Abstract
The Geropathology Research Network has established a plan to identify and use pathology-based surrogate endpoints for aging intervention in preclinical drug studies to provide a predictable and short-term anti-aging drug response in line with clinical trials. The plan involves pathological assessment of tissues and organs from strains of old mice, by independent pathology groups in a concurrent manner in order to characterize the changes in lesion incidence and severity in response to anti-aging drugs at specific time points. This approach allows for connection with translational endpoints of aging, such as serum factors and physiological parameters, between mice and humans. Preclinical drug testing is a critical component of the plan, designed to shorten testing times from lengthy lifespan studies by comparing lesion grades and composite scores in treated and placebo cohorts at cross-sectional time points. In conclusion, a geropathology-based preclinical testing program is a step toward assuring maximum utilization of translational resources and increasing predictability of efficacy of new or repurposed drugs for clinical aging intervention studies.
Keywords: geropathology, translational research, anti-aging drugs
The Geropathology Research Network sponsored a workshop at the Gerontological Society of America Annual Conference in Orlando, FL, in November 2016. The theme was based on ways of using pathology to assess translational endpoints in preclinical aging studies. A number of presentations were made describing aged mouse physiology and pathology and therapeutic intervention projects. By the end of the workshop, a consensus was established on a plan to identify and use pathology-based surrogate endpoints for aging intervention in preclinical drug studies. The critical objective was to provide a predictable and short-term anti-aging drug response in line with clinical trials.
The plan involves three overarching approaches. The first approach is to determine the incidence and severity of histological lesions in strains of mice at certain time points in older life in order to establish a baseline for a particular genetic background or strain. The timing would be cross-sectional, and an example would be during a 6-month window of time preceding historical 50% survival. This approach involves establishing guidelines for using histological lesions as endpoints for preclinical drug studies, including a scoring system that addresses multi-morbidities (1). A Geropathology Grading Committee is currently working to develop these guidelines, using the foundational structure and organization already established in the field of toxicological pathology. Strains of mice to evaluate include B6, CB6F1, HET3 (and possibly a progeria strain), using both genders.
The second approach is to characterize the changes in lesion incidence and severity in response to anti-aging drugs given over a specific period of time in older-aged mice. The example of a 6-month window of time preceding historical 50% survival would be worthy of consideration. This approach would also involve connecting with translational endpoints of aging between mice and humans, including molecular endpoints in mutually accessible tissues and body fluids such as serum, as well as physiological endpoints such as grip strength. Drugs for testing would be selected on a theme basis in alignment with other programs or randomly from proposals by the scientific community. This second approach would also provide an excellent opportunity to evaluate any areas of drug toxicity.
The third approach is to assemble, organize, and disseminate information and training tools for geropathology. Extensive data will be generated, so a bioinformatics approach is critical to enable the organization of data into usable sets. These sets need to be easily accessible from a user-friendly website.
A number of critical components, bundled with the necessary expertise and resources, are needed to drive the three approaches into successful outcomes (2). The critical components include anatomic pathology, serum molecular pathology, preclinical drug testing, and a bioinformatics-based infrastructure.
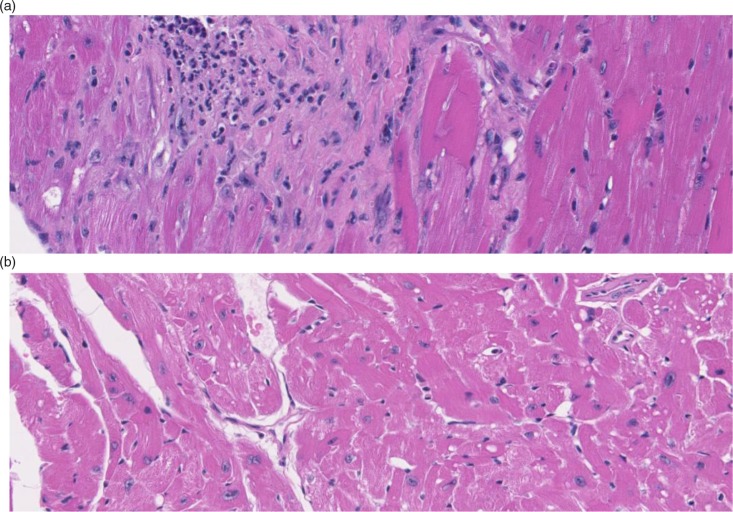
Anatomic pathology will be assessed in tissues and organs from three to four different strains of older-aged mice, by independent pathology groups in a concurrent manner. Several pathology groups, including the University of Washington, Oklahoma State University, University of Michigan, and the University of Texas, with expertise in the pathology of older-aged mice, have already been identified, but more groups would help strengthen this aspect. Representatives from these groups are participants in the Geropathology Grading Committee and have already shown that C57BL/6 and C57BL/6×BALB/c (CB6) F1 mice have robust and consistent age-dependent lesions that correlate with physiological decline. For example, CB6F1 male mice show evidence of cardiac dysfunction at 32 months of age, as determined by an increase in echocardiography-recorded left ventricular myocardial index, compared with sex-matched CB6F1 mice at 8 months of age (Table 1). This observation correlates with an increase in heart weight at necropsy and an increased incidence and severity of lesions on histological evaluation (Table 1 and Fig. 1a and 1b).
Table 1.
Heart function can be connected with cardiac pathology and levels of serum factors by comparing these parameters in 8- and 32-month-old CB6F1 male mice
| Feature | 8 months | 32 months |
|---|---|---|
| Echo-left ventricular myocardial index (mm) | 3.7 | 4.9 |
| Heart weight (g/tibial length) | 0.89 | 1.11 |
| Histo-cardiomyopathy (average grade) | 0.31 | 1.83 |
| Histo-fibrosis (average grade) | 0 | 0.92 |
| Serum cytokine array 200 | ||
| Growth factors | 12% decrease | |
| Inflammatory factors | 26% increase |
Fig. 1.
Hematoxylin- and eosin-stained heart tissue shows striking histological changes in 32-month-old CB6F1 male mice (a) compared with strain and gender-matched 8-month-old mice (b). The heart section from the old mouse shows an increased cellular infiltrate indicative of localized reactive sites, and extensive areas of fibrosis. These lesions help explain the cardiac dysfunction and increased heart weight observed in the older-aged mice.
A second component of the plan consists of looking at molecular endpoints in serum (and possibly other tissues readily accessible in both mice and humans) that can be connected with lesion assessment in tissues from old mice. For example, aging leads to a loss of physiological function such as cognition, mobility, and cardiovascular performance. One possibility for this loss is an age-dependent decrease in the generation of proteins necessary to support these functions when young. Many of these protein factors are secreted into the general circulation and can be detected in the serum of blood samples, thus providing the opportunity to monitor their levels at various ages. Antibody arrays are now available (RayBiotech and others) for assessing the serum levels of up to 200 proteins per sample. We have identified 23 proteins, involved in supporting blood vessels, the immune response, brain cells, skin, muscle, and bones, which are significantly decreased in old CB6F1 mice compared with young CB6F1 mice (Table 1), correlating with an increase in frailty, fatigue, heart failure, and cognitive impairment. This preliminary observation suggests that specific serum proteins, alone or in combination, may be useful markers for aging, and they provide the rationale to investigate the correlation between the incidence and severity of histological lesions and physiological phenotype. Histological pathology therefore is a critical validation to identify translational markers found in serum, a body fluid easily collected from both mice and humans. This is highly relevant because autopsies are rarely done in humans, while it is routine standard procedure in mice. Serum factors therefore are potential endpoints to investigate the ability of anti-aging drugs to restore specific protein factors to their youthful levels as a way to monitor any delay or reversal of age-related functional decline. It is of interest that a number of proteins involved in inflammatory activity were increased in 32-month-old mice (Table 1), suggesting that decreases in some of these factors could also be useful as drug–response endpoints.
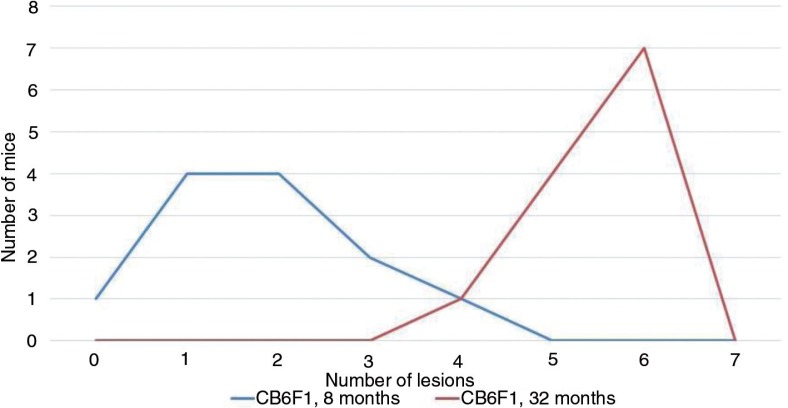
The third component needed for a preclinical geropathology-based program is an active drug-testing venue in mice using cross-sectional time points. Tentatively, a 6-month window of time preceding historical 50% survival specific for strain and gender is being considered. This would significantly shorten the drug-testing time compared with lifespan studies. Pathology would be used in several ways. First, lesion grades in drug-treated mice would be compared with lesion grades in placebo-treated mice. Second, composite scores for lesion incidence or grade would be compared between treated and placebo cohorts. An example of a composite incidence-scoring configuration is shown in Fig. 2, comparing the number of histological lesions observed in multiple organs in 32-month-old CB6F1 males to that observed in 8-month-old CB6F1 males. The objective is to see if a test drug could drive the 32-month-old lesion curve back toward the curve of the 8-month-old mice. Composite scores for middle-aged cohorts would greatly increase the sensitivity of this strategy.
Fig. 2.
The occurrence of multiple histological lesions increases with increasing age as shown by a dramatic shift of the curve to the right in 32-month-old CB6F1 male mice compared with the respective 8-month-old mice.
The fourth component entails building a bioinformatics infrastructure. There is a critical need for data management and dissemination of data, methodology, and training tools in geropathology for readily available access by the scientific community.
In conclusion, the lack of detailed pathological assessment in long-term preclinical aging studies has prevented the ability to maximize the generation of translational data. In the context of the enormous amounts of time and money needed for these types of investigations, it is disconcerting that they are not leveraged for the additional information to be gleaned from pathological evaluation. A geropathology-based preclinical testing program is a step toward assuring the maximum utilization of research dollars in the testing of new or repurposed drugs for aging intervention studies.
Acknowledgements
Echocardiography and heart weight data were generated by Dr. Christina Pettan, and the histological lesion incidence and grades were evaluated by Dr. Jessica Snyder. The serum cytokine array data were generated by Dr. Xuan Ge. All three scientists are members of the Department of Comparative Medicine at the University of Washington School of Medicine.
The Geropathology Research Network is supported by NIA grant R24 AG047115-02.
Conflict of interest and funding
The author has not received any funding or benefits from industry to conduct this study.
References
- 1.Ladiges W, Ikeno Y, Liggitt D, Treuting PM. Pathology is a critical aspect of preclinical aging studies. Pathobiol Aging Age Relat Dis. 2013;3:22451. doi: 10.3402/pba.v3i0.22451. doi: http://dx.doi.org/10.3402/pba.v3i0.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladiges W. Geropathology Research Network Symposium 2015. Pathobiol Aging Age Relat Dis. 2015;5:28866. doi: 10.3402/pba.v5.28866. doi: http://dx.doi.org/10.3402/pba.v5.28866. [DOI] [PMC free article] [PubMed] [Google Scholar]