Abstract
Purpose
We report long-term disease control, survival, and toxicity for patients with locally advanced non-small cell lung cancer prospectively treated with concurrent proton therapy and chemotherapy on a nonrandomized case-only obervational study.
Methods
All patients received passive-scatter proton therapy, planned with 4D-CT–based simulation; all received proton therapy concurrent with weekly chemotherapy. Endpoints were local and distant control, disease-free survival (DFS), and overall survival (OS).
Results
The 134 patients (21 stage II, 113 stage III; median age 69 years) had a median gross tumor volume (GTV) of 70 cm3 (range, 5-753 cm3); 77 patients (57%) received 74 Gy(RBE), and 57 (42% received 60–72 Gy(RBE) (range, 60-74.1 Gy(RBE)). At a median follow-up time of 4.7 years, median OS times were 40.4 months (stage II) and 30.4 months (stage III). Five-year DFS rates were 17.3% (stage II) and 18.0% (stage III). OS, DFS, and local and distant control rates at 5 years did not differ by disease stage. Age and GTV were related to OS and DFS. Toxicity was tolerable, with 1 grade 4 esophagitis and 16 grade 3 events (2 pneumonitis, 6 esophagitis, 8 dermatitis).
Conclusion
This report of outcomes after proton therapy for 134 patients indicated that this regimen produced excellent OS with tolerable toxicity.
Keywords: Proton beam therapy, passive scattering, survival, disease control, carboplatin, paclitaxel
INTRODUCTION
The standard of care for locally advanced inoperable non-small cell lung cancer (NSCLC) is concurrent chemotherapy and photon-based radiation therapy. However, disease recurrence, both local and distant, remains problematic and more effective treatments are actively being sought. One such treatment currently being investigated involves proton therapy, which has been shown in treatment-planning comparisons to deliver high-dose, highly conformal radiation to targets while minimizing damage to surrounding normal tissues. The potential for improving the therapeutic ratio by escalating the radiation dose to control the tumor while minimizing toxicity, even when concurrent chemotherapy is given, presumably would improve patient outcomes.
Although proton therapy has been used for decades, its early use in physics research facilities had numerous restrictions on field size, beam energy, and beam direction, which in turn limited the types of disease that could be treated at such facilities. However, as the numbers of proton facilities dedicated to clinical care increase across the world, proton beam therapy is increasingly being used to treat more diverse malignancies, including lung cancer. The experience with proton therapy for NSCLC to date has largely been confined to small (stage I) tumors treated with proton therapy alone; such therapy has resulted in high rates of local control even at long-term follow-up (1,2). Although several groups are testing proton therapy with concurrent chemotherapy for locally advanced (stages II and III) NSCLC (e.g., 3,4, and our own phase II study [5]), little is known of the long-term outcomes of such treatment.
Herein we report a series of patients enrolled in a prospective, nonrandomized case-only observational protocol to assess normal tissue toxicity arising from treatment with protons. Our intent is to report long-term outcomes, including disease control, survival, and toxicity, for patients with locally advanced lung cancer treated definitively with proton therapy to doses of up to 74 Gy(RBE), given with concurrent chemotherapy.
MATERIALS AND METHODS
Patient characteristics
In this prospective study, 134 patients diagnosed with stage II or III NSCLC received definitive chemotherapy and proton beam radiation therapy at a single institution from 2006 through 2010. All patients were enrolled on a protocol specifically designed to assess normal tissue effects (registered with the National Cancer Institute as NCT00991094). None had participated in the phase II trial reported by Chang et al. (5), and participation in a randomized trial was an exclusion criterion for this analysis.
Disease was staged according to the 6th (2002) edition of the American Joint Commission on Cancer staging system. NSCLC was histopathologically confirmed in all cases. Patients were evaluated with thoracic computed tomography (CT), positron emission tomography (PET)/CT, bronchoscopy with endobronchial ultrasonography or mediastinoscopy to stage mediastinal involvement. All patients were evaluated by a thoracic surgeon and were deemed to have either medical conditions that precluded surgery or unresectable disease. All patients received concurrent chemotherapy, most often with weekly carboplatin and paclitaxel as weekly intravenous infusions during proton therapy. The choice of chemotherapy, timing, and dose was at the discretion of the treating physician. In most cases, paclitaxel was administered at 50 mg/m2 of body surface area, and carboplatin was given at an area under the curve level of 2 mg/nL/min; about 10% of patients received etoposide and paclitaxel because of renal insufficiency. Chemotherapy was withheld at the discretion of the treating physician in the event of abnormal laboratory values or toxicity. Treatment-related toxicity was assessed according to the Common Terminology Criteria for Adverse Effects version 3.0.
Proton beam treatment delivery
All patients were treated at the MD Anderson Proton Therapy Center-Houston by using a passive scattering method, the standard of care during the study period; a scanning beam system suitable for lung cancer did not become available until 2010. The passive scattering system uses a double-scatttering range-modulating wheel to spread out the Bragg peak, a range shifter to determine the maximum depth of the proton beam, custom-made brass apertures to shape the field laterally, and customized compensators to conform the distal edge of the beam to the target volume. The treatment planning process for lung tumors involves the use of published formulas for calculating distal and proximal, range, and Hounsfield unit uncertainties (6). The compensators used for passive-scatter proton planning shape the dose distally to conform to the tumor boundaries; the compensators were designed with a drill bit with a 5-mm radius. Brass aperture blocking was used for the lateral margins, and a 1.3-cm uniform expansion from the clinical target volume (CTV) was used as a starting margin. The margins were adjusted slightly to increase or decrease coverage of the tumor. The proton beam energies available for the passive scattering system range from 100 MeV to 250 MeV.
Treatment plans were designed with Varian's Eclipse proton therapy system (Varian Medical Systems, Palo Alto, CA). All patients underwent 4D CT-based simulation to account for tumor motion. The internal gross tumor volume (IGTV) was defined as the envelope of motion of the GTV on a reconstructed maximum intensity projection image and verified across all phases of the 4-dimensional CT dataset (7). The internal clinical target volume (ICTV) was defined as the IGTV plus an 8-mm isotropic margin edited to cover possible tumor microextension. Nodes suspected of harboring disease on PET or mediastinoscopy were included in the high-dose region (≥70-Gy(RBE)) within the ICTV. Two or three treatment fields were delivered daily. All patients received 5 consecutive daily fractions per week at 2 Gy(RBE) per fraction. Prescription doses were determined by the treating physician, with an intended dose up to 74 Gy(RBE), based on our institutional upper limit for proton therapy for lung cancer. If dosimetric parameters could not be met, the dose was reduced to meet normal tissue constraints. Those dose constraints were as follows: spinal cord: Dmax <45 Gy; lung: mean dose ≤20 Gy; V20 (total lung volume minus GTV) ≤35%, V10 ≤45%, V5 ≤ 65%; heart: V30 ≤45%, mean dose <26 Gy; esophagus: Dmax ≤80 Gy, V70 <20%, V50 <40%, mean dose <34 Gy; kidney: 20 Gy to <32% of bilateral kidney; liver: V30 ≤40%, mean dose <30 Gy.
Treatment evaluations
Posttreatment evaluations took place every 3-6 months and consisted of interim history and physical examination, lab work, and CT or PET/CT scans. Toxicity was assessed at each follow-up visit and scored with the NCI's Common Terminology Criteria for Adverse Events, version 3.0.
Statistical analyses
Outcomes in this study were local, marginal (regional), and distant control (measured from the completion of proton therapy) and disease-free and overall survival (measured from the beginning of proton therapy). Local recurrence was defined as radiographic or histologic evidence of disease progression within the high-dose [≥70-Gy(RBE)] region. Marginal (regional) recurrence was defined as radiographic evidence of recurrence within the 60- to 70-Gy(RBE) region adjacent to the high-dose region. Distant failure was failure at any other site. Disease-free survival was defined as survival without evidence of lung cancer at local, marginal, or distant sites. Overall survival was defined as the time to death from any cause; deaths from lung cancer were considered an event in this analysis, and patients who were alive or lost to follow-up were censored. . The Kaplan-Meier method was used for all time-to-event analyses, and log-rank tests were used to compare curves. All statistical analyses were done with Stata release 9 (StataCorp, College Station, TX). A Cox proportional hazards model was used for univariate and multivariate analysis of factors potentially associated with disease control or survival.
RESULTS
Patient, disease, and treatment characteristics are summarized in Table 1. Twenty-one patients (16%) had stage II disease and 113 (84%) had stage III disease. The median age at diagnosis was 69 years (range, 28-95 years), the median Karnofsky performance status (KPS) score was 80 (range, 60-100), the median radiation dose was 74.0 Gy(RBE) [range, 60-74.1 Gy(RBE)], and the median GTV was 70 cm3 (range, 5-753 cm3). The median follow-up time was 4.6 years (range 1.5-6.7 years).
Table 1.
Patient characteristics
| Characteristic | Value or No. of Patients (%) |
|---|---|
| Sex | |
| Male | 73 |
| Female | 61 |
| Age, years | |
| <70 | 76 |
| ≥70 | 58 |
| Performance status | |
| KPS <80 | 36 |
| KPS ≥80 | 98 |
| Disease stage | |
| IIA | 6 |
| IIB | 15 |
| IIIA | 70 |
| IIIB | 43 |
| Tumor histology | |
| Squamous | 59 |
| Nonsquamous | 75 |
| Gross tumor volume, cm3 | |
| Median (range) | 70 (5-753) |
| Radiation dose | |
| 60-69.6 Gy(RBE) | 24 (18%) |
| 70-72 Gy(RBE) | 33 (25%) |
| 74 Gy(RBE) | 77 (57%) |
| Concurrent chemotherapy | 134 |
| Median follow-up time | 4.7 yrs |
Abbreviation: KPS, Karnofsky performance score
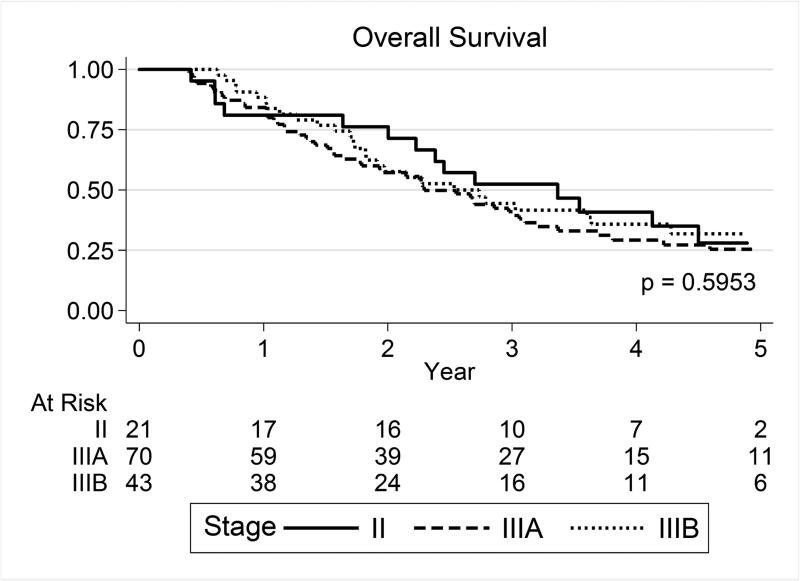
The median overall survival times were 40.4 months for patients with stage II disease and 30.4 months for those with stage III disease. Neither overall nor disease-free survival rates differed by disease stage at 3 years or at 5 years (Table 2; Fig. 1A,B). Similarly, local and distant control did not differ by disease stage at 3 years or at 5 years (local+marginal [regional] failure-free survival rates for all patients were 55.8% at 3 years and 54.4% at 5 years [P=0.762], and distant metastasis–free rates were 50.3% at 3 years and 45.8% at 5 years [P=0.861]) (Table 2; Fig. 1C).
Table 2.
Survival and Disease Control Outcomes by Disease Stage and Radiation Dose
| Survival Rates, % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disease Stage | Overall | Disease-Free | Local+Marginal Failure-Free | Distant Metastasis-Free | ||||
| 3-y | 5-y | 3-y | 5-y | 3-y | 5-y | 3-y | 5-y | |
| Disease Stage | ||||||||
| II | 52.3 | 30.0 | 23.8 | 19.0 | 49.7 | 49.7 | 52.4 | 52.4 |
| IIIA | 41.0 | 25.3 | 24.3 | 17.4 | 58.7 | 55.7 | 50.0 | 43.3 |
| IIIB | 44.5 | 31.8 | 24.2 | 14.1 | 49.2 | 49.2 | 48.0 | 43.1 |
| P Values | 0.595 | 0.955 | 0.762 | 0.861 | ||||
| Radiation Dose, Gy(RBE) | ||||||||
| 60-72 | 45.1 | 32.7 | 22.8 | 12.2 | 49.5 | 46.4 | 49.1 | 39.2 |
| 74 | 42.9 | 23.8 | 25.3 | 17.8 | 58.6 | 58.6 | 50.7 | 50.7 |
| P Values | 0.804 | 0.687 | 0.227 | 0.429 | ||||
Figure 1.
(A) Overall survival, (B) local+marginal (regional) failure-free survival, and (C) distant metastasis-free survival by disease stage at diagnosis. No significant differences were found according to disease stage.
Variables evaluated for potential relationship with outcomes (local control, distant metastasis, disease-free survival, and overall survival) after proton-based chemoradiation were age, GTV, radiation dose, sex, tumor histology, KPS, and disease stage. Because relatively few patients had stage II disease (n=21), we analyzed potential associations for the entire cohort (n=134) and for those with stage III disease (n=113) separately. On univariate analysis, GTV was related to both overall and disease-free survival, both for all patients and for the subset of patients with stage III disease. Age at diagnosis was related to overall survival both for all patients and for the subset of patients with stage III disease; however, age at diagnosis was associated with disease-free survival for only for the entire group (Table 3). Multivariate analysis with these variables produced findings similar to those of the univariate analysis (Table 3).
Table 3.
Univariate and multivariate analyses of factors affecting outcome
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n=134) | Patients with Stage III Disease (n=113) | All Patients (n=134) | Patients with Stage III Disease (n=113) | |||||
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Univariate | ||||||||
| 100-cm3 increase in GTV | 1.433 (1.1641-1.7654) | 0.00069 | 1.437 (1.1531-1.7918) | 0.00124 | 1.297 (1.0754-1.5631) | 0.0065 | 1.277 (1.0506-1.5631) | 0.0140 |
| Sex (male) | 0.818 ((0.542-1.233) | 0.3366 | 0.637 (0.436-0.93) | 0.0196 | ||||
| Age (continuous) | 1.032 (1.0092-1.0558) | 0.0123 | 1.023 (1.0053-1.0472) | 0.0135 | ||||
| Age (≥70) | 1.436 (0.953-2.165) | 0.0837 | 1.351 (0.928-1.968) | 0.1164 | ||||
| KPS 80-100 | 1.027 (0.645-1.637) | 0.9091 | 1.245 (0.81-1.914) | 0.3172 | ||||
| KPS 90-100 | 0.829 (0.509-1.35) | 0.4513 | 0.723 (0.456-1.147) | 0.1687 | ||||
| Tumor histology (squamous) | 1.385 (0.922-2.081) | 0.1171 | 1.24 (0.853-1.801) | 0.2598 | ||||
| Dose (70-92 Gy(RBE)) | 1.183 (0.602-2.324) | 0.6259 | 0.917 (0.516-1.629) | 0.7676 | ||||
| Dose (74 Gy(RBE)) | 1.172 (0.64-2.145) | 0.6076 | 0.879 (0.531-1.456) | 0.6013 | ||||
| Multivariate | ||||||||
| Age (continuous) | 1.032 (1.008-1.057) | 0.0099 | 1.026 (1.001-1.052) | 0.433 | 1.025 (1.003-1.048) | 0.0284 | — | — |
| 100-cm3 increase in GTV | 1.485 (1.198-1.1.841 | 0.0003 | 1.474 (1.177-1.845) | 0.0007 | 1.34 (1.104-1.628) | 0.0031 | 1.277 (1.0506-1.5631) | 0.0140 |
Abbreviations: HR, hazard ratio; CI, confidence interval; GTV, gross tumor volume; KPS, Karnofsky performance status score
Toxicity was mild overall, with only 1 patient experiencing grade 4 toxicity (esophagieal stricture) and 16 experiencing grade 3 toxicity (2 radiation pneumonitis, 6 esophagitis, and 8 dermatitis). Toxicity is summarized in Table 4.
Table 4.
Toxicity
| No. of Patients Experiencing Toxicity (%) | ||||||
|---|---|---|---|---|---|---|
| Type of Toxicity | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P Value* |
| Dermatitis | 74 (55) | 33 (25) | 19 (14) | 8 (6) | 0 | |
| 60-66 Gy(RBE) | 14 (61) | 5 (22) | 2 (9) | 2 (9) | 0 | 0.5469 |
| 70-72 Gy(RBE) | 19 (56) | 9 (26) | 4 (12) | 2 (6) | 0 | |
| 74 Gy(RBE) | 41 (52) | 19 (25) | 13 (17) | 4 (5) | 0 | |
| Esophagitis | 69 (51) | 25 (19) | 33 (25) | 6 (4) | 1 (1)† | |
| 60-66 Gy(RBE) | 10 (43) | 3 (13) | 8 (35) | 2 (9) | 0 | 0.4162 |
| 70-72 Gy(RBE) | 24 (71) | 6 (18) | 3 (9) | 1 (3) | 0 | |
| 74 Gy(RBE) | 35 (45) | 16 (21) | 22 (29) | 3 (4) | 1 (1) | |
| Radiation pneumonitis | 68 (51) | 35 (26) | 29 (22) | 2 (1.5) | 0 | |
| 60-66 Gy(RBE) | 14 (61) | 5 (22) | 4 (17) | 0 | 0 | 0.525 |
| 70-72 Gy(RBE) | 20 (6) | 9 (26) | 5 (15) | 0 | 0 | |
| 74 Gy(RBE) | 34 (44) | 21 (27) | 20 (26) | 2 (3) | 0 | |
Two-sided Fisher's exact test
Esophageal stricture
DISCUSSION
To our knowledge, this study is the first report of long-term outcomes after proton-based chemoradiation therapy for a relatively large number of patients with locally advanced inoperable lung cancer. Our results indicated that this regimen was tolerable, with a grade 4 toxicity rate of 0.7% and grade 3 toxicity rate of 12%, and produced excellent median overall survival times (40.4 months for stage II and 30.4 months for stage III). Disease stage was not associated with 5-year rates of overall survival or local control, but not suprisingly, larger GTV and older age were associated with poorer prognosis.
Some evidence exists to support higher radiation doses being beneficial in terms of local disease control and survival, although this is still a topic of debate. An analysis of seven RTOG trials for locally advanced NSCLC showed that higher radiotherapy doses were associated with improved local-regional control and survival when radiation was given with chemotherapy (8). Several prospective trials ensued to evaluate the feasibility and benefit of delivering 74 Gy thoracic radiation therapy with concurrent chemotherapy for patients with inoperable stage I-III NSCLC (9,10)). Dose escalation up to 84 Gy seemed to indicate improved survival for patients receiving higher radiation doses if delivered safely to minimize toxicity (11). Phase I/II studies further demonstrated that higher radiation doses produced favorable outcomes, with improved median survival times (>20 months) and tolerable toxicity for patients with inoperable NSCLC (9-13).
This evidence of a benefit from dose escalation makes the results of the recent Radiation Therapy Oncology Group (RTOG) protocol 0617 somewhat perplexing (14). The lack of an observed dose-response relationship for local control or survival was disappointing and was counter to findings of previous RTOG studies. More significantly, the poorer survival in the high-dose (74 Gy) group relative to in the lower-dose group (60 Gy) seems counterintuitive to basic radiobiology principles. Although further analysis of these findings is pending, one likely explanation for the lack of benefit in the higher dose arm is subclinical (in terms of adverse events reported) cardiopulmonary toxicity (15). Such toxicity has been hypothesized to reduce survival in other studies of outcomes after receipt of postoperative radiation, (16,17), particularly when that radiation is given with older, 2D techniques (18). Minimizing cardiopulmonary toxicity is particularly relevant to treating thoracic malignancies with proton therapy. The near-absence of treatment-related pneumonitis and the low frequency of esophageal toxicity are reasonable surrogates for lower subclinical doses to lung and to the heart, which could translate to improvements in survival.
Thus we believe that proton therapy may be one way in which radiation dose can be safely escalated, as dose escalation with photons in RTOG 0617 led to compromised survival. However, other groups have raised the possibility that local control is a function of not only dose but also GTV and treatment time. Although the probability of local control by radiation is known to decrease as tumor volume increases, one group has posited that higher doses may effectively minimize the adverse outcomes associated with large GTVs in early-stage disease (19). Findings from the the CHARTWEL (ARO 97-1) trial showed that treatment intensification via highly accelerated radiation therapy led to improved local control relative to conventionally fractionated radiation, particularly for patients with advanced disease or those who received induction chemotherapy (20). A subgroup analysis of the CHARTWEL trial revealed that the negative effect of GTV on tumor control after radiotherapy was also influenced by treatment schedule; specifically, larger tumors were associated with greater risk of local failure after conventionally fractionated radiotherapy, but dose-intensification via highly accelerated radiotherapy could miminize that risk, particularly among patients with advanced disease or those who are not eligible for concurrent chemotherapy (21). Differences between the CHARTWEL study and that reported here include the design (randomized vs. nonrandomized data collection), the fractionation schedule (60 Gy was given in forty 1.5-Gy fractions three times a day over 2.5 weeks vs. 74 Gy(RBE) in thirty-seven 2-Gy(RBE) fractions, 5 days per week, over 7.5 weeks), the radiation type (3D-planned conformal X-ray therapy vs. proton therapy), the radiation dose (60 Gy vs. up to 74 Gy(RBE)), and the use of sequential vs. concurrent chemotherapy. Nevertheless, such a regimen may be effective for patients with advanced disease or those who cannot tolerate concurrent chemoradiation therapy.
Studies of proton therapy to date have been criticized for the relatively small numbers of patients with NSCLC treated in various series and the lack of sufficiently long follow-up to provide some measure of efficacy. Nakayama et al. reported that 35 patients with stage II-III NSCLC treated with a mean proton dose of 78 Gy(RBE) without chemotherapy had good local control, with 65.9% local progression-free survival at 2 years, and low toxicity (3). Investigators at the University of Florida treated 19 patients with locally advanced NSCLC with concurrent chemotherapy and proton therapy to a median dose of 74 Gy(RBE). At a median follow-up interval of 16 months, only 1 patient in that study had experienced local progression (4). One prospective randomized trial of proton therapy with concurrent chemotherapy vs. photon (X-ray) therapy and chemotherapy, combining the efforts of investigators from the Massachusetts General Hospital and The University of Texas MD Anderson Cancer Center, recently completed accrual. Another, the multi-institutional trial RTOG 1308, is randomly assigning patients with inoperable stage II or III NSCLC to 70 Gy of proton or photons; this trial is currently accruing patients. While we await the results of both of these trials, it is important to report our experience with patients who were not enrolled on those trials, either because they had been treated before the randomized trial was begun or because they had declined randomization in those trials owing to difficulties with insurance coverage, unwillingness to participate in a randomized trial, or other patient or physician preferences.
Our study had some limitations, chief among them being its design. This was not, nor was it designed to be, a randomized study; rather, it was a nonrandomized, data collection/single-case observation study, and our methods of statistical analysis were applied accordingly. We specifically excluded patients who were enrolled in randomized trials in an attempt to minimize selection bias; we acknowledge that this may complicate interpretation of our findings. Other limitations are that only 77 patients (57%) received 74 Gy(RBE) (33 received 70-72 Gy(RBE) and 24 received 60-69.6 Gy(RBE)) and that this study included only patients treated at a single institution, with relatively strict normal-tissue constraints. Conversely, the strengths of this study also include its relatively large number of patients and long-term follow-up; exclusion of patients who were participating in randomized trials; and its conduct at a single ‘high-throughput’, high-volume center in which dose constraints were both strict and consistently adhered to. Indeed, every patient undergoing proton therapy at this institution is required to participate in a protocol, to gather sufficient data to document treatment-related toxicity and to generate realistic, reproducible normal-tissue dose constraints.
This series is the largest reported to date to include the standard treatment paradigm of concurrent chemotherapy and radiation with proton beam therapy. We found very good median survival times (40.4 months for patients with stage II disease and 30.4 months for those with stage III disease) and promising 5-year overall survival rates (25.3% for patients with stage IIIA disease and 31.8% for those with stage IIIB disease). This apparent difference in overall survival was not statistically significant, perhaps because fewer patients in this study had stage IIIB disease. Nevertheless, this improved survival relative to other reports of patients with stage III NSCLC could be explained by our ability to safely deliver a relatively high radiation dose of 74 Gy(RBE) with protons even to large bulky inoperable tumors. The potential advantage of proton beam therapy for lung cancer is the ability to safely deliver radiation to large tumors while minimizing cardiopulmonary toxicity, which could be contributing to mortality in patients with lung cancer. Findings from prospective studies, such as the Mass General–MD Anderson trial and RTOG 1308, will help to resolve open questions regarding these potential advantages.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement: The authors declare no conflicts of interest.
REFERENCES
- 1.Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12 year experience at Loma Linda University Medical Center. Int J Radiat Biol Phys. 2013;86(5):964–968. doi: 10.1016/j.ijrobp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Kanemoto A, Okumura T, Ishikawa H, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non-small cell lung cancer. Clin Lung Cancer. 2014;15(2):7–12. doi: 10.1016/j.cllc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama H, Satoh H, Sugahara S, et al. Proton beam therapy of stage II and III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):979–984. doi: 10.1016/j.ijrobp.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Hoppe BS, Flampouri S, Henderson RH, et al. Proton therapy with concurrent chemotherapy for non-small cell lung cancer: technique and early results. Clin Lung Cancer. 2012;13(5):352–358. doi: 10.1016/j.cllc.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117(20):4707–4713. doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Zhu R, Park PC, et al. Comprehensive analysis of proton range uncertainties related to patient stopping-power-ratio estimation using the stoichiometric calibration. Phys Med Biol. 2012;57(13):4095–4115. doi: 10.1088/0031-9155/57/13/4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3(2):177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 8.Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2012;82(1):425–434. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26(15):2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 10.Bradley JD, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I-III non-small cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77(2):367–372. doi: 10.1016/j.ijrobp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable non small cell lung carcinoma. Cancer. 2005;103(10):2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B non-small cell lung carcinoma: a modified phase I/II trial. Cancer. 2001;92:1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Bradley JD, Paulus P, Komaki R, et al. for the Radiation Therapy Oncology Group Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82(3):1042–1044. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 16.PORT Meta-analysis Trialists Group Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352(9124):257–263. [PubMed] [Google Scholar]
- 17.PORT Meta-analysis Trialists Group Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;(2):CD002142. doi: 10.1002/14651858.CD002142.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Billiet C, Decaluwé H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol. 2014;110(1):3–8. doi: 10.1016/j.radonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, West BT, Hayman JA, Lyons S, Cease K, Kong FM. High radiation dose may reduce the negative effect of large gross tumor volume in patients with medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:103–110. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Baumann M, Herrmann T, Koch R, et al. CHARTWEL-Bronchus study group Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol. 2011 Jul;100(1):76–85. doi: 10.1016/j.radonc.2011.06.031. doi: 10.1016/j.radonc.2011.06.031. Epub 2011 Jul 13. PMID: 21757247. [DOI] [PubMed] [Google Scholar]
- 21.Soliman M, Yaromina A, Appold S, et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: subgroup analysis of the prospective randomized CHARTWEL trial. Radiother Oncol. 2013 Mar;106(3):299–304. doi: 10.1016/j.radonc.2012.12.008. doi: 10.1016/j.radonc.2012.12.008. Epub 2013 Jan 17. PMID: 23333018. [DOI] [PubMed] [Google Scholar]