Abstract
This study was conducted with objective of evaluating the toxic effects of nickel chloride (NiCl2) on development of bursa of Fabricius in broilers fed on diets supplemented with 0, 300, 600 and 900 mg/kg of NiCl2 for 42 days by using the methods of experimental pathology, flow cytometry (FCM), and quantitative real-time polymerase chain reaction (qRT-PCR). The results showed that dietary NiCl2 in 300 mg/kg and over induced toxic suppression in the bursal development, which was characterized by decreasing lymphocytes histopathologically and relative weight, increasing G0/G1 phase (a prolonged nondividing state), reducing S phase (DNA replication) and proliferating index, and increasing percentages of apoptotic cells. Concurrently, the mRNA expression levels of bax, cytochrome c (cyt c), apoptotic peptidase activating factor 1 (Apaf-1), caspase-3, caspase-6, caspase-7 and caspase-9 were increased and the bcl-2 mRNA expression levels were decreased. The toxic suppression of bursal development finally impaired humoral immunity duo to the reduction of B lymphocyte population and B lymphocyte activity in the broiler chicken. This study provides new evidences for further studying the effect mechanism of Ni and Ni compoundson B-cell or bursa of Fabricius.
Keywords: NiCl2, lesion, relative weight, cell cycle, apoptosis, Immunology and Microbiology Section, Immune response, Immunity
INTRODUCTION
Nickel (Ni), an essential element of more than one hundred compounds used widely in industry and commerce, is also considered to be a nutritionally essential trace metal for several animal species, microorganisms and plants [1-4]. However, there have been more reports on Ni or Ni compound toxicity than on Ni nutritional deficiency at present. Ni or Ni compounds have been proved to be potentially hazardous to living organisms due to their genotoxicity, immunotoxicity, mutagenicity and carcinogenicity [5-7]. In rats, nickel chloride (NiCl2) at a level of 1200 mg/kg can cause weight loss and reduction of food intake [8]. Ling and Leach [9] have reported that diets supplemented with Ni 300 mg/kg or over are toxic to male chicks. It has been also proved that long-term exposure to Ni is deleterious to the upper respiratory tract, skin, kidney, immune system [10-13], embryos, and the breeding system [14-16], and that Ni causes DNA damage and inhibits DNA repair in mammalian cells [17-19]. Ni (II) can directly generate ROS, activate caspase-3 expression, increase caspase-3-like protease activity, and then cause cell death in mice [20, 21]. Our previous studies have shown that dietary NiCl2 in excess of 300 mg/kg causes oxidative damage, lesions, immunotoxicity, cytotoxicity, genotoxicity, cytokine content reduction, apoptosis and inflammatory response in the cecal tonsil, spleen, thymus, kidney and intestine [22-37]. In humans, persons are exposed to Ni or Ni compounds via food, water, and air produced from sources such as mining, extraction, refining, electroplating, food processing, and waste disposal [38]. After accidently drinking water contaminated with Ni sulfate (NiSO2) and NiCl2 (1.63g Ni/L), workers can develop acute gastrointestinal and neurological symptoms [39]. Based on the above-mentioned reports, Ni and Ni compound toxic effects on humans and animals, or/and Ni and Ni compound contamination of food and water have been a big problem in the environmental safety and public health.
The bursa of Fabricius has been found only in the bird and is located between the cloaca and the sacrum. It is the primary lymphoid organ, and is necessary for B-cell differentiation, development and maturation, and is responsible for the establishment and maintenance of the B-cell compartment in avian species [40-42]. Thus, bursae may be used as a good model for studies on effects of many factors on B-cell function. However, there have been no reports focused on the toxic effects of NiCl2 on bursa of Fabricius in avian species at present.
Therefore, this study was conducted to evaluate toxic effects of NiCl2 on bursal development, including histopathological lesions and changes of relative weight, cell cycle phase, percentages of apoptotic bursa cells and the mRNA expression levels of mitochondrial apoptotic pathway-related factors [bcl-2, bax, cytochrome c (cyt c), apoptotic peptidase activating factor 1 (Apaf-1), caspase-3, caspase-6, caspase-7 and caspase-9] by using the methods of experimental pathology, flow cytometry (FCM), and quantitative real-time polymerase chain reaction (qRT-PCR).
RESULTS
Clinical observation
From 14 to 42 days of age during the experiment, the feed intake of broilers in the 300 mg/kg, 600 mg/kg, and 900 mg/kg groups began to decline when compared with those in the control group, except the 300 mg/kg group at 14 days of age. From 21 to 42 days of age during the experiment, broilers in the 300 mg/kg, 600 mg/kg, and 900 mg/kg groups showed poor appetite, poor growth and depression. A few broilers showed polypnea. No death was found during the experiment.
Histopathological observation in the bursa of Fabricius
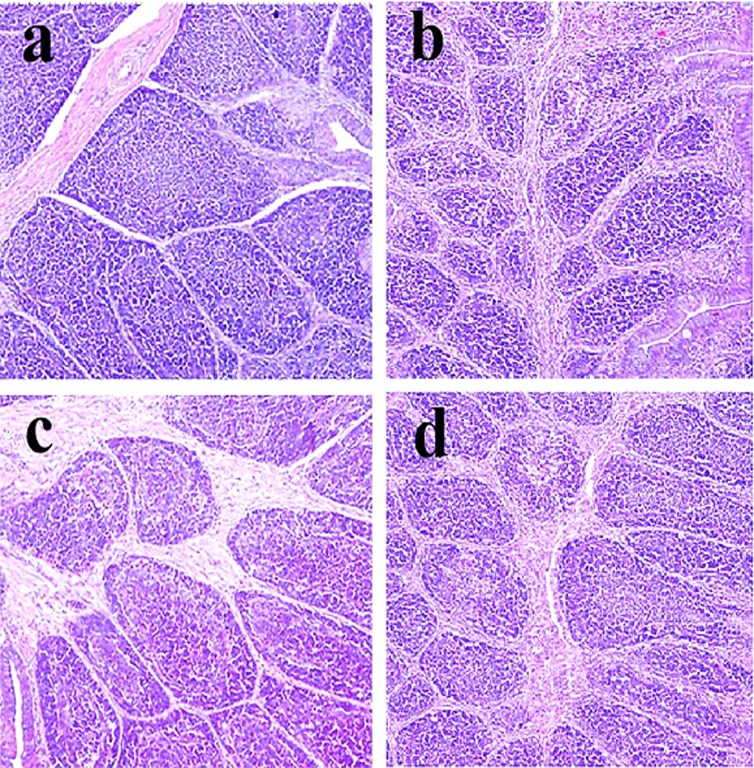
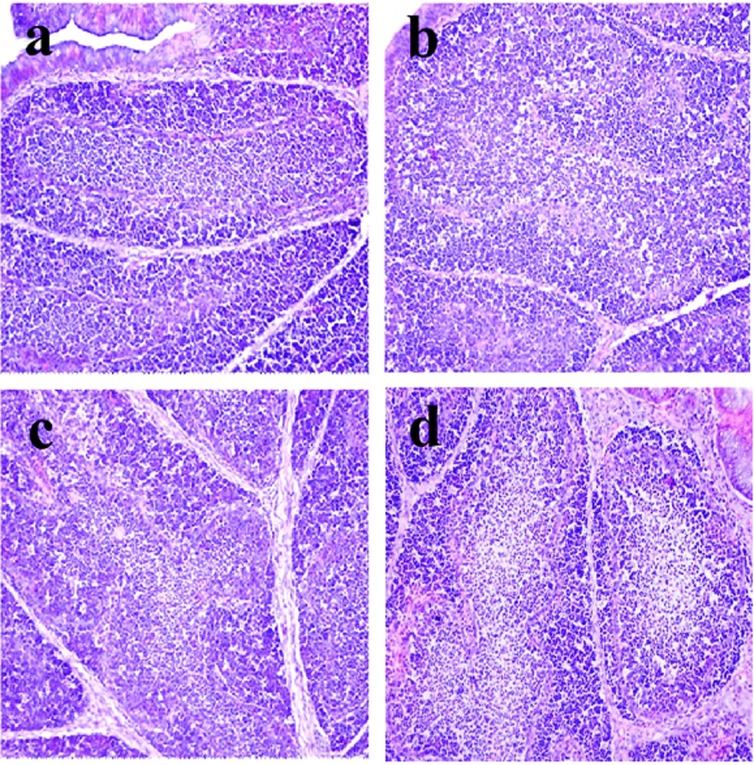
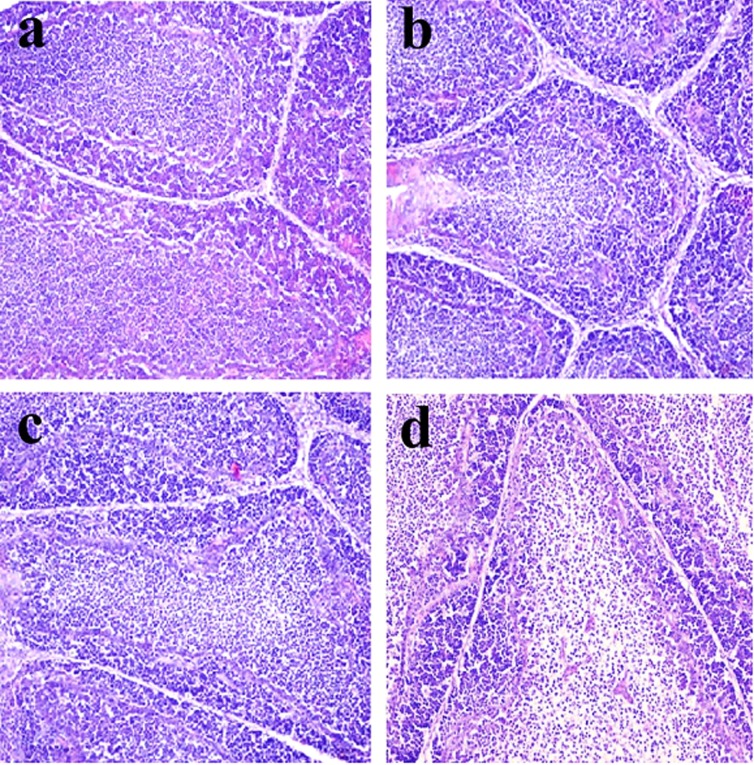
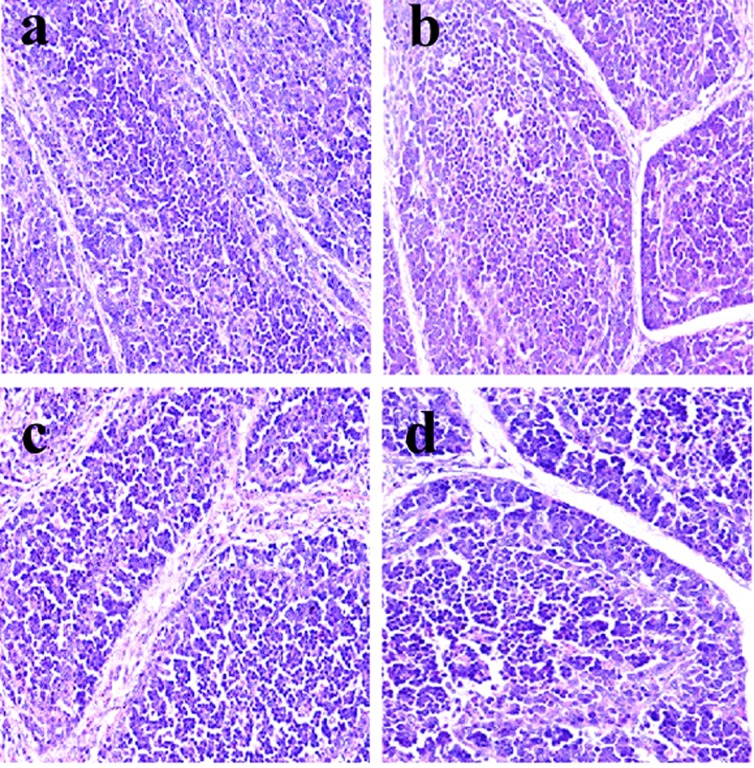
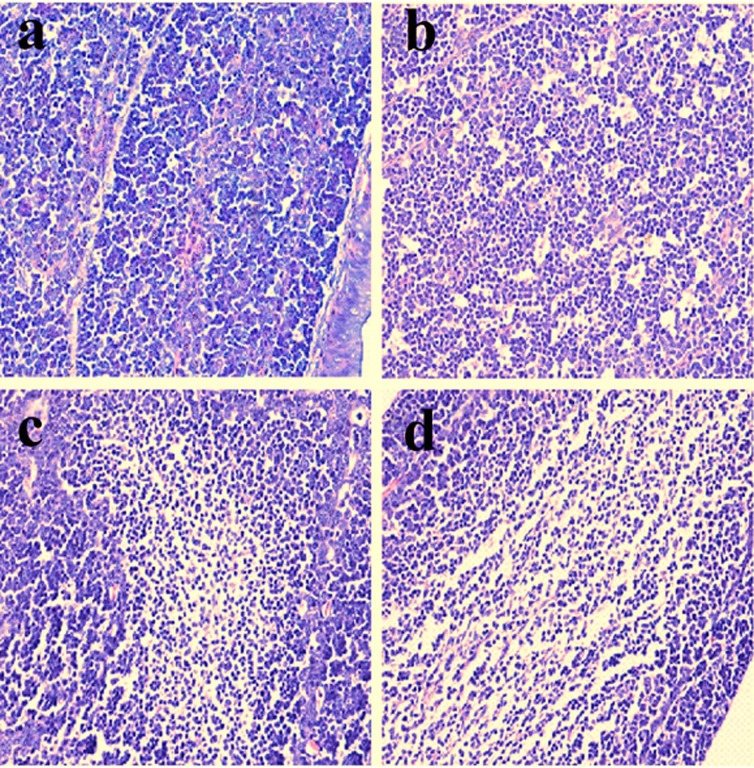
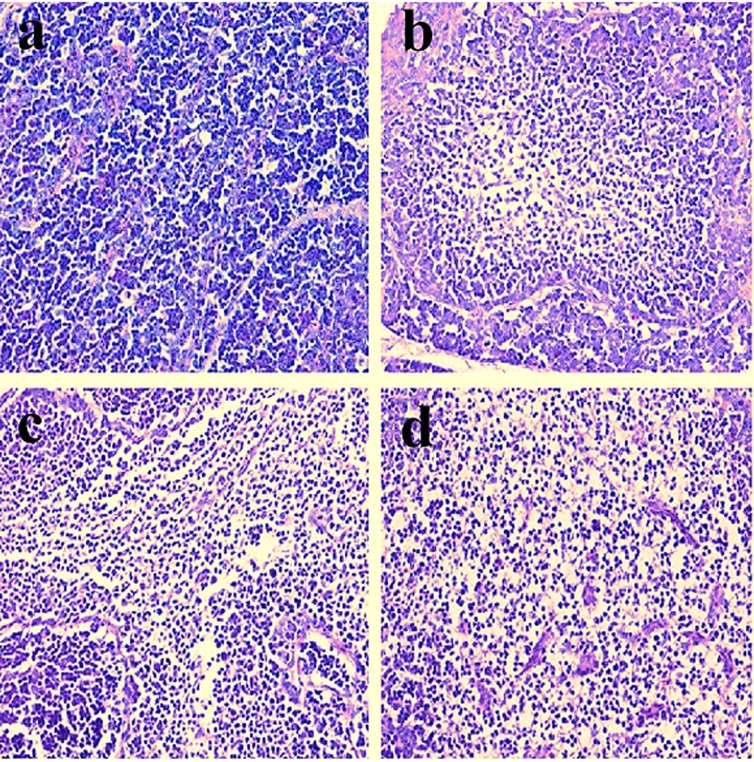
Histopathological changes were observed in the three NiCl2-treated groups when compared with those in the control group from 14 to 42 days of age. Also, histopathological changes showed obvious time- and dose-characterization. At 14 days of age, lymphocytes were slightly reduced in lymphoid follicles. At 21 and 28 days of age, lymphocytes were obviously decreased in lymphoid follicles with thinner cortices and wider medullae. At 35 and 42 days of age, lymphocytes were significantly decreased in lymphoid follicles with thinner cortices and wider medullae. The results were shown in Figures 1, 2, 3, 4, 5, 6.
Figure 1. Histopathological changes in the bursa of Fabricius at 14 days of age.
a. Control group. Lymphocytes are slightly reduced in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×200.
Figure 2. Histopathological changes in the bursa of Fabricius at 28 days of age.
a. Control group. Lymphocytes are reduced in lymphoid follicles of the 300mg/kg group b. and are obviously decreased in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×200.
Figure 3. Histopathological changes in the bursa of Fabricius at 42 days of age.
a. Control group. Lymphocytes are obviously reduced in lymphoid follicles of the 300mg/kg group b. and are significantly decreased in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×200.
Figure 4. Histopathological changes in the bursa of Fabricius at 14 days of age.
a. Control group. Lymphocytes are slightly reduced in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×400.
Figure 5. Histopathological changes in the bursa of Fabricius at 28 days of age.
a. Control group. Lymphocytes are reduced in lymphoid follicles of the 300mg/kg group b. and are obviously decreased in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×400.
Figure 6. Histopathological changes in the bursa of Fabricius at 42 days of age.
a. Control group. Lymphocytes are obviously reduced in lymphoid follicles of the 300mg/kg group b. and are significantly decreased in lymphoid follicles of the 600 c. and 900 d. mg/kg groups when compared with those in the control group. H•E ×400.
Changes of relative weight in the bursa of Fabricius
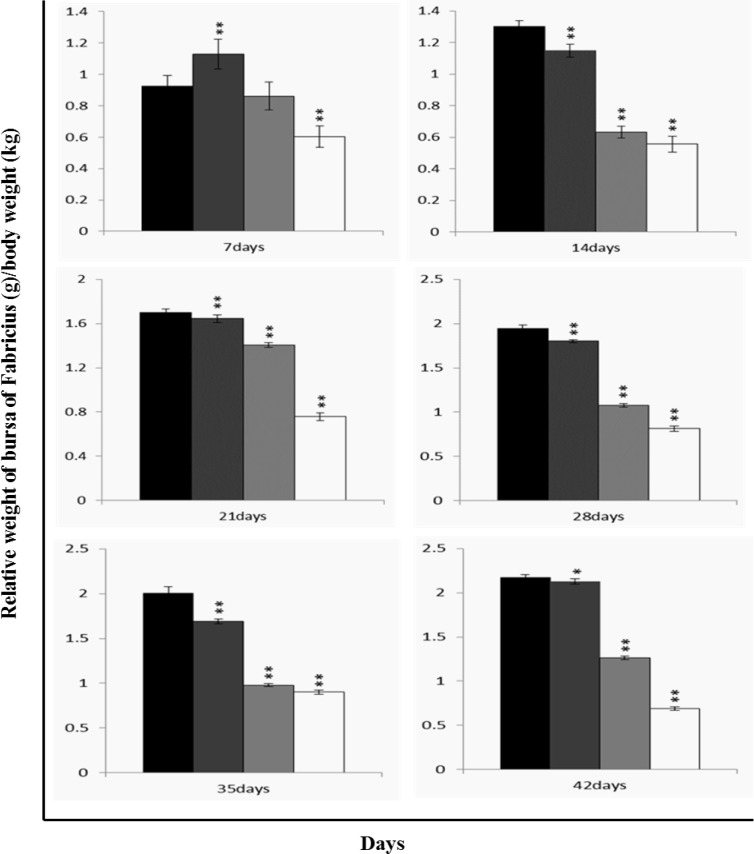
The relative weight of the bursa was significantly lower (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups than those in the control group from 7 to 42 days of age, as shown in Figure 7.
Figure 7. Changes of relative weight [bursa (g)/body weight (kg)] in the bursa of Fabricius.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Changes of cell cycle in the bursa of Fabricius
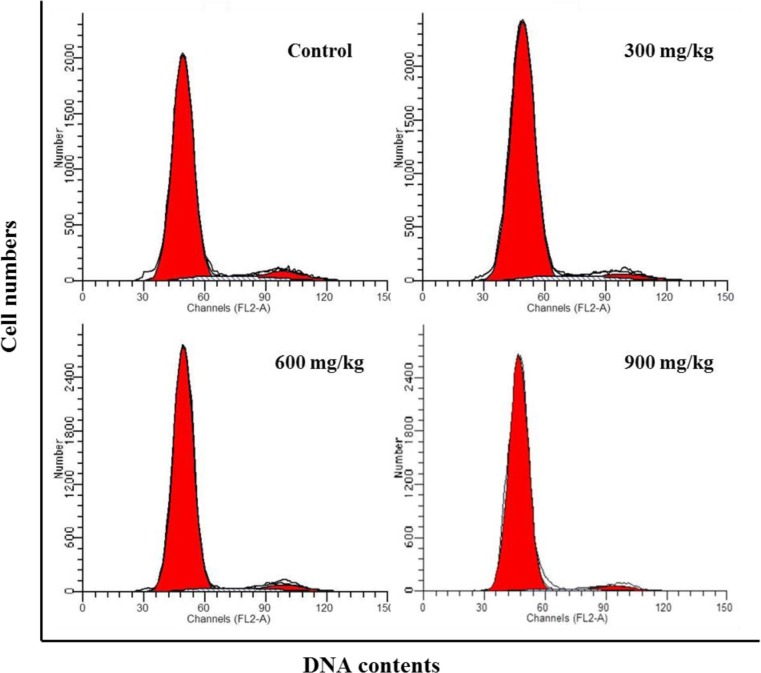
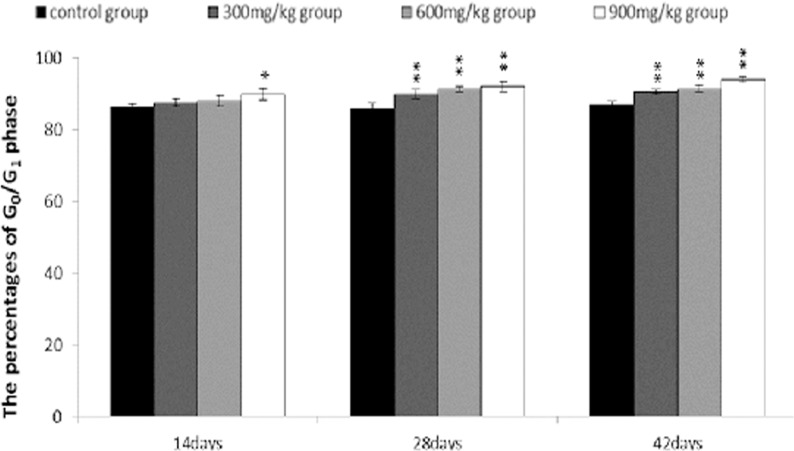
From 14 to 42 days of age during the experiment, the percentages of G0/G1 phase (a prolonged nondividing state) were significantly increased (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups.
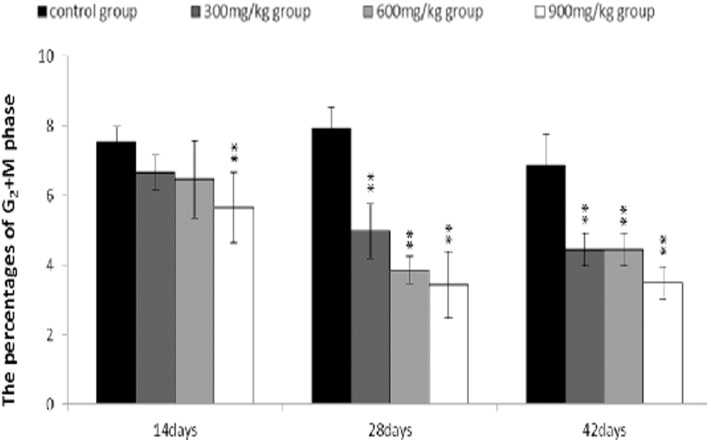
The percentages of the G2+M phase were lower (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups than those in the control group from 14 to 42 days of age.
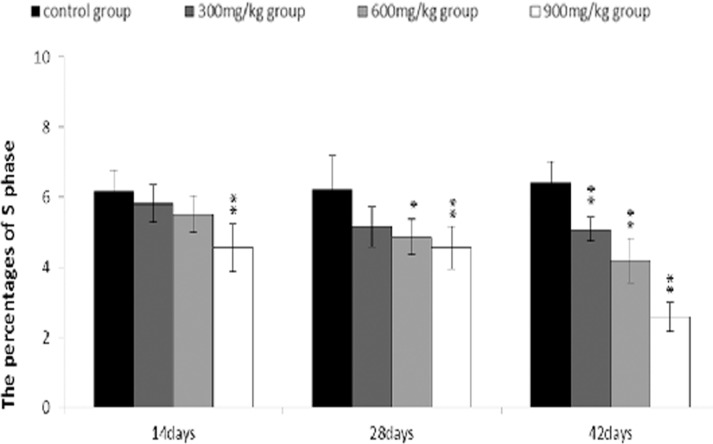
The percentages of S phase (DNA replication) were significantly decreased (P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups when compared with those in the control group at 14, 28 and 42 days of age.
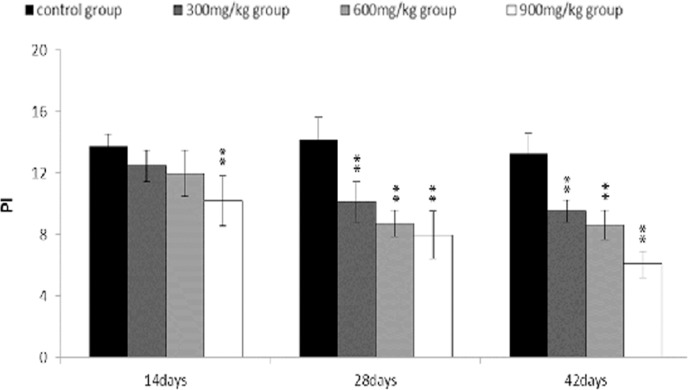
The proliferating index (PI) value was markedly lower (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups than that in the control group from 14 to 42 days of age.
The abovementioned results were shown in Figures 8, 9, 10, 11 and 12.
Figure 8. Changes of cell cycle in the bursa of Fabricius by FCM at 42 days of age.
Figure 9. Changes of the percentages of G0/G1 phase in the bursa of Fabricius.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Figure 10. Changes of the percentages of G2+M phase in the bursa of Fabricius.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Figure 11. Changes of the percentages of S phase in the bursa of Fabricius.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Figure 12. Changes of proliferating index (PI) of bursa of Fabricius.
PI = [S + (G2 + M)]/[(G0/G1) +S + (G2 + M)] Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Changes of apoptosis in the bursa of Fabricius
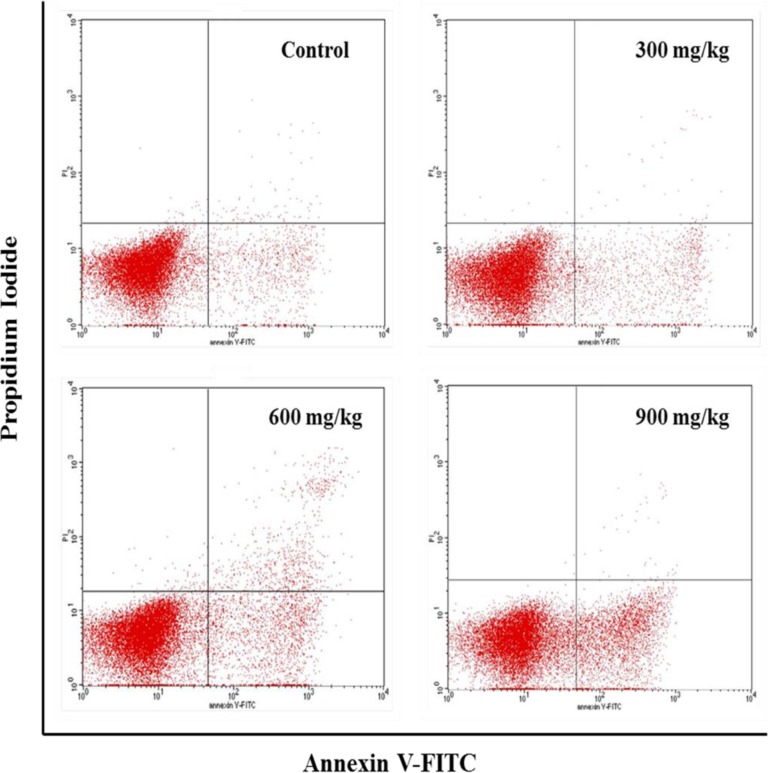
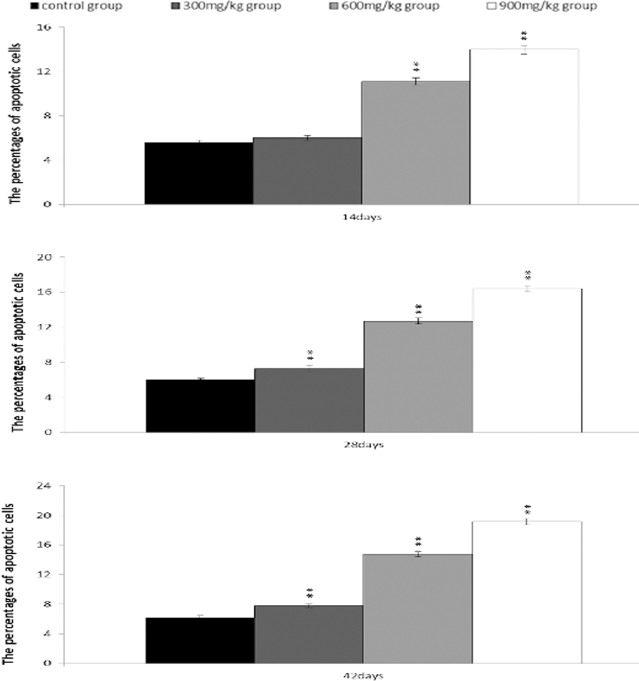
From 14 to 42 days of age during the experiment, the percentages of apoptotic cells in the bursa were increased in the NiCl2-treated groups. The apoptotic percentages were significantly higher (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups than those in the control group, as shown in Figures 13 and 14.
Figure 13. Changes of the apoptosis in the bursa of Fabricius by FCM at 42 days of age.
Figure 14. Changes of the percentages of apoptotic cells in the bursa of Fabricius by FCM.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
Changes of bcl-2, bax, cyt c, Apaf-1, caspase-3, caspase-6, caspase-7 and caspase-9 mRNA expression levels
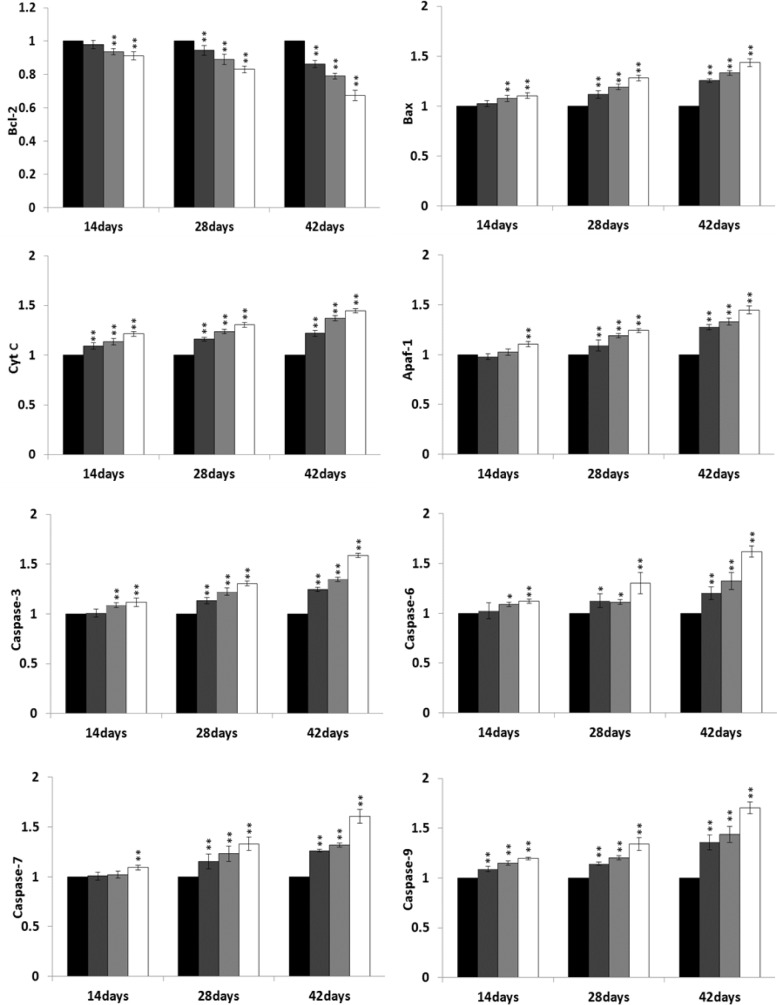
At 14, 28 and 42 days of age during the experiment, the bax, cyt c, Apaf-1, caspase-3, caspase-6, caspase-7 and caspase-9 mRNA expression levels were significantly increased (P < 0.05 or P < 0.01) in the 300 mg/kg, 600 mg/kg and 900 mg/kg groups when compared with those in the control group. However, the bcl-2 mRNA expression levels were significantly lower (P < 0.05 or P < 0.01) in the NiCl2-treated groups than those in the control group from 14 to 42 days of age. The results were shown in Figure 15.
Figure 15. Changes of mRNA expression levels of mitochondrial apoptotic pathway-related factors in the bursa of Fabricius.
Data are the means ± standard deviation (n=5) *p < 0.05, compared with the control group **p < 0.01, compared with the control group.
DISCUSSION
The objective of this study was to evaluate the toxic effects of dietary NiCl2 on bursal growth. In the present study, dietary NiCl2 in 300 mg/kg and over was indeed found to have toxic effects on bursa of Fabricius, including histopathological lesions (Figures 1, 2, 3, 4, 5, 6), reduced relative weight (Figure 7), arrested cell-cycle (Figures 8, 9, 10, 11 and 12) and increased apoptosis (Figures 13 and 14).
It is well known that relative weight, as a satisfactory measure of nutritive value, can also represent the growth state of organs. In the present study, the relative weight of bursa was used to judge the bursal growth. The relative weight of bursa in NiCl2-treated groups was lower than that in control group, implying that dietary NiCl2 in 300 mg/kg and over inhibited the bursal development, and then impaired the bursal function, which was consistent with the cell cycle arrest.
In order to reveal how NiCl2 induced bursal relative weight reduction or growth suppression, we used FCM to measure the cell cycle of bursal lymphocytes. The results showed that NiCl2 induced cell cycle arrest at the G0/G1 phase, which inhibited damaged cells to stop DNA replication at G1 phase, and ultimately resulted in apoptosis of the cells when damaged cells could not be repaired. The above-mentioned observation was supported by the findings that NiCl2 decreased bursal lymphocyte numbers in the S phase and reduced proliferating index, and increased percentages of apoptotic lymphocytes of the bursa. It is clear that the cell cycle arrest and apoptosis of bursal lymphocytes are direct evidences of toxic suppression of the bursa of Fabricius induced by dietary NiCl2.
Apoptosis is a process of programmed cell death morphologically characterized by chromatin condensation, DNA and nuclear fragmentation, cytoplasmic shrinkage and formation of apoptotic bodies [43]. Inappropriately regulated apoptosis is implicated in an extensive variety of diseases [44]. In our study, the increased apoptotic lymphocytes in the bursa of Fabricius were observed in the three NiCl2-treated groups by FCM, which was consistent with the mRNA expression alteration of apoptotic proteins. It was found in the present study that dietary NiCl2 increased bax, cyt c, Apaf-1, caspase-3, caspase-6, caspase-7 and caspase-9 mRNA expression levels, and decreased bcl-2 mRNA expression levels, which explained the occurrence of mitochondrial apoptosis pathway in the bursa of Fabricius because increase of pro-apoptotic or reduction of anti-apoptotic genes could push cells down the apoptotic pathway.
The apoptotic process is mediated by the bcl-2 family proteins and performs in Fas pathway or caspase-independent apoptotic pathway which relies on the mitochondrial active control [45]. Bcl-2 family is classified into anti-apoptotic proteins such as bcl-2 and bcl-xL, which reduces the level of cyt c release [46] and pro-apoptotic proteins such as bax and bak, which induces the release of cyt c and a loss of the mitochondrial membrane potential [47]. The cyt c release is the main steps in the mitochondrial apoptosis pathway [48-50]. After being released, cyt c is immediately combined with Apaf -1, and then activates caspase family to induce apoptosis [51]. Caspases are central mediators of apoptosis [52]. Nevertheless, bcl-2-like proteins can prevent bax-induced cell death by blocking cyt c release [53]. The present study showed that mRNA expression levels of bcl-2, bax, cyt c, Apaf1, caspase-3, caspase-6, caspase-7, caspase-9 in the mitochondrial apoptotic pathways had significant difference between control group and NiCl2-treated groups. The results of our study are similar to reports that exposure of A549 cells to Ni ferrite nanoparticles [54] results in significant increase in mRNA expression levels of cell cycle checkpoint protein p53 and apoptotic proteins (bax, caspase-3, and caspase-9).
Cell cycle arrest and increased apoptotic percentages contributed to the decrease in bursal lymphocytes, which was supported strongly and directly by histopathological evidences. Histopathologically, bursal lymphocytes were decreased with obvious time- and dose-characterization. Simultaneously, dietary NiCl2 has been reported to induce toxicity in the immune organ or tissue, such as spleen, thymus and cecal tonsil [22, 29, 31-33]. B lymphocytes take part in humoral immunity. Thus, the humoral immune function is finally impaired due to the decreased B lymphocyte numbers and reduced B lymphocyte activities in the peripheral blood and lymphatic organ or tissue caused by dietary NiCl2.
In conclusion, dietary NiCl2 in 300 mg/kg and over induces toxic suppression in the bursal growth by decreasing lymphocytes histopathologically and relative weight, arresting cell cycle, and increasing apoptosis percentage. The toxic suppression of bursal growth finally impairs humoral immunity duo to the reduction of B lymphocyte population and B lymphocyte activity in the chicken. This study provides new evidences for further studying the effect mechanism of Ni and Ni compounds on B-cell or bursa of Fabricius.
MATERIALS AND METHODS
Broilers and diets
A total of 280 one-day-old healthy avian broilers were randomly divided into four groups of 70 broilers in each group. The broilers were housed in separate cages with electrically heated units and were provided with water and the control or experimental diets ad libitum for 42 days. The commercial broilers' growth cycle is about 42 days, and then they will be put into use for consumption. In this period they grow rapidly and a lot of diet will be consumed, and broilers will easily affected by diet containing metal pollutants (such as Ni). The aim of our study is to evaluate the effect of dietary NiCl2 on the broilers in the period of growth.
All experimental procedures involving broilers were approved by Animal Care and Use Committee, Sichuan Agricultural University.
A corn-soybean basal diet formulated by the National Research Council [55] was the control diet. NiCl2·6H2O (Cheng Du Kelong Chemical Co., Ltd., Chengdu, China) was mixed into the corn-soybean basal diet to produce experimental diets with 300, 600, and 900 mg/kg of NiCl2, respectively.
Clinical signs and the relative weight of bursa of Fabricius
Clinical signs were observed and recorded daily. At 7, 14, 21, 28, 35 and 42 days of age during the experiment, five broilers in each group were humanely killed. Bursa was taken from each broiler and weighed after dissecting connective tissue around the organ. Relative weight of bursa was calculated by the following formula:
Histopathological observation
At 7, 14, 21, 28, 35 and 42 days of age, five broilers in each group were humanely killed, and the bursae were fixed in 4% buffered formaldehyde and routinely processed in paraffin. Thin sections (5 μm) of each tissue were sliced from each block and mounted on glass. Slices were stained with hematoxylin and eosin (H.E), and then were examined under an Olympus light microscope.
Determination of cell cycle in the bursa of Fabricius by FCM
At 14, 28, and 42 days of age, the bursae of five broilers in each group were selected for determination of the cell-cycle by using FCM, as described by Cui et al. [56].
Bursae were immediately removed and ground to form a cell suspension that was filtered through 300-mesh nylon screen. The cells were suspended in 1×binding buffer at a concentration of 1×106 cells/mL after washing the cells twice with cold phosphate buffer solution (PBS) (pH 7.2-7.4). Five hundred microliters of the solution was transferred to a 5mL culture tube and centrifuged (500-1,000 rpm). After removing the supernatant, 5μL 0.25% Triton X-100 and 5μL propidium iodide were added to the tube. Then the cells were gently vortexed and incubated for 30 min at 25°C in the dark. Finally, 500μL PBS was added to each tube, and the contents were analyzed by FCM (BD FACSCalibur) within 45 min.
Determination of apoptosis in the bursa of Fabricius by FCM
At 14, 28, and 42 days of age, the bursae of five broilers in each group were selected for determination of the bursal apoptotic lymphocytes using FCM, as described by Peng et al. [57].
The cell suspension was prepared as described in the method of cell of cycle. One hundred microliters of the solution was transferred to a 5 mL culture tube, then subsequently adding 5 μl of Annexin V-FITC and 5 μl of propidium iodide to the tube. The cells were gently vortexed and incubated for 15 min at room temperature (25°C) in the dark. Four hundred microliters of 1×binding buffer was added to each tube and analyzed by FCM within 1 h.
Detection of apoptotic protein mRNA expression levels by qRT-PCR
At 14, 28, and 42 days of age during the experiment, the bursae from five broilers in each group were respectively stored in liquid nitrogen. Adding liquid nitrogen, the samples were homogenized using a mortar and pestle. Total RNA was extracted from the powder of bursa of Fabricius by RNA isolate (RNAiso) Plus (9108/9109, Takara, Japan). Then the total RNA reverse transcribed into cDNA using a Prim-Script™ RT reagent Kit (RR047A, Takara, Japan) according to the manufacture's introduce. The cDNA was used as a template for quantitative real-time PCR analysis. Sequences for primers of bcl-2, bax, cyt c, Apaf-1, caspase-3, caspase-6, caspase-7 and caspase-9 and β-Actin were obtained from Genbank and NCBI. Primers were designed with Primer 5 software and synthesized at BGI Tech (Shenzhen, China) (Table 1).
Table 1. A list of primers in qRT-PCR analysis of mRNA expression of the apoptotic proteins.
| Gene symbol | Accession number | Primer | Primer sequence(5′-3′) | Product size | Tm (°C) |
|---|---|---|---|---|---|
| Bax | XM422067 | Forward Reverse |
TCCTCATCGCCATGCTCAT CCTTGGTCTGGAAGCAGAAGA |
69bp | 62 |
| Bcl-2 | NM205339 | Forward Reverse |
GATGACCGAGTACCTGAACC CAGGAGAAATCGAACAAAGGC |
114bp | 62 |
| Cytc | NM001079478 | ForwardReverse | TGTCCAGAAATGTTCCCAGTGC CCTTTGTTCTTATTGGCATCTGTG |
138bp | 61 |
| Apaf-1 | XM416167 | ForwardReverse | AAGGGCATAAGGAAGCAATCAA CAGCACAAGAAAGAACAGCACC |
156bp | 61 |
| Caspase-3 | NM204725 | ForwardReverse | TGGCCCTCTTGAACTGAAAG TCCACTGTCTGCTTCAATACC |
139bp | 62 |
| Caspase-6 | AF469049 | Forward Reverse |
TCAGAGGAGACAAGTGCCAGAGT TACTGAATCCTGAACGAGAACTGG |
107bp | 59 |
| Caspase-7 | XM421764 | ForwardReverse | CCGAAGTCCTCACTCAGTAACCA TTGCGTGTACCCATTCCTGTT |
137bp | 59 |
| Caspase-9 | AY057940 | ForwardReverse | CGAAGGAGCAAGCACGACAG CCGCAGCCCTCATCTAGCAT |
130bp | 61 |
| β-actin | L08165 | ForwardReverse | TGCTGTGTTCCCATCTATCG TTGGTGACAATACCGTGTTCA |
178bp | 62 |
For qRT-PCR reactions, 25 μL mixtures were made by using SYBR® Premix Ex TaqTM II (DRR820A, Takara) containing 12.5 μL SYBR® Premix Ex TaqTM II, 1.0μL of forward and 1.0 μL of reverse primer, 8.5 μL ribonuclease (RNase)-free water and 2 μL cDNA. The real-time PCR reaction conditions were consisted of 3 min at 95°C (first segment, one cycle), 10 s at 95°C and 30 s at Tm of a specific primer pair (second segment, 44 cycles) followed by 10 s at 95°C, and 72°C for 10 s (dissociation curve segment) using Thermal Cycler (C1000, BIO RAD, USA). Gene expression values of control group at 14, 28, and 42 days of age were respectively used for gene expression calibration respectively. The results from the qRT-PCR were analyzed with 2−ΔΔCT assay [58] and β-Actin was used as an internal control gene.
Statistical analysis
The significance of difference among four groups was analyzed by variance analysis, and results presented as mean ± standard deviation (X±SD). The variation was measured by one-way analysis of variance (ANOVA) test of SPSS 16.0 for windows. Statistical significance was considered at P < 0.05.
Acknowledgments
The study was supported by the program for Changjiang scholars and innovative research team in university (IRT0848) and the Shuangzhi project of Sichuan Agricultural University (03570327; 03571198).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Scott-Fordsmand JJ. Reviews of environmental contamination and toxicology. Springer; 1997. Toxicity of nickel to soil organisms in Denmark; pp. 1–34. [Google Scholar]
- 2.Haber LT, Erdreicht L, Diamond GL, Maier AM, Ratney R, Zhao Q. Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharm. 2000;31:210–230. doi: 10.1006/rtph.2000.1377. [DOI] [PubMed] [Google Scholar]
- 3.Doreswamy K, Shrilatha B, Rajeshkumar T, Muralidhara Nickel-induced oxidative stress in testis of mice: evidence of DNA damage and genotoxic effects. J Androl. 2004;25:996–1003. doi: 10.1002/j.1939-4640.2004.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 4.Cempel M, Nikel G. Nickel: a review of its sources and environmental toxicology. Pol J Environ Study. 2006;15:375–382. [Google Scholar]
- 5.Alarifi S, Ali D, Alakhtani S, Al Suhaibani ES, Al-Qahtani AA. Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biol Trace Elem Res. 2014;157:84–93. doi: 10.1007/s12011-013-9871-9. [DOI] [PubMed] [Google Scholar]
- 6.Das KK, Das SN, Dhundasi SA. Nickel, its adverse health effects & oxidative stress. Indian J Med Res. 2008;128:412–425. [PubMed] [Google Scholar]
- 7.Kubrak OI, Husak VV, Rovenko BM, Poigner H, Mazepa MA, Kriews M. Tissue specificity in nickel uptake and induction of oxidative stress in kidney and spleen of goldfish Carassius auratus, exposed to waterborne nickel. Aquat Toxicol. 2012;118:88–96. doi: 10.1016/j.aquatox.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Cempel M, Janicka K. Distribution of nickel, zinc, and copper in rat organs after oral administration of nickel(II) chloride. Biol Trace Elem Res. 2002;90:215–226. doi: 10.1385/BTER:90:1-3:215. [DOI] [PubMed] [Google Scholar]
- 9.Ling J, Leach R. Studies on nickel metabolism: interaction with other mineral elements. Poultry Sci. 1979;58:591–596. doi: 10.3382/ps.0580591. [DOI] [PubMed] [Google Scholar]
- 10.Sunderman FW., Jr. Mechanism of nickel carcinogenesis. Scand J Work Environ Health. 1989;15:1–12. doi: 10.5271/sjweh.1888. [DOI] [PubMed] [Google Scholar]
- 11.Kasprzak KS, Sunderman FW, Jr, Salnikow K. Nickel carcinogenesis. Mutat Res. 2003;533:67–97. doi: 10.1016/j.mrfmmm.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Haley PJ, Bice DE, Muggenburg BA, Hann FF, Benjamin SA. Immunopathologic effects of nickel subsulfide on the primate pulmonary immune system. Toxicol Appl Pharmacol. 1987;88:1–12. doi: 10.1016/0041-008x(87)90264-x. [DOI] [PubMed] [Google Scholar]
- 13.Donskoy E, Donskoy M, Forouhar F, Gillies C, Marzouk A, Reid M, Zaharia O, Sunderman F. Hepatic toxicity of nickel chloride in rats. Ann Clin Lab Sci. 1986;16:108–117. [PubMed] [Google Scholar]
- 14.Doreswamy K, Shrilatha B, Rajeshkumar T. Nickel-Induced oxidative stress in testis of mice: Evidence of DNA damage and genotoxic effects. J Androl. 2004;25:996–1003. doi: 10.1002/j.1939-4640.2004.tb03173.x. [DOI] [PubMed] [Google Scholar]
- 15.Anke M, Grun M, Dittrich G, Groppel B, Hennig A. Low nickel rations for growth and reproduction in pigs. International Symposium on Trace Element Metabolism Inanimal; Madison, WI, USA: University Park Press; 1974. pp. 715–718. [Google Scholar]
- 16.Costa M, Klein CB. Nickel carcinogenesis, mutation, epigenetics, or selection. Environ Health Persp. 1999;107:A438–A439. doi: 10.1289/ehp.99107a438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perminova I, Sinel'shchikova T, Alekhina N, Perminova E, Zasukhina G. Individual sensitivity to genotoxic effects of nickel and antimutagenic activity of ascorbic acid. Bull Exp Biol Med. 2001;131:367–370. doi: 10.1023/a:1017912521543. [DOI] [PubMed] [Google Scholar]
- 18.Lee-Chen SF, Wang MC, Yu CT, Wu DR, Jan KY. Nickel chloride inhibits the DNA repair of UV-treated but not methyl methanesulfonate-treated Chinese hamster ovary cells. Biol Trace Elem Res. 1993;37:39–50. doi: 10.1007/BF02789400. [DOI] [PubMed] [Google Scholar]
- 19.Dally H, Hartwig A. Induction and repair inhibition of oxidative DNA damage by nickel (II) and cadmium (II) in mammalian cells. Carcinogen. 1997;18:1021–1026. doi: 10.1093/carcin/18.5.1021. [DOI] [PubMed] [Google Scholar]
- 20.Au A, Ha J, Hernandez M, Polotsky A, Hungerford DS, Frondoza CG. Nickel and vanadium metal ions induce apoptosis of T-lymphocyte Jurkat cells. J Biomed Mater Res. 2006;79:512–521. doi: 10.1002/jbm.a.30811. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Lee SH, Seo YR, Perkins SN, Kasprzak KS. Nickel (II)-induced apoptosis in murine T cell hybridoma cells is associated with increased fas ligand expression. Toxicol Appl Pharmacol. 2002;185:41–47. doi: 10.1006/taap.2002.9513. [DOI] [PubMed] [Google Scholar]
- 22.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem Toxicol. 2014;63:18–29. doi: 10.1016/j.fct.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Toxicological effects of nickel chloride on the cytokine mRNA expression and protein levels in intestinal mucosal immunity of broilers. Environ Toxicol. 2015;30:1309–1321. doi: 10.1002/tox.22001. [DOI] [PubMed] [Google Scholar]
- 24.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Dietary nickel chloride restrains the development of small intestine in broilers. Biol Trace Elem Res. 2013;155:236–246. doi: 10.1007/s12011-013-9792-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Dietary nickel chloride induces oxidative intestinal damage in broilers. Int J Environ Res Public Health. 2013;10:2109–2119. doi: 10.3390/ijerph10062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Huang J. Toxicological Effects of Nickel Chloride on IgA+ B Cells and sIgA, IgA, IgG, IgM in the Intestinal Mucosal Immunity in Broilers. Int J Environ Res Public Health. 2014;11:8175–8192. doi: 10.3390/ijerph110808175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu B, Cui H, Peng X, Pan K, Fang J, Zuo Z, Deng J, Wang X, Huang J. Effects of Dietary Nickel Chloride on the Intestinal Microbiota. Ecotoxicology and Environmental Safety. 2014;109:70–76. doi: 10.1016/j.ecoenv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J. Analysis of the toll-like receptor 2-2 (TLR2-2) and TLR4 mRNA expression in the intestinal mucosal immunity of broilers fed on diets supplemented with nickel chloride. Int J Environ Res Public Health. 2014;11:657–670. doi: 10.3390/ijerph110100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wu B. Effect of dietary nickel chloride on splenic immune function in broilers. Biol Trace Elem Res. 2014;159:183–191. doi: 10.1007/s12011-014-0003-y. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B. Downregulation of TLR4 and 7 mRNA Expression Levels in Broiler's Spleen Caused by Diets Supplemented with Nickel Chloride. Biol Trace Elem Res. 2014;158:353–358. doi: 10.1007/s12011-014-9938-2. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wu B. The Association between Splenocyte Apoptosis and Alterations of Bax, Bcl-2 and Caspase-3 mRNA Expression, and Oxidative Stress Induced by Dietary Nickel Chloride in Broilers. Int J Environ Res Public Health. 2013;10:7310–7326. doi: 10.3390/ijerph10127310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang K, Guo H, Deng J, Cui H, Peng X, Fang J, Zuo Z, Wang X, Li J, Yin S. Inhibitive Effects of Nickel Chloride (NiCl2) on Thymocytes. Biol Trace Elem Res. 2015;164:242–252. doi: 10.1007/s12011-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 33.Tang K, Li J, Yin S, Guo H, Deng J, Cui H. Effects of Nickel Chloride on Histopathological Lesions and Oxidative Damage in the Thymus. Health. 2014;6:2875. [Google Scholar]
- 34.Guo H, Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Deng J, Yin S, Li J, Tang K. NiCl2-down-regulated antioxidant enzyme mRNA expression causes oxidative damage in the broiler's kidney. Biol Trace Elem Res. 2014;162:288–295. doi: 10.1007/s12011-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Tang K, Yin S. Effects of Nickel Chloride on the Erythrocytes and Erythrocyte Immune Adherence Function in Broilers. Biol Trace Elem Res. 2014;161:173–179. doi: 10.1007/s12011-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Deng H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Chen K. Nickel chloride (NiCl2)-caused inflammatory responses via activation of NF-κB pathway and reduction of anti-inflammatory mediator expression in the kidney. Oncotarget. 2015;6:28607–28620. doi: 10.18632/oncotarget.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo H, Deng H, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wang X, Wu B, Chen K. Dietary NiCl2 causes G2/M cell cycle arrest in the broiler's kidney. Oncotarget. 2015;6:35964–35977. doi: 10.18632/oncotarget.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen FH, Shuler TR, McLeod TG, Zimmerman TJ. Nickel influences iron metabolism through physiologic, pharmacologic and toxicologic mechanisms in the rat. J Nutr. 1984;114:1280–1288. doi: 10.1093/jn/114.7.1280. [DOI] [PubMed] [Google Scholar]
- 39.Sunderman FW, Dingle B, Hopfer SM, Swift T. Acute nickel toxicity in electroplating workers who accidently ingested a solution of nickel sulfate and nickel chloride. Am J Ind Med. 1988;14:257–266. doi: 10.1002/ajim.4700140303. [DOI] [PubMed] [Google Scholar]
- 40.Wu B, Cui H, Peng X, Fang J, Cui W, Liu X. Pathology of Bursae of Fabricius in Methionine-Deficient Broiler Chickens. Nutrients. 2013;5:877–886. doi: 10.3390/nu5030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciriaco E, Píñera PP, Díaz-Esnal B, Laurà R. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius) Microsc Res Technol. 2003;62:482–487. doi: 10.1002/jemt.10416. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Cao J, Wang Z, Dong Y, Chen Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of Fabricius in broilers via monochromatic lights. Journal of Photochemistry and Photobiology B: Biology. 2015;142:29–34. doi: 10.1016/j.jphotobiol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Lossi L, Merighi A. In vivo cellular and molecular mechanisms of neuronal apoptosis in the mammalian CNS. Prog. Neurobiol. 2003;69:287–312. doi: 10.1016/s0301-0082(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 44.Chamond RR, Carracedo J, Moreno AF, Guerra P. Apoptosis and disease. Alergol Inmunol Clin. 1999;14:367–374. [Google Scholar]
- 45.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard S, Bottino C, Brooke S. Neuroprotective effects of Bcl-2 overexpression in hippocampal cultures: interactions with pathways of oxidative damage. J Neurochem. 2002;83:914–923. doi: 10.1046/j.1471-4159.2002.01198.x. [DOI] [PubMed] [Google Scholar]
- 47.Starkov A, Polster B, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 48.Stevens J M. Cytochrome c as an experimental model protein. Metallomics. 2011;3:319–322. doi: 10.1039/c0mt00089b. [DOI] [PubMed] [Google Scholar]
- 49.Gustavsson T, Trane M, Moparthi VK. A cytochrome c fusion protein domain for convenient detection, quantification, and enhanced production of membrane proteins in Escherichia coli—expression and characterization of cytochrome-tagged Complex I subunits. Protein Sci. 2010;19:1445–1460. doi: 10.1002/pro.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huttemann M, Helling S, Sanderson TH. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012;1817:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Kim C N, Yang J. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 52.Denault JB, Salvesen GS. Apoptotic caspase activation and activity. Apoptosis Cancer Humana Press. 2008;414:191–220. doi: 10.1007/978-1-59745-339-4_15. [DOI] [PubMed] [Google Scholar]
- 53.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 54.Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J, Musarrat J, Al-Khedhairy AA. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicol. 2011;283:101–108. doi: 10.1016/j.tox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 55.NRC. Nutrient requirements of poultry. National Research Council. National Academy Press; Washington eUSA USA: 1994. [Google Scholar]
- 56.Cui W, Cui H, Peng X, Fang J, Zuo Z, Liu Dietary excess vanadium induces lesions and changes of cell cycle of spleen in broilers. Biol Trace Elem Res. 2011;143:949–956. doi: 10.1007/s12011-010-8938-0. [DOI] [PubMed] [Google Scholar]
- 57.Peng X, Cui Y, Cui W, Deng J, Cui H. The decrease of relative weight, lesions, and apoptosis of bursa of fabricius induced by excess dietary selenium in chickens. Biol Trace Elem Res. 2009;131:33–42. doi: 10.1007/s12011-009-8345-6. [DOI] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen T D. Analysis of relative gene expression data using real-time PCR and the 2 (−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]