Abstract
Background
The purpose of this study was to collect data regarding breast cancer profiles and factors that affect local recurrence and distant metastasis after breast-conserving surgery (BCS) in Chiang Mai University Hospital.
Materials and methods
This study was a retrospective review in a single institution of newly diagnosed invasive breast cancer patients who were treated with BCS between April 9, 2001 and December 25, 2011.
Results
A total of 185 patients treated with BCS were included in this study, with an average age of 46.83 years. The average recurrence age was 41.1 years and the average nonrecurrence age was 47.48 years, with a recurrence rate of 10.27%. Premenopause was significant in recurrence (P=0.047), as well as non-estrogen-expression patients (P=0.001) and patients who did not receive antihormonal treatment (P=0.011).
Conclusion
The recurrence rate in our institute was 10.27%. Factors affecting recurrence after BCS included young age, premenopausal status, nonexpression of the estrogen receptor, and patients who had not received antihormonal treatment. The recurrence rate was higher in the first 90 postoperative months.
Keywords: breast-conserving surgery, breast cancer surgery, invasive breast cancer, factor, recurrence
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of death from cancer in women. The mainstay treatment of early breast cancer is surgery. Previously, mastectomy was the standard operation, but recently breast-conserving surgery (BCS; wide local excision or lumpectomy) has been becoming one of the primary surgical treatments. Several studies that compared the outcomes of BCS followed by whole-breast irradiation and mastectomy showed that the two operation techniques were equivalent with regard to long-term survival1–4 if there were no contraindications, and thus should be the treatment of choice for women with relatively small breast cancers.1 Local recurrence after treatment with BCS occurs in about 10%–20% of patients for early invasive breast cancer.5 Studies reporting recurrence factors after BCS5–7 have come from the US and Europe, but seldom from Asia. Chiang Mai University Hospital had not collected data regarding BCS, so the purpose of this study was to collect data of breast cancer profiles and factors affecting local recurrence and distant metastasis after BCS.
Materials and methods
This study was a retrospective review from digital medical records of Chiang Mai University Hospital. The study protocols were approved by the Chiang Mai University Institutional Ethical Committee, Thailand. Patient consent was not required in this retrospective study according to the ethics committee of the Chiang Mai University hospital. The data collected included the following: patients diagnosed with invasive breast cancer without documentation for distant metastasis at the first diagnosis; and with regard to treatment characteristics and follow-up, patients who had undergone treatment with BCS plus axillary lymph-node (ALN) evaluation that included sentinel lymph-node (SLN) and axillary lymph-node dissection (ALND) in Chiang Mai University Hospital from April 9, 2001 until December 25, 2011.
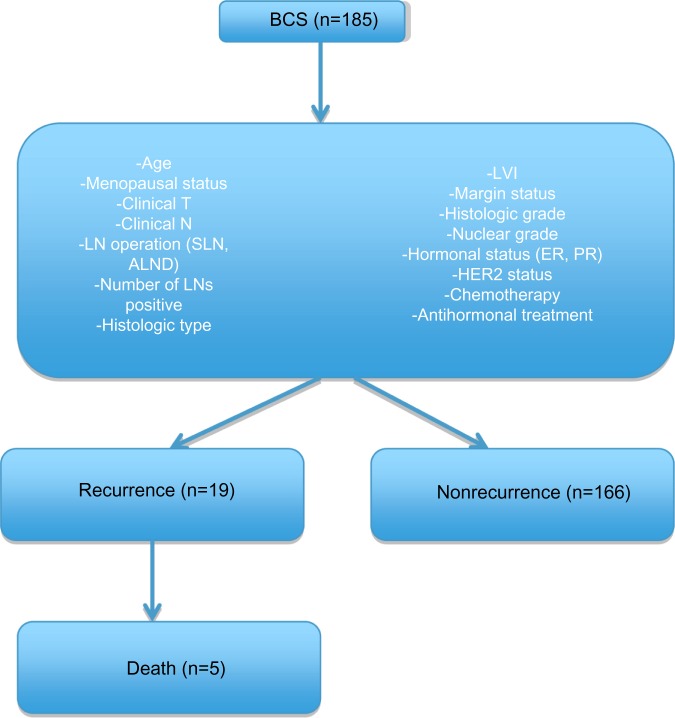
A total of 185 patients treated with BCS were included in this study. Collected data analyzed included age, menopausal status, clinical T (tumor), clinical N (node involvement), LN (lymph-node) operation that included SLN and ALND, number of positive LNs, histologic type, lymphovascular invasion, margin status, histologic grade, nuclear grade, hormonal status, HER2 status, chemotherapy, and antihormonal treatment. Patients were followed up every 3 months at the Head, Neck, and Breast Unit, Outpatient Department by physical examination. Chest imaging and mammography were performed annually. The patients were divided into two groups: recurrence including locoregional and distant metastases, and the nonrecurrence group (Figure 1).
Figure 1.
Flowchart shows data collection of breast-conserving surgery patients and divided subgroups.
Abbreviations: BCS, breast-conserving surgery; LVI, lymphovascular invasion; LN, lymph-node; SLN, sentinel LN; ALND, axillary LN dissection; T, tumor; N, node involvement; ER, estrogen receptor; PR, progesterone receptor; HER2: human epidermal growth factor receptor 2.
Statistical analysis
Statistical analysis was done with commercial statistical software (Stata 11.0; StataCorp LP, College Station, TX, USA). We report descriptive categorical data with percentage and analysis with Fisher’s exact test, descriptive numerical data with mean and standard deviation, and analysis with Student’s t-test. P-values less than 0.05 were considered statistically significant. Survival was calculated by the Kaplan–Meier method.
Results
General patient characteristics
A total of 185 female patients were treated with BCS. The median age at diagnosis of invasive breast cancer was 46.83 years (range 45.37–48.29 years). The nonrecurrence group comprised 166 patients, and the recurrence group consisted of 19 patients (10.27%). The median age in the nonrecurrence group was 47.48 years and in the recurrence group 41.11 years (P<0.01). In premenopausal status patients, we found that the recurrence rate was 10.27% (19 patients), but there was no recurrence in the menopausal status patients (P=0.047) (Table 1).
Table 1.
Patient characteristics
| Recurrence (n) | Nonrecurrence (n) | P-value | |
|---|---|---|---|
| Patients (n=185) | 19 (10.27%) | 166 (89.73%) | |
| Average age, years (range) | 41.11 (36.98–45.23) | 47.48 (45.94–49.02) | 0.0085 |
| Menopausal status | 0.047 | ||
| Premenopausal | 19 (10.27%) | 137 (74.1%) | |
| Menopausal | 0 | 29 (15.68%) |
Abbreviation: SD, standard deviation.
Clinical T, N, and tumor histologic types
At the time of diagnosis the majority of clinical T patients in the nonrecurrence group were clinical T1, as were the patients in recurrence group. Clinical N patients were N0 in both nonrecurrence and recurrence groups (75.14% and 7.57%, respectively). Clinical N was N0 in both groups (75.14% and 7.57% in nonrecurrence and recurrence, respectively). The histologic type was invasive ductal carcinoma in both groups (167 patients [90.27%]) (Table 2). There was no statistical difference in clinical T and clinical N between the recurrence and nonrecurrence groups (P=0.219 and 0.227, respectively) (Table 3).
Table 2.
Histologic type of invasive breast cancer
| Recurrence (n) |
Nonrecurrence (n) |
P-value | |
|---|---|---|---|
| Histologic type | 0.323 | ||
| Ductal carcinoma | 17 (9.19%) | 149 (80.54%) | |
| Lobular carcinoma | 0 | 4 (2.16%) | |
| Papillary carcinoma | 1 (0.54%) | 5 (2.70%) | |
| Medullary carcinoma | 0 | 3 (1.62%) | |
| Mucinous carcinoma | 0 | 4 (2.16%) | |
| Tubular carcinoma | 0 | 1 (0.54%) | |
| Neuroendocrine | 1 (0.54%) | 0 |
Table 3.
Clinicopathologic characteristics
| Recurrence (n) | Nonrecurrence (n) | P-value | |
|---|---|---|---|
| Clinical T | 0.219 | ||
| T1 | 18 (9.73%) | 162 (87.57%) | |
| T2 | 0 | 3 (1.62%) | |
| T3 | 1 (0.54%) | 1 (0.54%) | |
| Clinical N | 0.227 | ||
| N0 | 14 (7.57%) | 139 (73.13%) | |
| N1 | 4 (2.16%) | 23 (12.43%) | |
| N2 | 0 | 3 (1.62%) | |
| N3 | 1 (0.54) | 1 (0.54%) | |
| Histologic grade | 0.611 | ||
| 1 | 0 | 8 (4.32%) | |
| 2 | 8 (4.32%) | 80 (43.24%) | |
| 3 | 9 (4.86%) | 63 (34.05%) | |
| Nuclear grade | 0.458 | ||
| 1 | 0 | 12 (6.49%) | |
| 2 | 8 (4.32%) | 74 (40%) | |
| 3 | 10 (5.41%) | 66 (35.68%) | |
| ER status | 0.001 | ||
| 0 | 10 (5.62%) | 53 (29.78%) | |
| 1+ | 3 (1.69%) | 11 (6.18%) | |
| 2+ | 4 (2.25%) | 11 (6.18%) | |
| 3+ | 2 (1.12%) | 84 (47.19%) | |
| PR status | 0.003 | ||
| 0 | 11 (6.18%) | 68 (38.20%) | |
| 1+ | 2 (1.12%) | 18 (10.11%) | |
| 2+ | 5 (2.81%) | 12 (6.74%) | |
| 3+ | 1 (0.56%) | 61 (34.27%) | |
| HER2 status | 0.224 | ||
| Negative | 10 (5.41%) | 89 (48.11%) | |
| Equivocal (2+) | 3 (1.62%) | 35 (18.92%) | |
| 3+ | 6 (3.24%) | 32 (17.3%) | |
| Triple-negative | 0.337 | ||
| No | 126 (73.68%) | 13 (7.60%) | |
| Yes | 27 (15.79%) | 5 (2.92%) | |
| LVI | 0.583 | ||
| Negative | 93 (54.07%) | 11 (6.39%) | |
| Positive | 61 (35.47%) | 7 (4.07%) |
Abbreviations: LVI, lymphovascular invasion; T, tumor; N, node involvement; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Nuclear and histologic grade
The majority of nuclear grade 2 occurred in the nonrecurrence group (40%), and nuclear grade 3 occurred more frequently in the recurrence group (5.41%). No statistical difference was determined for nuclear grade between the recurrence and nonrecurrence groups (P=0.458).
Histologic grade 2 was primarily in the nonrecurrence group (43.24%), and histologic grade 3 occurred more frequently in the recurrence group 4.86%). There was no statistical difference in nuclear grade between the recurrence and nonrecurrence groups (P=0.611) (Table 3).
Hormonal and HER2 status
Hormonal expression was divided into estrogen receptor (ER) and progesterone receptor (PR). In ER, most patients in the nonrecurrence group were ER 3+, while ER was not expressed in the recurrence group. There was a significant statistical difference in ER expression between the two groups. While consideration of PR in the nonrecurrence group revealed that patients who had PR 3+ and negative PR were similar in number (61 and 68 patients), in the recurrence group we found that patients with negative PR had a higher recurrence rate, with statistical significance. There was HER2 overexpression in 76 patients (41.08%) and nonexpression in 99 patients (58.92%). No statistical difference in HER2 status was shown between the recurrence and nonrecurrence groups (P=0.224). Triple-negative patients trended toward more recurrence without a statistical difference (P=0.337) (Table 3).
Lymphovascular invasion
There was no statistical difference between the groups (P=0.583) (Table 3).
Lymph-node operation
We have performed SLN biopsy since 1999. A total of 96 of the BCS patients had undergone SLN. Seven SLN-positive patients were divided into six patients in the nonrecurrence group and one patient in the recurrence group and further ALND. ALND was performed in 80 patients. No statistical difference was found in LN operations between the two groups (Table 4). In the subgroup analysis of positive LNs, we found that having more than two positive LNs trended toward recurrence (P=0.066) (Table 4).
Table 4.
Lymph-node operation and margin status
| Recurrence (n) |
Nonrecurrence (n) |
P-value | |
|---|---|---|---|
| Lymph-node operation | 0.107 | ||
| SLN | 13 (7.10%) | 83 (45.36%) | |
| ALND | 4 (2.19%) | 76 (41.53%) | |
| SLN-positive, then ALND | 1 (0.55%) | 6 (3.28%) | |
| Number of positive LNs | 0.066 | ||
| LN-positive 0–2 | 13 (7.10%) | 146 (79.78%) | |
| LN-positive ≥3 | 5 (2.73%) | 19 (10.38%) | |
| Margin status | 0.63 | ||
| Negative | 17 (9.34%) | 152 (83.52%) | |
| Positive | 2 (1.1%) | 11 (6.04%) |
Abbreviations: LN, lymph-node; SLN, sentinel LN; ALND, axillary LN dissection.
Margin status
A positive margin was found in 7.14% of overall patients who refused reexcision, and a negative margin was obtained in 92.86%. No statistical difference was determined between the recurrence and nonrecurrence groups (P=0.63) (Table 4).
Chemotherapy treatment
We analyzed groups divided into neoadjuvant chemotherapy and adjuvant chemotherapy. Neoadjuvant chemotherapy was administered 11.11% of the time in the recurrence group and 7.88% of the time in the nonrecurrence group. In the recurrence group, adjuvant chemotherapy was received 88.89% of the time, and in the non-recurrence group 92.12% of the time. There were no statistical differences regarding chemotherapy treatments (Table 5).
Table 5.
Type of chemotherapy and antihormonal treatment
| Recurrence (n) |
Nonrecurrence (n) |
P-value | |
|---|---|---|---|
| Type of chemotherapy | 0.646 | ||
| Neoadjuvant | 2 (1.09%) | 13 (7.10%) | |
| Adjuvant | 16 (8.74%) | 152 (83.06%) | |
| Trastuzumab | 0.252 | ||
| Yes | 2 (1.10%) | 9 (4.97%) | |
| No | 14 (7.73%) | 156 (86.19%) | |
| Hormonal treatment | 0.011 | ||
| None | 12 (6.59%) | 56 (30.77%) | |
| Tamoxifen | 5 (2.75%) | 84 (46.15%) | |
| Aromatase inhibitor | 0 | 22 (12.09%) | |
| Switching | 1 (0.55%) | 2 (1.10%) |
Antihormonal treatment
In the nonrecurrence group, 84 of the patients received tamoxifen. Most of the recurrence group did not receive antihormonal treatment, due to their non-hormone-expression status, with a statistically significant difference in both groups (P=0.011) (Table 5).
Length of follow-up
From Kaplan–Meier analysis, we found that the recurrence rate after BCS decreased after 90 months (7.5 years) from the date of operation (Figure 2). Premenopausal women were the only group with recurrence.
Figure 2.
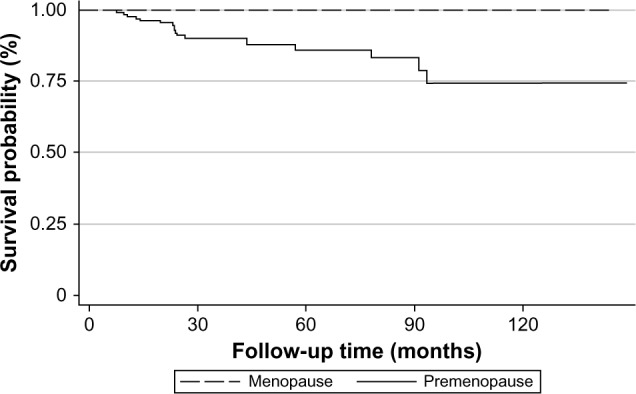
Kaplan–Meier survival estimates.
Discussion
In this study, our purpose was to find factors that affected recurrence (locoregional and distant metastasis) after BCS in patients who were diagnosed with invasive breast cancer in Chiang Mai University Hospital. The local recurrence rate after BCS ranges from 10% to 20%,5 and from our study 10.27% included local and distant metastasis. Univariate analyses indicated that younger age and premenopausal status were risk factors for recurrence. This result was similar to previous studies.6–9 With regard to the tumor size, our study was in contrast to the results of Martínez-Ramos et al, who found that patients who received BCS with a tumor size more than 2 cm had a higher local recurrence rate.10 None of the menopausal women had a recurrence in this study. Estrogen-overexpression patients demonstrated less recurrence than patients who had negative or minimal ER expression, with significant statistical difference, which was the same as other studies.5,11 The luminal A subtype locoregional recurrence occurred less than other subtypes. Consequently, patients who received antihormonal treatment had less recurrence than the nontreatment group. One reason that patients who had positive ER had less recurrence could be the benefit of endocrine therapy, which has been shown in many studies to reduce the risk of recurrence significantly.12–14 Several studies have reported that the locoregional recurrence rate is higher if there is LN metastasis, positive margin status and lymphovascular invasion,5,15–18 but this is opposite to our results, because there was no effect on recurrence. Conversely, HER2 overexpression is one of the risk factors for breast cancer recurrence,19–21 but in this study HER2 did not affect recurrence. This result may have been due to fewer patients developing recurrence and needing longer follow-up, which may in itself have been a result of too few patients being enrolled for there to be a statistically significant difference.
Nineteen patients had a recurrence after BCS, with nine patients having locoregional recurrence and ten patients with distant metastasis. Five patients died within 2 years after diagnosis of distant metastasis. Pulmonary metastasis was found in three of five patients who died from breast cancer. Four in ten patients had more than one site of metastasis (one patient developed lung, liver, and bone metastasis, and three patients developed lung and bone metastasis). The most common site of distant metastasis was bone (seven patients). Finally, the recurrence rate decreased after 90 months (7.5 years) of follow-up from the date of BCS (Figure 2).
Several potential limitations to this study included the small number of patients in both groups, and some data were missed due to the retrospective review. Previously, our hospital did not report about Ki-67 in pathological reports so in this study Ki-67 was not included in the statistical analysis.
Conclusion
The recurrence rate at our institute was 10.27%. Factors that affected recurrence after BCS included young age, premenopausal status, nonexpression of ER and PR, and patients who did not receive antihormonal treatment. The recurrence rate was also higher in the first 90 months after the operation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 4.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. 2003;98(4):697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 5.Neri A, Marrelli D, Rossi S, et al. Breast cancer local recurrence: risk factors and prognostic relevance of early time to recurrence. World J Surg. 2007;31(1):36–45. doi: 10.1007/s00268-006-0097-2. [DOI] [PubMed] [Google Scholar]
- 6.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29(29):3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollet MA, Sigal-Zafrani B, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol. 2007;82(3):272–280. doi: 10.1016/j.radonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Sonke JJ, Stroom J, Bartelink H, Verheij M, Gilhuijs K. The effect of age in breast conserving therapy: a retrospective analysis on pathology and clinical outcome data. Radiother Oncol. 2015;114(3):314–321. doi: 10.1016/j.radonc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z, Wang X, Lin H, et al. Breast-conserving therapy: a viable option for young women with early breast cancer – evidence from a prospective study. Ann Surg Oncol. 2014;21(7):2188–2196. doi: 10.1245/s10434-014-3620-y. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Ramos D, Fortea-Sanchis C, Escrig-Sos J, Prats-de Puig M, Queralt-Martín R, Salvador-Sanchis JL. Local recurrence based on size after conservative surgery in breast cancer stage T1-T2: a population-based study. Cir Cir. 2014;82(3):252–261. Spanish. [PubMed] [Google Scholar]
- 11.Dominici LS, Mittendorf EA, Yu TK, Bedrosian I. Impact of breast cancer subtypes on local-regional outcomes. Curr Breast Cancer Rep. 2010;2(2):107–113. [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 14.Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103(11):2241–2251. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- 15.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18(8):1668–1675. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 16.Jobsen JJ, Van Der Palen J, Ong F, Meerwaldt JH. Differences in outcome for positive margins in a large cohort of breast cancer patients treated with breast-conserving therapy. Acta Oncol. 2007;46(2):172–180. doi: 10.1080/02841860600891325. [DOI] [PubMed] [Google Scholar]
- 17.Houssami N, MacAskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46(18):3219–3232. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Freedman GM, Li T, Polli LV, et al. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J. 2012;18(5):415–419. doi: 10.1111/j.1524-4741.2012.01271.x. [DOI] [PubMed] [Google Scholar]
- 19.Carr JA, Havstad S, Zarbo RJ, Divine G, Mackowiak P, Velanovich V. The association of HER-2/neu amplification with breast cancer recurrence. Arch Surg. 2000;135(12):1469–1474. doi: 10.1001/archsurg.135.12.1469. [DOI] [PubMed] [Google Scholar]
- 20.Andrulis IL, Bull SB, Blackstein ME, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16(4):1340–1349. doi: 10.1200/JCO.1998.16.4.1340. [DOI] [PubMed] [Google Scholar]
- 21.Rouanet P, Roger P, Rousseau E, et al. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: results from a French regional cohort. Cancer Med. 2014;3(1):134–142. doi: 10.1002/cam4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]