Abstract
The goal of this study was to investigate the human ability to stabilize the trunk in space during pelvic tilt. Upper body sway was evoked in kneeling-seated healthy subjects by angular platform perturbations with a rotation around a virtual low-back pivot point between the L4 and L5 vertebrae. To investigate motor control modulation, variations in task instruction (balance naturally or minimize trunk sway), vision (eyes open or closed), and perturbation bandwidth (from 0.2 up to 1, 3, or 10 Hz) were applied. Cocontraction and proprioceptive muscle spindle feedback were associated with minimizing low-back flexion/extension (trunk-on-pelvis stabilization), while vestibular and visual feedback were supposed to contribute to trunk-in-space stabilization. Trunk-in-space stabilization was only observed with the minimize trunk sway task instruction, while the task instruction to balance naturally led to trunk-on-pelvis stabilization with trunk rotations even exceeding the perturbations. This indicates that vestibular feedback is used when minimizing trunk sway but has only a minor contribution during natural trunk stabilization in the sagittal plane. The eyes open condition resulted in reduced global trunk rotations and increased global trunk reflexive responses, demonstrating effective visual contributions to trunk-in-space stabilization. On the other hand, increasing perturbation bandwidth caused a decreased feedback contribution leading to deteriorated trunk-in-space stabilization.
Keywords: lumbar spine, postural control, system identification, muscle spindles, visual and vestibular feedback
low-back motor control enables humans to keep an upright trunk posture by stabilizing the trunk with cocontraction and reflexes. In some conditions or activities people may stabilize their trunk in space, for example, providing a stable base for visual perception during movement, while in other conditions they may stabilize their trunk relative to their pelvis, for example, to maintain balance or control spinal movement (e.g., during mogul skiing vs. power lifting). Improper low-back motor control may be associated with chronic low-back pain (Cholewicki et al. 2005; Radebold et al. 2001; van Dieën et al. 2003) with diverse changes in motor control in relation to pain including strategies to limit lumbar bending (Hodges et al. 2009; Roland 1986).
Intrinsic low-back stiffness and damping, comprising passive tissue and passive muscle properties, can be altered by muscle cocontraction, while reflexes include proprioceptive (muscle spindle), vestibular, and visual feedback (Day et al. 1997; Goodworth and Peterka 2009, 2010; van Drunen et al. 2013, 2015). The function of intrinsic properties and muscle spindle feedback is to minimize lumbar bending and thus to contribute to stabilizing the trunk with respect to the pelvis (e.g., in power lifting), which we define as trunk-on-pelvis stabilization. On the other hand, vestibular and visual feedback are involved in trunk-in-space stabilization as part of head-in-space stabilization (Goodworth and Peterka 2009, 2010; van Drunen et al. 2015).
Vestibular feedback was found to have a substantial effect on lateral trunk stabilization during lateral pelvic rotations in a modeling study (Goodworth and Peterka 2009) and studies on vestibular loss patients (Goodworth and Peterka 2010). In addition, galvanic vestibular stimuli in both standing and seated subjects resulted in lateral low-back flexion/extension (Andreopoulou et al. 2015; Day et al. 1997; Maaswinkel et al. 2014). However, pelvic translations in the sagittal plane lead primarily to trunk-on-pelvis stabilization with eyes closed (van Drunen et al. 2015), suggesting only minor vestibular contributions to trunk stabilization in the sagittal plane.
To further investigate the vestibular and visual contribution to trunk stabilization in the sagittal plane, an experiment was designed in which trunk-in-space stabilizing feedback was needed to keep an upright posture. This experiment involved pelvic rotational perturbations around a virtual low-back pivot point (between the vertebrae L4 and L5; van Drunen et al. 2015). During such perturbations, cocontraction and proprioception hamper trunk-in-space stabilization by stiffening the lumbar spine, leaving visual and vestibular feedback as potential contributors for active trunk-in-space stabilization. These kind of perturbations resemble pelvic tilt during for instance walking (Callaghan et al. 1999; Vink and Karssemeijer 1988).
To be able to operate in altering conditions or perform different tasks, modulation of motor control is of vital importance. Low-back motor control modulation has been found for changing task instructions, perturbation amplitude and frequency bandwidth, and the availability of feedback such as vision or vestibular information (Buchanan and Horak 1999; Goodworth and Peterka 2009, 2010; van Drunen et al. 2013, 2015). This study focused on modulation due to task instruction, vision, and perturbation bandwidth. With instructions to minimize trunk sway (MS) or to balance naturally (BN), the modulation between maximal trunk-in-space stabilization (during the MS task) and natural trunk stabilization (BN) was assessed. In Goodworth and Peterka (2009), the visual contribution was found to be small for trunk stabilization in the frontal plane, while vision resulted in a consistent change towards a trunk-in-space strategy in the sagittal plane (Buchanan and Horak 1999; van Drunen et al. 2015). Perturbation bandwidths exceeding the natural frequency have been shown to attenuate reflexive behavior in postural control of upper and lower extremities (Stein and Kearney 1995; van der Helm et al. 2002).
Thus the goal of this study was to investigate the human ability to stabilize the trunk-in-space during pelvic tilt. Task instruction, perturbation bandwidth, and the presence of visual feedback were varied to investigate motor control modulation with changed conditions. First, it was hypothesized that both the minimize trunk sway and the balance naturally task instructions will lead to trunk-in-space stabilization using vestibular and visual feedback, with the best performance during the task to minimize sway. Secondly, vision was hypothesized to improve trunk-in-space performance, while increased trunk-in-space performance was also hypothesized with perturbation bandwidth below the natural frequency.
METHODS
Subjects.
Six healthy adults (age: 23–25 yr, 4 male) participated in this study and gave informed consent according to the guidelines of the ethical committee of Delft University of Technology. Subjects did not experience lower back pain in the year before the experiments.
Experiments.
The subjects were placed in a kneeling-seated posture (Fig. 1; 90° knee and ankle angle, 135° angle between upper body and upper leg) in a rigid chair, while being restrained at the pelvis by clamping the anterior superior and posterior iliac spine from the side. The chair was placed on top of a hydraulic motion platform, which was position controlled through a dedicated computer system (dSpace, Paderborn, Germany) with a custom-made controller (Matlab/Simulink; Mathworks, Natick, MA). The seat and clamps were foam covered for subject comfort. To avoid extra dynamics of the arms, subjects were instructed to cross their arms in front of their chest.
Fig. 1.
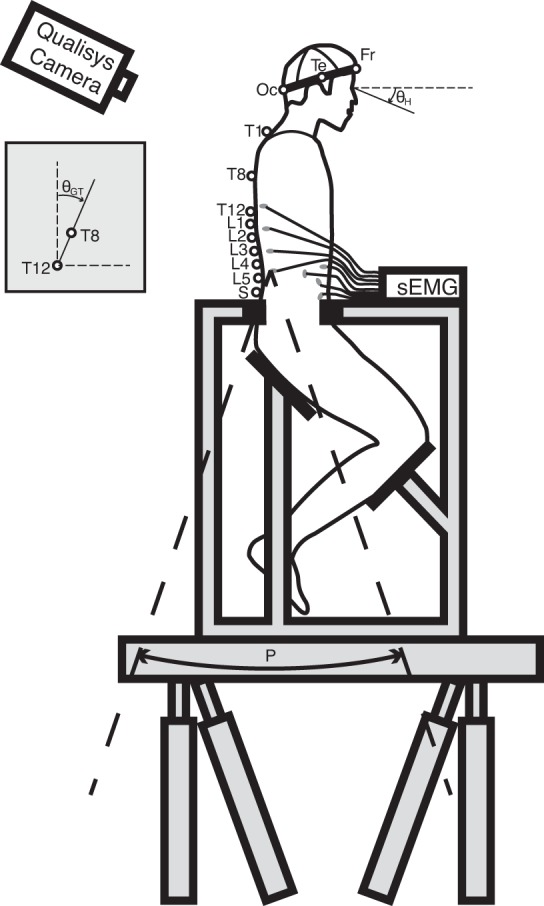
Experimental setup. Markers (open circles) were placed on the sacrum (S), the lumbar vertebrae (L1–L5), the thoracic vertebrae (T1, T8, and T12), the head [occipital bone (Oc), temple (Te), frontal bone (Fr), the left ear, and the left eye socket], the acromion, and the chair. Angular perturbations (P) were rotating around a virtual low-back pivot point between L4 and L5. Kinematics were described in global trunk rotations (θGT), relative trunk rotations (θRT = θGT − P), and global head rotations (θGH). Muscle activity was measured using surface electromyography (sEMG).
Upper body sway was evoked by swing-like platform perturbations in anterior-posterior directions [P(t)] around a virtual rotation point between the vertebrae L4 and L5, being defined as the virtual dynamic rotation point of the low back, where van Drunen et al. (2015) showed that this rotation point adequately captured thorax kinematics up to 5 Hz in anterior-posterior pelvic perturbations. Task instructions were alternately to minimize upper body sway (MS) or to behave naturally (BN) during the swing perturbations. Both tasks were expected to result in trunk-in-space stabilization, where MS would provide “maximum” performance. All tasks were performed both with eyes open (EO) and eyes closed (EC).
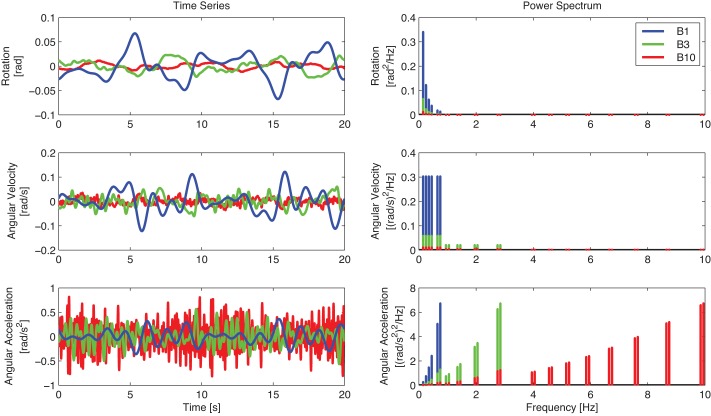
The perturbations P(t) were random-appearing multi-sine signals of 20-s duration (Fig. 2). Each experimental trial had a duration of 80 s consisting of a 10-s fade-in period, three repetitions of the same perturbation signal P(t), and a 10-s fade-out period. The fade-in and fade-out period were applied to minimize transient behavior and prevent abrupt platform motions. To investigate the effect of bandwidth on trunk stabilization, three disturbances with different bandwidths were included containing frequencies between 0.2 Hz and 1, 3, or 10 Hz (B1, B3 and B10, respectively). The excited frequencies consisted of pairs of two adjacent frequency points, which were linearly (<1 Hz) and logarithmically (>1 Hz) spaced. To reduce modulation due to high-frequency perturbations, the perturbation power was reduced to 60% (>1 Hz) and 40% (>4 Hz), with flat power in angular velocity in these three bands (Mugge et al. 2007). To create a similar perturbation amplitude, perturbations for the three perturbation bandwidths were scaled to have equal maximal power in the angular acceleration, equivalent to perturbation force. For the safety and comfort of the subjects, the maximum angular accelerations were kept <0.85 rad/s2. All 12 conditions (Table 1) were repeated twice, resulting in a total of 24 trials per subject performed in a randomized sequence.
Fig. 2.
Perturbation signal as time series (left) and power spectrum (right) for the perturbation bandwidths B1 (blue), B3 (green), and B10 (red). The perturbation signal is shown as position (top), angular velocity (middle), and angular acceleration (bottom) signal.
Table 1.
Overview of the 12 experimental conditions
| Task |
||||
|---|---|---|---|---|
| Perturbation | Bandwidth, Hz | Frequency Points (pairs) | Balance naturally | Minimize sway |
| B1 | 0.15–0.75 | 6 (3) | EO/EC | EO/EC |
| B3 | 0.15–2.85 | 14 (7) | EO/EC | EO/EC |
| B10 | 0.15–9.95 | 30 (15) | EO/EC | EO/EC |
Overview of the 12 experimental conditions, with the bandwidths B1, B3, and B10, the tasks to balance naturally (BN) and minimize trunk sway (MS), and eyes opened (EO) or closed (EC).
Data recording and processing.
Three-dimensional (3D) kinematics of the low-back, trunk, and head were measured at 200 Hz using an Oqus 6-camera 3D motion capture system (Qualisys, Gothenburg, Sweden). Markers were placed at the sacrum, the lumbar vertebrae (L1–L5), the thorax (T1, T8, and T12), the left acromion, the head (occipital bone, temple, frontal bone, immediately in front of the left ear in line with the tragion, and on the lower border of the left eye socket), and the chair. The global trunk rotations θGT(t) were defined in the sagittal plane by the angle of the link between the T8 and T12 markers with respect to the vertical axis, while the relative trunk rotations θRT(t) representing the low-back flexion/extension angle were calculated by subtracting P(t) from θGT(t). The global head rotations θGH(t) were derived using the Veldpaus-algorithm (Veldpaus et al. 1988) applied to all markers on the head. Rotations in flexion direction were considered positive.
Activity of 20 muscles [10 bilateral pairs; m. longissimus (at T9 and L4), m. Iliocostalis (at T12 and L2), m. rectus abdominus, m. obliquus externus (lateral and anterior), m. obliquus internus, m. gluteus maximus, and m. rectus femoris] was measured at 2,000 samples/s [surface electromyography (sEMG); Porti 17, TMSi, The Netherlands] as described in Willigenburg et al. (2010). The EMG data ej(t) (with j = #muscle) were digitally filtered (zero phase, first-order, and high-pass) at 250 Hz (Staudenmann et al. 2007) and then rectified. A lumped muscle activation E(t) with positive flexion activity was derived with the 16 back and abdominal muscles by optimizing the weight factors wj for a maximum coherence of the disturbance to EMG (Kiemel et al. 2008). Weight factors were derived per subject optimizing over all conditions:
System identification.
System identification techniques (Guitton et al. 1986; Pintelon and Schoukens 2001; van der Helm et al. 2002) were used to describe the low-back kinematics and reflexes as frequency response functions (FRFs). The kinematic FRFs describe the overall response of the subjects including cocontractive and reflexive behavior. Because trunk kinematics did not influence the platform perturbations, the kinematic FRFs were defined as the open-loop response to the perturbation [P(t)] of the global trunk rotations [θGT(t)], the relative trunk rotations [θRT(f)], and the global head rotations [θGH(t)]:
with SPθ(f) representing the estimated cross-spectral density between perturbation signal P and rotations θ, etc. The cross-spectral densities were only evaluated at the frequencies containing power in the input disturbance signal. For improved estimates and noise reduction, the cross-spectral densities were averaged across the six time segments per condition (2 trials each containing 3 segments of 20 s) and over two adjacent frequency points (Jenkins and Watts 1969).
Cocontraction levels (ERMS) were estimated with the root-mean-square-value (RMS) of all back and abdominal muscles [ej(t)] averaged:
The reflexive contribution was estimated with the reflexive FRFs, which were obtained using the joint input-output approach (van der Kooij et al. 2005). This approach uses the external perturbation [P(t)] to identify the closed-loop response of the lumped EMG [E(t)] to the relative trunk rotations [θRT(t)] and the global trunk rotations [θGT(t)], representing respectively the proprioceptive feedback and a combination of vestibular and visual feedback:
with SPE(f) representing the estimated cross-spectral density between perturbation signal P and the lumped EMG E.
Coherence was calculated to evaluate input-output relationship:
Coherence ranges from zero to one, where one reflects a perfect, noise-free relation. Since spectral densities were averaged over 12 points, a coherence >0.24 is significant with P < 0.05 (Halliday et al. 1995).
Statistics.
A parametric linear mixed model was applied to assess the effect of vision, bandwidth, and task instruction. The statistical model took into account the main and the two-way interaction effects of the different conditions. Statistics were performed on the averaged gain and phase of the kinematic and reflexive FRFs at the lowest frequency points (<1 Hz; see Fig. 5) and the cocontractive levels ERMS (see Fig. 6). A Bonferroni-corrected P < 0.05 was considered significant.
Fig. 5.
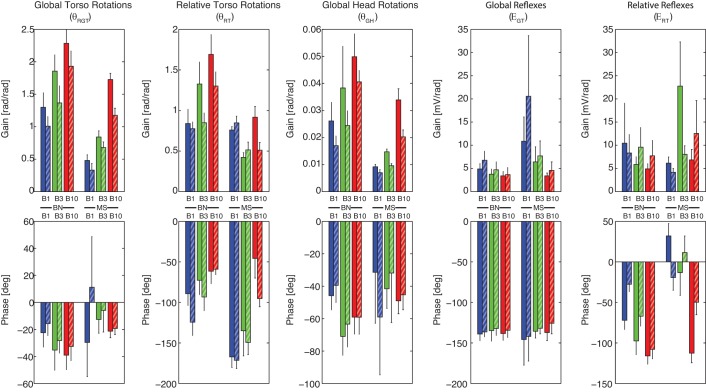
Gain and phase for all experimental conditions. Modulation occurred due to task instruction [to BN (left) and to MS (right)], vision [EC (solid) and eyes open (EO; striped)], and perturbation bandwidth [B1 (blue), B3 (green), and B10 (red)]. Gains and phases were averaged over the three lowest frequency pairs (<1 Hz). Values are the mean (bars) and standard deviation (error bars) over subjects. Kinematics are described by the FRFs of the global trunk rotations (θGT; left), relative trunk rotations (θRT), and global head rotations (θGH), while reflexive EMG is described with respect to the global torso rotations (EGT) and the relative trunk rotations (ERT; right). A smaller θGT gain and phase lag describes a modulation towards trunk-in-space stabilization. Modulation towards trunk-on-pelvis stabilization is illustrated by a θGT closer to 1 (gain) and 0° (phase) in combination with a smaller θRT gain and phase lag. The results indicate modulation towards trunk-in-space stabilization and increased global reflexes (EGT) with the MS task, EO, and low bandwidths.
Fig. 6.
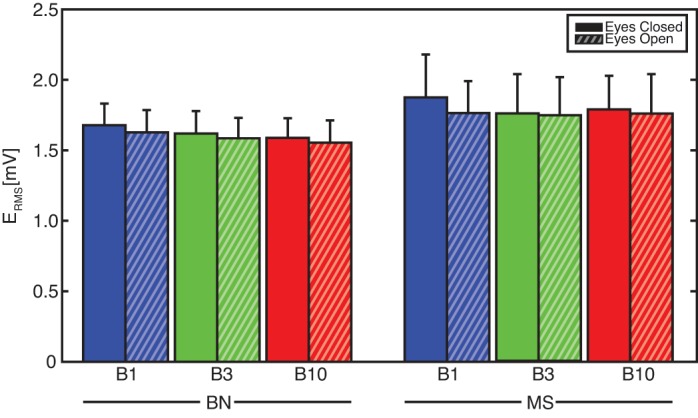
The EMG root-mean-square-value (ERMS) representing cocontraction during all conditions: frequency bandwidths up to 1 Hz (B1; blue), 3 Hz (B3; green), and 10 Hz (B10; red); Task instruction to BN (left) and to MS (right); with EC (solid) and EO (striped). Bandwidth and vision did not affect cocontraction. Effects due to task instruction were found, with MS resulting in higher ERMS and thus higher cocontraction levels (P < .001).
RESULTS
Frequency response functions.
Human trunk stabilizing behavior is described by the FRFs of the kinematics and EMG (Figs. 3, 4, and 5). Kinematics are described in global trunk rotations [θGT(f)], trunk rotations relative to the platform [θRT(f)] and global head rotations [θGH(f)]. In the lumped EMG [E(t)], the contributions of abdominal and back muscles were roughly equal (back muscles 58%), with the lumbar (29%) and thoracic (17%) part of the Longissimus muscle and the lateral part of the Oblique Externus muscle (16%) as main contributors (Table 2). The EMG response was described relative to the global trunk rotations [EGT(f)], representing a trunk-in-space (vestibular and visual) reflexive response, and the relative trunk rotations [ERT(f)], representing a trunk-on-pelvis (proprioceptive) reflexive response.
Fig. 3.
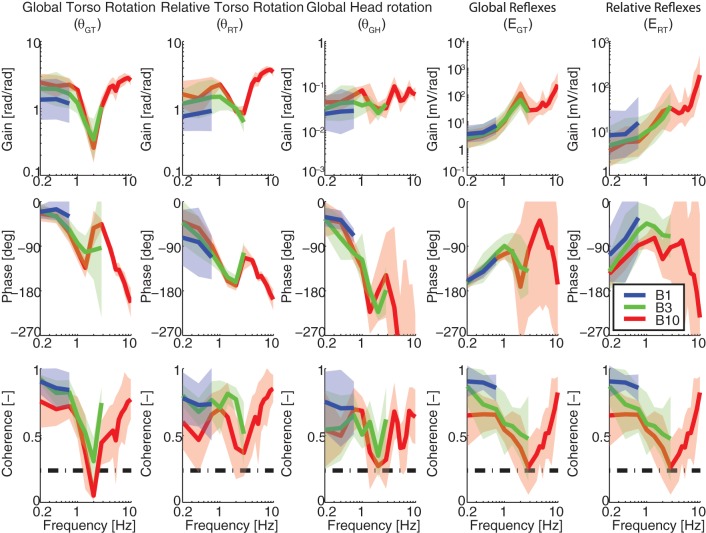
Task instruction to balance naturally (BN) with eyes closed (EC): frequency response functions (FRFs) of the trunk and head kinematics and reflexes. Kinematics are described by the FRFs of the global trunk rotations (θGT; left), relative trunk rotations (θRT), and global head rotations (θGH), while reflexive EMG is described with respect to the global torso rotations (EGT) and the relative trunk rotations (ERT; right). The effects of perturbation bandwidth are shown for bandwidths B1 (blue), B3 (green), and B10 (red).
Fig. 4.
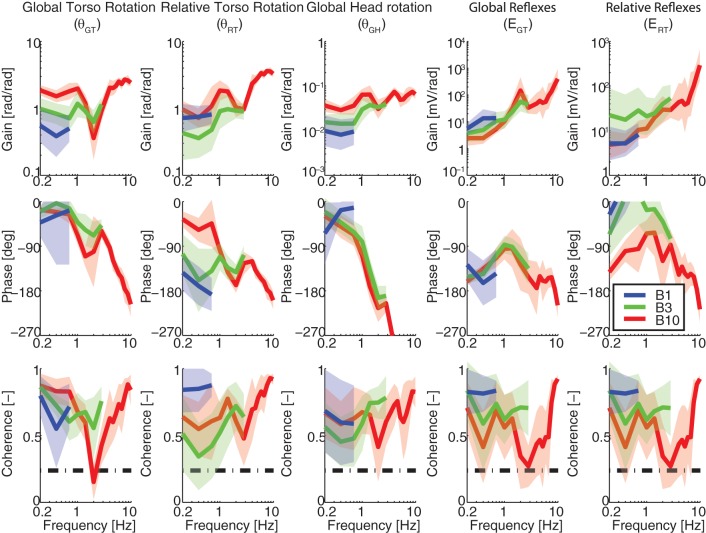
Task instruction to minimize trunk sway (MS) with EC: FRFs of the trunk and head kinematics and reflexes. Kinematics are described by the FRFs of the global trunk rotations (θGT; left), relative trunk rotations (θRT), and global head rotations (θGH), while reflexive EMG is described with respect to the global torso rotations (EGT) and the relative trunk rotations (ERT; right). The effects of perturbation bandwidth are shown for bandwidths B1 (blue), B3 (green), and B10 (red).
Table 2.
The resulting weighting factors wj describing the contribution to the lumped EMG signal of all individual muscles
| Subjects |
|||||||
|---|---|---|---|---|---|---|---|
| Muscles | 1 | 2 | 3 | 4 | 5 | 6 | Mean |
| Back muscles | |||||||
| Longissimus at trunk level | |||||||
| Right | 12 | 15 | 4 | 9 | 8 | 10 | 10 |
| Left | 5 | 3 | 5 | 10 | 19 | 7 | |
| Iliocostalis muscle at trunk level | |||||||
| Right | 3 | 10 | 5 | 1 | 3 | ||
| Left | 9 | 10 | 3 | ||||
| Iliocostalis muscle at lumbar level | |||||||
| Right | 8 | 6 | 2 | ||||
| Left | 3 | 4 | 10 | 4 | 4 | ||
| Longissimus muscle at lumbar level | |||||||
| Right | 23 | 30 | 6 | 19 | 13 | 9 | 17 |
| Left | 12 | 17 | 9 | 8 | 22 | 8 | 12 |
| Abdominal muscles | |||||||
| Rectus abdominus muscle | |||||||
| Right | 10 | 1 | 2 | 1 | 4 | 9 | 4 |
| Left | 7 | 5 | 1 | 10 | 4 | ||
| Oblique externus muscle anteriorly | |||||||
| Right | 6 | 6 | 10 | 5 | 1 | 5 | |
| Left | 5 | 5 | 12 | 2 | 4 | ||
| Oblique internus muscle | |||||||
| Right | 6 | 11 | 8 | 1 | 4 | ||
| Left | 13 | 4 | 13 | 5 | |||
| Oblique externus muscle laterally | |||||||
| Right | 5 | 7 | 7 | 13 | 5 | ||
| Left | 10 | 9 | 2 | 25 | 16 | 10 | |
Contributions were separated in left and right side muscles. For better overview, all values <1% were omitted.
All conditions resulted in consistent kinematic and reflexive responses with a relatively high coherence for the kinematics (with exception of a frequency band ∼2 Hz) and reflexes up to 2 Hz (given the noisy character of EMG). Therefore, the system identification techniques were justified indicating FRF estimates of high quality.
Bandwidth, vision, and task instruction effects appeared predominantly below the low-back natural frequency (∼1 Hz), while FRFs coincided at higher frequencies. The gains and phases averaged over the perturbed frequencies below 1 Hz are presented in Fig. 5 for all conditions and were tested for significant differences.
Kinematic FRFs could be interpreted as trunk-in-space (perfectly stationary orientation in space; global gain of 0; relative gain and phase of 1 and −180°) and trunk-on-pelvis (perfectly moving in line with the pelvis and platform; global gain and phase of 1 and 0°; relative gain of 0). However, such ideal behaviors were not observed, indicating that subjects did not realize either strategy perfectly. For example, during the BN task θGT(f) resembles a critically damped, second-order low-pass system up to 2 Hz with a natural frequency of ∼1 Hz. Below 1-Hz rotations up to two times larger than the platform were found, while at ∼2 Hz a tendency towards stationary orientation in space was observed with an ∼60% reduction of the global movement. Above 2 Hz, increased rotations in opposite direction of the perturbations (θGT gain and phase of >1 and −180°) were found, which could be explained by the virtual low-back pivot point between L4 and L5 being unable to capture complex spinal bending modes (see discussion).
The reflexive FRFs describe the contribution of different feedback systems. For example, during the BN task ERT(f) shows a combination of position feedback (flat gain, −180° phase) and velocity feedback (slope of +1 in gain and −90° phase) up to ∼5 Hz. At higher frequencies, higher order feedback (acceleration/force/trunk-in-space feedback) was present (slope of +2 in gain, 0° phase). The phase decreases at the higher frequencies due to reflex delays. However, a clear distinction between separate feedback systems is hard to make, suggesting multiple sensory contributions (muscle spindle, Golgi tendon organ, and vestibular) to the reflexive behavior.
Fairly small global head rotations were observed with <6% of the perturbation and ∼2% of the global trunk rotations appearing in the head kinematics. The kinematic characteristics were comparable for the head and trunk rotations with only a slightly larger phase lag for the head. Hence, the rotational stabilization of the head in space seems to be dominated by the neck, while trunk stabilization efforts seem hardly effective for head-in-space stabilization.
Effects of task instruction, bandwidth, and vision.
The effects of task instruction, bandwidth, and vision on the kinematic and reflexive FRFs below the low-back natural frequency (∼1 Hz; Fig. 5) and on the cocontraction levels (Fig. 6) are shown. The effects were predominantly present <1 Hz as shown for the BN and MS tasks with EC (Figs. 3 and 4), while FRFs at higher frequencies coincided.
Effective trunk-in-space stabilization was only found for the MS task during the B1 and B3 conditions with θGT(f) gains <1 in combination with a θRT(f) phase towards −180°. All other conditions resulted in global trunk rotations larger than or in line with the platform [θGT(f) gains ≥ 1]. More effective trunk-in-space stabilization coincided with higher EGT(f) gains and the increase of ERT(f) phase towards 0°, indicating increased vestibular (and potentially visual) feedback during these conditions.
Effect of task instruction.
As expected, the MS task resulted in more effective trunk-in-space stabilization than the BN task with reduced θGT(f) (P < 0.001) and θGH(f) gains (P < 0.001), while the θRT(f) phase lag increased (P < 0.001). An overall decrease of low-back flexion/extension [θRT(f) gains; P < 0.001] was observed, which was in line with the higher cocontraction levels (P < 0.001). Thereby, a combination of increased EGT(f) gains (P < 0.01) and decreased ERT(f) (P < 0.001) phase lag described an increase in globally oriented (vestibular and visual) feedback.
None of the conditions with the BN task did result in θGT(f) gains <1, thereby rejecting the hypothesis of trunk-in-space stabilization during natural trunk stabilization.
Effect of vision.
Significant effects of vision were found, which all supported the global stabilizing role of vision. The EO condition reduced global rotations of the trunk [θGT(f) gain; P < 0.01] and head [θGH(f) gain; P < 0.005]. This was achieved by a tendency of higher global reflexive feedback [EGT(f) gain; P < 0.13], leading to a larger phase lag in the relative trunk rotations [θRT(f) phase; P < 0.03]. Cocontraction levels were not influenced by vision.
Effect of bandwidth.
Increasing perturbation bandwidth led to deteriorated trunk-in-space stabilization, as shown by the increased θGT(f) gains (P < 0.02) and decreased the phase lag of θRT(f) (P < 0.001). During the BN task, even the trunk-on-pelvis stabilization deteriorated with increasing bandwidth described by higher θRT(f) gains. On the other hand, during the MS task, the B3 perturbation led to reduced trunk-in-space stabilization with respect to the B1 perturbation, which resulted from a decrease in θRT(f) gains with a smaller phase lag. Bandwidth effects were fairly similar in the head and trunk global rotations with increasing θGH(f) gains (P < 0.001) as well.
Bandwidth effects were also present in the reflexive behavior, with decreasing EGT(f) gains with increasing bandwidth (P < 0.005). During the BN task, the increasing phase lag in ERT(f) described a tendency towards more regular muscle spindle feedback with velocity (phase of −90°) and position (phase of −180°) feedback. Different feedback behavior (such as global vestibular/visual feedback) was found during the MS task with B1 and B3 perturbations described by a ERT(f) phase ∼0°. Cocontraction levels were not influenced by perturbation bandwidth.
DISCUSSION
The goal of this study was to investigate the human ability to stabilize the trunk in space during pelvic tilt, while motor control modulation was elicited by varying task instruction, vision, and perturbation bandwidth conditions. The first hypothesis that both task instructions would lead to trunk-in-space behavior was partly rejected, since only the task instruction to MS did result in trunk-in-space stabilization. The task instruction to BN led to trunk rotations larger or in line with the platform perturbations (trunk-on-pelvis). This indicates that vestibular feedback is used when minimizing sway but has only a minor contribution during natural trunk stabilization in the sagittal plane. The presence of visual feedback and decreasing perturbation bandwidth resulted in reduced global rotations, thereby confirming the other hypotheses that vision and perturbation bandwidths below the natural frequency would improve trunk-in-space stabilization.
The most effective trunk-in-space stabilization was found during the MS task with vision and with the lowest bandwidth. This makes this an interesting condition to investigate deviating control in low back pain, where this condition could show patients to limit lumbar bending in relation to pain (Hodges et al. 2009; Roland 1986) and being unable to perform trunk-in-space stabilization.
With the principle of minimizing effort in mind, trunk-in-space stabilization during rotational perturbations was expected to be achieved by a combination of minimized effective stiffness (intrinsic stiffness + proprioceptive position feedback) only counteracting the gravitational negative stiffness for stability and by vestibular and visual feedback providing trunk-in-space information. The EO condition resulted in reduced global trunk rotations supporting this theory. However, the increased cocontraction levels observed during the MS task contradicted this expectation for trunk stabilization during sagittal pelvic tilt. The trunk-in-space stabilization coincided with an increase of vestibular feedback as described by the increased global reflexive gains [EGT(f)] and the shift towards 0° phase lag in the relative reflexes [ERT(f)]. Probably, the trunk-on-pelvis destabilizing effect of these global reflexes elicited an increase in cocontraction to maintain local stability at the individual intervertebral joints.
During the BN task, trunk rotations greater than or in line with the platform (trunk-on-pelvis) were found. During such kinematic behavior, muscle spindle responses have the same direction as the vestibular and visual feedback. Since muscle spindle responses are more directly involved with trunk stabilization (vestibular and visual feedback are filtered by the neck kinematics) with shorter reflex delays, we conclude that vestibular feedback had only a minor contribution to natural trunk stabilization in combination with sagittal pelvic tilt. This is in line with findings during translations in the sagittal plane (van Drunen et al. 2015) but in contradiction with lateral pelvic tilt (Goodworth and Peterka 2009, 2010) or laterally unstable sitting in combination with GVS (Andreopoulou et al. 2015). Therefore, the vestibular contributions seem to be affected by perturbation direction, with substantial lateral and minor anterior-posterior vestibular contribution, but not affected by perturbation type. It shall be noted that we only tested between 0.2 and 10 Hz and cannot exclude a prominent role of vestibular feedback in the BN task <0.2 Hz.
Increasing perturbation bandwidth resulted in decreased trunk-in-space stabilization [θGT(f)] and reflexes [EGT(f)], while during the BN task also decreased trunk-on-pelvis stabilization [θRT(f)] was observed. During translational perturbation to the low-back, neck, and standing posture (Buchanan and Horak 1999; Forbes et al. 2013; van Drunen et al. 2015), reduced trunk-on-pelvis stabilization, describing reduced performance of the stabilization objective, was found due to increasing perturbation bandwidths. This was in line with the results in this study, where trunk-in-space (during the MS task) and trunk-on-pelvis (during the BN task) performance decreased with increasing bandwidth. In human limb control, bandwidth effects are related to perturbations that either remain below the natural frequency or exceed the natural frequency (Stein and Kearney 1995; van der Helm et al. 2002). Surprisingly, bandwidth effects in this study were not only present between perturbation bandwidths below (B1) and exceeding (B3 and B10) the natural low-back frequency (∼1 Hz) but occurred between the bandwidths B3 and B10 as well. This could, in combination with the high-frequency behavior during B10, be a result of the perturbation center of rotation, which was defined between the vertebrae L4 and L5. With translational perturbations, van Drunen et al. (2015) estimated a virtual low-back pivot point between the vertebrae L4 and L5 up to 5 Hz, while >5 Hz the location of the low-back virtual pivot point was moving downwards. Assuming that this happened during the rotational perturbations as well, the perturbation bandwidth B10 was not rotating purely around the virtual low-back pivot point, thereby introducing additional high-frequency translational perturbations.
Vision enhanced trunk-in-space stabilization as evidenced by reduced global trunk and head rotations. This was in line with findings in stabilization of the trunk (van Drunen et al. 2015), the neck (Forbes et al. 2013), and standing posture (Buchanan and Horak 1999) during translational perturbations. The improved trunk-in-space stabilization was achieved by an increased contribution of global reflexes [higher EGT(f) gains and smaller ERT(f) phase lag]. The increased contribution could be explained by the extra (visual) feedback loop but could also be the result of the increased reliability of the information of global head orientation. However, although vision realizes a better trunk-in-space performance in all conditions, adding vision did not lead to effective trunk-in-space stabilization during natural trunk stabilization.
In conclusion, during the task to minimize trunk sway trunk-in-space stabilization was found, while global rotations larger than or in line with the platform were found during task instruction to balance naturally. This indicates that trunk-in-space feedback systems (vestibular and visual) have a minor contribution to natural trunk stabilization in the sagittal plane, while only with the instruction to minimize trunk sway, the contribution of vestibular (and visual) feedback became large enough to realize trunk-in-space stabilization.
GRANTS
This research is supported by the Dutch Technology Foundation STW, which is part of The Netherlands Organization for Scientific Research (NWO) and which is partly funded by the Ministry of Economic Affairs (see www.neurosipe.nl: Project 10732: QDISC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.v.D., F.C.T.v.d.H., J.H.v.D., and R.H. conception and design of research; P.v.D. performed experiments; P.v.D. analyzed data; P.v.D., F.C.T.v.d.H., J.H.v.D., and R.H. interpreted results of experiments; P.v.D. prepared figures; P.v.D. drafted manuscript; P.v.D., F.C.T.v.d.H., J.H.v.D., and R.H. edited and revised manuscript; P.v.D., F.C.T.v.d.H., J.H.v.D., and R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We express sincere gratitude to Yorick Koumans, MSc, for contributions in preparing and performing the measurements.
REFERENCES
- Andreopoulou G, Maaswinkel E, Cofré Lizama LE, van Dieën JH. Effects of support surface stability on feedback control of trunk posture. Exp Brain Res 233: 1079–1087, 2015. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol 81: 2325–2339, 1999. [DOI] [PubMed] [Google Scholar]
- Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech 14: 203–216, 1999. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Silfies SP, Shah RA, Greene HS, Reeves NP, Alvi K, Goldberg B. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine 30: 2614–2620, 2005. [DOI] [PubMed] [Google Scholar]
- Day BL, Séverac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol 500: 661–672, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes PA, de Bruijn E, Schouten AC, Helm FC, Happee R. Dependency of human neck reflex responses on the bandwidth of pseudorandom anterior-posterior torso perturbations. Exp Brain Res 226: 1–14, 2013. [DOI] [PubMed] [Google Scholar]
- Goodworth AD, Peterka RJ. Contribution of sensorimotor integration to spinal stabilization in humans. J Neurophysiol 102: 496–512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodworth AD, Peterka RJ. Influence of bilateral vestibular loss on spinal stabilization in humans. J Neurophysiol 103: 1978–1987, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitton D, Kearney RE, Wereley N, Peterson BW. Visual, vestibular and voluntary contributions to human head stabilization. Exp Brain Res 64: 59–69, 1986. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Progr Biophys Mol Biol 64: 237–278, 1995. [DOI] [PubMed] [Google Scholar]
- Hodges PW, van den Hoorn W, Dawson A, Cholewicki J. Changes in the mechanical properties of the trunk in low back pain may be associated with recurrence. J Biomech 42: 61–66, 2009. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. San Francisco, CA: Holden-Day, 1969, p. 525. [Google Scholar]
- Kiemel T, Elahi AJ, Jeka JJ. Identification of the plant for upright stance in humans: multiple movement patterns from a single neural strategy. J Neurophysiol 100: 3394–3406, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel E, Veeger HE, Dieen JH. Interactions of touch feedback with muscle vibration and galvanic vestibular stimulation in the control of trunk posture. Gait Posture 39: 745–749, 2014. [DOI] [PubMed] [Google Scholar]
- Mugge W, Abbink DA, van der Helm FC. Reduced power method: how to evoke low-bandwidth behaviour while estimating full-bandwidth dynamics. In: IEEE 10th International Conference on Rehabilitation Robotics (ICORR). Noordwijk aan Zee, The Netherlands: IEEE, 2007, p. 575–581. [Google Scholar]
- Pintelon R, Schoukens J. System Identification: a Frequency Domain Approach. New York: John Wiley & Sons, 2001, p. 648. [Google Scholar]
- Radebold A, Cholewicki J, Polzhofer GK, Greene HS. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 26: 724–730, 2001. [DOI] [PubMed] [Google Scholar]
- Roland MO. A critical review of the evidence for a pain-spasm-pain cycle in spinal disorders. Clin Biomech 1: 102–109, 1986. [DOI] [PubMed] [Google Scholar]
- Staudenmann D, Potvin JR, Kingma I, Stegeman DF, van Dieën JH. Effects of EMG processing on biomechanical models of muscle joint systems: sensitivity of trunk muscle moments, spinal forces, and stability. J Biomech 40: 900–909, 2007. [DOI] [PubMed] [Google Scholar]
- Stein RB, Kearney RE. Nonlinear behavior of muscle reflexes at the human ankle joint. J Neurophysiol 73: 65–72, 1995. [DOI] [PubMed] [Google Scholar]
- van der Helm FC, Schouten AC, de Vlugt E, Brouwn GG. Identification of intrinsic and reflexive components of human arm dynamics during postural control. J Neurosci Methods 119: 1–14, 2002. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, van Asseldonk E, van der Helm FC. Comparison of different methods to identify and quantify balance control. J Neurosci Methods 145: 175–203, 2005. [DOI] [PubMed] [Google Scholar]
- van Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol 13: 333–351, 2003. [DOI] [PubMed] [Google Scholar]
- van Drunen P, Koumans Y, van der Helm FC, van Dieën JH, Happee R. Modulation of intrinsic and reflexive contributions to low-back stabilization due to vision, task instruction, and perturbation bandwidth. Exp Brain Res 233: 735–749, 2015. [DOI] [PubMed] [Google Scholar]
- van Drunen P, Maaswinkel E, van der Helm FC, van Dieën JH, Happee R. Identifying intrinsic and reflexive contributions to low-back stabilization. J Biomech 46: 1440–1446, 2013. [DOI] [PubMed] [Google Scholar]
- Veldpaus FE, Woltring HJ, Dortmans LJ. A least-squares algorithm for the equiform transformation from spatial marker co-ordinates. J Biomech 21: 45–54, 1988. [DOI] [PubMed] [Google Scholar]
- Vink P, Karssemeijer N. Low back muscle activity and pelvic rotation during walking. Anat Embryol 178: 455–460, 1988. [DOI] [PubMed] [Google Scholar]
- Willigenburg N, Kingma I, van Dieën J. How is precision regulated in maintaining trunk posture? Exp Brain Res 203: 39–49, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]