Abstract
Medial olivocochlear (MOC) neurons provide an efferent innervation to outer hair cells (OHCs) of the cochlea, but their tonotopic mapping is incompletely known. In the present study of anesthetized guinea pigs, the MOC mapping was investigated using in vivo, extracellular recording, and labeling at a site along the cochlear course of the axons. The MOC axons enter the cochlea at its base and spiral apically, successively turning out to innervate OHCs according to their characteristic frequencies (CFs). Recordings made at a site in the cochlear basal turn yielded a distribution of MOC CFs with an upper limit, or “edge,” due to usually absent higher-CF axons that presumably innervate more basal locations. The CFs at the edge, normalized across preparations, were equal to the CFs of the auditory nerve fibers (ANFs) at the recording sites (near 16 kHz). Corresponding anatomical data from extracellular injections showed spiraling MOC axons giving rise to an edge of labeling at the position of a narrow band of labeled ANFs. Overall, the edges of the MOC CFs and labeling, with their correspondences to ANFs, suggest similar tonotopic mappings of these efferent and afferent fibers, at least in the cochlear basal turn. They also suggest that MOC axons miss much of the position of the more basally located cochlear amplifier appropriate for their CF; instead, the MOC innervation may be optimized for protection from damage by acoustic overstimulation.
Keywords: outer hair cell, efferent, afferent, characteristic frequency, cochlear amplifier
medial olivocochlear (MOC) neurons respond to sound and act on outer hair cells (OHCs) of the cochlea (Ryugo et al. 2011). The action of MOC endings is to hyperpolarize the OHC (Fuchs 2002), which is thought to reduce the gain of the “cochlear amplifier,” the process by which the hair cell electromotility enhances motion of the receptor organ, the organ of Corti (Ashmore et al. 2010). Cochlear amplification increases the sensitivity of hearing by at least 100 times (Liberman et al. 2002). The MOC action on the cochlear amplifier is beneficial because it shifts the dynamic range of auditory nerve fibers (ANFs) to higher levels (Wiederhold and Kiang 1970) and decreases the effects of masking by steady background noise (Jennings et al. 2011; Kawase et al. 1993; Winslow and Sachs 1988). MOC action also protects the inner ear from damage due to high-level sound (Maison and Liberman 2000; Reiter and Liberman 1995). However, the cochlear amplifier, protection from damage, and how the MOC neurons influence these processes are all incompletely understood, so studies of their mechanisms may offer valuable insight into the hearing process.
In particular, the present study aims to determine whether MOC neurons target the OHCs most involved in the cochlear amplifier for the sound frequency to which they are most sensitive, the characteristic frequency (CF). The CF is the lowest point on the tuning curve; these curves for MOC responses are sharply tuned to sound frequency (Fex 1962, 1965; Liberman and Brown 1986; Robertson and Gummer 1985). High-CF MOC axons project to the cochlear base and lower-CF neurons project more apically (Brown 1989, 2014; Liberman and Brown 1986; Robertson and Gummer 1985), in general alignment with the tonotopic mapping observed for ANFs (Liberman 1982a; Tsuji and Liberman 1997). If the CF mappings are in exact alignment, though, this would mean that MOC axons miss the position of OHCs involved in the cochlear amplifier (Brown 2014), because the amplifier extends basally from the CF position. The cochlear amplifier's extent is about one-quarter octave in the basal turn and larger in the apical turns as demonstrated in lesion/perturbation studies (Cody 1992; Fisher et al. 2012), masking and suppression studies (Pang and Guinan 1997; Prijs 1989), and modeling studies (Neely and Kim 1986; Patuzzi 1996; Shera 2007). This amount of basal offset in projections would be expected if an individual MOC neuron's action is to reduce the gain of the amplifier appropriate to its CF. A key factor in the above argument is our confidence in the mappings of MOC axons. There are several reasons to suggest incomplete confidence. The previous studies have exclusively used single-unit, intracellular labeling, which is difficult and low yielding, so that the number of physiologically characterized axons traced to their endings is low. Also, because the labeled axons had more than the average number of endings (Brown 2014), the sample of labeled axons could be biased toward those with the largest diameters. Conceivably, axons of more average diameters might target the site of the amplifier.
A different approach is used in the present work to investigate this issue without such biases. This approach takes advantage of the pathway of the MOC axons as they enter and innervate the cochlea (Gacek 1961; Portmann and Portmann 1954; Rasmussen 1953). As schematized in Fig. 1, individual axons progress apically from their entrance in the lower basal turn and successively turn out to innervate OHCs (Brown 1987b, 1989, 2014; Liberman and Brown 1986). The axonal progression is always in the apical direction, never “hooking back” basally, as shown by reconstructions of 40 single MOC axons labeled by spiral ganglion injections in the guinea pig (Brown 1987a, 1987b, 1989, 2014). Thus, at a single site along this course, MOC axons are recorded that innervate adjacent to the site and at more apical locations, but not those that innervate more basal locations because these have already turned out (e.g., MOC 3 in Fig. 1). A prediction from this pattern is that the recorded CFs should be broadly distributed but have an upper limit, or “edge” (see scale on Fig. 1). In fact, previous studies have indicated a paucity of MOC units with very high CFs when recordings are from the spiral ganglion (Brown 1989; Robertson and Gummer 1985) versus when they are made from the entire OC bundle (Liberman 1988; Liberman and Brown 1986). What is new in the present study is to determine the upper limit of the MOC CFs and compare it with CFs of ANFs at the recording site. Such a comparison is strong if the MOC distribution has a sharp edge, but this requires a large number of units and thus pooling of data from different animal preparations. Thus another new facet of the present study is to normalize CFs so that data can be compared across preparations. Overall, equivalence of the CFs of the MOC edge and nearby ANFs would mean that the mappings of these two neural populations are in alignment at this point in the cochlea, without the bias accompanying the previous labeling experiments.
Fig. 1.
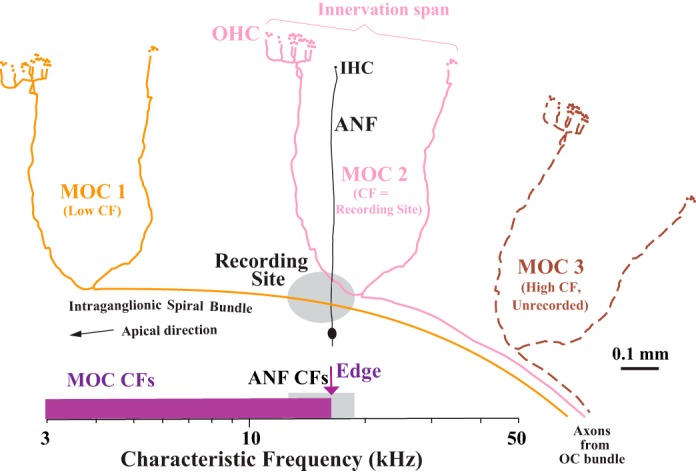
Schematic of the cochlear innervation by medial olivocochlear (MOC) axons (colors) and auditory nerve fibers (ANFs; black), shown in relation to a recording site (gray oval) in the spiral ganglion. Three MOC axons are shown entering the cochlea from the OC bundle at right. The axons run apically in the intraganglionic spiral bundle and terminate onto outer hair cells (OHC) at different positions along the cochlea. For simplicity, the drawings are duplicates of an axon from a single-unit labeling study (Brown 2014). Axons MOC 1 and MOC 2 can be sampled because they take a spiral course through the recording site, but axon MOC 3 cannot be sampled because before it reaches the site it turns out to terminate basally at a location appropriate for its high characteristic frequency (CF). The ANF can be sampled because it takes a radial course through the recording site from a single inner hair cell (IHC) to its cell body in the spiral ganglion. The scale at bottom shows the expected CF distributions: for MOC units (purple), a broad pattern with an upper limit, or “edge,” and for ANFs (gray), a narrow pattern. The CF scale is exaggerated and would span much of the basal turn, rather than just the small portion schematized here. The type of ANF shown is termed “type I”; not shown are unmyelinated ANFs, termed “type II,” which provide contacts on OHCs. Unmyelinated lateral OC fibers that innervate the IHCs also are not shown.
Furthermore, the edge of CFs may be determined separately for the types of MOC units, which are distinguished according to which ear drives the response to sound (Liberman and Brown 1986; Robertson and Gummer 1985). Thus 1) ipsi units are driven by sound in the ipsilateral ear (where the ipsilateral cochlea is the one where the MOC axons project); 2) contra units are driven by sound in the contralateral ear; and 3) either-ear units are driven by sound in either ear. This issue is of interest because very limited data on contra units suggest that their mapping is somewhat apical to that for ipsi units and ANFs (Brown 2014).
A complicating factor in this approach is the fact that MOC axons have considerable spans of innervation as a result of branching (e.g., MOC 2 in Fig. 1). If recordings from branches of MOC axons are possible, then the upper limit of CFs may be blurred by higher CF axons projecting some of their apical branches through the recording site. This anatomy, as well as the MOC endings pattern and its relationship to ANFs, is studied here with extracellular deposits of tracer into the sites. In fact, the blurring of the edge of labeling is used in this study to provide a measure of the innervation span of MOC axons that complements measures obtained by single-unit labeling.
MATERIALS AND METHODS
Experimental procedures on guinea pigs were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were performed under approved protocols at the Massachusetts Eye and Ear Infirmary. Guinea pigs were of the Hartley strain and were of either sex. They were anesthetized with either 1) urethane (1,100 mg/kg) plus fentanyl (0.2 mg/kg) and droperidol (10 mg/kg), or 2) pentobarbital sodium (Nembutal; 15 mg/kg) plus the same doses of fentanyl and droperidol. Supplemental doses of anesthesia (about one-third of the original dose) were administered as needed. After anesthesia, a tracheal cannula was inserted, both pinnae were removed, and the animal's head was placed in a head holder. A rectal thermometer was used in conjunction with the heated air temperature in the chamber and a heating pad to control the rectal temperature to 38°C.
For single-unit recordings, the database of 133 guinea pigs was partly from earlier studies (Brown 1989, 2014). These experiments were carried out within a sound-attenuating and electrically shielded chamber in which the air temperature was heated. Sound stimuli were produced by half-inch condenser microphones driven as sound sources that were placed into the right and left ear canals using couplers. The bulla on the left side was surgically opened posterior to the ear canal, and a silver wire was placed on the round window of the cochlea to record the compound action potential with respect to a reference electrode in the neck muscles. Cochlear sensitivity was tested using tone pips (duration 5 ms, rise/fall time 0.5 ms, repeated 10/s in alternate polarity pairs, frequency 4–32 kHz). Responses were amplified (10,000×) and averaged with artifact rejection, and a 15-μV criterion was used for threshold. The thresholds were checked periodically during the experiment, and only preparations with minimal threshold shifts (<10 dB) relative to the beginning of the experiment were used. Surgical methods to access the cochlear structures via the scala tympani were as described previously (Brown 1989; Robertson and Gummer 1985; Sellick and Russell 1979). A fenestration of the otic capsule bone over scala tympani in the basal turn was made, and the spiral ganglion was visualized. A recording site opening was made with a Minutien insect pin to pierce the bone overlying the spiral ganglion, usually at the ganglion's peripheral edge where the MOC axons run in the intraganglionic spiral bundle. The pin was coated with pencil graphite to mark the circumference of the opening for aiming electrodes and for postexperiment histological recovery of the recording site. In a few preparations in which the yield of MOC units was low, a second site was made apical to the first, but then only data from the second site were used. ANF CFs from the second site averaged 0.42 (range 0.18–0.67, n = 8 preparations) octave lower than the first. None of these preparations had MOC units with CFs higher than those of ANFs. Sharp electrodes for recording single units were glass micropipettes, usually filled with 2 M KCl in 0.05 M Tris buffer, 4% biocytin plus 0.45 M KCl in 0.05 M Tris buffer, or 5% horseradish peroxidase (HRP) plus 0.15 M KCl in 0.05 M Tris buffer; the latter two solutions were used to label single units that were reported previously (Brown 2014).
All sound stimuli for single-unit responses were 50-ms in duration and repeated 10/s, and search stimuli were binaural noise bursts at 80 dB SPL. After a unit was obtained, it was first classified as either an ANF (short-latency response to sound and irregular interspike intervals) or MOC unit (long-latency response to sound and regular interspike intervals) (Brown 1989; Fex 1962; Liberman and Brown 1986; Robertson and Gummer 1985). MOC units were further classified as ipsi units [responsive to 65 dB SPL noise bursts presented to the ipsilateral ear but not to noise bursts presented to the contralateral ear, the ipsilateral ear being the (left) ear where the recordings were made], contra units (responsive to noise bursts presented to the contralateral ear but not to noise bursts presented to the ipsilateral ear), or either-ear units (responsive to noise bursts presented to either ear and further classified as either-ear ipsi or either-ear contra according to the lower threshold). An automated tuning curve algorithm (Kiang et al. 1970; Liberman 1978) was used to obtain the tuning curve (for MOC units, the response window was delayed 15 ms to compensate for the longer latency relative to ANFs). The CF was obtained from the tuning curve. For either-ear units, the assigned CF was for the ear with the lower threshold.
Labeling experiments used 27 guinea pigs of a different group from those described above; however, similar surgical methods were used. These guinea pigs were part of previous studies in which the methods for injections and processing were described (Brown 1987a, 1987b). Briefly, extracellular injections of HRP (30% in 0.1 M Tris buffer, pH 8.6) were made by iontophoresis (3-μA positive current, 10 s on-10 s off for 10 min) into the intraganglionic spiral bundle using a pipette with a broken tip (diameter 15–40 μm). After a survival time (see below), the guinea pigs were perfused intravascularly with physiological saline followed by fixative (2.5% glutaraldehyde and 1% paraformaldehyde in 0.065 M phosphate buffer, pH 7.3, with 0.008% CaCl2) and postfixed 2–24 h at 4°C. Decalcification was by 0.1 M EDTA at 4°C. Cochleas were embedded in a gelatin-albumin mixture and then sectioned (80- to 100-μm thickness) perpendicular to the modiolus to form quasi-surface preparations and processed with CoCl2 and diaminobenzidine (Adams 1977). The thick, type I ANFs and the thick MOC axons were documented using a compound light microscope with ×10 or ×20 objectives and a drawing tube. MOC endings were distinguished from terminals of type II ANFs, because the latter are smaller and given off by fibers spiraling beneath the OHCs that are only present immediately basal to the injection site (Brown 1987a). Cochlear segments were drawn with a ×4 objective, and lengths were measured using ImageJ. Basal labeling and other labeling close to the injection site were present for all survival times of 1–24 h; apical labeling was present only for the longer survival times (e.g., 12–24 h, Fig. 7).
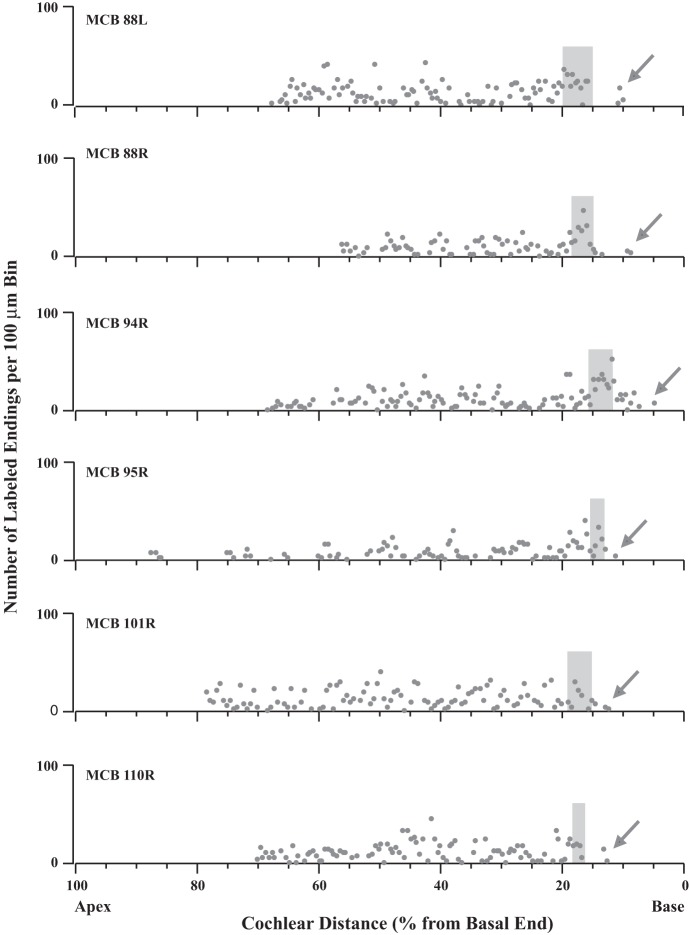
Fig. 7.
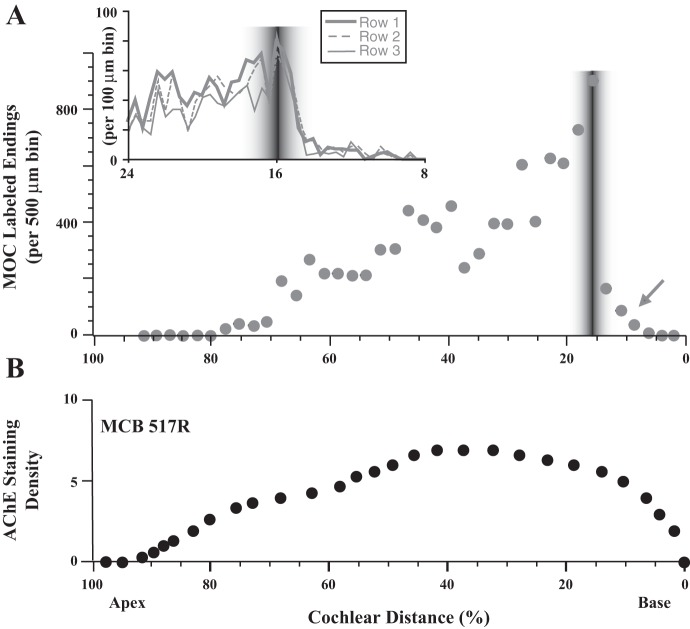
Data illustrating that MOC labeling is distributed in a broad pattern approaching a basal limit, or edge, where it drops off at the position of the labeling of ANFs (gray shading). Each graph shows the labeling in an individual cochlea. Points represent the number of MOC endings in 100-μm bins; bins with no endings are not plotted. Arrows indicate MOC labeling more basal than the ANF labeling. Survival times were the longest of the present data set to ensure the most apical labeling and are (from top to bottom) 23.5, 22.5, 13.25, 24, 24, and 12 h. Case 94R was illustrated in Fig. 6B.
In three other guinea pigs, four cochleas were selected and stained for acetylcholinesterase (Churchill and Schuknecht 1959). Methods used were similar to those described above for tracer injections, except that no injections were made and the fixative was 1.25% glutaraldehyde and 1% paraformaldehyde in 0.065 M phosphate buffer (pH 7.3) with 0.008% CaCl2. In two of the guinea pigs, instead of sectioned material, microdissected pieces of the organ of Corti and associated osseous spiral lamina were processed to better resolve the extreme basal end. Previously described staining methods (Osen and Roth 1969) were used, except that after incubation for 60 min with acetylthiocholine iodide, the sections were treated with 4% sodium sulfide (pH 7.8) for 1 min.
RESULTS
ANFs: high CFs in a narrow band.
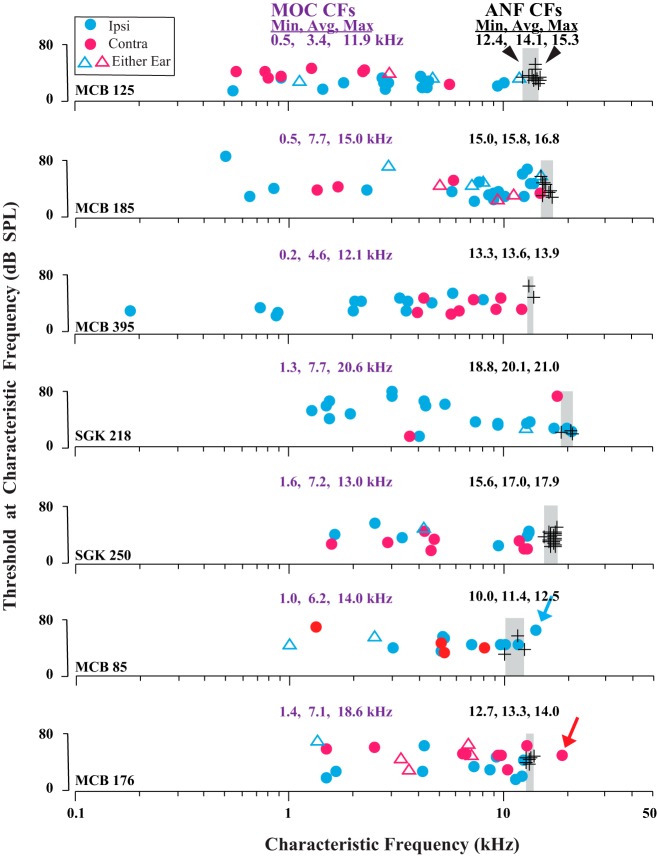
Recordings from sites in the lower basal turn of the cochlea obtained both ANFs and MOC axons having distinct distributions of CFs (Fig. 2). As expected from the location of the site, the CFs of ANFs (black symbols) were high (overall average CF: 15.8 kHz, range: 9.64–22.6 kHz, 640 units from 133 guinea pigs). At a single site, the range of CFs obtained was narrow. For example, in the case shown in Fig. 2, top, CFs ranged from a minimum of 12.4 kHz to a maximum of 15.3 kHz (from 0.18 octave below to 0.12 octave above the recording site average of 14.1 kHz, which is a range of 0.30 octave). For the 13 preparations in which substantial numbers of ANFs were recorded per animal (6–25), the average range of CFs was 2.15 kHz, or 0.2 octave (SD 0.71 kHz).
Fig. 2.
Data illustrating that MOC CFs (colored symbols) are distributed in a broad pattern up to an upper limit, or edge, at the CF of ANFs (black symbols). Data are from 7 individual guinea pigs; CF minimums, averages, and maximums (Min, Avg, Max) are given for each animal. For ANFs, the narrow range of CFs (gray shading) differs from case to case because of differences in placement of the recording site in each cochlea. The guinea pigs in the 2 lowermost plots had exceptional MOC units with CFs above those of ANFs (colored arrows). Ipsi units are driven by sound in the ipsilateral ear, contra units by sound in the contralateral ear, and either-ear units by sound in either ear.
MOC units: broad distribution of CFs up to an edge near those of ANFs.
Recordings from the same sites obtained MOC units with a large range of CFs (Fig. 2, colored symbols). For example, in the case shown in Fig. 2, top, the minimum CF was 0.5 kHz (4.82 octaves below the ANF CF of 14.1 kHz) and the maximum CF was 11.9 kHz (0.24 octave below), a huge range of 4.58 octaves. For the 10 preparations in which substantial numbers of units were recorded per animal (14–28), the range of CFs averaged 9.51 kHz, or 4.07 octaves (SD 3.3 kHz). In pooled data (Fig. 3), the MOC CFs were broadly distributed, with the most common CFs being between 5 and 10 kHz and a usual upper limit of about 16 kHz, the approximate CF of ANFs in these preparations.
Fig. 3.
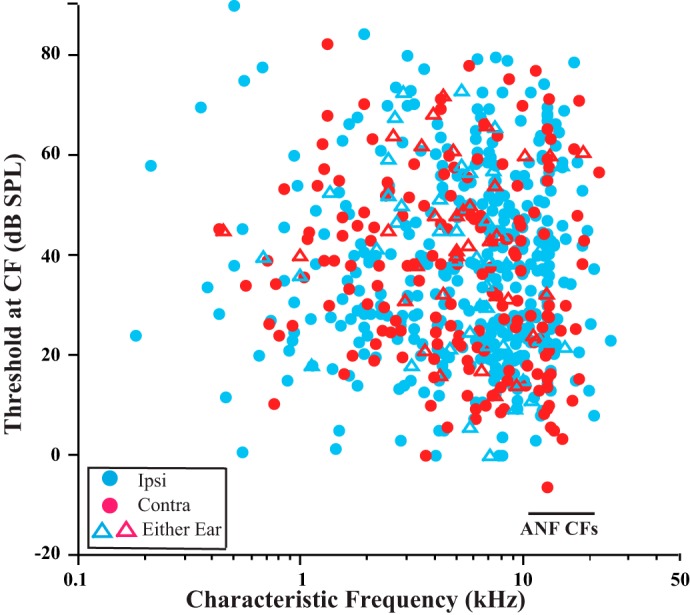
Pooled distribution of CFs of all MOC units. Data are 639 MOC units from 133 guinea pigs. Horizontal bar indicates the overall range for CFs of ANFs (9.64 to 22.6 kHz).
A more precise comparison of the upper limit of MOC CFs and the CFs of ANFs, though, must take into account small individual differences in the ANF CFs (Fig. 2) that result from differences in recording site placement. To align the individual preparations, data were normalized to the ANF CFs in each preparation (Fig. 4). The normalization (see Fig. 4 legend) was done separately according to the following three measures of CFs of the ANFs within each preparation: 1) minimum (Fig. 4A), 2) maximum (Fig. 4B), and 3) average (Fig. 4, C and D). An MOC edge is best seen in the average-normalized data (Fig. 4, C and D). This may occur because the distribution of CFs of ANFs is narrow and bell-shaped with a peak near the average (Fig. 4D, black). In contrast, there are very few ANF CFs near the edges. The MOC CF at the edge matches the peak CF of the ANFs (Fig. 4D, black vs. purple), so if MOC fibers at the edge terminate locally near ANFs, then these two fiber populations have the same tonotopic organization there.
Fig. 4.
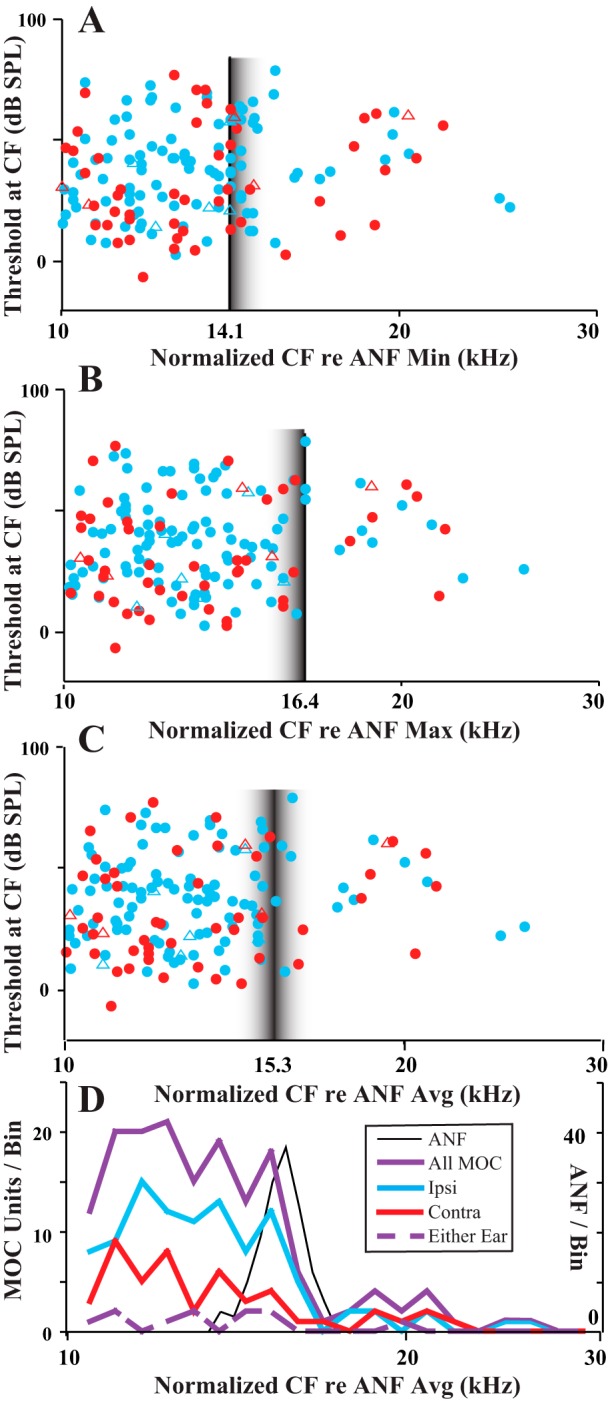
Normalized MOC data to allow for comparisons of the CF edge of all units across preparations. Normalization was done for each particular guinea pig according to 3 measures of the CFs of ANFs in that guinea pig. A: CFs multiplied by 14.1/(ANF CFmin), where ANF CFmin is the minimum CF of the ANF from the preparation in which the MOC unit was recorded. B: CFs multiplied by 16.4/(ANF CFmax), where ANF CFmax is the maximum CF of the ANF from the preparation. C: CFs multiplied by 15.3/(ANF CFavg), where ANF CFavg is the average CF of the ANF from the preparation. The multipliers (14.1, 16.4, or 15.3) were chosen because they are the average minimum CF, average maximum CF, and average CF (in kHz) across the preparations of the study. Vertical lines indicate the points of alignment based on ANF measures, and fuzzy borders are meant to indicate nonalignment of ANF CFs there. D: binned data using average normalized MOC CFs (same x-axis as C) and ANF data consisting of 145 units from the 12 guinea pigs in which 8 or more ANFs were recorded.
The CF distributions were plotted separately for the different MOC response type (Fig. 4D). Ipsi units (Fig. 4D, blue) have a clear edge or cutoff at the maximum ANF peak. By contrast, contra units (Fig. 4D, red) have a more gradual decline at this peak position, although this is less clear because of fewer data points. Finally, the data for either-ear units (Fig. 4D, dashed purple) are too sparse to allow a meaningful comparison.
A few MOC units had CFs higher than those of ANFs, blurring the edge.
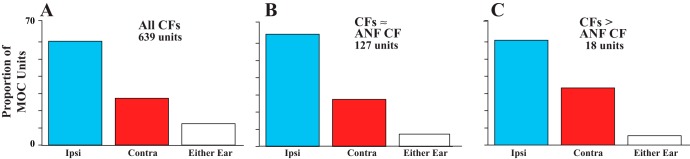
A few MOC units had CFs equal to or higher than the maximum ANF CF in the same preparation. These data are indicated for individual guinea pigs in Fig. 2 (arrows) and for all data of the study in Fig. 4 (where in Fig. 4B, the maximum ANF-CF data, they appear on and to the right of the vertical line). Such units were found in 14 (10.5%) of 133 guinea pigs, and they totaled only 18 of 639 (2.8%) of all MOC units. The CFs of these units ranged from 14.0 to 24.7 kHz (average 19.0 kHz). Relative to the maximum ANF CFs, these MOC CFs were higher by up to 0.65 octave. The other characteristics of these units, though, were similar to other MOC units with high CFs. For example, tuning curve thresholds at CF averaged 44.14 dB (SD 14.6 dB), not significantly different from other high-CF units (37.8 dB, SD 19.8 dB; P = 0.235 for CFs >12 kHz). The width of their tuning curves, as measured by Q10, averaged 4.77 (SD 2.76, range 1.69–10.1), similar to other MOC units with CFs almost as high (Brown 1989). Their breakdown according to response type was 11 ipsi, 6 contra, and 1 either-ear unit (Fig. 5C), which is a proportion of response types not significantly different from those of another group of high-CF MOC units (Fig. 5B). It is about the same as the overall proportions for MOC units (Fig. 5A; ipsi: 60.4%, contra: 27.1%, and either-ear: 12.5% of all units). Also, ipsi and contra units did not greatly differ in the highest CFs recorded: the highest ipsi unit CF was 24.7 kHz (from a preparation that had a highest auditory nerve CF of 17.9 kHz), and the highest contra unit CF was 22.0 kHz (from a preparation that had a highest auditory nerve CF of 17.5 kHz). For those units that had a higher CF than the corresponding ANF CF, ipsi units were an average of 3.62 kHz greater and contra units were an average of 3.40 kHz greater.
Fig. 5.
Proportions of units for each response type. A: all MOC units. B and C: MOC normalized CFs over the range 10–16.4 kHz (B) and for the range above16.4 kHz (C). For normalization, the maximum CF for ANFs was used so that all points in C are MOC units with CFs greater than that of any ANF in that preparation. Numbers of units are indicated for each histogram. A z-test showed no significant difference in proportion of ipsi units in these 2 regions (z = 0.3572, P = 0.71884).
Labeling patterns: broad MOC terminations up to an edge near a band of ANFs.
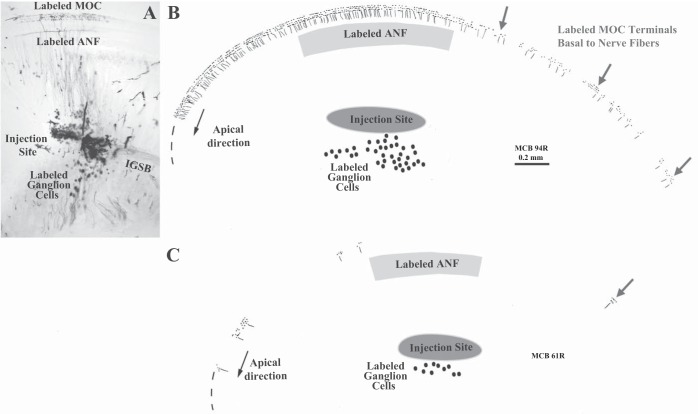
Extracellular injections into sites similar to those used for recordings were used to test the innervation plan schematized in Fig. 1. The MOC projections near the injection site were examined closely to determine whether the edge of labeling was near the ANFs or whether it showed a basal offset toward the location of the cochlear amplifier. These injections labeled both ANFs and MOC fibers. For the former, the labeling consisted of a cluster of spiral ganglion cells on the modiolar side of the injection site and a band of labeled ANFs on the organ of Corti side immediately radial to the injection site (Fig. 6). The width of the ANF labeling was narrow (average width from the apicalmost to basalmost labeled ANF = 648 μm; range 310-1,676 μm, SD 353 μm, for 17 preparations), equivalent to 0.28 octave of cochlear distance. This result is consistent with the observed narrow band of CFs recorded from ANFs.
Fig. 6.
Patterns of MOC labeling resulting from extracellular injections of neural tracer. Micrograph of the injection site in 1 case (A) and reconstructions of 2 other cases (B and C). In B and C, the dots and fibers represent all labeled MOC endings and tunnel-crossing branches within a half-turn from the injection site. In B, a case with many labeled fibers, the MOC labeling is dense adjacent and apical to the injection site, but it diminishes adjacent to the injection site to become sparse basally (gray arrows). Labeling extends along the organ of Corti for 1,429 μm from the basalmost edge of the labeled peripheral ANFs (gray shaded band). In C, a case with few labeled fibers, the basal labeling consists of only 1 cluster of MOC endings (gray arrow) located 731 μm basal to the ANFs. Ovals indicate labeled cell bodies of the spiral ganglion that give rise to the ANFs. IGSB, intraganglionic spiral bundle.
In contrast, labeled MOC axons took a spiral course in the intraganglionic spiral bundle (IGSB; Fig. 6A), and apical to the injection site the axons branched to form endings over a broad extent of the cochlea (Figs. 6A, 7, and 8A). Basal to the injection site, however, the axons rarely branched so that at the injection site there was an abrupt decline in labeling in the basal direction (Figs. 7 and 8A). The decline was at the same position of the labeled ANFs with no indication that the bulk of projections was offset basally. The labeling edge was closely matched to the CF edge as shown by plotting both measures on a logarithmic scale (Fig. 9), a result consistent with the innervation plan with high-CF axons being unlabeled (Fig. 1). The edge was not observed in acetylcholinesterase staining (Fig. 8B), presumably because there is staining of all MOC axons without regard to an injection site. Instead, the staining plot forms a smooth curve beginning at a cochlear distance of about 95%, rising to a broad peak located in the middle of the cochlea, and smoothly declining toward the base (Fig. 8B).
Fig. 8.
Comparison of MOC labeled endings (A) and staining for acetylcholinesterase (AChE; B). Labeling in A is the same as cases shown in Fig. 7, aligned on the basis of the middle of the ANF labeling. Inset shows an expanded view of endings tabulated separately for the OHC rows. AChE staining used a qualitative scale ranging from 0 (no staining) to 5 (darkest staining) because staining was too dense to resolve individual endings. Results for 1 cochlea are shown in B; the other 3 AChE-stained cochleas had similar patterns.
Fig. 9.
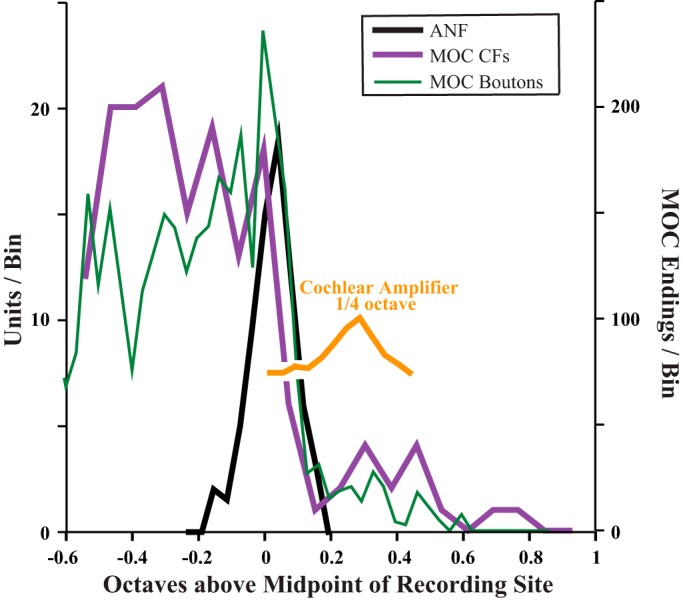
Comparison of distributions CFs and MOC endings as plotted on an octave scale. CFs are binned and normalized as in Fig. 4D, and y-axis at left indicates MOC units/bin, or (ANF units/bin)/2 (half the scale used in Fig. 4D). Boutons are binned and aligned as in Fig. 8A, and the CF correlate of their position was computed using the cochlear frequency mapping (Brown 2014). The extent of the cochlear amplifier (yellow curve) is indicated by displacing ANF distribution by 650 μm (one-quarter octave).
Further basal to the edge of MOC labeling, 18 of 27 injected cochleas had a limited amount of labeling (arrows in Figs. 6, 7, and 8A). Overall, this basal labeling was uncommon and consisted of only 267 (3.1%) of 8,567 total endings (in the 11 cases analyzed in detail). The basal labeling extended an average of 746 μm (range 314-1,429 μm) basal to the ANF labeling, corresponding in these cases to an average cochlear distance of 3.63% (0.33 octave) with a range of 1.47–7.72% (0.13–0.69 octave). Figure 6B shows the case with the highest amount of such labeling, whereas Fig. 6C shows a case with just one very small patch of such labeling. This labeling was evenly distributed on all three rows of OHCs (Fig. 6A, inset), without targeting OHCs in row 1, which is the most important row for the cochlear amplifier as demonstrated by noise-induced damage (Liberman and Dodds 1984). In terms of its rarity and distance basal from the edge, the basal labeling corresponds fairly well to the rare CFs above the edge of CFs (Fig. 9), suggesting that both result from axons injected or recorded within their branching pattern (e.g., MOC 2 in Fig. 1). However, this idea could not be tested because axons could not be traced through the injection site because of debris and extracellular reaction product (Fig. 6A).
DISCUSSION
In the current study, extracellular recording and labeling in the spiral ganglion were used to compare the CF distributions and labeling patterns of ANFs and MOC units. The data support the innervation plan of Fig. 1, which has radially coursing ANFs and spirally coursing MOC axons, with an edge of labeling and CFs because higher-CF MOC axons turn out basally before passing through the site. The MOC CFs at the edge matched the ANF CFs there, which, coupled with the lack of a basal offset in MOC projections, demonstrates the alignment of ANF and MOC tonotopic mappings at this site.
Mappings of MOC units and ANFs are in CF alignment.
Mappings of MOC axons onto the cochlea have previously been studied with single-unit labeling, in which an axon is characterized by its response to sound (including CF and response type) and then injected with neural tracer. The labeled axon is then traced to its endings on the OHCs along the cochlea. These labeling studies indicate that 1) the MOC projections are tonotopic, with high-CF axons projecting to the cochlear base and low-CF axons projecting more apically, and 2) the projection is in general alignment with the mapping for ANFs (Brown 1989, 2014; Liberman and Brown 1986; Robertson and Gummer 1985). An advantage of those studies is that the mapping can be investigated for a variety of CFs. However, those experiments are difficult, and only a handful of MOC axons have been reconstructed to all their terminations (Brown 1989, 2014; Liberman and Brown 1986). Data from the present study (Figs. 4D and 9), with a completely different approach, support the idea of the alignment of MOC edge CFs and ANF CFs in the basal turn of the cochlea. The labeling pattern shows an edge at the injection site at the position of the labeled ANFs. Such combined data indicate similar tonotopic mappings.
The range of CFs of ANFs from a single site in the ganglion, which was on average 0.20 octave, is reported here for the first time. These physiological data are consistent with the labeled band of ANFs, averaging 668 μm in cochlear distance, which corresponds to 0.28 octave of cochlear distance. These data suggest some variability in the radial course of the peripheral axon of spiral ganglion cells. The larger measurement of the labeling may indicate spread of the HRP to axons that are not recordable in the site. The labeled peripheral axons studied presently emanate from type I ganglion cells and contact inner hair cells (Liberman 1982b; Perkins and Morest 1975; Smith 1961; Tsuji and Liberman 1997). Thinner diameter ANFs that emanate from type II ganglion cells and contact OHCs (Brown 1987b; Kiang et al. 1982) are apparently not recorded with micropipette electrodes.
MOC units with CFs above the “edge.”
It is assumed that labeling basal to the injection site represents axonal branches that are connected to others passing through the site (Fig. 1, MOC 2). This idea follows from the considerable branching observed in MOC axons, some of which takes place in the spiral ganglion area (Brown 1989, 2014; Liberman and Brown 1986). It is also consistent with labeling of very small patches of endings (Fig. 6C, arrow) that are almost certainly parts of axons terminating mainly apical to the site of injection. The largest basal extent of this labeling was 7.72% of the cochlear distance; this distance is a measure of the span of MOC axons. Previous labeling work showed spans up 24% of the cochlear distance in the guinea pig (Brown 2014). Present data did not show basal extents that great, so such spans may be rare and may stem from single-unit labeling of only the largest diameter MOC axons (Brown 2014), which may have the largest spans. The distance at which the endings appeared basal to the injection site was variable (Fig. 7). The variability may be due to 1) axons not always being labeled at a particular point within their branching and 2) the variability in span of MOC axons (Brown 2014).
In the current study, it was also assumed that the few MOC units with higher CFs (relative to those of ANFs) represent recordings from an axon branch passing through the recording site that is part of an axon with other branches terminating basal to the recording site (Fig. 1, MOC 2). The largest CF difference, 0.65 octave, corresponds to a cochlear distance of 8.93%. If MOC mappings are via the midpoint of innervation (Brown 2014), then twice this distance would predict a maximal span of 17.86%. This measure is larger than present labeling results and more in line with single-unit labeling data (Brown 2014). Axonal span as measured by recordings may be different from span as measured by labeling due to branches of MOC axons that spiral peripheral to the ganglion such as in the inner spiral bundle (e.g., Fig. 5 in Brown 1987b). Also, another difference may result from thinner branches, especially to the edges of the axon's span, which are not recordable.
An alternate explanation for the very high-CF MOC units, though, is that they represent axons terminating off the average mapping. For example, an MOC unit with an 18-kHz CF might terminate at the 16-kHz location and thus run through the 16-kHz recording site. Substantial variability has been observed in the CF mapping of MOC axons (Brown 2014). However, a large amount of variability is not supported by the present study's observation of a sharp edge found in the MOC CF distribution (Fig. 4D). Overall, some variability in terminations along with broad innervation extents are possible anatomical correlates of broad MOC actions on stimulus frequency otoacoustic emissions (Chery-Croze et al. 1993; Lilaonitkul and Guinan 2012; Veuillet et al. 1991).
Response type differences.
Axonal spans of contra axons have been reported to be greater than those for ipsi axons (Brown 2014). Correspondingly, physiological effects of contralateral sound are integrated over a broader frequency range compared with those of ipsilateral sound (Lilaonitkul and Guinan 2012). The longer spans of contra axons may explain the more gradual decline in the pattern of CFs observed near the edge of MOC CFs (Fig. 4D), because it may be possible to sample contra axons over a longer distance within their innervation span. For example, at the recording site, locally terminating contra axons of CFs over a broad range (e.g., 12 -20 kHz) might be sampled, whereas sampling of ipsi axons might be over a narrower range (e.g., 14–18 kHz). Another measure that might relate to span is the sample of MOC units with very high CFs, above those of ANFs. However, ipsi and contra units did not significantly differ in their relative numbers (compared with the general population, Fig. 5) or in their maximum CFs. Limited data from single-unit labeling studies suggest that MOC contra units project somewhat apically to ipsi units (Brown 2014). Although the biggest difference was for low-CF units, if this is the case for high-CF units, then contra units with CFs of 18 kHz could project through a recording site tuned to 16 kHz. The more gradual decline in contra CFs near the ipsi edge, though, prevents us from investigating whether the mappings of ipsi and contra units are different.
MOC innervation along the length of the cochlea.
Present data clearly show the drop-off in MOC units tuned to low CFs (Fig. 3) and drop-off in innervation toward the apex of the cochlea (Figs. 7 and 8). Many previous studies have shown this decrease toward the apex (Brown 1989, 1987b; Guinan et al. 1984; Ishii and Balough 1968; Liberman and Brown 1986; Maison et al. 2003; Rama et al. 1980; Robertson and Gummer 1985; Smith and Sjostrand 1961). There is also a decrease in MOC innervation toward the base as observed with acetylcholinesterase stains (Fig. 8B), but it is much less abrupt than the decrease basal to the edge of labeling seen after injections into the ganglion (Fig. 8A). The acetylcholinesterase data suggest that if recordings had been made at a site more basal, or from the entire OC bundle, there would be a larger number of high-CF MOC units.
Effect of alignment of tonotopy vs. misalignment with the cochlear amplifier.
The CF alignment of MOC axons and ANFs shown in the present study means that there is a CF misalignment for the MOC axon and the corresponding cochlear amplifier location (Fig. 9), because the position of the amplifier is basal to the CF position for ANFs. The effect of this misalignment does not fit with the simple idea that the MOC reflex pathway responds to a tone and activates the MOC neurons to turn down the amplifier gain (present data and Brown 2014). Rather, to maximally affect the amplifier, and thus the tip region of the ANF's tuning curve tip (the region generated by the cochlear amplifier), MOC neurons activated by a tone at CF should project more basally than they do. MOC effects on stimulus frequency otoacoustic emissions (SFOAEs), the most frequency-specific OAEs, sometimes show offsets, but they can be in either direction (Lilaonitkul and Guinan 2013). Such SFOAE data are from humans, and it is not clear whether the MOC innervation data is the same in the species used presently (guinea pig). Perhaps, however, thinking about MOC neurons acting in a frequency specific way is incorrect, given their excellent responses to broadband signals such as noise (Liberman 1988), their relatively broad projection onto the OHCs, and their presumed antimasking functions for broadband maskers.
The MOC tonotopy is better positioned for protection from acoustic overstimulation. Although the mechanisms by which MOC activity protects from damage are not understood, this protection may not be due to changes in the cochlear amplifier, because at very high sound levels there is much less influence of the amplifier. Acoustic overstimulation at high frequencies causes damage about a half-octave above the exposure frequency (Zhang and Zwislocki 1996). This is because in response to a high-level tone, the largest basilar membrane motion occurs at a place about a half-octave basal to the tone's CF place (Ruggero 1992). Consider a particular position along the cochlea that has a CF of 11 kHz, which, at threshold levels, has basilar membrane vibration that is maximal for 11-kHz tones. However, this same 11-kHz tone, at high levels, produces the largest basilar membrane vibration at a position a half-octave basal (about 16-kHz CF) and potentially causes damage there. For MOC activity to reduce damage at the 16-kHz place, MOC axons that innervate that place should be activated. This comes about because the large motion at the 16-kHz place causes the largest response in ANFs coming from that place, in turn activating maximally the 16-kHz MOC reflex pathway. As shown in the present study, that pathway feeds back tonotopically to the 16-kHz place, and thus it is well-positioned to prevent damage there. Such a theory predicts that the pattern of damage would change its frequency dependence when the OC bundle is removed. Although the peak of the damage pattern does not seem to shift (Rajan 1995), the pattern is broad and a shift would be hard to observe. Additionally, there may be involvement of lateral OC neurons, which also provide protection (Darrow et al. 1997). Selective MOC lesion data would be helpful in investigating this idea. This innervation pattern does not mean that the MOC system cannot also control cochlear amplification. For instance, if the only effects of MOC neurons were at high levels, then it would be expected that MOC neurons would have high acoustic thresholds, as do motoneurons of the middle ear muscles (Kobler et al. 1992). Overall, the full effect of the MOC innervation pattern, whether it is to provide protection and/or affect the cochlear amplifier, remains to be worked out.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grant DC01089.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.C.B. conception and design of research; M.C.B. performed experiments; M.C.B. analyzed data; M.C.B. interpreted results of experiments; M.C.B. prepared figures; M.C.B. drafted manuscript; M.C.B. edited and revised manuscript; M.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. Sharon Kujawa for participating in some of the experiments, Dr. M. Charles Liberman for generous help throughout the project, and Dr. John J. Guinan, Jr., for helpful comments on an earlier version of the manuscript.
REFERENCES
- Adams JC. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience 2: 141–145, 1977. [DOI] [PubMed] [Google Scholar]
- Ashmore J, Avan P, Brownell WE, Dallos P, Dierkes K, Fettiplace R, Grosh K, Hackney CM, Hudspeth AJ, Jülicher F, Lindner B, Martin P, Meaud J, Petit C, Santos-Sacchi J, Canlon B. The remarkable cochlear amplifier. Hear Res 266: 1–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–110, 1989. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J Comp Neurol 260: 591–604, 1987a. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol 260: 605–618, 1987b. [DOI] [PubMed] [Google Scholar]
- Brown MC. Single-unit labeling of medial olivocochlear neurons: the cochlear frequency map for efferent axons. J Neurophysiol 111: 2177–2186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chery-Croze A, Moulin A, Collet L. Effect of contralateral sound stimulation on the distortion product 2f1-f2 in humans: evidence of a frequency specificity. Hear Res 68: 53–58, 1993. [DOI] [PubMed] [Google Scholar]
- Churchill JA, Schuknecht HF. The relationship of acetylcholinesterase in the cochlea to the olivocochlear bundle. Henry Ford Hosp Med Bull 7: 202–210, 1959. [PubMed] [Google Scholar]
- Cody AR. Acoustic lesions in the mammalian cochlea: Implications for the spatial distribution of the ‘active process’. Hear Res 62: 166–171, 1992. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol 97: 1775–1785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex J. Auditory activity in centrifugal and centripetal cochlear fibres in cat. A study of a feedback system. Acta Physiol Scand Suppl 189: 1–68, 1962. [PubMed] [Google Scholar]
- Fex J. Auditory activity in uncrossed centrifugal cochlear fibres in cat. A study of a feedback system. II. Acta Physiol Scand 64: 43–57, 1965. [DOI] [PubMed] [Google Scholar]
- Fisher JA, Nin F, Reichenbach T, Uthaiah RC, Hudspeth AJ. The spatial pattern of cochlear amplification. Neuron 76: 989–997, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. The synaptic physiology of cochlear hair cells. Audiol Neurootol 7: 40–44, 2002. [DOI] [PubMed] [Google Scholar]
- Gacek RR. The efferent cochlear bundle in man. Arch Otolaryngol 74: 690–694, 1961. [DOI] [PubMed] [Google Scholar]
- Guinan JJ Jr, Warr WB, Norris BE. Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226: 21–27, 1984. [DOI] [PubMed] [Google Scholar]
- Ishii D, Balough K Jr. Distribution of efferent endings in the organ of Corti. Their graphic reconstruction in cochleae by localization of acetylcholinesterase activity. Acta Otolaryngol 66: 282–288, 1968. [DOI] [PubMed] [Google Scholar]
- Jennings SG, Heinz MG, Strickland EA. Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot. J Assoc Res Otolaryngol 12: 345–360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol 70: 2533–2549, 1993. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural Hearing Loss: a Ciba Foundation Symposium, edited by Wolstenholme GE, Knight J. London: Churchill, 1970, p. 241–273. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Rho JM, Northrop CC, Liberman MC, Ryugo DK. Hair-cell innervation by spiral ganglion cells in adult cats. Science 217: 175–177, 1982. [DOI] [PubMed] [Google Scholar]
- Kobler JB, Guinan JJ Jr, Vacher SR, Norris BE. Acoustic reflex frequency selectivity in single stapedius motoneurons of the cat. J Neurophysiol 68: 807–817, 1992. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve responses from cats raised in a low-noise chamber. J Acoust Soc Am 63: 442–455, 1978. [DOI] [PubMed] [Google Scholar]
- Liberman MC. The cochlear frequency map for the cat: Labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am 72: 1441–1449, 1982a. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: Monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60: 1779–1798, 1988. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in the cat auditory nerve. Science 216: 1239–1241, 1982b. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res 16: 55–74, 1984. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 419: 300–304, 2002. [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ Jr. Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol 107: 1598–1611, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol 455: 406–416, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci 20: 4701–4707, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely ST, Kim DO. A model for active elements in cochlear biomechanics. J Acoust Soc Am 79: 1472–1480, 1986. [DOI] [PubMed] [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res 16: 165–185, 1969. [DOI] [PubMed] [Google Scholar]
- Pang XD, Guinan JJ Jr. Growth rate of simultaneous masking in cat auditory-nerve fibers: relationship to the growth of basilar-membrane motion and the origin of two-tone suppression. J Acoust Soc Am 102: 3564–3575, 1997. [DOI] [PubMed] [Google Scholar]
- Patuzzi R. Cochlear micromechanics and macromechanics. In: The Cochlea, edited by Dallos P, Popper AN, and Fay RR. New York: Springer, 1996, p. 186–257. [Google Scholar]
- Perkins RE, Morest DK. A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarski optics. J Comp Neurol 163: 129–158, 1975. [DOI] [PubMed] [Google Scholar]
- Portmann M, Portmann C. Efferent nerve fibers of cochlea. Arch Otolaryngol 59: 543–554, 1954. [DOI] [PubMed] [Google Scholar]
- Prijs VF. Lower boundaries of two-tone suppression in the guinea pig. Hear Res 42: 73–82, 1989. [DOI] [PubMed] [Google Scholar]
- Rajan R. Frequency and loss dependence of the protective effects of the olivocochlear pathways in cats. J Neurophysiol 74: 598–615, 1995. [DOI] [PubMed] [Google Scholar]
- Rama J, Morales J, Sanchez G. Comparative study of the nerve endings of the outer and inner hair cells in the organ of Corti. J Laryngol Otol 94: 1125–1143, 1980. [DOI] [PubMed] [Google Scholar]
- Rasmussen GL. Further observations of the efferent cochlear bundle. J Comp Neurol 99: 61–94, 1953. [DOI] [PubMed] [Google Scholar]
- Reiter ER, Liberman MC. Efferent mediated protection from acoustic overexposure: relation to “slow” effects of olivocochlear stimulation. J Neurophysiol 73: 506–514, 1995. [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morphological characterization of efferent neurons in the guinea pig cochlea. Hear Res 20: 63–77, 1985. [DOI] [PubMed] [Google Scholar]
- Ruggero M. Responses to sound of the basilar membrane of the mammalian cochlea. Curr Opin Neurobiol 2: 449–456, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Fay RR, Popper AN. Auditory and Vestibular Efferents. New York: Springer Science+Business Media, 2011. [Google Scholar]
- Sellick PM, Russell IJ. Intracellular studies of the receptor potentials of inner hair cells of the guinea pig cochlea: techniques. In: Auditory Investigation: The Scientific and Technological Basis, edited by Beagley HA. Oxford: Oxford University Press, 1979, p. 368–381. [Google Scholar]
- Shera CA. Laser amplification with a twist: traveling-wave propagation and gain functions from throughout the cochlea. J Acoust Soc Am 122: 2738–2758, 2007. [DOI] [PubMed] [Google Scholar]
- Smith CA. Innervation pattern of the cochlea. The internal hair cell. Ann Otol Rhinol Laryngol 70: 504–528, 1961. [PubMed] [Google Scholar]
- Smith CA, Sjostrand FS. Structure of the nerve endings on the external hair cells of the guinea pig cochlea as studied by serial sections. J Ultrastruct Res 5: 523–556, 1961. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of the auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol 381: 188–202, 1997. [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65: 724–735, 1991. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am 48: 950–965, 1970. [DOI] [PubMed] [Google Scholar]
- Winslow R, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of crossed olivocochlear bundle. Hear Res 35: 165–190, 1988. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zwislocki JJ. Intensity-dependent peak shift in cochlear transfer functions at the cellular level, its elimination by sound exposure, and its possible underlying mechanisms. Hear Res 96: 46–58, 1996. [DOI] [PubMed] [Google Scholar]