Abstract
The nucleus tractus solitarii (nTS) is the initial central termination site for visceral afferents and is important for modulation and integration of multiple reflexes including cardiorespiratory reflexes. Glutamate is the primary excitatory neurotransmitter in the nTS and is removed from the extracellular milieu by excitatory amino acid transporters (EAATs). The goal of this study was to elucidate the role of EAATs in the nTS on basal synaptic and neuronal function and cardiorespiratory regulation. The majority of glutamate clearance in the central nervous system is believed to be mediated by astrocytic EAAT 1 and 2. We confirmed the presence of EAAT 1 and 2 within the nTS and their colocalization with astrocytic markers. EAAT blockade with dl-threo-β-benzyloxyaspartic acid (TBOA) produced a concentration-related depolarization, increased spontaneous excitatory postsynaptic current (EPSC) frequency, and enhanced action potential discharge in nTS neurons. Solitary tract-evoked EPSCs were significantly reduced by EAAT blockade. Microinjection of TBOA into the nTS of anesthetized rats induced apneic, sympathoinhibitory, depressor, and bradycardic responses. These effects mimicked the response to microinjection of exogenous glutamate, and glutamate responses were enhanced by EAAT blockade. Together these data indicate that EAATs tonically restrain nTS excitability to modulate cardiorespiratory function.
Keywords: astrocytes, sympathetic nervous system, glutamate transporters, phrenic nerve activity, synaptic currents
as the first central synapse for visceral sensory afferents, the nucleus tractus solitarii (nTS) is the initial target for modulation of synaptic, autonomic, and cardiorespiratory reflex function. Glutamate, the primary excitatory neurotransmitter in the nTS, evokes both phasic and tonic excitatory synaptic currents to induce neuronal depolarization (Andresen and Kunze 1994; Machado 2001; Kline 2008). Such excitatory actions profoundly influence cardiorespiratory regulation (Burton and Kazemi 2000; Baude et al. 2009). Microinjection of glutamate into the nTS induces hypotension, bradycardia, and apnea (Talman et al. 1980; Vardhan et al. 1993a; Foley et al. 1998), and glutamate antagonists block most cardiorespiratory reflexes (Talman et al. 1981; Andresen and Kunze 1994). While glutamate is required for basal synaptic, autonomic, and cardiorespiratory function, prolonged glutamate receptor activation of nTS neurons produces excitotoxicity, eliminating cardiorespiratory reflexes (Talman et al. 1981; Cheng et al. 2002). Glutamatergic signaling is tempered by the removal of transmitter from the extracellular milieu by excitatory amino acid transporters (EAATs) (Danbolt 2001; Shigeri et al. 2004). Such transporter uptake is essential to prevent excessive excitation, receptor desensitization, and normal function of the synapse and the system as a whole.
Astrocytes within the tripartite synapse [presynaptic terminal, postsynaptic neuron, and adjacent astrocyte(s)] contribute significantly to synaptic transmission in the central nervous system (CNS) (Perea et al. 2009) including the nTS (Accorsi-Mendonça et al. 2013; Huda et al. 2013; Vance et al. 2015). They can release gliotransmitters that modulate neuronal pre and postsynaptic function and overall synaptic activity (Araque et al. 1999; Halassa et al. 2007, 2009; Vance et al. 2015). Astrocytes also potently modulate synaptic function through their uptake of neurotransmitters in the synaptic cleft (Campbell and Hablitz 2004; Potapenko et al. 2012; Medrano et al. 2013). The majority of glutamate uptake, and receptor inactivation, is due to astrocyte transporters (Rothstein et al. 1996; Anderson and Swanson 2000), with EAAT 1 and 2 subtypes as their primary mediators.
The glial EAAT 1 and 2 have been identified in the nTS (Chounlamountry and Kessler 2011). Reducing EAAT glutamate uptake potentiates the slow excitation observed following repeated afferent stimulation of nTS neurons (Huda et al. 2013; Zhao et al. 2015). Nevertheless, little is known about the role of EAATs within the nTS in relation to basal synaptic and neuronal function or EAAT influence on autonomic and cardiorespiratory regulation. We hypothesized that EAATs tonically modulate nTS excitability, which influences cardiorespiratory regulation. Using patch-clamp electrophysiology and whole animal assessment of cardiorespiratory function, we demonstrate that blocking EAATs increases nTS activity, leading to sympathoinhibition, hypotension, bradycardia, and apnea. Thus EAATs within the nTS restrain neuronal excitability to modulate cardiorespiratory function.
METHODS
Animals.
All experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals guidelines and protocols were approved by the University of Missouri Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan, Indianapolis, IN) aged 4–8 wk were used for all experiments. Animals were housed with a 12:12-h light-dark cycle with food and water available ad libitum.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (Kline et al. 2010; Clark et al. 2011; King et al. 2012). Briefly, isoflurane anesthetized rats were transcardially perfused with heparinized, oxygenated Dulbecco's modified Eagle's medium (125 ml, pH 7.4; Sigma, St. Louis, MO) followed by 4% paraformaldehyde (500 ml, pH 7.4; Sigma). The brainstems were removed and sectioned in the horizontal plane at 30 μm using a vibratome (VT 1000S; Leica). Antigen retrieval was performed on free floating sections with citrate buffer solution (30 min, pH 6.0). Sections were rinsed (3 × 10 min) in 0.01 M phosphate-buffered saline (PBS) and then blocked by 10% normal donkey serum (NDS; S30; Millipore) in 0.3% Triton-PBS. Tissue sections were rinsed and subsequently incubated with primary antibodies against EAAT 1 (rabbit anti-EAAT 1; Abcam ab416, 1:250) or EAAT 2 (rabbit anti-EAAT 2; Abcam ab41621; 1:250) in 3% NDS in 0.01 M PBS. To identify astrocytes, we used either the astrocyte marker S100B (mouse anti-S100B; Abcam ab41548; 1:500), which labels both somas and processes, or glial fibrillary acidic protein (GFAP; guinea pig anti-GFAP; Synaptic Systems 173004; 1:500) to label major astrocytic processes. Localization with neurons was assessed by the neuronal marker NeuN (mouse anti-NeuN; Millipore MAB377; 1:500). The next day, sections were rinsed and incubated for 2 h in Alexa Fluor 647-conjugated donkey anti-rabbit IgG, Alexa Fluor 488-conjugated donkey anti-mouse IgG, and Cy3-conjugated donkey anti-guinea pig IgG (all from Jackson ImmunoResearch) in 3% NDS and 0.3% Triton-PBS. Sections were rinsed, mounted on gelatin-coated slides, air dried, and coverslipped. Slides were then sealed with nail polish. One section per run was incubated without primary antibody and served as negative control. No fluorescent staining was present on negative controls.
Immunoreactivity was examined with a conventional fluorescence microscope (BX51; Olympus) equipped with a digital monochrome camera (ORCA-ER; Hamamatsu) and a spinning disk confocal unit (Olympus). Appropriate filter sets and excitation wavelengths were used to visualize the different fluorophores. For each fluorophore used, z-stacks (0.5-μm separation) were taken in the same focal planes. Images were postprocessed for contrast and brightness and background subtracted (ImageJ; National Institutes of Health).
In vitro brain slice preparation, electrophysiology, and protocols.
Rats (n = 10) were anesthetized with isoflurane and decapitated, and the brainstem rapidly removed and placed in ice-cold NMDG-HEPES cutting solution (in mM: 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 10 MgSO4, 30 NaHCO3, 20 HEPES, 25 d-glucose, 5 l-ascorbic acid, 2 thiourea, 3 sodium pyruvate, and 0.5 CaCl2, aerated with 95% O2-5% CO2, pH 7.4, and 300–310 mosM). The brainstem was ventrally trimmed and affixed with cyanoacrylate onto the chuck of a vibratome. Horizontal slices (∼280 μm) were generated that contained the nTS and a long length of the tractus solitarii (TS) in the same plane.
Slices were secured with a nylon mesh harp in a heated recording chamber (TC-344B; Warner Instruments, Hamden, CT). Slices were superfused with normal recording artificial cerebrospinal fluid (aCSF; in mM: 124 NaCl, 3 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 d-glucose, 0.4 l-ascorbic acid, and 2 CaCl2, aerated with 95% O2-5% CO2, pH 7.4, and 300–310 mosM) at 32–35°C and allowed to recover a minimum of 30 min following slicing before recording.
Recording electrodes (3–5 MΩ) were pulled from borosilicate glass (no. 8250; King Precision Glass, Claremont, CA) on a horizontal pipette puller (Sutter Instruments P-97, Novato, CA). Neurons were visualized on an Olympus BX51WI fixed stage upright microscope with DIC optics. Recording electrodes were positioned on the neuron using a piezoelectric micromanipulator (Burleigh PCS-6000; Thor Labs) and recorded in the whole cell configuration. Signals were filtered at 2 kHz and acquired at 10 kHz using a Multiclamp 700B amplifier controlled by Clampex 10 software by a Digidata 1440 interface (Molecular Devices, Sunnyvale, CA).
Recordings were targeted to the caudal nTS and regions comparable to our in vivo microinjections [∼400 μm rostral to calamus scriptorius (Ostrowski et al. 2014)]. Recording electrodes were filled with the following (in mM): 130 K+ gluconate, 10 NaCl, 11 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 2 MgATP, and 0.2 NaGTP. To examine synaptic and holding currents, neurons were voltage clamped at −60 mV. TS-evoked excitatory postsynaptic currents (TS-EPSCs) were induced by placing a concentric bipolar stimulating electrode (FHC, Bowdoin, ME) on the TS afferent bundle and stimulating at 0.5 Hz. Spontaneous EPSCs (sEPSCs) and holding currents were recorded in gap-free mode without stimulation. Membrane potential (Vm) and spontaneous action potential (AP) discharge frequency were recorded in current-clamp mode with no holding current. Spiking was induced by depolarizing current injection (ramp of −20 to +120 pA over 500 ms) from the cells' resting potential. Subsequently, the role of EAAT blockade on cell properties was examined.
Recorded cells, typically one to two cell layers deep, were exposed to 5 min of the general EAAT antagonist DL-threo-β-benzyloxyaspartic acid (TBOA; 100, 200, or 500 μM) followed by an aCSF wash. TBOA is a nontransportable selective antagonist of EAATs (Shimamoto et al. 1998; Shigeri et al. 2001; Campiani et al. 2003). Concentrations were selected based on the literature (Jabaudon et al. 1999; Anderson et al. 2001; Potapenko et al. 2012) and preliminary experiments. Drugs were bath applied via superfusion by means of a valve controller (Warner Instruments VC-6).
In vivo surgical preparation and cardiorespiratory function protocol.
Rats were anesthetized with isoflurane (5%, induction; 2–2.5% maintenance, in 100% O2) as previously described (Mueller and Hasser 2006; Clark et al. 2011). Core temperature was monitored and maintained at ∼38°C (Tele-Thermometer, Simpson Electric, Lac du Flambeau, WI). Femoral arterial and venous catheters (PE-10 fused to PE-50; A-M Systems, Sequim, WA) were implanted for measuring arterial pressure and drug or fluid administration, respectively. A tracheostomy was performed and rats were mechanically ventilated with O2-enriched room air. Arterial O2 saturation was monitored continuously (MouseOx; Starr Life Sciences, Oakmont, PA) and maintained between 95 and 100%. Arterial blood gases were measured periodically (Osmetech; OPTI CCA, Roswell, GA), and ventilatory parameters were adjusted if necessary to maintain baseline blood gas values.
The left splanchnic sympathetic nerve was isolated via a retroperitoneal approach, placed on bipolar Teflon-coated silver recording electrodes (0.005″; A-M Systems), and covered in silicone elastomer (Kwik-Cast; WPI, Sarasota, FL). The left phrenic nerve was exposed using a ventral cervical approach, crushed distally, placed on a bipolar recording electrode, and coated with silicone as above. The contralateral phrenic nerve was cut and a bilateral cervical vagotomy was performed to prevent entrainment of phrenic motor output with the ventilator. Ground wires were sutured to the surrounding muscle to reduce noise and the incisions were closed.
Nerve activity was amplified (×1,000), filtered (30-3,000 Hz; P511; Grass Technologies, Warwick, RI), rectified, and integrated using a root mean square (RMS) converter (time constant: phrenic = 100 ms; splanchnic = 28 ms); sympathetic nerve activity was electronically averaged. Background noise was determined from the signal between bursts of activity and verified following euthanasia. Splanchnic sympathetic nerve activity (SSNA) and phrenic nerve activity (PhrNA) were defined as the respective recorded nerve activity minus background noise. Mean arterial pressure (MAP) and heart rate (HR) were determined from the pulsatile arterial pressure signal (PowerLab data acquisition system; ADInstruments, Colorado Springs, CO). Minute phrenic activity (Min PhrNA) is defined as the product of phrenic amplitude and frequency. SSNA responses were evaluated as a percentage of baseline activity before a given intervention.
Rats were placed prone in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) and a partial occipital craniotomy was performed to expose the brainstem as previously described (Mueller and Hasser 2006; Clark et al. 2011; Ostrowski et al. 2014). Following completion of surgical procedures, anesthesia was gradually converted from isoflurane to inactin (100 and 20 mg/kg iv supplements as required). Animals underwent neuromuscular blockade with gallamine (12.5 and 0.3–0.45 mg/h iv maintenance). Adequate plane of anesthesia was verified regularly by lack of cardiovascular responses (<5-mmHg increase in arterial pressure) to firm tail pinch. Cardiorespiratory parameters were allowed to stabilize for ∼60 min before initiating experimental procedures. Multibarrel glass micropipettes (2–3 barrels, ∼10 μm per barrel, borosilicate glass; WPI) filled with appropriate solutions were advanced into the nTS under visual guidance using surface landmarks. Microinjections [relative to calamus scriptorius (in mm): 0.0 and 0.4 rostral, 0.2 and 0.4 lateral, 0.4 ventral to the dorsal surface of the medulla] were targeted to the caudal nTS in areas comparable to in vitro recordings as previously (Ostrowski et al. 2014). Volume of injection was monitored using a compound microscope (×150). Subsequently, the role of astrocyte EAAT blockade on baseline cardiovascular parameters and response to glutamate was examined.
Baseline responses to unilateral nTS microinjections of glutamate (3–5 mM, 30 nl) were evaluated, which induced depressor and sympathoinhibitory responses that also served to functionally identify the nTS (Foley et al. 1998; Clark et al. 2011). To assess the overall cardiorespiratory effects of nTS glutamate transporters, rats (n = 9) were subjected to bilateral injections of TBOA (1 mM; ≤90 nl; Oldenziel et al. 2006). Similar volumes of aCSF served as vehicle controls. Five minutes after injection of TBOA or aCSF, unilateral injections of glutamate were repeated (n = 6). In three rats, glutamate was not injected after TBOA to determine the time course of TBOA responses.
Drugs.
TBOA was purchased from Tocris Bioscience (R&D Systems, Minneapolis, MN). TBOA stocks were made in double distilled water and diluted in aCSF to reported concentrations. All other chemicals were purchased from Sigma-Aldrich and Fisher Scientific (Pittsburgh, PA).
Data analysis.
In vitro electrophysiological data were analyzed with pClamp10 (Molecular Devices) and Microsoft Excel software. Only nTS cells connected to TS afferents with a single synapse (i.e., monosynaptic) were included in this study (Ostrowski et al. 2014). Neurons were determined to be monosynaptic second-order by low jitter (148 ± 15 μs) and latency (4.3 ± 0.3 ms). sEPSC detection was set at two times the RMS noise level, and currents were manually confirmed. TS-EPSC properties per experimental treatment (baseline, TBOA, wash) were determined for each individual event over 30 sweeps at 0.5 Hz and then averaged. TS-EPSC decay time was determined from a single exponential function of the averaged trace for each treatment. Decay time for sEPSCs was determined by taking the mean of 20 sEPSC events per treatment. Group data were taken as the mean of all respective experimental treatments. Baseline values are shown indicating the variability around the normalized mean. TBOA responses were normalized and expressed relative to their individual baseline (defined as “1”).
In vivo cardiorespiratory responses to nTS microinjection of TBOA were analyzed as the average of 15 s at the peak response to TBOA, and values at recovery were evaluated 5 min following microinjection. Responses to aCSF were taken at similar times as those examined for TBOA. Cardiorespiratory effects of glutamate before (pre) and after (post) TBOA or aCSF were examined as 1-s averages of a given parameter for 5 s before and 30 s after glutamate injection. To account for differences in nerve activities following TBOA or aCSF, SSNA and PhrNA were normalized to baseline values immediately before glutamate injection (defined as “1”). Because the duration of the response to glutamate varied with treatment, the overall effect was also evaluated by determining the area under the curve with respect to baseline for the 20 s following glutamate injection.
The effects of TBOA on synaptic or cardiorespiratory parameters were statistically evaluated by SigmaPlot 12.5 (Systat Software) and GraphPad Prism 6 (GraphPad Software). For in vitro protocols, the group effect of TBOA on holding current, membrane potential, RMS noise, and AP discharge was examined using one-way repeated-measures ANOVA. TBOA concentration effects on changes in these parameters were compared via one-way ANOVA. Effects of TBOA on sEPSC frequency and amplitude and TS-EPSC amplitude were determined using repeated-measures (within a concentration) two-way ANOVA. sEPSC amplitude and interevent intervals within an individual cell between baseline and TBOA were tested with Kolmogorov-Smirnov two-sample test. In vivo data evaluating the effects of TBOA on cardiorespiratory parameters were analyzed using one-way repeated-measures ANOVA. Time course of glutamate responses pre- and post-TBOA or aCSF was examined using two-way repeated measures ANOVA. The overall response (area under the curve) to glutamate under control conditions and following TBOA was compared using paired t-tests. For both in vitro and in vivo data, Fisher least significant difference post hoc test was used to identify individual differences among groups where appropriate. All group data are presented as means ± SE. Results were considered significantly different at P < 0.05.
RESULTS
EAAT 1 and 2 are present in the nTS and associated with astrocytes.
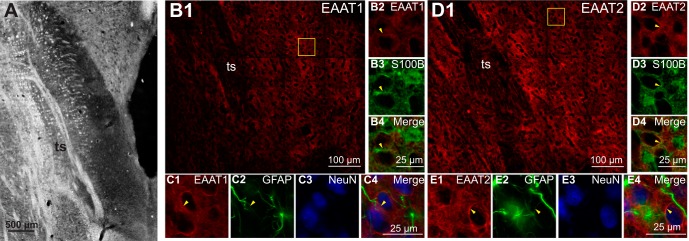
The presence of EAAT 1 and 2 in the nTS was confirmed by immunohistochemistry (Fig. 1). Figure 1A shows a bright-field photomicrograph of the horizontal nTS brainstem slice representative of the regions recorded from electrophysiologically and microinjected in in vivo experiments. EAAT 1 and 2 were found throughout the caudal nTS (Fig. 1, B1 and D1). At higher magnification it can be seen that both EAAT 1 and 2 colocalized (arrowheads) with the glial markers S100B (Fig. 1, B4 and D4) and GFAP (Fig. 1, C4 and E4) and are surrounding but not colocalized with neurons marked with NeuN.
Fig. 1.
Excitatory amino acid transporters (EAAT) 1 and 2 are present in astrocytes in the nucleus tractus solitarii (nTS). A: bright-field photomicrograph of a horizontal nTS section. B1: representative example of EAAT 1 expression in a horizontal nTS section. B2–B4: higher magnification of the boxed area in B1 indicating colocalization of EAAT 1 with S100B. C1–C4: high-magnification photomicrographs from a separate but similar nTS section demonstrating colocalization of EAAT 1 with glial fibrillary acidic protein (GFAP). Neither EAAT 1 nor GFAP were colocalized with NeuN. D1: representative example of EAAT 2 expression in a horizontal nTS section. D2–D4: higher magnification of the boxed area in D1 showing colocalization of EAAT 2 with S100B. E1–E4: high-magnification photomicrographs from a separate but similar nTS section indicating colocalization of EAAT 2 with GFAP. Neither EAAT 2 nor GFAP was colocalized with NeuN. Arrowheads denote colocalization; ts, solitary tract.
EAAT blockade depolarizes nTS neurons.
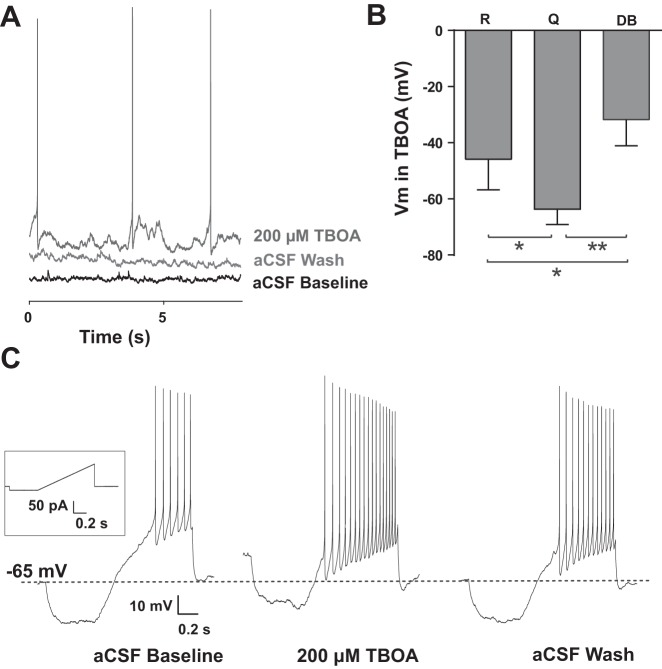
We examined the effect of EAATs in modulating overall nTS neuronal properties. EAATs were blocked with the antagonist TBOA (100, 200, and 500 μM, 5 min). TBOA induced an inward current that was greater with increasing concentrations (Fig. 2A). Compared with aCSF baseline, 100 μM TBOA evoked a significant inward current (Fig. 2B) that readily reversed. This inward current was augmented at 200 μM and increased further at 500 μM. The inward current produced by TBOA at 500 μM was significantly greater than currents produced with 100 or 200 μM. Corresponding with this inward current, TBOA depolarized the membrane (Fig. 2C). Application of 500 μM TBOA (baseline −64 ± 4 mV) evoked a significant and reversible depolarization. The TBOA-induced depolarization with 500 μM TBOA was significantly greater than the depolarization due to 100 or 200 μM (Fig. 2C). RMS of noise, an indicator of background membrane activity (Traynelis and Jaramillo 1998), increased with TBOA compared with aCSF baseline and was readily reversible. The RMS increase did not reach significance for 100 and 200 μM but was significant for 500 μM TBOA (Fig. 2D).
Fig. 2.
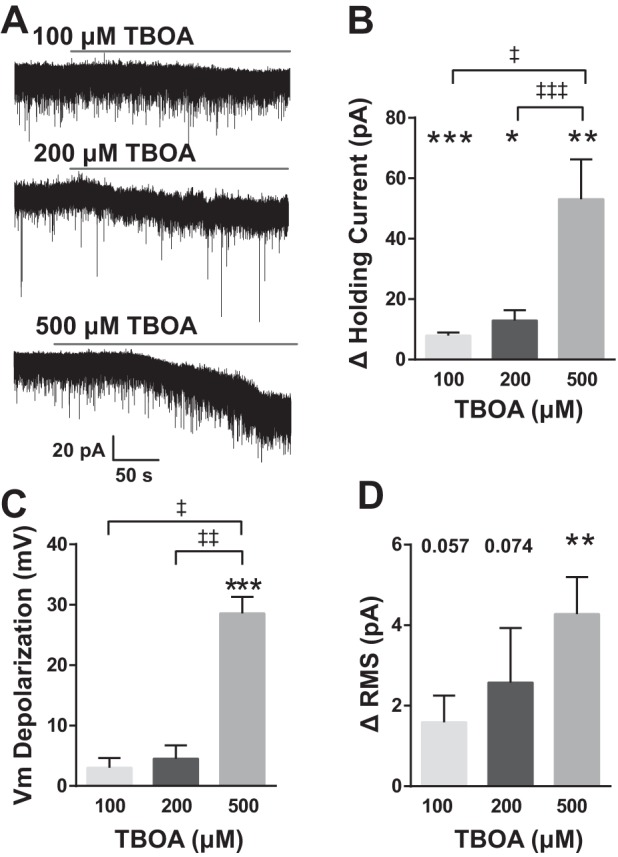
EAAT blockade depolarizes nTS neurons. A: representative traces of spontaneous activity showing the inward current produced in response to increasing concentrations of dl-threo-β-benzyloxyaspartic acid (TBOA). Cells were voltage clamped at −60 mV. B: group data showing the total change in holding current. All 3 concentrations were significantly different from aCSF baseline. Among concentrations, responses to 500 μM TBOA were significantly greater than both 100 and 200 μM. C: membrane potential (Vm) depolarized in response to TBOA although only 500 μM was significantly different from baseline. The amount of depolarization was significantly greater for 500 μM compared with either 100 or 200 μM. D: all 3 concentrations of TBOA increased root mean square (RMS) noise but only 500 μM differed significantly from baseline and 100 and 200 μM, n = 6; 500 μM, n = 7. *vs. individual baseline; ‡vs. TBOA concentration as indicated. *‡P < 0.05; **‡‡P < 0.01; ***‡‡‡P < 0.001.
TBOA-induced depolarization resulted in increased spontaneous AP discharge in a large portion of neurons. A representative example of the enhanced spontaneous AP discharge after 200 μM TBOA is shown in Fig. 3A. In response to 100 μM TBOA, 83% (5/6) of neurons significantly increased AP frequency (baseline 2 ± 1 Hz vs. TBOA 5 ± 1 Hz, P = 0.03). Following both 200 and 500 μM TBOA, 50% (3/6 each concentration) of neurons increased AP frequency (200 μM, 0.89 ± 0.55 to 2.2 ± 1.1 Hz, P = 0.15; 500 μM, 0.0 ± 0.0 to 5.9 ± 3.5 Hz, P = 0.23). A small number of neurons were quiescent during baseline and did not change following TBOA (100 μM, n = 1; 200 μM, n = 2). During EAAT blockade these non-responding neurons had a significantly hyperpolarized Vm compared with those that responded to TBOA with increased AP frequency, regardless of concentration [Fig. 3B, quiescent (Q), n = 3 vs. responders (R), n = 11, P = 0.015]. Likewise, a number of neurons (n = 4) in response to 500 μM TBOA either did not spike or decreased spontaneous AP firing. These neurons had a significantly depolarized Vm compared with TBOA-responders (Fig. 3B, P = 0.0008), suggesting lack of spiking was due to depolarizing block (DB) following EAAT blockade.
Fig. 3.
EAAT blockade increases nTS action potential (AP) discharge. A: representative example of membrane potential (Vm) during baseline (−68 mV), 200 μM TBOA, and wash. Note the TBOA-induced depolarization and AP discharge. Recordings were made in current-clamp mode with no holding current. aCSF, artificial cerebral spinal fluid (aCSF). B: membrane potential (Vm) during TBOA. Those neurons that responded to TBOA with an increase in AP discharge [responders (R)] were more depolarized that those that remained quiescent (Q). Neurons responding to TBOA with depolarizing block (DB), which eliminated AP discharge, had the most depolarized Vm. C: representative discharge from an nTS neuron using the indicated ramp protocol (inset) showing Vm depolarization and increased AP frequency in response to 200 μM TBOA. Dashed line indicates baseline resting Vm. *P < 0.05; **P < 0.01.
To further examine the influence of EAAT inhibition on AP discharge, we evoked spiking with a depolarizing ramp. The majority of nTS neurons increased AP frequency in response to TBOA compared with their aCSF baseline. Figure 3C is a representative example of the augmented AP discharge during 200 μM TBOA. For 100 μM TBOA, 60% (3/5) of cells increased the frequency of APs (aCSF: 13 ± 7 vs. TBOA: 18 ± 6 Hz), while 200 μM TBOA significantly augmented discharge in 67% (4/6) of cells (19 ± 9 vs. 32 ± 8 Hz, P = 0.02). During 500 μM TBOA, 66% (4/6) of cells showed evidence of depolarizing block consistent with our spontaneous AP data and the large depolarization, whereas the remainder (2/6) increased AP discharge.
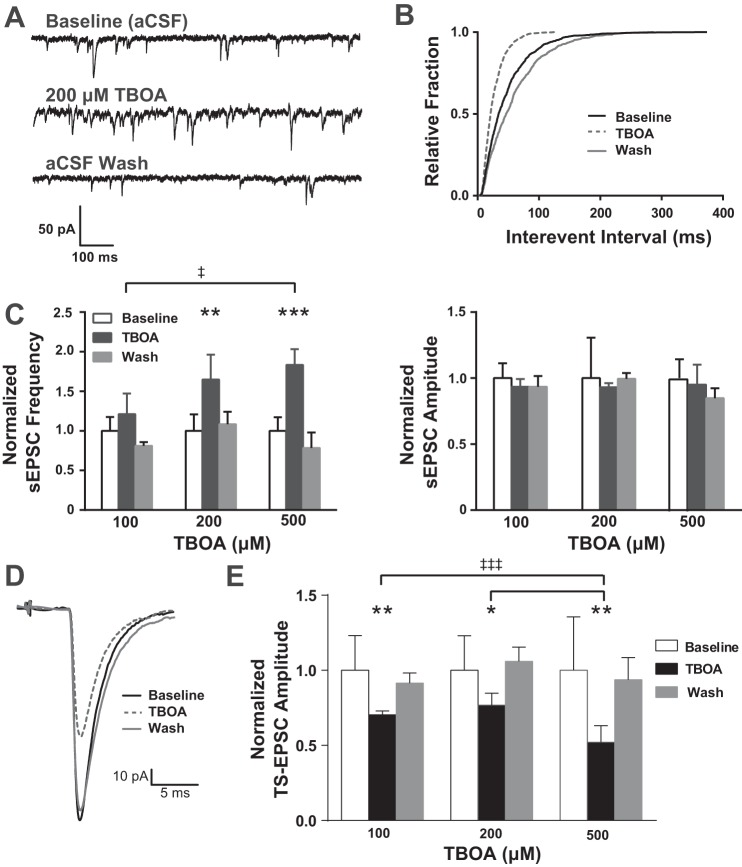
EAAT blockade increases sEPSCs frequency but reduces TS-EPSCs amplitude.
To determine the role of EAATs in nTS network activity we examined sEPSCs in response to increasing concentrations of TBOA (100, 200, and 500 μM, 5 min). As shown in the representative example (Fig. 4A), 200 μM TBOA increased sEPSC frequency with little effect on amplitude or decay time. In this neuron, following TBOA sEPSC interevent intervals were significantly shifted to the left (Fig. 4B, i.e., increased frequency, Kolmogorov-Smirnov test, P < 0.001). Quantitatively, the frequency of sEPSCs was augmented with increasing concentrations of TBOA (Fig. 4C, left). There were nonsignificant increases in frequency compared with baseline for 100 μM TBOA. For 200 and 500 μM TBOA, sEPSC frequency significantly increased. The effect of 500 μM was significantly greater than that of 100 μM. TBOA had no effect on the amplitude (Fig. 4C, right) or decay time (not shown) of sEPSCs at any concentration of TBOA.
Fig. 4.
EAAT blockade alters synaptic properties of nTS neurons. A: example of spontaneous synaptic activity showing the reversible increase in spontaneous excitatory postsynaptic current (sEPSC) frequency due to TBOA. Cells were voltage clamped at −60 mV. B: cumulative distribution plot of interevent interval. Note the leftward shift in duration of interevent interval of the cell in A, indicating an increase in event frequency. C: group data showing sEPSC frequency (left) and amplitude (right) normalized to aCSF baseline. Note that frequency increased with increasing concentration, reaching significance from baseline at 200 and 500 μM TBOA. The effect of 500 μM was greater than the effect at 100 μM. Amplitude did not change with any concentration of TBOA tested. D: representative example of TS-EPSCs showing a reversible decrease in amplitude with 200 μM TBOA. E: group data showing amplitude normalized to aCSF baseline. All TBOA concentrations significantly reduced peak amplitude of TS-EPSCs. The effect of 500 μM was greater than the effect at 100 and 200 μM. *vs. individual baseline; ‡vs. TBOA concentration as indicated. *‡P < 0.05; **‡‡P < 0.01; ***‡‡‡P < 0.001.
To examine the role of EAATs in modulating the synapse between the sensory afferent and second-order nTS neuron, we examined TS-EPSCs evoked at 0.5 Hz before and after blocking EAATs with TBOA. As shown in the representative example, 200 μM TBOA reversibly attenuated TS-EPSC amplitude (Fig. 4D). Quantitatively, for all concentrations of TBOA examined there was a significant reduction in peak amplitude of TS-EPSCs (Fig. 4E). The effect of 500 μM was significantly greater than both 100 and 200 μM. TBOA increased TS-EPSCs decay time compared with baseline, with a significant increase at 200 μM (4.1 ± 0.7 vs. 5.7 ± 1.63 ms, P = 0.02).
EAAT blockade produces depressor, sympathoinhibitory and apneic responses.
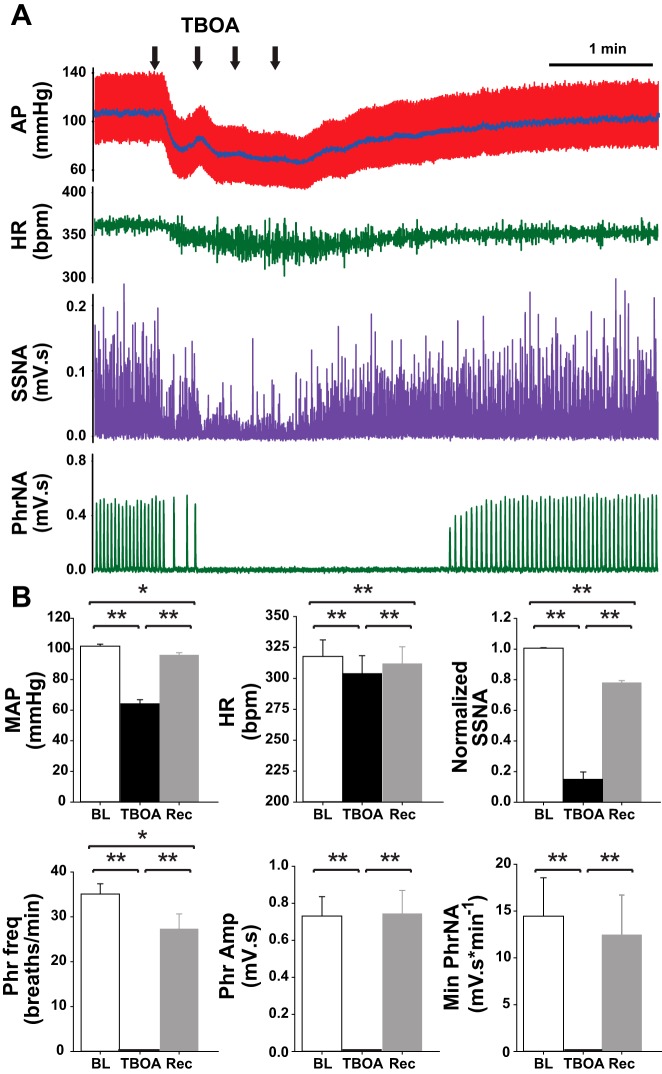
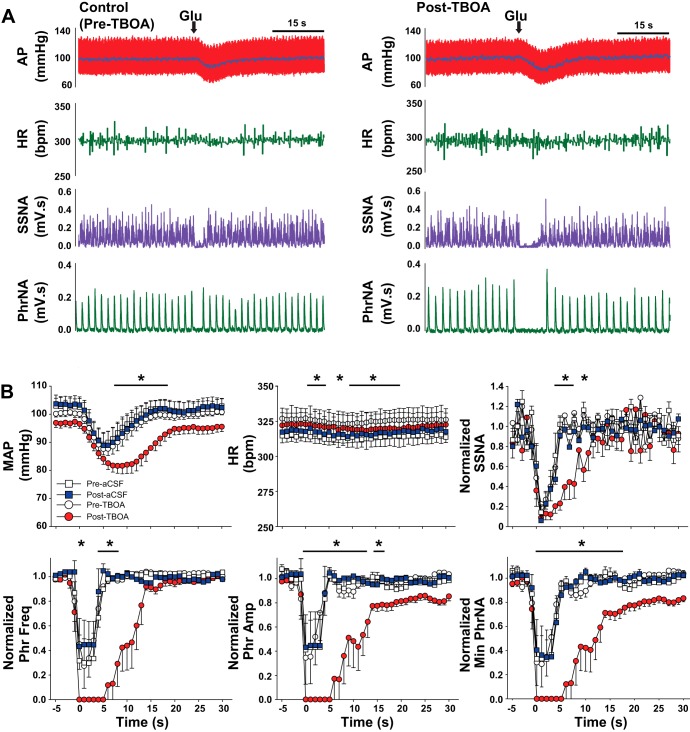
To determine if the tonic influence of EAATs on synaptic and neuronal properties within the nTS contributes to autonomic and cardiorespiratory function, we inhibited EAATs by bilateral microinjection of TBOA (1 mM) into the medial-commissural nTS, in regions from which in vitro recordings were made (Clark et al. 2011; Ostrowski et al. 2014). Baseline cardiorespiratory parameters before any interventions are presented in Table 1. Figure 5A is a representative example from one rat showing depressor, bradycardic, and sympathoinhibitory responses to TBOA that were accompanied by cessation of PhrNA. Microinjection of equal volumes of aCSF (vehicle) did not reduce any cardiorespiratory parameters measured (Table 2). Quantitative data (Fig. 5B) indicate bilateral nTS microinjection of TBOA significantly decreased MAP, HR, and SSNA and abolished PhrNA, responses consistent with increasing glutamate in the nTS (Bonham and McCrimmon 1990; Vardhan et al. 1993a,b; Foley et al. 1998; Braga et al. 2006; Ostrowski et al. 2014). Phr amplitude and Min PhrNA recovered within 5 min after TBOA injection. Phr frequency, MAP, HR, and SSNA partially recovered but remained lower than baseline at 5 min. In three animals in which cardiorespiratory function was monitored for longer than 5 min after TBOA without additional interventions, all parameters had returned to baseline within 10–15 min.
Table 1.
Baseline cardiorespiratory parameters
| Parameter | Baseline |
|---|---|
| MAP, mmHg | 103 ± 1.5 |
| HR, beats/min | 314 ± 11.5 |
| SSNA, mV·s | 0.037 ± 0.001 |
| Phr Freq, bursts/min | 35 ± 1.9 |
| Phr Amp, mV·s | 0.411 ± 0.064 |
| Minute PhrNA, mV·s·min−1 | 14 ± 3.3 |
Values are means ± SE; n = 10. Cardiorespiratory parameters in all animals are before any other interventions.
MAP, mean arterial pressure; HR, heart rate; SSNA, splanchnic sympathetic nerve activity; Phr Freq, phrenic nerve frequency; Phr Amp, phrenic nerve amplitude; PhrNA, phrenic nerve activity.
Fig. 5.
EAAT inhibition in the nTS decreases baseline cardiorespiratory function. A: representative example showing decreases in arterial pressure (AP), mean AP (MAP, superimposed on pulsatile AP trace), heart rate (HR), integrated splanchnic sympathetic nerve activity (SSNA), and integrated phrenic nerve activity (PhrNA) following microinjection of TBOA into the nTS. B: group data indicate that TBOA significantly decreased all cardiorespiratory parameters relative to baseline (BL). Note the partial to full recovery (Rec). *P < 0.05; **P < 0.01.
Table 2.
Cardiorespiratory response to aCSF
| Parameter | Baseline | aCSF |
|---|---|---|
| MAP, mmHg | 103 ± 3.0 | 105 ± 3.1 |
| HR, beats/min | 316 ± 16.2 | 317 ± 15.9 |
| SSNA, mV·s | 102 ± 1.9 | 105 ± 1.8 |
| Phr Freq, bursts/min | 38 ± 2.1 | 38 ± 2.2 |
| Phr Amp, mV·s | 0.398 ± 0.148 | 0.410 ± 0.152 |
| Minute PhrNA, mV·s·min−1 | 15 ± 5.9 | 15 ± 6.1 |
Values are means ± SE; n = 5. Cardiorespiratory parameters before (baseline) and 5 min after nucleus tractus solitarii (nTS) microinjection of artificial cerebral spinal fluid (aCSF).
EAAT blockade potentiates cardiorespiratory responses to nTS microinjection of glutamate.
To determine whether EAAT inhibition in the nTS augments autonomic and cardiorespiratory responses to glutamate, we microinjected glutamate (30 nl, 3–5 mM) before and after administration of the EAAT inhibitor TBOA (1 mM) or, as a control, after similar volumes of aCSF. As indicated in the representative recording from one rat (Fig. 6A), nTS glutamate produced a rapid decrease in MAP, HR, and SSNA and inhibited PhrNA (Fig. 6A, left). These responses were augmented or prolonged by TBOA injection (Fig. 6A, right). Group data showing the time course of the response to glutamate are displayed in Fig. 6B. Because recovery of PhrNA and SSNA following TBOA injection was not complete in all animals, responses are shown as a percentage of baseline (before glutamate injection). Under control conditions (open symbols) glutamate produced depressor, bradycardic, and sympathoinhibitory responses, and decreased PhrNA, effects that returned toward control values within 15–20 s. Responses to glutamate were similar following injection of aCSF (blue squares). Five minutes after administration of TBOA (red circles), glutamate produced responses that were prolonged and significantly greater, particularly at the later time points in the response. Because the effects of glutamate were more sustained following EAAT inhibition, we examined the overall response by integrating the changes with respect to time (20 s following glutamate injection). As shown in Fig. 7, the integrated changes in MAP, HR, Phr frequency, Phr amplitude, and Min PhrNA were all significantly augmented by EAAT inhibition, and there was a nonsignificant augmentation of SSNA. Integrated responses following administration of aCSF were not altered compared with control (data not shown).
Fig. 6.
EAAT inhibition augments cardiorespiratory responses to glutamate. A: original recordings from 1 rat showing the response to nTS glutamate (Glu) microinjection under control (pre-TBOA) conditions and 5 min after (post) microinjection of TBOA. B: group data showing time course of the response to glutamate (at time = 0) before (pre; open symbols) and 5 min after (post) injection of TBOA (red circles) or aCSF (blue squares). After EAAT inhibition, cardiorespiratory responses were augmented and/or prolonged. *P < 0.05, post-TBOA compared with pre-TBOA.
Fig. 7.
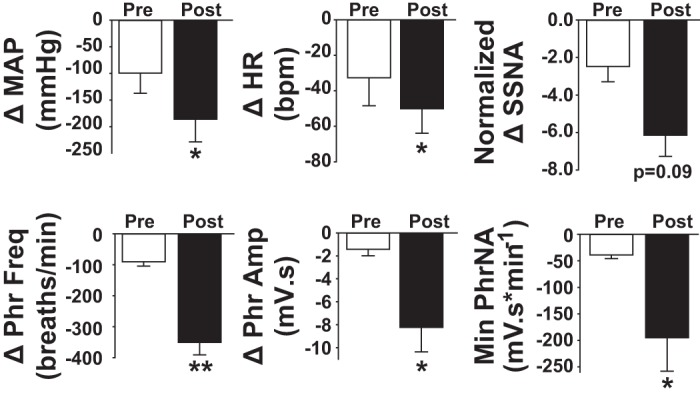
EAAT inhibition enhances the overall cardiorespiratory response to glutamate. Overall (area under the curve, expressed as total change over 20 s) change in cardiorespiratory variables due to nTS microinjection of glutamate under control (pre-TBOA, open bars) conditions and 5 min after nTS injection of TBOA (post-TBOA, closed bars). *P < 0.05, relative to control (pre-TBOA) response.
DISCUSSION
In the present study, we confirm the location of EAATs in the nTS and demonstrate that EAAT inhibition increased excitation and modulated cardiorespiratory function. EAAT block in vitro produced an inward depolarizing current, depolarized Vm, increased RMS noise, and elevated AP discharge in second-order nTS neurons. These effects were reversible and concentration related. EAAT block also decreased the amplitude of sensory afferent-evoked EPSCs yet increased the frequency of spontaneous, network-driven synaptic currents. Consistent with increased nTS excitation, EAAT block in vivo induced depressor, bradycardic, sympathoinhibitory, and apneic responses. TBOA effects were mimicked by exogenous glutamate, and glutamate responses were enhanced by EAAT inhibition. These results demonstrate nTS EAATs tonically modulate synaptic, neuronal, and cardiorespiratory function.
Glutamate clearance in the CNS occurs via EAATs, primarily EAAT 1 and 2, which are localized in astrocytes (Anderson and Swanson 2000). Immunohistochemistry showed that both EAAT 1 and 2 were present in the caudal nTS, consistent with other studies (Chounlamountry and Kessler 2011). Furthermore, both EAATs were present in astrocytes, as indicated by colabeling with the astrocyte markers S100B and GFAP and were more evident in astrocyte processes. There was no EAAT 1 or 2 found within neurons as shown by NeuN. Thus, similar to other brain regions (Rothstein et al. 1994, 1996; Chaudhry et al. 1995), EAAT 1 and 2 within the nTS are primarily found in glia.
EAAT blockade induced nTS neuronal excitation, indicated by an inward (depolarizing) tonic current, membrane depolarization, increased RMS noise, and enhanced action potential discharge. EAATs maintain the basal level of glutamate within the extracellular space (Anderson and Swanson 2000; Danbolt 2001); their block rapidly elevates glutamate concentration (Jabaudon et al. 1999, 2000). As in other brain regions (Potapenko et al. 2012), TBOA-induced excitation of nTS neurons likely results from activation of somal extrasynaptic receptors that are normally not active when EAATs are functional. Upon block of EAATs, these unbound receptors are activated and increase background conductance to excite neurons. The elevation in RMS noise, indicative of background stochastic membrane activity (Traynelis and Jaramillo 1998; Fleming et al. 2011), further supports increased activation of somal receptors following EAAT inhibition. TBOA augmented AP discharge in the majority of nTS neurons likely due to the observed depolarization and increased network activity, similar to that seen in the locus coeruleus (Medrano et al. 2013).
Astrocyte processes are dynamic (Hirrlinger et al. 2004; Rodnight and Gottfried 2013), contain EAATs (Fig. 1; see also Zhou and Sutherland 2004; Murphy-Royal et al. 2015), and partially or completely ensheathe nTS synapses (Chounlamountry and Kessler 2011). Thus astrocytic EAATs within the nTS likely shape and modify phasic glutamatergic signaling, including circuit-driven events. For example, in spinal sensory (Nie et al. 2010) and neocortex (Campbell and Hablitz 2004) synapses, EAAT blockers enhance spontaneous currents. Consistent with this, we observed that EAAT inhibition augmented the frequency of network-driven sEPSCs. This increase in sEPSC frequency is likely due to TBOA-induced depolarization of neurons within the nTS network resulting in an increase in glutamate release. Inhibition of EAATs did not alter sEPSC amplitude or decay times, indicating neither quantal size nor postsynaptic glutamate receptor conductance or sensitivity was altered.
In contrast to spontaneous currents, sensory afferent (TS)-EPSC amplitude decreased following EAAT blockade. This was unexpected given that TBOA likely elevated extracellular glutamate, which would activate postsynaptic receptors. Several mechanisms could account for this observation. Excess glutamate may activate metabotropic (Glaum and Miller 1993; Chen et al. 2002) or NMDA (Ohi et al. 2015) glutamate receptors on the TS terminal to inhibit TS-EPSCs. Distinct presynaptic effects could be mediated by differentially modifying the distinct vesicle pools responsible for TS-evoked and spontaneous synaptic currents (Kline et al. 2007; Fawley et al. 2014, 2015; Hofmann and Andresen 2015). Activation of metabotropic receptors on nearby GABAergic terminals may also inhibit afferent glutamate release through GABA acting on GABAB receptors on the TS (Fernandes et al. 2011). Blockade of hippocampal glutamate transporters activates inhibitory interneurons via metabotropic glutamate receptors (Huang et al. 2004). Postsynaptic desensitization of glutamate receptors potentially could account for decreased TS-EPSC amplitude; however, this mechanism is not supported because spontaneous (low quantal) EPSC amplitudes and decay times were not altered by EAAT blockade. Additionally, responses to exogenous glutamate in vivo were augmented following TBOA, which further argues against receptor desensitization. Future experiments will be required to determine the exact mechanism that decreased the current amplitude.
Also in contrast to spontaneous EPSCs, block of glutamate transporters prolonged afferent-evoked (TS) multiquantal EPSC decay in the nTS, as is also observed with cerebellar parallel fiber-Purkinje cell (Barbour et al. 1994) and mossy fiber-unipolar brush cell (Kinney et al. 1997) synapses. EPSC decay is governed, in part, by the affinity of the receptor for glutamate, and the rate of its removal by diffusion and uptake (Otis et al. 1996). Therefore, block of glutamate uptake likely prolongs receptor activation and subsequently enhances cardiorespiratory responses by increasing or maintaining glutamate concentration, reducing its diffusion away from the cleft, and promoting continued binding at the receptor. The lack of effect on decay time in spontaneous EPSCs may be due to the smaller neurotransmitter release compared with afferent-evoked EPSCs.
Despite reduced afferent-driven EPSC amplitude, EAAT block overall increased network currents and depolarized neurons. The latter events contribute predominantly to increased neuronal nTS discharge indicating an increase in excitatory drive within the nTS. Depolarization also likely relieves neurons, specifically via postsynaptic NMDA receptors (Zhao et al. 2015), of voltage-dependent block. We suggest that the net effect of EAAT blockade is to increase nTS network excitability, which then induces the depressor response, sympathoinhibition and apnea we observed in vivo.
The nTS is critical in basal and reflex cardiorespiratory regulation. Visceral afferents, including afferents for lung stretch, arterial and cardiopulmonary baroreceptors, and peripheral chemoreceptors, project to the caudal nTS (Haxhiu et al. 2005; Kubin et al. 2006; Travagli et al. 2006; Grill and Hayes 2009; Accorsi-Mendonça and Machado 2013). Glutamate signaling in nTS contributes importantly to cardiorespiratory function. Visceral afferents release glutamate as their primary excitatory neurotransmitter (Andresen and Kunze 1994) to produce cardiorespiratory reflex effects and activation of both ionotropic and metabotropic glutamate receptors influences autonomic outputs and breathing (Foley et al. 1999; Burton and Kazemi 2000). Blockade of glutamate transporters with TBOA produced depressor responses, bradycardia, sympathoinhibition, and inhibition of PhrNA. These effects likely are due to increasing endogenous nTS glutamate as a result of reduced uptake with subsequent activation of baroreflex and other reflex pathways such as the Hering-Breuer reflex. This concept is supported by our results showing that TBOA mimics responses to nTS microinjection of glutamate (Fig. 3 and see Bonham and McCrimmon 1990; Vardhan et al. 1993a,b; Foley et al. 1999; Machado 2001; Mueller et al. 2005). Thus these data suggest that EAATs in the nTS tonically take up sufficient glutamate in vivo to influence basal autonomic and respiratory function.
EAAT blockade also augmented and/or prolonged the cardiovascular, autonomic, and respiratory responses to nTS glutamate microinjection. Glutamate alone nearly eliminated sympathetic nerve activity, so it cannot be determined if TBOA enhanced the maximum sympathoinhibitory effect of glutamate. Nevertheless, inhibition of sympathetic and phrenic nerve activity and depressor responses was prolonged or enhanced by TBOA, and the overall integrated responses (Fig. 7) were augmented. Interestingly, responses to glutamate were greater even at a time when the cardiorespiratory effects of EAAT blockade had largely recovered. This suggests that, although output function (MAP, HR, SSNA, and PhrNA) had recovered, residual EAAT blockade remained or there were longer lasting downstream effects that accounted for subsequent enhancement of glutamatergic signaling. Alternatively, mechanisms independent of EAAT blockade could account for the functional recovery. Regardless of the mechanisms for recovery, responses to subsequent glutamate application were enhanced, and this enhancement may have been even greater if glutamate had been applied at an earlier time. Augmented responses to glutamate are in agreement with our in vitro experiments indicating increased excitability of nTS neurons in association with depolarization and augmented action potential discharge following EAAT block. Furthermore, as indicated above, glutamate receptor desensitization does not predominate after EAAT blockade since responses to glutamate were prolonged following TBOA. Together, the data suggest that inhibiting glutamate uptake prolonged synaptic and extrasynaptic nTS glutamate receptor activation, increasing neuronal activity and thus influencing cardiorespiratory function.
This study used the general EAAT antagonist TBOA to block all EAATs present within the nTS (EAAT 1, 2, and 3). EAAT 3 is located primarily on nTS dendrites but not at the synapse and may function to import cysteine rather than participating in glutamate clearance (Chounlamountry et al. 2014). This is supported by a lack of nTS neuronal EAAT transport of a glutamate analog whereas astroglia exhibit EAAT currents that were attenuated by TBOA (Huda et al. 2013). Postsynaptic neuronal glutamate transport has not been shown to be significant in the CNS outside of the cerebellum (Bergles and Jahr 1997; Bergles et al. 1999). Thus most of the effects observed in this study were likely due to EAAT 1 and 2. In most regions of the brain, including the nTS, EAAT 2 is the predominant EAAT subtype in both expression and function (Otis and Kavanaugh 2000; Danbolt 2001; Chounlamountry and Kessler 2011). TBOA has a greater affinity for EAAT2 than EAAT 1 (Shimamoto et al. 1998; Anderson et al. 2001). Therefore we suggest that most of the effects observed in our study were due to EAAT 2.
In summary, our data show that uptake of glutamate through EAATs tonically limits nTS synaptic and neuronal excitability and thus modulates cardiorespiratory function. This is most likely due to astrocytic EAATs. Although studies have shown that nTS astrocytes modulate synaptic and neuronal function via release of gliotransmitters (Hermann and Rogers 2009; McDougal et al. 2011; Accorsi-Mendonça et al. 2015), the overall role of astrocytes in cardiorespiratory function is not completely understood (Accorsi-Mendonça et al. 2013; Costa et al. 2013; Lin et al. 2013). Our data demonstrate that nTS EAATs are important in maintaining basal function of both the nTS network and the cardiorespiratory system as a whole. Furthermore, by limiting neuronal excitation they may also play a critical role in preventing excitotoxicity.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant R01-HL-098602 (to D. D. Kline and E. M. Hasser) and American Heart Association Grant 15GRNT25710159 (to D. D. Kline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P.M., E.M.H., and D.D.K. conception and design of research; M.P.M. and B.C.R. performed experiments; M.P.M., B.C.R., E.M.H., and D.D.K. analyzed data; M.P.M., B.C.R., E.M.H., and D.D.K. interpreted results of experiments; M.P.M. and B.C.R. prepared figures; M.P.M., E.M.H., and D.D.K. drafted manuscript; M.P.M., B.C.R., E.M.H., and D.D.K. edited and revised manuscript; M.P.M., B.C.R., E.M.H., and D.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Heather A. Dantzler and Sarah A. Friskey for technical expertise.
REFERENCES
- Accorsi-Mendonça D, Almado CE, Bonagamba LG, Castania JA, Moraes DJ, Machado BH. Enhanced firing in NTS induced by short-term sustained hypoxia is modulated by glia-neuron interaction. J Neurosci 35: 6903–6917, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi-Mendonça D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton Neurosci Basic Clin 175: 3–8, 2013. [DOI] [PubMed] [Google Scholar]
- Accorsi-Mendonça D, Zoccal DB, Bonagamba LG, Machado BH. Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats. Physiol Rep 1: e00080, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Bridges RJ, Chamberlin AR, Shimamoto K, Yasuda-Kamatani Y, Swanson RA. Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J Neurochem 79: 1207–1216, 2001. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32: 1–14, 2000. [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215, 1999. [DOI] [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron 12: 1331–1343, 1994. [DOI] [PubMed] [Google Scholar]
- Baude A, Strube C, Tell F, Kessler JP. Glutamatergic neurotransmission in the nucleus tractus solitarii: structural and functional characteristics. J Chem Neuroanat 38: 145–153, 2009. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 9: 293–298, 1999. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19: 1297–1308, 1997. [DOI] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol 427: 261–280, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VA, Antunes VR, Machado BH. Autonomic and respiratory responses to microinjection of l-glutamate into the commissural subnucleus of the NTS in the working heart-brainstem preparation of the rat. Brain Res 1093: 150–160, 2006. [DOI] [PubMed] [Google Scholar]
- Burton MD, Kazemi H. Neurotransmitters in central respiratory control. Respir Physiol 122: 111–121, 2000. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Hablitz JJ. Glutamate transporters regulate excitability in local networks in rat neocortex. Neuroscience 127: 625–635, 2004. [DOI] [PubMed] [Google Scholar]
- Campiani G, Fattorusso C, De Angelis M, Catalanotti B, Butini S, Fattorusso R, Fiorini I, Nacci V, Novellino E. Neuronal high-affinity sodium-dependent glutamate transporters (EAATs): targets for the development of novel therapeutics against neurodegenerative diseases. Curr Pharm Des 9: 599–625, 2003. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15: 711–720, 1995. [DOI] [PubMed] [Google Scholar]
- Chen CY, Ling Eh E, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol 538: 773–786, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Guo SZ, Lipton AJ, Gozal D. Domoic acid lesions in nucleus of the solitary tract: time-dependent recovery of hypoxic ventilatory response and peripheral afferent axonal plasticity. J Neurosci 22: 3215–3226, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chounlamountry K, Castets F, Tell F, Kessler JP. The excitatory amino acid carrier 1 (EAAC1) in the rat nucleus of the solitary tract: subcellular localization suggests no major role in glutamate clearance. Brain Struct Funct 2014. Dec 11 [Epub ahead of print]. [DOI] [PubMed]
- Chounlamountry K, Kessler JP. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: Comparison between single synapses and multisynaptic arrangements. Glia 59: 655–663, 2011. [DOI] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci 31: 12318–12329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KM, Moraes DJ, Machado BH. Acute inhibition of glial cells in the NTS does not affect respiratory and sympathetic activities in rats exposed to chronic intermittent hypoxia. Brain Res 1496: 36–48, 2013. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol 65: 1–105, 2001. [DOI] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Andresen MC. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J Neurosci 34: 8324–8332, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Largent-Milnes TM, Andresen MC. Temperature differentially facilitates spontaneous but not evoked glutamate release from cranial visceral primary afferents. PLoS One 10: e0127764, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes LG, Jin YH, Andresen MC. Heterosynaptic crosstalk: GABA-glutamate metabotropic receptors interactively control glutamate release in solitary tract nucleus. Neuroscience 174: 1–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol 589: 3929–3941, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley CM, Moffitt JA, Hay M, Hasser EM. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol 275: R1858–R1866, 1998. [DOI] [PubMed] [Google Scholar]
- Foley CM, Vogl HW, Mueller PJ, Hay M, Hasser EM. Cardiovascular response to group I metabotropic glutamate receptor activation in NTS. Am J Physiol Regul Integr Comp Physiol 276: R1469–R1478, 1999. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol 70: 2669–2672, 1993. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 33, Suppl 1: S11–S15, 2009. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13: 54–63, 2007. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57: 343–346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Kc P, Moore CT, Acquah SS, Wilson CG, Zaidi SI, Massari VJ, Ferguson DG. Brain stem excitatory and inhibitory signaling pathways regulating bronchoconstrictive responses. J Appl Physiol 98: 1961–1982, 2005. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: implications for autonomic control. Brain Res 1273: 72–82, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger J, Hülsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci 20: 2235–2239, 2004. [DOI] [PubMed] [Google Scholar]
- Hofmann ME, Andresen MC. Vanilloids selectively sensitize thermal glutamate release from TRPV1 expressing solitary tract afferents. Neuropharmacology 101: 401–411, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci 24: 4551–4559, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, McCrimmon DR, Martina M. pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 110: 368–377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, Scanziani M, Gähwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci USA 97: 5610–5615, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA 96: 8733–8738, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 302: R1219–R1232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GA, Overstreet LS, Slater NT. Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells. J Neurophysiol 78: 1320–1333, 1997. [DOI] [PubMed] [Google Scholar]
- Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience 167: 510–527, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27: 4663–4673, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Moore SA, Jones SY, McGlashon J, Talman WT. Astrocytes in the Rat Nucleus Tractus Solitarii Are Critical for Cardiovascular Reflex Control. J Neurosci 33: 18608–18617, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci 940: 179–196, 2001. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14037–14045, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano MC, Gerrikagoitia I, Martínez-Millán L, Mendiguren A, Pineda J. Functional and morphological characterization of glutamate transporters in the rat locus coeruleus. Br J Pharmacol 169: 1781–1794, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PJ, Foley CM, Vogl HW, Hay M, Hasser EM. Cardiovascular response to a group III mGluR agonist in NTS requires NMDA receptors. Am J Physiol Regul Integr Comp Physiol 289: R198–R208, 2005. [DOI] [PubMed] [Google Scholar]
- Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006. [DOI] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci 18: 219–226, 2015. [DOI] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Bidirectional neuron-glia interactions triggered by deficiency of glutamate uptake at spinal sensory synapses. J Neurophysiol 104: 713–725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Kimura S, Haji A. Modulation of glutamatergic transmission by presynaptic N-methyl-d-aspartate mechanisms in second-order neurons of the rat nucleus tractus solitarius. Neurosci Lett 587: 62–67, 2015. [DOI] [PubMed] [Google Scholar]
- Oldenziel WH, Dijkstra G, Cremers TI, Westerink BH. In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res 1118: 34–42, 2006. [DOI] [PubMed] [Google Scholar]
- Ostrowski TD, Ostrowski D, Hasser EM, Kline DD. Depressed GABA and glutamate synaptic signaling by 5-HT1A receptors in the nucleus tractus solitarii and their role in cardiorespiratory function. J Neurophysiol 111: 2493–2504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci 20: 2749–2757, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci 16: 1634–1644, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32: 421–431, 2009. [DOI] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303: R291–R300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnight RB, Gottfried C. Morphological plasticity of rodent astroglia. J Neurochem 124: 263–275, 2013. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron 13: 713–725, 1994. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev 45: 250–265, 2004. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Shimamoto K, Yasuda-Kamatani Y, Seal RP, Yumoto N, Nakajima T, Amara SG. Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5). J Neurochem 79: 297–302, 2001. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol 53: 195–201, 1998. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for l-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209: 813–815, 1980. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Acute hypertension after the local injection of kainic acid into the nucleus tractus solitarii of rats. Circ Res 48: 292–298, 1981. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Jaramillo F. Getting the most out of noise in the central nervous system. Trends Neurosci 21: 137–145, 1998. [DOI] [PubMed] [Google Scholar]
- Vance KM, Rogers RC, Hermann GE. PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. J Neurosci 35: 776–785, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol 264: R41–R50, 1993a. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in the nucleus tractus solitarius mediate the responses to the stimulation of cardio-pulmonary vagal afferent C fiber endings. Brain Res 618: 23–31, 1993b. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol 511: 733–745, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Peters JH, Zhu M, Page SJ, Ritter RC, Appleyard SM. Frequency-dependent facilitation of synaptic throughput via postsynaptic NMDA receptors in the nucleus of the solitary tract. J Physiol 593: 111–125, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci 24: 6301–6306, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]