Aminoacyl-tRNA synthetases (AARSs) are well known for catalyzing the selective attachment of amino acids to their cognate tRNAs to ensure accurate protein translation. Evidence has accumulated in recent years that AARSs are involved in additional complex eukaryotic processes, including recruitment of immune cells and regulation of angiogenesis [1-4] . In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Castranova et al. report an additional link between angiogenesis regulation and two specific AARSs, threonyl tRNA synthetase (TARS) and isoleucyl tRNA synthetase (IARS)[Reference]. In a genetic screen for altered vasculature, mutations in the tars and iars zebrafish genes were identified by classical genetic mapping and sequencing. The dysregulated vessel formation was associated with a premature stop codon in the tars gene and a point mutation in the iars gene. The phenotype caused by these mutations could be rescued by transgenes encoding the wild type alleles. Further mechanistic studies showed that loss of TARS or IARS function leads to upregulation of genes in the unfolded protein response (UPR) as well as vascular endothelial growth factor (vegfaa), which encodes the angiogenic growth factor VEGF. The induction of vegfaa was significantly reduced by mopholino knockdown of the UPR-mediated transcription factor gene atf4, leading to the conclusion that the connection between loss of AARS activity and angiogenesis was through the UPR.
The UPR is a collection of signaling pathways that is activated in response to the accumulation of unfolded proteins in the endoplasmic reticulum (ER). These pathways result in the slowing of protein translation, an increase in protein degradation and an increase in expression of protein translation and folding mediators. If these adaptations fail to reduce the level of unfolded proteins, continued activation of the UPR results in mitochondrial-mediated apoptosis [5]. UPR malfunctions have been associated with numerous pathologies including diabetes, cancer and immune disorders [6]. The finding by Castranova et al. that loss of function mutations in AARSs can elicit the UPR is consistent with related work indicating that natural products that bind tightly to AARS also elicit ER stress and UPR. Notably, the two most dramatic examples that link synthetase inhibition with induction of ER stress are inhibition of glutamyl-prolyl tRNA synthetase (EPRS) by halofuginone [7, 8] and inhibition of TARS by borrelidin [9]. There have also been previous connections made between the UPR pathways and VEGF induction, but not in the context of vascular phenotype mapping [10]. The hypothesis emerging from these observations is that loss of synthetase function (the result of nutrient depletion, genetic mutation or small molecule inhibition) produces ER stress, a subsequent increase in ATF4 levels, and rising VEGF levels to promote increased vascular branching.
While this model accounts for much of what is reported in Castranova et al., the role of AARSs in stress-mediated angiogenesis is likely more complex. For example, zebrafish mutations in other AARS genes (vars, qars, and mars) do not universally affect angiogenesis during development [11]. In addition, seryl-tRNA synthetase (SARS) mutants also exhibit the ectopic branching vascular phenotype through a VEGF pathway, but this effect is independent of aminoacylation activity and ATF4 upregulation. Evidence for the association of other AARSs with angiogenesis through a secretion mechanism, such as tyrosyl-trna synthetase (YARS) and TARS, has also been reported [12, 13]. In the case of TARS, the UPR- and extracellular-mediated angiogenic functions can be separated, and the extracellular angiogenic function does not require aminoacylation activity [14]. These various studies all point to a more complicated model in which a number of aminoacyl-tRNA synthetases can promote angiogenesis either by detecting nutrient depletion, which triggers UPR and VEGF production, or through extracellular mechanisms (Figure 1). Despite the missing pieces in the puzzle, the work of Castranova et al. adds to the emerging picture that the AARSs play a key role as direct signaling molecules that integrate nutrient status with regulation of angiogenic pathways via the UPR and other stress mechanisms.
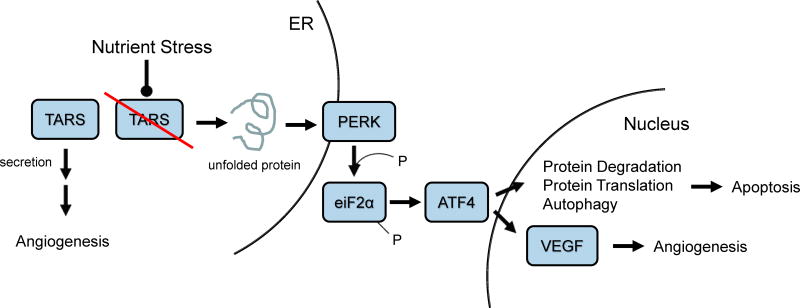
Figure 1. TARS, an example of AARS regulation of Angiogenesis through the UPR.
Nutrient stress or loss of TARS activity leads to an increase in unfolded proteins which promotes the activation of PERK (protein kinase RNA-like endoplasmic reticulum kinase) in the ER membrane. PERK transduces the signal through phosphorylation of eukaryotic initiation factor 2α (eiF2α) and activating transcription factor 4 (ATF4) translation. ATF4 induces the transcription of genes important in protein degradation, translation and autophagy. Extended UPR activity leads to apoptosis. Castranova et al. made a direct link between loss of TARS synthetase activity and induction of angiogenesis through an ATF4-VEGF pathway. A separate pathway includes secretion of TARS and angiogenic signaling that is independent of TARS synthetase activity.
Footnotes
Disclosures: None
References
- 1.Howard OM, Dong HF, Yang D, Raben N, Nagaraju K, Rosen A, Casciola-Rosen L, Hartlein M, Kron M, Yang D, Yiadom K, Dwivedi S, Plotz PH, Oppenheim JJ. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196(6):781–791. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park MC, Kang T, Jin D, Han JM, Kim SB, Park YJ, Cho K, Park YW, Guo M, He W, Yang X-L, Schimmel P, Kim S. Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. PNAS. 2012;109(11):E640–E647. doi: 10.1073/pnas.1200194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ofir-Birin Y, Fang P, Bennett SP, Zhang HM, Wang J, Rachmin I, Shapiro R, Song J, Dagan A, Pozo J, Kim S, Marshall AG, Schimmel P, Yang XL, Nechushtan H, Razin E, Guo M. Structural switch of lysyl-tRNA synthetase between translation and transcription. Mol Cell. 2013;49(1):30–42. doi: 10.1016/j.molcel.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirando AC, Francklyn CS, Lounsbury KM. Regulation of Angiogenesis by Aminoacyl-tRNA Synthetases. International journal of molecular sciences. 2014;15(12):23725–23748. doi: 10.3390/ijms151223725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarti A, Chen AW, Varner JD. A Review of the Mammalian Unfolded Protein Response. Biotechnology and bioengineering. 2011;108(12):2777–2793. doi: 10.1002/bit.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. The Journal of Cell Biology. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone Inhibits TH17 Cell Differentiation by Activating the Amino Acid Starvation Response. Science. 2009;324(5932):1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, Yum J, Kim Y-J, Lee H-K, Cortese JF, Wirth DF, Dignam JD, Rao A, Yeo C-Y, Mazitschek R, Whitman M. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat Chem Biol. 2012;8(3):311–317. doi: 10.1038/nchembio.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidhu A, Miller JR, Tripathi A, Garshott DM, Brownell AL, Chiego DJ, Arevang C, Zeng Q, Jackson LC, Bechler SA, Callaghan MU, Yoo GH, Sethi S, Lin H-S, Callaghan JH, Tamayo-Castillo G, Sherman DH, Kaufman RJ, Fribley AM. Borrelidin Induces the Unfolded Protein Response in Oral Cancer Cells and Chop-Dependent Apoptosis. ACS Medicinal Chemistry Letters. 2015;6(11):1122–1127. doi: 10.1021/acsmedchemlett.5b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional Regulation of VEGF-A by the Unfolded Protein Response Pathway. PloS one. 2010;5(3):e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg Y, King M, Kiosses WB, Ewalt K, Yang X, Schimmel P, Reader JS, Tzima E. The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. Faseb J. 2008;22(5):1597–1605. doi: 10.1096/fj.07-9973com. [DOI] [PubMed] [Google Scholar]
- 13.Williams TF, Mirando AC, Wilkinson B, Francklyn CS, Lounsbury KM. Secreted Threonyl-tRNA synthetase stimulates endothelial cell migration and angiogenesis. Scientific reports. 2013;3:1317. doi: 10.1038/srep01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirando AC, Fang P, Williams TF, Baldor LC, Howe AK, Ebert AM, Wilkinson B, Lounsbury KM, Guo M, Francklyn CS. Aminoacyl-tRNA synthetase dependent angiogenesis revealed by a bioengineered macrolide inhibitor. Scientific reports. 2015;5:13160. doi: 10.1038/srep13160. [DOI] [PMC free article] [PubMed] [Google Scholar]