Abstract
Young children are often hyperreactive to somatosensory inputs hardly noticed by adults, as exemplified by irritation to seams or labels in clothing. The neurodevelopmental mechanisms underlying changes in sensory reactivity are not well understood. Based on the idea that neurodevelopmental changes in somatosensory processing and/or changes in sensory adaptation might underlie developmental differences in somatosensory reactivity, high-density electroencephalography was used to examine how the nervous system responds and adapts to repeated vibrotactile stimulation over childhood. Participants aged 6–18 yr old were presented with 50-ms vibrotactile stimuli to the right wrist over the median nerve at 5 blocked interstimulus intervals (ranging from ∼7 to ∼1 stimulus per second). Somatosensory evoked potentials (SEPs) revealed three major phases of activation within the first 200 ms, with scalp topographies suggestive of neural generators in contralateral somatosensory cortex. Although overall SEPs were highly similar for younger, middle, and older age groups (6.1–9.8, 10.0–12.9, and 13.0–17.8 yr old), there were significant age-related amplitude differences in initial and later phases of the SEP. In contrast, robust adaptation effects for fast vs. slow presentation rates were observed that did not differ as a function of age. A greater amplitude response in the later portion of the SEP was observed for the youngest group and may be related to developmental changes in responsivity to somatosensory stimuli. These data suggest the protracted development of the somatosensory system over childhood, whereas adaptation, as assayed in this study, is largely in place by ∼7 yr of age.
Keywords: tactile, development, ERP, gating, habituation
young children are often overresponsive to sensory inputs that are hardly noticed by adults, as exemplified by irritation from items of clothing, sensitivity to the brightness of lights, or reactivity to loud sounds (Dunn and Westman 1997). In fact, studies have found a high incidence of children that experience sensory stimuli strongly enough to affect aspects of their everyday life: ∼1 in 6 children ranging from kindergarten age to 11 yr old are negatively affected by their sensitivity to sensory inputs (Ahn et al. 2004; Ben-Sasson et al. 2009; Carter et al. 2011). Overresponsivity to somatosensory stimuli is particularly common and spans across cultures (Royeen and Mu 2003), suggesting that this sensitivity may reflect typical neurodevelopmental changes in somatosensory processing that reduce with age (Verrillo 1980). The neurobiology underlying changes in reactivity to such stimulation may originate in part from the process of synapse elimination. Following the early “exuberant synaptogenesis” phase in which an overabundance of synapses are formed, redundant or inhibited synaptic connections are pruned through childhood and adolescence, and connections are refined with genetic and environmental modulation (Huttenlocher and Dabholkar 1997). Changes in the pathway of activation (which occurs through age 17; Allison et al. 1984), along with reduced redundancy, may parallel the diminished sensory sensitivity (Verrillo 1980). In addition, changes in the somatosensory pathway are likely to occur as a consequence of sensory experience and the development of modulatory feedback connections between prefrontal and sensory cortices, which would have meaningful consequences for higher order modulation of the sensory response (Sehatpour et al. 2008).
Based on intracranial and scalp-recorded electrophysiological recordings in adult humans, the spatiotemporal dynamics of the cortical somatosensory evoked response (SEP) have been quite thoroughly delineated. From this literature, which has traditionally used strong single-pulse electrical activity to activate the somatosensory pathway, early cortical processing of stimulus features begins in the primary somatosensory cortex (SI) at ∼20 ms and projects throughout all areas of contralateral SI through ∼80 ms (Allison et al. 1982, 1984, 1992, 1989a; Broughton et al. 1981; Butler et al. 2012; Foxe et al. 2000; Kekoni et al. 1992; Mauguiere et al. 1997). Further processing of tactile information occurs in higher order cortical areas: in bilateral secondary somatosensory cortices (SII), which typically begins at ∼100–120 ms (Allison et al. 1992, 1989b; Forss et al. 1999); in contralateral posterior parietal cortex, which typically begins at ∼100 ms (Allison et al. 1989b; Forss et al. 1994, 1999); and in bilateral frontal cortices, which typically begins at ∼140–190 ms (Allison et al. 1992). These higher order cortical areas also continually provide feedback to SI (Hari et al. 1984) and other upstream areas of the somatosensory processing network. Whereas the nature of the tactile stimulation will necessarily impact the timing of initial cortical activation, it can be assumed that different types of tactile stimulation will follow a similar temporal evolution of the cortical pathway. It should also be noted that this delineation represents the center of mass of the major neural generators underlying the electrical responses within these timeframes and is relatively insensitive to the loci of smaller contributions from other cortical regions. Indeed, given the rapid dynamics of information flow and the role of extensive feedback projections in sensory information processing (Foxe and Simpson 2002; Girard et al. 2001; Hackett et al. 2014; Hupe et al. 2001; Schroeder et al. 1998, 2001), it is almost certain that there is extensive engagement of the greater somatosensory system as early as 30 ms following the initial afferent volley of cortical input.
The extensive synaptic pruning that occurs throughout childhood development would be expected to impact the cortical somatosensory response (Picton and Taylor 2007). Several studies have now assessed the developmental trajectory of somatosensory processing using SEPs. The overwhelming majority of these have found that the trajectory is represented by a “U-shaped curve,” with higher SEP amplitudes at a young age (childhood to mid-20s), which gradually reduce through middle age (30 to early 40s) and then increase again in older age (mid-40s and above) (Desmedt et al. 1976; Dustman and Beck 1969; Luders 1970; Shagass and Schwartz 1965; Tamura et al. 1972). These early studies were pivotal in providing the foundational work in this domain, but they were also necessarily limited by the available technology in what they tell us about the neurodevelopment of somatosensory processing due to the use of low-density electroencephalography (EEG) montages (sometimes only 2 channels were recorded from), which limits characterization of the underlying neural generators and changes therein as a function of development, and consideration of the sensory response as a homogeneous process rather than examining successive stages of sensory processing.
Another area that impacts how somatosensory stimulation is processed, and therefore experienced, is the nervous system's automatic adaptation to repeated stimulation, commonly referred to as sensory adaptation (Wark et al. 2007). That is, whereas the nervous system is highly responsive to novel stimulation, it is much less so to constant or repeated stimulation (for example, see Fruhstorfer et al. 1970; Ritter et al. 1968; Shipley and Hyson 1977). Adaptation is represented by this diminished response to repeated stimulus presentations (Webster 2012), and generally speaking, the faster the presentation rate (i.e., the shorter the interstimulus interval; ISI), the more dramatic the reduction of response strength (Angel et al. 1985). This would appear to be crucial to directing limited cortical processing bandwidth toward novel, and therefore potentially more informative, incoming stimuli (Andrade et al. 2015). Such response modulation has been interpreted as due to neural refractory effects and/or higher order dampening of the response when stimulation is highly predictable or repetitive (Grill-Spector et al. 2006). Sensory adaptation has been extensively studied in the auditory system (e.g., Davies et al. 2009) and also has been examined in the visual (e.g., Andrade et al. 2015) and somatosensory systems (e.g., Angel et al. 1985; Kekoni et al. 1992). Surprisingly, however, to our knowledge there are no published studies on the development of somatosensory adaptation. Thus, although it is clear that the refinement of somatosensory processing occurs throughout development, how somatosensory adaptation is affected by development, and by implication, its potential role in developmental changes in sensory reactivity, is not yet known.
Given these gaps in the literature and the potential significance of this information for understanding both typical and neuropathological processes, the present study set out to use high-density EEG in response to vibrotactile stimulation to more thoroughly characterize the neurodevelopment of somatosensory processing, over successive stages of sensory information processing, for different stages of childhood (from 6 to 17 yr of age). Additionally, use of a high-density montage and group-level dipole modeling allowed for the characterization of potential developmental changes in underlying neuronal generators and extent of lateralization of the SEP. This approach contrasts with previous developmental studies, which have often focused on data from only a very few electrodes and have not considered successive stages of sensory processing (Desmedt et al. 1976; Dustman and Beck 1969; Luders 1970; Shagass and Schwartz 1965; Tamura et al. 1972). Furthermore, many of these considered only very early stages of processing (<20 ms) in response to somewhat unnatural and noxious tactile stimulation (such as a single pulse of electricity) or did not separate children from young adults (Desmedt et al. 1976; Dustman and Beck 1969; Luders 1970; Shagass and Schwartz 1965; Tamura et al. 1972).
MATERIALS AND METHODS
Participants
Fifty-three children with typical development (ages 6.1–17.8 yr; mean 11.59 ± 3.19 yr; 29 males) participated in this study; 4 participants were excluded from the analysis because of noisy data, leaving a total of 49 participants. The children were separated into three groups: younger (ages 6.1–9.8 yr, mean age 7.94 ± 1.07 yr; n = 17, 7 males, 14 right handed), middle (ages 10.1–12.9 yr, mean age 11.81 ± 0.86 yr; n = 14, 8 males, 11 right handed), and older (ages 13.0–17.8 yr, mean age 14.83 ± 1.48 yr; n = 18, 12 males, 15 right handed). Over 90% of the participants were right handed as assessed by the Edinburgh Handedness Inventory (Oldfield 1971); therefore, handedness was not considered as an additional factor during analysis, since the samples of left handed and ambidextrous participants were too small and were proportionately similar across age groups. Participants were recruited from the local Bronx area and from our research participant database. Exclusion criteria included a history of head trauma, psychopathologies, and behavioral problems, a history of learning disabilities or developmental disabilities, and age-inappropriate Intelligence Quotient (IQ) level. IQ was measured by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999). As a whole, the participants had an average IQ of 112.94 ± 14.19; when stratified by age, the younger group had an average of 112.64 ± 11.22, the middle group had an average of 116.25 ± 16.37, and the older group had an average of 110.57 ± 14.89. IQ levels were not significantly different between groups. IQ data were unavailable for three participants in the younger group and two participants in the middle group, and were left out of IQ calculations.
In accordance with the Declaration of Helsinki, prior consent was obtained from either the participants' parents or the participant (when age appropriate) following a full explanation of the procedure and experiment. Participants were paid $12 per hour for their time. All procedures and consent forms were approved by the Albert Einstein College of Medicine Institutional Review Board.
Apparatus
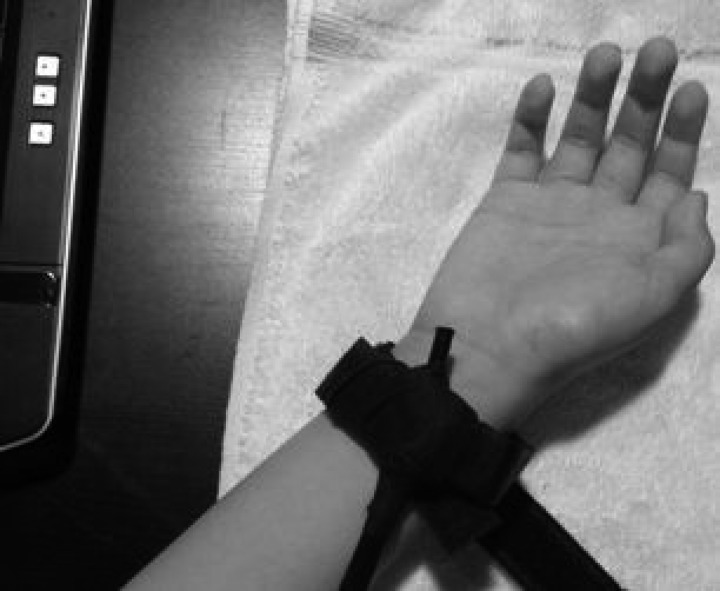
Somatosensory mechanical stimuli were generated using an in-house custom-built vibrotactile stimulator. Vibration was generated using a 4-mm-diameter × 8-mm-long counterweight vibration motor (model 304-108; Precision Microdrives), similar to those typically found in cell phones or pager devices. The 304-108 spring vibration motor is rated at 3 V DC, with a working range between 1.2 and 3.6 V. The motor runs at a speed of ∼10,000 rpm (167 Hz) at 3 V while producing a typical 0.6 g (5.87 m/s2) of vibration output. The current draw is typically 85 mA, and this equates to a vibration efficiency of 3.5 g/W output. Audible noise level is less than 40 dBA when the motor is not encased in a housing. The motor has a 25-ms onset lag time, a 49-ms rise time, and a 76-ms stopping time at rated voltage, as per the manufacturer's specifications. The 304-108 vibrating motor was encased in a 23-mm length of semirigid polyethylene tubing (6.3-mm internal diameter, 9.5-mm external diameter; Watts model SPGE25), which provided a very tightly fitting housing. In turn, the encased motor, polyethylene tubing, and wiring of the device were encased in black polyvinyl chloride heat shrink tubing that fully enclosed the mechanism in a tightly sealed casing (product no. HSPVC0375; BuyHeatShrink). This sealed device was then affixed to the interior surface of a 21.5 × 2.5-cm nonelastic nylon wrist band using surgical tape. The wrist band was secured to the participant's right wrist over the median nerve using Velcro (see Fig. 1). A custom-built 1.5-V amplifier supplied power to the vibrotactile stimulator, which was controlled directly by the parallel port on a computer running Presentation software (Neurobehavioral Systems, Berkeley, CA). Presentation software also controlled the stimulus duration and ISI. The participant's hand and forearm were placed on a towel on the table slightly to their right. To maintain constant pressure of stimulation across participants, right wrist circumference was measured, and the length of the wristband was adjusted to be 2 mm longer than the right wrist diameter measurement. This led to a comfortable but secure placement of the stimulator. It is likely that the vibrotactile stimulation at 167 Hz resulted in stimulation of Pacinian and/or Meissner's corpuscle mechanoreceptors, with Pacinian receptors dominating given the higher frequency (167 Hz) of the stimulus.
Fig. 1.
Image showing encased and secured vibrotactile stimulator, placed on the right wrist over region of the median nerve.
The median nerve area was selected for stimulation for several reasons. The present work was designed with the ancillary goal of providing a crucial benchmark for work in clinical populations. We accordingly chose this region because we found it to be relatively nonintrusive for individuals who are hypersensitive to touch (such as children with sensory processing disorders and individuals with autism). In addition, stimulation of the median nerve area follows precedent of much of the published SEP work (although using electrical instead of vibrotactile stimulation; e.g., Allison et al. 1989a, 1989b; Andrew et al. 2015; Brodie et al. 2014; Foxe et al. 2000).
To ensure that the somatosensory stimulus was inaudible under the experimental conditions, the stimulator was insulated with rubber, the stimulated wrist was placed on a towel, and a movie was played during the experiment. The volume of the movie (measured for random segments of movies that the participants watched and listened to from our video library) ranged from ∼55 to 70 dB for a given movie. The ambient noise in the recording booth was measured at 19–20 dB, which increased to 20–22 dB when the somatosensory stimulator was on. No audible sound from the stimulator was detected in a pilot study in which participants (experimenters, naive lab members, and children) listened for the presence of sound from the tactile stimulator while a movie played. Following a reviewer suggestion, we also made EEG recordings while the stimulator and a movie were on, but the stimulator was not placed on the participant and processed the EEG data to the “somatosensory” events. There was no indication of an auditory evoked response, providing further evidence that the insulation combined with presentation of a movie was highly effective at masking any possible stimulus-related sounds.
Procedure
Participants were seated in a sound-attenuated, electrically shielded, double-walled room (Industrial Acoustics, Bronx, NY) and watched a movie of their choice, presented with the sound set to a comfortable audible volume (∼70 dB SPL) with the screen 80 cm away. Task-irrelevant vibrotactile stimuli, 50 ms in duration, were presented in 5 single blocks of ISIs: 150, 250, 350, 550, and 1,050 ms. The experiment was 1 h in duration, with the block length duration varying from 4:13 to 9:20 min. More trials were presented in the shorter ISI blocks (150 ms, ∼1,492; 250 ms, ∼1,493; and 350 ms, ∼984) than the longer blocks (550 ms, ∼473; and 1,050 ms, ∼472)1. With the use of Biosemi Active amplifiers, continuous high-density EEG data were recorded from 64 scalp electrodes mounted on a nylon cap, as well as 6 external electrodes, followed by analog-to-digital conversion and fiber-optic pass-through to a dedicated acquisition computer (digitized at 512 Hz; DC to 150-Hz pass band). Biosemi replaces the ground electrodes that are used in conventional systems with two separate electrodes: a common mode sense active electrode and a driven right leg passive electrode. These two electrodes form a feedback loop, which drives the average potential of the participant as close as possible to the reference voltage of the analog-to-digital converter, thus rendering them references.
Data Analysis
MATLAB (The MathWorks, Natick, MA) was used for offline processing and analysis. Digital filtering with a low-pass filter of 45 Hz and a high-pass filter of 0.1 Hz was applied to the continuous EEG data using a fourth-order zero-phase Butterworth filter. The EEG was segmented on the basis of stimulus onset into epochs of 600 ms, including a 100-ms prestimulus onset period. An artifact rejection criterion of ± 120 μV was used to remove artifacts defined as blinks and movements, and bad channels were interpolated using the nearest neighbor spline (Perrin et al. 1987, 1989). Bad channels, which did not tend to cluster, were determined before interpolation and hence excluded from this process. Because we acquired the data on a high-density electrode cap, all the channels of interest in our study are surrounded by at least eight channels, meaning there would always be good channels from which to interpolate. If more than five channels contained bad data, the trial was rejected. Artifact-clean epochs were averaged according to ISI condition, and the resulting ERPs were re-referenced to the average of all electrodes.
Selection of Channels
The spatial and temporal profiles of the major components of the SEP were identified on the basis of visualization of the scalp topography of the SEP over time for each of the age groups and guided by SEP literature (Angel et al. 1985; Wang et al. 2008). Given that the response maxima were relatively stable across ISI conditions, the spatial and temporal profiles of the components assessed were determined using the 1,050-ms ISI condition, for which the SEP had the strongest signal. Scalp topographies were highly consistent across age groups, and three time windows of activity were identified: 45–80 ms, 85–105 ms, and 135–195 ms. Maximal responses within these windows had inverted patterns over frontal/frontocentral and parietal/centroparietal scalp sites. Electrodes selected for analyses were centered on these maximal SEP voltage responses and are described in detail in results. Additionally, scalp topographic maps were created for each time period of analysis, and dipole source modeling was conducted (as described below).
Statistical Analysis
Statistical analyses were performed using custom MATLAB scripts, the Fieldtrip toolbox for EEG analysis (Oostenveld et al. 2011), EEGLAB (Delorme and Makeig 2004), and the SPSS software package (IBM SPSS Statistics, Armonk, NY). The maximum amplitude within the window encompassing the peak was taken as the representative amplitude measure for each participant. A mixed-model repeated-measures analysis of variance (rmANOVA) was conducted with a between-subjects factor of age (younger, middle, older) and within-subjects factors of ISI (150, 250, 350, 550, and 1,050 ms) and electrode location (e.g., frontal, contralateral centroparietal, ipsilateral centroparietal) to assess the relationship between these factors and the amplitude of the SEP response (a 3 × 5 × 3 ANOVA). Separate mixed-model rmANOVAs were performed for each time period of the SEP (45–80, 85–105, and 135–195 ms). The Huynh-Feldt correction was used to adjust F values when sphericity was violated (Huynh and Feldt 1976). Effects were significant when P < 0.05; these significant effects were further examined through follow-up ANOVAs when appropriate. As necessary, post hoc Bonferroni-corrected comparisons were conducted. When further probing the effect of ISI on SEP amplitude (i.e., adaptation effects), we narrowed the comparison of short vs. long ISIs to representative fast and slow conditions: 250 and 1,050 ms, respectively.
Exploratory Analysis
Statistical cluster plots were generated to fully characterize age-related differences in the SEP for the longest ISI (1,050 ms) with regard to the entire data matrix. Pointwise, unpaired two-tailed t-tests between SEPs for the older vs. younger groups, the middle vs. younger group, and the older vs. middle group were generated for each time point for each electrode. As outlined in previous studies (Butler et al. 2012; De Sanctis et al. 2009; Molholm et al. 2002), we minimized type I errors by only considering a comparison statistically significant if P < 0.05 for 11 consecutive data points, because it is highly unlikely for a false positive result to occur simultaneously at adjacent electrodes or to occur in clusters across several time points (Guthrie and Buchwald 1991). This approach allowed us to consider effects that our highly constrained a priori analyses might have been blind to and served as a hypothesis generation tool for future studies.
Source Localization
Brain Electrical Source Analysis (BESA version 6.0; BESA, Gräfelfing, Germany) was employed to estimate the intracranial generators underlying the scalp-recorded activity for grand-averaged data from each of the age groups. BESA models the best-fit location and orientation of dipoles, adjusting their location iteratively until the minimal residual variance is reached for the data. To model the intracranial sources of the SEP, models were generated for group-averaged data from the 1,050-ms ISI condition, in which the signal strength was greatest. When modeling each age group, we used manufacturer standard electrode coordinates and selected the BESA head model most consistent with the age group being assessed.
To model the temporal dynamics of the neural generators, a sequential approach was taken in which each temporally successive time window was fit with symmetrically constrained dipole pairs until their addition no longer markedly improved the solution. Once a maximal fit was achieved for a given time window, the next time window was modeled, maintaining the earlier dipoles and adding additional pairs as necessary. It is important to note, however, that when dipoles are modeled, the source localization is an oversimplification of the center of the activity of the contributing neural sources in those regions and is not a precise location of exact generators (Dias et al. 2003; Molholm et al. 2004; Murray et al. 2002; Sehatpour et al. 2006).
These analyses are intended to be purely descriptive and to provide a plausible model of the general loci of the underlying neural generators. Future investigation in which anatomical MRIs are also acquired will allow for a more precise and individualized approach to examination of the SEP neural generators, which can be used to characterize expected interparticipant variability.
RESULTS
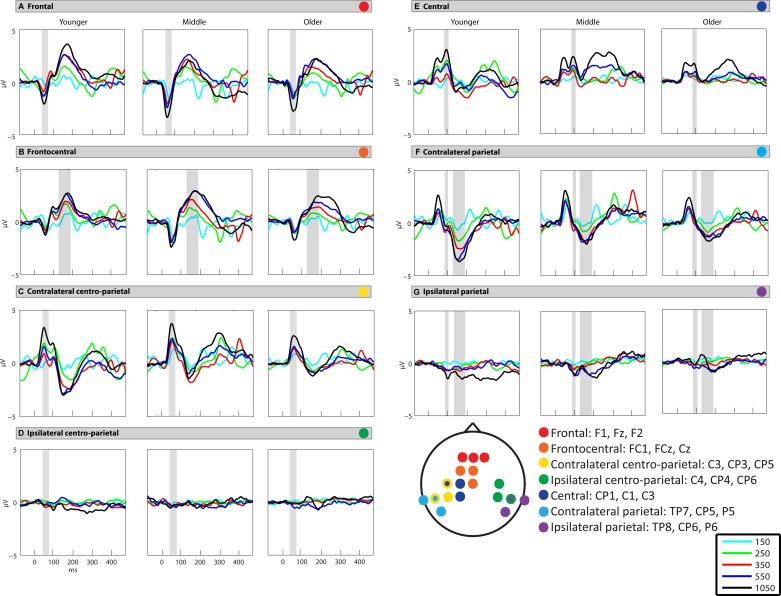
In this work we examined the neurodevelopment of somatosensory processing, and tested whether somatosensory adaptation modulates as a function of age. The vibrotactile stimuli evoked robust SEPs with highly similar spatiotemporal patterns of activation across the three age groups (Fig. 2)2. Three main periods of activity peaked at ∼55, 95, and 175 ms. Robust ISI effects were observed for all age groups, with greatest amplitude SEPs to the slowest ISI conditions of 550 and 1,050 ms. Below are the results of the mixed-model rmANOVA for each analyzed time window.
Fig. 2.
Somatosensory evoked potential (SEP) waveforms at each analyzed region. Gray shaded boxes represent the time periods of analysis for a given region: 45–80 ms (A, C, D), 85–105 ms (E, F, G), and 135–195 ms (B, F, G). All interstimulus interval (ISI) conditions are depicted: 150 ms (light blue), 250 ms (light green), 350 ms (red), 550 ms (dark blue), and 1,050 ms (black).
Time Period: 45–80 ms
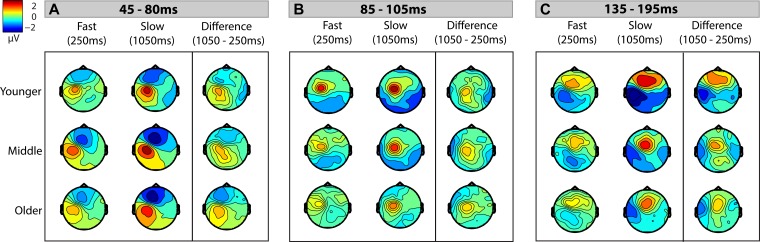
The initial response began at ∼25 ms and peaked at ∼55 ms (except for the 150-ms ISI condition, during which the peak was delayed to ∼70 ms). This response inverted across frontal and contralateral centroparietal scalp (Fig. 3A), consistent with generators in contralateral somatosensory cortex. Data were therefore analyzed from frontal (F1, Fz, F2) and centroparietal (contralateral: C3, CP3, CP5; ipsilateral: C4, CP4, CP6) scalp regions (Fig. 2, A, C, and D). The middle group showed a stronger response than the younger and older groups (Figs. 2 and 3A), which was verified by the main effect of age (F2, 46 = 4.843, P = 0.012). Statistical analysis revealed significant main effects of region (F2, 92 = 112.52, P < 0.001) and ISI (F3.8, 174.92 = 43.67, P < 0.001), as well as a significant interaction between ISI and region (F7.09, 326.1 = 7.68, P < 0.001). There was also a statistically significant interaction between age and region (F4, 92 = 3.47, P = 0.011) due to the differing effects of age on SEP amplitude in the frontal vs. contralateral centroparietal regions.
Fig. 3.
Topographic maps of activation across the scalp. Topographic maps of response to vibrotactile stimulation are shown for the representative fast condition (250 ms; left) and the representative slow condition (1,050 ms; middle) and the difference in response between the 2 conditions (right) for each of the 3 analyzed time windows: 45–80 ms (A), 85–105 ms (B), and 135–195 ms (C). Amplitude is indicated by heat map; see color bar at top left.
Developmental effects.
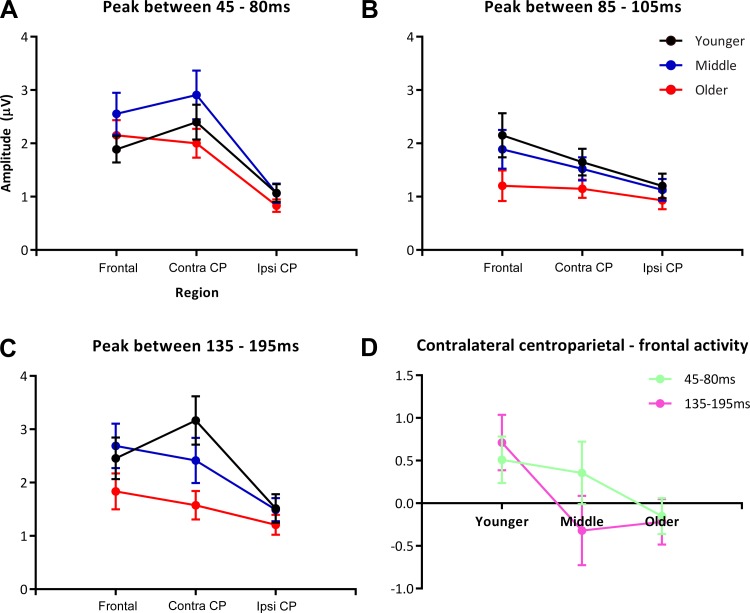
To unpack the significant interaction between age and region, one-way ANOVAs with a factor of region were performed for each of the age groups with the data collapsed across ISI. This yielded a significant main effect of region (F2, 252 = 36.53, P < 0.001), which appeared to be driven by the differing regional pattern of activation in the younger group compared with the middle and older groups. In younger children, contralateral centroparietal SEP responses were significantly greater than frontal SEP responses (P = 0.004), which were significantly greater than ipsilateral centroparietal responses (P < 0.001). In the middle and older groups, however, contralateral centroparietal and frontal responses were not significantly different (middle, P = 0.749; older, P = 1.000), although both the frontal (middle, P < 0.001; older, P < 0.001) and contralateral centroparietal (middle, P < 0.001; older, P < 0.001) responses were significantly greater than the ipsilateral centroparietal responses at this time. Thus, in all age groups, the response was greater over contralateral compared with ipsilateral regions, and in the younger group contralateral centroparietal activity was greater than frontal activity (Fig. 3A). These results are illustrated in Fig. 4, A and D.
Fig. 4.
A–C: graphs depicting absolute SEP amplitude at each region, separated by age. A: 45–80 ms. Note the increased amplitude response in the middle age group compared with the younger and older age groups at both the frontal and contralateral centroparietal regions. B: 85–105 ms. Graph depicts the relative similarity in response amplitude for the younger and middle age groups and the reduced response for the older age group. C: 135–195 ms. There is a clear stratification by age in the last window of analysis, with the younger group showing the greatest activity in the contralateral parietal region and the older group showing the lowest amplitude response. D: graph of the difference in amplitude between more posterior vs. more anterior regions of interest. For the early time window (45–80 ms) there is a relatively linear trajectory with age, representing activity weighted toward the more posterior region in the younger group, vs. relatively equal activity across the anterior and posterior regions of activation foci in the older group. For the 135–195 ms time period, the younger group shows this same differential weighting toward the posterior region, whereas the middle and older groups have responses that are slightly weighted toward the anterior region of the activation foci. CP, centroparietal; contra, contralateral; ipsi, ipsilateral.
Adaptation effects.
To further assess the interaction between ISI and region, we performed a two-way rmANOVA. We narrowed our analysis to two ISI conditions (250 and 1,050 ms) in the two regions representing the majority of the SEP activity (frontal and contralateral centroparietal) to determine whether there was a significant difference in adaptation between the regions in which the response was greatest. This yielded a significant effect of ISI (F1, 48 = 57.75, P < 0.001) due to a larger SEP amplitude elicited for the 1,050-ms ISI condition and a significant effect of region (F1, 48 = 5.74, P = 0.021) due to larger SEP amplitudes in the contralateral centroparietal region, but no interaction between the two factors. Thus adaptation effects were statistically comparable between the two regions where the response was greatest. Visualization of the topography of adaptation effects at this time window can be seen in Fig. 3A, which illustrates the overall similarity in adaptation between the two regions across ages. To provide a more comprehensive picture of the adaptation effects in this time window, paired t-tests between all ISIs were performed for the frontal and contralateral centroparietal regions of interest. These are presented in Table 1 and confirm and extend the findings of the primary analyses.
Table 1.
Paired t-tests comparing all ISI conditions
|
t-Test Comparison: ISI1, ISI2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ISI1: |
150 ms |
250 ms |
350 ms |
550 ms |
||||||
| ISI2: | 250 ms | 350 ms | 550 ms | 1,050 ms | 350 ms | 550 ms | 1,050 ms | 550 ms | 1,050 ms | 1,050 ms |
| Frontal/central region | ||||||||||
| 45–80 ms | 0.029* | 0.001* | <0.001* | <0.001* | 1.000 | 0.483 | <0.001* | 1.000 | <0.001* | <0.001* |
| 85–105 ms | 0.027* | 0.008* | 0.051 | <0.001* | 1.000 | 1.000 | 0.004 * | 1.000 | 0.176 | 0.078 |
| 135–150 ms | 0.002* | <0.001* | <0.001* | 0.374 | 0.002* | 1.000 | ||||
| Contralateral centroparietal/parietal region | ||||||||||
| 45–80 ms | 0.012* | 0.002* | 0.005* | <0.001* | 1.000 | 1.000 | <0.001* | 1.000 | <0.001* | <0.001* |
| 85–105 ms | 0.587 | 1.000 | 1.000 | <0.001* | 1.000 | 1.000 | 0.008* | 1.000 | <0.001* | 0.002* |
| 135–150 ms | 0.001* | 0.039* | <0.001* | 1.000 | 0.228 | 0.498 | ||||
Data are P values determined by paired t-tests comparing all interstimulus interval (ISI) conditions. Significant P values are marked by an asterisk. Specific regions of interest vary by time window, as detailed in text.
Dipole modeling of the SEP.
Source analysis, in which dipoles were modeled by freely fitting a location-unconstrained and orientation-unconstrained pair of symmetric dipoles to the 1,050-ms condition, localized to bilateral postcentral areas at all ages in the 1,050 ms condition [goodness of fit (GoF) in the younger group = 93%, middle = 98%, and older = 98%; Fig. 5A, red and royal blue dipoles], although the contralateral postcentral dipole accounted for the majority of the activity (GoF for contralateral dipole: younger = 80%, middle = 95%, older = 92%; Fig. 5B, waveform 1). Additional dipoles did not greatly affect the solution (younger = +3%, middle = +0.2%, older = +0.2%). The general loci of these dipoles are consistent with activity from the vicinity of SI, as would be expected for the initial somatosensory-driven cortical activity. Clearly, however, one must be highly cautious in interpreting the underlying intracranial sources of this signal on the basis of dipole modeling. First, the models are based on group data. Second, the signal modeled was for a window of time from 45 to 80 ms that was centered around the peak of the initial activity. Given that the cortical response onset begins at ∼25 ms and the analysis window is from 45 to 80 ms, the underlying neural generators very likely included an already extended network of primary somatosensory cortex, and possibly secondary somatosensory cortex as well (Allison et al. 1989a, 1989b; Foxe and Simpson 2002).
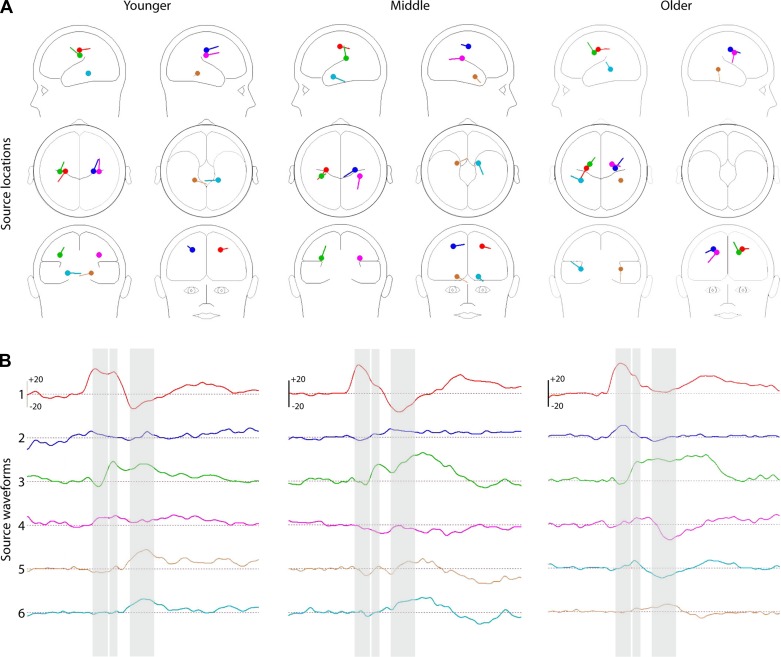
Fig. 5.
Source modeling of the SEP. Six-dipole model of the SEP, based on sequential modeling of the 3 major periods of activity within the first 195 ms following stimulus onset, for the young (left), middle (middle) and older (right) groups. A: head maps indicate relative regions of neural sources for each age group. Right and left are in radiological orientation, with left and right reversed. B: waveforms indicate the overall activity over time for each of the dipoles. Waveforms 1–6 correspond with dipoles by color.
In the interest of understanding the possible timing of engagement of secondary somatosensory cortices, in an exploratory exercise, we modeled the early and late portions of the initial response separately, with the first segment spanning 25–55 ms and the second spanning 55–85 ms. For all age groups, dipole modeling of the first segment resulted in dipoles very similar to those seen for the time window as a whole, whereas modeling of the later portion resulted in inferiorly placed dipoles. Bearing in mind the caveats above, these models are consistent with the rapid engagement of SII within this initial phase of processing.
Time Period: 85–105 ms
The next major response peaked at ∼95 ms and had a positive focus over contralateral central scalp that was accompanied by opposite polarity activity over bilateral parietal scalp (Fig. 3B). To most accurately capture this activity, data were analyzed from central (CP1, C1, C3), contralateral parietal (TP7, CP5, P5), and ipsilateral parietal (TP8, CP6, P6) scalp regions (Fig. 2, E–G). The response was most prominent in the younger and middle groups, with progressively reduced response with increased age (Fig. 4B). Scalp topographic mapping suggested the presence of low-amplitude activity over ipsilateral parietal scalp at the longest ISI (Fig. 3B; Fig. 5B, waveform 4), suggestive of bilateral activity in somatosensory cortices. At this latency, there was a significant effect of ISI (F3.52, 161.96 = 28.66, P < 0.001) and region (F1.51, 69.49 = 18.97, P < 0.001) on SEP amplitude, and the effects of these two factors were dependent on each other (F5.25, 241.34 = 4.7, P < 0.001).
Adaptation effects.
The ISI-by-region interaction was followed up with an rmANOVA comparing 250- and 1,050-ms ISI conditions for central and contralateral parietal regions. Through this analysis, we confirmed that the 1,050-ms ISI condition elicited a larger SEP response than the 250-ms ISI condition (F1, 48 = 24.66, P < 0.001), and that the central region had significantly greater activity than the contralateral parietal region (F1, 48 = 13.26, P = 0.001; Fig. 3B). Additionally, there was a significant interaction between ISI and region (F1, 48 = 4.28, P = 0.044), reflecting a greater adaptation effect over the central region (Fig. 3B). To provide a more comprehensive picture of adaptation effects in this time window, we also performed paired t-tests between all ISIs for the central and contralateral parietal regions of interest. These are presented in Table 1 and confirm and extend findings from the primary analyses.
Dipole modeling of the SEP.
Source analysis of the SEP indicated that at this latency, the addition of a symmetrically constrained pair of dipoles localized to bilateral somatosensory areas ventral to the original pair of somatosensory dipoles (Fig. 5A, green and pink dipoles; GoF: younger = 94%, middle = 95%, older = 78%). This is consistent with engagement of the secondary somatosensory cortices in this time frame. Although a large portion of the activity is from contralateral dipoles, removing ipsilateral dipoles greatly reduces the GoF (GoF of contralateral dipoles: younger = 76%, middle = 78%, older = 56%), suggesting the engagement of the ipsilateral somatosensory regions as would be expected at this time period. The lower GoF in the older group is likely due to the relatively smaller signal; when 2 additional dipole pairs were added to the solution, the GoF increased by <10% for a given age group, leading to the conclusion that the current 4-dipole solution was the best fit for this period of the SEP.
Time Period: 135–195 ms
The next major response in the SEP began at ∼105 ms and peaked at ∼175 ms. This response inverted across frontocentral scalp and contralateral parietal scalp regions (Fig. 3C). Data were analyzed from frontocentral (FC1, FCz, Cz), contralateral parietal (TP7, CP5, P5), and homologous ipsilateral parietal (TP8, CP6, P6) regions (Fig. 2, B, F, and G). The 150-ms condition was excluded because this time frame of analysis overlaps with the SEP to the subsequent stimulus for this condition. The rmANOVA revealed a main effect of age at this latency (F2, 46 = 9.23, P < 0.001), with the response decreasing progressively as a function of age (Fig. 2, B, F, and G; Fig. 4C). There were also main effects of ISI (F2.65, 121.71 = 44.07, P < 0.001) and region (F2, 92 = 45.03, P < 0.001). Developmental effects were seen through a significant interaction between region and age (F4, 92 = 6.76, P < 0.001).
Developmental effects on the SEP.
As Fig. 2 depicts, activity in the younger group was greater over contralateral parietal scalp compared with frontocentral scalp regions, whereas this pattern reversed for the middle and older groups. This observation was confirmed through follow-up analyses on the region-by-age interaction, for which we performed one-way ANOVAs for each age group, collapsing across ISIs with region as the factor. In the younger group, there was a main effect of region (F2, 252 = 23.72, P < 0.001), with the contralateral parietal region having significantly greater activity than the frontocentral region (P = 0.01), which in turn had significantly greater activity than the ipsilateral parietal region (P < 0.001). The middle group also showed a main effect of region (F2, 222 = 15.16, P < 0.001) due to significantly greater activity in both frontocentral and contralateral parietal regions compared with the ipsilateral parietal region (P < 0.001 for both comparisons). The older group also had a main effect of region (F2, 252 = 5.97, P = 0.003) and showed a pattern of regional activation similar to that of the middle group: response amplitude of the frontocentral region did not significantly differ from that of the contralateral parietal region, and the ipsilateral parietal region had the least activity (significantly less than the frontocentral region; P = 0.002). A graphical depiction of these results can be seen in Fig. 4D.
Adaptation effects.
To zero in on the interaction of ISI and region, we performed an rmANOVA on the 250- and 1,050-ms ISI conditions in the frontal and contralateral parietal regions. This interaction was driven by the significant effect of ISI on SEP amplitude (F1, 48 = 99.34, P < 0.001), with higher SEP amplitudes evoked by the 1,050-ms ISI than the 250-ms ISI. The lack of an interaction effect suggested that these adaptation effects were not significantly different across the two regions where the SEP response was the greatest (Fig. 3C). To provide a more comprehensive picture of adaptation effects for this time window, we also performed paired t-tests between all ISIs for the frontocentral and contralateral parietal regions of interest. These are presented in Table 1 and confirm and extend findings from the primary analyses.
Dipole modeling of the SEP.
At this time period, the two pairs of existing dipoles accounted for most of the activity (younger = 94%, middle = 90%, older = 86%). An additional pair of freely fitting symmetrically constrained dipoles, which localized to areas in and around the temporal lobe, improved the solution slightly (younger = 97%, middle = 94%, older = 96%), but more than six total dipoles did not substantially increase the GoF (younger = +0.7%, middle = +0.2%, older = +0.2%). In the younger group, the third set of dipoles approximately localized to the lateral globus pallidus, whereas dipoles in the middle group localized closer to the inferior frontal gyrus, and dipoles in the older group localized to an area neighboring the insula.
Exploratory Analysis of SEP Developmental Effects
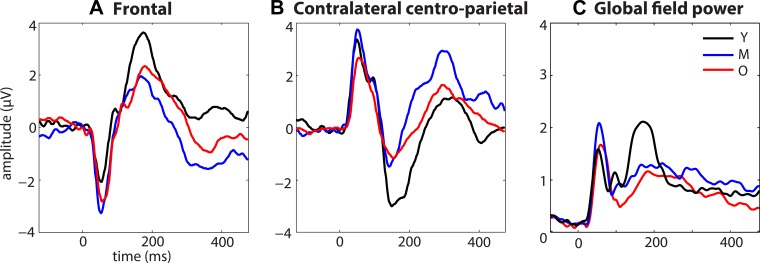
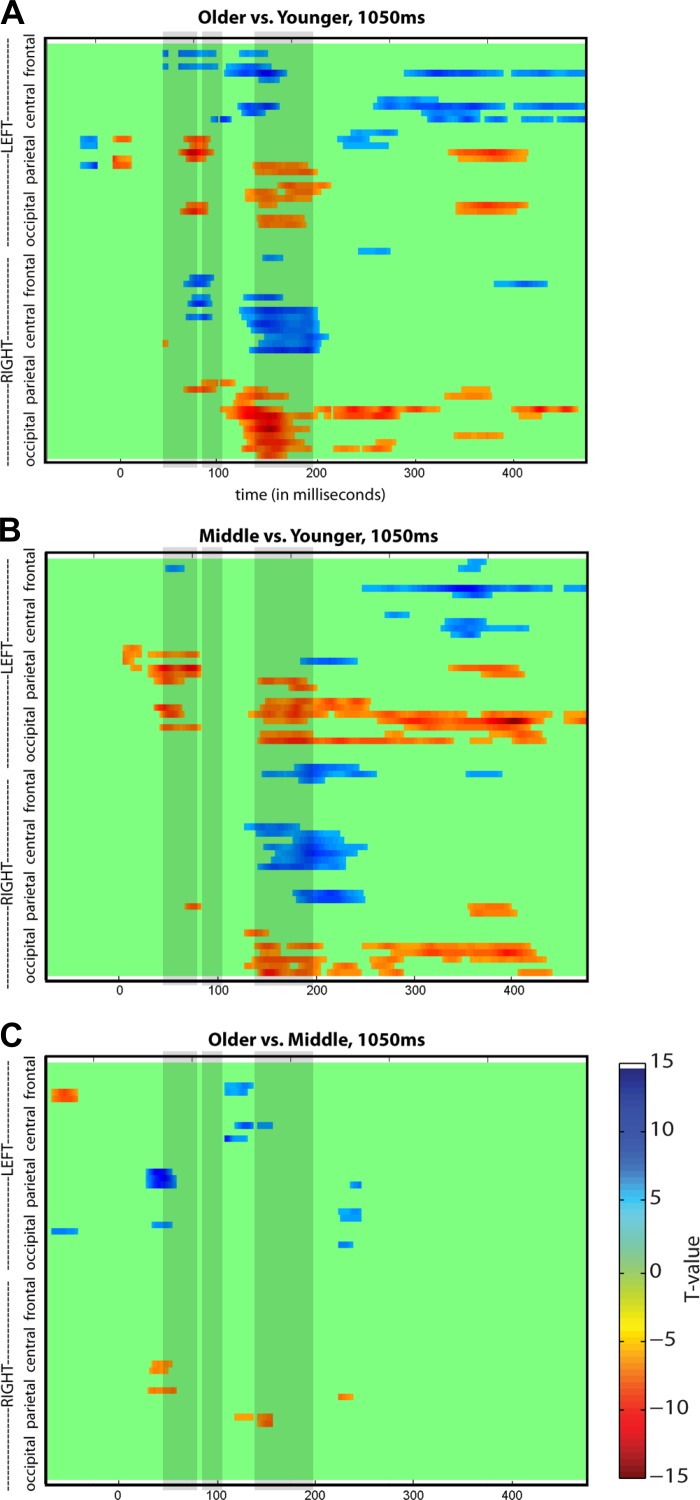
The extent of age-related differences in the SEP can be seen in Fig. 6, which shows the SEP for the 1,050-ms condition for the younger (black), middle (blue), and older (red) groups over frontal (A) and contralateral centroparietal sites (B), and in terms of global field power (C), which quantifies net signal power without regard for scalp topography. Exploratory statistical cluster plots (SCPs) were generated to fully capture these age-related differences. Examination of Fig. 7 makes it clear that the SEP of the younger group differs from that of the middle and older groups most dramatically around 75 ms and from ∼125 to 205 ms. This is illustrated by focusing on the younger vs. older SCP (Fig. 7A), in which differences are observed bilaterally in parts of the frontal and parietal regions at ∼75 ms (see shaded box over first time period) and over the contralateral frontocentral and occipitoparietal regions as well as the ipsilateral central and occipitoparietal regions at 200 ms (see shaded box over third time period). Figure 7B depicts SEP differences between younger and middle groups for the 1,050-ms condition that are generally in the same areas as in the younger vs. older groups. This is largely consistent with our planned analyses; however, the SCPs also revealed lateralization differences that were not as clear in our primary analyses. For example, when the younger and older groups (Fig. 7A) and the younger and middle groups (Fig. 7B) are compared, there appear to be more developmentally based SEP differences in ipsilateral compared with contralateral scalp regions in the third time period (note bottom halves of SCP maps in A and B). For example, the younger group has a greater increased response in the central region on the ipsilateral side than on the contralateral side (compared with older and middle groups, Fig. 7, A and B, respectively). The SCPs also clearly illustrate the lack of change between the SEPs of the middle and older groups (Fig. 7C), suggesting that the majority of developmental changes occur before the age of 10 yr.
Fig. 6.
Developmental changes in overall SEP activity. A: developmental changes in frontal activity are apparent across age groups, with the middle group showing the most activity in the initial negative-going response at ∼55 ms and the younger group showing the most activity in the later positive-going response at ∼175 ms. B: the same pattern of differential activation as in A was seen, in inverted form, over the contralateral centroparietal region. C: global field power was also calculated to examine amplitude of the signal without regard to scalp topography. This confirmed the age-related differences in signal power, with the middle group showing increased activity in the early period and the younger group relatively increased activity in the later time window. Y, younger; M, middle; O, older.
Fig. 7.
Statistical cluster plots illustrating developmental differences in SEP amplitude over time and across electrodes. The x-axis represents time, the y-axis represents scalp region, and the z-axis represents the t-test result (indicated by a color value from red to blue) at each data point. Areas represented in green do not meet criteria for statistical significance. Shaded boxes cover the 3 time periods used in the primary analyses, for easy reference.
Secondary Analysis of Effects of ISI and Age on Amplitude of the SEP
Our primary analysis was followed up with a potentially more sensitive approach to exploring developmental effects on adaptation, in which age was considered as a continuous function. Multiple linear regression analysis was performed to test for the relationship of the absolute amplitude of the evoked response as a function of age and ISI and their interaction. The 150-ms condition was excluded from the analysis of the last time window because this time frame of analysis overlaps with the SEP to the subsequent stimulus for this condition, and separate tests were performed for data from contralateral posterior and frontal scalp regions. For the contralateral posterior region, the results were as follows: for the earliest time window of 45–80 ms, the regression equation was significant (F3, 241 = 22.24, P < 0.001) with an R2 of 0.216. Only ISI was a significant predictor of amplitude (P < 0.05), with SEP amplitude increasing by 0.0023 mV with increasing ISI. For the middle time period of 85–105 ms, the regression equation was significant (F3, 241 = 15.01, P < 0.001) with an R2 of 0.1574. Only age was a significant predictor of amplitude (P < 0.001), with participants' evoked response decreasing by 0.134 mV per year. For the last time window of 135–195 ms, the regression equation was significant (F3, 193 = 35.66, P < 0.001) with an R2 of 0.3578. Both ISI and age were significant predictors of amplitude (P < 0.001), with participants' SEP amplitude decreasing by 0.234 mV per year and increasing by 0.002938 as a function of increasing ISI. Thus, for the contralateral posterior region, for both the early and the late time windows of analysis, as ISI increased, so did the amplitude of the response, and for the middle and late time windows of analysis, as age increased, this response decreased. There were no significant age-by-ISI interactions. For the frontal region, the results were as follows: for the earliest time window of 45–80 ms, the regression equation was significant (F3, 241 = 6.123, P < 0.001) with an R2 of 0.05925, but none of the coefficients were significant. For the middle time period of 85–105 ms, the regression equation was significant (F3, 241 = 17.01, P < 0.001) with an R2 of 0.1645, and only age was a significant predictor of amplitude (P < 0.001), with participants' evoked response decreasing by 0.064 mV per year. For the last time window of 135–195 ms, although the regression equation was significant (F3, 192 = 14.03, P < 0.001) with an R2 of 0.1819, none of the coefficients were significant.
These results align well with our primary analysis insofar as ISI and age affect SEP amplitude, whereas there is no support for developmental effects on adaptation. The results also suggest that the contralateral posterior region is more sensitive to these effects than the frontal region.
DISCUSSION
Reactivity to tactile stimulation appears to change dramatically over childhood development; however, very little work has been done to thoroughly map the developmental trajectory of the neural processing of somatosensory inputs over the entirety of childhood. In this study, high-density SEPs were recorded from children ranging in age from 6 to 18 yr of age to determine the developmental course of somatosensory processing in response to vibrotactile stimulation and to test the hypothesis that there are developmental changes in sensory adaptation. To probe adaptation to somatosensory stimulation, we varied the presentation rate of the somatosensory stimulus from ∼1 stimulus per second to ∼7 stimuli per second. In many respects the somatosensory response was found to be very similar across the age groups. However, analyses also revealed clear developmental changes for both early and later phases of the SEP. In contrast, adaptation effects were already highly robust in the youngest age group and appeared to be stable over childhood. These findings and their implications are discussed below.
Neurodevelopment of Somatosensory Processing
The first detectable cortical response to the somatosensory stimulus began at about 25 ms and was clearly present in all age groups for all presentation rates, although it was of greatest amplitude for the middle age group (Fig. 6). This response was strongly lateralized, with a robust response that inverted between contralateral centroparietal and frontal scalp regions (Figs. 3A and 6), suggestive of neural generators in contralateral somatosensory cortex. Previous work by Allison et al. (1992) corroborates these findings, showing that early somatosensory processing (∼70 ms) was generally restricted to somatosensory cortices, with a frontal negativity and contralateral parietal positivity observed in scalp recordings. This initial response likely reflects early cortical processing of the physical attributes of the stimulus in SI (Allison et al. 1982, 1984; Broughton et al. 1981). Whereas the middle and older groups showed similar response amplitudes over the two major scalp foci (Fig. 4A), the response was significantly larger over contralateral centroparietal compared with frontal scalp in younger children (see Figs. 4A and 6). What is more, the middle group had the largest overall response in this time period (Figs. 4A and 6). The middle group's response amplitude approximated that of the younger group over contralateral centroparietal scalp and that of the older group over frontal scalp. Although certainly highly speculative, this may reflect that the middle age group represents a transitional stage whereby both “immature” and “mature” somatosensory processing networks are engaged. The differential weighting by age of the response across these two regions could reflect 1) a different configuration of neural generators in somatosensory cortices due to developmental changes in the microcircuitry within somatosensory cortices; 2) that developmental changes in anatomy and/or skull thickness/shape influenced the scalp recorded signal, but the underlying neuronal response was the same (Casey et al. 2005; Matsuzawa et al. 2001); or 3) a combination of these two factors. As indicated through developmental studies of synaptogenesis and cortical development, somatosensory areas continue developing through age 17 (Allison et al. 1984), and these organizational changes likely affected the responses measured in this study (Picton and Taylor 2007). Although one might expect all age groups to show the same overall response if these response differences were simply due to slightly reorienting dipolar fields, consideration of the global field potential (Fig. 6C) argues against this, illustrating that the middle group has the larger amplitude response overall. Nevertheless, dipole modeling of the initial cortical response localized to approximately the same region in all age groups and is consistent with neural generators from contralateral somatosensory cortex accounting for the majority of this response (Fig. 5B, waveforms 1 and 3; Table 2).
Table 2.
Goodness of fit for dipole source models
| Goodness of Fit, % |
||||
|---|---|---|---|---|
| Dipole | Period of Analysis, ms | Younger | Middle | Older |
| 1, 2 | 45–80 | 92.725 | 97.895 | 98.236 |
| 1 | 45–80 | 80.324 | 94.899 | 92.337 |
| 2 | 45–80 | 36.364 | 50.954 | 57.858 |
| 1, 2 | 85–105 | 40.564 | 12.226 | 31.496 |
| 1, 2, 3, 4 | 85–105 | 94.216 | 95.057 | 77.304 |
| 1, 3 | 85–105 | 76.099 | 78.333 | 60.118 |
| 2, 4 | 85–105 | 29.225 | 31.130 | 24.745 |
| 1, 2, 3, 4 | 135–195 | 94.156 | 90.389 | 89.018 |
| 1, 2, 3, 4, 5, 6 | 135–195 | 97.569 | 94.255 | 96.099 |
| 1, 3, 5 | 135–195 | 93.129 | 84.105 | 85.286 |
| 2, 4, 6 | 135–195 | 70.911 | 40.526 | 57.700 |
| 1, 2, 3, 4, 5, 6 | Overall SEP | 84.233 | 84.834 | 92.670 |
SEP, somatosensory evoked potential.
The relatively late latency of the initial somatosensory response is likely due to a combination of factors, including stimulation type (vibrotactile rather than electrical), use of a stimulation frequency that may have been suboptimal for Pacinian mechanoreceptors, and the relatively low receptor density of the region stimulated. For example, if one considers the latency of the response typical for electrical median nerve stimulation, the initial response tends to peak at about 20 ms (Allison et al. 1992; Foxe et al. 2000; Lee and Seyal 1998; Wood et al. 1985), some 35 ms earlier than we observed in the present study. A primary difference between electrical and vibrotactile stimulation of the median nerve area is that the former is thought to primarily engage the deeper median nerve, whereas vibrotactile stimulation is biased toward cutaneous receptors. Thus stimulation of different somatosensory receptor types might partially account for such latency differences. At the same time, however, it is notable that vibrotactile stimulation between thumb and forefinger in another study peaked at ∼50 ms (De Santis et al. 2007), for vibration frequencies of 22.5 and 110 Hz, whereas air puffs to the same region (the pad of the forefinger) have produced peak responses as early as 24 ms (Bast et al. 2007) and as late as ∼50 ms (Cascio et al. 2015). Given the variance that is observed across studies, it is clear that the temporal dynamics of the SEP need to be considered in light of the specific stimulation variables as well as participant characteristics.
By the second major phase of processing, somatosensory stimulation appeared to engage ipsilateral somatosensory cortex. Although the SEP response was always dominant over contralateral scalp regions in this passive tactile stimulation experiment, in this time frame removal of the ipsilateral dipoles led to a GoF that was an average of 19% poorer than the full 4-dipole solution. The more inferior placement of the dipoles for this later time period in all age groups is consistent with generators in SII. This pattern of findings is in line with previous work in adults, in which the bilateral SII is engaged at ∼120 ms (Allison et al. 1992). Since the representation of sensory information tends to increase in complexity as it travels along the temporal and anatomical information processing hierarchy (Felleman and Van Essen 1991; Iwamura 1998; Lucan et al. 2010; Rauschecker and Scott 2009), for this temporally later stage it can be assumed that there is more integrated/complex processing and representation of the features of the stimulus and its somatotopic placement (e.g., respectively, the object's texture and its integrated placement on the body). This may occur along parallel ventral and dorsal what/where pathways, insofar as it has been proposed that information flow from SI to SII follows “ventral” and “dorsal” streams (De Santis et al. 2007) involved in the processing of “what” and “where” information, respectively. This ventral/dorsal what/where division is seen in auditory and visual cortices, as well (Felleman and Van Essen 1991; Leavitt et al. 2011; Mishkin and Ungerleider 1982; Rauschecker and Tian 2000; Romanski et al. 1999) and may represent an organizational principle shared across the major sensory systems.
In the final time period considered, whereas a robust response was observed for the younger group, the amplitude of the SEP was substantially reduced for the middle and older groups. Delineation of the SEP pathway by Allison et al. (1992) suggests that somatosensory processing already includes engagement of bilateral frontal regions by 140–190 ms following stimulus onset. Given the timing and temporal stage of this last phase of activity, it is then reasonable to assume that it includes the engagement of frontal regions and modulatory feedback processing from frontal to somatosensory cortices (Allison et al. 1992; Sehatpour et al. 2008). In older children (the middle and older groups), the substantially reduced response could signify an experience-dependent reduction of frontal involvement in the processing of stimuli that are wholly task irrelevant. Engagement of frontal regions, however, was not clearly supported by dipole modeling. When an additional pair of dipoles was added for the third time period, for which the first two pairs of dipoles already provided a very good fit for all age groups, they localized to more medial cortical regions. Given that most of the signal was already accounted for, we are reluctant to speculate on the functional significance of these localizations. Future investigation focused on localizing neural sources of activity using methods with high anatomical resolution (e.g., functional magnetic resonance imaging), and on linking brain activity to sensory reactivity, will be needed to satisfactorily interpret the neural sources of this last period of activity and to determine its role in increased reactivity to sensations at younger ages or in clinical groups (Ahn et al. 2004; Ben-Sasson et al. 2009; Carter et al. 2011; Royeen and Mu 2003).
Overall, our SEP data suggest that all age groups followed the same basic spatiotemporal patterns of activity within the somatosensory processing pathway while also revealing age-related differences at early and later stages of processing. Consistent with the notion of neural pruning and synaptogenesis impacting the ERP (Picton and Taylor 2007), both auditory and visual evoked responses also vary over childhood in school-age individuals (Brandwein et al. 2011) and do so even more dramatically than seen here in the SEP. Additional work is needed to determine how developmental trajectories of somatosensory processing differ as a function of stimulation type and stimulated body region. Our finding of age effects on the SEP contrast with a recent report from Casio et al. (2015) in which age was not associated with any of the probed ERP variables, in a cohort of similarly aged school-age children (ranging in age from 5–17). Different analytic approaches, participant numbers, and stimulation parameters likely contributed to this difference in findings.
Neurodevelopment of Somatosensory Adaptation
A major goal of this study was to characterize hypothesized developmental changes in somatosensory adaptation. As would be expected from previous studies (Angel et al. 1985) and from the adaptation literature more broadly, faster presentation rates led to smaller SEP responses. Furthermore, adaptation effects were apparent for the three major phases of sensory processing observed in our data, and scalp topographic maps of the adaptation effects resembled those for the SEP. This suggests that adaptation processes are propagated throughout the cortical somatosensory processing network during passive stimulation, rather than selectively manifesting within a given region. Counter to our prediction of reduced adaptation function in younger children, adaptation effects were highly robust in all age groups considered and, as assessed by comparing exemplar fast and slow ISI conditions, did not differ as a function of age. Undoubtedly, one must be cautious in generalizing this finding to all types of somatosensory stimulation. The developmental time course of adaptation might well differ as a function of both the submodality of the somatosensory system engaged and the stimulation parameters used.
Study Caveats and Limitations
There are several limitations to this study that bear mention. Although the stimulator apparatus was systematically applied to all participants, because force was not measured for individual participants, it is possible that there was some small degree of variation across our sample. There is, however, no reason that this variability would occur systematically as a function of age group, and thus it is highly unlikely to account for developmental differences in the SEP. Attention, which can impact the SEP (Desmedt et al. 1976; Dustman and Beck 1969; Luders 1970; Shagass and Schwartz 1965; Tamura et al. 1972), was not explicitly controlled. Rather, participants watched a movie of their choice and were instructed to look at the monitor and to ignore the vibrotactile stimulus. Given the monotony of the experiment, and the welcome distraction of a movie, we do not expect that attention was frequently directed at the stimulus. Still, it is possible that participants' attention was occasionally directed toward the somatosensory events, and this could have small effects on the SEP (Desmedt et al. 1976; Dustman and Beck 1969; Karns and Knight 2009; Luders 1970; Shagass and Schwartz 1965; Tamura et al. 1972). A related issue is that in watching and listening to a movie, attention was directed toward the auditory and visual systems and away from the somatosensory system. The data might look quite different were attention not biased in this manner (Karns and Knight 2009). Clearly, further work needs to be done to better understand the impact of such cross-sensory attentional biases on the somatosensory response. Inclusion of a task, either directed at the stimuli (e.g., duration or intensity change judgments) or toward another set of unrelated stimuli, would be useful for controlling attention.
There might be concern that in the shorter ISI conditions, there is not sufficient time for the neural response to a given stimulus to resolve before presentation of the next stimulus. However, in considering adaptation effects, it is just this influence on brain processing that we are interested in. How does a recent prior stimulation influence the response to the present stimulation, and are these effects constant across childhood or do they differ? Use of a jittered ISI might affect this outcome (Loizides et al. 2015), making the occurrence of the next stimulus less predictable, and would be useful for teasing apart the contribution of predictability to adaptation effects.
Conclusion
The present data make clear that to accurately characterize developmental effects on the neurodynamics of tactile processing, it is necessary to assess changes in the topography as well as in the amplitude of the response. We also find that a much more complex relationship presents across childhood development than is characterized by the previously reported simple U-shaped function over the lifespan and that before ∼10 yr of age may represent a particularly crucial period of development, especially with regard to later sensory-perceptual processing stages. This latter point may seem surprising insofar as the somatosensory system is one of the first sensory systems to develop (e.g., Kostovic and Rakic 1990), and this may be thought of therefore as relatively late in development for basic sensory processing changes to present. However, the refinement of the response to tactile inputs has been argued to continue in newborns and school-age children to achieve a mature adultlike response (Nevalainen et al. 2014). This may be achieved through changes in microcircuitry of somatosensory cortices (Picton and Taylor 2007) and changes in connectivity with regions such as insula and/or frontal cortex. Such changes may also lead to developmental effects on the strength of coupling of the components of the SEP, presenting another potentially fruitful approach to understanding important changes in somatosensory processing over the course of development. In contrast to the presence of a literature on SEP development in children, to our knowledge our study is the first to characterize whether developmental changes in somatosensory adaptation occur in childhood. Counter to our expectation, tactile adaptation appears to be well developed and largely matured by 6–9 yr of age. One might ask why there is this juxtaposition of findings, with differences present in the processing of somatosensory stimuli, but not in somatosensory adaptation, across development. The neurobiological bases of adaptation effects remain a subject of inquiry (Kohn 2007), with refractoriness, ongoing response to the adapter, and prediction-based reduction of the response all possibly contributing to adaptation effects (Friston 2005; Lanting et al. 2013). What is clear, however, is that although the processes underlying adaptation are largely in place by ∼7 yr of age, the pruning and wiring of the extended somatosensory system is a relatively protracted process that is still evolving over childhood. This work clearly needs to extend into younger ages and clinical groups where adaptation deficits have been observed behaviorally (Puts et al. 2014; Tommerdahl et al. 2007), and the relationship between SEP amplitude and tactile reactivity needs to be further examined (see, e.g., work of Casio et al. 2015) to more fully understand the implications of the processing differences we observe.
GRANTS
Primary funding for this work was provided by National Institute of Mental Health Grant R01MH085322 (to S. Molholm and J. J. Foxe). The Human Clinical Phenotyping Core, where the children enrolled in this study were clinically evaluated, is a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center, which is funded through Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P30HD071593. N. Uppal received additional support from the Maternal & Child Health Interdisciplinary Leadership Education in Neurodevelopmental & Related Disorders Grant 31529F.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.F., J.S.B., and S.M. conception and design of research; J.S.B. and F.A. performed experiments; N.U. and J.S.B. analyzed data; N.U., J.J.F., and S.M. interpreted results of experiments; J.S.B. prepared figures; N.U. drafted manuscript; J.J.F. and S.M. edited and revised manuscript; N.U., J.J.F., J.S.B., F.A., and S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Gregory Peters for tremendous help with data collection, Drs. Jeremy Murphy and Gizely Andrade for guidance in data analysis and interpretation, and Dr. Adam Snyder for assistance in building the vibrotactile stimulator. Finally, we are incredibly grateful for the time and effort of the participants and their families, without whom this study would not have been possible.
Footnotes
The different number of trials for fast vs. slow conditions was not expected to impact analysis of adaptation effects. First, the minimum number of trials is quite high at ∼400 and should therefore already have excellent signal-to-noise ratio (SNR). Indeed, SNR analysis performed for the 250- and 1,050-ms conditions showed that SNR was >10 (a very good value) in both cases and for all age groups. SNR did not differ significantly between age groups, and if anything, much as one would expect, tended to be slightly larger for the 1,050-ms condition for which there were fewer trials in the average, but bigger, signal. We further tested whether the lesser number of trials might impact the averaged response comparing the average of the first 400 trials with the average of the full set of trials, for a random subset of 20 participants, using data from the 250-ms ISI condition. The two waveforms were near identical, and any minimal differences did not exceed the standard errors of the responses. SNR was measured from the global field power (GFP) of the 1,050- and 250-ms ISI conditions of the 64 scalp channels for each participant. The background noise was estimated from the prestimulus period of the GFP (−35 to 0 ms). To represent the signal in the SEP, GFP was taken from 45 to 80 ms. The squared signal was divided by squared noise and converted to decibels to be made scale invariant. The resulting SNRs were compared between groups using an independent sample Kruskal-Wallis test. These differences were not significant between groups for either the 250-ms ISI condition (P = 0.130) or the 1,050-ms ISI condition (P = 0.117). A Wilcoxon signed-rank test found that, much as would be expected because of the larger amplitude response of the 1,050-ms condition, SNR approached or was significantly greater for the 1,050-ms vs. the 250-ms ISI condition for the younger (P = 0.055) and older groups (P < 0.005). In contrast, SNR did not differ significantly between these ISI conditions for the middle group (P = 0.900).
The peak latency of the initial cortical response tended to increase with age (see e.g., Figs. 2 and 6), much as one would expect because of increased distance to the cortex with a corresponding increase in height with age (Desmedt et al. 1976). However, these differences were minimal: 6 ms at most, and typically 3 ms or less. These latency differences due to age should not impact our results, because in our analytic approach the peak amplitude was detected within a temporal window that encompassed all of the groups' peak latencies and that was cushioned on either side to allow for variability in the latency of the peak amplitude across participants.
REFERENCES
- Ahn RR, Miller LJ, Milberger S, McIntosh DN. Prevalence of parents' perceptions of sensory processing disorders among kindergarten children. Am J Occup Ther 58: 287–293, 2004. [DOI] [PubMed] [Google Scholar]
- Allison T, Hume AL, Wood CC, Goff WR. Developmental and aging changes in somatosensory, auditory and visual evoked potentials. Electroencephalogr Clin Neurophysiol 58: 14–24, 1984. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC. The relationship between human long-latency somatosensory evoked potentials recorded from the cortical surface and from the scalp. Electroencephalogr Clin Neurophysiol 84: 301–314, 1992. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62: 694–710, 1989a. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 62: 711–722, 1989b. [DOI] [PubMed] [Google Scholar]
- Allison T, Wood CC, McCarthy G, Hume AL, Goff WR. Short-latency somatosensory evoked potentials in man, monkey, cat, and rat: comparative latency analysis. Adv Neurol 32: 303–311, 1982. [PubMed] [Google Scholar]
- Andrade GN, Butler JS, Mercier MR, Molholm S, Foxe JJ. Spatio-temporal dynamics of adaptation in the human visual system: a high-density electrical mapping study. Eur J Neurosci 41: 925–939, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Yielder P, Murphy B. Do pursuit movement tasks lead to differential changes in early somatosensory evoked potentials related to motor learning compared with typing tasks? J Neurophysiol 113: 1156–1164, 2015. [DOI] [PubMed] [Google Scholar]
- Angel RW, Quick WM, Boylls CC, Weinrich M, Rodnitzky RL. Decrement of somatosensory evoked potentials during repetitive stimulation. Electroencephalogr Clin Neurophysiol 60: 335–342, 1985. [DOI] [PubMed] [Google Scholar]
- Bast T, Wright T, Boor R, Harting I, Feneberg R, Rupp A, Hoechstetter K, Rating D, Baumgartner U. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin Neurophysiol 118: 1721–1735, 2007. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Carter AS, Briggs-Gowan MJ. Sensory over-responsivity in elementary school: prevalence and social-emotional correlates. J Abnorm Child Psychol 37: 705–716, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Russo NN, Altschuler TS, Gomes H, Molholm S. The development of audiovisual multisensory integration across childhood and early adolescence: a high-density electrical mapping study. Cereb Cortex 21: 1042–1055, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SM, Villamayor A, Borich MR, Boyd LA. Exploring the specific time course of interhemispheric inhibition between the human primary sensory cortices. J Neurophysiol 112: 1470–1476, 2014. [DOI] [PubMed] [Google Scholar]
- Broughton R, Rasmussen T, Branch C. Scalp and direct cortical recordings of somatosensory evoked potentials in man (circa 1967). Can J Psychol 35: 136–158, 1981. [DOI] [PubMed] [Google Scholar]
- Butler JS, Foxe JJ, Fiebelkorn IC, Mercier MR, Molholm S. Multisensory representation of frequency across audition and touch: high density electrical mapping reveals early sensory-perceptual coupling. J Neurosci 32: 15338–15344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AS, Ben-Sasson A, Briggs-Gowan MJ. Sensory over-responsivity, psychopathology, and family impairment in school-aged children. J Am Acad Child Adolesc Psychiatry 50: 1210–1219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Gu C, Schauder KB, Key AP, Yoder P. Somatosensory event-related potentials and association with tactile behavioral responsiveness patterns in children with ASD. Brain Topogr 28: 895–903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol 15: 239–244, 2005. [DOI] [PubMed] [Google Scholar]
- Davies PL, Chang WP, Gavin WJ. Maturation of sensory gating performance in children with and without sensory processing disorders. Int J Psychophysiol 72: 187–197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis P, Molholm S, Shpaner M, Ritter W, Foxe JJ. Right hemispheric contributions to fine auditory temporal discriminations: high-density electrical mapping of the duration mismatch negativity (MMN). Front Integr Neurosci 3: 5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis L, Spierer L, Clarke S, Murray MM. Getting in touch: segregated somatosensory what and where pathways in humans revealed by electrical neuroimaging. Neuroimage 37: 890–903, 2007. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Brunko E, Debecker J. Maturation of the somatosensory evoked potentials in normal infants and children, with special reference to the early N1 component. Electroencephalogr Clin Neurophysiol 40: 43–58, 1976. [DOI] [PubMed] [Google Scholar]
- Dias EC, Foxe JJ, Javitt DC. Changing plans: a high density electrical mapping study of cortical control. Cereb Cortex 13: 701–715, 2003. [DOI] [PubMed] [Google Scholar]
- Dunn W, Westman K. The sensory profile: the performance of a national sample of children without disabilities. Am J Occup Ther 51: 25–34, 1997. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Beck EC. The effects of maturation and aging on the wave form of visually evoked potentials. Electroencephalogr Clin Neurophysiol 26: 2–11, 1969. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. [DOI] [PubMed] [Google Scholar]
- Forss N, Hari R, Salmelin R, Ahonen A, Hamalainen M, Kajola M, Knuutila J, Simola J. Activation of the human posterior parietal cortex by median nerve stimulation. Exp Brain Res 99: 309–315, 1994. [DOI] [PubMed] [Google Scholar]
- Forss N, Hietanen M, Salonen O, Hari R. Modified activation of somatosensory cortical network in patients with right-hemisphere stroke. Brain 122: 1889–1899, 1999. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Morocz IA, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Brain Res Cogn Brain Res 10: 77–83, 2000. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res 142: 139–150, 2002. [DOI] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 360: 815–836, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhstorfer H, Soveri P, Jarvilehto T. Short-term habituation of the auditory evoked response in man. Electroencephalogr Clin Neurophysiol 28: 153–161, 1970. [DOI] [PubMed] [Google Scholar]
- Girard P, Hupe JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J Neurophysiol 85: 1328–1331, 2001. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10: 14–23, 2006. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology 28: 240–244, 1991. [DOI] [PubMed] [Google Scholar]
- Hackett TA, de la Mothe LA, Camalier CR, Falchier A, Lakatos P, Kajikawa Y, Schroeder CE. Feedforward and feedback projections of caudal belt and parabelt areas of auditory cortex: refining the hierarchical model. Front Neurosci 8: 72, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Reinikainen K, Kaukoranta E, Hamalainen M, Ilmoniemi R, Penttinen A, Salminen J, Teszner D. Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalogr Clin Neurophysiol 57: 254–263, 1984. [DOI] [PubMed] [Google Scholar]
- Hupe JM, James AC, Girard P, Lomber SG, Payne BR, Bullier J. Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol 85: 134–145, 2001. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387: 167–178, 1997. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomised block and split-plot designs. J Educ Stat 1: 69–82, 1976. [Google Scholar]
- Iwamura Y. Hierarchical somatosensory processing. Curr Opin Neurobiol 8: 522–528, 1998. [DOI] [PubMed] [Google Scholar]
- Karns CM, Knight RT. Intermodal auditory, visual, and tactile attention modulates early stages of neural processing. J Cogn Neurosci 21: 669–683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekoni J, Tiihonen J, Hamalainen H. Fast decrement with stimulus repetition in ERPs generated by neuronal systems involving somatosensory SI and SII cortices: electric and magnetic evoked response recordings in humans. Int J Psychophysiol 12: 281–288, 1992. [DOI] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol 97: 3155–3164, 2007. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297: 441–470, 1990. [DOI] [PubMed] [Google Scholar]
- Lanting CP, Briley PM, Sumner CJ, Krumbholz K. Mechanisms of adaptation in human auditory cortex. J Neurophysiol 110: 973–983, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt VM, Molholm S, Gomez-Ramirez M, Foxe JJ. “What” and “where” in auditory sensory processing: a high-density electrical mapping study of distinct neural processes underlying sound object recognition and sound localization. Front Integr Neurosci 5: 23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EK, Seyal M. Generators of short latency human somatosensory-evoked potentials recorded over the spine and scalp. J Clin Neurophysiol 15: 227–234, 1998. [DOI] [PubMed] [Google Scholar]
- Loizides C, Achilleos A, Iannetti GD, Mitsis GD. Assessment of nonlinear interactions in event-related potentials elicited by stimuli presented at short interstimulus intervals using single-trial data. J Neurophysiol 113: 3623–3633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucan JN, Foxe JJ, Gomez-Ramirez M, Sathian K, Molholm S. Tactile shape discrimination recruits human lateral occipital complex during early perceptual processing. Hum Brain Mapp 31: 1813–1821, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders H. The effects of aging on the wave form of the somatosensory cortical evoked potential. Electroencephalogr Clin Neurophysiol 29: 450–460, 1970. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex 11: 335–342, 2001. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R. Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 104: 281–289, 1997. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res 6: 57–77, 1982. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Javitt DC, Foxe JJ. Multisensory visual-auditory object recognition in humans: a high-density electrical mapping study. Cereb Cortex 14: 452–465, 2004. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res 14: 115–128, 2002. [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J Neurosci 22: 5055–5073, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen P, Lauronen L, Pihko E. Development of human somatosensory cortical functions - what have we learned from magnetoencephalography: a review. Front Hum Neurosci 8: 158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: value and estimation from potential data. IEEE Trans Biomed Eng 34: 283–288, 1987. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol 72: 184–187, 1989. [DOI] [PubMed] [Google Scholar]
- Picton TW, Taylor MJ. Electrophysiological evaluation of human brain development. Dev Neuropsychol 31: 249–278, 2007. [DOI] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol 111: 1803–1811, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci 12: 718–724, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA 97: 11800–11806, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter W, Vaughan HG Jr, Costa LD. Orienting and habituation to auditory stimuli: a study of short term changes in average evoked responses. Electroencephalogr Clin Neurophysiol 25: 550–556, 1968. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci 2: 1131–1136, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royeen CR, Mu K. Stability of tactile defensiveness across cultures: European and American children's responses to the touch inventory for elementary school aged children. Occup Ther Int 10: 165–174, 2003. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Foxe JJ. Determinants and mechanisms of attentional modulation of neural processing. Front Biosci 6: D672–D684, 2001. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex 8: 575–592, 1998. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Javitt DC, Foxe JJ. Spatiotemporal dynamics of human object recognition processing: an integrated high-density electrical mapping and functional imaging study of “closure” processes. Neuroimage 29: 605–618, 2006. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, Javitt DC, Stanton PK, Foxe JJ. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc Natl Acad Sci USA 105: 4399–4404, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagass C, Schwartz M. Age, personality, and somatosensory cerebral evoked responses. Science 148: 1359–1361, 1965. [DOI] [PubMed] [Google Scholar]
- Shipley T, Hyson M. Amplitude decrements in brain potentials in man evoked by repetitive auditory, visual, and intersensory stimulation. Sens Processes 1: 338–353, 1977. [PubMed] [Google Scholar]
- Tamura K, Luders H, Kuroiwa Y. Further observations on the effects of aging on the wave form of the somatosensory cortical evoked potential. Electroencephalogr Clin Neurophysiol 33: 325–327, 1972. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116–123, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrillo RT. Age related changes in the sensitivity to vibration. J Gerontol 35: 185–193, 1980. [DOI] [PubMed] [Google Scholar]
- Wang AL, Mouraux A, Liang M, Iannetti GD. The enhancement of the N1 wave elicited by sensory stimuli presented at very short inter-stimulus intervals is a general feature across sensory systems. PLoS One 3: e3929, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol 17: 423–429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA. Evolving concepts of sensory adaptation. F1000 Biol Rep 4: 21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Psychological, 1999. [Google Scholar]
- Wood CC, Cohen D, Cuffin BN, Yarita M, Allison T. Electrical sources in human somatosensory cortex: identification by combined magnetic and potential recordings. Science 227: 1051–1053, 1985. [DOI] [PubMed] [Google Scholar]