Abstract
Introduction: An ongoing Zika virus pandemic in Latin America and the Caribbean has raised concerns that travel-related introduction of Zika virus could initiate local transmission in the United States (U.S.) by its primary vector, the mosquito Aedes aegypti.
Methods: We employed meteorologically driven models for 2006-2015 to simulate the potential seasonal abundance of adult Aedes aegypti for fifty cities within or near the margins of its known U.S. range. Mosquito abundance results were analyzed alongside travel and socioeconomic factors that are proxies of viral introduction and vulnerability to human-vector contact.
Results: Meteorological conditions are largely unsuitable for Aedes aegypti over the U.S. during winter months (December-March), except in southern Florida and south Texas where comparatively warm conditions can sustain low-to-moderate potential mosquito abundance. Meteorological conditions are suitable for Aedes aegypti across all fifty cities during peak summer months (July-September), though the mosquito has not been documented in all cities. Simulations indicate the highest mosquito abundance occurs in the Southeast and south Texas where locally acquired cases of Aedes-transmitted viruses have been reported previously. Cities in southern Florida and south Texas are at the nexus of high seasonal suitability for Aedes aegypti and strong potential for travel-related virus introduction. Higher poverty rates in cities along the U.S.-Mexico border may correlate with factors that increase human exposure to Aedes aegypti.
Discussion: Our results can inform baseline risk for local Zika virus transmission in the U.S. and the optimal timing of vector control activities, and underscore the need for enhanced surveillance for Aedes mosquitoes and Aedes-transmitted viruses.
Introduction
Zika virus (ZIKAV), a flavivirus, was first isolated from a primate in 1947 in the Zika forest of Uganda and in 1948 from Aedes (Ae.) africanus mosquitos in the same location1. Serological evidence of ZIKAV transmission in humans has been reported since 1951, but until recently, transmission was confined to ecological niches in the equatorial regions of Africa and Asia2. In 2007, an outbreak of ZIKAV in Yap State, Federated States of Micronesia, was the first documented epidemic outside Africa or Asia3 , 4. Over 28,000 cases were reported in 2013-2014 in French Polynesia, and nearby island nations followed5 , 6. Introduced into Brazil in 2015, ZIKAV has spread explosively across Latin America and the Caribbean, and is now pandemic, with over 20 countries reporting autochthonous transmission7 , 8 , 9. This follows the introduction and subsequent pandemic of chikungunya virus (CHIKV) in the Western Hemisphere in late 201310 , 11. Aedes mosquitoes transmit ZIKAV and CHIKV, as well as dengue (DENV) and yellow fever (YFV) viruses. Over the past several decades the range of Aedes mosquitoes has expanded across the Americas12 , 13. Although Ae. albopictus is thought to be a competent vector of ZIKAV14, Ae. aegypti has been implicated as the primary transmitter of the virus in human populations in the ongoing outbreak in the Americas15.
ZIKAV infections are commonly characterized by fever, maculopapular rash, arthralgia or conjunctivitis. Clinical illness is usually mild, occurs in approximately 20% of people infected with ZIKAV, and symptoms typically last for a week8. However, in mid-November 2015, the Pan American Health Organization issued an epidemiological alert16 based on an unusual increase in the number of children born with microcephaly in northeast Brazil that coincided with the arrival of ZIKAV17. Although no causal links have been established, in the aftermath of a previous outbreak of ZIKAV in French Polynesia during 2014-2015, a notable increase in central nervous system malformations in newborns and infants was also registered18. Additionally, in both Brazil and French Polynesia, following recent illnesses with ZIKAV-like symptoms, authorities detected patients with neurological syndromes such as Guillain-Barré18.
As of 22 February 2016, all cases of ZIKAV reported in the contiguous United States were associated with travel or sexual transmission72. Given the potential link between exposure to ZIKAV during pregnancy and microcephaly, the U.S. Centers for Disease Control and Prevention (CDC) has recommended pregnant women consider postponing non-essential travel and urges all travelers to take enhanced precautions in areas where ZIKAV is circulating19. The risk for local transmission of ZIKAV has been discussed widely in the media and scientific and public health communities. Sporadic outbreaks of DENV and CHIKV have already occurred in the United States and there is concern that local outbreaks of ZIKAV could follow20 , 21 , 22. Prior DENV outbreak investigations in the U.S.-Mexico border region suggest improved socioeconomic conditions in the United States may minimize vector-human contact and reduce the risk of Aedes-transmitted arboviruses20 , 23 , 24. Aside from sexual transmission that has been reported in comparatively few instances25, for local transmission to occur ZIKAV would have to be introduced by infected travelers or infected mosquitoes and become sustained in local Aedes mosquito populations. While Ae. aegypti and Ae. albopictus are established across much of the southern United States (Fig. 1), mosquito populations in many areas are only present seasonally. This seasonality varies according to local meteorological conditions, and thus can provide one measure of potential for ZIKAV transmission (i.e., local transmission may not be sustained if competent vector mosquitoes are not present). In addition, assuming transmission dynamics are similar to DENV, risk of ZIKAV introduction can be modulated by population travel patterns, human exposure to Aedes mosquitoes, and vector control response to travel-associated cases26 , 27. To better understand the risk for local ZIKAV transmission in the United States, we simulated the potential abundance of adult Ae. aegypti mosquitoes across fifty cities using meteorologically-driven life cycle models, and identified proxies of travel-related introduction and of human exposure to vectors to provide context for the model results.
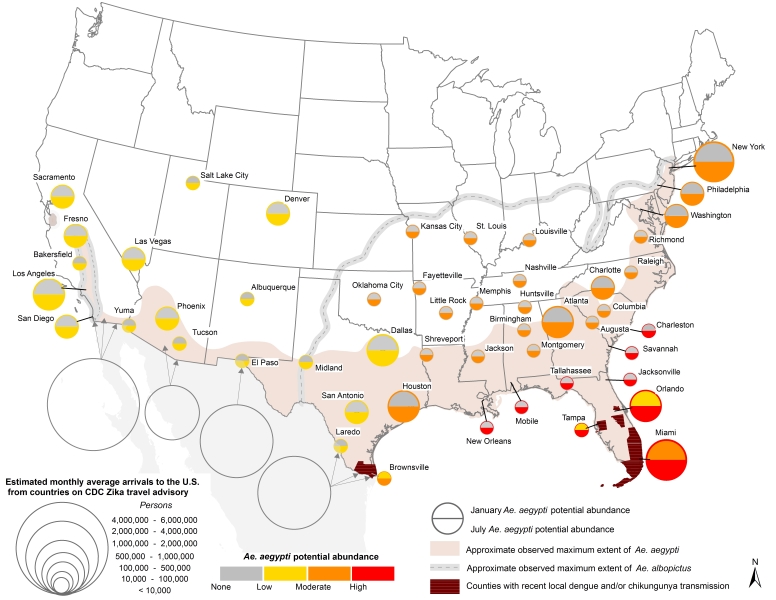
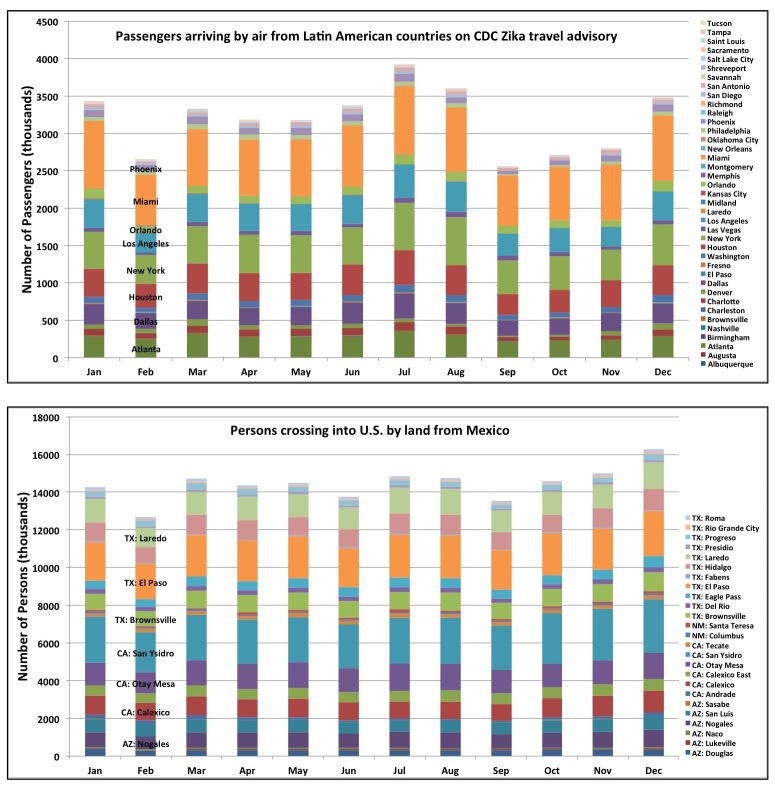
Fig. 1. U.S. map showing 1) Ae. aegypti potential abundance for Jan/July (colored circles), 2) approximate maximum known range of Ae. aegypti (shaded regions) and Ae. albopictus (gray dashed lines), and 3) monthly average number arrivals to the U.S. by air and land from countries on the CDC Zika travel advisory. Additional details can be found in the text.
Data and Methods
Cities
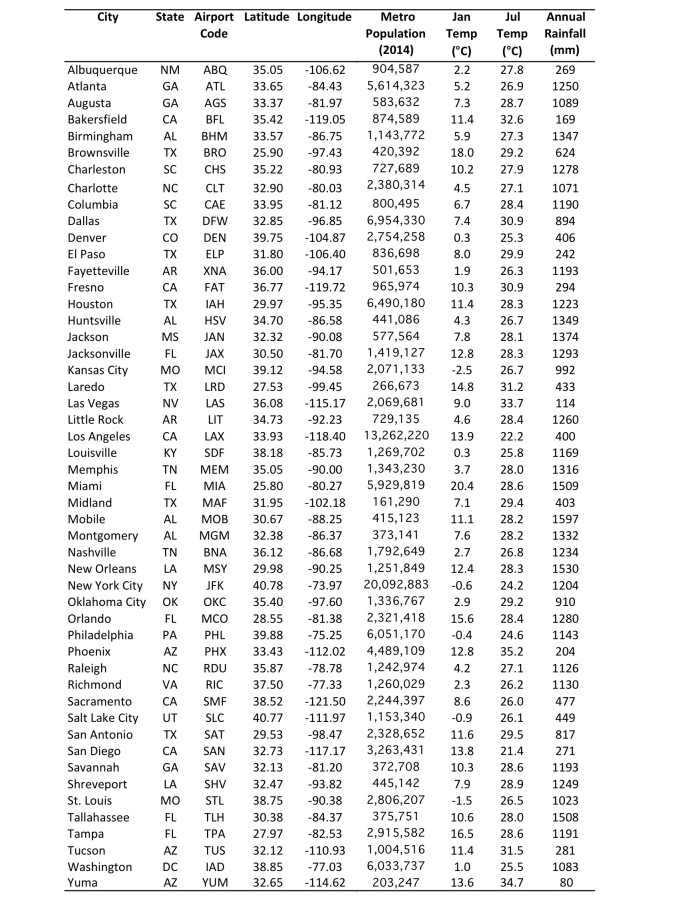
We chose fifty U.S. cities within and near the known range of Ae. aegypti (Fig. 1, Table 1). These cities were selected because they may be meteorologically suitable for Ae. aegypti for at least part of the year, though not all have recorded the presence of Ae. aegypti to date. In general, seasonal and annual temperatures within the study cities decrease from south-to-north, and precipitation decreases from east-to-west. These gradients are modified in the vicinity of the Appalachian and Rocky Mountains, and by proximity to the ocean.
Table 1. Coordinates, population28 and climate characteristics30 , 31 for the 50 study cities.
Ae. aegypti life cycle modeling
To estimate the seasonal abundance of adult Ae. aegypti we employed two life cycle models, Skeeter Buster32 and DyMSiM33. Both models simulate the egg, larval, pupal and adult life stages as a function of daily meteorological inputs and user specified information about container habitats. Using two models enabled the quantification and reduction of model uncertainty; previous studies have shown that model ensembles provide a more accurate result than those from individual models34 , 35 , 36. Both models simulated daily counts of adult Ae. aegypti mosquitoes per 1 m2 of water surface for a multi-year period, as described below. The amount of water in containers was modulated primarily by rainfall and evaporation, but a fraction of water representing about 5-10% of the maximum due to rainfall was made available in containers year-round to account for the possibility that water may be available even during periods of little or no rainfall due to humans filling containers intentionally (e.g., via birdbaths, kiddie pools, etc.) or unintentionally (e.g., during lawn irrigation). Human-filled containers can represent an important source of water for Ae. aegypti habitat in arid and semi-arid regions73. For both models, releasing new cohorts of eggs each month prevented extinction of mosquitoes, or (in the case of DyMSiM) when the population of any life stage reduced to zero. Releases ranged from 80-100 eggs per container. This choice essentially assumes that Aedes eggs are frequently reintroduced into areas through transportation networks37 or that eggs are able to survive through periods when weather is unfavorable for development. There is strong evidence that Ae. aegypti eggs are capable of surviving even in harsh winter conditions as a result of being laid in favorable cryptic environments such as subterranean habitats38.
The DyMSiM and Skeeter Buster models differ primarily in their treatments of containers and stochasticity. DyMSiM assumes one large water-filled container per city33; in this study a surface area of 1 m2 was specified for each DyMSiM container, and water volume fluctuated as a function of rainfall and evaporation but did not drop below 0.001 m3 or rise above 0.02 m3. Skeeter Buster operates at the level of individual containers, and therefore was configured to simulate 10 adjacent households per city, each having numerous containers with a surface area totaling 1 m2. Each container had a surface area of 0.01 m2 and could be filled to a maximum depth of 0.2 m. As with DyMSiM, Skeeter Buster water depth was allowed to vary as a function of rainfall and evaporation, and manual filling was performed randomly for 5% of containers on average every 7 days. The resulting simulated Ae. aegypti adult counts were then averaged over the 10 homes. In this manner, the initial adult mosquito abundance results for both DyMSiM and Skeeter Buster were expressed for each 1 m2 of water surface area per city; these values were then normalized for presentation, as described below. Sheltering of containers (via shading) was also considered for both models because Ae. aegypti prefers shaded environments29. DyMSiM treated sheltering by reducing evaporation among 50% of containers and limiting the maximum water temperature to <34oC. Skeeter Buster assumed a sun exposure of 0.1 (i.e., 90% shade) for containers. Additional descriptions of both models can be found in the publications cited above, and model inputs and other study-specific details are described below.
Simulations were conducted with both models for the most recent eleven years, 2005-2015. The meteorological fields needed to drive the models – daily precipitation, humidity and maximum and minimum near-surface temperature – were derived from the 1/8th degree hourly meteorological forcing dataset for the North American Land Data Assimilation Phase 2 (NLDAS-2)30 , 31. The widely used NLDAS-2 forcing data have been employed for numerous health-related applications over North America requiring high fidelity meteorological data39 , 40. The NLDAS-2 data were bilinearly interpolated to the coordinates of each city from the four nearest grid points.
Once the simulations were complete, the results for year 2005 were discarded to allow for model burn-in. Then, for each city (n=50) and each day of the year (n=365) the mean adult Ae. aegypti abundance was computed from the remaining 10-year sample (2006-2015) for each model. Ensemble mean values were computed by taking the geometric mean of the daily mosquito abundance values from both models. Using a geometric mean rather than an arithmetic mean allows the two models to be weighted equally despite having different mean Ae. aegypti abundance values. Next, the average daily abundance was computed for Miami for May-October (Julian days 121-304) for both models and the ensemble mean, and then the mean daily abundance for each city and day of year was expressed as a percentage of this average. In this manner, the potential abundance patterns across cities and for each day were normalized with respect to the average warm season conditions for Miami. Finally, these normalized daily values were averaged for each month and city for both models and the ensemble mean. We chose Miami and the May-October period because it is the warmest and wettest time of year in south Florida, a time when Ae. aegypti is known to be abundant41 and when most transmission of Ae. aegypti-borne viruses has occurred in the past42. May-October in Miami thus represents high environmental suitability for Ae. aegypti.
The normalized daily values of adult Ae. aegypti potential abundance were placed into four categories to facilitate comparison across space and time: 1) “None to low” for normalized abundances <2% the value of the May-Oct Miami mean; 2) “Low to moderate” (2-25%); 3) “Moderate to high” (25-75%); and 4) “High” (>75%). These cut points were chosen to provide a proxy of the lower quartile, the interquartile range, and the upper quartile with respect to Miami values. The “None to low” cut-point of 2% rules out extremely low abundance values that are unlikely to be sufficient to generate substantial risk for human contact.
Results of simulations of adult Ae. aegypti populations are referred to in terms of potential abundance in order to emphasize that they indicate meteorological suitability for the mosquito but not necessarily that it is present or will occur in a given location. For example, we find that Denver, CO has low-to-moderate potential abundance for the mosquito during the summer months, however as of this writing presence of the mosquito has not been detected there. Possible reasons that Ae. aegypti does not occur in some cities that exhibit meteorological suitability during part of the year include a lack of eggs being introduced during the warm months, or a lack of water availability (particularly in western U.S. cities).
Model validation data
Validating the simulations of potential seasonal abundance of Ae. aegypti was challenging because there are few observational records of seasonally-resolved Ae. aegypti abundance (for any life stage) that can provide comparative benchmarks. Focks et al.43 present graphical records of weekly ovitrap accumulations of Ae. aegypti eggs for 1981-1985 from a number of southeastern U.S. cities, based on unpublished data from CDC. We were unable to obtain the ovitrap data from Focks et al.43 at the time this manuscript was submitted, which limited us to a qualitative comparison of our simulated adult abundance to the ovitrap data. However, we did find quantitative seasonal records for two cities, in two different climatic settings and compared the seasonality of observed vs. modeled Ae. aegypti abundance. The first dataset contains Ae. aegypti immature abundance collected from 30 sites covering a 675 km2 area of Palm Beach, FL, collected over 27 four-week periods from 2006-200841. Aedes aegypti immature abundance was determined from samples collected in the field from ovitraps (eggs) or from water in containers (larvae), and reared in a laboratory to fourth-instar larvae or pupal stages for identification to species. A comparison of results from a variety of adult and egg trapping methods for Ae. aegypti 44 showed that the seasonal cycle of abundance for trapped adults versus trapped eggs was similar, suggesting that validating our normalized simulated adult abundance results to normalized egg/larval abundance results41 is a reasonable approach. Palm Beach is located just north of Miami, FL and has similar climatic conditions; therefore we consider this dataset to be representative of Miami. The second dataset contains unpublished monthly adult abundance for 750 trapping sites across Phoenix, AZ collected over 10 years from 2006-2015 by co-author KAS and colleagues. Samples were collected with CDC light traps baited with CO2 and laboratory-identified to species. Miami has a warm humid climate, and Phoenix has a warm desert climate. Both cities have distinct wet and dry seasons.
Transportation data
To understand the potential for human introduction of ZIKAV, we determined the number of air travelers arriving in the U.S. from airports in Latin American countries and territories listed on the CDC Zika travel advisory as of 29 January 201645. These countries and territories included the U.S. Virgin Islands, Dominican Republic, Commonwealth of Puerto Rico, Barbados, Bolivia, Brazil, Colombia, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Martinique, Mexico, Panama, Paraguay, Saint Martin, Suriname, and Venezuela. We did not include Cape Verde and Samoa, two countries not in Latin America that account for a very small fraction of passengers into the United States. Monthly estimated passenger counts on outbound international flights to airports in these countries originating from the U.S. were obtained for year 2014 from a database maintained by the U.S. Department of Transportation46. Inbound passenger counts were not available, however the data curator confirmed that they are approximately equal to outbound counts (personal communication, Michael Lane, USDOT); we confirmed this by analyzing international flight data for domestic carriers only (for which both inbound and outbound counts exist) and found that inbound and outbound passenger counts differ by <1% for monthly and longer timescales47. Passenger data were only available for the international leg of each flight and therefore do not indicate the number of passengers traveling on to secondary and tertiary U.S. destinations. Therefore, it is likely that passenger numbers are overestimated for large international hubs (e.g., Miami, Atlanta, New York, Los Angeles) and underestimated for smaller airports that primarily serve domestic passengers (e.g., New Orleans, Nashville, Jacksonville). Additionally, it is probable that some passengers were from Latin American countries not on the CDC ZIKAV travel advisory (e.g., Argentina, Chile) who had traveled through major international airports in countries on the advisory.
Because Mexico shares a border with the U.S. and is on the CDC ZIKAV travel advisory, we also obtained year 2014 estimates of the monthly number of persons crossing into the U.S. from Mexico along the land border by passenger vehicle, bus, train or as pedestrians48.
We did not obtain data for cruise ship arrivals, though a number of ports along the Gulf of Mexico and the eastern seaboard depart to areas of the Caribbean with ongoing Zika transmission49.
DENV and CHIKV transmission data
To provide information about the location of Aedes-transmitted virus outbreaks we mapped counties with confirmed local DENV and CHIKV transmission in the contiguous U.S. from 1 January 2010–6 February 2016. Data were obtained from The Florida Department of Health42 and from the CDC ArboNET via the United States Geological Survey Disease Mapper50. It is noteworthy that DENV transmission occurred in the contiguous U.S. prior to 2010, however those reported cases were more difficult to confirm and additionally were mainly from counties in Florida and Texas already identified as having DENV transmission using the (more reliable) data from 2010 onward.
Human exposure data
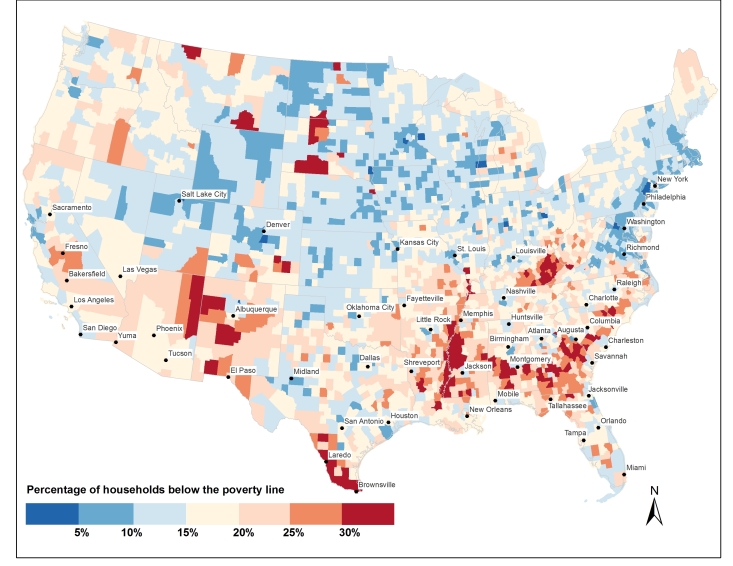
Vector-human contact is likely modulated by socioeconomic factors. Impoverished communities are at higher risk of introduction of infectious diseases51. Poverty has been linked to a number of indicators of elevated exposure to mosquitoes such as lower usage rates of air conditioning and less efficient cooling options52, poorer housing infrastructure such as screening of windows53, as well as decreased access to safe water and sanitation54. To better understand potential human exposure to adult Ae. aegypti, we obtained and mapped 2014 county-level data for the percentage of households living below the poverty line from the U.S. Census Bureau55. The poverty line varies according to the size of a family and ages of its members. Thresholds do not vary geographically but they are updated annually for inflation. In 2014, the poverty line was (for example) defined by an annual income of $12,316 for a household with a single adult under 65 years, and $24,008 for a household with two adults and two children under 18 years.
Maximum Aedes geographic range data
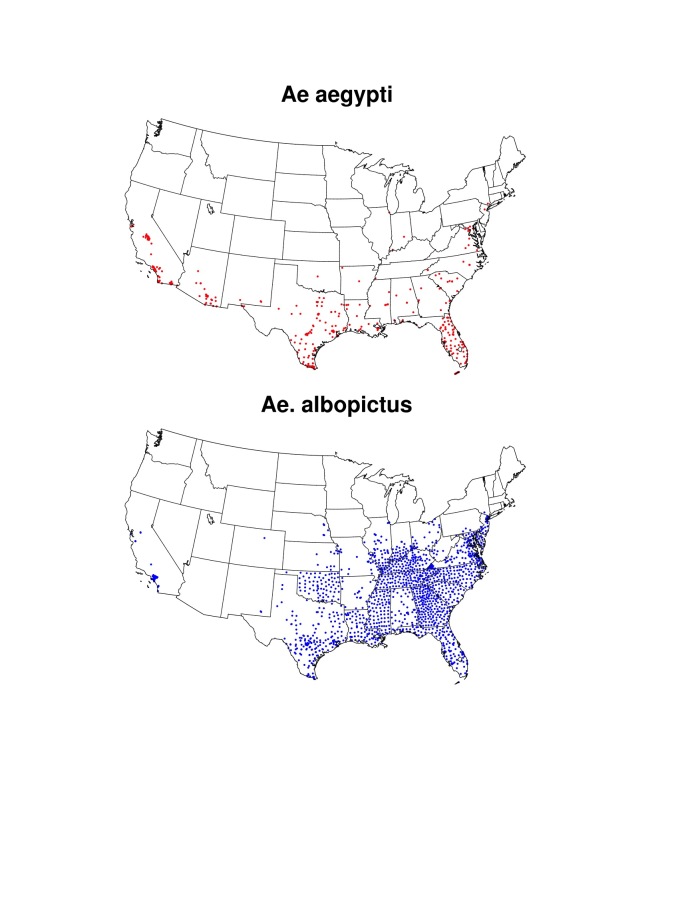
A recent compendium of observed Ae. aegypti and Ae. albopictus presence locations collected between 1960-201456 , 57 , 58 was used to provide background information on the maximum known geographic range of both mosquitoes in the U.S. (Fig. S1). These data were augmented by surveillance data of both Aedes species across southern and central California59. Known ranges for both species were then established by outlining areas in which presence locations were recorded (Fig. 1). Such information is useful for placing our results for simulated seasonal potential abundance of Ae. aegypti into context, as the mosquito may not exist in some cities where seasonally suitable meteorological conditions exist, for example because eggs are unable to overwinter60. Additionally, mapping known occurrences of Aedes mosquitoes enables comparison of the simulated potential range of Ae. aegypti to the known range of Ae. albopictus, which is also a competent vector of ZIKAV in some regions14. This is important because Ae. albopictus (which was not simulated in this study) has greater cold tolerance than Ae. aegypti, and therefore could facilitate seasonal ZIKAV transmission risk in more northerly U.S. cities where Ae. aegypti is not found61.
Fig. S1. Locations of know occurrences of Ae. aegypti and Ae. albpictus in the U.S. for 1960-2014 reproduced from Kraemer et al.56 , 57 , 58 and updated with collections in California for 2011-201559 .
Results
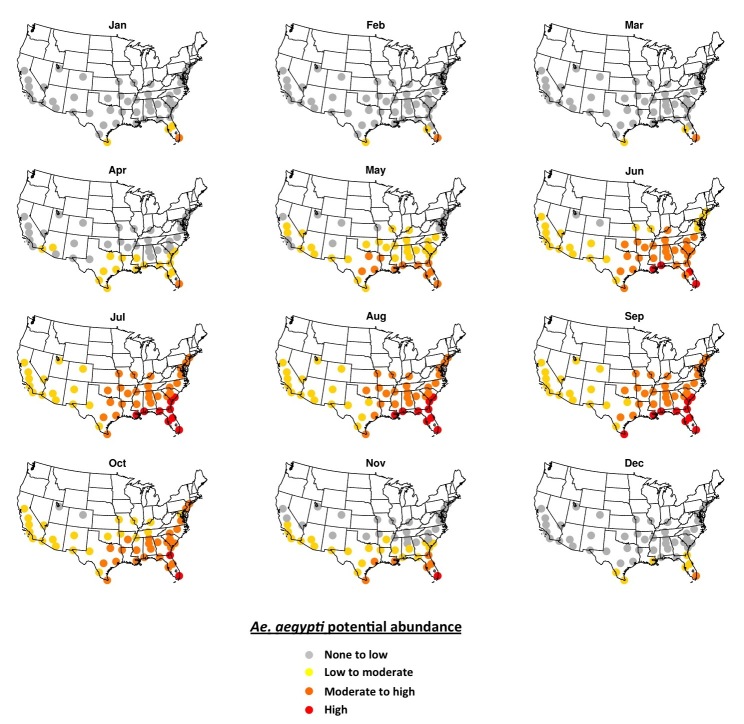
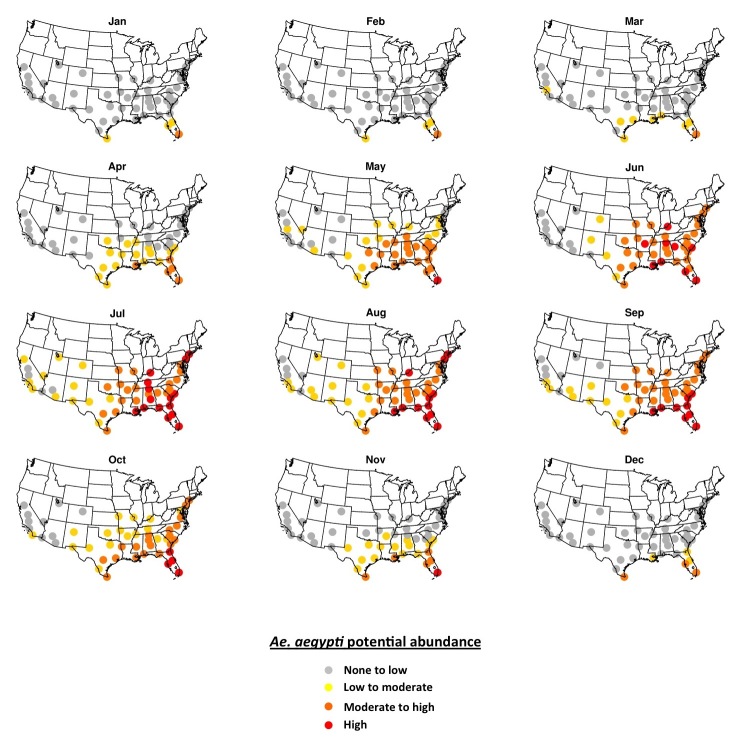
Simulated potential abundance of Ae. aegypti adults is zero or near-zero throughout the U.S. in January except in southern Florida and Texas (Fig. 1). By mid-July, all fifty cities are meteorologically suitable for Ae. aegypti, with far southeastern cities being suitable for high abundance, and other eastern cities being suitable for moderate-to-high abundance. Cities in the western U.S. are suitable for low-to-moderate abundance of Ae. aegypti. Some cities that are suitable in mid-summer are not within the present known range for Ae. aegypti (e.g., Denver, Albuquerque, Louisville). As the season progresses from winter to summer Ae. aegypti potential abundance begins to increase in April in the southeastern U.S. and some cities in Arizona (Fig. 2). By June nearly all cities exhibit the potential for at least low-to-moderate abundance, and most eastern cities are suitable for moderate-to-high abundance. Conditions are most suitable in July, August and September during the warmest (and in many cities wettest) time of year. Conditions in the southern and western states remain suitable through November, and by December are largely unsuitable again, except for southern Florida and Texas.
Fig. 2.The 2006-2015 ensemble mean monthly average Ae. aegypti potential abundance.
Model results demonstrated a similar seasonal cycle compared to the observed Ae. aegypti abundance at our two validation sites (Fig. 3). However, in Miami the observed onset and cessation of peak summer abundance in June-July occurs more rapidly than in the simulations, which have a broader peak that extends later into the summer. Interestingly, our climate data indicate that daily maximum and minimum temperatures and rainfall in August-September are similar to June-July conditions, suggesting that very high abundance might otherwise be expected to extend into these later summer months. The difference between peak and off-season abundance in Miami is also more pronounced in the observations. A qualitative comparison of simulated adult abundance to observed average weekly ovitrap accumulations in Tampa43 (not shown) similarly indicates a more pronounced difference between peak and off-season abundance than is simulated. However, for Southeast cities with somewhat cooler winters like Jacksonville and New Orleans the seasonal cycle of simulated adult Ae. aegypti abundance is similar to the observed ovitrap accumulations. It is possible that the broader, lower amplitude seasonality of Ae. aegypti abundance that is simulated in the warmer Southeast cities like Miami and Tampa occurs because the models do not account for interspecies competition between Ae. aegypti and Ae. albopictus, which may favor Ae. albopictus in areas with longer warm and wet periods41. Or, it is also possible that monthly egg introductions specified in the model simulations may lead to broader peaks in adult Ae. aegypti abundance (i.e., more eggs may be available in the models compared to reality). In Phoenix the observed peak abundance in August, during the monsoon season, is resolved by the simulations, as is the substantial difference between peak and off-season abundance (off-season abundance is basically zero in both the observations and simulations). An early-season peak in the simulations during April-May, which is not observed, is due to the small amount of water maintained in the simulated containers during dry periods to accommodate the potential for manual filling of containers. In reality manually filled containers may be relatively uncommon in Phoenix, though allowing for a modest amount of human-mediated water availability in simulations during the dry season can be useful for determining when conditions are warm enough for Ae. aegypti development and survival. Additionally, the models may underestimate the effects of low humidity (i.e., desiccation) during the dry season on adult survival, which may be particularly important in arid environments62. Vector control activities likely also lead to differences between observed and simulated Ae. aegypti populations in both cities (the simulations do not account for mosquito control). Given the uncertainty in the observations and the limitations of the models, the objective of the comparison in Fig. 3 is to evaluate whether the models can simulate the general seasonal cycle including higher abundances during observed peak months, and lower abundances during observed off-peak months, for these two cities that represent different climatic settings; we conclude that the model simulations meet this basic objective.
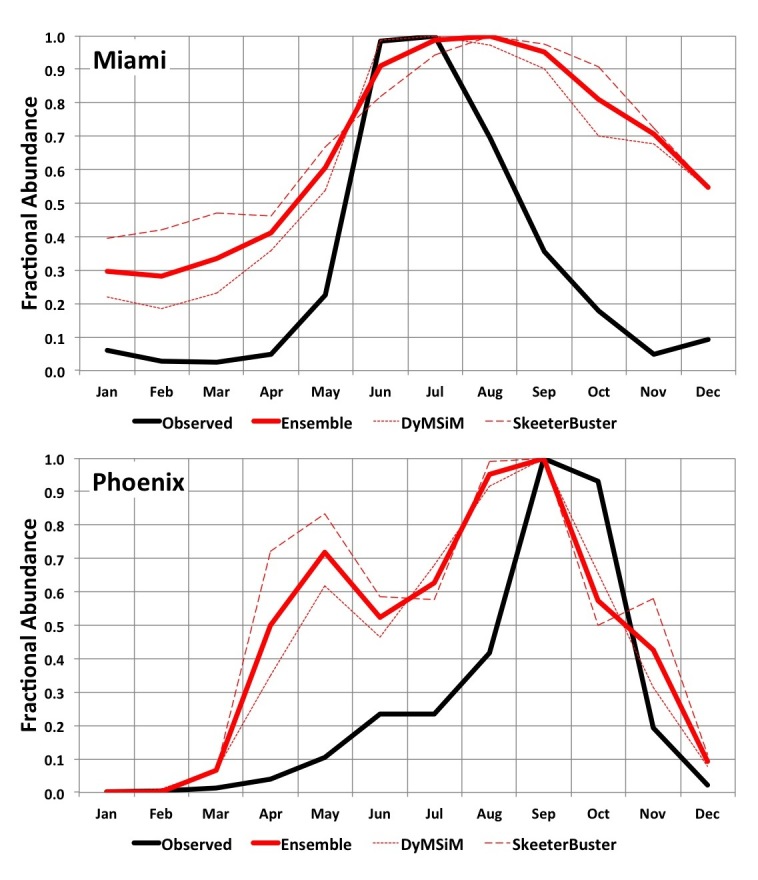
Fig. 3. 2006-2015 average simulated monthly Ae. aegypti potential abundance versus average observed abundance for Miami (2006-2008; top) and Phoenix (2006-2015; bottom). Results are expressed as a fraction of the maximum monthly abundance for each dataset in order to facilitate comparison (otherwise abundance measures will vary widely due to how simulated versus observed mosquitoes are quantified and the areal extents they represent).
The estimated monthly average number of persons arriving by air from countries on the CDC Zika virus travel advisory – which provides a proxy for the potential for virus introduction – is greatest in large cities with major international airports (Fig. 1). Cities having both high potential Ae. aegypti abundance and large volumes of air passengers include Miami, Houston and Orlando. It is also noteworthy that nearly five times as many persons cross the U.S.-Mexico border per month than arrive by air in all fifty cities combined.
Fig. 4. 2014 monthly number of persons arriving by air from Latin American countries on the CDC Zika travel advisory (top), and by land from Mexico (bottom). Sources for data can be found in the text.
Travel patterns fluctuate seasonally (Fig. 4). Importantly, July and August are the two months with the highest estimated number of passengers arriving by air from countries on the CDC Zika travel advisory. These months fall within the season of highest meteorological suitability for Ae. aegypti across the fifty cities. These months also have comparatively high traffic across the U.S.-Mexico border. The holiday months of December and January also have relatively high estimated numbers of persons arriving both by air and across the U.S.-Mexico border; these are months having no-to-low Ae. aegypti potential abundance in most cities, although there may be low-to-moderate potential abundance in cities like Brownsville, Miami and Orlando. Although rare in winter, one case of locally-acquired DENV was reported in January 2011 in Miami-Dade county42, indicating that in some years off-season Aedes populations may be abundant enough in these cities to enable virus transmission.
High poverty rates, an indicator of potential vector-human contact, are particularly prevalent in the southern U.S. where seasonal abundance of Ae. aegypti is simulated to be highest (Fig. 5). Many of the poorest counties across the geographic area under study are rural and unlikely to be inhabited by Ae. aegypti given its proclivity for urban areas55. Ae. albopictus, however, is present across the rural South (Fig. S1) and may be a competent vector of Zika virus14. The poorest urban areas include the counties that encompass Laredo, TX and Brownsville, TX, where poverty rates are >30%, as well as Yuma, AZ and Miami, FL (>20%). A number of other southern cities in this study are situated within counties having poverty rates > 15%.
Fig. 5. 2014 percentage of households below the poverty line by U.S. county. Source of data can be found in text.
Discussion
Numerous studies have produced global maps of general climatic suitability for Ae. aegypti 56 , 63 , 64. Our results for peak warm season Ae. aegypti potential abundance over the U.S. compare reasonably with the maps published in these previous studies. Fewer studies have explored seasonal suitability for Ae. aegypti. Brady et al.60 mapped weekly temperature-based suitability for Ae. aegypti and Ae. albopictus oviposition globally (they also examined weekly temperature suitability for introduction and persistence of DENV transmission for both mosquitoes). Here, we employ temperature, precipitation and humidity fields to drive two process-based life cycle models and simulate the daily potential abundance of Ae. aegypti for the most recent ten years at fifty cities across the U.S. While these simulations are subject to limitations as discussed below, they are an important step toward improved understanding of the spatial and seasonal variability of Ae. aegypti in the U.S. and the periods of higher risk of ZIKAV introduction.
We find that meteorological conditions are largely unsuitable for Ae. aegypti over the U.S. during winter months (December-March), except in southern portions of Florida and Texas that can sustain low-to-moderate potential mosquito abundance compared to summer (Fig. 2). Meteorological conditions are suitable for Ae. aegypti across all fifty cities during peak summer months (July-September), though the mosquito has not been observed in all cities. Highest potential mosquito abundances is simulated in the Southeast and south Texas where local cases of other Aedes-transmitted viruses have been reported previously, a result that is consistent with the suitability mapping studies noted above.
Introduction of the virus into local Ae. aegypti populations is more likely in cities with high volumes of people arriving from areas where transmission is ongoing (Fig. 1). This could include introduction by persons arriving in the U.S. by air, land (cross-border traffic by vehicles or walking) and sea (cruise ship passengers). Higher risk is thus implied for U.S.-Mexico border cities and neighboring areas, where population movement is high, and larger air traffic hubs such as Houston, Los Angeles or Miami. In Mexico, most reported Zika cases are in southern states, however cases have been reported further north in Sinaloa and Nuevo Leon by the Health Ministry of Mexico as of 22 February 2016. The potential for introduction in the U.S. may be highest during summer months when Ae. aegypti populations are abundant, particularly if Zika transmission increases in the northern Mexican states.
Multiple factors determine when and where arboviruses emerge; vector presence is only one factor that needs to be evaluated to assess the potential for disease emergence. Socioeconomic conditions that modulate human exposure to vectors play a role in population vulnerability to arboviruses23. Poverty is associated with lower air-conditioning use and less efficient cooling options52; such conditions can lead to open windows and doors and raise the probability of human-vector contact. Disparities in income have been associated with intermittent or no access to piped water in unregulated communities (colonias) in the U.S.-Mexico border region65; such conditions can necessitate water storage that in turn provides oviposition opportunities for Ae. aegypti. Intermittent garbage collection may also contribute to the provision of important oviposition sites along the U.S.-Mexico border. For example, discarded tires provide ideal habitat for Ae. aegypti due to their dark color, water holding capacity, and thermal insulation66. Additionally, the condition of housing infrastructure such as door and window screens may be lower in impoverished communities53. Areas in the southern parts of Texas demonstrated higher rates of poverty than some other regions under study (Fig. 3). Coupled with the higher levels of travel from areas where transmission is occurring and the history of Ae. aegypti –borne virus outbreaks, this suggests that southern Texas may be a vulnerable region for Zika transmission.
Even if Zika virus arrives in the United States, transmission can be mitigated and reduced through the use of vector control strategies. As part of our investigation we initially undertook a review of all vector control activities being conducted within these fifty cities. Due to the lack of standardization of reporting of Ae. aegypti-related activities in each jurisdiction we have not presented those results in this paper. It is clear, however, that vector control and public health interventions should consider tailoring their messages to the local communities. The provision of geographically referenced Ae. aegypti data to the public could assist in raising public awareness about the vector and motivate action when neighboring communities have Ae. aegypti activity67.
This study has numerous limitations related to the model simulations. We do not account for vector control practices in the simulations and thus abundance may be overestimated (as suggested during the off-peak months in Miami and Phoenix in the validation). There is incomplete understanding of how temperature may limit population dynamics at the geographic margins of Ae. aegypti survival68, where sensitivity to poorly-constrained meteorological thresholds may hamper model performance. For example, because knowledge of egg survival during winter in marginal areas is limited68, we artificially introduce eggs into the simulations each month, which keeps eggs from becoming extinct in cities that have seasonal Ae. aegypti populations, but likely causes egg availability/viability to be overestimated. Cryptic habitats that are not represented in models may enhance environmental suitability during winter; an overwintering Ae. aegypti population was recently found in Washington, D.C.38, suggesting that Aedes mosquitoes are adept at ovipositing in semi-concealed places that stay relatively warm year-round (e.g., subterranean habitats). Likewise, during the warm season it is likely that mosquitoes are adept at finding suitable microclimates in sheltered areas that aren’t explicitly accounted for in the models29, particularly in challenging environments such as desert cities where high temperatures and low humidity may otherwise inhibit mosquito survival. The influence of humidity in the simulations may not be adequately represented, which could be an important issue in arid regions where desiccation may substantially impact adult survival62. Little is known of the geographic distribution of container characteristics such as type, quantity and degree of shading, all factors that regulate water temperature and amount available for immature development. Additionally, the degree and frequency of manual filling of potential container habitats by humans is virtually unknown and may be over- or understated in the models. Intra-city climatic variability is not accounted for, which may be important in cites with sharp elevation gradients or heterogeneous urban land use patterns. Furthermore, the models do not allow for interaction between Ae. aegypti and other container dwelling invertebrates, including the competitor Ae. albopictus. This competitive interaction has been shown to determine the distribution of Ae. aegypti in Florida at scales of less than 5km41 , 69, and suggests the importance of understanding the local distribution of these mosquitoes in assessing risk. The competitive interaction may also affect the seasonality of Ae. aegypti due to increased competition late in the wet season41 , 70. The limitations noted here are the primary reason we refer to simulated Ae. aegypti abundance as potential abundance. Despite these limitations, our validation indicates that overall the Skeeter Buster and DyMSiM simulations, as well as their ensemble mean, resolve the seasonal cycle of Ae. aegypti abundance in Phoenix and Miami. Importantly, the simulations capture the months of peak abundance in both cities. We also find that Skeeter Buster and DyMSiM have similar seasonal cycles of abundance at most U.S. cities (Figs. S2 and S3).
Fig. S2. The 2006-2015 Skeeter Buster mean monthly average Ae. aegypti potential abundance.
Fig. S3. The 2006-2015 DyMSiM mean monthly average Ae. aegypti potential abundance.
Study limitations not related to the model simulations include incomplete information about travel from areas of ongoing Zika transmission. In particular, our air travel data set does not provide information on “thru-travelers” proceeding on to secondary and tertiary destinations from their U.S. port-of-entry. This likely leads to overestimated numbers for large international airports and underestimated numbers at smaller domestic airports. Additionally, though there are high numbers of travelers crossing the U.S.-Mexico border, many of the same individuals cross regularly, so actual unique individuals crossing is lower. As of February 2016 no Mexican cities along the border had local ZIKAV transmission, which suggests a low probability of introduction. Finally, our study region does not include non-contiguous U.S. states and territories where risk for transmission of Aedes-transmitted viruses is high: Hawaii, Puerto Rico, and areas in the South Pacific.
Though we do not explicitly address whether the potential abundance of Ae. aegypti may be lower or higher than normal in the forthcoming months of 2016 due to anomalous meteorological conditions (perhaps linked to the strong El Niño conditions occurring at the time of writing), it is noteworthy that a seasonal climate forecast initialized in February 2016 and valid for June-August 2016 suggests a 40-45% probability of above-normal temperatures over the entire contiguous U.S. for the upcoming summer of 201671. This is compared to a 20-25% probability of below-normal temperatures, and a 35% probability of normal conditions. Therefore, it is possible that above-normal temperatures will lead to increased suitability for Ae. aegypti throughout much of the U.S. in summer 2016, though in some of the hottest regions of Texas, Arizona and California above-normal temperatures may lead to decreased suitability.
Despite the limitations, our analysis is a step towards simultaneously mapping the geographic and seasonal suitability of the vector mosquito Ae. aegypti in the contiguous United States. There is a need for enhanced, long-term, nationally-coordinated, local-level surveillance of both Aedes mosquitoes and Aedes-transmitted viruses, particularly in areas where simulations indicate Ae. aegypti populations may be high and coincide with more frequent travel between the U.S. and countries where Zika is circulating.
Competing Interests
The authors declare no competing interests.
Appendix
Acknowledgments
The authors thank Dr. Fred Gould (North Carolina State University) and Dr. Chet Moore (Colorado State University) for useful comments and suggestions.
Biographies
Dr. Andrew Monaghan is an atmospheric scientist at the National Center for Atmospheric Research (NCAR) in Boulder, Colorado. He is a guest researcher with the U.S. Centers for Disease Control and Prevention and a co-leader of the NCAR Weather, Climate and Health Program. His research interests include a broad range of interdisciplinary regional climate topics, with an emphasis on the use of model-based techniques to study climate-sensitive health and disease issues.
Dr. Mary Hayden (NCAR) is a behavioral scientist with over 15 years of experience working on weather, climate and health related linkages. She received her PhD in Health and Behavioral Sciences from the University of Colorado and is adjoint faculty at the University of Colorado School of Public Health as well as a Guest Researcher with the U.S. Centers for Disease Control and Prevention’s Division of Vector-Borne Diseases. Her primary research emphasis is on the human behavioral component of climate-sensitive health and disease issues, including community participatory research and the characterization of population vulnerability to weather and climate related health threats.
Geographer and Senior Research Scientist pursuing research on the applications of NASA remote sensing data to public health and climate change.
Kacey Ernst is an Associate Professor of Epidemiology and Biostatistics at the University of Arizona. Her research focuses on understanding the dynamics of vector-borne diseases in an effort to improve prevention and control strategies. Her work focuses primarily on malaria in western Kenya and dengue and Ae. aegypti dynamics in Mexico
Funding Statement
This research was supported by the National Institutes of Health (R01 AI091843/AI/NIAID; AI044793/AI/NIAID; R01 AI091980/AI/NIAID; P01 AI098670/AI/NIAID) and the National Aeronautics and Space Administration via an Early Career Investigator Award (NNX14AI89G) and a NASA Postdoctoral Program Fellowship. Funding was also provided by the NASA Marshall Space Flight Center Science Innovation Fund and NASA Center Innovation Fund projects. The National Science Foundation sponsors the National Center for Atmospheric Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Andrew J. Monaghan,
Cory W. Morin,
Daniel F. Steinhoff,
Olga Wilhelmi, .
Mary Hayden, .
Dale A. Quattrochi,
Michael Reiskind, .
Alun L. Lloyd,
Kirk Smith, .
Chris A. Schmidt,
Paige E. Scalf,
Kacey Ernst, .
References
- 1.Dick, G. W. A., Kitchen, S. F., & Haddow, A. J. (1952). Zika virus (I). Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene, 46(5), 509-520. [DOI] [PubMed]
- 2.Pierson, T. C., Diamond, M. S. (2013). Flaviviruses In: Knipe DM, Howley PM, editors. Fields Virology.
- 3.Lanciotti, R. S., Kosoy, O. L., Laven, J. J., Velez, J. O., Lambert, A. J., Johnson, A. J., ... & Duffy, M. R. (2008). Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging infectious diseases, 14(8), 1232. [DOI] [PMC free article] [PubMed]
- 4.Hayes, E. B. (2009). Zika virus outside Africa. Emerging infectious diseases, 15(9), 1347. [DOI] [PMC free article] [PubMed]
- 5.Cao-Lormeau, V.M., Roche, C., Teissier, A. et al. Zika virus, French polynesia, South pacific, 2013. Emerging Infectious Diseases, 20,1084-1086. [DOI] [PMC free article] [PubMed]
- 6.Besnard, M., Lastère, S., Teissier, A., Cao-Lormeau, V., & Musso, D. (2014). Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill, 19(13), 20751. [PubMed]
- 7.Zanluca, C., Melo, V. C. A. D., Mosimann, A. L. P., Santos, G. I. V. D., Santos, C. N. D. D., & Luz, K. (2015). First report of autochthonous transmission of Zika virus in Brazil. Memórias do Instituto Oswaldo Cruz, 110(4), 569-572. [DOI] [PMC free article] [PubMed]
- 8.Centers for Disease Control and Prevention (2016a, Jan 30). Zika virus. Retrieved from www.cdc.gov/zika.
- 9.Fauci, A. S., & Morens, D. M. (2016). Zika Virus in the Americas—Yet Another Arbovirus Threat. New England Journal of Medicine. doi:10.1056/NEJMp1600297. [DOI] [PubMed]
- 10.Cauchemez, S., Ledrans, M., Poletto, C., Quenel, P., De Valk, H., Colizza, V., & Boëlle, P. Y. (2014). Local and regional spread of chikungunya fever in the Americas. Euro surveillance: bulletin Europeen sur les maladies transmissibles= European communicable disease bulletin, 19(28), 20854. [DOI] [PMC free article] [PubMed]
- 11.Fischer, M., & Staples, J. E. (2014). Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR Morb Mortal Wkly Rep, 63(22), 500-1. [PMC free article] [PubMed]
- 12.Gubler, D. J. (2002). Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in microbiology, 10(2), 100-103. [DOI] [PubMed]
- 13.Lambrechts, L., Scott, T. W., & Gubler, D. J. (2010). Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis, 4(5), e646. [DOI] [PMC free article] [PubMed]
- 14.Grard, G., Caron, M., Mombo, I. M., Nkoghe, D., Ondo, S. M., Jiolle, D., ... & Leroy, E. M. (2014). Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus?. PLoS Negl Trop Dis, 8(2), e2681. [DOI] [PMC free article] [PubMed]
- 15.Hennessey, M., Fischer, M., & Staples, J. E. (2016). Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR. Morbidity and Mortality Weekly Report, 65(3), 5-58. [DOI] [PubMed]
- 16.Pan American Health Organization (2016, Jan 30). Epidemiological alert. Increase in microcephaly in the northeast of Brazil—epidemiological alert. Retrieved from http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en.
- 17.Schuler-Faccini, L. (2016). Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR. Morbidity and Mortality Weekly Report, 65(3), 59-62. [DOI] [PubMed]
- 18.Pan American Health Organization (2015, Dec 1). Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas—epidemiological alert. Retrieved from http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en.
- 19.Petersen, E. E. (2016). Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR. Morbidity and mortality weekly report, 65(2), 30-33. [DOI] [PubMed]
- 20.Kendrick, K., Stanek, D., Blackmore, C. (2014). Notes from the field: transmission of chikungunya virus in the continental United States—Florida, 2014. MMWR Morb Mortal Wkly Rep, 63(48), 1137. [PMC free article] [PubMed]
- 21.Trout, A., Baracco, G., Rodriguez, M., Barber, J., Leal, A., Radke, E., ... & Sauber-Schatz, E. (2010). Locally Acquired Dengue-Key West, Florida, 2009-2010. Morbidity and Mortality Weekly Report, 59(19), 577-581. [PubMed]
- 22.Ramos, M. M., Mohammed, H., Zielinski-Gutierrez, E., Hayden, M. H., Lopez, J. L. R., Fournier, M., ... & Waterman, S. H. (2008). Epidemic dengue and dengue hemorrhagic fever at the Texas–Mexico border: results of a household-based seroepidemiologic survey, December 2005. The American Journal of Tropical Medicine and Hygiene, 78(3), 364-369. [PubMed]
- 23.Reiter, P., Lathrop, S., Bunning, M., Biggerstaff, B., Singer, D., Tiwari, T., ... & Hayes, E. (2003). Texas lifestyle limits transmission of dengue virus. Emerging infectious diseases, 9(1), 86-89. [DOI] [PMC free article] [PubMed]
- 24.Brunkard, J. M., Robles Lopez, J. L., Ramirez, J., Cifuentes, E., Rothenberg, S. J., Hunsperger, E. A., ... & Haddad, B. M. (2007). Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis, 13(10), 1477-1483. [DOI] [PMC free article] [PubMed]
- 25.McCarthy, M. (2016). Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ, 352, i720. [DOI] [PubMed]
- 26.Koopman, J. S., Prevots, D. R., Mann, M. A. V., Dantes, H. G., Aquino, M. L. Z., Longini, I. M., & Amor, J. S. (1991). Determinants and predictors of dengue infection in Mexico. American journal of epidemiology, 133(11), 1168-1178. [DOI] [PubMed]
- 27.Eisen, L., & Lozano-Fuentes, S. (2009). Use of mapping and spatial and space-time modeling approaches in operational control of Aedes aegypti and dengue. PLoS Negl Trop Dis, 3(4), e411. [DOI] [PMC free article] [PubMed]
- 28.United States Census Bureau (2016a, Feb 20). Population Estimates: Metropolitan and Micropolitan Statistical Areas. Retrieved from: http://www.census.gov/popest/data/metro/totals/2014/.
- 29.Russell, R. C., Webb, C. E., Williams, C. R., & Ritchie, S. A. (2005). Mark–release–recapture study to measure dispersal of the mosquito Aedes aegypti in Cairns, Queensland, Australia. Medical and veterinary entomology, 19(4), 451-457. [DOI] [PubMed]
- 30.Cosgrove, B. A., Lohmann, D., Mitchell, K. E., Houser, P. R., Wood, E. F., Schaake, J. C., ... & Ming, J. (2003). Real‐time and retrospective forcing in the North American Land Data Assimilation System (NLDAS) project. Journal of Geophysical Research: Atmospheres, 108(D22).
- 31.Xia, Y., Mitchell, K., Ek, M., Sheffield, J., Cosgrove, B., Wood, E., ... & Mocko, D. (2012). Continental‐scale water and energy flux analysis and validation for the North American Land Data Assimilation System project phase 2 (NLDAS‐2): 1. Intercomparison and application of model products. Journal of Geophysical Research: Atmospheres, 117(D3).
- 32.Magori, K., Legros, M., Puente, M. E., Focks, D. A., Scott, T. W., Lloyd, A. L., & Gould, F. (2009). Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis, 3(9), e508. [DOI] [PMC free article] [PubMed]
- 33.Morin, C. W., Monaghan, A. J., Hayden, M. H., Barrera, R., & Ernst, K. (2015). Meteorologically driven simulations of dengue epidemics in San Juan, PR. PLoS Negl Trop Dis, 9(8), e0004002. [DOI] [PMC free article] [PubMed]
- 34.Semenov, M. A., & Stratonovitch, P. (2010). Use of multi-model ensembles from global climate models for assessment of climate change impacts. Climate research (Open Access for articles 4 years old and older), 41(1), 1.
- 35.Tebaldi, C., & Knutti, R. (2007). The use of the multi-model ensemble in probabilistic climate projections. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 365(1857), 2053-2075. [DOI] [PubMed]
- 36.Xu, C., Legros, M., Gould, F., & Lloyd, A. L. (2010). Understanding uncertainties in model-based predictions of Aedes aegypti population dynamics. PLoS Negl Trop Dis, 4(9), e830. [DOI] [PMC free article] [PubMed]
- 37.Tatem, A. J., Hay, S. I., & Rogers, D. J. (2006). Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences, 103(16), 6242-6247. [DOI] [PMC free article] [PubMed]
- 38.Lima, A., Lovin, D. D., Hickner, P. V., & Severson, D. W. (2016). Evidence for an Overwintering Population of Aedes aegypti in Capitol Hill Neighborhood, Washington, DC. The American journal of tropical medicine and hygiene, 94(1), 231-235. [DOI] [PMC free article] [PubMed]
- 39.Hahn, M. B., Monaghan, A. J., Hayden, M. H., Eisen, R. J., Delorey, M. J., Lindsey, N. P., ... & Fischer, M. (2015). Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. The American journal of tropical medicine and hygiene, 92(5), 1013-1022. [DOI] [PMC free article] [PubMed]
- 40.Moore, S. M., Eisen, R. J., Monaghan, A., & Mead, P. (2014). Meteorological influences on the seasonality of Lyme disease in the United States. The American journal of tropical medicine and hygiene, 90(3), 486-496. [DOI] [PMC free article] [PubMed]
- 41.Reiskind, M. H., & Lounibos, L. P. (2013). Spatial and temporal patterns of abundance of Aedes aegypti L.(Stegomyia aegypti) and Aedes albopictus (Skuse)[Stegomyia albopictus (Skuse)] in southern Florida. Medical and veterinary entomology, 27(4), 421-429. [DOI] [PubMed]
- 42.Florida Department of Health (2016, Feb 6). Mosquito-Borne Disease Surveillance. Retrieved from: http://www.floridahealth.gov/diseases-and-conditions/mosquito-borne-diseases/surveillance.html.
- 43.Focks, D. A., Haile, D. G., Daniels, E., & Mount, G. A. (1993). Dynamic life table model for Aedes aegypti (Diptera: Culicidae): simulation results and validation. Journal of Medical Entomology, 30(6), 1018-1028. [DOI] [PubMed]
- 44.Codeço, C. T., Lima, A. W., Araújo, S. C., Lima, J. B. P., Maciel-de-Freitas, R., Honório, N. A., ... & Valle, D. (2015). Surveillance of Aedes aegypti: Comparison of house index with four alternative traps. PLoS Negl Trop Dis, 9(2), e0003475. [DOI] [PMC free article] [PubMed]
- 45.Centers for Disease Control and Prevention (2016b, Jan 29). Zika Travel Information. Retrieved from: http://wwwnc.cdc.gov/travel/page/zika-information.
- 46.United States Department of Transportation (2016a, Jan 29). U.S. International Air Passenger and Freight Statistics Report. Retrieved from https://www.transportation.gov/policy/aviation-policy/us-international-air-passenger-and-freight-statistics-report.
- 47.United States Department of Transportation (2016b, Feb 24). T-100 International Segment Database (U.S. Carriers Only). Retrieved from: http://www.transtats.bts.gov/Fields.asp?Table_ID=307.
- 48.United States Department of Transportation (2016c, Feb 10). Border Crossing/Entry Data. Retrieved from: http://transborder.bts.gov/programs/international/transborder/TBDR_BC/TBDR_BC_Index.html.
- 49.United States Department of Transportation (2016d, Feb 24). North America Cruise Detail Data. Retrieved from: http://www.marad.dot.gov/wp-content/uploads/xls/north_america_cruise_detail_data.xls.
- 50.United States Geological Survey (2016, Feb 6). Disease Maps Dynamic Map Application. Retrieved from: http://diseasemaps.usgs.gov/mapviewer/.
- 51.Hotez, P. J., Murray, K. O., & Buekens, P. (2014). The Gulf Coast: a new American underbelly of tropical diseases and poverty. PLoS Negl Trop Dis, 8(5), e2760. [DOI] [PMC free article] [PubMed]
- 52.United States Energy Information Administration (2016, Feb 6). 2009 Residential Energy Consumption Survey Data. Retrieved at: https://www.eia.gov/consumption/residential/data/2009/.
- 53.Hotez, P. J. (2011). America’s most distressed areas and their neglected infections: the United States Gulf Coast and the District of Columbia. PLoS Negl Trop Dis, 5(3), e843. [DOI] [PMC free article] [PubMed]
- 54.Wescoat, J. L., Headington, L., & Theobald, R. (2007). Water and poverty in the United States. Geoforum, 38(5), 801-814.
- 55.United States Census Bureau (2016b, Feb 6). Small Area Income and Poverty Estimates: State and County Estimates 2014. Retrieved from: http://www.census.gov/did/www/saipe/data/statecounty/data/2014.html.
- 56.Kraemer, M. U., Sinka, M. E., Duda, K. A., Mylne, A. Q., Shearer, F. M., Barker, C. M., ... & Hay, S.I. (2015a). The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife, 4, e08347. [DOI] [PMC free article] [PubMed]
- 57.Kraemer, M. U., Sinka, M. E., Duda, K. A., Mylne, A., Shearer, F. M., Brady, O. J., ... & Hay, S. I. (2015b). The global compendium of Aedes aegypti and Ae. albopictus occurrence. Scientific data, 2. [DOI] [PMC free article] [PubMed]
- 58.Kraemer, M. U., Sinka, M. E., Duda, K. A., Mylne, A., Shearer, F. M., Brady, O. J., ... & Hay, S. I. (2015c). Data from: The global compendium of Aedes aegypti and Ae. albopictus occurrence. Dryad Digital Repository. Retrieved from http://dx.doi.org/10.5061/dryad.47v3c. [DOI] [PMC free article] [PubMed]
- 59.California Department of Public Health (2016, Feb 17). Aedes aegypti and Aedes albopictus mosquitoes detection sites in California, 2011-2015. Retrieved from https://www.cdph.ca.gov/HealthInfo/discond/Documents/AedesDistributionMap.pdf.
- 60.Brady, O. J., Golding, N., Pigott, D. M., Kraemer, M. U., Messina, J. P., Reiner Jr, R. C., ... & Hay, S. I. (2014). Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors, 7(338), 1-4. [DOI] [PMC free article] [PubMed]
- 61.Bogoch, I. I., Brady, O. J., Kraemer, M. U., German, M., Creatore, M. I., Kulkarni, M. A., ... & Khan, K. (2016). Anticipating the international spread of Zika virus from Brazil. The Lancet. [DOI] [PMC free article] [PubMed]
- 62.Lucio, P. S., Degallier, N., Servain, J., Hannart, A., Durand, B., de Souza, R. N., & Ribeiro, Z. M. (2013). A case study of the influence of local weather on Aedes aegypti (L.) aging and mortality. Journal of Vector Ecology, 38(1), 20-37. [DOI] [PubMed]
- 63.Capinha, C., Rocha, J., & Sousa, C. A. (2014). Macroclimate determines the global range limit of Aedes aegypti. EcoHealth, 11(3), 420-428. [DOI] [PubMed]
- 64.Khormi, H. M., & Kumar, L. (2014). Climate change and the potential global distribution of Aedes aegypti: spatial modelling using GIS and CLIMEX. Geospatial health, 8(2), 405-415. [DOI] [PubMed]
- 65.Parcher, J. W., & Humberson, D. G. (2007). CHIPS: a new way to monitor colonias along the United States-Mexico border (No. 2007-1230). Geological Survey (US).
- 66.Uejio, C. K., Hayden, M. H., Zielinski-Gutierrez, E., Lopez, J. L. R., Barrera, R., Amador, M., ... & Waterman, S. H. (2014). Biological Control of Mosquitoes in Scrap Tires in Brownsville, Texas, USA and Matamoros, Tamaulipas, Mexico. Journal of the American Mosquito Control Association, 30(2), 130-135. [DOI] [PubMed]
- 67.Gubler, D. J., & Clark, G. G. (1996). Community involvement in the control of Aedes aegypti. Acta tropica, 61(2), 169-179. [DOI] [PubMed]
- 68.Eisen, L., & Moore, C. G. (2013). Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range. Journal of medical entomology, 50(3), 467-478. [DOI] [PubMed]
- 69.Rey, J. R., Nishimura, N., Wagner, B., Braks, M. A., O'Connell, S. M., & Lounibos, L. P. (2006). Habitat segregation of mosquito arbovirus vectors in south Florida. Journal of Medical entomology, 43(6), 1134-1141. [DOI] [PMC free article] [PubMed]
- 70.Leisnham, P. T., LaDeau, S. L., & Juliano, S. A. (2014). Spatial and temporal habitat segregation of mosquitoes in urban Florida. PloS one, 9(3), e91655. [DOI] [PMC free article] [PubMed]
- 71.International Research Center for Climate and Society (2016, Feb 19). Seasonal Climate Forecasts. Retrieved from http://iri.columbia.edu/our-expertise/climate/forecasts/seasonal-climate-forecasts/.
- 72.Hills, S. L., Russell, K., Hennessey, M., Williams, C., Oster, A. M., Fischer, M., and Mead, P. (2016). Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission—Continental United States, 2016. MMWR. Morbidity and Mortality Weekly Report, 65. [DOI] [PubMed]
- 73.Beebe, N. W., Cooper, R. D., Mottram, P., & Sweeney, A. W. (2009). Australia's dengue risk driven by human adaptation to climate change. PLoS Negl Trop Dis, 3(5), e429. [DOI] [PMC free article] [PubMed]