Abstract
Background
To assess the prognostic value of progesterone receptor (PR) expression in patients with hormone receptor-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer subgroups.
Methods
A retrospective review of breast cancer patients who underwent mastectomy or breast-conserving surgery between January 1998 and December 2007 was performed. The prognostic impact of PR status on disease-free survival (DFS) was analyzed.
Results
Of the 1,301 patients included in this study, the median follow-up time was 64 months, and the median age was 46 years. There were 18.4% of patients (n=219) with PR negative (PR−) cancer. Women with PR–breast cancer were more likely to be postmenopausal (P<0.001) and have pN3 stage (P=0.031) and Stage III (P=0.049) cancer. Cox regression univariate and multivariate analysis showed that PR status was a significant prognostic factor for DFS. Patients with PR− status had poorer DFS (hazard ratio =1.626, 95% confidence interval =1.060–2.497, P=0.026). The 5-year DFS for patients with PR− and PR+ breast cancer was 79.4% and 86.2%, respectively, and the 8-year DFS for patients with PR− and PR+ breast cancer was 69.6% and 78.1%, respectively (P=0.012). A significant difference in DFS was observed between PR− and PR+ disease in patients with node-negative cancer, but was not for patients with lymph node metastasis (P=0.242). In premenopausal patients, DFS varied significantly by PR status (P=0.049). A marginally significant difference in DFS between the PR− and PR+ disease was seen in postmenopausal patients (log rank P=0.065).
Conclusion
Lack of PR expression is associated with worse survival in patients with hormone receptor-positive and HER2-negative breast cancer subgroups.
Keywords: breast cancer, breast cancer subtype, progesterone receptor, prognosis, recurrence
Introduction
Breast cancer can be divided into at least four subtypes based on gene expression profile; there are different breast cancer subtypes with distinct prognosis, and these subtypes can predict therapeutic efficacy.1–5 Hormone receptor-positive and human epidermal growth factor receptor 2-negative (HER2−) breast cancer subgroup is a subtype of breast cancer that is estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+), and HER2−. Nearly 20% of invasive breast cancers have a mixed hormone receptor status as either ER+/PR− or ER−/PR+. ER+/PR− is the most common mixed hormone receptor subtype. Studies have confirmed that the risk factors associated with ER+/PR− breast cancer are similar to those for ER+/PR+ disease.6,7
The role of the PR in breast cancer remains controversial. While the rate of ER+ breast cancer increases with age, no such pattern is seen with the PR, and the PR+ rate is constant in all age-groups.8 PR gene expression is dependent on estrogen, and consequently, PR expression has been considered to indicate an intact estrogen–ER response pathway. This raises the question as to whether ER+/PR− breast cancers are a heterogeneous disease in the hormone receptor-positive and HER2− subtype. Therefore, in this study, we evaluated the prognostic value of PR expression in hormone receptor-positive and HER2− breast cancer patients and also investigated whether it is necessary to further subtype hormone receptor-positive and HER2− breast cancer.
Patients and methods
Patients
The records of patients with breast cancer who were treated at Sun Yat-sen University Cancer Center (SYSUCC) from January 1998 to December 2007 were retrospectively analyzed. Criteria for inclusion in the analysis were: 1) females with pathologically confirmed unilateral invasive breast cancer; 2) underwent mastectomy or breast-conserving surgery, and axillary lymph node dissection; 3) cancer stage was T1-4N1-3M0 according to the (2009) 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control Tumor–Node–Metastasis (TNM) staging system; 4) the tumor was completely resected with no positive margins; 5) complete immunohistochemistry results including ER, PR, and HER2 (patients who were ER+ and/or PR+, and HER2− were included in the analysis); and 6) corresponding therapies (chemotherapy, radiotherapy, or endocrine therapy) were given after surgery according to TNM stage and hormone receptor status. The study was approved by the Ethics Committee of SYSUCC. All patients provided written consent for storage of their medical information in the hospital database and for research use of this information.
Patient characteristics and lymph node status
Patients’ clinicopathological and immunohistochemical factors, including age, menstrual status, pathologic tumor (pT) stage, pathologic node (pN) stage, ER status, PR status, Ki-67, and lymphovascular invasion were used to assess the risks of relapse. ER+ and PR+ were defined as >1% positive cells on immunohistochemical staining. Breast cancer subtypes were not determined according to the criteria developed at the St Gallen International Breast Cancer Conference, because some patients did not have immunohistochemistry testing for Ki-67.9 The expression of Ki-67 was determined according to our previous report, and 25% positivity was used as the cutoff point.10
Follow-up and survival end points
Follow-up was performed every 3–6 months. Because all patients in this study received adjuvant treatment according to stage and hormone receptor status, the end point was disease-free survival (DFS). DFS was defined as the absence of locoregional or distant recurrence. For patients with recurrence, survival time was determined from the date of surgery to the date of locoregional recurrence and/or distant metastasis.
Statistical analysis
All data were analyzed using the SPSS statistical software package (version 16.0; SPSS Inc., Chicago, IL, USA). The χ2 and Fisher’s exact probability tests were used to analyze the differences between qualitative data. Survival rates were determined and plotted by the Kaplan–Meier method, and compared using the log-rank test. Univariate and multivariate Cox regression model analyses were performed. A value of P<0.05 was considered statistically significant.
Results
Clinicopathological factors and relationship with PR status
A total of 1,301 patients with hormone receptor-positive and HER2− breast cancer who met the inclusion criteria were included in this study, and their characteristics are summarized in Table 1. The median age was 46 years (range: 21–92 years); 66.6% of patients (866/1,301) were premenopausal, 52.3% of patients (681/1,301) were node-negative, and 18.4% of patients (219/1,301) were PR−. PR status was associated with menopausal status (P<0.001), pN stage (P=0.031), TNM stage (P=0.049), and ER status (P<0.001; Table 1). Women with PR− breast cancer were more likely to be postmenopausal and have pN3 stage and Stage III cancer.
Table 1.
Correlation between PR and clinicopathologic characteristics
| Characteristic | N (%) | PR-negative (%) | PR-positive (%) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| ≤35 | 132 (10.1) | 10 (9.1) | 122 (10.2) | 0.702 |
| >35 | 1,169 (89.9) | 100 (90.1) | 1,069 (89.8) | |
| Menopausal status | ||||
| Premenopausal | 866 (66.6) | 51 (46.3) | 815 (68.4) | <0.001 |
| Postmenopausal | 435 (33.4) | 59 (53.7) | 376 (31.6) | |
| Tumor stage | ||||
| pT1 | 496 (38.1) | 38 (34.5) | 458 (38.4) | 0.193 |
| pT2 | 703 (54.0) | 58 (52.7) | 645 (54.2) | |
| pT3 | 59 (4.5) | 7 (6.4) | 52 (4.4) | |
| pT4 | 43 (3.4) | 7 (6.4) | 36 (3.0) | |
| Node status | ||||
| Negative | 681 (52.3) | 55 (50.0) | 626 (52.6) | 0.607 |
| Positive | 620 (47.7) | 55 (50.0) | 565 (47.4) | |
| Nodal stage | ||||
| pN0 | 681 (52.3) | 55 (50.0) | 626 (52.6) | 0.031 |
| pN1 | 417 (32.1) | 30 (27.3) | 387 (32.5) | |
| pN2 | 113 (8.7) | 10 (9.1) | 103 (8.6) | |
| pN3 | 90 (6.9) | 15 (13.6) | 75 (6.3) | |
| TNM stage | ||||
| I | 327 (25.1) | 23 (20.9) | 304 (25.5) | 0.049 |
| II | 731 (56.2) | 57 (51.8) | 674 (56.6) | |
| III | 243 (18.7) | 30 (27.3) | 213 (17.9) | |
| ER status | ||||
| Negative | 219 (16.8) | 0 (0) | 219 (18.4) | <0.001 |
| Positive | 1,082 (83.2) | 110 (100) | 972 (81.6) | |
| Ki-67 (n=834) | ||||
| ≤25% | 586 (70.3) | 48 (64.9) | 538 (70.8) | 0.287 |
| >25% | 248 (29.7) | 26 (35.1) | 222 (29.2) | |
| Lymphovascular invasion | ||||
| Negative | 1,264 (97.2) | 107 (97.3) | 1,157 (97.1) | 0.939 |
| Positive | 37 (2.8) | 3 (2.7) | 34 (2.9) | |
Abbreviations: ER, estrogen receptor; pN, pathologic node; PR, progesterone receptor; pT, pathologic tumor; TNM, tumor–node–metastasis.
Prognosis
Results of the univariate and multivariate analysis are listed in Table 2. In the univariate Cox analysis, age, tumor stage, node stage, TNM stage, and PR status were prognostic factors for DFS (all, P<0.05).
Table 2.
Cox regression analysis of prognostic factors influencing the disease-free survival of breast cancer patients
| Characteristic | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (continuous variable) | 0.980 | 0.967–0.994 | 0.004 | 0.984 | 0.971–0.998 | 0.022 |
| Menopausal status | ||||||
| Premenopausal vs postmenopausal | 0.869 | 0.640–1.178 | 0.364 | – | – | – |
| Tumor stage | ||||||
| pT2 vs pT1 | 2.211 | 1.569–3.114 | <0.001 | 1.855 | 1.306–2.635 | 0.001 |
| pT3 vs pT1 | 3.097 | 1.722–5.560 | <0.001 | 1.944 | 1.047–3.612 | 0.035 |
| pT4 vs pT1 | 3.804 | 1.964–7.367 | <0.001 | 2.893 | 1.464–5.719 | 0.002 |
| Node stage | ||||||
| pN1 vs pN0 | 2.020 | 1.459–2.797 | <0.001 | 1.861 | 1.341–2.583 | <0.001 |
| pN2 vs pN0 | 2.543 | 1.607–4.024 | <0.001 | 1.953 | 1.213–3.144 | 0.006 |
| pN3 vs pN0 | 3.783 | 2.420–5.914 | <0.001 | 2.646 | 1.653–4.234 | <0.001 |
| TNM stage | ||||||
| II vs I | 2.436 | 1.558–3.811 | <0.001 | 0.955 | 0.476–1.913 | 0.896 |
| III vs I | 4.214 | 2.602–6.826 | <0.001 | 0.628 | 0.192–2.051 | 0.441 |
| ER status | ||||||
| Negative vs positive | 1.373 | 0.982–1.921 | 0.064 | – | – | – |
| PR | ||||||
| Negative vs positive | 1.700 | 1.118–2.586 | 0.013 | 1.626 | 1.060–2.497 | 0.026 |
| Ki-67 | ||||||
| ≤25% vs >25% | 1.146 | 0.753–1.744 | 0.525 | – | – | – |
| Lymphovascular invasion | ||||||
| Negative vs positive | 1.518 | 0.903–2.551 | 0.115 | – | – | – |
Abbreviations: HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; pN, pathologic node; PR, progesterone receptor; pT, pathologic tumor; TNM, tumor–node–metastasis.
Multivariate Cox analysis adjusted for significant factors from the univariate analysis was used to examine DFS. Age, tumor stage, node stage, and PR status were significant prognostic factors for DFS (all, P<0.05). Patients with PR− disease had poorer DFS (hazard ratio [HR] =1.626, 95% confidence interval [CI] =1.060–2.497, P=0.026) than patients with PR+ disease.
Relationship between PR status and survival
The median follow-up time was 64 months (range: 6–144 months), recurrence occurred in 198 patients, and the 5- and 8-year DFS were 85.6% and 77.3%, respectively. PR− status correlated with disease recurrence. The 5-year DFS for patients who were PR− and PR+ was 79.4% and 86.2%, respectively; the 8-year DFS for patients who were PR− and PR+ was 69.6% and 78.1%, respectively (log rank P=0.012; Figure 1).
Figure 1.
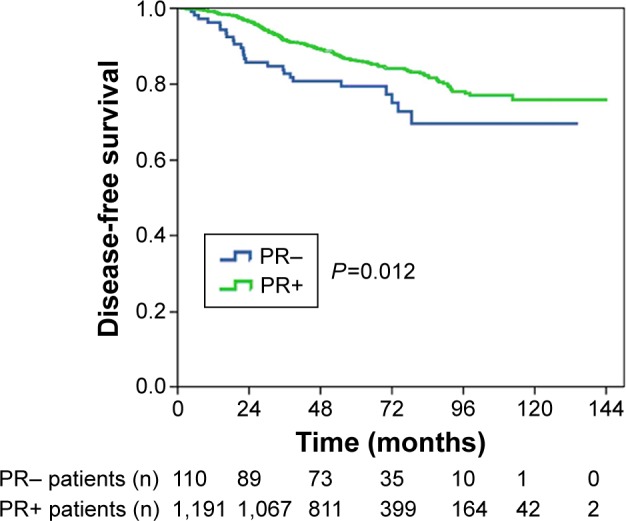
Kaplan–Meier plot of disease-free survival by PR status.
Abbreviation: PR, progesterone receptor.
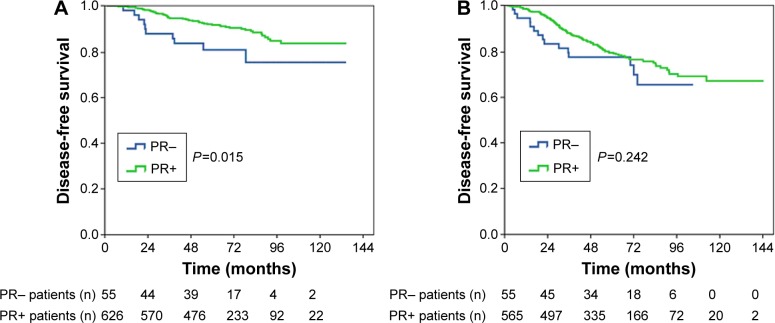
The prognostic effect of PR status in patients with and without lymph node metastasis was examined. A significant difference in DFS based on PR status was observed in patients with lymph-node-negative disease; the 5-year DFS for patients with PR− and PR+ disease was 81.1% and 91.8%, respectively, and the 8-year DFS for patients with PR− and PR+ disease was 75.3% and 84.9%, respectively (log rank P=0.015; Figure 2A). However, for patients with lymph node metastasis, PR status was not associated with DFS (log rank P=0.242; Figure 2B).
Figure 2.
Kaplan–Meier plot of disease-free survival by PR status in (A) lymph-node-negative patients and (B) lymph-node-positive patients.
Abbreviation: PR, progesterone receptor.
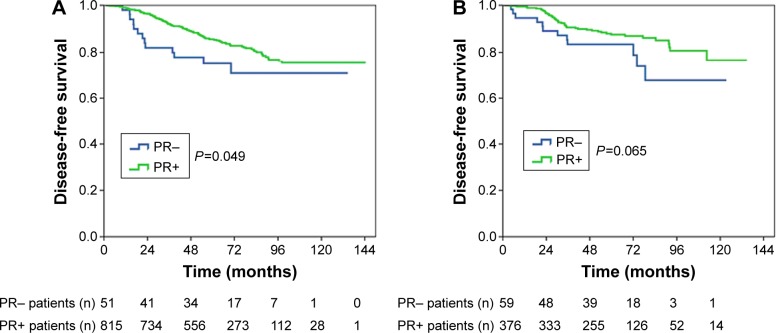
In premenopausal patients, a significant difference in DFS based on PR status was observed; the 5-year DFS for patients with PR− and PR+ disease was 75.0% and 85.6%, respectively, and the 8-year DFS for patients with PR− and PR+ disease was 70.8% and 74.8%, respectively (log rank P=0.049; Figure 3A). A marginally significant difference in DFS between the PR− and PR+ disease was seen (5-year DFS 83.4% vs 87.6%, 8-year DFS 67.7% vs 80.7%, log rank P=0.065; Figure 3B).
Figure 3.
Kaplan–Meier plot of disease-free survival by PR status in (A) premenopausal patients and (B) postmenopausal patients.
Abbreviation: PR, progesterone receptor.
Discussion
In this study, the prognostic value of PR status was evaluated in hormone receptor-positive and HER2−breast cancer patients, and the results showed that PR status was associated with survival of patients with hormone receptor-positive and HER2− breast cancer.
The molecular biological and clinical significance of PR in hormone receptor-positive and HER2− breast cancer are still controversial. The 14th St Gallen Breast Cancer Conference defined hormone receptor-positive breast cancer as ER+ and/or PR+ cells of >1%.11 However, there is evidence showing that the prognosis of ER+ breast cancer patients is different between those who are PR+ and PR−.12–15 Bae et al12 investigated ER+ and HER2–breast cancer patients and found that PR− patients had poorer DFS (HR =2.123, 95% CI =1.201–3.755, P=0.010) and overall survival (OS) (HR =4.779, 95% CI =1.874–12.189, P=0.001) as compared to PR+ patients. Prat et al13 also found that luminal A breast cancer patients with <20% PR+ cells had a significantly poorer prognosis when compared with those with >20% PR+ cells. Bal et al14 found that the OS (8.7 vs 15.3 years, P=0.032) and DFS (5.7 vs 10.5 years, P=0.022) in ER+/PR−/HER2− patients were significantly shorter than in patients with ER+/PR+/HER2− breast cancer. Our results showed that ER+/PR− patients had poorer DFS as compared to ER+/PR+ patients, which is consistent with the aforementioned findings. It was also found that the PR negativity was a prognostic factor in patients with luminal B/HER2− breast cancer.16,17 Therefore, it is better to subtype hormone receptor-positive and HER2− breast cancer according to PR status.
Loss of the PR is associated with an activated growth factor pathway, such as for HER2, epidermal growth factor, and insulin-like growth factor-1, hypermethylation of the PR promoter, loss of heterozygosity at the PR gene locus, and resistance to tamoxifen therapy.18–20 This means that ER+/PR− breast cancer exhibits a more aggressive phenotype. Patients with ER+/PR− tumors had a higher recurrence rate in the tamoxifen and combination arm of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial, but recurrence rates were similar in both ER+/PR+ and ER+/PR− tumors that had only been treated with anastrozole.21 This was because of the diminished efficiency of tamoxifen in PR− subgroup. Yu et al18 found that in ER+/PR− breast cancer patients, the risk of recurrence in patients treated with aromatase inhibitors was lower than in those treated with tamoxifen.22
In our study, most of the PR− breast cancer patients were postmenopausal, which is consistent with previous reports.14,23 This is a result of ovarian shutdown in the elderly, which causes insufficient levels of estrogen to transcribe PRs.19 Thus, we further evaluated whether the PR status affected the prognosis of breast cancer patients with different menopausal status. The results indicated that PR− premenopausal patients had a poorer prognosis, and a negative PR status was also detrimental to the prognosis of postmenstrual patients. Nishimukai et al23 also found that postmenstrual patients with a low PR expression (<20%) had marginally significant differences in distant relapse-free survival compared to patients with higher PR expression (P=0.060). These findings suggest that PR status not only affects the efficacy of endocrine therapy in postmenstrual patients but also has prognostic value in premenopausal patients. Thus, clinicians should emphasize PR status in both pre- and postmenopausal patients, because PR status is able to not only predict prognosis but is also useful for guiding the selection of adjuvant therapy.
Whether lymph node status affects the prognostic value of PR status in breast cancer patients is still controversial. There is evidence showing that PR− patients have a higher risk for lymph node metastasis.14,24 However, our results showed that PR status had no relationship with nodal status, but with the number of lymph node metastasis (pN3 stage). Further analysis showed the PR status only affected the survival of patients without lymph node metastasis and had no influence on the prognosis of patients with positive lymph nodes. However, a study by Park et al24 revealed that PR status affected the prognosis of luminal A breast cancer patients with positive lymph nodes, but failed to influence the prognosis of lymph-node-negative patients. Purdie et al25 found that PR expression in primary breast cancer was strongly and independently associated with worse prognosis in all subgroups, including ER+/lymph-node-negative patients; however, the study did not further evaluate HER2 status.25
There are a few limitations of this study. First, this was a single-center retrospective study, and the results might not extend to all breast cancer patients. Second, we did not stratify patients according to treatment with tamoxifen or aromatase inhibitors. In addition, this study spans a 10-year period, and thus there may be some methodological problems with ER/PR assessment.
Conclusion
Our findings suggest that lack of PR expression is associated with worse survival in hormone receptor-positive and HER2− breast cancer patients. We recommend further subdividing hormone receptor-positive and HER2− breast cancer subgroups according to PR status, which may be helpful to predict prognosis and guide treatment selection. Further prospective trials and larger studies are needed to confirm the aforementioned results and to better define subgroups of patients with hormone receptor-positive and HER2− breast cancer.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (number 81402527), the Sci-Tech Office of Guangdong Province (number 2013B021800157), the Youth Foundation of Fujian Provincial Health and Family Planning Commission (number 2014-2-63), and the Natural Science Foundation of Fujian Province (number 2015J01550).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Martín M, Rodríguez-Lescure A, Ruiz A, et al. Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Res Treat. 2010;123(1):149–157. doi: 10.1007/s10549-009-0663-z. [DOI] [PubMed] [Google Scholar]
- 4.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu SG, He ZY, Li Q, et al. Predictive value of breast cancer molecular subtypes in Chinese patients with four or more positive nodes after postmastectomy radiotherapy. Breast. 2012;21(5):657–661. doi: 10.1016/j.breast.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96(3):218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 8.Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10(14):2293–2301. doi: 10.2217/fon.14.110. [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li FY, Wu SG, Zhou J, et al. Prognostic value of Ki-67 in breast cancer patients with positive axillary lymph nodes: a retrospective cohort study. PLoS One. 2014;9(2):e87264. doi: 10.1371/journal.pone.0087264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies – improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor-positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138. doi: 10.1186/s12885-015-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat A, Cheang MC, Martín M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bal O, Yalcintas Arslan U, Durnali A, et al. Progesterone receptor status in determining the prognosis of estrogen receptor positive/HER2 negative breast carcinoma patients. J BUON. 2015;20(1):28–34. [PubMed] [Google Scholar]
- 15.Feeley LP, Mulligan AM, Pinnaduwage D, et al. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Mod Pathol. 2014;27(4):554–561. doi: 10.1038/modpathol.2013.153. [DOI] [PubMed] [Google Scholar]
- 16.Zong Y, Zhu L, Wu J, et al. Progesterone receptor status and Ki-67 index may predict early relapse in luminal B/HER2 negative breast cancer patients: a retrospective study. PLoS One. 2014;9(8):e95629. doi: 10.1371/journal.pone.0095629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancello G, Maisonneuve P, Rotmensz N, et al. Progesterone receptor loss identifies luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. 2013;24(3):661–668. doi: 10.1093/annonc/mds430. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Thakkar JP, Mehta DG. A review of an unfavorable subset of breast cancer: estrogen receptor positive progesterone receptor negative. Oncologist. 2011;16(3):276–285. doi: 10.1634/theoncologist.2010-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 21.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (arimidex, tamoxifen alone or in combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 22.Yu KD, Liu GY, Di GH, et al. Progesterone receptor status provides predictive value for adjuvant endocrine therapy in older estrogen receptor-positive breast cancer patients. Breast. 2007;16(3):307–315. doi: 10.1016/j.breast.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Nishimukai A, Yagi T, Yanai A, et al. High Ki-67 expression and low progesterone receptor expression could independently lead to a worse prognosis for postmenopausal patients with estrogen receptor-positive and HER2-negative breast cancer. Clin Breast Cancer. 2015;15(3):204–211. doi: 10.1016/j.clbc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Park BW, Kim TH, et al. Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol. 2013;20(5):1505–1513. doi: 10.1245/s10434-012-2772-x. [DOI] [PubMed] [Google Scholar]
- 25.Purdie CA, Quinlan P, Jordan LB, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. 2014;110(3):565–572. doi: 10.1038/bjc.2013.756. [DOI] [PMC free article] [PubMed] [Google Scholar]