Abstract
To predict short-term outcome in acute ischemic stroke, we analyzed somatosensory evoked potentials (SEP) and biochemical parameters [neuron-specific enolase (NSE) and S100 protein] in a prospective study with serial measurement. In 31 patients with 1st middle cerebral artery infarction, serum NSE and S100 protein were measured daily between days 1 and 6 poststroke. The N20 and N70 components of the SEP (SEP20 and SEP70) were determined on days 1 and 6. SEP and biochemical markers in stroke patients were compared with a control group. Short-term outcome was assessed by the modified Rankin Scale (mRS) at days 7-10 and was dichotomized between good (mRS 0–2) and poor (mRS ≥3) outcome. Specificity and positive predictive value (PPV) were high at day 1 for SEP (SEP20: 100% for both; SEP70: 93 and 88%, respectively) compared with lower values for NSE (67 and 50%) and S100 (23 and 57%). In contrast, S100 showed the highest sensitivity at day 1 with 77% compared with a relatively low sensitivity of NSE (31%) and SEP (SEP20: 35%, SEP70: 47%). The biochemical markers showed an improving sensitivity over time with best values (>90%) between days 3 and 4 at the expense of a lower specificity. Specificity and PPV of SEP on day 6 was still 100% with sensitivity increasing up to 53% (SEP20) and 60% (SEP70). SEP could early differentiate between good and poor outcome and reliably predict poor outcome. Since biochemical markers and SEP complement each other in the prognosis of stroke, a combined application of these markers seems promising.
Keywords: ischemic stroke, somatosensory evoked potentials, S100, NSE, SEP, prediction, outcome
early prediction of short-term outcome after ischemic stroke is essential for the planning of acute and rehabilitative therapeutic strategies during the first days of hospital care. Neuroimaging holds a pivotal role for the infarct detection and the estimation of prognosis. Accordingly, the association of imaging measures with outcome parameters has been shown to be robust and clinically relevant. However, serial imaging might be difficult to perform in critically ill patients. Therefore, the use of additional outcome parameters that could simply and repeatedly be obtained and that reflect different aspects of stroke damage may help to specify the prognosis in the early phase of stroke.
The measurement of median-nerve somatosensory evoked potentials (SEP) is a standard technique for the noninvasive neurophysiological assessment of brain tissue function in stroke-related central impairment. It is an important part of neurological assessment in patients treated in the intensive care unit, where its prognostic value has been well-demonstrated (Haupt et al. 2000, 2006; Zeman and Yiannikas 1989; Zhang et al. 2011). Other investigators have approached this problem using a combination of median- and tibial-nerve SEP and motor function [Medical Research Council (MRC) Scale for Muscle Strength and Barthel Index; Tzvetanov et al. 2005].
Serum biochemical markers on the other hand are of current interest in stroke research since they may become a simple and rapid bedside test for the extent of irreversible tissue damage after ischemic stroke. They have been used for the prediction of outcome, infarct size, and treatment-associated complications. Among them, the S100 protein (Dassan et al. 2009; Foerch et al. 2005; Jauch et al. 2006; Lynch et al. 2004; Nash et al. 2008; Wunderlich et al. 2004) and neuron-specific enolase (NSE; Anand and Stead 2005; Jauch et al. 2006; Schaarschmidt et al. 1994; Wunderlich et al. 2004, 2006) showed promising results to determine clinical outcome in ischemic stroke.
The combination of electrophysiological and biochemical markers is of major interest since they depict different aspects of stroke-related cerebral impairment, i.e., the cellular damage and the resulting functional damage. Our prospective study, therefore, aimed to evaluate the prognostic value of easily assessable electrophysiological (SEP) and biochemical (NSE and S100) markers in patients with ischemic stroke with respect to short-term clinical outcome.
MATERIALS AND METHODS
Patients
In this study, consecutive patients with acute middle cerebral artery (MCA) stroke were included after informed, written consent. The diagnosis was based on clinical assessment and native computed tomography (CT) or MRI. Exclusion criteria were concomitant brain diseases as previous ischemic strokes, evidence of a second stroke episode during the study, nonischemic strokes, brain tumors, infectious cerebral diseases, and previous cerebral trauma. The study was approved by the local ethics committee.
Twenty-six patients served as a control group for the assessment of serum biomarkers. To account for a possible effect of hospitalization, we chose hospitalized patients suffering from diseases of the peripheral nervous system such as Bell's palsy or polyneuropathies that are not known to affect NSE and S100 values.
SEP normal values were assessed according to internal laboratory normal values established in another control group of 20 healthy volunteers.
Clinical Examination and Outcome
National Institutes of Health Stroke Scale (NIHSS) was evaluated at day 1 and between days 6 and 7 poststroke. Short-term outcome was assessed by the modified Rankin Scale (mRS) at days 7-10 (van Swieten et al. 1988). Patients were dichotomized for functional independence: favorable outcome (mRS 0–2) and poor outcome (mRS 3–6).
Serum Biomarkers
The day of stroke onset was termed day 0. The blood samples were taken at day 1 (i.e., 24 h after onset) and days 2, 3, 4, 5, 6, and 7. The Monovettes were processed within 90 min (i.e., centrifugation and freezing at −70°C). The S100 samples were stored at −20°C. Analysis of serum biomarkers was blinded from clinical information.
NSE.
The NSE activity was measured using the Elecsys 1010/2010/MODULAR ANALYTICS E170 (Roche). This is a two-sided immunoradiological assay where monoclonal antibodies bind to the γ-subunit of the enzyme and thereby register the γγ-dimer and the αγ-dimer. The 95th percentile of the values of our control group was 15.8 μg/l. All values above this level were considered pathological.
S100.
The S100 protein was determined with the LIAISON system (Sangtec). This is a two-sided immunoradiological assay where the monoclonal antibodies bind to the β-subunit of the enzyme and register the ββ-dimer and the αβ-dimer. The 95th percentile of the values of our control group was 0.16 μg/l. All values above this level were considered pathological.
Electrophysiological Biomarkers
Testing of median-nerve SEP was performed according to standard techniques. SEP were recorded after stimulation of the median nerve at the wrist with 0.2-ms square-wave pulses and a repetition rate of 3 Hz at an intensity above the motor threshold of the thumb muscles. Recording and reference electrodes were placed according to the International 10-20 EEG system at C3′ and C4′ (2 cm posterior to C3 and C4) with an FZ reference. Cervical SEP were simultaneously recorded at the C7 level. Two traces of two hundred fifty-six averaged responses were recorded for each side. The SEP were measured between days 1 and 2 (first assessment: SEP-1) and days 7 and 10 (second assessment: SEP-2). Normal values were measured in twenty healthy volunteers without signs of neurological disease. The 95th percentile was determined for latencies. Values above this value were considered pathological. For the N20 component of the SEP (SEP20), the cutoff value was 19.985 ms, and for the N70 midlatency component (SEP70), the cutoff value was 86.25 ms. The SEP values were classified in four grades (see Table 1). Grades 1 and 2 were considered favorable SEP, and grades 3 and 4 were considered to indicate unfavorable SEP. Analysis and categorization of the SEP were blinded from clinical information.
Table 1.
Classification of SEP findings
| Grade 1 | Normal findings | Favorable SEP |
| Grade 2 | Uni- or bilaterally, pathologically delayed latencies | |
| Grade 3 | Unilaterally lost cortical response | Unfavorable SEP |
| Grade 4 | Bilaterally lost cortical response |
SEP, somatosensory evoked potentials.
Imaging
In most of the patients, cerebral imaging was performed by native CT scans. Thus infarct size could reliably be estimated only in the follow-up imaging between days 2 and 7. Infarct size was classified as <1/3 of MCA territory, between 1/3 and 2/3 of MCA territory, and >2/3 of MCA territory. Analysis of imaging data was blinded for clinical information.
Statistical Methods
The significance of differences at P < 0.05 was tested between sequential measurements (NIHSS, S100, and NSE) and between the outcome groups using ANOVA. For group differences of all other parameters, we used a t-test for independent variables after testing for normal distribution. To determine the prognostic value of the studied parameters, four-field tables for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. To yield the cutoff value for NIHSS and infarct size, which best discriminates between good and poor outcome, we calculated the receiver operating characteristic (ROC curve). Cutoff value for initial NIHSS was 10, for follow-up NIHSS 7. For infarct size, cutoff value was 1/3 of MCA territory.
RESULTS
In this study, thirty-one patients were included. For patient data, see Table 2. Nine of thirty-one were female, and the age ranged from 37 to 81 yr. Three out of thirty-one died during the 1st 7 days of observation. Five out of thirty-one patients were intubated due to a decrease in consciousness and were assigned an mRS of 5 (poor outcome). NIHSS on day 1 was not significantly different between the two groups, whereas follow-up NIHSS was higher in the group with poor outcome. Predictive values of early NIHSS showed a higher sensitivity of 0.6 compared with SEP but lower than for S100. Specificity, PPV, and NPV of early NIHSS were inferior to early SEP. Sensitivity of follow-up NIHSS was inferior to NSE but showed a specificity and PPV of 100%.
Table 2.
Patient data
| Good Outcome (n = 13) | Poor Outcome (n = 18) | P | |
|---|---|---|---|
| Mean age, yr | 59.6 ± 12.9 | 60.5 ± 11.8 | NS |
| Mean NIHSS on admission | 9.8 ± 1.9 | 10.2 ± 4.4 | NS |
| Mean NIHSS on day 6 | 6.9 ± 2.7 | 12.2 ± 7.3 | <0.05 |
| Thrombolysis (%) | 4/13 (23) | 4/18 (22) | NS |
| mRS (median) | 1.1 | 4.4 | <0.01 |
Values are means ± SD.
NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; NS, not significant.
Patients with larger infarctions covering >1/3 of the MCA territory were more likely to have a poor outcome (Fig. 1). An infarction of >1/3 of MCA territory estimated within the 1st wk after infarction showed a comparingly high sensitivity of 0.78 but a low specificity and PPV compared with SEP (Table 3).
Fig. 1.
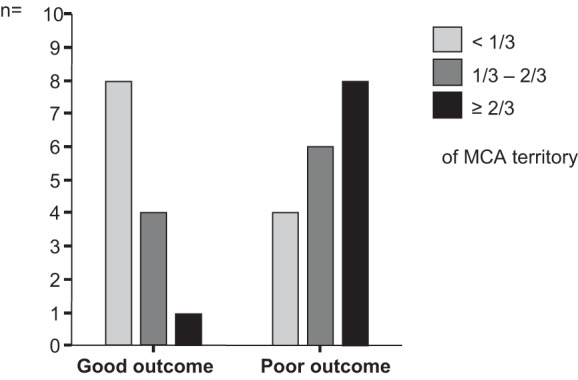
Size of middle cerebral artery (MCA) infarction stratified by outcome groups. Patients with large MCA infarction were more likely to have a poor outcome. However, infarct size on computed tomography (CT) scan could be reliably estimated only in the follow-up imaging.
Table 3.
Predictive values of the various parameters
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | ||
|---|---|---|---|---|---|---|---|
| Sensitivity | NSE | 0.31 | 0.46 | 0.75 | 0.92 | 0.75 | 0.91 |
| S100 | 0.77 | 0.88 | 0.94 | 0.87 | 0.87 | 0.75 | |
| N20 SEP | 0.35 | 0.53 | |||||
| N70 SEP | 0.47 | 0.6 | |||||
| NIHSS | 0.60 | 0.77 | |||||
| Imaging | 0.78 | ||||||
| Specificity | NSE | 0.67 | 0.77 | 0.67 | 0.5 | 0.67 | 0.39 |
| S100 | 0.23 | 0.29 | 0.23 | 0.43 | 0.29 | 0.43 | |
| N20 SEP | 1.0 | 1.0 | |||||
| N70 SEP | 0.93 | 1.0 | |||||
| NIHSS | 0.70 | 1.0 | |||||
| Imaging | 0.62 | ||||||
| Positive predictive value | NSE | 0.5 | 0.67 | 0.69 | 0.65 | 0.69 | 0.56 |
| S100 | 0.57 | 0.60 | 0.60 | 0.62 | 0.57 | 0.60 | |
| N20 SEP | 1.0 | 1.0 | |||||
| N70 SEP | 0.88 | 1.0 | |||||
| NIHSS | 0.80 | 1.0 | |||||
| Imaging | 0.74 | ||||||
| Negative predictive value | NSE | 0.47 | 0.59 | 0.73 | 0.86 | 0.73 | 0.83 |
| S100 | 0.43 | 0.67 | 0.75 | 0.75 | 0.67 | 0.60 | |
| N20 SEP | 0.56 | 0.70 | |||||
| N70 SEP | 0.62 | 0.70 | |||||
| NIHSS | 0.47 | 0.77 | |||||
| Imaging | 0.67 | ||||||
NSE, neuron-specific enolase; S100, S100 protein.
Electrophysiological Parameters
SEP20.
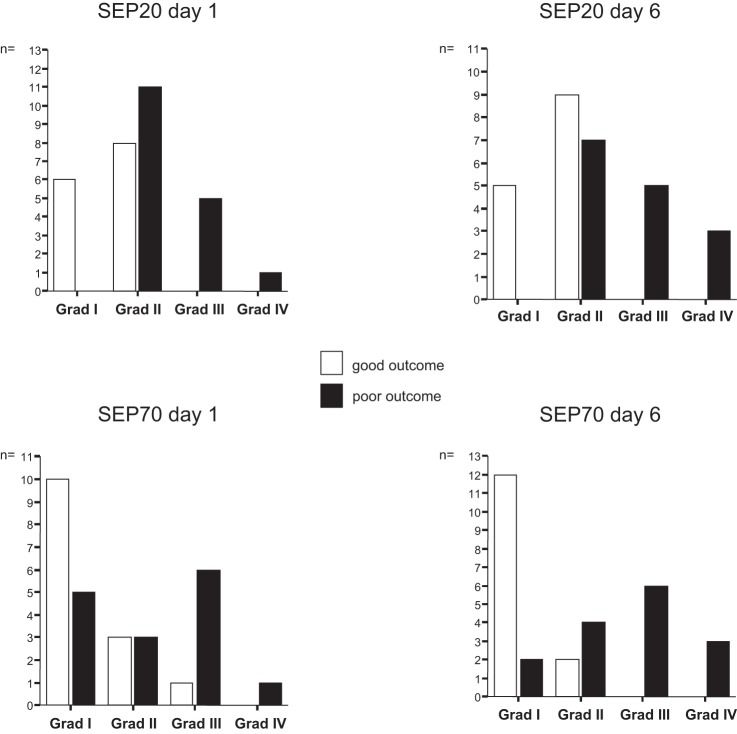
The distribution of the SEP20 groups is shown in Fig. 2. At baseline, favorable SEP detected all patients with good outcome (i.e., specificity 100%) but included 11 patients with poor outcome showing grade 2 SEP (NPV 56%). Unfavorable SEP detected 6/17 patients with poor outcome (sensitivity 35%), but all patients with unfavorable SEP at day 1 had a poor outcome (PPV 100%). Thus early unfavorable SEP20 was invariably associated with poor outcome and could thereby reliably predict such poor clinical course. The prognostic values of the follow-up measurement were unaltered robust with a slightly increased sensitivity (53%) and NPV (70%)
Fig. 2.
Unfavorable N20 components of the somatosensory evoked potentials [SEP20; grades 3 and 4 (Grad III and Grad IV)] were invariably associated with poor outcome already at day 1, whereas grade 1 SEP20 were invariably associated with good outcome. Discriminatory power of SEP70 was high but inferior to SEP20.
SEP70.
The distribution of the SEP70 groups is shown in Fig. 2. At baseline, favorable SEP detected 13/14 patients with good outcome (specificity 92%) but included 8/15 patients with poor outcome (NPV 62%). Those patients with favorable SEP and poor outcome showed both grade 1 and grade 2 SEP. Unfavorable SEP70 detected 7/15 patients with poor outcome (sensitivity 47%) and included 1 patient with good outcome (PPV 88%). In the follow-up measurement, favorable SEP70 detected 14/14 patients with good outcome (specificity 100%) but included 6/14 patients with poor outcome (NPV 70%), which again showed grade 1 as well as grade 2 SEP. Unfavorable follow-up SEP70 detected 9/14 patients with poor outcome (sensitivity 60%), but all patients with unfavorable SEP70 had a poor outcome. Thus the predictive value of unfavorable follow-up SEP for poor outcome was 100%.
The prognostic values of SEP are listed in detail in Table 3.
Biochemical Markers
The mean values of all stroke patients were higher than the mean of the control group at all days (mean value of control group: 12.0 μl/l). Within the group with poor outcome, there was a continuous increase of NSE values, whereas NSE values peaked at day 4 in the group with good outcome. Although patients with poor outcome generally had higher NSE values, there was no significant difference of the NSE values between the outcome groups at any time point (Fig. 3A). Sensitivity of NSE analysis was low at day 1 with 31% showing an increase over time to >90% at day 4, whereas specificity was 67% at day 1. PPV and NPV were comparatively low at day 1 with increasing values over time (Table 3).
Fig. 3.
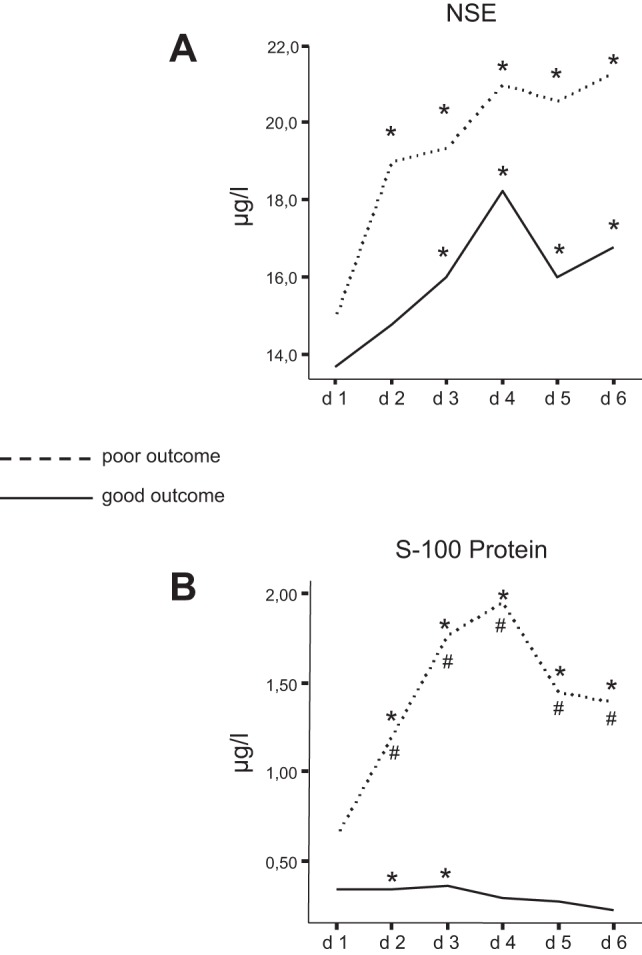
A: comparison of mean neuron-specific enolase (NSE) values between the 2 outcome groups. NSE values increased over time in both groups and were generally higher in the group with poor outcome, but the difference between the groups did not reach statistical significance. *Statistically different from control group (P < 0.05). B: comparison of mean S100 protein values between the 2 outcome groups. S100 values were significantly higher in the group with poor outcome and peaked at day 4 (d 4). In contrast, S100 values were stable and did not increase over time in the group with good outcome. *Significantly different from control group (P < 0.05); #significant difference between outcome groups (P < 0.05).
S100.
Mean value of the control group was 0.11 μl/l. The mean values of the patients were higher at any day compared with the mean values of the control group, but this difference was not significant at all days. Mean values of S100 were generally higher in patients with poor outcome and peaked at day 4. In contrast, patients with good outcome showed relatively stable values with no rise (Fig. 3B). S100 analysis showed a sensitivity of 77% at day 1, increasing to >90% at day 3, whereas specificity and PPV were comparatively low. The NPV reached 75% at day 3.
DISCUSSION
Our study aimed to compare the prognostic value of simple, easily and repeatedly accessible parameters for short-term outcome after MCA infarction. The target of prediction was to differentiate between good and poor outcome early after onset of stroke. Particularly, we aimed to identify patients with poor outcome reliably and unequivocally since such categorization as poor outcome might lead to substantial therapeutic decisions.
Already on the 1st day after stroke, SEP20 could identify all patients with good clinical course by favorable SEP and could invariably identify patients with poor prognosis by unfavorable SEP and was thus superior to clinical examination by NIHSS. In contrast, sensitivity was best for S100. As for the electrophysiological parameters, the follow-up measurement showed improved predictive values over the first days after stroke. With regard to the biochemical markers, highest sensitivity was reached with S100 and highest specificity with NSE, but both markers did not reach the specificity and PPV of early SEP or NIHSS.
The prediction of short-term outcome after stroke is of great importance to identify patients at risk for secondary deterioration early and to define early strategies for further therapeutic interventions but also for advanced diagnostic steps and early rehabilitation. Such early information might also help to plan the therapeutic strategies in the increasing number of old and very old stroke patients with health care directives. Presently, there is no standardized integrated approach that is used among the stroke centers. Additionally, it has to be kept in mind that a considerable proportion of stroke patients is treated in centers that do not have access to repetitive, if any, sophisticated methods (e.g., stroke MRI). This implies the need of simple and easily accessible predictors.
Biochemical parameters are tempting since they are easy to obtain. Serial measurements are feasible, and the possibility of a bedside test approach offers a promising perspective. They are thought to be a surrogate marker of tissue loss and infarct volume (Ahmad et al. 2012; Foerch et al. 2005), although the detailed pathophysiology may differ between the markers.
In previous studies, NSE increased within 24 h after ischemic stroke compared with controls and correlated to infarct volume (Ahmad et al. 2012; Schaarschmidt et al. 1994). The association to clinical outcome, however, was shown to be equivocal (Anand and Stead 2005). In our study, NSE values in stroke patients were higher than in controls and increased throughout the measuring period of the 1st 6 days. However, NSE values alone could not distinguish reliably between the outcome groups.
S100, in contrast, has been described as a more promising marker of final infarct volume, of risk of early complications, and of functional outcome (Dassan et al. 2009; Foerch et al. 2005; Kim et al. 1996; Lynch et al. 2004). The assessment beyond 24 h after stroke is supposed to reflect astroglial necrosis as an estimate of infarct size. We found that S100 values increased significantly in patients with poor outcome and peaked at days 3 and 4. These findings are in line with previous studies that found the best prediction around day 3 (Elting et al. 2000). In our study, however, S100 could not reliably predict poor outcome, although the sensitivity was high with 77–95% in the first days poststroke.
Electrophysiological parameters are a different approach to assess stroke-associated damage. They represent the integrity of functional circuits, which does not necessarily depend on the infarct volume since small strategic lesions will impair both function and electrophysiological parameters but not necessarily biochemical parameters (Zeman and Yiannikas 1989). The additive value of these markers can thus be described as a “weighted” parameter that includes functional aspects more important for outcome than infarct size alone. Another difference from biochemical markers is the absence of time dependency. In a previous study on a large sample of cerebrovascular patients that require intensive care therapy, it was shown that serial measurements add only little to the prognostic value of early SEP measurements to predict clinical outcome (Haupt et al. 2000, 2006). This is in accordance with the results of our sample where both components of the SEP, SEP20 and SEP70, but also the serial measurements showed comparable results. Favorable SEP detected all patients with good outcome, thus reaching an excellent specificity, whereas unfavorable SEP invariably identified patients with poor prognosis. However, unfavorable SEP missed several patients with poor outcome and had, therefore, a low sensitivity. Since the sensitivity of S100 and NSE increased up to >90% during the first days after stroke, they might be used as a complementary parameter in combination with SEP and clinical examination to maximize prognostic precision.
A limitation of our study with a relatively small sample size is that predictive values were not corrected for multiple comparisons and we did not calculate a combined statistical approach of all parameters with the inclusion of other confounders. Additionally, the dichotomization of the parameters did not take into account absolute values of, e.g., the biomarkers that may neglect additional information but may better reflect a simple clinical stratification. Our aim was a first descriptive analysis in a small sample to detect a possible additive value of SEP.
In summary, we could show that early SEP are a reliable and early indicator of short-term outcome in ischemic stroke. Unfavorable SEP20 were invariably associated with a poor outcome, i.e., mRS ≥3. The disadvantage of low sensitivity in SEP measurement may be balanced by the combination with biochemical markers and clinical assessment by NIHSS between days 1 and 4 after stroke. These findings are encouraging since they support the use of simple and easily accessible bedside predictors and may prompt a further combined prospective approach in a larger sample of patients.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.F.H. and C.D. conception and design of research; G.C., J.S., and W.-C.L. performed experiments; W.F.H., G.C., J.S., W.-C.L., and C.D. analyzed data; W.F.H. and C.D. interpreted results of experiments; G.C., J.S., W.-C.L., and C.D. prepared figures; W.F.H. and C.D. drafted manuscript; W.F.H. and C.D. edited and revised manuscript; W.F.H., G.C., J.S., and C.D. approved final version of manuscript.
REFERENCES
- Ahmad O, Wardlaw J, Whiteley WN. Correlation of levels of neuronal and glial markers with radiological measures of infarct volume in ischaemic stroke: a systematic review. Cerebrovasc Dis 33: 47–54, 2012. [DOI] [PubMed] [Google Scholar]
- Anand N, Stead LG. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis 20: 213–219, 2005. [DOI] [PubMed] [Google Scholar]
- Dassan P, Keir G, Brown MM. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis 27: 295–302, 2009. [DOI] [PubMed] [Google Scholar]
- Elting JW, de Jager AE, Teelken AW, Schaaf MJ, Maurits NM, van der Naalt J, Sibinga CT, Sulter GA, De Keyser J. Comparison of serum S-100 protein levels following stroke and traumatic brain injury. J Neurol Sci 181: 104–110, 2000. [DOI] [PubMed] [Google Scholar]
- Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol 62: 1130–1134, 2005. [DOI] [PubMed] [Google Scholar]
- Haupt WF, Birkmann C, Halber M. Serial evoked potentials and outcome in cerebrovascular critical care patients. J Clin Neurophysiol 17: 326–330, 2000. [DOI] [PubMed] [Google Scholar]
- Haupt WF, Pawlik G, Thiel A. Initial and serial evoked potentials in cerebrovascular critical care patients. J Clin Neurophysiol 23: 389–394, 2006. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR; NINDS rt-PA Stroke Study Group . Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 37: 2508–2513, 2006. [DOI] [PubMed] [Google Scholar]
- Kim JS, Yoon SS, Kim YH, Ryu JS. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke 27: 1553–1557, 1996. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke 35: 57–63, 2004. [DOI] [PubMed] [Google Scholar]
- Nash DL, Bellolio MF, Stead LG. S100 as a marker of acute brain ischemia: a systematic review. Neurocrit Care 8: 301–307, 2008. [DOI] [PubMed] [Google Scholar]
- Schaarschmidt H, Prange HW, Reiber H. Neuron-specific enolase concentrations in blood as a prognostic parameter in cerebrovascular diseases. Stroke 25: 558–565, 1994. [DOI] [PubMed] [Google Scholar]
- Tzvetanov P, Rousseff RT, Atanassova P. Prognostic value of median and tibial somatosensory evoked potentials in acute stroke. Neurosci Lett 380: 99–104, 2005. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604–607, 1988. [DOI] [PubMed] [Google Scholar]
- Wunderlich MT, Lins H, Skalej M, Wallesch CW, Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin Neurol Neurosurg 108: 558–563, 2006. [DOI] [PubMed] [Google Scholar]
- Wunderlich MT, Wallesch CW, Goertler M. Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J Neurol Sci 227: 49–53, 2004. [DOI] [PubMed] [Google Scholar]
- Zeman BD, Yiannikas C. Functional prognosis in stroke: use of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry 52: 242–247, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su YY, Ye H, Xiao SY, Chen WB, Zhao JW. Predicting comatose patients with acute stroke outcome using middle latency somatosensory evoked potentials. Clin Neurophysiol 122: 1645–1649, 2011. [DOI] [PubMed] [Google Scholar]