Abstract
Modulation of phosphorylation states of ion channels is a critical step in the development of hyperalgesia during inflammation. Modulatory enhancement of channel activity may increase neuronal excitability and affect downstream targets such as gene transcription. The specificity required for such regulation of ion channels quickly occurs via targeting of protein kinases and phosphatases by the scaffolding A-kinase anchoring protein 79/150 (AKAP79/150). AKAP79/150 has been implicated in inflammatory pain by targeting PKA and PKC to the TRPV1 channel in peripheral sensory neurons, thus lowering threshold for activation by multiple inflammatory reagents. However, the expression pattern of AKAP79/150 in peripheral sensory neurons is unknown. In this study we use immunofluorescence microscopy to identify in DRG sections the peripheral neuron subtypes that express the rodent isoform AKAP150, as well as the subcellular distribution of AKAP150 and its potential target ion channels. We found that AKAP150 is predominantly expressed in a subset of small DRG sensory neurons where it is localized at the plasma membrane of the soma, axon initial segment and small fibers. The majority of these neurons is peripherin positive and produces c-fibers, though a small portion produces Aδ-fibers. Furthermore, we demonstrate that AKAP79/150 colocalizes with TRPV1 and CaV1.2 in the soma and axon initial segment. Thus AKAP150 is expressed in small, nociceptive DRG neurons where it is targeted to membrane regions and where it may play a role in the modulation of ion channel phosphorylation states required for hyperalgesia.
Keywords: Pain, inflammation, ion channel, PKA, calcineurin, calcium signaling
Introduction
The transition from acute pain to longer lasting inflammatory pain requires specific protein modulation in peripheral nociceptive neurons. Prolonged exposure to noxious stimuli or tissue injury results in this switch by release of inflammatory agents that cause sensitization and increased excitation of the nociceptors. Sensitization occurs by multiple pathways, i.e. direct modulation of protein receptors (TRP family of channels) and ion channels (calcium, sodium and potassium channels), upregulation of protein expression and changes in gene transcription (Caterina et al., 1997; Cardenas et al., 1997; Gould et al., 1998; Novakovic et al., 1998; Gould et al., 1999; Gould et al., 2000; Rasband et al., 2001; Jung and Miller, 2008; Staaf et al., 2009; Hagenacker et al., 2010).
The mechanisms by which protein kinases specifically target a protein receptor or ion channel were uncertain until the discovery of A-kinase anchoring proteins (AKAPs). AKAPs are a group of functionally related proteins that are defined by their ability to bind PKA and to anchor both the kinase and target proteins into multicomponent scaffolds (Colledge and Scott, 1999). Through formation of these complexes, AKAPs provide means by which neurons can rapidly and specifically modulate target proteins.
AKAP79 (human ortholog) and AKAP150 (rat ortholog) differ by a large amino acid repeat of unknown function in the rat ortholog and will be referred to as AKAP79/150 unless specifically referring to our AKAP150 antibody results. The AKAP79/150 isoform targets both kinases and phosphatases to ion channels by co-assembling them as a multicomponent complex at the membrane (Carr et al., 1992; Hirsch et al., 1992; Colledge and Scott, 1999; Dell’Acqua et al., 2002; Rathee et al., 2002; Oliveria et al., 2003; Dell’Acqua et al., 2006; Gardner et al., 2006; Zhang et al., 2008; Sanderson JL and Dell’Acqua, 2010), where they can respond to activation signals such as the influx of calcium or cAMP (Oliveria and others, 2003).
Recent studies also show that AKAP79/150 plays an important role in the encoding and integration of pain. In this role AKAP79/150 is required for the proper phosphorylation of the ion channel TRPV1 (transient receptor potential vanilloid1) in sensory neurons in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) (Rathee and others, 2002; Schnizler et al., 2008; Zhang and others, 2008; Jeske et al., 2009). TRPV1 is a member of the large TRP channel family of ion channels that are non-selective cation channels permeable to both Ca++ and Na+, and which play a role in detecting a wide range of sensory stimuli (Vriens et al., 2009). TRPV1 itself is a polymodal receptor activated by noxious heat (>43C), protons, and vanilloids such as capsaicin, the irritant in “hot” peppers (Caterina and others, 1997; Tominaga et al., 1998; Szallasi and Blumberg, 1999; Caterina et al., 2000; Vriens and others, 2009). Overall, activation of inflammatory pathways can lead to the phosphorylation of TRPV1 by PKA and PKC and reduction in the thermal threshold of the channel, leading to increased excitation of sensory neurons (Sugiura et al., 2002; Bhave et al., 2003; Mohapatra and Nau, 2003; Luo et al., 2004; Dai et al., 2004).
Multiple studies have demonstrated that direct AKAP79/150 mediated anchoring of PKA and PKC to TRPV1 is required for phosphorylation and dephosphorylation of the channel. Thus Rathee et.al. (2002) initially showed that forskolin-potentiated TRPV1 currents in mouse DRG were not only inhibited by PKA inhibition, but currents were inhibited by the presence of an AKAP inhibitor. Furthermore, prostaglandin E2 (PGE2) and PKA dependent modulation of TRPV1 was inhibited in d36 mice, which have a 36-amino acid c-terminal truncation in AKAP150 that deletes the PKA binding site (Schnizler and others, 2008). Significantly, this truncation also resulted in a decrease in thermal hyperalgesia. Additional studies in the trigeminal ganglion confirmed that AKAP150 is required for PKA, as well as PKC phosphorylation of TRPV1 (Jeske et al., 2008; Jeske and others, 2009), while desensitization of the TRPV1 ion channel via the phosphatase calcineurin is dependent upon interactions with AKAP79/150 (Zhang and others, 2008). Lastly, capsaicin-induced desensitization of the channel was decreased when AKAP79/150 was knocked down or the CaN binding site on AKAP79/150 was deleted (Zhang and others, 2008). Together, these data demonstrate that regulation of TRPV1 activity is highly dependent upon forming a macromolecular complex with AKAP79/150.
However, in contrast to the molecular interactions between AKAP79/150 and TRPV1 in cultured DRG neurons, little is known about the distribution of AKAP79/150 in peripheral neurons and other proteins that may interact with this scaffolding macromolecule. In the central nervous system, AKAP79/150 has been shown to interact with the L-type calcium channel, CaV1.2, voltage gated potassium channels, and NMDARs and AMPARs in the brain (Colledge and Scott, 1999; Oliveria and others, 2003; Dell’Acqua and others, 2006; Oliveria et al., 2007; Lu et al., 2008). Thus there is potential for AKAP79/150 to be playing a larger role in peripheral sensory neurons beyond modulation of TRPV1. In the present set of studies, we performed an in depth analysis of AKAP150 expression in vivo and show that AKAP150 exhibits a unique expression pattern in small, primary sensory neurons where it potentially plays a role in nociceptive signaling.
Materials and Methods
Animals and tissue processing
This study was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Eight to twelve week old mice and rats were used according to institutionally approved animal care and use protocols. Sprague Dawley rats (n-5) and C57/Bl6 (n=4) and AKAP150 null mice (n=4) (Tunquist et al., 2008) were bred onsite.
Mice and rats were anesthetized with chloral hydrate and perfused with 0.1 phosphate buffered saline (PBS) and then 2% paraformaldehyde in PBS. DRG were dissected and rinsed in PBS followed by a 30 minute post-fixation incubation in 2% paraformaldehyde. DRG were rinsed in 3–10 minute washes to remove excess paraformaldehyde. The DRG were cryoprotected for 12 hours in 30% Sucrose in PBS and 40% sucrose for four hours at 4°C. 30μm cryosections were collected using a Microm HM-550 cryostat (Microm international GmbH, Walldorf, Germany) and mounted on Fisherbrand Colorfrost/Plus slides (Fisher Scientific, Waltham, MA).
AKAP150 antibody generation
Site-directed polyclonal antibodies for AKAP150 were raised is a similar manner as described by (Dugandzija-Novakovic et al., 1995). The peptide synthesis and antisera were produced by Sigma-Aldrich Laboratories. The target 18 amino acid peptide (TTVGQAEEATVGQAEEA) was found in a large repeat segment of the rat AKAP150 scaffolding protein and conserved in the mouse AKAP150 sequence (Colledge et al., 2000). The peptide was synthesized with the addition of an N-terminal cysteine required for keyhole limpet hemocyanin (KLH) conjugation and for affinity purification of antisera. Antisera to KLH-AKAP150 conjugates were raised by immunizing rabbits at 4 week intervals and collected by Sigma Labs.
AKAP150 antibodies were purified from Sigma generated antisera in lab using affinity chromatography on a peptide-coupled column (ImmunoPure Ag/Ab Immobilization Kit #2, Pierce). Antibody was eluted and collected in fractions equal to the column volume. Fraction concentrations were calculated by measuring absorbance levels at 280 nm. Peak fractions were combined and concentrated to 1–3 μg/μl. The 15 amino acid peptide was diluted to 0.3 μg/μl and used as a peptide-block control for all immunological studies using the AKAP150 antibody.
Immunocytochemistry
Immunostaining was performed as described previously (Dugandzija-Novakovic and others, 1995). Briefly, tissue was permeabilized and non-specific protein binding sites were blocked by a two hour preincubation in a blocking solution (2% bovine γ-globulin (Sigma, St. Louis, MO), 4% normal goat serum (Sigma) and 0.3% Triton X-100 (Fisher Scientific) in 0.1 M PBS). All antibodies were diluted in the blocking solution. Primary and secondary antibodies used and dilutions of antibodies are described in Table 1. Tissues were incubated overnight in the primary antibody in a humidifier at room temperature. Slides were rinsed in PBS for 3–10 minute washes, followed by a 90 minute incubation with secondary, fluorescent antibodies (Table 1) and another 3–10 minute PBS wash. After a brief rinse in high purity, deionized water, slides were dried and mounted with Vectashield antifade mounting medium (Vector Labs, Burlingame, CA).
Table 1.
ANTIBODIES USED IN IMMUNOCYTOCHEMISTRY EXPERIMENTS
| Antibody | Immunogen | Source, Cat #, host, type, dilution |
|---|---|---|
| AKAP150 | TTVGQAEEATVGQAEEA Corresponding to a large repeat in the rat AKAP150 sequence |
Produced by authors, rabbit, polyclonal |
| AKAP150 | TTVGQAEEATVGQAEEA Corresponding to a large repeat in the rat AKAP150 sequence |
Produced by authors, rabbit, polyclonal |
| Substance P | Synthetic peptide NH2-CRPKPQQFFGLM-CONH2-Corresponding to residues 1-11 |
Neuromics, GP14110, guinea pig, polyclonal, 1:200 |
| Kv1.2 | Fusion protein Amino acids 428–499 for the c-terminus of rat Kv1.2 |
NeuroMab, 443-1KS-37, mouse, monoclonal, 1:200 |
| VR-1 C-terminus | Synthetic peptide YTGSLKPEDAEVFKDSMVPGEK |
Neuromics, GP14100/400765, guinea Pig, polyclonal, 1:200 |
| CGRP | Synthetic peptide: LQAVPLRSTLESSPG, corresponding to C terminal amino acids 23–37 of Rat conjugated to BSA by a Glutaraldehyde linker | AbCam, Ab22560, sheep, polyclonal, 1:100 |
| Isolectin GS-IB4 | N/A | Invitrogen, L21411/488745, Griffonia simplicifolia, 1:400 |
| Ankyrin G | Fusion protein Amino acids 990-2622 |
NeuroMab, 75-146/441-4BK-91B, mouse, monoclonal, 1:100 |
| Pan Specific Sodium Channel | Synthetic peptide CTEEQKKYYNAMKKLGSKK from the intracellular III–IV loop of Na+ channels | Invitrogen, S8809/045K6083, mouse, polyclonal, 1:100 |
| Peripherin | Recombinant Rat Peripherin purified from E. coli | AbCam, ab4653/595343, mouse, polyclonal, 1:100 |
| NF-200 | Purified mid-size rat neurofilament (NF-M) subuint | Sigma, N2912, mouse, polyclonal, 1:200 |
| Caspr | Fusion protein of extracellular domain and human IgG Fc | Elior Peles, mouse monoclonal, 1:200 |
| TRPc7 | Synthetic peptide FGKNLNKDHLRVNKGKDI | NeuroMab, 75–123/443.1Ks.43, mouse, polyclonal, 1:100 |
| CaV1.2 | Fusion Protein Amino acids 1507–1773, intracellular carboxyl terminus |
NeuroMab, 440-7hk-01, mouse, monoclonal, 1:100 |
| Alexa Fluor 568 | Invitrogen, A11036/531813, Goat anti-Rabbit, 1:100 | |
| Alexa FLuor 488 | Invitrogen, A11015/423310, Donkey anti-Sheep, 1:100 | |
| Alexa FLuor 488 | Invitrogen, A11073/400881, Goat anti-Guinea Pig, 1:100 | |
| Alexa FLuor 488 | Invitrogen, A11029/727756, Goat anti-Mouse, 1:100 | |
| Alexa FLuor 488 | Invitrogen, A11039/399709, Goat anti-Chicken, 1:100 |
Antibody use
Three different controls were used to test antibody specificity for immunocytochemistry. First, when available, specificity of antibody binding was controlled by staining parallel slides with antibodies that had been preincubated with a 100-fold molar excess of corresponding peptide antigens. Second, controls for the AKAP150 antibody also included the use of AKAP150-null mouse tissue. The third control for antibody specificity was the use of the fluorescently-tagged secondary antibody only. This control was only used in cases where neither the corresponding peptide nor a null transgenic animal was available.
We used a number of highly characterized antibodies. The antibodies for Peripherin, CGRP and substance P stained small DRG neurons in the cytoplasm and axons. IB4 stained the membrane of small neurons and regions within the cytoplasm. NF-200 and Kv1.2 were expressed in small to large diameter neurons, and could be detected only in myelinated fibers and Kv1.2 was detected in juxtaparanodal regions. Cspr was detected only in paranodal regions. Cav1.2 was found in the cytoplams, membrane and axon initial segment. The monoclonal pan specific sodium channel was compared to a highly characterized rabbit polyclonal antibody (Gould and others, 1998) and expressed in the soma and nodes of Ranvier of DRG neurons but not in the axon initial segment.
AKAP150 expression in DRG was compared to a variety of neuronal markers to identify which DRG neurons contain AKAP150. DRG that expressed AKAP150 as well as a marker were said to coexpress together in the neuron. AKAP150 and a marker found in the same subcellular region of a DRG neuron were said to colocalize to that region.
Image analysis and quantification
16-bit, 0.5-μm stacks of images were collected along the “z” axis of DRG cryosections using using a 40X/N.A.1.30 oil immersion objective and a Nikon PCM-2000 laser scanning confocal microscope. Image acquisition was performed using SimplePCI software, v4.6 (Compix Inc., Cranberry Township, PA). Individual images were saved as a 16 bit files with 1024 pixels2 resolution, in which each square pixel was 0.3 μm in dimension and represented an area of 0.09 μm2. Image quantification and processing was performed using NIH Image J software and Corel PhotoPaint X3 (Corel Corporation, Ottawa, Canada). Background fluorescence for individual antibodies was determined from tissue stained with peptide-blocked antibodies or with secondary antibody only when no peptide antigens were available or by using AKAP knockout tissue.
The “region of interest” or “ROI” feature of Image J was used measure cell area and protein marker intensity. Area measurements were collected by drawing outlines of DRG cells containing visible nuclei within image stacks for AKAP150 alone and proteins marker alone. Non-nucleated DRG were excluded from the cell profiles to prevent inaccurate measurements of elliptical shaped DRG soma measured in cross section. Cells were classified as protein marker positive if staining intensities were three standard deviations above background (excluding the nucleus).
Membrane intensity levels for AKAP150 were collected by outlining regions of the membrane for each neuron. DRG neurons were classified at AKAP150 positive if the membrane levels of AKAP150 were three standard deviations above background as measured by using DRG probed with preabsorbed antibody. Neurons were classified as “AKAP150 high” expressing if membrane intensity levels were 15 standard deviations above background.
Statistical analysis
A student T-test was used to determine significance between the mean areas of DRG populations. Analysis was performed using SigmaPlot (Systat Software, Inc.).
Western blot
Brain and DRG were dissected from rat, wild-type (WT) mice and AKAP150-null mice and flash frozen on dry ice. Tissue was thawed in homogenization buffer (20mM tetrasodium pyrophosphate, 20mM sodium phosphate, 1 mM MgCl2, 0.5 mM EDTA 300 mM sucrose, 0.8 mM benzamidine, 1.0 nM iodoacetamide, 1.1 μM leupeptin, 0.7 μM pepstatin A, 76.8 nM aprotinin, 0.23 mM PMSF) and homogenized on ice for thirty seconds. Tissue homogenates were centrifuged at 20,000 RPM (50k × g) using a J2-21 centrifuge and JA-20 rotor (Beckman-Coulter, Inc., Brea, CA). Protein concentrations were determined using Pierce BCA protein assay kits according to manufacturer’s directions (BCA Protein Assay Kit, #23225, Pierce, Rockford, IL).
10 μg total protein extract was loaded per lane on precast 4–15% gradient gels (BioRad, Hercules, CA). Gels were run at −80V. Protein bands were transferred to nitrocellulose membranes using semidry transfer, which was then blocked in a solution of 5% nonfat milk and 0.1% Tween in PBS. The membranes were incubated overnight in a 1:1000 dilution of primary antibody in the blocking solution. A secondary antibody conjugated to HRP (Jackson ImmunoResearch, West Grove, PA) was applied for two hours. The membrane was washed with PBS for thirty minutes between each antibody application. SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, #34096) was applied to the blot for 5-minutes; excess solution was removed before imaging. Protein bands were visualized using a Kodak ImageStation 440cf.
Results
AKAP150 immunoreactivity in rat DRG
To examine the expression patterns of AKAP150 in DRG, we probed rat DRG sections with a well-characterized rabbit anti-AKAP150 antibody. Specificity of the antibody was tested by probing tissue with antibody preincubated with peptide as well as DRG tissue from AKAP150 null mice. AKAP150 staining was abolished in slices probed with preincubated antibody (Fig 1D) and slices generated from the AKAP150 null mice (Fig 1C). Non-specific binding of the antibody was occasionally found in satellite cells surrounding large DRG soma (Fig 1A, asterisk). Specificity of the AKAP150 antibody was further confirmed by immunoblots using brain and DRG homogenates from rat, WT mouse and AKAP150 null mouse (Fig. 1 E). Protein bands at 150kD for AKAP150 were apparent in lanes containing the rat and WT mouse brain and DRG but were absent in the lane containing AKAP150 null DRG and the lane containing rat DRG probed with peptide-blocked antibody.
Figure 1. AKAP150 expression in adult rat DRG neurons is confirmed by immuonhistochemistry and western blot analysis.
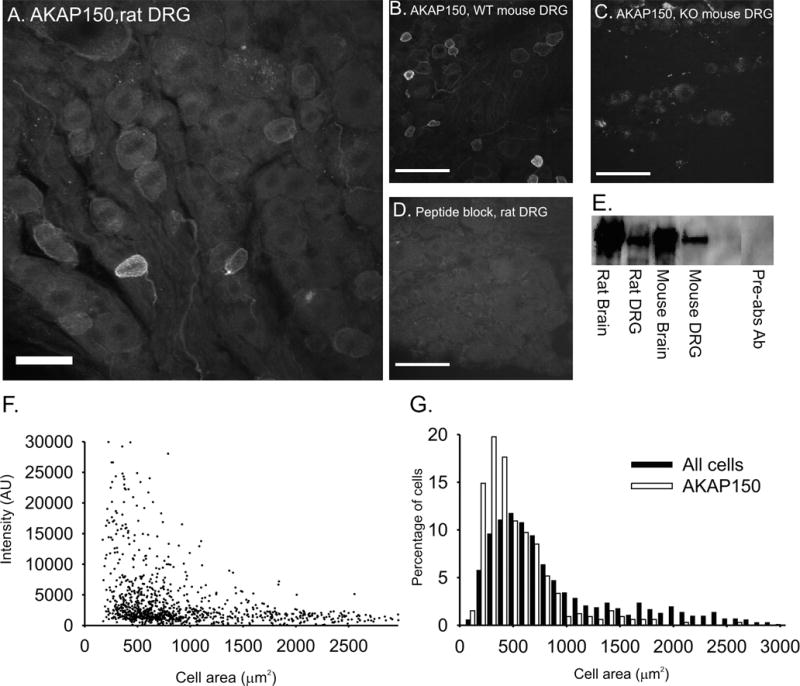
Confocal images of AKAP150 expression in (A.) adult rat DRG, scale = 100 μM, (B.) WT adult mouse DRG, (C.) AKAP150 null mouse DRG and (D.) preabsorbed antibody probe of rat DRG. Scale bars = 50 μM (B–D). E. Bands at 150 kD in a western blot of tissue probed with AKAP150 antibody and preabsorbed antibody, 10μg tissue/lane. F. Distribution of AKAP150 membrane intensity based on DRG soma area in all DRG neurons. G. Size distribution of AKAP150 positive DRG neurons and all DRG neurons.
In Figure 1A, a rat DRG slice probed with AKAP150 antibody demonstrates that AKAP150 is expressed in DRG neurons of a variety of sizes and at varying intensity levels. While DRG could contain AKAP150 at levels too low to detect immunocytochemically, cells were considered AKAP150 positive if they contained AKAP150 levels in the membrane three standard deviations above the mean background calculated from slides probed with the pre-incubated antibody. On this basis, 36% (381/1055) of the DRG neurons measured were classified as AKAP positive.
Based upon the cell area histogram of all DRG neurons (Figure 1G), cells with cross-sectional area less than 1200 μm2 were considered small, cells with areas between 1200 and 1900 μm2 were considered medium and cell with areas above 1900 μm2 were considered large (Swett et al., 1991). AKAP150 was primarily expressed in small DRG neurons (93.6% of positive neurons) while only 5.4% of AKAP150 positive cells were found to be medium neurons and less than 1% to be in large neurons. The average size of AKAP150 positive DRG neurons was 574.67 +/− 18.45 μm2. Examples of DRG of different areas expressing AKAP150 are shown in figure 2.
Figure 2. Characterization of levels of AKAP150 expression in rat DRG neurons using confocal microscipic analysis.
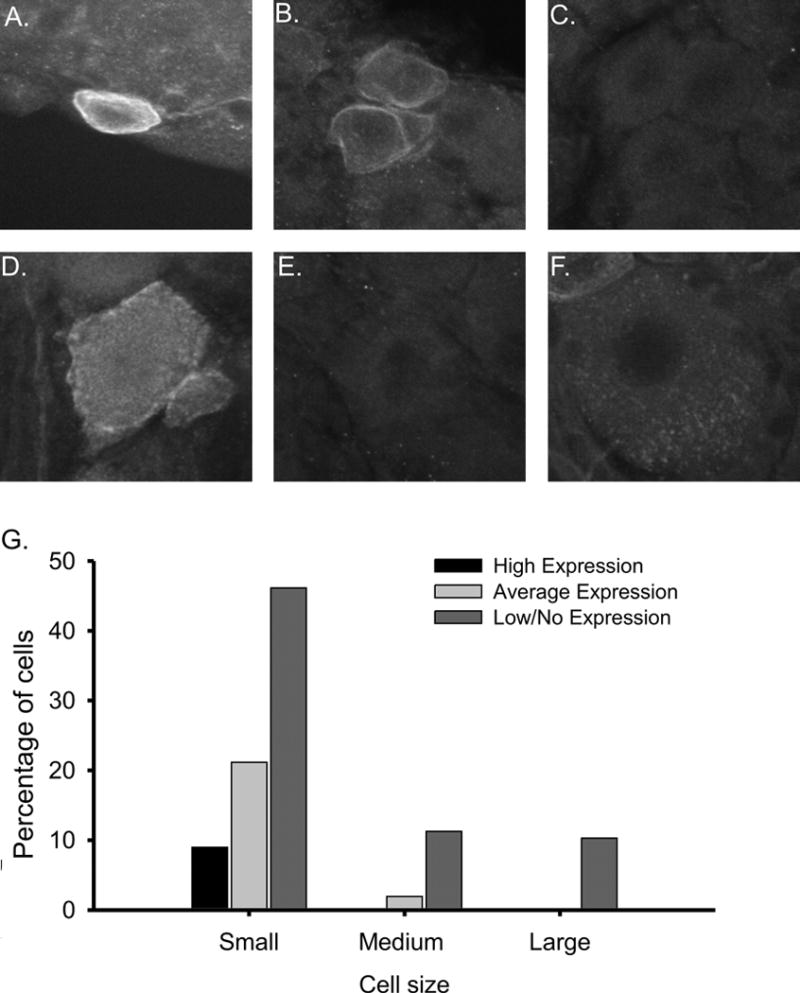
Scale bars = 50 μM. A. AKAP150 is highly expressed at the membrane of the soma and axon initial segment (AIS) in a subpopulation of small diameter DRG neurons morphologically resembling small-sized nociceptors. AKAP150 is expressed at the membrane of the soma and AIS of these neurons. B. Small diameter DRG neuron expressing moderate levels of AKAP150. C. A small DRG neuron displaying little to no AKAP150 expression. D. A small subset of medium diameter neurons expressed AKAP150, but the majority of medium and large diameter DRG neurons lacked any AKAP150 immunoreactivity (E, F). G. Histogram of cell area and AKAP150 intensity in small, medium and large DRG neurons. Cell size and AKAP150 intentiAKAP150 expression levels where highest in small diameter DRG neurons. Within the small diameter neurons, a subset displayed significantly higher levels of AKAP150.
AKAP150 immunoreactivity was observed to be on or near the cell membrane of the DRG soma, axon initial segment (AIS), stem axon and in thin processes (figure 2A,B,D). The membrane patches of AKAP150 are similar in appearance to those found in cultured hippocampal neurons (Dell’Acqua and others, 2006). While high levels of AKAP150 were detected at the membrane, little AKAP150 was found within the cytoplasm domain of the cell body or axon.
A subset of the AKAP150 positive neurons expressed significantly higher (>15 STDs above background) levels of AKAP150. In these neurons, AKAP150 formed distinct patches near the cell membrane of the soma, AIS and stem axon (figure 2A). This subset of exclusively small, high intensity AKAP150 positive DRG comprised 28% of AKAP positive DRG neurons and 9% of all neurons as shown in figure 2G. Together, these data demonstrate that AKAP150 has variable expression in DRG neurons, favoring a subset of the small, presumptively nociceptive neurons that expresses a significantly higher level of AKAP150 with an unusual membrane associated heterogeneous distribution.
AKAP150 expression in rat DRG subpopulations
In the peripheral nervous system, the primary sensory neuron cell bodies residing in the DRG can be divided into subtypes that produce either unmyelinated C-fibers or myelinated A-fibers. DRG were probed with peripherin, a marker for small DRG neurons that produce unmyelinated C-fibers, and neurofilament-200 (NF-200), a marker for DRG neurons that produce the myelinated larger, A-beta and smaller A-delta fibers (Starkey et al., 2010). Levels of AKAP150 co-expression with cell type markers is shown in Table 2. Cell size histograms correspond to the area of individual DRG neurons that express a given protein, but do not correspond to colocalization of proteins.
Table 2.
EXPRESSION OF PROTEIN MARKERS WITH AKAP150 IN DRG CELL BODIES
| Protein Marker | %AKAP pos. neurons pos for marker | %high intensity AKAP neurons pos. for marker | Ave. size marker positive DRG | Average size marker and AKAP150 pos. DRG |
|---|---|---|---|---|
| Peripherin | 62.5 | 60.0 | 544.58 +/− 24.82 | 485.27 +/− 29.44 |
| NF-200 | 31.6 | 10.5 | 1579.74+/− 125.91 | 570.88+/− 49.52 |
| IB4 | 55.6 | 52.4 | 571.86 +/− 18.25 | 489.27 +/− 26.01** |
| CGRP | 35.5 | 35.3 | 571.38 +/− 41.18 | 544.28 +/− 65.60 |
| SubP | 20.3 | 0.0 | 681.85 +/−41.18 | 695.13 +/− 124.02 |
| VGSC | 57.6 | 52.9 | 602.22 +/− 39.76 | 503.08 +/− 71.97 |
| CaV1.2 | 63.9 | 62.5 | 802.56 +/− 44.56 | 646.18 +/− 84.86 |
| Kv1.2 | 11.8 | 0.0 | 1827.53 +/− 123.12 | 933.52 +/− 45.76** |
| TRPV1 | 67.3 | 66.7 | 506.72 +/−37.24 | 400.75 +/− 35.27** |
| TRPc4 | 11.1 | 0.0 | 1091.61 +/− 95.23 | 858.48 +/− 63.82** |
The distribution of peripherin positive and AKAP150 positive DRG neurons was confined to the range of small cell bodies and fibers. In contrast, the NF-200 positive DRG neurons displayed a broad range of cell areas, ranging from small to large cell bodies and axons. Peripherin co-expressed with AKAP150 in 62.5% of AKAP150-positive DRG neurons (Fig.3, A–D). While 31.6% of AKAP150 positive DRG neurons also co-expressed NF-200, these neurons were significantly smaller than the average NF-200 containing neurons (570.88 +/− 18.45 vs. 1579.74 +/− 125.91 μm2, p<0.01). As the DRG neurons that expressed both AKAP150 and NF-200 were classified as small neurons, we surmise that these neurons produced the small, myelinated A-delta fibers. Within the subset of high intensity AKAP150 DRG neurons, 60.0% co-expressed peripherin while only 10.5% co-expressed NF-200. Thus, the majority of AKAP150 expressing DRG neurons were small neurons that produce thin C-fibers with a lesser population that produces the small, myelinated a-delta fibers.
Figure 3. Characterization of AKAP150 in myelinated and unmyelinated DRG neurons using confocal microscopy.
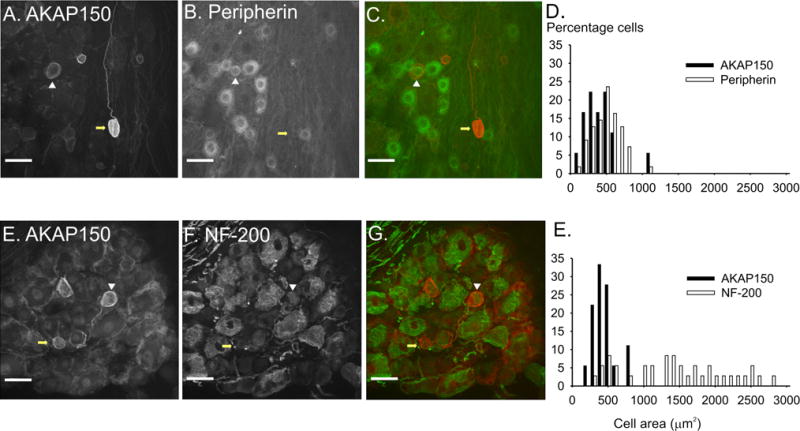
Scale bars = 50 μM. AKAP150 (A.) and Peripherin immunoreactivity (B.) in DRG neurons. C. Overlay of AKAP150 (red) and peripherin (green) demonstrates that a subpopulation of AKAP150 expressing DRG neurons coexpress with peripherin, suggesting they are small, unmyelinated c-fiber producing neurons (white arrowhead), while another population of AKAP150 expressing neurons do not coexpress peripherin (yellow arrow). AKAP150 (E.) and NF-200 (F.) immunoreactivity in DRG neurons. G. Overlay of AKAP150 (red) and NF-200 (green) shows that a subset of AKP150 expressing neurons produce myelinated fibers. D,H. Size histograms demonstrate that AKAP150 and peripherin are expressed in small diameter DRG neurons while NF-200 is expressed in a broader range of neurons.
The C-fiber producing DRG neurons can be further subdivided in populations of peptidergic and non-peptidergic neurons. The peptidergic class of DRG that responds to NGF was identified by probing for the neuropeptides CGRP and substance P (Averill S et al., 1995). The non-peptidergic class of small DRG neurons that responds to GDNF were identified by the binding of Griffonia simplicifolia isolectin B4 (IB4) to surface glycoconjugates (Kashiba et al., 2001). The coexpression of IB4 (55.6%, Fig 4 A–D) was higher than that of the peptides in the small, AKAP150 positive neurons (CGRP: 35.5% coexpression, Fig 4 E–G; substance P: 20.3% coexpression, Fig 4H–I). However, the subset of AKAP150/SubP positive neurons had a broader range of soma areas, including the rare AKAP150 positive, medium sized DRG neuron (Fig 4 I–K, white arrowhead). Additionally, a subset of neurons expressing IB4 or CGRP, but not substance P, displayed high levels of AKAP150. In any case, we can conclude that while AKAP150 is expressed in multiple types of small diameter DRG neurons, the majority are non-peptidergic and give rise to unmyelinated C-fibers.
Figure 4. AKAP150 expression in subpopulations of small rat DRG neurons.
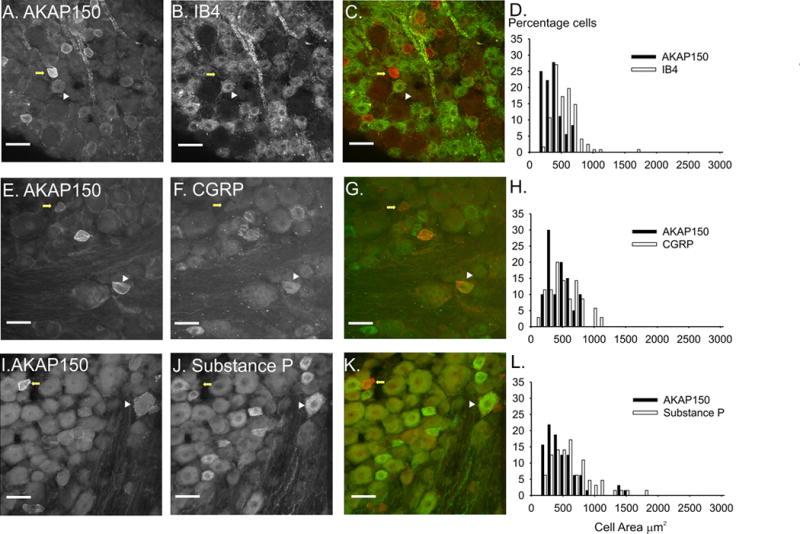
Scale bars = 50 μM. The left panel of images shows AKAP150 expression (A,E,I), the middle panel shows the expression of IB4 (B), CGRP (F) and substance P (J) and the right panel of images (C,G,K) is an overlay of AKAP150 (red) and the protein marker (green). White arrowheads mark DRG in which AKAP150 coexpresses with the protein marker. Yellow arrows mark DRG in which only AKAP150 is expressed. Histograms (D,H,L) demonstrate that the majority of AKAP150 expressing DRG neurons are small neurons with the rare medium sized neurons. IB4 and CGRP (D, H) are expressed only in small DRG neurons and SP is expressed in a small subset of medium DRG neurons (L).
AKAP150 coexpression with voltage gated ion channels
The phosphorylation state of ion channels in neuronal membranes regulates the excitability of the cell. Multiple studies have shown that AKAPs interact with ion channels in the CNS (Tibbs et al., 1998; Colledge and others, 2000; Hulme et al., 2002; Gardner and others, 2006; Oliveria and others, 2007; Lu and others, 2008; Schnizler and others, 2008). Both AKAP79/150 and AKAP15, a small member of the AKAP family, interact with the L-type calcium channel, CaV1.2, through a modified leucine zipper on the channel (Hulme and others, 2002; Oliveria and others, 2007). As L-type calcium channels have also been implicated in inflammatory pain we determined whether AKAP150 could be expressed with CaV1.2 in DRG neurons. Thus CaV1.2 co-expressed with 63.0% of AKAP150 positive neurons and was found both in the cytoplasm domain of the DRG neuron and in the neuron plasma membrane (Fig 5 A–C). Although the majority of the CaV1.2 appeared in the cytoplasmic region, high levels of CaV1.2 and AKAP150 colocalization were also visible the membrane of the soma and AIS. Thus, we conclude that AKAP150 and L-type calcium channels colocalize in multiple membrane domains of small, nociceptive DRG.
Figure 5. Characterization of AKAP150 expression with voltage gated ion channels and TRPV1 in rat DRG neurons.
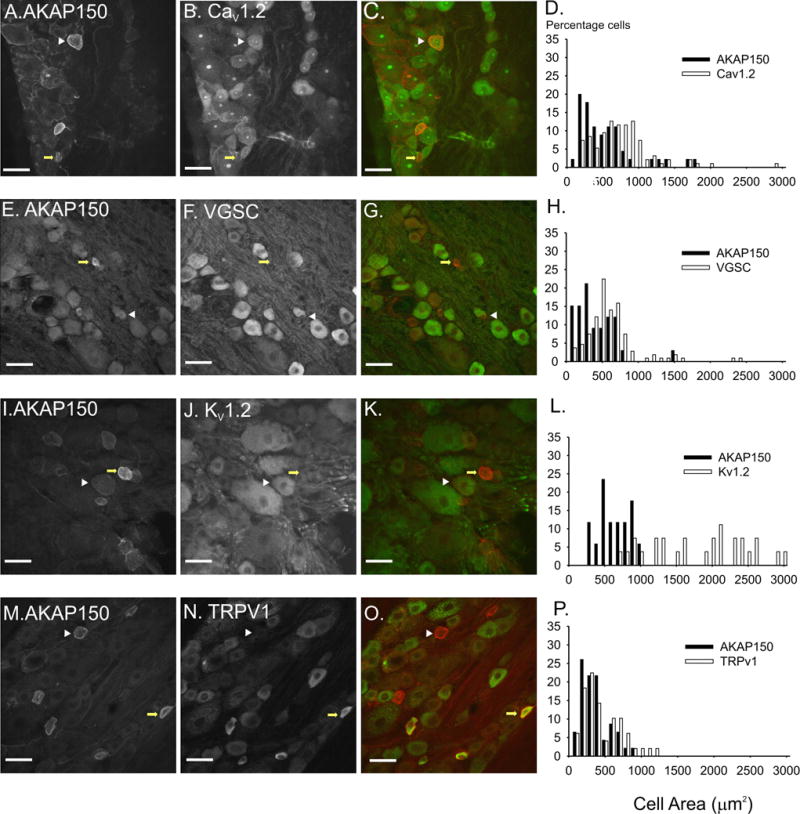
The left panel of images shows AKAP150 expression (A,E,I,M), the middle panel shows the immunolocalization of ion channels CaV1.2 (B), Voltage gated sodium channel (VGSC) (F), Kv1.2 (J) and TRPV1 (N) and the right panel of images (C,G,K,O) is an overlay of AKAP150 (red) and the protein marker (green). White arrowheads mark DRG in which AKAP150 coexpresses with the protein marker. Yellow arrows mark DRG in which on AKAP150 is expressed. Scale bars = 50 μM. The histograms (D,H,L,P) demonstrate the narrow distritbution of AKAP150 positive DRG neuron areas and the braod areas of DRG expressing specific ion channels.
As well as interacting with L-type calcium channels, AKAP15 has been shown to interact with Nav1.2 in brain tissue through a similar leucine zipper, targeting PKA and PKC to the channel and resulting in phosphorylation of the channel and reduction of its peak current (Tibbs and others, 1998; Hulme and others, 2002; Oliveria and others, 2007). As other VGSC isoforms are also phosphorylated by PKA and PKC and contain leucine zippers, it is possible that AKAP150 targets the enzymes to VGSCs in DRG neurons. We probed DRG using both the AKAP150 antibody and a mouse monoclonal antibody directed towards a sequence conserved in all voltage gated sodium channel isoforms. We found that 57.6% of AKAP150 positive neurons co-expressed with voltage gated sodium channels in the soma of DRG neurons (Fig 5 E–G). However, while we demonstrated that there was an overlap in AKAP150 and VGSC expression in DRG neurons, we found that AKAP150 was not co-expressed with VGSCs at the nodes of Ranvier.
As with L-type calcium channels and voltage gated sodium channels, isoforms of the voltage gated (Kv) potassium channel are differentially expressed in the DRG (Ishikawa et al., 1999; Rasband and others, 2001). The voltage gated potassium channel, Kv1.2, was expressed in the soma, AIS and the juxta-paranodal regions of DRG neurons. Similar to the distribution of NF-200, Kv1.2 was expressed on small to large neurons. We found that only 11.8% of AKAP150 expressing neurons also express significant levels of Kv1.2 (Fig 5 I–K). The area of cells expressing both AKAP150 and Kv1.2 fell into the medium range of DRG neurons and thus were significantly larger than the average, small AKAP150 positive DRG neuron (933.52 +/− 45.76 vs 574.67 +/− 18.45 μm2, p<0.01). As with VGSC staining in DRG neurons, we could not determine whether the two proteins colocalize at the membrane due to the high levels of Kv1.2 immunoreactivity in the cytosol.
AKAP150 expression with transient receptor potential ion channels
Multiple studies have demonstrated that AKAP79/150 targets PKA and PKC to TRPV1 channels in DRG neurons after neuronal stimulation by inflammatory factors such as bradykinin (Schnizler and others, 2008; Zhang and others, 2008; Jeske and others, 2008). Furthermore, AKAP79/150 was shown to co-immunopreciptitate with TRPV1 in DRG cultures (Schnizler and others, 2008; Zhang and others, 2008). However, no research had yet determined AKAP150 and TRPV1 coexpression in DRG in vivo or whether these proteins co-localized to specific subcellular regions. In the present study, we found that 67.3% of AKAP150-positive DRG neurons coexpress with TRPV1 and 63% of TRPV1-positive neurons coexpress AKAP150. Furthermore, DRG neurons that produce high levels of AKAP150 also displayed similarly high levels of co-expression with TRPV1 (66.7%). Furthermore, in DRG neurons that co-express AKAP150 and TRPV1, the two proteins colocalized in the soma, AIS, stem axon and in thin fibers (Fig 5 M–O). DRG neurons that co-expressed AKAP150 and TRPV1 were also significantly smaller than the average AKAP150 positive neurons (400.75 +/− 37.24 vs 506.72 +/− 37.24 μm2, P< 0.01).
Neurons were stained with TRPc4 to determine whether AKAP150 co-expressed with other families of TRP channels. Only 11.1% of AKAP positive neurons co-expressed TRPc4 (data not shown). These neurons were also significantly larger than the average AKAP positive neuron. Thus, we can conclude that AKAP150 is coexpressed with the TRPV1 channel isoform in the soma and AIS of a unique subpopulation of small, nociceptive DRG neurons.
AKAP150 membrane expression in the soma and axon initial segment
As described above, we found that AKAP150 was primarily expressed in small diameter DRG neurons producing C-fibers and Aδ-fibers. To further elucidate the subcellular regions in which AKAP150 was expressed, we probed DRG neurons with markers for subcellular regions as well as markers for neuronal subtypes. Ankyrin G (AnkG), a scaffolding molecule important in the development and maintenance of axon initial segments and nodes of Ranvier, is expressed at the cell membrane of these subcellular regions (Lustig et al., 2001; Grubb and Burrone, 2010). AnkG is expressed in the AIS of both myelinated and unmyelinated fibers. We found that AKAP150, while co-expressed with AnkG at the AIS (Fig 6 A–C), is not present at nodes of Ranvier (Fig 6 D–E). To confirm that AKAP150 is not present at the nodes of Ranvier, we probed DRG slices with caspr, a cell adhesion molecule found at the paranodal regions of myelinated fibers (Einheber et al., 1997). As shown in figure 6 G–H (arrows), AKAP150 is not present at the nodes of Ranvier or paranodal regions.
Figure 6. Magnified confocal images of AKAP150 expression in the membrane of AKAP150 soma and axon initial segment and nerve fibers.
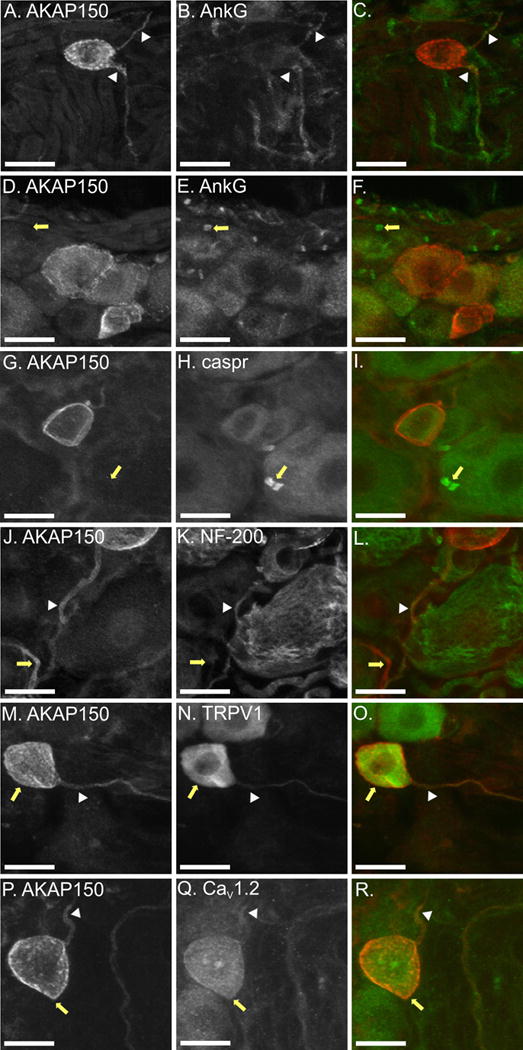
In each image row, AKAP150 is on the left, protein markers in the middle and overlays are on the right with AKAP150 in red and cell marker in green. A–C. AKAP150 (A) and Ankyrin G (AnkG) (B.) are both expressed in the axon initial segment (AIS), with moderate levels of colocalization (white arrowhead, (C.)). D–F. AnkG (E.), but not AKAP150 (D.), is highly expressed at nodes of Ranvier (white arrow, (F.)). G–I. AKAP150 (G.) and CASPR (H.) do not colocalize at nodes of Ranvier in rat DRG neurons (white arrowhead, (I.)). J–L. AKAP150 (J.) is expressed in the AIS of DRG expressing NF-200 (K.). M–N. AKAP150 (M.) coexpresses with TRPV1 (N.) at the membrane of the soma (yellow arrow, (O.)) and axon initial segment (white arrowhead, (O.)) of small DRG neurons. P–R. AKAP150 (P.) colocalizes with CaV1.2 (Q.) in the soma (yellow arrow (R.)) and in the AIS (white arrowhead (R.)) of small DRG neurons.
Although the majority of DRG neurons expressing AKAP150 produce unmyelinated C-fibers, a small subset form aδ fibers identified by colabeling with NF-200. In figure 6 J–L (arrowhead), an example is shown of a DRG neuron expressing both AKAP150 and NF-200 in the AIS. This coexpression does not occur in the larger, Aβ-fiber producing DRG neuron, or in the small, C-fibers (yellow arrow). Thus, AKAP150 can be expressed in the AIS of both myelinated and unmyelinated neurons, but is found distally only in C-fibers.
The high levels of AKAP150 co-expression with TRPV1 and CaV1.2 led us to examine further where colocalization of the proteins occurred in DRG neurons. While the majority of somal TRPV1 is cytosolic and, therefore, did not colocalize with AKAP150 (Fig 6 N), the channel did appear to colocalize with AKAP150 at the cell membrane (Fig 6 M–O, yellow arrow). Colocalization of TRPV1 and AKAP150 was more apparent in the AIS (Fig 6 M–O, arrowhead) of small DRG neurons and in unmyelinated fibers in the DRG.
Regulation of L-type calcium channels has been shown to be required for inflammation-induced gene transcription (Graef et al., 1999; Hogan et al., 2003; Jackson et al., 2007; Jung and Miller, 2008; Kim and Usachev, 2009). Thus, we examined subcellular DRG neuron regions in which AKAP150 could be colocalizing with CaV1.2. AKAP150 was found to be highly colocalized with CaV1.2 at the membrane of the soma (Fig 6P–R, yellow arrow). CaV1.2 was expressed in similar, high-density, patchy regions as was AKAP150. AKAP150 and CaV1.2 also showed high levels of colocalization in the AIS (Fig 6 P–R, arrowhead). We conclude that AKAP150 colocalizes with TRPV1 and CaV1.2 in the soma and AIS of small DRG neurons.
Discussion
This study demonstrates that AKAP150 has a unique expression pattern in a small subset of nociceptive DRG neurons where it is highly expressed at the membrane of the cell soma and in the axon initial segment. Based on co-localization in the membrane, we suggest that AKAP150 interacts with TRPV1 and Ca1.2 channels in these areas.
Ours is the first study examining the expression of AKAP150 in fixed DRG slices. Due to the potential changes in protein expression and variations in cell type survival in cell culture, it is important to analyze the expression pattern of AKAP150 in vivo as well as in culture. Thus, we found that 36% of DRG neurons express AKAP150, a significant reduction in expression compared to the 77% AKAP150 positive neurons found in cultured DRG neurons (Schnizler and others, 2008), where no antibody control was reported. By using peptide blocked AKAP150 antibody and an AKAP150 null mouse line as controls, we were able to exclude neurons non-specifically labeled with the AKAP150 antibody. Lacking proper controls, Schnitzler et.al. (2008) may also have included neurons whose cell bodies exhibited non-specific immunoreactivity.
We have also demonstrated that AKAP150 has a unique expression pattern within a small subset of small, nociceptive DRG neurons that produce either thinly myelinated Aδ-fibers involved in sensation of the quick “first pain” after injury or the small, more numerous, unmyelinated C-fibers that produce long lasting, dull pain. Based on immunoreactivity, AKAP150 was expressed on the membrane of the soma, AIS and stem axon of the C- and Aδ–fiber producing DRG and was largely absent within the cytoplasm. Within the small nociceptive DRG neurons, there is a subset of small neurons that has a significantly higher level of AKAP150 expression, potentially for increased regulation of target proteins and, thus, increased regulation of excitability in the soma and AIS. The expression pattern of AKAP150 in the soma and AIS indicates that AKAP150 is functionally targeted to the cell membrane, in contrast to proteins that are packaged for transport to distal regions of the neuron.
To determine what sensory modality the AKAP150 positive DRG neurons might serve, we examined co-expression of AKAP150 with proteins involved in specific sensory pathways. TRPV1 is activated by noxious heat and is essential for the development of inflammatory hyperalgesia (Caterina and others, 1997; Tominaga and others, 1998; Caterina and others, 2000; Amaya et al., 2003; Zhang and others, 2008). Inflammation induces phosphorylation of TRPV1, thus reducing the thermal activation threshold of TRPV1 and sensitizing the receptor to capsaicin, protons and vanilloids (Rathee and others, 2002; Amaya et al., 2004; Moriyama et al., 2005; Yu et al., 2008; Schnizler and others, 2008; Zhang and others, 2008; Jeske and others, 2008; Jeske and others, 2009). We found that AKAP150 and TRPV1 expression in DRG neurons overlapped in two thirds of the neurons expressing AKAP150. This is a somewhat higher percentage of coexpression compared to the 50% coexpression of AKAP150 and TRPV1 found by to Schnitzler (2008) in rat DRG culture. Though the high level of cytosolic TRPV1 inhibits the ability to see whether AKAP150 and TRPV1 colocalize in the soma, multiple studies have suggested that AKAP150 and TRPV1 do indeed physically associate(Schnizler and others, 2008; Zhang and others, 2008; Jeske and others, 2008; Jeske and others, 2009). Zhang (2008) demonstrated that TRPV1 associates with AKAP79/150 via a 14 amino acid sequence on the C-terminal tail of the ion channel.
Thus, phosphorylation of TRPV1 requires AKAP79/150 anchored PKA and PKC. In cultured DRG neurons, FSK induced phosphorylation of TRPV1 was shown to be inhibited by the application of St-Ht31, a non-specific AKAP inhibitor, demonstrating a requirement for AKAP anchoring of PKA (Schnizler and others, 2008). Schnizler, et al (2008) also demonstrated that the anchoring of PKA by AKAP79/150 specifically is required for the potentiation of heat activated currents in mouse DRG, since in d36 transgenic mice having a truncation in the PKA binding site at the C-terminus of AKAP150, PKA sensitization of TRPV1 is reduced, but PKC-dependent sensitization is unaltered. Additional studies demonstrated that AKAP150 anchoring to TRPV1 was required for not only PKA, PKC and CaN modulation of the channel, but for capsaicin induced sensitivity and trafficking of the channel to the plasma membrane (Zhang and others, 2008).
While AKAP150 has been shown to be required for TRPV1 modulation during thermal hyperalgesia, we found that only 63% of TRPV1 containing DRG also expressed AKAP150 which is lower than the 90% coexpression found by Zhang (2008). Though, again, these data were gathered from cultured rat DRG neurons and contained no controls for AKAP150 expression. Perhaps the small percentage of TRPV1 that does not associate with AKAP150 associates with another AKAP isoform, which could target PKA, PKC and CaN to the channel. Alternatively, in a subset of cells TRPV1 might not be modulated during inflammation. A recent study demonstrated that about 1/3 of TRPV1 channels were inaccessible to PKC, possibly because the channels did not assemble with AKAP79/150 (Studer and McNaughton, 2010).
We have also found that AKAP150 co-expresses with voltage gated ion channels. In particular, small diameter DRG expressed both AKAP150 and CaV1.2. Multiple studies have shown that Ca2+-dependent gene regulation controls many aspects of neuronal plasticity. Studies in hippocampal neurons demonstrated that AKAP79/150 interacts directly with CaV1.2, anchoring PKA and calcineurin to the channel (Oliveria and others, 2003; Oliveria and others, 2007). PKA phosphorylation of L-type channels increases activity of the L-type channel in dendrites and dendritic spines. By targeting both PKA and CaN to the CaV1.2, AKAP79/150 can regulate L-type current amplitude in a bidirectional manner. CaN bound AKAP79/150 dominantly suppresses PKA modulation of the channel.
The AKAP-mediated modulation of CaV1.2 channels could be important in activating downstream signaling pathways essential to the establishment of chronic pain mechanisms in DRG neurons. Thus the influx of Ca2+ through L-type channels activates the transcription factor NFATc4 in a calcineurin-dependent manner (Oliveria and others, 2007). A study in the DRG neuronal cell line F11 showed that NFAT initiates transcription of pronociceptive molecules such as chemokine receptor 2 (CCR2) in a calcium dependent manner (Jung and Miller, 2008). Upregulation of CCR2, which is not expressed in DRG under normal conditions, leads to increased excitability of neurons. Ca2+ influx through both LTCC and TRPV1, inducing mitochondrial calcium release, lead to dephosphorylation of NFATc4 and translocation of the transcription factor from the cytoplasm to the nucleus (Kim and Usachev, 2009). As we show, AKAP150 is expressed in a high proportion of CaV1.2 containing neurons. The interactions between the two proteins could mediate gene transcription pathways in inflammatory pain.
This unique expression pattern of AKAP150 in the AIS and soma raises the question of why AKAP150 and its ion channel targets are transported and colocalized to these regions. Action potentials in DRG neurons are generated at the free nerve endings and then propagate along the peripheral axon, past the T-junction to synapse on second order neurons in the dorsal horn of the spinal cord. Such a straightforward description of the conduction of nociceptive conduction raises several important issues, namely why are there electrically excitable proteins in the membrane of the AIS and soma and why is there a need for the anchoring of protein kinases and phosphatases by AKAP79/150 to ion channels in the membrane?
The majority of DRG soma produce little afferent activity. However, subthreshold oscillations in membrane potential intrinsic to the neuron have been detected in a subpopulation of large, Aβ DRG neurons, but not in the small DRG that give rise to Aδ- and C-fibers (Liu et al., 2000; Liu et al., 2002). These oscillations are thought to arise from interactions between Na++ inward current and K+ outward current (Amir et al., 2002). When Aβ-fibers were damaged in animal models of neuropathic pain, depolarization of the neuron resulted in increased oscillations in the soma and spike initiation in the AIS. Increase excitability in the DRG soma also lead to cross-excitation of small DRG neurons (Amir et al., 1999; Amir and Devor, 1999; Liu and others, 2000; Amir and others, 2002). Thus, somal and AIS excitability in small DRG neurons could arise from cross-excitation from the large DRG neurons (Amir and Devor, 1999).
Could AKAP79/150 targeting of PKA, PKC and CaN to TRPV1 and CaV1.2 in the soma and AIS be important for cross-excitation? Potentially, AKAP150 mediated phosphorylation of TRPV1 by PKA and/or PKC would lower the threshold for activation of TRPV1. Increasing the excitability of the soma and AIS could result in the amplification or distortion of action potentials generated from nociceptors in the free nerve endings. Furthermore, increased influx of Ca2+ through CaV1.2 could lead to changes in gene transcription through NFATc4, resulting in long-term increases in excitability of small, nociceptive DRG neurons. Thus, we suggest that AKAP79/150 is targeted to specialized membrane regions of a subpopulation of small, nociceptive DRG where is has the potential to regulate neuronal excitability and gene expression important to the coordination of nociceptor responses to chronically painful stimuli.
Acknowledgments
We would like to thank Dr. Karen Smith for her help in breeding the AKAP150 knockout mice.
Support: R01 MH080291 (to MLD), R01 DE015576 (subcontract to SRL), gift of Allergan Corporation (SRL).
Footnotes
Associate editors: Oswald Steward, University of California–Irvine: Neuronal Plasticity and Response to Injury, Hippocampal Formation, and Auditory System
Literature Cited
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- Amaya F, Shimosato G, Nagaono M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience. 1999;95:189–195. doi: 10.1016/s0306-4522(99)00388-7. [DOI] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane Potential Oscillations in Dorsal Root Ganglion Neurons: Role in Normal Electrogenesis and Neuropathic Pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Burst Discharge in Primary Sensory Neurons: Triggered by Subthreshold Oscillations, Maintained by Depolarizing Afterpotentials. J Neurosci. 2002;22:1187–1198. doi: 10.1523/JNEUROSCI.22-03-01187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical Localization of trkA Receptors in Chemically Identified Subgroups of Adult Rat Sensory Neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Scroggs RS. Two Parallel Signaling Pathways Couple 5HT1A Receptors to N- and L-Type Calcium Channels in C-Like Rat Dorsal Root Ganglion Cells. J Neurophysiol. 1997;77:3284–3296. doi: 10.1152/jn.1997.77.6.3284. [DOI] [PubMed] [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. Journal of Biological Chemistry. 1992;267:16816–16823. [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to Glutamate Receptors through a MAGUK-AKAP Complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Colledge M, Scott JD. AKAPs: from structure to function. Trends in Cell Biology. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-Activated Receptor 2-Mediated Potentiation of Transient Receptor Potential Vanilloid Subfamily 1 Activity Reveals a Mechanism for Proteinase-Induced Inflammatory Pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the Protein Phosphatase-2B Anchoring Site on AKAP79. Journal of Biological Chemistry. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Regulation of neuronal PKA signaling through AKAP targeting dynamics. European Journal of Cell Biology. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci. 1995;15:492–503. doi: 10.1523/JNEUROSCI.15-01-00492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The Axonal Membrane Protein Caspr, a Homologue of Neurexin IV, Is a Component of the Septate-like Paranodal Junctions That Assemble during Myelination. The Journal of Cell Biology. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated Targeting of the Cyclic AMP-dependent Protein Kinase to the +¦1-Adrenergic Receptor Promotes Recycling and Functional Resensitization of the Receptor. Journal of Biological Chemistry. 2006;281:33537–33553. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Gould TN, Paul D, England JD, Liu ZP, Reeb SC, Levinson SR. Development of inflammatory hypersensitivity and augmentation of sodium channels in rat dorsal root ganglia. Brain Research. 1999;824:296–299. doi: 10.1016/s0006-8993(99)01218-4. [DOI] [PubMed] [Google Scholar]
- Gould HJ, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund’s adjuvant. Brain Research. 1998;802:69–74. doi: 10.1016/s0006-8993(98)00568-x. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Gould TN, England JD, Paul D, Liu ZP, Levinson SR. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Research. 2000;854:19–29. doi: 10.1016/s0006-8993(99)02216-7. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Building and maintaining the axon initial segment. Current Opinion in Neurobiology. 2010;20:481–488. doi: 10.1016/j.conb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenacker T, Czeschik JC, Schafers M, Busselberg D. Sensitization of voltage activated calcium channel currents for capsaicin in nociceptive neurons by tumor-necrosis-factor-[alpha] Brain Research Bulletin. 2010;81:157–163. doi: 10.1016/j.brainresbull.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Hirsch AH, Glantz SB, Li Y, You Y, Rubin CS. Cloning and expression of an intron-less gene for AKAP 75, an anchor protein for the regulatory subunit of cAMP-dependent protein kinase II beta. Journal of Biological Chemistry. 1992;267:2131–2134. [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes & Development. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A Novel Leucine Zipper Targets AKAP15 and Cyclic AMP-dependent Protein Kinase to the C Terminus of the Skeletal Muscle Ca2+ Channel and Modulates Its Function. Journal of Biological Chemistry. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Jackson JG, Usachev YM, Thayer SA. Bradykinin-Induced Nuclear Factor of Activated T-Cells-Dependent Transcription in Rat Dorsal Root Ganglion Neurons. Molecular Pharmacology. 2007;72:303–310. doi: 10.1124/mol.107.035048. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry MA, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain. 2009;146:301–307. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: A possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Molecular and Cellular Neuroscience. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Molecular Brain Research. 2001;95:18–26. doi: 10.1016/s0169-328x(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Kim MS, Usachev YM. Mitochondrial Ca2+ Cycling Facilitates Activation of the Transcription Factor NFAT in Sensory Neurons. J Neurosci. 2009;29:12101–12114. doi: 10.1523/JNEUROSCI.3384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Devor M, Waxman SG, Kocsis JD. Subthreshold Oscillations Induced by Spinal Nerve Injury in Dissociated Muscle and Cutaneous Afferents of Mouse DRG. J Neurophysiol. 2002;87:2009–2017. doi: 10.1152/jn.00705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M. Spinal Nerve Injury Enhances Subthreshold Membrane Potential Oscillations in DRG Neurons: Relation to Neuropathic Pain. J Neurophysiol. 2000;84:205–215. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. AKAP150-anchored PKA activity is important for LTD during its induction phase. The Journal of Physiology. 2008;586:4155–4164. doi: 10.1113/jphysiol.2008.151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Cheng J, Han JS, Wan Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. Neuroreport. 2004;15:655–658. doi: 10.1097/00001756-200403220-00016. [DOI] [PubMed] [Google Scholar]
- Lustig M, Zanazzi G, Sakurai C, Blanco C, Levinson SR, Lambert S, Grumet JL, Salzer JL. Nr-CAM and neurofascin interactions regulate ankyrin G and sodium channel clustering at the node of Ranvier. Current Biology. 2001;11:1864–1869. doi: 10.1016/s0960-9822(01)00586-3. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of Capsaicin-activated Currents in the Vanilloid Receptor TRPV1 Is Decreased by the Cyclic AMP-dependent Protein Kinase Pathway. Journal of Biological Chemistry. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the Tetrodotoxin-Resistant Sodium Channel PN3 in Rat Sensory Neurons in Normal and Neuropathic Conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 Anchoring of Calcineurin Controls Neuronal L-Type Ca2+ Channel Activity and Nuclear Signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Gomez LL, Dell’Acqua ML. Imaging kinase–AKAP79–phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. The Journal of Cell Biology. 2003;160:101–112. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 Module: A Common Link of Gs-Mediated Signaling to Thermal Hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Dell’Acqua ML. AKAP Signlaing Complexes in Regulation of Excitatory Synaptic Plasticity. The Neuroscientist. 2010;16 doi: 10.1177/1073858410384740. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein Kinase A Anchoring via AKAP150 Is Essential for TRPV1 Modulation by Forskolin and Prostaglandin E2 in Mouse Sensory Neurons. J Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144:187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Davies M, Yip PK, Carter LM, Wong DJ, McMahon SB, Bradbury EJ. Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury. J Comp Neurol. 2010;513:51–68. doi: 10.1002/cne.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, McNaughton PA. Modulation of single-channel properties of TRPV1 by phosphorylation. The Journal of Physiology. 2010;588:3743–3756. doi: 10.1113/jphysiol.2010.190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin Lowers the Threshold Temperature for Heat Activation of Vanilloid Receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Experimental Neurology. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tibbs VC, Gray PC, Catterall WA, Murphy BJ. AKAP15 Anchors cAMP-dependent Protein Kinase to Brain Sodium Channels. Journal of Biological Chemistry. 1998;273:25783–25788. doi: 10.1074/jbc.273.40.25783. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proceedings of the National Academy of Sciences. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B. Pharmacology of Vanilloid Transient Receptor Potential Cation Channels. Molecular Pharmacology. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Molecular Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. Proinflammatory Mediators Modulate the Heat-Activated Ion Channel TRPV1 via the Scaffolding Protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]


