Abstract
Introduction
Prior studies have suggested that low baseline quality of life (QOL) scores predict worse survival in patients undergoing non-small cell lung cancer (NSCLC) surgery. However, these studies involved average-risk patients undergoing lobectomy. We report QOL results from a multicenter trial, ACOSOG Z4032, which randomized high-risk operable patients to sublobar resection (SR) or sublobar resection with brachytherapy (SRB) and included longitudinal QOL assessments.
Methods
Global QOL using the SF-36, and dyspnea score using the University of California San Diego (UCSD) scale were measured at baseline, 3, 12 and 24 months. The SF36 physical component (PCS) and mental component (MCS) scores were standardized and adjusted for age/gender normals, with scores below 50 indicating below average health status. UCSD scores were transformed to 0-100 (poor-excellent) scale. Aims were to determine 1) the impact of baseline scores on recurrence-free (RFS), overall survival (OS) and 30-day adverse events (AE), and 2) identify subgroups (surgical approach, resection type. tumor location, tumor size, respiratory function) with a ≥10-point decline or improvement in QOL following SR.
Results
212 randomized eligible patients were included. There were no significant differences in baseline QOL scores between arms. Median baseline PCS, MCS and UCSD scores were 42.7, 51.1 and 70.8 respectively. There were no differences in Grade 3+ AEs, OS or RFS in patients with baseline scores ≤ median versus >median values, except for a significantly worse OS for patients with baseline UCSD scores ≤ median value. There were no significant differences between the study arms in percent change of QOL scores from baseline to 3, 12, or 24 months. Further comparison combining the two arms demonstrated a higher percentage of patients with a 10-point or worse decline in UCSD scores with segmentectomy compared to wedge resection (40.5% vs. 21.9%, p=0.03) at 12 months, with thoracotomy versus VATS (38.8% vs. 20.4%, p=0.03) at 12 months, and T1b vs. T1a tumors (46.9% vs. 23.5%, p=0.020 at 24 months. A 10-point or greater improvement in PCS was seen at 3 months with VATS versus thoracotomy (16.5% vs. 3.6%, p=0.02).
Conclusions
In high-risk operable patients, poor baseline QOL scores did not predict for worse OS or RFS or for higher risk for AEs following SR (with or without brachytherapy). VATS was associated with improvement in physical function at 3 months and improved dyspnea scores at 12 months, lending support for the preferential use of VATS when SR is undertaken.
Keywords: Lung Cancer, surgery, quality of life
Background
Sublobar resection (SR) has traditionally been used for high-risk operable patients with non-small cell lung cancer (NSCLC) when lobectomy is not considered feasible. More recently non-operative treatments such as stereotactic body radiation therapy (SBRT) or radiofrequency ablation (RFA) have been applied to this population, after successful application in medically inoperable patients (1,2). Standard outcome measures such as survival and recurrence rates are undoubtedly the most helpful measures to guide physicians in making treatment recommendations. Quality of life (QOL), however, is an important variable that has rarely been measured in these clinical trials, but is of tremendous significance, particularly when treating a high-risk operable patient, often with emphysema and early stage lung cancer. American College of Surgeons Oncology Group (ACOSOG) Z4032 (Alliance) was a randomized trial that was undertaken to compare SR alone to sublobar resection with brachytherapy (SRB) for high-risk operable patients with early stage NSCLC. The primary endpoint was time to local recurrence between these two arms that utilized sublobar resection only in this high-risk operable population. This was not significantly different and has been reported elsewhere (3). A secondary aim of this study was to measure longitudinal QOL, and self-reported functional health status. We now report these outcomes in this manuscript.
Methods
Eligible patients for this study included patients with biopsy proven stage I lung cancers 3 cm or less in maximum diameter. Patients were defined as high-risk for lobectomy if they met at least one major criterion or two minor criteria (4). Patients were required to be evaluated by an ACOSOG-approved thoracic surgeon and considered either to not be a candidate for lobectomy (standard–risk operable patients), or to not be a candidate for any form of pulmonary resection (medically inoperable patients). To confirm that patients did not have nodal involvement, all suspicious lymph nodes seen on PET or CT scan required biopsy by mediastinoscopy, endobronchial ultrasound, or lymph node sampling at the time of resection. Wedge or segmental resection was allowed, and could be performed by video-assisted thoracic surgery (VATS) or thoracotomy. Two methods of brachytherapy were allowed (5, 6). In the first technique, polyglactin sutures containing 125I seeds [Oncura, Princeton, NJ] were placed parallel to and 5 mm away from the staple line on each side of the resection margin. The suture strands were fixed to the lung surface with several 3.0 silk or polyglactin sutures placed 1-2 cm apart. With the second brachytherapy technique a polyglycolic mesh implant was created. The same 125I suture strands were woven into a piece of vicryl mesh. The strands were placed at 1-cm intervals. The mesh was then sutured over the staple line. The dosimetry goal of the brachytherapy was to deliver 100 Gy at 5-7mm along the central axis of the resection margin.
Adverse events (AEs) were recorded using the Common Terminology Criteria (CTC) for Adverse Events Version 3.0 (7). A report of 30- and 90-day adverse events has previously been published from this study (8). There were no significant differences in grade 3 or higher AEs between the study arms.
Global QOL was measured using the short-form-36 (SF36), an instrument which has previously been reported and validated (9). This instrument is a 36-item survey that provides a measure of overall health status. Scores can be reported as 8 domains of functional health and well-being or transformed into a physical component summary (PCS) score and a mental component summary (MCS) score. In this study, SF36 results were expressed as PCS and MCS scores. Dyspnea was evaluated using the University of California San Diego Shortness of Breath Questionnaire (UCSD). This self-reported instrument that measures functional health status has also been validated in other studies (10). This is a 24-item disease-specific questionnaire that assesses self-reported shortness of breath while performing activities of daily living. QOL assessments using the SF36 and functional health status assessed using the UCSD were administered at baseline and at 3, 12, and 24 months.
All patients provided written informed consent before trial enrollment in accordance with applicable guidelines. At each participating site, Institutional Review Board approval was obtained in accordance with an assurance filed with and approved by the United States Department of Health and Human Services. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
Statistical Analysis
All randomized and eligible patients are included in the QOL analysis. The UCSD scores were converted to a percentage of theoretical range 0-100, with 0=poor, and 100=excellent. Eight subscale scores of SF36 were calculated by adding the subscale-related individual items and transforming to 0-100, with 0=poor, and 100=excellent. Standardized scores of SF36 physical component (PCS) and mental component (MCS) scores were calculated using the mean, standard deviation (SD) and scoring coefficients from the U.S. general population. The SF36 PCS and SF36 MCS standardized scores were then adjusted for age and gender using the mean and SD of U.S. general population according to age/gender grouping and employing a linear transformation (11, 12). Scores below 50 indicate below average health status. Compliance for SF36 and UCSD at each time point of assessment was defined as the percentage of eligible patients who filled out the questionnaire (any item on the SF36 and UCSD) among all evaluable (still on treatment) patients.
A clinically significant decline (improvement) in QOL was defined as a 10-point or greater decrease (increase) from baseline (13). In addition to considering the scores on a continuous scale, scores were also dichotomized using the sample median (≤median vs. > median). Scores at baseline, 3, 12, and 24 months as well as percent change in scores from baseline to 3, 12 and 24 months were compared between the arms using a Wilcoxon rank sum test. Baseline scores ≤ median versus those > median were compared between patients who had any grade 3 or above adverse event (G3+ AE) within 30 days versus none using Fisher's exact test. The 10-point decline in scores between the SR and the SRB arms from baseline to 3, 12 and 24 months were compared using a Fisher's exact test. Similar analyses were also carried out regardless of arm by: 1) resection type (wedge versus segmentectomy), 2) surgical approach (VATS versus thoracotomy), 3) clinical tumor size (≤ 2 cm versus > 2 cm), 4) lobe (upper versus other), 5) any G3+ AE within 30 days (yes versus no), 6) median of baseline DLCO% (>45 versus ≤45) and 7) median of baseline FEV1% (>49 vs. ≤49), to identify potentially vulnerable or successful subgroups. Finally, a generalized estimating equation (GEE) model was utilized to assess the impact of intervention arm and other baseline factors on longitudinal PCS, MCS and USCD scores.
Overall survival (OS) was defined as the time from randomization to death due to any cause. Recurrence=free survival (RFS) was defined as the time from randomization to the first of any recurrence or death from any cause. The distribution of survival times was estimated using the method of Kaplan-Meier, and Cox proportional hazards models (adjusted and unadjusted for treatment arm) were used to evaluate the prognostic importance of baseline PCS, MCS and UCSD (both as continuous as well as categorized at the median value) on OS and RFS. A landmark analysis at 3 and 12 months was also utilized to assess the impact of 10-point decline in QOL scores from baseline to 3 months, and baseline to 12 months on subsequent OS and RFS. Two-sided P-values ≤ 0.05 were considered statistically significant.
Results
Data was frozen for this analysis on July 15, 2013. A total of 224 patients were registered to Z4032. One patient from the SR arm had the intervention at a non-IRB approved hospital and was deemed not evaluable. One patient randomized to the SRB arm did not have surgery and was also not evaluable. An additional 10 registered (6 SR and 4 SRB) patients were found to be ineligible (see consort diagram). Thus, 212 patients (108 SR and 104 SRB) are included in this analysis. The completion rates for questionnaires at baseline, 3, 12 and 24 months for SR and SRB arm were 97.2% versus 99.0%, 82.4% versus 83.7%, 63.9% versus 74.0% and 46.3% versus 53.8%, respectively. The drop in completion rates over time by study subjects may have been related to responder fatigue. Table 1 provides the baseline patient characteristics, by arm. Median length of follow-up on alive patients was 4.4 years (range: 0.04 to 5.59).
Patient Consort Diagram.
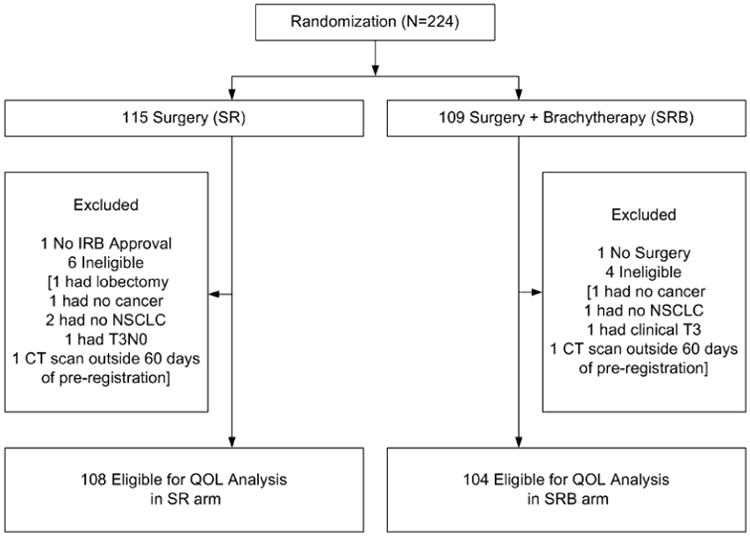
Table 1. Baseline patient characteristics.
| Factor | SR (N=108) | SRB (N=104) | P-value* |
|---|---|---|---|
|
| |||
| Age in years (median, range) | 70 (49 – 85) | 71 (50 – 87) | 0.47† |
| Sex | 0.89 | ||
| Female | 61 (56.5%) | 57 (54.8%) | |
| Male | 47 (43.5%) | 47 (45.2%) | |
| PS | 0.72 | ||
| 0 | 19 (17.6%) | 23 (22.1%) | |
| 1 | 63 (58.3%) | 58 (55.8%) | |
| 2 | 26 (24.1%) | 23 (22.1%) | |
| Clinical Nodule Size | 0.78 | ||
| <=2cm | 70 (64.8%) | 65 (62.5%) | |
| >2cm | 38 (35.2%) | 39 (37.5%) | |
| T Stage | 0.12 | ||
| T1 | 108 (100%) | 101 (97.1%) | |
| T2 | 0 (0%) | 3 (2.9%) | |
| T3 | 0 (0%) | 0 (0%) | |
| M Stage: M0 | 108 (100%) | 104 (100%) | NA |
| N Stage: N0 | 108 (100%) | 104 (100%) | NA |
| ASA class on surgery day** | 0.05 | ||
| I/II | 10 (9.3%) | 20 (19.2%) | |
| III/IV | 98 (90.7%) | 83 (79.8%) | |
| Baseline FEV1% (median, range)** | 48 (22 – 117) | 53 (25 – 110) | 0.31† |
| Baseline DLCO% (median, range)** | 46 (18 – 97) | 44 (8 -83) | 0.25† |
SR=Sublobar Resection; SRB=Sublobar Resection with Intraoperative Brachytherapy
: Fisher's Exact test;
: Wilcoxon rank sum test;
1 SRB with missing data;
3 SR and 2 SRB with missing data
The baseline median PCS, MCS and UCSD scores for the 212 patients were 42.7, 51.1 and 70.8, respectively. Sixty-five percent and 46.5% of patients in our study had baseline PCS and MCS scores that were at least 1 SD below the U.S. general population. Table 2 shows the standardized PCS and MCS scores based on age and sex grouping. PCS scores were at least 0.5 to 2 SD lower than U.S. general values for all groups. MCS scores were similar for most groups except for two groups where differences of 0.5 SD were seen.
Table 2.
Mean scores of subscales comparison by age/sex between Z4032 and normative data (Based on 1990 U.S. General Population Sample conducted by the National Opinion Research Center (11)). The PCS and MCS scores are standardized to a mean of 50 and standard deviation of 10.
| Physical Component Means* | Mental Component Means* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normative data (Mean) |
Z4032 data (Mean) |
Z4032 data (SD) |
Z4032 data (N) |
Difference in Means |
Normative data (Mean) |
Z4032 data (Mean) |
Z4032 data (SD) |
Z4032 data (N) |
Difference in Means |
||
| Male | 45-<55 | 50.40 | 28.68 | 2.82 | 3 | 2 SD | 51.03 | 45.36 | 2.32 | 3 | ½ SD |
| Male | 55-<65 | 46.90 | 38.02 | 11.18 | 21 | 1 SD | 51.60 | 50.99 | 8.32 | 21 | About the same |
| Male | >=65 | 41.95 | 36.55 | 10.73 | 64 | ½ SD | 52.51 | 51.47 | 10.19 | 64 | About the same |
| Female | 45-<55 | 48.95 | 34.07 | 10.68 | 7 | 1 ½ SD | 50.07 | 48.93 | 12.50 | 7 | About the same |
| Female | 55-<65 | 45.03 | 35.00 | 10.89 | 26 | 1 SD | 50.56 | 45.98 | 11.89 | 26 | ½ SD |
| Female | >=65 | 41.02 | 34.88 | 9.47 | 83 | ½ SD | 51.44 | 51.19 | 10.40 | 83 | About the same |
Standardized scores were provided, which involved the following: 1) Z scores was calculated for each of the eight subscales utilized the mean and SD of U.S. general population; 2) raw scores of physical component/mental component were calculated by the summation of the eights subscales' Z scores after multiplying each subscale score by the scoring coefficients from U.S. general population; 3) standardized scores were calculated by multiplying the raw scores by 10, then adding 50 to the scores.
Comparison of SRB and SR arms
Median PCS, MCS and UCSD scores at each time point for each arm are depicted in Figure 1 (a, b and c). There were no significant differences between arms at baseline, or at the 3-, 12- and 24-month time intervals. Based on the GEE models, while there was a significant trend over time in PCS (p = 0.05) and USCD (p < 0.01) scores, no significant differences by arm were observed for any of the scores (p for PCS = 0.74; p for MCS = 0.66; p for USCD = 0.48). The time trend was not significant when using data only up to 12 months. Additionally, there were no significant changes in the median % change in PCS, MCS and UCSD scores from baseline during follow-up. Therefore the arms are combined for further analysis.
Figure 1. Comparison of QOL scores at each time point, by arm.
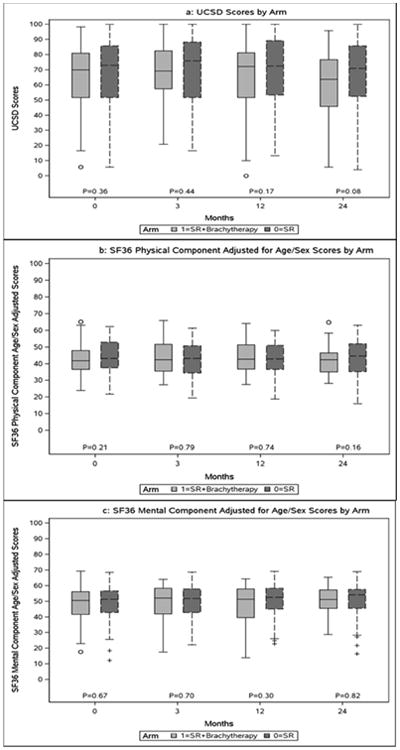
Longitudinal QOL (SF36) and functional health status (UCSD) for all patients
At 12 months, there was a significantly greater 10-point decline in UCSD scores for patients having segmental resection (40.5%) compared to wedge resection (21.9%) (p = 0.03), and for patients having a thoracotomy (38.8%) versus a VATS resection (20.4%) (p = 0.03). At 24 months, resection of tumors >2 cm (46.9%) was associated with a greater 10-point decline in UCSD scores than ≤2-cm (23.5%) tumors (p = 0.02). No subgroup was associated with any significant 10-point decline in PCS and MCS scores; however a10-point or greater improvement in PCS was seen at 3 months with VATS versus thoracotomy (16.5% vs. 3.6%, p=0.02). There were no significant (10-point or greater) declines in QOL scores during 3, 12, and 24 months of follow-up for patients with upper versus lower lobe resections, any G3+ AE within 30 days versus none, and for patients with baseline pulmonary function test scores below versus above median values.
Based on the GEE models, a significant time trend for PCS (p = 0.05) and USCD (p < 0.01) scores was still observed. However, this trend was not significant when using data only up to 12 months. In addition, patients with baseline DLCO% ≤45 had worsening of PCS (p < 0.01) and USCD (p = 0.01) scores over time.
Using baseline scores dichotomized at sample median (≤ median versus > median), there were no differences in the occurrence of grade 3 or higher AEs at 30 days. Overall survival was significantly worse for patients with baseline UCSD scores ≤ median value (see Figure 2). There were no significant different in OS or RFS by baseline PCS or MCS scores (both as a continuous as well as categorical at the median value). Table 3 shows the results of the landmark analysis for OS and RFS for 10-point decline in UCSD, PCS, and MCS scores from baseline to 3 and 12 months. Patients with a 10-point or higher decline in UCSD at 12-months had worse subsequent OS (HR: 2.10; 95%CI: 1.16, 3.81; p = 0.01). None of the others were significantly associated with subsequent OS or RFS.
Figure 2. Kaplan-Meier curve for overall survival by median values of baseline UCSD scores.
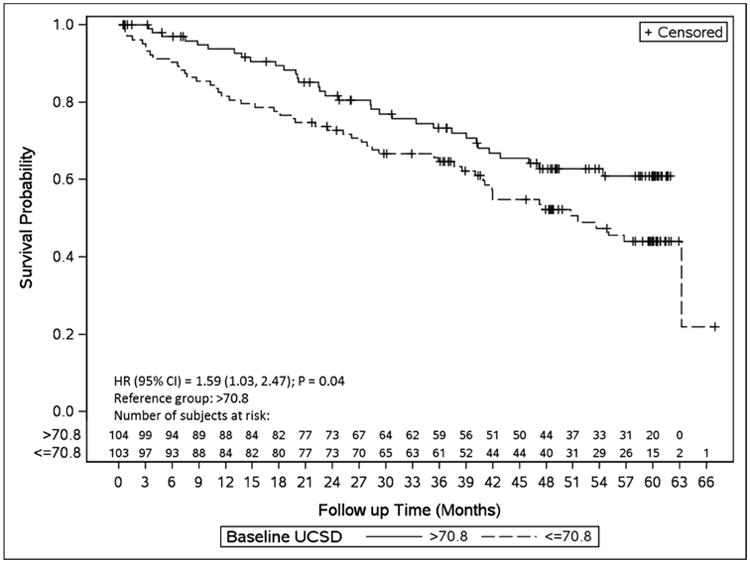
Table 3. Results of a landmark analysis for overall survival and recurrence-free survival using month 3 and month 12 QOL scores.
| UCSD | ||||
|---|---|---|---|---|
| Outcome | Month 3 | Month 12 | ||
| 10 point decline | *No 10 point decline | 10 point decline | *No 10 point decline | |
| Overall Survival | ||||
| N | 45 | 127 | 38 | 104 |
| Median (months) (95% CI) | 47.7 (30.4, NA) | 60.2 (53.7, NA) | 43.0 (16.3, NA) | NA (NA, NA) |
| HR (95% CI) | 1.57 (0.93, 2.65) | 2.10 (1.16, 3.81) | ||
| P-value | 0.09 | 0.01 | ||
| 3-year rate (95% CI) ** | 65. 2% (52.3, 81.1) | 78.3% (71.3, 86.1) | 63.9% (49.9, 81.8) | 87.2% (81.0, 94.0) |
| Recurrence free Survival | ||||
| N | 44 | 125 | 31 | 98 |
| Median (months) (95% CI) | 47.7 (34.1, NA) | 50.7 (38.1, NA) | 43.0 (23.4, NA) | 49.6 (41.7, NA) |
| HR (95% CI) | 1.03 (0.63, 1.68) | 1.39 (0.74, 2.61) | ||
| P-value | 0.90 | 0.30 | ||
| 2-year rate (95% CI) ** | 75.1% (63.4, 89.0) | 74.2% (66.9, 82.3) | 62.7% (49.0, 80.3) | 86.5% (80.2, 93.4) |
| SF36 Physical Component Sex/Age Adjusted | ||||
| Overall Survival | ||||
| N | 17 | 148 | 22 | 119 |
| Median (months) (95% CI) | NA (NA, NA) | 60.2 (50.6, NA) | NA (NA, NA) | 119NA (NA, NA) |
| HR (95% CI) | 1.06 (0.46, 2.47) | 1.51 (0.73, 3.13) | ||
| P-value | 0.89 | 0.26 | ||
| 3-year rate (95% CI) ** | 68.2% (48.6, 95.7) | 75.7% (68.9, 83.2) | 62.2% (44.6, 86.8) | 85.3% (79.0, 92.0) |
| Recurrence free Survival | ||||
| N | 15 | 147 | 15 | 113 |
| Median (months) (95% CI) | NA (NA, NA) | 50.6 (37.9, 58.6) | 34.6 (10.5, NA) | 49.6 (42.4, NA) |
| HR (95% CI) | 0.71 (0.29, 1.77) | 1.52 (0.68, 3.39) | ||
| P-value | 0.47 | 0.30 | ||
| 2-year rate (95% CI)** | 70.1% (51.2, 96.0) | 75.8% (69.1, 83.1) | 54.2% (36.8, 79.8) | 86.5% (80.6, 92.9) |
| SF36 Mental Component Sex/Age Adjusted | ||||
| Overall Survival | ||||
| N | 27 | 138 | 20 | 121 |
| Median (months) (95% CI) | 60.2 (21.6, 60.2) | NA (NA, NA) | NA (NA, NA) | NA (NA, NA) |
| HR (95% CI) | 1.40 (0.74, 2.65) | 1.37 (0.62, 3.07) | ||
| P-value | 0.30 | 0.44 | ||
| 3-year rate (95% CI) ** | 62.0.% (45.0, 85.5) | 77.3% (70.4, 84.8) | 72.9% (54.9, 96.6) | 83.2% (76.7, 90.2) |
| Recurrence free Survival | ||||
| N | 27 | 135 | 17 | 111 |
| Median (months) (95% CI) | 45.4 (14.8, NA) | 50.7 (38.6, NA) | NA (NA, NA) | 49.6 (41.7, NA) |
| HR (95% CI) | 1.38 (0.79, 2.42) | 0.80 (0.32, 2.01) | ||
| P-value | 0.26 | 0.63 | ||
| 2-year rate (95% CI) ** | 65.2% (49.1, 86.4) | 77.1% (70.3, 84.5) | 74.7% (57.7, 96.6) | 82.6 % (76.2, 89.7) |
Reference group; HR=Hazard ratio; CI=Confidence interval; P-value from Cox Model
Kaplan-Meier estimates using all data
Discussion
QOL and functional health status is rarely reported in surgical publications, yet it is an important metric that can be of use to physicians and patients when making treatment decisions. Previous reports in the thoracic literature have usually involved standard-risk operable patients (14, 15). A recent study of 245 patients treated with lobectomy or pneumonectomy measured QOL using the SF36 (15). In that study PCS score of less than 50, as well as age greater than 70 and DLCO less than 70% were associated with poor overall survival. The patients in our study represented a high-risk operable group who were considered poor candidates for lobar resection. It is noteworthy that the median ages of our patients were 70 and 71 years, and the median DLCO% were 46% and 44% for the sublobar and sublobar resection with brachytherapy arms, respectively.
Previous studies that measured QOL after lung resection have shown that thoracotomy is associated with a slower return to normal quality of life compared with VATS (14, 16). Studies have also shown that more complex resections such as pneumonectomy are also associated with worse postoperative QOL (16, 17). Although none of our patients underwent such complex resections, the use of a segmental resection rather than wedge, as well as a thoracotomy rather than VATS, were associated with a larger proportion of patients with significant declines in UCSD scores at the 12-month follow-up. In addition, in our series VATS was associated with more rapid improvement in PCS scores at 3 months following surgery. Although our results suggest that wedge resection may be preferential with respect to post-operative dyspnea, this has to be weighed against the oncological benefits that have been reported with segmentectomy over wedge in other studies (18).
Another finding from previous studies has been that QOL will usually fall, at least with respect to physical functioning, in the early postoperative period and then improve with time (16, 19). In one study, QOL decreased at 1 month but improved to baseline values by 3 months for lobectomy patients (16). This improvement was not seen after pneumonectomy. In another study that involved 156 patients treated with lobectomy or pneumonectomy, SF36 was measured preoperatively, and at 1 and 3 months postoperatively (19). PCS scores were significantly lower compared to baseline at 1 month. At 3 months scores had recovered. MCS scores were unchanged. In our study, no significant decline was seen. However, this may have been related to QOL being measured at 3 months rather than earlier after surgery.
We analyzed our data to see if low baseline QOL predicted poor survival. While low baseline PCS and MCS scores did not predict poor survival, low baseline UCSD scores did. As discussed above, previous studies in standard-risk operable patients have suggested that low PCS and DLCO% are associated with poor overall survival (15). A prospective study of 173 patients with clinical stage I or II NSCLC measured QOL preoperatively and serially after surgery for 2 years (17). Recurrence occurred in 36% at two years. QOL improved in those patients without recurrence, whereas in patients with recurrence there was some early recovery in QOL that subsequently deteriorated significantly after 1 month. As discussed above, our results suggest that in high-risk patients, baseline PCS and MCS are not good predictors of outcome.
The occurrence of post-operative complications could be postulated to predict for poor QOL scores at longer follow-up. Certainly this has been demonstrated in patients undergoing curative colorectal surgery (20). Interestingly in our study, there were no significant (10 point or greater) decline in PCS, MCS or UCSD scores at 3, 12 and 24 months between patients with and without grade 3 or higher AE within 30 days. We also performed a landmark analysis to determine whether post-operative scores could predict for poor outcome. A significant decline in PCS, MCS or UCSD at 3 months did not predict recurrence-free survival. However a 10-point drop in UCSD at 12 months did predict for poor subsequent overall survival.
Although QOL measurement may help surgeons decide optimal surgical therapy, it has even more relevance when considering surgical versus nonsurgical therapies for the high-risk patient with early stage lung cancer. A recent study of stereotactic body radiation therapy for medically inoperable lung cancer patients measured QOL before treatment and at 6 weeks and serially for 12 months. Interestingly, the mean FEV1 and DLCO percentages were 62.2% and 61.5%, respectively. A 10-point change or higher was considered significant. There was no significant decline in QOL measurements that included UCSD. However, the mean DLCO did drop significantly after SBRT from 61.5% to 44.8% (21).
In conclusion, we report QOL results from a prospective multicenter study of high-risk operable patients treated with sublobar resection. Global QOL measured with the SF36 and dyspnea measured with the UCSD did not deteriorate significantly after sublobar resection. Low baseline SF36 scores did not predict poor survival; however low UCSD scores at baseline as well as a significant decline in UCSD at 12 months did predict subsequent poor overall survival. There were advantages with respect minimizing postoperative dyspnea as measured by the UCSD, when VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy) were used. VATS was also superior to thoracotomy with improved PCS at 3-months lending support to the preferential use of VATS when sublobar resection is performed.
Acknowledgments
We thank the ACOSOG and Alliance staff, in particular the leadership of Heidi Nelson and David Ota for assistance in the completion of this project. We also thank all of the investigators and their site research teams who enrolled patients in this study. These were; University of Pittsburgh (Pittsburgh, PA), Mayo Clinic (Rochester, MN), Washington University (St Louis, MO), University of Virginia (Charlottesville, VA), Benedictine Hospital (Kingston, NY), University of Cincinnati (Cincinnati, OH), Jameson Hospital (New Castle, PA), University of Michigan (Ann Arbor, MI), Latter Day Saints Hospital (Salt lake City, UT), Memorial Medical Center (Springfield, IL), Rhode Island Hospital (Providence, RI), Valley Hospital (Ridgewood, NJ), William Beaumont Hospital (Royal Oak, MI), Northwestern University (Chicago, IL), Medical City Dallas (Dallas, TX), Allegheny Cancer Center Network (Pittsburgh, PA), Boston Medical Center (Boston, MA), City of Hope Medical Center (Duarte, CA), Portland Veterans Administration Medical Center (Portland, OR), University of Philadelphia (Philadelphia, PA), Virginia Mason Medical Center (Seattle, WA), Medical University of South Carolina (Charleston, SC), Memorial Hospital (Chattanooga, TN), South Nassau Community Hospital (Oceanside, NY), Southern Illinois University School of Medicine (Springfield, IL), Swedish Hospital (Seattle, OR), University of Tennessee(Knoxville, TN), Dartmouth Hitchcock Medical Center (Lebanon, NH), Emory University (Atlanta, GA), Fox Chase Cancer Center (Philadelphia, PA), Oregon Health Sciences University (Portland, OR), Vanderbilt University Medical Venter (Nashville, TN), Intermountain Medical Center (Murray, UT), London Health Sciences Centre (London, ON), Methodist Hospital (Houston, TX), Miami Valley Hospital (Dayton, OH), Monmouth Medical Center (Long Branch, NJ), Northshore University Health System (Evanston, IL), Providence Medical Center (Portland, OR), Roswell Park Cancer Institute(Buffalo, NY), Thomas Jefferson University Hospital (Philadelphia, PA)
Finally, we wish to thank the brave patients with non-small cell lung cancer and their caregivers who participated in this study.
Supported by NCI U10 grant # CA076001, and by an additional Grant from Oncura, Princeton, NJ. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). Presented at ASCO 2013, Chicago, IL
Footnotes
Presented at AATS 2014, Toronto, Canada
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Timmerman R, Paulus R, Galvin J, et al. Sterotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–45. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 3.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of brachytherapy on local recurrence rates after sublobar resection. Results from Z4032 (Alliance) a multicenter randomized trial. 2013 doi: 10.1200/JCO.2013.53.4115. Presented at ASCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of brachytherapy on local recurrence rates after sublobar resection. Results from ACOSOG Z4032 (Alliance), a Phase III randomized trial for high-risk operable non-small cell lung cancer (NSCLC) doi: 10.1200/JCO.2013.53.4115. Submitted to JCO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.d'Amato TA, Galloway M, Szydlowski G, et al. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest. 1998;114:1112–5. doi: 10.1378/chest.114.4.1112. [DOI] [PubMed] [Google Scholar]
- 6.Lee W, Daly BDT, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: Observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75:237–43. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 7.Common Terminology Criteria for Adverse Events (CTCAE) Version 3. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Jul, 2003. [Google Scholar]
- 8.Fernando HC, Landreneau RJ, Mandrekar SJ, Hillman SL, Nichols FC, Meyers B, DiPetrillo TA, Heron DE, Jones DR, Daly BD, Starnes SL, Tan A, Putnam JB. Thirty and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg. 2011;142(5):1143–51. doi: 10.1016/j.jtcvs.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware JE, Gandek B. Overview of the SF36 Health Survey and the International Quality of Life Assesment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 10.Swigris JJ, Han M, Vij R, et al. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respir Med. 2012;106:1447–55. doi: 10.1016/j.rmed.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thalji L, Haggerty CC, Rubin R, et al. 1990 National Survey of Functional Health Status, Final Report. Chicago, Ill: National Opinion Research Center; 1991. [Google Scholar]
- 12.Ware JE, Jr, Gandek B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) Project. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 13.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005;58:1217–9. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Handy JR, Jr, Asaph JW, Douville EC, et al. Does video assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg. 2010;37(2):451–5. doi: 10.1016/j.ejcts.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli A, Salati M, Refai M, et al. Development of patient-centered aggregate score to predict survival after lung resection for non-small cell lung cancer. J Thorac Cardiovasc Surg. 2013;146(2):385–90. doi: 10.1016/j.jtcvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Balduyck B, Hendriks J, lauwers P, et al. Quality of life evolution after lung cancer surgery : A prospective study in 100 patients. Lung Cancer. 2007;56(3):423–31. doi: 10.1016/j.lungcan.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non-small cell lung cancer. J Clin Oncol. 2008;26:233–241. doi: 10.1200/JCO.2006.07.7230. [DOI] [PubMed] [Google Scholar]
- 18.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14(8):2400–5. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 19.Brunelli A, Socci L, Refai M, et al. Quality of life before and after major lung resection for lung cancer: A prospective follow-up analysis. Ann Thorac Surg. 2007;84(2):410–6. doi: 10.1016/j.athoracsur.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Brown SR, Mathew R, Keding A, et al. The impact of post-operative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–23. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 21.Videtic GMM, Chandana AR, Sorenson L. A postoperative study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer. 2013;21:211–18. doi: 10.1007/s00520-012-1513-9. [DOI] [PubMed] [Google Scholar]


