Abstract
Images that move rapidly across the retina of the human eye blur because the retina has sluggish temporal dynamics. Voluntary smooth pursuit eye movements are modeled as matching object velocity to minimize retinal motion and prevent retinal blurring. However, “catch-up” saccades that are ubiquitous during pursuit interrupt it and disrupt clear vision. But catch-up saccades may not be a common feature of ocular pursuit, because their existence has been documented with a small moving spot, the classic pursuit stimulus, which is a weak motion stimulus that may poorly emulate larger pursuit objects. We found that spot pursuit does not generalize to that of larger objects. Observers pursued a spot or a larger virtual object with or without a superimposed spot target. Single-spot targets produced lower pursuit acceleration than larger objects. Critically, more saccadic intrusions occurred when stimuli had a central dot, even when position and velocity errors were equated, suggesting that catch-up saccades result from pursuing a single, small object or a feature on a large one. To determine what differentiates a large object from a small one, we progressively shrank the featureless virtual object and found that catch-up saccade frequency was highest when it fit in the fovea. The results suggest that pursuit of a small target or an object feature recruits a saccade mechanism that does not compensate for a weak motion signal; rather, the target compels foveation. Furthermore, catch-up saccades are likely generated by neural circuitry typically used to foveate small objects or features.
Keywords: smooth pursuit, catch-up saccades, fovea, saccade
the smooth pursuit eye movement system is used to view moving objects, presumably to improve image clarity by minimizing the blur that occurs when retinal motion is excessive (Barmack 1970; Westheimer and McKee 1975). Prevailing models of pursuit operate to stabilize retinal motion by smoothly matching eye velocity to that of a moving target (Krauzlis and Lisberger 1989; Robinson et al. 1986). However, catch-up saccades, which are rapid, jerky eye movements that interrupt the smooth response, are not captured by the models. This seems strange, because catch-up saccades are ubiquitous during ocular pursuit, occurring several times a second (Jin et al. 2014). Despite this, both the models and the bulk of pursuit research ignore catch-up saccades, as they are routinely excised from pursuit records and excluded from subsequent analysis. This omission is even more puzzling given evidence that saccadic intrusions may be an integral part of the pursuit response, as they are thought to boost pursuit gain (Lisberger 1998) and correct errors between the target and the eye for which the smooth component of pursuit fails to compensate (de Brouwer et al. 2002). Yet if the goal of pursuit is to minimize image motion for clear vision, the prevalence of catch-up saccades is perplexing, as they exacerbate retinal image motion and likely suppress image visibility, given that suppression occurs for saccades to static objects (Zuber and Stark 1966).
It is possible that the frequent occurrence of catch-up saccades is a function of the small spot (usually <1°) used to study smooth pursuit and that they do not occur as often during pursuit of larger objects, such as a dog running through the bedroom (∼5–20° depending on the breed). This is because the size of the spot makes it a poor motion stimulus to drive pursuit, as small stimuli produce weak motion signals in area MT (Britten and Heuer 1999), the primary motion processing region in the brain, which directly feeds the pursuit system (Dursteler and Wurtz 1988; Komatsu and Wurtz 1988). Catch-up saccades might therefore supplement the weak motion drive. In support of this argument, higher pursuit gain during pursuit of large (10°) random-dot stimuli is accompanied by a significant reduction in catch-up saccades (Heinen and Watamaniuk 1998; Watamaniuk and Heinen 1999). Alternatively, the spot might create demands on the pursuit system different from those the models predict: namely, the system might be compelled to foveate a small target rather than match its velocity. Catch-up saccades might then be used for foveation, analogous to how volitional saccades bring the fovea to objects of interest.
In the present study, we tested between these alternatives with simple stimuli that covaried in their motion information content and the presence or absence of a central target. Pursuit stimuli were a single central dot, four peripheral dots arranged as the vertices of a virtual diamond, or a composite of these stimuli, with both central and peripheral elements. Pursuit acceleration increased with the number of dots, consistent with a stronger motion signal boosting the pursuit response. However, catch-up saccades were more frequent for stimuli with central targets, regardless of stimulus size, even though all of our stimuli produce the same retinal velocity and position errors when moving at the same speed with respect to the eye and at the same distance from the fovea. Furthermore, fewer saccades occurred for stimuli without central targets despite the fact that the velocity and position errors reached the same magnitudes that elicited catch-up saccades when central targets were present. Finally, when the size of the virtual diamond was manipulated, saccade frequency was highest when the diamond was smaller than the fovea, indicating that the oculomotor system differentiates between large and small objects based on whether they fit in the fovea. The results suggest that ocular pursuit operates to match the velocity of large moving objects but attempts to foveate small objects or features, and recruits saccade circuitry to do so.
MATERIALS AND METHODS
Subjects.
Five healthy human observers (3 men, 2 women), three naive to the purpose of the study, participated in the experiments. All had normal or corrected to normal vision and were 24–50 yr old. All experimental protocols were approved by the Smith-Kettlewell Institutional Review Board, and all observers gave informed consent before participating.
Apparatus and stimuli.
Visual stimuli were generated with functions from PsychToolbox (Brainard 1997; Pelli 1997) in MATLAB on a Macintosh G4 computer and were presented on a 17-in. high-resolution Nanao color monitor (1.76 min arc/pixel) at a rate of 60 Hz. Horizontal and vertical eye position was sampled at 1,000 Hz by an EyeLink 1000 video-based eye tracker (SR Research, Mississauga, ON, Canada). The EyeLink was calibrated and validated with the standard nine-point method included with the system. Observers used a chin and forehead rest to stabilize the head and maintain a constant viewing distance of 48 cm.
Stimuli and experimental procedure.
In one experiment, stimuli were 1, 4, or 5 dots (0.2° in diameter, luminance 2.63 cd/m2) presented on a dark background (luminance 0.3 cd/m2). The 4- and 5-dot stimuli were arranged in a diamond configuration (Fig. 1A), with a radius of 3°. The radius of the 4-dot stimulus was manipulated in a second experiment and varied between 0.5°, 1°, 3°, and 6° (Fig. 1B). In each trial, a fixation spot appeared at the left side of the screen for a randomly variable fixation period (500-1,000 ms), after which the full stimulus appeared at the fixation point location and translated from left to right at a randomly selected constant velocity of 10, 20, or 30°/s. Observers followed the stimulus with their eyes while maintaining their gaze at the center of the stimulus (see Fig. 4). Participants controlled the pace of the experiment by pressing the “Enter” key to initiate each trial. To avoid predictive effects, all stimuli (1-, 4-, and 5-dot and 4-dot stimuli of different radii) appeared in a pseudorandom order within a block of trials. All permutations of dot configuration and speed appeared an equal number of times within a block. Each observer performed two blocks of 210 trials each. Note that all data were pooled across speeds unless otherwise stated, as with individual eye traces.
Fig. 1.
Stimulus configurations. A: experiment 1: stimuli varied in number and distribution of dots. Pursuit stimuli were either a single dot, 4 dots arranged in a diamond configuration, or a 5-dot stimulus in a diamond configuration with a central dot. Configurations were randomized within a block. A trial began when a fixation spot appeared at the left of the screen. After a random fixation period (0.5–1 s), the full stimulus appeared, centered on the fixation spot location, and moved rightward at 10, 20, or 30°/s. B: experiment 2: possible sizes of the 4-dot stimulus.
Fig. 4.
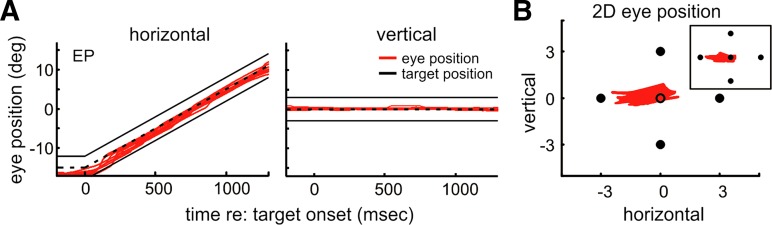
Eye position during pursuit of the 4-dot stimulus. A: raw horizontal (left) and vertical (right) eye position traces (red lines) for a representative observer pursuing the 4-dot stimulus. Ten random trials are shown. Solid black lines indicate dot positions, and dashed line indicates center position of the stimulus. B: 2D eye position superimposed on the stimulus for the same trials as in A indicate that gaze was maintained centrally on the target. Inset: 2D eye position during pursuit of the 5-dot stimulus for comparison. Note that the central circle in the main image shows the centroid of the 4-dot stimulus and was not present in the stimulus.
Eye movement analysis.
Horizontal and vertical eye velocities were calculated off-line from the recorded eye position signals by differentiating and filtering the raw position data (2-pole Butterworth noncausal filter, cutoff = 50 Hz). Saccades were detected off-line with an eye velocity threshold of 50°/s. Missed and false positive saccades were corrected manually. Saccades were removed from the velocity trace, and adjoining points were interpolated. Pursuit onset was first detected automatically with interpolated mean velocity and variance thresholds of 5°/s and was subsequently inspected visually and adjusted manually when needed. Eye acceleration was computed by differentiating and filtering the eye velocity data (2-pole Butterworth noncausal filter, cutoff = 10 Hz), and the maximum acceleration between 50 and 130 ms after pursuit onset was used to characterize peak open-loop acceleration. We used peak open-loop acceleration as our main measure of the smooth ocular response, as in our previous studies (e.g., Heinen and Watamaniuk 1998). Steady-state pursuit gain was computed by dividing average eye velocity, 500-2,700 ms after stimulus motion onset, by stimulus velocity. Anticipatory pursuit velocity was computed by calculating the mean velocity from 50 ms before to 50 ms after target onset. All statistical tests used an α level of 0.05.
Catch-up saccade analysis.
Catch-up saccades during pursuit initiation were analyzed according to the method of de Brouwer et al. (2002). Position error (PE) and retinal slip (RS) were calculated 125 ms before saccade onset. On trials in which saccades did not occur, average PE and RS were calculated from shifting a 50-ms sliding window in 25-s increments along a 130-ms (length of the open-loop period) window starting 125 ms prior to pursuit onset. This is approximately the temporal interval during which PE and RS would generate a saccade in the open-loop period. PE was computed as the difference between target and eye position at that time and RS as the difference between target and eye velocity. For multidot targets, target position was calculated as the centroid of all dot positions. The proportion of times a saccade occurred for a given ratio of PE magnitude to RS magnitude (PE/RS) was determined by dividing the number of saccade-generating trials by the total number of trials in which that PE/RS combination occurred. By the convention of de Brouwer et al. (2002), the PE/RS at which the proportion of saccade-generating trials for all stimuli dropped significantly below 50% (PE/RS = 0.5, 26.47% saccade trials, P = 0.0043, 1-sample t-test) defined the boundary of the “RS saccade zone” and was used to determine the slopes of the diagonal lines in Fig. 3A with the equation m = y/x, where m is the slope, y is the RS, and x is the PE. Note that we modified our saccade analysis from that of de Brouwer et al. (2002) in one key way: we used a simple ratio of the magnitudes of PE to RS instead of eye crossing time (TXE), because TXE confounds the relative contributions of position and velocity error.
Fig. 3.
Analysis of catch-up saccades in the open-loop period. A: open-loop saccades (colored dots) plotted as a combination of absolute position error (PE) and retinal slip (RS) for the 1-, 4-, and 5-dot stimuli. Gray dots represent position error and retinal slip on non-saccade-generating trials. Points above the line, defined by the equation RS = 2.22 × PE, fall within the “retinal slip saccade zone.” B: relative % of trials generating open-loop saccades for a given ratio of position error to retinal slip (PE/RS), plotted separately for 1-, 4-, and 5-dot stimuli. Dashed line represents the boundary of the retinal slip saccade zone and represents the PE/RS at which the % of saccade trials for all stimuli combined drops significantly below 50% (PE/RS = 0.45). To aid comparison, the orange outline appearing in the middle and bottom panels shows the saccade data during pursuit of the 1-dot stimulus. C: proportion of saccades that occur outside the retinal slip saccade zone for the 1-, 4-, and 5-dot stimuli. Error bars indicate SE.
RESULTS
Catch-up saccades do not depend on stimulus motion.
Large random-dot cinematogram (RDC) stimuli have been shown to increase pursuit gain and reduce the frequency of saccadic intrusions (Heinen and Watamaniuk 1998; Watamaniuk and Heinen 1999). A simple hypothesis as to why large RDCs produce fewer catch-up saccades is that the motion signals generated by individual dots spatially summate to produce a stronger integrated motion signal, which boosts smooth pursuit gain and thereby renders catch-up saccades less necessary to assist in following the target (Fig. 2A). To test this hypothesis, observers pursued a single dot, 4 peripheral dots arranged as the vertices of a virtual diamond (3° radius), or a 5-dot conglomerate of the 1- and 4-dot stimuli (see Fig. 1A). Consistent with the summation hypothesis, initial smooth eye acceleration increased with the number of dots (Fig. 2B). However, catch-up saccades did not follow the predicted trend. While saccade frequency was lower with 4 dots than with 1, it increased again during pursuit of 5 dots (Fig. 2C). Figure 2D summarizes peak acceleration and catch-up saccade frequency during the open-loop period. While peak acceleration as a function of dot number conformed to the summation hypothesis, catch-up saccade frequency did not. Peak open-loop acceleration increased with dot number over all observers [1-way repeated-measures ANOVA: F(2,8) = 19.14, P = 0.0009]. However, the 4-dot stimulus produced fewer saccades than the 1- and 5-dot stimuli during the open-loop period (2-sample t-test: 4 vs. 1 dot: t = −4.93, P < 0.0001; 4 vs. 5 dot: t = −5.18, P < 0.0001; 1 vs. 5 dot: t = 0.19, P = 0.85). The same trend was also evident during steady-state pursuit (not shown) (2-sample t-test: 4 vs. 1 dot: t = −4.30, P < 0.0001; 4 vs. 5 dot: t = −2.54, P = 0.01; 1 vs. 5 dot: t = −1.77, P = 0.07). Steady-state pursuit gain and pursuit latency were also analyzed but showed no significant differences between dot configurations [1-way repeated-measures ANOVA: steady-state gain: F(2,8) = 1.91, P = 0.21; latency: F(2,8) = 1.74, P = 0.24]. Note that the predictability of target direction produced a significant anticipatory response prior to pursuit onset (see Fig. 2B), which may have affected velocity and position errors, and therefore the peak acceleration and number of saccades, in the open-loop period. However, anticipatory velocity did not differ between stimulus conditions [1-way repeated-measures ANOVA: F(2,8) = 0.35, P = 0.71], indicating that differences in anticipatory pursuit cannot account for the differences seen in peak acceleration and catch-up saccades in the open-loop period.
Fig. 2.
Number of saccades and peak open-loop acceleration. A: summation hypothesis: if simple spatial summation of stimulus elements accounts for better pursuit performance on multidot stimuli, then smooth acceleration should increase as a function of dot number (dashed line). If higher initial acceleration reduces retinal slip and thereby the need for a catch-up saccade, then the number of saccades should decrease as a function of dot number (solid line). B: mean open-loop velocity traces for 1 observer (EP) pursuing a stimulus moving at 20°/s, with saccades removed, aligned on pursuit onset (note that the target began to move 80–100 ms before pursuit onset in this observer). Gray shaded area shows analysis period (50–130 ms after pursuit onset). Open-loop acceleration (slope of the trace) increases with dot number. C: raw horizontal eye velocity traces for 1 observer (GM) pursuing a stimulus moving at 20°/s (10 trials per condition). Saccade frequency is higher when a central dot is present. D: no. of saccades per second and peak acceleration in the open-loop (OL) period. Saccade frequency is lowest in the absence of a central stimulus, while acceleration increases with dot number. Peak acceleration follows the motion-summation hypothesis, but saccades appear to be dependent on the presence of a foveal element in the stimulus. Black markers show average data, while colored markers show individual observers. Error bars indicate SE.
Catch-up saccades are not triggered only by position and velocity errors.
We next attempted to determine why fewer catch-up saccades occurred during pursuit of the 4-dot stimulus. Catch-up saccades during spot pursuit are purportedly triggered by a mathematical combination of PE and RS (de Brouwer et al. 2002). It may be that the pursuit system was better able to follow the 4-dot stimulus and thus position and velocity errors necessary to trigger a saccade did not occur as frequently. To assess this, we characterized the combinations of PE and RS that preceded catch-up saccades for our three stimuli, as has been done during pursuit of a spot stimulus (de Brouwer et al. 2002). In Fig. 3A, we plot the combination of absolute PE and RS at the time when a catch-up saccade in the open-loop period would be generated (see materials and methods). The data show that most catch-up saccades are generated by errors that fall in the so-called “RS saccade zone,” where RS predominantly contributes to their genesis. For the 1- and 5-dot stimuli, quite a few other saccades fall outside the zone, where PE is relatively high. Saccades outside the zone are much rarer for the 4-dot stimulus, indicating that PE was a less potent saccade trigger in this case. Figure 3B shows the percentage of saccades generated by different ratios of PE to RS. The 1- and 5-dot stimuli produce similar catch-up saccade patterns. Although the 4-dot stimulus generates the same amount of PE and RS as the other stimuli [1-way repeated-measures ANOVA: PE: F(2,8) = 0.54, P = 0.6; RS: F(2,8) = 3.43, P = 0.08], the 4-dot stimulus produces not only a smaller percentage of saccades overall but also a smaller proportion of saccades outside the RS saccade zone (2-sample t-test: 4 vs. 1 dot: t = −2.32, P < 0.05; 4 vs. 5 dot: t = −2.14, P < 0.05; 1 vs. 5 dot: t = 0.29, P = 0.77) (Fig. 3C). Therefore, PEs sufficient to trigger catch-up saccades with the 1- and 5-dot stimuli do not trigger them with the 4-dot stimulus.
The eyes do not drift freely on featureless objects.
Another possible explanation for the paucity of saccades during pursuit of the 4-dot stimulus is that without a central spot there was no anchor to keep the eyes centered. Therefore, the eyes may have drifted freely within the 6° area of the stimulus, without the need for compensation from catch-up saccades. However, this was not the case, as gaze was maintained at a reasonably constant location on the 4-dot stimulus and was no more variable than it was during pursuit of the 5-dot stimulus. Figure 4 shows horizontal and vertical eye position as a function of time for a sample of trials from a representative observer pursuing a 4-dot stimulus and two-dimensional spatial eye position for the same 10 trials. The eyes remained aligned with the center of the stimulus throughout the trial, despite the absence of a central target. Gaze position during pursuit of the 4-dot stimulus remained precisely located across all observers, as confirmed by a one-way repeated-measures ANOVA showing no difference between the standard deviation of eye position during pursuit of the 4- and 5-dot stimuli [horizontal: F(1,4) = 0.84, P = 0.40; vertical: F(1,4) = 0.44, P = 0.53]. Moreover, a separate one-way repeated-measures ANOVA conducted on eye position error (eye position − stimulus center) showed that the eyes remained as equally centered during pursuit of the 4-dot and 5-dot stimuli [horizontal: F(1,4) = 1.75, P = 0.21; vertical: F(1,4) = 2.07, P = 0.19]. This is consistent with previous work showing that observers efficiently locate the centroids of dot clusters (Morgan and Glennerster 1991).
Small pursuit stimuli compel foveation.
We found that catch-up saccade frequency increased during pursuit of small targets. But what determines whether the pursuit system treats an object as large or small? To determine the size at which an object transitions from large to small, we incrementally varied the radius of the 4-dot stimulus from 6.0° to 0.5° (see Fig. 1B). Saccade frequency was low with both 6.0° and 3.0° stimuli but increased dramatically for stimuli with radii of 1.0° and 0.5° (Fig. 5). A one-way repeated-measures ANOVA shows that stimulus radius significantly impacted saccade frequency in both the open-loop [F(3,12) = 20.03, P < 0.0001] and steady-state [F(3,12) = 4.36, P = 0.027] periods. Interestingly, the transition from large to small occurs when a stimulus fits within the 2.0° rod-free fovea (Polyak 1941). Therefore, the mechanism generating catch-up saccades during pursuit of small stimuli appears to be linked to the fovea, and may be recruited to foveate a pursuit target. On the other hand, stimulus size did not have an effect on peak eye acceleration during the open-loop period [1-way repeated-measures ANOVA: F(3,12) = 1.21, P = 0.35], providing evidence that the mechanism driving smooth acceleration is insensitive to the retinal location stimulated and summates motion information equally from peripheral and foveal retina.
Fig. 5.
Foveal contribution to pursuit. Peak acceleration and number of saccades per second in the open-loop (OL) period plotted as a function of the stimulus radius (eccentricity of stimulation). The number of saccades per second decreases significantly with radius and is highest for stimuli that fit within the fovea, suggesting that the saccade trigger is dependent on the presence of a foveal target. Peak open-loop acceleration is not significantly affected by stimulus radius. Black markers show average data, while colored markers show individual observers. Error bars indicate SE.
DISCUSSION
Natural pursuit objects are often larger than the spot used in most laboratory pursuit studies. It could be that results gleaned from spot pursuit research generalize to larger stimuli either directly or in a linear fashion, i.e., the larger the object, the greater the pursuit response. Our results suggest that neither is true. In the present study, we characterized the ocular pursuit response to single-dot and multidot stimuli with or without foveal targets. As the number of dots increased, peak eye acceleration in the open-loop period increased, consistent with the activation of a mechanism that spatially integrates motion information to rapidly acquire a moving stimulus. In contrast, foveal targets, either alone or within a larger stimulus, increased the incidence of catch-up saccades, indicating that the amount of motion information, and thus the smooth velocity gain, did not predict saccade frequency. When we varied the diameter of the 4-dot stimulus peak acceleration was constant across all stimulus sizes, but catch-up saccade frequency was high only when the stimulus fit in the fovea. Our results are consistent with the idea that an isolated single spot is a weak motion stimulus and that motion information is summed across the retina to drive the smooth component of pursuit. Catch-up saccades, on the other hand, appear to be largely a consequence of foveating a small pursuit target, or a feature on a large pursuit object.
Previous work in other laboratories investigated pursuit of targets using peripheral retina. In one of the earliest studies, observers pursued illusory motion created by peripheral stimuli, demonstrating that the pursuit system can follow a motion percept that does not correspond to motion on the retina (Steinbach 1976). Another study tested pursuit of a single target in peripheral retina and found that it was pursued with gain that was almost as high as that attained during pursuit of a foveal target (Winterson and Steinman 1978). Like us, these authors concluded that pursuit was not purely a foveal behavior. In other work, observers pursued a pair of targets that flanked the fovea and demonstrated gain comparable to that produced by pursuit of a foveal target (Barnes and Hill 1984; Collewijn and Tamminga 1986). Another study found that pursuit of a pair of targets that flanked the fovea was superior to pursuit of a pair of targets whose virtual midpoint was positioned off to one side (Wyatt et al. 1994). This result suggests that the pursuit system prefers to keep objects centered on the fovea, even when peripheral retina is used. One study did find that targets that flanked the fovea but only stimulated peripheral retina produced lower initial acceleration than objects that also stimulated the fovea (Ilg and Their 1999). However, the stimulus used in that study, a large hourglass figure, stimulated substantially less total retina when it lacked the foveal component, consistent with our results showing that total motion information contributes to initial pursuit acceleration, regardless of foveal stimulation. The results of these previous studies are consistent with our finding that pursuit is not an exclusively foveal function.
The spot has been used for over 50 years to characterize smooth pursuit behavior, to chart its neural pathways (for reviews see Keller and Heinen 1991; Krauzlis 2004; Lisberger et al. 1987), and to construct pursuit system models (Krauzlis and Lisberger 1989; Robinson et al. 1986). A single spot was successful in studying saccades, another principal voluntary eye movement (e.g., see Liversedge and Findlay 2000; Wurtz and Goldberg 1989), but is it appropriate for studying ocular pursuit? Considering that the purpose of saccades is to orient the fovea to objects to resolve image detail, while the purpose of pursuit is to match eye velocity to that of a moving object (Krauzlis and Lisberger 1989; Robinson et al. 1986), it is questionable as to whether these different goals can be probed equally well by the same stimulus. We describe here a fundamentally different ocular response during pursuit of larger stimuli without a single, foveal pursuit target, namely, higher initial eye acceleration and fewer saccades. Our results suggest that the research using a spot may not fully generalize to pursuit of larger objects.
Our data violate current models of catch-up saccade generation, which posit that position and velocity error are the main triggers of catch-up saccades (de Brouwer et al. 2001, 2002). Catch-up saccades occur mostly at pursuit initiation, when the eye lags the target in both speed and position (Rashbass 1961), and are thought to compensate for sluggish dynamics of the pursuit system. However, our results imply that position and velocity error alone cannot account for saccade incidence, since saccade frequency varied significantly although these errors were comparable among the different stimuli. It appears that the composition of a pursuit stimulus, irrespective of position or velocity error, in part determines the frequency of catch-up saccades (see Fig. 3A). Specifically, while stimuli without foveal components trigger saccades only below a threshold of PE/RS = 0.45, targets with foveal elements seem to trigger catch-up saccades regardless of PE/RS. We think that foveal targets trigger catch-up saccades because attention is directed toward them, and attended objects can trigger saccades (Kowler et al. 1995). Evidence supporting our idea that attention is directed toward central pursuit targets is the finding that a single spot requires more attention to pursue than a large stimulus with predominantly peripheral elements (Heinen et al. 2011). However, we acknowledge that while attention may play a role in triggering catch-up saccades, the mechanism by which they are triggered is not fully characterized. Many factors likely contribute to catch-up saccade generation; for example, even verbal instructions can influence catch-up saccade frequency (Puckett and Steinman 1969).
Interestingly, while the fovea plays a special role in catch-up saccades during pursuit, peripheral and foveal stimulation appear to contribute equally to motion signals that drive smooth eye acceleration. This is surprising given the long-standing perception that peripheral retina has superior motion processing capabilities (Purkinje 1825). However, while some data support better flicker sensitivity in peripheral retina (Brown 1965; Tyler 1981), other data show that velocity discrimination, while varying across the retina, becomes homogeneous when corrected for the higher resolution of the fovea (McKee and Nakayama 1984), consistent with our results. Several other studies looked at smooth pursuit of stimuli that began moving at different retinal locations and found higher initial acceleration for foveal than peripheral stimuli (Lisberger and Westbrook 1985; Tychsen and Lisberger 1986), seemingly contradicting our results. However, in those studies, a single spot target was initially placed at a peripheral location before it began to move, creating position error for which observers had to compensate to acquire the target. In contrast, our single-dot and multidot stimuli were always centered on the fovea when they began to move, and peripheral elements remained peripheral throughout the trial. It could be argued that our peripheral 4-dot stimulus, which produces the fewest catch-up saccades, is a less natural pursuit stimulus than a spot. However, both practiced and naive observers pursued this stimulus easily, and as accurately and precisely as the other stimuli (see Fig. 4), whereas primates must be trained to pursue a single spot (Bourrelly et al. 2013; Heinen and Keller 1989). Pursuit of the 4-dot stimulus also showed higher open-loop acceleration than spot pursuit. We therefore feel that the 4-dot stimulus is a suitable surrogate for studying pursuit of larger natural objects because it creates a virtual object that lacks a single, prominent foveal target. When the foveating mechanism is released from driving pursuit, it could be used instead to inspect object features, even those requiring saccades directed opposite the motion of the pursuit object. Consistent with this idea, preliminary work from our laboratory shows that pursuit velocity is maintained during a saccade between two features on a large object but disrupted during an identical saccade from one target moving in isolation to another (Watamaniuk et al. 2015).
In the natural arena many moving objects are larger than the foveal spot, and our work suggests that pursuit of large objects elicits a different pattern of neural activation than pursuit of small ones. As a consequence, many structures that lie outside the classic motion pathways but are implicated in both spot pursuit and saccade generation may be little, if at all, involved in pursuit of large stimuli. These structures include the superior colliculus (SC) (Basso et al. 2000; Krauzlis et al. 2000), the frontal eye field (FEF) (MacAvoy et al. 1991), the supplementary eye field (SEF) (Heinen 1995), and possibly the vermis of the cerebellum (Suzuki and Keller 1988). While a common test to confirm that a saccadic structure is pursuit related is to show activation during saccade-free pursuit (e.g., Heinen 1995; Krauzlis et al. 2000), saccade structures might be activated during pursuit by position error that is subthreshold for generating catch-up saccades. Conversely, large stimuli may activate classic motion-processing structures to a greater degree than the spot does. These structures include the medial temporal (MT) and medial superior temporal (MST) cortical regions (Dursteler and Wurtz 1988; Komatsu and Wurtz 1988; Newsome et al. 1988), the paraflocculus of the cerebellum (Noda and Mikami 1986; Rambold et al. 2002), and, possibly, the nucleus of the optic tract (NOT) (Mustari and Fuchs 1990).
Smooth pursuit is impaired in many psychiatric and motor disorders (Leigh and Zee 2006), and oculomotor deficits often appear before the onset of other symptoms (Leigh and Zee 2006). A potential benefit of understanding normal pursuit system operation is that deficits in smooth pursuit performance could provide early diagnosis of disease in predisposed individuals, such as relatives of patients, or people with particular genetic markers, leading to early preventative treatment. In many psychiatric disorders, smooth pursuit gain is reduced and saccadic intrusions are frequent [e.g., schizophrenia (Abel et al. 1991; Karoumi et al. 2001), Parkinson's disease (Helmchen et al. 2012; Pinkhardt et al. 2009), and Alzheimer's disease (Zaccara et al. 1992)]. Our results could help interpret these deviations, and our stimuli could aid in more specific diagnosis of disorders that have differential effects on saccadic and smooth dynamics of ocular pursuit. For example, Alzheimer's disease may adversely affect motion processing, producing low smooth pursuit gain that leads to a necessary compensatory increase in catch-up saccades (Fletcher and Sharpe 1988). If this is true, then increasing stimulus size should decrease catch-up saccades. Alternatively, the disease process may leave the foveating system hyperactive, producing more saccades regardless of pursuit gain impairments. In this case, the difference in catch-up saccades between the 4-dot and the 1- and 5-dot stimuli should be exaggerated in the patient, regardless of stimulus size.
GRANTS
This work was funded by National Eye Institute Grant 1R01 EY-021286.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.H., E.P., and S.N.J.W. conception and design of research; S.J.H., E.P., and S.N.J.W. performed experiments; S.J.H., E.P., and S.N.J.W. analyzed data; S.J.H., E.P., and S.N.J.W. interpreted results of experiments; S.J.H., E.P., and S.N.J.W. prepared figures; S.J.H., E.P., and S.N.J.W. drafted manuscript; S.J.H., E.P., and S.N.J.W. edited and revised manuscript; S.J.H., E.P., and S.N.J.W. approved final version of manuscript.
REFERENCES
- Abel LA, Friedman L, Jesberger J, Malki A, Meltzer HY. Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol Psychiatry 29: 1063–1072, 1991. [DOI] [PubMed] [Google Scholar]
- Barmack NH. Dynamic visual acuity as an index of eye movement control. Vision Res 10: 1377–1391, 1970. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Hill T. The influence of display characteristics on active pursuit and passively induced eye movements. Exp Brain Res 56: 438–447, 1984. [DOI] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol 84: 892–908, 2000. [DOI] [PubMed] [Google Scholar]
- Bourrelly C, Quinet J, Goffart L. Equilibria and transitions during visual tracking: learning to track a moving visual target in the monkey (Abstract). Soc Neurosci Abstr 2013: 363.01/ZZ22, 2013. [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Britten KH, Heuer HW. Spatial summation in the receptive fields of MT neurons. J Neurosci 19: 5074–5084, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL. Flicker and intermittent stimulation. In: Vision and Visual Perception, edited by Graham CH. New York: Wiley, 1965, vol. 1, p. 251–320. [Google Scholar]
- Collewijn H, Tamminga EP. Human fixation and pursuit in normal and open−loop conditions: effects of central and peripheral retinal targets. J Physiol 379: 109–129, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S, Missal M, Barnes G, Lefèvre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87: 1772–1780, 2001. [DOI] [PubMed] [Google Scholar]
- de Brouwer S, Yuksel D, Blohm G, Missal M, Lefèvre P. What triggers catch-up saccades during visual tracking? J Neurophysiol 87: 1646–1650, 2002. [DOI] [PubMed] [Google Scholar]
- Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol 60: 940–965, 1988. [DOI] [PubMed] [Google Scholar]
- Fletcher WA, Sharpe JA. Smooth pursuit dysfunction in Alzheimer's disease. Neurology 38: 272–277, 1988. [DOI] [PubMed] [Google Scholar]
- Heinen SJ. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res 104: 357–361, 1995. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Jin Z, Watamaniuk SN. Flexibility of foveal attention during ocular pursuit. J Vis 11: 9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen SJ, Keller EL. Precision smooth pursuit eye movements require motor learning (Abstract). Soc Neurosci Abstr 15: 1205, 1989. [Google Scholar]
- Heinen SJ, Watamaniuk SN. Spatial integration in human smooth pursuit. Vision Res 38: 3785–3794, 1998. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Pohlmann J, Trillenberg P, Lencer R, Graf J, Sprenger A. Role of anticipation and prediction in smooth pursuit eye movement control in Parkinson's disease. Mov Disord 27: 1012–1018, 2012. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. Eye movements of rhesus monkeys directed towards imaginary targets. Vision Res 39: 2143–2150, 1999. [DOI] [PubMed] [Google Scholar]
- Jin Z, Watamaniuk S, Khan AZ, Potapchuk E, Heinen SJ. Motion integration for ocular pursuit does not hinder perceptual segregation of moving objects. J Neurosci 34: 5835–5841, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoumi B, Saoud M, d'Amato T, Rosenfeld F, Denise P, Gutknecht C, Gaveau V, Beaulieu FE, Dalery J, Rochet T. Poor performance in smooth pursuit and antisaccadic eye-movement tasks in healthy siblings of patients with schizophrenia. Psychiatry Res 101: 209–219, 2001. [DOI] [PubMed] [Google Scholar]
- Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res 11: 79–107, 1991. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol 60: 580–603, 1988. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res 35: 1897–1916, 1995. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84: 876–891, 2000. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A control systems model of smooth pursuit eye movements with realistic emergent properties. Neural Comput 1: 116–122, 1989. [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements (4th ed). New York: Oxford Univ. Press, 2006. [Google Scholar]
- Lisberger SG. Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79: 1918–1930, 1998. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10: 97–129, 1987. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci 5: 1662–1673, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liversedge SP, Findlay JM. Saccadic eye movements and cognition. Trends Cogn Sci 4: 6–14, 2000. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex 1: 95–102, 1991. [DOI] [PubMed] [Google Scholar]
- McKee SP, Nakayama K. The detection of motion in the peripheral visual field. Vision Res 24: 25–32, 1984. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Glennerster A. Efficiency of locating centres of dot-clusters by human observers. Vision Res 31: 2075–2083, 1991. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J Neurophysiol 64: 77–90, 1990. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol 60: 604–620, 1988. [DOI] [PubMed] [Google Scholar]
- Noda H, Mikami A. Discharges of neurons in the dorsal paraflocculus of monkeys during eye movements and visual stimulation. J Neurophysiol 56: 1129–1146, 1986. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Pinkhardt EH, Kassubek J, Sussmuth S, Ludolph AC, Becker W, Jurgens R. Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson's disease. J Neurol 256: 1438–1446, 2009. [DOI] [PubMed] [Google Scholar]
- Polyak SL. The Retina: the Anatomy and the Histology of the Retina in Man, Ape, and Monkey, Including the Consideration of Visual Functions, the History of Physiological Optics, and the Histological Laboratory Technique. Chicago, IL: Univ. of Chicago Press, 1941. [Google Scholar]
- Puckett JD, Steinman RM. Tracking eye movements with and without saccadic correction. Vision Res 9: 695–703, 1969. [DOI] [PubMed] [Google Scholar]
- Purkinje JE. Beobachtungen und Versuche zur Physiologie der Sinne, 2.53. Prague: J. G. Calve, 1825. [Google Scholar]
- Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG. Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol 87: 912–924, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern 55: 43–57, 1986. [DOI] [PubMed] [Google Scholar]
- Steinbach MJ. Pursuing the perceptual rather than the retinal stimulus. Vision Res 16: 1371–1376, 1976. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. J Neurophysiol 59: 1–18, 1988. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Lisberger SG. Visual motion processing for the initiation of smooth-pursuit eye movements in humans. J Neurophysiol 56: 953–968, 1986. [DOI] [PubMed] [Google Scholar]
- Tyler CW. Specific deficits of flicker sensitivity in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 20: 204–212, 1981. [PubMed] [Google Scholar]
- Watamaniuk SN, Heinen SJ. Human smooth pursuit direction discrimination. Vision Res 39: 59–70, 1999. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SN, Potapchuk E, Heinen SJ. Different mechanisms for pursuing a spot target versus a feature on a large object (Abstract). Soc Neurosci Abstr 2015: 110.01, 2015. [Google Scholar]
- Westheimer G, McKee SP. Visual acuity in the presence of retinal-image motion. J Opt Soc Am 65: 847–850, 1975. [DOI] [PubMed] [Google Scholar]
- Winterson BJ, Steinman RM. The effect of luminance on human smooth pursuit of perifoveal and foveal targets. Vision Res 18: 1165–1172, 1978. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. The Neurobiology of Saccadic Eye Movements. Amsterdam: Elsevier, 1989. [Google Scholar]
- Wyatt HJ, Pola J, Fortune B, Posner M. Smooth pursuit eye movements with imaginary targets defined by extrafoveal cues. Vision Res 34: 803–820, 1994. [DOI] [PubMed] [Google Scholar]
- Zaccara G, Gangemi PF, Muscas GC, Paganini M, Pallanti S, Parigi A, Messori A, Arnetoli G. Smooth-pursuit eye movements: alterations in Alzheimer's disease. J Neurol Sci 112: 81–89, 1992. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L. Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol 16: 65–79, 1966. [DOI] [PubMed] [Google Scholar]