Abstract
Down syndrome cell adherence molecule (DSCAM) contributes to the normal establishment and maintenance of neural circuits. Whereas there is abundant literature regarding the role of DSCAM in the neural patterning of the mammalian retina, less is known about motor circuits. Recently, DSCAM mutation has been shown to impair bilateral motor coordination during respiration, thus causing death at birth. DSCAM mutants that survive through adulthood display a lack of locomotor endurance and coordination in the rotarod test, thus suggesting that the DSCAM mutation impairs motor control. We investigated the motor and locomotor functions of DSCAM2J mutant mice through a combination of anatomical, kinematic, force, and electromyographic recordings. With respect to wild-type mice, DSCAM2J mice displayed a longer swing phase with a limb hyperflexion at the expense of a shorter stance phase during locomotion. Furthermore, electromyographic activity in the flexor and extensor muscles was increased and coactivated over 20% of the step cycle over a wide range of walking speeds. In contrast to wild-type mice, which used lateral walk and trot at walking speed, DSCAM2J mice used preferentially less coordinated gaits, such as out-of-phase walk and pace. The neuromuscular junction and the contractile properties of muscles, as well as their muscle spindles, were normal, and no signs of motor rigidity or spasticity were observed during passive limb movements. Our study demonstrates that the DSCAM mutation induces dystonic hypertonia and a disruption of locomotor gaits.
Keywords: DSCAM, EMG, locomotion, mutant, posture
signaling pathways are important in the patterning of neural circuits. Although the role of DCC (deleted in colorectal cancer) and its ligand, netrin-1, in the development of the spinal locomotor network has been described (Rabe et al. 2009; Rabe Bernhardt et al. 2012; Rybak et al. 2013; Vallstedt and Kullander 2013), very little is known about the contribution of DSCAM (Down syndrome cell adhesion molecule). However, given its wide expression throughout the central nervous system during development (Yamakawa et al. 1998) and its interactions with DCC and UNC5 on netrin-1-driven axonal growth in vitro (Liu et al. 2009; Purohit et al, 2012; Qu et al. 2013), DSCAM likely plays an important role in neural circuit formation.
At the cellular level, DSCAM mutation prevents synapse formation and transmission between sensory neurons and motoneurons in Aplysia (Li et al. 2009) and impairs the dendritic arborization and spine formation of cortical pyramidal tract neurons in the developing mouse cortex (Maynard and Stein 2012). At the synaptic level, ectopic DSCAM expression prevents synaptic targeting in Drosophila (Cvetkovska et al. 2013) and lamina-specific synaptic connections in the chick retina (Yamagata and Sanes 2008). At the circuit level, knockdowns of DSCAM impair the axonal growth of spinal commissural interneurons in Drosophila (Ly et al. 2008). Nevertheless, no defects have been reported in spinal commissural axons in embryonic and neonatal DSCAM mutant mice (Palmesino et al. 2012). Functionally, DSCAM mutation impairs the normal synchronization of preinspiratory neurons to their motoneuronal targets in the DSCAM mutant mouse, thus leading to an irregular rhythm and respiration with frequent apneas, eventually causing death at birth (Amano et al. 2009). Moreover, mutant mice that survive through adulthood exhibit a hunched posture and limb rigidity, and they perform badly on the rotarod test (Xu et al. 2011), thus raising the possibility that the DSCAM mutation might impair the neural control of movement at the central and peripheral level.
On one hand, in vitro mouse studies have shown that DSCAM interacts with DCC and UNC5 to either promote or prevent netrin-1-induced axonal growth of cortical neurons or spinal commissural interneurons (Ahmed and Cash 2013; Liu et al. 2009; Purohit et al. 2012). Interestingly, DCC and netrin-1 knockout mice exhibit an aberrant projection of the corticospinal tract (Finger et al. 2002) and spinal commissural axons (Rabe et al. 2009; Rabe Bernhardt et al. 2012; Vallstedt and Kullander 2013), which are associated with an aberrant left-right motor alternation leading to bilateral mirror movements. Therefore, the DSCAM mutation might impair the central motor system.
On the other hand, peripheral changes have been previously reported with other CAM mutations. Indeed, N-CAM mutant mice show an abnormal morphology, physiology, and function of their neuromuscular junction (Chipman et al. 2010, 2014; Polo-Parada et al. 2001; Rafuse et al. 2000). Although there is no information about neuromuscular transmission in DSCAM mutant mice, DSCAM is expressed in motoneurons and therefore could be involved in the normal functioning of the neuromuscular junction and the contractile properties of muscles. As such, changes in the peripheral motor system could lead to the aberrant motor phenotype previously reported in DSCAM mutants (Xu et al. 2011).
Our results are presented in two companion studies. With the use of a mutant mouse lacking DSCAM with an inbred genetic background to prevent the phenotypic variability of other DSCAM mutants (Fuerst et al. 2010; Xu et al. 2011; Schramm et al. 2012), the goal of the first study is to evaluate the locomotor and motor dysfunctions in DSCAM2J mice, in addition to characterizing the nature of the motor tone deficits and identifying the central vs. peripheral neural origin of the motor phenotype. The companion study, using neonatal spinal cord preparations, describes the role of DSCAM in the development of spinal locomotor and sensorimotor circuits (Thiry et al. in press).
MATERIALS AND METHODS
Experiments were conducted on wild-type (WT) and DSCAM2J mutant mice with a C3H/HeDiSn background (Fuerst et al. 2010) purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Mice of either sex were 2–5 mo old. All experiments were performed according to the guidelines of the Canadian Council on Animal Care, and all procedures were approved by the local councils on animal care of Université Laval and the research center of the CHU de Québec.
Magnetic Resonance Imaging and Analysis
Magnetic resonance imaging (MRI) studies were performed on a M2 compact high-performance MRI system. A subset of mice (n = 6 WT; n = 5 DSCAM2J) were anesthetized with isoflurane and placed in an MRI-adapted stereotaxic holder. Heartbeat and body temperature were continuously monitored. A first scan was performed to center the field of view, followed by a scan of 20 slices covering the head (TE/TR = 76.8/3924.7 ms; flip angle = 60°; field of view = 40 mm; 0.7-mm slices, 0.1-mm inter-slices, 142.8571429 × 142.8571429 μm; 8 excitations; 15-min acquisition time).
For analysis, the brain areas, neural tissues, and ventricles were processed and quantified using the NeuronJ plug-in in ImageJ software (National Institutes of Health, Bethesda, MD) and Adobe Photoshop. The reconstructed volume was inferred from the distance between each frame.
Kinematic Recordings and Analysis During Locomotion
All mice were walked on a single-lane mouse treadmill belt (Panlab) at various speeds (5, 10, 15, 20, 30, 40, 50, 60, 70, 80, and 90 cm/s). Once trained to walk at 0.4 m/s for 10–20 consecutive steps, mice were videotaped with high-frequency cameras (90 frames/s; Genie HM640; Dalsa Teledyne) placed on the left and the right sides of the treadmill. Videos were digitized on a personal computer with StreamPix 6 (NorPix).
For kinematic analysis, videos were analyzed offline by a custom-designed software (Mr. Philippe Drapeau) to determine the timing of foot contacts and lifts of forelimbs and hindlimbs at all investigated treadmill speeds. With these values, we computed the duration of the step cycle, as well as the stance and the swing phase, with custom-written routines in MATLAB (The MathWorks). The step cycle was defined as the interval between two successive foot contacts. The duration of the stance phase corresponds to the interval between the foot contact and the subsequent foot lift and the swing phase to the interval between the foot lift and the next foot contact. The duty cycle is the percentage of the step cycle occupied by the stance phase (Table 1).
Table 1.
Step cycle properties
| Gait | Step Frequency, Hz | Stance Duration, s | Swing Duration, s | Duty Cycle, % |
|---|---|---|---|---|
| Hop | ||||
| WT | 0.9 ± 0.9 | 1.39 ± 0.63 | 0.10 ± 0.04 | 91.1 ± 4.9 |
| DSCAM2J | 1.5 ± 1.4 | 1.15 ± 0.89 | 0.14 ± 0.09 | 82.0 ± 18.1 |
| P value | 0.061 | 0.062 | 0.0051 | 0.0064 |
| Asymmetrical | ||||
| WT | 2.3 ± 1.2 | 0.47 ± 0.29 | 0.09 ± 0.02 | 79.6 ± 10.8 |
| DSCAM2J | 2.7 ± 1.5 | 0.39 ± 0.37 | 0.12 ± 0.06 | 66.6 ± 16.2 |
| P value | 0.0033 | 1.09 × 10−7 | 1.1 × 10−22 | 9.3 × 10−21 |
| Pace | ||||
| WT | 2.0 ± 0.9 | 0.58 ± 0.32 | 0.09 ± 0.03 | 87.1 ± 5.5 |
| DSCAM2J | 3.3 ± 1.3 | 0.24 ± 0.16 | 0.14 ± 0.06 | 61.0 ± 12.6 |
| P value | 5.0 × 10−4 | 5.2 × 10−6 | 0.00036 | 1.3 × 10−8 |
| Lateral walk | ||||
| WT | 3.4 ± 1.1 | 0.24 ± 0.29 | 0.09 ± 0.02 | 70.9 ± 9.3 |
| DSCAM2J | 3.6 ± 1.2 | 0.20 ± 0.11 | 0.11 ± 0.05 | 60.2 ± 9.2 |
| P value | 0.0058 | 1.4 × 10−14 | 1.1 × 10−16 | 6.2 × 10−49 |
| Trot | ||||
| WT | 4.1 ± 1.1 | 0.18 ± 0.09 | 0.09 ± 0.07 | 64.2 ± 8.0 |
| DSCAM2J | 3.9 ± 1.6 | 0.27 ± 0.27 | 0.10 ± 0.04 | 66.9 ± 12.0 |
| P value | 0.89 | 0.66 | 0.18 | 0.36 |
Data are means ± SD for wild-type (WT) and Down syndrome cell adherence molecule mutant (DSCAM2J) mice; no. in parentheses indicates sample size. P values indicate significance of statistical difference tested with the Mann-Whitney test.
Circular statistics was used to evaluate the phase values of homologous forelimbs and hindlimbs, as well as the left homolateral limbs (Batschelet 1981; Drew and Doucet 1991; Kjaerulff and Kiehn 1996; Zar 1984). A phase value corresponded to the time of foot contact relative to the reference limb step cycle (see Fig. 3). Phase values range from 0 to 1: a phase of 0 (or 1) indicates a perfect synchrony, whereas a phase of 0.5 denotes a strict alternation. These phase values were used to attribute a gait to each step cycle. In this study, we observed five gaits: lateral walk, pace, trot, hopping at low step frequency, and asymmetrical walk.
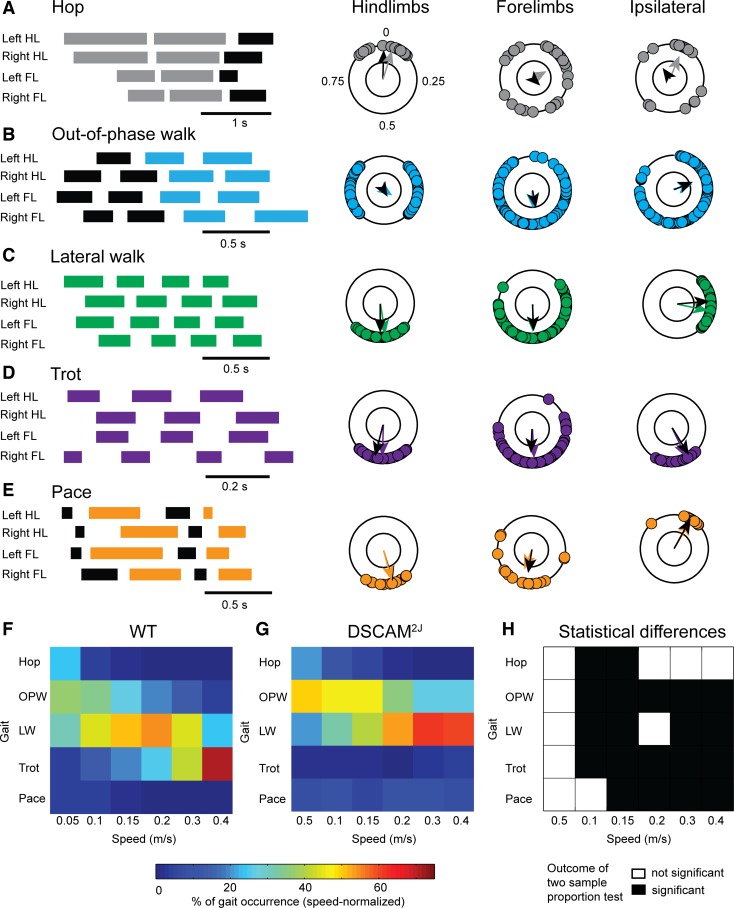
Fig. 3.
DSCAM2J mice use different locomotor gaits according to the treadmill belt speed. A–E: gait diagrams (left) and polar plots (right) were used to define gaits. At walking speeds, we identified hop, out-of-phase walk (OPW), lateral walk (LW), trot, and pace. HL, hindlimb; FL, forelimb. F and G: color-coded matrix of gait occurrence (in %) for each speed. Note in WT (F) a change from out-of-phase walk to lateral walk, and then from lateral walk to trot. Changes were delayed in DSCAM2J (G) and trot barely emerged. H: matrix of statistical differences of gait occurrence between WT and DSCAM2J mice, tested with a 2-sample proportion test.
Reflective markers were placed on joints for a subset of mice (n = 3 DSCAM2J and 3 WT mice). Only the hindlimb joints were studied: iliac crest, knee, ankle, metatarsophalangeal (MTP) joint, and extremity of the fourth digit (henceforth referred as the toe). We calculated the stride length and height of fore and hind foot, as well as the maximal speed and acceleration for the limb trajectory. For each joint, we analyzed the angular excursion (maximal minus minimal angle formed at the joint). The maximal and minimal limb placement was calculated by the angle formed by the iliac crest and the toe axis with an axis perpendicular to the ground. The posture of the animal was defined as the maximal vertical distance between its toe and its iliac crest.
Force and Kinematic Recordings and Analysis
Evaluation of rigidity and spasticity.
Mice were held at the thoracic level by the skin of the back to prevent any disruption of the forearm movement. Mice were approached to a triangular bar attached to a force transducer (FT03 C; Grass, Quincy, MA) until they grabbed it. The forelimb was flexed or extended in various directions and speeds along a sagittal plan by the experimenter. Mice were filmed on both sides to evaluate angular excursion of the elbow. Forearms were shaved and markers placed on the shoulder and the metacarpophalangeal joint. By knowing the exact X-Y position of the shoulder and wrist joints, as well as the length of the forearm and the arm, the position of the elbow could be obtained by triangulation (see Fig. 8).
Fig. 8.
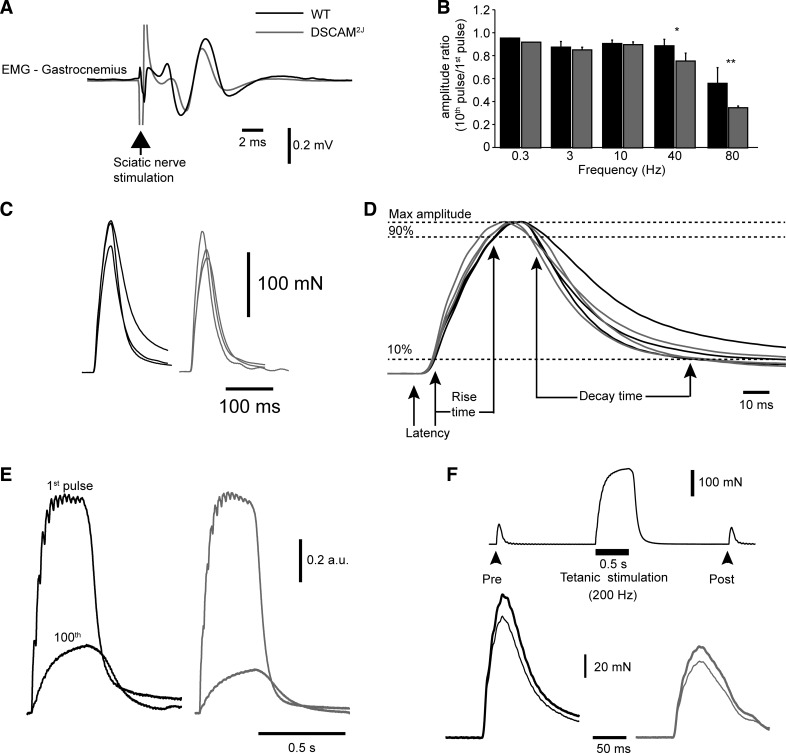
DSCAM2J mice have normal neuromuscular and contractile properties. A: averaged electromyographic (EMG) response to a single stimulation of the sciatic nerve. Note similarity of waveforms. B: bar graph of the ratio of the 10th and 1st pulses during repetitive nerve stimulation for WT (black bars) and DSCAM2J mice (gray bars). Statistical differences were tested with the Mann-Whitney test: *P < 0.05; **P < 0.01. C: averaged force response to a suprathreshold single stimulation of the sciatic nerve. Each trace corresponds to a mouse (n = 3 WT and 3 DSCAM2J). D: enlargement of traces in C to show latency, rise time, and decay time. E: averaged force traces (n = 5 trials) of the 1st and 100th trains (40 Hz, 300 ms, every second, suprathreshold). F: protocol of posttetanic plasticity (top trace) and averaged force response (bottom trace; n = 5 trials) before (thick trace) and after tetanus (thin trace).
Muscle properties.
The force developed by the gastrocnemius was recorded under isoflurane anesthesia (2–3%). The fascia surrounding the muscle was removed, and the distal tendon was cut and attached to a force transducer (FT03 C; Grass) with 6-0 suture. Signal was amplified with a signal condition amplifier (model 2310; Measurements Group, Raleigh, NC). To evoke muscle contractions, two stainless steel wires were wrapped around the sciatic nerve and spaced by 2–3 mm, and voltage pulses were delivered with an isolated pulse stimulator (model 2100; A-M Systems). The proximal end of the sciatic nerve was cut to prevent spinal reflex or back-propagation from the motoneurons. The peroneal nerve was also cut to prevent contraction of the tibialis anterior.
For analysis, force signals were exported to MATLAB and analyzed with a custom routine. The angular movement was extracted with custom-designed software and exported to MATLAB to correlate with force signals. To calculate velocity of force variation and angular movement of the elbow, the ascending phase before grip release was identified. The duration and amplitude corresponded to the 10–90% period of the maximal amplitude.
EMG Recordings and Analysis
EMG recordings were performed using single-strand nickel-chromium wire arranged in a duplex configuration (catalog no. CFW2027239; California Fine Wire) on four WT and four DSCAM2J mice. As previously described (Ritter et al. 2014), 2–4 mm were cut from one of the two wires to achieve bipolar recording of gross EMG signals. These wires were inserted into the tibialis anterior (TA), gastrocnemius lateralis (GL), and vastus lateralis (VL). EMG signals were amplified (1,000×) and bandpass filtered (0.1–10 kHz), digitally converted (Power 1401; CED, Cambridge, UK), and acquired with Spike2 version 8 (CED). Force or EMG recordings were synchronized with the video acquisition to correlate EMG activities with the locomotor behavior during offline analysis.
EMG signals were high-pass filtered and bursts were identified. Analyzed epochs were selected on the basis of a steady locomotion evaluated with our video recordings. The duration and area under the amplitude curve of rectified EMG bursts were quantified with custom-designed software for EMG analysis.
Immunohistochemistry
Fiber typing.
For fiber typing, gastrocnemius medialis, GL, TA, and soleus muscles were carefully dissected out, embedded in a tissue-freezing medium (Triangle Biomedical Sciences), and frozen for cryosectioning. Transverse sections (10 μm thick) of the mid-belly portion of muscles were incubated overnight with primary antibodies for myosin heavy chain I or II (1:30 dilution; Leica Biosystems), 1 h with biotinylated anti-mouse IgG secondary antibodies, and 30 min with horseradish peroxidase-labeled avidin D (Vector Laboratories). Immunoreactivity was revealed by diaminobenzidine chromogen (Dako Cytomation).
For anatomical analysis of the fiber typing, three images of each section were acquired with an inverted light microscope (Nikon Eclipse TE300) and a Nikon D5000 monitor. Approximately 500 fibers per section were analyzed. To assess whole muscle cross-sectional area and fiber cross-sectional area, three images of each muscle section were randomly acquired. Approximately 100 fibers per section were analyzed. Cross-sectional areas were calculated using ImageJ software version 1.46r (NIH).
Neuromuscular junction.
To label the neuromuscular junction, the TA and the GL muscles of WT and DSCAM2J mice were dissected out and fixed for 10 min in freshly prepared 4% paraformaldehyde in 0.1 M PBS. Once fixed and washed with 1× PBS, individual fibers were carefully teased apart to facilitate the penetration of antibodies. Muscle fibers were permeabilized with 2% Triton X-100 in PBS for 30 min and blocked with 5% donkey serum-1% Triton X-100 in PBS for 30 min. Samples were incubated overnight at 4°C in the blocking solution with primary antibodies against axonal neurofilaments (2H3; dilution 1:50) and presynaptic synaptic vesicles (SV2; dilution 1:100). Fibers were incubated for 2 h with Alexa Fluor 488 secondary antibody (1:250) and 1.5 μg/ml tetramethylrhodamine α-bungarotoxin (α-BTX) and mounted in Mowiol on slides with coverslips.
Images were taken on an Axio Imager M2 microscope connected to an AxioCam camera and using ZEN software (Zeiss). Muscle fibers were imaged using a ×63 oil objective (z stacks, 0.6-μm intervals). Z stacks were projected into single images using ImageJ. The resulting images were used for measuring the end-plate area.
Muscle spindle.
The TA and GL muscles were dissected free from mice transcardially perfused with 4% paraformaldehyde. The muscles were cryoprotected for 24 h in 30% sucrose in PBS and cut in 20-μm cryostat sections. Muscle spindles were labeled overnight with antibodies against the peripheral axon marker protein gene product 9.5 (PGP9.5; AbD Serotec 7863-0504; dilution 1:200) and vesicular glutamate transporter 1 (VGluT1; Chemicon-Millipore MAB5502; dilution 1:1,000). The following day, sections were incubated for 3 h with the following secondary antibodies: donkey anti-rabbit Alexa Fluor 488 (Invitrogen; dilution 1:1,000) and donkey anti-mouse Alexa Fluor 594 (Invitrogen; dilution 1:1,000).
Muscle spindles were counted on three muscle cross sections in three WT and three DSCAM2J mice. For each muscle section, the number of muscle spindles was counted and normalized by the surface (mm2) of the muscle section.
Statistics
Mean and SD are presented for each gait. Because data were not normally distributed (assessed by Kolmogorov-Smirnov test), the statistical significance of differences between DSCAM2J and WT mice was tested with a nonparametric Mann-Whitney test for paired comparison. When speed was considered, we used a Kruskal-Wallis test followed by a post hoc Tukey's honest significant difference (HSD) test. For contiguous speeds showing the same statistical outcome between WT and DSCAM2J mice, data were pooled for purpose of clarity. For proportions, we used a two-sample proportion test. We verified the significance of the mean phase of interlimb coupling with a Rayleigh test, and we compared mean phases with the Watson-William test (Batschelet 1981; Zar 1984). We compared slopes with a Student's t-test, using the squared standard error of the linear regression as an estimator of variance (Andrade and Estevez-Perez 2014).
RESULTS
DSCAM2J Mice Display no Severe Hydrocephalus
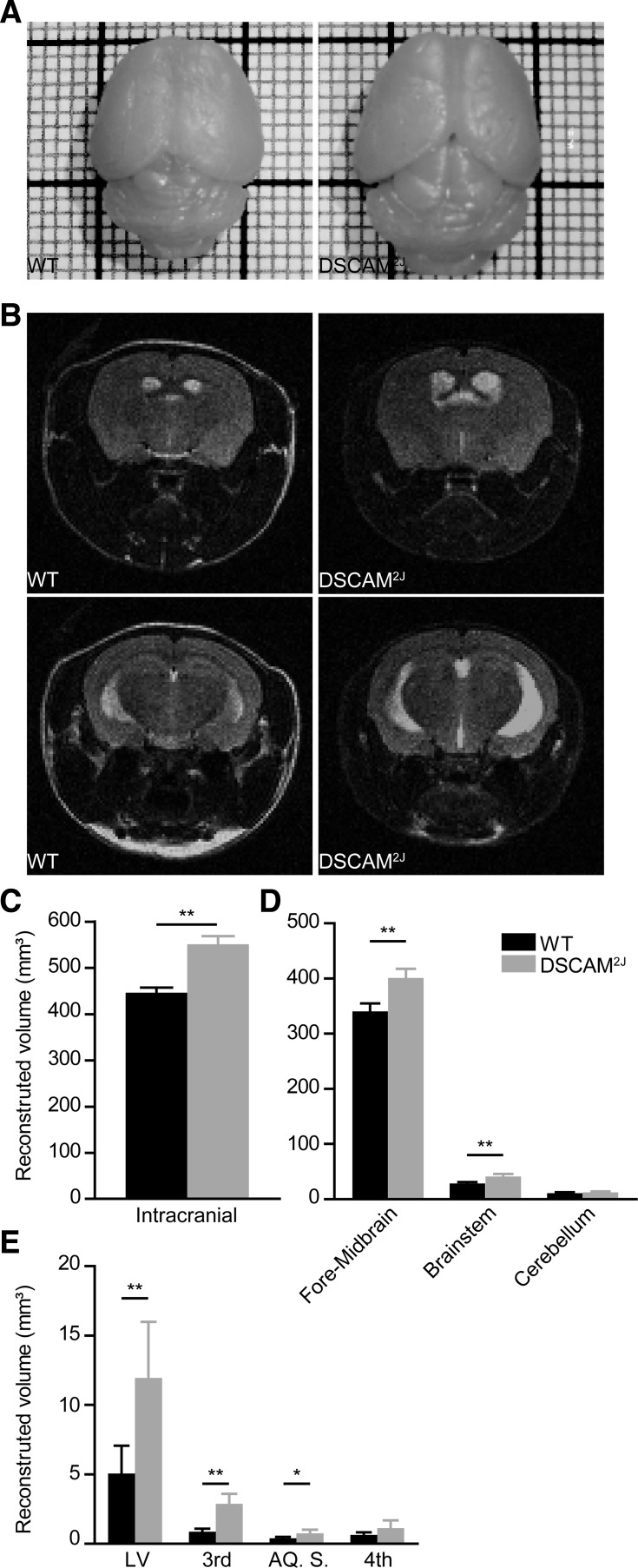
As shown by the pictures of the brain of both mouse lines, DSCAM2J mutants displayed a significantly larger brain size (Fig. 1A). Although there was no overt disorganization in their gross anatomy, MRI imaging of DSCAM2J mice showed a larger skull and brain size with slight ventricle dilation, illustrated by white regions (Fig. 1B). Quantification and reconstruction from MRI images revealed a significant increase in the volume occupied by neural tissues in DSCAM2J mice (mean ± SD: 551.30 ± 17.12 for DSCAM2J vs. 446.00 ± 11.19 mm3 for WT, P = 0.0043; Fig. 1C) with a significant increased volume in brain (401.00 ± 16.65 vs. 340.00 ± 14.40 mm3, P = 0.0043) and brain stem (40.98 ± 4.46 vs. 28.38 ± 1.07 mm3, P = 0.0043), but no differences in the cerebellum (Fig. 1D). DSCAM2J mutants also showed larger volumes in the lateral ventricles (11.94 ± 4.04 vs. 5.05 ± 2.02 mm3, P = 0.0043), the third ventricle (2.868 ± 0.729 vs. 0.8738 ± 0.2136 mm3, P = 0.0043), and the aqueduct of Sylvius (0.7615 ± 0.2533 vs. 0.3925 ± 0.1013 mm3, P = 0.019). Compared with previous DSCAM mutant mouse models, DSCAMdel17 and DSCAM3J (Maynard and Stein 2012; Schramm et al. 2012; Xu et al. 2011), DSCAM2J mice displayed no collapsing cortex and no severe hydrocephalus.
Fig. 1.
Down syndrome cell adherence molecule (DSCAM)2J mice display bigger brains and ventricle dilations. A: photographs of brains from wild-type (WT; left) and DSCAM2J mice (right). Note the larger size of the DSCAM2J brain. B: MRI scans from WT (left) and DSCAM2J mice brains (right) taken at the forebrain and midbrain levels. Note larger ventricles (white regions) of the DSCAM2J brain. C–E: means and SD of the total brain volume (C), brain regions (D), and ventricles (E: LV, lateral ventricles; 3rd, third ventricle; AQ. S., Sylvius aqueduct; 4th, fourth ventricle). Significance of differences was tested by Mann-Whitney test: *P < 0.05; **P < 0.01.
Locomotor Gaits
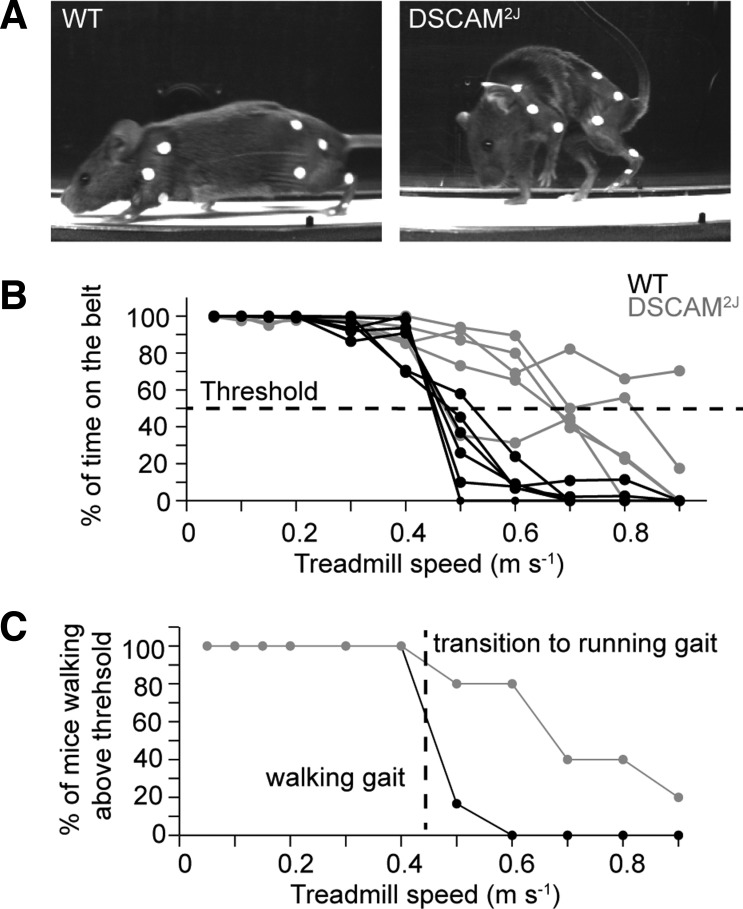
Despite a pronounced hunched posture marked by a forward flexion of the cervicothoracic spine (Fig. 2A), DSCAM2J mice walked as well and even longer than their WT littermates at high walking speed (Fig. 2B). Using a threshold of 50% for the time spent on the treadmill belt, we evaluated the percentage of animals walking at each speed (Fig. 2C). Whereas most mutants walked up to 0.6 m/s, most WT mice failed to sustain a walk above 0.4 m/s. For comparison purposes, we therefore limited our investigations of the locomotor and postural control of DSCAM2J mice to treadmill speeds up to 0.4 m/s.
Fig. 2.
DSCAM2J mice display a higher locomotor endurance. A: photographs of a WT (left) and DSCAM2J mouse (right) during locomotion on a treadmill belt. Markers were placed on hindlimb and forelimb joints. B: plot of percentage of time during the test period that mice were walking on the belt vs. treadmill speed. Each line corresponds to an animal. A threshold of 50% of the time on the belt (horizontal dashed line) was used to decide whether a mouse was walking at a given speed. C: plot of the percentage of mice walking above threshold. Vertical dashed line indicates the cutoff treadmill speed used in this study.
Using the interlimb coupling instead of the footfall pattern, we identified and characterized five locomotor gaits at walking speed. Figure 3 illustrates the representative gait diagrams and polar plots for each locomotor gait. Except for the pace, all gait diagram examples were taken from WT mice. In polar plots, the direction of arrows indicates the mean phase of the interlimb coupling (colored arrows for WT mice and black arrows for DSCAM2J mice). The mean phase was similar for both WT and DSCAM2J, thus revealing that gait patterns observed in WT were conserved in DSCAM2J mutants.
With regard to locomotor gaits, hop was characterized by an in-phase coupling of hindlimbs (i.e., synchronized hindlimbs), whereas forelimbs and ipsilateral limbs did not show any coupling preferences (Fig. 3A). Out-of-phase walk was defined by an out-of-phase coupling of hindlimbs and a loose anti-phase coupling of forelimbs (Fig. 3B). Lateral walk, trot, and pace were marked by a robust anti-phase coupling (i.e., strong alternation) of the left-right hindlimbs and forelimbs but a distinct ipsilateral forelimb-hindlimb coupling. Indeed, the forelimb ipsilateral to the hindlimb was out of phase during lateral walk (Fig. 3C), anti-phase during trot (Fig. 3D), and in phase during pace (Fig. 3E). Noteworthy, this last gait, pace, common in camels and long-legged dogs (Abourachid et al. 2007; Hildebrand 1976), has never before been reported in the mouse.
Although the pattern of locomotor gaits was conserved, its occurrence varied according to the walking speed. WT mice mainly used an out-of-phase walk at slow walking speed below 0.1 m/s (mean step frequency of 2.3 Hz), lateral walk at intermediate speed (mean step frequency of 3.4 Hz), and trot at 0.3–0.4 m/s (mean step frequency of 4.1 Hz; Fig. 3F and Table 1). Except at very slow walking speed (0.05 m/s; Fig. 3H), DSCAM2J mice preferentially used different locomotor gaits over different range of speed. Mutants predominantly adopted an out-of-phase walk peaking at low speed and lateral walk preferentially at high walking speed (Fig. 3, G and H). Interestingly, DSCAM2J preferred lateral walk and never shifted to trot at the highest walking speed assessed. Moreover, they paced for 5–9% of the step cycles at all speeds, whereas WT mice only paced below 0.15 m/s and for less than 5% of step cycles, thus suggesting that pace was likely an aberrant gait in the mouse.
Except for hop and trot, DSCAM2J mice showed a longer swing phase, a shorter stance phase, and, conversely, a higher step frequency than WT mice (Table 1). These differences might imply some disruption in the activity of the neuronal circuit generating the locomotor rhythm and pattern.
Hind Foot Placement and Trajectory
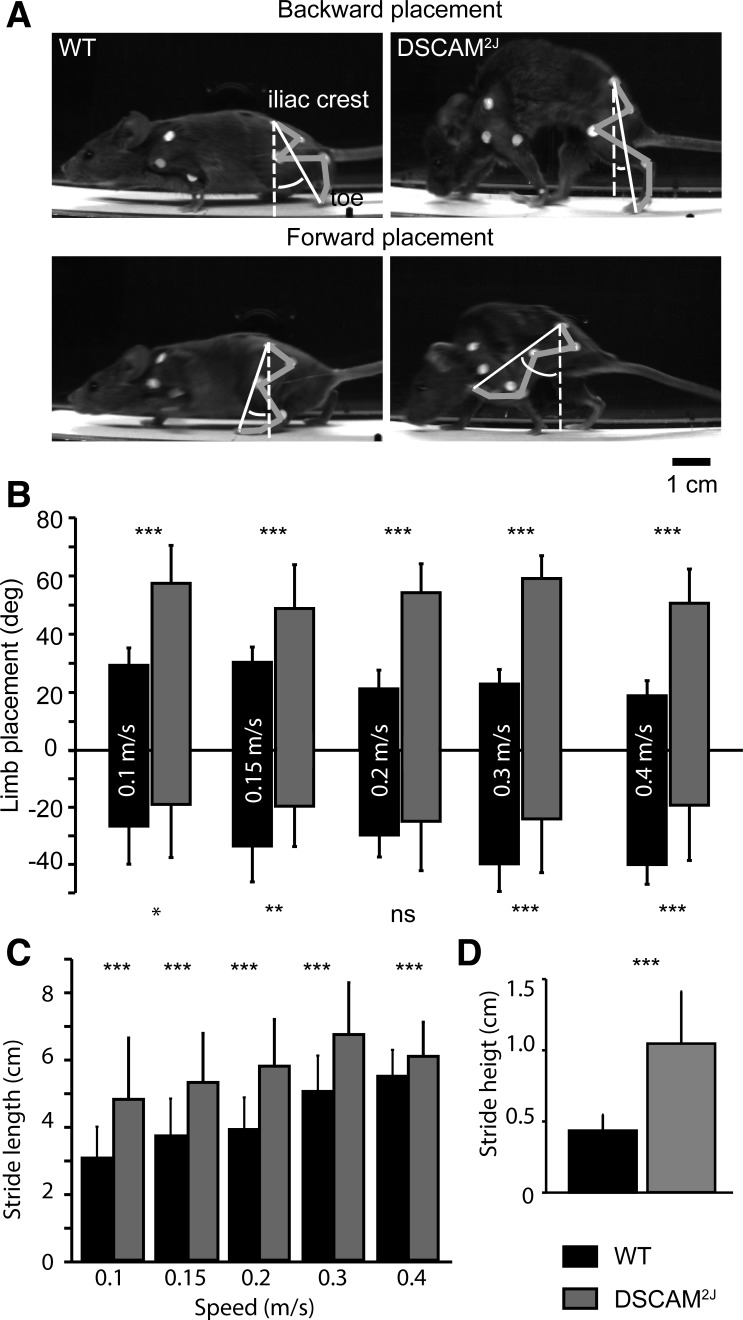
Given the discrepancies in locomotor gait usage in DSCAM2J vs. WT mice, we next wondered if the hind foot placement and trajectory might be impaired upon the DSCAM mutation. To assess the foot placement, we measured the angle formed by the toe and the iliac crest in relation to an axis perpendicular to the ground (Fig. 4A). Because there was an effect of speed on the hind foot placement in WT mice, we compared the maximal forward and backward foot placement of WT and DSCAM2J mice as a function of speed. In contrast to their WT littermates, the maximal forward placement was much more advanced for DSCAM2J (Fig. 4B), which shows that the longer swing phase was associated with an exaggerated forward projection of the hind foot. Conversely, the backward placement was more restricted at all speeds, except at 0.2 m/s, interestingly a speed at which both WT and DSCAM2J relied equally on lateral walk (Fig. 3, F–H).
Fig. 4.
DSCAM2J mice present an aberrant limb placement and trajectory. A: photographs of WT (left) and DSCAM2J mice (right) at their maximal backward (top) and forward limb placement (bottom). B–D: bar graphs of limb placement (B), stride length (C), and stride height (D). Because there was no effect of speed for the stride height, all data were pooled. Statistical differences between WT and DSCAM2J mice were tested at each speed with a Mann-Whitney test: *P < 0.05; **P < 0.01; ***P < 0.001 (ns, not significant).
To quantify the real stride length, we measured the distance covered from the onset of the swing phase to the onset of the stance phase by taking into consideration the speed of the treadmill belt and the duration of the swing phase (Fig. 4C). Both the stride length and height were higher in DSCAM2J mice at all speeds (Fig. 4, C and D). This suggests that the longer swing phase was associated with a higher forward placement and trajectory of the hind foot in DSCAM2J mice.
Intralimb Coordination
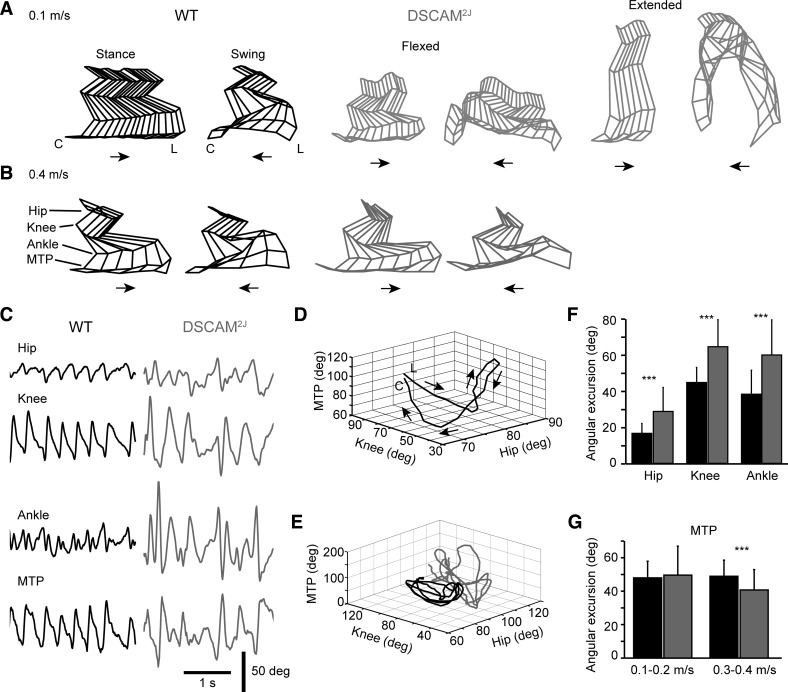
To assess whether changes in the hind foot placement and trajectory we reported (Fig. 4) could result from an impaired multijoint coordination of the hindlimb, we digitally reconstructed the hindlimb by using the X and Y positions of reflective markers placed on the joints during treadmill locomotion. Compared with WT mice, DSCAM2J mice presented either a flexed or extended posture at slow walking speed (Fig. 5A), with a marked hyperflexion of the limb during the swing phase, especially in the extended posture. That latter extended posture disappeared at high walking speed (Fig. 5B).
Fig. 5.
DSCAM2J mice display an increased angular excursion in the hindlimb joints. A and B: stick diagrams of the hindlimb during the stance and swing phases at low (A) and high walking (B) treadmill speeds. Note the occurrence of 2 postures for DSCAM2J mice (flexed vs. extended). C: traces illustrating the angular excursion of each joint for several subsequent step cycles. Note larger and irregular joint movements for DSCAM2J mice. D: 3-dimensional plot of the angle of the hip, knee, and metatarsophalangeal (MTP) joint from foot lift (L) to contact (C), or swing phase, of a WT mice. E: 3 subsequent step cycles of a WT and a DSCAM2J mouse reveal a higher and more variable angular movement of joints for the DSCAM2J mouse. F: bar graph of the angular excursion of proximal and intermediate joints. Data were pooled because differences between WT and DSCAM2J mice were similar at all speeds. G: bar graph of the angular excursion at the distal MTP joint. Data at speeds showing similar statistical differences were pooled for sake of clarity as in F. ***P < 0.001.
To quantify the multijoint coordination, we evaluated the maximal angular excursion of the proximal (hip), intermediate (knee and ankle), and distal (MTP) joints of the hindlimb. The angular excursion of the four joints tended to be larger and more variable in DSCAM2J than in their WT littermates (Fig. 5C). As shown for a single step cycle of a WT mouse (Fig. 5D), the knee started to flex and the hip to extend at the onset of the swing (foot lift indicated by L); the MTP then opened, and once it reached a maximum, the hip initiated a flexion phase associated with a forward propulsion of the limb. The flexion of the hip was associated with a flexion of the MTP. As the MTP angle reached a minimum, the knee extended and the MTP once again extended until the onset of the stance phase (hind foot contact indicated by C). As shown by the superposition of three successive step cycles (Fig. 5E, black traces), this sequence was very stable in the WT. In contrast, in the DSCAM2J mice the hip was constantly more in extension, and the angular excursion of the three joints was more variable across successive step cycles (Fig. 5, C–E, gray traces).
We quantified the angular excursion of the joints according to the treadmill belt speed. Because the angular excursion observed in DSCAM2J was significantly higher than in WT at all speeds, we pooled the data for the hip, knee, and ankle (Fig. 5F). In contrast to the other joints, the angular excursion of the MTP was similar at 0.1–0.2 m/s but significantly smaller at 0.3–0.4 m/s. Despite a reduced flexibility in their distal joint, DSCAM2J mice displayed an exaggerated flexibility in both proximal and intermediate joints during locomotion.
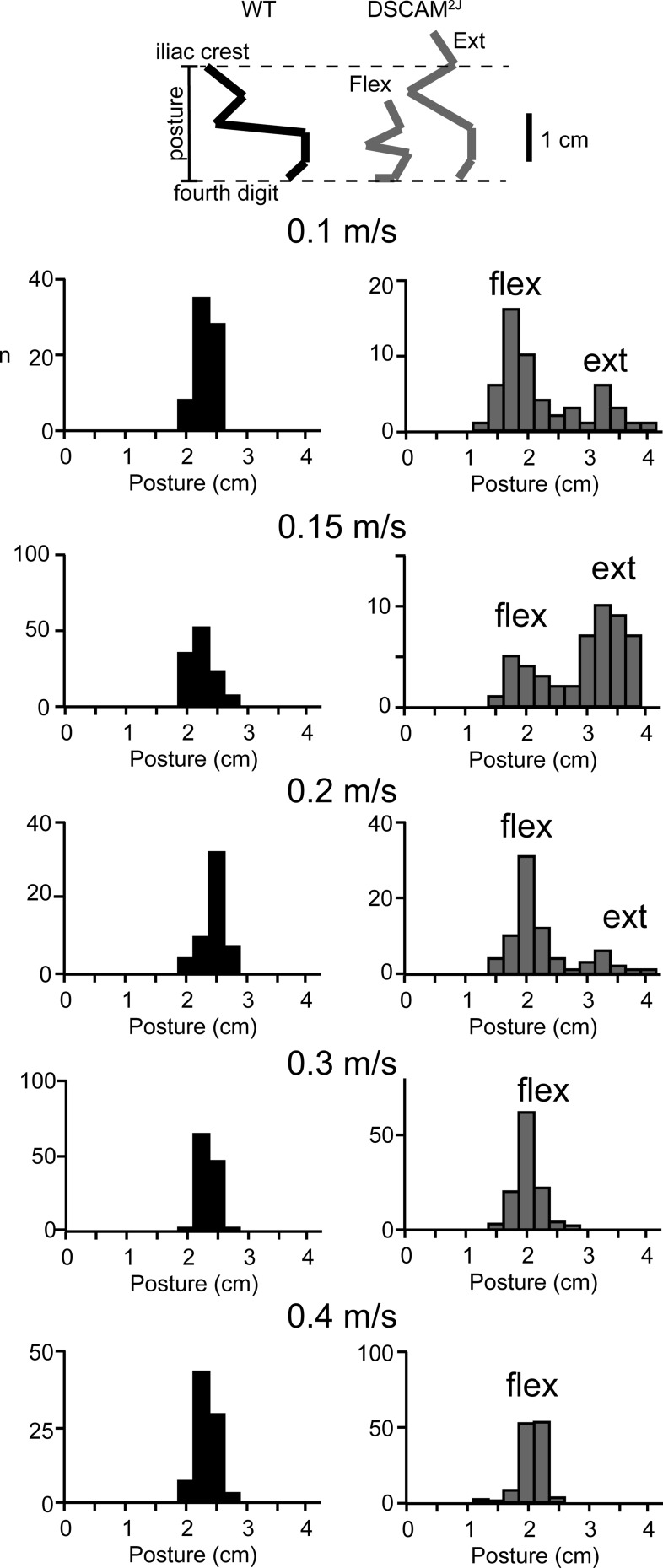
Extended and Flexed Postures
One striking feature of DSCAM2J mice was their aberrant transition of postures while walking (Fig. 6). To evaluate whether DSCAM2J mice displayed distinct postures according to their speed, we measured the vertical distance from the toe to the iliac crest before the onset of the swing phase. Compared with the overall normal posture seen in WT mice (Fig. 6, black bars), our analysis revealed a bimodal distribution of either extended and flexed postures in DSCAM2J mice at slow speed, with a predominant extended posture at 0.15 m/s and a more flexed posture at high walking speed. With respect to the WT mouse, our results argue that the mutant shifts smoothly and progressively from an extended posture to a more flexed posture with increasing speed. Given the smaller body weight and size of the DSCAM2J mouse with respect to the WT, this flexed posture is likely normal.
Fig. 6.
Extended posture of DSCAM2J mice observed at low speed disappears at high walking speed. Stick diagrams (top) illustrate how posture was measured (vertical distance between the 4th digit and the iliac crest). Posture was measured before onset of the swing phase. Data are presented in histograms for WT (left) and DSCAM2J mice (right) at all speeds (flex, flexed; ext, extended). Note that the bimodal distribution for mutant mice at low speeds disappeared at high walking speeds.
Potential Changes in the Central vs. Peripheral Nervous System
Mutation of cell adherence molecules, such as N-CAM, has been previously reported to be important in the normal establishment and stabilization of the neuromuscular junction and the contractile properties of muscle fibers (Chipman et al. 2010, 2014; Polo-Parada et al. 2001; Rafuse et al. 2000). As such, peripheral changes could therefore contribute to the locomotor and postural functional impairments we observed in DSCAM2J mice (Figs. 2–6). To evaluate this possibility, we next investigated the anatomical, physiological, and functional properties of the neuromuscular junction, muscle fibers, and muscle spindles.
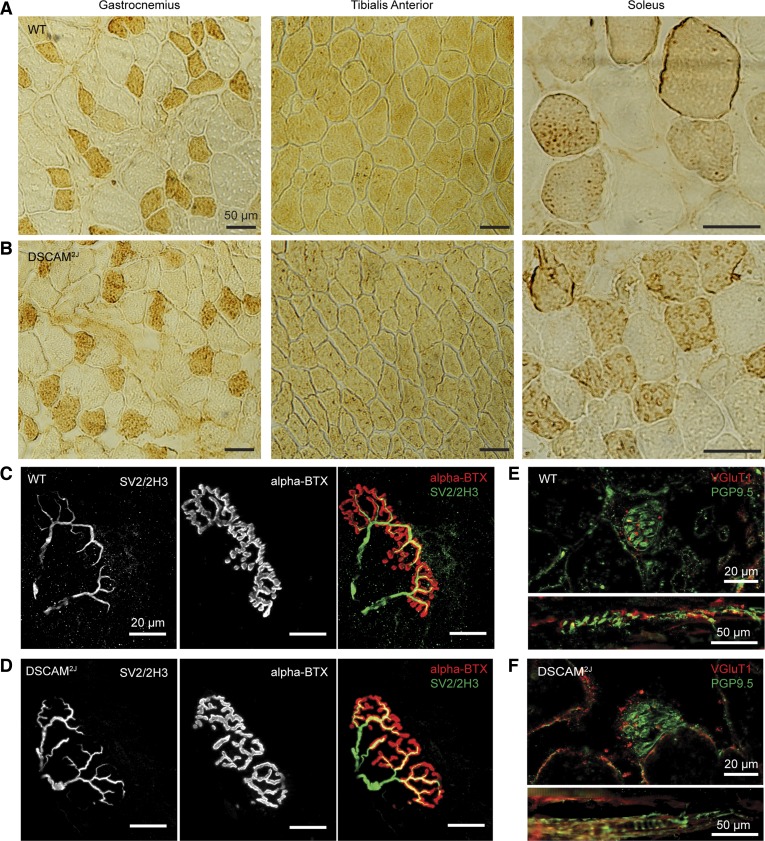
Anatomically, the weight of the muscles, the total number of fibers, and the proportion of fast vs. slow fiber types were very similar for the GL, TA, and soleus muscles of both WT and DSCAM2J mice (Fig. 7, A and B, Table 2). Moreover, although the cross-sectional area of the gastrocnemius and soleus muscles was smaller for DSCAM2J mice, only the cross-sectional area of the muscle fibers of the soleus was significantly smaller in DSCSAM2J mice, likely reflecting the smaller body weight (35.4 ± 10.3 for WT vs. 22.6 ± 2.4 for DSCAM2J, P = 0.0199, Mann-Whitney test) and size of the mutant. The neuromuscular junctions labeled with antibodies against SV2 for the presynaptic vesicles, 2H3 for the axonal terminals, and α-BTX for the postsynaptic cholinergic receptors were present and appeared normal for DSCAM2J mice (Fig. 7, C and D). Moreover, the end-plate area of the TA and the GL was similar for both WT and DSCAM2J mice (TA: 1,238 ± 511.4 μm2 for WT, 1,339 ± 633.7 μm2 for DSCAM2J, P = 0.6027; GL: 906.3 ± 365.2 μm2 for WT, 935.9 ± 403.8 μm2 for DSCAM2J, P = 0.81189, t-test, n = 18 end-plates for each group from 3 WT and 3 DSCAM2J mice).
Fig. 7.
Anatomical features of hindlimb muscles are intact in DSCAM2J mice. A and B: muscle fibers of the gastrocnemius (left), tibialis anterior (middle), and soleus muscle (right) of WT (A) and DSCAM2J mice (B) at high magnification. Fast-twitch fibers were revealed with myosin heavy chain antibodies (darker cytoplasm). Note that virtually every fiber is labeled in the tibialis anterior. C and D: representative examples of the neuromuscular junction in the tibialis anterior of WT (C) and DSCAM2J mice (D). Axons and presynaptic nerve terminals were labeled with antibodies against presynaptic synaptic vesicles (SV2) and axonal neurofilaments (2H3). Acetylcholine receptors on the postsynaptic membrane were evidenced by α-bungarotoxin (α-BTX). E and F: representative examples of coronal and longitudinal sections of the muscle spindle in the tibialis anterior of WT (E) and DSCAM2J mice (F). Muscle spindles and sensory nerve terminals were labeled with antibodies against protein gene product 9.5 (PGP9.5) and vesicular glutamate transporter 1 (VGluT1), respectively.
Table 2.
Muscle anatomical properties
| Gastrocnemius | Tibialis Anterior | Soleus | |
|---|---|---|---|
| Muscle weight, mg | |||
| WT | 158.0 ± 35.2 (5) | 39.2 ± 5.3 (4) | 11.2 ± 3.9 (4) |
| DSCAM2J | 130.7 ± 19.9 (6) | 35.7 ± 4.9 (5) | 11.9 ± 4.5 (5) |
| P value | 0.081 | 0.556 | 0.891 |
| Total CSA, mm2 | |||
| WT | 16.02 ± 2.35 (5) | 6.83 ± 1.65 (5) | 1.41 ± 0.56 (7) |
| DSCAM2J | 10.83 ± 1.94 (3) | 4.76 ± 1.22 (5) | 0.71 ± 0.28 (6) |
| P value | 0.0357 | 0.095 | 0.014 |
| Fiber CSA, μm2 | |||
| WT | 2,068 ± 268 (5) | 3,427 ± 281 (3) | 1,102 ± 274 (5) |
| DSCAM2J | 1,983 ± 360 (4) | 2,237 ± 273 (3) | 605 ± 50 (5) |
| P value | 0.5476 | 0.10 | 0.0079 |
| No. of fibers | |||
| WT | 7,899 ± 1,695 (5) | 1,678 ± 296 (3) | 931 ± 81 (3) |
| DSCAM2J | 5,813 ± 2,360 (3) | 2,290 ± 668 (3) | 1,080 ± 436 (3) |
| P value | 0.25 | 0.40 | 0.70 |
| Fast fiber, % | |||
| WT | 35.0 ± 5.1 (5) | 0.5 ± 0.8 (3) | 54.7 ± 11.1 (3) |
| DSCAM2J | 30.6 ± 3.1 (4) | 1.4 ± 1.2 (3) | 49.5 ± 3.5 (3) |
| P value | 0.19 | 0.40 | 0.70 |
| Slow fiber, % | |||
| WT | 65.0 ± 4.9 (5) | 99.5 ± 0.5 (3) | 45.3 ± 11.1 (3) |
| DSCAM2J | 69.4 ± 3.1 (4) | 98.6 ± 1.2 (3) | 50.5 ± 3.5 (3) |
| P value | 0.19 | 0.40 | 0.70 |
Data are means ± SD; no. in parenthesis indicates sample size. P values indicate significance of statistical difference tested with the Mann-Whitney test. CSA, cross-sectional area.
Assuming the possibility of peripheral sensory deficits upon the DSCAM mutation, we also investigated the morphology of muscle spindles. Our immunohistochemistry studies of DSCAM2J TA and GL muscle spindles showed a normal VGluT1-labeled neural innervation and PGP9.5-labeled annulospiral endings with respect to WT. Because of cross-sectional area differences, we measured the density of muscle spindles from TA and GL muscles, but no differences were observed in WT and DSCAM2J mice (TA: 2.203 ± 0.4822 spindles/mm2 for WT, 2.058 ± 0.5149 spindles/mm2 for DSCAM2J, P = 0.5444; GL: 2.585 ± 0.5121 spindles/mm2 for WT, 2.252 ± 0.4048 spindles/mm2 for DSCAM2J, P = 0.1463, t-test, n = 9 sections per muscle from 3 WT and 3 DSCAM2J mice; Fig. 7, E and F). This suggests a normal morphology and density of DSCAM2J muscle spindles.
Functionally, electrical stimulation of the motor axons of the tibial posterior nerve evoked a similar EMG waveform in both DSCAM2J and WT mice (Fig. 8A), and repetitive nerve stimulations evoked a similar synaptic depression with increasing stimulation frequencies (Fig. 8B). Figure 8C illustrates typical examples of force developed upon electrical stimulation of the tibial posterior nerve in WT and DSCAM2J mice. Figure 8D shows that the amplitude of the force (188.4 ± 22.9 mN for DSCAM2J vs. 191.4 ± 61.0 mN for WT, P = 0.486; Fig. 8C), the latency (7.7 ± 0.4 ms for DSCAM2J vs. 7.5 ± 0.4 ms for WT, P = 0.83), the rise time (18.7 ± 2.4 ms for DSCAM2J vs. 20.6 ± 5.3 ms for WT, P = 0.34), and the decay time (82.2 ± 26.6 ms for DSCAM2J vs. 79.0 ± 25.6 ms for WT, P = 0.89) were similar in both mouse types. Regarding fatigue, the ratio of the hundredth and first pulses was similar in both WT and mutant mice (0.22 ± 0.07 for DSCAM2J vs. 0.34 ± 0.13 for WT, P = 0.20; Fig. 8E). Although repetitive nerve stimulation depressed more strongly the EMG response in DSCAM2J (Fig. 8B), the amplitude of the muscle force upon a posttetanic depression was similar in both strains (post-to-prestimulation ratio: 0.83 ± 0.08 for DSCAM2J vs. 0.82 ± 0.09 for WT, P = 1.0; Fig. 8F). The absence of significant differences in the contractile properties of the muscle and in the electrical properties of the neuromuscular junction, as well as in the density and morphology of muscle spindles, argues that the aberrant locomotor and postural phenotype we reported in DSCAM2J mice has a central rather than peripheral origin.
Hypertonia, but No Signs of Motor Rigidity or Spasticity
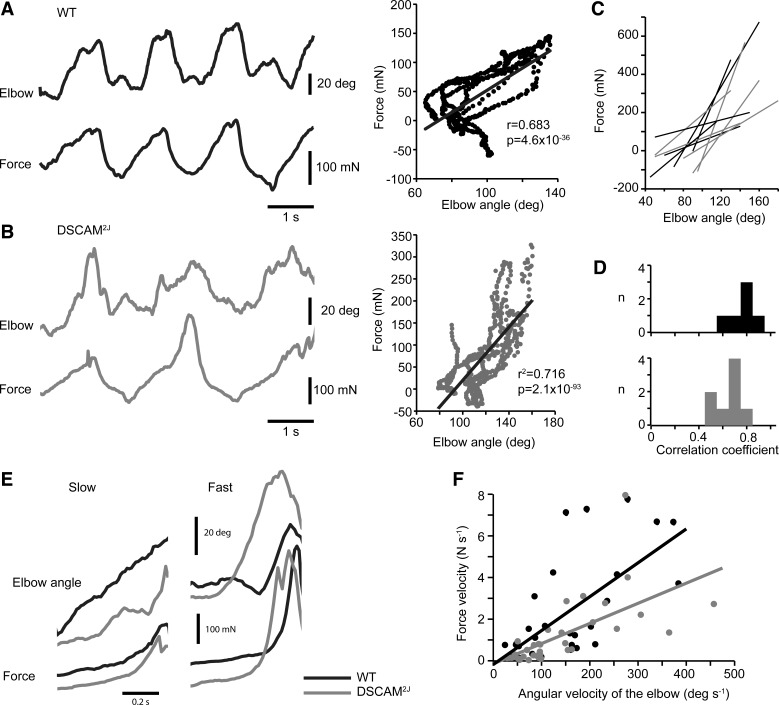
The exaggerated angular excursion (Fig. 5) and the aberrant extended posture at low speed (Fig. 6) suggest that DSCAM2J mice exhibit a motor hypertonia, which could be associated with rigidity, spasticity, or dystonia (Sanger et al. 2003). Rigidity is an intrinsic property to the muscle, which can be defined by the resistance of the muscle to passive or active movements. It can be therefore easily identified when the direction of the movement is reversed. To test whether DSCAM2J mice were rigid, the mouse was approached to a triangular bar attached to a force transducer that it had to grasp with its forepaws. Passive movements were then elicited by gently and as smoothly as possible pulling the mouse back and forth. The force was measured, and the angular movement of the elbow was monitored with two cameras placed on each side of the forelimb. For both WT and DSCAM2J, the elbow flexed and extended according to the direction of the movement. The force varied linearly with the angular excursion of the elbow (Fig. 9, A and B). The slope (Fig. 9C) and the correlation coefficients (Fig. 9D) of the relation between the force and the elbow angle varied from trial to trial, but no significant differences for the correlation coefficients were found between WT and DSCAM2J (P = 0.06, Mann-Whitney test), thus suggesting the absence of motor rigidity in DSCAM2J mice.
Fig. 9.
DSCAM2J mice do not show any signs of rigidity or spasticity. A and B, left: angular excursion of the elbow (top trace) and force signal (bottom trace) of WT (A) and DSCAM2J mice (B). Right, plots of force vs. elbow angle corresponding to the examples at left. Linear regressions are superimposed on plots. C: linear regressions of 5 trials for WT (black lines) and DSCAM2J (gray lines). D: histograms of correlation coefficients of the linear regression of force vs. elbow angular velocity (n = 6 trials from 3 WT mice and 8 trials from 4 DSCAM2J mice). E: angular excursion of the elbow and force signal when mice were pulled away slowly or rapidly from the force transducer. F: plot of force velocity vs. angular velocity of the elbow (n = 30 trials from 4 WT mice and 36 trials from 4 DSCAM2J mice). Slopes of the linear regression are not significantly different (P = 0.722, Student's t-test adapted for slopes of linear regression).
Since spasticity is a velocity-dependent form of hypertonia, if DSCAM2J mice were spastic, they should exhibit a sharp increase in force at high velocity during passive movement. To test this possibility, we varied the speed at which we pulled the mouse away from the force transducer. The angular velocity was computed from the angular motion of the elbow. As shown in Fig. 9E, we observed a slow increase in force developed while gently pulling back the mouse. In contrast, a fast increase was developed in force when WT and DSCAM2J mice were swiftly pulled away from the transducer (Fig. 9E). The velocity of the force was plotted as a function of the velocity of the elbow. Its linear regression revealed that the slope of the WT showed a trend toward steeper values in WT than in DSCAM2J mice (P = 0.051, Student's t-test for slopes comparison; Fig. 9F), thus arguing against spasticity in the mutant. Therefore, our combined force and kinematic study argues that DSCAM2J mice are unlikely rigid or spastic.
Episodes of Flexor-Extensor Coactivation in DSCAM2J Mice
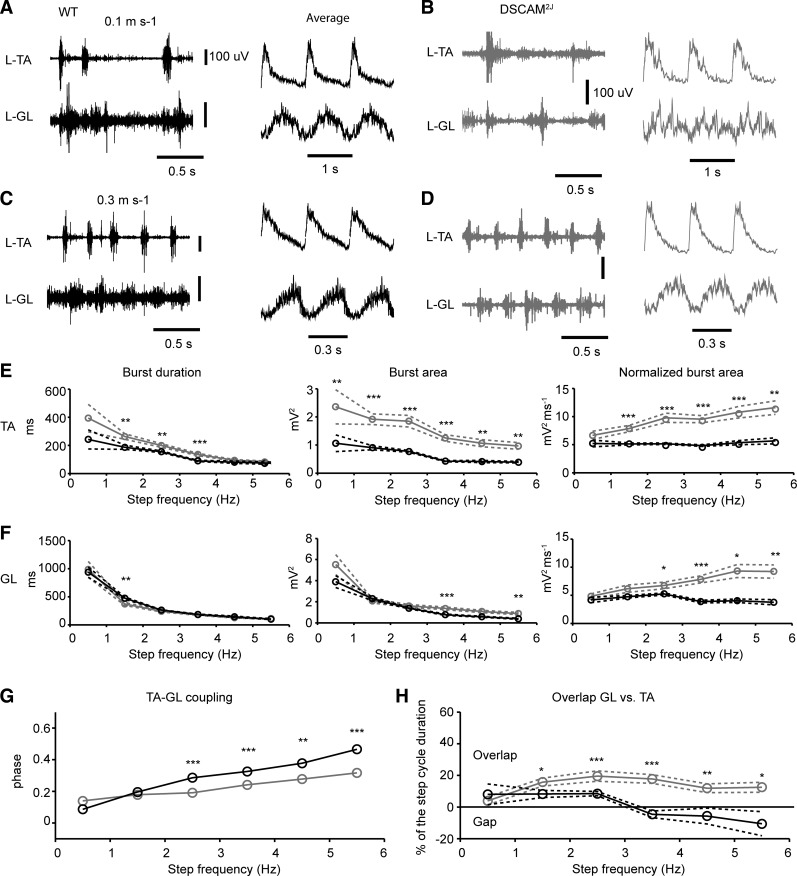
To better understand the neural mechanisms underlying kinematic and biomechanical changes observed in DSCAM2J mice, we recorded the EMG activity of hindlimb muscles during locomotion at low and high walking speeds (Fig. 10). We focused our EMG recordings on the ankle flexor (TA) and extensor (GL). EMG responses showed a normal alternation between flexor-extensor muscles in the WT mouse at both low and high walking speeds (Fig. 10, A and C). In contrast, when DSCAM2J mice ambulated at slow walking speed, we found numerous episodes of co-contractions in the TA and the GL. There was a poor coordination in flexor and extensor muscles, as shown by the EMG averaging (Fig. 10B), which likely contributed to deficits in the limb trajectory and intralimb coordination. Interestingly, this locomotor pattern was normalized at high walking speed, with a proper alternation between left-right extensor muscles (data not shown) and ipsilateral flexor-extensor muscles, resembling the locomotor pattern of the WT mouse (Fig. 10D).
Fig. 10.
Changes in the amplitude and timing of flexor and extensor muscles during locomotion in DSCAM2J mice. A–D: examples of EMG recordings (left) and averaged and normalized EMG recordings (right) for WT and DSCAM2J tibialis anterior (TA) and gastrocnemius lateralis (GL) muscles at low (A and B) and high (C and D) walking speeds. E and F: averaged burst duration, burst area, and normalized burst area of the TA (E) and GL (F). Data were averaged in bins of 1 Hz. Dashed lines denote SE. G: coupling of EMG activity onset between the TA and GL. H: overlap of EMG activity between the TA and GL (TA offset and GL onset). Black lines and circles correspond to WT, and gray ones to DSCAM2J. Statistical differences between WT and DSCAM2J were tested at each speed with a Mann-Whitney test, except for data in G, for which statistical differences were tested with a Watson-William test: *P < 0.05; **P < 0.01; ***P < 0.001.
The burst duration for the TA was longer for DSCAM2J mice at step cycles ranging from 1 to 4 Hz (Fig. 10E), which was consistent with the longer swing phase observed for all gaits but the trot (Table 1). The burst area for the TA (measured as the area under the curve of the rectified signal) was always larger for DSCAM2J than for WT mice. This larger burst area was not attributable to longer burst duration, because the normalized burst area showed a similar effect at all step frequencies beyond 1 Hz. In contrast, the burst duration for the GL was almost always similar between WT and mutant mice (Fig. 10F). The EMG area of the GL tended to be larger for the mutant at high step frequency, and the normalized area was always larger for DSCAM2J mice above 2 Hz (Fig. 10F, right). The enhanced EMG activities in flexor and extensor muscles confirm the motor hypertonia and the occurrence of limb hyperflexion during the swing phase of locomotion in DSCAM2J mice.
We next turned our attention to the coordination between flexor and extensor muscle activities. At very low step frequency, the coupling of the onset for the TA and GL was similar for WT and DSCAM2J mice (Fig. 10G). However, as the step frequency increased, the phase of the coupling did not increase in DSCAM2J mice with respect to the WT, thus suggesting an overlap in the EMG activity of both flexor and extensor muscles in the mutant. To evaluate this possibility, we measured the phase of the onset of the GL EMG burst in regard to the offset of the TA EMG burst (Fig. 10H). While at low step frequency, there was a slight overlap between the end of the flexor activity and the beginning of the extensor activity, and eventually the extensor was only active after the end of the flexor activity in the WT mouse, thus showing a gap and supporting a proper reciprocal inhibition between antagonist muscles. In contrast in the DSCAM2J mouse, the beginning of the extensor EMG activity always started before the end of the flexor EMG activity at all step frequencies, thus demonstrating an overlap and a coactivation in antagonist flexor extensor activities over 20% of the step cycle. Altogether, these findings demonstrate that the amplitude and timing of flexor and extensor locomotor activities were disrupted in DSCAM2J mice.
DISCUSSION
With an inbred genetic background, DSCAM2J mice exhibit a postural and locomotor gait phenotype consistent with that of other DSCAM mutants but without the severe hydrocephalus and phenotypic variability previously reported in DSCAM3J and DSCAMdel17 mice (Fuerst et al. 2010; Schramm et al. 2012; Xu et al. 2011). With respect to WT, DSCAM2J mice display a dystonic hypertonia, in absence of signs of rigidity or spasticity. The normal neuromuscular transmission also suggests that the motor phenotype is likely related to central neural mechanisms. The disruption in the intra- and interlimb coordination favors the emergence of less coordinated gaits in DSCAM2J mice. We discuss herein the potential neural mechanisms underlying these functional motor and locomotor impairments.
Central vs. Peripheral Neural Changes
To exclude the possibility that the motor phenotype observed in DSCAM2J mice might be due to peripheral neural changes, as in N-CAM mutant mice (Chipman et al. 2010, 2014; Polo-Parada et al. 2001; Rafuse et al. 2000), we performed a series of anatomical, histological, functional, EMG, and kinematic studies (Figs. 7 and 8). Despite a few differences in the cross-sectional area of the DSCAM2J soleus muscle and its fibers that could reflect the smaller muscle size and weight, there were no overt discrepancies in the morphology and contractile properties of hindlimb muscles. Moreover, the morphology, function, and physiology of the neuromuscular junction was normal with respect to WT, and the annulospiral endings showed a normal morphology, distribution, and innervation. Therefore, the aberrant locomotor and postural control observed in DSCAM2J mice results more likely from central rather than peripheral changes.
Hydrocephalus
Although DSCAM2J mice displayed a bigger brain volume associated with a light, but significant, dilation of their ventricles (Fig. 1), compared with the brain volume, the ventricle volume was not strikingly larger than that of WT mice and therefore appeared less enlarged than that of DSCAM3J (Schramm et al. 2012) or DSCAMdel17 mice (Xu et al. 2011), for which a marked thinning of the corpus callosum and a large dilation of the ventricle were reported.
With a less severe hydrocephalus than in other DSCAM mutant mouse models, DSCAM2J mutants showed a sustained locomotor endurance, an increased hind foot placement and trajectory, and an exaggerated flexibility in most limb joints. Taken together, all these features appear incompatible with the apraxia generally reported in patients with hydrocephalus (Hakim and Adams 1965; Knutsson and Lying-Tunell, 1985; Sudarsky and Simon 1987). Although we cannot definitively preclude that the hydrocephalus might not have indirectly impaired the locomotor and postural control in DSCAM2J mice, we think that the functional impairments reported in the current study more likely reflect central neural changes.
Motor Hypertonia Without Rigidity or Spasticity But Likely With Dystonia
In contrast to a previous study (Xu et al. 2011), our kinematic and EMG study (Figs. 4, 5, and 10) demonstrate motor hypertonia in DSCAM2J mice, in the absence of any signs of rigidity or spasticity. Compared with WT, DSCAM2J mice showed a longer stride length and height (i.e., hyperkinesia; Fig. 4) along with an increased angular excursion in the hindlimb joints (Fig. 5) during locomotion. Furthermore, the force developed by the forelimb increased linearly with the elbow angle during passive movements (Fig. 9), thus supporting the absence of motor rigidity, whereas the linear increase in the force developed by the forelimbs in response to an increase in passive movements (Fig. 9) demonstrated the lack of motor spasticity.
Nevertheless, DSCAM2J mice showed a striking hunched posture marked by a forward flexion of the spine at rest, at slow and high walking speeds, that persisted even under isoflurane or ketamine-xylazine anesthesia (Lemieux M, D. Laflamme O, and Bretzner F, unpublished observations), thus suggesting a permanent axial dystonia likely associated with a fusion of vertebrae during postnatal development.
On the other hand, DSCAM2J mice also switched from a combination of extended and flexed posture at slow walking speed, which eventually turned to a sole flexed posture at high walking speed (Fig. 6). It is important to note that although the angular excursion in the hindlimb joints was increased at all speeds in DSCAM2J mice, their smaller body size with respect to WT mice suggests that the predominant flexed posture in the mutant was likely normal. Furthermore, DSCAM2J mice displayed an increase in the amplitude of the EMG burst in the flexor muscle, and to a lesser degree in the antagonist extensor muscle, in addition to showing an antagonist flexor-extensor coactivation over 20% of the step cycle at almost all speeds (Fig. 10), thus arguing for the presence of dystonia in the appendicular musculature. Overall, our studies suggest that DSCAM2J mice display a motor hypertonia associated with an axial and appendicular dystonia over a wide range of speed.
Disruption in the Intralimb Coordination
DSCAM2J mice exhibited a longer stride length and height, a longer swing phase duration, and a marked limb hyperflexion (Figs. 4 and 5, Table 1), associated with an increase in the amplitude of the flexor EMG activity at all speeds (Fig. 10). In contrast, the stance phase was shorter (Table 1), although the amplitude of the extensor EMG activity was increased especially at high walking speed (Fig. 10). Therefore, these functional impairments point toward neural changes in the integration of central and peripheral inputs.
Given the DSCAM expression in the motor cortex and the transient alteration in the dendritic tree of developing cortical neurons in DSCAMdel17 mice (Maynard and Stein, 2012), it is tempting to speculate that the motor cortex and its corticospinal tract might be involved in the limb hyperflexion. Indeed, lesions of the corticospinal tract at the thoracic level lead to a hind paw dragging during the swing phase of locomotion (Jiang and Drew 1996), intracortical microstimulations of the motor cortex increase the activity of flexor muscles during the swing phase (Armstrong and Drew 1985; Bretzner and Drew 2005; Gibson et al. 1999), and single-unit recording studies have shown that corticospinal tract neurons fire phasically during locomotion and especially while stepping over obstacles (Armstrong and Drew 1984a, 1984b; Widajewicz et al. 1994). Therefore, an increased synaptic drive in supraspinal pathways such as the motor cortex, but also the red nucleus (Jiang and Drew 1996; Lavoie and Drew 2002; Rho et al. 1999), both involved in the control of flexor activities, might contribute to the limb hyperflexion during the swing phase in DSCAM2J mice.
Although the muscle spindles were normal in DSCAM2J mice (Fig. 7), an aberrant integration of primary sensory afferents might also contribute to the early initiation and prolongation of the swing phase at the expense of a shorter stance phase in DSCAM2J mice. Extensor muscle afferents are important for the initiation of the swing phase (Grillner and Rossignol 1978). The abnormal hunched posture (Fig. 5) and the more restricted backward placement of the DSCAM2J mouse's foot (Fig. 4) advance an abnormal stretching of the hip extensor muscle throughout the step cycle, which might contribute to an early and longer swing phase. Proprioceptive afferents from the knee and ankle are also likely involved. As previously shown (Gorassini et al. 1994; Hiebert et al. 1994), an unexpected loss of ground support in the intact animal walking on a treadmill with a hole inhibits its extensor activity and initiates a strong flexor activity from the knee and ankle to rapidly remove the foot from the hole. Deficits in the integration of proprioceptive afferents of the hip, knee, and ankle might therefore explain, in part, the hyperflexion observed in DSCAM2J mice. Our companion study addresses that point by evaluating spinal reflexes in neonatal and adult DSCAM2J mice (Thiry et al. in press). Although not exclusive, these supraspinal or spinal neural mechanisms likely contribute to the limb hyperflexion during the swing phase at the expense of a shorter stance phase in DSCAM2J mice.
Disruption of the Continuum of Locomotor Gaits
Changes in motor tone and intralimb coordination obviously might consequently impair interlimb coordination in DSCAM2J mice. To evaluate these functional changes, we integrated the interlimb couplings between hindlimbs, forelimbs, and lateral limbs to identify and characterize locomotor gaits. According to the footfall pattern (Grillner 1975; Hildebrand, 1977), symmetrical gaits were characterized by an anti-phase coupling in hindlimbs (e.g., pace, lateral walk, and trot), whereas asymmetrical gaits such as hop and out-of-phase walk were characterized by an in-phase and out-of-phase coupling in hindlimbs, respectively. WT mice with a C3H background adapted their gaits with a further increase in speed, thus switching from an asymmetrical pattern (hop and out-of-phase walk) to a symmetrical pattern (pace, lateral walk, and then trot) at the highest walking speed (Fig. 3).
The hop was one of the slowest gaits, with a locomotor frequency <1 Hz for both WT and DSCAM2J mice. It was characterized by a prolonged stance phase and a short swing phase, enabling the animal to explore and inspect its environment. To keep up with the moving treadmill belt, mice tended to synchronize their hindlimbs (i.e., in-phase coupling of hindlimbs), but rarely their forelimbs. This hindlimb synchronization at slow speed was reminiscent of the hopping locomotion in frogs and toads (Reilly and Jorgensen 2011). Hop occurred in a similar proportion in both mouse lines at very slow speed 0.05 m/s but rapidly disappeared in WT mice, whereas it persisted up to 0.15 m/s in DSCAM2J mice.
While the mouse increased its speed, it switched from hop to out-of-phase walk, characterized by a reduced coordination in hindlimbs (i.e., an out-of-phase coupling) and a loose coordination in forelimbs and lateral limbs. In contrast to WT mice that favored lateral walk at the slowest speeds, DSCAM2J mice preferred out-of-phase walk, thus suggesting a reduced bilateral left-right alternation that was confirmed in our neonatal isolated spinal cord studies (Thiry et al. in press).
The pace emerged with increasing speed. Pace is the usual gait in camels, long-legged dogs, and Icelandic horses (Andersson et al. 2012; Grillner 1975; Hildebrand 1976) and had never been reported in the mouse until now. Furthermore, we did not observe pace in C57/BL6 mice (Josset et al. 2015). Given that C3H mice are more anxious in response to novelty (Kopp et al. 1999) and display higher levels of activity across the circadian rhythm (Adamah-Biassi et al. 2013) than do C57/BL6 mice, the level of anxiety might explain, in part, the use of pace in C3H mice. Whereas WT mice paced rarely and only below 0.15 m/s, DSCAM2J mice paced over a wide range of speeds, thus suggesting the emergence of a new type of forelimb-hindlimb coordination in the mutant.
In contrast to WT mice that favored the trot at the highest walking speed, DSCAM2J mice preferred to use out-of-phase and lateral walk. This might be due to their axial and appendicular dystonia, which would prevent DSCAM2J mice from placing their opposite fore- and hindlimbs underneath their belly during trot. This could also reflect that both out-of-phase and lateral walk ensured a better weight support than trot. It is noteworthy that both trot and pace require a robust but opposite forelimb-hindlimb coordination; the lack of trot could hence reflect the higher occurrence of pace in the mutant, thus suggesting an impaired lateral coordination, through long propriospinal interneurons or supraspinal inputs. Overall, the DSCAM mutation seems to impair the emergence of the most reliable and coordinated locomotor gaits (i.e., lateral walk and trot) and may produce a less coordinated gait (out-of-phase walk) or even an unusual gait for the mouse (pace), thus suggesting a reorganization of spinal cervical and lumbar locomotor circuits and supraspinal and peripheral inputs.
In conclusion, the DSCAM mutation induces a motor hypertonia associated with an axial and appendicular dystonia and changes in intra- and interlimb coordination, thus disrupting the continuum of locomotor gaits with a prevalence of less coordinated gaits (e.g., out-of-phase walk and pace). Along with dystonia, these functional motor and locomotor impairments likely result from a reorganization within and between cervical, lumbar, and brain locomotor circuits. Our companion study describes in further detail the role of DSCAM in the development of spinal locomotor and sensorimotor circuits (Thiry et al. in press).
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant RGPIN/418635-2012 (to F. Bretzner). F. Bretzner is supported by a Fonds de Recherche du Quebec en Santé scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L. and F.B. conception and design of research; M.L., O.D.L., L.T., A.B.-P., and F.B. performed experiments; M.L., O.D.L., L.T., A.B.-P., and F.B. analyzed data; M.L., O.D.L., L.T., A.B.-P., J.F., and F.B. interpreted results of experiments; M.L., O.D.L., L.T., A.B.-P., and F.B. prepared figures; M.L., L.T., and F.B. drafted manuscript; M.L., L.T., and F.B. edited and revised manuscript; M.L., O.D.L., L.T., A.B.-P., J.F., and F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Philippe Drapeau and Hugo Delivet-Mongrain for technical help.
REFERENCES
- Abourachid A, Herbin M, Hackert R, Maes L, Martin V. Experimental study of coordination patterns during unsteady locomotion in mammals. J Exp Biol 210: 366–372, 2007. [DOI] [PubMed] [Google Scholar]
- Adamah-Biassi EB, Stepien I, Hudson RL, Dubocovich ML. Automated video analysis system reveals distinct diurnal behaviors in C57BL/6 and C3H/HeN mice. Behav Brain Res 243: 306–312, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed OJ, Cash SS. Finding synchrony in the desynchronized EEG: the history & interpretation of gamma rhythms. Front Integr Neurosci 7: 58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Fujii M, Arata S, Tojima T, Ogawa M, Morita N, Shimohata A, Furuichi T, Itohara S, Kamiguchi H, Korenberg JR, Arata A, Yamakawa K. DSCAM deficiency causes loss of pre-inspiratory neuron synchroneity and perinatal death. J Neurosci 29: 2984–2996, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjälm G, Imsland F, Petersen JL, McCue ME, Mickelson JR, Cothran G, Ahituv N, Roepstorff L, Mikko S, Vallstedt A, Lindgren G, Andersson L, Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488: 642–646, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JM, Estevez-Perez MG. Statistical comparison of the slopes of two regression lines: a tutorial. Anal Chim Acta 838: 1–12, 2014. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol 346: 471–495, 1984a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol 346: 497–517, 1984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. Forelimb electromyographic responses to motor cortex stimulation during locomotion in the cat. J Physiol 367: 327–351, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschelet E. Circular Statistics in Biology. London: Academic, 1981. [Google Scholar]
- Bretzner F, Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. J Neurophysiol 94: 657–672, 2005. [DOI] [PubMed] [Google Scholar]
- Chipman PH, Schachner M, Rafuse VF. Presynaptic NCAM is required for motor neurons to functionally expand their peripheral field of innervation in partially denervated muscles. J Neurosci 34: 10497–10510, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman PH, Franz CK, Nelson A, Schachner M, Rafuse VF. Neural cell adhesion molecule is required for stability of reinnervated neuromuscular junctions. Eur J Neurosci 31: 238–249, 2010. [DOI] [PubMed] [Google Scholar]
- Cvetkovska V, Hibbert AD, Emran F, Chen BE. Overexpression of Down syndrome cell adhesion molecule impairs precise synaptic targeting. Nat Neurosci 16: 677–682, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Doucet S. Application of circular statistics to the study of neuronal discharge during locomotion. J Neurosci Methods 38: 171–181, 1991. [DOI] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci 22: 10346–10356, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Harris BS, Johnson KR, Burgess RW. A novel null allele of mouse DSCAM survives to adulthood on an inbred C3H background with reduced phenotypic variability. Genesis 48: 578–584, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol 71: 603–610, 1994. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev 55: 247–304, 1975. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146: 269–277, 1978. [DOI] [PubMed] [Google Scholar]
- Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 2: 307–327, 1965. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Gorassini MA, Jiang A, Prochazka A, Pearson KG. Corrective responses to loss of ground support during walking. II. Comparison of intact and chronic spinal cats. J Neurophysiol 71: 611–622, 1994. [DOI] [PubMed] [Google Scholar]
- Hildebrand M. Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Neural Control of Movement, edited by Herman RM, Grillner S, Stein PSG, Stuart DG. New York: Plenum, 1976, p. 203–236. [Google Scholar]
- Hildebrand M. Analysis of asymmetrical gaits. J Mammal 58: 131–156, 1977. [Google Scholar]
- Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76: 849–866. 1996. [DOI] [PubMed] [Google Scholar]
- Josset N, Lemieux M, Roussel M, Couraud S, Bretzner F. Speed modulation of locomotor gait in the adult mouse. Program No. 519.01 2015 Neuroscience Meeting Planner Chicago, IL: Society for Neuroscience, 2015. Online. [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E, Lying-Tunell U. Gait apraxia in normal-pressure hydrocephalus: patterns of movement and muscle activation. Neurology 35: 155–160, 1985. [DOI] [PubMed] [Google Scholar]
- Kopp C, Vogel E, Misslin R. Comparative study of emotional behaviour in three inbred strains of mice. Behav Processes 47: 161–174, 1999. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol 88: 1791–1814, 2002. [DOI] [PubMed] [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron 61: 527–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA 106: 2951–2956, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133: 1241–1254, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard KR, Stein E. DSCAM contributes to dendrite arborization and spine formation in the developing cerebral cortex. J Neurosci 32: 16637–16650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmesino E, Haddick PC, Tessier-Lavigne M, Kania A. Genetic analysis of DSCAM's role as a Netrin-1 receptor in vertebrates. J Neurosci 32: 411–416, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Bose CM, Landmesser LT. Alterations in transmission, vesicle dynamics, and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron 32: 815–828, 2001. [DOI] [PubMed] [Google Scholar]
- Purohit AA, Li W, Qu C, Dwyer T, Shao Q, Guan KL, Liu G. Down syndrome cell adhesion molecule (DSCAM) associates with uncoordinated-5C (UNC5C) in netrin-1-mediated growth cone collapse. J Biol Chem 287: 27126–27138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Li W, Shao Q, Dwyer T, Huang H, Yang T, Liu G. c-Jun N-terminal kinase 1 (JNK1) is required for coordination of netrin signaling in axon guidance. J Biol Chem 288: 1883–1895, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe N, Gezelius H, Vallstedt A, Memic F, Kullander K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J Neurosci 29: 15642–15649, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe Bernhardt N, Memic F, Gezelius H, Thiebes AL, Vallstedt A, Kullander K. DCC mediated axon guidance of spinal interneurons is essential for normal locomotor central pattern generator function. Dev Biol 366: 279–289, 2012. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Polo-Parada L, Landmesser LT. Structural and functional alterations of neuromuscular junctions in NCAM-deficient mice. J Neurosci 20: 6529–6539, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Jorgensen ME. The evolution of jumping in frogs: morphological evidence for the basal anuran locomotor condition and the radiation of locomotor systems in crown group anurans. J Morphol 272: 149–168, 2011. [DOI] [PubMed] [Google Scholar]
- Rho MJ, Lavoie S, Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol 81: 2297–2315, 1999. [DOI] [PubMed] [Google Scholar]
- Ritter LK, Tresch MC, Heckman CJ, Manuel M, Tysseling VM. Characterization of motor units in behaving adult mice shows a wide primary range. J Neurophysiol 112: 543–551, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Kiehn O. Modelling genetic reorganization in the mouse spinal cord affecting left-right coordination during locomotion. J Physiol 591: 5491–5508, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 111: e89–e97, 2003. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Li S, Harris BS, Rounds RP, Burgess RW, Ytreberg FM, Fuerst PG. A novel mouse Dscam mutation inhibits localization and shedding of DSCAM. PLoS One 7: e52652, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol 44: 263–267, 1987. [DOI] [PubMed] [Google Scholar]
- Thiry L, Lemieux M, D. Laflamme O, Bretzner F. Role of DSCAM in the development of the spinal locomotor and sensorimotor circuits. J Neurophysiol (December 9, 2015). doi: 10.1152/jn.00557.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K. Dorsally derived spinal interneurons in locomotor circuits. Ann NY Acad Sci 1279: 32–42, 2013. [DOI] [PubMed] [Google Scholar]
- Widajewicz W, Kably B, Drew T. Motor cortical activity during voluntary gait modifications in the cat. II. Cells related to the hindlimbs. J Neurophysiol 72: 2070–2089, 1994. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ye H, Shen Y, Xu Q, Zhu L, Liu J, Wu JY. Dscam mutation leads to hydrocephalus and decreased motor function. Protein Cell 2: 647–655, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451: 465–469, 2008. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Huot YK, Haendelt MA, Hubert R, Chen XN, Lyons GE, Korenberg JR. DSCAM: a novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Hum Mol Genet 7: 227–237, 1998. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984. [Google Scholar]