Abstract
Power spectral analyses of electrical signals from respiratory nerves reveal prominent oscillations above the primary rate of breathing. Acute exposure to intermittent hypoxia can induce a form of neuroplasticity known as long-term facilitation (LTF), in which inspiratory burst amplitude is persistently elevated. Most evidence indicates that the mechanisms of LTF are postsynaptic and also that high-frequency oscillations within the power spectrum show coherence across different respiratory nerves. Since the most logical interpretation of this coherence is that a shared presynaptic mechanism is responsible, we hypothesized that high-frequency spectral content would be unchanged during LTF. Recordings of inspiratory hypoglossal (XII) activity were made from anesthetized, vagotomized, and ventilated 129/SVE mice. When arterial O2 saturation (SaO2) was maintained >96%, the XII power spectrum and burst amplitude were unchanged for 90 min. Three, 1-min hypoxic episodes (SaO2 = 50 ± 10%), however, caused a persistent (>60 min) and robust (>400% baseline) increase in burst amplitude. Spectral analyses revealed a rightward shift of the signal content during LTF, with sustained increases in content above ∼125 Hz following intermittent hypoxia and reductions in power at lower frequencies. Changes in the spectral content during LTF were qualitatively similar to what occurred during the acute hypoxic response. We conclude that high-frequency content increases during XII LTF in this experimental preparation; this may indicate that intermittent hypoxia-induced plasticity in the premotor network contributes to expression of XII LTF.
Keywords: respiratory, plasticity, hypoxia, motoneuron
when electrical signals from respiratory nerves are subjected to power spectral analyses, prominent oscillations are detected at frequencies well above the primary rate of breathing (Cohen et al. 1987). These oscillations are a consistent finding across multiple laboratories and are present in species ranging from mouse to human (Ackerson and Bruce 1983; Bruce and Ackerson 1986; Christakos et al. 1991; Cohen et al. 1987; Konig and Engel 1995; Marchenko et al. 2012; Morris et al. 2001; O'Neal et al. 2005; Seven et al. 2013). In phrenic and hypoglossal (XII) nerve recordings from the cat and rat, two dominant ranges of frequency content have been identified. These ranges or “bands” have been termed medium frequency oscillations (MFOs; occurring at ∼15–50 Hz) and high-frequency oscillations (HFOs; occurring at ∼50–120 Hz) (Cohen et al. 1987; Marchenko et al. 2012; Marchenko and Rogers 2009). An upper-HFO (UHFO) band, occurring at ∼200 Hz, has also been reported in the mouse (O'Neal et al. 2005).
Comparison of power spectra across multiple respiratory-related motor outputs (e.g., phrenic, XII, recurrent laryngeal, and others) reveals that the same HFO (Cohen et al. 1987; Funk and Parkis 2002; Morris et al. 2001) and UHFO bands (O'Neal et al. 2005) are present within each recording. That is, the higher frequency oscillations show coherence across different neural recordings. In contrast, the MFO is not coherent across different respiratory outputs but rather, appears to have a unique “signature” in each motor pool (Cohen et al. 1987). Funk and Parkis (2002) proposed that the HFO coherence across different respiratory nerves results from a common premotor input that originates in the brain stem, and HFOs are thus a signature of the overall respiratory network. The absence of MFO coherence across respiratory-related motor outputs suggests that the relevant mechanism(s) reside within motoneuron pools (Funk and Parkis 2002). One strong possibility is that MFOs recorded in respiratory nerves reflect the underlying motoneuron discharge rates (Christakos et al. 1991).
Although the power spectrum of respiratory-related neural recordings has been well characterized [reviewed in Funk and Parkis (2002)], the impact of neuroplasticity on respiratory power spectra has not been explored. In this study, we focused on a well-established form of respiratory neuroplasticity—intermittent hypoxia-induced long-term facilitation (LTF). Independent laboratories have confirmed that certain paradigms of intermittent hypoxia lead to a persistent increase (i.e., facilitation) of respiratory motor output (Fuller et al. 2000). Most studies report respiratory LTF as an increase in the amplitude of the inspiratory signal, but increases in breathing frequency can also occur (Baker-Herman and Mitchell 2008). For the present study, we reasoned that evaluation of the XII nerve power spectrum, before, during, and following intermittent hypoxia, could provide insight into the mechanisms underlying expression of LTF as follows. A body of work from Mitchell and colleagues [reviewed in Dale et al. (2014)] indicates that the primary mechanism of respiratory LTF resides at or near the motoneuron pool, and other laboratories have provided strong support for this hypothesis [e.g., McKay et al. (2004)]. That work, coupled with the aforementioned theories regarding the origin of power spectrum oscillations (Funk and Parkis 2002) led us to explore the frequency content of the XII signal during LTF. Specifically, we hypothesized that high-frequency content (HFO and UHFO) of the XII power spectrum would remain stable during expression of intermittent hypoxia-induced LTF. This hypothesis was tested using an anesthetized mouse model of XII LTF.
METHODS
Adult male 129/SVE mice (Taconic, Albany, NY) were studied using procedures that were approved by the University of Florida Institutional Animal Care and Use Committee. A time-control (TC) group was not exposed to hypoxia (n = 9; 28 ± 1 g), and the treatment (i.e., LTF) group received intermittent hypoxia, as described below (n = 9; 29 ± 1 g).
Experimental preparation.
The nerve recording procedures were adapted from prior reports (DeRuisseau et al. 2009; Lee et al. 2011). Mice were anesthetized by intraperitoneal urethane (1.0–1.6 g/kg; Sigma, St. Louis, MO) and placed supine. Body temperature was maintained at 37–38°C using a servo-controlled heating pad (model TC-1000; CWE, Ardmore, PA). Pulse oximetry (MouseOx; Starr Life Sciences, Oakmont, PA) was used to measure the saturation of hemoglobin [O2 saturation (SaO2); %]. The trachea was cannulated below the larynx to enable mechanical ventilation (MicroVent; Harvard Apparatus, Holliston, MA) with a hyperoxic gas mixture [inspired O2 fraction (FiO2) = 0.50]. End-tidal CO2 (ETCO2) was monitored (MicroCapStar; CWE) and maintained during surgical procedures at 35 ± 3 mmHg (means ± SD) by changing the ventilator settings as necessary. During this period, the limb-withdrawal response to toe pinch was monitored to ensure adequacy of anesthesia, and supplemental urethane was given if indicated (0.3 g/kg ip). After initiating mechanical ventilation, muscular paralyses were induced with pancuronium bromide (2.5 mg/kg ip; Hospira, Lake Forest, IL) to eliminate respiratory muscle contraction. Mice were bilaterally vagotomized to prevent entrainment of XII motor output with the ventilator. A FunnelCath catheter (Instech Laboratories, Plymouth Meeting, PA) was inserted into the carotid artery to measure mean arterial pressure (MAP; Statham P10EZ pressure transducer and CP122 AC/DC Strain Gage Amplifier; Grass Technologies, Natus Neurology, Warwick, RI). The XII nerves were isolated bilaterally, and the distal ends were cut ∼3 min before initiating the recordings. Nerve activity was recorded using tungsten wire electrodes following amplification (1,000×; Model 1700; A-M Systems, Carlsborg, WA) and band-pass filtering (0.01–10 KHz). Oscillations (60 Hz) were initially prominent in the raw recordings, and therefore, a notch filter was used. The filter had a cutoff of −35 dB at 60 Hz, which was extended to −10 dB at 65 Hz. Thus there was a prominent reduction in signal content at 60 ± 5 Hz that was present in all experiments (i.e., both TC and intermittent hypoxia-exposed mice).
Neurograms were integrated using a 100-ms time constant (model MA-1000; CWE). Data were digitized using a CED Power 1401 data acquisition interface and recorded on a personal computer using Spike2 v 7.10 software (Cambridge Electronic Design, Cambridge, UK).
Experimental protocols.
Before initiating the LTF experiment, an inspiratory apnea was first induced by gradually increasing the ventilator pump rate until inspiratory bursting ceased. Apnea was maintained for 3 min, and the ventilator rate was then gradually decreased until XII inspiratory bursting resumed. The ETCO2, associated with the onset of inspiratory bursting, was noted, and the ventilator rate was adjusted to maintain ETCO2 at 2 mmHg above this value for the remainder of the experiment. After a 10- to 20-min baseline period, the intermittent hypoxia group was exposed to 3, 1-min episodes of hypoxia (FiO2 = 0.14–0.16), separated by 3 min of hyperoxia (FiO2 = 0.5). In the control group, the FiO2 was held constant at 0.50 for a duration of 60 min, whereas ETCO2 was regulated at 35 ± 3 mmHg. The intermittent hypoxia protocol was based on prior studies in a rat model (Bach and Mitchell 1996; Baker-Herman and Mitchell 2002; Fuller et al. 2001a), but we used a shorter period of hypoxia (i.e., 1 vs. 5 min), because preliminary studies indicated that in this experimental preparation, mice could not tolerate a period of hypoxia >90 s. Moreover, preliminary studies showed that these shorter durations of hypoxia were sufficient to trigger robust LTF.
Analysis of neurograms.
Inspiratory burst amplitude and frequency were analyzed using Spike2 software, as described previously (DeRuisseau et al. 2009; Lee et al. 2011). Peak integrated amplitude (∫XII) and burst frequency were averaged for a stable, 3-min period just before hypoxia (baseline); for the last 10 s of each hypoxic challenge; for 20 s, 1 min following cessation of hypoxia; and for over minutes 25–30 and 55–60 following the final hypoxic challenge (or equivalent sham time point). The analysis was performed 1 min following cessation of the hypoxic challenge to ensure that the hypoxic gas had washed out, and only hyperoxic gas was being delivered to the mouse. The ∫XII amplitude data were expressed in millivolts (i.e., arbitrary units) and also normalized to values recorded during baseline. The spectral analyses of the XII burst was accomplished using Matlab software (MathWorks, Natick, MA), as described below.
Inspiratory event detection.
XII nerve recordings were first manually screened for regions of artificial disturbances; these regions were subsequently removed. The signal was then high-pass filtered to remove activity below 20 Hz and notch filtered at 60 Hz to remove line noise. All filtering was done using Matlab's “idealfilter” command (MathWorks). The peak inspiratory burst was then identified using an automated routine for detecting signal peaks, as follows. First, a filtered version of the signal, xinsp, was generated by convolving the original signal, x(t), with a Gaussian kernel of width σ = 75 ms: xinsp(t) = x(t)·e−(t/σ)2/. Areas where xinsp exceeded a set threshold (tuned manually) were designated as inspiratory events, and events separated by <100 ms were grouped together. The center of each event was identified by fitting a quadratic function to xinsp (Talathi et al. 2009). The resulting set of centered inspiratory events was then used for stationarity and power spectrum analysis as described below.
Stationarity analysis.
Power spectrum analysis of finite time windows assumes that the data within are stationary (Oppenheim and Willsky 1997). We used a modified version of the approach developed by Seven and colleagues (2013) to identify nonstationary regions of the inspiratory signal. Those authors used a nonparametric reverse arrangement test (Bendat and Piersol 2010) to identify regions where the mean and mean-squared values of their signal were stationary. This approach involved dividing data into short duration segments (3 ms; less than the XII nerve absolute refractory period) [see Seven et al. (2013)]. The root mean square (Curran et al. 1997) of each segment, i, was calculated and labeled xi. The reverse arrangement test uses the test statistic, R, which equals the number of “reverse arrangements” in the set xi; namely, this is the number of times xi > xj for all i < j.
We found that this test did not perform well when the input data were highly symmetric. That is, there would often be an absence of reverse arrangements during the ramping up of XII activity but an excess during the ramping down. Together, these would cancel and contribute to false positives in our stationarity test. Therefore, we modified the test to calculate the number of reverse arrangements separately on the left and right halves of the data, RL and RR. We used the null hypothesis that the joint distribution for (RL, RR) is drawn from stationary data. RL and RR were assumed to be independent for this test. Matlab code for performing the reverse arrangement test is available online (http://www.davearthurstanley.com/codes.html).
Power spectral analysis.
Power spectral densities (PSDs) were calculated using the Welch method, as implemented by Matlab (MathWorks), by which each inspiration was windowed with a Hamming window before applying the fast Fourier transform. Two cases were investigated. The first case used 60 ms windows, which were centered on the peak of inspiratory activity (defined as the peak of the quadratic fit to xinsp, described above) and were required to be stationary. The second used 200 ms windows, also centered on the peak of inspiratory activity but not enforcing stationarity. We chose a 60-ms window for enforcing stationarity, because our reverse arrangement testing showed that this was the largest window that did not test as “nonstationary” for the majority of inspiratory events. The increase in the window size beyond 60 ms resulted in a sharp increase in the fraction of inspirations, which were classified as nonstationary. For the case when stationarity was not enforced, we used a 200-ms window that was sufficient to capture the bulk of the activity in an inspiratory burst. The PSDs for all inspirations from a given animal in a given experimental condition were averaged for subsequent analysis.
Frequency band analysis.
Two values were calculated from the PSD to help quantify changes in the power spectrum. Centroid frequency (fc) was calculated as
where S(f) is the PSD, and all frequency values are in hertz. High/low ratio (H/L) was defined as the ratio of power in the last quartile over that in the first quartile, as follows
where f1 and f3 are the first and third frequency quartile endpoints, respectively.
Statistical analysis.
Statistical tests were performed using SigmaStat version 12.0 software. Differences in XII amplitude at baseline and 60 min after baseline between groups were determined using a two-way repeated-measure (RM) ANOVA (e.g., factor 1 = Group; factor 2 = Time). Similarly, differences in fc and H/L at baseline and 60 min between groups were compared using two-way RM ANOVA. In addition, ETCO2, SaO2, body temperature, and MAP were compared between the LTF and TC groups using a two-way RM ANOVA. The Student-Newman-Keuls test was used for post hoc analyses when the ANOVA revealed main effects. XII nerve activity during and immediately following the acute hypoxic responses was compared with baseline within the LTF group using a one-way RM ANOVA. Differences were considered statistically significant when P ≤ 0.05. All data are presented as the means ± 1 SE.
RESULTS
Body weight, SaO2, MAP, and ETCO2.
Rectal temperature did not change by >0.4°C over the course of the experimental protocol in any animal, and mean temperatures were similar between groups (Table 1; P = 0.67). The SaO2 was maintained ≥96% throughout all experiments, except during hypoxia when SaO2 reached 50 ± 10%. There were no significant differences in ETCO2, SaO2, or MAP between groups (Table 1; P > 0.5 for all). MAP increased during each hypoxic exposure (P < 0.02) and also tended to decrease over the post-hypoxic recording period (Table 1 and Fig. 1B).
Table 1.
Mean values for O2 saturation (SaO2), end-tidal CO2 (ETCO2), rectal temperature (Temp), and mean arterial blood pressure (MAP)
| Time | Group | SaO2, % | ETCO2, mmHg | Temp, °C | MAP, mmHg |
|---|---|---|---|---|---|
| Baseline | LTF | 98.9 ± 0.1 | 35.6 ± 0.5 | 37.3 ± 0.03 | 85 ± 2.9 |
| Baseline | TC | 98.2 ± 0.7 | 35.3 ± 0.6 | 37.4 ± 0.03 | 83.7 ± 4.5 |
| Hypoxia 1 | LTF | 49.5 ± 0.5* | 35.1 ± 0.1 | 37.3 ± 0.03 | 111.5 ± 8.5* |
| Hypoxia 2 | LTF | 50.3 ± 0.3* | 35.2 ± 0.4 | 37.3 ± 0.03 | 110.3 ± 8.7* |
| Hypoxia 3 | LTF | 51.8 ± 1.75* | 35.6 ± 0.4 | 37.4 ± 0.00 | 108.8 ± 10.5* |
| 30 min | LTF | 98.4 ± 0.5 | 35.3 ± 0.2 | 37.4 ± 0.03 | 84.1 ± 3.3 |
| 30 min | TC | 97.3 ± 0.5* | 35.2 ± 0.1 | 37.4 ± 0.03 | 72.5 ± 3.2* |
| 60 min | LTF | 97.8 ± 0.6* | 34.5 ± 0.3 | 37.4 ± 0.03 | 83.2 ± 3.1† |
| 60 min | TC | 97.3 ± 0.8* | 34.2 ± 0.7 | 37.3 ± 0.10 | 74.8 ± 6.9* |
Data are presented from groups receiving intermittent hypoxia [long-term facilitation (LTF)] and the time-control (TC) group that did not receive hypoxia. The time points are the prehypoxia baseline, each of 3 hypoxic episodes, and 30 and 60 min following the final hypoxic episode (or corresponding point in the TC group).
P < 0.05 compared with baseline data point; †P < 0.05 compared with corresponding TC data point.
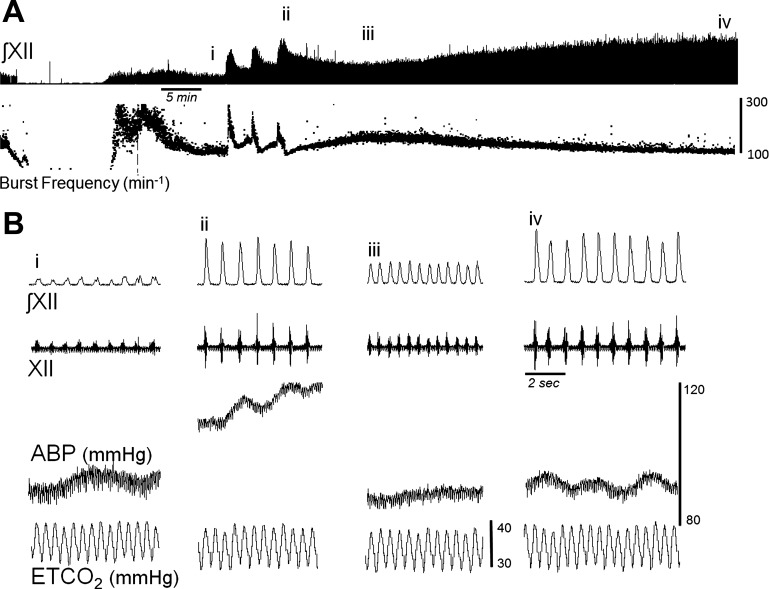
Fig. 1.
Representative example of hypoglossal (XII) nerve discharge before, during, and after intermittent hypoxia. A: an ∼90-min recording that includes hyperventilation-induced neural apnea, the baseline period, 3 bouts of hypoxia, and the progressive increase in amplitude following intermittent hypoxia. Instantaneous burst frequency was calculated for respiratory cycle and is included below the XII signal. B: expanded time-scale traces from the areas indicated by i–iv in A and includes the integrated XII (∫XII) and raw XII recordings, arterial blood pressure (ABP), and end-tidal CO2 (ETCO2).
Impact of intermittent hypoxia on XII nerve activity.
Figure 1 shows an example of XII nerve activity before, during, and after intermittent hypoxia in a urethane-anesthetized mouse. Inspiratory burst amplitude robustly increased during each exposure to hypoxia, as expected (Figs. 1 and 2). The XII burst amplitude also tended to show progressive augmentation (Fregosi and Mitchell 1994; Powell et al. 1998), as indicated by an increased response during each successive hypoxic exposure (Figs. 1 and 2A). In contrast, inspiratory burst frequency significantly decreased with each successive hypoxic episode (Fig. 2B) and was below the baseline value immediately post-hypoxia (Fig. 2D).
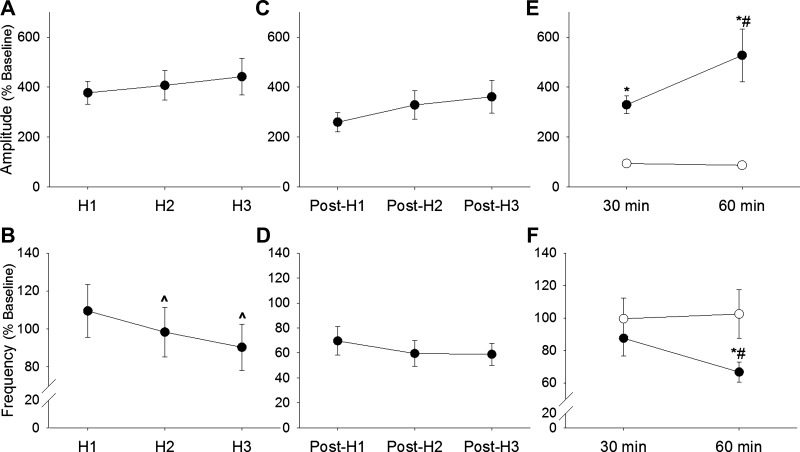
Fig. 2.
Mean XII inspiratory burst amplitude during and following intermittent hypoxia. The XII inspiratory burst amplitude was normalized to the baseline condition (% Baseline). A: burst amplitude robustly increased during each of the 3 hypoxic episodes (H1–H3). However, there was a progressive reduction in the burst frequency during each successive bout of hypoxia (B). Burst amplitude remained elevated above the baseline period at 1 min post-hypoxia (C), whereas burst frequency dropped below baseline immediately post-hypoxia (D). In mice that were not exposed to hypoxia (○; E and F), burst amplitude (E) and frequency (F) were maintained at baseline values for the entire recording period. However, after intermittent hypoxia (●; E and F), burst amplitude showed a robust and progressive increase above baseline values (E). Burst frequency was below baseline values following intermittent hypoxia (F). ^P < 0.05 vs. H1; *P < 0.05 vs. corresponding time point in the control group; #P < 0.05 vs. 30 min data point.
The control group, which was not exposed to hypoxia, had relatively little variance in XII burst amplitude over the course of the experiment (Fig. 2, E and F). In sharp contrast, at 30 and 60 min following intermittent hypoxia, XII inspiratory burst amplitude was considerably elevated above the baseline value, as shown in Fig. 2E. A progressive increase in XII burst amplitude was observed after intermittent hypoxia and by 60 min, actually exceeded the value seen during the initial acute hypoxic response.
In control mice, the burst frequency remained stable over the course of the experiment, and values were therefore similar at the onset and end of the recording (P = 0.39; Fig. 2F). However, there was a gradual decline of inspiratory burst frequency following exposure to intermittent hypoxia. Thus by 60 min post-hypoxia, burst frequency declined by ∼30% (P = 0.01 vs. baseline; Fig. 2F).
Impact of intermittent hypoxia on the inspiratory XII power spectrum.
The power spectrum was assessed for the stationary period of inspiratory discharge, as recently described (Seven et al. 2013), and also over the entire inspiratory burst, as in most prior reports (Cohen et al. 1987; Funk and Parkis 2002; O'Neal et al. 2005). Both approaches revealed bands of increased power that were similar to literature reports (Cohen et al. 1987; Funk and Parkis 2002; O'Neal et al. 2005). Thus bands with increased signal content were identified in the ranges of 20–50 Hz (i.e., MFO), 80–120 Hz (i.e., HFO), and 150–200 Hz (i.e., UHFO). Figure 3 provides examples of spectral content in XII nerve recordings in mice exposed to intermittent hypoxia (Fig. 3A) or TC (no hypoxia exposure; Fig. 3B). These examples show that intermittent hypoxia triggered a prominent increase in the UHFO range of the XII electrical signal and that there was minimal variance in the spectral content during the TC recordings.
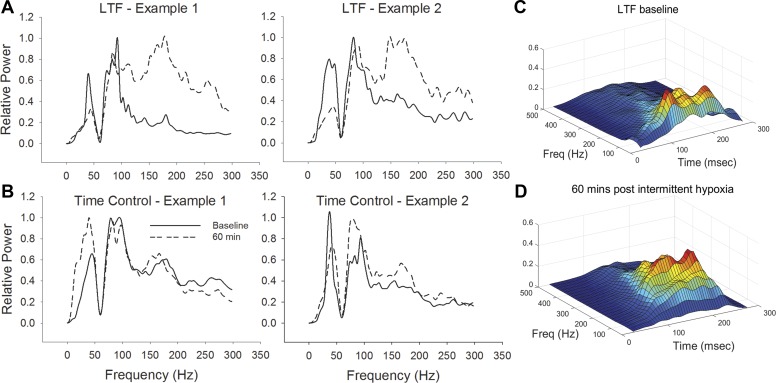
Fig. 3.
Representative examples of XII nerve power spectral density (PSD) following intermittent hypoxia. Two examples are provided from both the intermittent hypoxia [long-term facilitation (LTF; A)] and no-hypoxia [i.e., time-control (TC)] groups (B). For these examples, the power spectral analyses were done 60 min following the final bout of hypoxia (or the equivalent time point in the control groups). With the comparison of A and B, it is evident that there is a shift in the power spectrum following intermittent hypoxia, with more power in higher frequencies. Note that in these examples, the signals were band-passed filtered (0.01–10 KHz), and a 60-Hz notch filter was also applied. C and D: additional examples that include a time component. Thus these panels provide 3-dimensional plots presenting time [ms (msec)], frequency (Hz), and relative power (arbitrary units) across the respiratory cycle during baseline (C) and expression of LTF (D). The color of the plot is proportional to the amplitude of the oscillation at a given frequency and time (red = greatest amplitude).
The average of the normalized XII power spectrum during baseline, the first hypoxic episode, and 60 min following the final hypoxic episode is presented in Fig. 4. During hypoxia, the mean power spectrum shifted to the right with increased UHFO content (Fig. 4). A similar increase in UHFO power was also present following intermittent hypoxia (i.e., during LTF). In contrast, the MFO range showed a decrease in relative power during both the acute hypoxic challenge and LTF (Fig. 4). The control group that was not exposed to hypoxia continued to have prominent MFO and HFO peaks throughout the recording period and showed no apparent shift from baseline values (Fig. 4C).
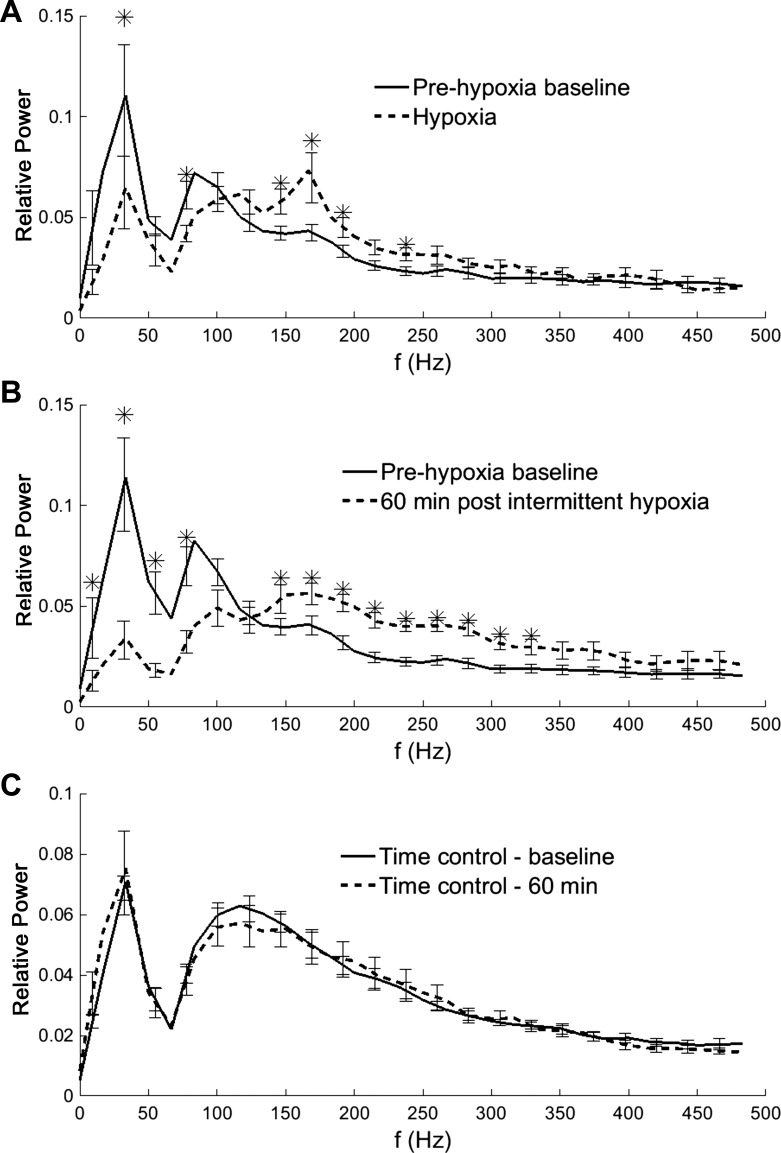
Fig. 4.
Mean PSD in XII recordings. During acute hypoxia (A), the power spectrum shifted with reduced content at low frequencies (f) and increased content at higher frequencies. At 60 min following the hypoxic episodes (B), there was a considerable “rightward shift” of the spectral curve. This was qualitatively similar to what occurred during the acute hypoxic response, but the overall magnitude was greater during LTF. C: there were no apparent changes in the power spectrum over time during the control (no-hypoxia) exposure group. *Baseline different than hypoxia data point (A) or baseline different than post-hypoxia data point (B).
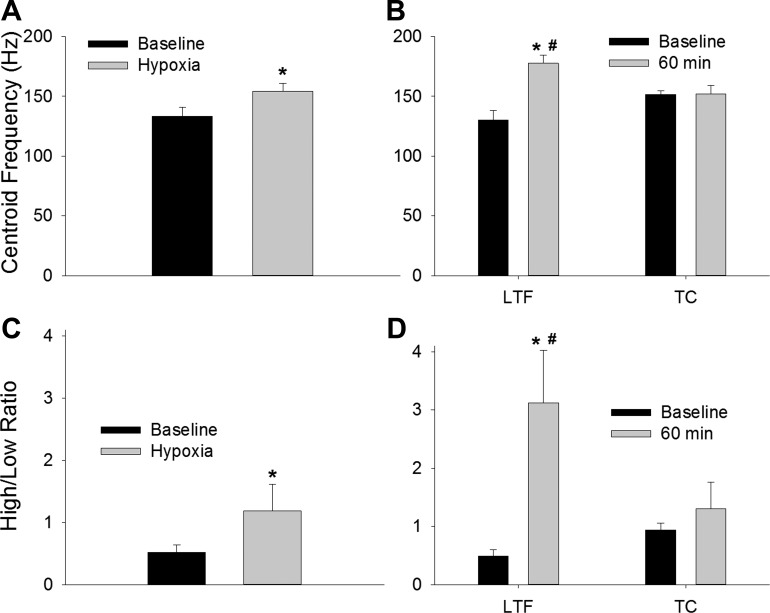
The fc was similar regardless of whether the XII power spectrum was calculated from the stationary portion of the inspiratory burst (baseline value = 140 ± 5 Hz) or the entire inspiratory cycle (baseline value = 138 ± 4 Hz). Indeed, the centroid values were not different between the stationary- and nonstationary-derived data at any point during the protocol (P > 0.70 for all). The subsequently presented data are all derived from the stationary analyses. During hypoxia, the fc increased by ∼15% compared with the baseline value (P = 0.004; Fig. 5A). At 60 min following intermittent hypoxia, the fc showed an even more robust increase (36%) that was significantly different than both the baseline (P = 0.005) and corresponding control data point (P = 0.003; Fig. 5B). In contrast, shifts in the fc did not occur in the control group (P = 0.95 vs. baseline; Fig. 5B). The XII power spectrum was also evaluated by binning the frequency content into quartiles (Seven et al. 2013). Consistent with the fc data, the ratio of power in the fourth quartile vs. the first quartile (i.e., “H/L”) was increased during hypoxia (Fig. 5C; P = 0.027), and this shift was even more robust during LTF. Figure 5D shows that the ratio of power in the high vs. low quartile was substantially increased at 60 min following intermittent hypoxia (P = 0.001 vs. baseline; P = 0.01 vs. corresponding control data), and the quartile distribution change did not occur at the corresponding time point in the control group (P = 0.49; Fig. 5D).
Fig. 5.
Mean centroid frequency (fc) and ratio of high:low (H/L) power in XII recordings. There was a significant increase in the fc during hypoxia (A) and during LTF following intermittent hypoxia (B). The fc did not change during the TC experiments in which mice were not exposed to hypoxia. The H/L was evaluated after binning the frequency content into quartiles and showed an increase during hypoxia (C). The increase in the H/L was even more profound during LTF following intermittent hypoxia (D). *P < 0.05 vs. the baseline value; #P < 0.05 vs. the corresponding TC data point.
DISCUSSION
There are two primary observations from this study. First, the relative magnitude of XII LTF in the urethane-anesthetized mouse model was considerably greater than previous reports in the cat (Jiang et al. 1991; Mateika and Fregosi 1997) and rat (Bach and Mitchell 1996). A prior study of LTF in mice (Toyama et al. 2009) also demonstrated robust respiratory LTF compared with other species, and accordingly, the mouse appears to have a particularly high capacity for this form of respiratory neuroplasticity. Second, LTF was associated with a shift in XII power to higher frequencies, and therefore, we reject our original hypothesis that high-frequency content would remain stable during LTF. Previous interpretations of the respiratory power spectrum [reviewed in Funk and Parkis (2002)] suggest that presynaptic mechanisms drive HFOs within efferent respiratory discharge, although this has not been proven.
Respiratory LTF in the mouse.
The most well-studied model of LTF in the XII (tongue) motor system is the anesthetized rat (Bach and Mitchell 1996; Baker-Herman and Strey 2011; Fuller 2005; Fuller et al. 2001a), although the response has also been described in cats (Jiang et al. 1991; Mateika and Fregosi 1997), humans (Harris et al. 2006; Lee et al. 2009; Syed et al. 2013), and the rat in vitro medullary slice preparation (Bocchiaro and Feldman 2004). Following a 3 × 5-min intermittent hypoxia paradigm in the urethane-anesthetized rat, XII burst amplitude typically shows a progressive increase to values ranging from ∼60 to 150% baseline (Bach and Mitchell 1996; Baker-Herman and Mitchell 2002; Fuller 2005; Fuller et al. 2001a). The study that most closely aligns with the current work examined intermittent hypoxia-induced phrenic LTF in wild-type mice compared with orexin-deficient mice (Toyama et al. 2009). Similar to our initial observations, the authors indicated that mice could tolerate only brief hypoxia and were thus exposed to five, 45-s hypoxic episodes (FiO2 = 0.10–0.15). This paradigm induced robust phrenic LTF in the wild-type mice, such that by 60 min post-hypoxia, the inspiratory burst amplitude actually exceeded the value recorded during the initial acute hypoxic response. That observation is consistent with the current data (e.g., Figs. 1 and 2), and the similarity between these two studies indicates that (comparatively) the mouse has a particularly robust capacity for intermittent hypoxia-induced respiratory neuroplasticity.
Significance of the XII power spectrum.
The power spectrum of respiratory-related neural recordings has been well characterized (Funk and Parkis 2002), although the mechanisms responsible for the occurrence of spectral peaks and the functional significance of these peaks cannot be stated with certainty. One logical hypothesis is that MFOs arise as a direct result of the discharge frequency of the motoneurons contributing to the extracellularly recorded compound action potentials from respiratory nerves or muscles (Christakos et al. 1991). We are unaware of reports of inspiratory-related XII motoneuron discharge frequency in the in vivo mouse, but in the rat, we have observed XII motoneuron bursting ranging from ∼20 to 70 Hz (Lee and Fuller 2010b). John et al. (2005) recorded genioglossus motor-unit discharge during spontaneous breathing in urethane-anesthetized rats and observed a linear increase in discharge from ∼55 to 85 Hz, as inspired CO2 was raised from 0 to 12%. The approximate range of the MFO oscillations in the current study was 20–50 Hz, similar to the prior report of anesthetized mice from Solomon's laboratory (O'Neal et al. 2005), but our use of the 60-Hz notch filter to reduce recording noise is likely to have directly attenuated at least a portion of the signal resulting from motoneuron discharge rates.
Experimental and theoretical data in other neural systems suggest that HFOs facilitate synchrony of neuronal activation (Konig and Engel 1995), and in turn, this contributes to more efficient activation of neuronal networks (Stevens and Zador 1998). In regards to the neural control of breathing, several different theories have been put forth regarding the significance of HFOs. First, HFOs may represent synchronization within brain stem respiratory neurons and networks (Cohen et al. 1987). Richardson and Mitchell (1982) first suggested this hypothesis, based on their auto-correlation studies of the PSD of inspiratory activity in cat recurrent laryngeal and phrenic nerves. Their experiments showed significant synchrony between inspiratory high-frequency spectral peaks in recurrent laryngeal and phrenic nerve recordings that were eliminated by a brain stem lesion. Cohen and colleagues (1987) subsequently confirmed that HFOs showed coherence across brain stem and spinal (phrenic) inspiratory motor output. A second possibility is that HFOs may play a role in determining the precise timing of motoneuron discharge and the “efficiency” with which presynaptic input is transformed into action potential output (Funk and Parkis 2002; Liu et al. 1990; Parkis et al. 2003). HFOs may result in greater recruitment of motoneurons by causing synchronized excitatory postsynaptic currents (EPSCs) (Funk and Parkis 2002; Marchenko et al. 2012; Stevens and Zador 1998). By altering the action-potential threshold, EPSCs can increase the “input-output efficiency” of synaptic communication and thereby amplify synaptic inputs (Funk and Parkis 2002). Lastly, it has been proposed that HFOs may act at the motor-unit level to enable synchronous myofiber activation, thereby improving mechanical force generation by the respiratory muscles (Cairns and Road 1998).
Changes in the XII power spectrum during and following intermittent hypoxia.
During baseline (prehypoxia) conditions, the frequency bands that demonstrated increased power were consistent with prior reports in the mouse (O'Neal et al. 2005) and other species (Cohen et al. 1987; Richardson and Mitchell 1982). That is, the expected MFO, HFO, and UHFO bands were identified in XII neurograms (e.g., Figs. 3 and 4). During the initial acute exposure to hypoxia, the XII power spectrum shifted, such that a greater proportion of the signal content was present at higher frequencies (i.e., a rightward shift). This finding is consistent with a report showing a shift to higher-frequency spectral content in phrenic and recurrent laryngeal nerve recordings during hypoxia or hypercapnia (Bruce 1988). In a study in piglets, Sica et al. (1991) found that hypoxia triggered a shift in the inspiratory power spectrum to higher frequencies in phrenic but not XII nerve output. However, these authors did observe that hypoxia induced an increase in total power in the XII PSD (Sica et al. 1991). Marchenko and Rogers (2006) reported that hypoxia induced an increase in both phrenic and XII HFO in the rat, with comparatively lesser change in MFO, and we have observed the same (K. A. Streeter and D. M. Baekey, unpublished observations).
During the robust LTF of XII burst amplitude that followed the exposure to intermittent hypoxia, both the HFO and UHFO bands became more prominent, and there was a decrease in MFO content. Accordingly, we reject our original hypothesis that the high-frequency content of the XII power spectrum (i.e., HFO and UHFO) would remain stable during LTF. In the next section, we review the potential significance of these observations.
Is the shift in power spectrum related to XII LTF?
The first possibility to consider is that the inspiratory power spectrum and the changes observed after intermittent hypoxia may not be directly (or even indirectly) related to expression of respiratory LTF. However, the “TC” experiments (i.e., prolonged recordings with no intermittent hypoxia exposure; Fig. 4C) demonstrated a very stable XII spectral content over the duration of the experiment. Accordingly, we conclude that the intermittent hypoxia exposure was responsible for the persistent changes in the XII power spectrum seen during LTF. Nevertheless, this does not establish a mechanistic link between a rightward shift of the power spectrum and the development of XII LTF. If, however, MFOs arise from motoneuron discharge rates (Christakos et al. 1991), then the apparent decrease in MFO content following intermittent hypoxia suggests that increased inspiratory XII bursting during LTF does not reflect rate modulation (i.e., increased discharge rates) of previously active motoneurons. The XII motor pool contains ∼1,000 motoneurons (ElMallah et al. 2012; Sturrock 1991), and only a fraction of these cells is likely to be active during the baseline (prehypoxia) conditions in the current experiments. Thus there is a large pool of inactive XII motoneurons that can be recruited during acute respiratory challenge and/or respiratory neuroplasticity. In the rat, a brief bout of hypoxia causes recruitment of inactive XII motoneurons, and these cells continue to discharge during the first few minutes post-hypoxia (Lee and Fuller 2010a). To our knowledge, however, the discharge rate of XII motoneurons (or any respiratory motoneurons) has never been directly evaluated during intermittent hypoxia-induced LTF.
During hypoxia, increased respiratory motor output is associated with an increase in the strength of presynaptic inputs to respiratory motoneurons due to stimulation of carotid chemoafferents. In our experiments, the rightward shift of the spectral curve during LTF was qualitatively similar to what occurred during the acute hypoxic response, but the relative magnitude of the shift was actually greater during LTF. The similarity in spectral content alterations during acute hypoxia and following intermittent hypoxia (i.e., during LTF) may indicate similar underlying mechanisms, and this may offer some explanation for the large magnitude of XII LTF in the mouse vs. other species. Thus we speculate that the large XII LTF in our murine preparation is associated with increased presynaptic drive. There is one report of increased activity in the medullary network activity following intermittent hypoxia (e.g., consistent with the possibility of a presynaptic mechanism contributing to LTF) (Morris et al. 2001). The majority of evidence, however, supports the hypothesis that postsynaptic mechanisms are primarily regulating respiratory LTF (Bach and Mitchell 1996; Baker-Herman et al. 2004; Devinney et al. 2013; Fuller et al. 2001b). Perhaps the most direct evidence for this comes from an in vitro study, in which serotonin was intermittently applied to XII motoneurons, and the result was a persistent LTF-like increase in XII discharge (Bocchiaro and Feldman 2004). In addition, a body of work from Mitchell and colleagues (Dale et al. 2014) points toward a “local” mechanism driving phrenic LTF in the anesthetized rat model, and the proposed mechanistic (molecular) models of LTF all point toward a postsynaptic motoneuron mechanism. Nevertheless, since the increased XII high-frequency content was also present during the acute hypoxic response (i.e., when increases in presynaptic drive can be certain), we speculate that presynaptic respiratory drive contributes to LTF in this particular experimental preparation. This interpretation is consistent with the view that the respiratory neural control network is capable of plasticity at multiple sites. The primary locus of the intermittent hypoxia-induced respiratory neuroplasticity may vary depending on the species and/or details of the experimental or clinical paradigm (Mateika and Sandhu 2011; Mitchell et al. 2001; Morris et al. 2001).
Conclusion.
In the urethane-anesthetized mouse, intermittent hypoxia produced a consistently large-magnitude LTF of XII motor output. The murine LTF model thus provides a future platform for mechanistic studies of respiratory neuroplasticity in genetically engineered mice (e.g., targeted mutations in genetic disease models) (DeRuisseau et al. 2009; Ittner et al. 2015). The current data also establish that LTF in the mouse is associated with a considerable change in the power spectral content of inspiratory XII nerve activity. Functionally, the power spectral shift during LTF could result in increased efficiency of synaptic transmission and increased coordination between different respiratory motor nuclei (Funk and Parkis 2002). Whereas the precise physiological significance of the power spectra in respiratory neural recordings is not established, the significant shift in the spectral content during LTF indicates that the mechanisms responsible for the oscillations in the power spectrum are influenced by exposure to intermittent hypoxia.
GRANTS
Support for this work was funded by the Parker B. Francis Fellowship (to M. K. ElMallah) and the National Institute of Child Health and Human Development, Grants K08 HD077040-01A1 (to M. K. ElMallah) and R01HD052682-06A1 (to D. D. Fuller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.K.E., K.-Z.L., and D.D.F. conception and design of research; M.K.E. performed experiments; M.K.E., D.A.S., K.A.S., and D.M.B. analyzed data; M.K.E., D.A.S., K.-Z.L., S.M.F.T., K.A.S., D.M.B., and D.D.F. interpreted results of experiments; M.K.E. and D.A.S. prepared figures; M.K.E. drafted manuscript; M.K.E., D.A.S., K.-Z.L., S.M.F.T., K.A.S., D.M.B., and D.D.F. edited and revised manuscript; M.K.E., D.A.S., K.-Z.L., S.M.F.T., K.A.S., D.M.B., and D.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of M. K. ElMallah: Dept. of Pediatrics and Gene Therapy, Division of Pulmonary Medicine, Univ. of Massachusetts Medical School, 55 Lake Ave. North, Worcester, MA 01655.
REFERENCES
- Ackerson LM, Bruce EN. Bilaterally synchronized oscillations in human diaphragm and intercostal EMGs during spontaneous breathing. Brain Res 271: 346–348, 1983. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179: 48–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random Data: Analysis and Measurement Procedures. New York: John Wiley & Sons, 2010. [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA 101: 4292–4295, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce EN. Correlated and uncorrelated high-frequency oscillations in phrenic and recurrent laryngeal neurograms. J Neurophysiol 59: 1188–1203, 1988. [DOI] [PubMed] [Google Scholar]
- Bruce EN, Ackerson LM. High-frequency oscillations in human electromyograms during voluntary contractions. J Neurophysiol 56: 542–553, 1986. [DOI] [PubMed] [Google Scholar]
- Cairns AM, Road JD. High-frequency oscillation and centroid frequency of diaphragm EMG during inspiratory loading. Respir Physiol 112: 305–313, 1998. [DOI] [PubMed] [Google Scholar]
- Christakos CN, Cohen MI, Barnhardt R, Shaw CF. Fast rhythms in phrenic motoneuron and nerve discharges. J Neurophysiol 66: 674–687, 1991. [DOI] [PubMed] [Google Scholar]
- Cohen MI, See WR, Christakos CN, Sica AL. High-frequency and medium-frequency components of different inspiratory nerve discharges and their modification by various inputs. Brain Res 417: 148–152, 1987. [DOI] [PubMed] [Google Scholar]
- Curran A, Eastwood P, Harms C, Smith C, Dempsey JA. Superior laryngeal nerve section alters responses to upper airway distortion in sleeping dogs. J Appl Physiol (1985) 83: 768–775, 1997. [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology 29: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH Jr, Mah C, Reier PJ, and Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci USA 106: 9419–9424, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci 1279: 143–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElMallah MK, Falk DJ, Lane MA, Conlon TJ, Lee KZ, Shafi NI, Reier PJ, Byrne BJ, Fuller DD. Retrograde gene delivery to hypoglossal motoneurons using adeno-associated virus serotype 9. Hum Gene Ther Methods 23: 148–156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol (1985) 98: 1761–1767, 2005. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics 4: 175–181, 2001a. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006; discussion 2000, 2001b. [DOI] [PubMed] [Google Scholar]
- Funk GD, Parkis MA. High frequency oscillations in respiratory networks: functionally significant or phenomenological? Respir Physiol Neurobiol 131: 101–120, 2002. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Halliday GM, Kril JJ, Gotz J, Hodges JR, Kiernan MC. FTD and ALS—translating mouse studies into clinical trials. Nat Rev Neurol 11: 360–366, 2015. [DOI] [PubMed] [Google Scholar]
- Jiang C, Mitchell GS, Lipski J. Prolonged augmentation of respiratory discharge in hypoglossal motoneurons following superior laryngeal nerve stimulation. Brain Res 538: 215–225, 1991. [DOI] [PubMed] [Google Scholar]
- John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig P, Engel AK. Correlated firing in sensory-motor systems. Curr Opin Neurobiol 5: 511–519, 1995. [DOI] [PubMed] [Google Scholar]
- Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol 587: 5451–5467, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Hypoxia-induced short-term potentiation of respiratory-modulated facial motor output in the rat. Respir Physiol Neurobiol 173: 107–111, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J Appl Physiol (1985) 108: 1187–1198, 2010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Qiu K, Sandhu MS, Elmallah MK, Falk DJ, Lane MA, Reier PJ, Byrne BJ, Fuller DD. Hypoglossal neuropathology and respiratory activity in pompe mice. Front Physiol 2: 31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol 64: 423–436, 1990. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Ghali MG, Rogers RF. Motoneuron firing patterns underlying fast oscillations in phrenic nerve discharge in the rat. J Neurophysiol 108: 2134–2143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko V, Rogers RF. GABAAergic and glycinergic inhibition in the phrenic nucleus organizes and couples fast oscillations in motor output. J Neurophysiol 101: 2134–2145, 2009. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Rogers RF. Selective loss of high-frequency oscillations in phrenic and hypoglossal activity in the decerebrate rat during gasping. Am J Physiol Regul Integr Comp Physiol 291: R1414–R1429, 2006. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol (1985) 82: 419–425, 1997. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol 176: 1–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol 557: 13–18, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol 532: 483–497, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal MH 3rd, Spiegel ET, Chon KH, Solomon IC. Time-frequency representation of inspiratory motor output in anesthetized C57BL/6 mice in vivo. J Neurophysiol 93: 1762–1775, 2005. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Willsky AS. Signals and Systems. Upper Saddle River, NJ: Prentice-Hall, 1997. [Google Scholar]
- Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci 23: 8152–8158, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Mitchell RA. Power spectral analysis of inspiratory nerve activity in the decerebrate cat. Brain Res 233: 317–336, 1982. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica AL, Gandhi MR, Steele AM. Central patterning of inspiratory activity in the neonatal period. Brain Res Dev Brain Res 64: 77–86, 1991. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Zador AM. Input synchrony and the irregular firing of cortical neurons. Nat Neurosci 1: 210–217, 1998. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Stability of motor neuron and interneuron number in the hypoglossal nucleus of the ageing mouse brain. Anat Anz 173: 113–116, 1991. [PubMed] [Google Scholar]
- Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol (1985) 114: 52–65, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talathi SS, Hwang DU, Ditto WL, Mareci T, Sepulveda H, Spano M, Carney PR. Circadian control of neural excitability in an animal model of temporal lobe epilepsy. Neurosci Lett 455: 145–149, 2009. [DOI] [PubMed] [Google Scholar]
- Toyama S, Sakurai T, Tatsumi K, Kuwaki T. Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol 168: 295–302, 2009. [DOI] [PubMed] [Google Scholar]