Abstract
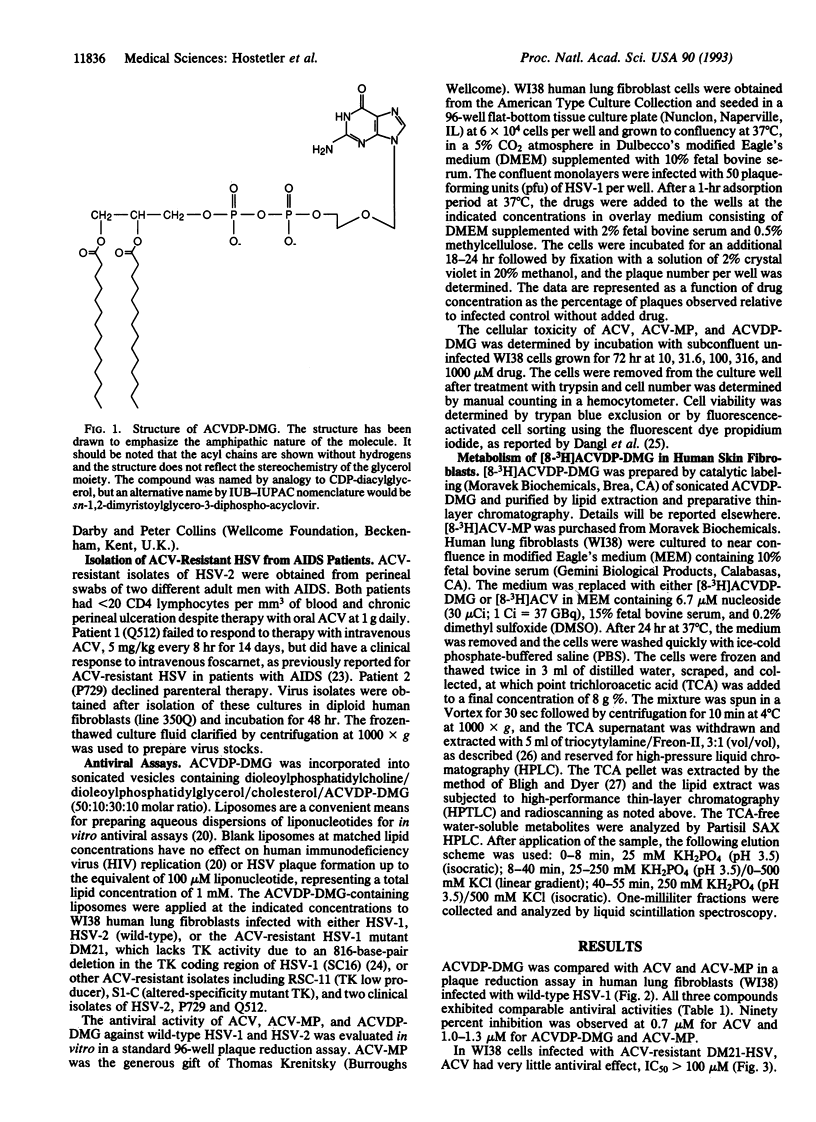
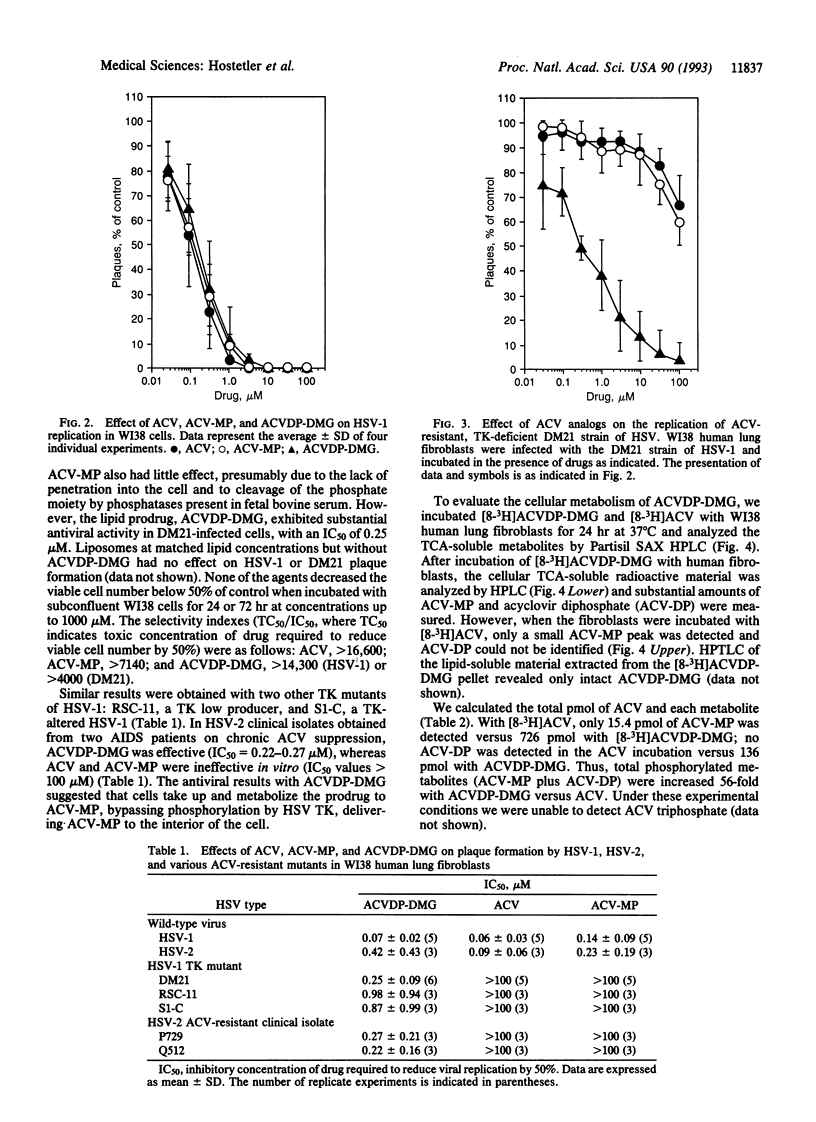
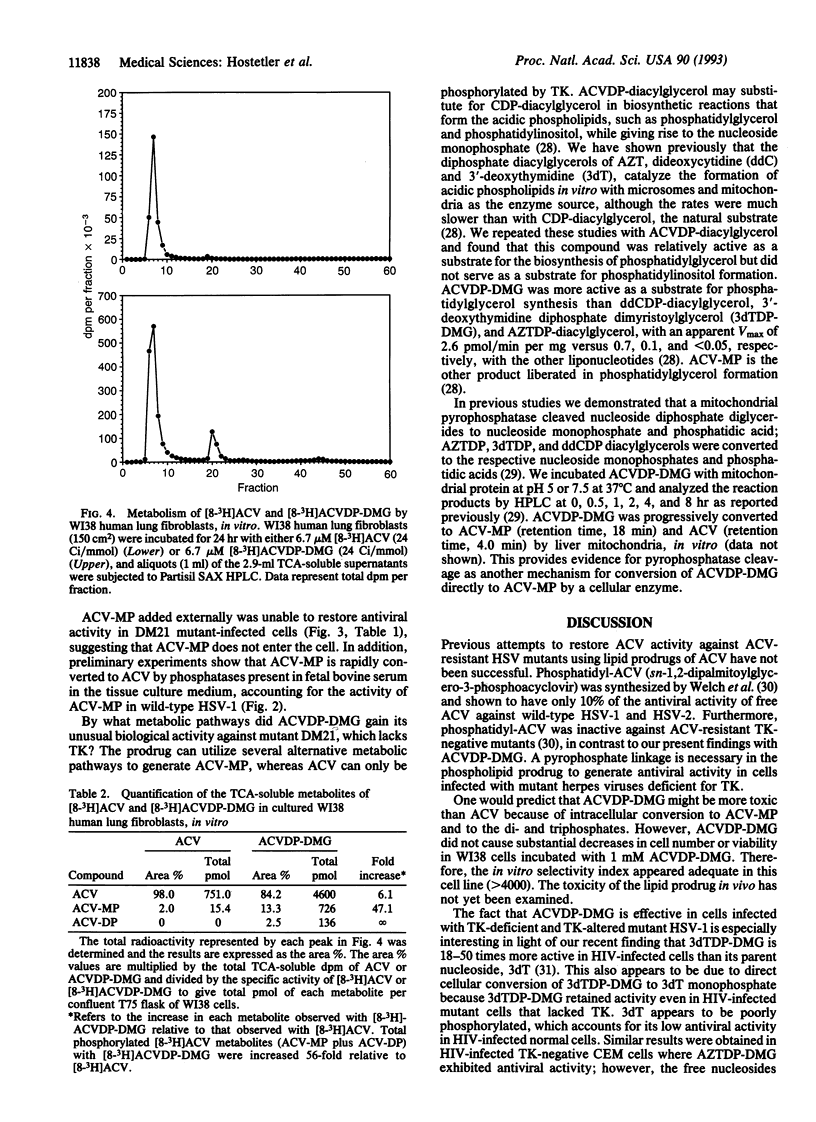
Infection with herpes simplex viruses (HSVs) resistant to treatment with acyclovir (9-[(2-hydroxyethoxy)-methyl]guanine, Zovirax) is a growing clinical problem in patients with AIDS and other immunosuppressed states. Most virus isolates resistant to acyclovir are deficient or defective in virally coded thymidine kinase (TK), which converts acyclovir to acyclovir monophosphate in virus-infected cells. To restore acyclovir efficacy, we synthesized acyclovir diphosphate dimyristoylglycerol, an analog of a naturally occurring phospholipid, CDP-diacylglycerol. Its biological activity was tested in WI38 human lung fibroblasts infected with the acyclovir-resistant DM21 strain of HSV, which is TK negative due to an 816-base-pair deletion in the TK coding region. Acyclovir diphosphate dimyristoylglycerol has substantial activity in DM21-infected cells (IC50 = 0.25 microM), whereas acyclovir and acyclovir monophosphate were ineffective (IC50 > 100 microM). Similar results were obtained in TK-altered and TK-deficient strains of HSV-1 and in acyclovir-resistant isolates of HSV-2 obtained from two AIDS patients. The phospholipid prodrug is active by means of TK-independent metabolic pathways that liberate acyclovir monophosphate inside the host cell. Acyclovir phosphates were 56 times greater in WI38 human lung fibroblasts incubated for 24 hr with [8-3H]acyclovir diphosphate dimyristoylglycerol relative to acyclovir. Acyclovir monophosphate added to the culture medium (outside the cell) did not circumvent the acyclovir resistance of the TK-negative DM21 mutant, presumably due to its conversion to acyclovir by phosphatases. Acyclovir diphosphate diacylglycerol prodrugs may be useful in treating TK-deficient mutant and wild-type strains of HSV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barry D. W., Nusinoff-Lehrman S., Ellis M. N., Biron K. K., Furman P. A. Viral resistance, clinical experience. Scand J Infect Dis Suppl. 1985;47:155–164. [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Collins P., Larder B. A., Oliver N. M., Kemp S., Smith I. W., Darby G. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989 Feb;70(Pt 2):375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Parks D. R., Oi V. T., Herzenberg L. A. Rapid isolation of cloned isotype switch variants using fluorescence activated cell sorting. Cytometry. 1982 May;2(6):395–401. doi: 10.1002/cyto.990020607. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Kemp S., Darby G., Minson A. C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989 Apr;70(Pt 4):869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Gateley A., Gander R. M., Johnson P. C., Kit S., Otsuka H., Kohl S. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J Infect Dis. 1990 Apr;161(4):711–715. doi: 10.1093/infdis/161.4.711. [DOI] [PubMed] [Google Scholar]
- Hill E. L., Hunter G. A., Ellis M. N. In vitro and in vivo characterization of herpes simplex virus clinical isolates recovered from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1991 Nov;35(11):2322–2328. doi: 10.1128/aac.35.11.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Carson D. A., Richman D. D. Phosphatidylazidothymidine. Mechanism of antiretroviral action in CEM cells. J Biol Chem. 1991 Jun 25;266(18):11714–11717. [PubMed] [Google Scholar]
- Hostetler K. Y., Richman D. D., Carson D. A., Stuhmiller L. M., van Wijk G. M., van den Bosch H. Greatly enhanced inhibition of human immunodeficiency virus type 1 replication in CEM and HT4-6C cells by 3'-deoxythymidine diphosphate dimyristoylglycerol, a lipid prodrug of 3'-deoxythymidine. Antimicrob Agents Chemother. 1992 Sep;36(9):2025–2029. doi: 10.1128/aac.36.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Stuhmiller L. M., Lenting H. B., van den Bosch H., Richman D. D. Synthesis and antiretroviral activity of phospholipid analogs of azidothymidine and other antiviral nucleosides. J Biol Chem. 1990 Apr 15;265(11):6112–6117. [PubMed] [Google Scholar]
- Huff J. C., Bean B., Balfour H. H., Jr, Laskin O. L., Connor J. D., Corey L., Bryson Y. J., McGuirt P. Therapy of herpes zoster with oral acyclovir. Am J Med. 1988 Aug 29;85(2A):84–89. [PubMed] [Google Scholar]
- Ljungman P., Ellis M. N., Hackman R. C., Shepp D. H., Meyers J. D. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis. 1990 Jul;162(1):244–248. doi: 10.1093/infdis/162.1.244. [DOI] [PubMed] [Google Scholar]
- Marks G. L., Nolan P. E., Erlich K. S., Ellis M. N. Mucocutaneous dissemination of acyclovir-resistant herpes simplex virus in a patient with AIDS. Rev Infect Dis. 1989 May-Jun;11(3):474–476. doi: 10.1093/clinids/11.3.474. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Reed E. C., Shepp D. H., Thornquist M., Dandliker P. S., Vicary C. A., Flournoy N., Kirk L. E., Kersey J. H., Thomas E. D. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med. 1988 Jan 14;318(2):70–75. doi: 10.1056/NEJM198801143180202. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Norris S. A., Kessler H. A., Fife K. H. Severe, progressive herpetic whitlow caused by an acyclovir-resistant virus in a patient with AIDS. J Infect Dis. 1988 Jan;157(1):209–210. doi: 10.1093/infdis/157.1.209. [DOI] [PubMed] [Google Scholar]
- Redfield D. C., Richman D. D., Albanil S., Oxman M. N., Wahl G. M. Detection of herpes simplex virus in clinical specimens by DNA hybridization. Diagn Microbiol Infect Dis. 1983 Jun;1(2):117–128. doi: 10.1016/0732-8893(83)90041-x. [DOI] [PubMed] [Google Scholar]
- Reichman R. C., Badger G. J., Mertz G. J., Corey L., Richman D. D., Connor J. D., Redfield D., Savoia M. C., Oxman M. N., Bryson Y. Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA. 1984 Apr 27;251(16):2103–2107. [PubMed] [Google Scholar]
- Safrin S., Crumpacker C., Chatis P., Davis R., Hafner R., Rush J., Kessler H. A., Landry B., Mills J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1991 Aug 22;325(8):551–555. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Furman P. A., Lubbers C. M., Elion G. B. Inhibition of cellular alpha and virally induced deoxyribonucleic acid polymerases by the triphosphate of acyclovir. Antimicrob Agents Chemother. 1980 Nov;18(5):741–745. doi: 10.1128/aac.18.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Seidlin M., Takiff H., Jacobs D., Bowen D., Smith H. A. Oral acyclovir to suppress recurring herpes simplex virus infections in immunodeficient patients. Ann Intern Med. 1984 Apr;100(4):522–524. doi: 10.7326/0003-4819-100-4-522. [DOI] [PubMed] [Google Scholar]
- Welch C. J., Larsson A., Ericson A. C., Oberg B., Datema R., Chattopadhyaya J. The chemical synthesis and antiviral properties of an acyclovir-phospholipid conjugate. Acta Chem Scand B. 1985;39(1):47–54. doi: 10.3891/acta.chem.scand.39b-0047. [DOI] [PubMed] [Google Scholar]
- van Wijk G. M., Hostetler K. Y., Schlame M., van den Bosch H. Cytidine diphosphate diglyceride analogs of antiretroviral dideoxynucleosides: evidence for release of dideoxynucleoside-monophosphates by phospholipid biosynthetic enzymes in rat liver subcellular fractions. Biochim Biophys Acta. 1991 Oct 15;1086(1):99–105. doi: 10.1016/0005-2760(91)90160-j. [DOI] [PubMed] [Google Scholar]
- van Wijk G. M., Hostetler K. Y., van den Bosch H. Antiviral nucleoside diphosphate diglycerides: improved synthesis and facilitated purification. J Lipid Res. 1992 Aug;33(8):1211–1219. [PubMed] [Google Scholar]
- van Wijk G. M., Hostetler K. Y., van den Bosch H. Lipid conjugates of antiretroviral agents: release of antiretroviral nucleoside monophosphates by a nucleoside diphosphate diglyceride hydrolase activity from rat liver mitochondria. Biochim Biophys Acta. 1991 Jul 30;1084(3):307–310. doi: 10.1016/0005-2760(91)90074-r. [DOI] [PubMed] [Google Scholar]



