Abstract
In many brain areas, repetition of a stimulus usually weakens the neural response. This “adaptation” or repetition suppression effect has been observed with mass potential measures such as event-related potentials (ERPs), in fMRI BOLD responses, and locally with local field potentials (LFPs) and spiking activity. Recently, it has been reported that macaque F5 mirror neurons do not show repetition suppression of their spiking activity for single repetitions of hand actions, which disagrees with human fMRI adaptation studies. This finding also contrasts with numerous studies showing repetition suppression in macaque inferior temporal cortex, including the rostral superior temporal sulcus (STS). Since the latter studies employed static stimuli, we assessed here whether the use of dynamic action stimuli abolishes repetition suppression in the awake macaque STS. To assess adaptation effects in the STS, we employed the same hand action movies as used when examining adaptation in F5. The upper bank STS neurons showed repetition suppression during the approaching phase of the hand action, which corresponded to the phase of the action for which these neurons responded overall the strongest. The repetition suppression was present for the spiking activity measured in independent single-unit and multiunit recordings as well as for the LFP power at frequencies > 50 Hz. Together with previous data in F5, these findings suggest that adaptation effects differ between F5 mirror neurons and the STS neurons.
Keywords: adaptation, hand action stimuli, inferior temporal cortex, multiunit activity, single-unit activity
in many areas of the brain and for all sensory modalities, repetition of a stimulus usually weakens the evoked response. This “adaptation” or repetition suppression effect can be observed at the macroscale, with mass potential measures such as EEG or event-related potential (ERP), in functional magnetic resonance imaging (fMRI) BOLD responses (for reviews see Grill-Spector et al. 2006; Malach 2012), and more locally with local field potentials (LFPs; De Baene and Vogels 2010; Kaliukhovich and Vogels 2012; Wang et al. 2011) and spiking activity (e.g., De Baene and Vogels 2010; Krekelberg et al. 2006; Solomon and Kohn 2014). Repetition suppression has aroused interest because of the widespread use of adaptation paradigms in human fMRI studies (Malach 2012). Furthermore, the changes in neural responses with adaptation show the impact of stimulus history on the neural representation of a stimulus. Given that stimulus history affects perception (e.g., Bar and Biederman 1999; Clifford 2002; Jordan et al. 2006; Kohn and Movshon 2004; Muller et al. 2009; Noudoost and Esteky 2013; Troje et al. 2006), a better understanding of adaptation is necessary to gain a deeper insight into the neural mechanisms of perception (Clifford et al. 2007).
Under some stimulus conditions, stimulus repetition can induce an enhancement instead of a suppression of the response. For instance, adaptation to stimuli that stimulate the surround of macaque primary visual cortical (V1) neurons can enhance instead of suppress single-unit responses (Wissig and Kohn 2012). Recently, Caggiano et al. (2013) and Kilner et al. (2014) reported that a single repetition of a hand action does not cause suppression of the spiking activity of single macaque premotor (F5) mirror neurons. In fact, both groups noted a tendency toward an enhancement of the response to the repeated stimulus. This was a surprising observation since fMRI studies in humans reported repetition suppression of the fMRI BOLD response for single repetitions of hand actions in premotor cortex (Dinstein et al. 2007; Kilner et al. 2009; Majdandzic et al. 2009; Press et al. 2012). It is also surprising because single neurons of the superior temporal sulcus (STS), which is connected, indirectly via parietal and prefrontal cortex, to F5 (Nelissen et al. 2011), demonstrate consistent repetition suppression for single repetitions of static stimuli (Kaliukhovich and Vogels 2012).
One potential difference that causes the discrepant adaptation effects between the studies of repetition suppression in single neurons in the premotor cortex F5 and the STS might be the kind of stimuli used, i.e., sequences of hand actions in F5 and static stimuli in the STS. A hand grasping an object is a rather complex stimulus: it consists of the simultaneous presentation of two stimuli (the hand and the object), the shape of the hand varies during the grasping action, and repetition of one phase of the action (e.g., the approaching, reaching phase) is interrupted by the presence of another action phase (e.g., withdrawal of the hand). All these factors can potentially affect the degree of repetition suppression. First, the simultaneous presence of the object and the hand can reduce repetition suppression by adaptation of broadly tuned divisive normalization inputs that counteract the adapted excitatory, driving input. Second, the hand shape transformations during the action sequence can interfere with the adaptation to a hand pose to which a neuron is tuned, and this “disadaptation” can reduce the overall repetition suppression. Third, neurons responsive to a particular action snippet (e.g., reaching phase) can become disadapted when another snippet (e.g., hand withdrawal) intervenes between the presentations of the preferred snippet. Fourth, adaptation effects can recover to some degree over a brief delay (Patterson et al. 2013). If neurons respond to a snapshot or a short segment of the action movie, the rest of the action movie may serve as a delay, thus reducing the overall adaptation effect. Because of these considerations, it is unclear whether STS neurons would show repetition suppression to hand action stimuli such as those employed in the F5 studies. In fact, to our knowledge, thus far no single-unit study had assessed whether STS neurons show repetition suppression to hand action stimuli. To address this, we recorded the spiking activity (single and multiunit) and LFPs in the STS of two monkeys during the presentation of hand action movies. LFPs represent a population measure of neuronal, mainly synaptic, activity in the local cortical network (for recent review see Einevoll et al. 2013). In previous studies, we consistently observed repetition suppression for static stimuli of the STS LFP power for frequencies above 50 Hz but not for lower frequencies (De Baene and Vogels 2010; Kaliukhovich et al. 2013; Kaliukhovich and Vogels 2011, 2012, 2014), but it is unknown whether the same holds for dynamic action stimuli. To compare the STS data with those of the F5 studies, we employed the same hand actions and object as those used by Caggiano et al. (2013). We recorded from the upper bank of the STS, since this region is part of the action observation network (Nelissen et al. 2011; Vangeneugden et al. 2009, 2011).
MATERIALS AND METHODS
Subjects
The experiments were carried out in two rhesus macaques (Macaca mulatta; male monkey P, left hemisphere, and female monkey K, right hemisphere, weighing 9 and 6 kg, respectively). Animal care and experimental procedures met the national and European guidelines and were approved by the Ethical Committee of the KU Leuven Medical School. During experiments, the monkey's head was restrained by a plastic headpost that was fixed to the monkey's skull with acrylic cement and ceramic screws. The recording chamber was positioned with the guidance of preoperative MRI. The surgical implants were performed under aseptic conditions and isoflurane gas anesthesia.
Recordings
Extracellular single-unit recordings were performed in both animals with tungsten electrodes (FHC, 1- to 2-MΩ impedance) lowered through a guide tube positioned in a Crist grid and connected to a Narishige microdrive. The guide tube was grounded and served as a reference. Amplification and filtering were performed by a Plexon data acquisition system (Plexon). Recorded signals were preamplified with a headstage having an input impedance of >1 GΩ. The signal was band-pass filtered between 250 and 8,000 Hz for spikes and between 0.7 and 170 Hz for LFPs. Action potentials from single cells were isolated online with the “time-window discrimination” tool provided by the Plexon data acquisition system. Triggered spike waveforms were saved at 40 kHz for later off-line analysis (Offline Sorter, Plexon) in which single-unit isolation was rechecked. In monkey P, we recorded multiunit activity (MUA) with the same apparatus, except that we lowered the impedance of the electrode to 0.2–0.5 MΩ. LFPs were recorded simultaneously with the same low-impedance electrodes. MUA and LFPs were recorded in the other animal with a 16-channel laminar electrode (U-Probe, Plexon). The intercontact (channel) spacing was 100 μm with electrode sites linearly arranged on a single shaft (outer diameter of 185 μm). The U-Probe was lowered with a Narishige microdrive through a guide tube that was fixed in a Crist grid. The grounded guide tube and metal shaft served as the reference. The depth of the U-Probe was adjusted based on gray-white matter transitions, and visually driven MUA needed to be present on at least some of the channels. After positioning the U-Probe in the STS, we waited for ∼1–1.5 h before performing the recordings to ensure good recording stability. The spiking activity was thresholded online, and Offline Sorter was used to remove noise or electrical artifacts from the spiking activity, when necessary, but no further spike sorting was performed for the MUA.
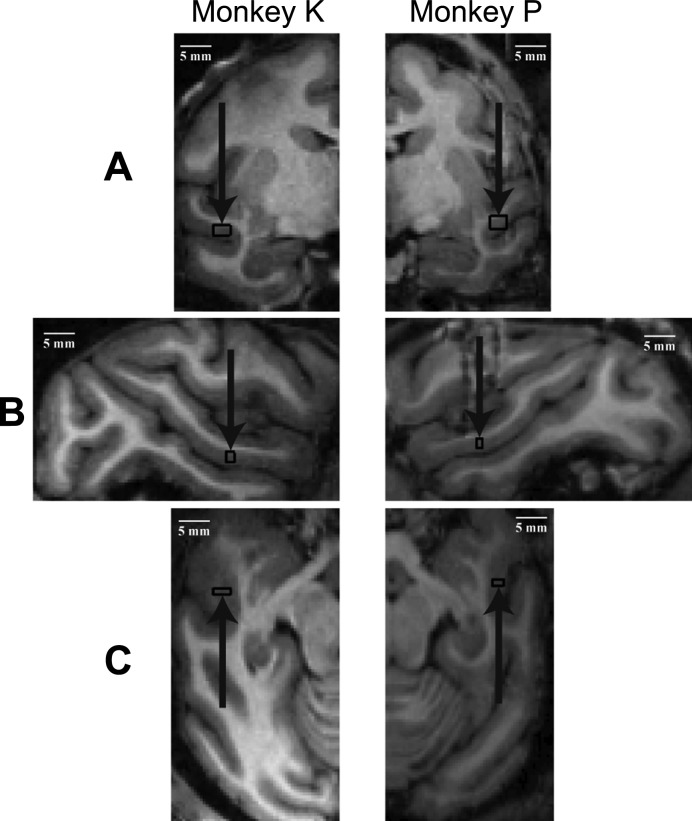
The estimations of the recording positions were obtained by MRI visualization of capillary tubes filled with a MRI opaque substance, copper sulfate (CuSO4), that were inserted into the recording chamber grid (Crist Instruments) at predetermined positions. The depths of the recording positions were estimated with the microdrive (Narishige Group) depth readings relative to the grid base and the white-gray matter transitions based on the recorded single-unit or multiunit activity. We aimed to record from the medial parts of the upper bank of the STS (21 and 22 mm lateral to the midline in monkeys P and K, respectively) since these regions showed activations to hand grasping actions in a previous monkey fMRI study (Nelissen et al. 2011). The recordings were at 16 and 17 mm anterior to the auditory meatus in the male monkey P and from 10 to 12 mm in the female monkey K (Fig. 1). The recording locations were based on an exploration of the upper bank of the STS for sites in which single neurons showed responses to the hand-action stimuli. In this exploration phase, we found several locations at which no or poor responses were present to hand-action stimuli. Neither the presence of repetition suppression nor the response pattern of the spiking activity during the course of the action served as a criterion to select the recording sites.
Fig. 1.
Recording sites. MRI images showing the estimated range of recording sites (boxes) in each monkey in the 3 anatomical planes. A: coronal section. B: sagittal section. C: horizontal section. Recordings were made in the right and left hemisphere of monkeys K and P, respectively. Scale bar, 5 mm. Arrows indicate the region recorded from.
Eye positions were measured online with an infrared-based eye tracking system (ISCAN EC-240A, ISCAN; 120 Hz sampling rate). The analog eye movement signal was saved with a sampling frequency of 1 kHz. Eye positions, stimulus, and behavioral events were stored for later off-line analysis on a computer that was synchronized with the Plexon data acquisition system.
Stimuli and Tests
The visual stimuli included movies depicting actions performed by a human hand. The movies were identical to those employed by Caggiano et al. (2013). The movies were recorded by a Canon XLI-S video camera with a frame rate of 25 Hz and resolution of 640 × 480 pixels in an uncompressed format. The video was recorded from above the left shoulder of the person who used the right hand to reach for or grasp the object. The recorded video sequences were edited by MATLAB to generate video clips with a resolution of 320 × 250 pixels in a noncompressed format. Homogeneous Gaussian noise estimated from the original background replaced the original background pixel values to ensure homogeneity of the background. Finally, each frame was smoothed with a normalized Gaussian kernel function. More details about the stimuli can be found in Caggiano et al. (2013).
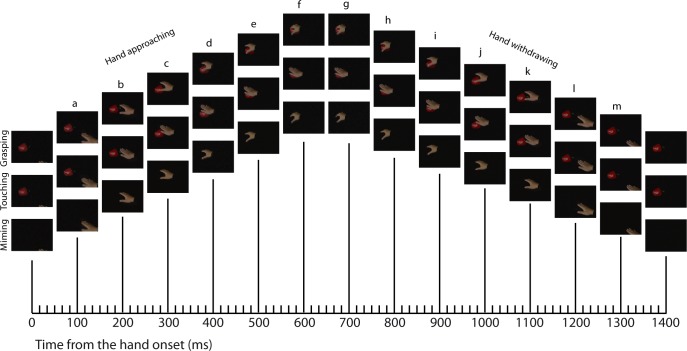
The present study included six video clips (Fig. 2): one in which the hand moves toward a dark red sweet pepper, grasps the pepper, releases the pepper, and withdraws from the pepper (grasping action); a second clip in which the hand moves toward the pepper and touches it with a flat hand, followed by a withdrawal of the hand (touching action); and a third clip in which the grasping is performed without the presence of the pepper (miming action). For each of these three versions we also created a version that was mirrored along the vertical axis. Including mirror versions of the actions increased the variation among the stimuli and prolonged the time span between across-trial stimulus repetitions. The pepper was presented at the center of the display, and thus the hand was coming either from the lower left or from the lower right of the visual field. The eccentricity of the starting point of the hand was 6.2° (4.8° to the left/right and 3.8° down). The pepper had a maximum extent of 3.2°. Each clip lasted 1,360 ms. The clips were presented in 4-s-long movies that consisted of a sequence of two clips. The following sequences were included: 1) repetition of the grasping action, 2) repetition of the touching action, 3) repetition of the miming, 4) grasping followed by touching, and 5) touching following by grasping. Each of these five conditions was shown with the hand starting from the left and the right visual field, thus defining 10 action sequences in total. The sequences started with the presentation of the pepper for 340 ms, except in the case of the miming sequences, where only the background was presented for that duration. After this presentation of the background or pepper only, a 1,360-ms-long clip (S1) was presented (grasping, touching, or miming) and was followed by a 640-ms-long “interclip” interval during which only the pepper was presented (or the background in the case of the miming sequences). After this “interclip” interval, the second clip (S2) of the sequence was presented. This clip was then followed by the presentation of the pepper (or the background only in the case of the miming sequence) for another 300 ms. A bright red fixation target (size 0.17°) was presented continuously on top of the pepper or background during the 4-s-long movie. The duration of the clips and the “interclip” interval were identical to those used by Caggiano et al. (2013).
Fig. 2.
Hand action stimuli. Movie snapshots, separated by 100 ms, i.e., every 6th frame, are shown for the grasping, touching, and miming actions with the hand starting in the right hemifield. The shifts in vertical position of the frames are for illustration purposes only. Letters indicate snapshots. The actual movies included all 240 frames.
In addition to the 10 action sequences, the test also included four other conditions with static stimuli. The first two conditions consisted of a 4-s-long presentation of the pepper, i.e., the same sequences as for the grasping and the touching conditions but without the moving hand. The difference between these two conditions was the orientation of the pepper (mirror reversal; see above). The other two conditions consisted of a sequence in which a snapshot of the grasping hand (without pepper) was presented twice. The two conditions differed in the orientation of the hand (mirror reversal). The sequences of the static hand conditions started with the presentation of the background for 500 ms, followed by a 1-s-long presentation of the static hand. This was followed by the background for 500 ms, which was followed again by the static hand for 1 s. After the second presentation of the hand, the background was shown for another 500 ms. All stimuli were displayed with a frame rate of 60 Hz on a CRT monitor in front of the animal.
The main adaptation test consisted in total of 14 conditions, of which 7 conditions were the mirror reversals of the other 7 conditions. The different actions and movement directions, with and without object, ensured variation in stimulus properties across sequences. Furthermore, the use of the grasping and touching actions allowed the presentation of “repetition” (same 2 actions in a sequence) and alternation (different actions in a sequence) trials to assess action-specific adaptation. The miming conditions tested the influence of the presence of the object (pepper) on the responses of the neurons and on the adaptation effect. The single-object (pepper) condition tested whether the neuron responded to the pepper alone and the degree to which the response suppression during the other sequences could result from adaptation to the object itself. The static hand sequences tested whether repetition suppression was present for a static stimulus. The different conditions were presented randomly interleaved.
A single trial started with the onset of the fixation target. After fixation of 200 ms the 4-s-long movie was presented, and this was followed by another fixation period of 50 ms. Continuous fixation during this 4,250-ms-long interval was then rewarded by a drop of apple juice. The size of the fixation window was 4°. This differed from Caggiano et al. (2013), since in that experiment the fixation window spanned the whole area of the movie. We wished to restrict eye movements, since these may affect the responses of STS neurons. Between trials, we presented a 500-ms movie of spatially and temporally scrambled images that filled the entire display. We constructed 15 spatially scrambled color images of natural scenes, and each frame of the 60-Hz movie consisted of a randomly drawn image of that pool of 15 images. During the presentation of the scrambled movie, the subject was allowed to make eye movements outside the fixation window. This movie was inserted between trials in order to reduce across-trial cross-adaptation.
A smaller number of neurons were tested with another adaptation test to examine stimulus-specific adaptation. This “flicker”-adaptation test included three new conditions. In each of these conditions, S2 was the grasping, touching, or miming clip with the hand starting in the hemifield that was contralateral to the hemisphere from which we recorded. The S1 in these movies consisted of randomly ordered frames (60 Hz) of the same clip as was presented as S2 of that sequence. These three conditions replaced one of the two single-object (pepper) conditions and the two conditions with alternation trials in which the hand started in the ipsilateral field. The other 11 conditions were identical to the main adaptation test.
Data Analysis: Spiking Activity
Data were analyzed with custom software written in MATLAB. Neurons or MUA sites with a minimum of seven unaborted trials per condition were considered for further analysis. The baseline activity was defined as the average firing rate in a window from 200 ms before movie onset until movie onset. Additionally, we defined six analysis windows during movie presentation, corresponding to each of three action phases for both S1 and S2. The three action phases included the hand approaching the object (duration 580 ms), the hand interacting with the object (or miming it; duration 200 ms), and the hand withdrawing from the object (duration 580 ms). These analysis windows were identical for all the conditions that included a hand action. The average firing rate was computed for each of these six windows for each trial of the 10 conditions that included a hand action. For each of the six analysis windows, we ran a split-plot ANOVA with as repeated, within-trial factor the baseline vs. analysis window and as between-trial factor stimulus condition (10). Only neurons or MUA sites showing a significant main effect of baseline vs. stimulus activity (split-plot ANOVA, P < 0.05/6; corrected for testing multiple windows) or a significant interaction between the two factors (split-plot ANOVA, P < 0.05/6) in at least one of the six windows were considered for further analysis. Cells passing the ANOVA test were considered for a nonparametric test Wilcoxon signed-rank test. For this test, we defined two analysis windows with duration of 1,360 ms each, one window for S1 and a second window for S2. The windows started at the onset of the hand movement. Wilcoxon tests compared the average firing rate in the baseline window and the analysis windows. For each condition, two tests were run, one for the S1 window and one for the S2 window. Significance level was set at P < 0.05. The purpose of the Wilcoxon testing was to select those conditions for which there was a response during the hand action for either S1 or S2. Furthermore, we required that the maximal net average firing rate computed with bins of 50 ms should be at least 10 spikes/s during either S1 or S2.
For each neuron and MUA site, an adaptation contrast index was computed by taking the difference between the firing rate to S1 and to S2 and normalizing that difference by the sum of the firing rate of both presentations. The adaptation indexes were computed for each condition with multiple analysis windows. One set of adaptation indexes were computed with the average firing rates in the 1,360-ms-long windows for S1 and S2. A second set of adaptation indexes, labeled peak adaptation indexes, were computed on the firing rates during the approaching or withdrawal phase of the action with a window of maximally 300 ms and of which the timing was defined by the peak of the response for each neuron and condition separately. For the main analysis, we chose the approaching phase since the majority of neurons had their response peak during the approaching phase of the action (see results). Thus we selected for each condition the neurons and MUA sites with a significant response in the approaching phase (580 ms) with respect to baseline (Wilcoxon signed-rank test, P < 0.05) for either S1 or S2. The net peak firing rate within a 50-ms bin also should exceed 10 spikes/s in the approaching phase for either S1 or S2. To define the time of the peak of the response, t(peak), we first convolved the spike trains with a Gaussian filter having a standard deviation of 50 ms. Then we averaged the convolved responses of S1 and S2, triggering on hand movement onset. The time t(peak) was then defined as the time (relative to hand movement onset) at which the convolved response (averaged across S1 and S2) was maximal. The averaging across S1 and S2 avoided a bias for S1 or S2. The firing rates for S1 and S2 were computed with the original unconvolved data and with a bin of 300 ms that was centered at t(peak). When t(peak) was <200 ms, the window started at 50 ms after movement onset and ended at 150 ms after t(peak). This avoided the inclusion of activity before 50 ms after movement onset. The maximal duration of the window (300 ms) was based on an inspection of the population peristimulus time histograms (PSTHs) across the S1 and S2 responses. It was a compromise between shorter, noisier windows and a longer window that would include nonresponsive later parts of the activity of the majority of the neurons. In control analyses, we varied the duration of the analysis window between 100 and 500 ms in 100-ms steps and obtained results qualitatively similar to those obtained with the standard 300-ms window. The median peak adaptation indexes for the single-unit data (n = 79 condition-neuron combinations) were all, except for the 100-ms window duration, statistically significantly higher than 0 (Wilcoxon signed-rank test, P < 0.05). For the MUA, the median peak adaptation indexes (n = 121 condition-site combinations) were statistically significantly higher than 0 for all five tested windows. Using a window equal to the entire approaching phase that started 50 ms after hand action onset also produced adaptation indexes that were significantly higher than 0 for the single-unit and multiunit activity, respectively. In results, we report the peak adaptation indexes since these windows are adapted on a cell-by-cell basis to the neuron/site and thus reflect the responses more reliably than a fixed window that lasts the entire approaching phase of the action. A similar analysis was conducted for the withdrawal phase of the action.
Data Analysis: LFPs
LFPs were filtered off-line with a digital 50-Hz notch filter (48- to 52-Hz fourth-order Butterworth FIR filter; Fieldtrip Toolbox, F.C. Donders Centre for Cognitive Neuroimaging, Nijmegen, The Netherlands). Trials in which the signal was <1 or >99% of the total input range were excluded. We employed the same method for spectral analysis of the LFP as De Baene and Vogels (2010) and Kaliukhovich and Vogels (2011). By convolving single-trial data with complex Morlet wavelets (Tallon-Baudry et al. 1997) and taking the square of the convolution between the wavelet and signal, the time-varying power of the signal for every frequency was obtained. Averaging spectral maps (power as a function of frequency and time) across trials for a given condition and site produced a spectral map of that condition and site. The complex Morlet wavelets had a constant center frequency-spectral bandwidth ratio (f0/σf) of 7, with f0 ranging from 1 to 170 Hz in steps of 1 Hz. The spectral maps of the sites were normalized at each frequency by the average power within the baseline window of 50 ms before movie onset. We considered only those sites for which the split-plot ANOVA analyses of the spiking activity showed a significant response (see above).
To quantify the adaptation effect for the average power for different spectral frequencies, we defined a high and a low frequency band in each animal. These two bands were based on inspection of the time-frequency plots, averaged across the actions and S1 and S2. As for the single-unit and MUA responses, the LFP responses to the actions were mainly present in the approaching phase. Thus taking a window as long as the movie presentations would have produced noisy data, since these would include unresponsive phases. To avoid that, we selected the analysis windows for each monkey separately by visual inspection of the mean of the time-frequency plots averaged across S1 and S2. The windows differed slightly between the two animals in both the temporal and frequency domains, given differences in power across time and frequency between the animals as was evident from the averaged time-frequency plots. For monkey P, the analysis window for the high frequency band started at 100 ms (postmovement onset) and ended at 270 ms and included the frequencies between 50 and 100 Hz. The window for the low frequency band started at 165 ms and ended at 580 ms, including frequencies from 5 Hz to 35 Hz. For monkey K, the analysis window for both bands started at 150 ms and ended at 580 ms and for the high and low spectral bands included the frequencies between 60 and 100 Hz and between 5 Hz and 20 Hz, respectively. The normalized power was averaged in each time-frequency window, and this was done separately for S1 and S2 of each action. Adaptation contrast indexes for each frequency band were computed with the thus-averaged power.
All statistical tests were two-tailed.
RESULTS
We examined whether macaque upper bank STS neurons show repetition suppression for the same hand action stimuli that failed to show repetition suppression in area F5 mirror neurons (Caggiano et al. 2013).
Spiking Activity
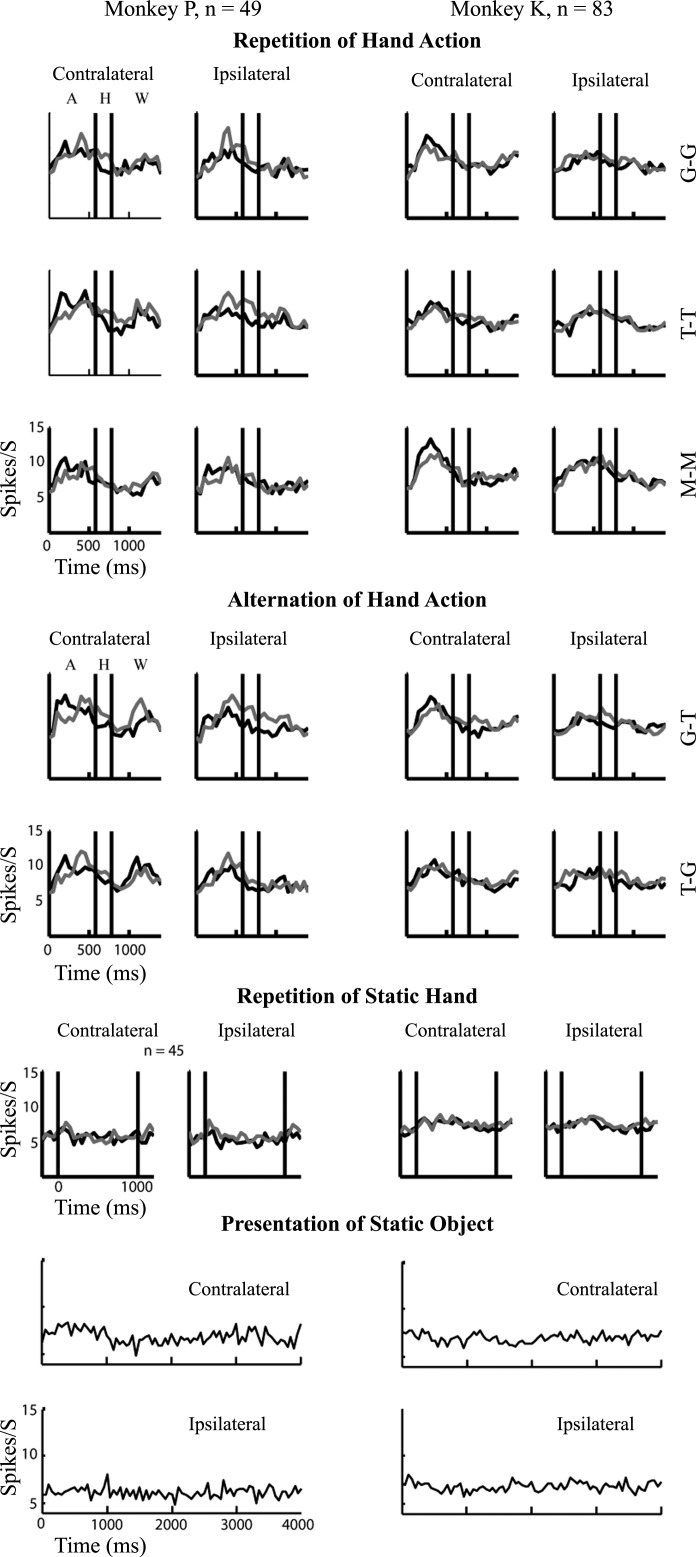
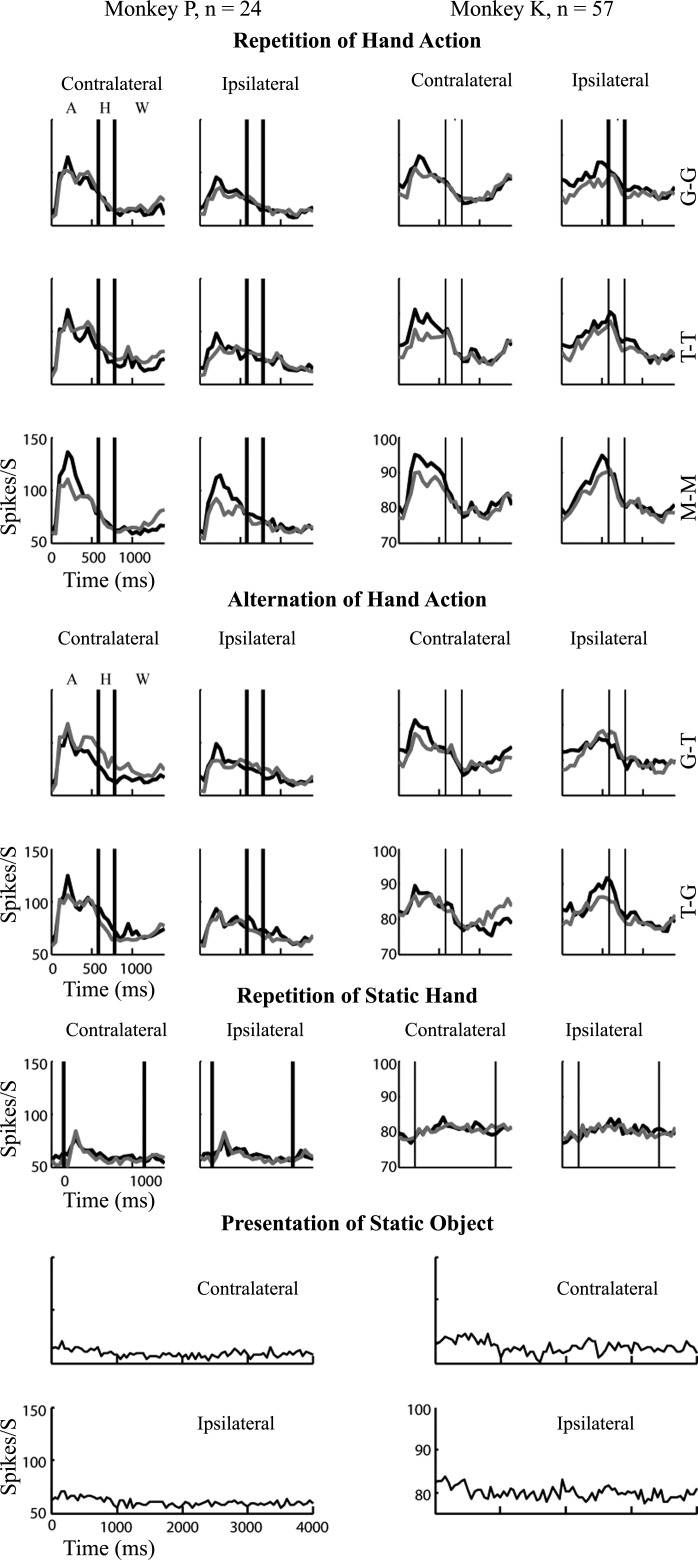
We recorded in separate recording sessions from 216 well-isolated single neurons (110 in monkey K) and from 108 MUA sites (80 in monkey K) located in the medial part of the rostral upper bank STS of two monkeys. The main test consisted of 14 randomly interleaved conditions: 6 conditions in which a hand action was repeated, 4 conditions in which the movies consisted of a sequence of two different hand actions, 2 conditions in which a static hand was repeated, and 2 in which the grasped or touched object was presented. We presented each action, object, and hand from two mirror-reflected perspectives. The object was presented centrally, and the hand was approaching the object from either the left or right lower visual field. We only included single neurons or MUA sites when they showed a significant response during at least one of the action sequences, as assessed with an ANOVA (see materials and methods). This selection yielded 83 single units and 57 MUA sites in monkey K and 49 single units and 24 MUA sites in monkey P. To obtain a general view of the responses of the selected neurons and sites in the different stimulus conditions, we show the averaged PSTHs for the 14 conditions of each animal in Fig. 3 and Fig. 4, respectively. Note that because we recorded from different hemispheres in the two animals the ipsi- and contralateral stimuli differed between the animals. Overall, the responses to the action stimuli were stronger when the hand started to move in the contralateral compared to the ipsilateral visual field, reflecting the known contralateral receptive field bias of STS neurons (Vangeneugden et al. 2009). Therefore we analyzed initially only the contralateral responses (as was done also by Caggiano et al. 2013). Close inspection of Figs. 3 and 4 shows that the average peak firing rate to S1 was larger than to S2 for several (but not all) repetition conditions in which the hand started contralaterally.
Fig. 3.
Spiking activity averaged across the population of upper bank superior temporal sulcus (STS) single units. Population peristimulus time histograms (PSTHs) are plotted for the neurons in monkeys K and P separately for each starting position of the hand (hemifield ipsilateral or contralateral with respect to the hemisphere from which recordings were made). Top: hand action repetition trials; action types are indicated by letters (G, grasping; T, touching; M, miming). Middle: hand action alternation trials (e.g., row 4: grasping followed by touching). The PSTHs for the S1 and S2 presentations are shown by black and gray lines, respectively, and 0 corresponds to hand movement onset. Solid vertical lines indicate the period during which the hand remains relatively still (H period in top left panels). Approaching and withdrawing phases of the action are indicated by A and W, respectively, in top left panels. Bottom: responses to presentations of static stimuli: repetition of a snapshot of the hand (H-H) and presentation of the object, split for the contralateral and ipsilateral conditions (i.e., mirror images; see materials and methods). Solid vertical lines for static hand PSTHs (H-H) indicate the onset and offset of the hand with 0 corresponding to hand onset. The H-H condition was tested in 45 of the 49 neurons in monkey P but in all 83 neurons of monkey K. Responses to the static object are plotted for the full 4-s stimulus duration, with 0 corresponding to the onset of the object. Bin width 50 ms; unsmoothed.
Fig. 4.
Spiking activity averaged across the population of upper bank STS multiunit activity (MUA) sites. Same conventions as in Fig. 3.
Whole action duration analysis: contralateral visual field.
The PSTHs shown in Figs. 3 and 4 represent average responses of all neurons that showed a significant response during at least one of the action conditions. In other words, the responses shown in these figures for a particular action condition will include neurons that did not respond to that particular action condition. This approach dilutes the overall response and repetition effects. Thus, to examine the adaptation effects quantitatively, we selected for each neuron and MUA site the repeated action conditions that produced a significant response for either S1 or S2 (see materials and methods). This analysis was performed first for the three action repetition conditions for which the hand started in the contralateral field. This analysis yielded 69 and 50 condition-neuron combinations for monkeys K and P, respectively, and 88 and 48 condition-site combinations for K and P, respectively. We quantified the degree of adaptation by computing an adaptation contrast index (see materials and methods) for each condition-neuron and condition-site combination. This index can vary between −1 and 1, with negative and positive indexes indicating repetition enhancement and suppression, respectively, and 0 corresponding to an equal response to S1 and S2. Computing this index for the whole duration of the action (1,360-ms clip) yielded mean values of 0.01 and −0.04 for the single units of monkeys K and P, respectively, and 0.02 and −0.01 for the MUA in K and P, respectively. When pooling across the two monkeys, the mean adaptation contrast index was −0.01 (Wilcoxon test, P = 0.77; n = 119) for the single units and 0.01 (P < 0.0005; n = 136) for the MUA. Thus, similar to Caggiano et al. (2013), no repetition suppression was present for single units when considering the whole action duration. There was significant repetition suppression for the MUA when pooling across animals, but this suppression was present only in one animal (monkey K), with the other monkey showing slight enhancement instead of suppression. The absence of an overall repetition suppression might result from some neurons showing a suppressed activity with repetition and a similar number of other neurons showing an enhanced activity. We addressed this by testing for each neuron and site that showed a significant response to either S1 or S2 for at least one action type in repetition trials the significance of the response difference between S1 and S2 in those trials with a Wilcoxon signed-rank test. To increase power, we pooled for each neuron the trials of the action types that produced a significant response for either S1 or S2 in repetition trials. Of 57 responsive single neurons, 16 showed a significant difference in the response between S1 and S2, with the response to S1 being higher in 9 neurons. Of the 66 MUA sites, 19 of 24 sites with a significant difference between S1 and S2 showed a suppressed response to the repeated stimulus. Thus the majority of MUA sites that showed a significant difference between S1 and S2 decreased their response with repetition, but this tendency was negligible for the single units.
Approaching action phase: contralateral visual field.
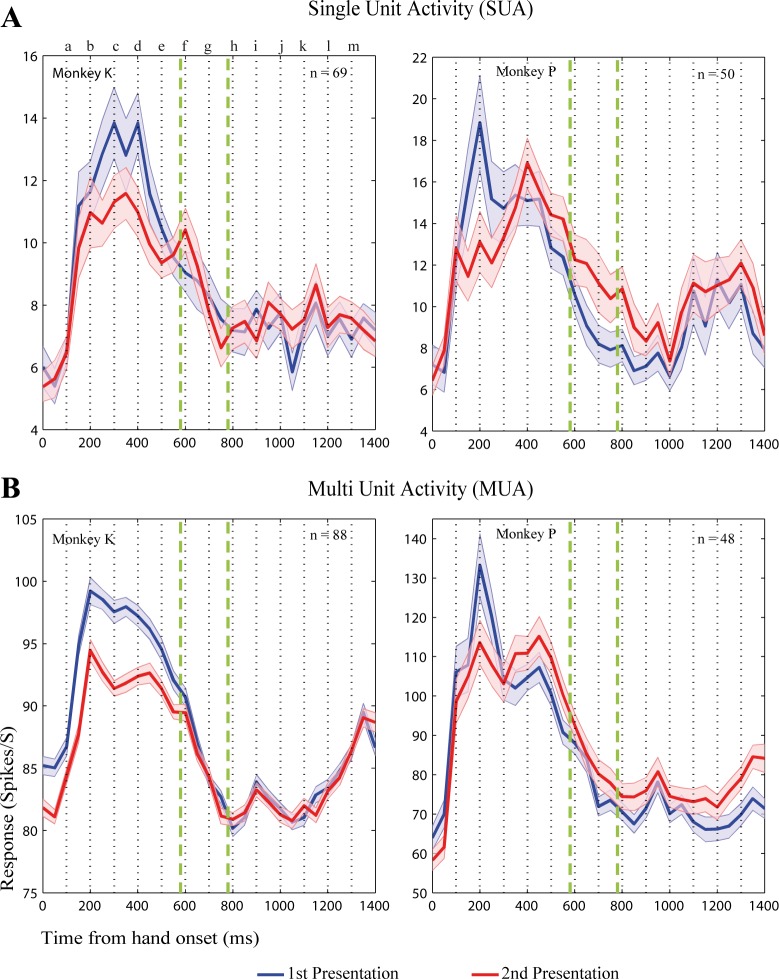
Closer examination of the data showed that this overall negative result was because the large majority of the neurons responded mainly during the approaching phase of the hand action and thus averaging the responses across the whole action period may have strongly underestimated, if not abolished, the repetition suppression that was present during the early phase of the action. This can be seen clearly in Fig. 5, which shows the mean PSTHs for S1 and S2, triggered on hand movement onset, for the condition-neuron and condition-site combinations for which there was a significant response (note that these differ from those shown in Figs. 3 and 4, because for those figures we did not require that the neuron demonstrate a response in each of the shown action conditions). In each animal and for both single units and MUA, the response to S1 was clearly greater than to S2 during the approaching phase of the action. There was a trend toward a repetition enhancement during the hand withdrawal phase in monkey P, but this was not present in the other animal.
Fig. 5.
Repetition suppression for hand action stimuli of upper bank STS spiking activity. A: single-unit activity (SUA). B: MUA. Solid blue line: population PSTHs for S1 (1st presentation of the action in a trial). Solid red line: PSTHs for the same neurons or sites for the repeated action (S2; second presentation of the same action as S1 with the trial). Shaded band indicates the SE, computed following the procedure by Loftus and Masson (1994), which removes the variance due to the differences in the overall mean response across neurons or sites. PSTHs were triggered on the onset of the hand action motion (= 0 ms). Dotted vertical lines and letters correspond to the timing of the snapshots shown in Fig. 1. Green vertical dashed lines indicate the period during which the hand remains relatively still. Responses were averaged across the condition-neuron (A) or condition-site (B) combinations for which there was a significant response during either the S1 or S2 presentation. Bin width 50 ms; unsmoothed.
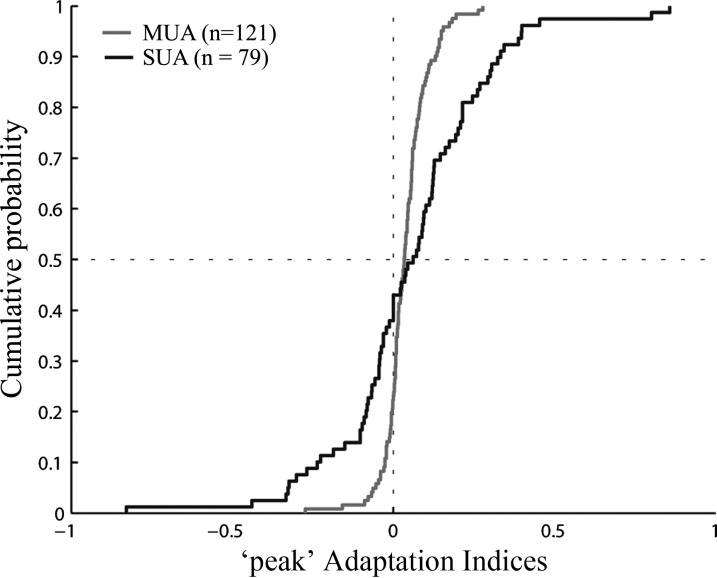
To quantify the degree of adaptation during the approaching phase of the action, we averaged the firing rate in a window of maximally 300 ms that was centered on the peak of the responses averaged across S1 and S2 (results were similar when applying shorter or longer window durations; see materials and methods). Thus the timing of the window could vary across conditions and neurons, depending on when the peak firing rate occurred during the approaching phase of the action, but was identical for S1 and S2. This peak adaptation index was only computed for those condition-neuron (n = 79; 66%) and condition-site (n = 121; 89%) combinations for which the peak response happened during the approaching phase of the hand actions with a minimum delay of 50 ms. The medians of thus computed peak adaptation contrast indexes were positive in each animal for both single-unit activity (median monkey P: 0.08; median monkey K: 0.04) and MUA (P: 0.02; K: 0.04). The overall mean peak adaptation index was 0.06 and 0.04 for the single-unit and multiunit data, respectively (Fig. 6). For the single-unit data, this corresponds to an 11% suppression of the response during the approaching action phase when repeating the stimulus. The reason why the mean peak adaptation index was smaller for the MUA than for the single units is because the adaptation indexes were computed using raw responses, i.e., without subtracting the baseline activity, and these responses were much higher for the MUA than for the single units, thus increasing the denominator of the adaptation index. The average indexes were significantly higher than 0 for the single unit and the MUA data [after averaging indexes across conditions for each neuron (Wilcoxon test, P < 0.05; n = 47 neurons) or MUA site (P < 0.00001; n = 60 sites)].
Fig. 6.
Cumulative distribution of peak adaptation indexes for SUA and MUA, pooled across monkeys. The peak adaptation index is plotted for those condition-neuron (n = 79) and condition-site (n = 121) combinations for which the peak response happened during the approaching phase of the hand actions with a minimum delay of 50 ms. Only actions for which the hand started in the contralateral field were included.
Adaptation for approaching phase compared among action types: contralateral visual field.
To assess whether the degree of adaptation depended on the action type we split the peak adaptation indexes (computed during the approaching phase) per action type, and to increase power we pooled the single-unit and MUA data of the two animals. The mean peak adaptation indexes were significantly larger than 0 for each of the three action types [mean adaptation indexes (P values, Wilcoxon signed-rank test; n = number of neurons/sites): grasping: 0.05 (P = 0.03, n = 48), touching: 0.05 (P = 0.002, n = 66), miming: 0.04 (P = 0.00006, n = 86)]. Thus, overall, repetition suppression was present for each of the three action types. Furthermore, the degree of repetition suppression was correlated between the action types to which a neuron responded [Spearman rank correlation for the neurons that showed a significant response (to either S1 or S2) to both action types (e.g., response to both grasping and touching): r = 0.41 (P = 0.007; n = 41 pairs)].
The repetition suppression that we observed for the grasping and touching might have been caused by a response reduction to the pepper but not to the repeated action. To address this, we computed the response in the pepper condition using the same analysis windows as those that were employed to compute the peak adaptation contrast indexes for the action stimuli. We reasoned that if the repetition suppression in the action conditions was because of a suppression of the response to the action and not merely to the object, the difference in response between S1 and S2 for the actions should be larger than the differences in responses measured with the same analysis windows for the pepper. This was indeed the case for both the grasping and the touching, but the difference between the pepper and action conditions in the S1–S2 comparison reached significance for the touching only [Wilcoxon signed-rank test; grasping: P = 0.18 (n = 48), touching: P = 0.01 (n = 66)]. Note that the repetition suppression observed for the miming action cannot be attributed to an adaptation for the object since the latter was not present in that condition.
Stimulus specificity of adaptation: grasping vs. touching.
To assess whether the repetition suppression was stimulus specific, we selected those neurons and MUA sites that showed a significant responses to either the grasping or touching action when the hand moved in the contralateral field. To do this, we ran a split-plot ANOVA with baseline vs. analysis window as repeated, within-trial factor and stimulus condition as between-trial factor. We included four stimulus conditions: repetition of the grasping action, repetition of the touching action, and the two conditions in which the action differed between S1 and S2 (alternation conditions). The ANOVA was performed for each of the same six analysis windows as defined to test for the responsiveness of a neuron (see materials and methods). Only neurons or MUA sites showing a significant main effect of baseline vs. stimulus activity (split-plot ANOVA, P < 0.05/6 corrected for testing multiple windows) or a significant interaction between the two factors (split-plot ANOVA, P < 0.05/6) in at least one of the six windows were considered for further analysis. The responses were averaged across the two actions and the two animals. Both the single-unit and MUA data show a decreased response to S2 in repetition trials (Fig. 7), which tended to be less than the response to S2 when it differed from S1 in action type for the MUA (Fig. 7B). However, the difference between the S2 responses in repetition and alternation trials was small, providing little evidence for action-specific repetition suppression.
Fig. 7.
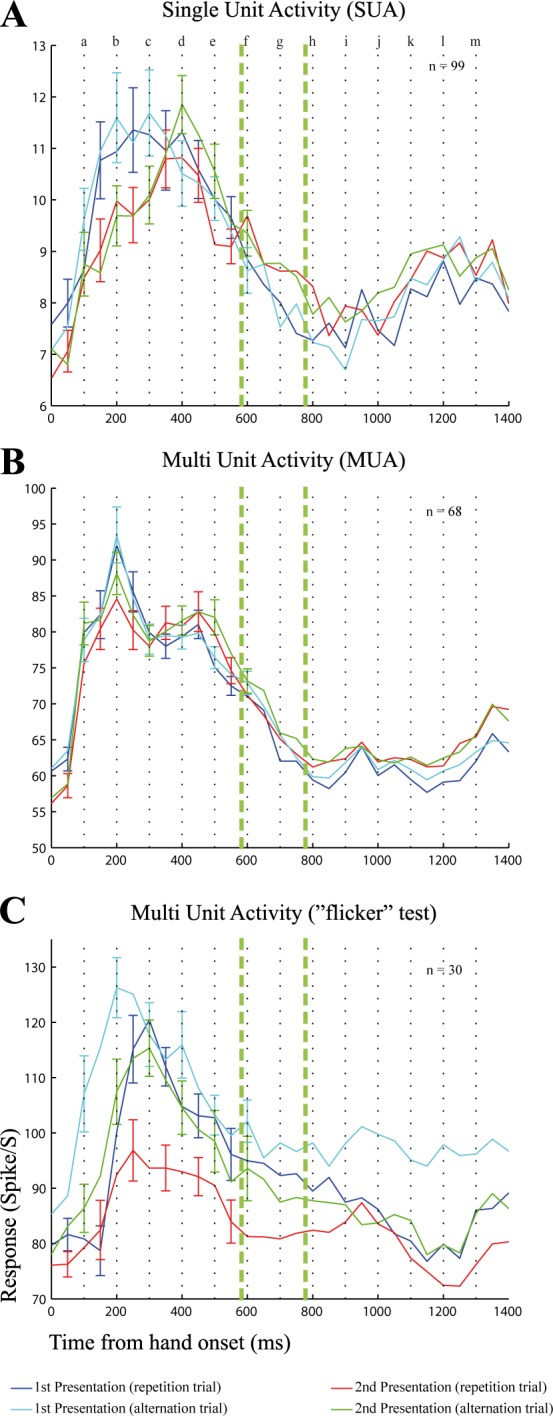
Comparison of spiking activity in repetition and alternation trials. A and B: population PSTHs for SUA (A; n = 99 neurons) and MUA (B; n = 68 sites) for 4 stimuli: S1 in repetition trials (blue line), S1 in alternation trials (cyan line), S2 in repetition trials (red line), and S2 in alternation trials (green line). Responses were averaged across the grasping and touching conditions and the 2 animals. C: population PSTHs of MUA to 4 stimuli: averaged MUA to S1 in repetition trials of the grasping, touching, and miming actions (blue line), averaged MUA to S2 in the same repetition trials (red line), averaged responses to S2 when these actions where preceded by the temporally scrambled clip of the same actions (“flicker” stimulus; green line), and averaged response to the “flicker stimulus” itself (cyan line). Error bars indicate the SE computed as in Fig. 5; error bars are shown for alternating data points for the sake of visibility. See results for more details. Same conventions as in Fig. 5.
The difference between touching and grasping might have been too small to show strong stimulus-specific repetition suppression in the STS. We compared the responses to these two action types, presented as S1 (pooling repetition and alternation trials for contralateral presentations; analysis window equal to the approaching phase with a 50-ms delay). Indeed, only 12 of the single neurons (n = 47) and 25% of MUA sites (n = 60) that responded significantly to either the grasping or touching action showed a significantly different response between these action types (Mann-Whitney U-test, P < 0.05).
Stimulus specificity of adaptation: “flicker” test.
To enhance the possibility of demonstrating stimulus-specific repetition suppression, in a subsequent experiment we increased the difference between S1 and S2 by replacing S1 by a clip of randomly ordered frames (60 Hz) of the same action that was presented as S2 of that sequence. This was done for the grasping, touching, and miming actions. We measured MUA in this “flicker”-adaptation test that included three conditions in which S2 was the grasping, touching, or miming clip with the hand starting in the hemifield that was contralateral to the hemisphere from which we recorded and S1s were temporally scrambled versions of those clips (see materials and methods). The responses were averaged for the 30 MUA sites (16 in monkey K) for which the MUA showed a significant responses with respect to baseline (split-plot ANOVA for 6 windows; see materials and methods). Note that the average response to the temporally scrambled movie tended to be at least as strong as that to the action movies. This could be due to the fact that the spatial features of the hand were still preserved in the scrambled movies since the scrambling was performed in the temporal domain only. The changes in hand position from frame to frame produced an impression of a random motion of the hand (which, however, is still along the 2 original movement directions). This seems to be sufficient to elicit responses of the STS neurons that we recorded from. This new sample of sites confirmed the presence of repetition suppression in repetition trials. Importantly, the response to S2 when it was preceded by scrambled S1 stimuli was similar to that obtained for S1 in repetition trials, indicating stimulus-specific repetition suppression in the repetition trials. We quantified the response differences to S1 and S2 by computing two indexes: one that contrasted the responses to S1 and S2 in repetition trials (adaptation index) and a second that contrasted the responses to S1 in repetition trials and to S2 when preceded by the scrambled S1 (cross-adaptation index). As before, these indexes were computed using a maximally 300-ms-long window that was centered on the time of the peak response during the approaching phase of the action (see materials and methods). The time of the peak response was defined on the smoothed responses (convolution with a Gaussian filter with standard deviation of 50 ms; see materials and methods), averaged across S1 of the repetition trials, S2 of the repetition trials, and S2 following the scrambled S1. The mean peak adaptation index was 0.03, which was significantly higher than 0 [Wilcoxon test, P = 0.001; n = 30 sites (peak adaptation indexes averaged across action conditions per site)]. The attenuation in response for the second stimulus presentation was absent for cross-adaptation trials (mean peak cross-adaptation index: −0.01, P = 0.73; n = 30 sites). Thus, despite the MUA response to the scrambled movies, the responses to the S2 presentation of the unscrambled hand actions that followed the scrambled S1 were significantly higher than for a repetition of the unscrambled hand actions (where S1 is identical to S2). This indicates that the suppression of the responses to S2 depended on the previous S1 and thus is stimulus specific. This stimulus specificity also shows that the decreased response to S2 in repetition trials is not related to the impending reward after S2 (which was also present in the scrambled S1 conditions) and is not due to an aspecific decrease of the response during the course of a trial.
Approaching action phase: ipsilateral visual field.
Analyzing the conditions in which the hand started in the ipsilateral field showed a significant enhancement of single-unit responses to S2 during the approaching phase in repetition trials in monkey P (mean peak adaptation index: −0.11, P < 0.05; n = 21 neurons; only neurons showing a significant response were included) and a similar but not significant trend in the other animal (−0.03, P = 0.51; n = 26 neurons). However, the MUA data showed a significant repetition suppression in each animal (mean peak adaptation indexes: monkey P: 0.05, P < 0.05; n = 16 sites; monkey K: 0.03, P < 0.00005; n = 35 sites). The discrepancy between the ipsi- and contralateral single-unit data and between the ipsilateral single-unit and MUA data could be due to the on average smaller ipsilateral responses.
Withdrawal action phase: contralateral visual field.
We performed the same analysis for the contralateral visual field data for the withdrawal phase as we reported above for the approaching phase (i.e., selecting neurons for which there was a significant response during the withdrawal phase and for which the peak response was during the withdrawal phase; 300-ms analysis window). There was no significant adaptation effect during the withdrawal phase, neither for the spiking activity (mean peak adaptation index = −0.10, P = 0.15; n = 32 condition-neuron combinations) or for the MUA (mean = −0.06, P = 0.10; n = 9) nor when pooling the single units and MUA (P = 0.06). Note the small sample size of the neurons with a significant response during the withdrawal phase, in agreement with the overall lower response strength during that action phase (Fig. 5). One reason for the absence of repetition suppression during the withdrawal phase is the lower response of the selected neurons responsive for that action phase [mean single unit raw firing rate to S1: 15 spikes/s (n = 32); mean raw MUA: 83 spikes/s (n = 9)] compared with those responding during the approaching phase (single units: 20 spikes/s; MUA 105 spikes/s). Another possibility is that repetition suppression mainly occurs during the initial part of the response or stimulus presentation, a tendency that was observed also for static stimuli in the STS (Kaliukhovich and Vogels 2012).
Local Field Potentials
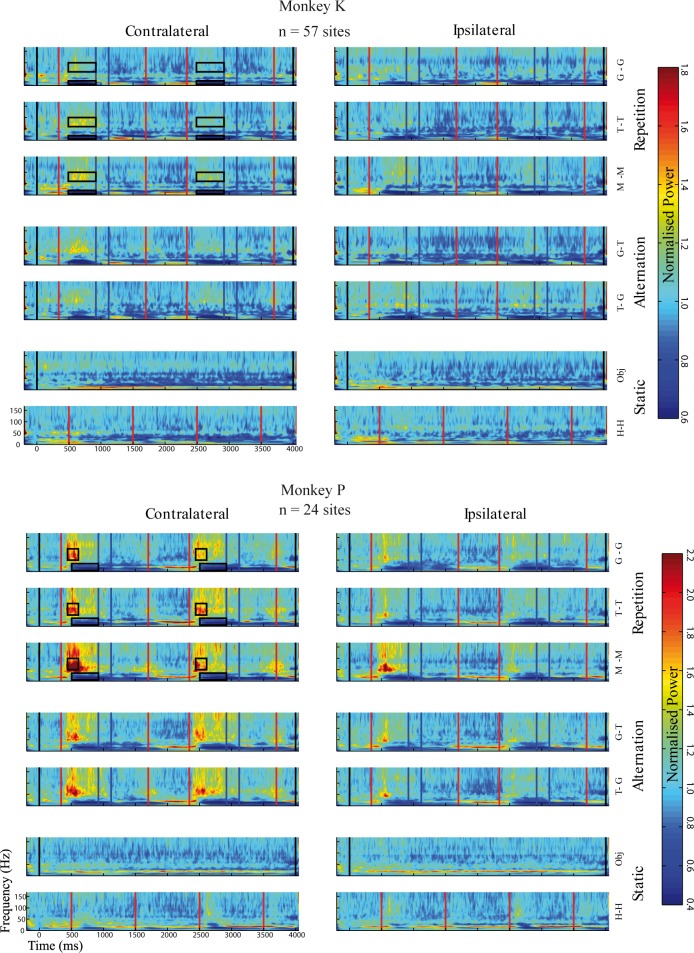
We performed a time-frequency power analysis of the LFP waveforms of each trial. For frequencies above 50 Hz, there was a strong increase in power during the approaching phase of the action, which reduced to close to baseline levels when the hand interacted with the object and when it was withdrawn from the object. This strong modulation of the LFP gamma power during the course of the action was present in both animals (Fig. 8) for each of the three actions in both hemifields. The power for frequencies in the alpha and beta bands, however, was less visual field specific, and the movement onset resulted mainly in a decrease of the power in those frequency bands.
Fig. 8.
Time-frequency plots of averaged normalized local field potential (LFP) power of the upper bank STS sites for all stimulus conditions. In top 6 rows, vertical black lines indicate the onset and offset of the static object (pepper). Red lines indicate the onset and offset of the hand actions (top 5 rows) or static hand presentation (7th row). Dark blue lines indicate the period during which the hand remains relatively still (touching or grasping the pepper). Boxes for the contralateral data show the time-frequency windows that were used to compute the average power for the high and low frequency bands, respectively (see results). The power was normalized by that obtained during the baseline period that preceded the onset of the pepper (normalized power of 1 indicates a power equal to the baseline power). Same conventions as in Fig. 3.
We defined in each monkey a low and a high frequency band (Fig. 8; see materials and methods), and we computed adaptation contrast indexes for the three contralateral action conditions (grasping, touching, and miming) for each band. Both animals showed mean adaptation indexes that were significantly larger than 0 for the gamma band [monkey P: mean adaptation contrast index = 0.08 (P = 0.00002; n = 24 sites; Wilcoxon test); monkey K: 0.04 (P < 0.00001; n = 57)], indicating repetition suppression. The repetition suppression was present and highly significant for each of the three action types [all P < 0.00001; pooled across the 2 animals (n = 81)]. The difference in average power between S1 and S2 for the grasping and touching actions was significantly greater than the difference in power computed for the same time windows of the pepper presentation (P < 0.0005 for each action type; pooled across monkeys). Thus the repetition suppression of the gamma power for the grasping and touching actions did not result from an adaptation to the continuously presented object (pepper).
The low frequency band showed a significant repetition suppression in monkey P only (mean adaptation contrast index: 0.04, P < 0.005; n = 24), while there was no effect of repetition in the other animal (mean = 0.01, P = 0.11; n = 57). In monkey P, the difference in average low-frequency power between S1 and S2 for the grasping and touching actions did not significantly differ from the difference in power computed for the same time windows of the pepper presentation (Wilcoxon signed-rank test, not significant). The suppression of the low-frequency power for the miming action was small (mean index = 0.02) and just significant (P = 0.02) in monkey P, suggesting repetition suppression for dynamic stimuli when no object was present in this monkey.
Analysis of Eye Movements
Both monkeys were required to fixate within a window of 4° during the entire presentation of the trial (4,250 ms). We analyzed eye positions along x and y directions and their respective velocities for each condition-neuron and condition-MUA site combination that showed a significant response to either S1 or S2 in the conditions in which the hand started to move in the contralateral visual field. Only repetition conditions were analyzed. For eye velocities we employed the same method as Engbert and Kliegl (2003) and Kaliukhovich and Vogels (2011). In short, the eye movement signals along the x and y directions were low-pass filtered (<40 Hz, 5th-order Butterworth filter) to remove high-frequency noise and then differentiated in time to obtain eye velocity.
The mean eye position, averaged across single units and MUA sites, differed only slightly between the S1 and S2 presentations, the maximal absolute difference during the course of the clip presentations being <0.55° for the horizontal and vertical direction in each monkey and repetition condition. These are rather small eye position differences compared with the large receptive field size of upper bank rostral STS neurons [larger than 40° according to Anderson and Siegel (1999)]. We performed several quantitative analyses of eye movements, but none demonstrated a clear relationship between differences in eye movements and neural responses. In a first analysis, we assessed whether the time course of the difference in neural responses to S1 and S2 covaried with the time course of the difference in eye movement metrics. To do so, we cross-correlated neural responses and eye movements across 28 nonoverlapping bins of 50-ms duration, starting at the hand movement onset. For each bin and trial, we computed the average firing rate, the average eye position for the x and y directions, and the average eye velocity for the x and y directions. Then we computed for each bin and condition-neuron/site combination d′s for each of these five metrics:
with M(S1) and M(S2) being the mean value of the metric across trials and σ(S1) and σ(S2) being the standard deviation for the S1 and S2 presentations, respectively. For each condition-neuron/site combination we then computed the cross-covariance between the ordinal values of the d′s for the neural responses and each of the four eye movement metrics (eye positions along x and y directions and their respective velocities). The statistical significance of the observed cross-covariance was assessed for each time lag by a two-sided permutation test (P < 0.05) in which null distributions were computed by permuting the order of the neural responses 1,000 times. Of a total of 79 single neuron-condition combinations, 17, 16, 5, and 8 showed a significant cross-covariance at 0 ms time lag for the x eye position, y eye position, x eye velocity, and y eye velocity, respectively. For the 121 condition-MUA site combinations, the numbers of significant effects were 37, 35, 5, and 18 for the four different eye movement metrics. For a 50-ms time lag (with the eye movements lagging the neural responses), the number of significant cross-covariances was 14, 15, 6, and 10 for single units and 35, 33, 5, and 23 for the MUA. Thus only a minority of the cases showed a significant covariance of the time course of the neural repetition effect and the eye movement signal. Importantly, the mean peak adaptation index was similar for the conditions in which there was no significant cross-covariance of the time courses of the neural responses and eye movements (mean peak adaptation index across conditions and monkeys: 0.05) and those for which the time courses covaried significantly (mean: 0.04). Similar results were obtained when employing adaptation contrast indexes instead of d′ as neural response metric or when computing an eye movement d′ using the length of the hypotenuse of the eye velocity along the x and y directions {“hypot[velocity(x), velocity(y)]”}.
In a second analysis, we correlated directly the differences in peak response (computed as average firing rates in 300-ms-long windows; for each condition, exactly the same windows as used to compute the peak adaptation indexes) between S1 and S2 with the differences in eye metrics. The differences in peak responses for each trial were normalized as follows:
with R(Sx) being the peak response in trial i for stimulus x and σ(Sx) being the standard deviation of the response to stimulus x for the trials of a particular condition. Thus the neural response difference was normalized by the variability of the responses (“noise”). The normalized response differences NR were correlated with the difference in mean eye position between S1 and S2, measured in the same time window as the neural responses. These correlations were performed across all trials of all conditions showing a significant response to either S1 or S2. The Pearson correlation coefficients between the differences in neural response and in eye position were all low, ranging between −0.03 and 0.05 for the x and y directions, monkeys, and single/MUA measures. Similar negligible correlations were present when taking the absolute (unsigned) response and absolute eye position differences (Pearson correlation coefficients ranging between −0.08 and 0.06; median 0.002).
DISCUSSION
We demonstrated repetition suppression for dynamic hand actions in the macaque STS for both spiking activity and LFP power at frequencies above 50 Hz. In the upper bank of the STS, repetition suppression was present in the approaching phase of the action, which was also the phase in which the response was the strongest overall.
Comparison with Human Adaptation Studies
It is likely that the human homolog of the macaque upper bank STS is the human STS, while the macaque lower bank of the STS corresponds to the more ventral human occipito-temporal cortex (Caspari et al. 2014; Fisher and Freiwald 2015). Human fMRI adaptation studies that employed actions produced a mixture of results: Shmuelof and Zohary (2005) reported repetition suppression in fusiform gyrus areas but not in the STS for 10 repetitions of a grasping action. In another study, a single repetition of a hand action did not produce repetition suppression in the STS but did so in lateral occipital complex (LOC) (Dinstein et al. 2007). Hamilton and Grafton (2006) found no repetition suppression for hand actions in the STS, while Lingnau et al. (2009) found repetition suppression for hand actions in LOC and the extrastriate body area. However, a range of occipito-temporal regions, including the STS, showed adaptation in a priming paradigm with many (>5) repetitions of a wide variety of actions (Kable and Chatterjee 2006). For point light displays of a wide range of actions, several studies reported repetition suppression in occipito-temporal cortex, again including the STS (Grossman et al. 2010; Jastorff et al. 2009), even for single repetitions. Thus it appears that the human STS shows repetition suppression for dynamic action stimuli, but less so for hand actions. However, repetition suppression for hand actions is present in more ventral occipito-temporal areas, likely homologs of monkey inferior temporal cortex proper. Our data do not disagree with the absence of repetition suppression for hand actions in the STS in fMRI adaptation studies. Indeed, when we averaged the spiking activity across the whole action, no repetition suppression was detectable for the spiking activity in the upper bank of the STS. It was only when we analyzed the approaching phase of the action separately that we were able to demonstrate repetition suppression. Isolating activation to the approaching phase of the action is difficult with a low-temporal resolution technique such as fMRI, and thus fMRI may miss repetition suppression that is limited to a particular phase of the action. The repetition suppression that we observed in the present study may underlie the behavioral adaptation effects observed for hand actions in human psychophysical studies (Barraclough et al. 2009). In those studies, the assumption was made that STS neurons show repetition suppression but without any physiological evidence to support this claim. Here we provide direct supporting evidence for such claim.
Factors Determining Adaptation Effects for Visual Action Stimuli
Several factors can reduce repetition suppression for a hand action: 1) the interaction between the presence of the hand and the object, the change of the 2) hand shape or 3) kinematics during the course of the action, and 4) an increase of the effective delay. The lack of a significant difference between the repetition suppression for the movies in which the object was present and the miming condition suggests that the presence of the object did not affect the adaptation effect, ruling out the first factor. The change of hand shape or kinematics may have reduced the adaptation effect. Indeed, although statistically significant, the degree of repetition suppression that we observed here for the hand actions appears to be smaller than those obtained for static stimuli in inferior temporal cortex, and this could be related to a disadaptation because of the presence of other snapshots or snippets between the action phases that drive the repetition suppression.
Responses of Upper Bank STS Neurons to Approaching Phase of a Hand Action
A striking feature of the present data, but unrelated to adaptation per se, was the predominant response of the upper bank STS neurons to the approaching phase of the hand action. This response pattern was observed in each monkey and when the hand started to move in either the lower contra- or ipsilateral hemifield. One explanation of this response pattern is a strong bias of upper bank STS neurons for centripetal motion. Rostral upper bank STS neurons are known to respond well to motion (Anderson and Siegel 1999; Bruce et al. 1981; Oram et al. 1993), with a preference for motion patterns more complex than translation (Anderson and Siegel 1999). The minority of rostral STS neurons that show direction selectivity for translation motion prefer the cardinal directions such as up or down (Anderson and Siegel 1999; Oram et al. 1993) but without any bias for upward motion (Anderson and Siegel 1999). Furthermore, most rostral STS neurons prefer expanding optic flow patterns (Anderson and Siegel 1999), which is opposite to the centripetal motion pattern postulated here to explain the response preference for the approaching phase of the hand action. Thus the cause of the latter response pattern is still unclear and was not investigated further since it was not the objective of the present adaptation study.
Comparison of STS and F5 Adaptation Effects
We demonstrated repetition suppression in the upper bank STS by analyzing the responses during the approaching phase of the action. Caggiano et al. (2013) found no evidence of repetition suppression in F5 mirror neurons with the same stimuli repeated up to four times (see Fig. 3 of Caggiano et al. 2013). Indeed, inspection of their population PSTHs (Fig. 2 of Caggiano et al. 2013) shows no trend toward repetition suppression during the initial phase of the action—in fact, if anything, repetition enhancement was present. Kilner et al. (2014) reported a subtle suppression of the spiking activity in F5 mirror neurons when 7–10 stimulus repetitions were employed. However, because in that study the experimenter was performing live actions in front of the animal and no eye movements were measured, it is unclear whether the rather small repetition suppression in that study was not caused by subtle variations in the natural stimulus or eye movement patterns across repetitions.
What is the cause of the discrepancy between the presence of repetition suppression in the STS and its absence in F5 when using a single repetition? Before speculating on this, we should note that there are a couple of minor differences between our study and the Caggiano et al. (2013) F5 study. First, the pepper was presented for 840 ms before the onset of the hand action in the F5 study, while this time period was 340 ms in the present study. It is difficult to see how this longer presentation of the pepper before the action could have abolished the repetition suppression in F5. Second, Caggiano et al. employed a larger fixation window than the present study. Thus the lack of repetition suppression in the F5 study may have resulted from a contamination of the responses to the stimuli by eye movement-related signals. However, we feel that this is unlikely because the authors found no systematic differences in eye movement patterns between their S1 and S2, or any detectable influence of the eye position on the F5 neuron's responses.
Caggiano et al. (2013) speculated that the absence of repetition suppression in their F5 study resulted from adaptation of the input of both excitatory and inhibitory inputs to the pyramidal neurons they recorded from. Recent work in the V1 indeed suggests that changing the relative contribution of adaptation of the excitatory vs. inhibitory input to a neuron can shift the adaptation effect from suppression to enhancement (Patterson et al. 2013). However, it is unclear why the relative weights of the excitatory and inhibitory inputs would differ between F5 mirror neurons and STS neurons. The STS connects indirectly via parietal cortex with F5 (Nelissen et al. 2011). Such a polysynaptic connection, in addition to the intrinsic processing within F5, leaves room for transformations of the adapted STS output so that the repetition suppression is negated or even changed into a slight response enhancement. If true, this would provide a counterexample to the principle of the inheritance of adaptation effects along successive hierarchical processing stages (Patterson et al. 2014).
GRANTS
This work was supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0582.12N and G.00007.12-Odysseus; R. Vogels), Interuniversitaire Attractiepool (R. Vogels), Programma Financiering (PF 10/008; R. Vogels), and the European Community's Seventh Framework Programme FP7/2007–2013 under grant agreement number PITN-GA-2008-290011 (ABC; R. Vogels and M. Giese). V. Caggiano and M. Giese were supported by DFG GI 305/4-1. M. Giese was supported also by DFG GZ: KA 1258/15-1 and FP7-ICT-GA604102 (HBP), DFG GZ: KA 1258/15-1, and BMBF, FKZ: 01GQ1002A.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.K., V.C., and R.V. conception and design of research; P.K. performed experiments; P.K. analyzed data; P.K., V.C., M.G., and R.V. interpreted results of experiments; P.K. prepared figures; P.K. and R.V. drafted manuscript; P.K., V.C., M.G., and R.V. edited and revised manuscript; P.K., V.C., M.G., and R.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical assistance of P. Kayenbergh, G. Meulemans, I. Puttemans, M. De Paep, W. Depuydt, and S. Verstraeten is gratefully acknowledged.
REFERENCES
- Anderson KC, Siegel RM. Optic flow selectivity in the anterior superior temporal polysensory area, STPa, of the behaving monkey. J Neurosci 19: 2681–2692, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Biederman I. Localizing the cortical region mediating visual awareness of object identity. Proc Natl Acad Sci USA 96: 1790–1793, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough NE, Keith RH, Xiao D, Oram MW, Perrett DI. Visual adaptation to goal-directed hand actions. J Cogn Neurosci 21: 1805–1819, 2009. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol 46: 369–384, 1981. [DOI] [PubMed] [Google Scholar]
- Caggiano V, Pomper JK, Fleischer F, Fogassi L, Giese M, Thier P. Mirror neurons in monkey area F5 do not adapt to the observation of repeated actions. Nat Commun 4: 1433, 2013. [DOI] [PubMed] [Google Scholar]
- Caspari N, Popivanov ID, De Maziere PA, Vanduffel W, Vogels R, Orban GA, Jastorff J. Fine-grained stimulus representations in body selective areas of human occipito-temporal cortex. Neuroimage 102: 484–497, 2014. [DOI] [PubMed] [Google Scholar]
- Clifford CW. Perceptual adaptation: motion parallels orientation. Trends Cogn Sci 6: 136–143, 2002. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O. Visual adaptation: neural, psychological and computational aspects. Vision Res 47: 3125–3131, 2007. [DOI] [PubMed] [Google Scholar]
- De Baene W, Vogels R. Effects of adaptation on the stimulus selectivity of macaque inferior temporal spiking activity and local field potentials. Cereb Cortex 20: 2145–2165, 2010. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J Neurophysiol 98: 1415–1427, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 14: 770–785, 2013. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res 43: 1035–1045, 2003. [DOI] [PubMed] [Google Scholar]
- Fisher C, Freiwald W. Contrasting specializations for facial motion within the macaque face-processing system. Curr Biol 25: 261–266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10: 14–23, 2006. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Jardine NL, Pyles JA. fMR-adaptation reveals invariant coding of biological motion on human STS. Front Hum Neurosci 4: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J Neurosci 26: 1133–1137, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastorff J, Kourtzi Z, Giese MA. Visual learning shapes the processing of complex movement stimuli in the human brain. J Neurosci 29: 14026–14038, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H, Fallah M, Stoner GR. Adaptation of gender derived from biological motion. Nat Neurosci 9: 738–739, 2006. [DOI] [PubMed] [Google Scholar]
- Kable JW, Chatterjee A. Specificity of action representations in the lateral occipitotemporal cortex. J Cogn Neurosci 18: 1498–1517, 2006. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, De Baene W, Vogels R. Effects of adaptation on object representation accuracy in macaque inferior temporal cortex. J Cogn Neurosci 25: 777–789, 2013. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition probability does not affect repetition suppression in macaque inferior temporal cortex. Cereb Cortex 21: 1547–1558, 2011. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition affects both strength and synchrony of macaque inferior temporal cortical activity. J Neurophysiol 107: 3509–3527, 2012. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Neurons in macaque inferior temporal cortex show no surprise response to deviants in visual oddball sequences. J Neurosci 34: 12801–12815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Kraskov A, Lemon RN. Do monkey F5 mirror neurons show changes in firing rate during repeated observation of natural actions? J Neurophysiol 111: 1214–1226, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. J Neurosci 29: 10153–10159, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat Neurosci 7: 764–772, 2004. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci 29: 250–256, 2006. [DOI] [PubMed] [Google Scholar]
- Lingnau A, Gesierich B, Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proc Natl Acad Sci USA 106: 9925–9930, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychon Bull Rev 1: 476–490, 1994. [DOI] [PubMed] [Google Scholar]
- Majdandzic J, Bekkering H, van Schie HT, Toni I. Movement-specific repetition suppression in ventral and dorsal premotor cortex during action observation. Cereb Cortex 19: 2736–2745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R. Targeting the functional properties of cortical neurons using fMR-adaptation. Neuroimage 62: 1163–1169, 2012. [DOI] [PubMed] [Google Scholar]
- Muller KM, Schillinger F, Do DH, Leopold DA. Dissociable perceptual effects of visual adaptation. PLoS One 4: e6183, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen K, Borra E, Gerbella M, Rozzi S, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Action observation circuits in the macaque monkey cortex. J Neurosci 31: 3743–3756, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Esteky H. Neuronal correlates of view representation revealed by face-view aftereffect. J Neurosci 33: 5761–5772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram MW, Perrett DI, Hietanen JK. Directional tuning of motion-sensitive cells in the anterior superior temporal polysensory area of the macaque. Exp Brain Res 97: 274–294, 1993. [DOI] [PubMed] [Google Scholar]
- Patterson CA, Duijnhouwer J, Wissig SC, Krekelberg B, Kohn A. Similar adaptation effects in primary visual cortex and area MT of the macaque monkey under matched stimulus conditions. J Neurophysiol 111: 1203–1213, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CA, Wissig SC, Kohn A. Distinct effects of brief and prolonged adaptation on orientation tuning in primary visual cortex. J Neurosci 33: 532–543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C, Weiskopf N, Kilner JM. Dissociable roles of human inferior frontal gyrus during action execution and observation. Neuroimage 60: 1671–1677, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron 47: 457–470, 2005. [DOI] [PubMed] [Google Scholar]
- Solomon S, Kohn A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr Biol 24: R1012–R1022, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci 17: 722–734, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troje NF, Sadr J, Geyer H, Nakayama K. Adaptation aftereffects in the perception of gender from biological motion. J Vis 6: 850–857, 2006. [DOI] [PubMed] [Google Scholar]
- Vangeneugden J, De Maziere PA, Van Hulle MM, Jaeggli T, Van Gool L, Vogels R. Distinct mechanisms for coding of visual action in macaque temporal cortex. J Neurosci 31: 385–401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangeneugden J, Pollick F, Vogels R. Functional differentiation of macaque visual temporal cortical neurons using a parametric action space. Cereb Cortex 19: 593–611, 2009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Iliescu BF, Ma J, Josiç K, Dragoi V. Adaptive changes in neuronal synchronization in macaque V4. J Neurosci 31: 13204–13213, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissig SC, Kohn A. The influence of surround suppression on adaptation effects in primary visual cortex. J Neurophysiol 107: 3370–3384, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]