Abstract
Olanzapine, an atypical antipsychotic, is widely prescribed for the treatment of schizophrenia and bipolar disorder despite causing undesirable metabolic side effects. A variety of mechanisms and brain sites have been proposed as contributors to the side effects; however, the role of the dorsal motor nucleus of the vagus nerve (DMV), which plays a crucial role in the regulation of subdiaphragmatic organs and thus governs energy and glucose homeostasis, is largely unknown. Identifying the effect of olanzapine on the excitability of DMV neurons in both sexes is thus crucial to understanding possible underlying mechanisms. Whole cell patch-clamp electrophysiological recordings were conducted in stomach- and liver-related DMV neurons identified with retrograde viral tracers and in random DMV neurons. The effect of olanzapine on the neuronal excitability of DMV neurons both in male and female mice was established. Our data demonstrate that olanzapine hyperpolarizes the DMV neurons in both sexes and this effect is reversible. The hyperpolarization is associated with decreased firing rate and input resistance. Olanzapine also decreases the excitability of a subset of stomach- and liver-related DMV neurons. Our study demonstrates that olanzapine has a powerful effect on DMV neurons in both sexes, indicating its ability to reduce vagal output to the subdiaphragmatic organs, which likely contributes to the metabolic side effects observed in both humans and experimental models. These findings suggest that the metabolic side effects of olanzapine may partially originate in the DMV.
Keywords: olanzapine, dorsal motor nucleus of vagus nerve, patch clamp, energy homeostasis, glucose homeostasis
olanzapine, an atypical antipsychotic, alleviates symptoms of schizophrenia and bipolar disorder while producing fewer extrapyramidal symptoms compared with first-generation antipsychotics (Leucht et al. 2013). Despite its positive impact on mental health, olanzapine has been associated with weight gain and dysregulated glucose homeostasis (Coccurello and Moles 2010; Lambert et al. 2006; Leucht et al. 2013; Ramankutty 2002; Weston-Green et al. 2011, 2012b). Initially, olanzapine-dependent weight gain and hyperglycemia, observed both in humans and in animal models, were believed to be associated with each other; however, recent studies suggest that glucose dysregulation occurs both in the presence and in the absence of weight gain, indicating a possible independent mechanism of action (Boyda et al. 2010b; Deng 2013; Houseknecht et al. 2007; Koller and Doraiswamy 2002; Ramankutty 2002). The brain-periphery disconnection hypothesis, which focuses on forebrain mechanisms, has been proposed as one of the underlying mechanisms of the metabolic side effects (Coccurello and Moles 2010). However, the contribution of autonomic brain stem nuclei, especially the dorsal motor nucleus of the vagus nerve (DMV), which projects to the majority of subdiaphragmatic organs and thus regulates feeding, gastrointestinal functions, energy, and glucose homeostasis (Berthoud 2008), has been mostly unknown.
The central nervous system, via the autonomic nervous system, is largely involved in the control of homeostatic processes, including food intake, energy homeostasis, and glucose metabolism (Carnethon et al. 2003; Gao et al. 2012; Sandoval et al. 2008; Zsombok and Smith 2009). The dorsal vagal complex (DVC), located in the hindbrain, consists of the nucleus of the solitary tract (NTS), the DMV, and the area postrema. The DVC has a pivotal role in energy and glucose homeostasis by integrating information from direct neural projections and from signals relevant to food intake and metabolic status (Berthoud 2002; Grill et al. 2002; Williams et al. 2007). After integration of the diverse signals, the DVC modulates the efferent motor vagal output to the subdiaphragmatic organs and plays a crucial role in determining gastric motility and acid secretion (Cruz et al. 2007; Ferreira et al. 2001; Krowicki et al. 2002; Pagani et al. 1985; Rogers et al. 1999; Williford et al. 1981; Zhang et al. 2006), insulin secretion (Ionescu et al. 1983; Mussa and Verberne 2008), and hepatic glucose production (Pocai et al. 2005). A variety of findings also demonstrated that the DVC plays an important role in olanzapine-related metabolic side effects. Quantitative autoradiography studies revealed that both short- and long-term treatment with olanzapine lead to decreased binding density of muscarinic M2 (Deng et al. 2007) and cannabinoid (Weston-Green et al. 2008) receptors, which led to the postulation that olanzapine likely modulates neurotransmission in the DVC (Deng et al. 2007; Weston-Green et al. 2008, 2012a). Moreover, AMP-activated protein kinase (AMPK) activation in the DVC was suggested to be associated with olanzapine-induced antagonistic effect on the histamine H1 receptors, which could contribute to the weight gain (He et al. 2014). Previous studies demonstrated that activation of NMDA receptors in the DVC lowers hepatic glucose production (Lam et al. 2010), reduced vagal tone to the pancreas suppresses insulin secretion (Frohman et al. 1967), and reduced excitability of DMV neurons due to perinatal high-fat diet exists in the absence of obesity, suggesting an altered vago-vagal reflex preceding the development of obesity (Bhagat et al. 2015). Therefore, we hypothesized that olanzapine reduces the activity of DMV neurons. Whole cell patch-clamp recordings were conducted to determine the effect of olanzapine on the neuronal excitability of DMV neurons in both sexes. In addition, the olanzapine-dependent modulation of stomach- and liver-related DMV neurons was also investigated. Our data demonstrate that application of olanzapine decreases the excitability of DMV neurons including subsets of liver- and stomach-related neurons. These observations suggest that olanzapine is able to directly modulate the activity of DMV neurons and thus has the ability to alter vagal output to the subdiaphragmatic organs, which in turn may have a profound effect on gastric function and glucose homeostasis.
MATERIALS AND METHODS
Experiments were performed on male and female C57BL/6J mice (8–18 wk old; Jackson Laboratory, Bar Harbor, ME) housed in a vivarium with food and water available ad libitum. Experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Tulane University's Institutional Animal Care and Use Committee.
Pseudorabies injection.
Retrogradely transported viral tracers expressing enhanced green (EGFP) or red (RFP) fluorescent protein [pseudorabies virus 152 (PRV-152), EGFP; PRV-614, RFP] were used to identify stomach- and liver-related neurons. Under anesthesia, the viscera was exposed and PRVs were injected as previously described (Derbenev et al. 2004; Gao et al. 2012). The animals were maintained in a biosafety level 2 facility up to 72 h after injection.
Brain stem slice preparation.
Acute brain stem slices were prepared from male and female C57BL/6J mice as described previously (Zsombok et al. 2011). Briefly, under anesthesia the mice were decapitated, and the brains were rapidly removed and immersed in ice-cold (0–4°C) oxygenated artificial cerebrospinal fluid (aCSF) containing the following (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 1.5 CaCl2, and 1.3 MgCl2, pH 7.2–7.4. Transverse brain stem slices (300 μm) were made with a vibrating microtome (Vibratome Series 1000; Technical Products, St. Louis, MO). Slices were maintained in an oxygenated bath at 34–36°C for at least 1 h before experiments were performed. Slices were then transferred to a recording chamber mounted on a fixed stage under an upright microscope, and the recordings were conducted at 34–36°C.
Whole cell patch-clamp recordings.
Neurons were identified under ×40 water-immersion objective with infrared illumination and differential interference contrast optics (IR-DIC). Epifluorescence was used to identify EGFP- or RFP-containing neurons and IR-DIC to target specific cells (Gao et al. 2012). The electrodes were filled with a solution containing the following (in mM): 130 K+ or Cs+ gluconate, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH or CsOH, and 2–4 Mg-ATP, with 0.2% biocytin, pH 7.2–7.4. Electrophysiological signals were recorded with an Axoclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and acquired by pCLAMP (Molecular Devices). Action potentials (APs) were examined at a holding current of 0 pA, unless specified otherwise. Tetrodotoxin (TTX; 1 μM) was used to block APs. Olanzapine (0.1–100 μM) was dissolved in ethanol and diluted in aCSF (final concentration of ethanol <0.1% by volume). All drugs were obtained from Tocris Bioscience (R&D Systems, Minneapolis, MN).
Data analysis.
Recordings were analyzed with pCLAMP 10 software (Molecular Devices). The effects of drugs across neuron groups were analyzed with a paired two-tailed Student's t-test or between different groups with an unpaired two-tailed Student's t-test. Values are expressed as means ± SE. IC50 values were calculated with GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS
Olanzapine hyperpolarizes DMV neurons.
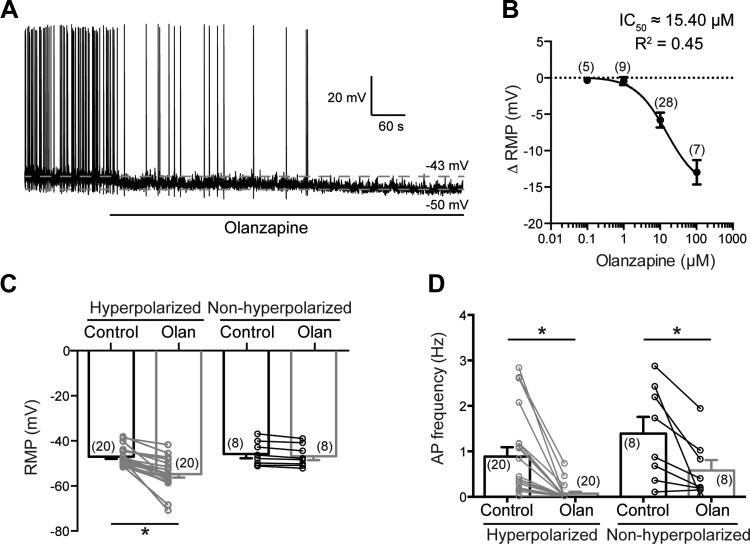
To reveal the effect of olanzapine on the excitability of DMV neurons, whole cell patch-clamp recordings in current-clamp mode were conducted without additional current injection. Previous studies used olanzapine in a variety of doses (Coccurello and Moles 2010), suggesting that olanzapine may alter neuronal excitability in a concentration-dependent manner. Therefore, we conducted experiments with olanzapine from 0.1 μM to 100 μM to determine the IC50 in DMV neurons. Our data demonstrate that olanzapine alters the resting membrane potential of DMV neurons in a concentration-dependent manner with an IC50 of 15.40 μM (Fig. 1B). Therefore, in the rest of the experiments, 10 μM olanzapine was used.
Fig. 1.
Olanzapine decreases the excitability of dorsal motor nucleus of the vagus nerve (DMV) neurons. A: continuous recording demonstrates that application of olanzapine (10 μM) hyperpolarizes DMV neurons. B: concentration-dependent effect of olanzapine on resting membrane potential (RMP) of DMV neurons. C: combined data showing effect of olanzapine on RMP. Neurons were considered hyperpolarized if RMP changed by >2.5 mV. D: combined data demonstrating effect of olanzapine on action potential (AP) frequency of DMV neurons. *Significance (P < 0.05). Olan, olanzapine.
Bath application of olanzapine (10 μM) reduced the overall resting membrane potential of the recorded DMV neurons (Table 1). The average resting membrane potential for all recorded DMV neurons in male mice was −46.69 ± 0.84 mV (range from −37.04 mV to −51.53 mV, n = 28) before and −52.23 ± 1.37 mV (range −39.08 mV to −70.74 mV, n = 28) after olanzapine application (P < 0.05). Neurons with a change in membrane potential of ≥2.5 mV were considered responders, and this value was used to separate the recorded neurons. Our data revealed that ∼70% of the recorded neurons were hyperpolarized (≥2.5 mV). The average resting membrane potential of DMV neurons was −47.03 ± 0.93 mV (range from −38.01 mV to −51.53 mV, n = 20) before and −54.80 ± 1.53 mV (range from −41.65 mV to −70.74 mV, n = 20) after olanzapine application (P < 0.05; hyperpolarized group). The resting membrane potential of the rest of the recorded DMV neurons was −45.85 ± 1.87 mV (range from −37.04 mV to −51.30 mV, n = 8) before and −46.85 ± 1.71 mV (range from −39.08 mV to −52.33 mV, n = 8) after olanzapine application (Fig. 1C; Table 1).
Table 1.
Electrophysiological properties of DMV neurons after olanzapine (10 μM) application
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| n Cells | Control | Olanzapine | n Cells | Control | Olanzapine | |
| AP frequency, Hz | ||||||
| All cells | 28 | 1.03 ± 0.18 | 0.22 ± 0.08* | 9 | 2.28 ± 0.35 | 0.07 ± 0.04* |
| Hyperpolarized | 20 | 0.88 ± 0.21 | 0.07 ± 0.04* | 7 | 2.54 ± 0.35 | 0.01 ± 0.01* |
| Nonhyperpolarized | 8 | 1.39 ± 0.37 | 0.58 ± 0.23* | 2 | 1.36 ± 0.83 | 0.29 ± 0.02 |
| RMP, mV | ||||||
| All cells | 28 | −46.69 ± 0.84 | −52.23 ± 1.37* | 9 | −49.00 ± 0.64 | −60.27 ± 2.78* |
| Hyperpolarized | 20 | −47.03 ± 0.93 | −54.80 ± 1.53* | 7 | −49.22 ± 0.81 | −63.23 ± 2.59* |
| Nonhyperpolarized | 8 | −45.85 ± 1.87 | −46.85 ± 1.71 | 2 | −48.24 ± 0.14 | −49.95 ± 0.57 |
| RMP in TTX, mV | ||||||
| All cells | 11 | −47.45 ± 2.07 | −55.91 ± 3.44* | 10 | −42.12 ± 1.97 | −57.63 ± 3.42* |
| Hyperpolarized | 8 | −47.02 ± 2.79 | −58.05 ± 4.47* | 7 | −41.33 ± 2.72 | −63.12 ± 2.79* |
| Nonhyperpolarized | 3 | −48.61 ± 2.25 | −50.21 ± 2.96 | 3 | −43.97 ± 2.03 | −44.83 ± 2.87 |
Values are means ± SE. DMV, dorsal motor nucleus of vagus nerve; AP, action potential; RMP, resting membrane potential; TTX, tetrodotoxin.
Significance (P < 0.05).
A similar response was observed in female mice. Olanzapine caused hyperpolarization in seven of nine recorded DMV neurons (Table 1). The resting membrane potential was −49.22 ± 0.81 mV (range from −44.83 mV to −51.14 mV, n = 7) before and −63.23 ± 2.59 mV (range from −53.58 mV to −70.72 mV, n = 7) after olanzapine application (P < 0.05; hyperpolarized group) (Table 1).
There was no significant difference in the resting membrane potentials between males and females (−47.03 ± 0.93 mV vs. −49.22 ± 0.81 mV, hyperpolarized groups); however, the average change following olanzapine application showed a greater hyperpolarization trend in females compared with males [14.01 ± 2.62 mV (range from 4.64 mV to 20.86 mV, n = 7) vs. 7.77 ± 1.17 mV (range from 2.63 mV to 22.37 mV, n = 20)].
Within the DVC, the NTS also plays a major role in transferring metabolic signals, and we conducted recordings to determine the effect of olanzapine application on NTS neurons. The recordings were conducted in the caudal NTS at the level of area postrema from nonidentified neurons (Glatzer et al. 2003). The average resting membrane potential of NTS neurons was −52.17 ± 3.78 mV (range from −45.4 mV to −65.1 mV, n = 5) before and −62.37 ± 3.16 mV (range from −54.8 mV to −66.9 mV, n = 5) after bath application of olanzapine (P < 0.05) (data not shown). The magnitude of hyperpolarization ranged from 6.3 to 16.3 mV, which was similar to the observed olanzapine effect in the DMV, suggesting that olanzapine is able to alter neuronal activity in both the DMV and NTS.
Olanzapine reduces excitability of DMV neurons.
The hyperpolarization was associated with a decrease of firing rate of DMV neurons in both male and female mice (Table 1). The recorded DMV neurons, with the exception of one DMV neuron from a male mouse, fired spontaneously in both sexes. The average firing rate of the hyperpolarized group of DMV neurons in male mice was 0.88 ± 0.21 Hz (range from 0 Hz to 2.82 Hz, n = 20) before and 0.07 ± 0.04 Hz (range from 0 Hz to 0.72 Hz, n = 20) after olanzapine application (hyperpolarized group, P < 0.05) (Fig. 1D). The spontaneous firing was abolished in 16 of 19 spontaneously firing neurons, demonstrating that olanzapine significantly reduces neuronal activity in the DMV (Fig. 1D; Table 1). Interestingly, after olanzapine administration, the firing rate was also decreased in the nonhyperpolarized group of DMV neurons (1.39 ± 0.37 Hz vs. 0.58 ± 0.23 Hz, n = 8, P < 0.05) (Fig. 1D), but seven of eight DMV neurons still had spontaneous firing. In female mice, olanzapine reduced the firing rate of DMV neurons to the same extent as in male mice (Table 1).
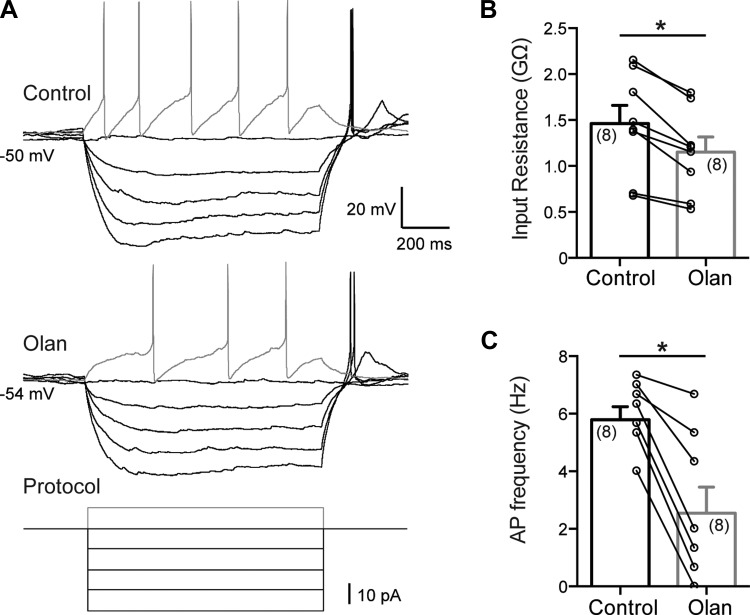
Recordings were then conducted to determine the input resistance and the firing rate after injection of current pulses before and after olanzapine application. Input resistance is a reflection of all ionic current passing through the cell membrane, and changes in input resistance indicate the opening or closing of channels. The recordings were conducted in current-clamp mode, and a series of current steps were applied to DMV neurons. The input resistance of DMV neurons in male mice was 1.46 ± 0.20 GΩ (range from 0.68 GΩ to 2.15 GΩ, n = 8) before olanzapine (10 μM) application and 1.15 ± 0.16 GΩ (range from 0.53 GΩ to 1.79 GΩ) after olanzapine application, indicating that olanzapine decreases input resistance of DMV neurons (P < 0.05) (Fig. 2, A and B). The firing rate of DMV neurons after injection of 10-pA depolarizing current pulses was used to further confirm that olanzapine (10 μM) decreases neuronal excitability. The firing rate was 5.79 ± 0.45 Hz (range from 4.00 Hz to 7.33 Hz, n = 8) before and 2.54 ± 0.91 Hz (range from 0 Hz to 6.66 Hz, n = 8) after bath application of olanzapine (P < 0.05) (Fig. 2, A and C). Together, these findings demonstrate that bath application of olanzapine results in hyperpolarization of DMV neurons, which reduces neuronal excitability independently of sex.
Fig. 2.
Olanzapine reduces input resistance and action potential firing rate of DMV neurons. A: representative current-clamp recordings showing firing rate of DMV neurons after injection of current steps before (top) and after (middle) olanzapine administration. Bottom: step protocol used. B: combined data demonstrating decreased input resistance after bath application of olanzapine. C: combined data illustrating decreased firing rate of DMV neurons in response to a 10-pA depolarizing current pulse. *Significance (P < 0.05).
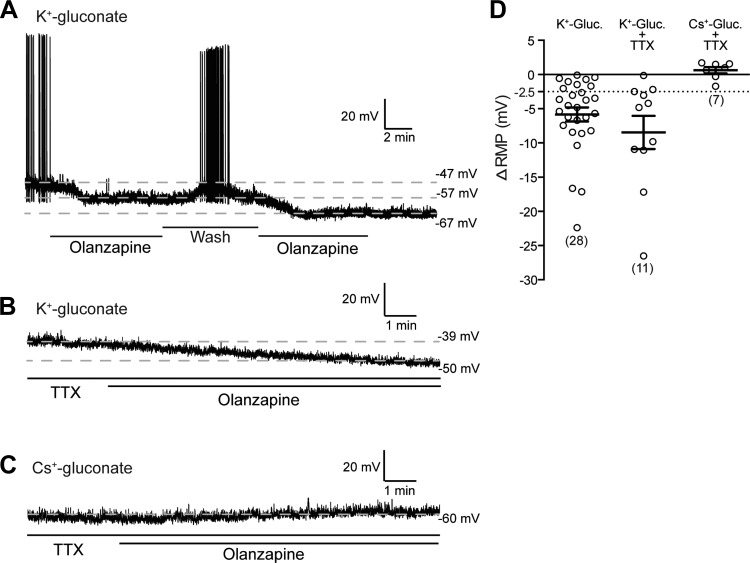
The experiments above demonstrated that olanzapine decreases the input resistance, hyperpolarizes the neurons, and reduces, in most cases abolishes, the firing rate of the majority of DMV neurons. Next, we revealed the reversibility of the olanzapine-dependent hyperpolarization of DMV neurons. In these experiments multiple olanzapine applications were separated with washout periods. Our data demonstrate that the hyperpolarization caused by acute olanzapine application was reversible (n = 6) (Fig. 3A).
Fig. 3.
Effect of olanzapine is reversible and network independent. A: continuous recording demonstrates that hyperpolarization caused by olanzapine (10 μM) was reversible and repeatable. B: olanzapine caused hyperpolarization in the presence of tetrodotoxin (TTX, 1 μM), indicating direct effect on DMV neurons. C: olanzapine did not cause hyperpolarization when recordings were conducted with Cs+ gluconate in the pipette. D: summarized data illustrating changes of RMP in the presence and absence of TTX.
Experiments were then conducted in the presence of TTX (1 μM) to block AP-dependent neurotransmitter release and to exclude network effect. In this condition, olanzapine was still able to hyperpolarize the majority of recorded neurons (8 of 11 in males and 7 of 10 in females) (Table 1). The resting membrane potential in the hyperpolarized group was −47.02 ± 2.79 mV (range from −30.67 mV to −53.53 mV, n = 8) before and −58.05 ± 4.47 mV (range from −34.88 mV to −77.14 mV) after olanzapine application in male mice (P < 0.05) (Table 1). The average hyperpolarization caused by olanzapine was 11.03 ± 2.83 mV (range from 3.04 mV to 27.1 mV) (Fig. 3, B and C). A similar effect was observed in female mice (Table 1). These data demonstrate that olanzapine has a direct effect on DMV neurons in both males and females.
In addition, to determine the contribution of K+ channels to olanzapine-induced hyperpolarization we conducted recordings with Cs+ gluconate in the pipette to block K+ channels. Olanzapine did not hyperpolarize DMV neurons when Cs+ gluconate was used. In the presence of TTX the resting membrane potential was −43.21 ± 3.38 mV (range from −33.52 mV to −62.21 mV, n = 7) before and −42.58 ± 3.11 (range from −35.24 mV to −60.64 mV) after olanzapine application (P > 0.05) (Fig. 3C). These data indicate that the olanzapine-dependent hyperpolarization involves K+ conductance.
Olanzapine decreases activity of liver- and stomach-related DMV neurons.
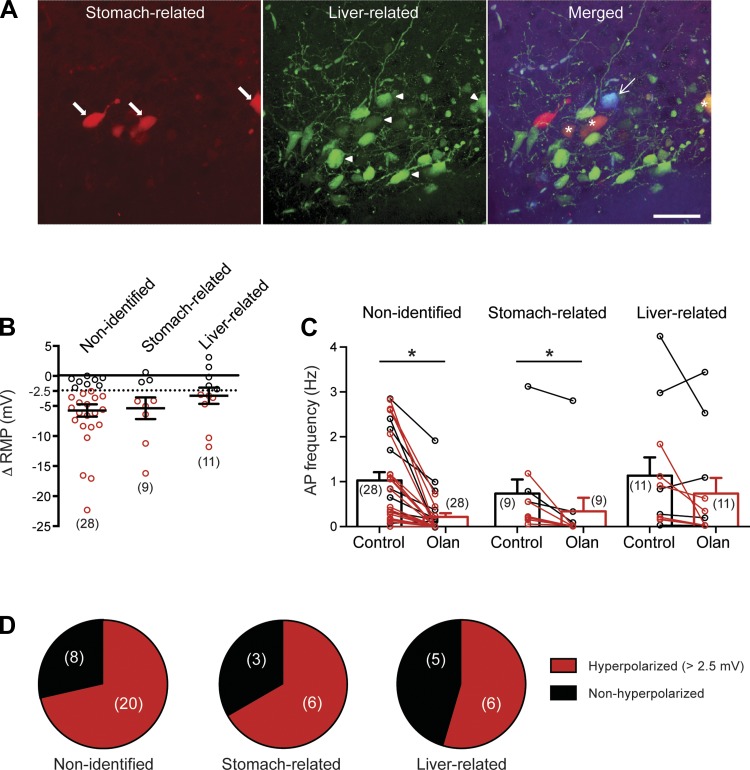
Since olanzapine treatment results in glucose dysregulation and weight gain in both humans and experimental models and DMV neurons have significant impact on glucose regulation (Lam et al. 2010) and stomach function (Ferreira et al. 2001; Pagani et al. 1985; Rogers et al. 1999; Williford et al. 1981), we investigated the effect of olanzapine on liver- and stomach-related DMV neurons. Organ-specific neurons were identified on the basis of their fluorescence after pseudorabies inoculation of the liver or the stomach as described previously (Derbenev et al. 2004; Gao et al. 2012) (Fig. 4A), and current-clamp recordings were conducted in the presence and absence of olanzapine. Recordings from liver- and stomach-related DMV neurons demonstrated a distribution of hyperpolarizing (≥2.5 mV) and nonhyperpolarizing neurons similar to that observed in nonidentified random DMV neurons (Fig. 4D).
Fig. 4.
Olanzapine effect on activity of liver- and stomach-related DMV neurons. A: retrograde transsynaptic labeling was used to identify stomach- and liver-related DMV neurons. Recordings from the organ-specific neurons were conducted ∼72 h after inoculation of the stomach and liver. Left: stomach-labeled DMV neurons identified on the basis of their red fluorescence (arrows). Center: liver-related DMV neurons identified on the basis of their green fluorescence (arrowheads). Right: merged image illustrating the recorded liver-related neuron after visualization with AMCA (arrow, blue). Stars indicate double-labeled (stomach and liver related) DMV neurons. Scale bar, 50 μm. B: summarized data illustrating RMP response of all nonidentified and stomach- and liver-related DMV neurons to olanzapine application. Red circles, hyperpolarized neurons (>2.5 mV); black circles, nonhyperpolarized DMV neurons. Average value is based on all cells. C: combined data demonstrating effect of olanzapine on action potential firing rate in all nonidentified and stomach- and liver-related DMV neurons. Red, firing rate of hyperpolarized neurons; black, firing rate of nonhyperpolarized DMV neurons. Average is based on all recorded neurons. D: in all groups similar % of DMV neurons showed hyperpolarization after olanzapine application. *Significance (P < 0.05).
The average resting membrane potential and the AP firing of the recorded stomach- and liver-related DMV neurons for all groups are shown in Table 2. The average resting membrane potential of stomach-related DMV neurons in the hyperpolarized group was −43.31 ± 1.33 mV (range from −39.04 mV to −47.86 mV, n = 6) before and −51.25 ± 1.12 mV (range from −49.49 mV to −56.55 mV, n = 6) after olanzapine application (P < 0.05). Hyperpolarization (>2.5 mV) was associated with diminished firing of stomach-related DMV neurons (0.39 ± 0.17 Hz vs. 0 ± 0 Hz, n = 6) (Fig. 4C). The remaining stomach-related DMV neurons were not hyperpolarized (−44.56 ± 0.85 mV vs. −45.02 ± 1.19 mV, n = 3).
Table 2.
Electrophysiological properties of stomach- and liver-related DMV neurons after olanzapine application
| Stomach-Related Neurons |
Liver-Related Neurons |
|||||
|---|---|---|---|---|---|---|
| n Cells | Control | Olanzapine | n Cells | Control | Olanzapine | |
| AP frequency, Hz | ||||||
| All cells | 9 | 0.73 ± 0.31 | 0.34 ± 0.30* | 11 | 1.13 ± 0.41 | 0.73 ± 0.35 |
| Hyperpolarized | 6 | 0.39 ± 0.17 | 0 ± 0 | 6 | 0.71 ± 0.28 | 0.15 ± 0.10 |
| Nonhyperpolarized | 3 | 1.42 ± 0.82 | 1.01 ± 0.86 | 5 | 1.65 ± 0.82 | 1.43 ± 0.66 |
| RMP, mV | ||||||
| All cells | 9 | −44.42 ± 1.07 | −49.55 ± 1.23* | 11 | −47.44 ± 1.41 | −50.82 ± 2.07* |
| Hyperpolarized | 6 | −43.31 ± 1.33 | −51.25 ± 1.12* | 6 | −47.83 ± 2.29 | −54.04 ± 3.03* |
| Nonhyperpolarized | 3 | −44.56 ± 0.85 | −45.02 ± 1.19 | 5 | −46.98 ± 1.73 | −46.95 ± 1.74 |
Values are means ± SE.
Significance (P < 0.05).
In liver-related DMV neurons, 6 of 11 recorded DMV neurons were hyperpolarized (>2.5 mV) after olanzapine (10 μM) application (Fig. 4). In the hyperpolarized group the resting membrane potential was altered from −47.83 ± 2.29 mV (range from −41.27 mV to −53.75 mV, n = 6) to −54.04 ± 3.03 mV (range from −44.44 mV to −64.15 mV, n = 6) (P < 0.05). Hyperpolarization was associated with a decreasing trend in firing rate of liver-related DMV neurons (0.71 ± 0.28 Hz vs. 0.15 ± 0.10 Hz, n = 6) (P = 0.09) (Fig. 4C). The remaining liver-related DMV neurons were not hyperpolarized (−46.98 ± 1.73 mV vs. −46.95 ± 1.74 mV, n = 5) (P > 0.05).
The magnitude of hyperpolarization in the responder neurons was similar in all three groups: 7.77 ± 1.17 mV (range from 2.63 mV to 22.37 mV, n = 20) in nonidentified, 7.94 ± 1.98 mV (range from 4.19 mV to 16.27 mV, n = 6) in stomach-related, and 6.22 ± 1.58 (range from 3.17 mV to 11.87 mV, n = 6) in liver-related DMV neurons of male mice. Together, these data demonstrate that olanzapine hyperpolarizes and decreases the excitability of a subset of both liver- and stomach-related DMV neurons.
DISCUSSION
Our study demonstrates that olanzapine has a powerful effect on DMV neurons, indicating its ability to alter vagal output to the subdiaphragmatic organs, which likely contributes to the metabolic side effects observed in humans and experimental models. The following novel findings emerged from our study: 1) olanzapine hyperpolarized the majority of DMV neurons in both sexes; 2) the hyperpolarization was associated with decreased firing rate and decreased input resistance of DMV neurons; 3) the effect was K+ dependent, network independent, and reversible; and 4) olanzapine decreased the excitability of a subset of liver- and stomach-related DMV neurons. These findings suggest that the metabolic changes observed in humans and experimental models after olanzapine treatment may partially originate in the DMV.
Atypical antipsychotic medications including olanzapine have undesirable, well-described side effects involving weight gain, dyslipidemia, and impaired glucose homeostasis leading to the development of type 2 diabetes mellitus (Barnard et al. 2013; Chiu et al. 2010; Coccurello and Moles 2010; Teff et al. 2013). The olanzapine-dependent weight gain and glycemic disturbances were believed to be associated with each other; however, a number of studies suggest that glucose dysregulation could occur independently of weight gain (Boyda et al. 2010b; Houseknecht et al. 2007; Koller and Doraiswamy 2002; Ramankutty 2002). These observations raise questions about the site of the mechanisms of action. The majority of previous investigations focused on the forebrain to determine which brain areas (e.g., hypothalamic nuclei), receptors (e.g., serotonin), and neuropeptides [e.g., neuropeptide Y (NPY), melanin-concentrating hormone (MCH)] are involved in the development of metabolic side effects (Huang et al. 2006a, 2006b), and these findings were reviewed previously in detail (Coccurello and Moles 2010; Miyamoto et al. 2005).
Our findings demonstrated that olanzapine decreases the excitability of DMV neurons and showed that acute olanzapine administration reduces the firing rate of vagal motor neurons. Our data revealed that olanzapine had an IC50 of ∼15.40 μM when its effect on the resting membrane potential was investigated in DMV neurons. The exact concentration of any type of drugs in the brain is always an intriguing and difficult question. In the case of olanzapine, Aravagiri and coworkers (Aravagiri et al. 1999) investigated olanzapine levels in plasma and a variety of tissues including the brain. Olanzapine was measured in rat plasma and tissues after a single oral dose of 6 mg/kg at multiple time points. Olanzapine levels were measurable in plasma up to 12 h and in the rat brain up to 48 h. Olanzapine peak concentration was ∼178 ng/ml in plasma and ∼1,162 ng/g in the brain. There was a strong relationship between plasma and tissue levels even after repeated administration. On average, the brain levels were ∼8–10 times higher compared with plasma levels, and the authors suggest that plasma concentration likely predicts concentrations in brain and other tissues (Aravagiri et al. 1999). A study by Pouzet et al. (2003) also demonstrated steady-state serum olanzapine levels in rats after 5 and 20 mg·kg−1·day−1 oral olanzapine administration. The authors found that serum olanzapine was ∼200 ng/ml in the 5 mg·kg−1·day−1 treated rats, which was similar to the previous study (Aravagiri et al. 1999), while it reached 800–900 ng/ml maximum levels in the 20 mg·kg−1·day−1 treated group. Based on the Aravagiri study, brain olanzapine levels could be ∼8–10 times higher than plasma levels. In this case, when plasma olanzapine levels are ∼200 ng/ml (∼0.6 μM) brain concentration can reach ∼1,200–2,000 ng/g (∼3–6 μM) after 5 mg·kg−1·day−1, or even higher (10 μM and above) when a higher dose of olanzapine is used and maximum serum levels are 800–1,000 ng/ml (∼3 μM serum) (Pouzet et al. 2003). These observations predict that the olanzapine level is in the micromolar range in rodent brain. We observed hyperpolarization in 2 of 9 recorded neurons after 1 μM and in 20 of 28 neurons after 10 μM olanzapine administration, which demonstrates that olanzapine hyperpolarizes a subset of DMV neurons at low micromolar range.
In patients with schizophrenia plasma olanzapine levels were also determined. A study by Aravagiri and coworkers (Aravagiri et al. 1997) determined mean plasma olanzapine levels at 19.9 ± 6.8 and 25.4 ± 9.7 ng/ml after 15 mg/day and 20 mg/day treatment, respectively. Perry and coworkers suggested that a plasma concentration above ∼23.2 ng/ml is a good predictor for therapeutic response following an average olanzapine treatment of 11.8 mg/day (Perry et al. 2001). A study by Olesen and Linnet (1999) found that plasma olanzapine concentrations ranged between 22 and 146 nM, and the suggested therapeutic reference range for olanzapine is between 20 and 80 ng/ml (Hiemke et al. 2011). These studies suggest that with a standard dose (≤20 mg/day) blood olanzapine concentration can reach 60–240 nM, which predicts a micromolar range in the brain. Furthermore, olanzapine is used in high and very high doses (40–100 mg/day) in treatment-resistant conditions (Batail et al. 2014; Lerner 2003; Qadri et al. 2006). The study by Batail et al. demonstrated a strong linear correlation between the daily oral dose of olanzapine and “trough olanzapine concentration” even at very high concentrations, indicating that a serum olanzapine concentration reaching ∼200 ng/ml (∼0.6 μM) may predict that brain olanzapine concentration reaches the 6–10 μM range in patients (Batail et al. 2014).
Our data also revealed that olanzapine hyperpolarizes NTS neurons. These data suggest that olanzapine has the ability to modulate brain stem neurons at the level of NTS and DMV. Altering NTS neuronal activity can modulate the excitability of DMV neurons, and we can speculate that olanzapine has the ability to alter neuronal excitability, and thus vagal outflow at more than one level. In our study we also observed that the effect of olanzapine was reversible. This is consistent with previous findings revealing that olanzapine withdrawal caused weight loss likely due to decreased food intake and feeding efficiency in rats (Goudie et al. 2002; Lian et al. 2014).
Activation of DMV neurons has been shown to decrease hepatic glucose production, and this effect was prevented by hepatic vagotomy (Lam et al. 2010). Our data revealed that olanzapine reduces the excitability of liver-related DMV neurons, and given that activation of DMV neurons decreases hepatic glucose production our data suggest that decreased activity of DMV neurons may contribute to increased hepatic glucose production. However, to confirm this scenario, further detailed investigations are required.
Olanzapine treatment is also associated with a biphasic effect on insulin secretion including an initial suppression of insulin secretion followed by overcompensation (Chiu et al. 2010). Our data revealed that acute olanzapine administration diminishes the excitability of the majority of DMV neurons, which likely includes a subset of pancreas-related DMV neurons. Since both electrical and chemical activation of the DMV increase insulin secretion (Ionescu et al. 1983; Mussa and Verberne 2008), we can speculate that the decreased excitability of DMV neurons will also result in reduced vagal tone to the pancreas, which in turn will likely suppress insulin secretion. This brain stem-based mechanism could explain the initial suppression of insulin secretion observed previously (Chiu et al. 2010); however, further studies are required to investigate the underlying details.
The DMV plays an essential role in the regulation of subdiaphragmatic organs, and various studies have revealed alteration of the vagal circuits during diet-induced obesity and high-fat diet. Furthermore, a recent study demonstrated that perinatal high-fat diet reduces the excitability of stomach-related DMV neurons and these changes occur before the development of obesity (Bhagat et al. 2015). Our data indicate that olanzapine diminishes the activity of DMV neurons including a subset of stomach-related DMV neurons, an effect similar to that described in the Bhagat study (Bhagat et al. 2015). These observations have particular importance because they indicate that attenuation of vago-vagal reflex signaling occurs before the development of weight gain and/or glucose dysregulation, and we can speculate that these changes contribute to the metabolic side effects of olanzapine treatment. Furthermore, we could assume that modulating the vagal activity would have beneficial effects on body weight. It has been observed that patients treated with vagus nerve stimulation showed weight loss without diet or exercise (Pardo et al. 2007), increased energy expenditure (Vijgen et al. 2013), and improved glucose tolerance (Huang et al. 2014). On the other hand, vagotomy also resulted in weight loss (Kral et al. 1993); however, some of the studies demonstrated conflicting results (Angrisani et al. 2009; Martin and Earle 2011; Perathoner et al. 2009). In general, these methods alter the vagal afferents and efferents and also modulate several hormonal signals (Abbott et al. 2005; le Roux et al. 2005), suggesting disruption of multiple physiological mechanisms. Therefore, additional studies are necessary to determine the exact underlying mechanisms and make the conclusion that increased or reduced vagal tone is more beneficial for the treatment of obesity and metabolic disturbances.
Our data also demonstrated that olanzapine caused hyperpolarization of DMV neurons in both sexes; however, the hyperpolarization showed a greater trend in female mice compared with male mice (14.01 ± 2.62 mV vs. 7.77 ± 1.17 mV). This potential sex difference in sensitivity of DMV neurons to olanzapine could provide a compelling explanation for why female rodents are more sensitive to antipsychotic-induced metabolic disturbances than their male counterparts (Boyda et al. 2010a; Mann et al. 2013). In agreement with the animal studies, sex differences following antipsychotic treatment are known in clinical practice. Women have higher risk for olanzapine-associated weight gain (Hakko et al. 2006), have higher plasma leptin levels (Kluge et al. 2009), and also have higher plasma drug levels (Kelly et al. 1999) compared with men. A systemic analysis of literature also revealed that women on antipsychotics are more susceptible to weight gain, obesity, and diabetes mellitus than men (Seeman 2009). The underlying mechanisms both in human and animal studies are just starting to be understood and require future detailed experiments including investigation of neuronal response in a sex-dependent manner.
Olanzapine interacts with a variety of receptors including dopaminergic, serotoninergic, and muscarinic receptors (Coccurello and Moles 2010; Miyamoto et al. 2005); however, the exact mechanism of action regarding the metabolic side effects of olanzapine is still being investigated. It has been suggested that acute olanzapine treatment disrupts glucose metabolism via hypothalamic AMPK activation (Ikegami et al. 2013) and this effect is relayed through the autonomic nervous system, likely through the sympathetic nervous system. On the other hand, a more recent study revealed time-dependent effects of olanzapine on AMPK signaling in the DVC that may suggest parasympathetic involvement (He et al. 2014). Short-term olanzapine treatment activated AMPK signaling in the DVC, whereas the activation of AMPK signaling was not detectable after long-term olanzapine treatment. The authors suggested that olanzapine prevents activation of histamine H1 receptors in the DVC contributing to AMPK activation that in turn can lead to increased food intake (He et al. 2014). This effect could also be an indirect effect caused by the hypothalamus, and it is likely that AMPK signaling in the hypothalamus and DVC are modulated through independent mechanisms (He et al. 2014). In addition, a recent study revealed that the hyperglycemia and increased hepatic glucose production caused by intragastric olanzapine administration were independent of the melanocortin system (Girault et al. 2013), which further suggests that olanzapine may modulate autonomic brain areas outside of the hypothalamus and thereby regulate glucose homeostasis. This hypothesis is supported by observation from studies by Weston-Green and coworkers, which revealed that the DVC is a possible site for olanzapine action (Weston-Green et al. 2008, 2012a). Their study demonstrated that short-term and chronic olanzapine administration decrease cannabinoid receptor binding density in the DVC, which was correlated with weight gain (Weston-Green et al. 2008). The authors proposed that treatment with antipsychotics like olanzapine may increase endogenous cannabinoid release, resulting in a compensatory decrease in cannabinoid receptor binding density, which may lead to decreased receptor expression. Another study from the same group revealed a reduction in muscarinic M2 receptor density in the DVC after short- and long-term olanzapine treatment (Deng et al. 2007). Model analysis based on occupancies determined significant correlation between histamine H1, serotonin 5-HT2C, and muscarinic receptor occupancies and antipsychotic-related weight gain and diabetes mellitus, and the authors suggest a critical role of H1 receptors (Matsui-Sakata et al. 2005).
It is noteworthy that gene analysis demonstrated that olanzapine upregulates Kcna1 gene and downregulates Kcnab1 and KChip3 genes in mouse brain after olanzapine treatment (Duncan et al. 2008). These genes are involved in regulation of potassium channels (An et al. 2000; Rettig et al. 1994). Potassium channels significantly contribute to the excitability of neurons, and our data demonstrate that acute olanzapine application hyperpolarizes DMV neurons; therefore, the involvement of potassium channels could be an intriguing underlying mechanism. In agreement with this, our data demonstrated that olanzapine-dependent hyperpolarization was observed in the presence of TTX but was absent with Cs+ in the pipette. These findings suggest that K+ conductance is involved in the hyperpolarization of DMV neurons by olanzapine. Interaction between serotonin, dopamine receptors, and potassium channels has been described previously (Albert et al. 1996; Greif et al. 1995; Kim et al. 1997; Zheng and Travagli 2007); however, further experiments are needed to determine the underlying mechanisms of olanzapine in the DMV including the interaction of olanzapine with potassium channels and the above-mentioned receptors.
Technical considerations.
To identify liver- and stomach-related DMV neurons, viral retrograde tracers (PRVs) for expression of green and red fluorescent proteins were used. The PRV spreads retrogradely; therefore, the EGFP or RFP labeling indicates liver- or stomach-related DMV neurons. The experiments were conducted ∼72 h after inoculation of the liver and stomach, and at this time point organ-specific neurons could be identified and used for electrophysiological recordings as shown in previous studies (Smith et al. 2000; Stanley et al. 2010), including ours (Gao et al. 2012). To date, inoculation with PRV remains one of the most widely used tools to study the excitability of organ-specific neurons. By using this technique, we were able to demonstrate modulation of neuronal excitability by olanzapine application, a goal that has remained elusive until now.
Conclusions.
In summary, our study demonstrates that acute olanzapine application has a powerful effect on brain stem neurons by hyperpolarizing and diminishing the excitability of the majority of vagal motor neurons including a subset of liver- and stomach-related neurons, which likely reduces the vagal output to a variety of subdiaphragmatic organs.
The vagal output plays a significant role in energy homeostasis, and reduced vagal activity is strongly associated with type 2 diabetes mellitus in humans (Carnethon et al. 2003; Liao et al. 1995). Furthermore, modulation of the DVC by hormones directly reduces feeding through the vagus nerve, suggesting that the vagal outflow is also a key regulator of satiety (Grill et al. 2002; Williams et al. 2000). Therefore, decreasing the neuronal excitability of DMV neurons, olanzapine could reduce the vagal output, potentially perturbing both glucose metabolism and energy homeostasis, indicating a brain stem-based mechanism. Taken together, besides the observed alteration in receptor binding following olanzapine administration, to date there was no direct evidence that olanzapine modulates the activity of brain stem neurons. Therefore, to the best of our knowledge, our study is the first to demonstrate that olanzapine has a direct acute effect on the vagal motor neurons.
GRANTS
Funding for this study was provided by Tulane University School of Medicine Pilot Funds and National Institutes of Health (NIH) R01 DK-099598 for A. Zsombok. The work utilized the Core facilities supported by COBRE in Hypertension (NIH P30 GM-103337). The authors express thanks for the PRV provided by the Center for Neuroanatomy with Neurotropic Viruses (NIH P40 RR-018604).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.J.A. and A.Z. conception and design of research; I.J.A., K.M., and A.Z. performed experiments; I.J.A. and K.M. analyzed data; I.J.A. interpreted results of experiments; I.J.A. and A.Z. prepared figures; I.J.A. and A.Z. edited and revised manuscript; I.J.A., K.M., and A.Z. approved final version of manuscript; A.Z. drafted manuscript.
REFERENCES
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- Albert AP, Spyer KM, Brooks PA. The effect of 5-HT and selective 5-HT receptor agonists and antagonists on rat dorsal vagal preganglionic neurones in vitro. Br J Pharmacol 119: 519–526, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403: 553–556, 2000. [DOI] [PubMed] [Google Scholar]
- Angrisani L, Cutolo PP, Ciciriello MB, Vitolo G, Persico F, Lorenzo M, Scarano P. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surg Obes Relat Dis 5: 435–438, 2009. [DOI] [PubMed] [Google Scholar]
- Aravagiri M, Ames D, Wirshing WC, Marder SR. Plasma level monitoring of olanzapine in patients with schizophrenia: determination by high-performance liquid chromatography with electrochemical detection. Ther Drug Monit 19: 307–313, 1997. [DOI] [PubMed] [Google Scholar]
- Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos 20: 369–377, 1999. [DOI] [PubMed] [Google Scholar]
- Barnard K, Peveler RC, Holt RI. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care 36: 3337–3345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batail JM, Langree B, Robert G, Bleher S, Verdier MC, Bellissant E, Millet B, Drapier D. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res 159: 411–414, 2014. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26: 393–428, 2002. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat R, Fortna SR, Browning KN. Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol 593: 285–303, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyda HN, Tse L, Procyshyn RM, Honer WG, Barr AM. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacol Sci 31: 484–497, 2010a. [DOI] [PubMed] [Google Scholar]
- Boyda HN, Tse L, Procyshyn RM, Wong D, Wu TK, Pang CC, Barr AM. A parametric study of the acute effects of antipsychotic drugs on glucose sensitivity in an animal model. Prog Neuropsychopharmacol Biol Psychiatry 34: 945–954, 2010b. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation 107: 2190–2195, 2003. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Chen CH, Chen BY, Yu SH, Lu ML. The time-dependent change of insulin secretion in schizophrenic patients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry 34: 866–870, 2010. [DOI] [PubMed] [Google Scholar]
- Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther 127: 210–251, 2010. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol 292: R291–R307, 2007. [DOI] [PubMed] [Google Scholar]
- Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am 42: 545–563, 2013. [DOI] [PubMed] [Google Scholar]
- Deng C, Weston-Green KL, Han M, Huang XF. Olanzapine treatment decreases the density of muscarinic M2 receptors in the dorsal vagal complex of rats. Prog Neuropsychopharmacol Biol Psychiatry 31: 915–920, 2007. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Stuart TC, Smith BN. Cannabinoids suppress synaptic input to neurones of the rat dorsal motor nucleus of the vagus nerve. J Physiol 559: 923–938, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CE, Chetcuti AF, Schofield PR. Coregulation of genes in the mouse brain following treatment with clozapine, haloperidol, or olanzapine implicates altered potassium channel subunit expression in the mechanism of antipsychotic drug action. Psychiatr Genet 18: 226–239, 2008. [DOI] [PubMed] [Google Scholar]
- Ferreira M Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, and Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol 536: 141–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman LA, Ezdinli EZ, Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes 16: 443–448, 1967. [DOI] [PubMed] [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61: 1381–1390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault EM, Toonen PW, Eggels L, Foppen E, Ackermans MT, la Fleur SE, Fliers E, Kalsbeek A. Olanzapine-induced changes in glucose metabolism are independent of the melanin-concentrating hormone system. Psychoneuroendocrinology 38: 2640–2646, 2013. [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Hasney CP, Bhaskaran MD, Smith BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol 464: 525–539, 2003. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Halford JC. Characterization of olanzapine-induced weight gain in rats. J Psychopharmacol 16: 291–296, 2002. [DOI] [PubMed] [Google Scholar]
- Greif GJ, Lin YJ, Liu JC, Freedman JE. Dopamine-modulated potassium channels on rat striatal neurons: specific activation and cellular expression. J Neurosci 15: 4533–4544, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002. [DOI] [PubMed] [Google Scholar]
- Hakko H, Komulainen MT, Koponen H, Saari K, Laitinen J, Jarvelin MR, Lindeman S, Longitudinal Northern Finland Birth Cohort Study. Are females at special risk of obesity if they become psychotic? The longitudinal Northern Finland 1966 Birth Cohort Study. Schizophr Res 84: 15–19, 2006. [DOI] [PubMed] [Google Scholar]
- He M, Zhang Q, Deng C, Wang H, Huang XF. Olanzapine-activated AMPK signaling in the dorsal vagal complex is attenuated by histamine H1 receptor agonist in female rats. Endocrinology 155: 4895–4904, 2014. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Muller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry 44: 195–235, 2011. [DOI] [PubMed] [Google Scholar]
- Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology 32: 289–297, 2007. [DOI] [PubMed] [Google Scholar]
- Huang F, Dong J, Kong J, Wang H, Meng H, Spaeth RB, Camhi S, Liao X, Li X, Zhai X, Li S, Zhu B, Rong P. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement Altern Med 14: 203, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Deng C, Zavitsanou K. Neuropeptide Y mRNA expression levels following chronic olanzapine, clozapine and haloperidol administration in rats. Neuropeptides 40: 213–219, 2006a. [DOI] [PubMed] [Google Scholar]
- Huang XF, Han M, Huang X, Zavitsanou K, Deng C. Olanzapine differentially affects 5-HT2A and 2C receptor mRNA expression in the rat brain. Behav Brain Res 171: 355–362, 2006b. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Ikeda H, Ishikawa Y, Ohsawa M, Ohashi T, Kai M, Kamei A, Kamei J. Olanzapine induces glucose intolerance through the activation of AMPK in the mouse hypothalamus. Eur J Pharmacol 718: 376–382, 2013. [DOI] [PubMed] [Google Scholar]
- Ionescu E, Rohner-Jeanrenaud F, Berthoud HR, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology 112: 904–910, 1983. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR, Tamminga CA. Differential olanzapine plasma concentrations by sex in a fixed-dose study. Schizophr Res 40: 101–104, 1999. [DOI] [PubMed] [Google Scholar]
- Kim KM, Nakajima S, Nakajima Y. Dopamine and GABA receptors in cultured substantia nigra neurons: correlation of electrophysiology and immunocytochemistry. Neuroscience 78: 759–769, 1997. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schuld A, Schacht A, Himmerich H, Dalal MA, Wehmeier PM, Hinze-Selch D, Kraus T, Dittmann RW, Pollmacher T. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology 34: 118–128, 2009. [DOI] [PubMed] [Google Scholar]
- Koller EA, Doraiswamy PM. Olanzapine-associated diabetes mellitus. Pharmacotherapy 22: 841–852, 2002. [DOI] [PubMed] [Google Scholar]
- Kral JG, Gortz L, Hermansson G, Wallin GS. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J Surg 17: 75–79, 1993. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Burmeister MA, Berthoud HR, Scullion RT, Fuchs K, Hornby PJ. Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol 283: G465–G472, 2002. [DOI] [PubMed] [Google Scholar]
- Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, Wang PY, Lai TY, Lam TK. Activation of N-methyl-d-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem 285: 21913–21921, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MT, Copeland LA, Sampson N, Duffy SA. New-onset type-2 diabetes associated with atypical antipsychotic medications. Prog Neuropsychopharmacol Biol Psychiatry 30: 919–923, 2006. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, Theodorou NA, Bloom SR. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 90: 4521–4524, 2005. [DOI] [PubMed] [Google Scholar]
- Lerner V. High-dose olanzapine for treatment-refractory schizophrenia. Clin Neuropharmacol 26: 58–61, 2003. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382: 951–962, 2013. [DOI] [PubMed] [Google Scholar]
- Lian J, Huang XF, Pai N, Deng C. Preventing olanzapine-induced weight gain using betahistine: a study in a rat model with chronic olanzapine treatment. PloS One 9: e104160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Cai J, Brancati FL, Folsom A, Barnes RW, Tyroler HA, Heiss G. Association of vagal tone with serum insulin, glucose, and diabetes mellitus—the ARIC Study. Diabetes Res Clin Pract 30: 211–221, 1995. [DOI] [PubMed] [Google Scholar]
- Mann S, Chintoh A, Giacca A, Fletcher P, Nobrega J, Hahn M, Remington G. Chronic olanzapine administration in rats: effect of route of administration on weight, food intake and body composition. Pharmacol Biochem Behav 103: 717–722, 2013. [DOI] [PubMed] [Google Scholar]
- Martin MB, Earle KR. Laparoscopic adjustable gastric banding with truncal vagotomy: any increased weight loss? Surg Endosc 25: 2522–2525, 2011. [DOI] [PubMed] [Google Scholar]
- Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet 20: 368–378, 2005. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104, 2005. [DOI] [PubMed] [Google Scholar]
- Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett 433: 71–76, 2008. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Linnet K. Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of comedication. Ther Drug Monit 21: 87–90, 1999. [DOI] [PubMed] [Google Scholar]
- Pagani FD, Norman WP, Kasbekar DK, Gillis RA. Localization of sites within dorsal motor nucleus of vagus that affect gastric motility. Am J Physiol Gastrointest Liver Physiol 249: G73–G84, 1985. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, Rittberg BR, Adson DE. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes 31: 1756–1759, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perathoner A, Weiss H, Santner W, Brandacher G, Laimer E, Holler E, Aigner F, Klaus A. Vagal nerve dissection during pouch formation in laparoscopic Roux-Y-gastric bypass for technical simplification: does it matter? Obes Surg 19: 412–417, 2009. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Lund BC, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response: acute phase results of the North American Olanzapine Trial. J Clin Psychopharmacol 21: 14–20, 2001. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Mow T, Kreilgaard M, Velschow S. Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacol Biochem Behav 75: 133–140, 2003. [DOI] [PubMed] [Google Scholar]
- Qadri SF, Padala PR, Strunk JC, Boust SJ. High-dose olanzapine orally disintegrating tablets for treatment-resistant psychosis. Prim Care Companion J Clin Psychiatry 8: 244–245, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramankutty G. Olanzapine-induced destabilization of diabetes in the absence of weight gain. Acta Psychiatr Scand 105: 235–237, 2002. [DOI] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature 369: 289–294, 1994. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol 514: 369–383, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 70: 513–535, 2008. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull 35: 937–948, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA 97: 9264–9269, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci USA 107: 7024–7029, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 62: 3232–3240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Leenen L, Rijkers K, Cornips E, Majoie M, Brans B, van Marken Lichtenbelt WD. Vagus nerve stimulation increases energy expenditure: relation to brown adipose tissue activity. PloS One 8: e77221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res 217: 337–346, 2011. [DOI] [PubMed] [Google Scholar]
- Weston-Green K, Huang XF, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PloS One 7: e33548, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston-Green K, Huang XF, Han M, Deng C. The effects of antipsychotics on the density of cannabinoid receptors in the dorsal vagal complex of rats: implications for olanzapine-induced weight gain. Int J Neuropsychopharmacol 11: 827–835, 2008. [DOI] [PubMed] [Google Scholar]
- Weston-Green K, Huang XF, Lian J, Deng C. Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur Neuropsychopharmacol 22: 364–373, 2012b. [DOI] [PubMed] [Google Scholar]
- Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141: 1332–1337, 2000. [DOI] [PubMed] [Google Scholar]
- Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148: 1868–1881, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford DJ, Ormsbee HS 3rd, Norman W, Harmon JW, Garvey TQ 3rd, DiMicco JA, Gillis RA. Hindbrain GABA receptors influence parasympathetic outflow to the stomach. Science 214: 193–194, 1981. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Ai HB, Cui XY. Effects of nucleus ambiguus and dorsal motor nuclei of vagus on gastric H+ and HCO3− secretion in rats. World J Gastroenterol 12: 3271–3274, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Travagli RA. Dopamine effects on identified rat vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 292: G1002–G1008, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31: 14024–14031, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta 1792: 423–431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]