Abstract
Biomaterials have played an increasingly prominent role in the success of biomedical devices and in the development of tissue engineering, which seeks to unlock the regenerative potential innate to human tissues/organs in a state of deterioration and to restore or reestablish normal bodily function. Advances in our understanding of regenerative biomaterials and their roles in new tissue formation can potentially open a new frontier in the fast-growing field of regenerative medicine. Taking inspiration from the role and multi-component construction of native extracellular matrices (ECMs) for cell accommodation, the synthetic biomaterials produced today routinely incorporate biologically active components to define an artificial in vivo milieu with complex and dynamic interactions that foster and regulate stem cells, similar to the events occurring in a natural cellular microenvironment. The range and degree of biomaterial sophistication have also dramatically increased as more knowledge has accumulated through materials science, matrix biology and tissue engineering. However, achieving clinical translation and commercial success requires regenerative biomaterials to be not only efficacious and safe but also cost-effective and convenient for use and production. Utilizing biomaterials of human origin as building blocks for therapeutic purposes has provided a facilitated approach that closely mimics the critical aspects of natural tissue with regard to its physical and chemical properties for the orchestration of wound healing and tissue regeneration. In addition to directly using tissue transfers and transplants for repair, new applications of human-derived biomaterials are now focusing on the use of naturally occurring biomacromolecules, decellularized ECM scaffolds and autologous preparations rich in growth factors/non-expanded stem cells to either target acceleration/magnification of the body's own repair capacity or use nature's paradigms to create new tissues for restoration. In particular, there is increasing interest in separating ECMs into simplified functional domains and/or biopolymeric assemblies so that these components/constituents can be discretely exploited and manipulated for the production of bioscaffolds and new biomimetic biomaterials. Here, following an overview of tissue auto-/allo-transplantation, we discuss the recent trends and advances as well as the challenges and future directions in the evolution and application of human-derived biomaterials for reconstructive surgery and tissue engineering. In particular, we focus on an exploration of the structural, mechanical, biochemical and biological information present in native human tissue for bioengineering applications and to provide inspiration for the design of future biomaterials.
Keywords: Raw materials, Biopolymers, Extracellular matrix, Tissue decellularization, Biomimetic design, Blood-derived biomaterials, Transplantation
1. Introduction
The human body has a limited ability to correctly auto-regenerate most, if not all, of its major tissues and organs in the event that the original tissue integrity has been seriously damaged as a result of medical disorders involving tissue dysfunction or devastating deficits [1,2]. Faced with an ever-increasing burden of trauma, congenital abnormalities and degenerative diseases, tissue engineering and regenerative medicine promise to develop new biological therapeutics to treat a diverse range of diseases that are currently intractable. Additionally, in most cases, this type of research seeks to assist and accelerate the regenerative process by stimulating the patient's own inherent healing potential or, alternatively, to create replacement biological tissues (or, more challengingly, whole organs) to replace damaged, deteriorated or lost body parts [3–5]. These therapeutic strategies regulate physiological conditions in a spatial and temporal manner and mimic the mechanisms of normal tissue repair and regeneration in different parts of the human body, and endeavors in this field have sparked a revolution in current and emerging trends in medical science [4].
Although bold steps have been made toward creating tissue constructs that could serve as integral parts of the clinical toolbox, many of these engineered tissues fail to fully match the functional properties of their native counterparts. This failure is partially due to our poor quantitative understanding of the mechanisms of the adaptive responses (i.e., the growth and remodeling processes) that modify the architecture of engineered tissues following in vivo transplantation [6]. Considering that most living tissues are composed of numerous repeating units that are hierarchically assembled across multiple length scales and possess well-defined three-dimensional (3D) microarchitectural features and tissue-specific functional properties, the production of micron-sized tissue modules has attracted increasing interest in the fast-growing field of tissue engineering [5,7]. These modules can be used alone as living materials (fillers) to repair wounded tissues at the sites of injury or can serve as building blocks for the generation of large tissue grafts or whole-organ implants through a so-called “bottom-up” approach [8]. In light of these applications, in vitro, it is indispensable to recapitulate not only the structural organization but also the cellular and molecular composition of a native tissue to enhance the biological performance and the overall therapeutic outcome of such engineered tissues upon in vivo transplantation [9]. Such modular tissues could be extraordinarily useful when used as injectable living microtissues for repair at sites of injury. Alternatively, if assembled into large 3D tissues, these modules could also be used as a patch for a large number of types of hitherto intractable extended damage to restore tissue function [7]. In the future, an increased availability of engineered “living” tissue or organ substitutes could significantly reduce the demand for organ replacement and dramatically expedite the development of new therapeutics that can cure patients with revivable organ failure, eliminating the need for organ allotransplantation altogether [10].
Although biotechnology that can produce complex organs de novo is not yet available [11], mounting evidence suggests that, at least to a certain degree, the body's innate powers of regeneration can be augmented by replacing sections of tissue and enhancing the regenerative cascade [4,12]. The current strategy for tissue engineering typically entails the ex vivo expansion of multipotential cell populations, such as mesenchymal stem cells (MSCs), followed by their transplantation into damaged areas [10]. Due to their unique regenerative potential and immunomodulatory properties, MSCs hold great promise in tissue engineering and reconstructive therapies, not only directly participating in wound healing and regeneration but also modulating the host foreign-body immunogenic reaction to transplants [13]. These cells are normally transplanted within a biomaterial-cell construct based on a biodegradable 3D matrix that provides the requisite extracellular milieu, which contains physical and chemical cues for cell-driven tissue development and regeneration [10,14]. Although a wide variety of therapeutic strategies based on different types of biomaterials and stem cells have been and are still being explored, in practice, modern tissue engineering is not an easily accessible approach to achieve regeneration in a clinical setting [15]. In particular, several biological (e.g., a poor understanding of underlying mechanisms), technical (e.g., the large-scale expansion of stem cells) and regulatory (e.g., cost and safety) hurdles relating to the use of exogenously manipulated stem cells and engineered constructs for human therapeutics have yet to be overcome [16,17]. In addition, a thorough understanding of the normal physiological processes in tissue development and of the mechanisms underlying the interactions between stem cells and biomaterials during the cascade of new tissue formation will be required to advance this field, as many crucial details remain unclear [18].
Biomaterials play a pivotal role in the success of tissue engineering, though this is not to say that traditional synthetic biomaterials must always be used [19]. However, to either create living neo-tissues in vitro that are similar or identical to their native body counterparts or facilitate in situ tissue regeneration by controlled presentation and on-demand release of specific chemokines at sites of injury, temporary biodegradable support matrices with natural, tissue-resembling structural and functional attributes are generally necessary, if not indispensable, for cell attachment and housing [20–23]. Similar to a blood clot serving as a natural polymeric scaffold in the cascade of wound healing events, these matrices should have a desirable shape that provides functionality and supports tissue regrowth until sufficient new tissue is formed [21]. Therefore, from a fundamental perspective, the goal of biomaterial design in tissue engineering is to identify or fabricate a substance that is innately able (or has been engineered) to assume a desirable form that can be applied to both synthesize a 3D cellular microenvironment for cell accommodation and guide new tissue formation [24,25]. The material should be able to maintain its structure and integrity for predictable periods of time to ensure new tissue formation and maturation, even under load-bearing circumstances [26,27]. In recent years, the development of regenerative biomaterials has rapidly evolved to allow the sequestration and controlled release of growth factors that work in concert with materials to achieve tailored biological properties and improved functionalities, which, in a precise and near-physiological fashion, can control stem cell fate under niche-mimicking, recognizable conditions both in vitro and in vivo [28–30]. The key concept of these designs is the recreation of myriad cellular and molecular events involved in the regeneration of a new tissue/organ [31]. Therefore, the design of material devices that approximate many of the critical features of normal cellular matrices in human tissue and thus foster and direct the formation of target tissues lies at the forefront of biomaterials science and tissue engineering and is indeed the epitome of the fields' present motivation [26].
No longer simply a non-viable material used in a medical device that is generally used as a “filler”, a biomaterial is now defined as “a substance that is able, or has been engineered, to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure, in human or veterinary medicine” [21,23]. The clinical benefit of bioengineering technologies, based to some extent on the use of biomaterials, in an increasing number of patients places exponentially growing demands on scaffolding materials. The search for an “excellent” tissue engineering template has remained a research hotspot as the rapidly growing multidisciplinary area of tissue engineering continues to advance, along with its intertwined field of “regenerative medicine” [20,22,32–34]. The term “regenerative medicine” was initially considered to encompass many more disciplines and fields of medicine than the traditional concept of “tissue engineering” did, but today, the two terms are often used interchangeably [10]. Therefore, the philosophy of the emerging discipline of current tissue engineering and, indeed, of regenerative medicine is that rather than aiming to develop a complex living-tissue replacement ex vivo, concerted efforts must focus on creating extracellular matrix (ECM)-mimicking biomaterials or modulating stem cell niches that recapitulate pivotal interactions with host cells to unlock the patient's own regenerative ability for organization and self-repair [22,24,25].
Although the structural, mechanical and biochemical information coded within the native ECM directs the design of new types of tissue-engineering templates, unfortunately, the biological properties and network architecture of currently available porous synthetic scaffolds fall short of the criteria for the creation of a complex human tissue [16,18,35,36]. It is now widely recognized that this gap has largely arisen because the study of porous scaffolds in vivo is often limited by the experimenter's inability to control all of the technical parameters, several of which rely on the systemic responses of the living organism [18,27,37]. Furthermore, the regulation of structural parameters in the development of fully synthetic biomaterials and their bioactivation, achieved through the integration of key biomacromolecules and signals capable of directing cell and tissue fate in vivo, represent a great challenge in practice [35,36]. Most importantly, in the hope of commercial success, regenerative biomaterials must be not only efficacious but also cost-effective to facilitate their translation into clinical settings to help the greatest number of patients, leading to an ineluctable dichotomy between the need for an appropriate level of sophistication (integrating complex information into scaffolds) and the ease of scaffold production (bypassing device over-engineering and keeping material complexity to a minimum) [15,20,38].
Unfortunately, if we wait for every aforementioned question to be answered, the timely introduction of novel treatments based on tissue engineering into human healthcare will be impossible [16]. In pursuing a perfect synthetic, tissue-engineered template, it is important to consider whether we have looked too far ahead and missed the readily available building blocks, such as naturally derived biomacromolecules required to create biomaterials for this purpose [39]. For decades, scientists have learned that the human body is a tremendous potential source of bioscaffolds and biopolymers for therapeutics; these biomaterials have attracted considerable attention in the tissue engineering and regenerative medicine communities [40,41]. For instance, certain therapeutic biomaterials may be produced from human blood. Several types of blood-derived bioscaffolds are utilized in clinical situations demanding a high fibrinogen content, whereas platelet-rich formulations are used because they contain multiple platelet-derived growth factors [42]. Based on the latest definition of biomaterials, from 2009 [21,23], in the present review, we define human-derived biomaterials much more broadly than we are accustomed to doing. We specifically define these biomaterials as those existing (e.g., donor organs and tissue grafts) or originally found (e.g., decellularized ECM materials and ECM components/constituents) within our bodies, along with a large variety of cell populations obtained from human materials and active proteins (e.g., mitogenic, chemotactic, adhesive, angiogenic and antiangiogenic proteins) of human origin (Fig. 1). In particular, the use of human-derived biomaterial scaffolds, naturally occurring proteins, ECM components and preparations rich in growth factors or non-expanded stem cells for tissue engineering provides new approaches for the redesign of clinically translatable regenerative therapies. Biomaterials scientists aim to recreate the intrinsic properties of human-derived biomaterials in next generation of regenerative biomaterials tailored to specific applications [39]. Those properties include, but are not limited to, the provision of a structural support for resident cells and the establishment of physical integrity in the tissue. Additionally, these properties have a profound influence on cell fate through the regulation of cell proliferation, migration and gene expression and the maintenance of functional homeostasis [26,43]. Research in this area is growing very rapidly thanks to the combined efforts of the multidisciplinary biomaterials and bioengineering communities. As will be detailed in this article, it is likely that the use of these biomaterials as biocompatible, biodegradable and versatile matrices in tissue engineering will circumvent many biological and technical problems in the design and development of synthetic biomaterials, hence opening medically exploitable avenues for the translational success of tissue engineering solutions [44]. In the future, these exciting human “raw materials” will be essential to help reconcile the pressures facing tissue engineering with respect to commercial production and clinical translation [39].
Fig. 1.
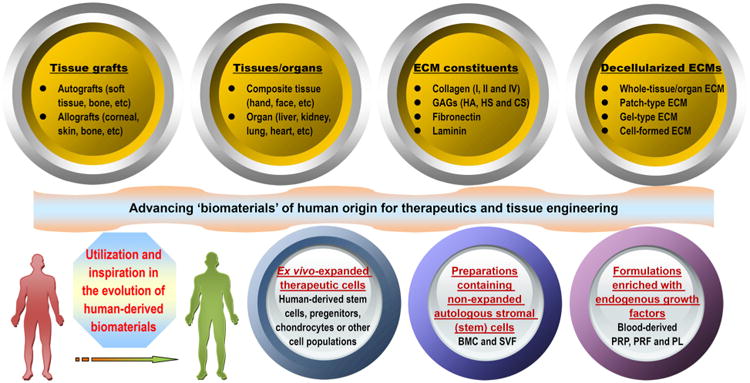
Schematic representation of the “biomaterials” of human origin frequently used as therapeutics in medicine or as potential building blocks in the specific context of advancing the “next generation” of tissue engineering templates for clinical use and commercial production. From tissue/organ transplantation to the use of either naturally occurring biopolymers or biomimetic materials inspired by nature in tissue engineering and regenerative medicine, the terms may change, but the essential goals remain the same. Examples are given where appropriate (please see the text for additional details). Although ex vivo-expanded cells derived from human tissues/organs for therapeutic use may be broadly classified as human-derived “biomaterials”, they are not included in the general definition of biomaterials and hence will not be detailed in this review. Abbreviations appearing in the figure: ECM, extracellular matrix; GAGs, glycosaminoglycans; HA, hyaluronic acid; HS, heparin sulfate; CS, chondroitin sulfate; PRP, platelet-rich plasma; PRF, platelet-rich fibrin; PL, platelet lysate; BMC, bone marrow concentrate; SVF, stromal vascular fraction.
Following an overview of tissue and organ auto-/allo-transplantation, which represents the original practice in this field, this review will discuss recent new insights into and expanding applications of human-derived biomacromolecules and biomaterials in tissue engineering for the management of human tissue-destructive conditions, recalcitrant chronic wounds and persistent/deteriorating organ failure. This review will also highlight recent approaches and expanding opportunities to exploit the molecular mechanisms of these “raw materials” to create bioscaffolds with a wide range of material properties and applications, even though these biomaterials are in their early stages of development. The potential challenges facing the field and the obstacles that must be addressed to explore and develop truly clinically viable biomaterials and regenerative therapies are also discussed in detail. Although concerted efforts have been and still are being made in the field of synthetic biomaterials in an attempt to develop advanced devices that mimic the critical aspects of natural ECMs and to shed light on their potential translation to clinical settings, we believe that the development and use of biomaterials of human origin is an equally inevitable trend. We therefore present a call to action for biological and materials scientists, funding agencies and professionals in reconstructive medicine to pool their resources to hasten this development through a highly multidisciplinary approach that addresses the largest limitations of current regenerative biomaterials. A major priority is to involve clinicians who practice regenerative medicine and who regularly encounter the problems that they aim to solve in the design and creation of advanced biomaterials and tissue-engineered constructs for clinical use. If more international research efforts are made in this direction, the unleashed potential of biomaterials of human origin will benefit more and more clinical patients each year.
2. Biomaterials for tissue engineering
The staggering potential of living tissues for auto-regeneration may be restricted/impaired by an age-related decline in the number and quality of host stem cell/progenitor populations, by the innately low regenerative capacity of certain tissues or by the negative effect of inflammation on wound repair [20]. In an effort to compensate for such poor healing capacity, tissue engineering has been established as a potential therapeutic option to recreate several of the biological processes that occur during tissue development and in the native wound healing cascade in microcosms [3–5]. For this purpose, a harmonious combination of a scaffold/supporting materials, adequate target cells and growth-stimulating bioactive factors is used to promote the regeneration of damaged tissues or to replace failing or malfunctioning/deteriorating organs [17]. Therefore, tissue-engineered constructs have to mimic a certain degree of the native complexity of a tissue to assist in the restoration of the full structure and functionality of the tissue. This approach is the first medical therapy wherein engineered tissues can potentially become fully integrated into the patient, thus conferring a permanent cure for a diverse range of diseases that are not curable today [45]. Notably, biomaterials that support and foster regenerative cell growth have played a considerable role in the tissue engineering design paradigm and in the success of numerous medical devices for clinical regenerative therapies [36,39].
Broadly speaking, biomaterials can be defined as material devices or implants used to repair/replace native body tissues or as scaffolding materials adopted to construct manmade tissues and organs [19]. Commonly, therapeutic biomaterials can be classified into two main categories: (I) living or once-living material of animal or human origin; and (II) other materials, including materials from vegetal sources and synthetic materials and their composites that are biocompatible and can be applied for tissue regeneration. For over two decades, progress in polymer science and tissue engineering has paved the way for the generation of sophisticated and ingenious biomaterials to optimize existing clinical treatments and to develop more safe and effective cures for a higher quality of human life.
2.1. Roles of biomaterials in tissue engineering
We preface the following discussion with a brief description of the multifaceted roles of biomaterials in tissue engineering, particularly their tasks as tissue-templates that home, foster and coax stem cells to form new tissue [23]. The basic role of biomaterials in tissue engineering is to provide temporary mechanical support and mass transport to encourage cell adhesion, proliferation, and differentiation and to control the size and shape of the regenerated tissue [45]. Moreover, biomaterials, usually described as scaffolds, may present physical and chemical signals with spatiotemporal accuracy, which are of great importance to the modulation of cell performance and function and in the guidance of correct tissue regeneration, as an ECM contains the intrinsic signals pivotal to communicating with and controlling niche cells [46]. Instead of an inert structure temporarily employed to construct inanimate objects, this new concept of a tissue engineering “template” incorporates the sense of a structure that is actively involved in delivering cues to cells and that takes part in the formation and characteristics of the engineered/regenerated tissue [47]. These design requirements stem from the recognition that mimicking the in vivo cell-supporting niche (i.e., the ECM) with regard to its structural, mechanical and biochemical properties will coax niche cells into behaving similarly to their natural in vivo counterparts [48]. Recent insights into ECM mimics have already enriched our understanding of how to explore/harness the regenerative potential of various cell types via a well-designed cellular matrix-scaffold to create an artificial tissue/organ and to dramatically enhance the engraftment of ex vivo-expanded progenitor/stem cells [35]. To this end, scaffolding templates provide a 3D matrix that replicates, as far as possible, the niche of the target cells, defining an artificial niche with complex and dynamic regulation, in which a target tissue can form [49,50].
Ideally, a scaffold should serve as a transient structure that, over an extended period, will be degraded or reabsorbed in a controlled manner that is in accordance with the regrowth rate of new tissue [51,52]. Consequently, the biomaterial template is correctly replaced with naturally deposited ECM and the newly formed tissue of interest. In the host environment, a biomaterial's ability to orchestrate human host responses to exogenously transplanted cells may positively influence cell behavior and function and ultimately dramatically affect the desired tissue formation [53]. Fortunately, advances in biomaterials science, combined with recently increasing knowledge of ECM biology and the role of environmental cues in tissue development, have led to the redesign of material templates that are modified to provide appropriate structural support and, in certain cases, biological and mechanical cues to promote the safe and effective reconstruction of a functional tissue in vivo [36,37,54]. Moreover, scaffolding biomaterials can be tailored to mobilize and present biologically active molecules, including cell adhesion peptides, cell homing factors and numerous growth/differentiation and mechanical signals; to expand or recreate the stem cell compartment to facilitate the recruitment of stem cells and their subsequent differentiation into a large number of daughter cells; and finally, to direct new tissue formation and integration [12,22,54–57]. For damaged sites featuring enough repair cells in the local microenvironment, scaffolds mainly serve to promote the homing of the host's own cells for in situ tissue regeneration, whereas other approaches leverage material templates for the delivery of exogenously expanded cell populations to supplement the body's cell niche [22,57,58]. In either case, tissue engineering scaffolds seek to mimic the natural ECM, at least partially, and to create a favorable microenvironment to support and induce tissue formation [35]. Therefore, the identification of adequate biomaterials for cell accommodation and mass transport is a pivotal step in any tissue engineering design. A wide range of options exist for designing a specific biomaterial to be used as a matrix template, including natural biomaterials, synthetic biomaterials, and composites composed of two or more material types/classes. The advantages and disadvantages of applying these biomaterials and their suitability for application must be determined [48,59] (Fig. 2).
Fig. 2.
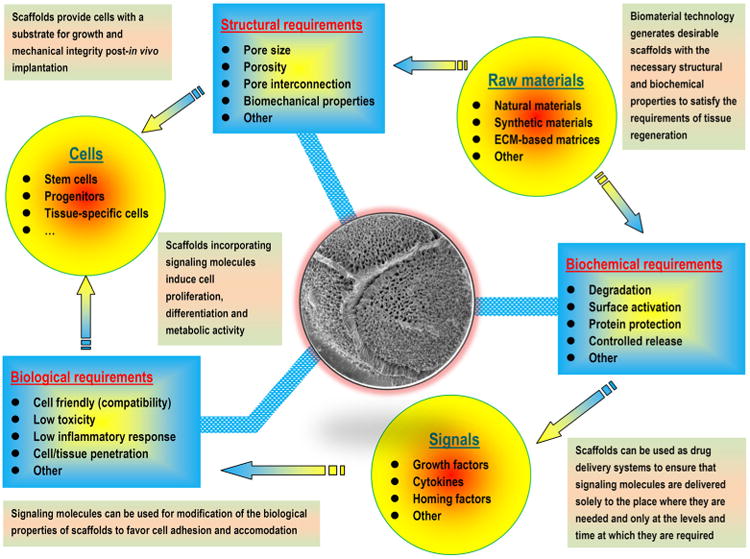
Schematic representation of pivotal factors (structural, mechanical, biochemical and biological) involved in the design of biomaterials (templates) for tissue engineering that coax cells to behave in the same or a similar manner as their natural in vivo counterparts.
2.2. Naturally derived biomaterials
Natural biomaterials present a crucial subset of biomaterials for use as tissue engineering templates due to their bioactivity, biocompatibility, tunable degradation and mechanical kinetics and their intrinsic structural resemblance of native tissue ECM. Natural biopolymers are often processed using environmentally–friendly aqueous-based methods. Upon application within biological systems, they do not release cytotoxic products during degradation, and their degradation rates may be adjusted by altering the starting formulation and/or processing conditions [60]. An advantage of natural biomaterials is their innate ability to promote biological recognition, which may positively support cell adhesion and function [39]. In addition, in nature, helical macromolecules such as collagen, cellulose and chitin are critical for the morphogenesis and functionality of a large variety hierarchically structured materials [61]. Naturally derived biomaterials may typically be divided into two groups: protein-based biomaterials (e.g., collagen, silk fibroin, gelatin, fibronectin, keratin, fibrin and eggshell membrane) and polysaccharide-based biomaterials (e.g., hyaluronan, cellulose, glucose, alginate, chondroitin, and chitin and its derivative, chitosan). Protein-based biomaterials are typically obtained from animal and human sources and include bioactive molecules that mimic the extracellular environment, whereas polysaccharide-based biomaterials are mostly obtained from algae, as in the case of agar and alginate, or from microbial sources, as in the case of dextran and its derivatives [40,41,52]. Another class of natural biomaterials is termed decellularized tissue-derived biomaterials, which are created by the elimination of all cellular and nuclear materials from native tissues/organs, as in decellularized dermis, heart valves, blood vessels, small intestinal submucosa (SIS) and liver, among others. These decellularized tissue-derived biomaterials contain a variety of different organic and/or inorganic components. If the tissue/organ is from a human, the resulting decellularized materials can be considered as human-derived biomaterials, which will be detailed in Section 4.2. Certain natural polymers also contain surface ligands or motifs required for cell adhesion and proliferation. In particular, cell adhesion and subsequent cell activity are mediated by specific integrin–ligand interactions between cells and their surrounding ECMs [62].
Due to the key advantage of these materials in supporting the attachment, proliferation and differentiation of cells, natural polymers have been extensively explored in the development of tissue engineering templates, often in combination with molecular and mechanical signals, for applications ranging from tissue repair to functional organ replacement [63]. For therapeutic applications, these polymers are generally processed for implantation as porous scaffolds, hydrogels, particulates or thin membranes and are typically enzymatically degradable into nontoxic end-products in vivo. Although the kinetics of the degradation of these biomaterials may not be easily controlled or predicted, they are still effective if local, short-term responsive action is sufficient. Additionally, special forms of natural polymers (e.g., injectable hydrogel) may be administered noninvasively to a target site of tissue damage [24,52,64,65].
The disadvantages of naturally derived biomaterials include generally weak mechanical strength and inconsistency in compositions and properties, which are associated with batch production due to their origin in living beings [66]. To overcome these limitations, recent advances in tissue engineering template redesign and fabrication have led to a paradigm shift toward the development of biomimetic scaffolds that incorporate ligands imitating the native ECM. These scaffolds are often utilized in vitro as analogs of the natural ECM to facilitate investigations of cell–ECM interplay and other intricate processes [67,68]. Another concern with naturally derived polymeric materials is the variability inherent in the production of the materials and the potential, albeit small, of the materials to evoke an immune response [35].
2.3. Synthetic polymer biomaterials
The use of synthetic polymers as matrices and templates in bioengineering presents several key advantages relative to naturally derived polymers, offering attractive options for the control of shape, architecture and chemistry to generate reasonable alternatives to or mimics of ECM systems of human origin that emulate or control biomaterial functions [69,70]. The most widely used synthetic polymers for tissue regeneration are poly(α-hydroxy acids), which include polylactic acid (PLA), polyglycolic acid (PGA) and their copolymer, poly(lactic-co-glycolic acid) (PLGA) [71,72]. These polymers' nontoxic degradation products (lactic acid and glycolic acid) are generated via simple chemical hydrolysis of the polymers and are cleared away by normal metabolic pathways [73]. Given the lack of dependence on local enzyme concentrations, chemical hydrolysis may be more readily predicted and controlled than enzymatic degradation in vivo [63,71]. The properties of synthetic polymers, such as tensile strength, the mechanical modulus and the degradation rate, can be easily tailored for target applications by altering the lactide/glycolide proportions and polymerization parameters. Indeed, these materials were successfully applied in the clinic for the creation of urethral tissue as well as for bladder replacement in patients with idiopathic detrusor or neurogenic bladder [74–77]. In addition, in situ-forming hydrogels based on synthetic polymers can be engineered to locally deliver a wide range of bioactive agents in a controlled and sustained manner to regulate stem cell fates encapsulated within the 3D polymer network, such as polyethylene glycol (PEG) [78]. Due to its exceptional qualities, such as its biocompatibility, low immunogenicity, hydrolysis under physiological conditions, and FDA approval for clinical use, poly(ε-caprolactone) (PCL) is another synthetic polyester based on hydroxyalkanoic acids that has attracted intense attention in tissue engineering. This polymer is used either alone, as hydrophobic PCL, or as a PCL-containing amphiphilic block copolymer when in combination with other agents, resulting in improved performance in certain applications [70,79,80].
Many synthetic polymers (e.g., PLGA, PEG, PCL, polyacrylic acid, polyvinyl alcohol and polyvinylpyrrolidone) owe their broad biomedical application to their biomimetic ECM-like micro/nanoscale fibers, attractive processability and biocompatibility. Although synthetic polymer biomaterials can be manufactured into scaffolds with fully interconnected pores, certain classes, such as poly(α-hydroxy esters), may produce acidic degradation products that can alter the pH of their surrounding tissues [51]. In turn, this pH change can affect cell behavior and survival and cause adverse tissue and inflammatory reactions [81]. Nevertheless, synthetic polymers themselves typically do not carry a risk of inducing an immune response because of a lack of biologically functional domains. This feature is also a limitation because the lack of peptide side-chain reactivity for binding regulatory peptides, growth factors and other biological signals does not allow the facilitation of cell adhesion or direct phenotypic expression, as a natural polymer would. However, various synthesis techniques have been developed and optimized to incorporate biologically active domains into synthetic polymer templates, thereby enabling the production of biomimetic scaffolds with a defined and tunable composition [82]. For example, synthetic polymeric scaffolds with a collagen or serum coating are usually sufficient to permit initial cell attachment and ECM deposition, whereas coating synthetic polymeric scaffolds with ceramic (calcium phosphate, or CaP) is crucial for bone tissue engineering applications [34,83]. In other cases, synthetic polymeric scaffolds have been fabricated and modified through the covalent immobilization of ECM-derived moieties to enable the presentation of biologics with spatiotemporal accuracy, to promote cell attachment and to enhance the directed differentiation of progenitor cell populations [84]. Presenting bioactive agents on synthetic polymer template surfaces is the most efficient way to elicit desired cell–material interactions [85]. The ability to devise these polymer systems to influence cell behaviors and interplay is another crucial feature that provides both fundamental insights into the chemistry of structure–function relationships and enormous potential to directly utilize these biomaterials as cellular scaffolds [86].
2.4. Challenges in biomaterial design
Each tissue commonly presents a unique cascade of wound healing processes following injury due to disease or trauma; however, common cellular and molecular events during tissue repair exist. Most tissue-healing phases involve multiple signaling components that coax cells under tight spatial and temporal control, leading to optimal tissue regeneration [2]. Ideally, a cellular scaffold, in addition to being biocompatible, should be a biomaterial device with physical and mechanical properties that match those of the target tissue and that contain a multitude of cytokines, growth factors and cell adhesion molecules that can promote a regenerative microenvironment for appropriate cell populations and induce their behavior [87]. Far more often than expected, a single-component template does not meet the requirements for a regenerative biomaterial matrix due to a lack of a controlled degradation rate; a lack of desired mechanical properties and bioactivity; and, more importantly, a lack of the desired cell–matrix interactions to control gene expression, cytoskeletal structure and dynamics [33,34]. A combination of two or more types of biomaterials into a medical device may overcome several of these limitations. Whereas composite biomaterials from the same class will generate a certain degree of regulation, mixing biomaterials from multiple classes may confer a greater level of control over the overall material properties for cell guidance. For example, hybrid hydrogel scaffolds synthesized from selected biopolymers may provide opportunities to closely mimic the key characteristics of the native ECM, including by displaying adhesion sites and presenting growth factors, which not only induces reparative cells but also triggers and governs specific events at the cellular and tissue levels [48,88]. In particular, the addition of natural components, with their natural ratios, into synthetic polymers, followed by the incorporation of biochemical and biophysical cues, mirroring the chemistry as well as the nanofibrous network of the native matrix, has emerged as a leading strategy in scaffolding design [52,89]. Such materials chemistry has made a fundamental and an increasingly crucial impact on materials science, showing significant promise in replicating the morphologies, nanostructures and functional building blocks of a large variety of human tissues or in fully recreating these building blocks using integrated reparative cell populations [90].
Alongside these positive developments based on biomimetic materials chemistry, there is growing recognition that the physical properties of the cell's environment are also crucial to a broad spectrum of cell biological functions that must be carefully taken into account in the design of biomaterials [34]. Unfortunately, however, there has been a surprising paucity of biomaterial templates that are designed to accurately mimic the architectures and functions of the structural fabric of native tissues, ensuring precise tissue regeneration [90]. Indeed, physical attributes, such as scaffold shape, size, architecture, structure, mechanics, porosity, surface texture and com-partmentalization, can profoundly affect the biological functions of biomaterials once they are placed into an in vivo cellular microenvironment [33,34]. For example, cells use compartmentalization to control various biochemical reactions in space and time [91], and the way in which cells migrate is directed by the physical aspects of their surroundings, and particularly the properties of the ECM [92]. Notably, the surprising properties of biomaterials largely result from their surfaces as well as their sophisticated hierarchical bulk structures [93,94]. For example, scaffolding biomaterials must possess biocompatible (and ideally antibacterial) surfaces to reduce or eliminate undesirable host responses, mimic the structure of the target living organism in one to three dimensions, exhibit interconnected porosity to support cell/tissue penetration and be capable of resorption over time to create space for new tissues [18,45,46]. Although fabrication techniques (e.g., scaffold sheet design strategies, particulate leaching techniques and electrospinning methods) have been proposed to enable the fabrication of porous 3D biomaterial templates with an appropriate porosity and pore size, controlling the pore geometry and architecture of these templates to match the native tissue has been a daunting, largely unpredictable task [95–97]. Moreover, the requisite mechanical and compositional standards of tissue engineering templates for clinical translation are complicated by the anisotropic nature of human tissues, such as the concentrically layered sheets of the intervertebral disc (IVD) and the parallel-arranged collagen fibers within tendons [33]. Recently, 3D printing methods have emerged to enable the fabrication of scaffolds with defined scaffold geometries while precisely controlling the arrangement of cells and bioactive nanomaterials throughout the structure; however, in certain cases, printing complex organ-targeted templates with clinically relevant dimensions, such as whole hearts or livers, maybe too time consuming for widespread application [98,99]. A growing goal in this field has been to explore new strategies that more effectively generate multi-material and cell-laden scaffolds with less effort. In this respect, polymer brushes with various structures and chemistries, as well as diverse brush-based strategies, which are both passive and bioactive, may be utilized for biomaterial modification. In particular, these features may make the material surfaces biocompatible and non-fouling, which passively prevents subsequent undesirable host responses [93]. In addition, fiber-assisted molding (FAM) has been shown to be a simple and robust method to create biomimetic 3D surfaces with controllable curvature and a helical twist. Such modified surfaces are able to guide cell alignment and the assembly of helically patterned ECM, demonstrating the potential of FAM for materials science and tissue engineering applications [100].
In addition to presenting interconnected pores with a tunable pore size and biomimetic surfaces with controllable curvature and a helical twist, scaffolds are commonly engineered and used for the presentation or controlled delivery of bioactive agents to accelerate and orchestrate tissue regeneration [22,101–103] (Fig. 3). For this purpose, significant efforts have been made to create biodegradable polymeric scaffolds with functional groups on the material surfaces that are coupled with biological cues and delivered to biological compartments, hence eliciting desired cell–material interactions [85]. The modification of biomaterials for the recapitulation of the native tissue healing cascade and other variables using a wide range of bioactive agents allows host cells to interface with the engineered environment and hence leads to better cell propagation and tissue regeneration [12,28,55].To be effective as therapeutics, bioactive agents have to reach their sites of action without damage or degradation. Additionally, they have to maintain their effective concentrations in the target area sufficiently long enough to exert desired biological functions [56]. When labile drugs are delivered in their native form, without control over their localization or rate of release, high doses are generally needed and are indeed frequently adopted to ensure the required therapeutic effect. Beyond generating additional waste and extra expense, these supraphysiological doses may result in increased toxic responses or undesirable side effects, such as inflammation, dangerous tissue over-growth and even tumor formation [104,105]. To this end, localized drug delivery systems have been developed to act as a depot of biologics targeted to damaged areas for controlled release, which can considerably improve therapeutic efficacy and safety and also offer protection to labile factors [105,106]. In contrast, a number of sophisticated drug delivery devices that circumvent challenges associated with traditional delivery systems have been engineered to exert control over the precise spatial and temporal presentation of a complex array of bioactive agents, including growth factors and therapeutic cells, in a tailored manner [28,29,101,105]. As our ever-expanding fundamental knowledge of the cell and molecular biology of human physiology and disease reveals new therapeutic targets that require more advanced strategies to control cell behavior and to address specific pathological situations, the importance of sophisticated material devices in medicine is expected to increase [30,107]. Through the development of smart biomaterials into a specific assembled system, a certain amount of cargo can be delivered to meet an individual patient's therapeutic requirements in spatially, temporally and dosage-controlled fashions [38,55,108,109]. To achieve such stimuli-responsive drug delivery systems requires the selection of biocompatible biomaterials that can respond to a specific stimulus or that are particularly susceptible to specific physical incitement, undergoing protonation, a (supra) molecular conformational change or hydrolytic cleavage [29,106,110]. In addition to templates for tissue engineering, many recent advances have shed light on more sophisticated devices with one or more characteristics, such as efficient drug protection, accurately controlled release, localized drug targeting, permeation enhancement, expanded self-modulated therapeutic action, enzyme inhibition, reporting or imaging [12,87,111].
Fig. 3.
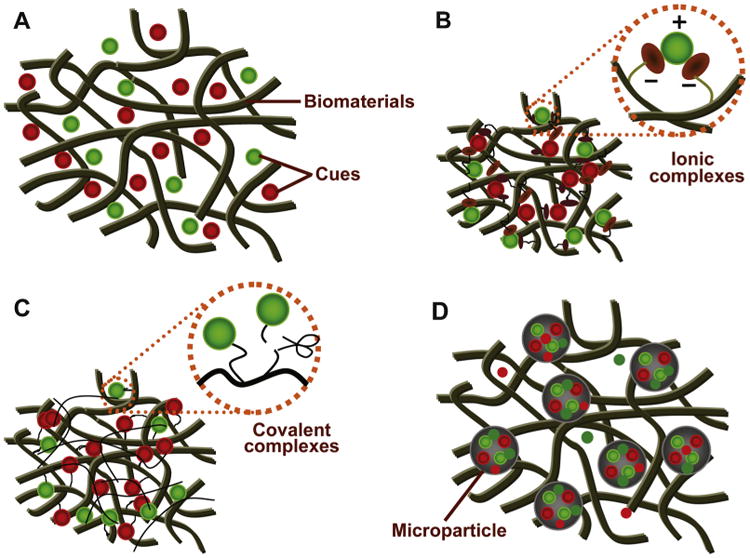
The presentation of growth factors or other therapeutic agents via biomaterials engineering. Cargo presentation via (A) adsorption or embedding. (B) Non-covalent immobilization (e.g., forming ionic complexes with the polymer backbone). (C) Covalent immobilization (e.g., tethering of cues to the polymer chains by linking via cleavable bonds). (D) Pre-encapsulation into a well-defined particulate system.
Source: [22], Copyright 2011. Reproduced with permission from Elsevier Ltd.
The crucial challenges related to drug delivery in the design of biomaterials arguably include the selection of not only the appropriate factor or combination of factors necessary to induce a desired response but also the dose and spatiotemporal delivery needed for proper tissue regeneration [55,105]. Furthermore, modulation of the exuberant host response to transplants and microbial contamination, which directs the in vivo milieu against tissue regeneration, has not attracted enough attention. Scientists therefore must explore the combined administration of anti-infective agents or host modifiers to optimize the overall outcomes of therapy [12]. For the implementation of these distinct requirements for tissue engineering use, biomaterial platforms must offer an increasing number of sophisticated strategies for controlled release, ensuring that under adverse conditions, an optimized ratio of multiple biologics, each acting in a specific spatiotemporal pattern, is delivered solely to the location where the factors are required and only at the levels, dosages and times at which they are needed [12,55,101,104]. Clearly, it is highly challenging to integrate all of these functionalities into a single medical device. Unfortunately, with respect to the dichotomy between the pursuit of sophistication and the feasibility of commercialization and regarding the critical aspects of the healing cascade, it remains unclear how much extrinsic physiochemical information is indispensable to coax endogenously residing or exogenously transplanted cells into generating a complex tissue for a specific purpose and, in particular, what minimum levels of biomaterial complexity are necessary for a given task [16,20,29,37,56,102].
Recently, however, a wealth of research has revealed that the development of tissue engineering templates might be experiencing the emergence of a diverse and powerful set of new concepts for biomaterials design [34]. Advanced biomaterial technologies for creating artificial refined cell-instructive platforms based on knowledge obtained from materials science, biology and engineering have heralded a new era in the redesign of cellular scaffolds [37,96]. In this era, the architecture of the stem cell milieu or niche can be replicated in terms of its biochemical, mechanical, structural and component details to manipulate cell fate, including cell migration, gene expression and the maintenance of functional homeostasis [48]. To design cell-based therapeutics for tissue defects with complex shapes, an injectable cell scaffold integrating an ECM-resembling structural feature for cell residence is desirable to achieve a precise anatomic fit and to minimize the complexity of the surgical procedure [65,112]. Unfortunately, although a superabundance of robust material devices exists to analyze the effects of the physical and chemical properties of stem cell microenvironments, these devices have only just started to be used to instruct stem cell behavior, and they often fail in regard to “biointegration”. Using the technologies established to date, it is still impossible to achieve an optimized protein-releasing mode that mimics naturally occurring events [34,113]. New developments stemming from biological disciplines are actively directing the redesign of ingenious biomaterials that work using nature's own mechanisms for regeneration; however, much remains unclear about the underlying events during which tissues heal and form [20]. Clearly, much work needs to be done before we can rationally design synthetic materials with attached functional groups, similar to native ECMs that are capable of providing autonomous direction to pluripotent stem cell populations in routine clinical therapies [37,114]. Crucial to current endeavors in this field is further collaboration between cell biologists and biomaterials scientists, which promises to foster intense effort in tissue engineering and to offer new insights into cell-instructive niches that will advance cell-based strategies for clinical tissue reconstruction [15,16]. Until ECM-mimicking material devices are successfully commercialized and readily available for widespread application, the use of biomaterials derived from human tissues/organs provides an option to clinicians, who have a moral obligation not only to address problems that may benefit patients in the future but also to develop therapeutics that can be immediately translated into routine clinical practice to assist patients today.
3. Tissue grafts of human origin
As described in Section 1, our original concept of biomaterials for use in the biomedical arena has changed; biomaterials can now include many substances, such as engineered constructs, therapeutic cells and indeed, a number of living tissues or organs used for transplantation [21,23], that may generally not be considered as biomaterials in the past. Consequently, as “living tissue replacements”, tissue grafts (autogenous or allogenic tissue grafts) and donor organs of human origin can be considered as the gold standard “biomaterials” for reconstructive therapies [115,116] (Fig. 4). The last century experienced remarkable advances in the science of reconstructive surgery, and over the span of the past two decades, surgical technology as well as graft safety and feasibility have largely improved, and soft- and hard-tissue grafts have grown in popularity for tissue reconstruction in routine clinics. This growth has been driven, in part, by a desire to restore the patients' impaired, damaged or lost body parts and hence to improve the patients' quality of life. Endeavors in the field of transplantation, alongside the shortage of tissue grafts and donated organs available for use in patients, have prompted the use of cells and biomaterials for the creation of lab-grown tissue/organ replacements that mimic, at least to a large extent, the complexity and functionality of a native tissue [117]. Human MSCs can be obtained from patient-derived tissue materials of mesenchymal origin or tissues derived there from, such as bone marrow, blood, adipose tissue, and, recently, clinically discarded dental-related tissues that have long been considered to be of no use [118–122]. In the last case, a broad spectrum of research has suggested that dental pulp tissue, periodontal ligaments and gingiva can be envisaged as suitable and the most accessible sources of stem cells, whether in a healthy or inflamed state [123–131]. At the same time, other cell populations, such as chondrocytes, can be separated from autologous cartilage and multiplied using a strictly controlled cell culture system for the development of new cartilage repair techniques. In this context, autologous chondrocyte implantation (ACI) and matrix-supported ACI have been demonstrated to be practical clinical techniques for the repair of full-thickness chondral defects in the knee [132–134]. Although tissue engineering emerged as a field at the intersection of numerous disciplines over 20 years ago, tissue engineers have largely stood on the shoulders of giants, relying on those who have worked in related fields, such as tissue/organ bioreactors, preservation and transplantation [135]. This previous work was conducted over several decades, and several of the principles established by those pioneering explorers are still followed by the scientists working in current bioengineering disciplines; be it tissue (or organ) auto-/allo-transplantation or tissue engineering (or regenerative medicine), the essential goals remain the same. Through the involvement of living substances in biomaterials science, and particularly native tissues and organs that exceed the traditional aspects of materials science, and the accompanying inspiration in and evolution of biomaterials, we are now able to illustrate the best characteristic for an engineered medical device to guarantee a maximally predictable outcome following clinical transplantation. The pivotal steps, or a roadmap, for device implementation also need to be carefully developed, hence providing an intellectual challenge that did not exist when we simply focused on material replacement for physical support and/or geometrical reconstruction. An overview of the advantages and disadvantages of each tissue resource (external or internal) and types of grafts will provide surgeons validated principles regarding graft choice during clinical practice. Furthermore, understanding the native tissue composition and structure and revisiting the observed clinical benefits related to tissue grafts can offer practicing tissue engineers important information on state-of-the-art biomaterial evolution and design inspiration [115,136].
Fig. 4.
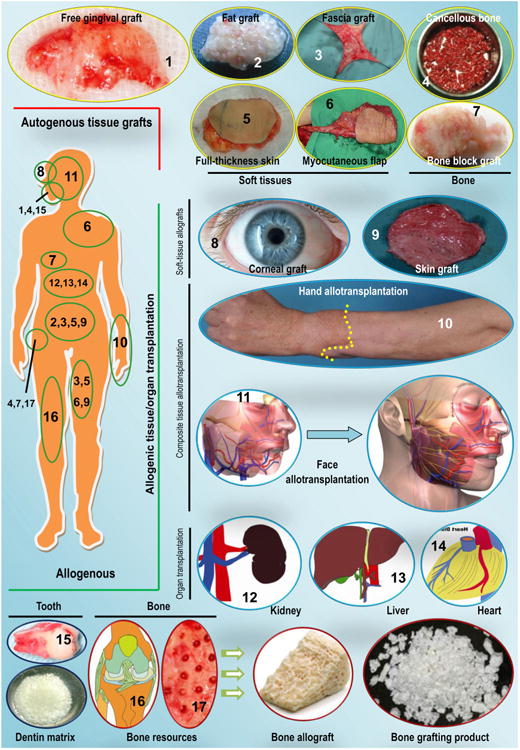
Schematic representation of tissue grafts and organs of human origin for clinical therapeutics (examples are given where appropriate; illustrations are not to scale). Autologous tissue grafts include soft tissues, such as free gingival grafts; fat, fascial, skin (partial-thickness or full-thickness) and myocutaneous flaps; and bone grafts, including block bone and cancellous bone. Allogenic tissues for transplantation include corneal grafts; skin grafts; and certain composite (organ-level) tissues, such as a full hand or a near-total face transplant. In addition, organ allotransplantation is often performed for the kidney, liver, lung and heart, among others. Bone grafting materials and dentin matrix can be produced from the bone and teeth of human cadavers. The images used here are selected samples for schematic representation only; they do not represent any particular preference by clinicians.
Source: Figure components (6) and (11): [115], Copyright 2011, and [116], Copyright 2009, respectively. Reproduced with permission from Elsevier Ltd; Component (10) courtesy of Jewish Hospital, Kleinert, Kutz and Associates Hand Care Center and University of Louisville; the remaining components are by the authors, or from unpublished resources from the corresponding author's institution (provided by Dr. Guicai Liu in the Department of Oral and Maxillofacial Surgery).
3.1. Autologous tissue grafts
The use of biomaterials of human origin for therapeutics was first rooted in tissue grafts transferred from one site to another site within the same individual. Even today, many clinicians still consider harvested autologous tissue to be the best material for the reconstruction of most, if not all, tissue defects (Fig. 4). Autologous tissue grafts, also called autografts, are the gold standard with which all other implantable biomaterials are compared because these grafts maintain large masses of living cells and possess all of the properties required for new tissue regrowth and structural reconstruction. Most importantly, an autologous graft, whether of hard or soft tissue, is taken from a patient's own body; hence, antigenicity is absent following transplantation [137,138]. Indeed, the ultimate goal for tissue engineering strategies is to develop a tissue construct that has biological performance identical or similar to that of an autologous tissue graft upon implantation.
3.1.1. Soft-tissue grafts
Regarding soft-tissue grafts, there has been considerable interest in the use of autologous adipose grafts for the management of cutaneous injuries, the treatment of soft-tissue volume deficiencies and the reconstruction of missing parts of the human body since the late 19th century. Indeed, autologous fat grafting has many clinical uses, ranging from routine facial rejuvenation, breast surgery, buttock augmentation and treatment for Romberg syndrome to a tool for treating liposuction sequelae. However, concerns about graft survival following in vivo transplantation have significantly limited the method's use. Although refinements in procuring and grafting technologies have considerably improved the overall clinical outcomes of autologous fat transplantation, the MSCs contained within adipose tissue (e.g., adipose stem cells) may offer unexpected opportunities for tissue repair and regeneration [139]. The placement of mature adipocytes and adipocyte-derived stem cells into the hormonally active environment of the breast for breast augmentation raises the possibility of inducing a breast tumor. However, no clinical trial has demonstrated this potential, and a consensus on the fundamental knowledge remains in development [140]. Nevertheless, given the relative abundance and accessibility of adipose tissue due to its proximity to the surface of the skin, this graft appears to be an option for the management of both acquired and congenital soft-tissue defects. Additionally, autologous fat grafting remains an appropriate material choice for myringoplasty, limited soft-tissue augmentation and the obliteration of frontal sinuses in head and neck surgery, albeit being associated with limitations such as unpredictability in certain situations [141]. In most medical uses, the expected resorption of adipose transplants can be estimated, and this phenomenon theoretically may be compensated for by initial overcorrection. Moreover, the use of adjuvants such as autologous platelet-rich formulations and cell-containing products, which will be addressed in Sections 5 and 6 in this review, may decrease the rate of adipose graft resorption and hence ameliorate the overall clinical outcome.
Human amniotic membrane (AM) has been used as a grafting material for over 100 years, either directly or following decellularization. This material exceeds several qualities of common materials, indicating great potential to treat a variety of medical conditions, including corneal defects, diabetic foot ulcers and severe skin burns [142,143]. For example, it has long been suggested that nonpreserved human AM transplantation in patients with acute chemical eye burns may reduce surface inflammation, increase patient comfort and decrease the extent and severity of vascularization [144]. Additionally, an autograft of amniotic tissue can be used as an autologous grafting material in a variety of pediatric neurosurgical procedures, such as for repair of myelomeningocele, with no risks of rejection, foreign-body reactions or transmission of slow virus infection [145]. For covering venous ulcers that do not respond to conventional treatment, human AM demonstrates excellent therapeutic potential for re-epithelialization but is less expensive than other skin substitutes [146]. Recently, it is becoming increasingly evident that human AM may also be used as a cost-effective wound dressing for split-thickness skin-graft donor sites [147]. When serving as an adjunctive therapy after primary pterygium excision, AM grafts have been demonstrated to be as effective as standard conjunctival autografts in preventing pterygium recurrence [148]. Moreover, as an effective procedure with a low rate of recurrence, sutureless human AM transplantation combined with a narrow-strip conjunctival autograft is considered as a preferred grafting strategy for primary pterygium, although further randomized controlled trials involving larger populations remain to be performed [149]. Additionally, to use this human material as an advanced biomedical product containing viable stem cells and bio-logics for reconstructive surgery, much more work remains to be conducted to shed light on the influences of tissue culture and/or cryopreservation conditions on cell viability, to identify easy and practical processes to store human AM containing robust cells and to verify the quality of the tissue transferred before its clinical application [143].
In dentistry, the management of gingival recessions is a universal request from patients due to its significant influence on both dentin hypersensitivity and esthetics. In this respect, a free gingival graft (FGG) can be used either alone or, most often, adjuvanted with a coronally positioned flap as an effective treatment for gingival recession. As first described by Sullivan and Atkins (1968), an FGG can be directly utilized to cover the denuded root and restore the gingival margin to its correct position [150]. Today, modified techniques based on FGGs have been demonstrated to be successful for the management of isolated and multiple gingival recessions along both the upper and the lower incisors and premolars to ameliorate root coverage potential and to improve mucogingival junction alignment [151,152]. This successful application is particularly true in the specialties of periodontics and implant surgery. When placing dental implants in partially edentulous areas, FGG, whether alone or in combination with another tissue augmentation technique, is the best-documented and most successful surgical procedure for increasing the width of keratinized mucosa and augmenting the soft-tissue volume around implants and in the esthetic zone [153].
The capacity of the skin to heal itself by intrinsic mechanisms after injury is vital to human survival, but the process of cutaneous wound repair is disrupted in a spectrum of disorders. Indeed, a large skin defect would not heal properly without a medical intervention such as skin transplantation [154]. Of note, either “full-thickness” or “partial-thickness”, accessible skin grafts can be harvested to treat small- to medium-sized superficial defects. Both types of skin grafts involve the entire epidermis and offer minimal postoperative pain and scar formation at the donor site; however, full-thickness grafts include all dermal components and appendages (e.g., hair follicles or, if present, sweat glands), whereas partial-thickness grafts leave the deeper reticular dermis (including dermal appendages) in place because these grafts are harvested at the level of the more superficial papillary dermis [155]. Full-thickness grafts are also esthetically superior and have less postoperative shrinkage. Recently, Thangavelu et al. (2011) reported the merit of using an autologous, full-thickness subcutaneous adipose composite graft isolated from the patient's abdomen as an interpositional biomaterial in the treatment of temporomandibular joint ankylosis in seven patients (eight joints) [156]. However, a careful examination of the literature soon reveals that following temporomandibular joint discectomy, there is still no perfect interpositional biomaterial that favors all of the criteria for the repair of a damaged/missing articular disc [157]. Nevertheless, experience with soft-tissue correction using dermal fat grafts in the temporal fossa to augment temporal hollowing has been expanding, suggesting a treatment that appears to have good long-term esthetic outcomes [158]. Future research endeavors, such as hormonal amendment of adipose grafts and advances in preadipocyte transplants, will perhaps significantly ameliorate the overall outcomes of soft-tissue transplantation [141].
In addition to fat or dermofat, very different autologous soft tissues, such as dermal fascia and muscle, used in the form of flaps according to the requirements of the tissue defects caused by trauma, autoimmune disease, cancer or infection, have also been used for facial augmentation. The survival of a graft relies on a well-vascularized recipient site, and the graft can remain immobile in its nutrient bed [155]. Clearly, none of the aforementioned soft-tissue grafts can satisfy all of the clinical requirements for an optimal transplant. Identifying the indications and each tissue's advantages and disadvantages will extend the clinician's armamentarium when soft-tissue correction using grafting materials is required [141]. The use of fascia grafts has proven very reliable for soft-tissue augmentation, particularly when tensile strength is a requirement for the transplant. Generally, a fascia graft permits more accurate and predictable reconstruction than does a fat graft, as evidenced by the observation that the majority of fascia grafts can survive as living tissue and retain their native characteristics. However, a relative lack of a blood supply or 3D bulk limits the biological potential of the fascia for healing and reconstruction [159]. In contrast, the transplantation of free muscle grafts leads to muscle cell death and subsequent partial fibrous tissue replacement in most, if not all, cases due to the enormous metabolic needs of this type of graft. Nonvascularized muscle grafts are therefore generally, if not only, used under conditions in which the desired result is the obliteration of fibrous tissue in a small defect (such as in the Eustachian tube or nasofrontal duct). If the bulk of the transplant or maintenance of the volume is of the utmost importance, the transfer of a vascularized tissue should instead be the first consideration. To this end, a wide variety of simple and composite flaps of vascularized fat, fascia, muscle and other tissues have been designed to meet the requirements of different specific applications [160]. In contrast to free tissue grafts, these flaps are harvested in such a way that their blood supply is maintained, and hence, they can maintain their structure following transfer to a recipient site. Importantly, tissue grafts can be advanced or rotated into position and can retain a good blood and nerve supply via their pedicle if the donor tissues for local flaps are located close to their recipient area. Many local flaps (e.g., the temporalis muscle flap) have been applied for the correction of facial and oral defects. Typical examples are flaps involving the lip (i.e., Abbe flaps) and those within the oral cavity (i.e., tongue flaps and palatal flaps) [155]. In contrast, for single-stage restoration of a complex soft-tissue defect, the anterolateral thigh flap with vascularized fascia lata may offer a relatively reliable fascial component [161]. However, vascularized tissue transfer is certainly not a solution to all reconstructive needs. For example, an Achilles tendon rupture is often complicated by skin substance loss around the tendon, which is a poorly vascularized site. Soft-tissue repair at this site is a crucial reconstructive problem and becomes very complex if skin reconstruction has to be associated with complex tendon repair [162]. Although each graft has its own limitations, the use of adipose, fascia and occasionally muscle tissue grafts remains a prevailing choice for soft-tissue reconstruction when properly selected and applied in head and neck surgery [141]. In addition to recipient-site characteristics, the function and esthetics of both the donor and the recipient sites must be taken into account in the selection of musculocutaneous or perforator flaps for application. Clinically, muscle flaps are applied for the obliteration of deep spaces because they offer a well-vascularized, pliable tissue replacement, whereas fasciocutaneous flaps are typically utilized for the treatment of flatter and more superficial wounds [163].
3.1.2. Bone grafts
Most, though not all, bone-devastating deficits can lead to significant alterations in function and appearance and can prove difficult to remedy, which may have significant implications for patients and pose serious clinical dilemmas for clinicians [164]. Bone tissue and materials derived from bone have a long and successful history of use as bone grafting materials to treat selected conditions, such as small- or medium-sized bone defects [165]. To replace bone tissue with a material that will eventually become bone, surgeons' first choice is to use pieces of the patient's own bone. Autologous bone grafts are the predominantly considered osteoconductive materials for bone replacement, with success rates of well over 90% [166]. Similar to autologous soft tissues, bone autografts from a patient's own body deliver no risk of immunological rejection; possess complete histocompatibility; and offer superior osteogenic, osteoconductive and osteoinductive performance compared with other clinically available grafting materials [167]. By their very nature, as mineralized scaffolds, living bone grafts can deliver an optimized combination of cellular components, including, but not limited to, differentiated osteoblasts, an appropriate matrix of cancellous bone, and a mixture of bone growth factors at a physiological level. This combination supports bone regrowth and integration into the surrounding bone, normally through creeping substitution, and ultimately rebuilds mechanically efficient bone structures [168]. However, clinical benefits are not guaranteed, and these autografts still suffer from drawbacks such as resorption, limited availability, short-term viability and unpredictable graft resorption. Most importantly, the extraction of an autograft is essentially a second surgery. The harvesting procedure can include pain following surgery and numbness at the extraction site, in addition to the potential attendant risks and postoperative complications [169]. Fortunately, recent minimally invasive and innovative harvesting tools and techniques have largely decreased historical issues such as donor site morbidity, making the acquisition of the required amount of bone simpler and easier [170]. Hence, clinical surgeons have renewed interest in choosing autologous bone grafts as a preferred source of bone reconstructive materials.
Native bone is a mineralized matrix consisting of biopolymers (mostly collagen I and certain minor but important noncollagenous proteins) and biominerals. The transplantation of a fresh bone autograft is an attempt to achieve rapid bone restoration because living bone can survive well and add to bone volume at a recipient site and eventually maintain bone strength. Compared with cortical bone grafts, cancellous bone autografts are considered to be more osteogenic because the existence of native spaces within their structure permits the diffusion of nutrients necessary for new bone formation and allows limited revascularization through the microanastomosis of circulating vessels [138,170,171]. The vascular response in autologous cancellous grafts is much greater than that in cortical autografts. As a result, within 1–2 weeks, the entire cancellous bed can be completely revascularized. Although cancellous grafts, as good space fillers, do not provide immediate structural support, they are rapidly revascularized and easily incorporated into the bone bed at the recipient site and ultimately achieve strength equivalent to that of cortical grafts by 6–12 months post-transplantation [138]. Theoretically, both the remaining viable cells within the graft itself and the recipient site cells participate in the incorporation of an autograft following transplantation. When an autologous fresh graft is transplanted into a recipient site, several osteoprogenitors that have acquired the ability to create sufficient daughter bone-forming cells (e.g., osteoblasts), and, hence, a significant amount of new bone, accompany the autograft and are transferred to the recipient site [170]. Because solely the osteoblasts and endosteal lining cells on the surface of the autograft may survive the transplant, a cancellous bone graft, acting primarily as an osteoconductive substrate upon application, may support the effective penetration of new osteoblasts and osteoblast precursors and facilitate ingrowth of new blood vessels [172]. Moreover, osteoinductive molecules and other growth signals released from the autograft during the resorptive process, as well as cytokines produced during the inflammatory phase, can contribute to healing of the autograft [173].
Based on the amount and shape of a bone autograft needed for a specific application, bone particulates or blocks are commonly harvested from non-essential bones, such as the iliac crest, tibia, fibula, chin, ribs, mandible and even parts of the skull [174]. However, the iliac crest is the most common area from which a cancellous autograft is harvested, although an iliac crest bone graft (ICBG) is occasionally also obtained from the distal part of the radius/tibia. Cancellous bone is widely utilized for the reconstruction of depressed fractures of the lateral tibial plateau and, more often, the delayed union of long-bone fractures [175,176]. The principal merits of cancellous grafts are their safety, including a low risk of transplant rejection and disease transmission, and their excellent clinical success rate. However, the supply of donor bone grafts is restricted, and donor site morbidity increases if a larger autograft is harvested, in addition to other disadvantages, such as increased blood loss, potential wound infection and the prolonged anesthetic time [137,177]. In particular, although the incidence is low, complications associated with the harvesting of iliac crest bone, such as persistent postoperative pain and nerve/arterial injury, have been reported [170].
Autologous cortical grafts are osteoconductive but lack osteoinductive properties; however, the surviving osteoblasts within the transferred bone do provide certain osteogenic properties [178,179]. Several parameters contribute to the success rate of such autografts, including the stability of the bone and the prevailing situation within the host recipient area [155]. A cortical graft may not be revascularized as rapidly or properly as cancellous bone. The structure of the cortical bone does not permit a large contact area between the autograft and the host for vascular penetration, so its revascularization generally requires approximately 2 months. However, nonvascularized bone autografts can provide a relatively reliable choice for osseous defect filling, and following bone remodeling, new bone growth and complete integration of the graft into the host site occur [155]. Compared with cancellous grafts, nonvascularized cortical autografts may offer immediate structural support, but they appear mechanically weak during the first 6 weeks post-transplantation due to resorption and revascularization [178–180]. Revascularization is completed through the old Haversian and Volkmann canals. After the revascularization of the periphery of the transplants, interior revascularization rapidly follows suit. The coverage of the nonvascularized bone and the presence of an optimized soft-tissue recipient bed are generally required to decrease healing complications with respect to infection and wound dehiscence and to ensure the survival of the osteogenic cells within the transplanted bone [181].
The shape and size of vascularized cortical autografts are largely dictated by the morphology of the donor site, but compared with nonvascularized grafts, vascularized cortical autografts are less dependent on sufficient soft-tissue bed vascularity at the recipient site. At the host–graft interface, vascularized cortical autografts heal quickly, and their remodeling is typically similar to the biological process of normal bone turnover [182]. Because vascularized autografts do not undergo revascularization and resorption, they may offer superior initial strength during the initial 6 weeks post-implantation. These grafts, however, still must be supported by external or internal fixation to protect them from fracture [179]. The excess soft tissue associated with the transferred vascularized cortical bone often must be removed by a second operation because such a graft is initially transplanted with a periosseous cuff of soft tissue containing its blood supply [183]. In oral and maxillofacial applications, additional adjuvant procedures may also be needed to increase the grafted bone volume to allow for immediate or subsequent dental implant rehabilitation [184]. In fact, vascularized bone transplants, along with soft-tissue grafts, are now routinely applied by clinical surgeons for the restoration of large composite tissue defects, through the more difficult and technically demanding method of microvascular composite tissue transfer [155]. In this regard, cortical bone autografts are good choices for the treatment of bone defects requiring immediate structural support, such as segmental bone defects of more than 5–6 cm.
In oral and maxillofacial therapies, historically, the best bone grafting procedures have been particulate cancellous bone/bone marrow autografts, which can offer a rich source of bone and marrow cells that have osteogenic potential [185]. Histologic findings from case reports substantiate the potential for autologous bone/bone marrow grafts to support periodontal regeneration in humans [186,187]. Multiple clinical concerns, however, have largely limited the transfer of extraoral autografts, particularly from the iliac crest, for intraoral therapies, including the possibility of surgical complications and pain associated with the donor site. Therefore, for the treatment of oral diseases, autologous bone is frequently harvested from intraoral sites, often in the same quadrant as the regenerative surgery [188]. Intraoral donor sites, however, typically yield comparatively limited graft volume. Harvesting sufficient donor bone, therefore, as an osseous coagulum of cortical or cortical-cancellous bone, can necessitate the creation of additional intraoral surgical sites, thereby increasing the potential for surgical morbidity and discomfort.
Not surprisingly, autologous ICBGs have been and will continue to be the gold standard for grafting in many surgical procedures, including procedures for repairing bone defects, treating bone fractures, promoting nonunion healing and alleviating severe back pain through spinal fusion, but their use necessitates harvesting autologous bone from a separate area, which may involve an additional surgery and donor site morbidity after harvesting [137,138]. In this respect, recombinant human bone morphogenetic protein-2 (rhBMP-2) on an absorbable collagen sponge (ACS) or other bone grafting materials have been suggested as an alternative to an autologous bone graft for bone reconstruction. In particular, several trials have reported statistically superior or similar clinical outcomes for these bone substitutes compared with autografts transferred from the iliac crest following lumbar fusion procedures or maxillary reconstruction in patients with a cleft lip and palate in terms of inducing new bone formation, achieving fusion success and avoiding reoperation [189–191]. Therefore, bone substitutes incorporating osteoinductive protein(s), whether alone or associated with autologous bone, have been considered as part of the orthopedic surgeon's treatment options [192,193]. Interestingly, in the treatment of long-bone nonunion, rhBMP-2/ACS mixed with a cancellous allograft showed possible advantages, including a shorter operative time and reduced intraoperative blood loss compared with an autologous iliac bone graft [192]. Similarly, rhBMP-2-aided bone tissue engineering has been demonstrated to be as effective as traditional autologous bone grafting for the treatment of tibial fractures associated with extensive traumatic diaphyseal bone loss [194] and for reconstruction of the alveolar cleft in patients with a cleft lip, alveolus and palate [193]. In implant dentistry, it appears that a variety of bone materials in association with growth factors may be an alternative/complement to autografts, leading to significant bone formation in the floor of the maxillary sinus or to lateral bone augmentation of the alveolar ridge [195]. Despite considerable interest, however, the clinical experience so far has been unsatisfactory, if not relatively disappointing. At the least, the field has not lived up to expectations, and to date, no well-documented prospective clinical studies have been performed. A clearly increased risk of cancer and other adverse events among patients receiving recombinant human protein treatments and a lack of reproducibility have been major problems. Most importantly, a carefully review of data published thus far, and particularly those data from the original industry-sponsored BMP trials, reveals a possible study design bias in the execution of these studies, thus weakening our confidence about the reported benefits of BMP administration in the treatment of orthopedic disorders [196]. Indeed, rhBMP-2 has no proven advantage over autografts based on critical reviews of currently available evidence, and more work remains to be done to standardize and optimize the technique. Additionally, more focused post-surgery evaluations of the complications and adverse events related to clinical BMP application are needed to prevent clinician and patient biases from affecting the functional outcome, thus providing surgeons and the public with more reliable and useful information [197].
3.2. Allogenic tissue grafts
Although an autograft is the best choice for the management of bone defects, the disadvantages of autologous grafts are the limited amount of available graft material and the morbidity associated with their harvest. The limitations related to the procurement of autologous tissue grafts may be addressed using a myriad of other grafting materials, which can be classified into the following categories: (i) allografts, also called allogenic or homologous grafts or homografts, which are composed of materials acquired from another individual of the same species (Fig. 4); (ii) xenografts, also known as heterografts orxenogenic grafts, which are materials acquired from another species; and (iii) alloplastic grafts or synthetic grafts, which are artificial or manufactured materials that can be subdivided based on their origin and chemical composition [198]. With respect to biomaterials of human origin, this review will focus its detailed discussion on allografts in this section. The successful transplantation of allogenic materials (e.g., tissues and organs) into patients depends on not only the benefits but also the mitigation of risks [199]. Rejection continues to be the main risk factor in allograft transplantation, and the immune response is one of the pivotal determinants of the development of rejection. However, the allograft outcome can be improved with an optimized immunosuppressive medication and enhanced human leukocyte antigen (HLA) matching between donors and recipients [200].
3.2.1. Corneal and skin grafts
Since the rise of modern corneal graft surgery, allogenic corneal transplantations have been successfully performed in human subjects for nearly a century and continue to be the most commonly performed allotransplants because they enjoy an immune privilege that is unrivaled in the field of allotransplantation [201]. The capacity of corneal allografts to evade immune rejection is attributable to multiple anatomical, physiological and immunoregulatory conditions that collaborate to escape the induction of alloimmunity [202]. To determine whether the donor or tissue characteristics of corneas for transplantation are predictive of adverse events reported to occur in the early postoperative period, Ple-Plakon et al. (2013) recently compared the preoperative donor and tissue characteristics of corneal tissues with or without reported adverse events from 2007 to 2011. It was observed that adverse events more commonly occurred after endothelial keratoplasty and that an increased rate of primary graft failure was associated with male donors and donors with a cancer history [203].
In patients under close observation, however, overall, corneal grafts have exhibited an excellent survival rate. Of course, proper donor cornea handling and patient review/selection and thorough preoperative and postoperative assessment are pivotal for the successful allotransplantation of corneal tissue and, indeed, of any other tissue [204]. Generally, orthotopic corneal allografts experience long-term survival in 50% to more than 90% of hosts, depending on the histocompatibility barriers that confront the host.
In contrast, skin allografts transplanted across various major histocompatibility complexes or minor histocompatibility barriers undergo rejection in approximately 100% of hosts [205]. Nevertheless, the temporary coverage of a third-degree facial burn with allograft skin following debridement has been evidenced to facilitate initial vascularization of the wound bed [206]. Six days later, the allograft skin is replaced with a split-thickness skin autograft because the allograft usually undergoes rejection within 2 weeks, although the possible repopulation of a skin allograft with host cells may make it persist in nonimmunosuppressed burn patients for as long as 7 weeks [207]. Further, recent evidence suggests that human dermal/epidermal cell fractions can be used directly from isolation and safely and conveniently transplanted in one single surgical intervention for wound closure toward improved healing [208]. Although allogenic skin grafts can be used directly for soft-tissue augmentation and have been described as having satisfactory efficacy when appropriately applied in optimized settings, a unique understanding of the components of human skin has led to the development of acellular human dermal matrices through decellularization [206,207,209,210]. These human-derived materials offer a viable and more feasible solution for soft-tissue reconstruction and the management of difficult wounds, especially in situations in which allografts are not readily available or extensive surgery for acquiring sufficient autograft material might be hazardous to the patient [211].
3.2.2. Composite tissue allotransplantation
Recent breakthroughs in the field of complex tissue allotransplantation (CTA) have enabled surgeons to address several complex problems that exceed the possibilities of traditional tissue transplantation [212]. As a pioneer of complex tissue allotransplantation (CTA), the hand allograft was successfully established near the end of the last century and, despite arguments related to its practicality and methods, has been unabatedly used for over 20 years [213]. Shortly after the report of the first case of hand allotransplantation, CTA, such as upper-extremity and face transplantation, has evolved into an exciting and promising subset of reconstructive transplant surgery, along with advances in immunotherapy. This subset involves a variable combination of upper-extremity (i.e., various levels) and facial (myocutaneous versus osteomyocutaneous) composite subtypes [214]. In particular, tissue damage/loss occurring in the craniofacial area may cause serious physiological and psychological consequences in affected patients. In November 2005, the first successful partial facial transplantation was performed in Amiens, France [215], and a similar case was also successfully performed in China 5 months later [216]. Furthermore, in December 2008, the first near-total face transplantation was performed in the United States, with 80% of the patient's traumatic facial deficit replaced by a composite allograft from a brain-dead donor [116,217]. Recent evidence suggests that the transplantation of an entire face, along with the auricles and scalp, is technically practical and feasible in humans [218]. However, reconstruction of the face to a functional and esthetic level presents a formidable challenge, even though it is desired by most, if not all, affected patients. Apart from the technical hurdles and difficult ethical/psychological concerns related to such a complex surgical procedure, the recipient patient must take immunosuppressive agents for his or her entire life, similar to those patients who have undergone solid-organ allotransplantation [219]. Although progress in CTA could provide new treatment for patients with severe tissue disfigurements, much remains to be done to make this technique more safe and practical. For example, it is arguable that logistical and immunologic challenges currently restrict the widespread clinical application of CTA. In addition to careful oversight and individualized screening procedures, further investigations are required for technical renovation to minimize the levels of surgery risk and to identify optimal immunomodulating protocols for specific patients as they seek improved quality of life [220,221]. Specifically, the use of bioreactors for tissue culture may extend ex vivo allograft survival times and enable allograft modulations that enhance graft function while mitigating immunogenicity following allotransplantation. Approaches utilizing bioreactor systems have expanded the reconstructive potential and applications of CTA and could one day enable organ-level engineering of customized complex tissue grafts and organs [212]. However, donor tissue/organ shortages and the adverse effects of chronic immunosuppression imply that alternatives to allogeneic organ transplantation (e.g., bioengineered organs based on patient-derived stem cells and decellularized organ templates) may hold greater promise for patients suffering from end-stage organ failure in the future [222]. Nevertheless, information gathered during the practice of CTA as well as solid-organ allotransplantation will definitely offer signposts in the future design and development of biological devices or tissue-engineered constructs or organs for transplantation.
3.2.3. Allogenic bone grafts
In contrast to complex tissues/organs, allogenic bone grafts that are cadaveric in origin and are available from commercial vendors (e.g., bone banks) have been widely used in reconstructive surgery. In this case, the expense and trauma associated with autograft harvesting, the quantity and size limitations of grafts and donor site morbidity are no longer concerns. Although lacking osteogenic properties due to the absence of living cells, allografts are in possession of both osteoconductive and weakly osteoinductive abilities upon implantation because they release bone morphogenetic proteins (BMPs) that coax bone-forming cells [223]. In addition to its advantages, such as its ready availability in the required sizes and shapes, its lack of donor site morbidity and its avoidance of the need to sacrifice host bone structures for harvesting autografts, this type of grafting material is attractive because it closely matches the recipient in constitutional elements and architecture and is theoretically available in unlimited quantities. The fundamental problems of this grafting material are antigenicity and the potential for infectious agent transmission, which is a major consideration that is in fact minimized by recent strategies associated with tissue processing, sterilization and freezing. However, these procedures in turn decrease graft properties with regard to osteoinduction, osteoconduction and mechanical strength, and indeed, real and perceived risks of disease transmission still exist; in particular, the risk of transmission of human immunodeficiency virus (HIV) is presumed to be 1 in 1.6 million [136,224]. Furthermore, there have been certain case reports of hepatitis B and C transmission through the transplantation of musculoskeletal allografts [225]. Despite these risks and given the advantages of bone grafting using allograft material, bone grafting procedures expanded from 5000 to 10,000 cases in 1985 to approximately 150,000 in 1996 and more than 1 million in 2004, and the number of cases continues to increase [141,226–228].
Both cortical and corticocancellous bone allografts are commercially available in various forms, such as particulates, chips and blocks, and these allografts have been elucidated by basic science and validated for clinical use; the industries built around these items are more successful and in demand than ever before [229]. Under United States Food and Drug Administration (FDA) regulations, facilities engaged in procuring and processing human tissues for transplantation must ensure that infectious disease testing (i.e., for HIV-1, HIV-2 and hepatitis B and C in the United States) and specified minimum medical screening have been performed and that records exist and are maintained to document the testing and screening of each human tissue. In addition, tissue processing (i.e., washing to ensure the removal of blood components, freeze drying or gamma irradiation) is applied by bone banks and other commercial vendors to ensure the safe clinical use of the resultant allografts [224]. Because of these measures, immunological rejection, depending on the method applied for graft preservation and the potential risk of viral, bacterial or prion transmission associated with allograft transplantation, has been well controlled at a clinically acceptable level. During the 30-year history of the use of freeze-dried allograft bone in reconstructive surgery, there have been very few reports of disease transmission [141,227,230]. Tissue banks process bone allografts using various methods, with several based on proprietary techniques; however, most are based on similar underlying concepts that address cleansing, decontamination, antimicrobial treatment, dehydration, graft sizing and terminal sterilization [230]. Allografts typically arrive in a form in one of two main categories, i.e., demineralized freeze-dried bone allografts (DFDBAs) or mineralized freeze-dried bone allografts (FDBAs). Both allograft bone types can be in the form of matrices, cancellous or morselized chips, cortical or corticocancellous grafts or whole-bone or osteochondral segments. It is suggested that FDBAs may yield a satisfactory outcome in socket and sinus augmentation, although a long time is needed to achieve a suitable amount of new bone formation [231,232]. However, large cortical allograft bone undergoes minimal remodeling and revascularization following implantation. The persistence of a nonvital graft at the site of a bone defect is incapable of physiological adaptation to functional loads and hence leads to the accumulation of microfractures over time [233].
DFDBAs exhibit the capacity to induce bone formation at nonorthotopic sites, such as muscle, and are considered to be osteoinductive. Levels of approximately 2% residual calcium in DFDBAs have been shown to provide maximum osteoinductive potential by assay systems [234], presumably due to the exposure of BMPs [235]. It was proposed that removal of the mineral component allows greater exposure of osteoinductive proteins [226,236,237]; however, allografts are predominately space-occupying osteoconductive lattices or frameworks. The rigorous processes involved in the removal of potential antigenicity and pathogenicity, e.g., ethylene oxide sterilization or higher levels of gamma irradiation, lead to a low concentration of bone growth factors, biological proteins and bioactive materials that are necessary for osteoinduction in the resultant grafts, and hence, the grafts present minimal osteoinductivity and no osteogenicity [238]. Additionally, certain processing techniques have been associated with detrimental impacts on the biological and mechanical properties of cortical bone allografts. For example, freeze drying may cause microcracks in the bone, and gamma irradiation increases bone brittleness [227].
The biological performance of bone allografts may be ameliorated by the incorporation of recombinant human growth factors, autologous bone (or exogenously cultured autologous bone-forming cells), enamel matrix derivative or various platelet-rich preparations [176,239–241]. Alternatively, the treatment of mineralized bone allografts with a 1:1 formic acid–citric acid mixture or with hydrochloric acid (0.5–0.6 M) can remove inorganic elements, yielding a natural polymer product that is generally referred to as demineralized bone matrix (DBM) [50,242]. As an acellular organic matrix, DBM mimics the microstructure of native bone but is less immunogenic and possesses good osteoinductive and osteoconductive performance [227,242]. DBM contains the major protein components of bone, such as adhesion ligands and osteoinductive growth factors that may contribute to new bone formation [243]. However, DBM cannot provide structural support, so its primary application is in structurally stable places such as the sites of bone defects. Although current clinical DBM delivery generally requires the incorporation of DBM particles within a carrier liquid, recent evidence suggests that it is possible to produce a soluble form of ECM materials or DBM product that could be induced to form a hydrogel scaffold [244]. These bone matrix-based materials with distinct structural, mechanical and biological properties can rapidly revascularize and act as suitable carriers for autologous bone marrow for medical use. As the antigenic surface structure of the graft is demolished during demineralization, the graft generally evokes no appreciable local foreign-body immunogenic reaction and facilitates cell attachment and growth (Fig. 5) [245]. Recently, it has become evident that DBM can be remineralized based on the alternating solution immersion (ASI) technique, resulting in mechanically stiff, strong and biocompatible allografts that facilitate tissue engineering and clinical applications [246]. The biological activity of these products may be attributed to the various proteins and growth factors present within the mineral component of the product and to the demineralization process, which makes these factors available in the host environment.
Fig. 5.
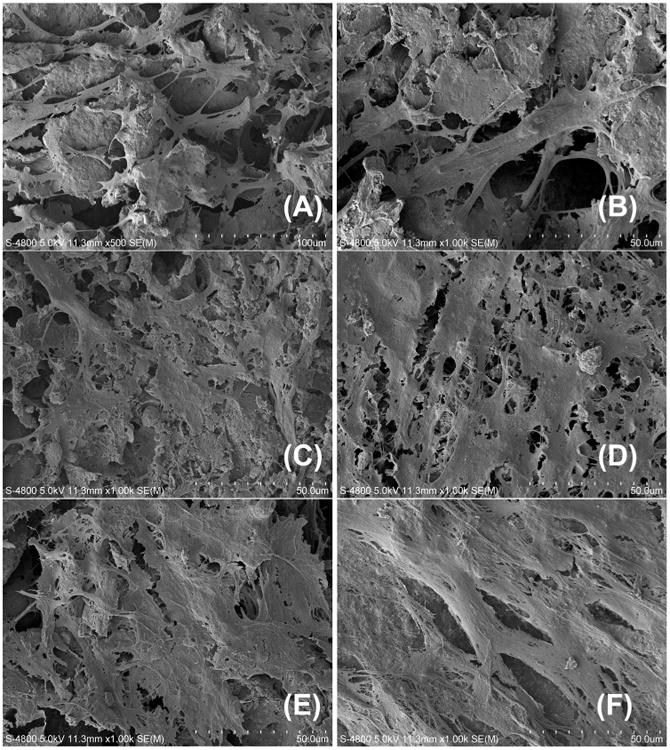
Representative scanning electron microscopy (SEM) images of the attachment and growth of bone marrow mesenchymal stem cells on demineralized bone matrix (DBM) for a 48-h period. (A) 12 h: cells attached onto the material; (B) 24 h: cells spread across the gap; (C) 48 h: constituting cell-cell interactions; (D) 72 h: protein and matrix production; (E) 96 h: cell penetration into the material and cell sheet formation; (F) 120 h: cell–matrix layer infiltration and enwrapping of the material.
The osteoinductive properties of DBM are not consistent; can be influenced by processing, sterilization and storage methods; and can change from donor to donor [247]. Of note, DBM has been successfully applied in a number of clinical circumstances to fill defects caused by bone cysts and cavities for craniomaxillofacial reconstruction and for the bridging of large bone defects, despite the potential to transmit disease [239,248–250]. When applied, DBM can be used in association with a cancellous bone autograft when the defect is very large and the supply of autologous bone is insufficient. Additionally, DBM has been combined with several different types of materials, such as glycerol (Grafton from Osteotech, USA), hyaluronate (i.e., DBX from Synthes, USA), poloxamer (DynaGraft from GenSci Regeneration Sciences, Canada), gelatin (Regenafil from Regeneration Technologies, USA) and calcium sulfate (Allomatrix from Wright Medical Technology, USA), to facilitate clinical handling and to improve surgical outcomes, leading to a variety of new products offered by various commercial vendors [251]. The use of patient-derived allografts, however, also introduces potential challenges regarding the potential transmission of infectious agents, the graft formats in which assets can be properly maintained and the ability to retrieve the grafts effectively, in addition to potential regulatory hurdles and damage to material components after months to years of storage.
3.2.4. Human dentin matrix
As a mineralized connective tissue, dentin is well adapted to its role as a major structural and functional component of the tooth. Although similar in composition to bone, the dentin matrix is not remodeled physiologically, and this matrix has been traditionally considered to be a relatively inert tissue [252]. In general, removed human teeth are considered as infective medical waste. However, biomaterials based on teeth, as an important native resource, contain native growth factors and several important functional sequences that support cell adhesion as an anchorage matrix. The use of human teeth removed for orthodontic, impaction-related or irreversible periodontic reasons as grafting materials addresses several of the problems with the bone grafting technique, such as the limited availability of bone mass, the risk of donor site infection and significant resorption of the grafted bone, although there are still concerns about the residual infective risks [253]. To assess the extent to which demineralized dentin matrix (DDM), prepared by a process similar to how DBM is obtained, induces osteochondral regeneration, DDM from bovine teeth was implanted into rabbit knees with surgery-created full-thickness articular cartilage defects. It was found that the DDM led to active new bone formation early in the postoperative period, indicating that the DDM acted as a suitable scaffolding material for osteochondral regeneration [254]. Recently, it was demonstrated that without causing an inflammatory reaction or infection, allogenic DDM significantly increased bone mass and improved bone quality when it was used as a bone grafting material to treat surgically created bone defects on the skull of rabbits [255]. Further evidence suggests that DDM increases the expression of vascular endothelial growth factors (VEGFs) and accelerates the healing process by stimulating bone deposition and vessel formation [256]. Based on these findings, DDM has been successfully applied as an osteoinductive/osteoconductive material for bone reconstruction in the clinic for many years (Fig. 6). Considering that the particulate morphology of DDM limits its applications at sites requiring structural support, collagen has been used in combination with DDM to form a composite DDM–collagen material that has a significant clinical advantage over DDM alone and the potential to be used in bone and orthopedic surgeries [257].
Fig. 6.
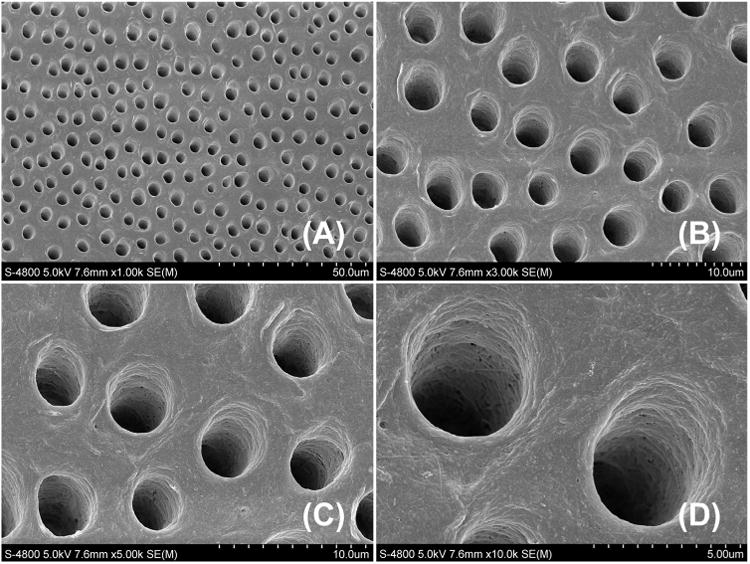
Representative scanning electron microscopy (SEM) images of demineralized dentin matrix (DDM) (images from a human tooth-derived sample). From (A) to (D), gradually magnified views of the DDM bioscaffold showing the arrangement of tubules in human dentin.
Accumulating laboratory studies have supported the use of human DDM in both bone regeneration and tooth tissue engineering. It is widely recognized that the dentin–pulp complex demonstrates strong regenerative potential due to the many bioactive molecules bound within the dentin matrix. Following dental injury, the release of dentin matrix components and many other signals can contribute to the angiogenic and cell-recruitment events necessary for regeneration of the dentin–pulp complex [258,259]. The outcomes of the interplay between a dentin scaffold and dentin-forming cells are important not only for biocompatibility but also for the potential for the material to skew the cell response toward correct differentiation [260]. In this regard, DDM has been evidenced to be an appropriate scaffold that provides an inductive environment for both dentin regeneration [261,262] and tooth root construction [263,264]. It is speculated that materials science will develop the next generation of regenerative procedures by using the human dentin matrix to treat patients in the near future.
3.3. Tissue engineering: the state of the art in transplantation
Although treatments by autogenic and allogenic tissue transplantation have been successfully applied in clinical procedures for numerous medical conditions, these therapies are largely impeded by their disadvantages, such as the limited tissue available, considerable donor site morbidity and risks of disease transmission [140,162,198,228]. A brief overview of conventional strategies for treating disfiguration and large composite tissue defects by applying human-derived tissue materials directly illustrates that in addition to autologous tissues, all of the grafts and techniques currently available for clinical application fall short of achieving the complete functional and esthetic replacement of lost/damaged tissues [137,157,178,182,187,218,219,265]. However, close coordination between biologists and surgeons offers a critical step for clinical success, and the implantation of specimens as rapidly as possible may improve the overall engraftment rate. Similarly, recent advances in quality management systems, coupled with new insights into the preservation and storage of human materials, have been shown to improve the safety and quality of human materials applied in transplantation [199]. As we continue to face severe shortages of organs (or certain specific tissues) for transplantation throughout the world, we need to consider innovative solutions to decrease the numbers of patients on waiting lists, which are ever growing because of the expanding aging population and the severe shortage of suitable donor tissues and/or organs available [266]. The approach that is most likely to make a real difference in transplantation in the long term is tissue engineering, a field that has emerged from the selective conjunction of stem cells, biomaterial scaffolds, gene therapy and chemical/mechanical molecules for new implantable tissue/organ production that may be used in either planned or emergency situations [115,136,266]. From the inception of tissue engineering as a field, research on the regrowth of most, if not all, types of human tissues has been and still is being conducted, and as a result of interdisciplinary endeavors by biologists, materials scientists, engineers and physicians, tissue-engineered products for bone, cartilage and skin repair have been approved for clinical application by the United States FDA. Several of these products have been produced using biomaterials of human origin as scaffolds [155]. Techniques for producing bioactive synthetic bone scaffolds have the potential to significantly improve the performance of bone graft replacement materials, and knowledge generated from tissue/organ allotransplantation, ranging from technique innovation to transplant design to immunomodulating protocols, will instruct the future development, optimization and application of tissue-engineered products [135,267,268]. Instead of the long-standing goal of merely replicating natural tissue regeneration, in the future, manmade materials and structures could be utilized to exceed the body's natural healing response and, for the first time, to offer a bone graft replacement material with a clear advantage over traditional autografts; however, current biotechnology for converting grafting materials to functional bone tissues remains in its infancy [136,165]. The recreation of more complex human tissue replacements remains challenging, although the production of complex tissues has recently appeared in the literature in many forms [5,35], such as in the form of a tissue-engineered bladder [74,269], rendering tissue engineering a potential candidate to revolutionize our current clinical strategies for reconstructive surgery and greatly enhance the lives of patients. The selection of a perfect biomaterial can significantly contribute to this innovation, not only by serving as a carrier device for signaling molecules and responsive cells but also by offering a platform that interacts with and guides tissue formation. Mass transport and the regulation of cell-material interplay are two of the crucial parameters that must be taken into consideration during design [31,50].The similarity of the microstructure, composition and biomechanical and biological properties of decellularized materials and substrates to those of native human tissues and organs is spurring interest in the direct use of these materials for tissue repair and in recreating human-derived tissues/organs in simplified forms for tissue engineering applications, such as the reduction of ECM into short functional domains to influence stem cell differentiation [222]. In particular, key ECM components, including chemical macromolecules, physical parameters (i.e., shear stress and tissue stiffness) and microenvironmental signals (i.e., hypoxia), can be advantageous for therapeutic applications because human cells already have a predisposition to recognize them and because these components' usage has a low potential to induce negative immune responses [270–273]. For many years, it has been recognized that both simple tissues and complicated organs may be decellularized for tissue engineering use, and decellularization methods have been optimized to completely remove cellular components while keeping the ECM intact [274,275]. The following section provides examples of human tissue ECM components that are currently being tested as scaffolds for tissue engineering and regenerative therapies. In particular, the section highlights different classes of ECM components and different strategies that are commonly applied or are promising in their possibility of future application and development in making the transition from a human tissue-derived ECM to a tissue engineering scaffold.
4. Human tissue ECM-based biomaterials
The ECM, or the extracellular macromolecule network between and around niche cells, is a multi-component structural element that is synthesized and assembled by the resident cells and that combines ubiquitous structural biomacromolecules, including an array of multidomain biomacromolecules (e.g., collagens, glycoproteins, proteoglycans and elastic fibers); its protein network remains in equilibrium with the surrounding cells and tightly regulates the fiber diameter, composition and organization [271]. As a complex, fibrillar 3D network of proteins and polysaccharides, the ECM has a highly regulated, tissue-specific composition and set of physical properties in the majority of tissues and organs in the human body, and the nature of its contact with stem cells also varies considerably [276]. The ECM of living organisms may be dispersed as an amorphous “ground substance” and/or organized into interacting fibrous structures arranged in a cell/tissue-specific manner. The general functionality of the native ECM is to impart physical cohesiveness on a tissue with regard to the provision of a structural function and an anchoring support for cells. Under certain conditions, the ECM also anchors and acts as a reservoir for various soluble molecules (i.e., growth factors and chemokines), increasing the local concentrations of agonists to which target cell populations in the niche are exposed [277]. According to recent findings, however, as a highly dynamic entity, the ECM has been increasingly shown to exert an intense impact on cell behavior and function via its matrix stiffness, its ligand types and its degree of coupling of fibrous protein to the surface of the underlying substrate (i.e., protein tethering and matrix porosity); such biological influence can be explored for use in the design of new ingenious biomaterials [278–283]. It is now well known that the living-tissue ECM not only acts as a reservoir for morphogens while providing mechanical support to resident cells but also participates in defining the stability and shape of tissues and in facilitating most cell communication activities associated with tissue development, turnover and regeneration [276,284] (Fig. 7).
Fig. 7.
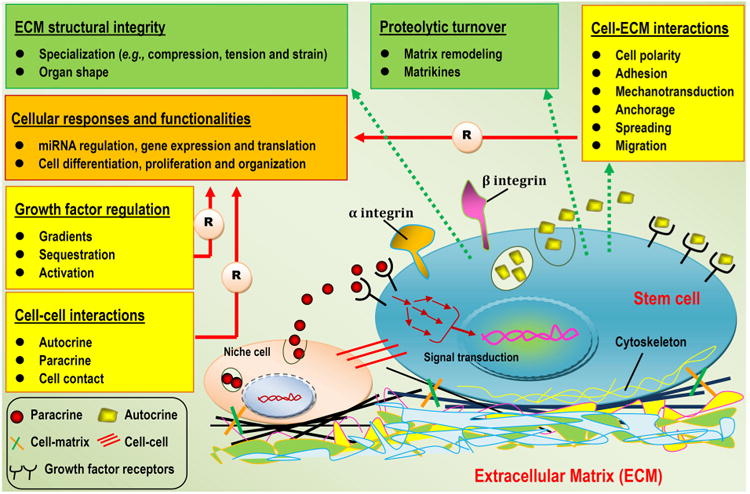
Schematic representation of the extracellular matrix (ECM) functions and crosstalk at the cell–cell and cell–matrix interfaces (illustrations are not to scale). Crosstalk between stem cells and niche cells is mediated by soluble ECM growth factors (secreted by producer cells via autocrine and/or paracrine routes) and the properties of the surrounding ECM. Through cell membrane growth factor receptors and a complex signal transduction network, the outside instructions are conveyed to the stem cells, resulting in specific biological cellular responses and functionalities, such as cell differentiation and gene expression [12]. Aside from acting as a mediator of mechanical constraints applied to cells, the ECM also affects cells via its architecture and overall biological and mechanical properties [278]. Soluble and matrix-binding factors combine with cell–matrix adhesion, cell–cell contact and signaling gradients to determine and control the most fundamental behaviors and characteristics of stem cells, including polarity, adhesion, anchorage, proliferation, migration, differentiation and apoptosis. In turn, cells contribute to the complex cell–matrix feedback loop, with their overall functionalities resulting in proteolytic turnover and ECM structural integrity [276]. The design of new ingenious biomaterials must consider these functions of the native ECM, at least to a certain degree, to mimic the natural environment to regulate stem cell fate decisions.
4.1. Biomaterials for tissue engineering inspired by the ECM
ECMs are the focus of intensive research endeavors worldwide that are directed not only at illustrating ECMs' nature and unique properties in biology and materials science but also at mining ECMs or ECM-like biomaterials for tissue engineering and regenerative medicine applications [276,285]. As a multi-component structural meshwork that is assembled into unique tissue-specific architectures, the ECM provides dynamic signaling cues that modulate various aspects of cell fate commitment in part through its physical, chemical and mechanical properties [26,286,287]. Over the past few decades, a considerable amount of attention has been focused on exploring the biological information and components of native ECM for biomaterials design. Based on its spatial patterning, chemical composition and functionality, ECM can typically be divided into 2 categories of components, namely, the basement membrane (BM) and the stromal matrix (SM) (Fig. 8). In epithelia, BMs are specialized ECM assemblies (containing type IV collagens, laminins, perlecan, agrin, nidogen and other macromolecules) that play a key organizing role, providing a film-like substrate for a tissue's peripheral cells, which includes wrapping around the vasculature as a supporting substratum for epithelial sheets and maintaining cell polarity [26]. However, in tissues such as tooth, bone, cartilage, muscle and tendons, in which BMs play an obvious mechanical role, the SM is comprised of larger, fibrous structures and constitutes the bulk of the ECM, serving as the main structural support of the ECM. Quantitatively, BM is a major component that dictates overall mechanical characteristics. The organization and composition of the ECM in these tissues reflect evolutionary adaptation to mechanical load, and each ECM component is unique in its interplay with the tissue-forming cells that have been studied for use in various regenerative procedures [288]. Indeed, in the development of new biomaterials by combining different molecular components at tailored concentrations and geometries, a wide range of tissue-unique structural requirements can be met [276]. Along with recent advances in ECM science and developmental biology, concentrated efforts on the exploration of human-derived biomaterials for therapeutics have now moved from the direct use of autogenic tissue grafts, allogenic tissues/organs from donors and a wide variety of allografts from cadavers toward the recreation of human-derived extracellular influences in simplified forms, the decellularization of tissues and organs for scaffolding use and the incorporation of short functional domains derived from human tissue into ECM-mimicking biomaterials to manipulate cell fate commitment [52,289,290].
Fig. 8.
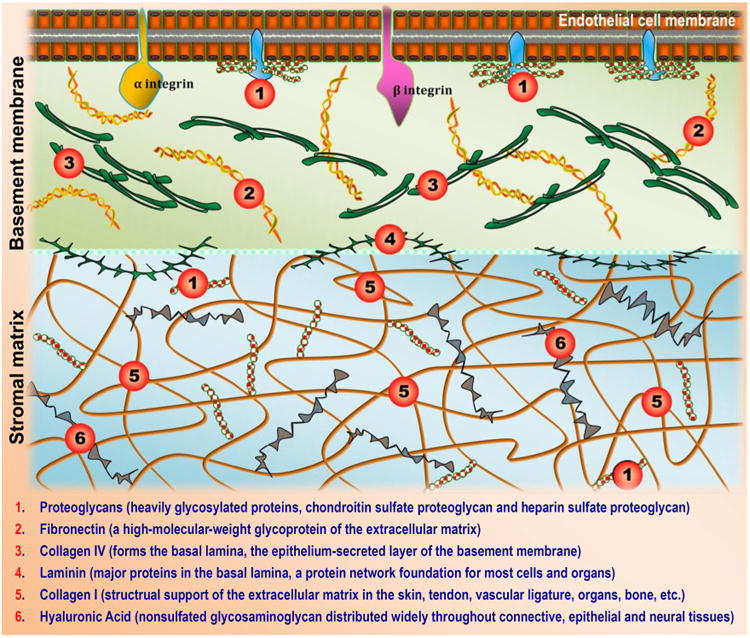
Schematic representation of the location of selected endogenous extracellular matrix (ECM) components within the basement membrane (BM) or stromal matrix (SM) that have been and are still being investigated for use in regenerative therapies and tissue engineering biomaterials (schematic is not to scale).
Source: modified from Ref. [288].
The selection and design of biomaterials are key steps in advancing medical devices for regenerative medicine. In general, excellent biomaterials, as mentioned in Section 2, should be of favorable biocompatibility and nontoxic, should coax appropriate cell-material interactions toward new tissue regeneration and should possess adequate physical and mechanical properties until the new tissue structure is constructed [52]. As noted previously in this section, the ECM was once considered to offer only structural support to tissues by serving as a substrate/template for cell binding; it is now, however, widely accepted that the ECM can additionally provide vital mechanical and chemical information to the cells that mediate cell–cell and cell–matrix interactions and communication [48,287]. A prefabricated material device must assume this instructive role to a certain degree to ensure cell viability and dictate cell behavior following cell seeding. Efforts to engineer such “cell-instructive” materials, regardless of their composition, by incorporating well-defined physical and chemical properties designed to affect surrounding cells, biological signals and tissues in a specific manner are therefore largely inspired by the native ECM of different tissues and organs [291]. In a recent study, non-collagen proteins (NCPs) from bone ECM combined with 3D nanofibrous gelatin (NF-gelatin) scaffolds were used to form a material device mimicking both the chemical composition and the nanostructured architecture of natural bone ECM. The incorporation of NCPs into the surfaces of the device was found to result in significant osteogenesis and mineralization, leading to new bone regeneration, suggesting that biomimics are a new signpost for future cell scaffolds and tissue engineering templates [292]. Of note, clues for how to construct ECM-mimicking biomaterials arise from ECM assemblies/components, including collagen types I and III, fibronectin, elastin, proteoglycans, laminin and many others. Naturally, the production and accumulation of these ECM assemblies at structural, chemical and physical levels yields an optimized milieu that mimics the actual in vivo microenvironment to instruct tissue formation and to maintain homeostasis. Taking inspiration from such physiological events, a practical paradigm has been to procure the main ECM constituents and to use them as building blocks for biomaterials after purification. Given recent advances in the fields of matrix biology, surface chemistry and biopolymer science, purified ECM macro-molecules of human or animal origin have served us well in the production of regenerative biomaterials for reconstructive surgery and tissue engineering [293].
During the same period, considerable efforts have been made to synthesize biomaterials based on the functionality of natural ECM molecules to guide morphogenesis in tissue engineering and regenerative medicine [267]. Decellularized ECMs can be used in tissue engineering directly, with or without further modifications, because (i) current methodologies are now able to remove nearly all cellular and nuclear material, while minimizing any adverse impacts on the composition, mechanical integrity and biological performance of the resultant ECM, and (ii) the maintenance of the ECM structural components allows the remaining matrix to offer biomechanical strength and structural integrity to newly formed tissues and to enable substantial cell rebinding [26]. The hypothesis here is that such a decellularized ECM, acting as a native scaffolding matrix, would preserve both biological information, which would play an instructive role in cell interactions, and other physicochemical features, which would maintain the correct spaces and microstructures for new tissue development following cell reseeding. These benefits would help to overcome one of the crucial disadvantages of synthetic polymers, namely, a lack of cell recognition signals, and could help to provide a tissue-specific template with natural microstructures that facilitate new tissue formation [45].
4.2. ECM constituents for scaffolding biomaterials
Recent decades have witnessed growing interest in the composition of the ECM of a given human tissue or organ as well as in the developmental and physiological roles of each matrix constituent (Fig. 9). In accordance with their unique molecular structures, these matrix components can generate biomaterials with various well-defined 3D configurations, such as fibrillar meshes, and the resulting physical and biological properties are linked to each macromolecule's structure–function relationships [294,295]. Many ECM-embedded and cell surface-associated assemblies/constituents have a highly organized spatiotemporal pattern, suggesting crucial structural and regulatory roles in tissue development and function and exceptionally strong relevance to and translational implications for human disease diagnosis and treatment [271,276,296,297]. In particular, the use of these macromolecules, whether natively derived or produced by decellularization, can mimic many features of the native ECM, offering a simple way to design and synthesize biomimetic materials for tissue engineering and regenerative medicine. Advanced materials either composed of naturally occurring macromolecular assemblies or including adjuvant ECM functional domains in a controlled 3D configuration have been demonstrated to regulate the healing cascade by modulating host immune responses, facilitating host cell homing, resisting bacterial infections and infiltrating and reestablishing homeostasis in the damaged areas targeted for regeneration [298]. Many ECM constituents and naturally derived proteins/substrates that could enable the application of research on scaffolding biomaterials have been characterized at the structural, chemical and physical levels, and the overall outcomes have been very successful in regenerative medicine. Broadly speaking, two classes of structural and functional macromolecules are yielded by the ECM: fibrous proteins (mainly collagen, laminin and elastin) and glycosaminoglycans (GAGs) [26,266]. Here, we provide a detailed discussion of several representative protein-based biomacromolecules (constituents) isolated from ECMs of human origin (Fig. 8), and their enormous potential for future scaffold design and development is detailed.
Fig. 9.
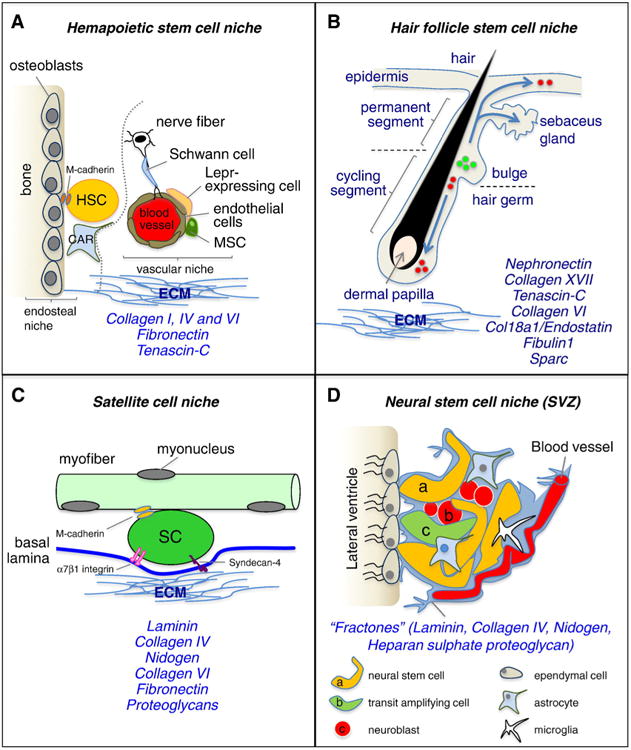
Schematic representation of four representative tissue-specific stem cell niches and their cellular and extracellular matrix (ECM) components. Examples are given where appropriate. (A) Hematopoietic stem cell (HSC) niche; (B) hair follicle stem cell niche; (C) satellite cell niche; and (D) neural stem cell niche (SVZ). Readers are directed to the original article for more information.
Source: [97], Copyright 2014, Reproduced with permission from Elsevier Ltd.
4.2.1. Collagen I
Collagen is the most abundant protein (approximately 30% of the total protein content) in the ECM and is arguably the most dominant in many types of human soft and hard connective tissues [299]. Collagen specifically comprises a right-handed bundle of three parallel, left-handed polyproline II-type helices (Fig. 10). This protein not only constitutes a key fibrous structural component in the human body but also helps in manufacturing the structural proteins necessary for the macromolecular composition and structural architecture of the skin, the skeletal systems (e.g., bone, cartilage, joints, ligaments, tendons and blood vessels) and various internal organs [300]. Collagen plays the role of a “mattress” that glues our body cells together, links all of the tissues/organs and supports the entire body framework, in which tough bundles of collagen are normally termed collagen fibers. Collagen, along with elastin and keratin, specifically constitutes fibrous extracellular networks that enable tissues or organs to withstand repetitive stresses and high tensile stress without plastic deformation or rupture [301]. At the cellular level, the intracellular bonds formed by collagen fibers provide the cementing mechanisms required for linkages, reinforcement and protection, and even the supply of oxygen and nutrients. Insufficiency of collagen will hence naturally result in an unhealthy cellular microenvironment, which eventually takes its toll on our overall health (http://vitaking.kurazmotorsports.com/what-is-collagen). Based on its functional and bioactive properties, collagen has been demonstrated to be a versatile biomaterial that can be formed into highly organized 3D matrices (e.g., sponges, films, skin grafts and dressings) endowed with high tensile strength and intrinsically biocompatible, biodegradable, and nontoxic properties upon exogenous application. These attributes make collagen the biopolymer of choice in many biomedical fields, such as drug delivery and regenerative medicine [302–304]. However, controversy persists about the perfect source of collagen for these applications, given possible disease transmission and immunogenicity related to animal or allogenic sources and reduced bioactivity due to production by recombinant techniques [305]. Thus far, most collagen has been extracted from tissues of animal or human cadaveric origin, such as the skin or tendons, or from discarded human tissues, such as the placenta or extracted adipose tissue; human collagen is more attractive for therapeutic applications [306]. Decades of research have uncovered more than 20 distinct forms of collagen, most of which have been purified for biomedical use. Various amount of types I, II, III, IV, V and VI are present in mammalian tissues, among which type I is the most abundant (approximately 90%) [267,306]. Other types, however, are only present in relatively minor amounts; their roles in the in vivo cell milieu remain poorly understood. The attractive properties of collagen for use in tissue engineering biomaterials include, but are not limited to, its good biocompatibility; low antigenicity; and tailored mechanical, degradation and water uptake properties due to its ability to be crosslinked through chemical glycation procedures or heat treatments [267]. Compared with protein constituents extracted from animal tissues, human-derived collagen for scaffold production in tissue engineering lowers the risk of hypersensitivity and immunogenicity, but the potential to cause pathogenic contamination and/or disease transmission still exists [307]. In this respect, recombinant human collagen based on plant materials provides an alternate natural collagen source without the risk of disease transmission or concerns regarding variability [308]. Currently, growing evidence suggests that synthetic polymer nanofibers may play the similar role as natural ECM collagen in the tissue regeneration process, thus allowing the design of biomimetic materials in a way that regulates cell incorporation and behavior toward desired overall differentiation and function [309–311]. The use of plant-derived and recombinant collagens for materials engineering has thus presented a new way of not only expanding our insights into raw materials science but also exploring the short functional domains of human proteins for the molecular design of biomimetic materials [312].
Fig. 10.
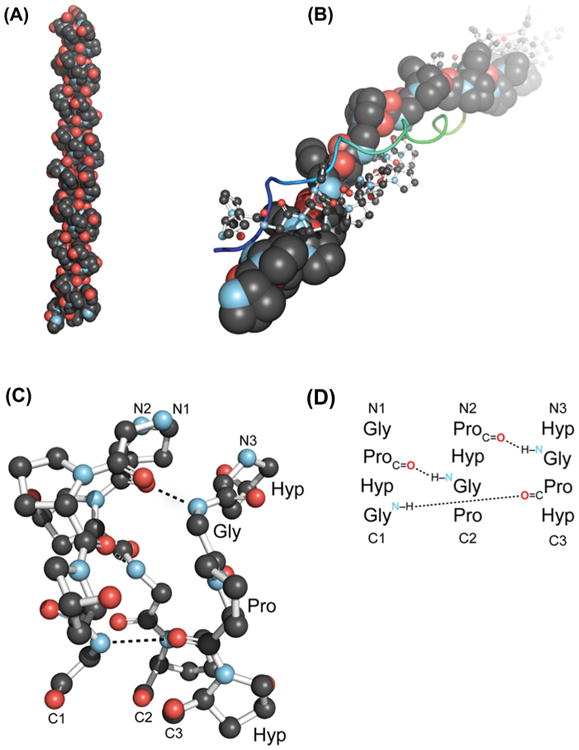
Overview of the collagen triple helix. (A) First high-resolution crystal structure of a collagen triple helix, formed from (ProHypGly)4–(ProHypAla)–(ProHypGly)5. (B) View down the axis of a (ProProGly)10 triple helix, with the three strands depicted in space-filling, ball-and-stick and ribbon representations. (C) Ball-and-stick image of a segment of a collagen triple helix, highlighting the ladder of interstrand hydrogen bonds. (D) Staggering of the three strands shown in panel (C).
Source: [300], Copyright 2009. Reproduced with permission from Annual Reviews.
Over the last few decades, accumulated knowledge about the complex hierarchical structure of native collagen molecules and the role of collagen's physicochemical properties in tissue development and regeneration has inspired biomaterials scientists to design innovative biomimetic materials that mirror the native nanofibrous collagen network to instruct cell behavior and guide tissue formation [61,89,313]. In particular, human tissue-derived collagen I is a ubiquitous ECM protein and one of the most common structural elements in a number of tissues. This protein adheres to various cell types and may provide a satisfactory scaffolding material upon which cells may thrive via capillary formation and cell chemotaxis [288]. Purified collagen I isolated from human tissues (e.g., connective tissue and the BM) has been an appropriate choice in a variety of restorative applications, and in recent years, there has been broad and intense interest in this protein's utility in a variety of biological and tissue engineering applications, partially due to its unique properties and relative abundance in living tissue [295]. Interestingly, this type of material is now also employed to generate 3D systems for the culture and directed differentiation of a number of cell populations and, indeed, to investigate the signaling interactions between these cells and the matrix, which is a fundamental issue in cell biology and materials research [314]. As a cell culture system, it has been suggested that collagen I hydrogel supports long-term in vitro cell expansion and the maintenance of fully elaborated human small intestinal epithelium for at least one month. This system gives rise to a new pattern of sheet-like growth at the gel-liquid interface as well as familiar enteroid structures, with polarized columnar cells with basolaterally located nuclei and apical brush borders [315]. Years of efforts to harness isolated collagen matrix as a substrate for cultivating cells have expanded into a large body of work focused on capitalizing on the protein's distinctive ability to form biomimetic hydrogels with well-defined fibrous architectures, biological properties, stable topographies that can withstand mechanical loading and the ability to modulate cell and tissue responses against inflammation and oxidative stress [316,317]. The reconstitution of collagen into hydrogel scaffolds can be specifically controlled to achieve desired hierarchical structures from the nanoscale to the microscale to the macroscale, leading to a highly relevant biomaterial for regulating cell function and mimicking tissue properties [61]. In this regard, collagen I offers a structural framework that determines the morphological characteristics of human connective tissues and plays a dominant role in the temporal cascade of myriad cellular and molecular events leading to the regeneration of new bone from osteoblastic progenitors [295].
If we reconsider specific molecular structures, the potential of the P-15 cell-binding domain of collagen I to create desirable biomimetic environments for osteoblasts offers the possibility of exploring ECM cues for osteogenesis and, subsequently, bone repair and regeneration. To this end, the introduction of this peptide into cell scaffolds has been demonstrated to largely enhance the attachment, proliferation and differentiation of human MSCs [318]. In an in vitro microenvironment without any other vertebrate ECM components of calcifying tissues, the collagen I matrix is able to initiate and orientate the generation of the mineral carbonated apatite based on a collagen/apatite self-assembly process. Additionally, collagen I can influence and control not only the structural characteristics of apatite on the atomic scale but also its size and the 3D distribution on a larger scale, likely through orchestrated signal cascades and cellular events modulated by the collagen I matrix [319]. Although collagen I does not signal MSC migration on its own, when cells are severely stressed, collagen I can induce a potent migratory response. Such findings suggest that cells may secrete a substance, which appears to be a protease, upon injury or when in a disease state. The substance interacts with the collagen matrix to release cell homing agents, and hence, a chemo-tactic signal emerges to recruit stem cells to damaged or injured areas to exert therapeutic function; the interplay appears to be due to the digestion of the protease into fragments that are chemotactic [273,320]. For several decades, it has been recognized that many other ECM components in addition to collagen I that also specifically modulate cell activities, including the migration, adhesion, proliferation and osteogenic differentiation of MSCs of human origin, can be used as a cell vehicle for bone tissue engineering applications (Fig. 11). For example, fibronectin facilitates cell adhesion, migration and proliferation, but not osteogenic differentiation, whereas fibrinogen may enhance cell proliferation and adhesion, but not migration [273]. These properties of ECM components offer the possibility of exploring naturally derived biomaterials that can instruct stem cell fate decisions.
Fig. 11.
Representative scanning electron microscopy (SEM) images of the attachment and growth of human bone marrow-derived stem cells on collagen fibers derived from human skin [58]. (A) Before cell seeding; (B) 8 h after cell seeding: cell attachment on collagen fibers; (C) 24 h after cell seeding: extracellular matrix formation and cell ingrowth into collagen fiber structures; (D) 48 h after cell seeding: cell–matrix–collagen integration.
Furthermore, collagen I-based biomaterials have been applied to cell therapeutics in vivo, and recently, for tissue engineering of whole menisci, a high-density form of type I collagen hydrogel has been used as an injectable scaffold [321]. In other applications, combinations of collagen with other biomaterials have also been tested as bone substitutes and skin replacements and, indeed, for the engineering of manmade valves and blood vessels, wherein collagen I and laminins are crucial for vessel structural integrity and deliver the contrasting signals necessary for angiogenesis [322]. To increase mechanical performance, collagen microsponges may be used for the modification of previously prepared synthetic polymeric materials; these sponges are easily impregnated into the structures of the scaffolds [52]. Meanwhile, biomimetic collagen-apatite scaffolds can be engineered via a self-assembly process in a simulated body fluid system. These scaffolds possess a unique multi-level lamellar structure characterized by tunable co-aligned micro- and macropores and have great potential to be applied in bone tissue engineering applications due to their biomimetic architectures, favorable mechanical strength and desirable biocompatibility [323]. More specifically, a three-component, biomimetic, injectable hydrogel composite composed of triblock PEG–PCL–PEG copolymer, collagen and nano-hydroxyapatite was found to have good thermo-sensitivity, biodegradability and biocompatibility, leading to favorable performance in guided bone regeneration applications [317,324]. Furthermore, growth factors and other bioactive agents can be incorporated into collagen-based systems, such as gels and scaffolds, to modulate their release rates and improve their therapeutic effects as tissue engineering strategies [52,325]. In this regard, a type I collagen matrix was found to be an effective vehicle for growth factor delivery in vascular tissue engineering [314,326], and a collagen-based biomatrix-scaffold composed of DBM and collagen-binding domain BMP-2 (CBD-BMP-2) has been used as a bioactive bone-inducing material (BBIM) for bone regeneration [327]. Additionally, collagen can be used to minimize unwanted progenitors localizing to non-target sites and causing undesirable tissue formation, addressing one major issue associated with stem cell therapy. The incorporation of MSCs into a collagen scaffold was found to reduce the dispersal of transplanted MSCs into the surrounding non-infarcted myocardium and the relocation of those cells to remote organs after intramuscular injection. More specifically, no relocated cells were found in the liver of animals receiving this combination treatment, which is, however, normally a sink for the relocation of injected cells [328]. Similarly, another study demonstrated improved early engraftment and directed localization of transplanted endothelial progenitor cells (EPCs) post-transplantation via enhancing progenitor cell retention and limiting distribution to nonspecific tissues when the cells were impregnated in an injectable collagen matrix [329]. ECM-mimetic hydrogels containing the collagen I-derived peptide GPQGIAGQ were also demonstrated to induce endothelial cell adhesion and capillary-like network formation [330]. Furthermore, the conjugation of a collagen-mimetic protein with a PEG hydrogel is able to generate a bioactive hydrogel that can bind to endothelial cells and resist platelet adhesion, forming a vascular graft with a potential multilayer design that can achieve rapid endothelialization of the conduit while minimizing the risk of thrombosis, intimal hyperplasia and mechanical failure [331]. Taken together, these findings suggest multiple important roles for collagen-containing materials in enhancing cell adhesion, locally retaining cells and regenerating capillary-like networks, which may have significant implications for both cell-based therapy and vessel tissue engineering paradigms.
4.2.2. Collagen II
Collagen II is the main protein present in the ECM of hyaline cartilage and the nucleus pulposus (NP), within which little to no collagen I is found. As a potential autoantigen in inflammatory synovial disease, collagen II may be useful for limited applications. For example, increasing evidence suggests that small doses of collagen II can modulate joint health in both rheumatoid arthritis and osteoarthritis [332,333]. The ingestion of non-denatured type II collagen (microgram quantities) in an animal model of collagen-induced arthritis specifically caused a dramatic reduction in the circulating levels of pro-inflammatory mediators (e.g., IL-2 and IL-17), along with an increase in anti-inflammatory molecule (e.g., IL-4 and transforming growth factor-β (TGF-β)) production, hence decreasing both the severity and the incidence of arthritis [334]. This finding indicates that collagen II plays an essential role in orchestrating the immune balance of Th17/Treg and Th1/Th2 in mice [334].
Due to its presence in significantly fewer ECMs of tissues in the body, collagen II has not been applied as frequently as collagen I in raw biomaterial approaches for generating tissue engineering constructs, even for cartilage tissue regeneration [335]. Nevertheless, collagen II has also been used for the production of matrix-scaffolds, including hydrogels, sponges and microspheres, but has mainly been utilized in cartilage and NP tissue engineering applications [39]. To mimic the native compositions associated with the transition of tissue types at the interface of cartilage, an osteochondral template based on collagen I/CaP, with an interfacial layer that connected to a collagen II/chondroitin sulfate (CS) layer, was designed and developed. These designs are combined to enable the resultant biomaterials to be embedded into an osteochondral defect site in the subchondral bone, with no demand for glue, sutures or screws [336]. To this end, a highly interconnected porous network can be inserted throughout the entire osteochondral defect. Due to the differential moduli of the cartilaginous and osseous compartments, these layered scaffolds are capable of exhibiting compressive deformation performance, similar to that observed in natural articular joints [337]. Similarly, with sufficient crosslinking, a composite hydrogel composed of collagen II and hyaluronic acid (HA) at a ratio equivalent to that in the native tissue ECM of the NP can serve as a potential candidate for IVD regeneration [338]. Recently, photocrosslinked carboxymethylcellulose (CMC) hydrogels have been demonstrated to direct human MSC differentiation with respect to chondrogenic or NP-like ECM elaboration; however, the mechanical properties of these IVD constructs need to be improved. Interestingly, the macromer concentration of photocrosslinked CMC hydrogels was found to direct functional NP-like matrix accumulation and organization, which is likely attributable to the diffusive properties of the various hydrogel formulations and the quantifiable differences in polymer crosslinking density. In particular, a lower polymer concentration allowed for a greater NP-like ECM assembly and hence improved the mechanical functionality of these IVD constructs, approaching the values of native NP over time [339].
The association between a microfracture of the subchondral plate and a coverage scaffold has emerged as a promising treatment for the management of cartilage lesions via a one-step procedure. Although a type I collagen scaffold is most often used for this purpose, biomatrix-scaffolds made of mixed type I and II collagen, which defied the immunological reaction of the synovial tissue, exhibited good biocompatibility in vivo and favored cartilage restoration by the collagen membrane when associated with a microfracture [340]. The limited use of collagen II in scaffolding materials may be potentially due to its limited availability and high cost, the lack of substantial data to support its application, limited consciousness of its utility or a combination of these factors [39]. However, the strategy for mimicking the native ECM composition in hyaline cartilage and the NP has increased the utility of collagen II, whether alone or in combination with other ECM components, in tissue engineering, although, more in-depth investigations are required to demonstrate its potential.
4.2.3. Collagen IV, laminin and entactin
BMs are cell surface-associated ECMs that are fundamental to tissue physiology and organization in all metazoans, with roles ranging from structuring, polarizing, protecting and compartmentalizing cells to supplying them with growth factors [341]. Although BMs are commonly composed of collagen IV and laminin networks that are stabilized by entactin/perlecan bridges, their precise composition is as unique as the tissues to which they are localized, with entactin, proteoglycans, perlecan and collagen VII and other macromolecules also present in the native membrane-like structures of BM [272,342]. Collagen IV and laminin are capable of connecting a cell to and procuring a cell from its microenvironment, suggesting that the production and maintenance of both macromolecules play a central role in endothelial cell function and the regeneration of different tissues [343]. Indeed, the BM is well known to support an atheroprotective endothelium, and collagen VII has been validated as a critical player in the physiological wound healing cascade in humans [344]. In addition, the presence of collagen IV and laminin has been found in mature and developing articular cartilage and has also been observed in tissue-engineered cartilaginous constructs ex vivo and in cartilage repair implants transplanted into in vivo defect sites [345]. Meanwhile, increasing evidence indicates that chondrocytes in articular cartilage are surrounded by a narrow pericellular matrix that serves as a transducer of microenvironmental signals to the chondrocyte and that is biochemically defined by distinct molecular components, such as type VI collagen and perlecan [346]. These findings may have implications for the tacit understanding of the function of human ECMs' BM molecules in chondrogenesis during cartilage repair and regeneration, in the control of the mechanical environment and mechanobiology of cells in articular cartilage and in the physiology and pathology of articular cartilage [345].
As a non-fibrillar collagen, collagen IV within small sheets represents a predominant component of the BM and plays a structural role in its assembly, suggesting an approach mimicking this protein's organization for vascular regeneration [347]. Collagen type IV is the main ECM constituent in the BM of blood vessels and plays crucial roles in regulating the cellular balance to minimize the risk involved in the use of vascular implants [348]. Two specific interacting domains of collagen IV, Hep I and Hep III, have been purified and investigated for their biological potential [349]. Although these peptides were not observed to induce migratory responses in vitro, treatment with the peptides resulted in improved functional recovery of cardiac muscle in the ischemic heart, without noted myocyte regeneration. The functional findings were considered to be caused by increased vascularity, indicating a pro-angiogenic impact of specific collagen IV domains that could be explored for the management of a large variety of ischemic vascular diseases. Similar to collagen I, as mentioned in Section 4.2.1, collagen IV by itself is not a chemotactic agent for MSCs; however, under stress conditions, collagen IV may also induce a potent migratory response [320].
Laminins are a large glycoprotein family that contains at least 16 isoforms; these isoforms associate with different heterotrimers composed of globular, laminin-type epidermal growth factor (EGF)-like repeats and α-helical domains. Laminins specifically consist of α, β and γ polypeptide chains, and the triple-helical coiled-coil domain in the center of each chain may combine to form distinct assembled structures that are involved in diverse cellular events, such as adhesion, survival, migration and differentiation [272,299]. The use of genetics to probe the functions of BM laminins with regard to their structure and binding and self-assembly activities has indicated that various laminin subunits profoundly influence tissue morphogenesis by inducing and maintaining cell polarity, establishing tissue compartment barriers, organizing cells into tissues and protecting adherent cells from detachment-induced cell death and anoikis, starting around the embryonic stage and extending through organogenesis and into the postnatal period [342]. It is evident that laminins are also crucial for the initial generation of the polymeric scaffolding structure of cell-attached matrices. As additional matrix components are gradually integrated into the scaffold, it becomes more and more mature in terms of ligand diversity, matrix stability and functional complexity. These findings suggest that diverse BM components differentially promote cell polarization, compartmentalize and organize developing tissues, and indeed maintain adult tissue function [342]. Interestingly, recent research found that a matrix metalloproteinase 2 (MMP2)-cleaved laminin-111 fragment was highly up-regulated during the early stage of stem cell differentiation, suggesting a previously unidentified role of laminin that goes far beyond BM assembly and a mechanism by which a biologically active laminin fragment modulates the dynamic epithelial-to-mesenchymal transition of embryonic stem cells (ESCs) [350].
For the regeneration of peripheral nerves after injury, the switching of Schwann cells to a proliferative state, the secretion of trophic factors and the presence of ECM molecules (such as laminin, collagen and fibronectin) in the distal stump are necessary elements in creating a permissive environment for axons to grow [270]. In this respect, bioscaffolds yielded by cryogelation of gelatin or dextran linked to laminin [351] or PCL-chitosan scaffolds with surfaces modified via the crosslinking of laminin [352] could serve as versatile substrates with excellent mechanical and surface properties for in vivo cell delivery, resulting in highly neuroregenerative properties for nerve tissue engineering applications. Furthermore, the application of laminin-modified linear ordered collagen biomaterials loaded with laminin-binding ciliary neurotrophic factor (CNTF) was beneficial for sciatic nerve regeneration and functional recovery when tested in a rat sciatic-nerve transection model [353]. Data obtained from this study suggest that the modification of linear ordered collagen scaffolds with laminin guided axon growth, as the laminin-binding domain fused to the N-terminus of CNTF retained more CNTF on the bioscaffolds and additionally enhanced nerve regeneration and functional recovery. Recently, a growing body of evidence has suggested that in addition to collagen IV, collagens V, VI and XV are all key components of peripheral nerves, in which they jointly not only provide structural support for neurite outgrowth but also affect Schwann cell function and myelination by triggering intracellular signals [354]. Therefore, the effects and molecular mechanisms of different collagens in peripheral nerve myelination and function must be carefully evaluated and taken into account when designing a scaffold for nerve tissue engineering application.
Because laminin-1 can induce endothelial differentiation in vitro and increase the formation of new blood vessels in vivo, it is considered to be a stimulator of angiogenesis [322,355] and may support vasculogenesis via the guidance of smooth muscle cell proliferation [356]. It is now is commonly accepted that a laminin-rich microenvironment plays a critical role in muscle regeneration. In this regard, the treatment of dystrophic muscles with the laminin-111 isoform in the mdx mouse model of Duchenne muscular dystrophy (DMD) significantly reduced the amount of muscle damage (protecting the muscle from exercised-induced damage) [357]. In addition to being a powerful stimulator of myoblast migration and proliferation in vitro, an intramuscular injection of laminin-111 resulted in increased strength and resistance in treated muscles. When laminin-111 was used as a coadjuvant for myoblast transplantation, this protein was found to considerably improve the overall therapeutic outcomes in the mdx mouse model of DMD [358]. These findings indicate that laminin-111 may serve as an unexpected and highly potent therapeutic agent for patients with congenital myopathies, representing a paradigm for the systemic administration of ECM proteins as therapeutics for genetic diseases [357,358]. Based on its capability to direct myoblast migration, laminin has been proven to be a powerful signaling molecule in endogenous regenerative therapies for guiding skeletal myoblasts to damaged areas to foster the in situ regeneration of skeletal muscles [288]. In this context, it has also been found that collagen VI is a key component of the satellite cell niche (including myofibers and ECM), where it plays essential roles in the regulation of satellite cell self-renewal and regeneration of skeletal muscles, as evidenced by reduced satellite cell self-renewal capability and impaired muscle regeneration after injury in Col6a1−/− mice as a result of the lack of collagen VI. Further investigation has revealed that collagen VI plays an unforeseen role in regulating satellite cell activity, through which the biomechanical properties of skeletal muscles are modulated and satellite cell homeostasis is regulated, suggesting a potential therapeutic strategy for the rescue of collagen VI-related muscular dystrophies [359].
Recently, the cell-laminin interplay has been demonstrated to be useful for biomaterial design, allowing a material to serve as an optimal platform by possessing inherent bioactive properties, retaining delivered cells, promoting cell survival and maintaining or promoting a specific cell phenotype in vivo. For example, a methylcellulose material functionalized with laminin-1 was found to provide a biomimetic microenvironment that may modulate neural stem cell survival, apoptosis, migration, differentiation and matrix production [360]. Similarly, when a PEG-based hydrogel was functionalized with the laminin-derived adhesion peptide YIGSR, the resulting biomaterial significantly enhanced intracellular triglyceride accumulation in encapsulated adipocytes and ultimately promoted the formation of coherent adipose tissue-like structures featuring many mature unilocular fat cells [361]. Although MSCs have been suggested to be a potential source for disc tissue regeneration, these cells neither differentiate into NP-like cells nor regenerate matrix with unique characteristics matching that of immature NP tissues of the IVD unless co-cultured with human NP cells or placed in a laminin-rich culture environment [362,363]. Indeed, substantial evidence suggests that NP cell-laminin interplay is unique to the immature disc because immature NP cells were found to express specific laminin isoforms and laminin-binding receptors [364,365]. This evidence includes higher expression of levels of the laminin α5 and γ1 chains, laminin receptors (the integrin α3, α6 and β4 subunits and CD239) and their related binding proteins in NP cells compared with cells from the neighboring annulus fibrosus [365,366]; soft-laminin-containing ECM substrates promoting immature NP cell morphology, cell-cell interactions and proteoglycan synthesis in the cells of the NP [367]; and immature porcine NP cells adhering to laminins in higher numbers compared with cells from the adjacent annulus fibrosus [368]. In this respect, a soft, laminin-functionalized hydrogel was developed as a biomaterial carrier for cell delivery to the pathological IVD to enhance IVD regeneration. The findings from this study demonstrate the ability of laminin-111-functionalized PEG (PEG-LM111) hydrogels to crosslink under physiological conditions, without the need for an initiator. Additionally, delivery within a PEG-LM111 hydrogel significantly improved primary NP cell retention in the disc space compared with the retention of cells delivered without a carrier in an organ culture model [369].
Entactin, also termed nidogen, is another widespread BM constituent of 150 kDa that primarily binds to laminin and collagen IV; multiple component interactions, consisting of inter-protein binding, self-polymerization and cell surface adhesion, facilitate BM assembly and integrity [342]. In humans as well as in all other mammals, there are two ubiquitous entactins (entactin-1 and entactin-2) encoded by distinct genes, whose specific interplay with collagen IV, laminin and perlecan is considered to be important for organizing basal laminae, including those in the muscle, the skin and the nervous system [370]. Structurally, entactins are sulfated glycoproteins that involve three globular domains (G1–G3) separated by rod-like domains. Whereas G3 is at the C-terminal domain, connected by a rod-like region that contains EGF repeats, G1 and G2 are at the N-terminus, connected by a small linkage region [322]. Entactin is reported to be responsible for the long-term maintenance and maturation of contractile skeletal myotubes, indicating a biological function for entactin in myogenesis [371]. Recent evidence also shows that entactin is an ECM protein that regulates the proliferation and migration of Schwann cells and induces elongation in the regenerative axon growth of adult sensory neurons, indicating a critical role of entactin in proper peripheral nerve regeneration [372].
The interactions between entactin and laminin are arguably of crucial importance to the assembly of BMs. Investigations using recombinant entactin to probe the calcium binding potential of various entactin domains and to examine the binding of entactin to various BM proteins indicated a large number of BM targets, demonstrating the capacity of entactin to perform a connective function and to mediate the formation of ternary complexes between laminin and collagen IV and between laminin and HS proteoglycan while integrating other BM members into the ECM [373]. For example, recombinant domain IV of perlecan, consisting of 14 immunoglobulin superfamily modules, was found to bind to entactins (entactin-1 andentactin-2),thelaminin-1/entactin-1 complex, fibronectin, fibulin-2 and heparin [374]. Entactin-1 is one of the central BM elements that play an essential role in hippocampal synaptic plasticity and excitability [375]. This entactin binds several BM macromolecules via domain-specific, well-defined interplay, and the highest-affinity cell-binding site of entactin-1 is positioned on one of the EGF repeats in the laminin γ1 chain, which is crucial for nerve guidance and for kidney development [376,377]. Entactin-2 is evidently more adhesive than entactin-1 in certain cell lines and is mainly mediated by α3β1 and α6β1 integrins, as shown by antibody inhibition [378]. The nature of the nidogen-binding epitope on the laminin γ1 chain suggests much more complementary functions of entactin-1 and nidogen-2 in modulation of the endothelial phenotype as well as vascularization and implies extensive co-regulation of the expression of the two nidogens than was previously recognized [377,379]. Further elucidation of the potential roles of entactin and specific laminin chains not only will clarify important aspects of BM regeneration but also may provide a better understanding of BM disorders (e.g., hepatic cirrhosis, skin-blistering diseases and inherited kidney syndromes) and even suggest therapeutic approaches [380–383].
Evidence that BM assembly and turnover depend on the composition and mechanical characteristics of the adjacent ECM and the dynamics of molecular self-polymerization is steadily accumulating [282]. In addition to collagen IV and laminin, minor local components such as perlecan and entactins are known to play crucial roles in the orchestration of matrix assembly and remodeling; herein, perlecan functions as a bridging stabilizer, whereas entactins largely serve as molecular adaptors or catalysts [384]. The BM composition and the functions of its macromolecules in embryonic development as well as in the homeostasis of adult tissues are increasingly being analyzed by structural investigations at atomic resolution and by recombinant techniques, opening the possibility of inducing distinct effects through changes in BM composition [272,341]. Investigations of mutated genes identified in inherited disorders and new insights obtained from gene-targeting studies will provide important information for the development of self-assembled biopolymer networks for tissue engineering with good biological compatibility as well as close chemical, structural and mechanical similarities to native ECMs [385–387].
4.2.4. Glycosaminoglycans
GAGs, including HA, heparin, heparin sulfate (HS) and CS, are linear, anionic and highly heterogeneous carbohydrate polymers composed of repeating disaccharide units, which are commonly a uronic acid component and a hexosamine (glucosamine or galactosamine). These polymers are ubiquitously present at the cell surface and in the ECM and may regulate matrix assembly and remodeling and cell–matrix and cell–cell interactions via interacting with various structural proteins (e.g., fibronectin and collagen) and signaling molecules (e.g., growth factors and chemokines) [388]. The supramolecular presentation of GAG chains, along with other cell surface or ECM molecules, is likely to be functionally important for cell adhesion, which is broadly applicable to the creation of multifunctional biomimetic surfaces in biomaterials [389]. The chemical characteristics of GAGs specifically enable them to be highly hydrated, endowing them with gelatinous properties or making them what has been termed a “ground substance” [322]. Except for HA, such chains are bound to a central protein, such as glypican, perlecan or syndecan, to form proteoglycans. In particular, heparin plays an important role in many biological processes via its interplay with various proteins, and hydrogels composed of heparin exhibit attractive properties, such as anticoagulant activity, growth factor binding and antiangiogenic and apoptotic effects, making them great candidates for emerging applications [390]. Whether the molecule is a heparin or CS proteoglycan is determined by the types of GAG side-chain residues, which are sulfated to different extents, depending on the location, source of production and physiological conditions; much of the proteoglycan heterogeneity may be attributed to the broad range of GAG sulfation patterns [288]. Because of their ionic properties, GAGs can absorb a mass of water, and this osmotic swelling confers compressive strength on an ECM product, within which the amount of GAGs present significantly depends on the decellularization methods applied for tissue processing. Additionally, sulfated GAGs are promising constituents for functional scaffolds because sulfate groups determine growth factor binding and thereby affect wound repair [391]. By immobilizing chemokines either in the ECM or on cell surfaces or by generating the stable haptotactic gradients required for directional cell migration under shear flow, GAGs also modulate the in vivo bioactivity of chemokines [392]. In this regard, positively charged chemokines not only bind to receptors whose different oligomeric forms can induce different but interrelated signaling responses but also interact with GAGs that are negatively charged. Mimicking this phenomenon has the potential to yield GAG-based biomatrices that induce desired cell activities. The degree of GAG sulfation within these matrices can be systematically manipulated via bioinspired alterations in the GAG content (increased sulfation from HA to CS to heparin) to regulate the sequestration of growth factor signals and, indeed, their subsequent function to influence cell fate within the biomatrix [393]. The strong interactions between chemokines and GAGs can protect chemokines from proteolysis and stabilize the formation of a large variety of chemokine oligomers and other structures that would not otherwise form in solution [394]. Thus, both the interaction of chemokines with GAGs (GAG binding) and the ability to form higher-order oligomers contribute significantly to the overall chemotactic function of specific chemokines [395]. Furthermore, interactions between GAGs and stromal cell-derived factor-1α (SDF-1α, or CXCL12) isoforms not only play a distinct role in the retention of hematopoietic stem cells (HSCs) in the bone marrow under homeostatic conditions but also contribute to stem cell recruitment and appropriate tissue revascularization after acute ischemia [392,396].
HA, also known as hyaluronan, is one of the primary components of the ECM and is present as high-molecular-weight chains. This linear GAG is synthesized by membrane-bound hyaluronan synthases and is composed of disaccharide units containing N-acetyl-d-glucosamine and glucuronic acid, which distinguish HA from other GAGs that are produced in the Golgi apparatus [397]. HA can be harvested from many tissues by enzymatic digestion or extraction and is increasingly being utilized in biomedical applications due to both its ability to serve as a blank slate and its biological activity. In particular, HA is commonly used to create hydrogel scaffolds for tissue engineering, which may in turn be applied for localized drug delivery purposes [398]. At the cellular level, high-molecular-weight HA and low-molecular-weight HA exhibit opposite effects on orchestrating cell function. In addition to inhibiting cell proliferation, high-molecular-weight HA is anti-angiogenic. For example, HA acts as a high-molecular-weight barrier that blocks endothelial cell migration and subsequent angiogenesis in the fetal development of rat follicles. However, cleaving the polymer into shorter fragments with hyaluronidase enables endothelial cells to migrate and activate angiogenesis [399]. HA is expressed on the BM of human sinusoidal endothelium and endosteum. Interestingly, SDF-1 is also constitutively expressed at high levels in these regions and plays a distinct role in maintaining the marrow HSC pool in a quiescent state. It has been suggested that as a major GAG component of bone marrow ECM, hyaluronan is an in vivo priming factor for the SDF-1-dependent transendothelial migration of human CD34+ stem/progenitor cells to sites with low CXCL12 concentrations and also contributes to the cells' final anchorage within specific niches in the BM [400]. It is now widely recognized that native HA exhibits a pro-survival effect on contacting cells by activation of cell anti-apoptotic Akt pathways [401] or by protection of the cells against toxic insults [402]. However, the HA-induced pro-survival effect on contacting cells is reversed when HA–receptor interactions are inhibited [403].
The design of HA-based hydrogel scaffolds to elicit highly controlled and tunable cell responses and behavior is a major area of interest in developing tissue engineering and regenerative medicine applications [398]. In particular, HA has been tested as a copolymer for silk fibroin (SF)-based biomaterials, allowing the combination of the biological characteristics of HA with the superior mechanical properties of SF. Following MSC seeding and in vitro culture, histological investigations of the constructs after a 3-week incubation revealed improved cellular penetration and ingrowth into SF/HA composites compared with plain SF materials. Furthermore, in vitro stem cell cultures on SF/HA composites in the presence of tissue-inductive stimuli showed more efficient tissue-forming potential in terms of GAG and collagen I/III gene expression compared with plain SF scaffolds, which were used as control materials [404]. Collectively, these findings indicate that HA may be an appropriate ECM biomaterial for use in revascularization techniques and may provide anti-apoptotic, protective functions while enhancing cell growth and differentiation [288].
Of note, during long-term in vitro culture, MSCs undergo cellular senescence, accompanied by a loss of cell migratory and homing abilities. It has been suggested that the migratory capability of ex vivo-expanded cells can be reversed if HA is used as an adjuvant supplement for cell cultures [405,406]. Because HA is a native element of cartilage, MSCs can interact with HA materials through cell surface receptors, leading to stem cell differentiation and, hence, cartilage formation [405]. Consequently, the incorporation of MSCs into HA-based biomaterials has been demonstrated to improve chondrogenic differentiation and cartilage-like ECM deposition. The use of chondrocytes in an HA material for the treatment of focal lesions of the articular cartilage of the knee has already been tested in clinical trials [407]. Additionally, recently, as a natural lubricant in the body, HA was used to enhance lubrication on tissue and biomaterial surfaces through a polymer-peptide surface-coating platform, offering a potential strategy to coat medical devices and to treat tissue-lubricating dysfunction [408]. In addition, HA hydrogels have been widely engineered and researched due to their biocompatibility and their ability to incorporate a wide variety of cues to modify cell-material interplay and to ultimately affect and adapt to greater control over the behavior of cells [398]. When the effect of HA on the physicochemical characteristics of collagen–gelatin composites and its cytocompatibility were investigated, the addition of HA at less than 15% to composites composed of collagen and gelatin (ratio of 1:9) resulted only in incremental improvement in the physical structure and cytocompatibility of the resultant biomaterials with a human intestinal epithelial cell line. However, increasing the proportion of HA in the scaffolds to 25% resulted in a dramatic improvement in the scaffolds' properties, including their support of cell adhesion, growth and viability, as well as their structural characteristics [409]. Recent studies have also demonstrated that incorporating various chemical, mechanical and spatial cues into HA hydrogels can lead to tuned unicellular and multicellular responses. The ability to control other HA gel-specific cellular functions, such as cell-mediated hydrogel degradation, is useful in designing systems via multipronged approaches that offer a range of applications. As research on HA hydrogels continues to progress, more intelligent systems with clearer, more cell-directed purposes will be developed [398].
HS, a carbohydrate–protein complex, is a highly sulfated proteoglycan that contains heparin chains or glucosamine and glucuronic/iduronic acid repeating disaccharide units. HS proteoglycans are major components of the ECM that are required for the insolubility, self-assembly and barrier characteristics of BMs. As a component of endothelial cell membranes and ECMs, HS proteoglycans are involved in many critical functions of the endothelium and of antigen-presenting cells [410]. A potent cell-mobilizing and cell-recruiting molecule used in biomaterial design, SDF-1, is well recognized to be bound to cell surfaces by HS proteoglycans and is believed to significantly affect the chemokine's biological properties, yet its role remains largely unexplored [411,412]. Interactions with HS proteoglycans are supposed to provide chemokines with the capacity to bind to the ECM and cell surface to trigger cell signaling, through which HS plays an important role in the functions of CXCL12 isoforms both during homeostasis and in physiopathological settings [396]. The polysaccharide side chains of HS proteoglycans differ in the structure and composition of their sulfated domains among various tissue types, resulting in selective protein binding. By selective accumulation on the cell's macro-molecular GAG coating, namely, the cell glycocalyx, HS may also enhance the specificity of chemokine function [392]. For example, when cellular HS from bone marrow endothelial cells and human umbilical vein endothelial cells is characterized, differences in the glycocalyx GAG pattern and in SDF-1 binding between the two cell types are observed. The highly sulfated domains present in HS chains from bone marrow endothelial cells are required to deliver chemokines that coax stem cells to roll during transendothelial migration. These proteoglycans are also critical for the binding of chemokines (e.g., SDF-1) and the adhesion of hematopoietic progenitor cells following cell transplantation [413]. Furthermore, soluble HS was observed to enhance SDF-1-driven migration in a dose-dependent manner, indicating that SDF-1 presentation may be optimized by using SDF-1–HS complexes instead of SDF-1 alone. These findings imply that in the subendothelial matrix, proteoglycans can create an SDF-1 gradient that directs hematopoietic progenitor cell homing to the bone marrow [414]. It is also believed that this strategy for SDF-1 immobilization can be used to recruit progenitor cells toward a targeted place (e.g., ischemic muscle), where angiogenic cells are required for the restoration of perfusion [415]. In addition to SDF-1, mounting evidence is also revealing the molecular mechanisms by which HS proteoglycans bind other cytokines, including, but not limited to, VEGFs, platelet-derived growth factors (PDGFs) and fibroblast growth factors (FGFs), to modulate progenitor cell migration, recruitment and angiogenesis. This evidence may result in a novel means to develop strategies that implement HS-induced control of cell fate commitments [416]. Following demonstration of the necessity of HS for the growth factor-stimulated differentiation of osteoprogenitor cells, the significance of HS for endogenous FGF-2 signaling (as a coreceptor) was also confirmed, suggesting that purified GAGs maybe promising alternatives to certain growth factors for enhancing the ex vivo proliferation and differentiation of MSCs [417]. Moreover, it is suggested that HS chains exhibit both gender- and tissue-specific diversity in biochemical composition that straightforwardly reflect their biological activities, as demonstrated by the potential benefit of gender-specific liver HS in manipulating human MSC properties, including cell expansion, multipotentiality and subsequent matrix production [418].
Considering their role in endothelial cell and skeletal muscle proliferation and progenitor cell mobilization, HS proteoglycans can play important roles in recruiting angiogenic stem cells to a site of ischemia for tissue repair [419]. Notably, a consistent body of in vivo evidence suggests the necessity of a specific HS proteoglycan for the successful regeneration of skeletal muscles, a highly regulated and complex process that involves muscle precursor proliferation and division and most likely demands the synergism of a vast wealth of heparin-binding growth factors, such as hepatocyte growth factor (HGF), FGFs and TGF-β [420]. Although a broad range of heparin-based biomaterials has been developed and used in the clinic, materials must be designed to maintain the desired level of stability in vivo and to be effectively eliminated from the body, without the formation of undesired metabolites. As these challenges are addressed, heparin-based materials are likely to have an increasingly significant impact on the management of various diseases and damaged tissues [390].
CS is a ubiquitous component of proteoglycans that is present both in the ECM and at the cell surface in various tissues. Arranged in an alternating unbranched sequence, CS is composed of hexuronic acid (d-glucuronic acid) and hexosamine (d-galactosamine) units. Functionally, CS is largely covalently bound to core proteins (i.e., proteoglycans), such that it displays specific interplay with proteoglycans in cell proliferation, differentiation and migration decisions. As a main component of the brain ECM, CS proteoglycans are involved in neural development; axon pathfinding and guidance; and post-injury nerve regeneration, plasticity and rehabilitation in the nervous system [421]. CS is well known as an integral cartilage component in addition to HA, whose physical and mechanical strength is due to its CS component. CS also has profound effects on proliferative and adhesive phenotypes in MSCs and in the chondrogenesis of various stem cells and chondrocytes [273]. To this end, CS is being extensively examined for use in cartilage tissue engineering approaches. Hydrogels containing CS may establish a niche-mimicking microenvironment that plays a morphogenetic role in directing cells toward a chondrogenic phenotype in terms of the temporal pattern of cartilage-specific gene expression and in subsequently promoting new matrix deposition during MSC chondrogenesis. In addition, this microenvironment may promote the inhibition of further MSC differentiation into hypertrophic chondrocytes [422].
In non-weight-bearing defects in a rat model, HA hydrogels were also demonstrated to support cartilage regeneration by human ESCs and to promote the integration of neocartilage into surrounding native cartilage [423]. Recently, the encapsulation of human chondrocytes in gelatin-methacrylamide-based hydrogels demonstrated that with the addition of a relatively small proportion of photocrosslinkable HA-methacrylate and, to a lesser extent, CS-methacrylate, chondrogenesis and mechanical properties may be potentiated for potential cartilage tissue regeneration. The encapsulated chondrocytes remain viable for as long as 8 weeks in culture, and the incorporation of HA-methacrylate into gelatin-methacrylamide cell constructs enhances chondrogenesis and facilitates matrix distribution, whereas the incorporation of CS-methacrylate enhances chondrocyte differentiation in certain ways. These phenomena highlight the potential for multiple-component photocrosslinkable hydrogels to enhance chondrocyte behavior and facilitate new matrix formation by incorporating GAGs into hydrogels [424]. Recent evidence suggests that biomaterials comprising a mixture of collagen, HA and CS can sequester and/or activate growth factors and thereby establish an even better biomimetic environment that mimics natural cartilage ECM, enhancing chondrogenesis and promoting cartilage-specific matrix deposition among loaded cells [425]. Therefore, the combination of collagen with HA and CS has the potential to result in biomaterials with appropriate scaffolding properties for cartilage bioengineering and hence warrants further scientific exploration [426]. This combination has also been successful in cell delivery and implantation within target ischemic tissue using a collagen I/CS tissue-engineered matrix [427]. Other such biomaterials with well-defined chemical, topographical, and mechanical cues and even gradients of these physicochemical cues may also enhance endogenous progenitor cell homing and engrafting to sites of ischemia [428] and may serve as novel substrates for human circulating angiogenic cells to augment angiogenesis for the revascularization of ischemic and infarcted tissue [290]. However, native GAGs derived from human tissue are heterogeneous and structurally complex, and specific GAG moieties have been demonstrated to trigger specific cellular responses during cell division, motility and migration. Although the specific structure–function relationships are difficult to clarify, the identification and use of selected sulfation patterns and chain lengths of GAG subfractions to enhance cell fate determination is an exciting new avenue for devising new biomaterials for target applications [429].
4.2.5. Fibronectin
Fibronectin, an elongated 45 nm protein, consists of 2 nearly identical outer globular domains (subunits of approximately 250 kDa), and an α-helical coiled-coil segment is covalently connected to each end of the central domain (C-terminus) by a pair of disulfide bonds. As a ubiquitous and important ECM protein, fibronectin is a multifunctional component residing in the SM that is known to regulate cell behavior via its cell-binding site and related synergy sites. In addition to being very crucial for vertebrate development, fibronectin regulates diverse cellular functions (e.g., cell adhesion, growth, migration and differentiation) among multiple cell types, as confirmed by the early embryonic lethality of targeted inactivation of the fibronectin gene in mice [430]. Based on the biological activity of its several modules, this glycoprotein may serve as a substrate for cell attachment and adhesion. Because of the numerous responses that fibronectin can elicit from diverse cell types, many investigations have opted to procure and apply specific bioactive domains that have been identified in the fibronectin molecule. Of note is the arginine–glycine–aspartic acid (RGD) tripeptide, or arginine–glycine–aspartic acid, in the tenth Fn3 module, which participates in multiple cell fate decisions [431]. The modification of 3D scaffolding materials with RGD sequences can increase the degree to which and rate at which MSCs migrate into and populate the constructs [432]. A 3D cell culture system was created and used to assess the effects of both substrate stiffness and integrin binding density on the morphology of human MSCs and on reconstruction of the microvascular network of endothelial cells. When endothelial cells were encapsulated into 3D hydrogel scaffolds without binding sites, no network formation and little cell elongation occurred, regardless of the matrix stiffness. However, hydrogels containing RGD binding sites induced robust microvascular network formation, the extent of which was inversely proportional to the matrix stiffness. In addition, the presence of the matrices attracted an increased number of MSCs and resulted in longer cellular projections at higher stiffness. In contrast, the absence of RGD induced round morphology at all rigidities. Overall, these findings reveal the potential to control both the binding site density and the substrate stiffness within 3D cell-populated hydrogels and demonstrate the crucial influence of both adhesion and stiffness on cell type-specific cellular behaviors [433].
Fibronectin-coated surfaces are important in bioengineering and other approaches involving cell contact; however, the random distribution of molecular orientations resulting from many immobilization strategies represents a problem. To address this issue, one potential strategy is the deposition of layers of oriented fibronectin, which could enhance the availability of the cell-binding sites in the layer. It was found that human umbilical vein endothelial cells spread much faster and in a more spherically symmetrical manner on an oriented fibronectin layer (i.e., in the presence of bound monoclonal antibodies) compared with a control fibronectin layer (i.e., in the absence of immobilized antibodies) [434].
Of note, the BM of the corneal epithelium presents biophysical cues in the form of compliance and topography, which can modulate cells' phenotypes and behaviors and their nuclei based on the presence of surface-associated ECM proteins [435]. Recently, the effects of a combination of exogenous fibronectin-collagen coatings with substratum topography on cytoskeletal architectures and on the migration and alignment of immortalized corneal epithelial cells were investigated. It was observed that in the absence of a fibronectin-collagen coating, a considerably greater number of cells aligned parallel with the long axis of the underlying anisotropically ordered topographic features, but their migratory capability was impaired. In addition, the surface area, orientation and elongation of cytoskeletal elements were variously affected by the absence or presence of fibronectin-collagen, suggesting that the impacts of topographic cues on cell behaviors are regulated by the presence of surface-associated ECM proteins [436].
Fibronectin has a remarkably broad variety of functional bioactivities, in addition to binding to cell surface integrins. Fibronectin binds to numerous biologically significant biomolecules, including heparin, fibrin and collagen/gelatin. These interactions are modulated by several distinct functional and structural domains, which have been determined by recombinant DNA analyses or proteolytic fragmentation [437]. As a provisional matrix during wound healing and tissue repair, fibrin is highly promiscuous in its growth factor-binding capacity, which may be one of fibrin's main physiological functions, and the coordinated interplay between the matrix and growth factors plays a crucial and ubiquitous role in regulating tissue regeneration. The reproduction of growth factor-ECM interactions within a fibrin-mimetic matrix could be clinically useful and has the significant benefit of a more direct regulatory pathway in relation to chemical synthesis, in contrast to human-sourced material [438]. Notably, fibronectin facilitates the migration, adhesion and proliferation of MSCs, but not osteogenic differentiation, whereas fibrinogen enhances cell proliferation and adhesion, but not migration. Consequently, the integrin expression pattern of MSCs on specific matrix components has been associated with cell fate decisions [273]. Similarly, RGD-modified fibronectin hydrogels have been demonstrated to offer anti-apoptotic properties to cells that migrate into the scaffolds. Apparent apoptosis was shown in unmodified scaffolds, indicating that cell adhesion via the fibronectin RGD sequence is one of the important methods for cell preservation and survival. In this respect, the immobilization of the RGD peptide on non-ECM component-based biomaterials, such as macroporous alginate scaffolds, has also proven to be a necessary parameter in cardiac tissue engineering, contributing to the better preservation of regenerated tissue in culture and the formation of functional cardiac muscle tissue [439]. These results suggest that RGD modification is an omnipotent strategy for the design of regenerative biomaterials. Recently, the incorporation of fibronectin into multilayer elastin-like protein biomaterials was observed to enhance overall cytocompatibility for tissue engineering; the high cell viability in the resultant 3D constructs indicated the applicability of fibronectin to the creation of resilient, strong manmade vessels and other soft-tissue replacements [440]. It would not be surprising if, in the future, fibronectin attracts increasingly concentrated attention in the design of biomaterial strategies.
4.2.6. Elastin
Elastin is a hydrophobic macromolecule of the ECM that is found throughout the vertebrate kingdom, including in humans. This macromolecule possesses a unique chemical composition that is rich in proline, glycine and hydrophobic amino acids, consonant with its characteristic physical properties [441]. The presence of highly crosslinked elastic fibers in the extracellular space, whose main components are elastin and microfibrils, allows a range of tissues, such as the large arteries, the ligaments, the dermis, the tendons, elastic cartilage and the lung parenchyma, to have the required elastic ability to transiently stretch [322,442]. Tropoelastin, a 60–72 kDa biosynthetic precursor form of the elastin protein that consists of hydrophobic domains (mainly valine, glycine and proline) and crosslinked hydrophilic domains (mainly lysine and alanine), is synthesized by cells into the extracellular space, where the polymerization of tropoelastin into a fibrillar biomatrix occurs in a process called elastogenesis [443]. The correct crosslinking and deposition of secreted tropoelastin as well as its temporal and spatial arrangement are thought to be vital steps in elastic fiber formation. Beginning with tethering to the elastogenic cell surface, water-soluble tropoelastin interacts with multiple proteins found in or colocalized with microfibrils. Subsequently, the macromolecule undergoes the complex stepwise process of elastogenesis (crosslinking, self-association and maturation) and finally aggregates into organized spheres for self-assembly and incorporation into growing elastic fibers in a rubber- or sheet-like network [444–446]. Based on ultrastructural and biochemical analyses, these fibers have been revealed to be primarily composed of two distinct types of small segments that alternate along a polypeptide chain. These segments are an abundant amorphous segment that is α-helix rich and composed of alanine and lysine chains, which are where the crosslinks form between the molecules, and a 10–12 nm microfibrillar segment that is mainly located around the periphery of the amorphous component and composed of highly insoluble amorphous segments that are responsible for the elastic properties [447]. The formation of tetravalent bonding between elastin and 3 lysine-derived products, namely, lysinonorleucine, desmosine and isodesmosine, via a crosslinking reaction leads to the polymerization of tropoelastin into insoluble elastin [444]. Although it is likely that the random coil structure of the molecules crosslinked into a network offers the ability to stretch similarly to a rubber band, the contribution of the elastin fiber structural conformation to the functional elasticity of the fibers remains unclear [447]. As another main component of elastic fibers, the microfibril segments found either in association with elastin or independently contain a variety of distinct and different glycoproteins [448]. Fibrillins, a family of such microfibril resident proteins, were first harvested from the media of human fibroblast cell cultures; its members precede elastin in developing tissues and yield a matrix scaffold to which elastin fibers subsequently attach [447,449]. Fibrillin is a 350 kDa protein that is periodically arrayed along individual microfibrils that maybe aligned within bundles. Type VI collagen interweaving among large, banded collagen fibers is not associated with the microfibril system identified by the presence of fibrillin, although it has been proposed as a possible microfibrillar component [449].
In major arteries, elastin is secreted and synthesized by vascular smooth muscle cells. Elastin is the most prominent ECM protein deposited in the arterial wall, comprising up to 50% of the nonhydrated mass of the vessel [450]. The elastic matrix imparts vessel integrity, extensibility and arterial elasticity with regard to the native structural configurations of vascular tissues and their ability to recoil after stretch under pulsatile flow. Via biomechanical transduction, elastin plays an equally critical role in regulating cell signaling pathways (e.g., those of smooth muscle cells and luminal endothelial cells in the arterial wall) involved in morphogenesis, inflammation and injury response [451,452]. Moreover, it has been demonstrated that elastin is an important autocrine factor that ensures vascular homeostasis via a combination of biologic signaling and biomechanical support. The elastic lamina specifically alternates with smooth muscle rings due to elastin-smooth muscle cell interaction to promote cellular synthesis and the assembly of elastic matrix superstructures, forming a flexible, strong part of the arterial wall [450,453].
For a tissue-engineered replacement to maintain vascular homeostasis, it is therefore important to ensure that the elastic matrix superstructures unique to its target tissue can be generated. However, the use of cells, scaffolds and growth factors based on strategies such as dynamic stretch and contact guidance to produce the vascular elastic matrix is particularly difficult because adult vascular cells inherently possess a very poor ability to synthesize elastin precursors. Replication of the process during development such that elastin precursors organize into the mature elastic matrix in terms of its structures and biocomplexity represents an even more challenging task [451]. However, the incorporation of elastin into engineered scaffolds is not a typical choice due to a lack of access to pure, homogeneous human elastin, although a limited yield of natural elastin can be extracted from tissues by harsh alkaline treatments [454]. Recently, it has been suggested that the crosslinking of tropoelastin in the developing elastin matrix may be simulated using short elastin-mimetic peptide sequences engineered to mimic the active motifs of human elastin. The potential for increased cell adhesion with appropriate cell-binding motifs and the engineering of elastin chains into synthetic polymer biomaterials is a new concept for elastin tissue engineering [455]. With remarkable advances in the field of genetic engineering, the design and biosynthesis of an ECM analog using human polypeptide sequences as its building blocks is possible [456]. To this end, recombinant human tropoelastin, the monomeric precursor of elastin, can be chemically crosslinked to form a polymer with unique properties, termed a recombinant human elastin-like polymer (rhELP); both the full-length monomer and elastin-like polypeptides can be applied to yield biomaterials with physical properties resembling those of native polymeric elastin [442,457–459]. In particular, artificial elastomeric proteins that mimic the molecular architecture of titin, a giant muscle protein in intact myofibrils that governs the passive elasticity of muscle, have been used to develop new muscle-mimetic biomaterials. Following photochemical crosslinking into solid form, the resulting biomaterial may behave as a shock-absorber-like material at high strain by effectively dissipating energy and as a rubber-like material showing high resilience at low strain. By adjusting the composition of the elastomeric proteins, the mechanical properties of such a biomaterial can be fine-tuned to develop biomaterials that mimic various muscle types [441]. Modeled after the naturally occurring tropoelastin, rhELPs have emerged as inspired synthetic biopolymers for the engineering of compliant, resilient soft tissues due to these polymers' non-immunogenic, biocompatible and biodegradable properties [460,461]. For example, based on recombinant DNA technology, recombinant silk-elastin polymers are engineered to be composed of tandem repeats of silk and elastin units. By alteration of the composition and length of these repeats, the mechanical properties of the biopolymers are tunable, meaning that these properties may be controlled for specific tissue engineering applications [462]. Notably, their mechanical stiffness, chemical composition and even fate within cells can also be controlled at the gene level [461]. The use of an rhELP containing the RGD tripeptide sequence can generate a 3D tissue equivalent derived from human oral epithelial cells and lamina propria fibroblasts. This tissue equivalent may contain as many highly proliferative and self-renewing cells as the native tissue itself and displays mechanical strength, stiffness and resilience resembling those of native tissue [463]. Moreover, recent evidence suggests that porous scaffolds with surface elastin and poly-l-lysine can maintain active chondrocytic proliferation and ECM secretion by cryopreserved chondrocytes [464]. Similarly, coating PLGA biomaterials with an elastin-like polypeptide has been demonstrated to improve neural progenitor cell adhesion and proliferation in an elastin concentration-dependent manner, and in combination with retinoic acid, these scaffolds may stimulate the differentiation of these progenitor cells into neuronal and astroglial lineages [465]. Additionally, although injectable hydrogels for tissue engineering biomaterials generally lack mechanical strength, synthetic human elastin scaffolds reinforced with collagen microfibers or injectable hydrogels modified by elastin incorporation have demonstrated tunable gelation and biodegradation properties, tailorable porosity and pore size, and favorable mechanical characteristics and/or structural stability, favoring various biomedical applications [466–469]. Interestingly, nanocarriers and injectable hydrogels based on silk-elastin-like proteins, a family of genetically engineered recombinant protein polymers, may possess properties allowing controlled therapeutic release due to their rational design, tunable structure–function relationships, stimuli-responsive features and target specificity [470]. Despite its growing utility as a scaffolding material in tissue engineering and its well-recognized function in the vasculature, the hemocompatibility of elastin is often overlooked. However, as elastin scaffolds and coatings display increased hemocompatibility [465,469], the potential of decellularized elastin and arterial elastin as nonthrombogenic biomaterials has begun to be recognized [452]. Thorough comprehension of developmental elastogenesis and the subsequent ability to mimic the spatiotemporal alterations that occur during that phase in the cellular environment will enable us to design elastogenic therapies to restore homeostasis in de-elasticized vessels and to develop clinically translatable elastic vascular tissue grafts [451].
4.2.7. ECM assemblies as scaffold building blocks
The ECM biomacromolecules, such as collagens, GAGs, laminins, entactin, fibronectin and elastin, involved in the matrix and the structure, organization and manner in which they are assembled determine the biochemical and biophysical properties of the resultant ECM [299]. The use of ECM assemblies directly or indirectly derived from human tissues/organs for tissue engineering applications opens a new route for scaffolding biomaterial design. On the one hand, efforts should in fact be made to establish or optimize new or combined processing approaches to yield robust biopolymers based on human ECM components that are likely to help to reconcile commercial and clinical pressures on regenerative medicine [471]. On the other hand, new insights into the physical and molecular information coded within the human ECM milieu are already informing the redesign of the “next generation” of advanced cell-instructive scaffolds for the clinical management of recalcitrant chronic wounds [20]. Investigations in highly regenerative organisms have revealed a specialized formulation of powerful ECM molecules that dynamically respond to cellular and soluble components to dictate the distribution and function of tissue-specific cells and to support repair and regeneration while avoiding acellular fibrotic scar tissue formation; several of these molecules have already been adopted in scaffolding science, whereas many others remain unidentified. By comparing matrix contexts that feature scarring resulting from fibrotic repair with matrices conducive to scar-less healing and full regeneration, biomaterials modified using specific ECM components were found to significantly enhance functional outcomes upon application [472]. This phenomenon is spurring interest in advancing our knowledge of tissue and ECM science, thus instructing the intentional design of porous biopolymer-based scaffolds incorporating ECM assemblies that constitute controlled morphologies at various scales (e.g., a combination of pores or micro- and nano-elements). These scaffolds comprise a variety of structural and matrix proteins that are spatially organized and have the ability to bind relevant informative signals, such as growth factors and cell homing agents, in a tailorable manner. However, exact control over the sequence composition of these biomacromolecules and their self-assembled superstructures to generate multitudes of well-defined protein-based biohybrid materials poses a major challenge in bionanotechnology and materials science [52,473]. Based on a careful review of the literature, a step forward could involve moving from the reduction of ECM into short functional domains and biomacromolecules for biomaterial functionalization to the exploration of basic material chemistries that dictate cell fate commitments and more closely reproduce the dynamically evolving in vivo milieu occurring in the natural ECM [92,282,299,474]. This field is wide open for new creative scientists to make certain approaches practical for actual clinical use.
4.3. Decellularized ECMs for biomaterials
Through the use of synthetic biomaterials in association with stem cells and/or growth factors, tissue engineering has adopted an interdisciplinary approach to the design of new biological therapeutics, and recent advances in this field have enabled the creation of certain functional tissue replacements in the laboratory [4]. Early attempts at engineering certain tissues (e.g., skin and cartilage) have achieved considerable success thanks to their simple architectures, which subsequently fueled enthusiasm for applying these same or similar approaches to the fabrication other complex tissues and organs [3]. However, many of these artificial scaffold-based constructs, at least in part, fail to match the sophisticated properties of their native counterparts in terms of structure, dynamics, biocompatibility and function. Clearly, we have thus far been unable to develop a cell-friendly bioactive material template with an architecture similar to that of a complex native tissue/organ, which involves an extensive vasculature system and contains intricate information within its physical and chemical structures [5,6]. Recent advances in tissue/organ decellularization offer acellular human-derived biomaterials that preserve the natural architecture from the whole-organ level to the microstructural scale and even down to the nanoscale [287,475]. The use of various forms of ECM scaffolds, with recent implementations including whole organs, derived from decellularized allogenic tissues/organs that retain structurally organized entities such as collagen, GAGs and fibronectin, is increasingly routine, enabling natural templates that accommodate tissue engineering and regenerative approaches [266,274] (Fig. 12). Even with a lack of living cellular components, the decellularized ECM may be regarded as a physiological depot for various signaling molecules, which retain their functionality, at least in part. Upon application, these bioactive agents are released and play their natural roles in cell regulation, thus offering the specific ECM the information necessary to conduct repair and regeneration [102]. The gentle removal of cells from human tissue or organs can leave behind a “footprint” within the ECM scaffold that directs cell ingrowth and repopulation; the biological components remaining in the intact matrix may specifically enhance cell activities such as adhesion, proliferation, migration and differentiation in a way that reflects the biological identity and functional requisites of the original tissues [274]. Specifically, human-derived decellularized matrices can also be considered biologically active scaffolds capable of recruiting endogenous host stem/progenitor cells and stimulating in vivo cell proliferation and differentiation toward the formation of a biointegrated tissue [476]. Decellularized ECM has thus attracted increased attention in tissue engineering as an “off-the-shelf” and immune-compatible biomaterial that may be used to create tissue-engineered alternatives to living tissue grafts for tissue replacement and repair [477,478].
Fig. 12.
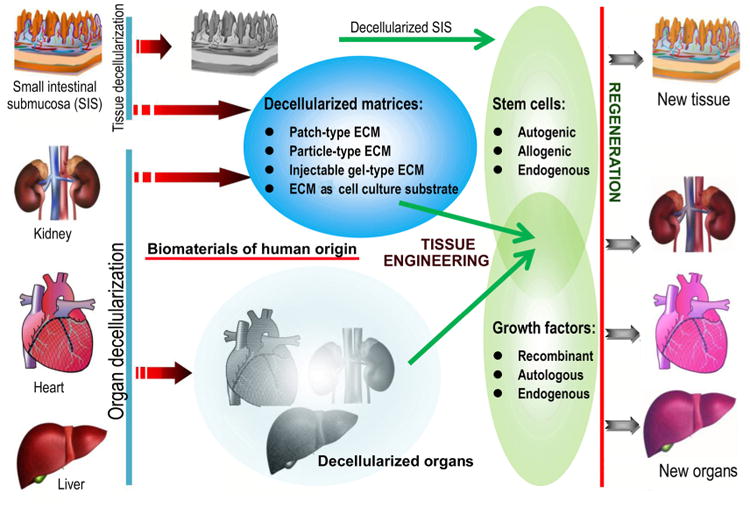
Decellularized matrices from tissues (e.g., small intestinal submucosa (SIS)) or organs (e.g., kidney, heart and liver) that have native-like extracellular matrix (ECM) microstructures, compositions and biomechanical properties (schematic is not to scale). These decellularized ECMs may maintain the shapes of the original tissues and organs when used as scaffolding materials in tissue engineering approaches for new tissue/organ regeneration. Alternatively, decellularized matrices derived from tissues and organs can be made into different types, such as a patch or particle, for tissue engineering scaffolding biomaterials or can be designed as an injectable gel for cell culture substrates [477].
4.3.1. Rationale and methods for decellularization
Decellularization is the process of stripping a donor tissue or organ of its resident cells while maintaining the native ultrastructure, ECM components and chemical cues that are essential for cell preservation and homeostasis in the remaining matrix template [475]. The removal of cells and a large proportion of the major histocompatibility complex from the ECM templates eliminates the inflammatory response, the foreign-body reaction and the potential for immune rejection and thus favors repopulation by new cells [274,479]. The effectiveness of a technique for the decellularization of a specific tissue or organ is dictated by many factors, such as tissue organization, cell density, the biological and geometric properties required in the post-processed product and the targeted tissue engineering and clinical uses (Fig. 13) [298]. Because human ECM molecules are highly conserved among tissues and organs, antigenic epitopes must be completely removed from intra- and extracellular components when preparing decellularized scaffolds. If this removal can be achieved, then adverse immunological responses can theoretically be circumvented [274,288]. Decellularized ECM is expected to direct cellular activities and regenerative events of breathtaking complexity not only via specific “organomorphic” structures but also via the physiological involvement of a vast wealth of regulatory factors in a physically and mechanically appropriate microenvironment [480,481]. Another prerequisite is the preservation of the complex composition and 3D ultrastructure of decellularized ECM and the activity of its functional components. Therefore, as mentioned previously in this review, the general purpose of all proposed and established decellularization methods is to minimize the cellular and nuclear materials in the matrix as efficiently as possible, while avoiding any potentially adverse impacts on the biological activity, composition and mechanical integrity of the ECM product [482]. Unfortunately, no decellularization methodology is absolutely effective in removing all cellular components and DNA materials from matrix constituents (1 mg dry weight of matrix material contains less than 50 ng of dsDNA), and in terms of the biochemical and structural properties of the resultant product, all decellularization strategies are highly variable in their results [275,483]. Moreover, long periods of decellularizing, as well as certain detergents, can adversely influence GAG and collagen components and then ECM antigenicity and/or integrity, resulting in both disruption of the construction and potential loss of the surface composition and structure [484,485].
Fig. 13.
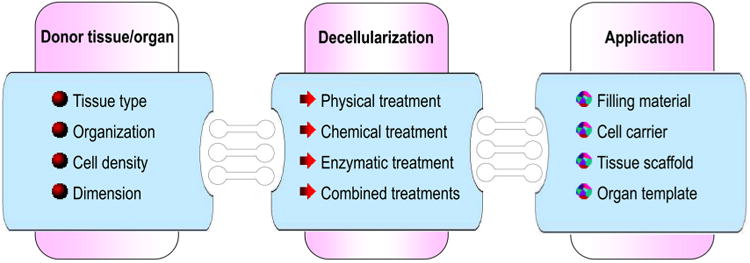
The production of extracellular matrix (ECM) products for targeted biomedical use is influenced by many factors, including the properties of the donor tissue or organ (e.g., tissue type, architecture, cellularity and dimension), the method, agent and protocol for decellularization (e.g., chemical, enzymatic and physical treatments) and the desired biological and geometric properties of the post-processed product. It is worth noting that every cell decellularization treatment will alter the ECM composition, damage the biochemical features and disrupt the native ultrastructure and architecture to some degree; the selection of an appropriate strategy for the decellularization of a particular tissue/organ for a target application is important to minimize rather than to completely avoid these undesirable effects [274].
The efficiency of cell elimination from a tissue is determined by not only the characteristics of the tissue and its origin but also the specific physical, chemical and enzymatic strategies that are applied for decellularization [486]. Decellularization strategies can be reasonably chosen if the principles of the disruptive process are thoroughly understood and contemplated [275,485,487]. The structures and arrangement of the various ECM proteins in the resulting acellular matrices are largely conserved by recent new technologies for decellularization, resulting in general retention of the mechanical properties of the original tissue (reviewed in Ref. [274]) (Fig. 14). The post-processed matrix may enable efficient cell reseeding and offer biomechanical strength and structural integrity for new tissue formation. For example, retrograde or antegrade perfusion has been applied as a method for whole-organ decellularization, in which the 3D architecture of the organ is largely preserved [93,486]. The decellularized matrices maintain the shape of the original organ and can be either directly used as scaffolding materials in tissue engineering approaches for organ regeneration or made into different types (e.g., patch, particle or gel) of tissue engineering scaffolding biomaterials [488].
Fig. 14.
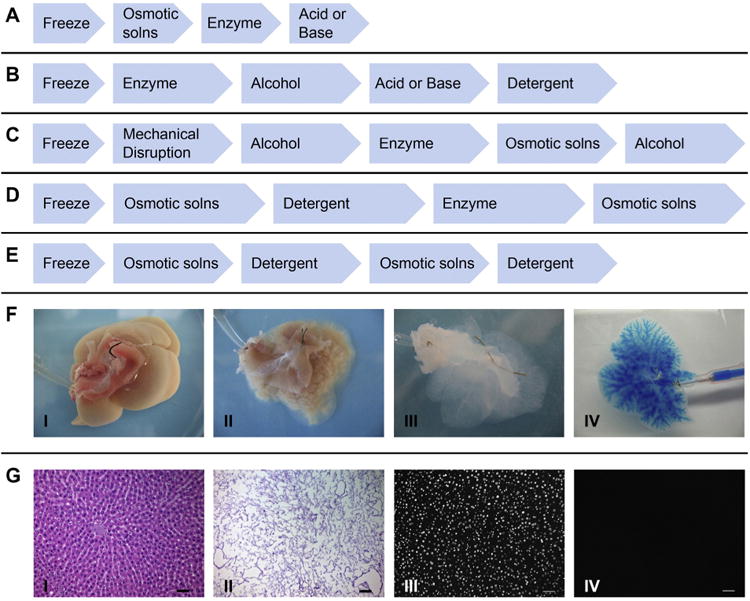
Representative decellularization protocols for (A) thin laminates (e.g., intestine, pericardium, amnion and urinary bladder); (B) thicker laminates (e.g., dermis); (C) amorphous, fatty tissues (e.g., adipose tissue, pancreas and brain); (D) composite tissues (e.g., trachea and urogenital tract) or whole simple organs (e.g., bladder and intestine); and (E) whole vital organs (e.g., heart, lung and liver). (F) Representative images of the gross appearance of an intact rat liver before (I), during (II), after (III) decellularization and of a decellularized liver following blue dye perfusion (IV). (G) Representative photomicrographs (scale bars are 50 μm) of hematoxylin-eosin staining of a native rat liver (I) and a decellularized liver-extracellular matrix (ECM) (II) or fluorescent staining with DAPI (4′,6-diamidino-2-phenylindole) in a native rat liver (III) and liver-ECM (IV).
Source: [274], Copyright 2011, Reproduced with permission from Elsevier Ltd.
Biochemical methodologies for tissue/organ decellularization include solvent extraction; osmotic shock; acid/alkaline treatments; ionic and nonionic detergent treatments; and enzymatic digestion with lipase, proteases, DNase and RNase. Recently, the pH of certain decellularization solutions was found to influence ECM retention (e.g., elastin, fibronectin and laminin) and cell-removal efficacy of the resulting product [489,490]. Direct force can also be used to aid in tissue decellularization. Commonly, biochemical agents are employed in combination with physical techniques to lyse cells, and the cell remnants are then removed by rinsing [298]. Of course, any biochemical procedure applied to remove cellular and nuclear materials may also slightly damage or alter the native 3D architecture of the resulting ECM template, and thus, during the decellularization process, achieving a balance between chemical and physical treatments is indispensable [274]. The optimal protocol for the use of agents for decellularization is determined by the characteristics and functions of the detergents commonly being applied, the thickness and density of the tissue targeted for decellularization and the intended use of the decellularized ECM in tissue engineering and reconstructive surgery [274,489] (Fig. 14). Each of these treatments can disrupt the tissue ultrastructure, biochemical composition and mechanical behavior of the end-product to a certain degree, which in turn affects host responses to the ECM scaffold [486]. These frequently used decellularization techniques and their impact on the structure and composition of the resulting ECMs have been reviewed elsewhere [274]. To simplify the cell removal process, undesirable excess tissue is generally removed prior to using decellularization agents. However, much attention should be given to retention of the main structure and ECM components, such as the BM. For thin tissue laminates, such as intestine, urinary bladder, amnion or pericardium, the most frequently applied decellularization technique is freezing and thawing [269]. Undesirable layers, such as submucosa or muscle, are first mechanically removed, and the resulting tissue is briefly exposed to easily removed acids or detergents for a relatively short time, followed by rinsing. More extensive biochemical exposure and longer rinse times are required for thicker tissue laminates, such as dermis or cardiac muscle tissue (Fig. 14). In contrast, the adjuvant use of lipid solvents such as alcohols is required for amorphous tissues and organs, such as adipose tissue, pancreas and brain (reviewed in Ref. [274]).
Although a wide range of decellularization techniques for both tissue parts and whole organs have been established and successfully used in many biomedical applications, routine protocols are inadequate for yielding an intact, elegant scaffolding ECM for the targeted tissue/organ that can then be revitalized by repopulation with ex vivo-expanded cells. By making educated decisions about the techniques and agents used during processing, the preservation of ECM bioactivity and integrity during tissue decellularization can be optimized to produce an application-specific standard product [298,487]. However, there is no established “gold standard” protocol for decellularization thus far, and scientists must therefore select an effective technique or different technique combinations based on the requirements for a targeted tissue/organ as well as continually develop new techniques to refine these protocols [475]. Not surprisingly, the utility of apoptosis as a decellularization method goes beyond the production of ECM materials with improved performance. Thanks to the potential to specifically target the cellular component of a tissue, the deliberate activation of programmed cell death is expected to better maintain the structural, biochemical and/or biomechanical features of the decellularized ECM [491]. In principle, by correlating regenerative potential with a particular ECM composition, this concept could also provide the unprecedented potential to observe the properties of decellularized but theoretically intact ECM and to identify a set of signals necessary to elicit specific functions. The identification of such a relationship would then subsequently allow a transition from the paradigm of decellularized tissue-engineered constructs to entirely synthetic scaffolding biomaterials devised to contain the sufficient and critical set of cues needed for specific tissue/organ regeneration [491].
Regardless of which decellularization method is used, cell residues remaining in ECM materials should be accurately assessed using a quantitative definition because of the expanding list of clinical applications and the rapid diversification of both decellularization techniques and source tissues [298]. It is reasonable to establish standards for tissue/organ decellularization based on readily determined quantitative criteria for the remaining cell remnants within ECM biomaterials, additional data related to the host tissue response upon the in vivo transplantation of these biological scaffolds and the observed regenerative capacity of a decellularized but theoretically intact ECM with a specific composition [274].The concept would also provide the unprecedented potential to evaluate the impacts of certain cell remnants and nuclear materials on the host response and to identify a set of cues critical to elicit certain functions [491].
4.3.2. Applications of decellularized ECMs in tissue engineering
Decellularized ECMs, such as those of SIS, arteries, the urinary bladder and heart valves, are common sources of instructive scaffold materials enriched in collagen and endogenous proteins [492–494]. Under the assumption that these matrices are capable of dictating the differentiation decision of seeded cells, ECM materials may be revitalized by living cells before implantation [495–497]. Alternatively, decellularized ECM can be directly applied to recruit resident host cells for endogenous tissue regeneration based on the leveraging principles of morphogenesis [22,55,477,498]. In this regard, scaffolds based on decellularized adipose tissue may be endowed with inherent adipo-inductive properties to facilitate adipose tissue growth in vivo, as demonstrated by a recent study that revealed that bFGF-binding, heparinized decellularized adipose tissue is an efficient, biocompatible and injectable adipogenic system for de novo adipogenesis and in vivo adipose tissue engineering [499]. Indeed, adipose ECM-based biomaterials may facilitate endogenous tissue regeneration, even without the delivery of exogenous cells, which is an attractive solution for the management of a number of soft-tissue defects [500]. The use of decellularized adipose tissues of allogenic origin, for example, may offer clinicians “off-the-shelf” products for corrective procedures to restore contour, offering an ideal alternative to autologous tissue transfer for patients in need of soft-tissue reconstruction [501]. Of note, acellular tissue matrices have recently evolved from space fillers and mechanical supports to biological tissue replacements that have been demonstrated to support the penetration of cells and tissues in several applications, without inducing a gross immune response, while guiding endogenous tissue regeneration [500,502]. Indeed, given the natural origin of the matrices, ECM scaffolds degrade slowly after implantation and are correctly replaced by or remodeled with a new matrix produced by cells [35].
ECM-based biomaterials that retain the native materials and proteins of a living tissue/organ, along with the innate spatial arrangements in certain cases, provide appealing tissue engineering templates on which either exogenously transplanted or endogenously recruited cells may adhere, proliferate, differentiate and ultimately integrate to form functional tissues [266,286,287]. The clinical use of these materials to replace missing and compromised tissues/organs has dramatically enhanced the practice and philosophy of reconstructive surgery. Currently, both SIS and human DBM have been approved by the FDA for clinical uses; DBM has been investigated for more than 30 years for application in bone grafting procedures (noted in Section 3.2.3). A number of ECM products based on tissues or organs of human origin are already commercially available (Table 1). Since the first use of decellularized bone as a prototype ECM grafting material [503], this fast-growing field has gained convincing proof-of-principle evidence of this approach's efficacy for bone [504], vaginal [505,506], epithelial [507], skin [206,207,209–211], musculoskeletal [508,509], corneal [201,204,510] and vascular [511] tissue repair as well as for tissue engineering of pulmonary [512–514], myocardial [488], airway [515,516], liver [517], renal [518] and pancreatic [519] implants. Most studies reported complete and functional organ regeneration in small-animal models, and early clinical successes with complex tissues in preclinical studies and certain individuals have served as proof of concept [266]. In particular, urinary bladder and lung matrices, arteries and heart valves from allogenic human sources have been applied in preclinical and clinical procedures over the last decade, although the outcomes of large-animal models and human clinical trials have varied (Table 2) (reviewed in Refs. [26,76,266,298,477,520,521]). However, we must be mindful of the fact that our understanding of the cellular processes involved in the recellularization and revitalization of these bioscaffolds to form practical tissues is still incomplete [522]. The last two decades have included quick translation of decellularized matrices from the bench to the clinic, and today, their clinical use in reconstruction is a reality. However, clinical experience has been far from successful, especially when longer-term benefits are taken into account. It behooves us to return to the bench in order to elucidate the macromolecular chemistry and biological roles of human tissue-derived ECM during decellularization–recellularization so that the ultimate goal of solving the problem of on-demand tissue/organ assembly can be successfully realized [17,26,286].
Table 1.
Partial list of commercially available extracellular matrix (ECM)-based products of human origin fortissue reinforcement or replacement.a
| Product | Company | ECM source | Application focus | Product form (brief description) |
|---|---|---|---|---|
| AlloDerm® | LifeCell Corp. | Skin | Soft-tissue augmentation, reinforcement or replacement (e.g., abdominal wall, breast) | Dry sheet (an intact acellular matrix of natural biological components) |
| GraftJacket® | Wright Medical Technology Inc. | Skin | Soft-tissue augmentation, chronic wound treatment | Dry sheet (a thin fenestrated acellular matrix) |
| Axis™ dermis | Mentor Worldwide LLC | Dermis | Pelvic floor repair, horizontal and vertical soft-tissue augmentation in thickness and length | Dry sheet (allograft dermis consisting of solvent-dehydrated, gamma-irradiated, preserved human collagen) |
| NeoForm™ | Mentor Worldwide LLC | Dermis | Breast augmentation | Dry sheet (acellular human dermal collagen retaining its constituent elastin fibers) |
| DermaMatrix™ | Synthes Inc. | Dermis | Replacement, repair, or reinforcement of soft tissue ingrafting procedures such as root coverage and soft-tissue ridge augmentation | Dry sheet (an allograft derived from donated human skin) |
| Suspend™ | Mentor Worldwide LLC | Fascia lata | Pelvic floor reconstruction | Dry sheet (a dense matrix of collagen bundles and transverse fasciculi) |
| AlloPatch® | Musculoskeletal Transplant Foundation | Fascia lata | Rotator cuff augmentation | Dry sheet (human fascia lata from iliotibial band) |
| AlloPatch HD™ | Musculoskeletal Transplant Foundation | Dermis | Tendon reconstruction | Dry sheet (an intact or fenestrated dermal graft that preserves and maintains the natural biomechanical and biochemical matrix properties) |
| FlexHD® | Musculoskeletal Transplant Foundation | Dermis | Breast augmentation, hernia repair | Fully hydrated matrix (a strong, versatile, ready-to-use acellular dermal matrix) |
| IOPatch™ | IOP Inc. | Pericardium | Ophthalmologic repair | Dry sheet (an acellular pericardial matrix) |
| Puros® DBM | Zimmer | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency) | Allograft demineralized bone matrix (DBM) putty (a natural polymer product produced by a mineralized bone allograft following the removal of its inorganic elements using a demineralizing agent; 100% derived from allograft tissue) |
| AlloMatrix® | Wright Medical Technology Inc. | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency, augmentation in situations of segmental bone loss and interbody spinal fusion) | Injectable or formable putty (DBM combined with surgical-grade calcium sulfate that can be formed into an onlay or injected directly into a defect site) |
| AlloFuse® | AlloSource | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency) | Injectable gel or putty (DBM combined with heat-sensitive copolymer) |
| InterGro® | Biomet Osteobiologics | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency and segmental bone loss) | Paste, putty or mix containing hydroxyapatite/calcium carbonate composite granules (DBM in a lecithin carrier) |
| Grafton® | Osteotech Inc. | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency) | Gel (DBM combined with glycerol) |
| Osteofil®/Regenafil® | Regeneration Technologies | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency, augmentation in situations of segmental bone loss and interbody spinal fusion) | Injectable paste or putty, strips and blocks with cortical cancellous chips (CCC) (DBM combined with a non-toxic natural gelatin carrier) |
| Optefil® | Exactech Inc. | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency and segmental bone loss) | Injectable bone paste/dry powder ready to be hydrated (DBM suspended in a gelatin carrier) |
| Opteform® | Exactech Inc. | Bone | Bone repair (e.g., reconstruction of cavitary bone deficiency and segmental bone loss) | Formable putty or dry powder ready to be hydrated (DBM and CCC suspended in a gelatin carrier) |
| Optecure® | Exactech Inc. | Bone | Bone repair (e.g., reconstruction and augmentation of deficient maxillary and mandibular alveolar ridges and dental intraosseous defects) | Dry mix delivered with buffer solution (an optimal concentration of DBM suspended in a resorbable hydrogel carrier) |
| Optecure® +CCC | Exactech Inc. | Bone | Bone repair (e.g., reconstruction of the spine, pelvis and extremities) | 3D matrix delivered with buffer solution (DBM and CCC suspended in a hydrogel carrier) |
The product list does not represent any particular preference by the authors.
Table 2.
Partial list of human tissue/organ-derived decellularized matrices that may be used for tissue engineering applications.
| Tissue source | Decellularized matrix | Application options | Partial list of Refs. |
|---|---|---|---|
| Skin | Dermal tissue | Abdominal wall, breast, ear, nose and throat/head and neck reconstruction, grafting | [206,207,209] |
| Bone | Bone matrix | Bone repair and regeneration | [245,247,248,503] |
| Cornea | Corneal stroma | Corneal transplantation | [201,204,510] |
| Adipose tissue | Adipose tissue | Adipose tissue regeneration | [499,501] |
| Vagina | Amniotic membrane | Vaginal/cervical reconstruction | [505,506] |
| Small intestine | Small intestinal submucosa | Reconstruction of integument, body wall, urinary bladder, rotator cuff, intestine, urethra, ureter and diaphragm | [26,286,477] |
| Trachea | Trachea matrix | Replacement of the main left bronchus | [515,516] |
| Heart | Heart matrix | Engineering of a bioartificial heart for heart tissue engineering | [488] |
| Lung | Lung matrix | Lung regeneration, orthotopic transplantation | [514] |
Of all potential tissues, SIS is one of the raw biomaterials that is most often investigated and is used as a prototypical ECM scaffold in a wide variety of applications because this material exhibits the key features of a highly supportive scaffold and presents growth factors and adhesion peptide sequences, which facilitate integration with surrounding tissue [26,477,492]. SIS tissue is degradable and is known to display excellent biocompatibility in clinical use; even xenogenic SIS possesses low or no immunogenicity, and when used as a wound-dressing material, SIS may provide defense against infections. In certain applications, SIS is crosslinked to reduce its hemo-compatibility or to modulate the mechanical properties or degradation rate of a scaffold [523]. Both SIS and another small-intestine-derived preparation termed the biological vascularized matrix (BioVaM) have been found to support endogenous cell homing after implantation, and the matrix may be subsequently remodeled to produce functional tissue [492]. By dry weight, an SIS-ECM biomaterial is composed of more than 90% collagen, and the collagen fiber orientation can be retained during the decellularization process [26]. Depending on the type of decellularization method used, the material can contain various GAGs that existed in the living tissue, including heparin, HS, CS and HA [298]. However, in the decellularization process, ionic detergents are commonly applied, which can remove GAGs from the post-processed ECM product [486,489]. Moreover, a pool of adhesion molecules (e.g., fibronectin and laminin), the proteoglycan decorin and the glycoproteins biglycan and entactin are present in a well-prepared SIS-ECM scaffold [26]. Most importantly, a pool of growth factors and other bioactive molecules also exists in a well-prepared SIS-ECM, such as TGF-β, b-FGF and VEGF. Even after terminal sterilization and long-term storage, these factors have been shown to maintain their bioactivity [492]. All of these inherent advantages have sparked strategies that apply SIS-ECM as a scaffolding material in applications for soft-tissue reconstruction and cardiovascular, neural and urogenital tissue engineering [39]. SIS is currently approved by the FDA for several urogenital uses, including bladder augmentation, hernia repair and urethral stricture repair [26,76,77]. Although SIS-ECM is evolving and slowly becoming a new standard for bladder augmentation [269], collagen-composite biomaterials that consist of nanofiber networks highly resembling natural ECMs populated with self-assembled fibroblast sheets or the patient's own muscle and urothelial cells are another attractive strategy that is showing optimistic clinical outcomes [74,524,525]. Other defects of the urogenital system, such as incontinence and vesicoureteral reflux, can also be managed by injection of collagen-based SIS-ECM biomaterials. To modify naturally derived SIS biomaterials in regenerative medicine, HA-PLGA nanoparticles were synthesized and were observed to stabilize the porous structure of SIS and hence to improve the surface biocompatibility and performance of the resultant composite scaffolds in tissue regeneration [526]. The presence of endogenous growth factors and aligned collagen fibers in an acellular SIS-ECM has also sparked considerable research interest in the bone tissue engineering community [39]. As an adjuvant material to DBM, SIS/DBM has a tendency to promote more cellular proliferation and osteoblast differentiation than does DBM alone. In one study, although SIS without cell seeding resulted in no new bone formation in vivo, whereas DBM alone demonstrated new bone formation along the edge of old DBM particles, an SIS/DBM composite exhibited higher osteoinductivity. Moreover, the residual SIS/DBM was surrounded by an osteoid-like matrix and newly formed bone [527]. The capacity for localized collagen formation and osteocalcin deposition by SIS-ECM was demonstrated in a full-thickness bilateral bone-defect model in rat crania; the defects were managed with SIS sponges, and the presence of bone marrow-derived stem cells (BMSCs) resulted in significantly greater bone formation [528]. Similarly, a tissue-engineered periosteum fabricated from osteoinduced rabbit BMSCs and porcine SIS was demonstrated to be superior to a structural allograft in the repair of an allogenic segmental bone defect [529]. Overall, the use of SIS-ECM may provide a new dimension to raw biomaterials in tissue engineering; the combination of various molecules and native aligned collagen fibers fosters the cellular processes necessary for the optimal function of the tissue and organs from which they are isolated. Although the essence of an ECM obtained from an individual tissue can function as a bioscaffold for the same tissue type because the compositions and structures of different tissues may vary from one another, the modification of an SIS biomatrix-scaffold with other materials may expand its use in diverse tissue engineering applications [39].
One scarcely explored decellularized ECM material is decellularized hyaline cartilage, which would be expected to offer a material rich in collagen type II, aggrecan and native growth factors [530]. As a significant source of morbidity in lower back pain, treatments for facet joint osteoarthritis have generally focused on reduction of the pain associated with this disease. However, recent efforts have shifted to the creation of decellularized articular cartilage that is utilized as a replacement for diseased facet cartilage [531]. When a decellularized porcine cartilage bone construct was used as an ECM material for cartilage substitution, the biomatrix-scaffold was observed to exhibit desirable compatibility in both in vitro and in vivo tests, offering a product for application in osteochondral defect repair [530,532]. In investigating the possibility of an acellular cartilage material for application as a scaffold in cartilage tissue engineering, this type of biomatrix displayed good biocompatibility with cultured rabbit BMSCs, with no indications of cytotoxicity in either contact or extraction assays. Compared with control groups at 6 and 12 weeks, the repair of cartilage defects in rabbit knees utilizing the cell–matrix constructs showed a significant improvement in histological scores [533]. Although cellular cartilage can offer a native ECM for cartilage regeneration, it is difficult, if not impossible, for cells to penetrate the biomatrix due to its nonporous structure. To address this limitation in engineering cartilage, a sandwich model containing chondrocytes and acellular cartilage slices was established; porcine chondrocytes were seeded in between each layer of cartilage, and the cartilage had a designed shape, achieved by pre-shaping the slices before in vivo implantation. This strategy for using nonporous matrices appears promising and warrants future deeper investigations [534]. Although many of the examples discussed herein describe animal sources, the principles may apply to human ECM-based biomaterials with variations depending on both the processing methods and the tissue source of the ECM.
Decellularized matrices may also be processed to form particulates that can be used either alone or in combination with other biomaterials to promote tissue repair, and scaffolds may be prepared in many forms, including sheets, powders and hydrogels [535]. Loai et al. (2010) combined particles of porcine bladder acellular biomatrix with polymeric materials (e.g., HA) loaded with VEGF in the fabrication of scaffolds with biological activity and tunable properties for the generation of a vascularized bladder in murine and porcine preclinical models [536]. Alternative approaches have seeded cell populations onto scaffolds and leveraged culture conditions to drive the differentiation of cells and the concomitant production of ECM. Along with fluid shear stresses, the inherent osteoinductive potential of bone-like ECM has recently been used to synergistically improve the osteodifferentiation of MSCs, which may have profound implications for bone tissue engineering uses [537]. Moreover, by culturing MSCs within an electrospun, biodegradable PCL fiber mesh material in a flow perfusion bioreactor, an osteogenic ECM construct with a temporal composition that may be useful for bone repair because of its ability to mineralize and capabilities for future remodeling may be generated [538]. More recently, thanks to advances in directing cell differentiation toward specific lineages, tissue-engineered constructs are also being used as a substrate for decellularization. This strategy opens a new route for the production of large quantities of customized and standardized ECM-based materials [491].
During regeneration, cells rely on environmental input for direction. ECM from an individual tissue therefore has a crucial role in aiding in skewing cell differentiation toward the specific cell phenotypes that normally reside in the native ECM of that tissue [539]. For example, myoblasts seeded in skeletal muscle ECM extract displayed accelerated proliferation and differentiation, potentially because they were in an environment in which they would be observed endogenously, offering an optimal and natural space for their maturation [509]. Based on this concept, a decellularized arterial scaffold that includes various ECM agents has been used to overcome limitations such as rejection, thrombosis, calcification, intimal hyperplasia, chronic inflammation, infection and a lack of growth potential associated with vessel scaffolds composed of synthetic polymers. In vitro, the endothelial cell matrix contains factors that are observed to instruct MSC differentiation into both endothelial cells and smooth muscle cells, and in turn, these factors are modified by MSC-secreted agents after the seeding of autologous bone marrow onto the scaffold. By studying the framework by which an endothelial cell matrix coaxes MSC differentiation, a feedback system has been uncovered, by which MSCs are able to alter the very matrix cues acting upon them [540]. Similarly, a tissue-engineered heart valve was developed utilizing umbilical cord blood-derived EPCs of human origin and decellularized valve scaffolds. The EPCs can differentiate into endothelial cells and form a functional endothelium atop the scaffolds, similar to that formed by normal endothelial cells on healthy human heart valves [541]. Currently, decellularized xenogenic heart valves have been used as a starter ECM material for the tissue engineering of valve grafts, with promising preclinical and clinical outcomes [520,542]. Interestingly, when seeded with cardiac progenitor cells, a human pericardium-derived biomatrix with well-defined architecture and interconnected pores, which mimics the natural myocardial extracellular surroundings, holds tremendous promise in the treatment of ischemic heart diseases [539]. Based on these investigations, it is concluded that the application of native decellularized tissues as biomaterials has the potential to open a new avenue for guiding seeded cells toward their normal activity and function.
4.3.3. Whole-organ decellularization
Techniques of tissue decellularization and their impacts on the properties of post-processed ECM materials were reviewed in 2006 by Gilbert et al. (2006) [486] and recently by He and Callanan (2013) [543]. In recent years, the development of novel decellularization methodologies for effective 3D composite tissue and whole-organ decellularization has attracted increasing attention [475,544]. Although the treatment of end-stage organ failure using organ transplantation is now a well-established procedure as a result of advances in various immunosuppressive drugs to control rejection, this paradigm has fallen victim to its own success given the growing disparity between the numbers of patients on the organ transplantation waiting list and the donor organ pool available for such procedures; this disparity leads to a substantial number of patient deaths each year [26,284]. In light of the shortage of donor organs, decellularized solid organs can be used to create potentially functional organ constructs in a short period of time, which may perform organ-specific functions following recellularization, and surgical implantation and preliminary animal studies have delivered encouraging outcomes for this proof-of-concept [11,43,222,545]. The use of bioreactor-based strategies for cell seeding of organ-level bioscaffolds provides a blueprint for the de novo fabrication of complex tissues and could allow the generation of customized organ grafts, irrespective of their natural geometry [212,546–548]. Although culturing cells within a decellularized organ prior to implantation offers scientists a high degree of control over the fates of the residing cells, in most cases, multiple cell types are required to recellularize a complex organ system (e.g., a mixture of cardiomyocytes, endothelial cells and smooth muscle cells is likely to be necessary for heart recellularization) [549]. The diverse range of different in vitro cell culture parameters is very difficult to balance, and culturing more than one cell type in the same decellularized ECM template presents its own unique pitfalls and challenges [475]. Recent studies suggest, however, that functional tissues or organs may also be obtained via the in vivo cell repopulation of an implanted decellularized matrix based on cell recruitment from the neighboring tissue and circulation, thus indicating significant clinical potential, although work in this field thus far has only resulted in short-term functionality [276,277,486,504,543]. For a deeper understanding of the interactions between the host cells and an implanted matrix to maximize recruiting efficacy and the propensity to spontaneous in body self-regeneration, more systematic studies of different types of decellularized matrix systems both in vitro and in vivo are required [550]. The complexity of the decellularization technique and the length of the decellularization protocol are commonly proportional to the degree of biological and geometric conservation required in the resultant tissue with respect to its composition (BM components and bioactive molecules) and architecture (macrostructure and ultrastructure), especially for composite tissues and whole organs [26,284,543,545]. Today, decellularization has become a reliable methodology for the production of complex 3D biomatrix scaffolds that maintains the intrinsic vascular network by removing residing cells from whole organs while retaining the original architecture [11,222]. This method is optimal for already existing biomaterials because the scaffolds do not only maintain the structure of the original organ but also contain native biomolecules within their ECM that direct correct cellular function [486]. For example, as a native and functional decellularized ECM biomaterial, ovine forestomach matrix has suitable biophysical properties for clinical uses in which the grafted scaffold is under load [486,543]. Clinical materials such as surgical mesh products based on ECM are obtained from diverse allogenic or xenogenic tissue sources, including the urinary bladder, dermis, small intestine, pericardium, mesothelium and heart valves, and from several different species [11,76,222]. In 2008, the main left bronchus in a young man was replaced with a decellularized cadaveric trachea seeded with the patient's own cells [515]. Since then, similar strategies have been clinically utilized to replace organs in patients with a tracheal/bronchial tumor or congenital tracheal stenosis [516,551], and many groups have investigated the production of decellularized organs for the replacement of the heart [488,520], liver [517] and lung [512,513] in small and large animals. Thus far, ECM and cells procured from the above-mentioned tissues and organs have been used to study the potential advantage of tissue specificity in maintaining selected cell functions and phenotypes, and these ECMs have been demonstrated to affect cell chemotaxis and mitogenesis, to guide cell differentiation and to induce constructive host tissue remodeling responses. The 3D ultrastructure, composition and surface topology of the organ ECM all likely contribute to these effects [284,476,552]. In contrast, the remaining residual cellular material within the biological scaffold materials attenuates or fully negates the constructive tissue remodeling advantages in vivo. Thus, tissue-processing strategies and decellularization techniques appear to be critical determinants of the clinical success of organ ECM products [274]. Although the general focus on structural relevance in the ECM research field is of fundamental importance, there is now a strong emphasis on several other new areas. In particular, the cell–matrix interface offers a pivotal signaling nexus that controls all aspects of cell functions [276].
The complete decellularization of most, if not all, organs commonly necessitates a synergism of physical, chemical and enzymatic treatments [274]. It is necessary to select the mildest approach that can result in an acellular scaffold without damage to the functional constituents and structural geometry of the ECM [43,543]. A typical progressive protocol would begin with treatment in a hypertonic or hypotonic solution, followed by a mild zwitterionic or nonionic detergent. If needed, enzymatic treatment with trypsin, either alone or in combination with a chelating agent such as ethylenediaminetetraacetic acid (EDTA) and ethylene glycol tetraacetic acid (EGTA), can be used as an adjuvant prior to the detergent treatment to aid in disrupting the bonds between cell membranes and the ECM. It is likely that EDTA or EGTA contributes to cell dissociation from ECM proteins and to subtle disruptions in protein–protein interactions by sequestering metal ion mechanisms. Finally, an ionic detergent such as sodium dodecyl sulfate (SDS), Triton X-100 or deoxycholate may be applied in the decellularization protocol if the prior treatments are still inadequate to remove all of the cellular and nuclear residues from the tissue or organ of interest [43,475,486]. An objective evaluation of the effects of the agents that are used to yield a particular ECM structure and composition can assist in the design of an optimal decellularization protocol [489]. For tissue delipidation, ionic detergents such as deoxycholate typically appear to be less effective than nonionic detergents. SDS appears to be more effective than other detergents, such as Triton X-100, for the removal of cell nuclei from dense tissues and organs while maintaining their native mechanics, but SDS is also more likely to induce ultra-structure disruption in and growth factor elimination from the resulting ECM [531]. Whether for a simple tissue or a complex whole organ, a standard protocol for tissue decellularization offers many benefits that aid in advancing biomaterials of human origin for tissue engineering. First, the standardization of post-processing ECM products enables the possibility of a congruous comparison of various ECM scaffolds and allows both researchers and manufacturers to appraise the efficacy and effectiveness of an established protocol when developing new decellularization methodologies or characterizing a particular ECM product derived from a particular decellularized tissue or organ. Second, standardized decellularization minimizes the existence of and variation in residual cell and DNA material in a post-processing product, thereby potentially eliminating adverse cell and host responses to ECM scaffolds and facilitating the comparison and interpretation of in vitro and in vivo findings [11,222,476]. Thus, the standardization of the current decellularization protocol and its post-processing product are prerequisites to expanding the clinical application of this technique and to promoting the rapid and effective development of more biomaterials of human origin for tissue engineering and regenerative medicine [274].
Overall, whole-organ decellularization provides a scaffolding platform on which to establish a strong translational pipeline for future organ tissue engineering, although organ-level decellularized matrices have not yet reached the stage of clinical adoption [545]. In principle, the integrity of the post-processed material following decellularization supports the reproducible, predictable and effective clinical application of the ECM product [544]. From a scientific standpoint, the constituents remaining in the ECM are instrumental to possibly identifying the mechanisms by which specific biomolecules and their organization elicit tissue regeneration processes [26]. However, in the production of readily available, patient-specific ECM equivalents, the typical procedures applied for the efficient removal of cellular and nuclear materials result in a simultaneous and at least partial impairment of ECM integrity and its constituents (e.g., a decline in soluble type II collagen and GAG content), especially in whole-organ template generation [274]. Although those bioactive materials have provided substantial evidence supporting recellularization and cell propagation, the cues from the “matrix footprint” that are critical in cell adhesion and viability remain unidentified. Additionally, the materials' allogenic nature has raised clinically relevant concerns, particularly with regard to both their safety and ethical issues, suggesting that host responses to residual cell debris and matrix constituents should be carefully characterized before therapies based on these matrices can be used in the clinic [475]. Upon application, critical challenges and hurdles remain associated with the following: sterilization without compromising the protein framework, selection of the optimal cell source required to restore tissue functionality, acquisition and growth of patient-specific cells on decellularized materials without contamination, reintroduction of these cells into their proper location (e.g., denuded vascular structures in a whole-organ scaffold) through seeding methods or induced migration, cell distribution or propagation following reseeding and subsequent organ revitalization, achievement of the functional properties of organs, characterization of regenerated organs and prevention of thrombosis [266,521,552]. Even when an organ ECM material is correctly recellularized with sufficient cell numbers, it is difficult to predict whether the engineered organ, as a defective transplant, is going to work with unpredictable fate or as a less-than-perfect transplant but with “self-repairing” and remodeling potential that will eventually restore the impaired bodily function [11,222]. Finally, in addition to the scientific and technical bases of decellularized matrices, the demand for clinical-grade bioreactors, the identification of the appropriate population of patients, regulatory issues and the clinical logistics of the transplantation approach should be considered to facilitate the clinical translation of these matrices for genuine real-world applications in the future [476]. From a therapeutic prospective, regulation of the production and management of donor tissues and organs, their effectiveness and safety evaluations, quality control, application protocols and guidelines, standardization and ethics are all critical issues that must be carefully taken into account in the development and commercialization of decellularized ECM templates of human origin as “off-the-shelf” substitutes or, following recellularization and revitalization, as fully functional organs similar to donor organs that can be transplanted into patients suffering from end-stage organ failure (Fig. 15) [274].
Fig. 15.
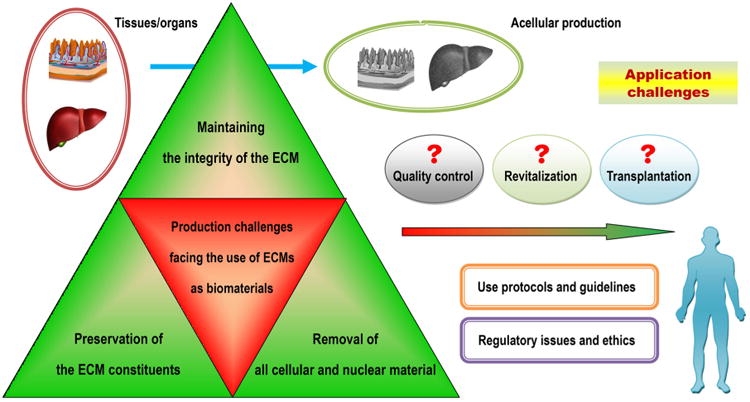
Schematic representation of the distinct and crucial challenges related to the production and application of extracellular matrix (ECM)-based biomaterials in regenerative medicine and tissue engineering.
5. Preparations containing non-expanded autologous stromal cells
Recently, autologous preparations, such as bone marrow concentrate (BMC) and the stromal vascular fraction (SVF), have been used in regenerative procedures because they contain uncultured stromal cells that may participate in the wound healing cascade during therapeutic regeneration (Fig. 16). As a new concept in using biomaterials of human origin, the use of non-expanded BMCs and SVFs offers interesting alternatives to therapeutic cell populations, carefully circumventing translational barriers regarding cell expansion and delivery. Although concerted efforts have been and are still being made in the identification and delivery of ex vivo-expanded cell populations for tissue engineering, these cell populations' economic and clinical feasibility continues to present formidable challenges. By providing an overview of BMC and the SVF, each an important but currently overlooked aspect of patient-derived biomaterials, we hope to present a call for action to develop these therapies for routine clinical use. For more detailed information, the reader is directed to many focused reviews published elsewhere, several of which are cited in this section [553–557].
Fig. 16.
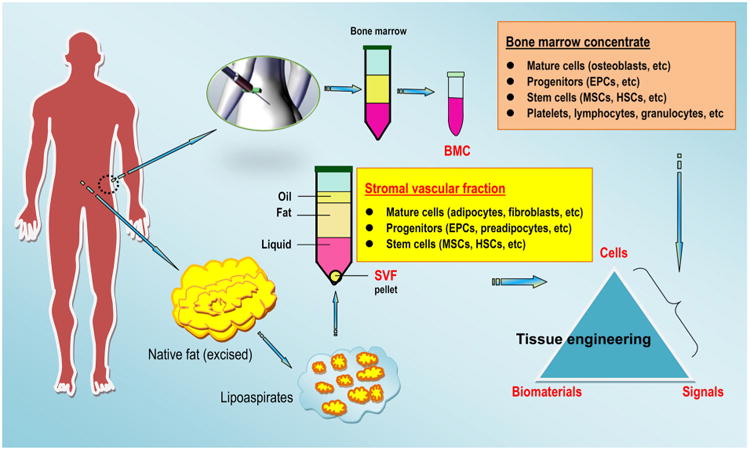
Schematic of the preparation of human-derived bone marrow concentrate (BMC) and a stromal vascular fraction (SVF) from non-expanded cell populations for cell therapy and tissue engineering applications (schematic is not to scale). BMC is a rich source of the regenerative cells needed for bone formation and angiogenesis, including mesenchymal stem cells (MSCs, which convert to osteoblasts in support of new bone formation), hematopoietic stem cells (HSCs, which orchestrate bone formation) and endothelial progenitor cells (EPCs, which stimulate angiogenesis). In addition, BMC includes platelets, which mediate cell-to-cell adhesion via the release of multiple growth factors; lymphocytes, which support the migration and proliferation of EPCs; and granulocytes, which release vascular endothelial growth factors (VEGFs) in support of angiogenesis. The use of BMC therefore offers the potential to bridge the gap between stem cells and signaling factors in a traditional tissue engineering triad. The SVF is the product of a lipoaspirate, which is obtained from liposuction of excess adipose tissue. The SVF contains a large population of mature cells, progenitors and stem cells. Adipose-derived stem cells (ASCs) share many similarities with bone marrow-derived stem cells, including self-renewal and multilineage differentiation capacity.
5.1. Bone marrow concentrate
MSCs represent a promising cell source for osteochondral regeneration because once obtained, e.g., from the iliac crest, these cells can differentiate into multiple types of tissue-forming cells, such as chondrocytes and osteoblasts. However, the in vitro expansion of MSCs encounters various problems, such as problems regarding the sterility of the cell culture; the risk associated with the use of fetal bovine serum (FBS) (this risk may be bypassed via the use of PL); and the length of time required for cell cultivation, as cell transplantation normally requires a time-delayed second operation. The need for advanced laboratory and technical support, as well as regulatory issues and high costs, are also major hurdles that need to be addressed. A possible alternative could be the use of a perioperative stem cell concentrate in a single-step procedure using density gradient centrifugation of autologous bone marrow [555]. Indeed, bone marrow has been directly applied to induce bone formation in skeletal defects and non-unions. The cells contained in the bone marrow may participate in the wound healing cascade, serving as building blocks for or directing regeneration via the secretion of growth or cellular signals instead of, or in addition to, directly participating in regrowth of the tissue [553]. The main advantage of using bone marrow is that this technique can be accomplished percutaneously in routine clinical practice, free of nearly any patient morbidity. Technically, the centrifugation of aspirated bone marrow at 400 times gravity for 10min may completely separate the marrow cells from the plasma to decrease the volume of material injected, resulting in the marrow product termed BMC. It has been demonstrated that the osteogenic potential of the cells can be well preserved in a well-prepared BMC product [558].
BMC contains a mixture of cell populations, such as MSCs and HSCs, as well as other substances, such as platelets and cytokines; all can facilitate the regeneration of numerous tissues as part of reconstructive surgery and tissue engineering strategies [176]. BMC represents a new frontier in cell-based therapeutics for an unexpectedly wide variety of human diseases, including those involving autoimmunity, inflammation and tissue damage, due to the multi-differentiation and the immunomodulatory and anti-inflammatory properties of BMC preparations. In particular, an intraoperative, one-step procedure for the clinical application of progenitor cells from bone marrow has shown promising results in musculoskeletal tissues [559] (Fig. 16). Following bone marrow aspiration, BMC is easily prepared using density gradient centrifugation and is available for a same-day procedure with minimal manipulation of the cells, thus complying with FDA restrictions. Future advances in this field will be the development of an easy procedure for harvesting (e.g., by vacuum aspiration) from the iliac crest to facilitate the availability of autologous bone marrow and the establishment of a standardized dose of stromal cells and mononuclear cells in a well-prepared BMC product [560]. For many years, it has been recognized that BMC may possess high potency in cartilage and osseous defect healing when used in combination with grafting materials and occasionally PRP [561–565]. However, there are no published randomized controlled trials on the efficacy of autologous BMC intra-articular injections performed as a same-day, in-office procedure for treating patients with cartilage or bone disease [566]. In any case, the exponential rate of progress in biotechnology has allowed for the immediate application of myriad novel therapies before clear evidence of benefit from randomized clinical trials. In addition to its fundamental science, the ease of on-site preparation of bone marrow-derived cells within the operating theater in the routine clinical setting, minimizing the specific risk of contamination and cell changes during ex vivo cell manipulation, suggests the great therapeutic potential of BMC for autologous cell-based therapy for bone or other tissue repair and regeneration [555,559,567].
5.2. Stromal vascular fraction
Human adipose tissue is becoming an increasing focus of tissue engineering due to the abundance of its tissue source, its relatively easy retrieval and the intrinsic biological properties of MSCs residing in its stroma [568,569]. Beyond its use as a fat grafting material, knowledge of the cell biology, isolation/manipulation and differentiation, and regenerative and immunomodulatory properties of adipose-derived cells has increasingly advanced in the past 10 years. In particular, concerted research efforts have yielded a wealth of basic science-based and preclinical evidence regarding the properties of both heterogeneous SVF cells and more homogeneous adipose-derived stem cells (ASCs) derived from the patient's own tissue [568]. There is also mounting evidence demonstrating that the human SVF compartment contains multipotent MSCs that may differentiate into smooth and cardiac muscle cells, osteocytes, neural cells, adipocytes and chondrocyte precursors and that are capable of generating tube-like cellular structures in 3D culture in vitro [569–571]. In addition to being a multipotent cell population amenable to soft-and hard-tissue repair, the human adipose SVF cell population represents a complex mixture of HSCs, endothelial cells, pericytes, T regulatory cells and anti-inflammatory M2 macrophages, indicating that this population is a useful source of cells for treating ischemic insults and autoimmune/inflammatory disease [571]. However, the reason why the adipose-tissue SVF represents a hot topic in stem cell research is that this non-expanded tissue compartment offers a rich source of multipotent ASCs (Fig. 16). Prior to cell transplantation, ASCs are readily accessible in human autologous fat tissue and have significant potential for tissue repair in scenarios of heart failure, myocardial infarction, hind limb ischemia and inflammation [572]. Indeed, ASCs display comparatively stable growth and proliferation kinetics and can differentiate into chondrogenic, osteogenic, adipogenic, myogenic or neurogenic lineages when cultivated under lineage-specific conditions. Moreover, ASCs have been proven to induce substantial tissue formation for several biomedical uses. In this regard, certain clinical trials on the utility of human ASCs in bone reconstruction have been concluded and have indicated efficacy [573].
Human SVF cells can be easily procured by centrifugation of collagenase-digested adipose tissue of human origin and are then ready for biomedical application, without the need for cell culture [556,557]. As such, non-expanded SVFs provide an alternative to cell products in regenerative medicine. This alternative may bypass many of the translational obstacles related to ex vivo cell cultivation and transplantation, allowing the development of one-step surgical procedures due to the high frequency and abundant availability of SVF cells [574]. Of note, these cells can be added to transplants of human origin, such as purified adipose tissue or bone grafts, or to a material carrier such as an ECM scaffold or an alloplastic material, stimulating long-term cell retention and subsequent colonization and providing a cure for various tissue injuries/damages. Based on this concept, non-expanded SVF cells (freshly procured without cell culture) were used to form new adipose tissue in vitro using a porous collagen/gelatin sponge as a scaffold [575]. Recent evidence also suggests that SVF transplantation combined with a chitosan conduit may be considered as a readily available stromal cell source for ameliorating the functional recovery of the sciatic nerve [576]. Furthermore, an intratendinous injection of uncultured adipose-derived SVF cells results in improved structural and mechanical properties in tendon repairs and could be an effective modality for the treatment of tendon injury [577]. In addition, the administration of SVF cells has been shown to ameliorate chronic experimental autoimmune encephalomyelitis in animal models, demonstrating the potential immunomodulatory, anti-inflammatory and regenerative effects of non-expanded SVF cells. Interestingly, it has also been revealed that SVF cells effectively inhibit disease severity and are significantly more effective than culture-expanded ASCs [578]. Similarly, when osteochondral defects in medial condyles and trochlear grooves in the knees of goats were treated with a freshly procured SVF or cultured ASCs, the SVF cells tended to perform better on all parameters, including the formation of type II collagen and hyaline-like cartilage and the elastic modulus [579]. These findings suggest that freshly procured, heterogeneous SVF cells, including a mixture of multiple cell types with both immunomodulatory and regenerative properties, can dictate a more effective cure upon application compared with culture-expanded and relatively homogeneous ASCs. More often than expected, bone tissue engineering based on the SVF has emerged as a promising approach to manage the structure and function of bone compromised by disease or injury [580,581]. Indeed, as a practical, promising candidate for cell-based therapy, the SVF has also attracted increasing attention for application in clinical reconstructive surgery [573].
Over the past several years, considerable advances have been made in the science and technology related to the use of SVF cells in tissue engineering and regenerative medicine. Of note, the clinical utility of cell-based therapeutics for tissue repair and regeneration has encountered numerous translational barriers [582]. In light of these barriers, an uncultured SVF in possession of a pool of regenerative cells may help to avoid an additional culture period, to reduce the risk of extensive cell contamination and to increase cost-effectiveness [583]. Recent data have revealed promising outcomes when a freshly procured SVF was used as a non-expanded stem cell source for advanced cartilage therapy [584] and adipose formation [568]. Furthermore, methods for the cryopreservation of the SVF in a serum-free freezing medium have been tested, and the findings indicated that the cell viability and differentiability of the SVF can be preserved [585]. Overall, the application of autologous SVFs in cell-based therapy not only is easy and effective but also facilitates their translation into human healthcare [574]. However, more investigations are required to identify whether the techniques described in recent studies will still work on the larger scale of tissue defect models and whether autologous SVF transplants can maintain their dimensions and shape over time at defect sites in humans. Additionally, the degree or longevity of engraftment of ASCs following SVF use has not been measured by external investigations independent of commercial organizations. It is also not clear whether the reported positive outcome of SVF cell administration was the result of local immunosuppression, paracrine expression of anti-apoptotic/angiogenic factors, adipose cell differentiation, or a combination of these or other unidentified effects [586]. Therefore, the stage has been set for the clinical translation of SVF cells from the bench to the bedside, but this process will involve “developmental” steps that fall outside of the traditional paradigm of the mechanism-based and hypothesis-driven experimental design common in the stem cell literature [587]. It is likely that several, if not all, of the above-mentioned questions must be addressed before clinicians can use SVF products, whether harvested from the patient or provided by biotechnology companies. It should be noted that although a variety of commercially available systems may yield measurable amounts of SVF cells in a clinical outpatient surgical environment, significant variability exists in the number, the identity and the safety profiles of the recovered viable cells. The lack of preclinical and clinical data reported in peer-reviewed manuscripts that can be used to objectively assess the overall performance of SVF products suggests that side-by-side clinical trials will be required to establish the relevance of these variations [588].
6. Formulations enriched with endogenous growth factors
In addition to the application of a human graft and its ECM, the concept that biomaterials of human origin may be useful for regenerative therapy has been supported by numerous pioneering studies that show that tissue regeneration can be accelerated using formulations enriched with growth factors from a patient's blood, such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) [589,590]. Although a vast wealth of recombinant proteins and growth factors are now commercially available for diverse applications, several of which have also been approved for testing and use in humans, unfortunately, their clinical implementation hitherto has been disappointing. One pivotal aspect of the development of preparations enriched with endogenous growth factors has been transforming human platelets, which are reservoirs of a spectrum of autologous growth factors, into therapeutic preparations that can be easily handled, evaluated and adopted by researchers and surgeons who practice regenerative medicine [44]. In particular, PRP and PRF have undergone clinical translation from bench to bedside in an easy, simple and predictable way; blood samples are generally harvested from an individual patient, and a personalized formulation rich in growth factors can be obtained by simply controlling the degree of coagulation of the samples and the elaboration protocol designed for production [591] (Fig. 17). Here, an overview of these products' roles and implications in future tissue engineering is intended to shed light on the various prospects of these formulations and on clinical insights into regenerative medicine.
Fig. 17.
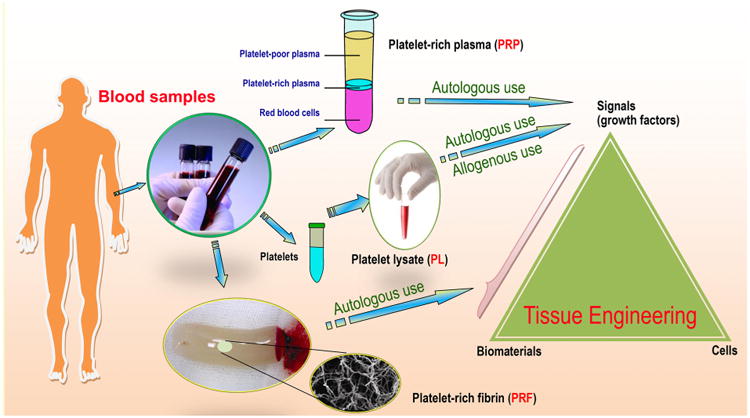
Schematic of the preparation of platelet-rich plasma (PRP), platelet lysate (PL) and platelet-rich fibrin (PRF) for tissue engineering applications (schematic is not to scale). PRP and PL may be used as autologous alternatives to recombinant human growth factors in a traditional tissue engineering triad, whereas PRF offers the potential to bridge the gap between biomaterials and signals.
6.1. Platelet-rich plasma
PRP is a platelet concentrate in a small volume of plasma that is typically developed from autologous blood [589,590]. Ranging from two- to several-fold above physiological levels, the platelet count in a PRP product varies according to the preparation protocol. Upon activation, the platelets contained within a PRP product release the contents of their granules, which consist of a complex array of growth factors, cytokines and chemokines, such as PDGFs (i.e., PDGF-αα, PDGF-ββ and PDGF-αβ), TGF-β (e.g., TGF-β1 and TGF-β2), insulin-like growth factor (IGF), VEGF, platelet factor-4 (PF-4), platelet-derived angiogenesis factor (PDAF), IL-1, epidermal growth factor (EGF), epithelial cell growth factor (ECGF), platelet-derived endothelial growth factor (PDEGF), osteonectin, osteocalcin, fibrinogen, fibronectin, vitronectin and thrombospondin-1 [592], many of which have been demonstrated to participate in the wound healing and tissue regeneration processes [593,594]. The interactions between these growth factors and their surface receptors on responsive cells activate the intracellular signaling pathways that enhance tissue regenerative processes, such as cell proliferation and differentiation, matrix deposition, collagen synthesis and osteoid production [595]. Thus, the potential to deliver these growth factors and matrix elements to a site of injury is the basis for using PRP in regenerative medicine (Fig. 17). In addition to its major roles in hemostasis and cell fate commitment, PRP is involved in the inflammatory and immunological aspects of wound healing. Platelets play a direct role in the inflammatory response through the production and release of a vast wealth of inflammatory mediators, including various cytokines, such as IL-1β, TGF-β and CD40L, as well as numerous chemokines, such as CXCL1, CXCL4, CXCL5, CXCL7, CXCL8, CXCL12, CCL2, CCL3, CCL5 and CXCL4L1. Moreover, platelets express a number of chemokine receptors, and particularly CCR1, CCR3, CCR4 and CXCR4 [596]. The collection of such bioactive molecules in well-prepared PRP plays a synergistic role in the fundamental processes of tissue repair, including inflammation, angiogenesis, cell migration and metabolism. In pathological conditions such as osteoarthritis, PRP exhibits anti-inflammatory properties through its impacts on the canonical nuclear factor-κB (NF-κB) signaling pathway in multiple cell types, including synoviocytes, macrophages and chondrocytes. Analyzing the effect that each biological factor can have on tissue-specific cells and understanding PRP in molecular terms could help us to exploit its therapeutic potential and could aid in the development of novel treatments and tissue engineering approaches [42,597].
An attractive advantage of PRP lies in its easy and rapid acquisition from a patient's own blood, thus theoretically circumventing the risk of disease transmission or an immunogenic reaction because the receptor and donor are the same [44]. Due to its autologous origin and low cost, PRP has significant advantages over other therapies including recombinant growth factors. Although appealing, the autologous nature of PRP introduces variability into plasma preparations, creating challenges for both the researcher and the clinician [590]. Differences in patients at the time of the blood draw result in plasma preparations that vary both within and between patients. This variability is compounded by the multitude of protocols and devices available for procuring PRP [598]. Criteria for PRP dosing and for tailoring preparations to each pathological situation have yet to be established. The common need for bovine plasma-derived thrombin, which triggers platelet activation through the thrombin receptor, present on the platelet surface, represents another limitation of current protocols for obtaining a PRP product [599]. Of note, the application of thrombin is associated with potential immunological reactions, and approximately 30% of patients exposed to bovine thrombin may develop cross-reacting antibodies to certain human plasma proteins. These antibodies have the potential to result in life-threatening coagulopathies [600,601].
An appealing strategy to circumvent several of the challenges is the use of an autologous plasma product obtained from an individual patient by plasmapheresis. This product, which is enriched in platelets, is termed “plasma rich in growth factor (PRGF)”, and calcium chloride is used to activate the platelets [591]. Because calcium chloride is adopted to activate the coagulation cascade, the use of potentially toxic materials, such as bovine thrombin, is avoided [602]. In addition, the calcium results in reduced burst protein release compared with thrombin, leading to a more sustained delivery of growth factors [603]. Furthermore, leukocytes can be removed from PRGF to eliminate the pro-inflammatory impacts of the proteases contained in white blood cells, such as acid hydrolases and metalloproteases, which may provoke tissue-destroying effects [604]. Most importantly, it is possible to regulate the platelet concentration and, therefore, the dose of endogenous growth factors within a target product by adjusting the processing parameters, among other variables. Finally, predictable platelet dosing also allows final control of the molecule/protein ratio because PRP contains a mixture of growth factors and bioactive agents involved in both plasma and platelets [605].
It has long been well demonstrated that platelet preparations promote tissue regeneration by inducing cell migration to and proliferation and differentiation in the area of an injury, which are essential processes for regeneration [590]. The ability of PRP preparations that mimic a native ECM milieu to recruit host progenitor/stem cells to enhance endogenous regenerative processes has also been proposed for further investigation [55]. In one study, when used as an adjuvant agent in arthroscopic microfracture surgery for the management of osteochondral lesions of the talus, PRP resulted in an improved functional status score in the medium term [606]. Additionally, when used in lumbar spine surgery for patients with posterolateral arthrodesis, a cancellous bone substitute soaked in PRP resulted in an enhanced fusion rate during the first 6 months after surgery and increased bone density, thus joining osteoinductive and osteoconductive effects [607]. Recently, it has also been shown that PRP derived from bone marrow aspirate promotes new cementum formation in rat periodontal fenestration defect models [608] and that a single autologous PRP injection combined with a rehabilitation program is an effective treatment for hamstring muscle injuries, one of the most common types of injury affecting athletes [609]. Although a growing body of evidence supports the use of PRP as a clinical treatment for bone, muscle, tendon, cartilage and periodontal injury in reconstructive surgery, with short-term clinical benefits, most of the studies published to date are of poor quality and at high risk of bias, and indeed, improvements in healing and clinical outcomes have not been universally reported [44,574,590,591]. One reason for this poor quality may be that each PRP product varies from the others and contains different cocktails of growth factors and regulatory molecules. Typically, the volume of whole blood collected, the efficacy of platelet recovery, the final concentration of platelets in the plasma, the absence or presence of white blood cells and the addition of xenogenic thrombin to activate the platelets or calcium chloride to induce fibrin formation can all influence the character and potential efficacy of the resulting PRP formulation [610]. It should be noted that the lack of standardized and optimized protocols might partly account for the outcome variability across patient populations receiving PRP-based therapies. Therefore, it is still impossible to compare data from different investigations to draw a general conclusion. Further high-quality comparative studies with longer follow-up periods are needed to ascertain whether PRP is beneficial, either alone or as an adjunct to other interventions during surgical procedures.
6.2. Platelet-rich fibrin
Although PRP use offers some efficacy in certain types of acute and chronic wounds, plasma-rich scaffold-like biomaterials, such as fibrin and PRF, might be the best choice if the aim is to retain growth factors from an excessive initial burst release upon implantation and to maintain the concentration of these therapeutic agents at a site of injury for a desired period (Fig. 17) [611]. As a major blood component responsible for hemostasis, fibrin has a long history of use for hemorrhage control. Thanks to fiber branching, fibrin fibers are able to auto-assemble into a mesh scaffold without the help of other proteins. However, this help is required if the purpose is to generate an intricate network based on collagen fibers [612]. At a damaged site in need of tissue regeneration, fibrin is a provisional matrix generated by fibrinogen polymerization in the presence of thrombin, which is not a regular component of the ECM [613]. Because different bioactive molecules are enclosed within the fibrin mesh, it offers a perfect tool to seal surgical defects while promoting the full re-epithelialization of soft tissues [44,591,604]. During the wound healing process, the mesh is progressively replaced by structurally stabilized ECM, and finally, new tissue is formed [613]. Thus, fibrin plays a pivotal role in the physiological wound healing cascade. Recently, fibrin has also been extensively applied, either alone or in combination with other materials, as a biopolymer material for scaffolding purposes in tissue engineering. To this end, fibrin is a prime material of human origin for producing auto-assembled 3D scaffolds for rapid clinical translation [611–614].
Along with the ECM assemblies (e.g., collagen and GAGs) noted in Section 4.2, fibrous-type proteins present in blood (e.g., fibrinogen and fibronectin) are currently considered the ideal components to prepare bioscaffolds for tissue engineering. As a unique group of blood-derived products including platelet- and cell-derived active components, fibrin polymers may be formed through self-assembly or following enzymatic activation (Fig. 18). The normal function of protein bioscaffolds in wound healing is to prevent the loss of body fluid and to provide stability to biological structures. In regenerative medicine, polymerized fibers elicit multiple physiological responses that ensure pivotal functions to provide mechanical and flexible support, as well as tensile strength, and hence reconstruct injured or pathologically abnormal tissues [42]. As a typical bioscaffold composed of platelet concentrates, PRF consists of a fibrin matrix polymerized in a tetra molecular structure that contains all the beneficial constituents of a blood sample that are favorable to tissue regeneration [42,614]. Because it features all the crucial parameters permitting optimal wound healing, the clinical experience confirms that PRF can be considered a healing biomaterial. PRF already has a list of clinical uses, and considering its advantages over traditionally prepared PRP, numerous bioengineering applications can also be imagined, increasing its popularity [615–617].
Fig. 18.
Representative scanning electron microscopy (SEM) images of a well-prepared platelet-rich fibrin (PRF) generated by fibrinogen polymerization. (A) A scaffold-like PRF derived from human blood; (B) a magnified view of a portion of (A) showing a dense crosslinked fibrin lattice; (C) a magnified view of a portion of (B) showing plenty of platelets (white arrows) enclosed within the fibrin mesh structures; (D) a magnified view of a portion of (C) showing the interconnected three-dimensional (3D) network of the PRF scaffold, which may facilitate cell penetration and accommodation.
Prepared at the bedside from a small volume of the patient's own blood, PRF, consisting of a dense crosslinked fibrin lattice itself, contains an abundant variety of signals and growth factors that facilitate tissue repair [614]. Simply by modulating the platelet activation process, PRGF and PRF technologies have been established to yield a 3D fibrin material for the controlled delivery of growth factors [605]. Because autologous fibrinogen can be acquired from plasma, PRF avoids the risk of a foreign-body response upon application [612]. To this end, scaffold-like PRF represents a new generation of formulations that have been demonstrated to possess several benefits over traditional PRP, such as their ease of production/application and their lack of a need for biochemical handling of blood [611]. Indeed, fibrin has been clinically employed as a safe and versatile biomaterial for stimulating and accelerating wound healing and tissue regeneration in numerous medical conditions [593,611,617]. Interestingly, recent evidence suggests that a 3D fibrin scaffold may mimic the main components of the hematopoietic niche, thus meeting the optimal requirements of clinical protocols for cord blood-hematopoietic stem cell expansion and transplantation [618].
PRF scaffolds containing a 3D macroscopic network are now easily generated from human blood by biotechnological methods without the use of exogenous thrombin and may be applied in diverse situations to aid in tissue regeneration and to facilitate wound healing [619]. It is recognized that the inherent properties of a prefabricated PRF can determine the pattern of growth factor release, including the establishment of a provisionally stable fibrin matrix; the platelet concentration in the PRF; the concentration and type of the platelet activator used; the rate and amount of individual growth factors released from the activated platelets; the fibrin network and degradation rate; and the specifications of the anatomical site targeted for implantation, such as the fluid turnover rate [620]. During the formation of a temporary matrix, several parameters can be modified to alter PRF's mechanical properties, structure and degradation. For instance, the ionic strength of or thrombin concentration in the solution may affect the crosslinking time of PRF, which in turn determines its fiber diameter and pore size [599]. Additionally, the tensile strength and shear modulus can be optimized by varying the concentration of two components, calcium and fibrinogen, respectively. The inclusion of antifibrinolytic agents in formulations of fibrin glue may delay or slow fibrinolysis, a process leading to the destruction of PRF gel by impairing blood clot formation and stability [617]. It cannot be ignored that, in addition to variation in PRF products, the quantity of PRF produced from an autologous blood sample is quite low, and only a small volume can be utilized. An optimized and reproducible protocol for the preparation of PRF with regard to its growth factor content and its structure for cell accommodation remains undefined.
In recent years, fibrin-based scaffolds have paved the way to the regeneration of a large variety of human tissues, such as bone, cartilage, adipose tissue, cardiac tissue, ocular tissue, nervous tissue, liver, skin, ligaments and tendons [614]. In tissue engineering and clinical applications, PRF has been demonstrated to offer the sustained release of various bioactive agents important for tissue repair while retaining the agents' bioactivity against proteolytic degradation [42,617]. Recently, evidence has shown that well-prepared PRF can gradually release a pool of endogenous growth factors for a period lasting more than 20 days, implying a potentially durable effect on wound repair [616]. Thus, autogenetic PRF may serve as a spacefilling matrix for implantable fillings or as a regenerative material. The best characteristics of a PRF matrix for the clinical demands of tissue engineering biomaterials include its autologous nature, low cost, good manageability, functional flexibility and absence of any allergic reaction or other adverse effects in the patient [612]. In this respect, PRF has been validated as an entirely autologous, injectable cell delivery system that overcomes the histocompatibility issues related to synthetic scaffolds while ensuring a stable 3D matrix for residing cells both in vivo and ex vivo [65,615]. Due to the non-cytotoxic, biocompatible and non-immunogenic nature of these fibrin scaffolds, the combination of PRF with exogenously manipulated cells and recombinant human growth factors has opened new avenues for various biomedical applications, especially for bone tissue engineering and the regeneration of cartilage and periodontal tissues [56,104,621].
When used alone, PRF, which is composed of cellular and fibrillar components, not only acts as a vehicle for proteins and growth factors but also permits cellular penetration and subsequent integration of newly formed tissue into the native tissue [605]. The versatility of PRF can be broadened when the patient's autologous tissues are transferred along with PRF for grafting or when PRF is used in association with other naturally derived materials, such as gelatin, alginate, collagen or CS, for scaffold modification [241,622–626]. PRF combined with additional natural or synthetic materials can yield scaffolds that offer tighter control over their cargo biodistribution and pharmacokinetics [44,605]. For instance, the growth factors released from PRF following its activation can be immobilized by the addition of an acidic gelatin material with an isoelectric point of 5.0; the physicochemical interplay substantially alters the growth factors' kinetic profile because release depends on hydrogel degradation [622]. Similar strategies have been reported using CS [627], alginate [623], collagen [624] or PCL-tricalcium phosphate (TCP) composites [625] as the underlying biomaterials. Interestingly, via a heparin-binding delivery system, the incorporation of exogenous growth factors or other bioactive molecules into its mesh structures may additionally improve the functionality of PRF as a scaffolding material [438]. To this end, recent technologies, such as magnetically influenced self-assembly and inkjet printing, are able to predict the most appropriate hierarchical structures of a fibrin structure for a given target application. Based on the inkjet printing technique, for example, PRF can be utilized as a printable gel to generate specific cell patterns in a 3D construct, offering a milieu that mimics the highly hydrated state of a native tissue, which renders this type of construct a promising candidate for cell delivery and tissue engineering [614].
In addition to the sustained release of growth factors with spatiotemporal accuracy, many materials can provide increased strength and stability to the fibrin scaffold, resulting in increased mechanical stability [612,614]. PRF in turn may facilitate the manipulation and handling of a wide variety of polymers. In oral implantology, for example, the handling and use of certain bone grafting biomaterials and even bone autografts are relatively challenging. Because PRF acts as a biological glue to hold matrix particles together, it is possible to improve the handling and adaptation of bone grafts by combining them with scaffold-like PRF prior to their transplantation into a defect site [44,591,605]. Moreover, the incorporation of PRF into alginate-based or hydrocolloid dressings for the management of chronic ulcers, the combination of PRF with augmentation materials applied in orthopedic surgery and the production and use of a hemostatic and elastic fibrin for a specific application have also been tested in recent years and have yielded promising results [599]. Although PRF offers a versatile tool with great potential for use in biomaterials science and regenerative medicine, more intensive investigations are required to illustrate the molecular roles that drive the varying biological performance of PRF and to identify potential new therapeutic indications for and applications of PRF technologies [620].
6.3. Platelet lysate
In most clinical trials, FBS is used as the main nutritional supplement in cell culture media. Although FBS is a widely accepted standard, its use is discouraged by regulatory authorities due to its high lot-to-lot variability even from the same manufacturer, coupled with concerns relating to its biosafety and clinical availability. Aside from ethical considerations, the most important concern is the risk of zoonoses due to the transmission of bacteria, viruses and/or prions and immunological reactions due to its xenogenic origin. Therefore, there is an increasing need to search for xeno-free agents to replace FBS [619,620]. Unfortunately, alternatives to FBS that yield competitive results for both cell isolation and cell expansion have not been identified thus far [628]. In addition, a chemically defined xeno-free nutritional supplement that is as good as FBS would perhaps be more expensive, which would ultimately hamper production on a large scale [629]. Human blood preparations, such as autologous and allogenic human sera; PRP; cord blood serum; and human platelet derivatives, including platelet lysate (PL) and platelet-released factors, have been efficiently implemented into MSC clinical manufacturing and increasingly introduced into stem cell therapy as a compelling substitute for FBS [630–632].
For the safe translation of stem cell therapy to the bedside, the standardized, large-scale propagation of MSCs in animal serum-free medium (without using animal-derived components) is quite important and profoundly impacts the overall safety of stem cell therapies [633]. It is suggested that cell culture with PL supplementation does not change the expression of surface markers on cultured cells compared to that in cultures supplemented with FBS [634]. Most importantly, these cells retain their biological features, such as their growth and differentiation potential favoring target tissue regeneration [634,635]. In recent years, cumulative evidence has strongly indicated that human PL-supplemented media not only preserve cell phenotypes and shorten culture time by increasing the cells' growth rate but also maintain the cells' multilineage differentiation capacity and can give rise to osteoblasts, chondrocytes, adipocytes, neurons and astrocytes, among others [630,634–638]. Interestingly, accompanied by a more compact colony formation and a more spindle-shaped morphology, PL-cultured BMSCs [639] and ADSCs [640] remained significantly smaller in size than did FBS-cultured cells. These small-sized cells occupied less space in the culture, and hence their intravenous administration would be safer and effective because smaller cells facilitate cell penetration through capillary vessel systems, while larger cells can create obstructions in the cardiac and pulmonary microcirculation [639,640]. Further evidence suggests that PRP cultures significantly inhibit serum starvation- or TNF-α/cycloheximide-induced apoptosis in multipotential preadipocytes. These results imply that cell culture with PL for clinical transplantation may increase the survival of stem cells [641]. In terms of chromosomal stability [637] and immunomodulatory capacity [630,633,635], no differences were observed between stem cells cultured under standard conditions and those cultured with human PL. All these findings recommend substituting FBS with human PL for cell expansion if the expanded cells are for clinical use. The functional capacity of PL-expanded cells, however, has only been partially explored. For example, impaired immunomodulatory properties have also been observed for MSCs cultured with human PL, implying some potential limitations in the utility of PL-expanded MSCs as immunomodulators in clinical applications [642]. Replacing FBS with human PL prevents bovine prion, viral and zoonotic contamination of the stem cell product, and there is evidence showing that good manufacturing practice (GMP)-compliant culture medium with human PL in a closed hollow-fiber-based bioreactor can be employed for large-scale MSC expansion for safe application in humans [643]. Recently, human PL was subjected to pathogen inactivation by psoralen to further eliminate the possibility of PL as a human-derived blood material to alleviate immunologic risks. The resulting product is an even safer alternative than PL to FBS, and is even more advantageous with regard to cellular growth and stemness [644]. The exploration of human platelet derivatives for clinical-scale MSC expansion may represent a major step toward promising new stem cell therapies [637].
As a culture substitute for clinical-scale human MSC expansion, PL may fully replace FBS, and its expansion-enhancing effect is likely due to the high concentration of native growth factors and bioactive molecules that PL may contain [636,637]. Human PL can be generated by subjecting common platelet units to several freeze and thaw cycles, which damages the membranes of platelets and releases their natural growth factors into the plasma. The platelet fragments are removed by centrifugation to deplete potential antigens and to avoid extensive aggregate formation. The major advantage of using human donor recipient-matched or autologous PL as an alternative to FBS is the elimination of any risk of secondary impacts that may be induced by FBS constituents in cell culture [645]. However, the potential contamination associated with adventitious agents in blood-derived substituents remains a risk. Fortunately, such a risk may be decreased by strict adherence to blood bank quality standards for blood collection, handling and processing. Another important point that should be noted is that the growth factor levels within human platelets vary significantly from donor to donor; pooling many prescreened individual samples may reduce the high variability among blood samples of human origin [646]. Although the removal of platelet fragments creates PL for allogenic application, it is safer if human PL from the same donor is used, which may further minimize the risk of immunological side effects and viral infections [647,648].
The transfusion of blood and blood components has been the standard of care for treating anemia for more than half a century. Platelets separated from the white and red blood cell fractions are of specific interest because they do not compete with the need for erythrocyte and plasma preparations for the limited number of available blood donations. The buffy coat-derived platelets used for PL preparation can be concentrated to at least 1 × 109 platelets/mL by centrifugation [646]. A cocktail of growth factors, cytokines and mitogens stored in the α granules of platelets can be released following platelet activation by thrombin or the disruption of platelets by freeze thawing, with the latter approach being more attractive because the use of commercially available thrombin derived from bovine plasma is bypassed [649]. Hence, human PL may be an adequate non-xenogenic alternative to FBS in many cell culture systems that have been previously thought to be solely dependent on the presence of FBS [629,650].
MSC therapies are limited by the loss of self-renewal and cell plasticity associated with ex vivo expansion in culture and, on transplantation, by increased immunogenicity due to xenogen exposure during culture. Recently, it has become increasingly clear that PL stimulation induces a transient increase in the inflammatory response in quiescent human osteoblasts during bone regeneration. This increase is mediated by NF-κB activation, cyclooxygenase-2 induction, prostaglandin E2 production and the secretion of pro-inflammatory cytokines. Furthermore, long-term PL stimulation enhances the proliferation of actively replicating osteoblasts without affecting their differentiation potential, along with changes in cell morphology, resulting in increased cell density at confluence [651]. Notably, human PL cultures of aged and senescent MSCs demonstrate cellular rejuvenation, reflected by a decreased doubling time and smaller cell size. These findings suggest that PL culture not only enhances MSC proliferation but also positively affects the physiological properties of MSCs [652]. Aside from its use in cell processing, human PL has been used to functionalize biomaterials for tissue engineering [653] (Fig. 18). For example, a PL coating directly increases the chemoattraction and adhesion of human MSCs and endothelial cells to a scaffold. Moreover, such a coating was demonstrated to induce human MSCs to produce and secrete pro-angiogenic proteins (e.g., placental growth factor and VEGF), which may in turn positively affect cell behavior, leading to synergistic effects that enhance in vivo neovascularization and new bone formation [654].
In sum, human PL has multiple beneficial effects on therapeutic cells. In addition to PL's clinical safety, the significant increases in proliferation in culture with PL compared with standard FBS culture allow more rapid culture expansion of MSCs to clinically relevant numbers ex vivo, without compromising their genomic stability or differentiation capacity [655]. Furthermore, PL cultures of high-passage and senescent MSCs may rejuvenate the cells, allowing them to be expanded to clinically relevant numbers ex vivo, even after being cultured for a short duration with conventional FBS supplementation [652]. The utility of native growth factors presented in well-prepared PL in therapeutics is revolutionizing many biomedical areas, cell biology and regenerative medicine. To succeed, scientists are actively working in this field to characterize the platelet secretome, to improve strategies for endogenous growth factor administration and to design new regenerative biomaterials that increase the versatility of human PL application.
7. Future directions and outlook
Taking inspiration from native ECMs, scientists have on one hand sought help from chemistry in designing regenerative biomaterials and, on the other hand, are seeking physics-derived solutions for controlling biological responses [34]. Recently, biomaterial strategies have considerably advanced to closely mimic the constituents and framework of a native tissue and to incorporate structural, biochemical and biomechanical signals that are able to communicate with cells and with the in vivo microenvironment in a biologically specific manner [48,656]. Future developments in biomaterials science, as well as in related fields (e.g., medicine, biology, chemistry and engineering), will need a thoughtful approach to ensure that tissue engineering fulfills its true clinical potential. To enter widespread clinical application, biomedical engineering and regenerative paradigms are required to be not only scientifically appropriate but also inexpensive, safe and clinically expeditious [16,582]. Expedited strategies for tissue regeneration based on human-derived biomaterials are compatible with existing clinical modalities and have dramatically accelerated the pace of the clinical translation of tissue engineering therapies. In parallel, the natural ECM, with its multitude of evolved cell-instructive and cell-responsive properties, provides inspiration and guidelines for the design of advanced biomaterials that recapitulate a 3D, ECM-mimicking environment to activate specific cell–material interactions for the organization of individual cells into functional tissues [279,293,398,471].
7.1. Design of ECM-mimicking biomaterials
Together with new insights into ECM assembly and its role during tissue development, expansion and regeneration, advanced biomaterials for tissue engineering have of late largely exceeded the requirements for passive biocompatibility that were previously considered acceptable for medical devices, and a new era of biomimicry has been unveiled to yield synthetic ECM-mimicking biomaterials that have both multi-component frameworks and high degrees of compositional and functional definition [37,471,657,658]. Cells residing in living systems are extremely sensitive to their surrounding physicochemical milieu, continually reading microenvironmental cues and responding to them to control the cellular phenotype and functions and to promote homeostasis [32,474]. Biomimicry, “a new science that studies nature's models and then imitates or takes inspiration from these designs and processes to solve human problems”, has recently been introduced as a term to describe design innovations in biomaterials science [24,25,659]. Cells that grow in a natural niche incorporating blood vessels continuously receive nutrients and oxygen, and their waste products, including CO2, are continuously removed [26,286,287]. In contrast, when cells are grown in a stagnant biomaterial-based scaffold without an ECM-mimicking nanoenvironment, nutrients and oxygen are not transported into the cells, and waste products are not removed from the cells. Thus, the nutrient concentration decreases and the concentration of waste products increases until the artificial scaffold is eventually noxious for cell viability and function [45,114,293]. Such an artificial biomaterial is far from the ideal environment that these cells experience in their natural state and hence is not suitable for new tissue growth and reconstruction. Although a particular cell behavior may be imitated in vitro by mimicking the corresponding in vivo conditions, the design of materials that closely mimic the hierarchical architecture and the biochemical and biophysical properties of the native ECM of human tissues remains an elusive goal and a great challenge [32,61,97,299]. The tools provided by synthetic biology and protein engineering, as well as electrospinning, have offered an unprecedented level of biomimicry, which is invaluable for the biomimetic design of the next generation of advanced cell-instructive biomaterials; these materials can provide attractive ECM conditions conductive to specific cell behaviors, including, but not limited to, the anchorage, migration and differentiation of different types of stem cells required for successful tissue regeneration [54,660–663].
In fact, biomimetic biomaterials inspired by the dynamics of the stem cell–niche interactions of the natural ECM have shown significant potential as instructive matrices or cell vehicles for tissue engineering that are also targeted for regeneration [67,112,292,294,661,664–666] (Fig. 19). Regarding the physical properties of such materials, they are biochemically and mechanically defined by the tissue of origin, and their physical architecture and chemical composition are organized in a nanoscale manner, in addition to having the required nanotopographical surface features [34,667]. In general, synthetic scaffolds exhibit uniformly distributed porosity, whereas biomimetic materials may not need to be uniformly porous because in natural tissue, porosity is not uniformly distributed [668]. Based on recent innovations in scaffold design and advanced scaffold-manufacturing technologies, a diverse distribution or gradient of porosity throughout the scaffold dimension can be achieved to mimic nature with respect to nanoscale topographical features, micro- and macroscale gradient structures, interconnectivity, pore size and size distribution [97] (Fig. 20). Recently, biocomposite nanofibrous scaffolds consisting of two or more polymeric blends were fabricated into uniform copolymers with interconnected pores to form flexible, cell-supporting substrates with desired biofunctional and biomechanical properties dependent on their applications [669]. The intricate and ingenious geometry is responsible for the overall performance of such tissue engineering templates [33,34]. As the primary role of biomaterials, a certain level of physical support from the moment of implantation must also be ensured to guarantee mechanical integrity and assist tissue function while new matrix is being deposited [34]. However, establishing a clear and direct mechanistic correlation between the nanomechanical properties of the individual constituent macromolecules and the emergent mechanical performance of the resulting bulk polymeric materials as a guide for the biomimetic design of advanced materials with well-defined bulk mechanical properties remains a challenge [33,670].
Fig. 19.
Design of extracellular matrix (ECM)-mimicking scaffolds. (A) Scanning electron microscopy (SEM) micrograph of a three-dimensional (3D) nanofibrous gelatin (NF-gelatin) scaffold that mimics the physical architecture and chemical composition of type I collagen in the ECM. (B) Higher-magnification image of (A), showing the nanofibrous pore walls of the gelatin scaffold. (C) Incorporation of bone-like apatite into the surface of the 3D NF-gelatin to further mimic the inorganic components of bone ECM and improve the mechanical strength of the scaffolding. (D) Incorporation of non-collagen proteins (NCPs) into the surface of the 3D NF-gelatin to form an artificial matrix (NF-gelatin-NCPs) mimicking both the nano-structured architecture and the chemical composition of natural bone ECM. The NCPs were labeled with fluorescein isothiocyanate (FITC). (E) Design and synthesis of nanofibrous hollow microspheres integrating the ECM-mimicking architecture that have a highly porous, injectable form, efficiently accommodating cells and enhancing tissue regeneration. (F) Higher-magnification image of (A), showing the nanofibers of the injectable hollow microspheres.
Source: (A–C) [67], Copyright 2009, (D) [292], Copyright 2013, (E and F) [112], Copyright 2011. Reproduced, respectively, with permission from Elsevier Ltd, Mary Ann Liebert Inc and Nature Publishing Group.
Fig. 20.
Representative scanning electron microscopy (SEM) images of glycidyl methacrylate-derivatized dextran (Dex-GMA)/gelatin scaffolds with intricate and ingenious hierarchical structures (a diverse distribution of porosity throughout the scaffold dimension), designed in our laboratory using a computer-aided technique, that resemble naturally derived extracellular matrix (ECM) with regard to geometry, interconnectivity, pore size and size distribution (images from unpublished results; a sample with similar architecture has been shown in Ref [56]). (A) Material niches with nano-and microscale features; (B) a magnified view of the central region shown in (A); (C) a magnified view of a portion of (B); and (D) a magnified view of the marginal region shown in (A).
Considering that the survival of cells following in vivo transplantation is often poor when the cells are placed in a suboptimal environment with an absence of necessary ECM macromolecules, such as a prefabricated scaffold without any biomimetic modifications, materials design has recently moved from simple cell delivery and physical support to the creation of an artificial niche inspired by tissue-specific niches [387,671]. Defining an artificial in vivo milieu with complex and dynamic regulation where cells interact and behave according to the surrounding environmental cues, ECM-mimicking substrates and scaffolds are excellent candidates for regenerative medicine because in our bodies, cells rest upon or are surrounded by ECM, which functions not only as a support material but also as a regulator of cellular events [18,37,297]. In this respect, today, the development of biomaterials for tissue engineering is increasingly considering the growing knowledge of and new insights into ECM components and structures that affect tissue development and regeneration [165,522,672]. To provide a strategy complementary to the ingenious provision of ECM-mimicking information to cells inside manmade scaffolds, structural, physical and biomechanical factors continue to be intensively exploited while attempting to effectively present all of the necessary influences found in the native milieu of a given tissue [20,113]. For clinical translation and success, however, the complexity of the resulting biomaterials should be reduced to a commercially acceptable level, regardless of the modes of action. To meet this prerequisite, to date, most efforts have concentrated on distillation of the essential chemical character of ECM influences into simple chemical functionalities for future scaffolding production [20].
In recent years, awareness that the ECM has a pivotal regulatory function, directly contributing to fundamental aspects of cell behavior and tissue formation, has increased [279]. The compositions, structures and biomechanical properties of ECM networks and molecules vary according to each specific tissue and organ and also dynamically change and remodel during tissue development and regeneration [26,276,474]. Ideally, the synthetic biomaterials used in tissue engineering and cell delivery should create the same or similar microenvironment for the seeded cells as that in an ECM existing in vivo. However, native ECMs have very intricate structures and are composed of numerous types of proteins, many of which remain unidentified thus far [37,280]. Therefore, using conventional physicochemical methods, it is difficult to obtain a scaffold or substratum that has the same composition and the complex microstructure and architecture of an in vivo ECM [477]. Biomimetic hydrogels formed by self-assembled biopolymer networks such as silk, keratin elastin, resilin and periodate oxidized alginate and gelatin display close chemical, structural and mechanical similarities with the native ECM and have therefore been widely used as artificial cell niches with tunable properties that favor cell functions similar to the events occurring in natural extracellular microenvironments [64,317,387,469,673,674]. In addition, these hydrogels, which are either derived from naturally occurring molecules or are synthetic polymers that recapitulate key motifs of biomolecules, typically have a highly interconnected porous network, good biological compatibility and maybe degraded by proteolytic enzymes in the body, holding great promise for various biomedical applications [88,675]. However, the reproducibility of cell constructs often remains complicated because of batch-to-batch variation and the sensitivity of cells (especially progenitor or stem cells) to these differences. Additionally, due to their variable printability, the implementation of these biomaterials via biofabrication may be challenging [676]. Furthermore, the changing biochemical cues in the hydrogels developed to date often induce simultaneous changes in the hydrogels' mechanical properties, which does not support mechanistic investigations of the stem cell-niche interplay under highly controlled conditions. To address this concern, a PEG-based interpenetrating network was recently developed as an artificial cell niche that resembles the micro- and nanoenvironment found in a natural tissue. The resulting hydrogel was composed of two polymer networks that could independently and simultaneously crosslink to form hydrogels in a cell-friendly manner. Hence, the hydrogel allowed independently tunable biochemical and mechanical properties and stable and more homogeneous presentation of biochemical ligands in 3D than allowed by currently available methods [677]. Upon presentation of appropriate biological cues, a transglutaminase factor XIII (FXIII)-crosslinked, PEG-based biomimetic 3D matrix was also found to mobilize mesenchymal progenitor cells from the amnion to proliferate and secrete native ECM proteins for fetal membrane healing [678]. More work must be performed to clarify the characteristics of a specific native tissue, to then design a scaffold with the proper mechanical properties and to ultimately define the cellular and molecular mechanisms behind the healing processes that are required for successful tissue repair and regeneration [48].
At the cellular level, recruiting cells out of their inherent niches demands comprehensive insight into the biophysical and biochemical cues that control stem cell actions. Instead of an individual entity that probes its surrounding milieu and responds to the players to which it is exposed, the cell is a key part of the living system, residing within a complex and dynamic architecture generated by itself [107,672]. The coordinated interplay between surrounding cells, soluble morphogens and growth factors, as well as insoluble biomacromolecules of the ECM, is orchestrated by spatiotemporal signaling patterns. In this context, cells obtain and process information from their surrounding microenvironment (i.e., the ECM) while they remodel its components and geometry [679]. A vital goal of tissue engineering and scaffolding science is therefore to generate a manmade ECM that at least partially resembles the most critical aspects of such a complex native scenario, such that the processes controlling cell function and cell commitment can be regulated and understood [294]. The degradation of scaffolding materials into macromolecules and the subsequent release of cell-instructive signals into a target place have opened a biomedically exploitable avenue toward modifying the in vivo cellular milieu into a more refined condition [680]. Nonetheless, our poor understanding of the signaling pathways that dictate cell biology, together with the complexity of the natural ECM system and its dynamic interactions with niche cells, makes the design of appropriate ECM-mimicking biomaterials exceedingly difficult [37,672,681,682].
Researchers have thus attempted to use ECM in tissues and organs or ECM proteins produced by in vitro-cultured cells after decellularization treatment [477]. Meanwhile, the technology of scaffolding science has provided an exciting opportunity to deconstruct this landscape to identify and evaluate the particular effects of a specific ECM component on the hosted cells [113]. Moreover, microfabrication and, more recently, nanofabrication are allowing the creation of appropriate investigating models in which the necessary biologics may be assessed on the supramillimeter to the nanometer scale [683–686]. Interestingly, following recent mining of the diversity of functional peptide modules, the modular design of ECM-mimetic protein-based biomaterials has become possible, which involves the combination of multiple protein domains with diverse functionalities into an individual, modular polymer sequence or motif (generally termed protein engineering), leading to a multifunctional matrix endowed with single functional domains that are independently tunable [661]. To this end, both decellularized scaffolds and synthetic matrices are being explored to satisfy the needs of the clinic, and the fundamental mechanisms by which stem cells contribute to regeneration and homeostasis are beginning to be clarified. To advance the field, multifaceted technologies will be increasingly necessary to examine and coax cells ex vivo, to engineer predictive cell and tissue constructs and to ultimately improve stem cell integration and tissue regeneration in vivo for therapeutic benefit [18].
7.2. Revisiting ECM influences for information
For all cells outside the circulation, the ECM constitutes the cellular milieu that is known to have a major instructive or regulatory effect on cell fate commitments [687]. Based on this principle, by introducing specific structural and molecular elements in varying geometries and at different concentrations, a range of biomaterials with tissue-specific structural properties and bioinspired polymeric surface close to those of the native ECM can be created [687,688]. Upon application, penetrating cells may be surrounded by a dynamic pericellular matrix, similar to a native matrix, that possesses considerable regulatory potential [689–692]. This approach is spurring interest in the use of natural ECM proteins for scaffolding production. To this end, most research has been focused on isolated ECM proteins and their combinations, although the ECM has a complex composition in each specific tissue. However, each tissue is often regionally specialized, regardless of its type and size. In this regard, a recent investigation of anatomically distinct cartilages presented substantial evidence for intriguing tropism in diverse patterns of joint pathology, highlighting distinct variations in protein patterns associated with different tissue mechanical properties [693]. Nevertheless, biomaterial strategies for the recreation of ECM influences in simplified forms, the reduction of biopolymers into short functional domains and the application of basic chemistries as well as biological mediators to control cell fate have paved the way for the development of a new generation of scaffolds and medical devices for tissue engineering [20,293,694]. In this context, for example, extracellular vesicles (EVs) that comprise a heterogeneous population of cell-derived lipid vesicles (e.g., exosomes, microvesicles and others) have recently emerged as patient-derived vehicles for targeted drug delivery in regenerative applications. Moreover, these vesicles are recognized as playing crucial roles in cell-based therapies because they are primary biological mediators of intercellular information transfer in multiple physiological systems. There is a growing need for biologists and material scientists to understand and exploit the bioactivity of EVs secreted by therapeutic cells, harness such information for the design of artificial ECM materials and specifically control their biological performance to enhance the efficacy of regenerative therapies [695].
If simplified into its essential mechanical elements, the ECM is composed of several secreted proteins (collagens, fibronectin, elastin and fibrillins) that constitute macromolecular structures in their functional embodiments, such as fibrils, microfibrils or fibers [285]. This category of ECM components also includes enzymes that post-translationally modify these biomacromolecules, such as proteinases, which cleave peptide bonds (e.g., the matrix metalloproteinases), and lysyl oxidase, which forms intermolecular crosslinks [20]. Another category of ECM molecules (termed matricellular proteins, such as thrombospondins and tenascins) is in charge of modulating cell functions and cell–matrix interplay, without a direct contribution to the generation or function of structural complexes [276]. The design of new ECM-like biomaterials should begin with exploring the simplest functional performance of several ECM components that are indispensable to addressing a defined clinical question and should then involve other elements and soluble factors that may act cooperatively or synergistically with these key macromolecules to ensure that the extracellular microenvironment can be properly recapitulated in 3D [28,279]. Of note, early ambitious approaches attempting to engineer whole organs have mostly given way to smaller, more accessible and practical goals. This change explains why clinical research on cardiac regeneration, for example, is now focusing on engineering coronary arteries, myocardium and valves individually, rather than trying to replace an entire heart [20,26,520].
The GAG hyaluronan is the main nonproteinaceous ECM component, and several core proteins of the ECM may be amended by the linkage of different types of GAG chains to form proteoglycans. These carbohydrate-rich components may be hydrated to exert a swelling pressure against the surrounding fibrous networks, which results in tissue turgidity and compressibility for molecular transport. Based on a systems-level bioinformatics view of ECM function and composition, Cromar et al. (2012) defined a set of 357 proteins that represent core components of the ECM, together with an additional 524 genes that modulate their associated functional roles, and generated a map illustrating their physical interactions [696]. In biomaterial design, there is immense current interest in the roles of the physical and mechanical properties of ECM, such as stiffness and elasticity, in affecting cell fate commitment, directly signaling to cells and modulating soluble signals [697]. One role is signaling via matrix adhesion proteins and receptors, such as discoidin domain and integrin receptors [698]. Another role is the activation and sequestration of signaling molecules, such as those in the TGF-β superfamily [699], and the modulation of morphogen gradients. With increasing elucidation of the potential roles of cytokines and growth factors and their interplay with ECM components, advanced materials are being devised that more closely mimic the healing microenvironments of native tissues, leading to increased efficacy in applications in wound healing and tissue regeneration [87]. The cell–matrix interface in particular offers a pivotal signaling nexus that controls all aspects of stem cell activity.
Alongside the traditional focus on the structural relevance of the ECM, which has not diminished, there is now a huge emphasis on several new fields. For instance, mimicking the organizational, biological and functional complexity of native tissue ECM at the molecular level is now regarded as the next challenge in biomaterial innovation [676]. This emerging interdisciplinary research area provides a platform on which to establish a tangible translational pipeline, such as by modifying chemical signals to responsive cells and by creating manmade tissues or organs using mechanical and adhesive molecules as raw building blocks [39]. It is plausible that in the near future, exciting developments that rise to the demands and realities of the marketplace will inform us how these goals can be realized at the clinical level and how even relatively practical and simple regenerative solutions may render considerable functional benefits [16,38,700].
7.3. Cell-formed decellularized matrices
Although decellularized matrices derived from tissues and organs have many advantages in terms of their composition, microstructure and biomechanical properties, their utility is limited by the availability, geometry and constitutive properties of the tissue or organ from which the ECM is harvested [701]. In particular, tissue and organ decellularization is not suitable for the preparation of decellularized matrices mimicking an ECM that is specific to a region of tissue, such as stem cell niches [477,671]. Knowing that the composition of the ECM depends on the types of residing cells, the tissue type and the organ type and that only native tissue-like matrices can have the greatest effect on cellular functions, matrices with unique physical and chemical attributes formed by a variety of cell lineages (e.g., skin fibroblasts, brain-derived astrocytes and MSCs) have been developed for a variety of research and medical applications [701–705]. This self-assembly strategy stems from the capability of MSCs to secrete, deposit and organize their own ECM. When these cells are cultured in vitro, ECM proteins are secreted from the cells and then deposited beneath the cells. The decellularization of these cell-formed matrices enables the production of autologous constructs from stem cells or from tissue-specific cells [243]. Recently, ECM derived from MSCs has been demonstrated to maintain the multipotent potential of MSCs during in vitro expansion and to rejuvenate the cell functions of aging MSCs, indicating that cell-formed ECM is an appropriate culture substrate to improve the bioactivity of scaffolding biomaterials to facilitate cell penetration [706]. These biomaterials can be stored until use in the engineering of autologous living tissues, such as blood vessels, using autologous vascular cells, accelerating the production of vascular substitutes [707]. After the formation of an ECM by cultured cells, cell-formed matrices can be extracted without the use of detergents, sterilized, and then used to coat tissue culture plates or can be directly used as a new cell culture substrate after a decellularization treatment; the resultant animal-free product can support the culture and differentiation of human stem cells [708].
Today, many types of predominantly cell-derived substrates and matrices are prepared under various culture conditions to satisfy specific application needs, including, but not limited to, cell expansion; tissue engineering; and, recently, growth factor delivery (Fig. 21). Along these lines, a fibroblast-derived, ECM-mediated platform was successfully engineered for VEGF delivery; its capacity to convey VEGF in a tailored way resulted in an advanced angiogenic response that promoted blood vessel ingrowth and maturation [709]. In another study, to create a cell-formed nanofibrous scaffold, a fibroblast cell sheet with highly aligned cells and ECM nanofibers was first generated by directing the growth of human dermal fibroblasts on synthetic nanogratings. A highly aligned nanofibrous ECM scaffold was then obtained after removing the cellular components from the sheet. Due to the preservation of the highly aligned elastin fibers, the elastic modulus was well maintained. Reseeding of cells indicated the excellent capacity of the scaffold to provide cell adhesion sites and to support and direct cell proliferation and alignment along the underlying fibers, suggesting that a specific cell-formed matrix may be harnessed to direct particular cell behaviors [710]. Indeed, when stem cells differentiate into somatic cells, their differentiation proceeds in step-by-step manner, and their cell-formed ECM is a crucial factor in instructing cell activities in tissue engineering design [711]. Far from being a static structure, during this stepwise differentiation of stem cells, the ECM surrounding the differentiating cells continues to undergo remodeling (i.e., assembly and degradation) according to the stage of development, differentiation and tissue regeneration [296]. The chemical and physical interactions of cells with the surrounding soluble and/or non-soluble messengers/components of the ECM play fundamental roles in these cellular processes. Moreover, cells can sense the stiffness and nanoscale features (e.g., ligand presentation and substrate topography) of the ECM and can deform it via generating cytoskeletal tension [712]. Given that mimicking the ECM at each maturational stage is one of the important approaches for directing stem cell differentiation, decellularized matrices mimicking the ECM during the osteogenesis or adipogenesis of MSCs were developed as stepwise tissue development-mimicking matrices [522,713]. These stepwise cell-formed, tissue development-mimicking matrices can be applied not only in regenerative medicine but also in basic biological studies. The fact that cells reside in their specific ECM within a complex in vivo milieu elicits the necessity to further illustrate the impacts of ECMs produced by various cell sources on overall cell fates [714]. More exciting than expected, a cohesive cell-formed sheet can be layered into 3D tissues or organs with physiological mechanical strength [715,716]; this concept has been demonstrated to be feasible in clinical sittings [717]. In addition, there is a wealth of evidence that cell-formed ECM products can be applied to enhance the large-scale expansion of highly functional adult human MSCs [718] or to decorate the surfaces of synthetic polymers and manufactured metals on which subsequent cell adhesion is regulated by adsorbed ECM proteins [96,184,719,720]. It is self-evident that the physical properties of synthetic biomaterials can be extensively tailored, but it is also clear that these materials often suffer from limited biological functionalities. Fortunately, their biological performance in in vivo application can be enhanced by ECM deposition by cultured cells in vitro and subsequent decellularization, as demonstrated by the resulting product's maintenance of its molecular functionality, retention of its structural integrity and enhancement of tissue regeneration following its in vivo transplantation [497]. Considering that polymers can be tailored for surface-driven ECM assembly, a microtextured polydimethylsiloxane scaffold was developed to induce cell-formed ECM deposition. When cultured on this scaffold, which had defined microtopography and chemistry, human fibroblasts underwent significant changes in their morphology, adhesion and actin cytoskeleton, which finally led to the generation of compacted units of fibronectin on the surface of the scaffold [721]. These findings consolidate the vista of in vitro bioreactor-based production of ECM-decorated materials as “off-the-shelf” hybrid scaffolds (i.e., carefully engineered synthetic polymers modified by naturally derived ECM) combining selected structural and mechanical properties with the physiological presentation of native cell-secreted ECM macromolecules and instructive biological signals [497,721]. One of the current strategies to enhance the osseointegrative performance of titanium and its related implants relies on the regulation of their surface cell receptor–ligand interactions, in which surface-bound ECM proteins act as ligands, whereas integrins act as cell receptors [665,722–724]. The surface modification technique has therefore been established as a reliable strategy for implant optimization. However, when linked to implant surfaces, specific ECM proteins and peptide sequences appear to be limited in their ability to trigger native cell responses. To cope with this limitation, native or synthetic ECM is applied to search for an optimal composition and structure that may help to overcome the difficulty [725].
Fig. 21.
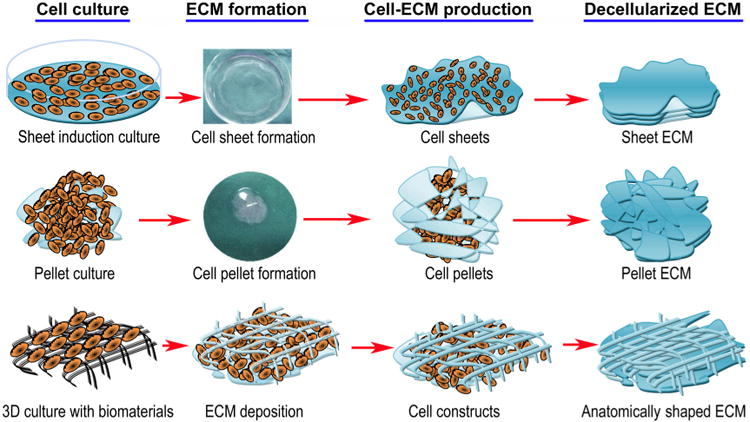
Schematic representation of several cell culture methods frequently used for the preparation of cell-formed decellularized extracellular matrices (ECMs) that satisfy specific application needs [477].
As a scaffold-free cell delivery approach, as well as a modern technique for the generation of functional tissue- or organ-like structures, cell sheet technology is also based on ECM formation by cultured cells [726]. In this regard, a “cell sheet” composed of living cells and their secreted ECM may be created and grafted onto the surface of a cell culture dish with the help of a temperature-responsive, intelligent polymer. By altering the temperature to below 32 °C, a contiguous cell sheet capable of maintaining intact ECM components and cell–cell interactions can be obtained [726]. Such strategies are relatively complicated and time-consuming. To simplify the process, modified protocols based on dexamethasone, vitamin C or osthole have been developed to create cell sheets that do not require special materials (Fig. 22). The resulting sheets can be used for cell transplantation, without the need for synthetic biomaterials, which has already paved the way to clinical therapeutics and clinically relevant animal experiments, such as studies on visual acuity, cardiomyopathy, esophageal ulcerations, periodontics and type 1 diabetes, confirming the safety and efficacy of the treatment [726–728]. Recently, decellularized cell sheets retaining ECM integrity have emerged as an interesting cell-derived ECM product for diverse biomedical uses because they offer a naturally occurring matrix with a complex set of physiologically functional biomolecules for cell repopulation and function [275,704,729,730]. More specifically, such cell-formed constructs may possess the ability to support allogenic recellularization; this direction is in need of investigation of both techniques and applications [730]. Compared with decellularized tissue- and organ-derived ECM templates, cell-formed decellularized matrices have the advantages of unlimited availability and adaptability to different developmental stages. However, in the self-assembly approach to ECM biomaterials, it is difficult to mimic the complicated native ECM-like architectures. Because the ECM secretion pattern of in vitro-cultured cells is different from that of cells in vivo, the potential difference in ECM composition and structure between cell-formed decellularized matrices and native ECMs should be considered when cell-formed decellularized matrices are used in tissue engineering applications [477].
Fig. 22.
Representative scanning electron microscopy (SEM) images of cell sheet formation. Bone marrow mesenchymal stem cells were plated into 6-well plates at a density of 1 × 106 cells/well and cultured until the cells reached 100% confluence, after which they were induced to form cell sheets using cell-sheet-inducing medium (i.e., basal medium supplemented with 50 μg/mL vitamin C) for a 10-day period. (A) Three days following cell sheet induction: extracellular matrix protein production; (B) 5 days following cell sheet induction: cell–cell and cell–matrix interactions established; (C) 5 days following cell sheet induction: sheet-like structure formation; (D) 10 days following cell sheet induction: complete cell sheet formation.
7.4. ECM–stem cell interactions: signposts in advanced biomaterials
The ECM–stem cell interface is a complex, dynamic milieu wherein the cells bind to and contract against the ECM, commonly via transmembrane receptors of the integrin family and by means of a feedback-signaling process. This binding cooperatively dictates their respective fates via the interactions of nanotopographical features with integrin receptors in the cells' focal adhesions [325,667,712]. Tissue engineering approaches integrating material nanotopography, integrin-matrix interactions and soluble growth factors (e.g., presented by the materials) could lead to the development of ingenious extracellular niches that can alter how cells adhere to material surfaces and regulate cellular differentiation commitment and functions through changes in both cell morphology and cell biochemistry [277,667,731,732]. Recent endeavors have tried to decipher the complex interaction between ECM niches and stem cells, and it is increasingly evident that inherent biomaterial properties (e.g., physicochemical properties or nanoscale surface features) can be exploited to control stem cell fate commitment [667]. Thus, the ability to harness nanoscale and nanotopographical design and material–stem cell interactions is poised to have substantial implications for the redesign of the next generation of stem cell delivery systems and tissue engineering templates [325,671,712,733]. Traditional cellular scaffolds served as inert matrices that merely acted as a support for the attached cells; however, recently, more emphasis has been placed on adding appropriate physical and chemical properties to these platforms for cell transplantation and tissue engineering [3]. In fact, several of these recent innovations have led to new insights into the design of artificial niches encompassing a wide range of biophysical, biochemical and biomechanical cues that are able to play a guiding role to elicit targeted cell functions [671]. More often than expected, the coordinated interplay between resident cells and their surrounding natural or synthetic scaffolds, soluble factors and other niche cells can define a local mechanical and biochemical microenvironment that has been and still is being studied and exploited to instruct an orchestrated set of cell events and reactions [297,664,734,735].
The last several decades have witnessed remarkable advances in the field of synthetic biology, with a significant increase in the number of patients benefiting annually from the therapeutics that have arisen from this field [107]. Today, synthetic biology is gaining increasing attention in materials science research, including research into the signals that cells directly acquire via interactions with scaffolds or bioactive molecules in their environment [20,70,736]. Notably, the inspiration that directs the development of new materials science strategies is commonly drawn from the elucidation of the biomaterials organized naturally by living cells in tissue on different scales [737]. Therefore, penetrating insight into thus far undiscovered areas motivates the development of new tools that allow for the more thorough exploration of cell–ECM interplay and its effects in a feedback manner [738–740].
As mentioned previously, molecular-scale interactions between stem cells and the surrounding ECM can be exploited to modulate the stem cell niche for facilitated tissue regeneration because an in vivo milieu represents a significant entry point for the therapeutic regulation of stem cell function [477,502,741] (Fig. 23). Given that potentially available progenitor/stem cells already exist in the body, a particularly exciting area involves the manipulation of the cellular components of the stem cell microenvironment to target specific aspects of the niche, normally by the administration of chemokines or other chemoattractants that act cooperatively or synergistically with chemokines, to induce the mobilization and homing of host reparative cells [277,741]. These cell populations can interpret and interact with multiple input signals and hence can be recruited to areas of tissue damage to acquire active biological functions, such as secreting a cocktail of immune-regulating signals or growth factors instead of, or in addition to, directly participating in regeneration of the tissue (Fig. 24) [22,277,498]. As an alternative or adjunct to cell transplantation therapy, which is inherently expensive and labor intensive, this strategy has the advantage of repairing damaged tissue with the patient's own stem/progenitor cells, without the need for specialized facilities for ex vivo cell isolation, expansion and transplantation [742]. Compared with strategies that rely on transplanting stem cells or their differentiated derivatives, this approach circumvents the current translational barriers associated with extensive cell manipulation steps and costly manufacturing challenges associated with the characterization and quality control of a living cell-based therapeutic product. The approach also significantly reduces the amount of time and effort required before implantation, thereby offering the possibility of fostering attractive new therapeutic paradigms [18,277]. Over the past decade, mounting data have revealed the ability of BMSCs to mobilize from the bone marrow to the peripheral blood and to eventually enter an injured site to replenish dying cells and regenerate damaged tissues. However, when the intrinsic biological response is inadequate, the stem cells naturally mobilized in response to injury (e.g., trauma and ischemic diseases) are generally quantitatively insufficient and hence do not offer a universal regenerative solution [277,392,743,744]. Worse, if the competing balance of processes associated with tissue remodeling and inflammation is perturbed, particularly irreversibly, the resulting positive feedback loops can continue to exacerbate tissue damage and deterioration and compromise cell recruitment and regeneration [745]. Therefore, in most cases, interventions such as the administration of biomaterials, small biomolecules, genes or other biological agents may be required to ensure a satisfactory therapeutic benefit. A question that arises here is thus how required clinical efficacy can be achieved while the complexity of the therapeutic interventions used is kept to a minimum; indeed, such cell-free interventions are less complex than the transplantation of cells manipulated ex vivo [277].
Fig. 23.
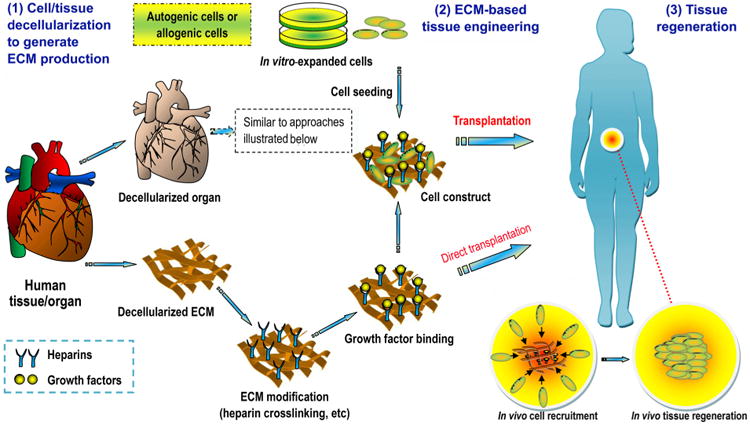
Concept of the use of decellularized tissue (organ) as a tissue engineering biomaterial (schematic is not to scale). Decellularized tissue (organ) can be obtained by the decellularization of living tissue (organ). The resulting cell-free ECM can be modified (e.g., by heparin crosslinking and growth factor binding) before implantation. To this end, the ECM scaffold can be reseeded with ex vivo-cultured cells that “prime” the biomaterial (e.g., to enhance its ability in vascularization or remodeling) and/or “get primed” toward a specific cell fate decision (e.g., to proliferate or differentiate). Such a cell–matrix construct could induce tissue regeneration by the combined action of seeded and recruited cells in a functionalized native matrix. Alternatively, modified ECM can be directly transplanted into a patient without cell seeding. In this case, tissue regeneration entirely relies on the capacity of the ECM material to instruct resident cells toward target recruitment, specific differentiation and subsequent tissue formation (endogenous tissue regeneration).
Fig. 24.
Schematic representation of cell migration mechanisms within the body that may be modulated to induce the recruitment or homing of endogenous stem cells for wound healing and regeneration (illustrations not to scale). (A) Stem cells located in cell niches neighboring an injury can recognize and obey the gradients of signals (e.g., directional cues) within the extracellular matrix (ECM) and reach the site of injury, independent of blood flow, normally via active amoeboid movement or chemotaxis-guided interstitial migration. (B) Stem cells from central cell niches (i.e., the bone marrow) can be mobilized to enter the blood (i.e., by signals produced in response to injury or systemically administered homing factors) and disseminated throughout the blood circulation system until they reach the local capillary vessel network surrounding the diseased site. Here, the cells recognize and interact with microvascular endothelial cells, after which they exit the blood to replenish and maintain the cell niche neighboring the injury and hence enhance the regenerative potential of the injured tissue. Alternatively, the cells can escape from the circulation and directly migrate to the injury site to participate in wound healing and tissue regeneration.
The quest for clinical strategies that might improve the body's natural regeneration mechanisms must be based on a thorough understanding of the cellular and molecular events underpinning tissue repair and its failure after surgery; trauma; and vascular disease, diabetes and other disease conditions that are frequently associated with healing pathologies [746]. To date, however, complete knowledge of in vivo cell repopulation has yet to be established with regard to what cell types need to be recruited; what chemoattractant/signaling molecules are required to modulate the niche therapeutically and ensure successful homing; whether a precise chronological sequence of events occurs during such homing processes; and, if so, how each biological event and the human immune system can be orchestrated to enable the successful regeneration and integration of the targeted tissue via materials design [268,476]. Nevertheless, there is emerging recognition that this approach is practical and effective in certain ideal clinical scenarios. A clearer picture is emerging that a target-specific biomaterial scaffolding system that can effectively control the host microenvironment and mobilize host stem/progenitor cells to the damaged areas is generally required to practice this concept [500]. Although damage to cells at a site of injury can result in neighboring cells dedifferentiating to replace the damaged cells, cell populations residing within tissues neighboring an insult are generally too low in number to have a clinically meaningful therapeutic effect. In most cases, it is therefore worthwhile to target stem cells from the circulation (such as BMSCs) to be mobilized into the peripheral blood system and, finally, directed to the target sites [747] (Fig. 24). A wide spectrum of cytokines and chemoattractants contribute to the whole-cell mobilization, homing and engraftment process, and the ability to control the spatiotemporal presentation of these bioactive agents may provide important clues as to how to devise biomaterials that ensure new tissues can reliably be generated in this way (reviewed in Refs. [22,500]). The use of biomaterials alone to coax host stem cell homing for in situ tissue regeneration is a new concept in tissue engineering. In this context, an appropriate combination of multiple growth factors in a cellular materials and their spatiotemporal coordination will provide a favorable microenvironment that leads to more successful outcomes in tissue regeneration [29,30,57,105,108,109,502,748]. The design of cell recruitment to biomaterials, how these biomaterials may establish the necessary interplay with endogenous cells in a way that unlocks the body's intrinsic capabilities of self-repair and organization and the implications in tissue engineering have been reviewed elsewhere [57,498].
New insights into the complex interplay and pathways of the biomolecules that are involved in targeted tissue repair are necessary to achieve effective therapeutic outcomes for translation into the clinic [684]. Nevertheless, the enhancement of endogenous tissue repair by a cellular materials or suitable chemokine delivery devices is not a replacement for stem cell therapy or transplantation. First, although an artificial increase in the concentrations of specific chemokines (such as SDF-1) at diseased sites offers an up-to-date paradigm to enhance and potentiate the homing of host reparative cells and to amplify in situ tissue regeneration processes, in vivo chemokine-guided recruitment of the whole spectrum of parenchymal and stromal cells for the reconstruction of complex tissues remains infeasible [392,476]. Second, endogenous regeneration may still not be sufficient in many situations or could be further enhanced by the delivery of exogenous cells; hence, the second strategy is the application of biomaterials as delivery vehicles for therapeutic cells [58]. In this context, exogenous cells are used for injection/transplantation or delivery via various types of material vehicles, either directly or in the form of cell sheets/pellets. The cells may be autologously sourced, with or without outside expansion, or may be allogeneic; regardless, they can be genetically manipulated to over-express and secrete selected factors or delivered in combination with bioactive agents. Upon transplantation, these cells may be able to reverse the typical course of cellular apoptosis and local inflammation within an injury site and markedly accelerate regeneration in certain types of tissue damage [105]. To engineer various tissues, such as liver or heart tissue, cell-dense structures that are similar to the native geometry and architecture are necessary. Developments in this field have considerable potential to synthesize “intelligent” materials that can communicate with the matrix, cells and multiple signaling factors [110]. Further investigations will be necessary to fully identify and capture the intricacy and role of this cell–scaffold–cell feedback loop [276,278]. It is notable that as an established clinical technique, cell sheet engineering has been based on the fact that it possible to obtain a sheet-like construct full of cells, along with their cell-formed ECM and natural cell-to-cell junctions [725].
Regardless of the application paradigm (with cells or with cell removal via decellularization), the utility of an individual ECM assembly or a known combination of ECM components can be more advantageous than utilizing whole ECM products due to the potential to selectively regulate biological activities [298]. Scaffold development in tissue engineering is rapidly advancing to display properties that, in a physiological and precise fashion, could manipulate stem cell fate by recreating native ECM function to elaborate the full complexity of biological signaling both in vitro and in vivo [297,311,749] (Fig. 25). In an in vitro environment mimicking the in vivo extracellular fluid inherent to living bone tissue in terms of three major features (i.e., monodispersed fibrils, high fibrillar density and long-range hierarchical organization), collagen mineralization can occur based on a collagen/apatite self-assembly process, even in the absence of Ca-rich NCPs, which were previously thought to play an important role in bone formation [319]. Therefore, in bone tissue engineering, the design and processing of synthetic hybrid materials in which multiple polymer constituents or phases are organized across multiple length scales, together with environmental cues, have allowed replication of the desirable biological activity of bone via easy-to-fabricate polymeric materials with nanoscale features such as substrate topography and surface ligand presentation [164,165,750,751]. The biological response to these new tissue engineering templates often exceeds that observed with scaffolds synthesized using biological materials, even if the scaffold's content and architecture, the host immune response and the scaffold's functional response to mechanical stimulation have all been carefully taken into consideration [165,666,752]. Based on the development of advanced biomaterials with defined micro- and nanostructures allowing for the presentation of endogenous and/or exogenous bioactive factors in a physiological manner, future advances in this discipline should aim to create cell–material and cell–cell interactions that encourage overall improvement following regeneration [12,55]. However, the pursuit of effective cell and protein (gene) delivery strategies remains hampered by the lack of noninvasive imaging approaches to monitor transplant survival, particularly over a long period [31]. Although recent material technologies enable the use of sophisticated scaffolds to direct or guide highly specific and coordinated cellular events, these reductionist strategies generally cannot satisfy the complexity of cell–ECM interactions and stimuli necessary for tissue regeneration under natural conditions [753]. Moreover, the bidirectional signaling between implanted biomatrices and their host environment, i.e., the dynamic reciprocity that drives the process of constructive remodeling to ensure successful graft integration, and how this signaling may be modulated and exploited to enhance tissue regeneration are far from fully characterized [754]. An increasing appreciation for the microstructure, biomechanical properties and functionality of manmade biological systems has led biomaterials scientists to reconsider nature, the master architect of biomaterials, for design inspiration [293,657]. By supporting microenvironmentally sensitive and cell-dependent binding affinity and integrin specificity, the natural ECM displays complex interactions with cells, leading to finely tuned, dynamically controlled and evolutionarily directed spatial periodicity and remodeling [755]. An area that warrants further attention is native matrix biology during tissue development, homeostasis and regeneration, including, but not limited to, dynamic properties, biological tubes and the active site architecture. Insights into this area will facilitate the generation of in vitro systems that accurately recapitulate the in vivo microenvironment and the subsequent the biomimetic design of biomaterials that can adapt to, or dynamically interact with, surrounding tissue, thereby promoting desirable cellular processes to ensure that natural function can be immediately replicated after the biomaterials' in vivo implantation [293,756,757]. Meanwhile, a fundamental understanding of ECM storage (cues and growth factors) could offer important cues about the nature of molecule–matrix interactions and improve the potency of current biopolymers in the presentation and delivery of multiple therapeutic agents for tissue engineering [101,102]. In the future, the design of next-generation bio-inspired materials should attempt to recapitulate the natural events involved in the secretion, clearance and interaction of endogenously produced molecules within tissues under pathological/healing conditions, as well as during the regeneration of new tissues or organs [31].
Fig. 25.
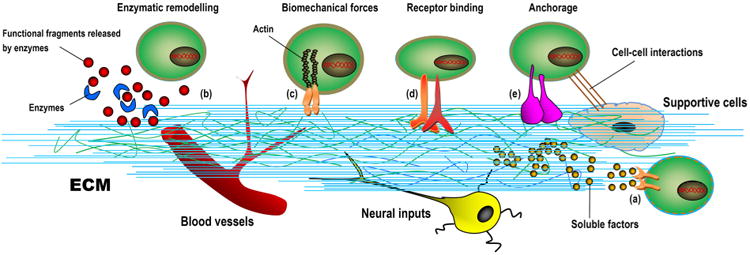
Schematic representation of the main players within the extracellular matrix (ECM), including inputs derived from neural and supportive cells, blood vessels and secreted and paracrine factors and ECM components that need to be captured in advanced biomaterial design to reestablish ECM-stem cell interactions [297]. The ECM offers a highly specialized and dynamic microenvironment encompassing different length scales; in this context, the matrix elasticity/topography and a number of inputs combine and control cell fate commitment. The ECM may act by soluble factor presentation (a) and be remodeled by the action of enzymes that produce functional fragments (b). More importantly, the ECM may directly bind cell surface receptors or co-receptors (c, d and e), thereby potentially regulating cell anchoring and mediating diverse pathways involved in mechanotransduction and intracellular signaling.
8. Conclusions
Increasing evidence suggests that biomaterials of human origin are yielding an ever-growing list of products and successful clinical approaches to maintain, enhance or restore tissues and organs. These “raw materials” continue to demonstrate great potential and will have an increasingly remarkable impact on synthetic biology, tissue engineering and clinical regenerative therapies in the future. Stem cells respond to these biomaterials containing native ECM information via self-recognition and interplay, which are very unlikely to elicit severe negative immune responses upon medical application. Decellularized materials are promising because they take advantage of nature's platform to create an intrinsically complex ECM template that is thought to better mimic the native extracellular surroundings. In addition, synthesized biomaterials are directing our nascent understanding of the cellular milieu and how the basic building blocks (e.g., biomacromolecules) of human systems are correctly integrated into the dynamic landscape that represents tissue physiology. Unfortunately, the science of human ECM biology remains in its infancy, with all current and emerging macromolecular assemblies or ECM mimics having serious limitations. As we advance the science of stem cell–ECM nanointeractions, we will become more precise in our ability to directly dictate regenerative processes based on tissue ECM materials or information coded within the ECM, which provides signposts for advanced biomaterial design. Elucidating the molecular pathways through which cells discriminate signals from their ECM will reveal important signposts in designing new biomimetic polymers tuned for a more defined cellular response, allowing for more controlled and efficient tissue regeneration if progress continues. Additionally, materials science strategies are bridging the gaps in various but interrelated scientific fields, and these strategies have become a comprehensive suite of essential tools in the fields of tissue engineering and regenerative medicine. Although several pivotal questions still need to be addressed, and particularly those concerning cell recruitment and functional integration of recruited cells, at least several responses of the components of these biomaterials to injury are remarkably similar to normal/natural wound healing and regeneration. Meeting the challenge of unraveling these complex mechanisms will be rewarded with therapeutic potential across the field of tissue engineering. A constant influx of new knowledge from biological systems and new structural, chemical, and physical insights into human-derived biomaterials, complemented by recent advances in synthetic technology and biological science, will yield new and more sophisticated biomaterial designs and inspiration in the future. The effect of the field will continue to grow and evolve with the collaborative development of tissue-engineered products that offer simple solutions to complex problems.
Acknowledgments
Due to space limitations, in certain areas, review papers are cited instead of individual contributions; in any case, representation of the entire literature on research and development in the broad field of human-derived biomaterials in the reference list would not be possible. The authors also apologize to those authors whose excellent work in this field is not discussed and cited here due to misunderstanding, oversight or limitation in knowledge. Figs. 8 and 20 were generously provided by Dr. Zhongshan Wang in the Department of Prosthetics, School of Stomatology, Fourth Military Medical University. The authors acknowledge the reuse of certain segments of text from resources by Kretlow et al. (2009) [50] and Kuraitis et al. (2012) [288] in the discussion of autologous/allogeneic materials (Sections 3.1 and 3.2) and ECM components (Section 4.2) due to content overlap, although this content has been rephrased in our own words. Special thanks go to the anonymous referees for their insight and truly stimulating and valuable remarks on a former version of this manuscript. Dr. Chen is supported by the Program for New Century Excellent Talents in University (NCET-12-1005) and the Ministry of Science and Technology Innovation Talent Promotion Plan of Shaanxi Province (Youth Scientific Innovation Talents, 2014SR2014). Dr. Liu is supported by NIH/NIDCRR03DE22838 and R41DE024343. Portions of the corresponding author's work discussed in this review were financially supported by the National Natural Science Foundation of China (Nos. 31170912 and 81471791) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT13051).
Abbreviations
- 3D
three-dimensional
- ACI
autologous chondrocyte implantation
- ACS
absorbable collagen sponge
- AM
amniotic membrane
- ASI
alternating solution immersion
- ASCs
adipose-derived stem cells
- BBIM
bioactive bone-inducing material
- BM
basement membrane
- BMC
bone marrow concentrate
- BMPs
bone morphogenetic proteins
- CaP
calcium phosphate
- CBD-BMP-2
collagen-binding domain bone morphogenetic protein-2
- CCC
cortical cancellous chips
- CMC
carboxymethylcellulose
- CNTF
ciliary neurotrophic factor
- CS
chondroitin sulfate
- CTA
complex tissue allotransplantation
- DBM
demineralized bone matrix
- DDM
demineralized dentin matrix
- Dex-GMA
glycidyl methacrylate-derivatized dextran
- DFDBAs
demineralized freeze-dried bone allografts
- DMD
Duchenne muscular dystrophy
- ECGF
epithelial cell growth factor
- ECM
extracellular matrix
- EDTA
ethylenediaminete-traacetic acid
- EGF
epidermal growth factor
- EGTA
ethylene glycol tetraacetic acid
- EPCs
endothelial progenitor cells
- ESCs
embryonic stem cells
- FAM
fiber-assisted molding
- FBS
fetal bovine serum
- FDA
Food and Drug Administration
- FDBAs
freeze-dried bone allografts
- FGG
free gingival graft
- GAGs
glycosaminoglycans
- GMP
good manufacturing practice
- HA
hyaluronic acid
- HGF
hepatocyte growth factor
- HIV
immunodeficiency virus
- HLA
human leukocyte antigen
- HS
heparin sulfate
- HSCs
hematopoietic stem cells
- ICBG
iliac crest bone graft
- IGF
insulin-like growth factor
- IVD
intervertebral disc
- MMP2
matrix metalloproteinase 2
- MSCs
mesenchymal stem cells
- NCPs
non-collagen proteins
- NF-κB
nuclear factor-κB
- NF-gelatin
nanofibrous gelatin
- NP
nucleus pulposus
- PCL
poly(ε-caprolactone)
- PDAF
platelet-derived angiogenesis factor
- PDEGF
platelet-derived endothelial growth factor
- PDGFs
platelet-derived growth factors
- PEG
polyethylene glycol
- PF-4
platelet factor-4
- PGA
polyglycolic acid
- PL
platelet lysate
- PLA
polylactic acid
- PLGA
poly(lactic-co-glycolic acid)
- PRF
platelet-rich fibrin
- PRGF
plasma rich ingrowth factor
- PRP
platelet-rich plasma
- RGD
arginine–glycine–aspartic acid
- rhBMP-2
recombinant human bone morphogenetic protein-2
- rhELR
recombinant human elastin-like polymer
- SDF-1
stromal cell-derived factor-1
- SDS
sodium dodecyl sulfate
- SEM
scanning electron microscopy
- SF
silk fibroin
- SIS
small intestinal submucosa
- SM
stromal matrix
- SVF
stromal vascular fraction
- TCP
tricalcium phosphate
- TGF-β
transforming growth factor-β
- VEGFs
vascular endothelial growth factors
References
- 1.Gurtner GC, Callaghan MJ, Longaker MT. Progress and potential for regenerative medicine. Annu Rev Med. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg AW. Engineered tissue grafts: opportunities and challenges in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2012;4:207–20. doi: 10.1002/wsbm.164. [DOI] [PubMed] [Google Scholar]
- 5.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan D, Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307–39. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varner VD, Nelson CM. Toward the directed self-assembly of engineered tissues. Annu Rev Chem Biomol Eng. 2014;5:507–26. doi: 10.1146/annurev-chembioeng-060713-040016. [DOI] [PubMed] [Google Scholar]
- 7.Urciuolo F, Imparato G, Totaro A, Netti PA. Building a tissue in vitro from the bottom up: implications in regenerative medicine. Methodist Debakey Cardiovasc J. 2013;9:213–7. doi: 10.14797/mdcj-9-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JS, Gartner ZJ. Directing the assembly of spatially organized multicomponent tissues from the bottom up. Trends Cell Biol. 2012;22:683–91. doi: 10.1016/j.tcb.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmiero C, Imparato G, Urciuolo F, Netti P. Engineered dermal equivalent tissue in vitro by assembly of microtissue precursors. Acta Biomater. 2010;6:2548–53. doi: 10.1016/j.actbio.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Griffith LG, Naughton G. Tissue engineering – current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 11.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest. 2012;122:3817–23. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson S, D'Souza RN, Hematti P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng Part A. 2014;20:2162–8. doi: 10.1089/ten.tea.2013.0359. [DOI] [PubMed] [Google Scholar]
- 14.Dawson JI, Oreffo RO. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys. 2008;473:124–31. doi: 10.1016/j.abb.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Fisher MB, Mauck RL. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng Part B Rev. 2013;19:1–13. doi: 10.1089/ten.teb.2012.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen FM, Zhao YM, Jin Y, Shi S. Prospects for translational regenerative medicine. Biotechnol Adv. 2012;30:658–72. doi: 10.1016/j.biotechadv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Harrison RH, St-Pierre JP, Stevens MM. Tissue engineering and regenerative medicine: a year in review. Tissue Eng Part B Rev. 2014;20:1–16. doi: 10.1089/ten.TEB.2013.0668. [DOI] [PubMed] [Google Scholar]
- 18.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanker JS, Giammara BL. Biomaterials and biomedical devices. Science. 1988;242:885–92. doi: 10.1126/science.3055300. [DOI] [PubMed] [Google Scholar]
- 20.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 21.Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189–209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 23.Williams DF. The biomaterials conundrum in tissue engineering. Tissue Eng Part A. 2014;20:1129–31. doi: 10.1089/ten.tea.2013.0769. [DOI] [PubMed] [Google Scholar]
- 24.Kim BS, Park IK, Hoshiba T, Jiang HL, Choi YJ, Akaike T, Cho CS. Design of artificial extracellular matrices for tissue engineering. Prog Polym Sci. 2011;36:238–68. [Google Scholar]
- 25.Fonseca KB, Granja PL, Barrias CC. Engineering proteolytically-degradable artificial extracellular matrices. Prog Polym Sci. 2014;39:2010–29. [Google Scholar]
- 26.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Guarino V, Causa F, Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 2007;4:405–18. doi: 10.1586/17434440.4.3.405. [DOI] [PubMed] [Google Scholar]
- 28.Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering – Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev. 2013;19:308–26. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering – Part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B Rev. 2013;19:327–52. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KW. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov Today. 2014;19:794–800. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino S, D'Angelo F, Armentano I, Kenny JM, Orlacchio A. Stem cell–biomaterial interactions for regenerative medicine. Biotechnol Adv. 2012;30:338–51. doi: 10.1016/j.biotechadv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Marino A, Filippeschi C, Mattoli V, Mazzolai B, Ciofani G. Biomimicry at the nanoscale: current research and perspectives of two-photon polymerization. Nanoscale. 2015;7:2841–50. doi: 10.1039/c4nr06500j. [DOI] [PubMed] [Google Scholar]
- 33.Meyers MA, McKittrick J, Chen PY. Structural biological materials: critical mechanics–materials connections. Science. 2013;339:773–9. doi: 10.1126/science.1220854. [DOI] [PubMed] [Google Scholar]
- 34.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4:160rv12/1–10. doi: 10.1126/scitranslmed.3004890. [DOI] [PubMed] [Google Scholar]
- 36.Fong EL, Watson BM, Kasper FK, Mikos AG. Building bridges: leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs. Adv Mater. 2012;24:4995–5013. doi: 10.1002/adma.201201762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2:824–6. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- 38.Holzapfel BM, Reichert JC, Schantz JT, Gbureck U, Rackwitz L, Nöth U, Jakob F, Rudert M, Groll J, Hutmacher DW. How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Renth AN, Detamore MS. Leveraging “raw materials” as building blocks and bioactive signals in regenerative medicine. Tissue Eng Part B Rev. 2012;18:341–62. doi: 10.1089/ten.teb.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow D, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A. Peptide-based biopolymers in biomedicine and biotechnology. Mater Sci Eng R Rep. 2008;62:125–55. doi: 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui FZ, Li Y, Ge J. Self-assembly of mineralized collagen composites. Mater Sci Eng R Rep. 2007;57:1–27. [Google Scholar]
- 42.Burnouf T, Goubran HA, Chen TM, Ou KL, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27:77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev. 2010;62:741–52. doi: 10.1016/j.addr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–24. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 46.Dutta RC, Dutta AK. Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv. 2009;27:334–9. doi: 10.1016/j.biotechadv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–56. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 48.Edalat F, Sheu I, Manoucheri S, Khademhosseini A. Material strategies for creating artificial cell-instructive niches. Curr Opin Biotechnol. 2012;23:820–5. doi: 10.1016/j.copbio.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elisseeff J, Ferran A, Hwang S, Varghese S, Zhang Z. The role of biomaterials in stem cell differentiation: applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295–303. doi: 10.1089/scd.2006.15.295. [DOI] [PubMed] [Google Scholar]
- 50.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368–93. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837–45. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 52.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants – a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 54.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6(Suppl.3):S311–24. doi: 10.1098/rsif.2008.0448.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 57.Chen FM, An Y, Zhang R, Zhang M. New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Rel. 2011;149:92–110. doi: 10.1016/j.jconrel.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33:6320–44. doi: 10.1016/j.biomaterials.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 59.Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–36. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 60.Stoppel WL, Ghezzi CE, McNamara SL, Iii LD, Kaplan DL. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43:657–80. doi: 10.1007/s10439-014-1206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, Wang E, Hexemer A, Lee SW. Biomimetic self-templating supramolecular structures. Nature. 2011;478:364–8. doi: 10.1038/nature10513. [DOI] [PubMed] [Google Scholar]
- 62.Murphy CM, O'Brien FJ, Little DG, Schindeler A. Cell–scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013;26:120–32. doi: 10.22203/ecm.v026a09. [DOI] [PubMed] [Google Scholar]
- 63.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–33. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels forbiomedical applications. Prog Polym Sci. 2013;38:672–701. [Google Scholar]
- 65.Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci. 2014;39:1973–86. [Google Scholar]
- 66.Barnes CP, Pemble CW, Brand DD, Simpson DG, Bowlin GL. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007;13:1593–605. doi: 10.1089/ten.2006.0292. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–8. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravindran S, Song Y, George A. Development of three-dimensional biomimetic scaffold to study epithelial–mesenchymal interactions. Tissue Eng Part A. 2010;16:327–42. doi: 10.1089/ten.tea.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng R Rep. 2001;34:147–230. [Google Scholar]
- 70.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 71.Pan Z, Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2:366–77. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okamoto M, John B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog Polym Sci. 2013;38:1487–503. [Google Scholar]
- 73.Liu X, Ma PX. The nanofibrous architecture of poly(l-lactic acid)-based functional copolymers. Biomaterials. 2010;31:259–69. doi: 10.1016/j.biomaterials.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 75.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin HK, Madihally SV, Palmer B, Frimberger D, Fung KM, Kropp BP. Biomatrices for bladder reconstruction. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2014.11.020. http://dx.doi.org/10.1016/j.addr.2014.11.020. [DOI] [PubMed]
- 77.Ribeiro-Filho LA, Sievert KD. Acellular matrix in urethral reconstruction. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2014.11.019. http://dx.doi.org/10.1016/j.addr.2014.11.020. [DOI] [PubMed]
- 78.Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials. 2014;35:6278–86. doi: 10.1016/j.biomaterials.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Z, Tan BH. Towards the development of polycaprolactone based amphiphilic block copolymers: molecular design, self-assembly and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2014;45:620–34. doi: 10.1016/j.msec.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Boffito M, Sirianni P, DiRienzo AM, Chiono V. Thermosensitive block copolymer hydrogels based on poly(ε-caprolactone) and polyethylene glycol for biomedical applications: state of the art and future perspectives. J Biomed Mater Res A. 2015;103:1276–90. doi: 10.1002/jbm.a.35253. [DOI] [PubMed] [Google Scholar]
- 81.Vacanti NM, Cheng H, Hill PS, Guerreiro JD, Dang TT, Ma M, Watson S, Hwang NS, Langer R, Anderson DG. Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules. 2012;13:3031–8. doi: 10.1021/bm300520u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J. Bioactive modification of polyethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, Xie J, Yuan X, Xia Y. Coating electrospun poly(epsilon-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir. 2008;24:14145–50. doi: 10.1021/la802984a. [DOI] [PubMed] [Google Scholar]
- 84.Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci U S A. 2012;109:10012–7. doi: 10.1073/pnas.1121605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12:911–9. doi: 10.1002/mabi.201100466. [DOI] [PubMed] [Google Scholar]
- 86.Rossi F, van Griensven M. Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng Part A. 2014;20:2043–51. doi: 10.1089/ten.tea.2013.0367. [DOI] [PubMed] [Google Scholar]
- 87.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 88.Resende RR, Fonseca EA, Tonelli FM, Sousa BR, Santos AK, Gomes KN, Guatimosim S, Kihara AH, Ladeira LO. Scale/topography of substrates surface resembling extracellular matrix for tissue engineering. J Biomed Nanotechnol. 2014;10:1157–93. doi: 10.1166/jbn.2014.1850. [DOI] [PubMed] [Google Scholar]
- 89.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Green DW, Goto TK, Kim KS, Jung HS. Calcifying tissue regeneration via biomimetic materials chemistry. J R Soc Interface. 2014:20140537/1–11. doi: 10.1098/rsif.2014.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakayama K. Membrane traffic: editorial overview. J Biochem. 2004;136:751–3. doi: 10.1093/jb/mvh183. [DOI] [PubMed] [Google Scholar]
- 92.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol. 2014;15:813–24. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 93.Yu YL, Shao YK, Ding YQ, Lin KZ, Chen B, Zhang HZ, Zhao LN, Wang ZB, Zhang JS, Tang ML, Mei J. Decellularized kidney scaffold-mediated renal regeneration. Biomaterials. 2014;35:6822–8. doi: 10.1016/j.biomaterials.2014.04.074. [DOI] [PubMed] [Google Scholar]
- 94.Zhao N, Wang Z, Cai C, Shen H, Liang F, Wang D, Wang C, Zhu T, Guo J, Wang Y, Liu X, Duan C, Wang H, Mao Y, Jia X, Dong H, Zhang X, Xu J. Bioinspired materials: from low to high dimensional structure. Adv Mater. 2014;26:6994–7017. doi: 10.1002/adma.201401718. [DOI] [PubMed] [Google Scholar]
- 95.Moroni L, de Wijn JR, van Blitterswijk CA. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials. 2006;27:974–85. doi: 10.1016/j.biomaterials.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Tran RT, Thevenot P, Zhang Y, Gyawali D, Tang L, Yang J. Scaffold sheet design strategy for soft tissue engineering. Nat Mater. 2010;3:1375–89. doi: 10.3390/ma3021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang T, Carbone EJ, Lo KWH, Laurencin CT. Electrospinning of polymer nanofibers for tissue regeneration. Prog Polym Sci. 2015 http://dx.doi.org/10.1016/j.progpolymsci.2014.12.001.
- 98.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338:921–6. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 99.O'Brien C, Holmes B, Faucett S, Zhang LG. 3D printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng Part B Rev. 2015;21:103–14. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosseini V, Kollmannsberger P, Ahadian S, Ostrovidov S, Kaji H, Vogel V, Khademhosseini A. Fiber-sssisted molding (FAM) of surfaces with tunable curvature to guide cell alignment and complex tissue architecture. Small. 2014;10:4851–7. doi: 10.1002/smll.201400263. [DOI] [PubMed] [Google Scholar]
- 101.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012;64:1257–76. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uebersax L, Merkle HP, Meinel L. Biopolymer-based growth factor delivery for tissue repair: from natural concepts to engineered systems. Tissue Eng Part B Rev. 2009;15:263–89. doi: 10.1089/ten.TEB.2008.0668. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen MK, Alsberg E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog Polym Sci. 2014;39:1235–65. doi: 10.1016/j.progpolymsci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen FM, Shelton RM, Jin Y, Chapple IL. Localized delivery of growth factors for periodontal tissue regeneration: role, strategies, and perspectives. Med Res Rev. 2009;29:472–513. doi: 10.1002/med.20144. [DOI] [PubMed] [Google Scholar]
- 105.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12:1004–17. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 106.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 107.Lee EJ, Tabor JJ, Mikos AG. Leveraging synthetic biology for tissue engineering applications. Inflamm Regen. 2014;34:15–22. [Google Scholar]
- 108.Azagarsamy MA, Alge DL, Radhakrishnan SJ, Tibbitt MW, Anseth KS. Photocontrolled nanoparticles for on-demand release of proteins. Biomacromolecules. 2012;13:2219–24. doi: 10.1021/bm300646q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Azagarsamy MA, Anseth KS. Wavelength-controlled photocleavage for the orthogonal and sequential release of multiple proteins. Angew Chem Int Ed. 2013;52:13803–7. doi: 10.1002/anie.201308174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vancoillie G, Frank D, Hoogenboom R. Thermoresponsive poly(oligo ethylene glycol acrylates) Prog Polym Sci. 2014;39:1074–95. [Google Scholar]
- 111.Chen FM, Lu H, Wu LA, Gao LN, An Y, Zhang J. Surface-engineering of glycidyl methacrylated dextran/gelatin microcapsules with thermo-responsive poly(N-isopropylacrylamide) gates for controlled delivery of stromal cell-derived factor-1α. Biomaterials. 2013;34:6515–27. doi: 10.1016/j.biomaterials.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10:398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cha C, Liechty WB, Khademhosseini A, Peppas NA. Designing biomaterials to direct stem cell fate. ACS Nano. 2012;6:9353–8. doi: 10.1021/nn304773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang CH, Chen SY, Fu JP, Dai NT, Chen SL, Chen TM, Chen SG. Reconstruction of trochanteric pressure sores with pedicled anterolateral thigh myocutaneous flaps. J Plast Reconstr Aesthet Surg. 2011;64:671–6. doi: 10.1016/j.bjps.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 116.Siemionow M, Papay F, Alam D, Bernard S, Djohan R, Gordon C, Hendrickson M, Lohman R, Eghtesad B, Coffman K, Kodish E, Paradis C, Avery R, Fung J. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009;374:203–9. doi: 10.1016/S0140-6736(09)61155-7. [DOI] [PubMed] [Google Scholar]
- 117.Daar AS. The future of replacement and restorative therapies: from organ transplantation to regenerative medicine. Transplant Proc. 2013;45:3450–2. doi: 10.1016/j.transproceed.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 118.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 119.Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005;46:1531–44. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- 120.Flynn A, Barry F, O'Brien T. UC blood-derived mesenchymal stromal cells: an overview. Cytotherapy. 2007;9:717–26. doi: 10.1080/14653240701584578. [DOI] [PubMed] [Google Scholar]
- 121.Minteer D, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: biology and potential applications. Adv Biochem Eng Biotechnol. 2013;129:59–71. doi: 10.1007/10_2012_146. [DOI] [PubMed] [Google Scholar]
- 122.Sanz AR, Carrión FS, Chaparro AP. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontol. 2015;2000(67):251–67. doi: 10.1111/prd.12070. [DOI] [PubMed] [Google Scholar]
- 123.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 125.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials. 2012;33:6974–86. doi: 10.1016/j.biomaterials.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 127.Gao LN, An Y, Lei M, Li B, Yang H, Lu H, Chen FM, Jin Y. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials. 2013;34:9937–51. doi: 10.1016/j.biomaterials.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 128.Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34:7033–47. doi: 10.1016/j.biomaterials.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Sun HH, Chen B, Zhu QL, Kong H, Li QH, Gao LN, Xiao M, Chen FM, Yu Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials. 2014;35:9459–72. doi: 10.1016/j.biomaterials.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 130.Mayo V, Sawatari Y, Huang CY, Garcia-Godoy F. Neural crest-derived dental stem cells – where we are and where we are going. J Dent. 2014;42:1043–51. doi: 10.1016/j.jdent.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Sedgley CM, Botero TM. Dental stem cells and their sources. Dent Clin North Am. 2012;56:549–61. doi: 10.1016/j.cden.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Vijayan S, Bartlett W, Bentley G, Carrington RW, Skinner JA, Pollock RC, Alorjani M, Briggs TW. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: a two- to eight-year follow-up study. J Bone Joint Surg Br. 2012;94:488–92. doi: 10.1302/0301-620X.94B4.27117. [DOI] [PubMed] [Google Scholar]
- 133.Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29:1872–8. doi: 10.1016/j.arthro.2013.07.271. [DOI] [PubMed] [Google Scholar]
- 134.Edwards PK, Ackland T, Ebert JR. Clinical rehabilitation guidelines for matrix-induced autologous chondrocyte implantation on the tibiofemoral joint. J Orthop Sports Phys Ther. 2014;44:102–19. doi: 10.2519/jospt.2014.5055. [DOI] [PubMed] [Google Scholar]
- 135.Atala A. Organ preservation, organ and cell transplantation, tissue engineering, and regenerative medicine: the terms may change, but the goals remain the same. Tissue Eng Part A. 2014;20:445–6. doi: 10.1089/ten.TEA.2013.0742. [DOI] [PubMed] [Google Scholar]
- 136.Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98:1317–75. doi: 10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 137.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am A. 2002;84:454–64. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 138.Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77–86. [PubMed] [Google Scholar]
- 139.Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–20. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 140.Parrish JN, Metzinger SE. Autogenous fat grafting and breast augmentation: a review of the literature. Aesthet Surg J. 2010;30:549–56. doi: 10.1177/1090820X10380859. [DOI] [PubMed] [Google Scholar]
- 141.Boyce RG, Nuss DW, Kluka EA. The use of autogenous fat, fascia, and nonvascularized muscle grafts in the head and neck. Otolaryngol Clin North Am. 1994;27:39–68. [PubMed] [Google Scholar]
- 142.Wilshaw SP, Kearney J, Fisher J, Ingham E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A. 2008;14:463–72. doi: 10.1089/tea.2007.0145. [DOI] [PubMed] [Google Scholar]
- 143.Laurent R, Nallet A, Obert L, Nicod L, Gindraux F. Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 2014;15:267–75. doi: 10.1007/s10561-014-9437-x. [DOI] [PubMed] [Google Scholar]
- 144.Uçakhan OO, Köklü G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002;21:169–72. doi: 10.1097/00003226-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 145.Hasegawa M, Fujisawa H, Hayashi Y, Yamashita J. Autologous amnion graft for repair of myelomeningocele: technical note and clinical implication. J Clin Neurosci. 2004;11:408–11. doi: 10.1016/j.jocn.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 146.Gutiérrez-Moreno S, Alsina-Gibert M, Sampietro-Colom L, Pedregosa-Fauste S, Ayala-Blanco P. Cost-benefit analysis of amniotic membrane transplantation for venous ulcers of the legs that are refractory to conventional treatment. Actas Dermosifiliogr. 2011;102:284–8. doi: 10.1016/j.ad.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Loeffelbein DJ, Rohleder NH, Eddicks M, Baumann CM, Stoeckel-huber M, Wolff KD, Drecoll E, Steinstraesser L, Hennerbichler S, Kesting MR. Evaluation of human amniotic membrane as a wound dressing for split-thickness skin-graft donor sites. Biomed Res Int. 2014;2014:572183/1–12. doi: 10.1155/2014/572183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Memarzadeh F, Fahd AK, Shamie N, Chuck RS. Comparison of de-epithelialized amniotic membrane transplantation and conjunctival autograft after primary pterygium excision. Eye (Lond) 2008;22:107–12. doi: 10.1038/sj.eye.6702453. [DOI] [PubMed] [Google Scholar]
- 149.Taylan-Şekeroğlu H, Erdem E, Dogan NC, Yagmur M, Ersoz R, Dogan A. Sutureless amniotic membrane transplantation combined with narrow-strip conjunctival autograft for pterygium. Int Ophthalmol. 2011;31:433–8. doi: 10.1007/s10792-011-9488-y. [DOI] [PubMed] [Google Scholar]
- 150.Sullivan HC, Atkins JH. Free autogenous gingival grafts I: Principles of successful grafting. Periodontics. 1968;6:121–9. [PubMed] [Google Scholar]
- 151.Cortellini P, Tonetti M, Prato GP. The partly epithelialized free gingival graft (pe-fgg) at lower incisors. A pilot study with implications for alignment of the mucogingivaljunction. J Clin Periodontol. 2012;39:674–80. doi: 10.1111/j.1600-051X.2012.01896.x. [DOI] [PubMed] [Google Scholar]
- 152.Cairo F, Nieri M, Pagliaro U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J Clin Periodontol. 2014;41(Suppl. 15):S44–62. doi: 10.1111/jcpe.12182. [DOI] [PubMed] [Google Scholar]
- 153.Thoma DS, Buranawat B, Hämmerle CH, Held U, Jung RE. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol. 2014;41(Suppl. 15):S77–91. doi: 10.1111/jcpe.12220. [DOI] [PubMed] [Google Scholar]
- 154.Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–5. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 155.Kretlow JD, Shi M, Young S, Spicer PP, Demian N, Jansen JA, Wong ME, Kasper FK, Mikos AG. Evaluation of soft tissue coverage over porous polymethylmethacrylate space maintainers within nonhealing alveolar bone defects. Tissue Eng Part C Methods. 2010;16:1427–38. doi: 10.1089/ten.tec.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thangavelu A, Santhosh Kumar K, Vaidhyanathan A, Balaji M, Narendar R. Versatility of full thickness skin-subcutaneous fat grafts as interpositional material in the management of temporomandibular joint ankylosis. Int J Oral Maxillofac Surg. 2011;40:50–6. doi: 10.1016/j.ijom.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 157.Dimitroulis G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermisfat graft. Int J Oral Maxillofac Surg. 2011;40:561–8. doi: 10.1016/j.ijom.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 158.McNichols CH, Hatef DA, Cole P, Hollier LH, Thornton JF. Contemporary techniques for the correction of temporal hollowing: augmentation temporoplasty with the classic dermal fat graft. J Craniofac Surg. 2012;23:e234–8. doi: 10.1097/SCS.0b013e31824de5b8. [DOI] [PubMed] [Google Scholar]
- 159.Burres S. Soft-tissue augmentation with Fascian. Clin Plast Surg. 2001;28:101–10. [PubMed] [Google Scholar]
- 160.Wong TY, Chung CH, Huang JS, Chen HA. The inverted temporalis muscle flap forintraoral reconstruction: its rationale andthe results of its application. J Oral Maxillofac Surg. 2004;62:667–75. doi: 10.1016/j.joms.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 161.Kuo YR, Yeh MC, Shih HS, Chen CC, Lin PY, Chiang YC, Jeng SF. Versatility of the anterolateral thigh flap with vascularized fascia lata for reconstruction of complex soft-tissue defects: clinical experience and functional assessment of the donor site. Plast Reconstr Surg. 2009;124:171–80. doi: 10.1097/PRS.0b013e3181a80594. [DOI] [PubMed] [Google Scholar]
- 162.Parodi PC, Moretti L, Saggin G, De Biasio F, Alecci V, Vaienti L. Soft tissue and tendon reconstruction after achilles tendon rupture: adipofascial sural turnover flap associated with cryopreserved gracilis tendon allograft for complicated soft tissue and achilles tendon losses. A case report and literature review. Ann Ital Chir. 2006;77:361–7. [PubMed] [Google Scholar]
- 163.Saint-Cyr M, Wong C, Buchel EW, Colohan S, Pederson WC. Free tissue transfers and replantation. Plast Reconstr Surg. 2012;130:858e–78e. doi: 10.1097/PRS.0b013e31826da2b7. [DOI] [PubMed] [Google Scholar]
- 164.Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, Paik KJ, Longaker MT, Wan DC. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93:1187–95. doi: 10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kane R, Ma PX. Mimicking the nanostructure of bone matrix to regenerate bone. Mater Today. 2013;16:418–23. doi: 10.1016/j.mattod.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon's point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosenberg E, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998;42:467–90. [PubMed] [Google Scholar]
- 168.Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma. 2010;24(Suppl 1):S36–40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 169.Sbordone C, Sbordone L, Toti P, Martuscelli R, Califano L, Guidetti F. Volume changes of grafted autogenous bone in sinus augmentation procedure. J Oral Maxillofac Surg. 2011;69:1633–41. doi: 10.1016/j.joms.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 170.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl. 1):S75–80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 171.Torroni A. Engineered bone grafts and bone flaps for maxillofacial defects: state of the art. J Oral Maxillofac Surg. 2009;67:1121–7. doi: 10.1016/j.joms.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 172.Bhatt RA, Rozental TD. Bone graft substitutes. Hand Clin. 2012;28:457–68. doi: 10.1016/j.hcl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 173.Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–81. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 174.Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25:2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Perlyn CA, Schmelzer R, Govier D, Marsh JL. Congenital scalp and calvarial deficiencies: principles for classification and surgical management. Plast Reconstr Surg. 2005;115:1129–41. doi: 10.1097/01.prs.0000156217.33683.2b. [DOI] [PubMed] [Google Scholar]
- 176.Johnson RG. Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine (Phila Pa 1976) 2014;39:695–700. doi: 10.1097/BRS.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 177.Putzier M, Strube P, Funk JF, Gross C, Mönig HJ, Perka C, Pruss A. Allogenic versus autologous cancellous bone in lumbar segmental spondylodesis: a randomized prospective study. Eur Spine J. 2009;18:687–95. doi: 10.1007/s00586-008-0875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Doi K, Tominaga S, Shibata T. Bone grafts with microvascular anastomoses of vascular pedicles: an experimental study in dogs. J Bone Joint Surg Am. 1977;59:806–15. [PubMed] [Google Scholar]
- 179.Dell PC, Burchardt H, Glowczewskie FP., Jr A roentgenographic, biomechanical, and histological evaluation of vascularized and nonvascularized segmental fibular canine autografts. J Bone Joint Surg Am. 1985;67:105–12. [PubMed] [Google Scholar]
- 180.Stevenson S. Biology of bone grafts. Orthop Clin North Am. 1999;30:543–52. doi: 10.1016/s0030-5898(05)70107-3. [DOI] [PubMed] [Google Scholar]
- 181.Smolka W, Iizuka T. Arthroscopic lysis and lavage in different stages of internal derangement of the temporomandibular joint: correlation of preoperative staging to arthroscopic findings and treatment outcome. J Oral Maxillofac Surg. 2005;63:471–8. doi: 10.1016/j.joms.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 182.Doi K, Hattori Y. Vascularized bone graft from the supracondylar region of the femur. Microsurgery. 2009;29:379–84. doi: 10.1002/micr.20671. [DOI] [PubMed] [Google Scholar]
- 183.Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res. 2010;132:15–30. [PubMed] [Google Scholar]
- 184.Liu X, Chu PK, Ding C. Surface nano-functionalization of biomaterials. Mater Sci Eng R Rep. 2010;70:275–302. [Google Scholar]
- 185.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: bone replacement grafts. Dent Clin North Am. 2010;54:55–71. doi: 10.1016/j.cden.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 186.Ross SE, Cohen DW. The fate of a free osseous tissue autograft. A clinical and histologic case report. Periodontics. 1968;6:145–51. [PubMed] [Google Scholar]
- 187.Hiatt WH, Schallhorn RG, Aaronian AJ. The induction of new bone and cementum formation IV. Microscopic examination of the periodontium following human bone and marrow allograft, autograft and nongraft periodontal regenerative procedures. J Periodontol. 1978;49:495–512. doi: 10.1902/jop.1978.49.10.495. [DOI] [PubMed] [Google Scholar]
- 188.Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16:219–55. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- 189.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superiorto autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16:113–22. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 190.Canan LW, Jr, da Silva Freitas R, Alonso N, Tanikawa DY, Rocha DL, Coelho JC. Human bone morphogenetic protein-2 use for maxillary reconstruction in cleft lip and palate patients. J Craniofac Surg. 2012;23:1627–33. doi: 10.1097/SCS.0b013e31825c75ba. [DOI] [PubMed] [Google Scholar]
- 191.Chen Z, Ba G, Shen T, Fu Q. Recombinant human bone morphogenetic protein-2 versus autogenous iliac crest bone graft for lumbar fusion: a meta-analysis often randomized controlled trials. Arch Orthop Trauma Surg. 2012;132:1725–40. doi: 10.1007/s00402-012-1607-3. [DOI] [PubMed] [Google Scholar]
- 192.Tressler MA, Richards JE, Sofianos D, Comrie FK, Kregor PJ, Obremskey WT. Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics. 2011;34:e877–84. doi: 10.3928/01477447-20111021-09. [DOI] [PubMed] [Google Scholar]
- 193.van Hout WM, Mink van der Molen AB, Breugem CC, Koole R, Van Cann EM. Reconstruction of the alveolar cleft: can growth factor-aided tissue engineering replace autologous bone grafting? A literature review and systematic review of results obtained with bone morphogenetic protein-2. Clin Oral Investig. 2011;15:297–303. doi: 10.1007/s00784-011-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431–41. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 195.Hallman M, Thor A. Bone substitutes and growth factors as an alternative/complement to autogenous bone forgrafting in implant dentistry. Periodontol. 2008;2000(47):172–92. doi: 10.1111/j.1600-0757.2008.00251.x. [DOI] [PubMed] [Google Scholar]
- 196.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 197.Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158:890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 198.Luciani GB, Santini F, Mazzucco A. Autografts, homografts, and xenografts: overview on stentless aortic valve surgery. J Cardiovasc Med (Hagerstown) 2007;8:91–6. doi: 10.2459/01.JCM.0000260208.98246.10. [DOI] [PubMed] [Google Scholar]
- 199.Herberts CA, Park MV, Pot JW, de Vries CG. Results from a horizon scan on risks associated with transplantation of human organs, tissues and cells: from donor to patient. Cell Tissue Bank. 2015;16:1–17. doi: 10.1007/s10561-014-9450-0. [DOI] [PubMed] [Google Scholar]
- 200.Gaafar A, Iqniebi A, Sheereen A, Eldali A, Turpeinen H, Adra C, Al Meshari K, Al Hussein K. Study of the cytokine polymorphisms in correlation to rejection and graft survival in renal allograft donors and recipients from a homogenous Saudi population. Transpl Immunol. 2014;30:34–9. doi: 10.1016/j.trim.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 201.Cassidy D, Beltz J, Jhanji V, Loughnan MS. Recent advances incorneal transplantation for keratoconus. Clin Exp Optom. 2013;96:165–72. doi: 10.1111/cxo.12047. [DOI] [PubMed] [Google Scholar]
- 202.Niederkorn JY. Corneal transplantation and immune privilege. Int Rev Immunol. 2013;32:57–67. doi: 10.3109/08830185.2012.737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ple-Plakon PA, Shtein RM, Musch DC, Blachley T, Saponara F, Woodward MA. Tissue characteristics and reported adverse events after corneal transplantation. Cornea. 2013;32:1339–43. doi: 10.1097/ICO.0b013e3182a0d154. [DOI] [PubMed] [Google Scholar]
- 204.Omar N, Bou Chacra CT, Tabbara KF. Outcome of corneal transplantation in a private institution in Saudi Arabia. Clin Ophthalmol. 2013;7:1311–8. doi: 10.2147/OPTH.S43719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Burd A, Chiu T. Allogenic skin in the treatment of burns. Clin Dermatol. 2005;23:376–87. doi: 10.1016/j.clindermatol.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 206.Bajnrauh R, Nguyen EV, Reifler DM, Wilcox RM. Dressing ignition and facial burns following orbital exenteration. Ophthal Plast Reconstr Surg. 2007;23:409–11. doi: 10.1097/IOP.0b013e318137a1a3. [DOI] [PubMed] [Google Scholar]
- 207.Banks ND, Milner S. Persistence of human skin allograft in a burn patient without exogenous immunosuppression. Plast Reconstr Surg. 2008;121:230e–1e. doi: 10.1097/01.prs.0000305391.98958.95. [DOI] [PubMed] [Google Scholar]
- 208.Cerqueira MT, da Silva LP, Santos TC, Pirraco RP, Correlo VM, Marques AP, Reis RL. Human skin cell fractions fail to self-organize within agellangum/hyaluronic acid matrix but positively influence early wound healing. Tissue Eng Part A. 2014;20:1369–78. doi: 10.1089/ten.tea.2013.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heim-bach D, Kagan R, Sittig K, Dimick A, Herndon D. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17:124–36. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 210.Bondioli E, Fini M, Veronesi F, Giavaresi G, Tschon M, Cenacchi G, Cerasoli S, Giardino R, Melandri D. Development and evaluation of a decellularized membrane from human dermis. J Tissue Eng Regen Med. 2014;8:325–36. doi: 10.1002/term.1530. [DOI] [PubMed] [Google Scholar]
- 211.Levin F, Turbin RE, Langer PD. Acellular human dermal matrix as a skin substitute for reconstruction of large periocular cutaneous defects. Ophthal Plast Reconstr Surg. 2011;27:44–7. doi: 10.1097/IOP.0b013e3181e2f85e. [DOI] [PubMed] [Google Scholar]
- 212.Rennert RC, Sorkin M, Wong VW, Gurtner GC. Organ-level tissue engineering using bioreactor systems and stem cells: implications for transplant surgery. Curr Stem Cell Res Ther. 2014;9:2–9. doi: 10.2174/1574888x113086660069. [DOI] [PubMed] [Google Scholar]
- 213.Ren X, Laugel MC. The next frontier in composite tissue allotransplantation. CNS Neurosci Ther. 2013;19:1–4. doi: 10.1111/cns.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gordon CR, Zor F, Cetrulo C, Jr, Brandacher G, Sacks J, Lee WP. Concomitant face and hand transplantation: perfect solution or perfect storm? Ann Plast Surg. 2011;67:309–14. doi: 10.1097/SAP.0b013e31822a2c8f. [DOI] [PubMed] [Google Scholar]
- 215.Devauchelle B, Badet L, Lengelé B, Morelon E, Testelin S, Michallet M, D'Hauthuille C, Dubernard JM. First human face allograft: early report. Lancet. 2006;368:203–9. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 216.Guo S, Han Y, Zhang X, Lu B, Yi C, Zhang H, Ma X, Wang D, Yang L, Fan X, Liu Y, Lu K, Li H. Human facial allotransplantation: a 2-year follow-up study. Lancet. 2008;372:631–8. doi: 10.1016/S0140-6736(08)61276-3. [DOI] [PubMed] [Google Scholar]
- 217.Siemionow MZ, Papay F, Djohan R, Bernard S, Gordon CR, Alam D, Hendrickson M, Lohman R, Eghtesad B, Fung J. First U.S. near-total human face transplantation: a paradigm shift for massive complex injuries. Plast Reconstr Surg. 2010;125:111–22. doi: 10.1097/PRS.0b013e3181c15c4c. [DOI] [PubMed] [Google Scholar]
- 218.Meningaud JP, Benjoar MD, Hivelin M, Hermeziu O, Toure G, Lantieri L. Procurement of total human face graft for allotransplantation: a preclinical study and the first clinical case. Plast Reconstr Surg. 2010;126:1181–90. doi: 10.1097/PRS.0b013e3181ec2089. [DOI] [PubMed] [Google Scholar]
- 219.Mundinger GS, Drachenberg CB. Chronic rejection in vascularized composite allografts. Curr Opin Organ Transplant. 2014;19:309–14. doi: 10.1097/MOT.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 220.Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation – state of the art. Clin Transplant. 2013;27:330–7. doi: 10.1111/ctr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaufman CL, Ouseph R, Marvin MR, Manon-Matos Y, Blair B, Kutz JE. Monitoring and long-term outcomes in vascularized composite allotransplantation. Curr Opin Organ Transplant. 2013;18:652–8. doi: 10.1097/MOT.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 222.Tapias LF, Ott HC. Decellularized scaffolds as a platform for bioengineered organs. Curr Opin Organ Transplant. 2014;19:145–52. doi: 10.1097/MOT.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Habibovic P, de Groot K. Osteoinductive biomaterials – properties and relevance in bone repair. J Tissue Eng Regen Med. 2007;1:25–32. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 224.Buck BE, Malinin TI. Human bone and tissue allografts. Preparation and safety. Clin Orthop Relat Res. 1994;303:8–17. [PubMed] [Google Scholar]
- 225.Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77-A:1742j–54j. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 226.Bolander ME, Balian G. The use of demineralized bone matrix in the repair of segmental defects: augmentation with extracted matrix proteins and a comparison with autologous grafts. J Bone Joint Surg. 1986;68:1264–74. [PubMed] [Google Scholar]
- 227.Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571–81. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 228.Precheur HV. Bone graft materials. Dent Clin North Am. 2007;51:729–46. doi: 10.1016/j.cden.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 229.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–24. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Holtzclaw D, Toscano N, Eisenlohr L, Callan D. The safety of bone allografts used in dentistry: a review. J Am Dent Assoc. 2008;139:1192–9. doi: 10.14219/jada.archive.2008.0334. [DOI] [PubMed] [Google Scholar]
- 231.Wang HL, Tsao YP. Mineralized bone allograft-plug socket augmentation: rationale and technique. Implant Dent. 2007;16:33–41. doi: 10.1097/ID.0b013e318031ece6. [DOI] [PubMed] [Google Scholar]
- 232.Soardi CM, Spinato S, Zaffe D, Wang HL. Atrophic maxillary floor augmentation by mineralized human bone allograft in sinuses of different size: an histologic and histomorphometric analysis. Clin Oral Implants Res. 2011;22:560–6. doi: 10.1111/j.1600-0501.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- 233.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang M, Powers RM, Jr, Wolfinbarger L., Jr Effect(s) of the demineralization process onthe osteoinductivity of demineralized bone matrix. J Periodontol. 1997;68:1085–92. doi: 10.1902/jop.1997.68.11.1085. [DOI] [PubMed] [Google Scholar]
- 235.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 236.Urist MR, Chang JJ, Lietze A, Huo YK, Brownell AG, DeLange RJ. Preparation and bioassay of bone morphogenic protein and polypetides fragments. Methods Enzymol. 1987;146:294–312. doi: 10.1016/s0076-6879(87)46031-x. [DOI] [PubMed] [Google Scholar]
- 237.Hopp SG, Dahners LE, Gilbert JA. A study of mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res. 1989;7:578–84. doi: 10.1002/jor.1100070416. [DOI] [PubMed] [Google Scholar]
- 238.Wozney JM. Bone morphogenic proteins and their gene expression. In: Masaki N, editor. Cellular and Molecular Biology of Bone. Tokyo: Academic Press, Inc.; 1993. pp. 131–67. [Google Scholar]
- 239.Ogihara S, Wang HL. Periodontal regeneration with or without limited orthodontics for the treatment of 2- or 3-wall infrabony defects. J Periodontol. 2010;81:1734–42. doi: 10.1902/jop.2010.100127. [DOI] [PubMed] [Google Scholar]
- 240.Yasuda H, Yano K, Wakitani S, Matsumoto T, Nakamura H, Takaoka K. Repair of critical long bone defects using frozen bone allografts coated with an rhBMP-2-retaining paste. J Orthop Sci. 2012;17:299–307. doi: 10.1007/s00776-012-0196-x. [DOI] [PubMed] [Google Scholar]
- 241.Anitua E, Tejero R, Alkhraisat MH, Orive G. Platelet-rich plasma to improve the bio-functionality of biomaterials. BioDrugs. 2013;27:97–111. doi: 10.1007/s40259-012-0004-3. [DOI] [PubMed] [Google Scholar]
- 242.Sandhu HS, Khan SN, Suh DY, Boden SD. Demineralized bone matrix, bone morphogenetic proteins, and animal models of spine fusion: an overview. Eur Spine J. 2001;10(Suppl. 2):S122–31. doi: 10.1007/s005860100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32:462–84. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sawkins MJ, Bowen W, Dhadda P, Markides H, Sidney LE, Taylor AJ, Rose FR, Badylak SF, Shakesheff KM, White LJ. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9:7865–73. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tuli SM, Singh AD. The osteoinductive property of decalcified bone matrix. An experimental study. J Bone Joint Surg Br. 1978;60:116–23. doi: 10.1302/0301-620X.60B1.342532. [DOI] [PubMed] [Google Scholar]
- 246.Soicher MA, Christiansen BA, Stover SM, Leach JK, Fyhrie DP. Remineralization of demineralized bone matrix (DBM) via alternating solution immersion (ASI) J Mech Behav Biomed Mater. 2013;26:109–18. doi: 10.1016/j.jmbbm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81:437–42. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 248.Tiedeman JJ, Garvin KL, Kile TA, Connolly JF. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18:1153–8. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- 249.Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomax-illofacial surgeon. J Craniofac Surg. 2005;16:981–9. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 250.Macisaac ZM, Rottgers SA, Davit AJ, 3rd, Ford M, Losee JE, Kumar AR. Alveolar reconstruction in cleft patients: decreased morbidity and improved outcomes with supplemental demineralized bone matrix and cancellous allograft. Plast Reconstr Surg. 2012;130:625–32. doi: 10.1097/PRS.0b013e31825dcb75. [DOI] [PubMed] [Google Scholar]
- 251.Acarturk TO, Hollinger JO. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006;118:862–73. doi: 10.1097/01.prs.0000232385.81219.87. [DOI] [PubMed] [Google Scholar]
- 252.Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR. Dentine as a bioactive extracellular matrix. Arch Oral Biol. 2012;57:109–21. doi: 10.1016/j.archoralbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 253.Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, Yamamoto G, Irie T, Tachikawa T, Maki K. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81:1264–72. doi: 10.1902/jop.2010.100016. [DOI] [PubMed] [Google Scholar]
- 254.Yagihashi K, Miyazawa K, Togari K, Goto S. Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcif Tissue Int. 2009;84:210–20. doi: 10.1007/s00223-008-9205-7. [DOI] [PubMed] [Google Scholar]
- 255.Bakhshalian N, Hooshmand S, Campbell SC, Kim JS, Brummel-Smith K, Arjmandi BH. Biocompatibility and microstructural analysis of osteopromotive property of allogenic demineralized dentin matrix. Int J Oral Maxillofac Implants. 2013;28:1655–62. [PubMed] [Google Scholar]
- 256.Reis-Filho CR, Silva ER, Martins AB, Pessoa FF, Gomes PV, de Araújo MS, Miziara MN, Alves JB. Demineralised human dentine matrix stimulates the expression of VEGF and accelerates the bone repair in tooth sockets of rats. Arch Oral Biol. 2012;57:469–76. doi: 10.1016/j.archoralbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 257.Li J, Yang J, Zhong X, He F, Wu X, Shen G. Demineralized dentin matrix composite collagen material for bone tissue regeneration. J Biomater Sci Polym Ed. 2013;24:1519–28. doi: 10.1080/09205063.2013.777227. [DOI] [PubMed] [Google Scholar]
- 258.Smith JG, Smith AJ, Shelton RM, Cooper PR. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res. 2012;318:2397–406. doi: 10.1016/j.yexcr.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 259.Zhang R, Cooper PR, Smith G, Nör JE, Smith AJ. Angiogenic activity of dentin matrix components. J Endod. 2011;37:26–30. doi: 10.1016/j.joen.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 260.Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning F, Zhang X, Xun W, Huo N, Tang L, Deng Z, Jin Y. Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng Part A. 2012;18:677–91. doi: 10.1089/ten.TEA.2011.0269. [DOI] [PubMed] [Google Scholar]
- 261.Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, Liu Y, Li Y, Zhou Y, Zhou J, Zhang M, Deng Z, Jin Y. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708–23. doi: 10.1016/j.biomaterials.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 262.Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, Li Y, Zou Q, Xie D, An X, Chen Y, Tian W. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525–38. doi: 10.1016/j.biomaterials.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 263.Guo W, Gong K, Shi H, Zhu G, He Y, Ding B, Wen L, Jin Y. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials. 2012;33:1291–302. doi: 10.1016/j.biomaterials.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 264.Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials. 2012;33:2449–61. doi: 10.1016/j.biomaterials.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 265.Johnson LL, Morrison KM, Wood DL. The application of arthroscopic principles to bone grafting of delayed union of long bone fractures. Arthroscopy. 2000;16:279–89. doi: 10.1016/s0749-8063(00)90052-5. [DOI] [PubMed] [Google Scholar]
- 266.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–52. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 267.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–8. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 268.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 269.Song L, Murphy SV, Yang B, Xu Y, Zhang Y, Atala A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng Part B Rev. 2014;20:163–72. doi: 10.1089/ten.TEB.2013.0103. [DOI] [PubMed] [Google Scholar]
- 270.Gonzalez-Perez F, Udina E, Navarro X. Extracellular matrix components in peripheral nerve regeneration. Int Rev Neurobiol. 2013;108:257–75. doi: 10.1016/B978-0-12-410499-0.00010-1. [DOI] [PubMed] [Google Scholar]
- 271.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 272.Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67:2879–95. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ode A, Duda GN, Glaeser JD, Matziolis G, Frauenschuh S, Perka C, Wilson CJ, Kasper G. Toward biomimetic materials in bone regeneration: functional behavior of mesenchymal stem cells on a broad spectrum of extracellular matrix components. J Biomed Mater Res A. 2010;95:1114–24. doi: 10.1002/jbm.a.32909. [DOI] [PubMed] [Google Scholar]
- 274.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lu H, Hoshiba T, Kawazoe N, Chen G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J Biomed Mater Res A. 2012;100:2507–16. doi: 10.1002/jbm.a.34150. [DOI] [PubMed] [Google Scholar]
- 276.Hubmacher D, Apte SS. The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol. 2013;25:65–70. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dado D, Levenberg S. Cell-scaffold mechanical interplay within engineered tissue. Semin Cell Dev Biol. 2009;20:656–64. doi: 10.1016/j.semcdb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 279.Keatch RP, Schor AM, Vorstius JB, Schor SL. Biomaterials in regenerative medicine: engineering to recapitulate the natural. Curr Opin Biotechnol. 2012;23:579–82. doi: 10.1016/j.copbio.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 280.Nakayama KH, Hou L, Huang NF. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv Healthc Mater. 2014;3:628–41. doi: 10.1002/adhm.201300620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 282.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13:979–87. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–32. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 285.Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med. 2010;5:961–70. doi: 10.2217/rme.10.61. [DOI] [PubMed] [Google Scholar]
- 286.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 287.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163:268–85. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kuraitis D, Giordano C, Ruel M, Musarò A, Suuronen EJ. Exploiting extracellular matrix–stem cell interactions: a review of natural materials for therapeutic muscle regeneration. Biomaterials. 2012;33:428–43. doi: 10.1016/j.biomaterials.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 289.Hammond JS, Gilbert TW, Howard D, Zaitoun A, Michalopoulos G, Shakesheff KM, Beckingham IJ, Badylak SF. Scaffolds containing growth factors and extracellular matrix induce hepatocyte proliferation and cell migration in normal and regenerating rat liver. J Hepatol. 2011;54:279–87. doi: 10.1016/j.jhep.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 290.Kuraitis D, Hou C, Zhang Y, Vulesevic B, Sofrenovic T, McKee D, Sharif Z, Ruel M, Suuronen EJ. Ex vivo generation of a highly potent population of circulating angiogenic cells using a collagen matrix. J Mol Cell Cardiol. 2011;51:187–97. doi: 10.1016/j.yjmcc.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 291.Viswanathan P, Chirasatitsin S, Ngamkham K, Engler AJ, Battaglia G. Cell instructive microporous scaffolds through interface engineering. J Am Chem Soc. 2012;134:20103–9. doi: 10.1021/ja308523f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sun Y, Jiang Y, Liu Q, Gao T, Feng JQ, Dechow P, D'Souza RN, Qin C, Liu X. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng Part A. 2013;19:1754–63. doi: 10.1089/ten.tea.2012.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bryksin AV, Brown AC, Baksh MM, Finn MG, Barker TH. Learning from nature-novel synthetic biology approaches for biomaterial design. Acta Biomater. 2014;10:1761–9. doi: 10.1016/j.actbio.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Custódio CA, Reis RL, Mano JF. Engineering biomolecular microenvironments for cell instructive biomaterials. Adv Healthc Mater. 2014;3:797–810. doi: 10.1002/adhm.201300603. [DOI] [PubMed] [Google Scholar]
- 295.Walters BD, Stegemann JP. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014;10:1488–501. doi: 10.1016/j.actbio.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 297.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–19. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Piterina AV, Cloonan AJ, Meaney CL, Davis LM, Callanan A, Walsh MT, McGloughlin TM. ECM-based materials in cardiovascular applications: inherent healing potential and augmentation of native regenerative processes. Int J Mol Sci. 2009;10:4375–417. doi: 10.3390/ijms10104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771–85. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Muiznieks LD, Keeley FW. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim Biophys Acta. 2013;1832:866–75. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 302.Gillette BM, Jensen JA, Tang B, Yang GJ, Bazargan-Lari A, Zhong M, Sia SK. In situ collagen assembly for integrating micro-fabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7:636–40. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 303.Pedraza CE, Marelli B, Chicatun F, McKee MD, Nazhat SN. An in vitro assessment of a cell-containing collagenous extracellular matrix-like scaffold for bone tissue engineering. Tissue Eng Part A. 2010;16:781–93. doi: 10.1089/ten.TEA.2009.0351. [DOI] [PubMed] [Google Scholar]
- 304.Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–33. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–54. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 306.Kim BS, Choi JS, Kim JD, Yoon HI, Choi YC, Cho YW. Human collagen isolated from adipose tissue. Biotechnol Prog. 2012;28:973–80. doi: 10.1002/btpr.1555. [DOI] [PubMed] [Google Scholar]
- 307.Bayrak A, Prüger P, Stock UA, Seifert M. Absence of immune responses with xenogeneic collagen and elastin. Tissue Eng Part A. 2013;19:1592–600. doi: 10.1089/ten.tea.2012.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Willard JJ, Drexler JW, Das A, Roy S, Shilo S, Shoseyov O, Powell HM. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng Part A. 2013;19:1507–18. doi: 10.1089/ten.tea.2012.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Beachley V, Wen X. Polymer nanofibrous structures: fabrication, biofunctionalization, and cell interactions. Prog Polym Sci. 2010;35:868–92. doi: 10.1016/j.progpolymsci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.He J, Jiang B, Dai Y, Hao J, Zhou Z, Tian Z, Wu F, Gu Z. Regulation of the osteoblastic and chondrocytic differentiation of stem cells by the extracellular matrix and subsequent bone formation modes. Biomaterials. 2013;34:6580–8. doi: 10.1016/j.biomaterials.2013.05.056. [DOI] [PubMed] [Google Scholar]
- 311.Jiang J, Papoutsakis ET. Stem-cell niche based comparative analysis of chemical and nano-mechanical material properties impacting ex vivo expansion and differentiation of hematopoietic and mesenchymal stem cells. Adv Healthc Mater. 2013;2:25–42. doi: 10.1002/adhm.201200169. [DOI] [PubMed] [Google Scholar]
- 312.Xu F, Li J, Jain V, Tu RS, Huang Q, Nanda V. Compositional control of higher order assembly using synthetic collagen peptides. J Am Chem Soc. 2012;134:47–50. doi: 10.1021/ja2077894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8:3191–200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 314.Pang Y, Greisler HP. Using a type 1 collagen-based system to understand cell-scaffold interactions and to deliver chimeric collagen-binding growth factors for vascular tissue engineering. J Investig Med. 2010;58:845–8. doi: 10.231/JIM.0b013e3181ee81f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martín MG, Dunn JC. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLOS ONE. 2014;9:e107814/1–9. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Alekseeva T, Hadjipanayi E, Abou Neel EA, Brown RA. Engineering stable topography in dense bio-mimetic 3D collagen scaffolds. Eur Cell Mater. 2012;23:28–40. doi: 10.22203/ecm.v023a03. [DOI] [PubMed] [Google Scholar]
- 317.Toh WS, Loh XJ. Advances in hydrogel delivery systems for tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2014;45:690–7. doi: 10.1016/j.msec.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 318.Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10:1148–59. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- 319.Wang Y, Azaïs T, Robin M, Vallée A, Catania C, Legriel P, Pehau-Arnaudet G, Babonneau F, Giraud-Guille MM, Nassif N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat Mater. 2012;11:724–33. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 320.Mauney J, Olsen BR, Volloch V. Matrix remodeling as stem cell recruitment event: a novel in vitro model for homing of human bone marrow stromal cells to the site of injury shows crucial role of extracellular collagen matrix. Matrix Biol. 2010;29:657–63. doi: 10.1016/j.matbio.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Puetzer JL, Bonassar LJ. High density type I collagen gels for tissue engineering of whole menisci. Acta Biomater. 2013;9:7787–95. doi: 10.1016/j.actbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 322.Rhodes JM, Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J Cell Mol Med. 2007;11:176–205. doi: 10.1111/j.1582-4934.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xia Z, Villa MM, Wei M. A biomimetic collagen-apatite scaffold with a multi-level lamellar structure for bone tissue engineering. J Mater Chem B Mater Biol Med. 2014;2:1998–2007. doi: 10.1039/C3TB21595D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fu S, Ni P, Wang B, Chu B, Zheng L, Luo F, Luo J, Qian Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33:4801–9. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 325.Murphy CM, Schindeler A, Gleeson JP, Yu NY, Cantrill LC, Mikulec K, Peacock L, O'Brien FJ, Little DG. A collagen-hydroxyapatite scaffold allows for binding and co-delivery of recombinant bone morphogenetic proteins and bisphosphonates. Acta Biomater. 2014;10:2250–8. doi: 10.1016/j.actbio.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 326.Pang Y, Wang X, Ucuzian AA, Brey EM, Burgess WH, Jones KJ, Alexander TD, Greisler HP. Local delivery of a collagen-binding FGF-1 chimera to smooth muscle cells in collagen scaffolds for vascular tissue engineering. Biomaterials. 2010;31:878–85. doi: 10.1016/j.biomaterials.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Han Q, Zhang B, Chen B, Dai J, Xu J, Wang C, Wang Z. Evaluation of a bioactive bone-inducing material consisting of collagen scaffolds and collagen-binding BMP2. J Biomed Mater Res A. 2014;102:3093–101. doi: 10.1002/jbm.a.34979. [DOI] [PubMed] [Google Scholar]
- 328.Dai W, Hale SL, Kay GL, Jyrala AJ, Kloner RA. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology. Regen Med. 2009;4:387–95. doi: 10.2217/RME.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhang Y, Thorn S, DaSilva JN, Lamoureux M, DeKemp RA, Bean-lands RS, Ruel M, Suuronen EJ. Collagen-based matrices improve the delivery of transplanted circulating progenitor cells: development and demonstration by ex vivo radionuclide cell labeling and in vivo tracking with positron-emission tomography. Circ Cardiovasc Imaging. 2008;1:197–204. doi: 10.1161/CIRCIMAGING.108.781120. [DOI] [PubMed] [Google Scholar]
- 330.Zhu J, He P, Lin L, Jones DR, Marchant RE. Biomimetic poly(ethylene glycol)-based hydrogels as scaffolds for inducing endothelial adhesion and capillary-like network formation. Biomacromolecules. 2012;13:706–13. doi: 10.1021/bm201596w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Browning MB, Dempsey D, Guiza V, Becerra S, Rivera J, Russell B, Höök M, Clubb F, Miller M, Fossum T, Dong JF, Bergeron AL, Hahn M, Cosgriff-Hernandez E. Multilayer vascular grafts based on collagen-mimetic proteins. Acta Biomater. 2012;8:1010–21. doi: 10.1016/j.actbio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 332.Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 333.Crowley DC, Lau FC, Sharma P, Evans M, Guthrie N, Bagchi M, Bagchi D, Dey DK, Raychaudhuri SP. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. IntJ Med Sci. 2009;6:312–21. doi: 10.7150/ijms.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tong T, Zhao W, Wu YQ, Chang Y, Wang QT, Zhang LL, Wei W. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res. 2010;59:369–77. doi: 10.1007/s00011-009-0109-4. [DOI] [PubMed] [Google Scholar]
- 335.Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, Wolf F, Jundt G, Marsano A, Farhadi J, Heberer M, Jakob M, Schaefer DJ, Martin I. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384:337–46. doi: 10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- 336.Hong Y, Fan H, Li B, Guo B, Liu M, Zhang X. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater Sci Eng R Rep. 2010;70:225–42. [Google Scholar]
- 337.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078–93. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 338.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O'Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel – a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–48. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 339.Gupta MS, Nicoll SB. Functional nucleus pulposus-like matrix assembly by human mesenchymal stromal cells is directed by macromer concentration in photocrosslinked carboxymethylcellulose hydrogels. Cell Tissue Res. 2014;358:527–39. doi: 10.1007/s00441-014-1962-1. [DOI] [PubMed] [Google Scholar]
- 340.Enea D, Guerra D, Roggiani J, Cecconi S, Manzotti S, Quaglino D, Pasquali-Ronchetti I, Gigante A. Mixed type I and type II collagen scaffold for cartilage repair: ultrastructural study of synovial membrane response and healing potential versusmicrofractures (a pilot study) Int J Immunopathol Pharmacol. 2013;26:917–30. doi: 10.1177/039463201302600410. [DOI] [PubMed] [Google Scholar]
- 341.Glentis A, Gurchenkov V, Vignjevic DM. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adh Migr. 2014;8:236–45. doi: 10.4161/cam.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–38. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 343.Anderson DE, Hinds MT. Extracellular matrix production and regulation in micropatterned endothelial cells. Biochem Biophys Res Commun. 2012;427:159–64. doi: 10.1016/j.bbrc.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 344.Nyström A, Velati D, Mittapalli VR, Fritsch A, Kern JS, Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498–509. doi: 10.1172/JCI68127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jeng L, Hsu HP, Spector M. Tissue-engineered cartilaginous constructs for the treatment of caprine cartilage defects, including distribution of laminin and type IV collagen. Tissue Eng Part A. 2013;19:2267–74. doi: 10.1089/ten.tea.2013.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Coelho NM, González-García C, Planell JA, Salmerón-Sánchez M, Altankov G. Different assembly of type IV collagen on hydrophilic and hydrophobic substrata alters endothelial cells interaction. Eur Cell Mater. 2010;19:262–72. doi: 10.22203/ecm.v019a25. [DOI] [PubMed] [Google Scholar]
- 348.Kanie K, Narita Y, Zhao Y, Kuwabara F, Satake M, Honda S, Kaneko H, Yoshioka T, Okochi M, Honda H, Kato R. Collagentype IV-specific tripeptides for selective adhesion of endothelial and smooth muscle cells. Biotechnol Bioeng. 2012;109:1808–16. doi: 10.1002/bit.24459. [DOI] [PubMed] [Google Scholar]
- 349.Mihardja SS, Gao D, Sievers RE, Fang Q, Feng J, Wang J, Vanbrocklin HF, Larrick JW, Huang M, Dae M, Lee RJ. Targeted in vivo extracellular matrix formation promotes neovascularization in a rodent model of myocardial infarction. PLoS ONE. 2010;5:e10384/1–8. doi: 10.1371/journal.pone.0010384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Horejs CM, Serio A, Purvis A, Gormley AJ, Bertazzo S, Poliniewicz A, Wang AJ, DiMaggio P, Hohenester E, Stevens MM. Biologically-active laminin-111 fragment that modulates the epithelial-to-mesenchymal transition in embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:5908–13. doi: 10.1073/pnas.1403139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Jurga M, Dainiak MB, Sarnowska A, Jablonska A, Tripathi A, Plieva FM, Savina IN, Strojek L, Jungvid H, Kumar A, Lukomska B, Domanska-Janik K, Forraz N, McGuckin CP. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials. 2011;32:3423–34. doi: 10.1016/j.biomaterials.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 352.Junka R, Valmikinathan CM, Kalyon DM, Yu X. Laminin functionalized biomimetic nanofibers for nerve tissue engineering. J Biomater Tissue Eng. 2013;3:494–502. doi: 10.1166/jbt.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao J, Zheng T, Wu W, Wu S, Wang J, Dai J. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials. 2011;32:3939–48. doi: 10.1016/j.biomaterials.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 354.Chen P, Cescon M, Bonaldo P. The role of collagens in peripheral nerve myelination and function. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-8862-y. http://dx.doi.org/10.1007/s12035-014-8862-y. [DOI] [PubMed]
- 355.Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107:714–22. doi: 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M, Ueda Y. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs. 2010;191:269–80. doi: 10.1159/000260061. [DOI] [PubMed] [Google Scholar]
- 357.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2009;106:7991–6. doi: 10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Goudenege S, Lamarre Y, Dumont N, Rousseau J, Frenette J, Skuk D, Tremblay JP. Laminin-111: a potential therapeutic agent for Duchenne muscular dystrophy. Mol Ther. 2010;18:2155–63. doi: 10.1038/mt.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964/1–13. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Stabenfeldt SE, Munglani G, García AJ, LaPlaca MC. Biomimetic microenvironment modulates neural stem cell survival, migration, and differentiation. Tissue Eng Part A. 2010;16:3747–58. doi: 10.1089/ten.tea.2009.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Brandl FP, Seitz AK, Tessmar JK, Blunk T, Göpferich AM. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials. 2010;31:3957–66. doi: 10.1016/j.biomaterials.2010.01.128. [DOI] [PubMed] [Google Scholar]
- 362.Ruan D, Zhang Y, Wang D, Zhang C, Wu J, Wang C, Shi Z, Xin H, Xu C, Li H, He Q. Differentiation of human Wharton's jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng Part A. 2011;18:167–75. doi: 10.1089/ten.TEA.2011.0186. [DOI] [PubMed] [Google Scholar]
- 363.Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res Ther. 2013;4:120/1–11. doi: 10.1186/scrt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Gilchrist CL, Chen J, Richardson WJ, Loeser RF, Setton LA. Functional integrin subunits regulating cell–matrix interactions in the intervertebral disc. J Orthop Res. 2007;25:829–40. doi: 10.1002/jor.20343. [DOI] [PubMed] [Google Scholar]
- 365.Chen J, Jing L, Gilchrist CL, Richardson WJ, Fitch RD, Setton LA. Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50:294–306. [PMC free article] [PubMed] [Google Scholar]
- 366.Nettles DL, Richardson WJ, Setton LA. Integrin expression in cells of the intervertebral disc. J Anat. 2004;204:515–20. doi: 10.1111/j.0021-8782.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Gilchrist CL, Darling EM, Chen J, Setton LA. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell–cell interactions. PLoS ONE. 2011;6:e27170/1–9. doi: 10.1371/journal.pone.0027170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Gilchrist CL, Francisco AT, Plopper GE, Chen J, Setton LA. Nucleus pulposus cell–matrix interactions with laminins. Eur Cell Mater. 2011;21:523–32. doi: 10.22203/ecm.v021a39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Francisco AT, Mancino RJ, Bowles RD, Brunger JM, Tainter DM, Chen YT, Richardson WJ, Guilak F, Setton LA. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34:7381–8. doi: 10.1016/j.biomaterials.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Durkin ME, Wewer UM, Chung AE. Exon organization of the mouse entactin gene corresponds to the structural domains of the polypeptide and has regional homology to the low-density lipoprotein receptor gene. Genomics. 1995;26:219–28. doi: 10.1016/0888-7543(95)80204-y. [DOI] [PubMed] [Google Scholar]
- 371.Funanage VL, Smith SM, Minnich MA. Entactin promotes adhesion and long-term maintenance of cultured regenerated skeletal myotubes. J Cell Physiol. 1992;150:251–7. doi: 10.1002/jcp.1041500205. [DOI] [PubMed] [Google Scholar]
- 372.Lee HK, Seo IA, Suh DJ, Park HT. Nidogen plays a role in the regenerative axon growth of adult sensory neurons through Schwann cells. J Korean Med Sci. 2009;24:654–9. doi: 10.3346/jkms.2009.24.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW. Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int. 1993;43:7–12. doi: 10.1038/ki.1993.3. [DOI] [PubMed] [Google Scholar]
- 374.Hopf M, Gohring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–25. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 375.Vasudevan A, Ho MS, Weiergräber M, Nischt R, Schneider T, Lie A, Smyth N, Köhling R. Basement membrane protein nidogen-1 shapes hippocampal synaptic plasticity and excitability. Hippocampus. 2010;20:608–20. doi: 10.1002/hipo.20660. [DOI] [PubMed] [Google Scholar]
- 376.Takagi J, Yang Y, Liu JH, Wang JH, Springer TA. Complex between nidogen and laminin fragments reveals a paradigmatic beta-propeller interface. Nature. 2003;424:969–74. doi: 10.1038/nature01873. [DOI] [PubMed] [Google Scholar]
- 377.Patel TR, Bernards C, Meier M, McEleney K, Winzor DJ, Koch M, Stetefeld J. Structural elucidation of full-length nidogen and the laminin-nidogen complex in solution. Matrix Biol. 2014;33:60–7. doi: 10.1016/j.matbio.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 378.Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 379.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–24. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 380.Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–10. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 381.Kunze A, Abari E, Semkova I, Paulsson M, Hartmann U. Deposition of nidogens and other basement membrane proteins in the young and aging mouse retina. Ophthalmic Res. 2010;43:108–12. doi: 10.1159/000247595. [DOI] [PubMed] [Google Scholar]
- 382.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9:470–7. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Varkey M, Ding J, Tredget EE. Superficial dermal fibroblasts enhance basement membrane and epidermal barrier formation in tissue-engineered skin: implications for treatment of skin basement membrane disorders. Tissue Eng Part A. 2014;20:540–52. doi: 10.1089/ten.tea.2013.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Breitkreutz D, Koxholt I, Thiemann K, Nischt R. Skin basement membrane: the foundation of epidermal integrity – BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int. 2013;2013:179784/1–17. doi: 10.1155/2013/179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Li Y, Zhu Y, Yu H, Chen L, Liu Y. Topographic characterization and protein quantification of esophageal basement membrane for scaffold design reference in tissue engineering. J Biomed Mater Res B Appl Biomater. 2012;100:265–73. doi: 10.1002/jbm.b.31949. [DOI] [PubMed] [Google Scholar]
- 386.Lv J, Chen L, Zhu Y, Hou L, Liu Y. Promoting epithelium regeneration for esophageal tissue engineering through basement membrane reconstitution. ACS Appl Mater Interfaces. 2014;6:4954–64. doi: 10.1021/am4059809. [DOI] [PubMed] [Google Scholar]
- 387.Silva R, Fabry B, Boccaccini AR. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 2014;35:6727–38. doi: 10.1016/j.biomaterials.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 388.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 389.Migliorini E, Thakar D, Sadir R, Pleiner T, Baleux F, Lortat-Jacob H, Coche-Guerente L, Richter RP. Well-defined biomimetic surfaces to characterize glycosaminoglycan-mediated interactions on the molecular, supramolecular and cellular levels. Biomaterials. 2014;35:8903–15. doi: 10.1016/j.biomaterials.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 390.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10:1588–600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.van der Smissen A, Samsonov S, Hintze V, Scharnweber D, Moeller S, Schnabelrauch M, Pisabarro MT, Anderegg U. Artificial extracellular matrix composed of collagen I and highly sulfated hyaluronan interferes with TGFβ(1) signaling and prevents TGFβ(1)-induced myofibroblast differentiation. Acta Biomater. 2013;9:7775–86. doi: 10.1016/j.actbio.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 392.Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32:483–92. doi: 10.1016/j.tibtech.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 393.Hortensius RA, Harley BA. The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity. Biomaterials. 2013;34:7645–52. doi: 10.1016/j.biomaterials.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Salanga CL, Handel TM. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp Cell Res. 2011;317:590–601. doi: 10.1016/j.yexcr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Rueda P, Richart A, Récalde A, Gasse P, Vilar J, Guérin C, Lortat-Jacob H, Vieira P, Baleux F, Chretien F, Arenzana-Seisdedos F, Silvestre JS. Homeostatic and tissue reparation defaults in mice carrying selective genetic invalidation of CXCL12/proteoglycan interactions. Circulation. 2012;126:1882–95. doi: 10.1161/CIRCULATIONAHA.112.113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 398.Lam J, Truong NF, Segura T. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014;10:1571–80. doi: 10.1016/j.actbio.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Tempel C, Gilead A, Neeman M. Hyaluronic acid as an anti-angiogenic shield in the preovulatory rat follicle. Biol Reprod. 2000;63:134–40. doi: 10.1095/biolreprod63.1.134. [DOI] [PubMed] [Google Scholar]
- 400.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 401.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 402.Ratliff BB, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, Mares J, Hatzopoulos AK, Goligorsky MS. Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am J Physiol Ren Physiol. 2010;299:F178–86. doi: 10.1152/ajprenal.00102.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–20. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 404.Garcia-Fuentes M, Meinel AJ, Hilbe M, Meinel L, Merkle HP. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials. 2009;30:5068–76. doi: 10.1016/j.biomaterials.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 405.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK, Oh DB. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochem Biophys Res Commun. 2011;404:463–9. doi: 10.1016/j.bbrc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 407.Jakobsen RB, Shahdadfar A, Reinholt FP, Brinchmann JE. Chondrogenesis in a hyaluronic acid scaffold: comparison between chondrocytes and MSC from bone marrow and adipose tissue. Knee Surg Sports Traumatol Arthrosc. 2010;18:1407–16. doi: 10.1007/s00167-009-1017-4. [DOI] [PubMed] [Google Scholar]
- 408.Singh A, Corvelli M, Unterman SA, Wepasnick KA, McDonnell P, Elisseeff JH. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat Mater. 2014;13:988–95. doi: 10.1038/nmat4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Shabafrooz V, Mozafari M, Köhler GA, Assefa S, Vashaee D, Tayebi L. The effect of hyaluronic acid on biofunctionality of gelatin-collagen intestine tissue engineering scaffolds. J Biomed Mater Res A. 2014;102:3130–9. doi: 10.1002/jbm.a.34984. [DOI] [PubMed] [Google Scholar]
- 410.Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14:500–5. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- 411.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–25. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 412.Mbemba E, Gluckman JC, Gattegno L. Glycan and glycosaminoglycan binding properties of stromal cell-derived factor (SDF)-1alpha. Glycobiology. 2000;10:21–9. doi: 10.1093/glycob/10.1.21. [DOI] [PubMed] [Google Scholar]
- 413.Netelenbos T, Dräger AM, van het Hof B, Kessler FL, Delouis C, Huijgens PC, van den Born J, van Dijk W. Differences in sulfation patterns of heparan sulfate derived from human bone marrow and umbilical vein endothelial cells. Exp Hematol. 2001;29:884–93. doi: 10.1016/s0301-472x(01)00653-1. [DOI] [PubMed] [Google Scholar]
- 414.Netelenbos T, Zuijderduijn S, Van Den Born J, Kessler FL, Zweegman S, Huijgens PC, Dräger AM. Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol. 2002;72:353–62. [PubMed] [Google Scholar]
- 415.Netelenbos T, van den Born J, Kessler FL, Zweegman S, Merle PA, van Oostveen JW, Zwaginga JJ, Huijgens PC, Dräger AM. Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia. 2003;17:175–84. doi: 10.1038/sj.leu.2402738. [DOI] [PubMed] [Google Scholar]
- 416.Cool SM, Nurcombe V. Heparan sulfate regulation of progenitor cell fate. J Cell Biochem. 2006;99:1040–51. doi: 10.1002/jcb.20936. [DOI] [PubMed] [Google Scholar]
- 417.Dombrowski C, Song SJ, Chuan P, Lim X, Susanto E, Sawyer AA, Woodruff MA, Hutmacher DW, Nurcombe V, Cool SM. Heparan sulfate mediates the proliferation and differentiation of rat mesenchymal stem cells. Stem Cells Dev. 2009;18:661–70. doi: 10.1089/scd.2008.0157. [DOI] [PubMed] [Google Scholar]
- 418.Murali S, Leong DF, Lee JJ, Cool SM, Nurcombe V. Comparative assessment of the effects of gender-specific heparan sulfates on mesenchymal stem cells. J Biol Chem. 2011;286:17755–65. doi: 10.1074/jbc.M110.148874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Kim KI, Cho HJ, Hahn JY, Kim TY, Park KW, Koo BK, Shin CS, Kim CH, Oh BH, Lee MM, Park YB, Kim HS. Beta-catenin overexpression augments angiogenesis and skeletal muscle regeneration through dual mechanism of vascular endothelial growth factor-mediated endothelial cell proliferation and progenitor cell mobilization. Arterioscler Thromb Vasc Biol. 2006;26:91–8. doi: 10.1161/01.ATV.0000193569.12490.4b. [DOI] [PubMed] [Google Scholar]
- 420.Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement ofsyndecan-3 for successful fiber formation. J Cell Sci. 2004;117:73–84. doi: 10.1242/jcs.00828. [DOI] [PubMed] [Google Scholar]
- 421.Kwok JC, Warren P, Fawcett JW. Chondroitin sulfate: a key molecule in the brain matrix. Int J Biochem Cell Biol. 2012;44:582–6. doi: 10.1016/j.biocel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 422.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 423.Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, Cao T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–80. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 424.Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014;10:214–23. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 425.Hu X, Li D, Zhou F, Gao C. Biological hydrogel synthesized from hyaluronic acid, gelatin and chondroitin sulfate by click chemistry. Acta Biomater. 2011;7:1618–26. doi: 10.1016/j.actbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 426.Guo Y, Yuan T, Xiao Z, Tang P, Xiao Y, Fan Y, Zhang X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med. 2012;23:2267–79. doi: 10.1007/s10856-012-4684-5. [DOI] [PubMed] [Google Scholar]
- 427.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(Suppl. 1):I138–44. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 428.Suuronen EJ, Zhang P, Kuraitis D, Cao X, Melhuish A, McKee D, Li F, Mesana TG, Veinot JP, Ruel M. An acellular matrix-bound ligand enhances the mobilization, recruitment and therapeutic effects of circulating progenitor cells in a hindlimb ischemia model. FASEB J. 2009;23:1447–58. doi: 10.1096/fj.08-111054. [DOI] [PubMed] [Google Scholar]
- 429.Ayerst BI, Day AJ, Nurcombe V, Cool SM, Merry CL. New strategies for cartilage regeneration exploiting selected glycosaminoglycans to enhance cell fate determination. Biochem Soc Trans. 2014;42:703–9. doi: 10.1042/BST20140031. [DOI] [PubMed] [Google Scholar]
- 430.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 431.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 432.Dong X, Wei X, Yi W, Gu C, Kang X, Liu Y, Li Q, Yi D. RGD-modified a cellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J Mater Sci Mater Med. 2009;20:2327–36. doi: 10.1007/s10856-009-3791-4. [DOI] [PubMed] [Google Scholar]
- 433.Stevenson MD, Piristine H, Hogrebe NJ, Nocera TM, Boehm MW, Reen RK, Koelling KW, Agarwal G, Sarang-Sieminski AL, Gooch KJ. A self-assembling peptide matrix used to control stiffness and binding site density supports the formation of microvascular networks in three dimensions. Acta Biomater. 2013;9:7651–61. doi: 10.1016/j.actbio.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Calonder C, Matthew HW, Van Tassel PR. Adsorbed layers of oriented fibronectin: a strategy to control cell-surface interactions. J Biomed Mater Res A. 2005;75:316–23. doi: 10.1002/jbm.a.30417. [DOI] [PubMed] [Google Scholar]
- 435.Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods. 2012;18:340–8. doi: 10.1089/ten.tec.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Raghunathan V, McKee C, Cheung W, Naik R, Nealey PF, Russell P, Murphy CJ. Influence of extracellular matrix proteins and substratum topography on corneal epithelial cell alignment and migration. Tissue Eng Part A. 2013;19:1713–22. doi: 10.1089/ten.tea.2012.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 438.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–8. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Shachar M, Tsur-Gang O, Dvir T, Leor J, Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011;7:152–62. doi: 10.1016/j.actbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 440.Ravi S, Caves JM, Martinez AW, Haller CA, Chaikof EL. Incorporation of fibronectin to enhance cytocompatibility in multilayer elastin-like protein scaffolds for tissue engineering. J Biomed Mater Res A. 2013;101:1915–25. doi: 10.1002/jbm.a.34484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li H. Designed biomaterials to mimic the mechanical properties of muscles. Nature. 2010;465:69–73. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- 442.Miao M, Sitarz E, Bellingham CM, Won E, Muiznieks LD, Keeley FW. Sequence and domain arrangements influence mechanical properties of elastin-like polymeric elastomers. Biopolymers. 2013;99:392–407. doi: 10.1002/bip.22192. [DOI] [PubMed] [Google Scholar]
- 443.Sato F, Wachi H, Ishida M, Nonaka R, Onoue S, Urban Z, Starcher BC, Seyama Y. Distinct steps of cross-linking, self-association, and maturation of tropoelastin are necessary for elastic fiber formation. J Mol Biol. 2007;369:841–51. doi: 10.1016/j.jmb.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 444.Francis G, John R, Thomas J. Biosynthetic pathway of desmosines in elastin. Biochem J. 1973;136:45–55. doi: 10.1042/bj1360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–61. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- 446.Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41:494–7. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 447.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–18. [PubMed] [Google Scholar]
- 448.Bashir MM, Abrams WR, Rosenbloom J, Kucich U, Bacarra M, Han MD, Brown-Augsberger P, Mecham R, Rosenbloom J. Microfibril-associated glycoprotein: characterization of the bovine gene and of the recombinantly expressed protein. Biochemistry. 1994;33:593–600. doi: 10.1021/bi00168a026. [DOI] [PubMed] [Google Scholar]
- 449.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Bio. 1986;103:2499–509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Brooke BS, Bayes-Genis A, Li DY. New insights into elastin and vascular disease. Trends Cardiovasc Med. 2003;13:176–81. doi: 10.1016/s1050-1738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 451.Bashur CA, Venkataraman L, Ramamurthi A. Tissue engineering and regenerative strategies to replicate biocomplexity of vascular elastic matrix assembly. Tissue Eng Part B Rev. 2012;18:203–17. doi: 10.1089/ten.teb.2011.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Waterhouse A, Wise SG, Ng MK, Weiss AS. Elastin as a nonthrombogenic biomaterial. Tissue Eng Part B Rev. 2011;17:93–9. doi: 10.1089/ten.TEB.2010.0432. [DOI] [PubMed] [Google Scholar]
- 453.Simionescu DT, Lu Q, Song Y, Lee JS, Rosenbalm TN, Kelley C, Vyavahare NR. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–13. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 454.Daamen WF, Hafmans T, Veerkamp JH, van Kuppevelt TH. Isolation of intact elastin fibers devoid of microfibrils. Tissue Eng. 2005;11:1168–76. doi: 10.1089/ten.2005.11.1168. [DOI] [PubMed] [Google Scholar]
- 455.Patel D, Menon R, Taite LJ. Self-assembly of elastin-based peptides into the ECM: the importance of integrins and the elastin binding protein in elastic fiber assembly. Biomacromolecules. 2011;12:432–40. doi: 10.1021/bm101214f. [DOI] [PubMed] [Google Scholar]
- 456.Girotti A, Reguera J, Rodríguez-Cabello JC, Arias FJ, Alonso M, Matestera A. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater Med. 2004;15:479–84. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 457.Betre H, Setton LA, Meyer DE, Chilkoti A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3:910–6. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 458.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–79. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 459.Nivison-Smith L, Rnjak J, Weiss AS. Synthetic human elastin microfibers: stable cross-linked tropoelastin and cell interactive constructs for tissue engineering applications. Acta Biomater. 2010;6:354–9. doi: 10.1016/j.actbio.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 460.Bozzini S, Giuliano L, Altomare L, Petrini P, Bandiera A, Conconi MT, Farè S, Tanzi MC. Enzymatic cross-linking of human recombinant elastin (HELP) as biomimetic approach in vascular tissue engineering. J Mater Sci Mater Med. 2011;22:2641–50. doi: 10.1007/s10856-011-4451-z. [DOI] [PubMed] [Google Scholar]
- 461.Jeon WB. Application of elastin-mimetic recombinant proteins in chemotherapeutics delivery, cellular engineering, and regenerative medicine. Bioengineered. 2013;4:368–73. doi: 10.4161/bioe.24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Gustafson JA, Price RA, Frandsen J, Henak CR, Cappello J, Ghandehari H. Synthesis and characterization of a matrix-metalloproteinase responsive silk-elastinlike protein polymer. Biomacromolecules. 2013;14:618–25. doi: 10.1021/bm3013692. [DOI] [PubMed] [Google Scholar]
- 463.Kinikoglu B, Rodríguez-Cabello JC, Damour O, Hasirci V. The influence of elastin-like recombinant polymer on the self-renewing potential of a 3D tissue equivalent derived from human lamina propria fibroblasts and oral epithelial cells. Biomaterials. 2011;32:5756–64. doi: 10.1016/j.biomaterials.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 464.Lyu SR, Kuo YC, Ku HF, Hsieh WH. Cryopreserved chondrocytes in porous biomaterials with surface elastin and poly-l-lysine for cartilage regeneration. Colloids Surf B Biointerfaces. 2013;103:304–9. doi: 10.1016/j.colsurfb.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 465.Choi SK, Park JK, Lee KM, Lee SK, Jeon WB. Improved neural progenitor cell proliferation and differentiation on poly(lactide-co-glycolide) scaffolds coated with elastin-like polypeptide. J Biomed Mater Res B Appl Biomater. 2013;101:1329–39. doi: 10.1002/jbm.b.32950. [DOI] [PubMed] [Google Scholar]
- 466.Caves JM, Kumar VA, Martinez AW, Kim J, Ripberger CM, Haller CA, Chaikof EL. The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts. Biomaterials. 2010;31:7175–82. doi: 10.1016/j.biomaterials.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Caves JM, Cui W, Wen J, Kumar VA, Haller CA, Chaikof EL. Elastin-like protein matrix reinforced with collagen microfibers for soft tissue repair. Biomaterials. 2011;32:5371–9. doi: 10.1016/j.biomaterials.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Rnjak-Kovacina J, Wise SG, Li Z, Maitz PK, Young CJ, Wang Y, Weiss AS. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials. 2011;32:6729–36. doi: 10.1016/j.biomaterials.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 469.Fathi A, Mithieux SM, Wei H, Chrzanowski W, Valtchev P, Weiss AS, Dehghani F. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials. 2014;35:5425–35. doi: 10.1016/j.biomaterials.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Huang W, Rollett A, Kaplan DL. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin Drug Deliv. 2015 doi: 10.1517/17425247.2015.989830. http://dx.doi.org/10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed]
- 471.Collier JH, Rudra JS, Gasiorowski JZ, Jung JP. Multi-component extracellular matrices based on peptide self-assembly. Chem Soc Rev. 2010;39:3413–24. doi: 10.1039/b914337h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar-free repair and regeneration: Insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol. 2014;56:47–55. doi: 10.1016/j.biocel.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 473.Huber MC, Schreiber A, Wild W, Benz K, Schiller SM. Introducing a combinatorial DNA-toolbox platform constituting defined protein-based biohybrid-materials. Biomaterials. 2014;35:8767–79. doi: 10.1016/j.biomaterials.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 474.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–12. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Fu RH, Wang YC, Liu SP, Shih TR, Lin HL, Chen YM, Sung JH, Lu CH, Wei JR, Wang ZW, Huang SJ, Tsai CH, Shyu WC, Lin SZ. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23:621–30. doi: 10.3727/096368914X678382. [DOI] [PubMed] [Google Scholar]
- 476.Moroni F, Mirabella T. Decellularized matrices for cardiovascular tissue engineering. Am J Stem Cells. 2014;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 477.Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717–28. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 478.Weber B, Dijkman PE, Scherman J, Sanders B, Emmert MY, Grünenfelder J, Verbeek R, Bracher M, Black M, Franz T, Kortsmit J, Modregger P, Peter S, Stampanoni M, Robert J, Kehl D, van Doeselaar M, Schweiger M, Brokopp CE, Wälchli T, Falk V, Zilla P, Driessen-Mol A, Baaijens FP, Hoerstrup SP. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013;34:7269–80. doi: 10.1016/j.biomaterials.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 479.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–32. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Toni R, Tampieri A, Zini N, Strusi V, Sandri M, Dallatana D, Spaletta G, Bassoli E, Gatto A, Ferrari A, Martin I. Exsitu bioengineering of bioartificial endocrine glands: a new frontier in regenerative medicine of soft tissue organs. Ann Anat. 2011;193:381–94. doi: 10.1016/j.aanat.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 482.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–9. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Baiguera S, Gaudio CD, Kuevda E, Gonfiotti A, Bianco A, Macchiarini P. Dynamic decellularization and cross-linking of rat tracheal matrix. Biomaterials. 2014;35:6344–50. doi: 10.1016/j.biomaterials.2014.04.070. [DOI] [PubMed] [Google Scholar]
- 484.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–49. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 485.Mirzarafie A, Grainger RK, Thomas B, Bains W, Ustok FI, Lowe CR. A fast and mild decellularization protocol for obtaining extracellular matrix. Rejuvenation Res. 2014;17:159–60. doi: 10.1089/rej.2013.1488. [DOI] [PubMed] [Google Scholar]
- 486.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 487.Tuan-Mu HY, Yu CH, Hu JJ. On the decellularization of fresh or frozen human umbilical arteries: implications for small-diameter tissue engineered vascular grafts. Ann Biomed Eng. 2014;42:1305–18. doi: 10.1007/s10439-014-1000-1. [DOI] [PubMed] [Google Scholar]
- 488.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 489.Tsuchiya T, Balestrini JL, Mendez J, Calle EA, Zhao L, Niklason LE. Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng Part C Methods. 2014;20:1028–36. doi: 10.1089/ten.tec.2013.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, D'Amore A, Badylak SF. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10:183–93. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Bourgine PE, Pippenger BE, Todorov A, Jr, Tchang L, Martin I. Tissue decellularization by activation of programmed cell death. Biomaterials. 2013;34:6099–108. doi: 10.1016/j.biomaterials.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 492.Andrée B, Bär A, Haverich A, Hilfiker A. Small intestinal submucosa segments as matrix for tissue engineering: review. Tissue Eng Part B Rev. 2013;19:279–91. doi: 10.1089/ten.TEB.2012.0583. [DOI] [PubMed] [Google Scholar]
- 493.Mancuso L, Gualerzi A, Boschetti F, Loy F, Cao G. Decellularized ovine arteries as small-diameter vascular grafts. Biomed Mater. 2014;9:045011/1–10. doi: 10.1088/1748-6041/9/4/045011. [DOI] [PubMed] [Google Scholar]
- 494.Teodori L, Costa A, Marzio R, Perniconi B, Coletti D, Adamo S, Gupta B, Tarnok A. Native extracellular matrix: a new scaffolding platform for repair of damaged muscle. Front Physiol. 2014;5:218/1–9. doi: 10.3389/fphys.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Decaris ML, Binder BY, Soicher MA, Bhat A, Leach JK. Cell-derived matrix coatings for polymeric scaffolds. Tissue Eng Part A. 2012;18:2148–57. doi: 10.1089/ten.TEA.2011.0677. [DOI] [PubMed] [Google Scholar]
- 496.Evan G. Getting one's Fak straight. Dev Cell. 2010;19:185–6. doi: 10.1016/j.devcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Sadr N, Pippenger BE, Scherberich A, Wendt D, Mantero S, Martin I, Papadimitropoulos A. Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials. 2012;33:5085–93. doi: 10.1016/j.biomaterials.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 498.Sun HH, Qu TJ, Zhang XH, Yu Q, Chen FM. Designing biomaterials for in situ periodontal tissue regeneration. Biotechnol Prog. 2012;28:3–20. doi: 10.1002/btpr.698. [DOI] [PubMed] [Google Scholar]
- 499.Lu Q, Li M, Zou Y, Cao T. Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novoadipogenesis. J Control Rel. 2013;174:43–50. doi: 10.1016/j.jconrel.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 500.Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013;45:e57/1–11. doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg. 2012;129:1247–57. doi: 10.1097/PRS.0b013e31824ec3dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ma T. Acellular biomaterials in mesenchymal stem cell-mediated endogenous tissue regeneration. J MaterChem B. 2014;2:31–5. doi: 10.1039/c3tb21369b. [DOI] [PubMed] [Google Scholar]
- 503.Freiberg RA, Ray RD. Studies of devitalized bone implants. Arch Surg. 1964;89:417–27. doi: 10.1001/archsurg.1964.01320030007002. [DOI] [PubMed] [Google Scholar]
- 504.Papadimitropoulos A, Scotti C, Bourgine P, Scherberich A, Martin I. Engineered decellularized matrices to instruct bone regeneration processes. Bone. 2015;70:66–72. doi: 10.1016/j.bone.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 505.Mhaskar R. Amniotic membrane for cervical reconstruction. Int J Gynaecol Obstet. 2005;90:123–7. doi: 10.1016/j.ijgo.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 506.Zafar M, Saeed S, Kant B, Murtaza B, Dar MF, Khan NA. Use of amnion in vaginoplasty for vaginal atresia. J Coll Physicians Surg Pak. 2007;17:107–9. [PubMed] [Google Scholar]
- 507.Doillon CJ. Porous collagen sponge wound dressings: in vivo and in vitro studies. J Biomater Appl. 1988;2:562–78. doi: 10.1177/088532828700200404. [DOI] [PubMed] [Google Scholar]
- 508.Franco B, Vincenzo V, Alessandro DV, Tonello C, Abatangelo G, Mazzoleni F. Tissue engineering approaches for the construction of a completely autologous tendon substitute. Indian J Plast Surg. 2008;41:38–46. doi: 10.4103/0970-0358.41109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, Soker S, Van Dyke M. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–9. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Choi JS, Williams JK, Greven M, Walter KA, Laber PW, Khang G, Soker S. Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma. Biomaterials. 2010;31:6738–45. doi: 10.1016/j.biomaterials.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 511.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 514.O'Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR, Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–55. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 516.Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, Cochrane L, Jephson C, Janes S, Beaumont NJ, Cogan T, Bader A, Seifalian AM, Hsuan JJ, Lowdell MW, Birchall MA. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bio-engineered kidney. Nat Med. 2013;19:646–51. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Daoud J, Rosenberg L, Tabrizian M. Pancreatic islet culture and preservation strategies: advances, challenges, and future outlook. Cell Transplant. 2010;19:1523–35. doi: 10.3727/096368910X515872. [DOI] [PubMed] [Google Scholar]
- 520.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Balestrini JL, Niklason LE. Extracellular matrix as a driver for lung regeneration. Ann Biomed Eng. 2015;43:568–76. doi: 10.1007/s10439-014-1167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Hoshiba T, Kawazoe N, Tateishi T, Chen G. Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells. Adv Mater. 2010;22:3042–7. doi: 10.1002/adma.201000038. [DOI] [PubMed] [Google Scholar]
- 523.Glynn HJ, Polsin EG, Hinds MT. Crosslinking decreases the hemocompatibility of decellularized, porcine small intestinal submucosa. Acta Biomater. 2015;14:96–103. doi: 10.1016/j.actbio.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Magnan M, Berthod F, Champigny MF, Soucy F, Bolduc S. In vitro reconstruction of a tissue-engineered endothelialized bladder from a single porcine biopsy. J Pediatr Urol. 2006;2:261–70. doi: 10.1016/j.jpurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 525.Bouhout S, Perron E, Gauvin R, Bernard G, Ouellet G, Cattan V, Bolduc S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng Part A. 2010;16:1539–48. doi: 10.1089/ten.TEA.2009.0473. [DOI] [PubMed] [Google Scholar]
- 526.Mondalek FG, Ashley RA, Roth CC, Kibar Y, Shakir N, Ihnat MA, Fung KM, Grady BP, Kropp BP, Lin HK. Enhanced angiogenesis of modified porcine small intestinal submucosa with hyaluronic acid-poly(lactide-co-glycolide) nanoparticles: from fabrication to preclinical validation. J Biomed Mater Res A. 2010;94:712–9. doi: 10.1002/jbm.a.32748. [DOI] [PubMed] [Google Scholar]
- 527.Honsawek S, Bumrungpanichthaworn P, Thanakit V, Kunrangseesomboonc V, Muchmee S, Ratprasert S, Tangchainavaphum P, Dechprapatsorn S, Prajuabtanyachat S, Suksamran A, Rojchanawatsirivech A. Osteoinductive potential of small intestinal submucosa/demineralized bone matrix as composite scaffolds for bone tissue engineering. Asian Biomed. 2010;4:913–22. [Google Scholar]
- 528.Kim KS, Lee JY, Kang YM, Kim ES, Kim GH, Rhee SD, Cheon HG, Kim JH, Min BH, Lee HB, Kim MS. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells. Biomaterials. 2010;31:1104–13. doi: 10.1016/j.biomaterials.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 529.Zhao L, Zhao J, Wang S, Wang J, Liu J. Comparative study between tissue-engineered periosteum and structural allograft in rabbit critical-sized radial defect model. J Biomed Mater Res B Appl Biomater. 2011;97:1–9. doi: 10.1002/jbm.b.31768. [DOI] [PubMed] [Google Scholar]
- 530.Sutherland AJ, Converse GL, Hopkins RA, Detamore MS. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv Healthc Mater. 2015;4:29–39. doi: 10.1002/adhm.201400165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Elder BD, Kim DH, Athanasiou KA. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery. 2010;66:722–7. doi: 10.1227/01.NEU.0000367616.49291.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Kheir E, Stapleton T, Shaw D, Jin Z, Fisher J, Ingham E. Development and characterization of an acellular porcine cartilage bone matrix for use in tissue engineering. J Biomed Mater Res A. 2011;99:283–94. doi: 10.1002/jbm.a.33171. [DOI] [PubMed] [Google Scholar]
- 533.Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, Tang J, Xiang J, Song C, Li G. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2010;16:865–76. doi: 10.1089/ten.TEC.2009.0444. [DOI] [PubMed] [Google Scholar]
- 534.Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–73. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 535.Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D'Amore A, Nagarkar SP, Velankar SS, Badylak SF. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–38. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, Farhat WA. Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A. 2010;94:1205–15. doi: 10.1002/jbm.a.32777. [DOI] [PubMed] [Google Scholar]
- 537.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–93. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Thibault RA, Mikos AG, Kasper FK. Protein and mineral composition of osteogenic extracellular matrix constructs generated with a flow perfusion bioreactor. Biomacromolecules. 2011;12:4204–12. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, Khademhosseini A, Baharvand H, Aghdami N. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. 2014;35:970–82. doi: 10.1016/j.biomaterials.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 540.Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y, Chen J, Qi Z. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31:296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 541.Fang NT, Xie SZ, Wang SM, Gao HY, Wu CG, Pan LF. Construction of tissue-engineered heart valves by using decellularized scaffolds and endothelial progenitor cells. Chin Med J (Engl) 2007;120:696–702. [PubMed] [Google Scholar]
- 542.Dijkman PE, Driessen-Mol A, Frese L, Hoerstrup SP, Baaijens FP. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno-and homografts. Biomaterials. 2012;33:4545–54. doi: 10.1016/j.biomaterials.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 543.He M, Callanan A. Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Eng Part B Rev. 2013;19:194–208. doi: 10.1089/ten.teb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater. 2013;8:014106/1–9. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 545.Faulk DM, Johnson SA, Zhang L, Badylak SF. Role of the extracellular matrix in whole organ engineering. J Cell Physiol. 2014;229:984–9. doi: 10.1002/jcp.24532. [DOI] [PubMed] [Google Scholar]
- 546.Khan AA, Vishwakarma SK, Bardia A, Venkateshwarulu J. Repopulation of decellularized whole organ scaffold using stem cells: an emerging technology for the development of neo-organ. J Artif Organs. 2014;17:291–300. doi: 10.1007/s10047-014-0780-2. [DOI] [PubMed] [Google Scholar]
- 547.Uzarski JS, Xia Y, Belmonte JC, Wertheim JA. New strategies in kidney regeneration and tissue engineering. Curr Opin Nephrol Hypertens. 2014;23:399–405. doi: 10.1097/01.mnh.0000447019.66970.ea. [DOI] [PubMed] [Google Scholar]
- 548.Zhou Q, Li L, Li J. Stem cells with decellularized liver scaffolds in liver regeneration and their potential clinical applications. Liver Int. 2015;35:687–94. doi: 10.1111/liv.12581. [DOI] [PubMed] [Google Scholar]
- 549.Yasui H, Lee JK, Yoshida A, Yokoyama T, Nakanishi H, Miwa K, Naito AT, Oka T, Akazawa H, Nakai J, Miyagawa S, Sawa Y, Sakata Y, Komuro I. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials. 2014;35:7839–50. doi: 10.1016/j.biomaterials.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 550.Iop L, Bonetti A, Naso F, Rizzo S, Cagnin S, Bianco R, Dal Lin C, Martini P, Poser H, Franci P, Lanfranchi G, Busetto R, Spina M, Basso C, Marchini M, Gandaglia A, Ortolani F, Gerosa G. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLOS ONE. 2014;9:e99593/1–15. doi: 10.1371/journal.pone.0099593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, Le Guyader S, Henriksson G, Hermanson O, Juto JE, Leidner B, Lilja T, Liska J, Luedde T, Lundin V, Moll G, Nilsson B, Roderburg C, Strömblad S, Sutlu T, Teixeira AI, Watz E, Seifalian A, Macchiarini P. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 552.Momtahan N, Sukavaneshvar SS, Roeder BL, Cook AD. Strategies and processes to decellularize and recellularize hearts to generate functional organs and reduce the risk of thrombosis. Tissue Eng Part B Rev. 2015;21:115–32. doi: 10.1089/ten.TEB.2014.0192. [DOI] [PubMed] [Google Scholar]
- 553.Connolly JL, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71:684–91. [PubMed] [Google Scholar]
- 554.Jäger M, Jelinek EM, Wess KM, Scharfstädt A, Jacobson M, Kevy SV, Krauspe R. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 555.Jäger M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29:173–80. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 556.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–85. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 558.Connolly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res. 1995;313:8–18. [PubMed] [Google Scholar]
- 559.Hendrich C, Franz E, Waertel G, Krebs R, Jäger M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev (Pavia) 2009;1:e32/1–5. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Jungbluth P, Hakimi AR, Grassmann JP, Schneppendahl J, Betsch M, Kröpil P, Thelen S, Sager M, Herten M, Wild M, Windolf J, Hakimi M. The early phase influence of bone marrow concentrate on metaphyseal bone healing. Injury. 2013;44:1285–94. doi: 10.1016/j.injury.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 561.Hakimi M, Grassmann JP, Betsch M, Schneppendahl J, Gehrmann S, Hakimi AR, Kröpil P, Sager M, Herten M, Wild M, Windolf J, Jungbluth P. The composite of bone marrow concentrate and PRP as an alternative to autologous bone grafting. PLOS ONE. 2014;9:e100143/1–18. doi: 10.1371/journal.pone.0100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Hart R, Komzák M, Okál F, Náhlík D, Jajtner P, Puskeiler M. Allograft alone versus allograft with bone marrow concentrate for the healing of the instrumented posterolateral lumbar fusion. Spine J. 2014;14:1318–24. doi: 10.1016/j.spinee.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 563.Rickert D, Vissink A, Slot WJ, Sauerbier S, Meijer HJ, Raghoebar GM. Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. Int J Oral Maxillofac Surg. 2014;43:243–7. doi: 10.1016/j.ijom.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 564.Steinwachs MR, Waibl B, Wopperer S, Mumme M. Matrix-associated chondroplasty: a novel platelet-rich plasma and concentrated nucleated bone marrow cell-enhanced cartilage restoration technique. Arthrosc Tech. 2014;3:e279–82. doi: 10.1016/j.eats.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Lee DH, Ryu KJ, Kim JW, Kang KC, Choi YR. Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res. 2014;472:3789–97. doi: 10.1007/s11999-014-3548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Sampson S, Botto-van Bemden A, Aufiero D. Autologous bone marrow concentrate: review and application of a novel intraarticular orthobiologic for cartilage disease. Phys Sportsmed. 2013;41:7–18. doi: 10.3810/psm.2013.09.2022. [DOI] [PubMed] [Google Scholar]
- 567.Gessmann J, Köller M, Godry H, Schildhauer TA, Seybold D. Regenerate augmentation with bone marrow concentrate after traumatic bone loss. Orthop Rev (Pavia) 2012;4:e14/1–5. doi: 10.4081/or.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, Sakai D, Kudoh T, Kawamoto K, Eguchi H, Satoh T, Tanemura M, Nagano H, Doki Y, Mori M, Ishii H. Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ. 2013;55:309–18. doi: 10.1111/dgd.12049. [DOI] [PubMed] [Google Scholar]
- 569.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Huang SJ, Fu RH, Shyu WC, Liu SP, Jong GP, Chiu YW, Wu HS, Tsou YA, Cheng CW, Lin SZ. Adipose-derived stem cells: isolation, characterization, and differentiation potential. Cell Transplant. 2013;22:701–9. doi: 10.3727/096368912X655127. [DOI] [PubMed] [Google Scholar]
- 571.Zhi K, Gao Z, Bai J, Wu Y, Zhou S, Li M, Qu L. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci (Landmark Ed) 2014;19:768–76. doi: 10.2741/4243. [DOI] [PubMed] [Google Scholar]
- 572.Ichim TE, Harman RJ, Min WP, Minev B, Solano F, Rodriguez JP, Alexandrescu DT, De Necochea-Campion R, Hu X, Marleau AM, Riordan NH. Autologous stromal vascular fraction cells: a tool for facilitating tolerance in rheumatic disease. Cell Immunol. 2010;264:7–17. doi: 10.1016/j.cellimm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 573.Barba M, Cicione C, Bernardini C, Michetti F, Lattanzi W. Adipose-derived mesenchymal cells for bone regereneration: state of the art. Biomed Res Int. 2013;2013:416391/1–11. doi: 10.1155/2013/416391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1:230–6. doi: 10.5966/sctm.2011-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Lin SD, Huang SH, Lin YN, Wu SH, Chang HW, Lin TM, Chai CY, Lai CS. Engineering adipose tissue from uncultured human adipose stromal vascular fraction on collagen matrix and gelatin sponge scaffolds. Tissue Eng Part A. 2011;17:1489–98. doi: 10.1089/ten.TEA.2010.0688. [DOI] [PubMed] [Google Scholar]
- 576.Mohammadi R, Sanaei N, Ahsan S, Rostami H, Abbasipour-Dalivand S, Amini K. Repair of nerve defect with chitosan graft supplemented by uncultured characterized stromal vascular fraction in streptozotocin induced diabetic R rats. Int J Surg. 2014;12:33–40. doi: 10.1016/j.ijsu.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 577.Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B. Adipose-derived stromal vascular fraction improves tendon healing in rabbits. Chin J Traumatol. 2011;14:329–35. [PubMed] [Google Scholar]
- 578.Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, Gimble JM, Bunnell BA. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med. 2013;2:789–96. doi: 10.5966/sctm.2013-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Jurgens WJ, Kroeze RJ, Zandieh-Doulabi B, van Dijk A, Renders GA, Smit TH, van Milligen FJ, Ritt MJ, Helder MN. One-step surgical procedure for the treatment of osteochondral defects with adipose-derived stem cells in a caprine knee defect: a pilot study. Biores Open Access. 2013;2:315–25. doi: 10.1089/biores.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Kim A, Kim DH, Song HR, Kang WH, Kim HJ, Lim HC, Cho DW, Bae JH. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy. 2012;14:296–305. doi: 10.3109/14653249.2011.627915. [DOI] [PubMed] [Google Scholar]
- 581.Rhee SC, Ji YH, Gharibjanian NA, Dhong ES, Park SH, Yoon ES. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011;20:233–42. doi: 10.1089/scd.2009.0525. [DOI] [PubMed] [Google Scholar]
- 582.Lu H, Xie C, Zhao YM, Chen FM. Translational research and therapeutic applications of stem cell transplantation in periodontal regenerative medicine. Cell Transplant. 2013;22:205–29. doi: 10.3727/096368912X656171. [DOI] [PubMed] [Google Scholar]
- 583.Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, Floris M, Fiaschetti V, Floris R, Cervell V. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1:341–51. doi: 10.5966/sctm.2011-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Chlapanidas T, Faragò S, Mingotto F, Crovato F, Tosca MC, Antonioli B, Bucco M, Lucconi G, Scalise A, Vigo D, Faustini M, Marazzi M, Torre ML. Regenerated silk fibroin scaffold and infrapatellar adipose stromal vascular fraction as feeder-layer: a new product for cartilage advanced therapy. Tissue Eng Part A. 2011;17:1725–33. doi: 10.1089/ten.TEA.2010.0636. [DOI] [PubMed] [Google Scholar]
- 585.Thirumala S, Gimble JM, Devireddy RV. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med. 2010;4:224–32. doi: 10.1002/term.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source ofstem cells for biotechnology. Trends Biotechnol. 2006;24:150–4. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 587.Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells. 2011;29:749–54. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 588.Aronowitz JA, Ellenhorn JD. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132:932e–9e. doi: 10.1097/PRS.0b013e3182a80652. [DOI] [PubMed] [Google Scholar]
- 589.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 590.Intini G. The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials. 2009;30:4956–66. doi: 10.1016/j.biomaterials.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 591.Anitua E, Sánchez M, Orive G, Andía I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials. 2007;28:4551–60. doi: 10.1016/j.biomaterials.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 592.Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(Suppl.1):3S–22S. [PubMed] [Google Scholar]
- 593.Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20:267–76. doi: 10.1089/ten.teb.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, Peng J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21:1627–37. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 595.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 596.Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents. 2012;26(Suppl. 1):35S–42S. [PubMed] [Google Scholar]
- 597.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9:721–30. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 598.Russell RP, Apostolakos J, Hirose T, Cote MP, Mazzocca AD. Variability of platelet-rich plasma preparations. Sports Med Arthrosc. 2013;21:186–90. doi: 10.1097/JSA.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 599.Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, Andia I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415–21. doi: 10.1002/jbm.b.30886. [DOI] [PubMed] [Google Scholar]
- 600.Zehnder JL, Leung LL. Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombin. Blood. 1990;76:2011–6. [PubMed] [Google Scholar]
- 601.Diesen DL, Lawson JH. Bovine thrombin: history, use, and risk in the surgical patient. Vascular. 2008;16(Suppl.1):S29–36. [PubMed] [Google Scholar]
- 602.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 603.Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R. Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg. 2005;63:521–8. doi: 10.1016/j.joms.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 604.Anitua EA. Enhancement of osseointegration by generating a dynamic implant surface. J Oral Implantol. 2006;32:72–6. doi: 10.1563/736.1. [DOI] [PubMed] [Google Scholar]
- 605.Anitua E, Sánchez M, Orive G, Andia I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008;29:37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 606.Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015 doi: 10.1007/s00167-013-2784-5. http://dx.doi.org/10.1007/s00167-013-2784-5. [DOI] [PubMed]
- 607.Tarantino R, Donnarumma P, Mancarella C, Rullo M, Ferrazza G, Barrella G, Martini S, Delfini R. Posterolateral arthrodesis in lumbar spine surgery using autologous platelet-rich plasma and cancellous bone substitute: an osteoinductive and osteoconductive effect. Global Spine J. 2014;4:137–42. doi: 10.1055/s-0034-1376157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Nagata MJ, de Campos N, Messora MR, Santinoni CS, Bomfim SR, Fucini SE, Pola NM, Neves AP, de Almeida JM, Theodoro LH, Ervolino E. Platelet-rich plasma derived from bone marrow aspirate promotes newcementum formation. J Periodontol. 2014;85:1702–11. doi: 10.1902/jop.2014.140083. [DOI] [PubMed] [Google Scholar]
- 609.Hamid A, Mohamed Ali MS, Yusof MR, George A, Lee JLP. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42:2410–8. doi: 10.1177/0363546514541540. [DOI] [PubMed] [Google Scholar]
- 610.Arnoczky SP, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21:180–5. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 611.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 612.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–29. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Fattahi T, Mohan M, Caldwell GT. Clinical applications of fibrin sealants. J Oral Maxillofac Surg. 2004;62:218–24. doi: 10.1016/j.joms.2003.01.005. [DOI] [PubMed] [Google Scholar]
- 614.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 615.Bryan N, Rhodes NP, Hunt JA. Derivation and performance of an entirely autologous injectable hydrogel delivery system for cell-based therapies. Biomaterials. 2009;30:180–8. doi: 10.1016/j.biomaterials.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 616.Liu B, Tan XY, Liu YP, Xu XF, Li L, Xu HY, An R, Chen FM. The adjuvant use of stromal vascular fraction and platelet-rich fibrin for autologous adipose tissue transplantation. Tissue Eng Part C Methods. 2013;19:1–14. doi: 10.1089/ten.TEC.2012.0126. [DOI] [PubMed] [Google Scholar]
- 617.Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Control Rel. 2010;148:49–55. doi: 10.1016/j.jconrel.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Ferreira MS, Jahnen-Dechent W, Labude N, Bovi M, Hieronymus T, Zenke M, Schneider RK, Neuss S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials. 2012;33:6987–97. doi: 10.1016/j.biomaterials.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 619.Anitua E, Prado R, Azkargorta M, Rodriguez-Suárez E, Iloro I, Casado-Vela J, Elortza F, Orive G. High-throughput proteomic characterization of plasma rich in growth factors (PRGF-Endoret)-derived fibrin clot interactome. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.1721. http://dx.doi.org/10.1002/term.1721. [DOI] [PubMed]
- 620.Anitua E, Alkhraisat MH, Orive G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J Control Rel. 2012;157:29–38. doi: 10.1016/j.jconrel.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 621.Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: a clinical case report. Int J Periodontics Restorative Dent. 2006;26:363–9. [PubMed] [Google Scholar]
- 622.Hokugo A, Ozeki M, Kawakami O, Sugimoto K, Mushimoto K, Morita S, Tabata Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005;11:1224–33. doi: 10.1089/ten.2005.11.1224. [DOI] [PubMed] [Google Scholar]
- 623.Lu HH, Vo JM, Chin HS, Lin J, Cozin M, Tsay R, Eisig S, Landesberg R. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A. 2008;86:1128–36. doi: 10.1002/jbm.a.31740. [DOI] [PubMed] [Google Scholar]
- 624.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 625.Rai B, Teoh SH, Ho KH. An in vitro evaluation of PCL-TCP composites as delivery systems for platelet-rich plasma. J Control Rel. 2005;107:330–42. doi: 10.1016/j.jconrel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 626.Sánchez-Ilárduya MB, Trouche E, Tejero R, Orive G, Reviakine I, Anitua E. Time-dependent release of growth factors from implant surfaces treated with plasma rich in growth factors. J Biomed Mater Res A. 2013;101:1478–88. doi: 10.1002/jbm.a.34428. [DOI] [PubMed] [Google Scholar]
- 627.Simson J, Crist J, Strehin I, Lu Q, Elisseeff JH. An orthopedic tissue adhesive for targeted delivery of intraoperative biologics. J Orthop Res. 2013;31:392–400. doi: 10.1002/jor.22247. [DOI] [PubMed] [Google Scholar]
- 628.Manello F, Tonti GA. Concise review, no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603–9. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 629.Govindasamy V, Ronald VS, Abdullah AN, Ganesan Nathan KR, Aziz ZA, Abdullah M, Zain RB, Kasim NH, Musa S, Bhonde RR. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13:1221–33. doi: 10.3109/14653249.2011.602337. [DOI] [PubMed] [Google Scholar]
- 630.Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–41. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 631.Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A. Platelet lysate consisting of a natural repair pro-teome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 632.Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol. 2004;32:1212–25. doi: 10.1016/j.exphem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 633.Anitua E, Prado R, Orive G. Endogenous morphogens and fibrin bioscaffolds for stem cell therapeutics. Trends Biotechnol. 2013;31:364–74. doi: 10.1016/j.tibtech.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 634.Shichinohe H, Kuroda S, Sugiyama T, Ito M, Kawabori M, Nishio M, Takeda Y, Koike T, Houkin K. Biological features of human bone marrow stromal cells (hBMSC) cultured with animal protein-free medium-safety and efficacy of clinical use for neurotransplantation. Transl Stroke Res. 2011;2:307–15. doi: 10.1007/s12975-011-0088-y. [DOI] [PubMed] [Google Scholar]
- 635.Pérez-Ilzarbe M, Díez-Campelo M, Aranda P, Tabera S, Lopez T, del Canizo C, Merino J, Moreno C, Andreu EJ, Prósper F, Pérez-Simón JA. Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion. 2009;49:1901–10. doi: 10.1111/j.1537-2995.2009.02226.x. [DOI] [PubMed] [Google Scholar]
- 636.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 637.Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–46. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 638.Ben Azouna N, Jenhani F, Regaya Z, Berraeis L, Ben Othman T, Ducrocq E, Domenech J. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther. 2012;3:6/1–14. doi: 10.1186/scrt97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Flemming A, Schallmoser K, Strunk D, Stolk M, Volk HD, Seifert M. Immunomodulative efficacy of bone marrow-derived mesenchymal stem cells cultured in human platelet lysate. J Clin Immunol. 2011;31:1143–56. doi: 10.1007/s10875-011-9581-z. [DOI] [PubMed] [Google Scholar]
- 640.Naaijkens BA, Niessen HW, Prins HJ, Krijnen PA, Kokhuis TJ, de Jong N, van Hinsbergh VW, Kamp O, Helder MN, Musters RJ, van Dijk A, Juffermans LJ. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348:119–30. doi: 10.1007/s00441-012-1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Fukaya Y, Kuroda M, Aoyagi Y, Asada S, Kubota Y, Okamoto Y, Nakayama T, Saito Y, Satoh K, Bujo H. Platelet-rich plasma inhibits the apoptosis of highly adipogenic homogeneous preadipocytes in an in vitro culture system. Exp Mol Med. 2012;44:330–9. doi: 10.3858/emm.2012.44.5.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Abdelrazik H, Spaggiari GM, Chiossone L, Moretta L. Mesenchymal stem cells expanded in human platelet lysate display a decreased inhibitory capacity on T- and NK-cell proliferation and function. Eur J Immunol. 2011;41:3281–90. doi: 10.1002/eji.201141542. [DOI] [PubMed] [Google Scholar]
- 643.Nold P, Brendel C, Neubauer A, Bein G, Hackstein H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochem Biophys Res Commun. 2013;430:325–30. doi: 10.1016/j.bbrc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 644.Castiglia S, Mareschi K, Labanca L, Lucania G, Leone M, Sanavio F, Castello L, Rustichelli D, Signorino E, Gunetti M, Bergallo M, Bordiga AM, Ferrero I, Fagioli F. Inactivated human platelet lysate with psoralen: a new perspective for mesenchymal stromal cell production in Good Manufacturing Practice conditions. Cytotherapy. 2014;16:750–63. doi: 10.1016/j.jcyt.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Hemeda H, Giebel B, Wagner W. Evaluation of human plateletlysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–80. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 646.Janetzko K, Kluter H, Waeg GV, Eichler H. Fully automated processing of buffy-coat-derived pooled platelet concentrates. Transfusion. 2004;44:1052–8. doi: 10.1111/j.1537-2995.2004.03296.x. [DOI] [PubMed] [Google Scholar]
- 647.Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion. 2009;49:2680–5. doi: 10.1111/j.1537-2995.2009.02346.x. [DOI] [PubMed] [Google Scholar]
- 648.Horn P, Bokermann G, Cholewa D, Bork S, Walenda T, Koch C, Drescher W, Hutschenreuther G, Zenke M, Ho AD, Wagner W. Impact of individual platelet lysates on isolation and growth of human mesenchymal stromal cells. Cytotherapy. 2010;12:888–98. doi: 10.3109/14653249.2010.501788. [DOI] [PubMed] [Google Scholar]
- 649.Chevallier N, Anagnostou F, Zilber S, Bodivit G, Maurin S, Barrault A, Bierling P, Hernigou P, Layrolle P, Rouard H. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2009;31:270–8. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 650.Shanskii YD, Sergeeva NS, Sviridova IK, Kirakozov MS, Kirsanova VA, Akhmedova SA, Antokhin AI, Chissov VI. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull Exp Biol Med. 2013;156:146–51. doi: 10.1007/s10517-013-2298-7. [DOI] [PubMed] [Google Scholar]
- 651.Ruggiu A, Ulivi V, Sanguineti F, Cancedda R, Descalzi F. The effect of Platelet Lysate on osteoblast proliferation associated with a transient increase of the inflammatory response in bone regeneration. Biomaterials. 2013;34:9318–30. doi: 10.1016/j.biomaterials.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 652.Griffiths S, Baraniak PR, Copland IB, Nerem RM, McDevitt TC. Human platelet lysate stimulates high-passage and senescent human multipotent mesenchymal stromal cell growth and rejuvenation in vitro. Cytotherapy. 2013;15:1469–83. doi: 10.1016/j.jcyt.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 653.Ito R, Morimoto N, Pham LH, Taira T, Kawai K, Suzuki S. Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Eng Part A. 2013;19:1398–405. doi: 10.1089/ten.TEA.2012.0375. [DOI] [PubMed] [Google Scholar]
- 654.Leotot J, Coquelin L, Bodivit G, Bierling P, Hernigou P, Rouard H, Chevallier N. Platelet lysate coating on scaffolds directly and indirectly enhances cell migration, improving bone and blood vessel formation. Acta Biomater. 2013;9:6630–40. doi: 10.1016/j.actbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 655.Trojahn Kølle SF, Oliveri RS, Glovinski PV, Kirchhoff M, Mathiasen AB, Elberg JJ, Andersen PS, Drzewiecki KT, Fischer-Nielsen A. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinicaluse. Cytotherapy. 2013;15:1086–97. doi: 10.1016/j.jcyt.2013.01.217. [DOI] [PubMed] [Google Scholar]
- 656.Sowmya S, Bumgardener JD, Chennazhi KP, Nair SV, Jayakumar R. Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog Polym Sci. 2013;38:1748–72. [Google Scholar]
- 657.Grafahrend D, Heffels KH, Beer MV, Gasteier P, Möller M, Boehm G, Dalton PD, Groll J. Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat Mater. 2011;10:67–73. doi: 10.1038/nmat2904. [DOI] [PubMed] [Google Scholar]
- 658.Zhang G. Biomimicry in biomedical research. Organogenesis. 2012;8:101–2. doi: 10.4161/org.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Benyus JM. Biomimicry: Innovation Inspired by Nature. New York: Harper Perennial Publication; 2002. Echoing nature; pp. 1–11. [Google Scholar]
- 660.Chen PY, McKittrick J, Meyers MA. Biological materials: functional adaptations and bioinspired designs. Prog Mater Sci. 2012;57:1492–704. [Google Scholar]
- 661.Cai L, Heilshorn SC. Designing ECM-mimetic materials using protein engineering. Acta Biomater. 2014;10:1751–60. doi: 10.1016/j.actbio.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Kim JH, Park CH, Perez RA, Lee HY, Jang JH, Lee HH, Wall IB, Shi S, Kim HW. Advanced biomatrix designs for regenerative therapy of periodontal tissues. J Dent Res. 2014;93:1203–11. doi: 10.1177/0022034514540682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Zhao J, Zhao X, Jiang Z, Li Z, Fan X, Zhu J, Wu H, Su Y, Yang D, Pan F, Shi J. Biomimetic and bioinspired membranes: preparation and application. Prog Polym Sci. 2014;39:1668–720. [Google Scholar]
- 664.Ma J, Wang J, Ai X, Zhang S. Biomimetic self-assembly of apatite hybrid materials: from a single molecular template to bi-/multi-molecular templates. Biotechnol Adv. 2014;32:744–60. doi: 10.1016/j.biotechadv.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 665.von der Mark K, Park J, Bauer S, Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339:131–53. doi: 10.1007/s00441-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 666.Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R Rep. 2014;80:1–36. [Google Scholar]
- 667.Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558–69. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 668.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Bhaarathy V, Venugopal J, Gandhimathi C, Ponpandian N, Mangalaraj D, Ramakrishna S. Biologically improved nanofibrous scaffolds for cardiac tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;44:268–77. doi: 10.1016/j.msec.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 670.Chung J, Kushner AM, Weisman AC, Guan Z. Direct correlation of single-molecule properties with bulk mechanical performance for the biomimetic design of polymers. Nat Mater. 2014;13:1055–62. doi: 10.1038/nmat4090. [DOI] [PubMed] [Google Scholar]
- 671.Choi YC, Choi JS, Woo CH, Cho YW. Stem cell delivery systems inspired by tissue-specific niches. J Control Rel. 2014;193:42–50. doi: 10.1016/j.jconrel.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 672.Tibbitt MW, Anseth KS. Dynamic microenvironments: the fourth dimension. Sci Transl Med. 2012;4:160ps24/1–4. doi: 10.1126/scitranslmed.3004804. [DOI] [PubMed] [Google Scholar]
- 673.Balakrishnan B, Joshi N, Jayakrishnan A, Banerjee R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–63. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 674.Hu X, Tang-Schomer MD, Huang W, Xia XX, Weiss AS, Kaplan DL. Charge-tunable silk-tropoelastin protein alloys that control neuron cell responses. Adv Funct Mater. 2013;23:3875–84. doi: 10.1002/adfm.201202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Wang Y, Zhao Q, Zhang H, Yang S, Jia X. A novel poly(amido amine)-dendrimer-based hydrogel as a mimic for the extracellular matrix. Adv Mater. 2014;26:4163–7. doi: 10.1002/adma.201400323. [DOI] [PubMed] [Google Scholar]
- 676.Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, Groll J, Hutmacher DW. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25:5011–28. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 677.Tong X, Yang F. Engineering interpenetrating network hydrogels as biomimetic cell niche with independently tunable biochemical and mechanical properties. Biomaterials. 2014;35:1807–15. doi: 10.1016/j.biomaterials.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 678.Kivelio A, Ochsenbein-Koelble N, Zimmermann R, Ehrbar M. Engineered cell instructive matrices for fetal membrane healing. Acta Biomater. 2015;15:1–10. doi: 10.1016/j.actbio.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 679.Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotechnol. 2007;18:448–53. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Yildirimer L, Seifalian AM. Three-dimensional biomaterial degradation – Material choice, design and extrinsic factor considerations. Biotechnol Adv. 2014;32:984–99. doi: 10.1016/j.biotechadv.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 681.DeForest CA, Anseth KS. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–44. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 682.Kirschner CM, Anseth KS. Hydrogels in healthcare: from static to dynamic material microenvironments. Acta Mater. 2013;61:931–44. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, Jin H, Parker KK, Langer R, Kohane DS. Nanowired three-dimensional cardiac patches. Nat Nanotechnol. 2011;6:720–5. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Huang H, Anker JN, Wang K, Kopelman R. Magnetically assisted and accelerated self-assembly of strawberry-like nano/microparticles. J Phys Chem B. 2006;110:19929–34. doi: 10.1021/jp062070j. [DOI] [PubMed] [Google Scholar]
- 685.Wong Po, Foo C, Patwardhan SV, Belton DJ, Kitchel B, Anastasiades D, Huang J, Naik RR, Perry CC, Kaplan DL. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc Natl Acad Sci U S A. 2006;103:9428–33. doi: 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, Pastorin G. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013;65:1964–2015. doi: 10.1016/j.addr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 687.Raza A, Ki CS, Lin CC. The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D. Biomaterials. 2013;34:5117–27. doi: 10.1016/j.biomaterials.2013.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Kroner E, Kaiser JS, Fischer SC, Arzt E. Bioinspired polymeric surface patterns for medical applications. J Appl Biomater Funct Mater. 2012;10:287–92. doi: 10.5301/JABFM.2012.10365. [DOI] [PubMed] [Google Scholar]
- 689.Bernard AB, Chapman RZ, Anseth KS. Controlled local presentation of matrix proteins in microparticle-laden cell aggregates. Biotechnol Bioeng. 2014;111:1028–37. doi: 10.1002/bit.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Cañas N, Kamperman M, Völker B, Kroner E, McMeeking RM, Arzt E. Effect of nano- and micro-roughness on adhesion of bioinspired micropatterned surfaces. Acta Biomater. 2012;8:282–8. doi: 10.1016/j.actbio.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 691.Celiz AD, Smith JG, Langer R, Anderson DG, Winkler DA, Barrett DA, Davies MC, Young LE, Denning C, Alexander MR. Materials for stem cell factories of the future. Nat Mater. 2014;13:570–9. doi: 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- 692.Tibbitt MW, Kloxin AM, Dyamenahalli KU, Anseth KS. Controlled two-photon photodegradation of PEG hydrogels to study and manipulate subcellular interactions on soft materials. Soft Matter. 2010;6:5100–8. doi: 10.1039/C0SM00174K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Önnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegård D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem. 2012;287:18913–24. doi: 10.1074/jbc.M111.298968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Jayakumar R, Prabaharan M, Nair SV, Tokura S, Tamura H, Selvamurugan N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog Mater Sci. 2010;55:675–709. [Google Scholar]
- 695.Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21:45–54. doi: 10.1089/ten.teb.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Cromar GL, Xiong X, Chautard E, Ricard-Blum S, Parkinson J. Toward a systems level view of the ECM and related proteins: a framework for the systematic definition and analysis of biological systems. Proteins. 2012;80:1522–44. doi: 10.1002/prot.24036. [DOI] [PubMed] [Google Scholar]
- 697.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction. J Cell Sci. 2012;125:3051–60. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–90. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 699.Doyle JJ, Gerber EE, Dietz HC. Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett. 2012;586:2003–15. doi: 10.1016/j.febslet.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Guthrie K, Bruce A, Sangha N, Rivera E, Basu J. Potency evaluation of tissue engineered and regenerative medicine products. Trends Biotechnol. 2013;31:505–14. doi: 10.1016/j.tibtech.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 701.Wolchok JC, Tresco PA. The isolation of cell derived extracellular matrix constructs using sacrificial open-cell foams. Biomaterials. 2010;31:9595–603. doi: 10.1016/j.biomaterials.2010.08.072. [DOI] [PubMed] [Google Scholar]
- 702.Costa-Almeida R, Granja PL, Soares R, Guerreiro SG. Cellular strategies to promote vascularisation in tissue engineering applications. Eur Cell Mater. 2014;28:51–67. doi: 10.22203/ecm.v028a05. [DOI] [PubMed] [Google Scholar]
- 703.Lü WD, Zhang L, Wu CL, Liu ZG, Lei GY, Liu J, Gao W, Hu YR. Development of an a cellulartumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLOS ONE. 2014;9:e103672/1–13. doi: 10.1371/journal.pone.0103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Xing Q, Yates K, Tahtinen M, Shearier E, Qian Z, Zhao F. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng Part C Methods. 2015;21:77–87. doi: 10.1089/ten.tec.2013.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Xu Y, Xu GY, Tang C, Wei B, Pei X, Gui JC, Min BH, Jin CZ, Wang LM. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2015;103:670–8. doi: 10.1002/jbm.b.33231. [DOI] [PubMed] [Google Scholar]
- 706.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–9. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 707.Bourget JM, Gauvin R, Larouche D, Lavoie A, Labbé R, Auger FA, Germain L. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials. 2012;33:9205–13. doi: 10.1016/j.biomaterials.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 708.Escobedo-Lucea C, Ayuso-Sacido A, Xiong C, Prado-López S, del Pino MS, Melguizo D, Bellver-Estellés C, Gonzalez-Granero S, Valero ML, Moreno R, Burks DJ, Stojkovic M. Development of a human extracellular matrix for applications related with stem cells and tissue engineering. Stem Cell Rev. 2012;8:170–83. doi: 10.1007/s12015-011-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Du P, Hwang MP, Noh YK, Subbiah R, Kim IG, Bae SE, Park K. Fibroblast-derived matrix (FDM) as a novel vascular endothelial growth factor delivery platform. J Control Rel. 2014;194:122–9. doi: 10.1016/j.jconrel.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 710.Xing Q, Vogt C, Leong KW, Zhao F. Highly aligned nanofibrous scaffold derived from decellularized human fibroblasts. Adv Funct Mater. 2014;24:3027–35. doi: 10.1002/adfm.201303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 712.Walters NJ, Gentleman E. Evolving insights in cell-matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 2015;11:3–16. doi: 10.1016/j.actbio.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Hoshiba T, Kawazoe N, Tateishi T, Chen G. Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions. J Biol Chem. 2009;284:31164–73. doi: 10.1074/jbc.M109.054676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Hoshiba T, Lu H, Yamada T, Kawazoe N, Tateishi T, Chen G. Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol Prog. 2011;27:788–95. doi: 10.1002/btpr.592. [DOI] [PubMed] [Google Scholar]
- 715.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 716.Dubé J, Bourget JM, Gauvin R, Lafrance H, Roberge CJ, Auger FA, Germain L. Progress in developing a living human tissue-engineered tri-leaflet heart valve assembled from tissue produced by the self-assembly approach. Acta Biomater. 2014;10:3563–70. doi: 10.1016/j.actbio.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 717.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L'heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–6. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 718.Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35:4046–57. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 719.Paital SR, Dahotre NB. Calcium phosphate coatings for bio-implant applications: materials, performance factors, and methodologies. Mater Sci Eng R Rep. 2009;66:1–70. [Google Scholar]
- 720.Pati F, Song TH, Rijal G, Jang J, Kim SW, Cho DW. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230–41. doi: 10.1016/j.biomaterials.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 721.Stanton MM, Parrillo A, Thomas GM, McGimpsey WG, Wen Q, Bellin RM, Lambert CR. Fibroblast extracellular matrix and adhesion on microtextured polydimethylsiloxane scaffolds. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33244. http://dx.doi.org/10.1002/jbm.b.33244. [DOI] [PubMed]
- 722.Bauer S, Schmuki P, von der Mark K, Park J. Engineering biocompatible implant surfaces. Part I: materials and surfaces. Prog Mater Sci. 2013;58:261–326. [Google Scholar]
- 723.Jagota A, Hui CY. Adhesion, friction, and compliance of bio-mimetic and bioinspired structured interfaces. Mater Sci Eng R Rep. 2011;72:253–92. [Google Scholar]
- 724.von der Mark K, Park J. Engineering biocompatible implant surfaces. Part II: cellular recognition of biomaterial surfaces: lessons from cell–matrix interactions. Prog Mater Sci. 2013;58:327–81. [Google Scholar]
- 725.Pham MT, Reuther H, Maitz MF. Native extracellular matrix coating on Ti surfaces. J Biomed Mater Res A. 2003;66:310–6. doi: 10.1002/jbm.a.10575. [DOI] [PubMed] [Google Scholar]
- 726.Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 727.Demirbag B, Huri PY, Kose GT, Buyuksungur A, Hasirci V. Advanced cell therapies with and without scaffolds. Biotechnol J. 2011;6:1437–53. doi: 10.1002/biot.201100261. [DOI] [PubMed] [Google Scholar]
- 728.Zhao YH, Zhang M, Liu NX, Lv X, Zhang J, Chen FM, Chen YJ. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials. 2013;34:5506–20. doi: 10.1016/j.biomaterials.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 729.Kim BS, Choi JS, Kim JD, Choi YC, Cho YW. Recellularization of decellularized human adipose-tissue-derived extracellular matrix sheets with other human cell types. Cell Tissue Res. 2012;348:559–67. doi: 10.1007/s00441-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 730.Farag A, Vaquette C, Theodoropoulos C, Hamlet SM, Hutmacher DW, Ivanovski S. Decellularized periodontal ligament cell sheets with recellularization potential. J Dent Res. 2014;93:1313–9. doi: 10.1177/0022034514547762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Hwang Y, Phadke A, Varghese S. Engineered microenvironments for self-renewal and musculoskeletal differentiation of stem cells. Regen Med. 2011;6:505–24. doi: 10.2217/rme.11.38. [DOI] [PubMed] [Google Scholar]
- 732.Dingal PC, Discher DE. Combining insoluble and soluble factors to steer stem cell fate. Nat Mater. 2014;13:532–7. doi: 10.1038/nmat3997. [DOI] [PubMed] [Google Scholar]
- 733.Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M, Lutolf MP. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater. 2013;12:1072–8. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 734.Horobin AJ, Shakesheff KM, Woodrow S, Robinson C, Pritchard DI. Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon interactions between human dermal fibroblasts and extracellular matrix components. Br J Dermatol. 2003;148:923–33. doi: 10.1046/j.1365-2133.2003.05314.x. [DOI] [PubMed] [Google Scholar]
- 735.Verhulsel M, Vignes M, Descroix S, Malaquin L, Vignjevic DM, Viovy JL. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials. 2014;35:1816–32. doi: 10.1016/j.biomaterials.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 736.Kong HJ, Mooney DJ. Microenvironmental regulation of biomacromolecular therapies. Nat Rev Drug Discov. 2007;6:455–63. doi: 10.1038/nrd2309. [DOI] [PubMed] [Google Scholar]
- 737.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Ghaemi SR, Harding FJ, Delalat B, Gronthos S, Voelcker NH. Exploring the mesenchymal stem cell niche using high throughput screening. Biomaterials. 2013;34:7601–15. doi: 10.1016/j.biomaterials.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 739.Hume PS, He J, Haskins K, Anseth KS. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials. 2012;33:3615–25. doi: 10.1016/j.biomaterials.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc. 2010;5:1867–87. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10:362–9. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 742.Mao JJ, Stosich MS, Moioli EK, Lee CH, Fu SY, Bastian B, Eisig SB, Zemnick C, Ascherman J, Wu J, Rohde C, Ahn J. Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev. 2010;16:257–62. doi: 10.1089/ten.teb.2009.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Park MS, Kim YH, Jung Y, Kim SH, Park JC, Yoon DS, Kim SH, Lee JW. In situ recruitment of human BMSCs using chemokines for articular cartilage regeneration. Cell Transplant. 2015 doi: 10.3727/096368914X681018. http://dx.doi.org/10.3727/096368914X681018. [DOI] [PubMed]
- 745.Glowacki AJ, Gottardi R, Yoshizawa S, Cavalla F, Garlet GP, Sfeir C, Little SR. Strategies to direct the enrichment, expansion, and recruitment of regulatory cells for the treatment of disease. Ann Biomed Eng. 2015;43:593–602. doi: 10.1007/s10439-014-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6/1–16. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Ko IK, Ju YM, Chen T, Atala A, Yoo JJ, Lee SJ. Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J. 2012;26:158–68. doi: 10.1096/fj.11-182998. [DOI] [PubMed] [Google Scholar]
- 748.Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, Moroni L, Dubruel P. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41:7147–94. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 749.Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31:654–68. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 750.Barrère F, Mahmood TA, de Groot K, van Blitterswijk CA. Advanced biomaterials for skeletal tissue regeneration: instructive and smart functions. Mater Sci Eng R Rep. 2008;59:38–71. [Google Scholar]
- 751.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB. Bone structure and formation: a new perspective. Mater Sci Eng R Rep. 2007;58:77–116. [Google Scholar]
- 752.Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, Ee PL, Ahn JH, Hong BH, Pastorin G, Özyilmaz B. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5:4670–8. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]
- 753.Baker BM, Handorf AM, Ionescu LC, Li WJ, Mauck RL. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev Med Dev. 2009;6:515–32. doi: 10.1586/erd.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Mauney JR, Adam RM. Dynamic reciprocity in cell–scaffold interactions. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2014.10.016. http://dx.doi.org/10.1016/j.addr.2014.10.016. [DOI] [PMC free article] [PubMed]
- 755.Barker TH. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials. 2011;32:4211–4. doi: 10.1016/j.biomaterials.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 756.Higuchi A, Ling QD, Kumar SS, Chang Y, Kao TC, Munusamy MA, Alarfaj AA, Hsu ST, Umezawa A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog Polym Sci. 2014;39:1585–613. [Google Scholar]
- 757.Dong B, Hayashi S. Shaping of biological tubes by mechanical interaction of cell and extracellular matrix. Curr Opin Genet Dev. 2015;32:129–34. doi: 10.1016/j.gde.2015.02.009. [DOI] [PubMed] [Google Scholar]


