Abstract
The oxytocin receptor is important in several domains of social behavior, and administration of oxytocin modulates social responding in several mammalian species, including humans. Oxytocin has both therapeutic and scientific potential for elucidating the neural and behavioral mechanisms governing social behavior. In the present study, operationally-defined aggressive behavior of six males with Antisocial Personality Disorder (ASPD) was measured following acute intranasal oxytocin dosing (12, 24, and 48 international units) and placebo, using a well-validated laboratory task of human aggression (Point-Subtraction Aggression Paradigm, or PSAP). The PSAP provides participants with concurrently available monetary-earning and operationally-defined aggressive response options, maintained by fixed ratio schedules of consequences. Shifts in response rates and inter-response time (IRT) distributions were observed on the aggressive response option following oxytocin doses, relative to placebo. Few changes were observed in monetary-reinforced responding. However, across participants the direction and magnitude of changes in aggressive responding were not systematically related to dose. No trends were observed between psychometric or physiological data and oxytocin dosing or aggressive behavior. While this report is to our knowledge the first to examine the acute effects of oxytocin in this population at high risk for violence and other forms of antisocial behavior, several limitations in the experimental design and the results cast the study as a preliminary report. Strategies for more extensive future projects are discussed.
Keywords: Aggression, Antisocial Personality Disorder, Social Behavior, Oxytocin
Introduction
Human social interaction involves complex and dynamic behavior patterns. One enduring and problematic form of social behavior is aggression. Aggression can be defined as the presentation of an aversive stimulus by one individual to another individual, who finds the aversive stimulus harmful and would seek to avoid it (Anderson & Bushman, 2002; Baron & Richardson, 1994). The consequences of aggression present considerable burdens on public health, criminal justice systems, and communities (Arseneault, Moffitt, Caspi, Taylor, & Silva, 2000; Kessler, Coccaro, Fava, Jaeger, Jin, & Walters, 2006; Rasmussen & Levander, 1996). Aggressive behavior is often heightened in individuals with Antisocial Personality Disorder (ASPD) and a history of substance use disorders (Alcorn III, Gowin, Green, Swann, Moeller, & Lane, 2013; Allen, Moeller, Rhoades, & Cherek, 1997; Bjork, Doughery, Moeller, Cherek, & Swann, 1999; Cherek, Moeller, Schann, & Doughterty, 1998; Cherek & Lane, 1999). Examination of behavioral and pharmacological modifiers of aggression in these high-risk individuals is of interest to scientific, therapeutic and public health endeavors (Patrick, 2008; Siever, 2008; Takahashi, Quadros, de Almeida, & Miczek, 2012).
In the search for effective interventions aimed at improving the social functioning of individuals with psychiatric disorders, the neuropeptide oxytocin (OT) and the oxytocinergic system may hold promise as an intervention strategy for promoting prosocial behaviors (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). OT is primarily synthesized in the nuclei of the hypothalamus (Lee, Macbeth, Pagani, & Young III, 2009a; Ludwig & Leng, 2006). Oxytocinergic axonal projections from the hypothalamus reach the prefrontal cortices, amygdala, and nucleus accumbens, which are important in modulating social behavior in human and nonhuman animals (Gimpl & Farhenholz, 2001; Lee et al., 2009a; Meyer-Lindenberg et al., 2011). OT administration increased prosocial behaviors of cooperation, trust, and generosity following acute administration to healthy adult humans (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Kosfeld, Neinrichs, Zak, Fischbacher, & Fehr, 2005; Rilling, DeMarco, Hackett, Thompson, Ditzen, Patel, & Pagnoni, 2012; Zak, Stanton, & Ahmadi, 2007). The prosocial effects of OT dosing in nonhuman animal studies suggest that this system underpins affiliative behaviors in both the social-bonds of monogamous rodents (Young, Gobrogge, Liu, & Wang, 2011) and parental care of rodents and primates (Alcorn III et al., 2013; Febo, Numan, & Ferris, 2005; Saltzman & Maestripieri, 2011).
In recent studies, OT was shown to facilitate social behavior in individuals with disorders characterized by deficits in social behavior and cognition (e.g. autism-spectrum disorders, frontotemporal-dementia, and schizophrenia) (Meyer-Lindenberg et al., 2011). Conversely, polymorphisms in OT receptor genes have been associated with extreme, persistent aggressive behavior during childhood (Malik, Zai, Abu, Nowrouzi, & Beitchman, 2012). To our knowledge, only one study has examined the potential impact of OT on human aggressive behavior. Campbell & Hausmann (2013) found evidence to suggest that OT administration might reduce aggressive behavior in women with high state anxiety. However, Campbell & Hausmann (2013) conducted a between groups design that used healthy women volunteers who were tested at one OT dose level and no main effect of dose was observed. Given the reported prosocial effects OT on the social behavior of in rodents, primates, healthy adults, and individuals with diagnosed psychiatric disorders, we sought to examine the potential impact of OT on aggressive behavior in individuals with ASPD and past substance use disorders. This combination of comorbidities represents the highest risk for violence and aggression (Alcorn III et al., 2013; Arseneault et al., 2000; Kessler et al., 2006; Rasmussen & Levander, 1996). Our study is the first placebo-controlled study using a well-established operant laboratory measure of human aggression (Point Subtraction Aggression Paradigm, PSAP) across a range of doses (12, 24, and 48 International Units, IU) to examine changes in aggressive behavior under OT dose. We hypothesized that OT would dose-dependently decrease aggressive responding compared to placebo, at both the molar and molecular level of behavior. To our knowledge this is the first study to address the possibility that the acute OT administration might alter aggressive behavior in individuals with ASPD and a history of substance use disorders (SUD).
Method
All experimental procedures within this study were reviewed and approved by the Institutional Review Board for the University of Texas Health Science Center-Houston, USA. Prior to study participation, informed consent was obtained from all participants.
Participants
Six adult males were recruited into the study via newspaper advertisements seeking male individuals on parole or probation. Newspaper advertisements were placed in freely distributed papers in the Houston metropolitan area. Individuals on parole or probation were recruited because the incidences of comorbid ASPD, past SUD and heightened aggressive are over represented in this population. Prior to study participation all participants underwent a physical exam to screen for exclusionary medical conditions (e.g. HIV, seizures, cardiovascular, kidney or endocrine diseases, diabetes, high blood pressure, and history of head trauma or loss of consciousness > 20 minutes) and current use of prescription medication. Female participants were excluded for the following reasons: (i) the male to female ratio for ASPD is 9 to 1, respectively (APA, 2000), thus female participants would be not representative of the target populations desired in this study, (ii) the neuropeptide oxytocin increases levels of luteinizing hormone (Evans, Reid, Wakeman, Croft, & Benny, 2003) which could potentially affect the regularly occurring menstrual cycles of female participants, and (iii) to our knowledge there are no reports of the interactions of oxytocin administration with oral contraceptives (i.e. birth control), thus behavioral and physiological side effects are unknown. Therefore, females were not recruited for both scientific and safety reasons.
Prior to study participation all participants underwent screening for current and past psychiatric illness using the Structured Clinical Interview of the DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1996) and the SCID-II NP (First, Spitzer, Gibbon, Williams, & Benjamin, 1997). The SCID-I and SCID-II were administered by a trained mental health professional. The SCID was used to screen for Axis I disorders and determine that participants met DSM-IV criteria for past SUD. Participants were excluded if they met DSM criteria for Axis I disorders other than past SUD. The SCID-II was used to ascertain that all participants met criteria for ASPD (i.e. childhood conduct disorder by age 15 and ASPD in adulthood). General cognitive capacity was assessed using The Shipley Institute of Living Scale-2 (Shipley, Gruber, Martin, & Klein, 2009) is a test of general cognitive aptitude consisting of a 40-item vocabulary test, a 26-item block test, and a 20-item abstraction test. Average composite verbal and block score on the Shipley-II was 176.16 (SD = ± 20.6: age-adjusted normative percentile with a Weschler Adult Intelligence Scale estimated mean of 86). All six participants were within two standard deviations of the Shipley-II normed means and had 12 or less years of education (range 9-12, see Table 1).
Participant demographics
| Participant | Age | Years of Education | Criminal History | Past SUDs |
|---|---|---|---|---|
| s13121 | 37 | 12 | aggravated assault, drug possession, theft |
a,b,e |
| s13146 | 24 | 9 | burglary parole/probation violation charge |
b |
| s13214 | 51 | 12 | Burglary | a,b |
| s13234 | 27 | 11 | Parole/probation violation |
a,c,d,g,h |
| s13246 | 28 | 12 | drug possession | b,d,g,h |
| s13285 | 46 | 11 | aggravated assault | a,b,c,h |
= alcohol;
= cannabis;
= cocaine;
= hallucinogen;
= inhalant;
= opiate;
= sedative
To avoid potential interactions between OT and extraneous drug use and alcohol intoxication during study participation, participants with any current SUD were excluded, and all participants had to provide clean urine and expire breath samples on testing days. Urine samples were screened for extraneous drugs using the Enzyme Multiple Immunoassay Technique Drug Abuse Urine Assay (Innovacon; San Diego, CA). Two positive detections were recorded during this study; one participant was positive for cannabis and another participant was positive for cocaine. Both participants were precluded from study participation until negative urine samples were obtained. Expired breath samples were collected each morning of testing to detect alcohol consumption.
Apparatus
Participants were seated in a 1.2m × 1.8m sound-attenuated testing room containing a video graph array (VGA) monitor and a 19cm × 43cm × 25cm USB-connected response panel containing four multicolored buttons (blue, yellow, green, and red). The blue, yellow, and green buttons represented the ‘A’, ‘B’, and ‘C’ response options displayed on the VGA monitor, respectively. The red button had no programmed consequence. The VGA monitor and response device were linked to Pentium-based computer located outside of the testing room which controlled and recorded all experimental sessions.
Procedure
The Point Subtraction Aggression Paradigm (PSAP) is a well-established and validated laboratory measure of state human aggression. The utility of the PSAP has been documented across many studies, with demonstrated sensitivity to acute drug administration (Lane, & Cherek, 2000) and aggressive response patterns in psychiatric populations, including ASPD and substance abuse (Allen et al., 1997; Bjork et al., 1999; Cherek et al., 1997; Cherek & Lane, 1999; Cherek, Pietras, & Lane, 2003). Participants were informed that they would be anonymously paired with another individual (who was actually fictitious) and participants were told that their task was to earn as much money as possible. To achieve this goal, participants were able to utilize three different button options labeled A, B, and C which correspond to monetary-reinforced, aggressive, and escape responses, respectively. The A, B, and C Buttons were non-reversible response options. Before the subject made a choice, Buttons A, B, and C were displayed across screen on the VGA monitor. The first response on A, B, or C resulted in the temporary removal of the other response options from the computer screen until the selected response option requirement was completed. Button A (monetary-reinforced response) was maintained on an FR100 (100 presses) schedule of responding. The subject gained $0.15 cents for each completed FR100 on button A. Completion of Button B (aggressive response) responding resulted in the ostensible loss of $0.15 from the fictitious other person’s counter. In accord with the operational definition of aggression, the aggressive response option is defined as the ostensible subtraction of money from the fictitious individual’s counter. Button B (the aggressive response) was maintained on an FR10 schedule of responding. Aggressive responding was elicited by provocation; which occurred probabilistically on average every 125 sec ± 20% and resulted in a loss of $0.15 from the participant’s counter. These monetary loses (subtractions) were attributed to the fictitious individual paired with the participant. Participants were told that the other (fictitious) individual kept the money subtracted from the subject’s counter. The subject was informed that money they subtract from the fictitious individual’s counter is not added to their own. Thus, the aggressive option was not maintained by monetary gain. Completion of Button C (FR10; escape response) responding resulted in the protection of subject’s earnings for a variable interval of time (a provocation-free interval averaging 125 sec ± 20%). Because both Button B (aggressive response) and Button C (escape response) option produce a provocation-free interval, they also provide support for the social deception that another person is present by reducing (but not preventing) subtractions throughout the PSAP sessions. In the present study, responding on Button C was not analyzed as because not all participants used Button C and those that did, did so sparingly.
To establish all PSAP contingencies and the social context, participants read a printed set of instructions describing the response requirements for all three response options and consequences on each option (the instructions only attribute the provocation free interval to Button C). If the participant asked questions pertaining to the PSAP, the instructions were repeated verbally from the printed instructions and clarified by a trained research assistant. Each study day, participants completed four PSAP sessions at 30min, 90min, 150min, and 210min post dose (Session 1, 2, 3, and 4, respectively). Each PSAP session lasted 25min. We chose to focus analysis of behavioral data on session 2, 90 min after dosing because the accumulation of neuropeptides in the cerebrospinal fluid (CSF) can be seen 30 min after intranasal administration, and levels continue to rise up to 80 min and remain stable 100-120 min after administration (Born, Lange, Kern, McGregor, Bickel, & Fehm, 2002).
The primary dependent variables in the PSAP were the overall response rate on the Button B (aggressive responses per minute; molar level of behavior), the distribution of inter-response times (IRT; molecular level of behavior) on the Button B option, and the response rate on the A button (monetary responses per second). Because Button A (monetary) responding occurs more frequently than Button B (aggressive) responding during PSAP sessions, the two response options are measured in different response rate units (Cherek, Tcheremissine, & Lane, 2006; Lane & Cherek, 2000). The overall response rate and IRT distribution on the Button B option were both analyzed because they represent two potentially unique response dimensions of the same behavior. Though overall response rate and IRT distributions may be independent of each other, we hypothesized that OT would affect aggressive responding on both the molar and molecular level. Specifically, we examined the possibility that acute OT administration would reduce the frequency of aggressive behavior during a PSAP session and for the IRT distributions, an increase in the interval between aggressive responses (i.e. rightward shift in IRT distribution) would demonstrate a decrease in the rate of responding during an aggressive bout (FR 10) and by extension suggest that the social salience of provocation from the “other person” (an aversive social stimulus) was altered. To analyze IRT data we took the difference in time between consecutive responses emitted during a bout of aggressive responding (FR 10). For each individual subject, we analyzed the cumulative distribution of IRTs on the Button B option in order to examine the shifts in IRT distribution as a function of OT dose. At the end of each experimental day, participants completed a short open-ended questionnaire to assess the veracity of the instructional deception in the PSAP. All participants reported being paired with another individual on every experimental day.
Dose order was counterbalanced across all six participants
Prior to dose administration, all participants were trained on the dosing procedures using 1.5ml of saline to ensure accurate self-administration and comfort with the self-administration procedures. Participants self-administered intranasal doses (12, 24, and 48 IU) of synthetic OT (Syntocinon, nasal spray: Novartis®). The drug administration apparatus was a 3cc (3 ml) needleless-syringe attached to a nasal atomizer for intranasal administration. Each dose (12, 24, and 48 IU) and placebo was self-administered at a total volume of 1.5 ml intranasal (≈0.75 ml per nostril). Participants’ self-administered the nasal-spray fluid of either OT or placebo dose at a volume of 0.75ml per nostril (1.5ml total) at their own pace under the supervision of a research assistant. All administrations were completed within 5min. One spray is 4 IU and each 4 IU spray is equivalent to 0.1 ml. Thus, 12, 24, and 48 IU of OT is equal to 0.3, 0.6, and 1.2 ml of nasal spray fluid. For drug preparation, OT doses were brought to their corresponding volume (ml) in a 3cc needleless-syringe, and then each syringe was brought to a full volume of 1.5 cc by adding placebo. This approach was used to blind participants to dose contents. The placebo dose contained saline, and was also administered at a total volume of 1.5 ml (0.75 ml per nostril). Participants self-administered the volume containing synthetic OT or just placebo two times (one spray per nostril).
Psychometric measures
Impulsive Premeditative Aggression Scale (IPAS; Stanford, Houston, Mathias, Villemarette-Pittman, Helfritz, & Conklin, 2003). The IPAS is a self-report measure of trait aggression, validated in prison populations, consisting of 30-items measuring aggressive acts within the last 6 months (Stanford et al., 2003). The Buss-Perry Aggression Questionnaire (BPAQ; Buss, & Perry, 1992) is a self-report measure of trait aggression consisting of 29-items. It is well validated in populations with both Axis I and Axis II disorders. The Barratt Impulsivity Scale (BIS-11; Patton, Stanford, & Barratt, 1995) is a self-report measure of the personality trait of impulsivity consisting of 30-items. The Self-Report Psychopathy Scale III (SRP-III; Mahmut, Menictas, Stevenson, & Homewood, 2011; Paulhus, Neuman, & Hare, 2010). The SRP-III is a self-report measure of the clinical construct of psychopathy consisting of 64-items developed from the well-established Psychopathy Checklist-Revised (Hare & Neumann, 2008). THE IPAS, BPAQ, BIS-11, and SRP-III were given a day prior to PSAP testing when consent was obtained. The Profile of Mood States: Short Form (POMs: Shacham, 1983) is a self-report measure of psychological distress consisting of 38-adjective items on a 0-4 Likert-rating scale ranging from “Not at all” to “Extremely”. The POMS provides a total mood disturbance score and six subscale scores measuring six distinct mood states: Depression-Dejection, Anger-Hostility, Tension-Anxiety, Fatigue, Vigor, and Confusion-Bewilderment. The POMS was given 5min prior to each PSAP session.
Previous studies have noted that OT administration increases heart rate variability, an index of parasympathetic activity (Kemp, Quintana, Kuhnert, Griffiths, Hickie, & Guastella, 2012). Heart rate and blood pressure (HR/BP) were measured using a sphygmomanometer (BpTru Vital Signs Monitor, Coquitlam, Canada) after each session of the PSAP. Cardiovascular data (HR/BP) were collected to examine changes in autonomic nervous system activity after OT dosing.
Results
In the first-level of analysis, statistical tests were conducted on both aggressive and monetary behavior at 90 min post dose (session 2, expected peak central nervous system levels) across each dose. The assumptions of normality and homogeneity of variance were tested for both aggressive responses/min and monetary responses/sec. For aggressive responses/min, Shapiro-Wilk’s test of normality (S-W = 0.928, df (22), p = 0.09) and Levene’s test of homogeneity of variance (F (3, 19) = 0.11, p = 0.95) were not significant. For monetary responses/sec, Shaprio-Wilk’s test of normality (S-W = 0.93 df (22), p = 0.11) and Levene’s test of homogeneity of variance (3, 19) = 2.14, p = 0.18) were not significant. A one-way repeated measures analysis of variance (ANOVA) on aggressive responses/min across dose levels was conducted and was not statistically significant (F (3, 19) = 0.62, ns). A one-way ANOVA on monetary responses/sec across all dose levels was conducted and was not found to be statistically significant (F (3, 19) = 2.14, ns).
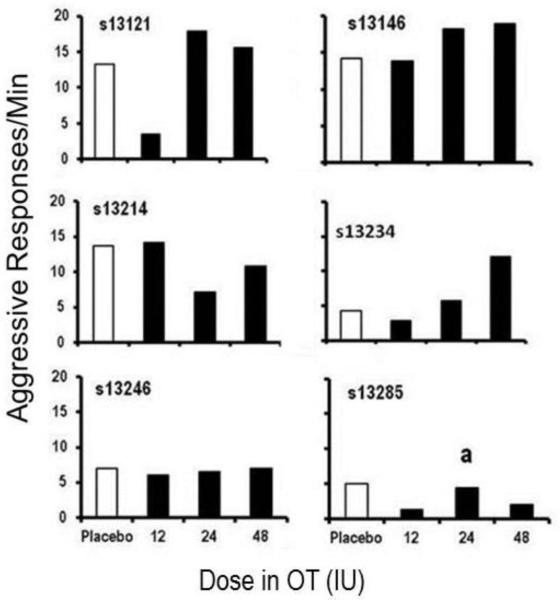
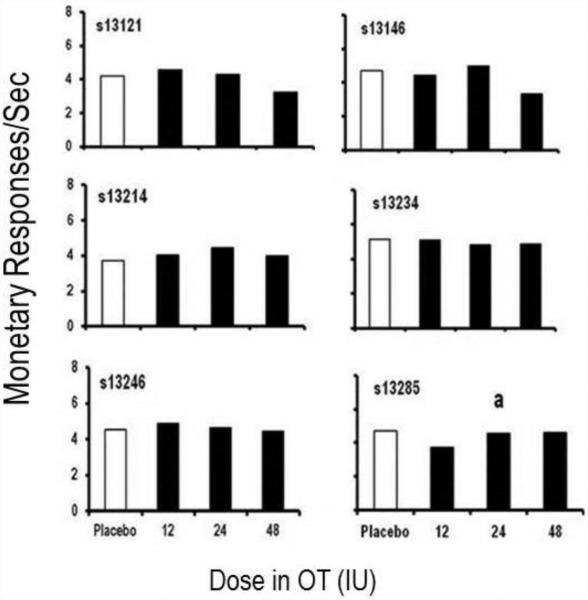
The second level of analysis focused on graphical inspection of each participant’s behavioral data (aggressive and monetary responding) at 90 min post dose. Overall aggressive response rates for each individual subject are presented in Figure 1. Compared to placebo, three participants (s13121, s13234, and s13285) showed decreases in aggressive responses per minute at the 12IU OT dose. All other participants showed no changes in aggressive responses per minute at the 12IU OT dose. At the 24IU dose, one participant (s13214) showed a decrease in aggressive responses per minute. Whereas, three participants (s13121, s13146, and s13234) showed increases in aggressive responses per minute there were two participants (s13246 and s13285) who showed no changes in aggressive responses per minute. Finally, at the 48IU OT dose two participants (s13214 and s13285) showed decreases in aggressive responses per minute, three participants (s13121, s13146, and s13234) showed increases in aggressive responses per minute, and one participant (s13246) showed no change in aggressive responses per minute. As shown in Figure 2, few notable changes in monetary responses/sec were observed across doses for any participants.
Figure 1.
Response rate (response/min) during session 2 (90 min post dose) on the aggressive response option (y-axis) across placebo and three doses of oxytocin (x-axis). a= Session 2 data is 15 min instead of 25 min, due to experimenter error.
Figure 2.
Response rate (response/sec) during session 2 (90 min post dose) on the monetary-earnng response option (y-axis) across placebo and three doses of oxytocin (x-axis). a= Session 2 data is 15 min instead of 25 min, due to experimenter error.
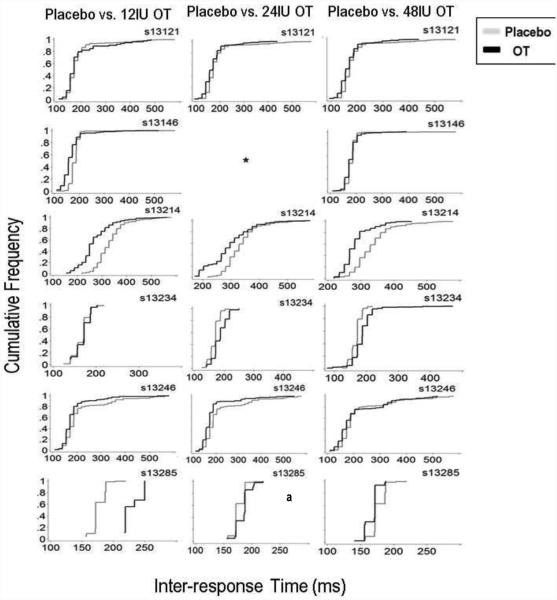
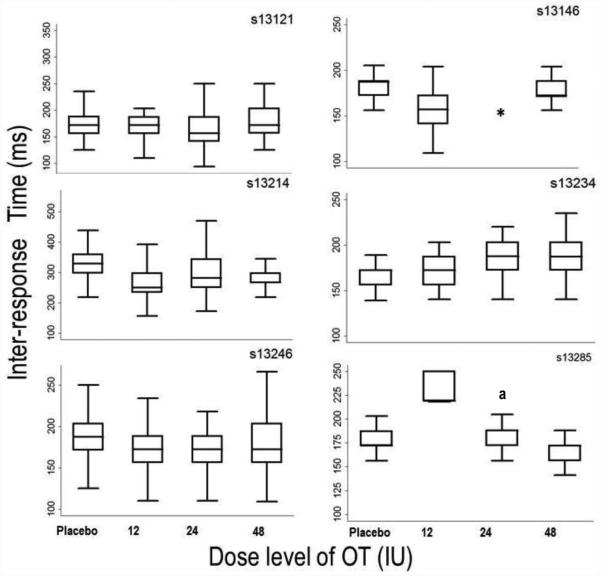
The third level of analysis employed statistical and graphical comparisons of the IRT distributions on the aggressive option. Kolmogorov-Smirnov (K-S) tests were conducted to compare IRT distributions under each OT dose to placebo. K-S tests were chosen because IRT distributions were not normally distributed, thus the K-S tests were used because they make no assumptions regarding underlying distributions (Corder & Foreman, 2009). To correct for multiple comparisons, the Benjamini–Yekutieli false discovery rate was employed (Benjamini & Yekutieli, 2001); the corrected critical p-value was p < 0.01. Figure 3 shows cumulative frequency distributions of IRTs on the aggressive response option (button B) for each subject at each dose. Twelve of the seventeen K-S tests of the IRT distributions under active OT doses were significantly different from placebo. For s13121, the distribution of aggressive IRTs was found to be significantly different from placebo only the 24IU OT dose (D = 0.179, p < 0.01). For s13146, the distribution of aggressive IRTs was found to be significantly different from placebo at the 12IU (D = 0.432, p < 0.01) OT dose. For s13214, the distributions of aggressive option IRTs were found to be significantly different from placebo under all OT doses: 12IU (D = 0.541, p < 0.01), 24IU (D = 0.35, p < 0.01), and 48IU (D = 0.49, p < 0.01). For s13234, the distributions of aggressive IRTs were found to be significantly different from placebo under 24IU (D = 0.36, p < 0.01) and 48IU (D = 0.413, p < 0.01) OT doses. For s13246, the distributions of aggressive IRTs were found to be significantly different from placebo under the 12IU (D = 0.218, p < 0.01) and 24IU (D = 0.581, p = p < 0.01) OT dose. For s13285, the distributions of aggressive IRTs were found to be significantly different from placebo under the 12IU (D = 0.99, p = p < 0.01) and 24IU (D = 0.33, p = p < 0.01). Measures of central-tendency for the aggressive option IRTs are presented in Figure 4 as box and whisker plots.
Figure 3.
Data shown are cumulative frequency distributions of IRT data on the aggressive response option across all doses from all participants. Note: different scaling on the x- and y-axes for each individual participant.
* = data lost due to experimenter error. a = session lasted 15min instead of 25min, due to experimenter error.
Figure 4.
Median (Inter-Quartile Range) Inter-response Times in milliseconds for each participant at each dose on the aggressive response option. Data are presented from session 2 (90min post dose). Note: different scaling on the y-axis for each individual participant.
* = data lost due to experimenter error. a = session lasted 15min instead of 25min, due to experimenter error.
To summarize of the molar and molecular behavioral data for each participant, in two participants (s13121 and s13146), a decrease in and no change in the overall aggressive response rates were seen under the 12IU OT dose, respectively. Conversely, for s13121 and s13146, increases in overall aggressive response rates were seen under the 24IU and 48IU OT doses. The IRT distributions under OT were only significantly different from placebo at the 24IU and 12IU dose for s13121 and s13146, respectively; the IRT distributions for both of these participants at the respective dose levels were both shifted leftwards. In two participants (s13214 and s13285), decreases in overall aggressive response rates were observed following OT dosing compared to placebo. For s13214, decrease in overall aggressive responses rate were observed under the 24 and 48IU OT doses. In s13214 IRT distributions under all three dose levels were significantly different from placebo and under all three doses IRT distributions were shifted leftward. For s13285, decrease in overall aggressive response rate was observed under the 12IU and 48IU OT doses, but not under the 24IU dose. In the same participant, IRT distributions were significantly different than placebo under the 12IU and 24IU OT doses, and under these doses IRT distributions were shifted leftward. For participant s13234, increase in the overall aggressive response rate was seen under the 24IU and 48IU OT doses. In the same participant, this increase in overall response rate was accompanied by significant rightward shifts in IRT distributions under the same dose levels. For participant s13246, we observed no change in overall aggressive response rate between OT doses and placebo. However, for s13246, IRT distributions were significantly different from placebo under the 12IU and 24IU OT doses; under both dose levels the IRT distributions were shifted leftward. In conclusion, while we observed several differences from placebo in overall aggressive response rates and IRT distributions (local or running response rate) following OT dosing, we found no overall systematic or orderly dose-response relationships. Thus, OT did appear to exert effects specifically on operationally-defined aggressive responding (rate changes were not observed for monetary-reinforced responding). However, these effects were marked by substantial individual differences.
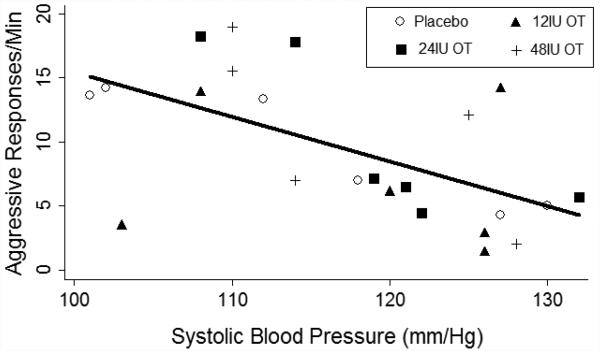
Cardiovascular data were analyzed using a two-way ANOVA with two within-subjects factors of dose and session and with significance set at p< 0.05. None of the cardiovascular measures (heart rate, systolic BP, diastolic BP) showed significant changes from placebo; all F values were < 1.5, and all p values were > .30. However, there was a significant negative relationship between aggressive responses per minute and systolic BP, depicted in Figure 4. This correlation was not different across OT doses, nor did OT dosing systematically alter any of the cardiovascular measures.
Analyses of the POMS across all doses and sessions revealed no systematic patterns or statistically significant outcomes. Analyses of the psychometric scores (SRP-III, BPAQ, BIS-11 and IPAS) did not reveal any notable associations or trends with overall aggressive response rates or IRT distributions. Psychometric scores were comparable across participants. Total scores from psychometric scales are presented in the Appendix material.
Discussion
In individuals with ASPD and part SUD, changes in aggressive response rates and IRT distributions were observed following OT dosing, but these were not systematic or dose related. Notably, the changes did appear specific to aggressive (social) behavior; OT dosing did not appear to alter monetary-reinforced (non-social) responding. This observation is consistent with reported role of OT in modulating social behavior (Meyer-Lindenberg et al., 2011). For three participants (s13121, s13146, s13234), increases in aggressive response rate were observed at the 24IU and 48IU OT doses. However, these changes were unrelated to shifts in IRT distributions. Shifts in IRT distributions reflect changes in local response rate during response bouts on the aggressive option. While OT doses appeared to modify local rates, changes were also not dose related. While most participants responded faster during aggressive bouts (typically at higher doses), rightward shifts (slower responding) were observed.
The neuropeptide OT has been reported to increase the saliency of both positive and negative social stimuli (Gamer, Zurowski, & Büchel, 2010; Guastella, Mitchell, & Dadds, 2008; Stripens, Scheele, Kendrick, Becker, Schäfer, Schwalba, Reul, Maier, & Hurlemann, 2012). This function of OT is thought to reflect promotion of prosociality (Bartz, Zaki, Bolger, & Ochsner, 2011) and decrease in avoidance, such as response to an angry face (Evans et al., 2010). Given that aggressive responding on the PSAP is maintained by avoidance from provocation (Cherek, Spiga, Steinberg, & Kelly, 1990) and occurs in bouts of responding; the time to complete the FR 10 on the aggressive response option on the PSAP (e.g. IRT distribution) could reflect the social saliency of provocations. Changes in the IRT distributions following OT administration may cautiously be interpreted as modification in the social salience of provocation used to elicit an aggressive response. However, the lack of orderly effects implies that OT effects on aggressive behavior were partially modulated by variables we did not measure or manipulate (e.g. personality traits, traumatic experiences). This possibility is supported by a study from Bartz et al. (2011a), in which empathic accuracy was selectively improved in less socially proficient individuals following oxytocin administration. Acute OT administration (24 IU) has been shown to interact with state anxiety level to reduce aggressive behavior of women with high state anxiety compared to women with high state anxiety given placebo (Campbell & Hausmann, 2013). Additionally, Norman, Cacioppo, Morris, Malarkey, Bernston et al. (2010) found that higher levels of loneliness (diminished social support) were significantly correlated with reduced cardiac reactivity following acute OT administration. It appears that on both behavioral and biological levels, the effects of OT administration are modulated by individual differences and the relationship between OT dose and neurobehavioral changes may be non-linear.
This study has several limitations. First, our sample of participants was small and homogenous. All six participants met criteria for ASPD and past SUD, had similar psychometric scores on personality assessments (see Appendix), and had criminal and drug use histories. Thus, we are limited in drawing inferences about the effects of OT on aggressive behavior to this subset of individuals. Future studies may wish to consider using larger sample of participants, including a separate group of healthy individuals to compare with a clinically relevant sample. A second limitation of the present study is that data analysis was limited mostly to qualitative rather than statistical analyses. While both approaches have relative advantages and disadvantages, expanding the present study to a larger sample would provide statistical power for future studies, which would allow greater generalizability and potentially better understanding of the direction and magnitude of effects following acute intranasal OT administration. Thirdly, our experimental design did not assess aggressive responding between OT doses (e.g., return to non-dosing baseline conditions between doses). Thus, we were unable to assess stability in aggressive responding across dosing days. Future studies may endeavor to implement designs that assess stability (or change) in social behavior across the study (i.e., an A-B-A-C-A-D design). Lastly, we observed no statistically significant result on cardiovascular data (HR/BP) or self-reported mood (POMS). Therefore, outside of the differences that were observed on aggressive responding, we do not have additional confirmation that OT was biologically or behaviorally active. Previous studies using intranasal OT dosing have observed cardiovascular changes (Kemp et al., 2012). The present lack of dose-related cardiovascular effects could be related to the fact that ASPD individuals have lowered cardiovascular arousal during rest (Lorber, 2004) or due to less sophisticated measurement techniques used in our lab relative to previous work. Given that we measured HR/BP after each PSAP session we cannot conclude that OT dose altered cardiovascular reactivity to provocation (i.e. lowered reactivity). However, the negative relationship between BP and overall aggressive response rate (across all doses) is roughly consistent with previous studies reporting that individuals with severe ASPD and clinically-defined psychopathology showed suppressed or lowered physiological reactivity to stimuli and lower resting heart rate (Lorber, 2004; Patrick, 2008). However, the relationship between BP and aggressive response rate was not altered by OT dosing. Generally, the cardiovascular data support the inverse relationship between physiological arousal and aggression in individuals who have higher levels of externalizing behavior (e.g. ASPD, psychopathy).
We hypothesized the OT would produce dose-dependent decreases in rates of aggressive responding. We tested this hypothesis in individuals who met criteria for ASPD and past SUD, believing these individuals are a clinically relevant group and perhaps uniquely sensitive to OT effects, as previous studies have observed diminished OT function in this population (Lee, Ferris, Van de Kar, & Coccaro, 2009b; Malik et al., 2012).The results imply that OT did have individual effects on aggressive responding. However, the direction and magnitude of the effect are not clear, and as such our results remain inconclusive. The results are best interpreted as preliminary information for future studies seeking to study OT effects on social behavior in high-risk populations. Further work is needed to understand individual differences that plausibly moderate OT effects on aggressive responding.
Future studies might examine the impact of childhood trauma. Ecological studies with rhesus monkeys showed that rearing conditions modulated CNS expression of OT. Specifically, Winslow et al (2003) found that lower CSF levels of OT were associated with rhesus monkeys that were not maternally reared, compared to rhesus monkeys that were maternally reared. These rhesus monkeys showed higher displays of aggressive behavior compared to rhesus monkeys that were maternally reared. In individuals with Borderline Personality Disorder, the acute effects of oxytocin on stress reactivity were predicted by childhood trauma (Simeon, Bartz, Hamilton, Crystal, Braun, Ketay, & Hollander, 2011). Another approach for future studies would be to examine the effect of OT dose on individuals with high versus low levels of aggression at baseline. Calcagnoli et al., (2013) reported anti- aggressive effects of OT in highly aggressive male rats as compared to low-aggressive rats. Lastly, it should be noted that while many studies have touted the prosocial effects of oxytocin (Bakermans-Kranenburg & van IJzendoorn, 2013), more nuanced experiments report that prosocial behaviors are not enhanced (or even decreased) in the absence of key social cues, and a broader range of behaviors may occur, e.g., competition and ratings of envy (De Dreu, Shalvi, Greer, Van Kleef, & Handgraaf, 2012, Declerck, Boone, & Kiyonari, 2010; Shamay-Tsoory, Fischer, Dvash, Harari, Perach-Bloom, & Levkovitz, 2009). Acute OT administration may actually decrease trust and cooperation in individuals with Borderline Personality Disorder, a behavior disorder that shares much common symptomatology with ASPD and SUD (Bartz, Simeon, Hamilton, Kim, Crystal, Braun, Vicens, & Hollander, 2011b). Thus, mixed or equivocal effects are not without precedent.
We observed some evidence of modulation of aggressive behavior via OT administration, but the effects were not systematic and the controlling variables remain unclear. Given that (i) OT receptors are prevalent in the cortico-striatal-limbic circuitry (Gimpl & Farhenholz, 2001; Ludwig & Leng, 2006; Lee et al., 2009a); (ii) that individuals with ASPD and past SUD have disrupted functioning in this same circuitry (Siever, 2008); and (iii) that OT appears to be evolutionarily conserved in regulating mammalian social and maternal behavior, the possibility that OT has anti-aggressive properties in individuals with deficiencies in affective control and social interaction remains a reasonable hypothesis and target for future experimentation.
Figure 5.
Scatterplot of the relationship between systolic blood pressure (x-axis) and aggressive responses per minute on the PSAP (y-axis). The solid line shows the line of best fit based on linear regression. There was a significant negative correlation between the variables (p < .05, see text for details).
ACKNOWLEDGEMENTS
This work was funded by NIH grants DA R01 003166 and DA P50 09262. We thank Ellen Desmarais, Nadeeka Dias, and Zahra Kamdar, for valuable technical assistance in conducting the research.
Appendix.
Mean (SEM) on the aggressive response option (responses/min) for each dose and each PSAP session.
| Session 1 | Placebo | 12IU OT | 24IU OT | 48IU OT |
|---|---|---|---|---|
| 1 | 7.5 (1.9) | 7.49 (1.1) | 6.04 (1.3) | 10.05 (1.4) |
| 2 | 9.58 (1.9) | 6.94 (2.3) | 9.96 (2.6) | 11.07 (2.5) |
| 3 | 8.46 (1.2) | 8.58 (1.9) | 12.11 (3.8) | 10.1 (1.8) |
| 4 | 13.3 (3.8)a | 13.88 (3.6)a | 10.13 (2.4)a | 8.63 (2.4)a |
= Session 2 data is 15 min instead of 25 min, due to experimenter error.
Mean (SEM) on the monetary-earning response option (responses/min) for each dose and each PSAP session.
| Session 1 | Placebo | 12IU OT | 24IU OT | 48IU OT |
|---|---|---|---|---|
| 1 | 4.54 (.2) | 4.57 (.12) | 4.59 (.18) | 4.1 (.28) |
| 2 | 4.49 (.2) | 4.45 (.2) | 4.62 (.11) | 4.08 (.27) |
| 3 | 4.75 (.2) | 4.76 (.15) | 4.41 (.12) | 4.64 (.31) |
| 4 | 4.5 (.2) a | 4.58 (.19) a | 4.6 (.17) a | 4.53 (.36) a |
= Session 2 data is 15 min instead of 25 min, due to experimenter error.
Psychometric total scores for all participants.
| Participant | SRP-III | BPAQ | BIS-11 | IPAS-IA | IPAS-PM |
|---|---|---|---|---|---|
| s13121 | 196 | 69 | 52 | 29 | 30 |
| s13146 | 169 | 77 | 63 | 27 | 24 |
| s13214 | 184 | 52 | 70 | 31 | 29 |
| s13234 | 166 | 88 | 61 | 39 | 22 |
| s13246 | 205 | 88 | 76 | 37 | 23 |
| s13285 | 178 | 88 | 67 | 22 | 28 |
SRP-III = Self Report of Psychopathy Scale – III Total Score; BPAQ = Buss-Perry Aggression Questionnaire Total Score; BIS-11 = Barratt Impulsiveness Scale – 11 Total Score; IPAS-IA = Impulsive Premeditated Aggression Scale – Impulsive Subscale Total Score; IPAS-PM = Impulsive Premeditated Aggression Scale – Premeditated Subscale Total Score
Median (Inter-Quartile Range) Inter-response Times in milliseconds for each participant at each dose on the aggressive response option. Data are presented from session 2 (90min post dose).
| Subject | Placebo | 12 IU | 24 IU | 48 IU |
|---|---|---|---|---|
| s13121 | ||||
| Median (IQR) | 172 (156-188) | 172 (156-187) | 156 (141-187) | 172 (157-203) |
| Range | 125-578 | 110-484 | 94-437 | 125-515 |
| s13146 | ||||
| Median (IQR) | 187(172-188) | 157 (141-172) | a | 172 (171-188) |
| Range | 110-580 | 109-515 | 110-391 | |
| s13214 | ||||
| Median (IQR) | 328 (297-359) | 250 (234-297) | 281 (250-343) | 266 (265-297) |
| Range | 218-579 | 156-563 | 172-578 | 218-453 |
| s13234 | ||||
| Median (IQR) | 172 (156-172) | 172 (156-187) | 187 (172-203) | 187 (172-203) |
| Range | 125-218 | 140-203 | 140-250 | 78-469 |
| s13246 | ||||
| Median (IQR) | 187 (171-203) | 172 (156-188) | 172 (156-188) | 172 (156-202) |
| Range | 109-578 | 110-578 | 109-548 | 109-516 |
| s13285 | ||||
| Median (IQR) | 172 (172-187) | 219 (219-250) b | 188 (172-188) | 172 (156-175) |
| Range | 156-219 | 218-250a | 156-220 | 141-188 |
= 24 IU dose data lost for this subject due to hardware malfunction
= session for this subject was 15 min instead of 25 min, due to experimental error
Footnotes
LOCATION: All work was conducted in the Department of Psychiatry & Behavioral Sciences, University of Texas Health Science Center – Houston, 1941 East Road, Houston, TX 77054.
References
- Alcorn JL, III, Gowin JL, Green CE, Swann AC, Moeller FG, Lane SD. Aggression, impulsivity, and psychopathic traits in combined antisocial personality disorder and substance use disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2013;25(3):229–232. doi: 10.1176/appi.neuropsych.12030060. Summer. doi: 10.1176/appi.neuropsych.12030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TJ, Moeller FG, Rhoades HM, Cherek DR. Subjects with a history of drug dependence are more aggressive than subjects with no drug use history. Drug Alcohol Dependence. 1997;6(1-2):95–103. doi: 10.1016/s0376-8716(97)00061-6. 46. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th. Author; Washington, DC: 2000. text rev. [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review Psychology. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA. Mental disorders and violence in a birth cohort: results from the Dunedin Study. Archives in General Psychiatry. 2000;57:979–986. doi: 10.1001/archpsyc.57.10.979. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. doi:10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RA, Richardson DR. Human aggression. Plenum Press; New York: 1994. [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011a Jul;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. 301-9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicen V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive Affective Neuroscience. 2011b Oct;6(5):556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008 May 22;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. The Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- Bjork JM, Doughtery DM, Moeller FG, Cherek DR, Swann AC. The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food-restricted control. Psychopharmacology. 1999 Feb;142(1):24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002 Jun;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Althaus M, den Boer JA, Koolhaas JM. Antiaggressive activity of central oxytocin in male rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3124-7. doi: 10.1007/s00213-013-3124-7. [DOI] [PubMed] [Google Scholar]
- Campbell A, Hausmann M. Effects of oxytocin on women’s aggression depend on state anxiety. Aggressive Behavior. 2013 Jul-Aug;39(4):316–322. doi: 10.1002/ab.21478. doi: 10.1002/ab.21478. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. Effects of d, l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology. 1999;146(4):473–481. doi: 10.1007/pl00005493. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Spiga R, Steinberg JL, Kelly TH. Human aggressive responses maintained by avoidance and escape from point loss. Journal of the Experimental Analysis of Behavior. 1990 Mar;53(2):293–303. doi: 10.1901/jeab.1990.53-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Moeller FG, Schnapp W, Dougherty DM. Studies of violent and non-violent male parolees I. Laboratory and psychometric measurements of aggression. Biological Psychiatry. 1997;41:514–522. doi: 10.1016/s0006-3223(96)00059-5. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. Effects of d,l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology. 1999 Oct;146(4):473–481. doi: 10.1007/pl00005493. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Pietras CJ, Lane SD. Laboratory Measures: Point Subtraction Aggression Paradigm (PSAP) In: Coccaro E, editor. Aggression: Assessment and Treatment. Marcel Dekker; New York: 2003. pp. 215–228. [Google Scholar]
- Cherek DR, Tcheremissine OV, Lane SD. Psychopharmacology of aggression. In: Nelson RJ, editor. Biology of Aggression. Oxford, UK; Oxford University Press: 2006. pp. 424–446. [Google Scholar]
- Corder GW, Foreman DI. Nonparametric Statistics for Non-Statisticians: A Step-by-Step Approach. Wiley; Somerset, NJ: 2009. [Google Scholar]
- De Dreu CK, Shalvi S, Greer LL, Van Kleef GA, Handgraaf MJ. Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS One. 2012;7(11):e46751. doi: 10.1371/journal.pone.0046751. doi:10.1371/journal.pone.004675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and Behavior. 2010 Mar;57(3):368–374. doi: 10.1016/j.yhbeh.2010.01.006. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Reid RA, Wakeman SA, Croft LB, Benny PS. Evidence that oxytocin is a physiological component of LH regulation in non-pregnant women. Human Reproduction. 2003 Jul;18(7):1428–31. doi: 10.1093/humrep/deg291. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain region associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005 Dec 14;35(50):11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. doi:10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders-patient edition, Biometrics Research Department. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, Benjamin L. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II), Biometrics Research Institute. New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceeding in the National Academy of Sciences U.S.A. 2010 May 18;107(20):9400–9405. doi: 10.1073/pnas.1000985107. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor System: structure, function, and regulation. Physiological Review. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. Doi: 10.10.16/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Kuhnert RL, Griffiths K, Hickie IB, Guastella AJ. Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One. 2012;7(8):e44014. doi: 10.1371/journal.pone.0044014. doi:10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Coccaro EF, Fava M, Jaeger S, Jin R, Walters E. The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey replication. Archive of General Psychiatry. 2006;63:669–678. doi: 10.1001/archpsyc.63.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Neinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005 Jun 2;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR. Biological and behavioral investigation of aggression and impulsivity. In: Fishbein DH, editor. The science, treatment and prevention of antisocial behavior: applications to the criminal justice system. 21. Vol. 5. Civic Research Institute; Kingston, NJ: 2000. pp. 1–5. [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: the great facilitator of life. Progress in Neurobiology. 2009a Jun;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009b;34:1567–1573. doi: 10.1016/j.psyneuen.2009.06.002. doi: 10.1016/j.psyneuen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychological Bulletin. 2004 Jul;130(4):531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Ludwig , Leg G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mahmut MK, Menictas C, Stevenson RJ, Homewood J. Validating the factor structure of the Self-Report Psychopathy scale in a community sample. Psychological Assessment. 2011;23:670–768. doi: 10.1037/a0023090. doi: 10.1037/a0023090. [DOI] [PubMed] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, Beitchman JH. The role of oxytocin receptor gene variants in childhood-onset aggression. Genes, Brain, and Behavior. 2012 Jul;11(5):545–551. doi: 10.1111/j.1601-183X.2012.00776.x. doi: 10.1111/j.1601-183X.2012.00776.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011 Aug 19;12(9):524–538. doi: 10.1038/nrn3044. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biological Psychology. 2011 Mar;86(3):174–180. doi: 10.1016/j.biopsycho.2010.11.006. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions in the Royal Society B: Biological Sciences. 2008 Aug 12;363(1503):2543–2555. doi: 10.1098/rstb.2008.0028. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulhus DL, Neuman CF, Hare RD. Manual for the Self-Report Psychopathy Scale. Toronto: Multi-Health Systems: 2010. [Google Scholar]
- Rasmussen K, Levander S. Symptoms and personality characteristics of patients in a maximum security psychiatric unit. International Journal of Law and Psychiatry. 1996;19:27–37. doi: 10.1016/0160-2527(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Maestripieri D. The neuroendocrinology of primate maternal behavior. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2011 Jul 1;35(5):1192–1204. doi: 10.1016/j.pnpbp.2010.09.017. doi: 10.1016/j.pnpbp.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacham S. A shortened version of the profile of mood states. Journal of Personality Assessment. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harai H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biological Psychiatry. 2009 Nov 1;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Shipley WC, Gruber CP, Martin TA, Klein AM. Western Psychological Services. Los Angeles: 2009. Shipley-2: Manual. [Google Scholar]
- Siever LJ. Neurobiology of violence. American Journal of Psychiatry. 2008 Apr;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D, Bartz J, Hamilton H, Crystal S, Braun A, Ketay S, Hollander E. Oxytocin administration attenuates stress reactivity in borderline personality disorder: a pilot study. Psychoneuroendocrinology. 2011 Oct;36(9):1418–1421. doi: 10.1016/j.psyneuen.2011.03.013. doi: 10.1016/j.psyneuen.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Houston RJ, Mathias CW, Villemarette-Pittman NR, Helfritz LE, Conklin SM. Characterizing aggressive behavior. Assessment. 2003;10:183–190. doi: 10.1177/1073191103010002009. [DOI] [PubMed] [Google Scholar]
- Striepens N, Scheele D, Kendrick KM, Becker B, Schäfer L, Schwalba K, Reul J, Maier W, Hurlemann R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proceedings in the National Academy of Sciences U.S. A. 2012 Oct 30;109(44):18144–18149. doi: 10.1073/pnas.1208852109. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, Miczek KA. Behavioral and pharmacogenetics of aggressive behavior. Current Topics in Behavioral Neuroscience. 2012 Feb;:2. doi: 10.1007/7854_2011_191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel T,R. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. advance online publication, 26 March 2003. doi:10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Frontiers in Neuroendocrinology. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007 Nov 7;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]