Abstract
Adaptation of fingertip forces to friction at the grasping surface is necessary to prevent use of inadequate or excessive grip forces. In the current study we investigated the effect of blocking tactile information from the fingertips noninvasively on the adaptation and efficiency of grip forces to surface friction during precision grasp. Ten neurologically intact subjects grasped and lifted an instrumented grip device with 18 different frictional surfaces under three conditions: with bare hands or with a thin layer of plastic (Tegaderm) or an additional layer of foam affixed to the fingertips. The coefficient of friction at the finger-object interface of each surface was obtained for each subject with bare hands and Tegaderm by measuring the slip ratio (grip force/load force) at the moment of slip. We found that the foam layer reduced sensibility for two-point discrimination and pressure sensitivity at the fingertips, but Tegaderm did not. However, Tegaderm reduced static, but not dynamic, tactile discrimination. Adaptation of fingertip grip forces to surface friction measured by the rate of change of peak grip force, and grip force efficiency measured by the grip-load force ratio at lift, showed a proportional relationship with bare hands but were impaired with Tegaderm and foam. Activation of muscles engaged in precision grip also varied with the frictional surface with bare hands but not with Tegaderm and foam. The results suggest that sensitivity for static tactile discrimination is necessary for feedforward and feedback control of grip forces and for adaptive modulation of muscle activity during precision grasp.
Keywords: tactile perception, sensorimotor adaptation, feedforward and feedback control, precision grasp, electromyography
we grasp objects of various textures with ease by adapting our fingertip (grip) forces to the friction at the grasping surface. The grip force must be optimized to prevent both excessive squeezing of the object (wasted force and/or object damage) and slipping of the object from grasp due to insufficient forces (Westling and Johansson 1984). Adaptation occurs by using sensory information at the grip surface to modify the rate of change of grip force such that smooth-surfaced objects elicit higher grip force rates than rougher textured ones (Johansson and Westling 1984). Tactile sensation is necessary to perceive the friction at the grasping surface and is obtained from four distinct kinds of mechanoreceptors that innervate the glabrous skin of the human hand (Hollins and Bensmaia 2007; Hsiao and Gomez-Ramirez 2011; Johnson and Hsiao 1992; Lederman 1974; Lederman et al. 1982; Yoshioka et al. 2007): 1) the slowly adapting type 1 (SAI) afferents innervating Merkel cells, 2) the rapidly adapting or fast-adapting type I (FAI) afferents that terminate in Meissner corpuscles, 3) the Pacinian afferents, also known as the fast-adapting type II afferents (FAII), and 4) the slowly adapting type 2 (SAII) afferents. Both the FAI and SAI receptors are located in clusters beneath the dermal papillary ridges of the fingertips that make up the fingerprints, whereas the FAII and SAII receptors terminate deeper in the skin and have a lower density than the type 1 receptors (Johansson and Vallbo 1979).
When tactile sensation is impaired, such as from peripheral nerve injury or central nervous system injury after a stroke, it is not clear how fingertip forces are adapted to different frictional surfaces. In this study we have used psychophysical and behavioral techniques to measure how human subjects adapt their fingertip forces to friction at the grip surface during precision grasp when tactile information from the fingertips is partially blocked.
Previous investigators have injected local anesthetic into the digital nerves (Johansson and Westling 1984; Westling and Johansson 1984) or have occluded blood flow to the nerves (Gandevia and Mccloskey 1977) to block cutaneous sensation from the fingertips. However, cutaneous anesthesia is invasive, and it may block sensation from the fingertips incompletely and inconsistently (Brutus et al. 2003). Furthermore, occlusion of blood flow can reduce both tactile and proprioceptive sensation. Other studies have shown that defective, unavailable, or inappropriate tactile information can result in a mismatch between incoming sensory signals and compromise function (Bloem et al. 2000).
For these reasons, we chose to partially block tactile sensation noninvasively in this study. Tactile sensation may be qualitatively reduced by placing a thin layer of plastic film on the fingertips, mimicking the use of a surgical glove. Similarly, plantar sensation is reduced in posturography studies with a layer of foam, which by its shock-absorbing property can dampen the vibrations at the skin-object interface (Patel et al. 2008a, 2008b). Both of these methods can also control the noise introduced by individual differences in skin thickness and moisture at the fingertip-object interface that contribute to intra- and inter-subject variability during grasping (André et al. 2009, 2010). We hypothesized that coating the fingertips with a thin layer of plastic or foam would show differential behavioral effects on grip force adaptation and efficiency during precision grasp compared with grasping with bare hands.
In this report, we demonstrate that placement of intermediate barriers between the hand and object surface reduces tactile sensation and impairs adaptation of fingertip forces to surface texture of grasped objects. The activation of task-specific muscles also varied with the frictional surface when assessed with the use of bare hands but not with partial blockage of tactile sensation. The results suggest that static tactile discrimination is necessary for feedforward and feedback control of grip forces and for adaptive modulation of activity in task-specific muscles during precision grasp.
METHODS
Subjects.
Ten neurologically intact, right-hand-dominant subjects (mean age = 26.5 ± 6.42 yr; 5 males and 5 females) without history of musculoskeletal or neurological problems participated in the study. Hand dominance of the subjects was assessed using the Edinburgh Handedness Inventory (Oldfield 1971). Subject recruitment and data collection took place at New York University School of Medicine. The protocols were submitted to and approved by the institutional review board, and informed consent was obtained from all subjects according to the Declaration of Helsinki.
Sensory blocking and testing.
To develop a noninvasive method to assess the role of tactile sensation in grip force control, we covered the glabrous surface of the thumb and index finger with a thin layer of plastic film (Tegaderm HP-9536HP; 3M Health Care, St. Paul, MN) or with a layer of foam (Polycushion Padding-A2911; Patterson Medical, Cedarburg, WI) with Tegaderm underneath and on top of it. The Tegaderm and foam had adhesive backing to enable fixation to the fingertip. Passive tactile sensitivity on the thumb and index finger was evaluated using standard neurological tests. The two-point discrimination test (Dellon et al. 1987) and the Semmes-Weinstein monofilament test of pressure sensitivity (Dannenbaum et al. 2002) were performed on the thumb and index fingers with vision occluded. Each test was performed twice and repeated over two separate sessions with bare hands, with Tegaderm, and with foam.
In addition, the subjects performed a modified version of the static and dynamic tactile discrimination test (Dunn et al. 2013) with the index finger using bare hands and with Tegaderm. Subjects were asked to discriminate five pairs of textured surfaces that differed in their coefficient of friction; some were relatively easy to discriminate, and others had relatively small differences in friction that were more difficult to discriminate. For each pair of surfaces, one surface was presented twice, and the subject had to verbally indicate which surface differed from the other two. Correct discrimination was given 1 point, for a maximum of 5 points, and the average score across the 5 pairs of surfaces was computed. For static tactile discrimination, the subjects were not allowed to move their index finger against the surface, whereas for dynamic tactile discrimination, they could move the finger against the surfaces. The tactile discrimination test was not performed with foam because the two-point discrimination and monofilament tests showed that tactile sensitivity was significantly impaired, and pilot trials with foam revealed that individuals could not discriminate between the textures. Eight of the 10 subjects were available to perform this test.
Grasp and lift task and experimental apparatus.
A custom-made grip device that measures the fingertip grip and load forces using 6-degree of freedom force-torque sensors (ATI, Apex, NC) at a sampling rate of 400 Hz was used in this study (Fig. 1A). The device was placed on a custom-made plate with electronic switches in it to detect the timing of object lift. The weight of the object was kept constant at 250 g.
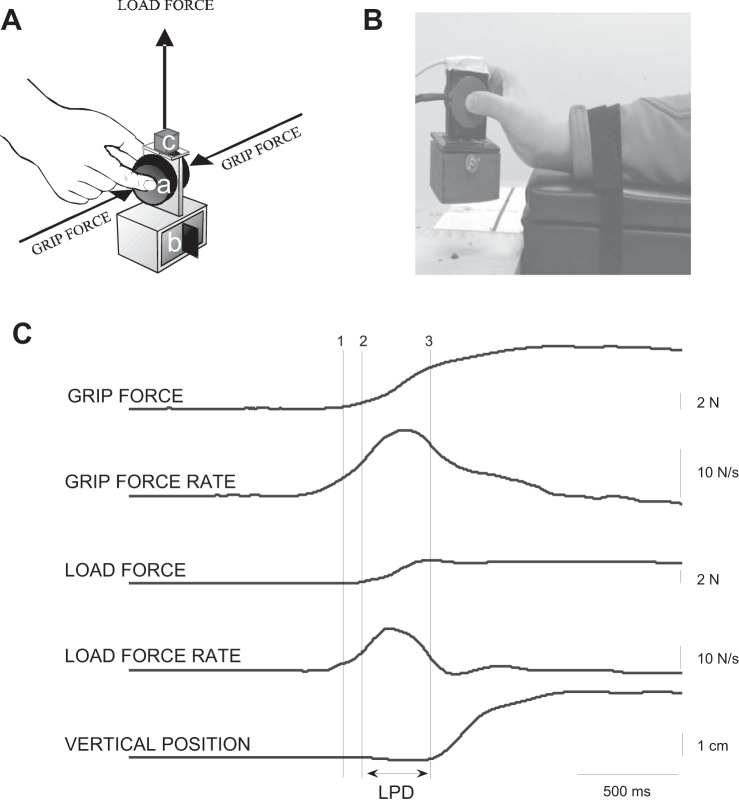
Fig. 1.
A: schematic of the instrumented grip device with 6-degrees of freedom strain-gauge force transducers to measure the horizontal grip and the vertical load forces (a), a compartment for an exchangeable mass (b), and a motion sensor (c) to verify timing of object lift and slip. B: depiction of the grasp and lift task using wrist extension. C: representative data from the 7th trial with surface T14 (sandpaper, Sp80; see Table 1) from 1 subject with bare hands showing the grip force, grip force rate, and object lift trajectory. The vertical lines represent the temporal events during grasp: object contact is made and the grip force begins to increase (1), the load force begins to increase (2), and object lift-off (3). The interval between 2 and 3 represents the loading phase duration (LPD).
Subjects grasped the handle of the grip device using a precision grasp and lifted it from the plate with their right hand using wrist extension (Fig. 1B) upon an auditory cue signal. They grasped and lifted the object under three conditions: with the use of bare hands, with a thin plastic film on the fingertips (Tegaderm HP-9536HP), and with a layer of foam on the fingertips (Polycushion Padding-A2911) with Tegaderm underneath and on top of it. A strap was loosely placed over the forearm to remind subjects not to move the forearm by flexing the elbow, but to only use wrist action to lift the object. Subjects were provided 7 s for each trial and were asked to complete the task at their preferred speed. On average, all subjects completed the task within 5 s and performed the task with full vision. The room temperature (23.17 ± 0.07°C) and humidity (51.87 ± 0.76%) were maintained across all sessions.
The friction at the grip surface was varied by changing the custom-made caps fitted snugly on the force sensors. Eighteen different frictional surfaces were affixed to the plastic caps. To avoid an order effect, the grip surfaces were presented randomly. Each new grip surface was unknown to the subject and was grasped in 7 consecutive trials; trials 2–7 were used for analysis because it takes at least 1 trial to learn the association between surface friction and the necessary grip force (Johansson and Westling 1984).
We assessed the static coefficient of friction (COF) for the 18 surfaces separately using a mass and pulley system (Table 1). A weighted block with a constant surface was pulled across each of the surfaces. The static COF, μs, of each surface was computed as the ratio of the average force, F (measured using ATI force sensors), required to initiate movement of a block across the surface to the weight of the block over 10 trials in each of the 4 cardinal directions, where μs = F/mg. Although two pairs of medical tape surfaces appear to have similar coefficients of friction (i.e., 0.44 for T3, T8, and 0.59 for T6, T9), their textures were very different and elicited different coefficients of friction at the fingertip-object interface (shown in Fig. 4).
Table 1.
Description of the grasping surfaces
| Sample No. | Product No./Name | Coefficient of Friction |
|---|---|---|
| 3M medical tape | ||
| T1 | 1527 | 0.36 ± 0.01 |
| T2 | 1529 | 0.40 ± 0.01 |
| T3 | 9860 | 0.44 ± 0.01 |
| T4 | 1776 | 0.50 ± 0.05 |
| T5 | 9916 | 0.57 ± 0.08 |
| T6 | 9904 | 0.59 ± 0.01 |
| T7 | 2475 | 0.63 ± 0.05 |
| T8 | 9926 | 0.44 ± 0.01 |
| T9 | 1538 | 0.59 ± 0.01 |
| Sandpaper | ||
| T10 | Sp400-PS8A-400/Klingspor | 0.85 ± 0.1 |
| T11 | Sp40-9322NA/3M | 0.86 ± 0.02 |
| T12 | Sp120-9320NA/3M | 0.88 ± 0.02 |
| T13 | Sp180-9319NA/3M | 0.94 ± 0.01 |
| T14 | Sp80-9321NA/3M | 1.00 ± 0.04 |
| T15 | Sp320-413Q 320/3M | 1.07 ± 0.03 |
| T16 | Sp500-500A Silicon carbide/Mercer Abrasives | 1.16 ± 0.02 |
| T17 | Sp36-PS31-36D/Klingspor | 0.73 ± 0.02 |
| T18 | Sp220-413Q 220/3M | 1.17 ± 0.02 |
The static coefficient of friction of each surface, defined as the ratio of the pulling force needed to initiate movement of an object across the surface and the weight of the object, was obtained using a mass and pulley system.
Fig. 4.
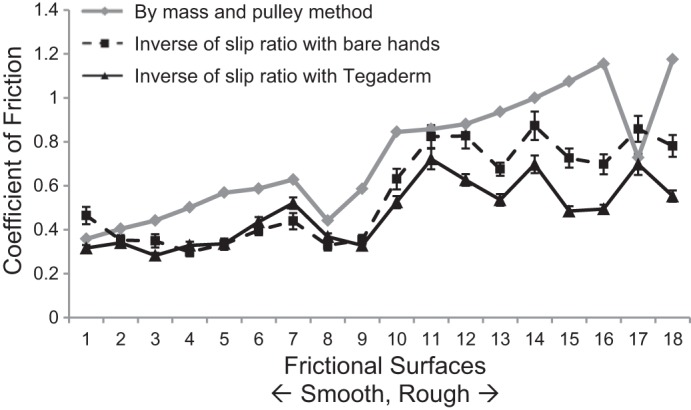
Comparison of the coefficient of friction (COF) of the 18 frictional surfaces measured using the mass and pulley method and by computation of the slip ratio using bare hands and Tegaderm. Error bars represent between-subject variability for the slip ratio.
Muscle activity was recorded synchronously with force data using bipolar surface electrodes (DE 2.1; Delsys) from eight muscles: abductor pollicis brevis (APB), first dorsal interosseous (FDI), flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), flexor carpi ulnaris (FCU), extensor carpi radialis longus (ECRL), upper trapezius (UT), and lower trapezius (LT). The electromyographic (EMG) signals were preamplified and sampled at 2,000 Hz and normalized to the maximum voluntary contraction of each muscle for each subject. An electromagnetic motion sensor (trakSTAR; Ascension technology, Shelburne, VT) was placed on the grip device to verify timing of object lift and slip. The force data were captured using Spike2 (Cambridge Electronic Design, Cambridge, UK), and the EMG and motion data were captured synchronously through the Motion Monitor (Innovative Sports Training, Chicago, IL).
Force and EMG data were imported to and processed with MATLAB (The MathWorks, Natick, MA). The grip force was computed as the average normal force applied by the thumb and index fingers, whereas the load force was computed as the sum of the tangential forces exerted by the two fingers. Object lift was determined from the plate signals and verified with the motion sensor. The data were smoothed using a moving average filter with a window of ±12.5 ms. Peak grip force rate was computed as the peak of the first-order derivative of the grip force between the onset of grip force (>0.1 N) and object lift (Fig. 1C). The slope at time t was calculated using a time window of ±12.5 ms. The loading phase was the time from onset of the vertical load force to lift off. Efficiency of grip force was computed by the grip-load force coordination (grip force/load force) at lift for each frictional surface. The EMG signals were filtered with a 5- to 500-Hz bandpass filter and a 60-Hz notch filter, and the root mean square of the signal during the loading phase was calculated and normalized using the maximum voluntary contraction for each muscle.
Slip experiment.
We quantified the person-specific COF at the fingertip-object interface by measuring the slip ratio, because it takes into account both the friction of the material (measured by the COF shown in Table 1) and the friction at the fingertip, which depends on the smoothness of the skin, moisture, and skin temperature, and varies across individuals. Each subject performed the slip experiment with each of the 18 grip surfaces separately from the grasping experiment. The subjects were asked to grasp and lift the grip device and slowly open their fingers to release it; this was repeated five times with each surface. The grip surfaces were presented randomly to avoid an order effect. The grip force and load force at the moment of object slip (detected using the motion sensor data on the grip device) were used to compute the slip ratio (grip force/load force) for each surface. The slip ratio indicates the minimum grip force needed to prevent slipping (Johansson and Westling 1984). The slip ratio derived in this manner was not affected by the mechanical factors during the experimental task described above, and vice versa. The inverse of the slip ratio provided a measure of the person-specific COF at the fingertip-object interface. The slip experiment was performed with bare hands and with Tegaderm. Since the foam coating had an additional layer of Tegaderm that contacted the grasping surface, the grip interface (Tegaderm-texture) was the same as with Tegaderm only.
Statistical analysis.
The statistical analyses were conducted using Rstudio (version 0.98). Package “nlme” was used to fit linear mixed-effects models (Fitzmaurice et al. 2011; Pinheiro and Bates 2000) to compare grip force adaptation and efficiency to the 18 different frictional surfaces under the 3 conditions. We computed the relationship between the person-specific COF, provided by the slip ratio, and the variables related to grip force adaptation and efficiency when grasping with bare hands, Tegaderm, and foam. The statistical model we adopted took the following form:
where Y refers to the logarithm of an outcome variable of interest. The following outcome variables were considered: the peak grip force rate (PGFR), the grip-load force ratio at lift off, and the normalized muscle activity of the eight muscles (APB, FDI, FDS, EDC, ECRL, FCU, LT and UT) during the loading phase. X is the log slip ratio; the higher the value of X, the smoother the surface, and vice versa. Note that although the inverse of slip ratio is a subject-specific measure of COF for the various frictional surfaces, for ease of interpretation, the slip ratio (at logarithm scale) was used as the predictor since we expect the primary outcomes, PGFR and grip-load force ratio, to increase for the smoother surface. In this model, subscript i refers to subject, i = 1, . . ., 10; subscript j refers to the jth condition (j = 1, bare hand; j = 2, Tegaderm; and j = 3, foam); subscript k refers to the kth frictional surface, k = T1–T18 (Table 1).
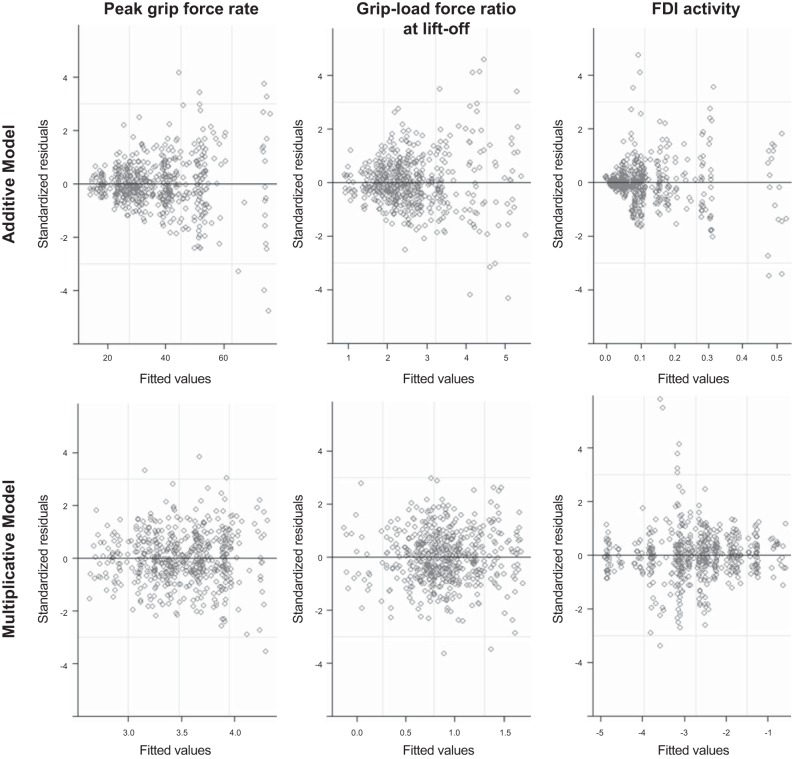
Since rates and ratio measures such as the PGFR, slip ratio, and normalized EMG tend to be highly skewed, it is more natural to consider a multiplicative effect model that can be conveniently fit with the use of a linear model by taking the logarithmic transformation of the variables (Lu et al. 2015). For example, the model assumes that, on average, the log PGFR varies as a linear function of log slip ratio. Exponentiating the linear intercept αj, eαj, we obtain the predicted mean value of PGFR under the jth condition. βj is the slope of the linear relationship under the jth condition, and (eβj − 1) × 100% captures the percent change of the outcome variable PGFR as log slip ratio increases by e = 2.37 times,11 when the surface becomes smoother. Furthermore, we conducted regression diagnostic checks to confirm that the multiplicative model fits the data better than the linear additive model. Under the linear additive model, the variance of the residuals increase as the value of the predicted outcome variable increases (Fig. 2), a pattern that suggests that logarithmic transformation of the variables is needed (Draper and Smith 1998). In addition, since the additive model and the multiplicative model have the same degrees of freedom, one can compare the pseudo R2 values [sum(values − predicted values)2/sum(values − mean value)2] under the two mixed-effects model specifications. For all the outcome variables we considered, the multiplicative model had larger pseudo R2 values: peak grip force rates (additive = 0.216 vs. multiplicative = 0.231), grip-load force ratio (additive = 0.149 vs. multiplicative = 0.151), and FDI activity (additive = 0.331 vs. multiplicative = 0.347). This confirms that the multiplicative model fits the data better.
Fig. 2.
Residual diagnostic plots comparing the linear additive model and the multiplicative model under logarithmic transformation for the variables of interest. For each plot, the standardized residuals are plotted against the fitted values of the outcome variable. FDI, first dorsal interosseous.
Considering the large amount of inter-subject variability in grasping behavior, we further include, in the model, the random effects (μij) for each individual's mean response under the three different conditions. This parsimonious approach allowed each subject to have his/her own intercept per condition and treated the deviations of each subject-specific intercept from a common population intercept as a zero-means random variable (the μij terms in the model, i = 1, . . ., 10; j = 1, . . ., 4) per condition (the αj term, j = 1, . . ., 4). The hierarchical model takes the subject-specific deviations into account by incorporating the variance of μij, σj (j = 1, . . ., 4) into the model estimation so that we are not simply pooling all the data into one model. Note that εijk is the residual term. The Wald test was used for establishing significant differences between slopes (and intercepts) of the regression. Last, to control for false positive rates, we report Bonferroni-adjusted P values whenever multiple hypotheses were tested.
RESULTS
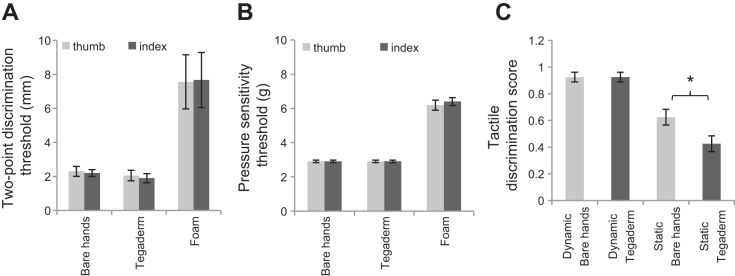
Standard neurological tests of tactile sensibility showed that the thresholds for two-point discrimination and pressure sensitivity did not differ with bare hands and Tegaderm but were significantly higher with foam (P < 0.05; Fig. 3, A and B). Tactile texture discrimination was significantly reduced with Tegaderm compared with bare hands (P < 0.05) when tested with static pressure, but not during dynamic scanning (Fig. 3C). These results suggest that tactile sensibility is reduced with a foam coating on the fingertips but is generally intact with a fine layer of plastic film except for static tactile discrimination.
Fig. 3.
A: threshold for 2-point discrimination. B: pressure sensitivity threshold measured using Semme's Weinstein monofilament. C: dynamic and static tactile discrimination test scores with bare hands and Tegaderm. *P < 0.05.
The COF is higher for rougher surfaces. Since it was obtained by pulling the object with different tactile surfaces against a common surface, it did not take into account individual differences in fingertip friction. In contrast, the slip ratio measures the smoothness of the grip surface, and it has an inverse relationship with the COF such that the slip ratio is higher for smoother surfaces. In Fig. 4, we plot the COF of the tactile surfaces used in the experiment and compare them with the inverse of the slip ratio obtained during the slip experiment (see methods) with bare hands and with Tegaderm. Note that, in general, the inverse of the slip ratio obtained with bare hands (averaged over all the subjects) is highly consistent with the COF values (r = 0.81). The standard error bars around the inverse of the slip ratio indicate the between-subject variability. In contrast, when Tegaderm is applied to the fingertip, the correlation between the inverse of the slip ratio and the COF is reduced (r = 0.68). Nevertheless, the correlation between the COF obtained using the slip ratio method with bare hands and with the fingertip coated with Tegaderm were very highly correlated (r = 0.91), suggesting that many of the features of the bare fingertip surface are preserved when the fingertip is coated with Tegaderm.
When we examined the adaptation and execution of fingertip grip forces to friction at the grasping surface, we found that the grip force and grip force-rate profiles differed for representative samples of smooth and rough grasping surfaces with bare hands, Tegaderm, and foam (Fig. 5). The grip force onset times were similar under the three conditions. The rough and smooth objects were lifted at approximately the same time with bare hands (Fig. 5A). However, Tegaderm delayed lift for the smooth object (Fig. 5B), whereas foam delayed lift for both the rough and smooth objects (Fig. 5C), reflecting a slow rate of change of grip force with Tegaderm and foam. Consequently, the peak grip force rate was higher for the smooth compared with the rough surface with bare hands but was relatively similar for the two surfaces with Tegaderm and foam.
Fig. 5.
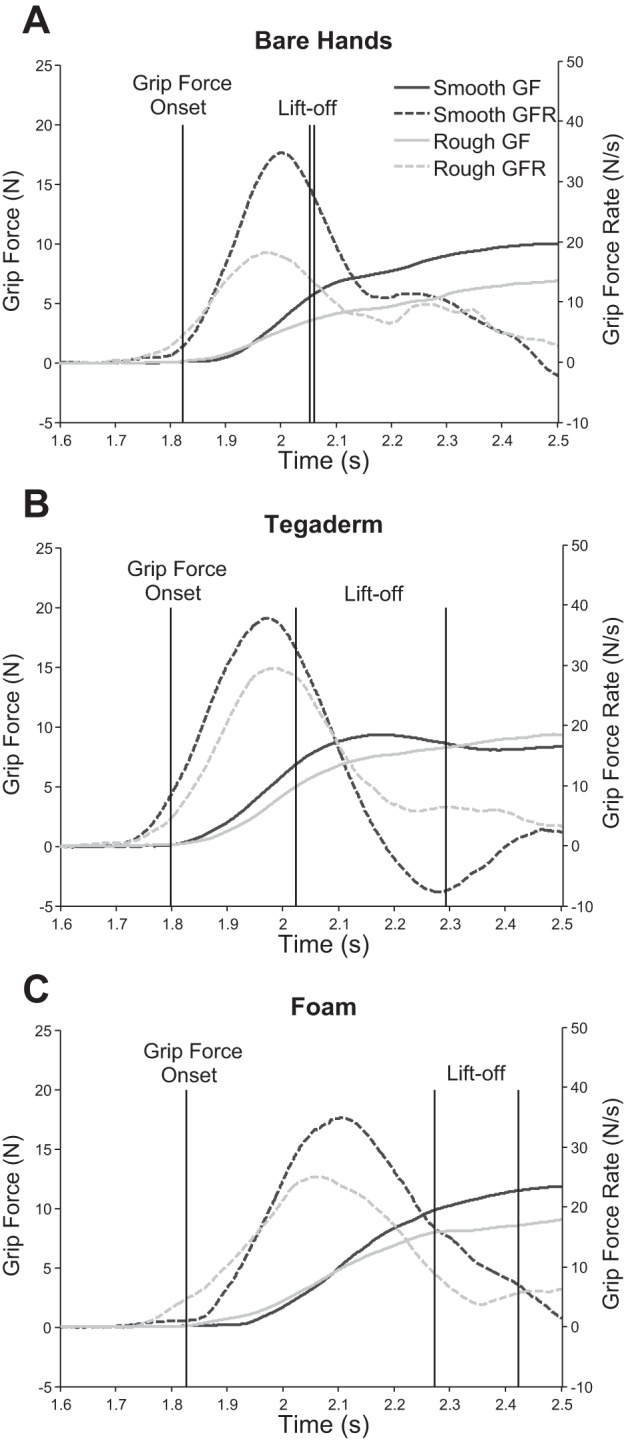
Representative data from 1 subject showing the grip forces (GF; solid lines) and grip force rates (GFR; dashed lines) on the 7th trial for lifting the grip device with a smooth surface, T4 (3M medical tape 1776; black), and a rough surface, T11 (sandpaper sp40; gray), with bare hands (A), Tegaderm (B), and foam (C).
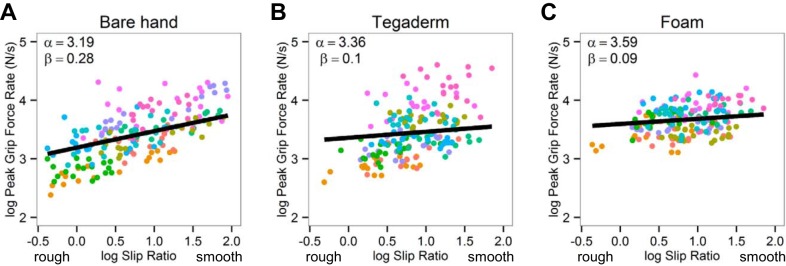
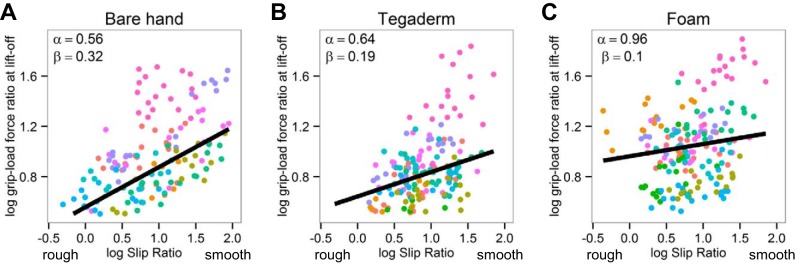
Figure 6 shows the relationship between the log peak grip force rate (log PGFR) and the log slip ratio across the 18 grip surfaces under the 3 experimental conditions; values for each subject are shown as different colored dots. When subjects grasped the handle with bare hands, the log PGFR increased linearly with the smoothness of the surface, since higher log slip ratios indicate a smoother surface (Fig. 6A, β = 0.28, P < 0.001). In contrast, adaptation to surface friction was impaired when Tegaderm and foam were applied to the fingertips, since the slopes with Tegaderm (Fig. 6B, β = 0.1, P < 0.001) and foam (Fig. 6C, β = 0.09, P < 0.001) were relatively flat and differed significantly from that obtained with bare hands (P < 0.001). Subjects seemed unable to scale the applied grip force rate to rough textures when their fingers were covered with Tegaderm or foam. In addition, the magnitude of log PGFR was higher when grasping with Tegaderm and foam than with bare hands. The intercept, α, reflects the level of the log PGFR and was not significantly different between bare hands and Tegaderm (P > 0.05), but it was significantly higher with foam than with bare hands (P < 0.001).
Fig. 6.
Adaptation of fingertip grip forces to friction at the grasping surface. Graphs show relationship between the log peak grip force rate and the log slip ratio across the 18 grip surfaces with bare hands (A), Tegaderm (B), and foam (C). Average values for each subject are shown using different colors.
The efficiency of grip force execution across the 18 grip surfaces was assessed by the ratio of the grip force to the load force at lift (Fig. 7). When tested with bare hands, the log grip-load force ratio increased proportionally, within subjects, with the smoothness of the grasping surface (Fig. 7A, β = 0.32, P < 0.001). In contrast, grip force efficiency was impaired when Tegaderm and foam were applied to the fingertips. The slopes β obtained with Tegaderm (Fig. 7B, β = 0.19, P < 0.001) and foam (Fig. 7C, β = 0.1, P < 0.001) were relatively flat and differed significantly from those obtained using bare hands (P < 0.001). These findings indicate that grip force efficiency is also impaired when Tegaderm and foam are applied to the fingertips.
Fig. 7.
Efficiency of fingertip grip forces. Graphs show relationship between the log grip-load force ratio at lift-off and the log slip ratio across the 18 grip surfaces with bare hands (A), Tegaderm (B), and foam (C). Average values for each subject are shown using different colors.
In addition, we note that the magnitude of the log grip-load force ratio was higher when grasping with Tegaderm and foam than with bare hands. The intercept, α, between bare hands and Tegaderm was not significantly different (P > 0.05), whereas the intercept was significantly higher with foam than with bare hands (P < 0.001), suggesting that individuals used a higher grip force for the given load, particularly for the roughest surfaces (lowest slip ratio), with foam than with bare hands.
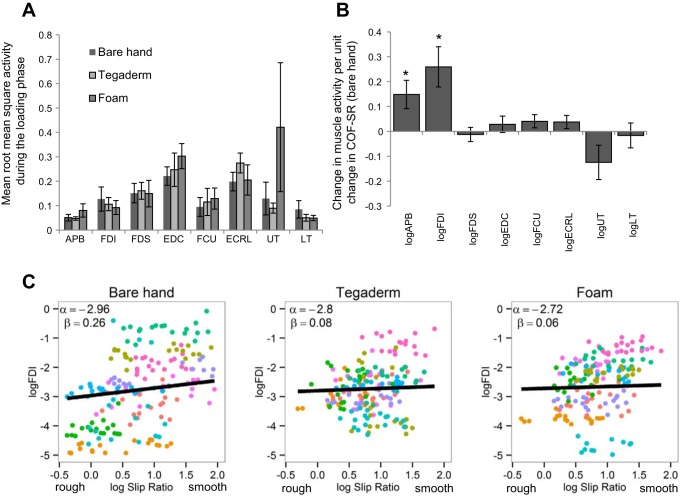
Finally, we examined the relationship between muscle activity and surface friction to understand the extent to which sensory input from the fingertips modulated motor output during precision grasp behavior. During the loading phase of grasp (Fig. 8A), the finger and wrist extensor muscles (EDC and ECRL) were strongly activated as the object was lifted by extending the wrist. There were no significant differences across the three conditions (bare hands, Tegaderm, or foam) in these or other muscles tested. However, activity in the muscles engaged in precision grip (APB and FDI) were significantly modulated by surface friction as measured by the log slip ratio (Fig. 8B, β = 0.15, P < 0.01 for APB; β = 0.26, P < 0.01 for FDI) with bare hands. In contrast, with Tegaderm and foam, even in these muscles the activity was not significantly related to the slip ratio of the surfaces being grasped (Fig. 8C; data for FDI are shown).
Fig. 8.
A: task-related muscle activity shown as the logarithmic root mean square of the electromyographic signal during the loading phase (from load force onset to lift-off), normalized with the maximum voluntary contraction, with bare hands, Tegaderm, and foam. B: relationship between the log change in muscle activity per unit change in the log COF obtained by the slip ratio method with bare hands for the abductor polices brevis (APB), FDI, flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), flexor carpi ulnaris (FCU), extensor carpi radialis longus (ECRL), upper trapezius (UT), and lower trapezius (LT) muscles for lift trials 2–7. C: relationship between the log FDI activity and the log slip ratio across the 18 grip surfaces during the loading phase with bare hands, Tegaderm, and foam. Average values for each subject are shown using different colors.
DISCUSSION
In this study we examined the adaptation and efficiency of fingertip grip forces to friction at the grasping surface with bare hands and when the fingertips were coated with a thin layer of plastic film (Tegaderm) and a layer of foam under full vision. Here, adaptation refers to the modification in the rate of change of grip force (grip force rate) before the object is lifted such that rougher surfaces elicit lower grip force rates than smoother surfaces. Efficiency refers to the grip-load force ratio at lift such that the grip force is minimized and is lower for a rougher than a smoother surface (Johansson and Westling 1984). The foam coating significantly reduced sensibility for two-point discrimination and pressure sensitivity at the fingertips compared with bare hands, but Tegaderm did not. However, static, but not dynamic, tactile texture discrimination was reduced with Tegaderm compared with bare hands, suggesting that subjects may be unable to discriminate the texture of the grasping surfaces when they hold the grip device with a precision grasp. Both Tegaderm and foam impaired the adaptation and efficiency of fingertip grip forces, but the magnitude of the grip force rates and the grip-load force ratios, particularly for the roughest surfaces, were preserved with Tegaderm but not with foam. Furthermore, fine-tuning of activity in the intrinsic hand muscles used to grip the object was poorer with the coated finger tips.
Selection of ecologically valid grasping surfaces with varying surface friction.
In this study, we used two different materials and nine different samples of each material that provided a wide range of ecologically valid frictional surfaces. Sandpaper of various grit sizes (Johansson and Westling 1984; Libouton et al. 2010) provided high-friction surfaces, and samples of 3M tape provided low-friction surfaces with particle size <200 μm (Scheibert et al. 2009). The materials showed a wide range in coefficient of friction. In previous studies, either a range of qualitatively different textured materials, for example, sandpaper, silk, suede, etc. (Johansson and Westling 1984), or a range of raised dots of different sizes on the same material (Blake et al. 1997a) were used to investigate the role of friction and roughness perception. Using qualitatively different materials can confound the relationship between surface friction and precision grasp variables, whereas using raised dot surfaces may not be ecologically valid and generalizable to every-day tasks. The coefficient of friction (inverse of the slip ratio) obtained during the fingertip-object interaction showed that the surfaces appeared functionally smoother when tested with bare hands, and rough surfaces were further smoothed by a layer of Tegaderm. Given that the task was performed with full vision of the hand and device, one might expect that the grip forces would be higher with Tegaderm. However, our results revealed that the level of the grip force rates and grip forces was preserved with Tegaderm (see Figs. 6 and 7). Since the grasping surfaces were presented in a random manner, we believe that the findings reflect tactile-motor control without significant interference by vision.
Differential effects of Tegaderm and foam on adaptation and efficiency of grip forces.
Even though tactile sensitivity was generally preserved with Tegaderm, with the exception of static tactile discrimination, the adaptation and efficiency of the fingertip grip forces in relation to surface friction was impaired. These results suggest that sensibility for static surface deformation is necessary to recognize the texture of the grasping surface during precision grasp. The addition of a foam layer significantly impaired tactile sensitivity and further reduced grip force efficiency, requiring the use of higher grip forces particularly for the rough surfaces.
The friction at the fingertip-object interface during precision grasp is sensed at contact and is thought to be mediated by the rapidly adapting or fast-adapting FAI receptors that are sensitive to low-frequency (5–50 Hz) skin deformations that occur during movement of a surface across the skin (Blake et al. 1997b; Johnson and Hsiao 1992; Lofvenberg and Johansson 1984; Talbot et al. 1968; Westling and Johansson 1987). However, the precise assessment of the grip force required is thought to occur during slips when the object is first lifted (Johansson and Westling 1987). The local spatial features of the surface, such as particle size responsible for surface roughness, are thought to be encoded by the SAI receptors (Johansson et al. 1982a, 1982b; Lofvenberg and Johansson 1984) that are excited by very low frequency skin deformations (<5 Hz) during slip. This information is then used to modify the rate of change of the grip force on subsequent grasping attempts such that smoother surfaces elicit higher grip force rates than rougher surfaces (Cadoret and Smith 1996; Johansson and Cole 1994; Johansson and Westling 1984; Westling and Johansson 1984). Furthermore, the fast-adapting Pacinian corpuscles (FAII) are sensitive to higher frequency vibrations (40–400 Hz) thought to be particularly important for the perception of roughness of fine textures (Bensmaia and Hollins 2003; Hollins and Risner 2000; Yoshioka et al. 2007).
Taken together with the known properties of the fingertip mechanoreceptors, our results suggest that Tegaderm may have effectively dampened sensitivity to low-frequency vibrations that are necessary for both feedback and feedforward control of grip forces. Both the FAI and SAI receptors are located in clusters underneath the papillary ridges that make up the fingerprint, and the ridges act as magnifying levers to increase the subsurface strain during deformation of the skin surface (Cauna 1954).
It is interesting to note that dynamic tactile discrimination was not impaired by Tegaderm; it may have been spared because the FAI and SAI receptors are more sensitive to dynamic skin deformation (Johnson 2001; Johnson et al. 2000). Although we cannot directly attribute the results to blocking of any specific receptor, because this was not directly measured, we measured consistent change at a behavioral level. One can speculate that Tegaderm occluded the papillary ridges and lowered the sensitivity of the underlying receptors. Thus even a thin layer of material over the fingertips, such as one might experience when wearing a surgical glove, may prevent tactile sensing needed for adjustment of the grip forces to surface friction, although the magnitude of the grip forces may not be compromised. In addition, foam can dampen high frequency vibrations (Patel et al. 2008b), and further reduce the reliability of sensory information from the fingertips compromising both the relationship of the grip forces to surface friction as well as the magnitude of the grip forces.
Grip force and modulation of muscle activity.
To further understand the extent to which the difference in precision grasp behavior was due to modulation of sensory input from the fingertips, we measured the activity from multiple muscles involved in the task (Johansson and Westling 1988a). The mean task-related muscle activation patterns during the loading phase of grasp were not significantly different across the three conditions, suggesting that the task was performed in an identical manner across the conditions. However, we found that the intrinsic finger muscles, the first dorsal interosseous and abductor polices brevis, but not the extrinsic finger flexors or extensors, were weakly, but significantly correlated with the slip ratio with bare hands, but not with Tegaderm or foam. The weak correlations reflect the large within-subject variation in EMG activity. Nevertheless, these results are consistent with a recent study which showed that adaptation of load forces to object weight is proportionally scaled to activity in the task-specific lifting muscle (Lu et al. 2015). Taken together, these results suggest that appropriate adaptation of forces is directly related to fine tuning of task-specific muscles during execution.
It has previously been shown that reactive muscle adjustments involve synergistically acting muscles, including the intrinsic and extrinsic muscles of the fingers, and flexors and extensors of the wrist and arm (Johansson and Westling 1988b; Macefield and Johansson 1996). It is possible that the extrinsic finger muscles would be additionally recruited when lifting heavier loads, and that other muscles may be selectively recruited for adaptation to different task conditions. The design of this study made it possible to isolate the muscles and the sensory inputs needed for selective muscle activation in response to changes in surface friction during precision grasp. Our results suggest that sensory information from the fingertips is tightly coupled with specific motor responses, and reduced sensitivity to static tactile discrimination can attenuate adaptive modulation of activity in the appropriate task-specific muscles.
Conclusion.
The comparisons of interest in this study were within-subject differences in the relationship between surface friction and grip forces during precision grasp with bare hands, Tegaderm, and foam. We employed a linear hierarchical model that allowed for individual level random intercepts and slopes to be controlled to account for the inter-subject variability. In addition, we also conducted residual diagnosis and found no obvious outliers. Therefore, despite the small sample size, we believe that the results are generalizable to a larger population of healthy individuals.
Several conclusions can be drawn from this study: 1) a layer of thin plastic film or foam coating on the fingertips can reduce tactile sensitivity noninvasively; 2) sensitivity to static surface deformation is necessary for feedback and feedforward control of grip forces; and 3) tactile information is necessary for adaptive modulation of activity in muscles engaged in precision grip. These results may assist in the development of tactile prostheses to substitute missing sensory information and enhance dexterity in individuals with sensory loss due to central and peripheral injury.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01 HD071978.
DISCLOSURES
P. Raghavan has a patent pending for a game-based sensorimotor rehabilitator for patients with stroke; the patent is not directly relevant to the present study, which was performed in healthy subjects.
AUTHOR CONTRIBUTIONS
S.B., Y.L., D.G., J.R.R., V.A., and P.R. conception and design of research; S.B., D.G., and V.A. performed experiments; S.B., Y.L., and P.R. analyzed data; S.B., Y.L., E.P.G., and P.R. interpreted results of experiments; S.B., Y.L., and P.R. prepared figures; S.B. and P.R. drafted manuscript; S.B., Y.L., E.P.G., and P.R. edited and revised manuscript; S.B., Y.L., D.G., J.R.R., V.A., E.P.G., and P.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Peter Thai, David Rhee, Qiang Lin, and Zena Moore for assistance with data collection.
Footnotes
To see this, β is the change in Y when X increases by 1 unit. If X = log slip ratio, then 1 unit increase in X corresponds to log slip ratio + 1 = log(e·slip ratio); if Y = log PGFR, then β unit increase in Y corresponds to log PFGR + β = log(eβ·PFGR), hence (eβ − 1) × 100% change in PGFR.
REFERENCES
- André T, Lefèvre P, Thonnard JL. Fingertip moisture is optimally modulated during object manipulation. J Neurophysiol 103: 402–408, 2010. [DOI] [PubMed] [Google Scholar]
- André T, Lefèvre P, Thonnard JL. A continuous measure of fingertip friction during precision grip. J Neurosci Methods 179: 224–229, 2009. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Hollins M. The vibrations of texture. Somatosens Mot Res 20: 33–43, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Hsiao SS, Johnson KO. Neural coding mechanisms in tactile pattern recognition: the relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J Neurosci 17: 7480–7489, 1997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Johnson KO, Hsiao SS. Monkey cutaneous SAI and RA responses to raised and depressed scanned patterns: effects of width, height, orientation, and a raised surround. J Neurophysiol 78: 2503–2517, 1997b. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Allum JH, Carpenter MG, Honegger F. Is lower leg proprioception essential for triggering human automatic postural responses? Exp Brain Res 130: 375–391, 2000. [DOI] [PubMed] [Google Scholar]
- Brutus J, Nikolis A, Baeten Y, Chahidi N, Kinnen L, Ledoux P, Moermans J. Reducing patient discomfort during digital blockade: the subcutaneous single injection digital block–a simple, safe and fast procedure. Can J Plast Surg 11: 33–35, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret G, Smith AM. Friction, not texture, dictates grip forces used during object manipulation. J Neurophysiol 75: 1963–1969, 1996. [DOI] [PubMed] [Google Scholar]
- Cauna N. Nature and functions of the papillary ridges of the digital skin. Anat Rec 119: 449–468, 1954. [DOI] [PubMed] [Google Scholar]
- Dannenbaum RM, Michaelsen SM, Desrosiers J, Levin MF. Development and validation of two new sensory tests of the hand for patients with stroke. Clin Rehabil 16: 630–639, 2002. [DOI] [PubMed] [Google Scholar]
- Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am 12: 693–696, 1987. [DOI] [PubMed] [Google Scholar]
- Draper NR, Smith H. Applied Regression Analysis. New York: Wiley, 1998. [Google Scholar]
- Dunn W, Griffith JW, Morrison MT, Tanquary J, Sabata D, Victorson D, Carey LM, Gershon RC. Somatosensation assessment using the NIH Toolbox. Neurology 80: S41–S44, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley, 2011. [Google Scholar]
- Gandevia SC, Mccloskey DI. Changes in motor commands, as shown by changes in perceived heaviness, during partial curarization and peripheral anesthesia in man. J Physiol 272: 673–689, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M, Bensmaia SJ. The coding of roughness. Can J Exp Psychol 61: 184–195, 2007. [DOI] [PubMed] [Google Scholar]
- Hollins M, Risner SR. Evidence for the duplex theory of tactile texture perception. Percept Psychophys 62: 695–705, 2000. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Gomez-Ramirez M. Touch. In: Neurobiology of Sensation and Reward, edited by Gottfried JA. Boca Raton, FL: CRC, 2011. [Google Scholar]
- Johansson RS, Cole KJ. Grasp stability during manipulative actions. Can J Physiol Pharmacol 72: 511–524, 1994. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Landstrom U, Lundstrom R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982a. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Landstrom U, Lundstrom R. Sensitivity to edges of mechanoreceptive afferent units innervating the glabrous skin of the human hand. Brain Res 244: 27–35, 1982b. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286: 283–300, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res 71: 59–71, 1988a. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res 71: 72–86, 1988b. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564, 1984. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res 66: 141–154, 1987. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci 15: 227–250, 1992. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol 17: 539–558, 2000. [DOI] [PubMed] [Google Scholar]
- Lederman SJ. Tactile roughness of grooved surfaces - touching process and effects of macrosurface and microsurface structure. Percept Psychophys 16: 385–395, 1974. [Google Scholar]
- Lederman SJ, Loomis JM, Williams DA. The role of vibration in the tactual perception of roughness. Percept Psychophys 32: 109–116, 1982. [DOI] [PubMed] [Google Scholar]
- Libouton X, Barbier O, Plaghki L, Thonnard JL. Tactile roughness discrimination threshold is unrelated to tactile spatial acuity. Behav Brain Res 208: 473–478, 2010. [DOI] [PubMed] [Google Scholar]
- Lofvenberg J, Johansson RS. Regional differences and interindividual variability in sensitivity to vibration in the glabrous skin of the human hand. Brain Res 301: 65–72, 1984. [DOI] [PubMed] [Google Scholar]
- Lu Y, Bilaloglu S, Aluru V, Raghavan P. Quantifying feedforward control: a linear scaling model for fingertip forces and object weight. J Neurophysiol 114: 411–418, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Johansson RS. Control of grip force during restraint of an object held between finger and thumb: responses of muscle and joint afferents from the digits. Exp Brain Res 108: 172–184, 1996. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Patel M, Fransson PA, Lush D, Gomez S. The effect of foam surface properties on postural stability assessment while standing. Gait Posture 28: 649–656, 2008a. [DOI] [PubMed] [Google Scholar]
- Patel M, Fransson PA, Lush D, Petersen H, Magnusson M, Johansson R, Gomez S. The effects of foam surface properties on standing body movement. Acta Otolaryngol (Stockh) 128: 952–960, 2008b. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer, 2000. [Google Scholar]
- Scheibert J, Leurent S, Prevost A, Debregeas G. The role of fingerprints in the coding of tactile information probed with a biomimetic sensor. Science 323: 1503–1506, 2009. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res 53: 277–284, 1984. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp Brain Res 66: 128–140, 1987. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Bensmaia SJ, Craig JC, Hsiao SS. Texture perception through direct and indirect touch: an analysis of perceptual space for tactile textures in two modes of exploration. Somatosens Mot Res 24: 53–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]