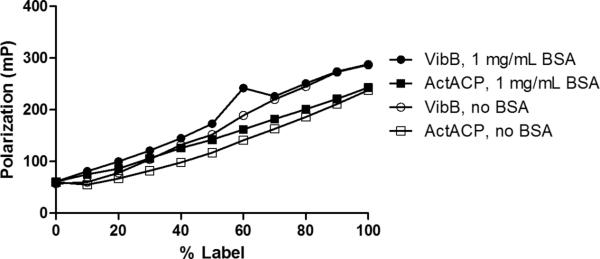
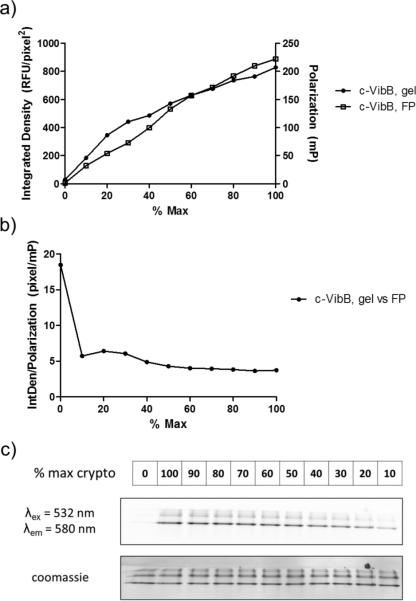
Throughout the development process, we found it important to work with Sfp PPTase since biochemical constants and parameters, as well as orthogonal detection methods already exist for this enzyme. To evaluate the ability of FP to distinguish between the substrate and product species, rhodamine-modified crypto- VibB and ActACP, were prepared by reaction with the corresponding rhodamine CoA in the presence of catalytic quantities of Sfp. These two proteins differ by more than 20 kDa in molecular weight, and allowed a comparison in the signal window between the two samples to inform future applications, since the small size of stand-alone CP-domains represents only a 10-fold increase in molecular weight vs. the corresponding CoA substrate. Following purification, these crypto-CP samples were used to assemble a series of solutions that contained a constant rhodamine fluorophore concentration, but varied by the degree to which the fluorescent species was attached to the protein; thus mimicking the enzymatic reaction at various states of conversion (Figure 4). We also compared the fluorescence polarization signal data for a linear dilution of crypto-VibB to its corresponding fluorescent gel densitometry data (Figure 5). In evaluating the data, a clear relationship was observed between the fluorescent polarization and densitometry data confirmed the feasibility of FP to detect the attachment of fluorescent phosphopantetheine arms to CP domains.
Figure 4. Crypto-VibB & ActACP linear dilution and fluorescence.
Complete fluorescently labeled crypto- carrier proteins were linearly diluted against unlabeled carrier protein (total carrier protein and probe concentrations constant) to demonstrate that higher amounts of carrier protein labeling corresponds to higher total fluorescent polarization signal. The common enzyme-stabilizing agent bovine serum albumin (BSA) was also determined to not interfere significantly with polarization signal.
Figure 5. Crypto-VibB monitoring by polarization vs. gel densitometry.
One particular strength of the fluorescence polarization assay lies in the ability to evaluate fluorescence polarization samples by gel electrophoresis if an EDTA quench is used to halt the reaction prior to analysis. Signal obtained from the same crypto-VibB (c-VibB) linear dilution scales with the percentage of labeling (a), as well as providing an almost static correlation above 10% labeling (b). Reference fluorescent gel imaging depicts the fluorescent intensity change with percent labeling (c).
We also evaluated PPTase and rhodamine-CoA association independently of carrier protein, using fluorescence polarization binding methodology instituted previously.31 Both Sfp and hPPTase demonstrated complete binding curves (Figure S15), which were used to calculate dissociation constants with Equations 1 & 2. Additionally, due to previous reports that Sfp co-purifies with coenzyme A when expressed in E. coli,35 we performed a calf-intestinal phosphatase treatment that resulted in a shift downward in the inflection point of the Sfp binding curve. This result suggests that competing endogenous CoA removal increases binding response to rhodamine-CoA.
Development of the FP-based assay for PPTase
Evaluation of suitability for high-throughput screening applications is an important precursor to large scale implementation of assay formats.36 HTS suitability is determined via the Z’ value calculated using Equation 3. We analyzed Sfp and hPPTase from 1 to 1000 nM with VibB (Figure S5) and human ACP (Figure S6) in order to determine optimal concentrations for assay implementation. The Z’ score was calculated throughout the reaction time course to depict Z’ data for VibB (Figure S16) and hACP (Figure S17) reactions. The lowest satisfactory Z’ value for a high-throughput assay is 0.5, whereas values above 0.8 are considered robust.36 Both Sfp and hPPTase displayed Z’ values above 0.6 with concentrations as low as 10 nM at 30 minute time points with VibB. However, Z’ values were much lower for both enzymes using hACP, such that Sfp only displayed Z’ values near 0.6 with 125 nM and hPPTase only approached Z’ = 0.6 at the highest 1000 nM concentration. We present sample Z’ data for Sfp and hPPTase at 30 and 60 minute timepoints for VibB (Table S1) and hACP (Table S2). We reason that the lower Z’ hACP values result from the significantly smaller molecular weight of hACP at ~ 10 kDa giving lower signal when fluorescently labeled by the rhodamine fluorophore, whereas the larger VibB at ~34 kDa gives higher signal. As such, we decided that the human ACP was not suitable for high-throughput inhibitor evaluation and VibB would be the primary screening substrate. As a compromise for further evaluation of both carrier proteins, we chose a working enzyme concentration of 100 nM for all other experiments unless otherwise noted.
We evaluated the effect of 0.01% v/v detergents Tween-20, Triton-X 100, NP-40 and 10% DMSO with respect to impact on Z’ scoring for PPTases and carrier proteins. PPTases evaluated with VibB (Figure S7) displayed similar performance at or above Z’ scores of 0.6 at 30 minutes for all detergents, with marginally higher Z’ scores (Figure S18) for 0.01% Tween-20. DMSO noticeably lowered Z’ scores for both Sfp and hPPTase with VibB, but maintained an acceptable Z’ score. However, analysis of hACP with these additives (Figure S8) once again displayed the lowest Z’ scores over time (Figure S19), only passing an acceptable Z’ score of 0.6 without DMSO at approximately 45 minutes. Z’ data at fixed timepoints of 30 and 60 minutes are supplied for VibB (Table S3) and hACP (Table S4). Since performance in the presence of DMSO is required for convenient inhibitor screening, this result disqualifies hACP for high-throughput screening without further optimization for increased Z’ score.
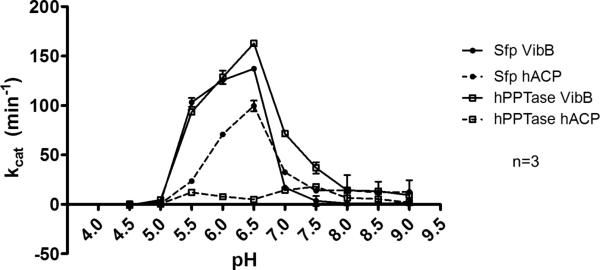
We evaluated pH-sensitivity of Sfp and hPPTase labeling in our experimental format (Figure 6) to analyze the flexibility of the assay format with variable enzyme reactions. Both PPTases demonstrated a preference for lower pH using VibB, which corresponds to previous results with Sfp.37 Upon testing human ACP at varied pH, we noticed the same low-pH preference for Sfp, but hPPTase lost its high activity at lower pH with this carrier protein. We may speculate that this could be due to truncation of the larger multi-domain synthase to a small carrier protein could impact substrate specificity. To maintain consistency with our previous screening work, we chose to conduct our inhibition studies at a pH of 7.6 that provides more biologically-relevant conditions.33
Figure 6. pH-dependent PPTase activity.
Sfp and hPPTase activities were determined over a broad pH range with both VibB and hACP carrier proteins using rhodamine-CoA. Both PPTases appear to prefer slightly acidic labeling conditions, with the exception of the hPPTase/hACP combination.
Applying known PPTase inhibitors to Sfp and hPPTase in FP assay
We evaluated a panel of inhibitors against the Sfp and human PPTases (Table 1). Typically, phosphoadenosine phosphate (PAP) is used as a product inhibitor for PPTase activity assays. In our case, we began our analysis with the analog 2’-deoxyadenosine-3,5-bisphosphate (2’-deoxy-PAP) (Figure S20a) due to the ease of synthesis, production cost, and its relatively close IC50 to PAP in other adenosine-binding systems such as P2Y receptors.32 In these experiments, hPPTase proved to be less sensitive to 2’-deoxy-PAP inhibition (IC50 = 50 μM) than Sfp (IC50 =11 μM). We next compared the effectiveness of the 2’-deoxy-PAP to the natural PAP (Figure S20b), finding the IC50 values to be similar for Sfp at 7 μM and approximately an order of magnitude lower for hPPTase at 6 μM. This implies that the 2’ hydroxyl group is more important in the binding of CoA for hPPTase. Additionally we utilized unlabeled CoA as an inhibitor of the assay because it will directly compete with rhodamine-CoA and the generation of the holo-CP will not increase polarization signal like the crypto-CP. The observed IC50 values for that unlabeled CoA displayed for hPPTase and Sfp were close to the active rhodamine-CoA concentrations in the assay (14 & 7 μM, respectively), and indicated that both enzymes displayed little preference for natural CoA over the rhodamine-CoA analog.
Table 1.
Analysis of Sfp-type PPTase standard inhibitors
| IC50 (μM) - VibB | ||
|---|---|---|
| Compound | Sfp | hPPTase |
| 2′-deoxy-3,5-phosphoadenosine | 11 ± 1 | 51 ± 1 |
| 3,5-phosphoadenosine | 7 ± 1 | 6 ± 1 |
| coenzyme A | 8 ± 1 | 14 ± 1 |
| Mitoxantrone 2HC1 | Inactive | Inactive |
| benserazide HCl | Inactive | NC |
| Bay 11-7085 | Activator | Inactive |
| SCH-202676 HBr | 0.2 ± 1 | NC |
| 6-nitroso-1,2-benzopyrone | 3 ± 1 | Inactive |
| PD 404,182 | 1 ± 1 | Inactive |
| guanidinyl-naltrindole ditrifluoroacetate | Activator | NC |
| sanguinarine Cl | 10 ± 1 | NC |
| calmidazolium Cl | 10 ± 1 | Inactive |
| (-)-ephedrine hemisulfate | Inactive | Inactive |
Legend: We subjected Sfp and human PPTase to inhibitors hits from previous assay formats. The two PPTases both demonstrate inhibition with product inhibition analog 2’-deoxy-phosphoadenosinephosphate (2’-deoxy-PAP), product inhibitor PAP and the competitive inhibitor coenzyme A. The compounds with previously identified Sfp inhibitory activity did not demonstrate any significant inhibition of human PPTase. However, some compounds slightly depressed human PPTase labeling, albeit not enough to calculate IC50 values. Compounds appearing to increase PPTase labeling are labeled as activators and were studied in further detail by gel analysis (Figure S22). Compounds displaying a reproducible but very small percent inhibition are not calculated “NC”.
Finally, we evaluated the ability of this assay to report on a panel of small molecule PPTase inhibitors that were identified from the library of pharmacologically active compounds 1280 (LOPAC1280) when it was screened against Sfp in a FRET assay format.33 Serial dilutions of these ten compounds were tested against both enzymes at 11 concentration points and the results summarized in Table 1 (full dose-response curves are presented in Figures S20c-g). In general, the activity profiles that these compounds displayed against Sfp in the FP assay were consistent with those observed previously in the FRET-based and gel methods, with SCH-202676 and PD 404,182 presenting the most potent inhibition. Interestingly, two compounds, BAY-11-7085 and guanidinyl-naltrindole, were apparent activators of Sfp but not hPPTase in the test. These findings were evaluated further by SDS-PAGE (Figure S22) and confirmed to increase fluorescent labeling of the carrier protein substrate, but the source of this inhibition discrepancy between assay conditions is unknown.
Given the unusual SCH-202676 inhibition profile of Sfp and hPPTase (Figure S20c), we further investigated the inhibition effect imparted by SCH-202676. Our initial investigation of fluorescent labeling in gel format focused on the suspected thiol-reactive mechanism for SCH-202676 proposed previously,38 and evaluated the presence or absence of additional 1 mM DTT above that which was present in protein preparations during VibB labeling. Gel results match those seen in fluorescent polarization as well as confirming significant inhibition in the case of free Ybbr (Figure S10). Given the good apparent inhibition of Ybbr labeling in gel format, we also investigated the effect of SCH-202676 inhibiting labeling of ActACP (Figure S11), with no apparent difference from the VibB results. We also investigated the effect BSA might have in blocking SCH-202676 activity due to its relatively higher concentration compared to PPTase in the reaction mixture, but no significant differences were seen (Figure S12) compared to the former experiments with BSA. Lastly, we wanted to confirm the mode of potential SCH-202676 modifications with L-cysteine (L-cys) as a model for protein inhibition, and DTT as a model for degradation. We observed the correct 269 m/z for SCH-202676 when incubated at room temperature alone (Figure S13a). However, incubation of SCH-202676 with one equivalent DTT provided both a clear new HPLC peak at 6.4 min and MS peak with a clear m/z of 271 (Figure S13b) not present in controls (Figure S14a), with the mass increase of 2 suggesting an opening of the SCH-202676 thiazole ring proposed previously.38 We observed a new small HPLC peak at 6 minutes upon incubating with one equivalent of L-cys (Figure S13c) not present in control with only L-cys (Figure S14b). These results indicate that SCH-202676 is a fragile inhibitor with regard to standard biochemical reagents, and identifies reducing agents and other free thiols as potential sources of inhibitor interference.
Additionally, we noted that some members of the panel displayed absorbance under the assay conditions, as evaluated the data for signs of perturbation via auto-fluorescence or inner filter effect; two common modes of nonspecific inhibition in FP assays.39 Evaluation of total intensity40 (Figure S21) revealed sanguinarine Cl and mitoxantrone impacted the raw signal at high concentrations; however, only sanguinarine Cl appeared to show inhibitory activity. We followed up on this with the SDS-PAGE analysis, where sanguinarine Cl displayed inhibitory behavior with Sfp (Figure S22).
In screening the Sfp inhibitors against hPPTase, we found that hPPTase was generally less susceptible to inhibition, as none of the Sfp LOPAC hit compounds presented IC50 values less than 100 μM against hPPTase. These results suggest we can expect a significant difference in inhibitory profiles of PPTase targets evaluated in the future, but presently allows facile detection of potential human side effects while pursuing these pathogenic PPTase drug targets using similar assay conditions.
Conclusion
We have developed and validated a novel fluorescence polarization PPTase activity assay for high throughput screening of PPTases utilizing fluorescently modified CoA to covalently label intact target apo-carrier protein. This assay format improves upon existing PPTase inhibitor screens because it measures actual labeling activity, thereby allowing identification of non-competitive and uncompetitive inhibitors while accommodating variation in the identity of the intact carrier protein and PPTase of interest. Our fluorescence polarization observations of Sfp inhibition activity indicate comparable IC50 values to existing FRET and gel-based techniques. Extension of this technique with the inhibitor panel to hPPTase generated notable differences in response compared to Sfp, underlying the necessity of an assay format capable of implementing the appropriate target PPTase and carrier protein directly into the assay for activity comparison. We seek to further extend this assay to various suitable drug target PPTases and scale up screening throughput in collaboration with the NIH Chemical Genomics Center. This new screening methodology furthers development and optimization of new inhibitor drug leads targeting PPTases conferring virulence to human pathogens.
Supplementary Material
Acknowledgements
Funding has been provided by NIH R21AI090213, NIH R01GM094924 and NIH R01 GM095970. We thank NIH Chemical Genomics Center (NCGC) for supplying LOPAC Sfp chemical inhibitors and human ACP plasmid, Udo Oppermann (Structural Genomics Consortorium, Oxford University) for gifting the human PPTase-containing plasmid, Susan Taylor Laboratory (UCSD) and Joe Noel (Salk Institute) for access to Tecan instruments for polarization measurements, Haydn Er Jun Cheng (UCSD) for synthesis of rhodamine-maleimide intermediate, Joris Beld (USCD) for performing LC/ESI-MS, and Mass Spectrometry Facility (UCSD) for performing ESI-MS & HR-MS analysis.
Acronyms
- PPTase
phosphopantetheinyl transferase
- CP
carrier protein
- ACP
acyl carrier protein
- PCP
peptidyl carrier protein
- FAS
fatty acid synthase
- PKS
polyketide synthase
- NRPS
non-ribosomal peptide synthase
- IPTG
Isopropyl-β-D-1-thiogalactoside
- HTS
high throughput screening
- FP
fluorescence polarization
- FRET
Fourier resonance energy transfer
- FITC
fluorescein isothiocyanate
- mP
millipolarization unit
- IC50
inhibitory concentration 50
- CoA
coenzyme A
- mCoA
modified coenzyme A
- PPant
phosphopantetheine
- MRSA
methicillin resistant Staphylococcus aureus
- Kd
dissociation constant
- Ni-NTA
nickel nitrilotriacetic acid
- EDTA
ethylene diaminetetraacetic acid
- PAP
phosphoadenosine phosphate
- LC
liquid chromatography
- ESI
electron-spray ionization
- MS
mass spectrometry
- TLC
thin layer chromatography
- DTT
dithiothreitol
- CIP
calf-intestinal phosphatase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MES
2-(N-morpholino)ethanesulfonic acid
- IMAC
immobilized metal affinity chromatography
References
- 1.World Health Organization . Global Tuberculosis Report. 2012. [Google Scholar]
- 2.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999-2005. Emerg. Infect. Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallen AJ, et al. Health care-associated invasive MRSA infections, 2005-2008. J. Am. Med. Assoc. 2010;304:641–648. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]
- 4.Marin MA, et al. Cholera outbreaks in Nigeria are associated with multidrug resistant atypical El Tor and non-O1/non-O139 Vibrio cholerae. PLoS Negl. Trop. Dis. 2013;7:e2049. doi: 10.1371/journal.pntd.0002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazeli H, et al. Pseudomonas aeruginosa infections in patients, hospital means, and personnel's specimens. J. Res. Med. Sci. 2012;17:332–337. [PMC free article] [PubMed] [Google Scholar]
- 6.Moran GJ, Rothman RE, Volturo GA. Emergency management of community-acquired bacterial pneumonia: what is new since the 2007 Infectious Diseases Society of America/American Thoracic Society guidelines. J. Am. Emerg. Med. 2013;31:602–612. doi: 10.1016/j.ajem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Dass Hazarika R, et al. Invasive Meningococcal Infection: Analysis of 110 cases from a tertiary care centre in north east India. Indian J. Pediatr. 2013;80:359–364. doi: 10.1007/s12098-012-0855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalut C, Botella L, de Sousa-D'Auria C, Houssin C, Guilhot C. The nonredundant roles of two 4'-phosphopantetheinyl transferases in vital processes of Mycobacteria. Proc. Natl. Acad. Sci. USA. 2006;103:8511–8516. doi: 10.1073/pnas.0511129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leblanc C, et al. 4'-Phosphopantetheinyl transferase PptT, a new drug target required for Mycobacterium tuberculosis growth and persistence in vivo. PLoS Pathog. 2012;8:e1003097. doi: 10.1371/journal.ppat.1003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barekzi N, et al. Genetic characterization of pcpS, encoding the multifunctional phosphopantetheinyl transferase of Pseudomonas aeruginosa. Microbiology. 2004;150:795–803. doi: 10.1099/mic.0.26823-0. [DOI] [PubMed] [Google Scholar]
- 11.Asghar AH, et al. The pobA gene of Burkholderia cenocepacia encodes a group I Sfp-type phosphopantetheinyltransferase required for biosynthesis of the siderophores ornibactin and pyochelin. Microbiology. 2011;157:349–361. doi: 10.1099/mic.0.045559-0. [DOI] [PubMed] [Google Scholar]
- 12.Allen G, et al. Functional analysis of a mitochondrial phosphopantetheinyl transferase (PPTase) gene pptB in Aspergillus fumigatus. Fungal Genet. Biol. 2011;48:456–464. doi: 10.1016/j.fgb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Horbach R, et al. Sfp-type 4'-phosphopantetheinyl transferase is indispensable for fungal pathogenicity. Plant Cell. 2009;21:3379–3396. doi: 10.1105/tpc.108.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quadri LEN, Sello J, Keating TA, Weinreb PH, Walsh CT. Identification of a Mycobacterium tuberculosis gene cluster encoding the biosynthetic enzymes for assembly of the virulence-conferring siderophore mycobactin. Chem. Biol. 1998;5:631–645. doi: 10.1016/s1074-5521(98)90291-5. [DOI] [PubMed] [Google Scholar]
- 15.McMahon MD, Rush JS, Thomas MG. Analyses of MbtB, MbtE, and MbtF suggest revisions to the mycobactin biosynthesis pathway in Mycobacterium tuberculosis. J. Bact. 2012;194:2809–2818. doi: 10.1128/JB.00088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peek ME, Bhatnagar A, McCarty NA, Zughaier SM. Pyoverdine, the major siderophore in Pseudomonas aeruginosa, evades NGAL recognition. Interdiscip. Perspect. Infect. Dis. 2012;2012:843509. doi: 10.1155/2012/843509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherr N, et al. Structure-activity relationship studies on the macrolide exotoxin mycolactone of Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2013;7:e2143. doi: 10.1371/journal.pntd.0002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, et al. Unique iron coordination in iron-chelating molecule vibriobactin helps Vibrio cholerae evade mammalian siderocalin-mediated immune response. J. Biol. Chem. 2012;287:8912–8919. doi: 10.1074/jbc.M111.316034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakin A, Schneider L, Podladchikova O. Hunger for iron: the alternative siderophore iron scavenging systems in highly virulent Yersinia. Front. Cell. Infect. Microbiol. 2012;2:151. doi: 10.3389/fcimb.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foley TL, Young BS, Burkart MD. Phosphopantetheinyl transferase inhibition and secondary metabolism. FEBS J. 2009;276:7134–7145. doi: 10.1111/j.1742-4658.2009.07425.x. [DOI] [PubMed] [Google Scholar]
- 21.Mootz HD, Finking R, Marahiel MA. 4'-phosphopantetheine transfer in primary and secondary metabolism of Bacillus subtilis. J. Biol. Chem. 2001;276:37289–37298. doi: 10.1074/jbc.M103556200. [DOI] [PubMed] [Google Scholar]
- 22.Finking R, et al. Characterization of a new type of phosphopantetheinyl transferase for fatty acid and siderophore synthesis in Pseudomonas aeruginosa. J. Biol. Chem. 2002;277:50293–50302. doi: 10.1074/jbc.M205042200. [DOI] [PubMed] [Google Scholar]
- 23.Joshi AK, Zhang L, Rangan VS, Smith S. Cloning, expression, and characterization of a human 4'-phosphopantetheinyl transferase with broad substrate specificity. J. Biol. Chem. 2003;278:33142–33149. doi: 10.1074/jbc.M305459200. [DOI] [PubMed] [Google Scholar]
- 24.Foley TL, Burkart MD. A homogeneous resonance energy transfer assay for phosphopantetheinyl transferase. Anal. Biochem. 2009;394:39–47. doi: 10.1016/j.ab.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley TL, et al. Preparation of FRET reporters to support chemical probe development. Org. Biomol. Chem. 2010;8:4601–4606. doi: 10.1039/c0ob00322k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J, Lin AJ, Golan DE, Walsh CT. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Prot. 2006;1:280–285. doi: 10.1038/nprot.2006.43. [DOI] [PubMed] [Google Scholar]
- 27.Marshall CG, Burkart MD, Meray RK, Walsh CT. Carrier protein recognition in siderophore-producing nonribosomal peptide synthetases. Biochemistry. 2002;41:8429–8437. doi: 10.1021/bi0202575. [DOI] [PubMed] [Google Scholar]
- 28.Haushalter RW, et al. Binding and “pKa” modulation of a polycyclic substrate analogue in a type II polyketide acyl carrier protein. ACS Chem. Biol. 2011;6:413–418. doi: 10.1021/cb200004k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano MM, Corbell N, Besson J, Zuber P. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 1992:313–321. doi: 10.1007/BF00280011. [DOI] [PubMed] [Google Scholar]
- 30.ExPASy ProtParam Tool http://web.expasy.org/protparam/
- 31.Duckworth BP, Aldrich CC. Development of a high-throughput fluorescence polarization assay for the discovery of phosphopantetheinyl transferase inhibitors. Anal. Biochem. 2010;403:13–19. doi: 10.1016/j.ab.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J. Med. Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasgar A, et al. A strategy to discover inhibitors of Bacillus subtilis surfactin-type phosphopantetheinyl transferase. Mol. Biosyst. 2010;6:365–375. doi: 10.1039/b913291k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Clair JJ, Foley TL, Schegg TR, Regan CM, Burkart MD. Manipulation of carrier proteins in antibiotic biosynthesis. Chem. Biol. 2004;11:195–201. doi: 10.1016/j.chembiol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Mofid MR, Marahiel MA, Ficner R, Reuter K. Crystallization and preliminary crystallographic studies of Sfp: a phosphopantetheinyl transferase of modular peptide synthetases. Acta Crystallogr. D Biol. Crystallogr. 1999;55:1098–1100. doi: 10.1107/s0907444999003674. [DOI] [PubMed] [Google Scholar]
- 36.Inglese J, et al. High-throughput screening assays for the identification of chemical probes. Nat. Chem. Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 37.Quadri LE, et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry. 1998;37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 38.Göblyös A, de Vries H, Brussee J, Ijzerman AP. Synthesis and biological evaluation of a new series of 2,3,5-substituted [1,2,4]-thiadiazoles as modulators of adenosine A1 receptors and their molecular mechanism of action. J. Med. Chem. 2005;48:1145–1151. doi: 10.1021/jm049337s. [DOI] [PubMed] [Google Scholar]
- 39.Lea WA, Simeonov A. Fluorescence polarization assays in small molecule screening. Expert Opin. Drug Discov. 2011;6:17–32. doi: 10.1517/17460441.2011.537322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turconi S, et al. Real experiences of uHTS: a prototypic 1536-well fluorescence anisotropy-based uHTS screen and application of well-level quality control procedures. J. Biomol. Screen. 2001;6:275–290. doi: 10.1177/108705710100600502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.