Abstract
Much research has been devoted to investigating response inhibition and the neuronal processes constituting this essential cognitive faculty. However, the nexus between cognitive subprocesses, behavior, and electrophysiological processes remains associative in nature. We therefore investigated whether neurophysiological correlates of inhibition subprocesses merely correlate with behavioral performance or actually provide information expedient to the prediction of behavior on a single-subject level. Tackling this question, we used different data-driven classification approaches in a sample of n = 262 healthy young subjects who completed a standard Go/Nogo task while an EEG was recorded. On the basis of median-split response inhibition performance, subjects were classified as “accurate/slow” and “less accurate/fast.” Even though these behavioral group differences were associated with significant amplitude variations in classical electrophysiological correlates of response inhibition (i.e., N2 and P3), they were not predictive for group membership on a single-subject level. Instead, amplitude differences in the Go-P2 originating in the precuneus (BA7) were shown to predict group membership on a single-subject level with up to 64% accuracy. These findings strongly suggest that the behavioral outcome of response inhibition greatly depends on the amount of cognitive resources allocated to early stages of stimulus-response activation during responding. This suggests that research should focus more on early processing steps during responding when trying to understand the origin of interindividual differences in response inhibition processes.
Keywords: EEG, response inhibition, machine learning, single-subject prediction
the ability to inhibit one's own responses is central to action control and indispensable for self-regulation as well as an important prerequisite to higher-order executive functions and cognitive flexibility (Diamond 2013). Poor or dysfunctional response inhibition may profoundly interfere with everyday life requirements and has been shown to be prevalent in a variety of neurological and psychiatric diseases (Aron and Poldrack 2005; Bari and Robbins 2013; Beste et al. 2008, 2009; Cavanna et al. 2009; Hanna-Pladdy 2007). On the behavioral level, different paradigms and parameters are used to measure response inhibition performance and abilities (Diamond 2013; Verbruggen and Logan 2008). While the rate of false alarms (i.e., the failed inhibition of the Go response in Nogo trials) is the main measure of inhibition in Go/Nogo tasks, stop-signal tasks estimate the stop signal reaction time (SSRT), which is thought to reflect a “race” between largely independent go and stop processes (Diamond 2013; Verbruggen and Logan 2008). Mostly based on these two kinds of inhibition paradigms, the functional neuroanatomical architecture and neuronal processes underlying the ability to inhibit prepotent responses have been subjected to intense research in cognitive neuroscience (Aron et al. 2014; Bari and Robbins 2013). In this context, electroencephalographic (EEG) techniques have long been applied to elucidate the neuronal mechanisms underlying this important executive control function (Bruin and Wijers 2002; Donkers and van Boxtel 2004; Huster et al. 2013; Kopp et al. 1996; Pfefferbaum et al. 1985; Sutton et al. 1965). With EEG techniques, it has been suggested that there are at least two distinct neurophysiological subprocesses that contribute to successful response inhibition: It has repeatedly been demonstrated that a frontal-midline N2 event-related potential (ERP) reflects premotor processes like conflict monitoring or updating of the response program, while a P3 ERP reflects evaluative processing of the successful outcome of inhibition (Beste et al. 2009, 2010, 2011; Falkenstein et al. 1999; Huster et al. 2013; Nieuwenhuis et al. 2003; Ramautar et al. 2004), or possibly of the inhibition process itself (Wessel and Aron 2015).
However, the relationship between the behavioral outcome of response inhibition and the neurophysiological responses reflecting underlying cognitive processes is still largely associative in nature. Importantly, this associative nexus does not imply that it is possible to predict behavior from electrophysiological data. It is still elusive whether electrophysiological correlates of response inhibition allow for prediction of the behavioral outcome of response inhibition on a single-subject level. In the present study, we hence examined the single-subject predictability of the behavioral outcome of response inhibition based on electrophysiological response inhibition substrates using data-driven classification approaches. In this context, it needs to be stressed that we do not confine our analyses to ERP data within the time frames of the N2 and P3 components. Instead, we examine different time frames over the entire poststimulus period. One of the main reasons for this is that response times are typically quite low in Go/Nogo tasks (often no more than 300–350 ms in healthy young subjects; e.g., Beste et al. 2010; Heilbronner and Münte 2013; Spronk et al. 2015; Stock et al. 2016). While the N2 might often still fall within this range, the P3 component usually peaks after the average response time. When rating performance based on hit rates and false alarm rates, whatever cognitive process allows prediction of performance should occur before any response is given (i.e., before the mean response time). In this context, some results suggest that early stages of either stimulus-response activation (Gajewski et al. 2013) and/or resource allocation processes, as reflected in a P2 component (Bonnefond et al. 2010; Campbell and Sharma 2013; Staub et al. 2014; Sugimoto and Katayama 2013), may also be important for response inhibition processes (Bonnefond et al. 2010; Gajewski and Falkenstein 2013; Staub et al. 2014). Others have suggested that the P2 reflects higher-order perceptual processing in terms of relevant stimulus features, which are enhanced while irrelevant ones are suppressed at the same time (Crowley and Colrain 2004). This is all the more relevant in the present context, considering that successful inhibition has been suggested to rely on the adjustment of attentional settings to optimize stimulus detection and evaluation (Dodds et al. 2011; Hampshire et al. 2010; Verbruggen et al. 2014). Given all this, we expect that an above-average allocation of resources as reflected by the P2 component should be one of the features allowing for the prediction of the behavioral outcome of response inhibition (as operationalized by group membership in this study).
Summing up our hypotheses, we expect the “classical” components (i.e., N2 and P3) to correlate with inhibition performance but deem earlier components such as the P2 to be similarly suitable to predict behavioral performance/group membership. We therefore take two complementary approaches: To provide results that can be compared to previous publications in the field, the P2, N2, and P3 components are analyzed by quantifying ERPs at midfrontal electrodes and using a regression analysis approach in order to explain behavior by (Nogo)-N2 and (Nogo)-P3 components. Furthermore, we additionally take a machine learning approach to identify potential predictors of behavioral performance/group membership performance among earlier, inhibition-related ERP components. Both of these approaches are complemented with source localization techniques (sLORETA) in order to determine the brain regions that can be used to predict inhibition performance. To be able to predict group membership based on behavioral measures of response inhibition, we investigated n = 262 healthy young subjects who were subdivided into two groups based on a median split of the false alarm rate. Subsequently, the nexus between groups, behavioral data [accuracy and reaction times (RTs)], classical ERPs (i.e., P2, N2, and P3), and different predictors identified by means of machine leaning (methods) is compared.
METHODS
Sample.
A sample of n = 262 healthy subjects between 18 and 30 yr of age (mean age 23.9 yr, SD = 3.06) was recruited for the study; 114 of the subjects were women. None of the participants enrolled in the study reported a history of neurological or mental illness. The study was approved by the Ethics committee of the Ruhr-Universität Bochum, Germany. The study was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent and received €10 reimbursement or course credits for their participation.
Task.
We used a standard Go/Nogo task, which has frequently been used by our group to assess response inhibition performance (Beste et al. 2010; Ocklenburg et al. 2011; Quetscher et al. 2015). Each trial started with the 200-ms presentation of either of the German words “DRÜCK” and “STOPP” (translating to “PRESS” and “STOP”) in the center of a 17-in. screen. Subjects were required to respond with their right index finger on a custom-made button as fast as possible whenever the Go stimulus (“DRÜCK”) was presented. Upon presentation of the Nogo stimulus (“STOPP”), the subjects were required to withhold their Go response. Seventy percent of all trials were Go trials, and subjects were instructed to respond as fast as possible, resulting in a relatively high rate of false alarms (i.e., a lower rate of correct Nogo trials). Each trial was terminated by the participant's first response (correct responses in Go trials or false alarms in Nogo trials) or ended after 2,200 ms had elapsed (missed Go responses or correct inhibition in Nogo trials). The intertrial interval (ITI) was jittered between 1,000 and 1,300 ms. In total, the paradigm comprised 450 trials.
EEG recording and analysis.
The EEG was recorded from 64 Ag/AgCl electrodes with the extended 10-20 system against a reference electrode placed at electrode FCz. The sampling rate was 1 kHz. Electrode impedances were kept below 5 kΩ. After recording, the data were downsampled to 256 Hz. Off-line, the EEG was digitally filtered with IIR bandwidth filters at 0.5 and 20 Hz (each with a slope of 48 dB/oct). The data were then visually inspected, and gross artifacts were manually removed from the EEG. Horizontal and vertical eye movements as well as pulse artifacts were removed with an independent component analysis (ICA) (infomax algorithm). After the rectified EEG was reconstructed from the remaining components, electrode FCz was topographically interpolated. The EEG was then segmented into epochs of 800-ms length starting 200 ms prior to the target stimulus onset, which was set to zero. Because of the large number of data files that underwent manual and semiautomatic correction, an automatic artifact rejection procedure was applied to eliminate any artifacts that might have survived the prior corrections. A value difference above 200 μV in a 100-ms interval as well as an activity below 0.5 μV in a 200-ms period were used as rejection criteria. Next, a current source density (CSD) transformation (Nunez and Pilgreen 1991) (order of splines m = 4, maximum degree of the Legendre polynomials n = 10, precision of 2.72−7) was applied to rereference the data. Because of this, the resulting CSD values are given in microvolts per square meter. A baseline correction was applied in the time range from −200 ms to 0 ms (i.e., prior to target onset) before the segments were separately averaged for Go and Nogo trials with correct responses on a single-subject level. The average number of epochs included in the data analysis was 285 ± 13 for Go trials and 115 ± 10 for Nogo trials. For the classical time-domain analysis of the ERP components, the Nogo-P2 was quantified by extracting the mean amplitude in the time interval from 175 to 180 ms. The Nogo-N2 was quantified by extracting the mean amplitude of the time interval from 250 to 310 ms. The Nogo-P3 was quantified by extracting the mean amplitude of the time interval from 350 to 440 ms. For the correctly answered Go trials, amplitudes of the Go-P2, Go-N2, and Go-P3 were measured in the corresponding time intervals of the individual averages. Because of quantifying the peak amplitudes by using mean amplitude values and the fact that no change in peak latencies could be visually detected in the mean averages of the groups, we refrained from determining peak latency values. The electrodes used for ERP classification were chosen based on scalp topographies of the averaged ERPs in Go and Nogo trials across the entire sample (see Fig. 1).
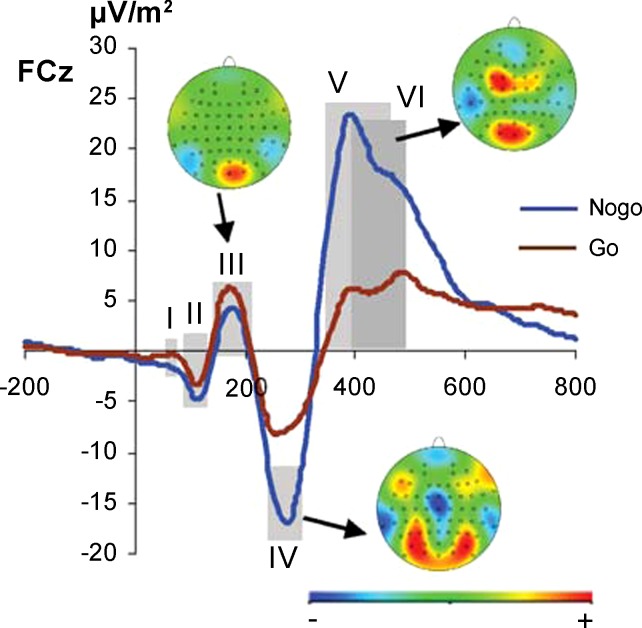
Fig. 1.
Illustration shows the mean potentials for n = 262 on Nogo (blue) and Go trials (brown) at electrode FCz including scalp topography maps. In the scalp topography plots, red colors denote positivity and blue colors negativity. Gray shadings show the different time bins used for data extraction for standard event-related potential (ERP) analysis and the data-driven analysis to predict performance on a single-subject level. Note that time bins V and VI overlap.
For the P2, this yielded electrodes Oz and O2. For the (No)Go-N2, this yielded electrodes FCz and Cz, while the (No)Go-P3 was quantified at electrodes FC1 and C1 (see map in Fig. 1). This choice of electrodes was validated by a statistical procedure previously described by Mückschel et al. (2014). For the standard (non-data-driven) ERP analysis, parametric statistics were used. To compare behavioral performance on Go and Nogo trials, independent-samples t-tests were used. The ERP data were analyzed with mixed-effects ANOVAs using “condition” (Go vs. Nogo) and “electrode” as within-subject factors and “performance group” (accurate/slow vs. less accurate/fast) as a between-subject factor. Greenhouse-Geisser correction was applied and performance post hoc tests were Bonferroni corrected whenever necessary. Separate ANOVAs were conducted for the P2, N2, and P3 components.
Data-driven ERP analysis.
The average ERP on Go and Nogo trials across the entire sample (n = 262) is shown in Fig. 1. Our machine learning approach was performed in four separate stages of analysis: data preparation, feature selection, classifier selection, and validation. The bulk of this analysis was done in MATLAB (MathWorks) with help from the open-source packages Weka (data mining software in Java, available online at http://www.cs.waikato.ac.nz/ml/weka/), LibSVM (support vector machine library, available online at http://www.csie.ntu.edu.tw/∼cjlin/libsvm/), and bolasso.m (bootstrap-enhanced least absolute shrinkage operator, available online at https://code.google.com). The goal of this analysis was to predict group membership in the Go/Nogo task, as based on the false alarm rate, from electrophysiological data on a single-subject level. As described above, participants were grouped based on a median split involving the Nogo condition. Irrespective of group membership, the whole sample was then randomly split into two groups to obtain a training set (2/3; n = 173) for hypothesis formation and another, albeit smaller, validation set (1/3; n = 89) for validation and discrimination, in order to minimize the chance of overfit.
In the first stage (data preparation), ERP data were reduced in dimensionality, which is basically done to ensure that the number of machine learning features does not exceed the number of participants, which would produce trivial classifiers. For this purpose, we first calculated topographical difference strength maps, i.e., bootstrapped scalp CSD differences between high and low performers averaged across all conditions. On the basis of these difference strength maps, we selected the following electrodes for further analyses: midline electrodes Fz, FCz, Cz, CPz, Pz, and Oz as well as lateral parieto-occipital electrodes P1, P2, P3, P4, PO7, PO8, PO9, PO10, O1, and O2. Next, ERPs were divided into different time bins that were specifically tailored to represent several ERPs reflecting perceptual and attentional processing (i.e., P1, N1, P2) as well as inhibition-related processes (N2 and P3) in the Go and Nogo conditions. These bins were 75–120 ms (I), 90–135 ms (II), 145–210 ms (III), 250–310 ms (IV), 350–440 ms (V), and 390–450 ms (VI). The bins were set on the basis of the waveform at electrode FCz (see Fig. 1), because this electrode was located at the center of the topographies in the Nogo-N2 (time bin IV) and Nogo-P3 (time bin V). As can be seen in Fig. 1, two time bins in the P3 range (i.e., V and VI) overlap. These two time bins were chosen for the P3 time window, as the peaks of the P3 differed in their latency between Nogo and Go trials (i.e., the P3 peaked later in Go than in Nogo trials). For the other time bins, we only used one time window for each ERP, as there were no differences in the latencies between the Go and Nogo conditions. Finally, ERP features were entered into machine learning analysis on the basis of selected electrodes (N = 16), time bins (N = 6), and experimental conditions (N = 2).
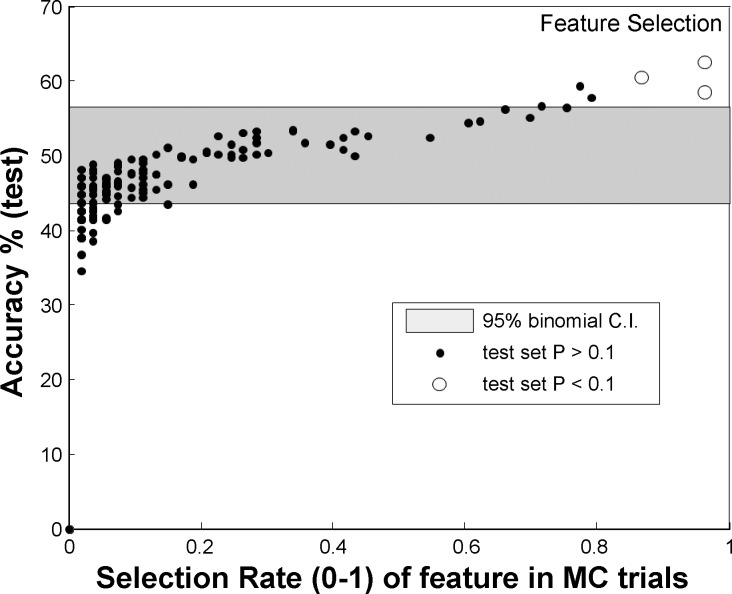
In the second stage (feature selection), 50 Monte Carlo simulations were performed using two equally sized random splits of the training set to cross-validate features with significant differences among the two conditions, using a two-sided Kolmogorov-Smirnoff (KS) test with a P < 0.05. For each Monte Carlo simulation, a different subset of features was selected. Within each simulation, features were further tested for significance of difference within test data in order of training set discrimination strength. Figure 2 shows the mean accuracy of linear threshold classification (simplest classifier) based on each individual feature.
Fig. 2.
Illustration of the feature selection process shows the mean accuracy of linear threshold classification based on each individual feature, trained on training sets and evaluated on corresponding test sets (y-axis) vs. the median P value of the Kolmogorov-Smirnoff test, calculated on the same test set sample for pre- and postselected features. As can be seen, the most frequent pre- and postselected features are also those that discriminate on test splits (the features pre- and postselected in >50% of the trials are marked with white dots); they are among the ones promising highest discrimination accuracy. Also shown is the 95% confidence interval (CI) of accuracy for random classifier for the same test set size based on the binomial distribution.
Of note, different feature selection processes are viable for dimension reduction, such as advanced principal component analyses (Dien et al. 2005), recursive feature elimination (Castro et al. 2011), or strong feature identification (Shen et al. 2014). We chose to use the KS test to identify strong features, as this method is firmly established and was shown to provide robust classification results in our previous studies (Neuhaus et al. 2014; Shen et al. 2014).
In the third stage of the analysis (classifier selection), we explored a hypothesis space that was the product of all combinations of predictive features (from the 3 listed above) and all the classifiers to be tested. The order in which these hypotheses were validated on (unseen) test data was based on the correlation between features and their estimated individual predictive ability (using a simple threshold classifier is the fundamental assumption of the KS test), based on the correlation-scaled conditional information. Suppose we have a series of predictors x1, x2, . . . xn of relevance to a binary variable y and these predictors have a correlation structure P with individual elements ρi,j. The basic concept is to prefer combinations of uncorrelated individual predictors over those who may be redundant. For this, we calculate the individual predicted accuracy for every single predictor, using a simple classifier (a binary threshold or range) r(xi), which for a single predictor is simply the value obtained on the training set. Then we define an intermediate utility function m:
| (1) |
| (2) |
and, based on that, the recursively defined predicted accuracy function:
| (3) |
| (4) |
where H is the Shannon entropy of a binary channel with accuracy r, H−1+ is the inverse of this function taken at the upper (of 2) roots, and .
The order of evaluation of multidimensional hypotheses (i.e., with >1 predictor combined) is then done in order of decreasing r.
The applied classifiers are common classification algorithms used in machine learning practice and include linear and quadratic discriminants (LDA/QDA and their diagonal variants), support vector machines (SVM with radial basis, linear, polynomial, sigmoid, and multilayer perceptron kernels), naive Bayes, k-nearest neighbors with Euclidean and cosine distance measures, and Mahalanobis with different types of regularization of the covariance estimator (no regularization, pseudoinverse, probabilistic reasoning model, BOLASSO, and robust regression).
In the fourth and final step (validation), hypotheses are evaluated (best to worst) on the validation subset, i.e., one-third remaining from the original split, which until this step has not played a part in any fitting or optimization. Hypotheses that resulted in tested overfit according to a two-sided binomial test were discarded.
RESULTS
Behavioral data.
For the descriptive statistics, means and SE are given. The mean rate of false alarms in the entire sample was 11.7% (0.5). Mean RTs were 349 ms (2.1) in Go trials and 289 ms (3.9) in erroneous Nogo trials. As expected, RTs were faster for false alarms (erroneous reactions on Nogo trials) than in Go trials (t257 = 18.02; P < 0.001). A comparison of common behavioral measures across groups is given in Table 1.
Table 1.
Common behavioral measures
| Measure | FA Rate, % | Omission Rate, % | GO RTs, ms | FA RTs, ms |
|---|---|---|---|---|
| Entire sample | 11.70 ± 0.53 | 1.12 ± 0.06 | 349 ± 2 | 289 ± 4 |
| Accurate/slow | 5.26 ± 0.22 | 0.97 ± 0.08 | 360 ± 3 | 305 ± 7 |
| Less accurate/fast | 18.05 ± 0.65 | 1.27 ± 0.09 | 339 ± 3 | 276 ± 3 |
| Group difference | P < 0.001 | P = 0.011 | P < 0.001 | P = 0.002 |
Even though the performance groups were based on differences in false alarm (FA) rates, all common behavioral measures differed between groups.
RT, reaction time.
As the high- and low-performance groups were based on the rate of false alarms, the rate of false alarms necessarily differed between groups (t260 = 14.92; P < 0.001). However, all other behavioral measures also differed between groups (all P ≤ 0.011; see Table 1). Of note, the RT data show that on both Go and Nogo trials, participants showing high false alarm rates show speeded responding compared with participants showing a lower false alarm rate. This shows that there is a speed-accuracy trade-off (SAT) between the groups. Therefore the groups were termed as “accurate/slow” and “less accurate/fast.”
Standard analysis of ERP data.
Figure 3A shows the ERPs on Go and Nogo trials for electrodes Cz, FC1, and FCz.
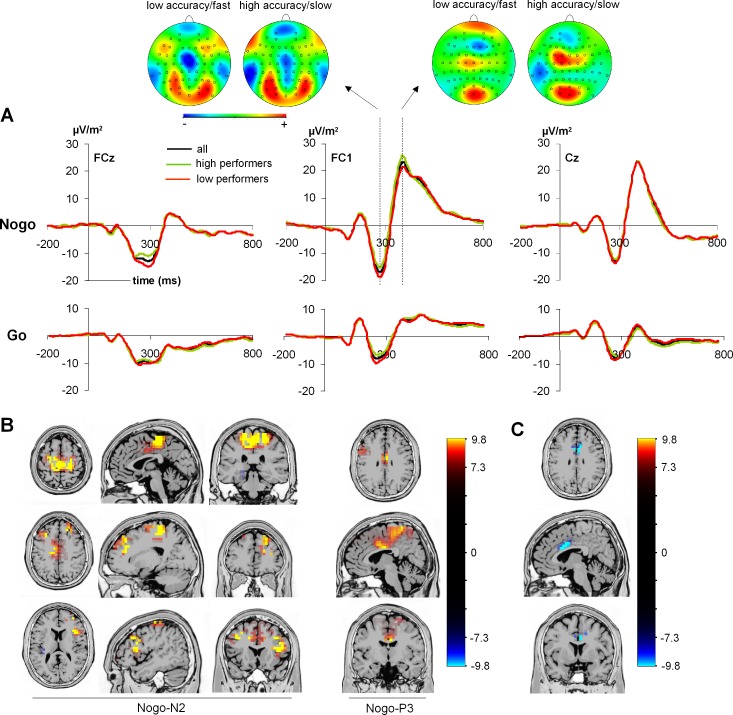
Fig. 3.
A: ERPs on Go and Nogo trials shown for the entire sample (all) as well as the high- and low-performance groups. Scalp topography plots show the Nogo-N2 and the Nogo-P3 for the high- and low-performance groups. In the scalp topography plots, red colors denote positivity and blue colors negativity. B: results from sLORETA analysis contrasting Go against Nogo trials (Nogo > Go) in the entire sample for the (Nogo)-N2 time window (left) and the (Nogo)-P3 time window (right). C: results from sLORETA analysis of Nogo trials between high and low performers in the N2 time window (accurate/slow < less accurate/fast).
Concerning the N2 data, the results showed significant main effects of “condition,” “electrode,” and “group” as well as a significant interaction of “condition” × “group.” The main effect of “condition” [F(1,260) = 32.93; P < 0.001; η2 = 0.112] showed that the N2 was larger in Nogo (−11.62 ± 0.5 μV/m2) than in Go (−9.1 ± 0.5 μV/m2) trials. The main effect of “electrode” [F(1,260) = 10.90; P = 0.001; η2 = 0.040] was based on a higher N2 amplitude at electrode FCz (−11.86 ± 0.6 μV/m2) compared with Cz (−8.84 ± 0.7 μV/m2). The main effect of “group” [F(1,260) = 7.86; P = 0.005; η2 = 0.029] relied on a larger N2 amplitude in the low-performance group (−11.73 ± 0.7 μV/m2) than in the high-performance group (−8.97 ± 0.6 μV/m2). Importantly, there was an interaction of “condition” × “group” [F(1,260) = 4.30; P = 0.025; η2 = 0.019]. Post hoc t-tests showed that there was a significant N2 difference between groups in Nogo trials (low performers: −13.40 ± 0.6 μV/m2; high performers: −9.84 ± 0.7 μV/m2; t260 = −3.12; P = 0.002) but not in Go trials (high performers: −8.10 ± 1.1 μV/m2; low performers: −10.06 ± 1.4 μV/m2; t260 = −1.13; P > 0.2). All other interaction effects were not significant (all F < 0.16; P > 0.6).
For the P3 data, there were main effects of “condition” and “electrode” as well as an interaction of “condition” × “electrode.” The main effect of “condition” [F(1,260) = 758.75; P < 0.001; η2 = 0.745] showed that the P3 at frontal electrode sites was larger in Nogo (19.86 ± 0.8 μV/m2) than in Go (4.57 ± 0.6 μV/m2) trials. The main effect of “electrode” [F(1,260) = 38.36; P < 0.001; η2 = 0.129] indicated that the P3 was larger at electrode C1 (13.78 ± 0.8 μV/m2) than at FC1 (10.65 ± 0.6 μV/m2). The interaction of “electrode” × “condition” [F(1,260) = 82.69; P < 0.001; η2 = 0.241] was based on a larger amplitude difference between Go and Nogo trials at electrode FC1 (18.06 ± 0.6 μV/m2) than at electrode C1 (12.53 ± 0.7 μV/m2), as shown by post hoc testing (t261 = 9.11; P < 0.001). There was no main effect of “group,” and there were also no interactions with this factor (all F < 1.6; P > 0.2).
Regression analyses were calculated to investigate how variance in the behavioral performance (i.e., rate of false alarms) is explained by the Nogo-N2 and Nogo-P3 amplitudes and whether there are differences between the performance groups. The rate of false alarms was used as the dependent variable, while group, as well as Nogo-N2 and Nogo-P3, served as predictors within the same model. The “inclusion” (enter) method was used. The regression model yielded a significant result [F(3,261) = 73.81; P < 0.001]. Only the factor “group” significantly contributed to the regression model (β = −0.679; t = −14.77; P < 0.001). This is, however, trivial because the groups were built upon a median split on the false alarm rate data. Neither the Nogo-N2 amplitude nor the Nogo-P3 amplitude was a significant predictor in the regression model (all β < −0.054; t < −1.16; P > 0.2).
sLORETA analyses were calculated to contrast the Go with the Nogo condition in the entire sample in the N2 and P3 time ranges; the results are shown in Fig. 3B. For the N2 time range, the analysis revealed stronger activity in Nogo trials in the left and right middle frontal gyrus [Brodmann area (BA) 9], left and right medial frontal gyrus (BA9, BA6), cingulate gyrus (BA24), and left and right precentral gyrus (BA6). For the P3 time range, the sLORETA analysis revealed stronger activity in Nogo trials in the anterior cingulate cortex (ACC; BA23, BA24), paracentral lobe (BA6), and middle frontal gyrus (BA9).
Because the interaction of “condition” × “group” had revealed differences between performance groups on Nogo trials in the N2 time range, we also contrasted the Nogo-N2 in the low-performance group to the Nogo-N2 in the high-performance group. This was, however, not done for the Nogo-P3 because there was no difference between performance groups. The sLORETA analysis suggests that activation differences between the groups were due to activation differences in the ACC (BA24, BA23) in the Nogo-N2 time window, with low performers displaying a larger activation than high performers (see Fig. 3C).
Aside from the analysis of these classical response inhibition-related ERPs [i.e., (No)Go-N2 and (No)Go-P3] we also analyzed the P2, which could be of importance, as some studies suggest that early stages of either stimulus-response activation (Gajewski et al. 2013) and/or resource allocation processes, as reflected in a P2 component (Bonnefond et al. 2010; Campbell and Sharma 2013; Staub et al. 2014; Sugimoto and Katayama 2013), may also be important for response inhibition processes (Bonnefond et al. 2010; Gajewski and Falkenstein 2013; Staub et al. 2014). The P2 was maximal at electrodes Oz and O2, as validated by statistical techniques (see methods). For the P2 at these electrodes, the mixed-effects ANOVA only revealed a main effect of “electrode” [F(1,260) = 12.19; P = 0.001; η2 = 0.045]. All other main or interaction effects were not significant (all F < 0.96; P > 0.3).
Single-subject classification (data-driven analysis).
The top hypotheses derived by machine learning are shown in Table 2, in terms of classification accuracy of both the training and (independent) test set.
Table 2.
Top hypotheses derived by machine learning in terms of classification accuracy of training and test sets
| Algorithm | Accuracy Training | Accuracy Test | 95% CI Test | P | Features |
|---|---|---|---|---|---|
| SVM lin | 0.56 | 0.64 | 0.571–0.709 | 0.0048 | PO7_Nogo_350–440 ms |
| PO9_Go_250–310 ms | |||||
| MAHA pinv | 0.57 | 0.63 | 0.546–0.714 | 0.0089 | PO7_Nogo_350–440 ms |
| PO9_Go_250–310 ms | |||||
| O1_Nogo_145–210 ms | |||||
| MAHA prm | 0.55 | 0.60 | 0.571–0.629 | 0.043 | PO7_Nogo_350–440 ms |
| PO9_Go_250–310 ms | |||||
| O1_Nogo_145–210 ms | |||||
| SVM lin | 0.59 | 0.60 | 0.532–0.668 | 0.066 | PO7_Nogo_350–440 ms |
| PO9_Go_250–310 ms | |||||
| O1_Nogo_145–210 ms |
Each algorithm is tested on a combination of selected event-related potential features and provides classification accuracies for the training set (2/3) and the test set (1/3). The final accuracy of the test set is complemented by a 95% confidence interval (CI). P value denotes the probability of the final accuracy of the test set being erroneously different from a random P.
SVM, support vector machine; MAHA, Mahalanobis distance classifier; lin, linear; pinv, pseudoinverse; prm, probabilistic reasoning model.
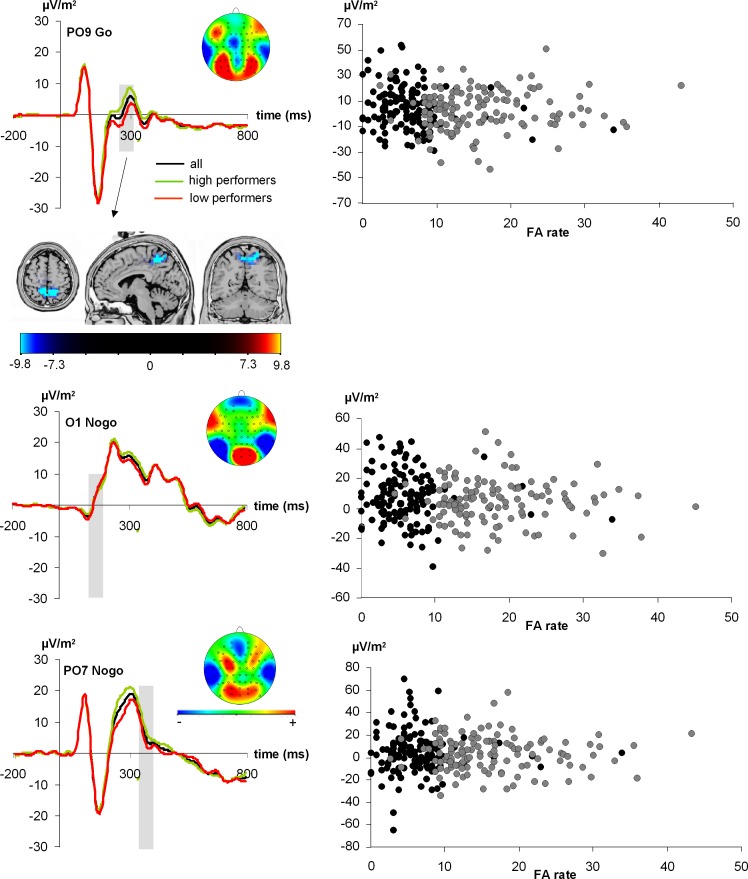
The first hypothesized estimate is the top row and has a 64% test accuracy rate (P < 0.07) on hitherto untested data (i.e., the validation set). Being evaluated independently of subsequently ranked hypotheses, this result is not dependent on any multiple-comparison correction and stands alone. Note that the predicted (training) and validated (test) accuracies are not only within the margin of error but are very similar, which is particularly convincing and strongly argues against overfitting the mathematical model to the data. Subsequent hypotheses would not retain their nominal P values (shown) after multiple-comparison correction and thus primarily serve as comparative algorithmic models. Of note, chosen features were identified by virtually all algorithms. The features most consistently selected were Go-P2 ERP 250–310 ms at electrode PO9 (feature 1) (Fig. 4, top), Nogo-P2 ERP 145–210 ms at electrode O1 (feature 2) (Fig. 4, middle), and Nogo-P3 ERP 350–440 ms at electrode PO7 (feature 3) (Fig. 4, bottom). Figure 4 shows the ERPs at the respective electrode sites, time bins, and conditions. It is important to note that in the EEG electrode setup employed, electrodes PO9/PO10 are not edge electrodes, as electrodes P11 and P12 were evident in this setup. This is important since CSD transformations can introduce distortions of activity at edge electrodes, which may compromise the reliability of data.
Fig. 4.
ERPs shown for the different features that best predicted task performance on a single-subject level. The entire sample as well as the high- and low performance groups are shown. Top: the extracted feature shows the P2 on Go trials. The sLORETA analysis contrasting the amplitude of the P2 on Go trials between high and low performers (accurate/slow < less accurate/fast) revealed a source in the precuneus (BA7). Middle and bottom: the other 2 extracted features. In all panels, gray shading shows the time bins that best predicted performance on a single-subject level. The scalp topography plot is also shown for these time bins. In the scalp topography plots, red colors show positivity and blue colors negativity. Right: scatterplots for the extracted feature as a function of the false alarm (FA) rate. Black dots denote the “accurate/slow” group and gray dots the “less accurate/fast” group.
Comparison of the “accurate/slow” to the “less accurate/fast” group on these extracted features using independent-samples t-tests revealed that for feature 1 there was a significant difference between the “accurate/slow” (5.3 ± 1.4) and the “less accurate/fast” (0.16 ± 1.4) group (t260 = −2.57; P = 0.005). For feature 2 and feature 3, there was no significant difference between the two performance groups (all t < −1.16; P > 0.2). This pattern of results highlights the nontrivial nature of machine learning analysis that allows identification of even those ERP features that are not significantly different on a group level if there is no explicit procedure to eliminate them (i.e., feature selection) as to increase inference specificity while controlling the (potentially combinatorially large) number of hypotheses such selection implies. This result also depends on the choice of statistical test used in the preselection of predictors, which either rely on differences of distribution (such as the KS test used during our feature selection approach) or on more narrowly defined and commonly used differences of mean values and their standard deviation (such as the t-test).
We further investigated feature 1 (Fig. 4, top), which likely reflects processes related to P2 amplitude variation. To examine this feature variation between the “accurate/slow” and the “less accurate/fast” group on a systems level, we ran an sLORETA analysis, contrasting the “accurate/slow” and the “less accurate/fast” group. This was done for the time point showing the most positive amplitude value of the P2 in the “accurate/slow” group, i.e., at 297 ms. The results show that group-dependent performance differences were due to activation differences in the precuneus (BA7) (see Fig. 4, top). Note that the result was identical when using the whole bin in the sLORETA analysis.
DISCUSSION
In the present study, we examined the predictability of behavioral performance by electrophysiological substrates of response inhibition processes using data-driven pattern recognition approaches. While electrophysiological data are widely used to study response inhibition processes, it has remained unclear whether it is possible to predict response inhibition performance (accuracy) on the basis of electrophysiological response inhibition indexes on a single-subject level. For this purpose, we median-split a sample of 262 participants into an “accurate/slow” and a “less accurate/fast” group. Our data analyses suggest that the prediction of group membership is indeed possible, but not on the basis of the electrophysiological correlates that are usually associated with response inhibition processes (i.e., the Nogo-N2 and Nogo-P3).
The standard analysis of response inhibition ERP-correlates with parametric tests revealed the usual and expected effects including higher N2 and P3 amplitudes on Nogo than on Go trials. The Nogo-N2, but not the Nogo-P3, was differentially modulated across groups, being smaller in the “accurate/slow” than in the “less accurate/fast” group. It therefore seems that especially premotor processes like conflict monitoring or updating of the response program (Donkers and van Boxtel 2004; Huster et al. 2013; Nieuwenhuis et al. 2004) distinguish between groups, while this is not the case for processes that either reflect evaluative processing of the successful outcome of the inhibition or the inhibition process itself (Huster et al. 2013). Given that requirements of the paradigm were rather simple, it seems logical, however, that response evaluation processes differed less between groups. Further supporting the importance of reduced premotor inhibition in the “less accurate/fast” group, we found that this group shows faster responding than the “accurate/slow” group. It is possible that compared with the “accurate/slow” group the “less accurate/fast” group seems to experience an enhanced conflict between responding and nonresponding when confronted with Nogo stimuli, which is reflected in the enhanced Nogo-N2. Source localization analyses revealed that in the N2 time window areas in the left and right middle frontal gyrus (BA9), left and right medial frontal gyrus (BA9, BA6), cingulate gyrus (BA24), and left and right precentral gyrus (BA6) were more activated in Nogo than in Go trials (Menon et al. 2001; Watanabe et al. 2002). In the context of our task, the heightened activation in BA6 most likely reflects the conflict to inhibit motor response plans, while activity in the dorsolateral prefrontal cortex (dlPFC, BA9) might reflect premovement-associated cognitive control processes (Disbrow et al. 2013; Obeso et al. 2013; Rushworth et al. 2004; Sumner et al. 2007). The heightened activity in the ACC (BA24) most likely depicts aspects of voluntary response inhibition and conflict monitoring (Beste et al. 2012; Fassbender et al. 2004; Hanna-Pladdy 2007; Rushworth et al. 2004; Watanabe et al. 2002). In the P3 time window, the sLORETA analysis revealed that areas encompassing the ACC (BA23, BA24), paracentral lobe (BA6), and middle frontal gyrus (BA9) were more activated in Nogo than in Go trials (Fallgatter et al. 2002, 2004; Menon et al. 2001; Watanabe et al. 2002). The finding that the P3 peak was largest over electrodes left from the midline might be explained by the response modality: Even though stimuli were centrally presented, all participants had been explicitly instructed to only use their right hand for responses, which might explain inhibition of motor plans in contralateral left premotor areas/left supplementary motor area (SMA) (i.e., BA6). Furthermore, it has previously been shown that important aspects of response evaluation may be shifted toward the hemisphere that is in charge of generating and sending the motor command of required responses (Stock et al. 2013; Stock and Beste 2014). In sum, the areas showing N2 and P3 differences between Go and Nogo trials have frequently been reported as elements of a response inhibition network (Bari and Robbins 2013; Menon et al. 2001; Swick et al. 2011; Watanabe et al. 2002), and electrophysiological studies using source localization approaches also report sources of the Nogo-N2 and Nogo-P3 in the brain regions identified in this study (Albert et al. 2012; Gonzalez-Rosa et al. 2013; Lavric et al. 2004). While these results clearly show that neurophysiological processes reflected by the Nogo-N2 and Nogo-P3 are associated with response inhibition processes, a regression analysis taking performance group into account showed that response inhibition performance could not be inferred on the basis of Nogo-N2 and Nogo-P3 amplitude modulations in the entire cohort. This suggests that performance was unrelated to modulations in components that are commonly suggested to reflect premotor processes like conflict monitoring/updating of the response program (Nogo-N2), evaluative processing of the successful outcome of the inhibition, or the inhibition process itself (Nogo-P3) (Albert et al. 2012; Beste et al. 2009, 2010, 2011; Falkenstein et al. 1999; Gonzalez-Rosa et al. 2013; Lavric et al. 2004; Neuhaus et al. 2010; Nieuwenhuis et al. 2003; Ramautar et al. 2004; for review see Huster et al. 2013). However, several other studies found correlations between performance and neurophysiological indexes (Beste et al. 2011, 2013; Quetscher et al. 2015; Sehlmeyer et al. 2010). One possible explanation might be that in many EEG studies the sample sizes are rather small, with often no more than 15–25 subjects per group. In such setups, it cannot be ruled out that some of the observed differences are due to special sample characteristics (Sehlmeyer et al. 2010).
Our data-driven analysis offers an alternative approach to predict group membership on the single-subject level. Furthermore, none of the employed algorithms showed an effect for classical response inhibition correlates, even though time windows used for ERP quantification were adjusted to Nogo-N2 and Nogo-P3 peaks. Instead, the data-driven analysis revealed that a small set of features that includes amplitude modulations in the P2 time range of Go trials predicted group membership on a single-subject level with up to 64% accuracy, i.e., well above chance. Yet both the electrode sites and time window of the P2 differed from the topography-based ones used in the standard ERP analysis. Given that healthy subjects usually display rather small interindividual differences in such a simple task and that the severe impairments caused by schizophrenia have been demonstrated to account for ∼75% of the interindividual neurophysiological variance (as compared with control subjects) in similar tasks (Neuhaus et al. 2011, 2013, 2014), the present results are in line with these rates of variance explanation. Even though there are some claims that the P2 largely reflects sensory processing (Barry 2009), the P2 ERP has been suggested to reflect either stimulus-response activation (Gajewski et al. 2013) and/or resource allocation processes (Bonnefond et al. 2010; Campbell and Sharma 2013; Staub et al. 2014; Sugimoto and Katayama 2013), which have already been shown to be important for response inhibition (Bonnefond et al. 2010; Staub et al. 2014). Especially on Go trials, the P2 amplitude was larger in the “accurate/slow” group than the “less accurate/fast” group, suggesting that the “accurate/slow” group shows enhanced resource allocation processes on Go trials. Given that Go trials were the most frequent trials in the task, we infer that stronger resource allocation processes, as reflected by the P2 component, are one of the main reasons for being rather accurate but slow. Such resource allocation processes may reflect the degree of top-down guided attention and the cognitive “effort” that the participants invest in performing the task. One possible explanation for the enhanced Go-P2 amplitude in the “accurate/slow” group would be that an increase in top-down attentional processes counteracts the formation of the automated response tendency induced by the large proportion of Go trials in the task. Following this logic, automatic response tendencies should be less intense in subjects belonging to the “accurate/slow” group. Alternatively, one could assume that automated response tendencies always form in a similar fashion but are subject to varying degrees of top-down cognitive control as reflected by the P2 amplitude. In this case, automatic response tendencies should be more or less similar for everyone, but subjects being more accurate but slow may exert more top-down control to modify this tendency. In line with this interpretation, sLORETA analysis suggests that activation differences in the precuneus (BA7) are related to the predictive P2 feature. The precuneus has been demonstrated to be involved in the orientation, allocation, and shifting of attention, as well as in conscious information processing (Cavanna and Trimble 2006; Lückmann et al. 2014), thus supporting the claim that the observed P2 differences might reflect the top-down allocation of cognitive resources to (Go) stimulus processing. Our findings hence suggest that in the context of inhibition one of the most predictive features for belonging to an “accurate/slow” group or a “less accurate/fast” group is the parietal allocation of (attentional) resources to the more frequent Go response as reflected by the Go-P2 component. Against this background, it would be logical to assume that the amount of attention allocated to regular responses modulates the automatization of responding, which should be reflected by the difficulty to inhibit prepotent response tendencies. Yet, aside from the Go-P2, there were also two time periods/ERP features in Nogo trials that also contributed to the prediction of performance. As can be seen in Fig. 4, these features fall in time frames preceding or following the P2 time window, suggesting that these processes may also be related to processes of resource allocation. It may be speculated that these reflect the speed with which resource allocation processes can be switched on and off. While three of our four top models included Nogo 145–210 ms as a predictor in the feature set, we chose to focus our discussion on the best model. The main reason for this is that this model stands out because of the best classification accuracy in the test sample, the best lower boundary of the 95% CI, and significant P when testing the final accuracy against a random distribution. Moreover, Nogo 145–210 ms does not seem to be a genuine component but a process directly preceding the P2 component (compare to Fig. 4, middle).
In summary, the present study shows that it is possible to predict on a single-subject level by means of ERPs whether subjects belong to an “accurate/slow” or a “less accurate/fast” response inhibition group. While “classical” neurophysiological correlates of response inhibition were clearly modulated by response inhibition performance, they were not suitable to predict group membership on a single-subject level. Instead, resource allocation processes associated with the function of the precuneus (BA7) seem to determine response inhibition performance and allow the prediction of group membership on a single-subject level. The results call for a change in the way we investigate inhibition-related neurophysiological data: Even though the Nogo-N2 and Nogo-P3 reflect cognitive processes involved in response inhibition, they may simply occur too late in the processing cascade to provide us with information useful for making predictions about behavioral performance. This information is, however, provided by the Go-P2 component, which occurs earlier and most likely reflects resource allocation and/or the attentional control exerted during stimulus-response activation. Consequentially, research should take more heed of early processing steps when investigating or drawing inferences about the neural mechanisms mediating inhibitory control in order to achieve a complete picture of all cognitive subprocesses relevant to response inhibition.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grant BE4045/10-2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.-K.S. and C.B. conception and design of research; A.-K.S. performed experiments; A.-K.S., F.P., A.H.N., and C.B. analyzed data; A.-K.S., F.P., A.H.N., and C.B. interpreted results of experiments; A.-K.S. and C.B. prepared figures; A.-K.S., F.P., A.H.N., and C.B. drafted manuscript; A.-K.S., F.P., A.H.N., and C.B. edited and revised manuscript; A.-K.S., F.P., A.H.N., and C.B. approved final version of manuscript.
REFERENCES
- Albert J, López-Martín S, Tapia M, Montoya D, Carretié L. The role of the anterior cingulate cortex in emotional response inhibition. Hum Brain Mapp 33: 2147–2160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 1285–1292, 2005. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185, 2014. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79, 2013. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Evoked activity and EEG phase resetting in the genesis of auditory Go/NoGo ERPs. Biol Psychol 80: 292–299, 2009. [DOI] [PubMed] [Google Scholar]
- Beste C, Dziobek I, Hielscher H, Willemssen R, Falkenstein M. Effects of stimulus-response compatibility on inhibitory processes in Parkinson's disease. Eur J Neurosci 29: 855–860, 2009. [DOI] [PubMed] [Google Scholar]
- Beste C, Konrad C, Uhlmann C, Arolt V, Zwanzger P, Domschke K. Neuropeptide S receptor (NPSR1) gene variation modulates response inhibition and error monitoring. Neuroimage 71: 1–9, 2013. [DOI] [PubMed] [Google Scholar]
- Beste C, Ness V, Falkenstein M, Saft C. On the role of fronto-striatal neural synchronization processes for response inhibition—evidence from ERP phase-synchronization analyses in pre-manifest Huntington's disease gene mutation carriers. Neuropsychologia 49: 3484–3493, 2011. [DOI] [PubMed] [Google Scholar]
- Beste C, Ness V, Lukas C, Hoffmann R, Stüwe S, Falkenstein M, Saft C. Mechanisms mediating parallel action monitoring in fronto-striatal circuits. Neuroimage 62: 137–146, 2012. [DOI] [PubMed] [Google Scholar]
- Beste C, Saft C, Andrich J, Gold R, Falkenstein M. Response inhibition in Huntington's disease—a study using ERPs and sLORETA. Neuropsychologia 46: 1290–1297, 2008. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia 48: 366–373, 2010. [DOI] [PubMed] [Google Scholar]
- Bonnefond A, Doignon-Camus N, Touzalin-Chretien P, Dufour A. Vigilance and intrinsic maintenance of alert state: an ERP study. Behav Brain Res 211: 185–190, 2010. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clin Neurophysiol 113: 1172–1182, 2002. [DOI] [PubMed] [Google Scholar]
- Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci 7: 71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro E, Martínez-Ramón M, Pearlson G, Sui J, Calhoun VD. Characterization of groups using composite kernels and multi-source fMRI analysis data: application to schizophrenia. Neuroimage 58: 526–536, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Eddy C, Rickards HE. Cognitive functioning in Tourette syndrome. Discov Med 8: 191–195, 2009. [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583, 2006. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol 115: 732–744, 2004. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol 64: 135–168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clin Neurophysiol 116: 1808–1825, 2005. [DOI] [PubMed] [Google Scholar]
- Disbrow EA, Sigvardt KA, Franz EA, Turner RS, Russo KA, Hinkley LB, Herron TJ, Ventura MI, Zhang L, Malhado-Chang N. Movement activation and inhibition in Parkinson's disease: a functional imaging study. J Parkinsons Dis 3: 181–192, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Morein-Zamir S, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb Cortex 21: 1155–1165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn 56: 165–176, 2004. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 101: 267–291, 1999. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Bartsch AJ, Herrmann MJ. Electrophysiological measurements of anterior cingulate function. J Neural Transm (Vienna) 109: 977–988, 2002. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, Herrmann MJ, Warnke A. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol 115: 973–981, 2004. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20: 132–143, 2004. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Effects of task complexity on ERP components in Go/Nogo tasks. Int J Psychophysiol 87: 273–278, 2013. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The functional tumor necrosis factor-α (308A/G) polymorphism modulates attentional selection in elderly individuals. Neurobiol Aging 34: 2694.e1–2694.e12, 2013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa JJ, Inuggi A, Blasi V, Cursi M, Annovazzi P, Comi G, Falini A, Leocani L. Response competition and response inhibition during different choice-discrimination tasks: evidence from ERP measured inside MRI scanner. Int J Psychophysiol 89: 37–47, 2013. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50: 1313–1319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B. Dysexecutive syndromes in neurologic disease. J Neurol Phys Ther 31: 119–127, 2007. [DOI] [PubMed] [Google Scholar]
- Heilbronner U, Münte TF. Rapid event-related near-infrared spectroscopy detects age-related qualitative changes in the neural correlates of response inhibition. Neuroimage 65: 408–415, 2013. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Enriquez-Geppert S, Lavallee CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol 87: 217–233, 2013. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalogr Clin Neurophysiol 99: 19–27, 1996. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When “go” and “nogo” are equally frequent: ERP components and cortical tomography. Eur J Neurosci 20: 2483–2488, 2004. [DOI] [PubMed] [Google Scholar]
- Lückmann HC, Jacobs HI, Sack AT. The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog Neurobiol 116: 66–86, 2014. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mückschel M, Stock AK, Beste C. Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb Cortex 24: 2120–2129, 2014. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH, Popescu FC, Bates JA, Goldberg TE, Malhotra AK. Single-subject classification of schizophrenia using event-related potentials obtained during auditory and visual oddball paradigms. Eur Arch Psychiatry Clin Neurosci 263: 241–247, 2013. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH, Popescu FC, Grozea C, Hahn E, Hahn C, Opgen-Rhein C, Urbanek C, Dettling M. Single-subject classification of schizophrenia by event-related potentials during selective attention. Neuroimage 55: 514–521, 2011. [DOI] [PubMed] [Google Scholar]
- Neuhaus AH, Popescu FC, Rentzsch J, Gallinat J. Critical evaluation of auditory event-related potential deficits in schizophrenia: evidence from large-scale single-subject pattern classification. Schizophr Bull 40: 1062–1071, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AH, Trempler NR, Hahn E, Luborzewski A, Karl C, Hahn C, Opgen-Rhein C, Urbanek C, Schaub R, Dettling M. Evidence of specificity of a visual P3 amplitude modulation deficit in schizophrenia. Schizophr Res 124: 119–126, 2010. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology 41: 157–160, 2004. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci 3: 17–26, 2003. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J Clin Neurophysiol 8: 397–413, 1991. [PubMed] [Google Scholar]
- Obeso I, Cho SS, Antonelli F, Houle S, Jahanshahi M, Ko JH, Strafella AP. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul 6: 769–776, 2013. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S, Güntürkün O, Beste C. Lateralized neural mechanisms underlying the modulation of response inhibition processes. Neuroimage 55: 1771–1778, 2011. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol 60: 423–434, 1985. [DOI] [PubMed] [Google Scholar]
- Quetscher C, Yildiz A, Dharmadhikari S, Glaubitz B, Schmidt-Wilcke T, Dydak U, Beste C. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct Funct 220: 3555–3564, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn 56: 234–252, 2004. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417, 2004. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Konrad C, Zwitserlood P, Arolt V, Falkenstein M, Beste C. ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia 48: 2488–2495, 2010. [DOI] [PubMed] [Google Scholar]
- Shen C, Popescu FC, Hahn E, Ta TT, Dettling M, Neuhaus AH. Neurocognitive pattern analysis reveals classificatory hierarchy of attention deficits in schizophrenia. Schizophr Bull 40: 878–885, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, De Bruijn ER, van Wel JH, Ramaekers JG, Verkes RJ. Acute effects of cocaine and cannabis on response inhibition in humans: an ERP investigation. Addict Biol (June 3, 2015). doi: 10.1111/adb.12274. [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Bacon É, Bonnefond A. The effects of aging on sustained attention ability: an ERP study. Psychol Aging 29: 684–695, 2014. [DOI] [PubMed] [Google Scholar]
- Stock AK, Beste C. Lateralization of spatial information processing in response monitoring. Front Psychol 5: 22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AK, Schulz T, Lenhardt M, Blaszkewicz M, Beste C. High-dose alcohol intoxication differentially modulates cognitive subprocesses involved in response inhibition. Addict Biol 21: 136–145, 2016. [DOI] [PubMed] [Google Scholar]
- Stock AK, Wascher E, Beste C. Differential effects of motor efference copies and proprioceptive information on response evaluation processes. PloS One 8: e62335, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto F, Katayama J. Somatosensory P2 reflects resource allocation in a game task: assessment with an irrelevant probe technique using electrical probe stimuli to shoulders. Int J Psychophysiol 87: 200–204, 2013. [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54: 697–711, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science 150: 1187–1188, 1965. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56: 1655–1665, 2011. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Stevens T, Chambers CD. Proactive and reactive stopping when distracted: an attentional account. J Exp Psychol Hum Percept Perform 40: 1295–1300, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage 17: 1207–1216, 2002. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. It's not too late: the onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology 52: 472–480, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]