Abstract
Background
Persistent use of secondary prevention therapies after acute myocardial infarction (MI) is critical to optimizing long-term outcomes.
Methods
Medication persistence was assessed among 7,955 MI patients in 216 hospitals participating in the TRANSLATE-ACS study from 2010 to 2012. Persistence was defined as continuation of aspirin, adenosine diphosphate receptor inhibitors (ADPRi), beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), and statins from discharge to 6 months post-MI. Multivariable logistic regression modeling was used to determine factors associated with non-persistence, defined as <80% persistence with all medication classes.
Results
Overall, 31% of MI patients stopped taking a least one medication by 6 months. The most common reasons cited for medications discontinuation were side effects and physician instruction (57%), while financial concerns were cited in 8% overall. After multivariable modeling, black race (odds ratio [OR] 1.36; 95% confidence interval [CI] 1.15–1.62), older age (OR 1.07; 95% CI 1.02–1.12), atrial fibrillation (OR 1.67, 95% CI 1.33–2.09), dialysis (OR 1.79; 95% CI 1.15–2.78), and depression (OR 1.22; 95% CI 1.02–1.45) were associated with lower likelihood of persistence. Private insurance (OR 0.85, 95% 0.76–0.95), prescription cost assistance (OR 0.63; 95% CI 0.54–0.75), and outpatient follow-up arranged prior to discharge (OR 0.89. 95% CI 0.80–0.99) were associated with higher persistence.
Conclusions
Nearly one-third of MI patients are no longer persistent with their prescribed medications by 6 months. Patients at high risk of non-persistence may be identified by clinical and sociodemographic features. These observations underscore key opportunities to optimize longitudinal use of secondary prevention therapies.
Keywords: acute myocardial infarction, medication adherence, antiplatelet therapy, percutaneous coronary intervention
The treatment of patients with acute myocardial infarction (MI) has improved dramatically over the past decade. Evidence-based medical therapies are now routinely used at very high rates during inpatient care.1 Most patients are prescribed appropriate secondary prevention therapies at discharge, yet persistent use of these therapies has been suboptimal. Studies have reported that as many as half of cardiac patients discontinue prescribed therapies soon after discharge from the hospital2,3; however, these studies are dated and reflect patterns in select patients and settings. Most secondary prevention medications have become generic,4 and the availability of prescription coverage has broadened.5,6 However, it is unknown whether these and other factors have combined to improve longitudinal persistence rates in routine community practice.
Persistence with medications is also known to vary as a function of patient, provider, and health system factors.7 Additionally, though several interventions have been identified to improve patient persistence with medications, their cost effectiveness has not been fully determined. As health care costs rise, improving patient persistence with medications will become a major priority of both policy makers and third-party payers. Suboptimal persistence with prescribed therapies has been identified as a driver of both healthcare costs and worse clinical outcomes, thereby making it a target for intervention and improvement.8,9 Nevertheless, it will be important to identify those who are at highest risk of non-persistence to allow these interventions to be tailored.10
The Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study is a contemporary multicenter observational registry of acute MI patients that captures detailed baseline clinical, sociodemographic, and provider characteristics along with downstream patient-reported medication persistence. TRANSLATE-ACS provides a unique opportunity to: 1) examine the patterns of, and reasons for, medication non-persistence after an acute MI; 2) compare differences in patient, sociodemographic, and economic factors between those who are persistent versus those who are less persistent; and 3) evaluate potentially modifiable factors associated with non-persistence.
Methods
Study design and population
TRANSLATE-ACS is an observational study of acute ST-segment elevation MI or non–ST-segment elevation MI patients treated with percutaneous coronary intervention (PCI) (clinical trial #NCT01088503). Inclusion and exclusion criteria, collected variables, and the details of data collection have been previously described.11 Briefly, the study was broadly inclusive, and excluded only patients who were not able or willing to provide written informed consent for longitudinal follow-up, and those who were also participating in a research study that directs the use of ADPRi within the first 12 months after acute MI. The individual institutional review board of each reporting hospital approved participation in TRANSLATE-ACS. All data was collected prospectively. Study follow-up was conducted via telephone interviews by trained personnel at the Duke Clinical Research Institute.
For the current analysis, we included all patients (n=8,654) enrolled in TRANSLATE-ACS between April 2010 and May 2012 who were eligible for 6-month post-MI follow-up to ascertain medication persistence. We excluded patients who died in the hospital (n=12), patients who were lost to follow-up for the 6-week and 6-month interviews (n=573), patients missing medication information at 6 weeks and 6 months post-MI (n=111), and patients who were not discharged on any of the five cardiovascular medication classes under investigation in this study (n=3). The final study population included 7,955 acute MI patients treated at 216 hospitals.
Study variables and definitions
Participating hospitals collected information on baseline demographic and clinical characteristics, processes of care, and in-hospital outcomes using a standardized set of data elements and definitions aligned with those used by the National Cardiovascular Data Registry®.12 Baseline patient data, including discharge medications, were captured prospectively via chart review at discharge and were entered by sites into a web-based data collection form. At discharge, enrolled patients were provided a medication diary to record any changes in medications and the rationale for each change. At each post-discharge interview, patients were instructed to collect all their current medication bottles and read back all medications to the interviewer. The lists were compared with the most recent medication list collected at either hospital discharge or at the last post-discharge interview. If a medication was continued, doses and frequency were confirmed. If a medication was not continued, the date of last use, as well as the reason for medication discontinuation, was collected from a list of options for a patient to choose from.
This analysis focused on five classes of secondary prevention medications with Class I recommendations by the American College of Cardiology/American Heart Association Guidelines including: 1) aspirin; 2) beta-blocker; 3) angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB); 4) statin; and 5) adenosine diphosphate receptor inhibitor (ADPRi).13 If any medication class was stopped since the last contact, the date of discontinuation and reason for this change was queried by the interviewer. Patients were prompted to refer to their study diary to provide this information. Medication persistence was defined as patient-reported continued use of a medication class prescribed at index hospital discharge at both 6-week and 6-month interviews. For analyzing use of all evidence-based medications, we stratified patients according to composite persistence14 into three groups: 1) high persistence (persistence with >80% of discharge medication classes); 2) moderate persistence (persistence with 40–80% of discharge medication classes); and 3) low persistence (persistent with <40% of discharge medication classes).
Statistical methods
Patient characteristics and outcomes in each group were described using frequencies and percentages for categorical variables and median (with 25th and 75th percentiles) for continuous variables. Missing data were rare (7.3%) for all variables considered in the regression models. Characteristics of patients in each group were compared using Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Multivariable logistic regression modeling was used to determine baseline demographic, clinical, and discharge factors associated with medication non-persistence at 6 months. Patient-level covariates examined in the model included age, gender, black race, non-Hispanic ethnicity, private insurance coverage, history of MI, coronary artery bypass graft surgery, PCI, prior heart failure, diabetes mellitus, hypertension, prior cerebrovascular accident/transient ischemic attack, atrial fibrillation or atrial flutter, end-stage renal disease requiring hemodialysis, dyslipidemia, peripheral artery disease, gastrointestinal or genitourinary bleed, in-hospital bleeding, current smoker, ejection fraction, referral to cardiac rehabilitation, and follow-up appointment made prior to discharge. Additional patient-level covariates collected by patient self-report included marital status, education achieved, employment status, EuroQol-5 Dimensions (EQ-5D) score, and Patient Health Questionnaire-2 (PHQ-2) score >3. The following variables were collected at the 6-week follow-up interview: written discharge medication list and instructions provided at hospital discharge, explanation for medication and potential side effects provided at hospital discharge, self-reported financial hardship due to medication, assistance with medications cost through insurance or other programs, and engages in at least 20 minutes of exercise weekly. Missing values of the continuous variables were imputed to the median.
The TRANSLATE-ACS study was funded by Eli Lilly and Company and Daiichi Sankyo, Inc. All data analyses were performed independently by statisticians at the Duke Clinical Research Institute using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Patterns of medication persistence by 6 months
Our final study population included 7,955 MI patients treated with PCI. Nearly all patients were discharged on aspirin (98%), an ADPRi (99.5%), statins (95%), beta-blockers (93%), and ACEIs/ARBs (74%). At 6 months post-MI, 5,509 patients (69%) were persistent with all evidence-based cardiovascular medications prescribed at discharge. Of those who discontinued at least one medication (31% of total), 2,265 patients were moderately persistent (continued use of 40–80% of all medications prescribed at discharge), and 181 patients had low persistence (continued use of <40% of all medications prescribed at discharge). Among individual medication classes, persistence was highest with aspirin (96%), followed by ADPRi (92%), beta-blockers (90%), statins (90%), and ACEIs/ARBs (84%). Among patients who stopped their medication prior to 6 months, the duration of persistence varied across the different medication classes. Patients remained on ADPRi for a median of 68 days (interquartile range [IQR] 31–116 days) before eventual discontinuation while they remained on aspirin for 55 days (IQR 7–119). This was followed by statins (51 days, IQR 7–117) and beta-blockers (49 days, IQR 13–108), while ACEIs/ARBs were discontinued the earliest after discharge at 37 days (IQR 9–92).
Patient characteristics and factors associated with non-persistence
Patients with high secondary prevention persistence were more often white, employed, and more likely to have private health insurance (Table I). Those with moderate and low persistence were more often unmarried, uninsured, current smokers, and reported symptoms of depression. Patients who were less persistent were also more likely to have atrial fibrillation or heart failure on presentation. After multivariable modeling, baseline demographic and socioeconomic factors independently associated with medication non-persistence at 6 months are presented in the Figure. Black race, as well as increasing age, remained independent demographic characteristics associated with non-persistence. Patients with private insurance coverage and those who had medication payment assistance through insurance or other programs were more likely to be persistent at 6 months. The presence of atrial fibrillation and hemodialysis was associated with non-persistence, whereas a prior history of coronary artery bypass graft surgery or hypertension was associated with higher persistence. Patients with signs of depression during the index hospitalization were less likely to be persistent, while those who engaged in regular exercise post-discharge were more likely to be persistent. Scheduling of outpatient follow-up prior to MI hospitalization discharge was significantly associated with increased medication persistence.
Table I.
Patient Characteristics
| High persistence (n=5,509) | Moderate persistence (n=2,265) | Low persistence (n=181) | p-value | |
|---|---|---|---|---|
| Age (years)* | 60 (52, 68) | 60 (53, 69) | 58 (51, 71) | 0.11 |
| Female | 27.1% | 29.3% | 26.5% | 0.13 |
| Race | ||||
| White | 90.1% | 86.9% | 86.2% | 0.0002 |
| Black | 7.4% | 9.7% | 12.7% | 0.0003 |
| Hispanic | 2.8% | 2.7% | 4.9% | 0.21 |
| Employed (full/part time) | 51.7% | 48.2% | 42.5% | 0.002 |
| Married | 66.5% | 64.2% | 49.7% | <.0001 |
| College education or higher | 53.7% | 52.8% | 45.9% | 0.12 |
| Insurance | ||||
| Private | 67.9% | 62.0% | 50.3% | <0.0001 |
| Medicare | 33.4% | 36.9% | 39.8% | 0.003 |
| Medicaid | 5.8% | 5.3% | 7.2% | 0.50 |
| Uninsured | 11.8% | 15.9% | 24.3% | <0.0001 |
| Comorbidities | ||||
| Prior MI | 19.0% | 18.9% | 19.3% | 0.99 |
| Prior PCI | 21.2% | 20.7% | 26.5% | 0.18 |
| Atrial fib/flutter | 3.9% | 5.5% | 12.2% | <0.0001 |
| Diabetes | 25.3% | 26.4% | 29.8% | 0.26 |
| Hypertension | 67.9% | 66.1% | 66.9% | 0.26 |
| Dyslipidemia | 68.3% | 65.9% | 59.1% | 0.006 |
| Current smoker | 36.0% | 38.9% | 47.5% | 0.0009 |
| Presentation features | ||||
| STEMI presentation | 51.7% | 51.4% | 50.3% | 0.42 |
| Cardiogenic shock | 2.2% | 2.0% | 2.2% | 0.88 |
| Heart failure symptoms/signs | 6.3% | 5.9% | 12.7% | 0.002 |
| ACTION risk score† | 30 (25, 34) | 30 (25, 35) | 29 (29, 24) | 0.81 |
| EQ-VAS score* | 75 (60, 85) | 75 (55, 85) | 75 (50, 90) | 0.13 |
| Depression: PHQ2 >3 | 7.0% | 8.9% | 8.3% | 0.02 |
Reported as median (25th and 75th percentiles).
Validated mortality risk prediction score15
ACTION, Acute Coronary Treatment and Interventions Outcomes Network; DC, discharge; EQ-VAS, EQ-5D visual analogue scale; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ2, Patient Health Questionnaire-2 (depression); STEMI, ST-segment elevation myocardial infarction
Figure. Forest Plot.
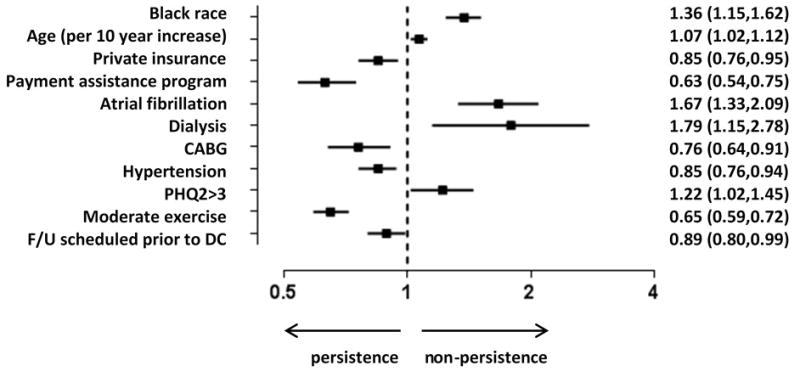
This figure displays significant factors associated with medication non-persistence. Other variables included in model: gender, ejection fraction, financial hardship with medication expenses, non-Hispanic, prior myocardial infarction, prior percutaneous intervention, prior heart failure, diabetes, hypertension, prior cerebrovascular accident/transient ischemic attack, smoker, dyslipidemia, gastrointestinal/genitourinary bleed, in-hospital bleeding, peripheral artery disease, discharge to home, cardiac rehab referral, EQ5D score, married, ≥ high school graduation, employed, written discharge medication list/instructions, explanation for medication and potential side effects, out of pocket med expenses. Moderate exercise = at least 1 day a week of ≥20 minutes of exercise. OR listed with 95% confidence intervals.
CABG, coronary artery bypass graft surgery; EQ-5D, EuroQol-5 Dimensions; OR, odds ratio
Reasons for medication non-persistence
Overall, physician direction accounted for approximately one-third of all reasons for medication discontinuation across medication classes. The remaining reasons cited were patient-related issues. For instance, medication side effect was reported in nearly 24% of patients, while cost was identified among 8.3% of patients. Patient self-discontinuation because of failure to refill after finishing the prior prescription was cited 5.6% of the time. Reasons for medication discontinuation varied according to medication class (Table II). Cost was cited as a factor in discontinuation more often with ADPRi (16%) compared with the other therapies which typically had generic options. Conversely, side effects were only identified in 12.6% of patients who discontinued ADPRi, though bleeding was cited more often.
Table II.
Medication Discontinuation by Medication Class*
| Reasons for discontinuation | ADPRi | Statins | Beta-blockers | ACEI/ARB | Overall |
|---|---|---|---|---|---|
| Physician instruction | 48.8% | 23.3% | 27.7% | 34.2% | 32.7% |
| Switched to alternative medication class | 7.9% | 13.3% | 12.8% | 12.6% | 11.9% |
| Physician felt no longer needed | 33.6% | 9.5% | 14.5% | 21.3% | 19.1% |
| Procedure/operation requiring stopping | 7.3% | 0.5% | 0.4% | 0.3% | 1.8% |
| Medication side effect | 12.6% | 26.0% | 25.3% | 28.3% | 23.8% |
| Bleeding/bruising | 6.0% | 0.6% | 0.6% | 1.0% | 1.8% |
| Other side effect | 6.6% | 25.4% | 24.7% | 27.3% | 22.0% |
| Cost | 15.9% | 9.5% | 6.1% | 3.9% | 8.3% |
| Other patient discontinuation reasons | 7.6% | 6.4% | 4.9% | 4.3% | 5.6% |
| Failure to refill after prescription ran out | 7.3% | 4.5% | 3.4% | 3.1% | 4.4% |
| Felt medication was not helping me | 0.3% | 1.4% | 1.5% | 1.1% | 1.1% |
| Friend/family advice | 0.0% | 0.5% | 0.0% | 0.1% | 0.2% |
| Other reason | 5.9% | 8.1% | 8.0% | 6.7% | 7.2% |
| Unknown | 9.3% | 26.8% | 28.0% | 22.7% | 22.4% |
Percentage of patients citing reasons for discontinuation of a medication class
ACEI, angiotensin-converting enzyme inhibitor; ADPRi, adenosine diphosphate receptor inhibitor; ARB, angiotensin receptor blocker
Transition of care factors
Patients were discharged with a median of 7 (IQR 6–10) medications; the number of medications at discharge was not significantly different between high, moderate, and low persistence groups (Table III). Most patients reported receiving written instructions for their medications at discharge; however, patients with high persistence more often stated that they had received explanations from the provider on the reasons for each medication and the potential side effects. Patients with higher medication persistence at 6 months more often had a follow-up visit with their outpatient providers shortly after discharge than patients with low persistence. Low persistence patients frequently reported financial hardship from medication expenses; only one-third of these patients had prescription coverage or were enrolled in a medication payment assistance program.
Table III.
Transition of Care Factors
| High persistence (n=5,509) | Moderate persistence (n=2,265) | Low persistence (n=181) | p-value | |
|---|---|---|---|---|
| Pre-discharge | ||||
| Number of discharge medications* | 7 (6, 10) | 7 (6, 10) | 7 (5, 10) | 0.17 |
| Received written discharge med list and instructions | 92.2% | 89.2% | 82.3% | 0.12 |
| Provider explained reason for each med | 83.0% | 80.0% | 69.6% | 0.008 |
| Provider explained side effect for each med | 59.4% | 56.6% | 45.9% | 0.008 |
| Primary in patient service cardiology | 88.5% | 87.8% | 82.9% | 0.03 |
| Cardiac rehab referral | 79.9% | 78.2% | 75.1% | 0.13 |
| Smoking cessation counseling | 51.9% | 54.6% | 56.4% | 0.08 |
| Follow-up appointment made before discharge | 67.3% | 65.0% | 57.5% | 0.27 |
| Post-discharge | ||||
| Cardiologist visit within 6 weeks after hospitalization | 69.4% | 65.8% | 51.4% | 0.0003 |
| Physician visit within 6 weeks after hospitalization | 60.4% | 60.8% | 48.1% | 0.045 |
| Prescription coverage or cost assistance program | 88.9% | 80.0% | 33.7% | <0.0001 |
| Financial hardship with meds | 37.3% | 40.1% | 44.8% | <0.0001 |
Reported as median (25th and 75th percentiles). Financial hardship meds = patient reported hardship with medication costs scored ≥3 on scale of 1 to 5 (1 = no hardship, 5 = extreme hardship).
Discussion
While the use of guideline-based therapies in the management of coronary artery disease is strongly recommended to improve morbidity and mortality, we found that up to one-third of contemporary patients are no longer persistent with therapies prescribed at discharge by 6 months post-MI. Reasons cited for discontinuation of medications vary according to medication class and include side effects, physician decision, and financial burden. Private insurance, prescription coverage insurance, and outpatient follow-up arranged prior to index discharge were associated with increased medication persistence, while increasing age, black race, and depression were markers of low persistence.
Poor medication persistence
We identified both modifiable and non-modifiable factors associated with a patient’s likelihood of persistence. Non-white race has been well established to be vulnerable to under-treatment,15 and we observed black race to be associated with non-persistence. Though our study was able to incorporate potential confounding factors such as such as household income, insurance status, educational attainment, and quality of life, black race remained an independent predictor of non-persistence. Additionally, patients with depression were also less likely to be persistent with medical therapy. Screening instruments, such as those employed in this study (EQ-5D16 and PHQ-217), are validated tools that are easy to administer and represent a feasible real-world option to identify patients at risk for low medication persistence once discharged from the hospital.
Evaluation of interventions to improve persistence by reducing the financial burden of medications have noted mixed results.18–21 In our study population, approximately 50% of patients reported paying more than $100 a month in out-of-pocket expenses for medications. When considering the median age of MI patients,22 costs are a strong concern in the face of fixed incomes and the likelihood of life-long medication needs. Though we found private insurance coverage continued to be associated with medication persistence after adjustment, this does not necessarily ensure adequate medication coverage.23 Patients who were enrolled in prescription coverage or payment assistance programs were additionally associated with higher persistence. Therefore, when considering the expansion of health insurance coverage, specific focus should be placed on patients’ out-of-pocket cost burden, and potentially providing incentives (financial and others) for evidence-based medication persistence.
Persistence rates in this study were higher than rates in previously published data. In a similar acute MI population, Ho et al. noted less than 60% of patients discharged on aspirin, beta-blockers, and statins were still on all three medications at 1 month post-discharge.3 By 6 months post-MI discharge, Kramer et al. noted only an approximate 50% adherence to beta-blockers.24 More recent studies demonstrate modest improvement with medication persistence approaching 70% for cardiovascular therapies.14,25 Possible explanations for the higher persistence noted in our contemporary study may be the greater proportion of patients covered by prescription drug coverage benefits, as well as the availability of generic options for most of these evidence-based therapies, with the exception of ADPRi. Our study may also reflect greater physician awareness of guideline-recommended therapies. 26 Targeted health system strategies deployed in recent years, such as remote monitoring,27 pharmacist interventions,28 and web-based patients education efforts,29,30 may also explain the higher rates of medication persistence found in our study. Finally, it should also be noted that we cannot exclude the possibility of overestimating true persistence rates, due to patient self-reporting.
Transition of care and downstream persistence
Current metrics for the inpatient treatment of MI patients have achieved almost perfect performance over the last decade, in part due to wide dissemination of guideline recommendations and incentivized quality improvement efforts focusing on team-based patient care.1,31–33 In contrast, our study found outpatient medication persistence post-MI to be considerably lower, underscoring the need for continued quality improvement efforts during the transition of patients from the hospital back into their communities. The processes for these transitions of care are likely fragmented and vary across health systems.34–37 For example, we found substantial variation in the degree of patient education received regarding medication indications and side effects between higher and lower persistence groups. Although time restraints during a hospitalization may at times limit patient education efforts,38 success has been noted in the care team model where physicians, discharge nurses, physician extenders, and social workers collectively educate the patient on post-discharge treatment goals, including medication adherence.39 We also observed that the arrangement of outpatient follow-up prior to discharge was associated with higher medication persistence.
Broad dissemination of care transition best practices, as well as team-based care quality surveillance and improvement initiatives, are likely to promote a more seamless transition of care from the inpatient to the community setting.40
Solutions
Our study has identified multiple reasons for non-persistence with medical therapy. Some of these factors appear valid, such as significant side effects or where medical therapy has deemed to no longer be indicated by a health care provider. Other reasons are more concerning, such as failing to refill medications or self-discontinuation due to lack of perceived benefit. A “one size fits all” approach to improve persistence is unlikely to be effective; therefore, any solution should have a multifaceted approach tailored to individual patients.41 For instance, patients with an increased risk of adverse events may need a higher provider threshold for discontinuation of therapies.42 Among patients with cost as an identified barrier, increased access to affordable medications would be an important component to improving persistence. Other patients, however, simply need a better understanding of their medication treatment regimen. Regardless of the reason, continued engagement with a patient by a member of the health care team allows for counseling as well as opportunities to reevaluate a patient’s changing needs. Identifying the specific needs of a patient via an understanding of both modifiable and non-modifiable risk factors can allow for a personalized multipronged intervention to increase medication use and ultimately improve outcomes.
Our study had several limitations. First, persistence with medications does not measure adherence to prescribed therapies and physician instructions. For example, changing clinical circumstances may result in new indications or contraindications prompting adjustments in medications. This is likely why clinical conditions such as dialysis are associated with nonpersistence. In the absence of direct observation or pharmacy validation, our data cannot address whether medications were actually taken by patients as prescribed or titrated to target doses in persistent patients. Additionally, data acquired through patient self-report may be subject to recall bias. Second, since this is an observational study, causal relationships cannot be inferred between persistence and patient or health-system factors. Even after adjustment, unmeasured confounding may persist. Finally, study subjects volunteered to participate in TRANSLATE-ACS, and therefore, may not be representative of all MI patients.
In conclusion, persistence with evidence-based therapies remains suboptimal among contemporary MI patients. Patients at high risk of non-persistence may be identified by clinical and sociodemographic factors. Additional modifiable factors include those associated with the transition of care from the hospital to the outpatient setting. A multifaceted approach to understand barriers at the patient, provider, and health system level, can allow for development of a tailored intervention based on an individual patient’s needs. Such an approach has the potential to improve persistence with secondary prevention therapies.
Acknowledgments
The authors would like to thank Erin Hanley, MS, for her editorial contributions to this manuscript. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Sources of Funding
TRANSLATE-ACS was funded by Daiichi Sankyo Ltd. and Eli Lilly USA (clinicaltrials.gov number, NCT01088503). The Duke Clinical Research Institute is the coordinating center for this study, which represents a collaborative effort with the American College of Cardiology. Robin Mathews is supported by grant number KM1CA156687 from the National Institute of Health/National Cancer Institute.
Footnotes
Conflict of Interest Disclosures
R Mathews: Dr. Mathews has no relevant disclosures to report.
TY Wang: Dr. Wang reports research funding from AstraZeneca, Gilead, Lilly, The Medicines Company, and Canyon Pharmaceuticals (all significant); educational activities or lectures (generates money for Duke) for AstraZeneca (modest); consulting (including CME) for Medco (modest) and American College of Cardiology (significant).
E Honeycutt: Ms. Honeycutt has no relevant disclosures to report.
TD Henry: Dr. Henry has no relevant disclosures to report.
M Zettler: Dr. Zettler reports being an employee of Eli Lilly & Company.
M Chang: Dr. Chang has no relevant disclosures to report.
GC Fonarow: Dr. Fonarow reports being a consultant to Novartis (significant) and Janssen (modest).
ED Peterson: Dr. Peterson reports research funding for the American College of Cardiology, American Heart Association, Eli Lilly & Company, Janssen Pharmaceuticals, and Society of Thoracic Surgeons (all significant); consulting (including CME) for Merck & Co. (modest), Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi-Aventis (all significant).
References
- 1.Hospital Compare. Data.Medicare.gov; the Official U.S. Government Site for Medicare web site. [Accessed March 27, 2015]; https://data.medicare.gov/data/hospital-compare.
- 2.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 3.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–7. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 4.ASPE Issue Brief, Office of the Assistant Secretary for Planning and Evaluation, Office of Science and Data Policy. U.S. Department of Health and Human Services; [Accessed March 27, 2015]. ASPE.hhs.gov web site. http://aspe.hhs.gov/sp/reports/2010/genericdrugs/ib.pdf. Updated December 1, 2010. [Google Scholar]
- 5.Schneeweiss S, Patrick AR, Pedan A, et al. The effect of Medicare Part D coverage on drug use and cost sharing among seniors without prior drug benefits. Health Aff (Millwood) 2009;28:w305–16. doi: 10.1377/hlthaff.28.2.w305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenberg FR, Sun SX. The impact of Medicare Part D on prescription drug use by the elderly. Health Aff (Millwood) 2007;26:1735–44. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- 7.Gellad WF, Grenard J, McGlynn EA. A Review of Barriers to Medication Adherence: A Framework for Driving Policy Options. [Accessed March 27, 2015];Rand Corporation web site. http://www.rand.org/content/dam/rand/pubs/technical_reports/2009/RAND_TR765.pdf. Updated 2009.
- 8.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 9.Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–30. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 10.Yong PL, Olsen L Roundtable on Evidence-Based Medicine; Institute of Medicine. National Academies of Sciences web site. Washington, DC: 2010. [Accessed March 27, 2015]. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. http://www.nap.edu/catalog.php?record_id=12750. Updated 2010. [PubMed] [Google Scholar]
- 11.Chin CT, Wang TY, Anstrom KJ, et al. Treatment with Adenosine Diphosphate Receptor Inhibitors—Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study design: expanding the paradigm of longitudinal observational research. Am Heart J. 2011;162:844–51. doi: 10.1016/j.ahj.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 12.National Cardiovascular Data Registry ACTION Registry-GWTG. [Accessed March 27, 2015];National Cardiovascular Data Registry web site. http://www.ncdr.com/webncdr/ACTION/Default.aspx. Updated July 22, 2013.
- 13.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–46. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 14.Bushnell CD, Olson DM, Zhao X, et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology. 2011;77:1182–90. doi: 10.1212/WNL.0b013e31822f0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Shrank WH, Avorn J, et al. Comparative cost-effectiveness of interventions to improve medication adherence after myocardial infarction. Health Serv Res. 2012;47:2097–117. doi: 10.1111/j.1475-6773.2012.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. World Health Organization web site. Geneva: World Health Organization; 2003. [Accessed March 27, 2015]. Adherence to long term therapies: evidence for action. http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Updated 2003. [Google Scholar]
- 17.Kind P, Dolan P, Gudex C, et al. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–41. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miedema MD, Cohn JN, Garberich RF, et al. Underuse of cardiovascular preventive pharmacotherapy in patients presenting with ST-elevation myocardial infarction. Am Heart J. 2012;164:259–67. doi: 10.1016/j.ahj.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. New Engl J Med. 2011;365:2088–97. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 20.Priebe S, Burton A, Ashby D, et al. Financial incentives to improve adherence to anti-psychotic maintenance medication in non-adherent patients - a cluster randomised controlled trial (FIAT) BMC Psychiatry. 2009;9:61. doi: 10.1186/1471-244X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient compliance. BMJ. 1997;315:703–7. doi: 10.1136/bmj.315.7110.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang V, Liu CF, Bryson CL, et al. Does medication adherence following a copayment increase differ by disease burden? Health Serv Res. 2011;46:1963–85. doi: 10.1111/j.1475-6773.2011.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chin CT, Wang TY, Li S, et al. Comparison of the prognostic value of peak creatine kinase-MB and troponin levels among patients with acute myocardial infarction: a report from the Acute Coronary Treatment and Intervention Outcomes Network Registry-get with the guidelines. Clin Cardiol. 2012;35:424–9. doi: 10.1002/clc.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry-Get With The Guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–22. e2. doi: 10.1016/j.ahj.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to β-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454, e1–8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–36. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- 27.Somma KA, Bhatt DL, Fonarow GC, et al. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:654–61. doi: 10.1161/CIRCOUTCOMES.111.963959. [DOI] [PubMed] [Google Scholar]
- 28.van Veldhuisen DJ, Maass AH. Telemonitoring of outpatients with heart failure: a search for the holy grail? Circulation. 2012;125:2965–7. doi: 10.1161/CIRCULATIONAHA.112.118141. [DOI] [PubMed] [Google Scholar]
- 29.Walker PC, Bernstein SJ, Jones JN, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169:2003–10. doi: 10.1001/archinternmed.2009.398. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;11:785–95. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 31.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1045–55. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:e215–367. doi: 10.1016/j.jacc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (Updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 35.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–87. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients--development of a discharge checklist for hospitalists. J Hosp Med. 2006;1:354–60. doi: 10.1002/jhm.129. [DOI] [PubMed] [Google Scholar]
- 37.Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143:121–8. doi: 10.7326/0003-4819-143-2-200507190-00011. [DOI] [PubMed] [Google Scholar]
- 38.Kociol RD, Peterson ED, Hammill BG, et al. National survey of hospital strategies to reduce heart failure readmissions: findings from the Get With The Guidelines-Heart Failure registry. Circ Heart Fail. 2012;5:680–7. doi: 10.1161/CIRCHEARTFAILURE.112.967406. [DOI] [PubMed] [Google Scholar]
- 39.Oates DJ, Paasche-Orlow MK. Health literacy: communication strategies to improve patient comprehension of cardiovascular health. Circulation. 2009;119:1049–51. doi: 10.1161/CIRCULATIONAHA.108.818468. [DOI] [PubMed] [Google Scholar]
- 40.Albert NM, Fonarow GC, Yancy CW, et al. Outpatient cardiology practices with advanced practice nurses and physician assistants provide similar delivery of recommended therapies (findings from IMPROVE HF) Am J Cardiol. 2010;105:1773–9. doi: 10.1016/j.amjcard.2010.01.360. [DOI] [PubMed] [Google Scholar]
- 41.Zullig LL, Peterson ED, Bosworth HB. Ingredients of successful interventions to improve medication adherence. JAMA. 2013;310:2611–2. doi: 10.1001/jama.2013.282818. [DOI] [PubMed] [Google Scholar]
- 42.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. New Engl J Med. 2014;371:2155–66. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]


