Abstract
N-myc downstream-regulated gene 2 (NDRG2) is a tumor suppressor and cell stress-related gene. NDRG2 is associated with tumor incidence, progression, and metastasis. NDRG2 regulates tumor-associated genes and is regulated by multiple conditions, treatments, and protein/RNA entities, including hyperthermia, trichostatin A and 5-aza-2′-deoxycytidine, which are promising potential cancer therapeutics. In this review, we discuss the expression as well as the clinical and pathological significance of NDRG2 in cancer. The pathological processes and molecular pathways regulated by NDRG2 are also summarized. Moreover, mechanisms for increasing NDRG2 expression in tumors and the potential directions of future NDRG2 research are discussed. The information reviewed here should assist in experimental design and increase the potential of NDRG2 as a therapeutic target for cancer.
Keywords: N-myc downstream-regulated gene 2, cancer, pathological processes, molecular pathways
INTRODUCTION
Cancer represents a large group of complex and multifactorial diseases that involve abnormal cell growth with the potential to invade other tissues [1]. Cancer accounted for approximately 8 million deaths in 2010, and invasive cancer was the leading cause of death in the developed world and the second leading cause of death in the developing world [2, 3]. Despite recent advances, effective clinical management remains elusive because of intra-tumoral heterogeneity and therapeutic resistance [4–6]. Therefore, it is essential to investigate the pathophysiology of cancer and identify novel therapies [7, 8]. Notably, associations between cancer (including lung cancer, prostate cancer, liver cancer, colorectal cancer and breast cancer) and N-myc downstream-regulated gene 2 (NDRG2) have been reported [2, 9–15]. Thus, NDRG2 may be a promising target for cancer.
The NDRG family consists of NDRG1, NDRG2, NDRG3 and NDRG4 [16, 17]. This family of proteins is characterized by an esterase/lipase/thioesterase active site serine and an α/β hydrolase fold of approximately 220 amino acids [16–19]. NDRG2 is an important member of the NDRG family and is located at chromosome 14q11.2. The structure and tissue distribution of NDRG2 have been previously studied and reviewed [20]. NDRG2 has been suggested to be a tumor suppressor and cell stress-related gene that is involved in cellular metabolic processes, such as hormone, ion, and fluid metabolism [21–23], and in stress responses, such as those to hypoxia and lipotoxicity [24, 25]. However, the associations between NDRG2 and cancer and the corresponding mechanistic details require intensive research.
This review focuses on the latest progress regarding the associations between NDRG2 and cancer. First, the expression as well as the clinical and pathological significance of NDRG2 in cancer is introduced. Then, we summarize how NDRG2 regulates pathological processes and molecular pathways in tumors and discuss mechanisms for increasing NDRG2 expression. Finally, potential directions for future NDRG2 research are discussed. The information compiled here comprehensively characterizes NDRG2 activity related to cancer, thus potentially aiding in the design of experimental research and promoting NDRG2 as a therapeutic target for cancer.
NDRG2 AND THE NDRG FAMILY
The term “NDRG” was first used by Shimono et al. [26] for the Ndr1 gene, which is up-regulated in N-Myc-knockout mouse embryos. The NDRG proteins (NDRG1, NDRG2, NDRG3 and NDRG4) are included within the α/β hydrolase group of enzymes, despite the lack of a hydrolytic catalytic site and a deficiency in enzyme function [16, 18, 27, 28]. Although the identity at the residue level is approximately 57-65% among members [17, 29], each NDRG family member forms a separate homology cluster across multiple species with specific and functionally divergent roles [16]. Phylogenetic analyses have revealed that NDRG1 and NDRG3 belong to one subfamily, whereas NDRG2 and NDRG4 belong to another subfamily [17]. The NDRG proteins are characterized by an esterase/lipase/thioesterase active site serine and an α/β hydrolase fold of approximately 220 amino acids [16–19]. The detailed structural features of human NDRG proteins have been discussed by Veerle et al. [16].
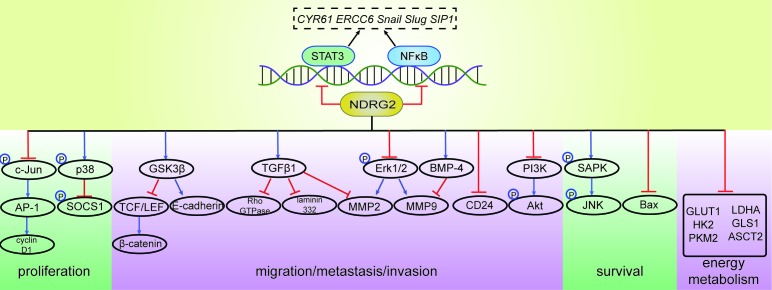
NDRG proteins have important roles in cell proliferation and differentiation. Notably, NDRG protein expression appears to positively correlate with progressive stages of differentiation. Low expression levels are detected at a relatively early embryonic stage, and expression levels are increased in postnatal and mature animals [16]. NDRG3 and NDRG4 respond to stress [32, 33] in addition to their roles as tumor-related genes [30, 31]. NDRG1 has been demonstrated to be negatively correlated with tumor progression [34–37]. Currently, there is no evidence demonstrating that NDRG1 acts as a transcription factor, and it lacks nuclear targeting sequence [38]. However, NDRG1 may affect other transcription factors, such as nuclear factor-kappa B (NF-κB), mothers against decapentaplegic homolog 4 (Smad4) and others [39–41]. The activation of NDRG1 and NDRG2 are activated in a similar manner related to phosphorylation [42–44]. NDRG2 has been shown to participate in ischemia-reperfusion injury [45], Alzheimer's disease [46, 47], depression [48, 49] and hypoxia [25, 50]. The role of NDRG2 in cancer has attracted increasing attention, and this topic will be discussed below (Figure 1 and Table 1).
Figure 1. The association between NDRG2 and cancer and NDRG2 regulation in tumors.
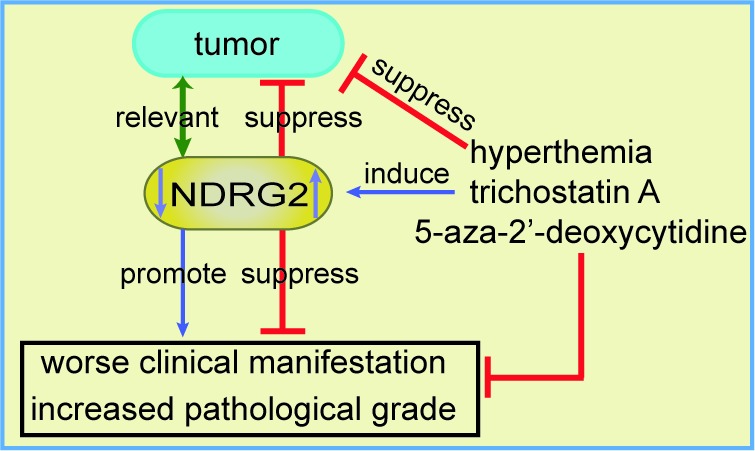
The down-regulation of NDRG2 is associated with tumor incidence, although there is insufficient evidence for a causal relationship, and NDRG2 down-regulation is associated with worse clinical manifestations and increased pathological grade. Hyperthermia, trichostatin A and 5-aza-2′-deoxycytidine up-regulate the expression of NDRG2, which may further inhibit tumor development.
Table 1. Role of NDRG2 in cancer.
| Tumor type | Expression level of NDRG2 | Correlation between the NDRG2 expression level with | Regulators | Effect of over-expression of NDGR2 | Reference No | |
|---|---|---|---|---|---|---|
| clinical significance | pathological significance | |||||
| Gastric cancer | mRNA and protein levels are lower | Survival rate of NDRG2-negative patients is lower. Silenced NDRG2 is associated with worse prognosis and shorter disease-free survival. | NDRG2 methylation and down-regulated NDRG2 are negatively related to depth of tumor invasion, Borrmann classification and TNM stage. | 5-aza-2′-deoxycytidine and trichostatin A | Inhibit invasion | [60, 61, 71, 75] |
| Hyperthermia | Increase apoptosis rate | |||||
| Colorectal cancer | mRNA and protein levels are lower | There is a trend for NDRG2 level to decrease with increasing Dukes' stage. Patients with reduced level of NDRG2 mRNA have a statistically significantly shorter disease-free survival and overall survival duration. | There is a trend for NDRG2 level to decrease with tumor invasion depth and histology grading. | -- | -- | [13, 14, 62–64, 68, 69] |
| ESCC | protein is lower | The expression of NDRG2 is inversely associated with clinical stage, patients' vital status and 5-year overall survival rate. | The expression of NDRG2 is inversely associated with TNM classification, and histological differentiation. | -- | Reduce cell proliferation, colony formation and DNA replication activity | [58, 70] |
| Hepatocellular carcinoma | mRNA and protein levels are lower | NDRG2 down-regulation in patients is accompanied with elevated AFP serum level, portal vein thrombi, recurrence and lower survival rate. | NDRG2 down-regulation in patients with late TNM stage, infiltrative growth pattern, poor differentiation grade, nodal/distant metastasis and tumor invasion. | Non-steroidal FXR agonists | Inhibit tumor growth and metastasis potential of corresponding cells | [9–11, 59, 73, 74] |
| Sh-NDRG2 | Enhance EMT | |||||
| Ad-NDRG2 | Increase apoptosis rate | |||||
| Gallbladder carcinoma | -- | Patients with NDRG2 negative expression correlate with worse prognosis and lower survival rate. | Down-regulation of NDRG2 tends to show deeper invasion depth and higher TNM stage. | -- | -- | [55] |
| Pancreatic cancer | mRNA is lower | There exists a significant association between poor prognosis and NDRG2-negative expression. | -- | -- | -- | [10, 72] |
| Glioblastoma | protein is lower | Survival rate of patients is significantly negative with NDRG2 expression level. | Glioma tumor grade is significantly negative with NDRG2 expression level. | cDNA encoding hNDRG2 | Reduce the cell proliferation | [19, 54–56] |
| Astrocytomas | mRNA and protein levels are lower | NDRG2 expression is positively correlated with the life span of astrocytoma patients. | NDRG2 expression is negatively correlated with pathological grading. | -- | -- | [51, 56] |
| MeningiomaA6:A6:B14 | mRNA and protein levels are lower | -- | -- | -- | -- | [52] |
| Neuroblastoma | -- | -- | -- | Inhibit cell proliferation | [57] | |
| Renal cell carcinoma | mRNA and protein levels are lower | The down-regulation of NDRG2 expression is associated with higher tumor recurrence and lower survival rate. | The down-regulation of NDRG2 expression is negatively associated with TNM stage, tumor magnitude, nuclear grade, Fuhrman's grade and tumor invasion. | -- | Inhibit tumor cell growth, migration and invasion | [79–82] |
| Prostate cancer | protein is lower | Low NDRG2 expression is significantly associated with short recurrence-free survival and overall survival. | The down-regulation of NDRG2 in prostate cancer tissues is significantly correlated with advanced pathological stage, positive metastatic status and high Gleason score. | Ad-NDRG2 | Inhibit tumor growth and invasion | [77, 78] |
| Bladder cancer | protein is lower | -- | The NDRG2 level is negatively correlated with tumor grade and pathologic stage. | LEN-NDRG2 | Inhibit cell proliferation | [12] |
| Breast cancer | mRNA is lower | Patients with high NDRG2 expression have better disease-free survival and overall survival. | NDRG2 overexpression suppresses breast cancer cell adhesion and invasion. | -- | -- | [21, 83, 84] |
| Lung cancer | protein is lower | NDRG2 level is negatively correlated with UICC stage, and positively correlated with survival time. | NDRG2 level is negatively correlated with pathological metastasis and TNM stage. | -- | -- | [15, 86] |
| Thyroid cancer | mRNA and protein levels are lower | -- | There is no significant correlation between NDRG2 expression and distant metastases. | -- | -- | [83, 87] |
| Fibrosarcoma | -- | -- | Tumor migration is significantly reduced by NDRG2. | Injected with NDRG2 cells | Inhibit cell proliferation | [88] |
| Oral squamous-cell carcinoma | mRNA is lower | -- | Induction of NDRG2 expression significantly inhibits cell proliferation. | -- | -- | [89] |
| Myeloid leukemia | protein is lower | -- | -- | -- | -- | [90] |
ASSOCIATIONS BETWEEN NDRG2 AND CANCER
The associations between NDRG2 and cancer have been reported in neurologic tumors [19, 51–57], gastrointestinal tumors [9–11, 13, 14, 55, 58–75], genitourinary tumors [12, 76–82], breast cancer [21, 83–85], lung cancer [15, 86], thyroid cancer [83, 87], fibrosarcoma [88], oral squamous-cell carcinoma [89], myeloid leukemia [90] and cervical cancer (Hela cells) [25] (Table 1). Collectively, NDRG2 expression is associated with the clinical features of tumors. NDRG2 levels are positively correlated with tumor differentiation but negatively correlated with lymph node metastasis and TNM stage. Furthermore, NDRG2 levels tend to decrease with tumor invasive depth and increasing grade (Table 1 and Figure 1). Epigenetic silencing of the NDRG2 promotor has been found in the majority of primary tumors, which may elicit resistance to anticancer drugs. However, whether NDRG2 down-regulation is a cause or a consequence of the progression from normal tissue to cancerous tissue remains unclear. NDRG2 down-regulation is associated with cancer development and progression, including such features as malignant clinical manifestations and increased pathological grade. NDRG2 is a relevant biomarker for predicting aggressive behavior, tumor recurrence and overall patient survival, independently or in combination with other factors, such as CD24, phospho-STAT3, and HOXD1. Ad-NDRG2, LEN-NDRG2, and B16F10-NDRG2 injections as well as other interventions that increase NDRG2 expression may control tumor progression. Therefore, NDRG2 up-regulation may be a promising therapeutic strategy for the treatment of cancer.
MOLECULAR TARGETS OF NDRG2
To understand the role of NDRG2 in cancer and provide insight into its mechanisms of action and potential applications, we have focused on the molecular basis of NDRG2 activity in this section. As a master switch for cell proliferation and differentiation, NDRG2 mainly exerts biological activity by modulating protein expression and phosphorylation.
Proliferation-associated proteins and pathways
Cyclin D1 belongs to the highly conserved cyclin family, whose members are characterized by a dramatic periodicity in protein abundance during the cell cycle [91]. The overexpression of cyclin D1 alters cell cycle progression, which may contribute to tumorigenesis; indeed, cyclin D1 overexpression has been observed in various cancers [91]. In SW620 colon carcinoma cells, the induction of NDRG2 decreases c-Jun phosphorylation at Ser63, which is followed by the attenuation of the transcriptional activator AP-1 (activator protein-1). This further down-regulates cyclin D1 and results in cell cycle arrest at G1/S [67]. In addition, NDRG2 siRNA can reverse the phenotype of NDRG2-expressing cells, recovering c-Jun phosphorylation and cyclin D1 expression as well as cell proliferation [67]. In conclusion, NDRG2 modulates intracellular signals to inhibit cell proliferation by suppressing c-Jun phosphorylation and cyclin D1 expression.
P38 mitogen-activated protein kinase (MAPK) plays an important role in key cellular processes related to cancer [92, 93]. Liu et al. [94] conducted a microarray study to determine the expression profile of NDRG2-overexpressing HepG2 cells and found that p38 phosphorylation was increased by NDRG2. Furthermore, in malignant breast cancer cells, NDRG2 overexpression specifically inhibits suppressor of cytokine signaling 1 (SOCS1) phosphorylation and induces the phosphorylation of p38 MAPK [95]. Inhibiting p38 MAPK activity blocks the induction of SOCS1 expression by NDRG2 [95]. Therefore, NDRG2 expression can increase the phosphorylation of p38 MAPK, which further inhibits the phosphorylation of SOCS1 and suppresses tumor proliferation. Interestingly, inhibitors of p38 MAPK have attracted attention in research related to cancer treatment [92, 93]; however, NDRG2 exerts anti-tumor effects via the activation of p38 MAPK.
Migration/metastasis/invasion-associated proteins and pathways
β-catenin is a dual-function protein that regulates cell-cell adhesion and gene transcription [96]. Mutation and overexpression of β-catenin are associated with the incidence of cancer. NDRG2 inhibits c-Myc expression by suppressing the expression of β-catenin [97], and the possible mechanisms for this effect have been investigated. The nuclear localization of β-catenin and the inappropriate activation of T-cell factor (TCF)/lymphoid enhancer factor (LEF)-mediated transcription appear to be important processes for establishing and maintaining cancer stem cells [98]. The introduction of wild-type, but not mutant, NDRG2 reduces the transcriptional activity of TCF/LEF [68]. Intracellular β-catenin levels are reduced in NDRG2-transfected SW620 cells, and the suppression of β-catenin stability and TCF/LEF activity is mediated through the activation of glycogen synthase kinase 3β (GSK-3β) by NDRG2. The attenuation of TCF/β-catenin signaling by NDRG2 contributes to the maintenance of healthy tissues and the suppression of tumor metastasis [68].
E-cadherin, a classical member of the cadherin superfamily, is a well-known tumor suppressor [99]. Positive correlations between the expression of E-cadherin and NDRG2 have been observed in cancer [66, 79]. Snail is a zinc-finger transcriptional repressor that has been shown to mediate the regulation of E-cadherin expression by NDRG2 [66]. The enhancement of GSK-3β activity by NDRG2 overexpression causes the proteasomal degradation of Snail followed by the transcriptional de-repression of E-cadherin. In renal cell carcinoma and colon cancer cells, NDRG2 can recover E-cadherin expression, and this effect can be reversed by NDRG2 siRNA [66, 79]. Through GSK-3β activation, NDRG2 promotes cell density-regulated E-cadherin expression and exerts anti-tumor effects.
Transforming growth factor beta 1 (TGFβ1), a member of the multifunctional set of TGFβ peptides, controls cell proliferation and differentiation [100]. Down-regulation of the TGFβ pathway is associated with cancer development and progression. Furthermore, dysregulation of TGFβ activation and signaling can result in apoptosis [101]. NDRG2 antagonizes TGFβ1-mediated tumor cell invasion by down-regulating the expression of matrix metalloproteinase 2 (MMP2) and laminin 332 pathways, with concomitant suppression of Rho GTPase activity [11].
Proteins in the MMP family are involved in the breakdown of the extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis [102]. There is evidence for an association between MMPs and cancer [103, 104]. NDRG2 overexpression inhibits the expression of MMP2 and MMP9 in clear cell renal cell carcinoma (CCRCC) and hepatocellular carcinoma (HCC) [11, 65, 81]. Moreover, NDRG2 knockdown increases cell invasion, which is rescued by treating HepG2 cells with the extracellular signal-regulated kinase (ERK) inhibitor PD98059, thus revealing that ERK1/2 phosphorylation is reduced in NDRG2-overexpressing cells and can further increase MMP expression [65]. In HCC cells, phospho-ERK1/2 levels were significantly decreased when NDRG2 was overexpressed [74]. There are several mechanisms by which NDRG2 suppresses MMP expression. In fibrosarcoma and murine melanoma, NDRG2 expression significantly suppresses tumor invasion by inhibiting MMP activity, which is regulated by NF-κB signaling [88]. The suppression of MMP2 can be reversed by the activation of TGFβ1 in response to NDRG2 overexpression [11]. MMPs, especially MMP9, can also be suppressed via the activation of bone morphogenetic protein-4 (BMP-4) [105], which will be discussed further in the next section. Thus, NDRG2 overexpression can suppress MMPs via various mechanisms, which further suppress tumor invasion.
BMP-4 is a member of the BMP family that stimulates tissue formation and differentiation, and the abnormal expression of BMP-4 may be associated with tumor development [106]. In breast cancer cells, the specific induction of active BMP-4 is exclusively observed in breast cancer cells expressing NDRG2 but not in control breast cancer cells, and NDRG2 expression inhibits the mRNA expression of several MMPs and the gelatinolytic activity of MMP9 [105]. Neutralization of BMP-4 in NDRG2-expressing breast cancer cells results in the rescue of MMP9 mRNA expression and migration capacity. Additionally, treatment with recombinant BMP-4 dramatically suppresses MMP9 mRNA expression and gelatinolytic MMP9 activity as well as the migration and invasion of MDA-MB-231 cells and PMA-treated MCF-7 cells [105]. Thus, the induction of BMP-4 by NDRG2 inhibits the metastatic potential of cancer cells, specifically by suppressing MMP9 activity.
CD24 mediates cell-cell interactions as a surface marker that is expressed on cancer cells [107]. Jaggupilli et al. [107] analyzed the significance of CD24 as a cancer stem cell surface marker. There is a negative correlation between NDRG2 expression and CD24 expression in gallbladder carcinoma (GBC), HCC, breast cancer and lung adenocarcinoma [55, 73, 84]. NDRG2 inhibits CD24 expression and further suppresses tumor adhesion, migration and invasion in HCC [73]. CD24 may be a downstream target of NDRG2 in cancer, and the combination of CD24 and NDRG2 is considered an effective biomarker of tumor behavior.
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway plays a critical role in malignant transformation as well as in tumor growth and metastasis [108, 109]. The majority of oral squamous cell carcinoma (OSCC) cell lines have activated PI3K/Akt signaling. Furthermore, positive p-Akt staining is inversely correlated with decreased NDRG2 expression in OSCC samples with moderate to poor differentiation [89]. Moreover, the enforced expression of NDRG2 in HSC-3 cells decreases the phosphorylation of Akt at Serine 473 [89]. In malignant breast cancer, NDRG2 overexpression has been demonstrated to specifically inhibit Akt phosphorylation [95]. Thus, NDRG2 contributes to the genesis and progression of OSCC and breast cancer partly through the inhibition of PI3K/Akt signaling.
Survival-associated proteins and pathways
Stress-activated protein kinase/c-Jun NH(2)-terminal kinase (SAPK/JNK) activation occurs in response to cellular stresses and extracellular signals. The activation of SAPK/JNK plays a key role in regulating cell survival, apoptosis, and proliferation [110]. NDRG2 overexpression in malignant breast cancer cells specifically induces the phosphorylation of SAPK and JNK, which contributes to the survival of normal cells and the apoptosis of tumor cells [95].
Bcl-2-associated X protein (Bax) is a member of the Bcl-2 gene family that functions as an apoptotic activator [111]. Bax has been demonstrated to be deregulated through mutation or the inhibition of expression, which increases resistance to chemotherapy and radiotherapy [111]. However, in Hela cells, NDRG2 has been shown to abolish the radiation-induced up-regulation of Bax, to contribute to the survival of Hela cells, and to prevent tumorigenesis [25].
Energy metabolism-associated proteins and pathways
Glucose transporter 1 (GLUT1), also known as solute carrier family 2, is a uniporter protein that is encoded by the SLC2A1 gene in humans. GLUT1 facilitates the transport of glucose across the plasma membrane of mammalian cells, and GLUT1 overexpression is a prognostic indicator for cancer [112]. NDRG2 expression is negatively correlated with GLUT1 expression in breast carcinoma tissues; NDRG2 promotes GLUT1 protein degradation but does not affect GLUT1 transcription [21]. In colorectal cancer cells, NDRG2 suppresses the expression of GLUT1 as well as of other glucose transporters and catalytic enzymes involved in glycolysis and glutaminolysis, including hexokinase 2 (HK2), pyruvate kinase M2 isoform (PKM2), lactate dehydrogenase A (LDHA), the glutamine transporter ASC amino acid transporter 2 (ASCT2) and glutaminase 1 (GLS1) [97].
Transcription factors and genes
Signal transducer and activator of transcription (STAT) activation within tumor cells contributes to pro-survival phenotypes [113, 114]. Signal transducer and activator of transcription 3 (STAT3) plays important roles in tumor cell proliferation, survival, invasion and immunosuppression [115, 116]. In addition to its established role as a transcription factor in cancer, STAT3 regulates mitochondrion function and gene expression through epigenetic mechanisms [115]. Moreover, STAT3 activation in both resting and IGF-stimulated cells is remarkably inhibited by NDRG2 expression [95]. The recovery of STAT3 phosphorylation can further block the inhibition of SOCS1 expression by NDRG2 [95]. When NDRG2 was overexpressed in HCC cells, STAT3 phosphorylation levels were significantly decreased [74]. These data demonstrate that NDRG2 expression inhibits STAT3 activation, thus affecting the expression of several genes and contributing to anti-tumor effects.
NF-κB constitutes a family of transcription factors involved in the regulation of a wide variety of biological responses, including cytokine production and cell survival. NF-κB regulates the expression of genes involved in many processes that play key roles in the development and progression of cancer, such as cell proliferation, migration and apoptosis. Aberrant or constitutive NF-κB activation has been observed in cancer [117, 118]. Kim et al. [88] found that NDRG2 suppresses NF-κB activity and affects cancer cell invasion by suppressing MMPs in colon carcinoma cells.
The Cockayne syndrome group B protein (ERCC6) gene is an NDRG2-inducible target gene in HCC [119]. ERCC6 gene expression is suppressed by Ad-NDRG2 in combination with rAd-p53, but the NDRG2-enhanced apoptosis is reversed after transfection with ERCC6 [57]. Cysteine-rich protein 61 (CYR61) is an important proliferation-related gene that can be inhibited by NDRG2 overexpression [59]. NDRG2 expression also inhibits the expression of epithelial-to-mesenchymal transition (EMT)-related genes, such as Snail, Slug, and Smad-interacting protein 1 (SIP1), and decreases EMT signaling in renal cell carcinoma [79].
Overall, NDRG2 regulates several transcription factors, which further impact the expression of downstream genes. NDRG2 has been reported to modulate tumor-related genes; however, whether NDRG2 is a transcription factor itself or acts via other transcription factors is unclear.
Thus, NDRG2 exerts anti-tumor effects through various mechanisms that are summarized in Figure 2.
Figure 2. Molecular targets of NDRG2.
NDRG2 acts on various proteins to inhibit tumor proliferation; suppress migration, metastasis and invasion; maintain normal cell survival; and interrupt energy metabolism in tumors. Several genes are regulated by NDRG2, which may function via interactions with transcription factors, such as NF-κB and STAT3, which are suppressed by NDRG2. The evidence supporting NDRG2 as a transcription factor itself is currently limited. NF-κB, nuclear factor-kappa B; STAT3, signal transducer and activator of transcription 3; CYR61, Cysteine-rich protein 61; ERCC6, Cockayne syndrome group B protein; SIP1, Smad interacting protein 1; AP-1, activator protein-1; SOCS1, suppressor of cytokine signaling 1; GSK-3β, glycogen synthase kinase 3β; TCF, T-cell factor; LEF, lymphoid enhancer factor; TGFβ1, transforming growth factor beta 1; Erk, extracellular signal regulated kinase; MMP, proteins of the matrix metalloproteinase; BMP-4, bone morphogenetic protein-4; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; SAPK, stress-activated protein kinase; NH(2)-terminal kinase, c-Jun; Bax, Bcl-2-associated X protein; GLUT1, glucose transporter 1; HK2, hexokinase 2; PKM2, pyruvate kinase M2 isoform; LDHA, lactate dehydrogenase A; ASCT2, ASC amino acid transporter 2; GLS1, glutaminase 1.
NDRG2-TARGETED PROCESSES
Promotion of apoptosis
Apoptosis is the process of programmed cell death that occurs in multicellular organisms and is characterized by blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation [120]. The anti-tumor effects of NDRG2 have been demonstrated to be closely related to the promotion of apoptosis. Substantially more apoptotic cells are observed in NDRG2-expressing CCRCC cells than in control cells [82]. In the ESCC cell lines EC9706 and EC109, NDRG2 overexpression markedly promotes apoptosis [70]. The phosphorylation of NDRG2 can be increased by hyperthermia (HT), which further promotes the apoptosis of MKN28 cells [71]. Cao et al. [59] found that Ad-NDRG2 enhances the p53-mediated apoptosis of HCC cells by attenuating nucleotide excision repair. NDRG2 has also been shown to protect Hela cells from radiation-induced apoptosis [25]. Collectively, NDRG2 promotes tumor cell apoptosis but inhibits the apoptosis of normal cells.
Resetting cell proliferation
One characteristic of a tumor is uncontrolled cell proliferation. Resetting of the cell cycle, including the G1, S, G2 and M phases, can be observed during tumorigenesis. A Gene Ontology biological process analysis revealed that NDRG2 overexpression elicits the up-regulation of genes related to the G protein signaling pathway and the down-regulation of gene sets related to the M phase of the cell cycle, which is consistent with cell cycle analyses [94]. A signaling pathway analysis demonstrated reduced glycosylphosphatidylinositol (GPI)-anchor biosynthesis and protein degradation [94]. Ma et al. [82] also found that NDRG2 expression can induce G1 arrest. Cell cycle arrest at G1/S was also observed after the introduction of NDRG2 into SW620 cells [67]. The resetting of the cell cycle in tumors can be effectively inhibited by NDRG2 expression.
Inhibition of angiogenesis
Intra- and peri-tumoral angiogenesis is critical for tumor growth and metastasis. Kim et al. [88] found that angiogenesis is clearly observed in tumors after injection with B16F10-mock cells, whereas angiogenesis is impaired in tumors after injection with NDRG2-expressing murine melanoma cells. Thus, the inhibition of angiogenesis may contribute to the anti-tumor effects of NDRG2.
Suppression of energy metabolism
The glucose transporter GLUT1 catalyzes the facilitative diffusion of glucose into erythrocytes and is responsible for glucose supply to the brain and other organs [112]. NDRG2 suppresses the expression of transporters and catalytic enzymes, which provide bioenergy and biomaterials for cancer cell proliferation and tumor progression, thereby playing an important role in inhibiting glycolysis and glutaminolysis and restraining tumor cell metabolism [21, 97].
Collectively, tumors exhibit numerous abnormal behaviors, including aberrant proliferation, angiogenesis and metabolism. NDRG2 exerts anti-tumor effects by regulating various processes, including promoting apoptosis, arresting cell proliferation by influencing cell cycle, inhibiting angiogenesis and suppressing energy metabolism, which may provide attractive strategies for therapeutic interventions in human cancer (Figure 3).
Figure 3. Processes targeted by NDRG2.
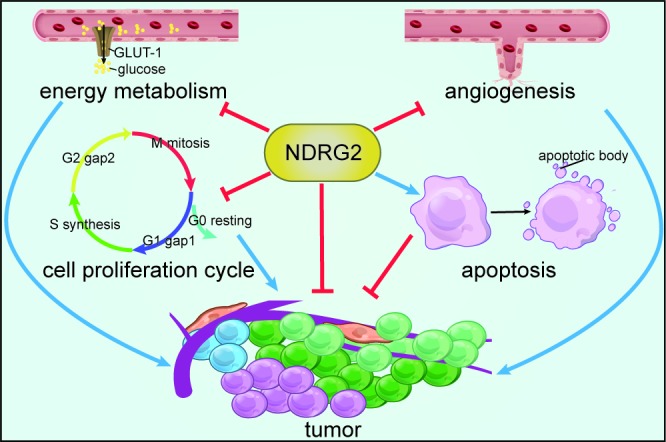
NDRG2 exerts anti-tumor effects by promoting apoptosis, arresting cell proliferation, inhibiting angiogenesis and suppressing energy metabolism.
REGULATION OF NDRG2 EXPRESSION IN CANCER
As a N-Myc downstream-regulated gene, NDRG2 expression can be suppressed by N-Myc [16]. In this section, we focus on the regulation of NDRG2 expression in cancer (Table 2).
Table 2. Regulation of NDRG2 expression in cancer.
| Factors | Tumor | Effect on NDRG2 | Reference No |
|---|---|---|---|
| DNA methylation | glioma, hepatocellular carcinoma, meningioma and gastric carcinoma | represses the expression of NDRG2 | [11, 52, 60, 64, 75, 121] |
| DNA histone deacetylase | pancreatic cancer cells | represses the expression of NDRG2 | [72] |
| p53 | clear cell renal cell carcinoma cells | upregulates the expression of NDRG2 | [82] |
| HIF-1 | Hela cells | upregulates the expression of NDRG2 | [25] |
| FXR | hepatoma cells | upregulates the expression of NDRG2 | [9] |
| Akt | gastric cancer cells | induces the phosphorylation of NDRG2 | [71] |
| Dp44mT | hepatocellular carcinoma cells | upregulates the expression of NDRG2 | [74] |
| miR-650 | colorectal cancer cells | represses the expression of NDRG2 | [64] |
Mutational analyses of the entire NDRG2 coding sequence have not revealed tumor-associated DNA sequence alterations [10, 56]. However, epigenetic alterations play a key role in tumorigenesis. The methylation rate of the NDRG2 promoter region is significantly higher in glioma, HCC, meningioma, and gastric carcinoma tissues compared with adjacent normal tissues, which may down-regulate NDRG2 [11, 52, 60, 64, 75, 121]. However, hyper-methylation was not detected in either pancreatic cancer cell lines or surgically resected specimens [72], whereas a histone deacetylase inhibitor up-regulates NDRG2 expression in pancreatic cancer cell lines that express low levels of NDRG2 [72]. These results demonstrate that altered NDRG2 expression levels during tumorigenesis are caused by epigenetic alterations such as increased methylation or histone deacetylase activity, not mutations in the coding region of NDRG2.
NDRG2 expression can be regulated by several factors. Ma et al. [82] found that p53 up-regulates NDRG2 expression in CCRCC. Hypoxia inducible factor 1 (HIF-1) is the key mediator of hypoxia signaling pathways and is involved in hypoxia-induced radioresistance [25]. NDRG2 is a target gene of HIF-1 and is up-regulated by hypoxia and radiation in an HIF-1-dependent manner, which decreases the sensitivity of Hela cells to radiation [25]. Farnesoid X receptor (FXR) directly increases NDRG2 transcription via IR1-type element(s) in the first introns of the human, mouse and rat NDRG2 genes [9]. NDRG2 mRNA can be induced by non-steroidal FXR agonists in the mouse liver and by the ectopic expression of human FXR [9]. Dp44mT, an iron chelator, up-regulates NDRG2, ultimately suppressing EMT and inhibiting metastasis in HCC [74]. NDRG2 phosphorylation affects NDRG2 protein activity and is induced by HT in an Akt-dependent manner in gastric cancer cells [71].
The transcriptional regulation by endogenous small noncoding RNA, including microRNA, may be a potential method for regulating gene expression in human cancer. MicroRNA-650 (miR-650) targets a homologous DNA region in the promoter region of the NDRG2 gene and represses its expression [64]. A reporter assay with the 3′ untranslated region of NDRG2 cloned downstream of the luciferase gene showed reduced luciferase activity in the presence of miR-650, indicating that miR-650 is a direct inhibitor of NDRG2 expression [64].
POTENTIAL FUTURE DIRECTIONS
The associations between NDRG2 expression and tumor incidence as well as clinical and pathological tumor behavior have been clarified. However, whether NDRG2 down-regulation is a cause or a consequence of the progression from normal tissue to carcinoma must be addressed.
NDRG2 has been identified as a specific tumor suppressor gene. However, whether NDRG2 can be used as a candidate biomarker for tumor incidence or prognosis requires further investigation. Detecting NDRG2 in combination with other molecules may contribute to the utility of NDRG2 in clinical settings. Wang et al. [15] detected NDRG2 and CD24 expression and found that the high NDRG2/low CD24 and low NDRG2/high CD24 combinations are independent prognostic indicators of lung adenocarcinoma that are also suitable for gallbladder carcinoma [55]. Jeschke et al. [85] identified the combination of HOXD1 and NDRG2 as the most sensitive (94%) and specific (90%) gene combination for detecting breast cancer. In HCC, the combination of low NDRG2/high phospho-STAT3 has prognostic value for adverse outcomes [74]. However, whether NDRG2 can be utilized as a biomarker for other types of cancer, with or without other molecules, requires additional research.
Epigenetic alterations play a key role in tumorigenesis, and inhibiting methylation or histone deacetylase processes with pharmaceutical interventions may have a benefit in cancer treatment. Trichostatin A is a histone deacetylase inhibitor, and NDRG2 expression is up-regulated by trichostatin A treatment via the inhibition of histone deacetylase [60, 72]. The methylation of NDRG2 is higher in primary gastric cancer specimens than in corresponding nonmalignant gastric tissues [60], and this pattern is also observed in OSCC [89]. Furthermore, upon treatment with a DNA demethylating agent, 5-aza-2′-deoxycytidine, NDRG2 expression is up-regulated in HGC27 cells, and MKN45 cell invasion is inhibited [60].
HT has been shown to alter the invasion capacity of cancer cells with few side effects [71]. Guo et al. [65] found that NDRG2 expression was induced by HT at 45°C. Moreover, the synergism between HT (43°C) and NDRG2 expression effectively reduces cytotoxicity and inhibits invasion compared with HT at 45°C. Thus, the combined application of constitutive NDRG2 expression with HT may yield an optimized therapeutic benefit. By increasing the phosphorylation of NDRG2, HT can also exert anti-tumor effects on MKN28 gastric cancer cells [71].
NDRG2 may decrease after antidepressant treatment and electroconvulsive therapy (ECT). Takahashi et al. [122] found that chronic treatment with imipramine, a tricyclic antidepressant, and sertraline, a selective serotonin reuptake inhibitor, reduced NDRG2 mRNA and protein expression in the rat frontal cortex. Moreover, repeated ECT significantly decreases NDRG2 expression in this region of the brain. These findings affirmed the important role of NDRG2 in the central nervous system and indicated that NDRG2 may be a candidate target of antidepressants and ECT, which suggests that NDRG2 can be induced by chronic stress. It is unknown whether cancer incidence and progression differs in people under chronic stress, which may contribute to a better understanding of the association between NDRG2 and cancer. The overexpression of NDRG2 in dorsal horn astrocytes contributes to their activation and plays a crucial role in diabetic mechanical allodynia. However, the intrathecal injection of RU486, a glucocorticoid receptor antagonist, reverses astrocyte reactivity and diabetic tactile allodynia by inhibiting NDRG2 overexpression [123]. Moreover, the use of glucocorticoids may induce NDRG2 expression [124], but whether this will contribute to the regulation of tumor development and progression remains unknown.
CONCLUSIONS
NDRG2 expression is down-regulated in human cancer, and NDRG2 overexpression inhibits the proliferation, migration, metastasis and invasion of cancer cells (Table 1). There is a negative correlation between NDRG2 expression levels and the clinical and pathological status of human cancer (Figure 1). NDRG2 may be a tumor biomarker, and the combination of NDRG2 with other molecules, such as CD24 and HOXD1, may yield more specific or sensitive biomarkers. The anti-tumor effects of NDRG2 are exerted via various mechanisms and pathways (Figures 2 and 3), and NDRG2 expression levels are regulated by numerous factors and treatments, which may provide insight into methods for successfully treating cancer (Figure 1).
Acknowledgments
This work was supported by National Natural Science Foundation of China (81500263) and China Postdoctoral Science Foundation (2015M572681).
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Chung CH, Bernard PS, Perou CM. Molecular portraits and the family tree of cancer. Nat Genet. 2002;32(Suppl):533–540. doi: 10.1038/ng1038. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Longo DL. Tumor heterogeneity and personalized medicine. N Engl J Med. 2012;366:956–957. doi: 10.1056/NEJMe1200656. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, McMichael JF, Wallis JW, Lu C, Shen D, Harris CC, Dooling DJ, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz C, Lenkiewicz E, Evers L, Holley T, Robeson A, Kiefer J, Demeure MJ, Hollingsworth MA, Shen M, Prunkard D, Rabinovitch PS, Zellweger T, Mousses S, Trent JM, Carpten JD, Bubendorf L, et al. Advancing a clinically relevant perspective of the clonal nature of cancer. Proc Natl Acad Sci U S A. 2011;108:12054–12059. doi: 10.1073/pnas.1104009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SS. Treatment options for hormone-refractory prostate cancer. Rev Urol. 2007;9(Suppl 2):S13–18. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Rosenberg JE, Taplin ME. Novel agents and new therapeutics in castration-resistant prostate cancer. Curr Opin Oncol. 2011;23:290–296. doi: 10.1097/CCO.0b013e3283449400. [DOI] [PubMed] [Google Scholar]
- 9.Deuschle U, Schuler J, Schulz A, Schluter T, Kinzel O, Abel U, Kremoser C. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS One. 2012;7:e43044. doi: 10.1371/journal.pone.0043044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu XL, Liu XP, Lin SX, Deng YC, Liu N, Li X, Yao LB. NDRG2 expression and mutation in human liver and pancreatic cancers. World J Gastroenterol. 2004;10:3518–3521. doi: 10.3748/wjg.v10.i23.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DC, Kang YK, Kim WH, Jang YJ, Kim DJ, Park IY, Sohn BH, Sohn HA, Lee HG, Lim JS, Kim JW, Song EY, Kim DM, Lee MN, Oh GT, Kim SJ, et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–4220. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 12.Li SJ, Wang WY, Li B, Chen B, Zhang B, Wang X, Chen CS, Zhao QC, Shi H, Yao L. Expression of NDRG2 in human lung cancer and its correlation with prognosis. Med Oncol. 2013;30:421. doi: 10.1007/s12032-012-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H, Jin H, Chu D, Wang W, Zhang J, Chen C, Xu C, Fan D, Yao L. Suppression of N-myc downstream-regulated gene 2 is associated with induction of Myc in colorectal cancer and correlates closely with differentiation. Biol Pharm Bull. 2009;32:968–975. doi: 10.1248/bpb.32.968. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Wang WZ, Yao LB, Zhang J, Yin Q, Xu CS, Chu DK, Dong GL, Zhang HW, Li JP. [Expression and significance of new candidate tumor suppressor gene N-Myc downstream-regulated gene 2 in colorectal cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12:281–284. [PubMed] [Google Scholar]
- 15.Wang H, Wang W, Wang X, Cai K, Wu H, Ju Q, Huang Z, Gao X. Reduced N-Myc downstream-regulated gene 2 expression is associated with CD24 upregulation and poor prognosis in patients with lung adenocarcinoma. Med Oncol. 2012;29:3162–3168. doi: 10.1007/s12032-012-0231-y. [DOI] [PubMed] [Google Scholar]
- 16.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruine AP, Baldwin HS, van Engeland M. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–4166. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 17.Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, He F. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem. 2002;229:35–44. doi: 10.1023/a:1017934810825. [DOI] [PubMed] [Google Scholar]
- 18.Shaw E, McCue LA, Lawrence CE, Dordick JS. Identification of a novel class in the alpha/beta hydrolase fold superfamily: the N-myc differentiation-related proteins. Proteins. 2002;47:163–168. doi: 10.1002/prot.10083. [DOI] [PubMed] [Google Scholar]
- 19.Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, Au WS, Wang J, Li F, Ji S, Han H, Nie X, Li Q, Kung HF, Leung SY, Lin MC. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003;106:342–347. doi: 10.1002/ijc.11228. [DOI] [PubMed] [Google Scholar]
- 20.Yao L, Zhang J, Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai) 2008;40:625–635. doi: 10.1111/j.1745-7270.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 21.Ma J, Liu W, Guo H, Li S, Cao W, Du X, Lei S, Hou W, Xiong L, Yao L, Li N, Li Y. N-myc downstream-regulated gene 2 expression is associated with glucose transport and correlated with prognosis in breast carcinoma. Breast Cancer Res. 2014;16:R27. doi: 10.1186/bcr3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Liu C, Hou W, Ma J, Lin K, Situ Z, Xiong L, Li S, Yao L. Retrograde ductal administration of the adenovirus-mediated NDRG2 gene leads to improved sialaden hypofunction in estrogen-deficient rats. Mol Ther. 2014;22:908–918. doi: 10.1038/mt.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Yang J, Li S, Zhang J, Zheng J, Hou W, Zhao H, Guo Y, Liu X, Dou K, Situ Z, Yao L. N-myc downstream-regulated gene 2, a novel estrogen-targeted gene, is involved in the regulation of Na+/K+-ATPase. J Biol Chem. 2011;286:32289–32299. doi: 10.1074/jbc.M111.247825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L, Liu X, Hou W, Yang G, Wu Y, Zhang R, Li X, Che H, Lu Z, Zhang Y, Yao L. NDRG2 is highly expressed in pancreatic beta cells and involved in protection against lipotoxicity. Cell Mol Life Sci. 2010;67:1371–1381. doi: 10.1007/s00018-010-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Zhang J, Wang X, Li Y, Chen Y, Li K, Yao L, Guo G. HIF-1 and NDRG2 contribute to hypoxia-induced radioresistance of cervical cancer Hela cells. Exp Cell Res. 2010;316:1985–1993. doi: 10.1016/j.yexcr.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Shimono A, Okuda T, Kondoh H. N-myc-dependent repression of ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N-myc mutant. Mech Dev. 1999;83:39–52. doi: 10.1016/s0925-4773(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 27.Hwang J, Kim Y, Kang HB, Jaroszewski L, Deacon AM, Lee H, Choi WC, Kim KJ, Kim CH, Kang BS, Lee JO, Oh TK, Kim JW, Wilson IA, Kim MH. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J Biol Chem. 2011;286:12450–12460. doi: 10.1074/jbc.M110.170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickok JR, Sahni S, Mikhed Y, Bonini MG, Thomas DD. Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J Biol Chem. 2011;286:41413–41424. doi: 10.1074/jbc.M111.287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, An L, Li X. NDRG3 and NDRG4, two novel tumor-related genes. Biomed Pharmacother. 2013;67:681–684. doi: 10.1016/j.biopha.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Kotipatruni RP, Ren X, Thotala D, Jaboin JJ. NDRG4 is a novel oncogenic protein and p53 associated regulator of apoptosis in malignant meningioma cells. Oncotarget. 2015;6:17594–604. doi: 10.18632/oncotarget.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto H, Kokame K, Okuda T, Nakajo Y, Yanamoto H, Miyata T. NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem. 2011;286:26158–26165. doi: 10.1074/jbc.M111.256446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park KC, Lee DC, Yeom YI. NDRG3-mediated lactate signaling in hypoxia. BMB Rep. 2015;48:301–302. doi: 10.5483/BMBRep.2015.48.6.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahni S, Krishan S, Richardson DR. NDRG1 as a molecular target to inhibit the epithelial-mesenchymal transition: the case for developing inhibitors of metastasis. Future Med Chem. 2014;6:1241–1244. doi: 10.4155/fmc.14.80. [DOI] [PubMed] [Google Scholar]
- 35.Fang BA, Kovacevic Z, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta. 2014;1845:1–19. doi: 10.1016/j.bbcan.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Bae DH, Jansson PJ, Huang ML, Kovacevic Z, Kalinowski D, Lee CS, Sahni S, Richardson DR. The role of NDRG1 in the pathology and potential treatment of human cancers. J Clin Pathol. 2013;66:911–917. doi: 10.1136/jclinpath-2013-201692. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Zhang D, Bae DH, Sahni S, Jansson P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z, Richardson DR. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis. 2013;34:1943–1954. doi: 10.1093/carcin/bgt163. [DOI] [PubMed] [Google Scholar]
- 38.Lachat P, Shaw P, Gebhard S, van Belzen N, Chaubert P, Bosman FT. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol. 2002;118:399–408. doi: 10.1007/s00418-002-0460-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Iiizumi-Gairani M, Okuda H, Kobayashi A, Watabe M, Pai SK, Pandey PR, Xing F, Fukuda K, Modur V, Hirota S, Suzuki K, Chiba T, Endo M, Sugai T, Watabe K. KAI1 gene is engaged in NDRG1 gene-mediated metastasis suppression through the ATF3-NFkappaB complex in human prostate cancer. J Biol Chem. 2011;286:18949–18959. doi: 10.1074/jbc.M111.232637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandyopadhyay S, Wang Y, Zhan R, Pai SK, Watabe M, Iiizumi M, Furuta E, Mohinta S, Liu W, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, et al. The tumor metastasis suppressor gene Drg-1 down-regulates the expression of activating transcription factor 3 in prostate cancer. Cancer Res. 2006;66:11983–11990. doi: 10.1158/0008-5472.CAN-06-0943. [DOI] [PubMed] [Google Scholar]
- 41.Kovacevic Z, Chikhani S, Lui GY, Sivagurunathan S, Richardson DR. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid Redox Signal. 2013;18:874–887. doi: 10.1089/ars.2011.4273. [DOI] [PubMed] [Google Scholar]
- 42.Matsugaki T, Zenmyo M, Hiraoka K, Fukushima N, Shoda T, Komiya S, Ono M, Kuwano M, Nagata K. N-myc downstream-regulated gene 1/Cap43 expression promotes cell differentiation of human osteosarcoma cells. Oncol Rep. 2010;24:721–725. doi: 10.3892/or_00000913. [DOI] [PubMed] [Google Scholar]
- 43.Chen WQ, Hoeger H, Diao WF, Pollak A, Lubec G. Mass spectrometrical characterization of NDRG2 protein (N-myc-downstream regulated gene 2) and description of two novel phosphorylation sites. Neurochem Res. 2007;32:1969–1977. doi: 10.1007/s11064-007-9397-7. [DOI] [PubMed] [Google Scholar]
- 44.Herskowitz JH, Seyfried NT, Duong DM, Xia Q, Rees HD, Gearing M, Peng J, Lah JJ, Levey AI. Phosphoproteomic analysis reveals site-specific changes in GFAP and NDRG2 phosphorylation in frontotemporal lobar degeneration. J Proteome Res. 2010;9:6368–6379. doi: 10.1021/pr100666c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Z, Tong G, Ma N, Li J, Li X, Li S, Zhou J, Xiong L, Cao F, Yao L, Wang H, Shen L. NDRG2: a newly identified mediator of insulin cardioprotection against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2013;108:341. doi: 10.1007/s00395-013-0341-5. [DOI] [PubMed] [Google Scholar]
- 46.Mitchelmore C, Buchmann-Moller S, Rask L, West MJ, Troncoso JC, Jensen NA. NDRG2: a novel Alzheimer's disease associated protein. Neurobiol Dis. 2004;16:48–58. doi: 10.1016/j.nbd.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, Zhong H, Li X, Peng Y, Kinden R, Liang W, Shi M, Liu L, Wang Q, Xiong L. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol Neurobiol. 2014;50:305–313. doi: 10.1007/s12035-013-8609-1. [DOI] [PubMed] [Google Scholar]
- 48.Nichols NR. Ndrg2, a novel gene regulated by adrenal steroids and antidepressants, is highly expressed in astrocytes. Ann N Y Acad Sci. 2003;1007:349–356. doi: 10.1196/annals.1286.034. [DOI] [PubMed] [Google Scholar]
- 49.Araya-Callis C, Hiemke C, Abumaria N, Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology (Berl) 2012;224:209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Liu N, Yao L, Li F, Zhang J, Deng Y, Liu J, Ji S, Yang A, Han H, Zhang Y, Han W, Liu X. NDRG2 is a new HIF-1 target gene necessary for hypoxia-induced apoptosis in A549 cells. Cell Physiol Biochem. 2008;21:239–250. doi: 10.1159/000113765. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Wang J, Shen X, Wang L, Li X, Liu Y, Shi M, Zhao G, Deng Y. Expression and prognostic value of NDRG2 in human astrocytomas. J Neurol Sci. 2011;308:77–82. doi: 10.1016/j.jns.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, Gutmann DH, Perry A. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005;65:7121–7126. doi: 10.1158/0008-5472.CAN-05-0043. [DOI] [PubMed] [Google Scholar]
- 53.Skiriute D, Tamasauskas S, Asmoniene V, Saferis V, Skauminas K, Deltuva V, Tamasauskas A. Tumor grade-related NDRG2 gene expression in primary and recurrent intracranial meningiomas. J Neurooncol. 2011;102:89–94. doi: 10.1007/s11060-010-0291-9. [DOI] [PubMed] [Google Scholar]
- 54.Skiriute D, Vaitkiene P, Asmoniene V, Steponaitis G, Deltuva VP, Tamasauskas A. Promoter methylation of AREG, HOXA11, hMLH1, NDRG2, NPTX2 and Tes genes in glioblastoma. J Neurooncol. 2013;113:441–449. doi: 10.1007/s11060-013-1133-3. [DOI] [PubMed] [Google Scholar]
- 55.Song SP, Zhang SB, Liu R, Yao L, Hao YQ, Liao MM, Zhang YD, Li ZH. NDRG2 down-regulation and CD24 up-regulation promote tumor aggravation and poor survival in patients with gallbladder carcinoma. Med Oncol. 2012;29:1879–1885. doi: 10.1007/s12032-011-0110-y. [DOI] [PubMed] [Google Scholar]
- 56.Tepel M, Roerig P, Wolter M, Gutmann DH, Perry A, Reifenberger G, Riemenschneider MJ. Frequent promoter hypermethylation and transcriptional downregulation of the NDRG2 gene at 14q11. 2 in primary glioblastoma. Int J Cancer. 2008;123:2080–2086. doi: 10.1002/ijc.23705. [DOI] [PubMed] [Google Scholar]
- 57.Zhang ZG, Li G, Feng DY, Zhang J, Qin HZ, Ma LT, Gao GD, Wu L. Overexpression of NDRG2 can inhibit neuroblastoma cell proliferation through negative regulation by CYR61. Asian Pac J Cancer Prev. 2014;15:239–244. doi: 10.7314/apjcp.2014.15.1.239. [DOI] [PubMed] [Google Scholar]
- 58.Cao W, Yu G, Lu Q, Zhang J. Low expression of N-myc downstream-regulated gene 2 in oesophageal squamous cell carcinoma correlates with a poor prognosis. BMC Cancer. 2013;13:305. doi: 10.1186/1471-2407-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao W, Zhang JL, Feng DY, Liu XW, Li Y, Wang LF, Yao LB, Zhang H, Zhang J. The effect of adenovirus-conjugated NDRG2 on p53-mediated apoptosis of hepatocarcinoma cells through attenuation of nucleotide excision repair capacity. Biomaterials. 2014;35:993–1003. doi: 10.1016/j.biomaterials.2013.09.096. [DOI] [PubMed] [Google Scholar]
- 60.Chang X, Li Z, Ma J, Deng P, Zhang S, Zhi Y, Chen J, Dai D. DNA methylation of NDRG2 in gastric cancer and its clinical significance. Dig Dis Sci. 2013;58:715–723. doi: 10.1007/s10620-012-2393-z. [DOI] [PubMed] [Google Scholar]
- 61.Choi SC, Yoon SR, Park YP, Song EY, Kim JW, Kim WH, Yang Y, Lim JS, Lee HG. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death. Exp Mol Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 62.Chu D, Zhang Z, Li Y, Wu L, Zhang J, Wang W. Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Mol Cancer Ther. 2011;10:47–56. doi: 10.1158/1535-7163.MCT-10-0614. [DOI] [PubMed] [Google Scholar]
- 63.Chu DK, Zhang J, Shi H, Dong GL, Liu XP, Wang WZ. [Expression of candidate tumor suppressor gene N-Myc downstream-regulated gene 2 in colon cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2008;11:354–357. [PubMed] [Google Scholar]
- 64.Feng L, Xie Y, Zhang H, Wu Y. Down-regulation of NDRG2 gene expression in human colorectal cancer involves promoter methylation and microRNA-650. Biochem Biophys Res Commun. 2011;406:534–538. doi: 10.1016/j.bbrc.2011.02.081. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, Ma J, Wu L, Wang Q, Li X, Zhang Y, Zhang J, Yao L, Liu W. Hyperthermia-induced NDRG2 upregulation inhibits the invasion of human hepatocellular carcinoma via suppressing ERK1/2 signaling pathway. PLoS One. 2013;8:e61079. doi: 10.1371/journal.pone.0061079. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Kim YJ, Kang HB, Yim HS, Kim JH, Kim JW. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncol Rep. 2013;30:1890–1898. doi: 10.3892/or.2013.2642. [DOI] [PubMed] [Google Scholar]
- 67.Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS, Kim JH, Song EY, Lee HG, Choi I, Kim JW. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int J Cancer. 2009;124:7–15. doi: 10.1002/ijc.23945. [DOI] [PubMed] [Google Scholar]
- 68.Kim YJ, Yoon SY, Kim JT, Song EY, Lee HG, Son HJ, Kim SY, Cho D, Choi I, Kim JH, Kim JW. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598–605. doi: 10.1093/carcin/bgp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lorentzen A, Vogel LK, Lewinsky RH, Saebo M, Skjelbred CF, Godiksen S, Hoff G, Tveit KM, Lothe IM, Ikdahl T, Kure EH, Mitchelmore C. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer. 2007;7:192. doi: 10.1186/1471-2407-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi H, Li N, Li S. Expression of NDRG2 in esophageal squamous cell carcinoma. 2010;101:1292–1299. doi: 10.1111/j.1349-7006.2010.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tao Y, Guo Y, Liu W, Zhang J, Li X, Shen L, Ru Y, Xue Y, Zheng J, Liu X, Yao L. AKT inhibitor suppresses hyperthermia-induced Ndrg2 phosphorylation in gastric cancer cells. Braz J Med Biol Res. 2013;46:394–404. doi: 10.1590/1414-431X20122211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamura A, Miura K, Karasawa H, Morishita K, Abe K, Mizuguchi Y, Saiki Y, Fukushige S, Kaneko N, Sase T, Nagase H, Sunamura M, Motoi F, Egawa S, Shibata C, Unno M, et al. Suppressed expression of NDRG2 correlates with poor prognosis in pancreatic cancer. Biochem Biophys Res Commun. 2013;441:102–107. doi: 10.1016/j.bbrc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 73.Zheng J, Li Y, Yang J, Liu Q, Shi M, Zhang R, Shi H, Ren Q, Ma J, Guo H, Tao Y, Xue Y, Jiang N, Yao L, Liu W. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC Cancer. 2011;11(251):251–259. doi: 10.1186/1471-2407-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Yin D, Xie C, Zheng T, Liang Y, Hong X, Lu Z, Song X, Song R, Yang H, Sun B, Bhatta N, Meng X, Pan S, Jiang H, Liu L. The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget. 2014;5:8478–8491. doi: 10.18632/oncotarget.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ling ZQ, Ge MH, Lu XX, Han J, Wu YC, Liu X, Zhu X, Hong LL. Ndrg2 promoter hypermethylation triggered by helicobacter pylori infection correlates with poor patients survival in human gastric carcinoma. Oncotarget. 2015;6:8210–8225. doi: 10.18632/oncotarget.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren GF, Tang L, Yang AQ, Jiang WW, Huang YM. Prognostic impact of NDRG2 and NDRG3 in prostate cancer patients undergoing radical prostatectomy. Histol Histopathol. 2014;29:535–542. doi: 10.14670/HH-29.10.535. [DOI] [PubMed] [Google Scholar]
- 77.Yu C, Wu G, Dang N, Zhang W, Zhang R, Yan W, Zhao Y, Gao L, Wang Y, Beckwith N, Yuan J, Yao L. Inhibition of N-myc downstream-regulated gene 2 in prostatic carcinoma. Cancer Biol Ther. 2011;12:304–313. doi: 10.4161/cbt.12.4.16382. [DOI] [PubMed] [Google Scholar]
- 78.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, Zhang G, Liu F, Yu CG, Yuan JL, Wang H, Yao LB. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;310:94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Liang ZL, Kang K, Yoon S, Huang SM, Lim JS, Kim JM, Lee HJ. NDRG2 is involved in the oncogenic properties of renal cell carcinoma and its loss is a novel independent poor prognostic factor after nephrectomy. Ann Surg Oncol. 2012;19:2763–2772. doi: 10.1245/s10434-011-2204-3. [DOI] [PubMed] [Google Scholar]
- 80.Ma J, Jin H, Wang H, Yuan J, Bao T, Jiang X, Zhang W, Zhao H, Yao L. Expression of NDRG2 in clear cell renal cell carcinoma. Biol Pharm Bull. 2008;31:1316–1320. doi: 10.1248/bpb.31.1316. [DOI] [PubMed] [Google Scholar]
- 81.Ma JJ, Kong LM, Liao CG, Jiang X, Wang Y, Bao TY. Suppression of MMP-9 activity by NDRG2 expression inhibits clear cell renal cell carcinoma invasion. Med Oncol. 2012;29:3306–3313. doi: 10.1007/s12032-012-0265-1. [DOI] [PubMed] [Google Scholar]
- 82.Ma JJ, Liao CG, Jiang X, Zhao HD, Yao LB, Bao TY. NDRG2 suppresses the proliferation of clear cell renal cell carcinoma cell A-498. J Exp Clin Cancer Res. 2010;29:103. doi: 10.1186/1756-9966-29-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lorentzen A, Lewinsky RH, Bornholdt J, Vogel LK, Mitchelmore C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer. 2011;11:14. doi: 10.1186/1471-2407-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng J, Liu Q, Li Y, Yang J, Ma J, Yu F, Shi H, Ren Q, Zhang R, Zhang J, Xue Y, Tao Y, Jiang N, Guo H, Yao L, Liu W. NDRG2 expression regulates CD24 and metastatic potential of breast cancer cells. Asian Pac J Cancer Prev. 2010;11:1817–1821. [PubMed] [Google Scholar]
- 85.Jeschke J, Van Neste L, Glockner SC, Dhir M, Calmon MF, Deregowski V, Van Criekinge W. Biomarkers for detection and prognosis of breast cancer identified by a functional hypermethylome screen. 2012;7:701–709. doi: 10.4161/epi.20445. [DOI] [PubMed] [Google Scholar]
- 86.Li R, Yu C, Jiang F, Gao L, Li J, Wang Y, Beckwith N, Yao L, Zhang J, Wu G. Overexpression of N-Myc downstream-regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PLoS One. 2013;8:e76689. doi: 10.1371/journal.pone.0076689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao H, Zhang J, Lu J, He X, Chen C, Li X, Gong L, Bao G, Fu Q, Chen S, Lin W, Shi H, Ma J, Liu X, Ma Q, Yao L. Reduced expression of N-Myc downstream-regulated gene 2 in human thyroid cancer. BMC Cancer. 2008;8:303. doi: 10.1186/1471-2407-8-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI, Lim JS. Suppression of NF-kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis. 2009;30:927–936. doi: 10.1093/carcin/bgp072. [DOI] [PubMed] [Google Scholar]
- 89.Furuta H, Kondo Y, Nakahata S, Hamasaki M, Sakoda S, Morishita K. NDRG2 is a candidate tumor-suppressor for oral squamous-cell carcinoma. Biochem Biophys Res Commun. 2010;391ss:1785–1791. doi: 10.1016/j.bbrc.2009.12.156. [DOI] [PubMed] [Google Scholar]
- 90.Tschan MP, Shan D, Laedrach J, Eyholzer M, Leibundgut EO, Baerlocher GM, Tobler A, Stroka D, Fey MF. NDRG1/2 expression is inhibited in primary acute myeloid leukemia. Leuk Res. 2010;34:393–398. doi: 10.1016/j.leukres.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 91.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 92.Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18:1893–1905. doi: 10.1517/13543780903321490. [DOI] [PubMed] [Google Scholar]
- 93.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 94.Liu X, Niu T, Hou W, Zhang J, Yao L. Microarray profiling of HepG2 cells ectopically expressing NDRG2. Gene. 2012;503:48–55. doi: 10.1016/j.gene.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 95.Park Y, Shon SK, Kim A, Kim KI, Yang Y, Cho DH, Lee MS, Lim JS. SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem Biophys Res Commun. 2007;363:361–367. doi: 10.1016/j.bbrc.2007.08.195. [DOI] [PubMed] [Google Scholar]
- 96.Mercer KE, Hennings L, Ronis MJ. Alcohol consumption, Wnt/beta-catenin signaling, and hepatocarcinogenesis. Adv Exp Med Biol. 2015;815:185–195. doi: 10.1007/978-3-319-09614-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li X, Yang G, Liu X, Yao L, Zhang J, Shen L. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget. 2015;6:26161–76. doi: 10.18632/oncotarget.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan RG, Ridsdale J, Tonks A, Darley RL. Factors affecting the nuclear localization of beta-catenin in normal and malignant tissue. J Cell Biochem. 2014;115:1351–1361. doi: 10.1002/jcb.24803. [DOI] [PubMed] [Google Scholar]
- 99.van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–134. doi: 10.1038/nrc3647. [DOI] [PubMed] [Google Scholar]
- 100.Santos JI, Teixeira AL, Dias F, Gomes M, Nogueira A, Assis J, Medeiros R. Restoring TGFbeta1 pathway-related microRNAs: possible impact in metastatic prostate cancer development. Tumour Biol. 2014;35:6245–6253. doi: 10.1007/s13277-014-1887-z. [DOI] [PubMed] [Google Scholar]
- 101.Al-Azayzih A, Gao F, Goc A, Somanath PR. TGFbeta1 induces apoptosis in invasive prostate cancer and bladder cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated activation of caspases. Biochem Biophys Res Commun. 2012;427:165–170. doi: 10.1016/j.bbrc.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, Adams SE, Gearing AJ. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Shi Q, Yuan TX, Song QL, Zhang Y, Wei Q, Zhou L, Luo J, Zuo G, Tang M, He TC, Weng Y. Matrix metalloproteinase 9 (MMP-9) in osteosarcoma: review and meta-analysis. Clin Chim Acta. 2014;433:225–231. doi: 10.1016/j.cca.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 104.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 106.Labeur M, Paez-Pereda M, Haedo M, Arzt E, Stalla GK. Pituitary tumors: cell type-specific roles for BMP-4. Mol Cell Endocrinol. 2010;326:85–88. doi: 10.1016/j.mce.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi: 10.1155/2012/708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Cheaib B, Auguste A, Leary A. The PI3K/Akt/mTOR pathway in ovarian cancer: therapeutic opportunities and challenges. Chin J Cancer. 2015;34:4–16. doi: 10.5732/cjc.014.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. J Biochem. 2004;136:123–126. doi: 10.1093/jb/mvh117. [DOI] [PubMed] [Google Scholar]
- 111.Pietrantonio F, Biondani P, Ciurlia E, Fanetti G, Tessari A, Bertarelli G, Bossi I, Musella V, Melotti F, Di Bartolomeo M, Valvo F, Pellegrinelli A, Milione M, Perrone F, de Braud F. Role of BAX for outcome prediction in gastrointestinal malignancies. Med Oncol. 2013;30:610. doi: 10.1007/s12032-013-0610-z. [DOI] [PubMed] [Google Scholar]
- 112.Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N. Crystal structure of the human glucose transporter GLUT1. Nature. 2014;510:121–125. doi: 10.1038/nature13306. [DOI] [PubMed] [Google Scholar]
- 113.Ferbeyre G, Moriggl R. The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim Biophys Acta. 2011;1815:104–114. doi: 10.1016/j.bbcan.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 114.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 116.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Bauer J, Namineni S, Reisinger F, Zoller J, Yuan D, Heikenwalder M. Lymphotoxin, NF-kB, and cancer: the dark side of cytokines. Dig Dis. 2012;30:453–468. doi: 10.1159/000341690. [DOI] [PubMed] [Google Scholar]
- 118.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 119.Hsu YL, Hung JY, Chou SH, Huang MS, Tsai MJ, Lin YS, Chiang SY, Ho YW, Wu CY, Kuo PL. Angiomotin decreases lung cancer progression by sequestering oncogenic YAP/TAZ and decreasing Cyr61 expression. Oncogene. 2015;34:4056–68. doi: 10.1038/onc.2014.333. [DOI] [PubMed] [Google Scholar]
- 120.Mohan S, Abdul AB, Abdelwahab SI, Al-Zubairi AS, Sukari MA, Abdullah R, Elhassan Taha MM, Ibrahim MY, Syam S. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: its activation coupled with G0/G1 phase cell cycle arrest. J Ethnopharmacol. 2010;131:592–600. doi: 10.1016/j.jep.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 121.Zhou B, Tang Z, Deng Y, Hou S, Liu N, Lin W, Liu X, Yao L. Tumor suppressor candidate gene, NDRG2 is frequently inactivated in human glioblastoma multiforme. Mol Med Rep. 2014;10:891–896. doi: 10.3892/mmr.2014.2237. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi K, Yamada M, Ohata H, Momose K, Higuchi T, Honda K. Expression of Ndrg2 in the rat frontal cortex after antidepressant and electroconvulsive treatment. Int J Neuropsychopharmacol. 2005;8:381–389. doi: 10.1017/S1461145705005134. [DOI] [PubMed] [Google Scholar]
- 123.Zuo ZF. Astrocytic NDRG2 is involved in glucocorticoid-mediated diabetic mechanical allodynia. 2015;108:128–136. doi: 10.1016/j.diabres.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi K, Saitoh A, Yamada M, Iwai T, Inagaki M. Dexamethasone indirectly induces Ndrg2 expression in rat astrocytes. J Neurosci Res. 2012;90:160–166. doi: 10.1002/jnr.22727. [DOI] [PubMed] [Google Scholar]