Abstract
Background
Skin sensitizers induce allergic reactions through the induction of reactive oxygen species. Allyl nitrile from cruciferous vegetables has been reported to induce antioxidants and phase II detoxification enzymes in various tissues. We assessed the effects of repeated exposure to allyl nitrile on sensitizer-induced allergic reactions.
Material/Methods
Mice were dosed with allyl nitrile (0–200 μmol/kg), and then received a dermal application of 1 of 3 sensitizers on the left ear or 1 of 2 vehicles on the right ear. Quantitative assessment of edema was carried out by measuring the difference in weight between the portions taken from the right and left ears. We tested enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and thiobarbituric acid reactive substances (TBARS) in ears.
Results
Repeated exposure to allyl nitrile reduced edemas induced by glutaraldehyde and by 2, 4-dinitrochlorobenzene (DNCB), but not by formaldehyde. The repeated exposure decreased levels of TBARS, a marker of oxidative stress, induced by glutaraldehyde and by DNCB, but not by formaldehyde. Allyl nitrile elevated SOD levels for the 3 sensitizers, and CAT levels for formaldehyde and DNCB. Allyl nitrile also increased GPx levels for formaldehyde and DNCB, but not for glutaraldehyde. The reduced edemas were associated with changes in oxidative stress levels and antioxidant enzymes.
Conclusions
Repeated exposure to allyl nitrile reduced allergic reactions induced by glutaraldehyde and by DNCB, but not by formaldehyde. This reduction was associated with changes in ROS levels and antioxidant enzyme activities.
MeSH Keywords: Allyl Compounds, Antioxidants, Edema, Haptens, Skin Abnormalities
Background
The skin, a major site of defense against pathogenic agents, is exposed to a large number of environmental chemicals, some of which induce allergic reactions (sensitizers). The potential for skin sensitization from workplace chemicals is an ongoing concern. To date, great efforts have been made to identify and quantitatively assess skin sensitizers [1,2], in view of the risk of unexpected exposure to identified and unidentified sensitizers in the workplace.
Skin sensitizers induce allergic reactions through the induction of reactive oxygen species (ROS) [3], a major source of toxicity [4]. After absorption, skin sensitizers induce oxidative stress in keratinocytes, leading to ROS formation. In the mouse ear swelling test, the sensitizer 2,4-dinitrochlorobenzene (DNCB) has been shown to induce a significant increase in ear thickness in Nrf2−/− compared with wild-type mice [5]. In a study on the mechanism involved in particulate matter contributing to the increased incidence of asthma and allergic conditions [6], H2O2 production was significantly higher in particulate-stimulated Nrf2−/− dendritic cells than in the Nrf2+/+ counterparts. This indicates an important role for Nrf2 in skin sensitization.
Nrf2 activation is known to induce antioxidants and phase II detoxification enzymes, and can be achieved by the intake of cruciferous vegetables. Recent studies have shown that cruciferous vegetables contain glucosinolates relevant to human health [7–10]. Sinigrin, one of the glucosinolates, is found in Brussels sprouts, horseradish, mustard, and broccoli [11,12]. The hydrolysis of sinigrin by the plant enzyme myrosinase results in the generation of bioactive compounds, including allyl isothiocyanate and allyl nitrile [7], and these products are thought to be responsible for a positive effect on health. Isothiocyanates have been shown to induce phase II enzymes [8,13,14], while allyl nitrile induces enzymes in the stomach, small intestine, urinary tract, kidneys, lungs, rectum, and brain [10,15,16]. Although allyl nitrile appears to upregulate the enzymes in the body, little is known about its effect on the skin.
In the present study, we hypothesized that allyl nitrile protects against sensitizer-induced allergic reactions to some extent. To test this, we examined the relationship between inflammation (mouse ear swelling) and antioxidant defense in the skin of mice that were exposed to allyl nitrile and then received a dermal sensitizer on the ear. We focused on oxidative stress and the antioxidants superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx): SOD catalyzes the dismutation of the superoxide anion to molecular oxygen and H2O2, playing a crucial role in the cellular antioxidant defense system; CAT efficiently promotes the conversion of H2O2 to water and molecular oxygen; and GPx scavenges H2O2 and organic hydroperoxides by using glutathione as a hydrogen donor. To explore the relationship, we used 3 skin sensitizers: formaldehyde (potency category, strong), glutaraldehyde (strong), and DNCB (extreme) [17].
Material and Methods
Materials
Allyl nitrile (3-butenenitrile, CAS 109-75-1) and DNCB (CAS 97-00-7) were purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan), and olive oil (CAS 8001-25-0) and formaldehyde solution (CAS 50-00-0) were from Wako Pure Chemical Industries (Osaka, Japan). Acetone (CAS 67-64-1), H2O2 (CAS7722-84-1), glutaraldehyde (CAS 111-30-8), and other chemicals were purchased from Nacalai Tesque (Kyoto, Japan).
Animals and treatment
Animal experiments were carried out according to the Guidelines of the Committee on Animal Experimentation of Kanazawa University. Male ddY mice weighing 26–30 g were obtained from Japan SLC Co. (Shizuoka, Japan), and were maintained at 22±2°C under a 12/12 h light/dark cycle with free access to tap water and laboratory food (CRF-1; Charles River Japan Inc., Yokohama, Japan). The ddY mice, a closed colony, have been used in our previous studies [10,15,16].
Groups of 6 animals were given subtoxic doses of allyl nitrile (50, 100, or 200 μmol/kg) or vehicle-distilled water (4 mL/kg) daily for 8 days by gastric intubation, on the basis of our previous findings [16]. On days 6, 7, and 8, the animals received 1 of 3 sensitizers (formaldehyde, glutaraldehyde, or DNCB) on the left ear and a vehicle (acetone or acetone-olive oil (4:1) [AOO]) on the right ear. The applications to the ear were 40 μL of 20% formaldehyde in acetone, 5% glutaraldehyde in acetone, and 1.5% DNCB in AOO, acetone, and AOO. The concentration of the sensitizers was determined such that each sensitizer would induce a similar ear edema (noted by a similar increase in weight). The animals were sacrificed on day 9 for all analyses.
Assessment of ear edema
Mice were anesthetized with 100 mg/kg sodium pentobarbital and a 63.3 mm2 tissue punch was taken from both the right and left ears to evaluate edema. Quantitative assessment of edema was carried out by measuring the difference in weight between the portions taken from the right and left ears. The ears were stored at −80°C until biochemical analyses.
Tissue preparation and biochemical assays
Tissue punches were homogenized in 1 mL of buffer (0.01 M Tris-HCl buffer, pH 7.4). The homogenates were centrifuged at 10 000 g at 4°C for 20 min, and the resulting cytosolic fractions were stored at −80°C until biochemical analyses. Protein concentrations were measured according to the Bradford method [18] using bovine serum albumin as the standard.
CAT activity was measured using the method of Abei [19], using H2O2 as the substrate. GPx activity was measured using the spectrophotometric method of Paglia and Valentine [20], using cumene hydroperoxide as the substrate and NADPH as the source of reducing equivalents. Thiobarbituric acid reactive substances (TBARS) and SOD levels were determined with commercial assay kits (Cayman Chemical Company, Ann Arbor, MI, USA).
Data analysis
We performed analysis of variance (ANOVA), followed by Fisher’s least significant difference test for multiple comparisons. The level of significance was set at p<0.05.
Results
Edematous responses to sensitizers in mice that received repeated exposure to allyl nitrile
Application of sensitizers (formaldehyde, glutaraldehyde, and DNCB) to the ear for 3 days induced edema, quantified by an increase in weight. No difference in the edema was observed between the 3 sensitizer groups and the controls (Figure 1A–1C). The repeated exposure to allyl nitrile had no observable effect on the edema induced by formaldehyde (Figure 1A). The repeated exposure, however, was correlated with a decrease in the edemas induced by glutaraldehyde and by DNCB at the levels of 50 and 100 μmol/kg/day (Figure 1B, 1C).
Figure 1.
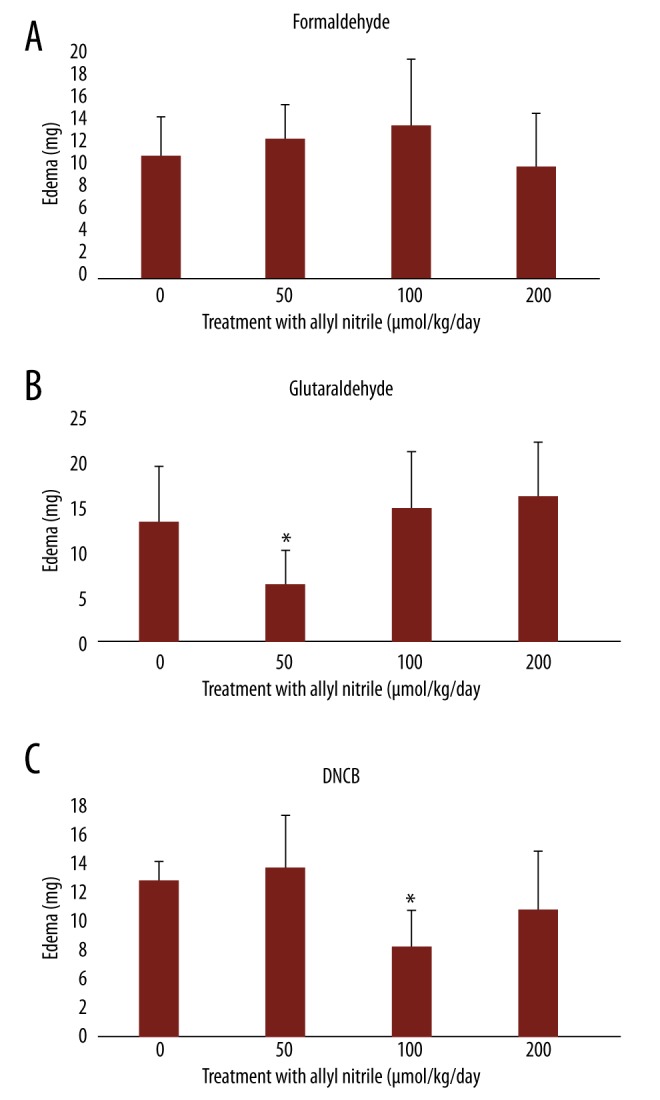
Edematous response to formaldehyde (A), glutaraldehyde (B), and DNCB (C) application to the ear. Values represent mean ±SD for 6 mice. Values with an asterisk are significantly different (p<0.05) from the control (0 μmol/kg/day).
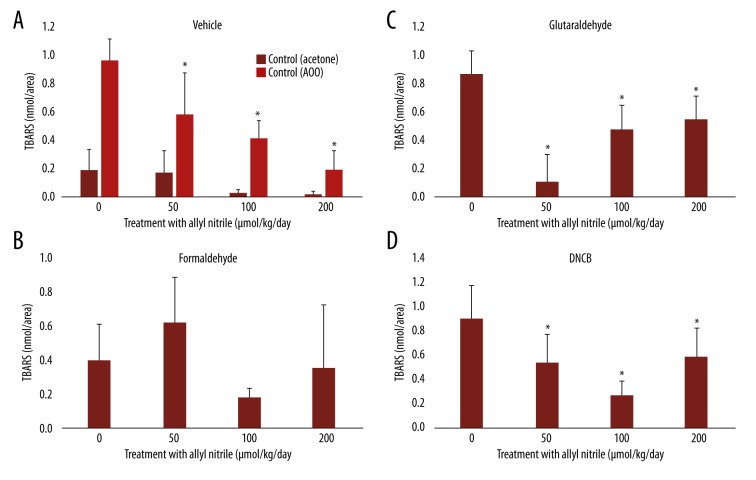
TBARS levels after sensitizer application
TBARS measurement was performed to assess oxidative stress after application of the vehicles and sensitizers. Lower levels were seen at 100 and 200 μmol/kg/day for the vehicle acetone, and at 50, 100, and 200 μmol/kg/day for the AOO vehicle, compared with the controls (p<0.05, vs. the vehicle acetone) (Figure 2A). The TBARS levels for formaldehyde did not vary at 0–200 μmol/kg/day (Figure 2B). The levels were decreased at 50, 100, and 200 μmol/kg/day for both glutaraldehyde and DNCB, the lowest being at 50 and 100 μmol/kg/day (Figure 2C, 2D). The level at 0 μmol/kg/day for glutaraldehyde was higher than that for acetone (p<0.05) (Figure 2A, 2C).
Figure 2.
Thiobarbituric acid reactive substances (TBARS) levels in the ears of allyl nitrile-treated mice after application of a vehicle (acetone or AOO) (A), or the sensitizers formaldehyde (B), glutaraldehyde (C), or DNCB (D). Values (nmol/63.3 mm2) represent mean ±SD for 6 animals. Values with an asterisk are significantly different (p<0.05) from the control (0 μmol/kg/day).
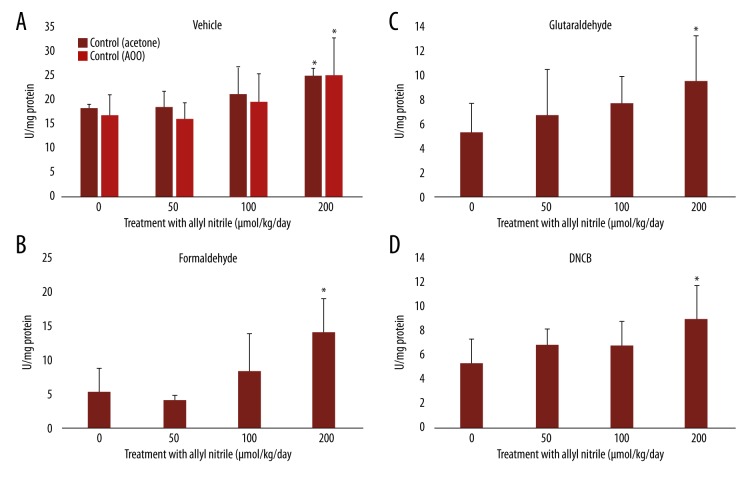
SOD levels after sensitizer application
Enhanced SOD activities were observed at 200 μmol/kg/day for both acetone and AOO (Figure 3A). Elevated activities were recorded at 200 μmol/kg/day for formaldehyde, glutaraldehyde, and DNCB, with lower activities at 0 μmol/kg/day (p<0.05, vs. corresponding vehicle at 0 μmol/kg/day) (Figure 3A–3D).
Figure 3.
Superoxide dismutase (SOD) levels in the ears of allyl nitrile-treated mice after application of a vehicle (acetone or AOO) (A), or the sensitizer formaldehyde (B), glutaraldehyde (C), or DNCB (D). One unit (U) of SOD is defined as the quantity of enzyme needed to manifest a 50% dismutation of the superoxide radicals. Values represent mean ±SD for 6 mice. Values with an asterisk are significantly different (p<0.05) from the control (0 μmol/kg/day).
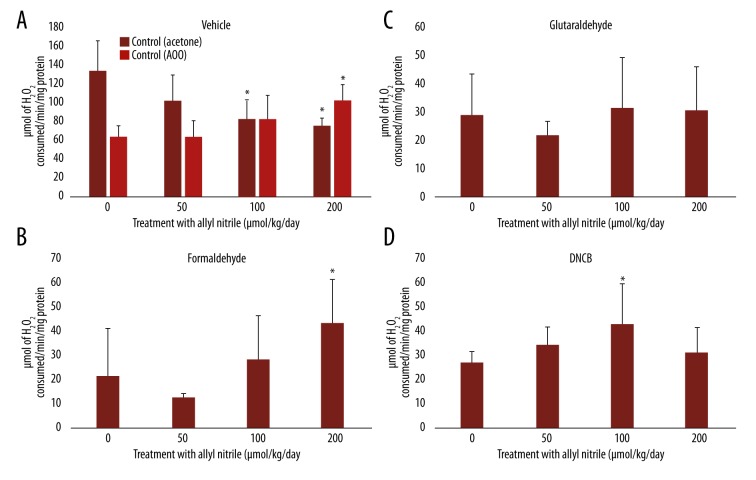
CAT levels after sensitizer application
Decreased CAT activities were observed at 100 and 200 μmol/kg/day for the vehicle acetone, with higher activity at 0 μmol/kg/day (p<0.05, vs. the vehicle AOO), while enhanced activity was recorded at 200 μmol/kg/day for the vehicle AOO (Figure 4A). For formaldehyde and DNCB, enhanced activities were observed at 200 and 100 μmol/kg/day, with lower activities at 0 μmol/kg/day (p<0.05, vs. corresponding vehicle at 0 μmol/kg/day) (Figure 4A, 4B, 4D). No change in activity was recorded for glutaraldehyde, with decreased levels at 0 μmol/kg/day (p<0.05, vs. the vehicle acetone at 0 μmol/kg/day) (Figure 4A, 4C).
Figure 4.
Catalase (CAT) levels in the ears of allyl nitrile-treated mice after application of a vehicle (acetone or AOO) (A), or the sensitizer formaldehyde (B), glutaraldehyde (C), or DNCB (D). Values represent mean ±SD for 6 mice. Values with an asterisk are significantly different (p<0.05) from the control (0 μmol/kg/day).
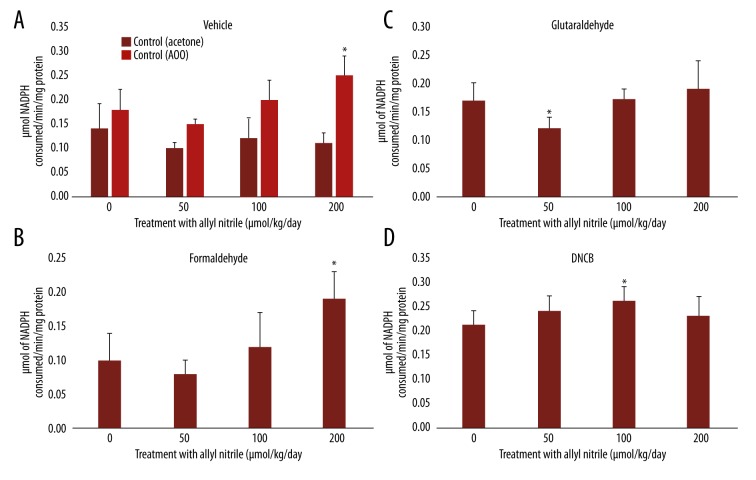
GPx levels after sensitizer application
No change in GPx activity was recorded at 0–200 μmol/kg/day for acetone, while an increase in activity was seen at 200 μmol/kg/day for AOO (Figure 5A). Elevated GPx activity was observed at 200 μmol/kg/day for formaldehyde (Figure 5B). Decreased activity was seen at 50 μmol/kg/day for glutaraldehyde (Figure 5C), and elevated levels at 100 μmol/kg/day for DNCB (Figure 5D).
Figure 5.
Glutathione peroxidase (GPx) levels in the ears of allyl nitrile-treated mice after application of a vehicle (acetone or AOO) (A), or the sensitizer formaldehyde (B), glutaraldehyde (C), or DNCB (D). Values represent mean ±SD for 6 mice. Values with an asterisk are significantly different (p<0.05) from the control (0 μmol/kg/day).
Discussion
We showed that repeated exposure to allyl nitrile can reduce allergic reactions to glutaraldehyde and DNCB, as measured by an edematous response, depending on exposure levels. Allyl nitrile did not reduce allergic reactions to formaldehyde. The protective effect was associated with decreased oxidative stress and with modified activities of antioxidant enzymes. These results suggest that edema induced by sensitizers can be reduced by allyl nitrile-modulated antioxidants. Additionally, we noted that AOO and acetone are not interchangeable.
Edemas induced by glutaraldehyde and DNCB were decreased by exposure to allyl nitrile, but not edemas induced by formaldehyde, although almost the same degree of edema was induced by each sensitizer. Allyl nitrile may be acting by modulating the activity of antioxidant enzymes. In glutaraldehyde-treated ears, allyl nitrile at 50 μmol/kg/day reduced edema, oxidative stress, and GPx levels, while levels of SOD increased and levels of CAT decreased. This may indicate the achievement of a particular H2O2 level. In DNCB-treated ears, allyl nitrile at 100 μmol/kg/day reduced edema and oxidative stress, and enhanced CAT, GPx, and SOD levels, indicating again that a particular H2O2 level was achieved. In formaldehyde-treated ears, we did not find any changes in edema, oxidative stress, or antioxidant enzymes, except at 200 μmol/kg/day; therefore, we assume that allyl nitrile could not affect the H2O2 levels.
ROS in high concentrations, including H2O2, promote cellular damage and tissue destruction, but recent findings suggest that ROS can act to fine-tune the inflammatory response [21]. H2O2 has been shown to have an effect on Nrf2, upregulating phase II enzyme expression [22]. After skin absorption, sensitizers may produce ROS. Repeated exposure to allyl nitrile may modify antioxidant activity, leading to a particular, low H2O2 level and reducing the edematous response.
Repeated exposure to allyl nitrile exhibited effects on CAT and GPx activities correlated by vehicle: Acetone decreased CAT activities while AOO increased them; GPx activities remained unchanged with acetone, but were enhanced under AOO. We encountered another difference between the vehicles in terms of their effects on TBARS and CAT levels in the control groups (Figure 2A, 4A): Acetone was correlated with lower levels of TBARS and higher levels of CAT, compared with AOO. The differences may be due to compounds in the olive oil [23]. Acetone and AOO have both been used as vehicles for sensitizers [17]. The vehicle itself may induce alterations in the allergic reaction process. We observed that the 2 vehicles contributed to different levels of CAT activity, which in turn would affect H2O2 levels.
Cruciferous vegetables have been shown in epidemiological studies to have a beneficial effect on health; their consumption may be inversely associated with the risk of various cancers [24–26]. Cruciferous allyl nitrile is thought to contribute to this preventive effect by upregulating antioxidants and phase II detoxification enzymes in various tissues [10,15,16]. The present study provides evidence that repeated exposure may protective against allergic reactions. In addition, it has been shown that allyl isothiocyanate has an anti-inflammatory potential in cultured macrophages, which may be mediated by Nrf2 and nuclear factor κB signaling pathways, but has little anti-inflammatory activity in mice [27]. Overall, higher intake of vegetables rich in sinigrin may be beneficial to individuals exposed to common industrial chemicals in the workplace [11,12].
There are some limitations to the study. The exposure was repeated for 8 days, but the study could be repeated with a one-time exposure to allyl nitrile. This could yield valuable information on the dose and frequency required for allyl nitrile consumption to have protective benefits. Further, this study measured the immune response solely by weight of edematous tissues. A future study could measure levels of specific immune factors to determine whether these factors exert a greater effect on edema reduction than the antioxidants do.
Conclusions
Repeated exposure to allyl nitrile reduced allergic reactions induced by glutaraldehyde and by DNCB, but not by formaldehyde. This reduction was associated with changes in ROS levels and antioxidant enzyme activities. The results suggest that SOD may be involved in allyl nitrile-modified reduction of the edematous response, while CAT and GPx may be involved in modulating the response. The 2 vehicles we used, acetone and AOO, did not result in comparable levels of oxidative stress and antioxidant enzymes.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: This work was supported by JSPS KAKENHI Grant Number 26460795
References
- 1.Dearden JC, Hewitt M, Roberts DW, et al. Mechanism-based QSAR modeling of skin sensitization. Chem Res Toxicol. 2015;28:1975–86. doi: 10.1021/acs.chemrestox.5b00197. [DOI] [PubMed] [Google Scholar]
- 2.OECD guidelines for the testing of chemicals No.442D. 2015. OECD: Test guideline on an in vitro skin sensitization: ARE-Nrf2 luciferase test method. [Google Scholar]
- 3.Corsini E, Galbiati V, Nikitovic D, Tsatsakis AFM. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol. 2013;61:74–81. doi: 10.1016/j.fct.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- 5.Ali ZE, Gerbeix C, Hemon P, et al. Allergic skin inflammation induced by chemical sensitizers is controlled by the transcription factor Nrf2. Toxicol Sci. 2013;134:39–48. doi: 10.1093/toxsci/kft084. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Rangasamy T, Bauer SM, et al. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–59. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey JW, Zelcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 8.Munday R, Munday C. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–71. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 9.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 10.Tanii H, Higashi T, Nishimura F, et al. Induction of detoxication enzymes in mice by naturally occurring allyl nitrile. J Agric Food Chem. 2005;53:8993–96. doi: 10.1021/jf0516282. [DOI] [PubMed] [Google Scholar]
- 11.Steinbrecher A, Linseisen J. Dietary intake of individual glucosinolates in participants of the EPIC-Heidelberg cohort study. Ann Nutr Metab. 2009;54:87–96. doi: 10.1159/000209266. [DOI] [PubMed] [Google Scholar]
- 12.Tanii H, Takayasu T, Higashi T, et al. Allyl nitrile: Generation from cruciferous vegetables and behavioral effects on mice of repeated exposure. Food Chem Toxicol. 2004;42:453–58. doi: 10.1016/j.fct.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Hintze KJ, Keck AS, Finley JW, Jeffery EH. Induction of hepatic thioredoxin reductase activity by sulforaphane, both in Hepa1c1c7 cells and in male Fisher 344 rats. J Nutr Biochem. 2003;14:173–79. doi: 10.1016/s0955-2863(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Wang S, Howie AF, et al. Sulforaphane, erucin, and iberin upregulate thioredoxin reductase 1 expression in human MCF-7 cells. J Agric Food Chem. 2005;53:1417–21. doi: 10.1021/jf048153j. [DOI] [PubMed] [Google Scholar]
- 15.Tanii H, Higashi T, Saijoh K. Preconditioning with subneurotoxic allyl nitrile: Protection against allyl nitrile neurotoxicity. Food Chem Toxicol. 2010;48:750–54. doi: 10.1016/j.fct.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Tanii H, Higashi T, Nishimura F, et al. Effects of cruciferous allyl nitrile on phase 2 antioxidant and detoxication enzymes. Med Sci Monit. 2008;14(10):BR189–92. [PubMed] [Google Scholar]
- 17.Gerberick GF, Ryan CA, Kern PS, et al. A chemical dataset for evaluation of alternative approaches to skin-sensitization testing. Contact Dermatitis. 2004;50:274–88. doi: 10.1111/j.0105-1873.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Abei H. Catalase in vitro. Methods Enzymol. 1984;105:121–26. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 20.Paglia D, Valentine W. Studies on the quantitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 21.Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends immunol. 2009;30:201–8. doi: 10.1016/j.it.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Holland R, Navamal M, Velayutham M, et al. Hydrogen peroxide is a second messenger in phase 2 enzyme induction by cancer chemopreventive dithiolethiones. Chem Res Toxicol. 2009;22:1427–34. doi: 10.1021/tx900110n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen RW, Giacosa A, Hull WE, et al. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000;1:107–12. doi: 10.1016/s1470-2045(00)00015-2. [DOI] [PubMed] [Google Scholar]
- 24.Graham S, Dayal H, Swanson M, et al. Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst. 1978;61:709–14. [PubMed] [Google Scholar]
- 25.Haenszel W, Locke FB, Segi M. A case-control study of large bowel cancer in Japan. J Natl Cancer Inst. 1980;64:17–22. [PubMed] [Google Scholar]
- 26.Verhoeven DT, Goldbohm RA, van Poppel G, et al. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;9:733–48. [PubMed] [Google Scholar]
- 27.Wagner AE, Boesch-Saadatmandi C, Dose J, et al. Anti-inflammatory potential of allyl isothiocyanate – role of Nrf2, NF-κB and microRNA-155. J Cell Mol Med. 2012;16:836–43. doi: 10.1111/j.1582-4934.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]